Unraveling the Role of Peroxisome Proliferator-Activated Receptor β/Δ (PPAR β/Δ) in Angiogenesis Associated with Multiple Myeloma
Abstract
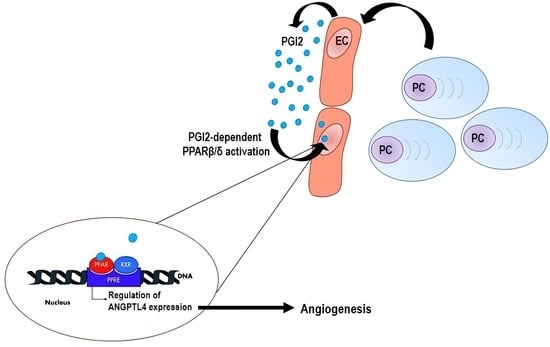
1. Introduction
2. Materials and Methods
2.1. Biological Samples
2.2. Cell Isolation and Culture
2.3. Functional Assays
2.4. Western Blot
2.5. Real-Time PCR
2.6. ELISA
2.7. PPAR β/δ Transcriptional Activity
2.8. In Vivo Experiments
2.9. Survival Analysis of the GSE9782 Multiple Myeloma Dataset
2.10. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marofi, F.; Tahmasebi, S.; Rahman, H.S.; Kaigorodov, D.; Markov, A.; Yumashev, A.V.; Shomali, N.; Chartrand, M.S.; Pathak, Y.; Mohammed, R.N.; et al. Any closer to successful therapy of multiple myeloma? CAR-T cell is a good reason for optimism. Stem Cell Res. Ther. 2021, 12, 217. [Google Scholar] [CrossRef]
- Fairfield, H.; Falank, C.; Avery, L.; Reagan, M.R. Multiple myeloma in the marrow: Pathogenesis and treatments. Ann. N. Y. Acad. Sci. 2016, 1364, 32–51. [Google Scholar] [CrossRef] [PubMed]
- Kyle, R.A.; Therneau, T.M.; Rajkumar, S.V.; Offord, J.R.; Larson, D.R.; Plevak, M.F.; Melton, L.J., 3rd. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N. Engl. J. Med. 2002, 346, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Leone, P.; Solimando, A.G.; Malerba, E.; Fasano, R.; Buonavoglia, A.; Pappagallo, F.; De Re, V.; Argentiero, A.; Silvestris, N.; Vacca, A.; et al. Actors on the Scene: Immune Cells in the Myeloma Niche. Front Oncol. 2020, 10, 599098. [Google Scholar] [CrossRef] [PubMed]
- Rollig, C.; Knop, S.; Bornhauser, M. Multiple myeloma. Lancet 2015, 385, 2197–2208. [Google Scholar] [CrossRef]
- Solimando, A.G.; Da Via, M.C.; Cicco, S.; Leone, P.; Di Lernia, G.; Giannico, D.; Desantis, V.; Frassanito, M.A.; Morizio, A.; Delgado Tascon, J.; et al. High-Risk Multiple Myeloma: Integrated Clinical and Omics Approach Dissects the Neoplastic Clone and the Tumor Microenvironment. J. Clin. Med. 2019, 8, 997. [Google Scholar] [CrossRef]
- Solimando, A.G.; Da Via, M.C.; Leone, P.; Borrelli, P.; Croci, G.A.; Tabares, P.; Brandl, A.; Di Lernia, G.; Bianchi, F.P.; Tafuri, S.; et al. Halting the vicious cycle within the multiple myeloma ecosystem: Blocking JAM-A on bone marrow endothelial cells restores angiogenic homeostasis and suppresses tumor progression. Haematologica 2021, 106, 1943–1956. [Google Scholar] [CrossRef]
- Eleutherakis-Papaiakovou, V.; Karali, M.; Kokkonouzis, I.; Tiliakos, I.; Dimopoulos, M.A. Bone marrow angiogenesis and progression in multiple myeloma: Clinical significance and therapeutic approach. Leuk Lymphoma 2003, 44, 937–948. [Google Scholar] [CrossRef]
- Leone, P.; Buonavoglia, A.; Fasano, R.; Solimando, A.G.; De Re, V.; Cicco, S.; Vacca, A.; Racanelli, V. Insights into the Regulation of Tumor Angiogenesis by Micro-RNAs. J. Clin. Med. 2019, 8, 2030. [Google Scholar] [CrossRef]
- Du, S.; Wagner, N.; Wagner, K.D. The Emerging Role of PPAR Beta/Delta in Tumor Angiogenesis. PPAR Res. 2020, 2020, 3608315. [Google Scholar] [CrossRef]
- La Paglia, L.; Listi, A.; Caruso, S.; Amodeo, V.; Passiglia, F.; Bazan, V.; Fanale, D. Potential Role of ANGPTL4 in the Cross Talk between Metabolism and Cancer through PPAR Signaling Pathway. PPAR Res. 2017, 2017, 8187235. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Colby, J.K.; Zuo, X.; Jaoude, J.; Wei, D.; Shureiqi, I. The Role of PPAR-delta in Metabolism, Inflammation, and Cancer: Many Characters of a Critical Transcription Factor. Int. J. Mol. Sci. 2018, 19, 3339. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.Y.; Lee, S.; Ghelani, D.; Matijevic-Aleksic, N.; Wu, K.K. Protection of endothelial survival by peroxisome proliferator-activated receptor-delta mediated 14-3-3 upregulation. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Piqueras, L.; Reynolds, A.R.; Hodivala-Dilke, K.M.; Alfranca, A.; Redondo, J.M.; Hatae, T.; Tanabe, T.; Warner, T.D.; Bishop-Bailey, D. Activation of PPARbeta/delta induces endothelial cell proliferation and angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 63–69. [Google Scholar] [CrossRef]
- Stephen, R.L.; Gustafsson, M.C.; Jarvis, M.; Tatoud, R.; Marshall, B.R.; Knight, D.; Ehrenborg, E.; Harris, A.L.; Wolf, C.R.; Palmer, C.N. Activation of peroxisome proliferator-activated receptor delta stimulates the proliferation of human breast and prostate cancer cell lines. Cancer Res. 2004, 64, 3162–3170. [Google Scholar] [CrossRef]
- Lim, H.; Dey, S.K. A novel pathway of prostacyclin signaling-hanging out with nuclear receptors. Endocrinology 2002, 143, 3207–3210. [Google Scholar] [CrossRef]
- Gupta, R.A.; Tan, J.; Krause, W.F.; Geraci, M.W.; Willson, T.M.; Dey, S.K.; DuBois, R.N. Prostacyclin-mediated activation of peroxisome proliferator-activated receptor delta in colorectal cancer. Proc. Natl. Acad. Sci. USA 2000, 97, 13275–13280. [Google Scholar] [CrossRef]
- Lim, H.; Gupta, R.A.; Ma, W.G.; Paria, B.C.; Moller, D.E.; Morrow, J.D.; DuBois, R.N.; Trzaskos, J.M.; Dey, S.K. Cyclo-oxygenase-2-derived prostacyclin mediates embryo implantation in the mouse via PPARdelta. Genes Dev. 1999, 13, 1561–1574. [Google Scholar] [CrossRef]
- Katusic, Z.S.; Santhanam, A.V.; He, T. Vascular effects of prostacyclin: Does activation of PPARdelta play a role? Trends Pharmacol. Sci. 2012, 33, 559–564. [Google Scholar] [CrossRef]
- Mangelsdorf, D.J.; Thummel, C.; Beato, M.; Herrlich, P.; Schutz, G.; Umesono, K.; Blumberg, B.; Kastner, P.; Mark, M.; Chambon, P.; et al. The nuclear receptor superfamily: The second decade. Cell 1995, 83, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.M.; Shah, Y.M.; Gonzalez, F.J. The role of peroxisome proliferator-activated receptors in carcinogenesis and chemoprevention. Nat. Rev. Cancer 2012, 12, 181–195. [Google Scholar] [CrossRef]
- Pedchenko, T.V.; Gonzalez, A.L.; Wang, D.; DuBois, R.N.; Massion, P.P. Peroxisome proliferator-activated receptor beta/delta expression and activation in lung cancer. Am. J. Respir. Cell Mol. Biol. 2008, 39, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.D.; Du, S.; Martin, L.; Leccia, N.; Michiels, J.F.; Wagner, N. Vascular PPARbeta/delta Promotes Tumor Angiogenesis and Progression. Cells 2019, 8, 1623. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Lu, J.; Xiao, J.; Upadhyay, G.; Umans, R.; Kallakury, B.; Yin, Y.; Fant, M.E.; Kopelovich, L.; Glazer, R.I. PPARdelta induces estrogen receptor-positive mammary neoplasia through an inflammatory and metabolic phenotype linked to mTOR activation. Cancer Res. 2013, 73, 4349–4361. [Google Scholar] [CrossRef]
- Zuo, X.; Deguchi, Y.; Xu, W.; Liu, Y.; Li, H.S.; Wei, D.; Tian, R.; Chen, W.; Xu, M.; Yang, Y.; et al. PPARD and Interferon Gamma Promote Transformation of Gastric Progenitor Cells and Tumorigenesis in Mice. Gastroenterology 2019, 157, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.M.; Foreman, J.E.; Gonzalez, F.J. Dissecting the role of peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta) in colon, breast, and lung carcinogenesis. Cancer Metastasis Rev. 2011, 30, 619–640. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.M.; Gonzalez, F.J.; Muller, R. Establishing the Role of PPARbeta/delta in Carcinogenesis. Trends Endocrinol Metab. 2015, 26, 595–607. [Google Scholar] [CrossRef]
- Wagner, N.; Wagner, K.D. PPAR Beta/Delta and the Hallmarks of Cancer. Cells 2020, 9, 1133. [Google Scholar] [CrossRef]
- Liu, S.; Ishikawa, H.; Li, F.J.; Ma, Z.; Otsuyama, K.; Asaoku, H.; Abroun, S.; Zheng, X.; Tsuyama, N.; Obata, M.; et al. Dehydroepiandrosterone can inhibit the proliferation of myeloma cells and the interleukin-6 production of bone marrow mononuclear cells from patients with myeloma. Cancer Res. 2005, 65, 2269–2276. [Google Scholar] [CrossRef]
- Otsuyama, K.I.; Ma, Z.; Abroun, S.; Amin, J.; Shamsasenjan, K.; Asaoku, H.; Kawano, M.M. PPARbeta-mediated growth suppression of baicalein and dexamethasone in human myeloma cells. Leukemia 2007, 21, 187–190. [Google Scholar] [CrossRef]
- Sha, Y.; Wu, J.; Paul, B.; Zhao, Y.; Mathews, P.; Li, Z.; Norris, J.; Wang, E.; McDonnell, D.P.; Kang, Y. PPAR agonists attenuate lenalidomide’s anti-myeloma activity in vitro and in vivo. Cancer Lett. 2022, 545, 215832. [Google Scholar] [CrossRef]
- Wu, J.; Chu, E.; Paul, B.; Kang, Y. Mechanistic Studies and a Retrospective Cohort Study: The Interaction between PPAR Agonists and Immunomodulatory Agents in Multiple Myeloma. Cancers 2022, 14, 5272. [Google Scholar] [CrossRef] [PubMed]
- International Myeloma Working, G. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: A report of the International Myeloma Working Group. Br. J. Haematol. 2003, 121, 749–757. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef] [PubMed]
- Leone, P.; Di Lernia, G.; Solimando, A.G.; Cicco, S.; Saltarella, I.; Lamanuzzi, A.; Ria, R.; Frassanito, M.A.; Ponzoni, M.; Ditonno, P.; et al. Bone marrow endothelial cells sustain a tumor-specific CD8(+) T cell subset with suppressive function in myeloma patients. Oncoimmunology 2019, 8, e1486949. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Giannico, D.; Leone, P.; Solimando, A.G.; Maiorano, E.; Caporusso, C.; Duda, L.; Tamma, R.; Mallamaci, R.; Susca, N.; et al. HB-EGF-EGFR Signaling in Bone Marrow Endothelial Cells Mediates Angiogenesis Associated with Multiple Myeloma. Cancers 2020, 12, 173. [Google Scholar] [CrossRef]
- Mulligan, G.; Mitsiades, C.; Bryant, B.; Zhan, F.; Chng, W.J.; Roels, S.; Koenig, E.; Fergus, A.; Huang, Y.; Richardson, P.; et al. Gene expression profiling and correlation with outcome in clinical trials of the proteasome inhibitor bortezomib. Blood 2007, 109, 3177–3188. [Google Scholar] [CrossRef]
- Dorris, S.L.; Peebles, R.S., Jr. PGI2 as a regulator of inflammatory diseases. Mediators Inflamm. 2012, 2012, 926968. [Google Scholar] [CrossRef]
- Spisni, E.; Bartolini, G.; Orlandi, M.; Belletti, B.; Santi, S.; Tomasi, V. Prostacyclin (PGI2) synthase is a constitutively expressed enzyme in human endothelial cells. Exp. Cell Res. 1995, 219, 507–513. [Google Scholar] [CrossRef]
- Pola, R.; Gaetani, E.; Flex, A.; Aprahamian, T.R.; Bosch-Marce, M.; Losordo, D.W.; Smith, R.C.; Pola, P. Comparative analysis of the in vivo angiogenic properties of stable prostacyclin analogs: A possible role for peroxisome proliferator-activated receptors. J. Mol. Cell Cardiol. 2004, 36, 363–370. [Google Scholar] [CrossRef]
- Chu, L.Y.; Liou, J.Y.; Wu, K.K. Prostacyclin protects vascular integrity via PPAR/14-3-3 pathway. Prostaglandins Other Lipid Mediat. 2015, 118–119, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, A.; Schwager, C.; Kleeff, J.; Esposito, I.; Domhan, S.; Peschke, P.; Hauser, K.; Hahnfeldt, P.; Hlatky, L.; Debus, J.; et al. Transcriptional network governing the angiogenic switch in human pancreatic cancer. Proc. Natl. Acad. Sci. USA 2007, 104, 12890–12895. [Google Scholar] [CrossRef] [PubMed]
- Le Jan, S.; Amy, C.; Cazes, A.; Monnot, C.; Lamande, N.; Favier, J.; Philippe, J.; Sibony, M.; Gasc, J.M.; Corvol, P.; et al. Angiopoietin-like 4 is a proangiogenic factor produced during ischemia and in conventional renal cell carcinoma. Am. J. Pathol. 2003, 162, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Staiger, H.; Haas, C.; Machann, J.; Werner, R.; Weisser, M.; Schick, F.; Machicao, F.; Stefan, N.; Fritsche, A.; Haring, H.U. Muscle-derived angiopoietin-like protein 4 is induced by fatty acids via peroxisome proliferator-activated receptor (PPAR)-delta and is of metabolic relevance in humans. Diabetes 2009, 58, 579–589. [Google Scholar] [CrossRef]
- Tan, M.J.; Teo, Z.; Sng, M.K.; Zhu, P.; Tan, N.S. Emerging roles of angiopoietin-like 4 in human cancer. Mol. Cancer Res. 2012, 10, 677–688. [Google Scholar] [CrossRef]
- Li, Y.J.; Sun, L.; Shi, Y.; Wang, G.; Wang, X.; Dunn, S.E.; Iorio, C.; Screaton, R.A.; Spaner, D.E. PPAR-delta promotes survival of chronic lymphocytic leukemia cells in energetically unfavorable conditions. Leukemia 2017, 31, 1905–1914. [Google Scholar] [CrossRef]
- Sun, L.; Shi, Y.; Wang, G.; Wang, X.; Zeng, S.; Dunn, S.E.; Fairn, G.D.; Li, Y.J.; Spaner, D.E. PPAR-delta modulates membrane cholesterol and cytokine signaling in malignant B cells. Leukemia 2018, 32, 184–193. [Google Scholar] [CrossRef]
- Gou, Q.; Zhang, W.; Xu, Y.; Jin, J.; Liu, Q.; Hou, Y.; Shi, J. EGFR/PPARdelta/HSP90 pathway mediates cancer cell metabolism and chemoresistance. J. Cell Biochem. 2021, 122, 394–402. [Google Scholar] [CrossRef]








| Variable | N. Patients (%) | Median Values | |
|---|---|---|---|
| MGUS | |||
| Median Age | 20/20 (100) | 62 years (49–75) | |
| Male | 12/20 (60) | ||
| Female | 8/20 (40) | ||
| IgG | 13/20 (65) | ||
| IgA | 5/20 (25) | ||
| Light chain | 2/20 (10) | ||
| Symptomatic newly diagnosed MM | |||
| Median Age | 20/20 (100) | 65.5 years (47–81) | |
| Male | 10/20 (50) | ||
| Female | 10/20 (50) | ||
| Stage I | 5/20 (25) | ||
| Stage II | 8/20 (40) | ||
| Stage III | 7/20 (35) | ||
| IgG | 17/20 (85) | ||
| IgA | 2/20 (10) | ||
| Light chain | 1/20 (5) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leone, P.; Solimando, A.G.; Prete, M.; Malerba, E.; Susca, N.; Derakhshani, A.; Ditonno, P.; Terragna, C.; Cavo, M.; Silvestris, N.; et al. Unraveling the Role of Peroxisome Proliferator-Activated Receptor β/Δ (PPAR β/Δ) in Angiogenesis Associated with Multiple Myeloma. Cells 2023, 12, 1011. https://doi.org/10.3390/cells12071011
Leone P, Solimando AG, Prete M, Malerba E, Susca N, Derakhshani A, Ditonno P, Terragna C, Cavo M, Silvestris N, et al. Unraveling the Role of Peroxisome Proliferator-Activated Receptor β/Δ (PPAR β/Δ) in Angiogenesis Associated with Multiple Myeloma. Cells. 2023; 12(7):1011. https://doi.org/10.3390/cells12071011
Chicago/Turabian StyleLeone, Patrizia, Antonio Giovanni Solimando, Marcella Prete, Eleonora Malerba, Nicola Susca, Afshin Derakhshani, Paolo Ditonno, Carolina Terragna, Michele Cavo, Nicola Silvestris, and et al. 2023. "Unraveling the Role of Peroxisome Proliferator-Activated Receptor β/Δ (PPAR β/Δ) in Angiogenesis Associated with Multiple Myeloma" Cells 12, no. 7: 1011. https://doi.org/10.3390/cells12071011
APA StyleLeone, P., Solimando, A. G., Prete, M., Malerba, E., Susca, N., Derakhshani, A., Ditonno, P., Terragna, C., Cavo, M., Silvestris, N., & Racanelli, V. (2023). Unraveling the Role of Peroxisome Proliferator-Activated Receptor β/Δ (PPAR β/Δ) in Angiogenesis Associated with Multiple Myeloma. Cells, 12(7), 1011. https://doi.org/10.3390/cells12071011