Abstract
Transcriptional regulation is fundamental to most biological processes and reverse-engineering programs can be used to decipher the underlying programs. In this review, we describe how genomics is offering a systems biology-based perspective of the intricate and temporally coordinated transcriptional programs that control neuronal apoptosis and survival. In addition to providing a new standpoint in human pathology focused on the regulatory program, cracking the code of neuronal cell fate may offer innovative therapeutic approaches focused on downstream targets and regulatory networks. Similar to computers, where faults often arise from a software bug, neuronal fate may critically depend on its transcription program. Thus, cracking the code of neuronal life or death may help finding a patch for neurodegeneration and cancer.
1. Introduction
A central focus in neurobiology and neurology is the study of neuronal survival and apoptosis, two processes that guarantee the appropriate development of the central nervous system (CNS) and its homeostatic maintenance throughout adulthood. During development, in fact, the brain produces more neurons that will eventually survive, and the interplay of pro-apoptotic and pro-survival signal is essential for sculpting the nervous system. Following this developmental window, inappropriate activation of these mechanisms can contribute to the development of pathological conditions, such as brain tumors and neurodegenerative diseases [1,2,3].
Similar to computers, where faults often arise from malfunctioning software, neuronal fate critically depends on its programs. The ability of neuronal cells to promote or evade apoptotic cell death, in fact, does not depend by the expression or activity of a single gene/protein (hardware), but is regulated by a transcriptional program that is activated by different extracellular signals, including the absence or presence of neurotrophic factors (NFs) [4].
Although several studies have investigated the contribution of individual genes at the crossroad of apoptosis and survival signaling pathways, the complex and coordinated temporal transcriptional programs that orchestrate neuronal cell fate decisions remain mostly unknown. Thus “cracking” the code of neuronal life or death may not only provide new insights into the sophisticated cellular and molecular events underlying these processes but, more importantly, may help finding new treatment strategies for neuronal degeneration and cancer.
The advances in the field of omics technologies, together with the increase in knowledge and research of computational analysis and modeling of neuronal networks are now offering a systems biology-based approach to experimentally interrogate the complexity of transcriptional programs controlling neuronal apoptosis and survival and their malfunctions, offering a novel strategy to modulate these cell states. In this review, we will describe how portraits of whole-genome expression analysis coupled to reverse engineering of regulatory networks are beginning to decode the complex transcriptional programs underlying neuronal cell fate and the implication of their perturbation in human pathology, which are paving the way to innovative therapeutic approaches focused on downstream targets and regulatory networks.
2. Systems Biology Approaches to Explore the Transcriptional Programs Underlying Neuronal Apoptosis and Survival
Neuronal turnover is not a static process and both survival and apoptosis rely on transcription. The condition for de novo gene expression and the activation of a “suicide” transcriptional program during neuronal apoptosis was postulated about four decades ago [5,6,7,8,9]. During the years, multiple genes or genetic pathways have been implicated in apoptosis; however, our inability to experimentally resolve and interrogate the full spectrum of genes operating in distinct temporal domains and their collective behavior has hampered the progress in this field and we had to wait the development of high-throughput technologies to begin its exploration from a system’s biology perspective. The advent of omics technologies has, in fact, dramatically revolutionized the current understanding of the molecular mechanisms mediating neuronal survival and apoptosis and the transition between these two cellular states in a variety of experimental paradigms [10,11,12,13,14,15,16].
Despite in vivo studies better reproduce human pathology, neurons undergo apoptosis asynchronously and it is difficult to time the sequential transcriptional changes of different cell states. On the other hand, in vitro models, although somewhat artificial, allow the study of transcriptional changes in homogeneous neuronal populations undergoing synchronous cell states. Over the past decades, a large number of studies reported whole-genome expression analysis in multiple in vitro paradigms of neuronal apoptosis, including cerebellar granule neurons (CGNs), as well as cortical and hippocampal neurons, implying the existence of universal transcriptional mechanisms regulating neuronal cell death [9,10,11,12,13,14,15,16,17,18,19,20,21]. Among these experimental paradigms, CGNs represent, both in vivo and in vitro, the election model for examining the signal transduction mechanisms underlying neuronal apoptosis and survival [5,6,9,20,22,23,24,25,26,27,28,29]. CGNs are the most abundant type of neurons in the mammalian brain and can be cultured in vitro up to 98% homogeneity. In vitro, CGNs undergo rapid and synchronous apoptotic cell death within 24 h after removal of serum and lowering of extracellular potassium concentration from 25 to 5 mM [9,30]. This cell death paradigm presumably mimics the naturally occurring death of 20–30% of granule cells, which is essential for harmonizing their number with Purkinje cells between the third and fifth week postnatally, whereas its pathological counterpart causes an in vivo lesion model of deafferentation in adult rats cerebellar cortex [31,32]. Apoptosis of CGNs requires transcription and protein synthesis and the process becomes irreversible throughout the first 6 hours succeeding its induction. Before this “commitment point”, apoptosis of CGNs can be rescued following the activation of specific signal transduction pathways or by the administration of NFs [9,30]. Over the past 20 years, our research groups and others have begun to explore the transcriptional changes during the pre-commitment phase of apoptosis and its rescue by different NFs [6,9,10,22,23,33,34,35,36,37,38,39,40,41,42]. Taken together, these studies highlighted that, although distinct NFs exert their survival effects by binding specific receptors and activating a plethora of intracellular second messengers, their signaling pathways share striking similarities and are propagated by common transcriptional cascades, suggesting the existence of a conserved transcriptional program at the intersection of apoptosis and survival.
Beside the evidence of a conserved transcriptional program, the key drivers of neuronal fate transitions remained enigmatic until we shifted our attention to transcriptional regulatory networks. In a recent work, we investigated the dynamic transcriptomic changes occurring during the early commitment phase (0.5 h, 1 h and 3 h) following CGNs apoptosis or their rescue by three different NFs (Pacap, Igf1 and SP). Overlapping these temporal transcriptome profiles, we identified a core set of genes (175 genes) exhibiting opposite mRNA expression trends during neuronal apoptosis and NFs-mediated rescue (Figure 1) [43]. Of note, this core set included both rapid and delayed transcriptional changes in response to NFs rescue (Figure 1) [43]. In particular, core set genes encoding proteins involved in transcriptional activity, neuronal proliferation and differentiation, exhibited rapid and transient transcriptional activation, suggesting their primary role in the immediate response to NF-treatment (Figure 1) [43]. Among these were genes known for their contribution in the regulation of neuronal development and survival (i.e., Pak4, Ntrk1, Twist2, Masp2, Hoxd9, Sstr2, Sstr3, Tep1, Tyrp1) (Figure 1) [44,45,45,46,47,48]. The p21 activated kinase Pak4 plays a pivotal role in neuronal pathophysiology, promoting the activity of transcription factors involved in cell survival, such as Akt and Cepbp and, thus, contributing to a wide range of intracellular processes including cytoskeletal dynamics, neuronal development, axonal outgrowth and neuronal survival [49,50,51]. Down-regulated expression of Pak4 has been associated with neurodegenerative disorders, including Amyotrophic Lateral Sclerosis and Parkinson’s disease, and it has been proposed as a therapeutic target against cancer and neurodegeneration [52,53,54,55]. Nerve growth factor receptor Ntrk1 promotes neuronal survival, proliferation and differentiation in neuronal populations, representing a clinically relevant target in neurology [56,57,58,59,60]. On the other hand, we also observed that delayed early response core set genes were, instead, enriched in different cellular processes, including cell adhesion, cytoskeleton organization, metabolic processes and oxidative damage, supporting their role as secondary effectors of the transcriptional program governing neuronal fate decision (Figure 1) [43].
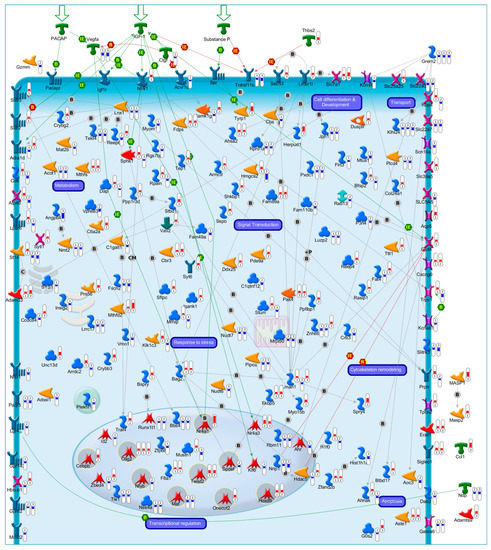
Figure 1.
Transcriptional Profiles of Cell Fate Transitions Reveal Early Drivers of Neuronal Apoptosis and Survival. In our recent work [43], we identified a core set of 175 genes exhibiting a significant opposite expression trend during the early commitment phase (0.5 h, 1 h and 3 h) of CGNs apoptosis or its rescue by three NFs (Pacap, Igf1 and SP). The illustrative map shows the biological function and sub-cellular localization of the encoded proteins of core set genes. Significant gene expression changes are shown with “thermometer-like” figures. Numbers indicate time points: ① 0.5 h, ② 1 h, and ③ 3 h following induction of CGNs apoptosis and rescue by NFs. For each time-point, the upward thermometers (red) indicate gene transcripts up-regulated by NFs treatment, while downward thermometers (blue) indicate genes down-regulated. The pathway map was created using MetaCore Pathway Map Creator tool (GeneGo). Further explanations are provided at https://portal.genego.com/legends/MetaCoreQuickReferenceGuide.pdf. (accessed on 14 February 2023).
Overall, these findings represent early portraits of the complex and coordinated temporal transcriptional programs underlying apoptosis and its rescue by NFs, further supporting the existence of a conserved transcriptional program governing neuronal life or death.
3. Apoptosis/Survival Switch and Human Diseases: At the Crossroads between Cancer and Neurodegenerative Diseases
As previously mentioned, while elimination of superfluous neuronal cells is essential for normal brain development, dysfunctions in the mechanisms leading to neuronal apoptosis or survival may play a role in different brain pathological conditions, including ischemia, cancer, neurodegenerative and neuropsychiatric disorders. In particular, defects in apoptotic cell death may promote development of brain cancers, while a significant increase in neuronal loss is associated with various psychiatric and neurodegenerative diseases, supporting these conditions may be considered the flip sides of the same coin and may derive from perturbations of the same regulatory mechanisms [4,61,62,63,64,65,66,67,68,69,70,71,72]. In light of these premises, it appeared evident that investigating molecular mechanisms regulating neuronal cell death or survival can be fundamental to explore the pathogenesis underlying pathological conditions and drive the development of targeted therapies.
To better investigate this aspect, in our previous work, we evaluated the clinical relevance of core set genes involved with CGNs apoptosis and survival [43]. Disease enrichment analysis revealed the core set genes may be relevant in human pathology since genetic defects in most of them (121/175) have been associated with different human diseases (Figure 2A) [43]. Of particular interest is the significant association of a group of core set genes with cognitive/mental diseases (anxiety, attention deficit, schizophrenia, bipolar disorder, depressive syndrome and disruptive behavior disorders), supporting that a dysregulation of these apoptotic-related genes may contribute to the pathophysiology of these disorders (Figure 2A,B) [43,65,73,74,75]. Among these genes were key transcriptional regulators (e.g., Ahr, Id2, Nr4a1, Nr4a3, Olig2, Zbed4), sustaining previous evidence that several severe cognitive disorders are associated with alterations in transcriptional regulatory activity [76,77,78] (Figure 2B).
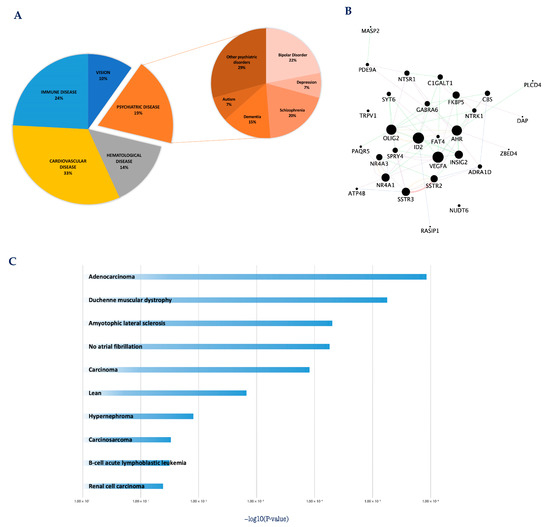
Figure 2.
Core set genes are involved in the etio-pathogenesis of multiple cancer and neurological disorders. (A) Pie chart representation of core set genes implicated in human diseases. Disease enrichment analysis was performed with DAVID bioinformatics resources, including OMIM, KEGG DISEASE, and GAD catalogs. (B) The protein–protein interaction network of the 29 core set genes previously associated with cognitive/mental diseases. The network was built using the STRING website and visualized by Cytoscape (version: 3.8.2), by mapping the ‘degree parameter’ to node size. As the node size increased, the value of the connectivity degree of node genes increased. Differently colored ‘edges’ indicate the type of evidence supporting each interaction: dark purple: co-expression; light purple: physical interaction; light blue: co-localization; light green: shared protein domain; orange: predicted; grey: other. (C) Histogram of the most significantly enriched transcriptional signatures from iLINCS positively correlated with apoptotic CGN-related expression changes of core set genes. The significance of each disease related signature is represented by the enrichment scores value (−log10 (p-value)). For more details, please refer to the original work [43].
The potential contribution of core set genes in human pathology was further explored by directly matching the transcriptional changes of core set genes with disease-specific gene expression signatures included in the integrative Library of Integrated Network-Based Cellular Signatures (iLINCS, http://www.ilincs.org/ilincs/signatures/main) (accessed on 29 March 2023) [43]. This integrative web-based platform facilitates mining and re-analysis of user-submitted omics signatures in the context of a large collection of pre-computed disease signatures [79]. Our in silico analysis showed that core set expression patterns overlapped with transcriptional signatures associated with different human diseases, including various types of cancers and neurodegenerative disorders (Figure 2C), further supporting these two disease categories may derive from perturbations of the same regulatory mechanisms and can be considered the flip sides of the same coin [43]. Taken together, these data support the possibility that the early transcription changes associated with CGNs apoptosis and survival may be conserved in other different cells, tissues and species, thus sustaining the existence of a universal program governing cellular life-and-death processes.
Given the implication of core set genes in human pathology, our findings open the possibility to identify new or already existent therapeutics that are able to modulate their activity. To this regard, we performed a transcriptional signature connectivity analysis in iLINCS to explore repurposing drugs that could revert the expression of the core set genes during neuronal apoptosis, representing putatively therapeutically useful candidates [43]. iLINCS, in fact, also includes a comprehensive large-scale drug perturbation databases containing transcriptomic profiles of dozens of cultivated cell lines treated with thousands of chemical compounds serving as reference databases. By overlapping these drug perturbation signatures with the expression patterns of our apoptotic-related gene set, we identified candidate repurposable drugs that may reverse apoptosis (Table 1) [43]. Of note, almost all the perturbagens we have found are established neuroprotective entities [43]. Taken together, this evidence further supports the implication of the core set genes in human pathology and highlight the utility of their perturbation as a therapeutical strategy.

Table 1.
List of the most enriched “repurposable” drug candidates with a potential to reverse apoptotic CGNs transcriptomic signature.
4. Cracking the Transcriptional Regulatory Programs of Neuronal Cell Fate May Orient New Therapeutic Strategies
Until today, different therapeutic strategies aimed at controlling neuronal apoptosis and survival have targeted the input (inducing signals) or output (executing machinery) underlying mechanisms, which can be considered the cellular “hardware”. Although many of these therapeutic strategies have been validated, most of them remain in the preclinical state because of lack of specificity and low efficacy [80]. An example is the use of NFs, whose therapy potential is hampered by the difficulty in delivering these proteins to the CNS and limiting their unwanted pleiotropic effects [81,82,83,84,85,86,87].
Similar to computers, where most of the problems commonly arise from buggy software, our neurons may also deal with malfunctions in the transcription regulatory program. Thus, cracking the code of neuronal fate may elicit novel pharmacological strategies, no longer oriented to the cellular hardware but, rather, the nuclear transcriptional regulatory mechanisms. Reconstruction of this cellular program by “reverse engineering” of gene regulatory networks (GRNs) poses great opportunities in systems biology [88,89,90,91,92,93,94,95,96,97] and allows to build accurate models of physiological and pathological processes, including those implicated in neuronal fate and development [98,99,100,101,102,103]. The impact of using these gene regulatory models to understand human diseases and find new treatments is profound, since they may allow to identify disease driver genes and promising biomarkers and therapeutic targets more efficiently and accurately [99,103,104,105,106].
Recently, we applied a “reverse engineering” method to identify candidate upstream regulators of early transcriptional changes observed following induction of CGNs apoptosis and its rescue by NFs [43]. In particular, we performed an in silico analysis to predict transcription factors (TFs) whose binding motifs are enriched in the promoter regions of core set genes [43]. Our analysis revealed that temporally distinct modules of core set genes are regulated by the coordinated action of nine TFs (Hoxd9, Maf, Nr4a1, Cebpb, Olig2, Onecut2, Spdef, Twist2, Nfyb) that may act as upstream regulators of neuronal cell fate, converging apoptosis and survival-inducing signals in a highly interconnected and temporally ordered manner (Figure 3) [43]. In particular, these results showed a high degree of cross-regulation among the nine TFs as well as a common early (0.5 h and 1 h) and transient peak of transcription for the almost all TFs, with the exception of Onecut2 that was activated after 3 h following NF treatment (Figure 3). Of note, some of these transcription factors encode previously tested molecular/pharmacological targets and their exploitation may interfere with the early stages of the apoptotic/survival transcriptional program and represent novel therapeutic strategies [43]. In the following paragraphs, we will discuss these master regulators in light of their potential role as therapeutic targets for neurological disorders.
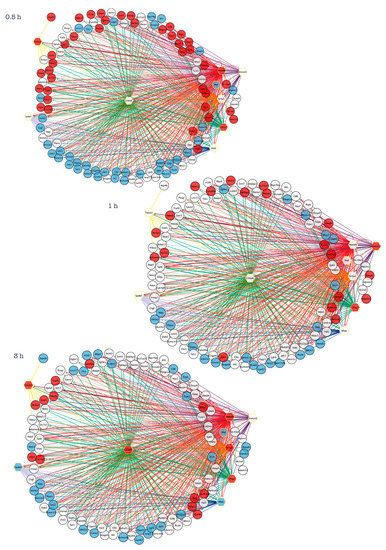
Figure 3.
Reverse-engineering of transcriptional changes identifies key transcription factors at the intersection of neuronal apoptosis and survival. Inferring transcriptional gene regulatory networks of core set genes identify nine transcription factors (Hoxd9, Maf, Nr4a1, Cebpb, Olig2, Onecut2, Spdef, Twist2, Nfyb), which may act as upstream regulators of neuronal cell fate [43]. Transcription regulatory network analysis generated at each time point (0.5 h, 1 h, 3 h) following induction of apoptosis or rescue by NFs emphasizes how temporally distinct apoptosis and survival-inducing signals are orchestrated by the action of interconnected and temporally ordered TFs. Regulatory networks are visualized by Cytoscape and for each time-point the node color is consistent with the expression logFC of each gene: genes in blue are down-regulated by NFs treatment, while genes in red are up-regulated. Transcription factors are represented as hexagon nodes, while gene targets are represented as circle nodes. Regulons for each transcription factor are represented by different edge colors.
4.1. Homeobox D9 (Hoxd9)
Hoxd9 belongs to an evolutionarily conserved family of homeodomain-containing transcription factors that plays an important role during development of the central nervous system and continue to be expressed into adulthood [107,108,109,110]. Following their initial discovery, a substantial amount of information has been gained regarding the roles Hox genes play in various physiologic and pathologic processes, including brain cancer and neurological disorders, suggesting their molecular/pharmacological modulation as a potential strategy for therapies of complex human disorders [109,111,112]. The importance of Hoxd9 as master regulator of neuronal cell fate is highlighted by its early transcriptional activation following NF-mediated rescue (Figure 1 and Figure 3) [43]. In accordance with these results, previous studies have demonstrated that Hoxd9 regulates the expression of several genes involved in neuronal apoptosis, displaying increased expression in unfavorable brain tumors, whereas its loss of function causes defects in axonal targeting and reduction in neural cell numbers, suggesting its utility as a potential therapeutic target at the crossroads between neurodegeneration and cancer [111,113,114,115]. To this regard, siRNA-induced silencing of Hoxd9 gene has been already employed to induce apoptosis in different types of brain tumors, including neuroblastoma [116,117].
4.2. Nuclear Receptor 4A1 (Nr4a1)
Nr4a orphan nuclear receptor are a family of transcription factors that are rapidly and strongly up-regulated in response to a diverse range of signals, including growth factors, cytokines, membrane depolarization, oxidative stress and excitotoxic insults to the central nervous system, which up-regulate neuroprotective genes and improve neuronal survival [118,119,120,121]. Nr4a sub-family members are categorized as early-response genes, are robustly induced in the CNS by pathological stimuli such as ischemia, seizures and focal brain injury and have pleiotropic physiological roles, including maintenance of neuronal integrity, regulation of the density and distribution of spines and synapses, suppression of apoptosis and induction of pro-survival genes [119,120,122]. Among the key components of this TF family is Nr4a1, whose gene expression levels were reverted (up-regulated) following NFs-induced rescue effects and that we found involved in the transcriptional regulation of a large number of core set genes, including other TFs (Maf, Nfyb and Spdef), implicated in system development, regulation of apoptotic process, chemotaxis and metabolic process (Figure 1 and Figure 3) [43]. In particular, as an immediate-early gene, Nr4a1 modulates cell fate by controlling mitochondrial functions and synaptic activity in response to a variety of stressors and sensory stimuli [123,124]. Notably, a marked decrease of Nr4a1 was associated with a variety of neurological conditions, including Alzheimer’s and Parkinson’s diseases, and its pharmacological activation exerts neuroprotective, anti-inflammatory and pro-survival effects, proposing Nr4a1 as a potential therapeutic target for multiple neurological disorders [120,122,123,125,126,127,128,129,130,131,132,133,134]. Within this context, previous studies have provided evidence for the implication of nuclear receptors (i.e., Nr4a1 and Nr4a3) in schizophrenia and bipolar disorders, demonstrating that down-regulated expression levels or sequence variations correlate with increased susceptibility to these cognitive disorders (Figure 2) [126,127,135,136].
4.3. Musculoaponeurotic Fibrosarcoma (Maf)
Maf (also known as c-Maf or v-Maf) is a member of a large group of b-Zip proteins that form a complex regulatory network, acting either as transcriptional activators or repressors of multiple cellular genes involved in immune response, apoptosis as well as neuronal outgrowth, maintenance and differentiation [19,109,122]. Despite little is known about the specific role of Maf in neuronal apoptosis and degeneration, recent reports suggest that over-expression of this gene can cause cell death probably through a p53-mediated signaling [122,137]. In agreement with these results, we found an early and transient peak of transcription for Maf following induction of CGNs apoptosis, while its expression decreased following treatment with NFs (Figure 1 and Figure 3) [43]. As other oxidative stress reactive proteins, Maf has been implicated in various neurological disorders, including Alzheimer’s and Parkinson’s diseases, and its role is emerging as a novel target in the treatment of these disorders [138,139,140].
4.4. CCAAT Enhancer Binding Protein Beta (Cebpb)
Cebpb encodes a basic-leucine zipper transcription factor that plays pivotal roles in development and synaptic plasticity of the nervous system, regulating the expression of genes involved in cell differentiation, neuronal development, immune response, neuronal apoptosis and metabolism [141,142,143,144,145,146]. In accordance with our study showing a transient activation of Cebpb during NFs-mediated rescue from apoptosis [43], previous studies demonstrated that up-regulation of this TF in rat primary cortical and cerebellar neuronal cultures plays neuroprotective and antiapoptotic effects, while its reduced neuronal levels may represent a pathogenic factor in neurodegenerative disorders, including Alzheimer’s and Parkinson’s diseases, supporting the potential of Cebpb as a pharmacological target in brain injury and neurodegenerative disorders (Figure 1 and Figure 3) [141,147,148,149,150,151,152].
4.5. Oligodendrocyte Transcription Factor2 (Olig2)
Among the enriched TFs in up-regulated core set genes, we identified the basic-helix-loop-helix (bHLH) transcription factor Olig2 that plays a key role in directing cell fate choices, promoting cell proliferation and controlling CNS development [43,153,154]. Several studies, in fact, have demonstrated that activation of Olig2 in response to different NFs (e.g., FGF, GDNF and PDGF) exerts protective and pro-survival effects in multiple neuronal types [155,156,157,158]. According with these findings, we observed an increased expression of Olig2 during CGNs rescue by Pacap, Igf1 and SP (Figure 1 and Figure 3) [43]. Despite the role elicited by Olig2 in the adult cerebral cortex under pathological conditions is not yet known, its reduced expression seems to switch cell fate from differentiation to death, contributing to the development of psychiatric disorders and acute/chronic neurodegenerative diseases, including Alzheimer’s disease and Amyotrophic Lateral Sclerosis, while its increased expression has been associated with different brain tumors, supporting its potential utility as therapeutic target for the treatment of both cancer and neurodegeneration [159,160,161,162,163,164,165,166,167,168,169,170,171]. Of note, several evidence showed that Olig2 deficiency as well as the presence of rare genetic polymorphisms in this gene (i.e., rs1059004) may represent risk factors for cognitive disorders and schizophrenia, through an effect on neuroanatomical connectivity (Figure 2) [159,164,172,173,174,175,176].
4.6. One Cut Homeobox 2 (Onecut2)
Onecut2 encodes a member of a family of transcription factors that function as transcriptional activators controlling cell differentiation and survival, as well as oxidative defense signaling, and that have only been recently proposed as regulators of neuronal differentiation [177,178,179,180,181,182,183,184]. Showing a consistent increased expression of this TF in CGNs during NF-mediated rescue, our results support the importance of Onecut2 for neuronal survival (Figure 1 and Figure 3) [43]. Although the expression of this factor is dysregulated in different types of tumors or following hypoxic insult to neurons, future studies are needed to further investigate the utility of Onecut2 as a target for brain tumors or neurodevelopmental disorders.
4.7. SAM Pointed Domain Containing ETS Transcription Factor (Spdef)
Spdef is an ETS (E26 transformation-specific) transcription factor, highly expressed in the prostate but also expressed in the brain and liver, where it regulates cellular differentiation, proliferation, cell-cycle control and apoptosis [185]. We observed an increased expression of Spdef throughout the time-course of CGNs apoptosis and its down-regulation following NFs treatment (Figure 1 and Figure 3) [43]. This evidence supports previous studies proposing its role as a tumor suppressor in various types of cancers [186,187]. In addition, altered expression of SPDEF has been found in Alzheimer’s disease patients and animal models, while a recent association was found between blood-based Spdef methylation and stress response, altered dopaminergic neurotransmission and increased vulnerability to substance abuse, suggesting its role as a biomarker for these pathological conditions [188,189,190,191].
4.8. Nuclear Transcription Factor Y Subunit Beta (Nfyb)
According to our recent findings showing increased expression of Nfyb during CGNs apoptosis [43], previous works demonstrated that its induction promotes neuronal apoptosis via the proapoptotic protein Bim and the activation of the p53 signaling pathway (Figure 1 and Figure 3) [192,193,194]. Nfyb regulates transcription of several genes that are related to cell cycle and its alterations contribute to neurodegeneration and apoptosis [195,196].
4.9. Twist Family BHLH Transcription Factor 2 (Twist2)
Twist2 is a highly conserved member of the Twist subfamily of basic Helix-Loop-Helix (bHLH) transcription factors that have been implicated in the transcriptional regulation of developmental programs in multiple cell lineages, and that are known to play important roles in cell migration, inflammation, protection of cells from apoptosis, and cellular response to oxidative stress [197,198,199]. According with the anti-apoptotic, anti-oxidative and anti-inflammatory effects of this TF, we observed its increased expression following NF-induced neuronal apoptotic rescue (Figure 1 and Figure 3) [43]. From a clinical point of view, Twist2 is upregulated in a variety of cancers, including glioma and neuroblastoma [181]. In addition, Twist2 dysfunctions are associated with human pathological conditions characterized by oxidative stress-induced neuronal death, supporting its potential role as therapeutical target for cancer and neurological diseases [199,200,200,201,202,203].
5. Conclusions
In this review, we highlighted how whole-genome gene expression analysis coupled to reverse engineering of gene regulatory networks are beginning to decode the complex transcriptional programs underlying neuronal apoptosis and survival [43]. This new experimental approach may foster an innovative pharmacology no longer oriented to influence the cellular hardware but focused on its regulatory transcriptional program.
Author Contributions
Conceptualization, writing—original draft preparation, G.M. and S.C.; review and editing, G.M., V.L.C., M.G., V.D. and S.C.; supervision, SC. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the IRIB-CNR project “A multi-omics approach for the study of neurodegeneration” (grant number: DSB.AD007.304 to S.C).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors gratefully acknowledge Cristina Calì, Alfia Corsino, Maria Patrizia D’Angelo and Francesco Marino for administrative and technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mattson, M.P. Apoptosis in neurodegenerative disorders. Nat. Rev. Mol. Cell Biol. 2000, 1, 120–129. [Google Scholar] [CrossRef]
- Datta, A.; Sarmah, D.; Mounica, L.; Kaur, H.; Kesharwani, R.; Verma, G.; Veeresh, P.; Kotian, V.; Kalia, K.; Borah, A.; et al. Cell Death Pathways in Ischemic Stroke and Targeted Pharmacotherapy. Transl. Stroke Res. 2020, 11, 1185–1202. [Google Scholar] [CrossRef] [PubMed]
- Erekat, N.S. Apoptosis and its therapeutic implications in neurodegenerative diseases. Clin. Anat. 2022, 35, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Neuronal Life-and-Death Signaling, Apoptosis, and Neurodegenerative Disorders. Antioxid. Redox Signal. 2006, 8, 1997–2006. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Yamada, M.; Nishio, C.; Konishi, A.; Hatanaka, H. SNRK, a member of the SNF1 family, is related to low K(+)-induced apoptosis of cultured rat cerebellar granule neurons. Brain Res. 2000, 873, 274–282. [Google Scholar] [CrossRef]
- Galli, C.; Meucci, O.; Scorziello, A.; Werge, T.M.; Calissano, P.; Schettini, G. Apoptosis in cerebellar granule cells is blocked by high KCl, forskolin, and IGF-1 through distinct mechanisms of action: The involvement of intracellular calcium and RNA synthesis. J. Neurosci. 1995, 15, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Valenzulela, E.; Gorczyca, W.; Darzynkiewicz, Z.; Sharma, S.C. Apoptosis in adult retinal ganglion cells after axotomy. J. Neurobiol. 1994, 25, 431–438. [Google Scholar] [CrossRef]
- Martin, D.P.; Schmidt, R.E.; DiStefano, P.S.; Lowry, O.H.; Carter, J.G.; Johnson, E.M. Inhibitors of protein synthesis and RNA synthesis prevent neuronal death caused by nerve growth factor deprivation. J. Cell Biol. 1988, 106, 829–844. [Google Scholar] [CrossRef]
- D’Mello, S.R.; Galli, C.; Ciotti, T.; Calissano, P. Induction of apoptosis in cerebellar granule neurons by low potassium: Inhibition of death by insulin-like growth factor I and cAMP. Proc. Natl. Acad. Sci. USA 1993, 90, 10989. [Google Scholar] [CrossRef]
- Cavallaro, S. Cracking the code of neuronal apoptosis and survival. Cell Death Dis. 2015, 6, e1963. [Google Scholar] [CrossRef]
- Cavallaro, S. Neuronal apoptosis revealed by genomic analysis: Integrating gene expression profiles with functional information. Neuroinformatics 2007, 5, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, S.; Calissano, P. A Genomic Approach to Investigate Neuronal Apoptosis. Curr. Alzheimer Res. 2006, 3, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, S.; D’Agata, V.; Alessi, E.; Coffa, S.; Alkon, D.L.; Manickam, P.; Ciotti, M.T.; Possenti, R.; Bonini, P.; Marlier, L.; et al. Gene expression profiles of apoptotic neurons. Genomics 2004, 84, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Paratore, S.; Parenti, R.; Torrisi, A.; Copani, A.; Cicirata, F.; Cavallaro, S. Genomic profiling of cortical neurons following exposure to β-amyloid. Genomics 2006, 88, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Hetmańczyk-Sawicka, K.; Iwanicka-Nowicka, R.; Fogtman, A.; Cieśla, J.; Włodarski, P.; Żyżyńska-Granica, B.; Filocamo, M.; Dardis, A.; Peruzzo, P.; Bednarska-Makaruk, M.; et al. Changes in global gene expression indicate disordered autophagy, apoptosis and inflammatory processes and downregulation of cytoskeletal signalling and neuronal development in patients with Niemann–Pick C disease. Neurogenetics 2020, 21, 105–119. [Google Scholar] [CrossRef]
- Ułamek-Kozioł, M.; Czuczwar, S.J.; Kocki, J.; Januszewski, S.; Bogucki, J.; Bogucka-Kocka, A.; Pluta, R. Dysregulation of Autophagy, Mitophagy, and Apoptosis Genes in the CA3 Region of the Hippocampus in the Ischemic Model of Alzheimer’s Disease in the Rat. J. Alzheimer’s Dis. 2019, 72, 1279–1286. [Google Scholar] [CrossRef]
- Calissano, P.; Matrone, C.; Amadoro, G. Apoptosis and in vitro Alzheimer’s disease neuronal models. Commun. Integr. Biol. 2009, 2, 163–169. [Google Scholar] [CrossRef]
- Parenti, R.; Paratore, S.; Torrisi, A.; Cavallaro, S. A natural antisense transcript against Rad18, specifically expressed in neurons and upregulated during β-amyloid-induced apoptosis. Eur. J. Neurosci. 2007, 26, 2444–2457. [Google Scholar] [CrossRef]
- Estus, S.; Tucker, H.M.; Van Rooyen, C.; Wright, S.; Brigham, E.F.; Wogulis, M.; Rydel, R.E. Aggregated Amyloid-β Protein Induces Cortical Neuronal Apoptosis and Concomitant “Apoptotic” Pattern of Gene Induction. J. Neurosci. 1997, 17, 7736. [Google Scholar] [CrossRef]
- Lossi, L.; Gambino, G. Apoptosis of the cerebellar neurons. Histol. Histopathol. 2008, 23, 367–380. [Google Scholar] [CrossRef]
- Jung, C.G.; Uhm, K.O.; Miura, Y.; Hosono, T.; Horike, H.; Khanna, K.K.; Kim, M.J.; Michikawa, M. Beta-amyloid increases the expression level of ATBF1 responsible for death in cultured cortical neurons. Mol. Neurodegener. 2011, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Maino, B.; Ciotti, M.T.; Calissano, P.; Cavallaro, S. Transcriptional analysis of apoptotic cerebellar granule neurons following rescue by gastric inhibitory polypeptide. Int. J. Mol. Sci. 2014, 15, 5596–5622. [Google Scholar] [CrossRef] [PubMed]
- Maino, B.; D’Agata, V.; Severini, C.; Ciotti, M.T.; Calissano, P.; Copani, A.; Chang, Y.C.; Delisi, C.; Cavallaro, S. Igf1 and pacap rescue cerebellar granule neurons from apoptosis via a common transcriptional program. Cell Death Discov. 2015, 1, 15029. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.-M.; Paul, S.M. Cultured Cerebellar Granule Neurons as a Model of Neuronal Apoptosis. Apoptosis Tech. Protoc. 1997, 47–66. [Google Scholar] [CrossRef]
- Chiang, L.W.; Grenier, J.M.; Ettwiller, L.; Jenkins, L.P.; Ficenec, D.; Martin, J.; Jin, F.; DiStefano, P.S.; Wood, A. An orchestrated gene expression component of neuronal programmed cell death revealed by cDNA array analysis. Proc. Natl. Acad. Sci. USA 2001, 98, 2814–2819. [Google Scholar] [CrossRef]
- Austdal, L.P.E.; Mathisen, G.H.; Løberg, E.M.; Paulsen, R.E. Calcium-induced apoptosis of developing cerebellar granule neurons depends causally on NGFI-B. Int. J. Dev. Neurosci. 2016, 55, 82–90. [Google Scholar] [CrossRef]
- Fernández-Suárez, D.; Krapacher, F.A.; Andersson, A.; Ibáñez, C.F.; Kisiswa, L. MAG induces apoptosis in cerebellar granule neurons through p75NTR demarcating granule layer/white matter boundary. Cell Death Dis. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- De Luca, A.; Weller, M.; Fontana, A. TGF-β-Induced Apoptosis of Cerebellar Granule Neurons Is Prevented by Depolarization. J. Neurosci. 1996, 16, 4174. [Google Scholar] [CrossRef]
- Padmanabhan, J.; Park, D.S.; Greene, L.A.; Shelanski, M.L. Role of Cell Cycle Regulatory Proteins in Cerebellar Granule Neuron Apoptosis. J. Neurosci. 1999, 19, 8747–8756. [Google Scholar] [CrossRef]
- Contestabile, A. Cerebellar granule cells as a model to study mechanisms of neuronal apoptosis or survival in vivo and in vitro. Cerebellum 2002, 1, 41–55. [Google Scholar] [CrossRef]
- Williams, R.W.; Herrup, K. The Control of Neuron Number. Annu. Rev. 2003, 11, 423–453. [Google Scholar] [CrossRef] [PubMed]
- Borsello, T.; Di Luzio, A.; Ciotti, M.T.; Calissano, P.; Galli, C. Granule neuron DNA damage following deafferentation in adult rats cerebellar cortex: A lesion model. Neuroscience 1999, 95, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Maino, B.; Spampinato, A.G.; Severini, C.; Petrella, C.; Ciotti, M.T.; D’Agata, V.; Calissano, P.; Cavallaro, S. The trophic effect of nerve growth factor in primary cultures of rat hippocampal neurons is associated to an anti-inflammatory and immunosuppressive transcriptional program. J. Cell. Physiol. 2018, 233, 7178–7187. [Google Scholar] [CrossRef]
- Pattarawarapan, M.; Burgess, K. Molecular basis of neurotrophin-receptor interactions. J. Med. Chem. 2003, 46, 5277–5291. [Google Scholar] [CrossRef]
- Cavallaro, S.; Copani, A.; D’Agata, V.; Musco, S.; Petralia, S.; Ventra, C.; Stivala, F.; Travali, S.; Canonico, P.L. Pituitary adenylate cyclase activating polypeptide prevents apoptosis in cultured cerebellar granule neurons. Mol. Pharmacol. 1996, 50, 60–66. [Google Scholar] [PubMed]
- Amadoro, G.; Pieri, M.; Ciotti, M.T.; Carunchio, I.; Canu, N.; Calissano, P.; Zona, C.; Severini, C. Substance P provides neuroprotection in cerebellar granule cells through Akt and MAPK/Erk activation: Evidence for the involvement of the delayed rectifier potassium current. Neuropharmacology 2007, 52, 1366–1377. [Google Scholar] [CrossRef] [PubMed]
- Vaudry, D.; Gonzalez, B.J.; Basille, M.; Pamantung, T.F.; Fontaine, M.; Fournier, A.; Vaudry, H. The neuroprotective effect of pituitary adenylate cyclase-activating polypeptide on cerebellar granule cells is mediated through inhibition of the CED3-related cysteine protease caspase-3/CPP32. Proc. Natl. Acad. Sci. USA 2000, 97, 13390–13395. [Google Scholar] [CrossRef]
- Linseman, D.A.; Phelps, R.A.; Bouchard, R.J.; Le, S.S.; Laessig, T.A.; McClure, M.L.; Heidenreich, K.A. Insulin-Like Growth Factor-I Blocks Bcl-2 Interacting Mediator of Cell Death (Bim) Induction and Intrinsic Death Signaling in Cerebellar Granule Neurons. J. Neurosci. 2002, 22, 9287–9297. [Google Scholar] [CrossRef]
- Gleichmann, M.; Weller, M.; Schulz, J.B. Insulin-like growth factor-1-mediated protection from neuronal apoptosis is linked to phosphorylation of the pro-apoptotic protein BAD but not to inhibition of cytochrome c translocation in rat cerebellar neurons. Neurosci. Lett. 2000, 282, 69–72. [Google Scholar] [CrossRef]
- Paratore, S.; Teresa Ciotti, M.; Basille, M.; Vaudry, D.; Gentile, A.; Parenti, R.; Calissano, P.; Cavallaro, S. Gastric inhibitory polypeptide and its receptor are expressed in the central nervous system and support neuronal survival. Cent. Nerv. Syst. Agents Med. Chem. 2011, 11, 210–222. [Google Scholar] [CrossRef]
- Seaborn, T.; Masmoudi-Kouli, O.; Fournier, A.; Vaudry, H.; Vaudry, D. Protective effects of pituitary adenylate cyclase-activating polypeptide (PACAP) against apoptosis. Curr. Pharm. Des. 2011, 17, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Schulz, J.B.; Weller, M.; Klockgether, T. Potassium Deprivation-Induced Apoptosis of Cerebellar Granule Neurons: A Sequential Requirement for New mRNA and Protein Synthesis, ICE-Like Protease Activity, and Reactive Oxygen Species. J. Neurosci. 1996, 16, 4696–4706. [Google Scholar] [CrossRef] [PubMed]
- Morello, G.; Villari, A.; Spampinato, A.G.; La Cognata, V.; Guarnaccia, M.; Gentile, G.; Ciotti, M.T.; Calissano, P.; D’agata, V.; Severini, C.; et al. Transcriptional profiles of cell fate transitions reveal early drivers of neuronal apoptosis and survival. Cells 2021, 10, 3238. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, L.; Zhang, Z.; Qin, C.; Yang, B.; Ke, Y. TYRP1 Protects Against the Apoptosis and Oxidative Stress of Retinal Ganglion Cells by Binding to PMEL. Ocul. Immunol. Inflamm. 2022. [Google Scholar] [CrossRef]
- Mercurio, D.; Oggioni, M.; Fumagalli, S.; Lynch, N.J.; Roscher, S.; Minuta, D.; Perego, C.; Ippati, S.; Wallis, R.; Schwaeble, W.J.; et al. Targeted deletions of complement lectin pathway genes improve outcome in traumatic brain injury, with MASP-2 playing a major role. Acta Neuropathol. Commun. 2020, 8, 174. [Google Scholar] [CrossRef] [PubMed]
- Gangwani, K.; Snigdha, K.; Kango-Singh, M. Tep1 Regulates Yki Activity in Neural Stem Cells in Drosophila Glioma Model. Front. Cell Dev. Biol. 2020, 8, 306. [Google Scholar] [CrossRef] [PubMed]
- Srikant, C.B. Cell Cycle Dependent Induction of Apoptosis by Somatostatin Analog SMS 201-995 in AtT-20 Mouse Pituitary Cells. Biochem. Biophys. Res. Commun. 1995, 209, 400–406. [Google Scholar] [CrossRef]
- Stumm, R.K.; Zhou, C.; Schulz, S.; Endres, M.; Kronenberg, G.; Allen, J.P.; Tulipano, G.; Höllt, V. Somatostatin Receptor 2 Is Activated in Cortical Neurons and Contributes to Neurodegeneration after Focal Ischemia. J. Neurosci. 2004, 24, 11404. [Google Scholar] [CrossRef]
- Yu, X.; Huang, C.; Liu, J.; Shi, X.; Li, X. The significance of PAK4 in signaling and clinicopathology: A review. Open Life Sci. 2022, 17, 586–598. [Google Scholar] [CrossRef]
- Civiero, L.; Greggio, E. PAKs in the brain: Function and dysfunction. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 444–453. [Google Scholar] [CrossRef]
- Gnesutta, N.; Qu, J.; Minden, A. The Serine/Threonine Kinase PAK4 Prevents Caspase Activation and Protects Cells from Apoptosis. J. Biol. Chem. 2001, 276, 14414–14419. [Google Scholar] [CrossRef] [PubMed]
- Cong, C.; Liang, W.; Zhang, C.; Wang, Y.; Yang, Y.; Wang, X.; Wang, S.; Huo, D.; Wang, H.; Wang, D.; et al. PAK4 suppresses motor neuron degeneration in hSOD1G93A-linked amyotrophic lateral sclerosis cell and rat models. Cell Prolif. 2021, 54. [Google Scholar] [CrossRef] [PubMed]
- Pütz, S.M.; Kram, J.; Rauh, E.; Kaiser, S.; Toews, R.; Lueningschroer-Wang, Y.; Rieger, D.; Raabe, T. Loss of p21-activated kinase Mbt/PAK4 causes Parkinson-like phenotypes in Drosophila. Dis. Model. Mech. 2021, 14, dmm047811. [Google Scholar] [CrossRef] [PubMed]
- Won, S.Y.; Park, M.H.; You, S.T.; Choi, S.W.; Kim, H.K.; McLean, C.; Bae, S.C.; Kim, S.R.; Jin, B.K.; Lee, K.H.; et al. Nigral dopaminergic PAK4 prevents neurodegeneration in rat models of Parkinson’s disease. Sci. Transl. Med. 2016, 8, 367ra170. [Google Scholar] [CrossRef] [PubMed]
- Won, S.Y.; Park, J.J.; Shin, E.Y.; Kim, E.G. PAK4 signaling in health and disease: Defining the PAK4–CREB axis. Exp. Mol. Med. 2019, 51, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Arévalo, J.C.; Waite, J.; Rajagopal, R.; Beyna, M.; Chen, Z.Y.; Lee, F.S.; Chao, M.V. Cell Survival through Trk Neurotrophin Receptors Is Differentially Regulated by Ubiquitination. Neuron 2006, 50, 549–559. [Google Scholar] [CrossRef]
- Majdan, M.; Walsh, G.S.; Aloyz, R.; Miller, F.D. TrkA mediates developmental sympathetic neuron survival in vivo by silencing an ongoing p75NTR-mediated death signal. J. Cell Biol. 2001, 155, 1275. [Google Scholar] [CrossRef] [PubMed]
- Culmsee, C.; Gerling, N.; Lehmann, M.; Nikolova-Karakashian, M.; Prehn, J.H.M.; Mattson, M.P.; Krieglstein, J. Nerve growth factor survival signaling in cultured hippocampal neurons is mediated through TrkA and requires the common neurotrophin receptor p75. Neuroscience 2002, 115, 1089–1108. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, T.J.; Xiong, Z.Q. NGF-dependent retrograde signaling: Survival versus death. Cell Res. 2009, 19, 525–526. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Moheban, D.B.; Conway, B.R.; Bhattacharyya, A.; Segal, R.A. Cell surface Trk receptors mediate NGF-induced survival while internalized receptors regulate NGF-induced differentiation. J. Neurosci. 2000, 20, 5671–5678. [Google Scholar] [CrossRef]
- Moujalled, D.; Strasser, A.; Liddell, J.R. Molecular mechanisms of cell death in neurological diseases. Cell Death Differ. 2021, 28, 2029–2044. [Google Scholar] [CrossRef] [PubMed]
- Wolozin, B.; Behl, C. Mechanisms of neurodegenerative disorders: Part 2: Control of cell death. Arch. Neurol. 2000, 57, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhao, S.; Li, Y.; Zhang, D.; Wang, B.; Xie, J.; Wang, J. Regulated cell death: Discovery, features and implications for neurodegenerative diseases. Cell Commun. Signal. 2021, 19, 1–29. [Google Scholar] [CrossRef]
- Tatton, W.G.; Olanow, C.W. Apoptosis in neurodegenerative diseases: The role of mitochondria. Biochim. Biophys. Acta 1999, 1410, 195–213. [Google Scholar] [CrossRef] [PubMed]
- Uribe, E.; Wix, R. Neuronal migration, apoptosis and bipolar disorder. Rev. Psiquiatr. Salud Ment. 2012, 5, 127–133. [Google Scholar] [CrossRef]
- Karlović, D. Apoptosis—The potential pathophysiological mechanism in mood disorders modifiable by lithium salts. Biochem. Med. 2008, 18, 291–310. [Google Scholar] [CrossRef]
- Margolis, R.L.; Chuang, D.M.; Post, R.M. Programmed cell death: Implications for neuropsychiatric disorders. Biol. Psychiatry 1994, 35, 946–956. [Google Scholar] [CrossRef]
- Andreone, B.J.; Larhammar, M.; Lewcock, J.W. Cell Death and Neurodegeneration. Cold Spring Harb. Perspect. Biol. 2020, 12, a036434. [Google Scholar] [CrossRef]
- Chi, H.; Chang, H.Y.; Sang, T.K. Neuronal Cell Death Mechanisms in Major Neurodegenerative Diseases. Int. J. Mol. Sci. 2018, 19, 3082. [Google Scholar] [CrossRef]
- Yuan, J.; Yankner, B.A. Apoptosis in the nervous system. Nature 2000, 407, 802–809. [Google Scholar] [CrossRef]
- Gorman, A.M. Neuronal cell death in neurodegenerative diseases: Recurring themes around protein handling. J. Cell. Mol. Med. 2008, 12, 2263–2280. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, S.R.; Chin, P.C. Treating neurodegenerative conditions through the understanding of neuronal apoptosis. Curr. Drug Targets CNS Neurol. Disord. 2005, 4, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Parul; Mishra, A.; Singh, S.; Singh, S.; Tiwari, V.; Chaturvedi, S.; Wahajuddin, M.; Palit, G.; Shukla, S. Chronic unpredictable stress negatively regulates hippocampal neurogenesis and promote anxious depression-like behavior via upregulating apoptosis and inflammatory signals in adult rats. Brain Res. Bull. 2021, 172, 164–179. [Google Scholar] [CrossRef]
- Bachis, A.; Cruz, M.I.; Nosheny, R.L.; Mocchetti, I. Chronic Unpredictable Stress Promotes Neuronal Apoptosis in the Cerebral Cortex. Neurosci. Lett. 2008, 442, 104. [Google Scholar] [CrossRef] [PubMed]
- Jarskog, L.F.; Glantz, L.A.; Gilmore, J.H.; Lieberman, J.A. Apoptotic mechanisms in the pathophysiology of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 846–858. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, A.; Gan, L.; Bao, Y.; Zhu, W.; Hu, Y.; Ma, L.; Wei, S.; Lan, Y. Screening of Potential Genes and Transcription Factors of Postoperative Cognitive Dysfunction via Bioinformatics Methods. Med. Sci. Monit. 2018, 24, 503. [Google Scholar] [CrossRef]
- Santos-Terra, J.; Deckmann, I.; Fontes-Dutra, M.; Schwingel, G.B.; Bambini-Junior, V.; Gottfried, C. Transcription factors in neurodevelopmental and associated psychiatric disorders: A potential convergence for genetic and environmental risk factors. Int. J. Dev. Neurosci. 2021, 81, 545–578. [Google Scholar] [CrossRef]
- Johnston, M.V.; Alemi, L.; Harum, K.H. Learning, Memory, and Transcription Factors. Pediatr. Res. 2003, 53, 369–374. [Google Scholar] [CrossRef]
- Stathias, V.; Turner, J.; Koleti, A.; Vidovic, D.; Cooper, D.; Fazel-Najafabadi, M.; Pilarczyk, M.; Terryn, R.; Chung, C.; Umeano, A.; et al. LINCS Data Portal 2.0: Next generation access point for perturbation-response signatures. Nucleic Acids Res. 2020, 48, D431–D439. [Google Scholar] [CrossRef]
- Fischer, U.; Schulze-Osthoff, K. Apoptosis-based therapies and drug targets. Cell Death Differ. 2005, 12 (Suppl. 1), 942–961. [Google Scholar] [CrossRef]
- Razavi, S.; Nazem, G.; Mardani, M.; Esfandiari, E.; Salehi, H.; Esfahani, S.H.Z. Neurotrophic factors and their effects in the treatment of multiple sclerosis. Adv. Biomed. Res. 2015, 4, 53. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, O.; Saarma, M. Neurotrophic Factors in Parkinson’s Disease: Clinical Trials, Open Challenges and Nanoparticle-Mediated Delivery to the Brain. Front. Cell. Neurosci. 2021, 15, 178. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shehadah, A.; Pal, A.; Zacharek, A.; Cui, X.; Cui, Y.; Roberts, C.; Lu, M.; Zeitlin, A.; Hariri, R.; et al. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef]
- Padmakumar, S.; Taha, M.S.; Kadakia, E.; Bleier, B.S.; Amiji, M.M. Delivery of neurotrophic factors in the treatment of age-related chronic neurodegenerative diseases. Expert Opin. Drug Deliv. 2020, 17, 323–340. [Google Scholar] [CrossRef]
- Chmielarz, P.; Saarma, M. Neurotrophic factors for disease-modifying treatments of Parkinson’s disease: Gaps between basic science and clinical studies. Pharmacol. Rep. 2020, 72, 1195–1217. [Google Scholar] [CrossRef]
- Alfonsetti, M.; D’Angelo, M.; Castelli, V. Neurotrophic factor-based pharmacological approaches in neurological disorders. Neural Regen. Res. 2023, 18, 1220–1228. [Google Scholar] [CrossRef]
- Reagents for Therapeutic Targets of Neurodegenerative Diseases: Get Quote, RFQ, Price or Buy. Available online: https://www.news-medical.net/Reagents-for-therapeutic-targets-of-neurodegenerative-diseases (accessed on 9 February 2023).
- Saint-André, V. Computational biology approaches for mapping transcriptional regulatory networks. Comput. Struct. Biotechnol. J. 2021, 19, 4884–4895. [Google Scholar] [CrossRef] [PubMed]
- Zak, D.E.; Gonye, G.E.; Schwaber, J.S.; Doyle, F.J. Importance of Input Perturbations and Stochastic Gene Expression in the Reverse Engineering of Genetic Regulatory Networks: Insights from an Identifiability Analysis of an In silico Network. Genome Res. 2003, 13, 2396–2405. [Google Scholar] [CrossRef]
- Zoppoli, P.; Morganella, S.; Ceccarelli, M. TimeDelay-ARACNE: Reverse engineering of gene networks from time-course data by an information theoretic approach. BMC Bioinform. 2010, 11, 1–5. [Google Scholar] [CrossRef]
- Stolovitzky, G.; Monroe, D.; Califano, A. Dialogue on reverse-engineering assessment and methods: The DREAM of high-throughput pathway inference. Ann. N. Y. Acad. Sci. 2007, 1115, 1–22. [Google Scholar] [CrossRef]
- van der Sande, M.; Frölich, S.; van Heeringen, S.J. Computational approaches to understand transcription regulation in development. Biochem. Soc. Trans. 2023, 51, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, J.; Wurtmann, E.J.; Baliga, N.S. Reverse engineering systems models of regulation: Discovery, prediction and mechanisms. Curr. Opin. Biotechnol. 2012, 23, 598. [Google Scholar] [CrossRef] [PubMed]
- De Bastiani, M.A.; Pfaffenseller, B.; Klamt, F. Master regulators connectivity map: A transcription factors-centered approach to drug repositioning. Front. Pharmacol. 2018, 9, 697. [Google Scholar] [CrossRef] [PubMed]
- Carré, C.; Mas, A.; Krouk, G. Reverse engineering highlights potential principles of large gene regulatory network design and learning. NPJ Syst. Biol. Appl. 2017, 3, 17. [Google Scholar] [CrossRef]
- Perkel, J.M. Smart software untangles gene regulation in cells. Nature 2022, 609, 428–431. [Google Scholar] [CrossRef]
- Villaverde, A.F.; Banga, J.R. Reverse engineering and identification in systems biology: Strategies, perspectives and challenges. J. R. Soc. Interface 2014, 11, 20130505. [Google Scholar] [CrossRef]
- Vohradsky, J. Neural model of the genetic network. J. Biol. Chem. 2001, 276, 36168–36173. [Google Scholar] [CrossRef]
- De Cegli, R.; Iacobacci, S.; Flore, G.; Gambardella, G.; Mao, L.; Cutillo, L.; Lauria, M.; Klose, J.; Illingworth, E.; Banfi, S.; et al. Reverse engineering a mouse embryonic stem cell-specific transcriptional network reveals a new modulator of neuronal differentiation. Nucleic Acids Res. 2013, 41, 711–726. [Google Scholar] [CrossRef]
- Acquaah-Mensah, G.K.; Taylor, R.C. Brain in situ hybridization maps as a source for reverse-engineering transcriptional regulatory networks: Alzheimer’s disease insights. Gene 2016, 586, 77–86. [Google Scholar] [CrossRef]
- Petrovskiy, E.D.; Saik, O.V.; Tiys, E.S.; Lavrik, I.N.; Kolchanov, N.A.; Ivanisenko, V.A. Prediction of tissue-specific effects of gene knockout on apoptosis in different anatomical structures of human brain. BMC Genom. 2015, 16, S3. [Google Scholar] [CrossRef]
- Cheng, Y.; Yin, Y.; Zhang, A.; Bernstein, A.M.; Kawaguchi, R.; Gao, K.; Potter, K.; Gilbert, H.Y.; Ao, Y.; Ou, J.; et al. Transcription factor network analysis identifies REST/NRSF as an intrinsic regulator of CNS regeneration in mice. Nat. Commun. 2022, 13, 4418. [Google Scholar] [CrossRef]
- Lefebvre, C.; Rieckhof, G.; Califano, A. Reverse-engineering human regulatory networks. Wiley Interdiscip. Rev. Syst. Biol. Med. 2012, 4, 311. [Google Scholar] [CrossRef] [PubMed]
- Carro, M.S.; Lim, W.K.; Alvarez, M.J.; Bollo, R.J.; Zhao, X.; Snyder, E.Y.; Sulman, E.P.; Anne, S.L.; Doetsch, F.; Colman, H.; et al. The transcriptional network for mesenchymal transformation of brain tumours. Nature 2010, 463, 318–325. [Google Scholar] [CrossRef]
- Dusonchet, J.; Li, H.; Guillily, M.; Liu, M.; Stafa, K.; Derada Troletti, C.; Boon, J.Y.; Saha, S.; Glauser, L.; Mamais, A.; et al. A Parkinson’s disease gene regulatory network identifies the signaling protein RGS2 as a modulator of LRRK2 activity and neuronal toxicity. Hum. Mol. Genet. 2014, 23, 4887–4905. [Google Scholar] [CrossRef] [PubMed]
- Łastowska, M.; Al-Afghani, H.; Al-Balool, H.H.; Sheth, H.; Mercer, E.; Coxhead, J.M.; Redfern, C.P.F.; Peters, H.; Burt, A.D.; Santibanez-Koref, M.; et al. Identification of a neuronal transcription factor network involved in medulloblastoma development. Acta Neuropathol. Commun. 2014, 2, 35. [Google Scholar] [CrossRef]
- Fromental-Ramain, C.; Warot, X.; Lakkaraju, S.; Favier, B.; Haack, H.; Birling, C.; Dierich, A.; Dolle, P.; Chambon, P. Specific and redundant functions of the paralogous Hoxa-9 and Hoxd-9 genes in forelimb and axial skeleton patterning. Development 1996, 122, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Domsch, K.; Papagiannouli, F.; Lohmann, I. The HOX-Apoptosis Regulatory Interplay in Development and Disease. Curr. Top. Dev. Biol. 2015, 114, 121–158. [Google Scholar] [CrossRef]
- Bhatlekar, S.; Fields, J.Z.; Boman, B.M. Role of HOX genes in stem cell differentiation and cancer. Stem Cells Int. 2018, 2018. [Google Scholar] [CrossRef]
- Briscoe, J.; Wilkinson, D.G. Establishing neuronal circuitry: Hox genes make the connection. Genes Dev. 2004, 18, 1643–1648. [Google Scholar] [CrossRef]
- Gonçalves, C.S.; Le Boiteux, E.; Arnaud, P.; Costa, B.M. HOX gene cluster (de)regulation in brain: From neurodevelopment to malignant glial tumours. Cell. Mol. Life Sci. 2020, 77, 3797–3821. [Google Scholar] [CrossRef]
- Finch, N.A.; Wang, X.; Baker, M.C.; Heckman, M.G.; Gendron, T.F.; Bieniek, K.F.; Wuu, J.; Dejesus-Hernandez, M.; Brown, P.H.; Chew, J.; et al. Abnormal expression of homeobox genes and transthyretin in C9ORF72 expansion carriers. Neurol. Genet. 2017, 3, e161. [Google Scholar] [CrossRef]
- Goodman, F.R.; Majewski, F.; Collins, A.L.; Scambler, P.J. A 117-kb microdeletion removing HOXD9-HOXD13 and EVX2 causes synpolydactyly. Am. J. Hum. Genet. 2002, 70, 547–555. [Google Scholar] [CrossRef]
- Sneha, N.P.; Dharshini, S.A.P.; Taguchi, Y.H.; Gromiha, M.M. Integrative Meta-Analysis of Huntington’s Disease Transcriptome Landscape. Genes 2022, 13, 2385. [Google Scholar] [CrossRef]
- Kocak, H.; Ackermann, S.; Hero, B.; Kahlert, Y.; Oberthuer, A.; Juraeva, D.; Roels, F.; Theissen, J.; Westermann, F.; Deubzer, H.; et al. Hox-C9 activates the intrinsic pathway of apoptosis and is associated with spontaneous regression in neuroblastoma. Cell Death Dis. 2013, 4, e586. [Google Scholar] [CrossRef]
- Zhu, H.; Dai, W.; Li, J.; Xiang, L.; Wu, X.; Tang, W.; Chen, Y.; Yang, Q.; Liu, M.; Xiao, Y.; et al. HOXD9 promotes the growth, invasion and metastasis of gastric cancer cells by transcriptional activation of RUFY3. J. Exp. Clin. Cancer Res. 2019, 38, 412. [Google Scholar] [CrossRef]
- Tabuse, M.; Ohta, S.; Ohashi, Y.; Fukaya, R.; Misawa, A.; Yoshida, K.; Kawase, T.; Saya, H.; Thirant, C.; Chneiweiss, H.; et al. Functional analysis of HOXD9 in human gliomas and glioma cancer stem cells. Mol. Cancer 2011, 10, 60. [Google Scholar] [CrossRef]
- Kuert, P.A.; Hartenstein, V.; Bello, B.C.; Lovick, J.K.; Reichert, H. Neuroblast lineage identification and lineage-specific Hox gene action during postembryonic development of the subesophageal ganglion in the Drosophila central brain. Dev. Biol. 2014, 390, 102–115. [Google Scholar] [CrossRef]
- Volakakis, N.; Kadkhodaei, B.; Joodmardi, E.; Wallis, K.; Panman, L.; Silvaggi, J.; Spiegelman, B.M.; Perlmann, T. NR4A orphan nuclear receptors as mediators of CREB-dependent neuroprotection. Proc. Natl. Acad. Sci. USA 2010, 107, 12317–12322. [Google Scholar] [CrossRef]
- Ly, L.L.; Yoshida, H.; Yamaguchi, M. Nuclear transcription factor Y and its roles in cellular processes related to human disease. Am. J. Cancer Res. 2013, 3, 339–346. [Google Scholar]
- Herring, J.A.; Elison, W.S.; Tessem, J.S. Function of Nr4a Orphan Nuclear Receptors in Proliferation, Apoptosis and Fuel Utilization Across Tissues. Cells 2019, 8, 1373. [Google Scholar] [CrossRef]
- Hale, T.K.; Myers, C.; Maitra, R.; Kolzau, T.; Nishizawa, M.; Braithwaite, A.W. Maf transcriptionally activates the mouse p53 promoter and causes a p53- dependent cell death. J. Biol. Chem. 2000, 275, 17991–17999. [Google Scholar] [CrossRef]
- Jeanneteau, F.; Barrè, C.; Vos, M.; De Vries, C.J.M.; Rouillard, X.; Levesque, D.; Dromard, Y.; Moisan, M.-P.; Duric, V.; Franklin, T.C.; et al. The Stress-Induced Transcription Factor NR4A1 Adjusts Mitochondrial Function and Synapse Number in Prefrontal Cortex. J. Neurosci. 2018, 38, 1335–1350. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Ertürk, A.; Kallop, D.; Jiang, Z.; Weimer, R.M.; Kaminker, J.; Sheng, M. Activity-induced Nr4a1 regulates spine density and distribution pattern of excitatory synapses in pyramidal neurons. Neuron 2014, 83, 431–443. [Google Scholar] [CrossRef]
- Jakaria, M.; Haque, M.E.; Cho, D.-Y.; Azam, S.; Kim, I.-S.; Choi, D.-K. Molecular Insights into NR4A2(Nurr1): An Emerging Target for Neuroprotective Therapy Against Neuroinflammation and Neuronal Cell Death. Mol. Neurobiol. 2019, 56, 5799–5814. [Google Scholar] [CrossRef]
- Corley, S.M.; Tsai, S.Y.; Wilkins, M.R.; Weickert, C.S. Transcriptomic analysis shows decreased cortical expression of nr4a1, nr4a2 and rxrb in schizophrenia and provides evidence for nuclear receptor dysregulation. PLoS ONE 2016, 11, e0166944. [Google Scholar] [CrossRef]
- Tsai, S.-Y.; Catts, V.S.; Fullerton, J.M.; Corley, S.M.; Fillman, S.G.; Weickert, C.S. Nuclear Receptors and Neuroinflammation in Schizophrenia. Mol. Neuropsychiatry 2017, 3, 181–191. [Google Scholar] [CrossRef]
- Xiao, G.; Sun, T.; Songming, C.; Cao, Y. NR4A1 enhances neural survival following oxygen and glucose deprivation: An in vitro study. J. Neurol. Sci. 2013, 330, 78–84. [Google Scholar] [CrossRef]
- Rouillard, C.; Baillargeon, J.; Paquet, B.; St-Hilaire, M.; Maheux, J.; Lévesque, C.; Darlix, N.; Majeur, S.; Lévesque, D. Genetic disruption of the nuclear receptor Nur77 (Nr4a1) in rat reduces dopamine cell loss and L-Dopa-induced dyskinesia in experimental Parkinson’s disease. Exp. Neurol. 2018, 304, 143–153. [Google Scholar] [CrossRef]
- Zhao, L.G.; Tang, Y.; Tan, J.Z.; Wang, J.W.; Chen, G.J.; Zhu, B.L. The effect of NR4A1 on APP metabolism and tau phosphorylation. Genes Dis. 2018, 5, 342–348. [Google Scholar] [CrossRef]
- Bao, X.J.; Wang, G.C.; Zuo, F.X.; Li, X.Y.; Wu, J.; Chen, G.; Dou, W.C.; Guo, Y.; Shen, Q.; Wang, R.Z. Transcriptome profiling of the subventricular zone and dentate gyrus in an animal model of Parkinson’s disease. Int. J. Mol. Med. 2017, 40, 771–783. [Google Scholar] [CrossRef]
- Chatterjee, S.; Walsh, E.N.; Yan, A.L.; Giese, K.P.; Safe, S.; Abel, T. Pharmacological activation of Nr4a rescues age-associated memory decline. Neurobiol. Aging 2020, 85, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Tello, P.; Lin, H.; Khan, P.; de Vera, I.M.; Kamenecka, T.; Kojetin, D. Assessment of NR4A Ligands that Directly Bind and Modulate the Orphan Nuclear Receptor Nurr1. bioRxiv 2020. [Google Scholar] [CrossRef] [PubMed]
- Bridi, M.S.; Hawk, J.D.; Chatterjee, S.; Safe, S.; Abel, T. Pharmacological Activators of the NR4A Nuclear Receptors Enhance LTP in a CREB/CBP-Dependent Manner. Nat. Publ. Gr. 2016, 42, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Sánchez, E.; Jiménez-Genchi, J.; Alcántara-Flores, Y.M.; Castañeda-González, C.J.; Aviña-Cervantes, C.L.; Yescas, P.; del Socorro González-Valadez, M.; Martínez-Rodríguez, N.; Ríos-Ortiz, A.; González-González, M.; et al. Working memory deficits in schizophrenia are associated with the rs34884856 variant and expression levels of the NR4A2 gene in a sample Mexican population: A case control study. BMC Psychiatry 2021, 21, 86. [Google Scholar] [CrossRef] [PubMed]
- Novak, G.; Zai, C.C.; Mirkhani, M.; Shaikh, S.; Vincent, J.B.; Meltzer, H.; Lieberman, J.A.; Strauss, J.; Lévesque, D.; Kennedy, J.L.; et al. Replicated association of the NR4A3 gene with smoking behaviour in schizophrenia and in bipolar disorder. Genes Brain Behav. 2010, 9, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Muto, A.; Tashiro, S.; Tsuchiya, H.; Kume, A.; Kanno, M.; Ito, E.; Yamamoto, M.; Igarashi, K. Activation of Maf/AP-1 repressor Bach2 by oxidative stress promotes apoptosis and its interaction with promyelocytic leukemia nuclear bodies. J. Biol. Chem. 2002, 277, 20724–20733. [Google Scholar] [CrossRef]
- Peng, S.; Lalani, S.; Leavenworth, J.W.; Ho, I.C.; Pauza, M.E. c-Maf interacts with c-Myb to down-regulate Bcl-2 expression and increase apoptosis in peripheral CD4 cells. Eur. J. Immunol. 2007, 37, 2868–2880. [Google Scholar] [CrossRef]
- Katsuoka, F.; Motohashi, H.; Tamagawa, Y.; Kure, S.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Small Maf compound mutants display central nervous system neuronal degeneration, aberrant transcription, and Bach protein mislocalization coincident with myoclonus and abnormal startle response. Mol. Cell. Biol. 2003, 23, 1163–1174. [Google Scholar] [CrossRef]
- Jiang, Q.; Mao, H.; He, G.; Mao, X. Targeting the oncogenic transcription factor c-Maf for the treatment of multiple myeloma. Cancer Lett. 2022, 543, 215791. [Google Scholar] [CrossRef]
- Balamurugan, K.; Sterneck, E. The Many Faces of C/EBPδ and their Relevance for Inflammation and Cancer. Int. J. Biol. Sci. 2013, 9, 917–933. [Google Scholar] [CrossRef]
- Pulido-Salgado, M.; Vidal-Taboada, J.M.; Saura, J. C/EBPβ and C/EBPδ transcription factors: Basic biology and roles in the CNS. Prog. Neurobiol. 2015, 132, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Kfoury, N.; Kapatos, G. Identification of neuronal target genes for CCAAT/Enhancer Binding Proteins. Mol. Cell. Neurosci. 2009, 40, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Canteli, M.; Aguilar-Morante, D.; Sanz-SanCristobal, M.; Megias, D.; Santos, A.; Perez-Castillo, A. Role of C/EBPβ transcription factor in adult hippocampal neurogenesis. PLoS ONE 2011, 6, e24842. [Google Scholar] [CrossRef] [PubMed]
- Meir, O.; Dvash, E.; Werman, A.; Rubinstein, M. C/EBP-β regulates endoplasmic reticulum stress-triggered cell death in mouse and human models. PLoS ONE 2010, 5, e9516. [Google Scholar] [CrossRef]
- Moore, F.; Santin, I.; Nogueira, T.C.; Gurzov, E.N.; Marselli, L.; Marchetti, P.; Eizirik, D.L. The transcription factor C/EBP delta has anti-apoptotic and anti-inflammatory roles in pancreatic beta cells. PLoS ONE 2012, 7, e31062. [Google Scholar] [CrossRef]
- Cortes-Canteli, M.; Luna-Medina, R.; Sanz-SanCristobal, M.; Alvarez-Barrientos, A.; Santos, A.; Perez-Castillo, A. CCAAT/enhancer binding protein β deficiency provides cerebral protection following excitotoxic injury. J. Cell Sci. 2008, 121, 1224–1234. [Google Scholar] [CrossRef]
- Wang, Z.H.; Gong, K.; Liu, X.; Zhang, Z.; Sun, X.; Wei, Z.Z.; Yu, S.P.; Manfredsson, F.P.; Sandoval, I.M.; Johnson, P.F.; et al. C/EBPβ regulates delta-secretase expression and mediates pathogenesis in mouse models of Alzheimer’s disease. Nat. Commun. 2018, 9, 1784. [Google Scholar] [CrossRef]
- Ramberg, V.; Tracy, L.M.; Samuelsson, M.; Nilsson, L.N.G.; Iverfeldt, K. The CCAAT/enhancer binding protein (C/EBP) δ is differently regulated by fibrillar and oligomeric forms of the Alzheimer amyloid-β peptide. J. Neuroinflamm. 2011, 8, 34. [Google Scholar] [CrossRef]
- Nadeau, S.; Hein, P.; Fernandes, K.J.L.; Peterson, A.C.; Miller, F.D. A transcriptional role for C/EBP β in the neuronal response to axonal injury. Mol. Cell. Neurosci. 2005, 29, 525–535. [Google Scholar] [CrossRef]
- Chen, C.M.; Wu, C.T.; Chiang, C.K.; Liao, B.W.; Liu, S.H. C/EBP Homologous Protein (CHOP) Deficiency Aggravates Hippocampal Cell Apoptosis and Impairs Memory Performance. PLoS ONE 2012, 7, e40801. [Google Scholar] [CrossRef]
- Nashine, S.; Liu, Y.; Kim, B.J.; Clark, A.F.; Pang, I.H. Role of C/EBP Homologous Protein in Retinal Ganglion Cell Death After Ischemia/Reperfusion Injury. Invest. Ophthalmol. Vis. Sci. 2015, 56, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Seto, Y.; Ishiwata, S.; Hoshino, M. Characterization of Olig2 expression during cerebellar development. Gene Expr. Patterns 2014, 15, 1–7. [Google Scholar] [CrossRef]
- Gaber, Z.B.; Novitch, B.G. All the embryo’s a stage, and Olig2 in its time plays many parts. Neuron 2011, 69, 833–835. [Google Scholar] [CrossRef] [PubMed]
- Esain, V.; Postlethwait, J.H.; Charnay, P.; Ghislain, J. FGF-receptor signalling controls neural cell diversity in the zebrafish hindbrain by regulating olig2 and sox9. Development 2010, 137, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.L.; Fragoso, G.; Miron, V.E.; Darlington, P.J.; Mushynski, W.E.; Antel, J.; Almazan, G. Response of human oligodendrocyte progenitors to growth factors and axon signals. J. Neuropathol. Exp. Neurol. 2010, 69, 930–944. [Google Scholar] [CrossRef]
- Allahdadi, K.J.; De Santana, T.A.; Santos, G.C.; Azevedo, C.M.H.; Mota, R.A.; Nonaka, C.K.; Silva, D.N.; Valim, C.X.R.; Figueira, C.P.; Dos Santos, W.L.C.; et al. IGF-1 overexpression improves mesenchymal stem cell survival and promotes neurological recovery after spinal cord injury. Stem Cell Res. Ther. 2019, 10, 146. [Google Scholar] [CrossRef] [PubMed]
- Furusho, M.; Kaga, Y.; Ishii, A.; Hébert, J.M.; Bansal, R. Fibroblast Growth Factor Signaling Is Required for the Generation of Oligodendrocyte Progenitors from the Embryonic Forebrain. J. Neurosci. 2011, 31, 5055–5066. [Google Scholar] [CrossRef]
- Sims, R.; Hollingworth, P.; Moskvina, V.; Dowzell, K.; O’Donovan, M.C.; Powell, J.; Lovestone, S.; Brayne, C.; Rubinsztein, D.; Owen, M.J.; et al. Evidence that variation in the oligodendrocyte lineage transcription factor 2 (OLIG2) gene is associated with psychosis in Alzheimer’s disease. Neurosci. Lett. 2009, 461, 54–59. [Google Scholar] [CrossRef]
- Komatsu, H.; Takeuchi, H.; Kikuchi, Y.; Ono, C.; Yu, Z.; Iizuka, K.; Takano, Y.; Kakuto, Y.; Funakoshi, S.; Ono, T.; et al. Ethnicity-Dependent Effects of Schizophrenia Risk Variants of the OLIG2 Gene on OLIG2 Transcription and White Matter Integrity. Schizophr. Bull. 2020, 46, 1619–1628. [Google Scholar] [CrossRef]
- Gouvêa-Junqueira, D.; Falvella, A.C.B.; Antunes, A.S.L.M.; Seabra, G.; Brandão-Teles, C.; Martins-de-Souza, D.; Crunfli, F. Novel Treatment Strategies Targeting Myelin and Oligodendrocyte Dysfunction in Schizophrenia. Front. Psychiatry 2020, 11, 379. [Google Scholar] [CrossRef]
- Tsigelny, I.F.; Mukthavaram, R.; Kouznetsova, V.L.; Chao, Y.; Babic, I.; Nurmemmedov, E.; Pastorino, S.; Jiang, P.; Calligaris, D.; Agar, N.; et al. Multiple spatially related pharmacophores define small molecule inhibitors of OLIG2 in glioblastoma. Oncotarget 2015, 8, 22370–22384. [Google Scholar] [CrossRef] [PubMed]
- Alton, G.; Kesari, S. Novel small molecule inhibitors of the OLIG2 transcription factor: Promising new therapeutics for glioblastoma. Future Oncol. 2016, 12, 1001–1004. [Google Scholar] [CrossRef]
- Chen, X.; Wang, F.; Gan, J.; Zhang, Z.; Liang, X.; Li, T.; Huang, N.; Zhao, X.; Mei, F.; Xiao, L. Myelin Deficits Caused by Olig2 Deficiency Lead to Cognitive Dysfunction and Increase Vulnerability to Social Withdrawal in Adult Mice. Neurosci. Bull. 2020, 36, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Johns, T.; Greenall, S.; Chen, S.; Stewart, R.; Alton, G.; Kesari, S. DDIS-19. CT-179: An Inhibitor of The Olig2 Transcription Factor with Potent Anti-Tumour Activity In Brain Cancer. Neuro. Oncol. 2018, 20, vi73. [Google Scholar] [CrossRef]
- Wegener, A.; Deboux, C.; Bachelin, C.; Frah, M.; Kerninon, C.; Seilhean, D.; Weider, M.; Wegner, M.; Nait-Oumesmar, B. Gain of Olig2 function in oligodendrocyte progenitors promotes remyelination. Brain 2015, 138, 120–135. [Google Scholar] [CrossRef]
- Szu, J.; Wojcinski, A.; Jiang, P.; Kesari, S. Impact of the Olig Family on Neurodevelopmental Disorders. Front. Neurosci. 2021, 15, 332. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y. Molecular mechanisms of regeneration in Alzheimer’s disease brain. Geriatr. Gerontol. Int. 2010, 10, S158–S168. [Google Scholar] [CrossRef]
- Stewart, S.E.; Platko, J.; Fagerness, J.; Birns, J.; Jenike, E.; Smoller, J.W.; Perlis, R.; Leboyer, M.; Delorme, R.; Chabane, N.; et al. A genetic family-based association study of OLIG2 in obsessive-compulsive disorder. Arch. Gen. Psychiatry 2007, 64, 209–215. [Google Scholar] [CrossRef]
- Satoh, J.-I.; Asahina, N.; Kitano, S.; Kino, Y. A Comprehensive Profile of ChIP-Seq-Based Olig2 Target Genes in Motor Neuron Progenitor Cells Suggests the Possible Involvement of Olig2 in the Pathogenesis of Amyotrophic Lateral Sclerosis. J. Cent. Nerv. Syst. Dis. 2015, 7, JCNSD.S23210. [Google Scholar] [CrossRef]
- Tan, B.T.; Yu, J.; Yin, Y.; Jia, G.W.; Jiang, W.; Yu, L.H. The Olig family affects central nervous system development and disease. Neural Regen. Res. 2014, 9, 329–336. [Google Scholar] [CrossRef]
- Zhou, B.; Zhu, Z.; Ransom, B.R.; Tong, X. Oligodendrocyte lineage cells and depression. Mol. Psychiatry 2020, 26, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Raabe, F.J.; Galinski, S.; Papiol, S.; Falkai, P.G.; Schmitt, A.; Rossner, M.J. Studying and modulating schizophrenia-associated dysfunctions of oligodendrocytes with patient-specific cell systems. NPJ Schizophr. 2018, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Fessel, J. Formulating treatment of major psychiatric disorders: Algorithm targets the dominantly affected brain cell-types. Discov. Ment. Health 2023, 3, 3. [Google Scholar] [CrossRef]
- Cai, S.; Lv, Y.; Huang, K.; Zhang, W.; Kang, Y.; Huang, L.; Wang, J. Association of rs1059004 polymorphism in the OLIG2 locus with whole-brain functional connectivity in first-episode schizophrenia. Behav. Brain Res. 2020, 379, 112392. [Google Scholar] [CrossRef] [PubMed]
- Valdés-Tovar, M.; Rodríguez-Ramírez, A.M.; Rodríguez-Cárdenas, L.; Sotelo-Ramírez, C.E.; Camarena, B.; Sanabrais-Jiménez, M.A.; Solís-Chagoyán, H.; Argueta, J.; López-Riquelme, G.O. Insights into myelin dysfunction in schizophrenia and bipolar disorder. World J. Psychiatry 2022, 12, 264. [Google Scholar] [CrossRef]
- Van Der Raadt, J.; Van Gestel, S.H.C.; Kasri, N.N.; Albers, C.A. ONECUT transcription factors induce neuronal characteristics and remodel chromatin accessibility. Nucleic Acids Res. 2019, 47, 5587–5602. [Google Scholar] [CrossRef]
- Patel, T.; Hammelman, J.; Closser, M.; Gifford, D.K.; Wichterle, H. General and cell-type-specific aspects of the motor neuron maturation transcriptional program. bioRxiv 2021. [Google Scholar] [CrossRef]
- Hahn, O.; Foltz, A.G.; Atkins, M.; Kedir, B.; Moran-Losada, P.; Guldner, I.H.; Munson, C.; Kern, F.; Pálovics, R.; Lu, N.; et al. A spatiotemporal map of the aging mouse brain reveals white matter tracts as vulnerable foci. bioRxiv 2022. [Google Scholar] [CrossRef]
- Chakrabarty, K.; Von Oerthel, L.; Hellemons, A.; Clotman, F.; Espana, A.; Koerkamp, M.G.; Holstege, F.C.P.; Pasterkamp, R.J.; Smidt, M.P. Genome wide expression profiling of the mesodiencephalic region identifies novel factors involved in early and late dopaminergic development. Biol. Open 2012, 1, 693–704. [Google Scholar] [CrossRef]
- Toch, M.; Harris, A.; Schakman, O.; Kondratskaya, E.; Boulland, J.L.; Dauguet, N.; Debrulle, S.; Baudouin, C.; Hidalgo-Figueroa, M.; Gow, A.; et al. Onecut-dependent Nkx6.2 transcription factor expression is required for proper formation and activity of spinal locomotor circuits. Sci. Rep. 2020, 10, 996. [Google Scholar] [CrossRef]
- Ulmke, P.A.; Sakib, M.S.; Ditte, P.; Sokpor, G.; Kerimoglu, C.; Pham, L.; Xie, Y.; Mao, X.; Rosenbusch, J.; Teichmann, U.; et al. Molecular Profiling Reveals Involvement of ESCO2 in Intermediate Progenitor Cell Maintenance in the Developing Mouse Cortex. Stem Cell Rep. 2021, 16, 968–984. [Google Scholar] [CrossRef]
- Francius, C.; Clotman, F. Dynamic expression of the Onecut transcription factors HNF-6, OC-2 and OC-3 during spinal motor neuron development. Neuroscience 2010, 165, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, S.; Chertkow, H.; Schipper, H.M.; Yuan, Z.; Shetty, V.; Jenkins, S.; Jones, T.; Wang, E. Increased microRNA-34c abundance in Alzheimer’s disease circulating blood plasma. Front. Mol. Neurosci. 2014, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Hin, N.; Newman, M.; Kaslin, J.; Douek, A.M.; Lumsden, A.; Nik, S.H.M.; Dong, Y.; Zhou, X.F.; Manucat-Tan, N.B.; Ludington, A.; et al. Accelerated brain aging towards transcriptional inversion in a zebrafish model of the K115fs mutation of human PSEN2. PLoS ONE 2020, 15, e0227258. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, M.; Cui, Y.; Tan, Z.; Jiang, Y. Differential Expression Profile of lncRNA in Glioma Cells and the Effect of lncRNA NKX3-1 on Glioma Cells Through Fem1b/SPDEF Pathway. Front. Oncol. 2021, 11, 2734. [Google Scholar] [CrossRef] [PubMed]
- Ducker, C.; Shaw, P.E. Ubiquitin-Mediated Control of ETS Transcription Factors: Roles in Cancer and Development. Int. J. Mol. Sci. 2021, 22, 5119. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Mou, X.; Deng, J.; Di, B.; Zhong, R.; Wang, S.; Yang, Y.; Zeng, W. Differences of immune disorders between Alzheimer’s disease and breast cancer based on transcriptional regulation. PLoS ONE 2017, 12, e0180337. [Google Scholar] [CrossRef]
- Tansey, K.E.; Cameron, D.; Hill, M.J. Genetic risk for Alzheimer’s disease is concentrated in specific macrophage and microglial transcriptional networks. Genome Med. 2018, 10, 14. [Google Scholar] [CrossRef]
- Arefin, A.S.; Mathieson, L.; Johnstone, D.; Berretta, R.; Moscato, P. Unveiling Clusters of RNA Transcript Pairs Associated with Markers of Alzheimer’s Disease Progression. PLoS ONE 2012, 7, e45535. [Google Scholar] [CrossRef]
- Tay, N.; Macare, C.; Liu, Y.; Ruggeri, B.; Jia, T.; Chu, C.; Biondo, F.; Ing, A.; Luo, Q.; Sarkysian, D.; et al. Allele-specific methylation of SpDEF: A novel moderator of psychosocial stress and substance abuse. Am. J. Psychiatry 2019, 176, 146–155. [Google Scholar] [CrossRef]
- Hughes, R.; Kristiansen, M.; Lassot, I.; Desagher, S.; Mantovani, R.; Ham, J. NF-Y is essential for expression of the proapoptotic bim gene in sympathetic neurons. Cell Death Differ. 2010, 18, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Gatta, R.; Dolfini, D.; Mantovani, R. NF-Y joins E2Fs, p53 and other stress transcription factors at the apoptosis table. Cell Death Dis. 2011, 2, e162. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, A.; Fuschi, P.; Martelli, F.; Manni, I.; Artuso, S.; Simonte, G.; Ambrosino, V.; Antonini, A.; Folgiero, V.; Falcioni, R.; et al. Transcription factor NF-Y induces apoptosis in cells expressing wild-type p53 through E2F1 upregulation and p53 activation. Cancer Res. 2010, 70, 9711–9720. [Google Scholar] [CrossRef]
- Olajide, O.J.; Suvanto, M.E.; Chapman, C.A. Molecular mechanisms of neurodegeneration in the entorhinal cortex that underlie its selective vulnerability during the pathogenesis of Alzheimer’s disease. Biol. Open 2021, 10, bio056796. [Google Scholar] [CrossRef]
- Yamanaka, T.; Tosaki, A.; Kurosawa, M.; Matsumoto, G.; Koike, M.; Uchiyama, Y.; Maity, S.N.; Shimogori, T.; Hattori, N.; Nukina, N. NF-Y inactivation causes atypical neurodegeneration characterized by ubiquitin and p62 accumulation and endoplasmic reticulum disorganization. Nat. Commun. 2014, 5, 3354. [Google Scholar] [CrossRef] [PubMed]
- Franco, H.L.; Casasnovas, J.; Rodríguez-Medina, J.R.; Cadilla, C.L. Redundant or separate entities?—Roles of Twist1 and Twist2 as molecular switches during gene transcription. Nucleic Acids Res. 2011, 39, 1177–1186. [Google Scholar] [CrossRef]
- Twist Is a Potential Oncogene That Inhibits Apoptosis. Available online: http://genesdev.cshlp.org/content/13/17/2207 (accessed on 9 February 2023).
- Puisieux, A.; Valsesia-Wittmann, S.; Ansieau, S. A twist for survival and cancer progression. Br. J. Cancer 2005, 94, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Floc’h, N.; Kolodziejski, J.; Akkari, L.; Simonin, Y.; Ansieau, S.; Puisieux, A.; Hibner, U.; Lassus, P. Modulation of Oxidative Stress by Twist Oncoproteins. PLoS ONE 2013, 8, e72490. [Google Scholar] [CrossRef]
- Jembrek, M.J.; Oršolić, N.; Mandić, L.; Sadžak, A.; Šegota, S. Anti-Oxidative, Anti-Inflammatory and Anti-Apoptotic Effects of Flavonols: Targeting Nrf2, NF-κB and p53 Pathways in Neurodegeneration. Antioxidants 2021, 10, 1628. [Google Scholar] [CrossRef]
- Pan, Y.; Zhu, Y.; Yang, W.; Tycksen, E.; Liu, S.; Palucki, J.; Zhu, L.; Sasaki, Y.; Sharma, M.K.; Kim, A.H.; et al. The role of Twist1 in mutant huntingtin-induced transcriptional alterations and neurotoxicity. J. Biol. Chem. 2018, 293, 11850–11866. [Google Scholar] [CrossRef]
- Yang, W.; Xiang, Y.; Liao, M.J.; Wu, P.F.; Yang, L.; Huang, G.H.; Shi, B.Z.; Yi, L.; Lv, S.Q. Presenilin1 inhibits glioblastoma cell invasiveness via promoting Sortilin cleavage. Cell Commun. Signal. 2021, 19, 1112. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).