Multicellular Liver Organoids: Generation and Importance of Diverse Specialized Cellular Components
Abstract
1. Introduction
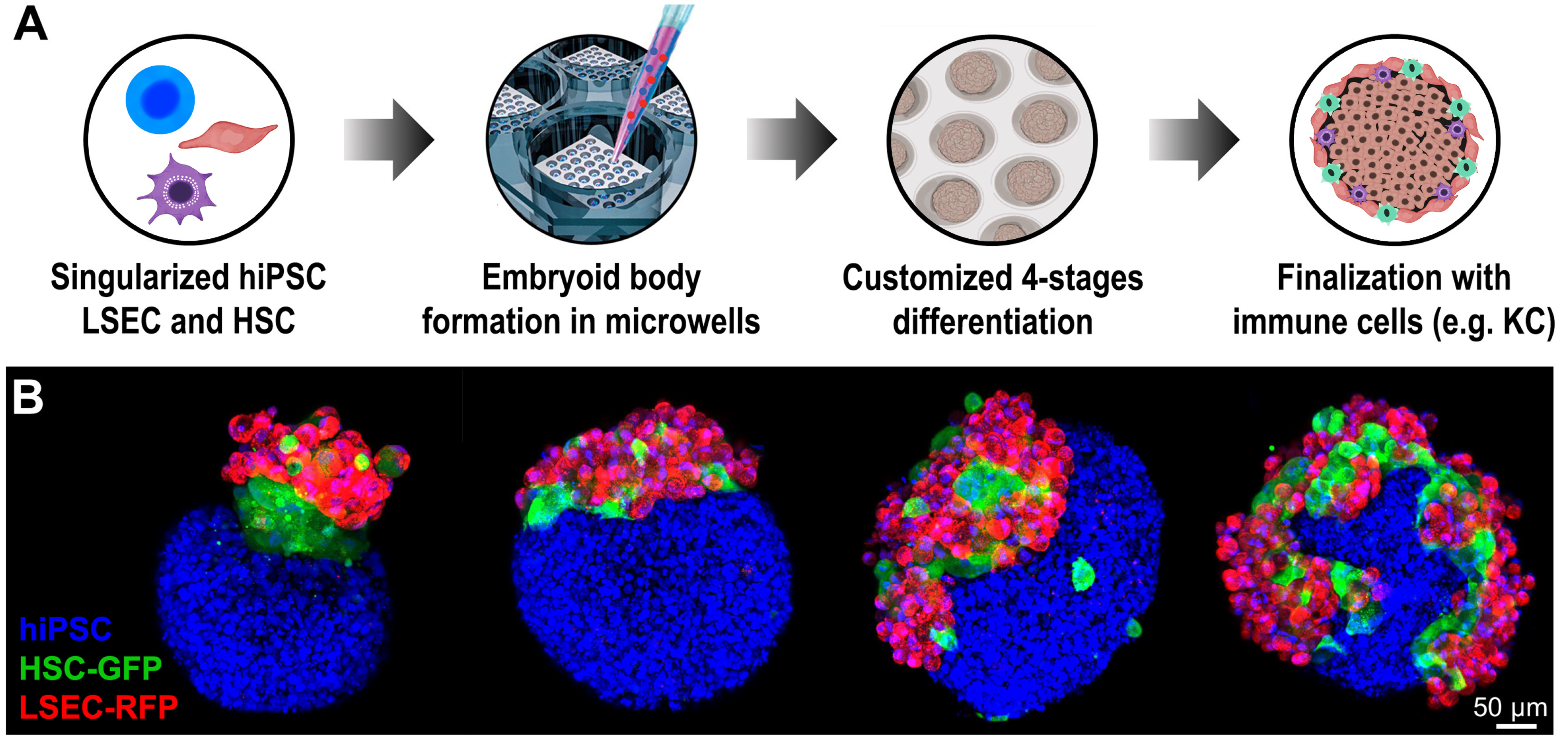
2. Generation of Embryoid Body for Three-Dimensional Culture
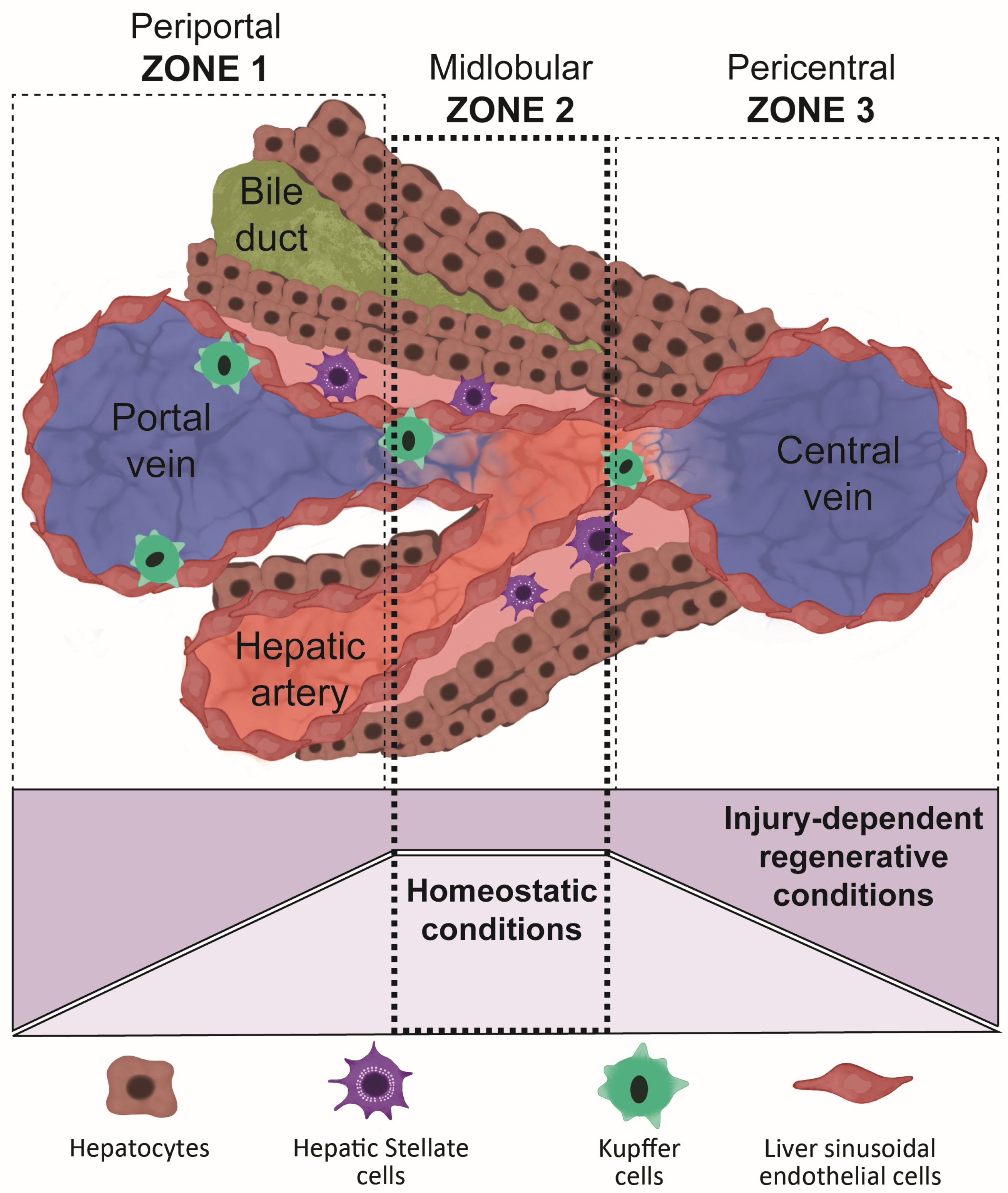
3. Liver Regeneration and the Importance of Replicating Its Structure When Differentiating Organoids
4. Differentiation Strategies
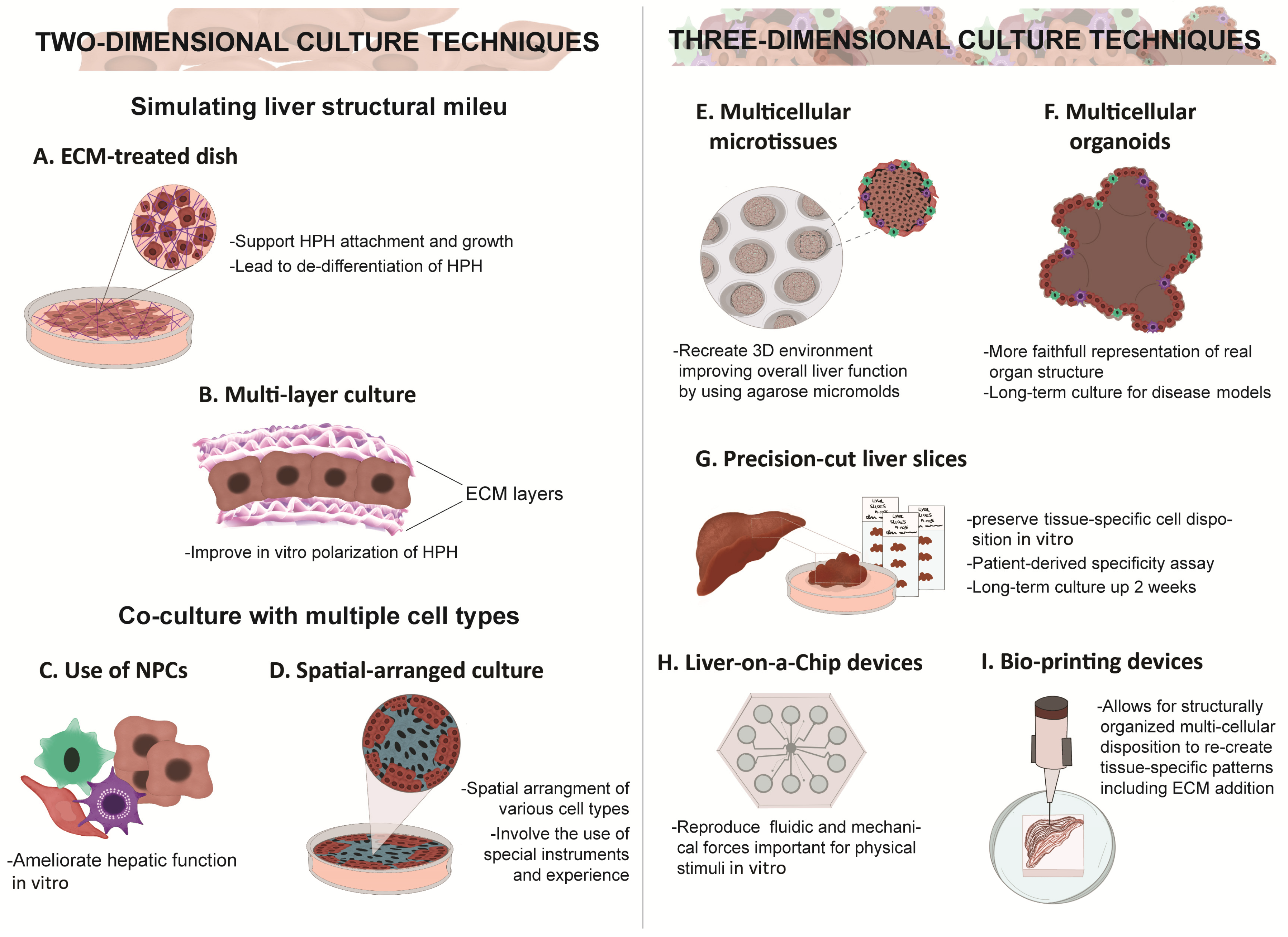
5. Two-Dimensional vs. Three-Dimensional Culture
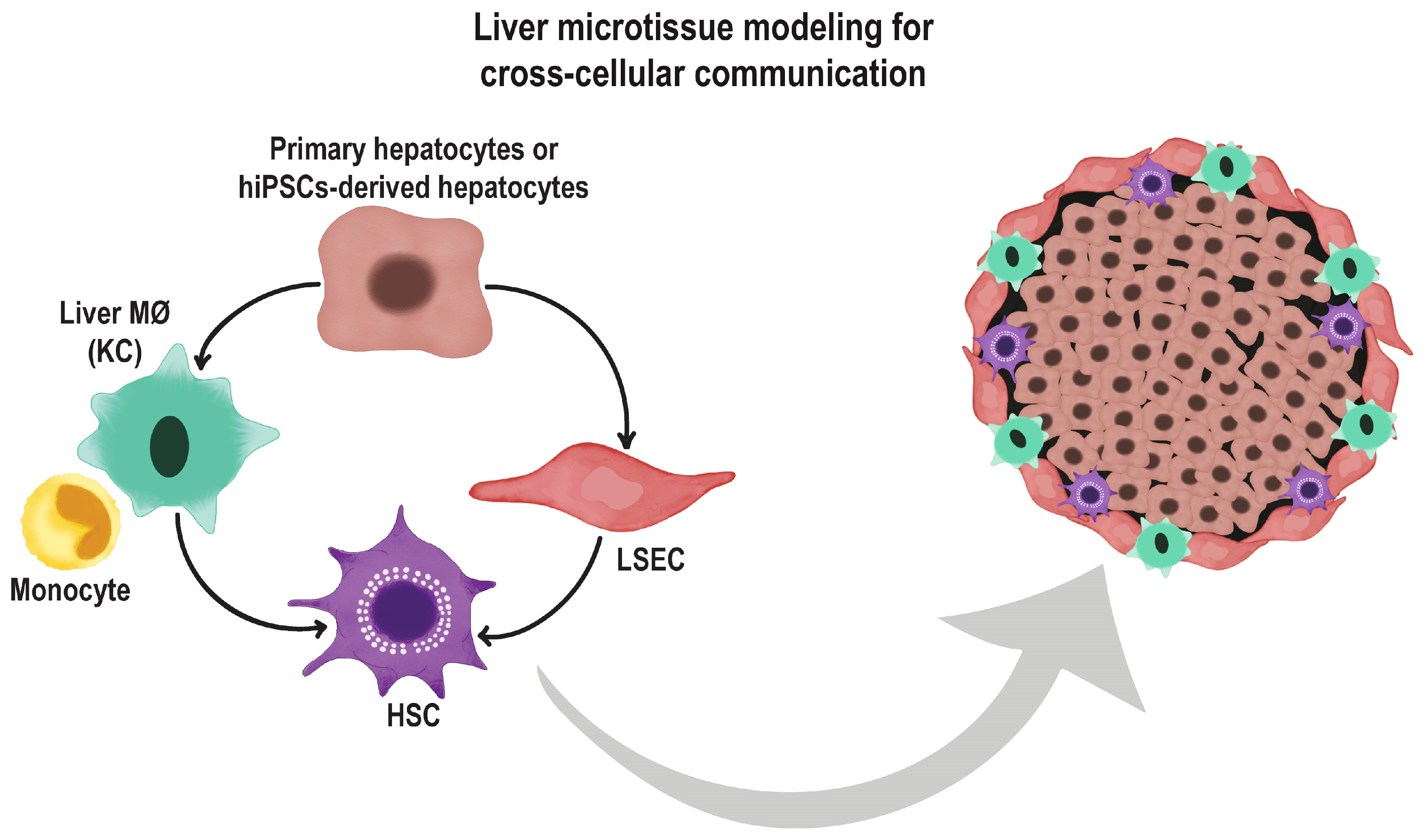
6. Co-Culture Methods and Use of Extracellular Matrices
7. The Role of Endothelial Cells in Organoid Differentiation and Vascularization
8. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alqahtani, S.; Larson, A.M. Adult liver transplantation in the USA. Curr. Opin. Gastroenterol. 2011, 27, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Dianat, N.; Steichen, C.; Vallier, L.; Weber, A.; Dubart-Kupperschmitt, A. Human Pluripotent Stem Cells for Modelling Human Liver Diseases and Cell Therapy. Curr. Gene Ther. 2013, 13, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Terry, C.; Dhawan, A.; Mitry, R.R.; Lehec, S.C.; Hughes, R.D. Optimization of the cryopreservation and thawing protocol for human hepatocytes for use in cell transplantation. Liver Transplant. 2010, 16, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Aasen, T.; Raya, A.; Barrero, M.; Garreta, E.; Consiglio, A.; Gonzalez, F.; Vassena, R.; Bilić, J.; Pekarik, V.; Tiscornia, G.; et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat. Biotechnol. 2008, 26, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Raju, R.; Chau, D.; Verfaillie, C.M.; Hu, W.-S. The road to regenerative liver therapies: The triumphs, trials and tribulations. Biotechnol. Adv. 2013, 31, 1085–1093. [Google Scholar] [CrossRef]
- Pettinato, G.; Ramanathan, R.; Fisher, R.A.; Mangino, M.J.; Zhang, N.; Wen, X. Scalable Differentiation of Human iPSCs in a Multicellular Spheroid-based 3D Culture into Hepatocyte-like Cells through Direct Wnt/β-catenin Pathway Inhibition. Sci. Rep. 2016, 6, 32888. [Google Scholar] [CrossRef]
- Si-Tayeb, K.; Noto, F.K.; Nagaoka, M.; Li, J.; Battle, M.A.; Duris, C.; North, P.E.; Dalton, S.; Duncan, S.A. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology 2010, 51, 297–305. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, B.; He, Y.; Bao, J. Liver Organoids: Formation Strategies and Biomedical Applications. Tissue Eng. Regen. Med. 2021, 18, 573–585. [Google Scholar] [CrossRef]
- De Rudder, M.; Dili, A.; Stärkel, P.; Leclercq, I.A. Critical Role of LSEC in Post-Hepatectomy Liver Regeneration and Failure. Int. J. Mol. Sci. 2021, 22, 8053. [Google Scholar] [CrossRef] [PubMed]
- Kosmacheva, S.M.; Seviaryn, I.N.; Goncharova, N.V.; Petyovka, N.V.; Potapnev, M.P. Hepatogenic Potential of Human Bone Marrow and Umbilical Cord Blood Mesenchymal Stem Cells. Bull. Exp. Biol. Med. 2011, 151, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-B.; Tao, R. Hepatocyte differentiation of mesenchymal stem cells. Hepatobiliary Pancreat. Dis. Int. 2012, 11, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Hannan, N.; Segeritz, C.-P.; Touboul, T.; Vallier, L. Production of hepatocyte-like cells from human pluripotent stem cells. Nat. Protoc. 2013, 8, 430–437. [Google Scholar] [CrossRef]
- Heslop, J.A.; Rowe, C.; Walsh, J.; Sison-Young, R.; Jenkins, R.; Kamalian, L.; Kia, R.; Hay, D.; Jones, R.P.; Malik, H.Z.; et al. Mechanistic evaluation of primary human hepatocyte culture using global proteomic analysis reveals a selective dedifferentiation profile. Arch. Toxicol. 2017, 91, 439–452. [Google Scholar] [CrossRef]
- Tong, J.Z.; De Lagausie, P.; Furlan, V.; Cresteil, T.; Bernard, O.; Alvarez, F. Long-term culture of adult rat hepatocyte spheroids. Exp. Cell Res. 1992, 200, 326–332. [Google Scholar] [CrossRef]
- Höpfl, G.; Gassmann, M.; Desbaillets, I. Differentiating Embryonic Stem Cells into Embryoid Bodies. In Germ Cell Protocols; Schatten, H., Ed.; Humana Press: Totowa, NJ, USA, 2004; Volume 254, pp. 79–98. [Google Scholar] [CrossRef]
- Khoo, M.L.; McQuade, L.R.; Smith, M.S.; Lees, J.G.; Sidhu, K.S.; Tuch, B.E. Growth and Differentiation of Embryoid Bodies Derived from Human Embryonic Stem Cells: Effect of Glucose and Basic Fibroblast Growth Factor. Biol. Reprod. 2005, 73, 1147–1156. [Google Scholar] [CrossRef]
- Mohr, J.C.; Zhang, J.; Azarin, S.M.; Soerens, A.G.; de Pablo, J.J.; Thomson, J.A.; Lyons, G.E.; Palecek, S.P.; Kamp, T.J. The microwell control of embryoid body size in order to regulate cardiac differentiation of human embryonic stem cells. Biomaterials 2010, 31, 1885–1893. [Google Scholar] [CrossRef]
- Messana, J.M.; Hwang, N.S.; Coburn, J.; Elisseeff, J.H.; Zhang, Z. Size of the embryoid body influences chondrogenesis of mouse embryonic stem cells. J. Tissue Eng. Regen. Med. 2008, 2, 499–506. [Google Scholar] [CrossRef]
- Van Winkle, A.P.; Gates, I.D.; Kallos, M.S. Mass Transfer Limitations in Embryoid Bodies during Human Embryonic Stem Cell Differentiation. Cell. Tissues Organs 2012, 196, 34–47. [Google Scholar] [CrossRef]
- Friedrich, J.; Seidel, C.; Ebner, R.; Kunz-Schughart, L.A. Spheroid-based drug screen: Considerations and practical approach. Nat. Protoc. 2009, 4, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Kelm, J.M.; Timmins, N.E.; Brown, C.J.; Fussenegger, M.; Nielsen, L.K. Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnol. Bioeng. 2003, 83, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.T.; Hughes-Fulford, M. Monolayer and Spheroid Culture of Human Liver Hepatocellular Carcinoma Cell Line Cells Demonstrate Distinct Global Gene Expression Patterns and Functional Phenotypes. Tissue Eng. Part A 2009, 15, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, J.; Sakai, Y.; Nakazawa, K. Novel hepatocyte culture system developed using microfabrication and collagen/polyethylene glycol microcontact printing. Biomaterials 2006, 27, 1061–1070. [Google Scholar] [CrossRef]
- Pettinato, G.; Wen, X.; Zhang, N. Formation of Well-defined Embryoid Bodies from Dissociated Human Induced Pluripotent Stem Cells using Microfabricated Cell-repellent Microwell Arrays. Sci. Rep. 2014, 4, 7402. [Google Scholar] [CrossRef]
- Pettinato, G.; Berg-Foels, W.S.V.; Zhang, N.; Wen, X. ROCK Inhibitor Is Not Required for Embryoid Body Formation from Singularized Human Embryonic Stem Cells. PLoS ONE 2014, 9, e100742. [Google Scholar] [CrossRef]
- Pettinato, G.; Wen, X.; Zhang, N. Engineering Strategies for the Formation of Embryoid Bodies from Human Pluripotent Stem Cells. Stem Cell. Dev. 2015, 24, 1595–1609. [Google Scholar] [CrossRef]
- Joannides, A.J.; Fiore-Hériché, C.; Battersby, A.A.; Athauda-Arachchi, P.; Bouhon, I.A.; Williams, L.; Westmore, K.; Kemp, P.J.; Compston, A.; Allen, N.D.; et al. A Scaleable and Defined System for Generating Neural Stem Cells from Human Embryonic Stem Cells. Stem Cell. 2007, 25, 731–737. [Google Scholar] [CrossRef]
- Lock, L.T.; Tzanakakis, E.S. Expansion and Differentiation of Human Embryonic Stem Cells to Endoderm Progeny in a Microcarrier Stirred-Suspension Culture. Tissue Eng. Part A 2009, 15, 2051–2063. [Google Scholar] [CrossRef]
- Nieden, N.I.Z.; Cormier, J.T.; Rancourt, D.E.; Kallos, M.S. Embryonic stem cells remain highly pluripotent following long term expansion as aggregates in suspension bioreactors. J. Biotechnol. 2007, 129, 421–432. [Google Scholar] [CrossRef]
- Taiani, J.T.; Krawetz, R.J.; Nieden, N.I.Z.; Wu, Y.E.; Kallos, M.S.; Matyas, J.R.; Rancourt, D.E. Reduced Differentiation Efficiency of Murine Embryonic Stem Cells in Stirred Suspension Bioreactors. Stem Cell. Dev. 2010, 19, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Ueno, M.; Kamiya, D.; Nishiyama, A.; Matsumura, M.; Wataya, T.; Takahashi, J.B.; Nishikawa, S.; Nishikawa, S.-I.; Muguruma, K.; et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 2007, 25, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Chaddah, R.; Arntfield, M.; Runciman, S.; Clarke, L.; Van Der Kooy, D. Clonal Neural Stem Cells from Human Embryonic Stem Cell Colonies. J. Neurosci. 2012, 32, 7771–7781. [Google Scholar] [CrossRef]
- Ng, E.S.; Davis, R.; Azzola, L.; Stanley, E.G.; Elefanty, A. Forced aggregation of defined numbers of human embryonic stem cells into embryoid bodies fosters robust, reproducible hematopoietic differentiation. Blood 2005, 106, 1601–1603. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, S.D.; Surampudi, V.; Rao, R.R. Analysis of Embryoid Bodies Derived from Human Induced Pluripotent Stem Cells as a Means to Assess Pluripotency. Stem Cell. Int. 2012, 2012, 738910. [Google Scholar] [CrossRef] [PubMed]
- Higgins, G.; Anderson, R.; Higgins, G.; Anderson, R. Experimental pathology of the liver: Restoration of the liver of the white rat following partial surgical removal. Arch. Pathol. 1931, 12, 186–202. [Google Scholar]
- Yanger, K.; Knigin, D.; Zong, Y.; Maggs, L.; Gu, G.; Akiyama, H.; Pikarsky, E.; Stanger, B.Z. Adult Hepatocytes Are Generated by Self-Duplication Rather than Stem Cell Differentiation. Cell Stem Cell 2014, 15, 340–349. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, Y.G.; Jia, Y.; Li, L.; Yoon, J.; Zhang, S.; Wang, Z.; Zhang, Y.; Zhu, M.; Sharma, T.; et al. Liver homeostasis is maintained by midlobular zone 2 hepatocytes. Science 2021, 371, eabb1625. [Google Scholar] [CrossRef]
- Tsomaia, K.; Patarashvili, L.; Karumidze, N.; Bebiashvili, I.; Az-Maipharashvili, E.; Modebadze, I.; Dzidziguri, D.; Sareli, M.; Gusev, S.; Kordzaia, D. Liver structural transformation after partial hepatectomy and repeated partial hepatectomy in rats: A renewed view on liver regeneration. World J. Gastroenterol. 2020, 26, 3899–3916. [Google Scholar] [CrossRef]
- Kholodenko, I.V.; Yarygin, K.N. Cellular Mechanisms of Liver Regeneration and Cell-Based Therapies of Liver Diseases. BioMed Res. Int. 2017, 2017, 8910821. [Google Scholar] [CrossRef]
- Sato, Y.; Koyama, S.; Tsukada, K.; Hatakeyama, K. Acute portal hypertension reflecting shear stress as a trigger of liver regeneration following partial hepatectomy. Surg. Today 1997, 27, 518–526. [Google Scholar] [CrossRef]
- Lory, J.; Schweizer, W.; Blumgart, L.H.; Zimmermann, A. The pathology of the atrophy/hypertrophy complex (AHC) of the liver. A light microscopic and immunohistochemical study. Histol. Histopathol. 1994, 9, 541–554. [Google Scholar] [PubMed]
- García, I.C.; Villalba, J.S.; Iovino, D.; Franchi, C.; Iori, V.; Pettinato, G.; Inversini, D.; Amico, F.; Ietto, G. Liver Trauma: Until When We Have to Delay Surgery? A Review. Life 2022, 12, 694. [Google Scholar] [CrossRef] [PubMed]
- Poisson, J.; Lemoinne, S.; Boulanger, C.; Durand, F.; Moreau, R.; Valla, D.; Rautou, P.-E. Liver sinusoidal endothelial cells: Physiology and role in liver diseases. J. Hepatol. 2017, 66, 212–227. [Google Scholar] [CrossRef]
- Currier, A.R.; Sabla, G.; Locaputo, S.; Melin-Aldana, H.; Degen, J.L.; Bezerra, J.A. Plasminogen directs the pleiotropic effects of uPA in liver injury and repair. Am. J. Physiol. Liver Physiol. 2003, 284, G508–G515. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.A.; Strom, S. Human Hepatocyte Transplantation: Worldwide Results. Transplantation 2006, 82, 441–449. [Google Scholar] [CrossRef]
- Forbes, S.J.; Gupta, S.; Dhawan, A. Cell therapy for liver disease: From liver transplantation to cell factory. J. Hepatol. 2015, 62, S157–S169. [Google Scholar] [CrossRef]
- Kadyk, L.C.; Collins, L.R.; Littman, N.J.; Millan, M.T. Proceedings: Moving Toward Cell-Based Therapies for Liver Disease. Stem Cell. Transl. Med. 2015, 4, 207–210. [Google Scholar] [CrossRef]
- Nicolas, C.T.; Wang, Y.; Nyberg, S.L. Cell therapy in chronic liver disease. Curr. Opin. Gastroenterol. 2016, 32, 189–194. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, X.; Nyberg, S.L. Potential and Challenges of Induced Pluripotent Stem Cells in Liver Diseases Treatment. J. Clin. Med. 2014, 3, 997–1017. [Google Scholar] [CrossRef]
- Singh, V.K.; Kalsan, M.; Kumar, N.; Saini, A.; Chandra, R. Induced pluripotent stem cells: Applications in regenerative medicine, disease modeling, and drug discovery. Front. Cell Dev. Biol. 2015, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- D’Amour, K.A.; Agulnick, A.D.; Eliazer, S.; Kelly, O.G.; Kroon, E.; Baetge, E.E. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 2005, 23, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Cai, J.; Liu, Y.; Zhao, D.; Yong, J.; Duo, S.; Song, X.; Guo, Y.; Zhao, Y.; Qin, H.; et al. Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell Res. 2009, 19, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Touboul, T.; Hannan, N.R.F.; Corbineau, S.; Martinez, A.; Martinet, C.; Branchereau, S.; Mainot, S.; Strick-Marchand, H.; Pedersen, R.; Di Santo, J.; et al. Generation of functional hepatocytes from human embryonic stem cells under chemically defined conditions that recapitulate liver development. Hepatology 2010, 51, 1754–1765. [Google Scholar] [CrossRef]
- Ogawa, S.; Surapisitchat, J.; Virtanen, C.; Ogawa, M.; Niapour, M.; Sugamori, K.S.; Wang, S.; Tamblyn, L.; Guillemette, C.; Hoffmann, E.; et al. Three-dimensional culture and cAMP signaling promote the maturation of human pluripotent stem cell-derived hepatocytes. Development 2013, 140, 3285–3296. [Google Scholar] [CrossRef] [PubMed]
- Iii, R.L.G.; Hannan, N.; Bort, R.; Hanley, N.; Drake, R.A.L.; Cameron, G.W.W.; Wynn, T.; Vallier, L. Maturation of Induced Pluripotent Stem Cell Derived Hepatocytes by 3D-Culture. PLoS ONE 2014, 9, e86372. [Google Scholar] [CrossRef]
- Pettinato, G.; Coughlan, M.F.; Zhang, X.; Chen, L.; Khan, U.; Glyavina, M.; Sheil, C.J.; Upputuri, P.K.; Zakharov, Y.N.; Vitkin, E.; et al. Spectroscopic label-free microscopy of changes in live cell chromatin and biochemical composition in transplantable organoids. Sci. Adv. 2021, 7, eabj2800. [Google Scholar] [CrossRef]
- Duncan, S.A. Transcriptional regulation of liver development. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2000, 219, 131–142. [Google Scholar] [CrossRef]
- Duboc, V.; Lapraz, F.; Saudemont, A.; Bessodes, N.; Mekpoh, F.; Haillot, E.; Quirin, M.; Lepage, T. Nodal and BMP2/4 pattern the mesoderm and endoderm during development of the sea urchin embryo. Development 2010, 137, 223–235. [Google Scholar] [CrossRef]
- Morrison, G.M.; Oikonomopoulou, I.; Migueles, R.P.; Soneji, S.; Livigni, A.; Enver, T.; Brickman, J.M. Anterior Definitive Endoderm from ESCs Reveals a Role for FGF Signaling. Cell Stem Cell 2008, 3, 402–415. [Google Scholar] [CrossRef]
- Gadue, P.; Huber, T.L.; Paddison, P.J.; Keller, G.M. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc. Natl. Acad. Sci. USA 2006, 103, 16806–16811. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Holton, K.L.; Lanza, R. Efficient Differentiation of Functional Hepatocytes from Human Embryonic Stem Cells. Stem Cell. 2008, 26, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Zheng, M.; Goldfarb, M.; Zaret, K.S. Initiation of Mammalian Liver Development from Endoderm by Fibroblast Growth Factors. Science 1999, 284, 1998–2003. [Google Scholar] [CrossRef] [PubMed]
- Rossi, J.M.; Dunn, N.R.; Hogan, B.L.; Zaret, K.S. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev. 2001, 15, 1998–2009. [Google Scholar] [CrossRef]
- Kamiya, A.; Kinoshita, T.; Ito, Y.; Matsui, T.; Morikawa, Y.; Senba, E.; Nakashima, K.; Taga, T.; Yoshida, K.; Kishimoto, T.; et al. Fetal liver development requires a paracrine action of oncostatin M through the gp130 signal transducer. EMBO J. 1999, 18, 2127–2136. [Google Scholar] [CrossRef]
- Huang, P.; He, Z.; Ji, S.; Sun, H.; Xiang, D.; Liu, C.; Hu, Y.; Wang, X.; Hui, L. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature 2011, 475, 386–389. [Google Scholar] [CrossRef]
- Nakamori, D.; Takayama, K.; Nagamoto, Y.; Mitani, S.; Sakurai, F.; Tachibana, M.; Mizuguchi, H. Hepatic maturation of human iPS cell-derived hepatocyte-like cells by ATF5, c/EBPα, and PROX1 transduction. Biochem. Biophys. Res. Commun. 2016, 469, 424–429. [Google Scholar] [CrossRef]
- Snykers, S.; De Kock, J.; Rogiers, V.; Vanhaecke, T. In Vitro Differentiation of Embryonic and Adult Stem Cells into Hepatocytes: State of the Art. Stem Cell. 2009, 27, 577–605. [Google Scholar] [CrossRef]
- Cadigan, K.M.; Nusse, R. Wnt signaling: A common theme in animal development. Genes Dev. 1997, 11, 3286–3305. [Google Scholar] [CrossRef]
- Hoeflich, K.P.; Luo, J.; Rubie, E.A.; Tsao, M.-S.; Jin, O.; Woodgett, J.R. Requirement for glycogen synthase kinase-3β in cell survival and NF-κB activation. Nature 2000, 406, 86–90. [Google Scholar] [CrossRef]
- Monga, S.; Monga, H.K.; Tan, X.; Mulé, K.; Pediaditakis, P.; Michalopoulos, G.K. β-catenin antisense studies in embryonic liver cultures: Role in proliferation, apoptosis, and lineage specification. Gastroenterology 2003, 124, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Nejak-Bowen, K.; Monga, S.P. Wnt/β-catenin signaling in hepatic organogenesis. Organogenesis 2008, 4, 92–99. [Google Scholar] [CrossRef] [PubMed]
- McLin, V.A.; Rankin, S.A.; Zorn, A.M. Repression of Wnt/β-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development 2007, 134, 2207–2217. [Google Scholar] [CrossRef] [PubMed]
- Decaens, T.; Godard, C.; de Reyniès, A.; Rickman, D.S.; Tronche, F.; Couty, J.-P.; Perret, C.; Colnot, S. Stabilization of β-catenin affects mouse embryonic liver growth and hepatoblast fate. Hepatology 2008, 47, 247–258. [Google Scholar] [CrossRef] [PubMed]
- So, J.; Martin, B.; Kimelman, D.; Shin, D. Wnt/β-catenin signaling cell-autonomously converts non-hepatic endodermal cells to a liver fate. Biol. Open 2013, 2, 30–36. [Google Scholar] [CrossRef]
- Sekiya, S.; Suzuki, A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature 2011, 475, 390–393. [Google Scholar] [CrossRef]
- Yahoo, N.; Pournasr, B.; Rostamzadeh, J.; Hakhamaneshi, M.S.; Ebadifar, A.; Fathi, F.; Baharvand, H. Forced expression of Hnf1b/Foxa3 promotes hepatic fate of embryonic stem cells. Biochem. Biophys. Res. Commun. 2016, 474, 199–205. [Google Scholar] [CrossRef]
- Yahoo, N.; Pournasr, B.; Rostamzadeh, J.; Fathi, F. Forced expression of Hnf4a induces hepatic gene activation through directed differentiation. Biochem. Biophys. Res. Commun. 2016, 476, 313–318. [Google Scholar] [CrossRef]
- Cao, H.; Yang, J.; Yu, J.; Pan, Q.; Li, J.; Zhou, P.; Li, Y.; Pan, X.; Li, J.; Wang, Y.; et al. Therapeutic potential of transplanted placental mesenchymal stem cells in treating Chinese miniature pigs with acute liver failure. BMC Med. 2012, 10, 56. [Google Scholar] [CrossRef]
- Hu, X.; Xie, P.; Li, W.; Li, Z.; Shan, H. Direct induction of hepatocyte-like cells from immortalized human bone marrow mesenchymal stem cells by overexpression of HNF4α. Biochem. Biophys. Res. Commun. 2016, 478, 791–797. [Google Scholar] [CrossRef]
- Bao, J.; Wu, Q.; Wang, Y.; Li, Y.; Li, L.; Chen, F.; Wu, X.; Xie, M.; Bu, H. Enhanced hepatic differentiation of rat bone marrow-derived mesenchymal stem cells in spheroidal aggregate culture on a decellularized liver scaffold. Int. J. Mol. Med. 2016, 38, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.-J.; Kang, S.-J.; Park, Y.-I.; Yang, Y.-H.; Bang, S.-I.; Park, Y.H.; So, B.; Cho, M.-H.; Kang, H.-G. Hepatic differentiation of human adipose tissue-derived mesenchymal stem cells and adverse effects of arsanilic acid and acetaminophen during in vitro hepatic developmental stage. Cell Biol. Toxicol. 2015, 31, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-Q.; Pan, X.-H.; Yao, L.; Li, W.; Cui, J.; Wang, G.; Mrsny, R.J.; Hoffman, A.R.; Hu, J.-F. Converting Skin Fibroblasts into Hepatic-like Cells by Transient Programming. J. Cell. Biochem. 2016, 117, 589–598. [Google Scholar] [CrossRef]
- De Kock, J.; Rodrigues, R.M.; Buyl, K.; Vanhaecke, T.; Rogiers, V. Human Skin-Derived Precursor Cells: Isolation, Expansion, and Hepatic Differentiation. Methods Mol. Biol. 2015, 1250, 113–122. [Google Scholar] [CrossRef]
- Chen, Z.; Kuang, Q.; Lao, X.-J.; Yang, J.; Huang, W.; Zhou, D. Differentiation of UC-MSCs into hepatocyte-like cells in partially hepatectomized model rats. Exp. Ther. Med. 2016, 12, 1775–1779. [Google Scholar] [CrossRef] [PubMed]
- Raut, A.; Khanna, A. High-throughput sequencing to identify microRNA signatures during hepatic differentiation of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells. Hepatol. Res. 2017, 47, 910–927. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, R.; Pettinato, G.; Beeston, J.T.; Lee, D.D.; Wen, X.; Mangino, M.J.; Fisher, R.A. Transplantation of human stem cell-derived hepatocytes in an animal model of acute liver failure. Surgery 2015, 158, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.G.; Mallon, B.S.; McKay, R.D.G.; Robey, P.G. Human Pluripotent Stem Cell Culture: Considerations for Maintenance, Expansion, and Therapeutics. Cell Stem Cell 2014, 14, 13–26. [Google Scholar] [CrossRef]
- Iwasawa, K.; Takebe, T. Organogenesis in vitro. Curr. Opin. Cell Biol. 2021, 73, 84–91. [Google Scholar] [CrossRef]
- Thompson, W.L.; Takebe, T. Human liver model systems in a dish. Dev. Growth Differ. 2021, 63, 47–58. [Google Scholar] [CrossRef]
- Thompson, W.L.; Takebe, T. Generation of multi-cellular human liver organoids from pluripotent stem cells. Methods Cell Biol. 2020, 159, 47–68. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A.; Hughey, R.; Lancin, P.; Larue, L.; Moghe, P.V. E-cadherin synergistically induces hepatospecific phenotype and maturation of embryonic stem cells in conjunction with hepatotrophic factors. Biotechnol. Bioeng. 2005, 92, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Frith, J.E.; Thomson, B.; Genever, P.G. Dynamic Three-Dimensional Culture Methods Enhance Mesenchymal Stem Cell Properties and Increase Therapeutic Potential. Tissue Eng. Part C Methods 2010, 16, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Vosough, M.; Omidinia, E.; Kadivar, M.; Shokrgozar, M.-A.; Pournasr, B.; Aghdami, N.; Baharvand, H. Generation of Functional Hepatocyte-Like Cells from Human Pluripotent Stem Cells in a Scalable Suspension Culture. Stem Cell. Dev. 2013, 22, 2693–2705. [Google Scholar] [CrossRef]
- Glicklis, R.; Shapiro, L.; Agbaria, R.; Merchuk, J.C.; Cohen, S. Hepatocyte behavior within three-dimensional porous alginate scaffolds. Biotechnol. Bioeng. 2000, 67, 344–353. [Google Scholar] [CrossRef]
- Ramaiahgari, S.C.; den Braver, M.W.; Herpers, B.; Terpstra, V.; Commandeur, J.N.M.; Van De Water, B.; Price, L.S. A 3D in vitro model of differentiated HepG2 cell spheroids with improved liver-like properties for repeated dose high-throughput toxicity studies. Arch. Toxicol. 2014, 88, 1083–1095. [Google Scholar] [CrossRef]
- Sivertsson, L.; Synnergren, J.; Jensen, J.; Björquist, P.; Ingelman-Sundberg, M. Hepatic Differentiation and Maturation of Human Embryonic Stem Cells Cultured in a Perfused Three-Dimensional Bioreactor. Stem Cell. Dev. 2013, 22, 581–594. [Google Scholar] [CrossRef]
- Ramasamy, T.S.; Yu, J.S.; Selden, A.; Hodgson, H.; Cui, W. Application of Three-Dimensional Culture Conditions to Human Embryonic Stem Cell-Derived Definitive Endoderm Cells Enhances Hepatocyte Differentiation and Functionality. Tissue Eng. Part A 2013, 19, 360–367. [Google Scholar] [CrossRef]
- Miki, T.; Ring, A.; Gerlach, J. Hepatic Differentiation of Human Embryonic Stem Cells Is Promoted by Three-Dimensional Dynamic Perfusion Culture Conditions. Tissue Eng. Part C Methods 2011, 17, 557–568. [Google Scholar] [CrossRef]
- Khetani, S.R.; Bhatia, S.N. Microscale culture of human liver cells for drug development. Nat. Biotechnol. 2008, 26, 120–126. [Google Scholar] [CrossRef]
- Subramanian, K.; Owens, D.J.; Raju, R.; Firpo, M.; O’Brien, T.; Verfaillie, C.M.; Hu, W.-S. Spheroid Culture for Enhanced Differentiation of Human Embryonic Stem Cells to Hepatocyte-Like Cells. Stem Cell. Dev. 2014, 23, 124–131. [Google Scholar] [CrossRef] [PubMed]
- An, S.Y.; Woo, D.-H.; Han, J.; Kim, J.H.; Jang, Y.J.; Son, J.S.; Yang, H.; Cheon, Y.P.; Wang, M.; Zhang, P.; et al. Engraftment Potential of Spheroid-Forming Hepatic Endoderm Derived from Human Embryonic Stem Cells. Stem Cell. Dev. 2013, 22, 1818–1829. [Google Scholar] [CrossRef]
- Zhang, R.-R.; Takebe, T.; Miyazaki, L.; Takayama, M.; Koike, H.; Kimura, M.; Enomura, M.; Zheng, Y.-W.; Sekine, K.; Taniguchi, H. Efficient Hepatic Differentiation of Human Induced Pluripotent Stem Cells in a Three-Dimensional Microscale Culture. Methods Mol. Biol. 2014, 1210, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Kawabata, K.; Nagamoto, Y.; Kishimoto, K.; Tashiro, K.; Sakurai, F.; Tachibana, M.; Kanda, K.; Hayakawa, T.; Furue, M.K.; et al. 3D spheroid culture of hESC/hiPSC-derived hepatocyte-like cells for drug toxicity testing. Biomaterials 2013, 34, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Stevens, K.R.; Ungrin, M.D.; Schwartz, R.E.; Ng, S.; Carvalho, B.; Christine, K.S.; Chaturvedi, R.R.; Li, C.Y.; Zandstra, P.W.; Chen, C.S.; et al. InVERT molding for scalable control of tissue microarchitecture. Nat. Commun. 2013, 4, 1847. [Google Scholar] [CrossRef]
- Gothard, D.; Roberts, S.J.; Shakesheff, K.M.; Buttery, L.D. Controlled embryoid body formation via surface modification and avidin–biotin cross-linking. Cytotechnology 2009, 61, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Buttery, L.D.K.; Bourne, S.; Xynos, J.D.; Wood, H.; Hughes, F.J.; Hughes, S.P.F.; Episkopou, V.; Polak, J.M. Differentiation of Osteoblasts and in Vitro Bone Formation from Murine Embryonic Stem Cells. Tissue Eng. 2001, 7, 89–99. [Google Scholar] [CrossRef]
- Yirme, G.; Amit, M.; Laevsky, I.; Osenberg, S.; Itskovitz-Eldor, J. Establishing a Dynamic Process for the Formation, Propagation, and Differentiation of Human Embryoid Bodies. Stem Cell. Dev. 2008, 17, 1227–1242. [Google Scholar] [CrossRef]
- Feraud, O.; Cao, Y.; Vittet, D. Embryonic Stem Cell-Derived Embryoid Bodies Development in Collagen Gels Recapitulates Sprouting Angiogenesis. Lab. Investig. 2001, 81, 1669–1681. [Google Scholar] [CrossRef]
- Pal, R.; Mamidi, M.K.; Das, A.K.; Gupta, P.K.; Bhonde, R. A Simple and economical route to generate functional hepatocyte-like cells from hESCs and their application in evaluating alcohol induced liver damage. J. Cell. Biochem. 2012, 113, 19–30. [Google Scholar] [CrossRef]
- Berger, D.R.; Ware, B.R.; Davidson, M.D.; Allsup, S.R.; Khetani, S.R. Enhancing the functional maturity of induced pluripotent stem cell–derived human hepatocytes by controlled presentation of cell–cell interactions in vitro. Hepatology 2015, 61, 1370–1381. [Google Scholar] [CrossRef]
- Soto-Gutiérrez, A.; Navarro-Alvarez, N.; Zhao, D.; Rivascarrillo, J.D.; Lebkowski, J.; Tanaka, N.; Fox, I.J.; Kobayashi, N. Differentiation of mouse embryonic stem cells to hepatocyte-like cells by co-culture with human liver nonparenchymal cell lines. Nat. Protoc. 2007, 2, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Dziedzic, N.; Gadue, P.; Keller, G.M.; Gouon-Evans, V. An Endothelial Cell Niche Induces Hepatic Specification Through Dual Repression of Wnt and Notch Signaling. Stem Cell. 2011, 29, 217–228. [Google Scholar] [CrossRef]
- Takebe, T.; Zhang, R.; Koike, H.; Kimura, M.; Yoshizawa, E.; Enomura, M.; Koike, N.; Sekine, K.; Taniguchi, H. Generation of a vascularized and functional human liver from an iPSC-derived organ bud transplant. Nat. Protoc. 2014, 9, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Gou, J.; Deng, N.; Shen, H.; He, T.; Zhang, B.-Q. Three-dimensional Co-culture of hepatic progenitor cells and mesenchymal stem cells in vitro and in vivo. Microsc. Res. Tech. 2015, 78, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Mizumoto, H.; Aoki, K.; Nakazawa, K.; Ijima, H.; Funatsu, K.; Kajiwara, T. Hepatic Differentiation of Embryonic Stem Cells in HF/Organoid Culture. Transplant. Proc. 2008, 40, 611–613. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Tuleuova, N.; Jones, C.N.; Ramanculov, E.; Zern, M.A.; Revzin, A. Directing hepatic differentiation of embryonic stem cells with protein microarray-based co-cultures. Integr. Biol. 2009, 1, 460–468. [Google Scholar] [CrossRef]
- Li, L.; Sharma, N.; Chippada, U.; Jiang, X.; Schloss, R.; Yarmush, M.L.; Langrana, N.A. Functional Modulation of ES-Derived Hepatocyte Lineage Cells via Substrate Compliance Alteration. Ann. Biomed. Eng. 2008, 36, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Amimoto, N.; Mizumoto, H.; Nakazawa, K.; Ijima, H.; Funatsu, K.; Kajiwara, T. Hepatic Differentiation of Mouse Embryonic Stem Cells and Induced Pluripotent Stem Cells During Organoid Formation in Hollow Fibers. Tissue Eng. Part A 2011, 17, 2071–2078. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, S.; Chen, X.; Li, G.; Wang, Y. Hepatic Differentiation of Mouse Embryonic Stem Cells in Three-Dimensional Polymer Scaffolds. Tissue Eng. Part A 2010, 16, 1115–1122. [Google Scholar] [CrossRef]
- Farzaneh, Z.; Pournasr, B.; Ebrahimi, M.; Aghdami, N.; Baharvand, H. Enhanced Functions of Human Embryonic Stem Cell-derived Hepatocyte-like Cells on Three-dimensional Nanofibrillar Surfaces. Stem Cell Rev. Rep. 2010, 6, 601–610. [Google Scholar] [CrossRef]
- Fang, S.; Qiu, Y.-D.; Mao, L.; Shi, X.-L.; Yu, D.-C.; Ding, Y.-T. Differentiation of embryoid-body cells derived from embryonic stem cells into hepatocytes in alginate microbeads in vitro. Acta Pharmacol. Sin. 2007, 28, 1924–1930. [Google Scholar] [CrossRef] [PubMed]
- Malinen, M.M.; Kanninen, L.K.; Corlu, A.; Isoniemi, H.M.; Lou, Y.-R.; Yliperttula, M.L.; Urtti, A.O. Differentiation of liver progenitor cell line to functional organotypic cultures in 3D nanofibrillar cellulose and hyaluronan-gelatin hydrogels. Biomaterials 2014, 35, 5110–5121. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Hexig, B.; Meng, Q.; Hossain, S.; Nagaoka, M.; Akaike, T. The effect of recombinant E-cadherin substratum on the differentiation of endoderm-derived hepatocyte-like cells from embryonic stem cells. Biomaterials 2011, 32, 2032–2042. [Google Scholar] [CrossRef] [PubMed]
- Kanninen, L.K.; Porola, P.; Niklander, J.; Malinen, M.M.; Corlu, A.; Guguen-Guillouzo, C.; Urtti, A.; Yliperttula, M.L.; Lou, Y.-R. Hepatic differentiation of human pluripotent stem cells on human liver progenitor HepaRG-derived acellular matrix. Exp. Cell Res. 2016, 341, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Kanninen, L.K.; Harjumäki, R.; Peltoniemi, P.; Bogacheva, M.S.; Salmi, T.; Porola, P.; Niklander, J.; Smutný, T.; Urtti, A.; Yliperttula, M.L.; et al. Laminin-511 and laminin-521-based matrices for efficient hepatic specification of human pluripotent stem cells. Biomaterials 2016, 103, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Michielin, F.; Giobbe, G.G.; Luni, C.; Hu, Q.; Maroni, I.; Orford, M.R.; Manfredi, A.; Di Filippo, L.; David, A.L.; Cacchiarelli, D.; et al. The Microfluidic Environment Reveals a Hidden Role of Self-Organizing Extracellular Matrix in Hepatic Commitment and Organoid Formation of hiPSCs. Cell Rep. 2020, 33, 108453. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Shi, Y.; Sun, H.; Yin, X.; Yang, R.; Li, L.; Chen, X.; Bu, H. Construction of a Portal Implantable Functional Tissue-Engineered Liver Using Perfusion-Decellularized Matrix and Hepatocytes in Rats. Cell Transplant. 2011, 20, 753–766. [Google Scholar] [CrossRef]
- Skardal, A.; Smith, L.; Bharadwaj, S.; Atala, A.; Soker, S.; Zhang, Y. Tissue specific synthetic ECM hydrogels for 3-D in vitro maintenance of hepatocyte function. Biomaterials 2012, 33, 4565–4575. [Google Scholar] [CrossRef]
- Geerts, S.; Ozer, S.; Jaramillo, M.; Yarmush, M.L.; Uygun, B.E. Nondestructive Methods for Monitoring Cell Removal During Rat Liver Decellularization. Tissue Eng. Part C Methods 2016, 22, 671–678. [Google Scholar] [CrossRef]
- Mazza, G.; Rombouts, K.; Hall, A.R.; Urbani, L.; Luong, T.V.; Al-Akkad, W.; Longato, L.; Brown, D.; Maghsoudlou, P.; Dhillon, A.P.; et al. Decellularized human liver as a natural 3D-scaffold for liver bioengineering and transplantation. Sci. Rep. 2015, 5, 13079. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, M.N. Mechanisms of human embryo development: From cell fate to tissue shape and back. Development 2020, 147, dev190629. [Google Scholar] [CrossRef]
- Harrison, S.E.; Sozen, B.; Christodoulou, N.; Kyprianou, C.; Zernicka-Goetz, M. Assembly of embryonic and extraembryonic stem cells to mimic embryogenesis in vitro. Science 2017, 356, eaal1810. [Google Scholar] [CrossRef] [PubMed]
- Al Reza, H.; Okabe, R.; Takebe, T. Organoid transplant approaches for the liver. Transpl. Int. 2021, 34, 2031–2045. [Google Scholar] [CrossRef]
- Tirziu, D.; Simons, M. Endothelium as master regulator of organ development and growth. Vasc. Pharmacol. 2009, 50, 1–7. [Google Scholar] [CrossRef]
- Belle, M.; Godefroy, D.; Couly, G.; Malone, S.A.; Collier, F.; Giacobini, P.; Chédotal, A. Tridimensional Visualization and Analysis of Early Human Development. Cell 2017, 169, 161–173.e12. [Google Scholar] [CrossRef]
- Matsumoto, K.; Yoshitomi, H.; Rossant, J.; Zaret, K.S. Liver Organogenesis Promoted by Endothelial Cells Prior to Vascular Function. Science 2001, 294, 559–563. [Google Scholar] [CrossRef]
- Rafii, S.; Butler, J.M.; Ding, B.-S. Angiocrine functions of organ-specific endothelial cells. Nature 2016, 529, 316–325. [Google Scholar] [CrossRef]
- Butler, J.M.; Kobayashi, H.; Rafii, S. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat. Rev. Cancer 2010, 10, 138–146. [Google Scholar] [CrossRef]
- Nolan, D.J.; Ginsberg, M.; Israely, E.; Palikuqi, B.; Poulos, M.G.; James, D.; Ding, B.-S.; Schachterle, W.; Liu, Y.; Rosenwaks, Z.; et al. Molecular Signatures of Tissue-Specific Microvascular Endothelial Cell Heterogeneity in Organ Maintenance and Regeneration. Dev. Cell 2013, 26, 204–219. [Google Scholar] [CrossRef]
- Takebe, T.; Sekine, K.; Enomura, M.; Koike, H.; Kimura, M.; Ogaeri, T.; Zhang, R.; Ueno, Y.; Zheng, Y.-W.; Koike, N.; et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013, 499, 481–484. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, L.; Fish, M.; Logan, C.Y.; Nusse, R. Self-renewing diploid Axin2+ cells fuel homeostatic renewal of the liver. Nature 2015, 524, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Michalopoulos, G.K.; Grompe, M.; Theise, N.D. Assessing the potential of induced liver regeneration. Nat. Med. 2013, 19, 1096–1097. [Google Scholar] [CrossRef] [PubMed]
- Pettinato, G.; Lehoux, S.; Ramanathan, R.; Salem, M.M.; He, L.-X.; Muse, O.; Flaumenhaft, R.; Thompson, M.T.; Rouse, E.A.; Cummings, R.D.; et al. Generation of fully functional hepatocyte-like organoids from human induced pluripotent stem cells mixed with Endothelial Cells. Sci. Rep. 2019, 9, 1–21. [Google Scholar] [CrossRef]
- Pettinato, G. Generation of Hepatocyte Organoids from Human iPS Cells. In Hepatocytes: Methods and Protocols; Springer: New York, NY, USA, 2022; Volume 2544. [Google Scholar] [CrossRef]
- Sun, X.; Aghazadeh, Y.; Nunes, S.S. Isolation of ready-made rat microvessels and its applications in effective in vivo vascularization and in angiogenic studies in vitro. Nat. Protoc. 2022, 17, 2721–2738. [Google Scholar] [CrossRef] [PubMed]
- Panwar, A.; Das, P.; Tan, L.P. 3D Hepatic Organoid-Based Advancements in LIVER Tissue Engineering. Bioengineering 2021, 8, 185. [Google Scholar] [CrossRef]
- Lee, H.; Mun, S.J.; Jung, C.; Kang, H.; Kwon, J.; Ryu, J.; Ahn, H.; Kwon, O.; Ahn, J.; Moon, K.; et al. In vitro modeling of liver fibrosis with 3D co-culture system using a novel human hepatic stellate cell line. Biotechnol. Bioeng. 2023, 120, 1241–1253. [Google Scholar] [CrossRef]
- Pastore, M.; Caligiuri, A.; Raggi, C.; Navari, N.; Piombanti, B.; Di Maira, G.; Rovida, E.; Piccinni, M.-P.; Lombardelli, L.; Logiodice, F.; et al. Macrophage MerTK promotes profibrogenic cross-talk with hepatic stellate cells via soluble mediators. JHEP Rep. 2022, 4, 100444. [Google Scholar] [CrossRef]
- Nam, D.; Park, M.R.; Lee, H.; Bae, S.C.; Gerovska, D.; Araúzo-Bravo, M.J.; Zaehres, H.; Schöler, H.R.; Kim, J.B. Induced Endothelial Cell-Integrated Liver Assembloids Promote Hepatic Maturation and Therapeutic Effect on Cholestatic Liver Fibrosis. Cells 2022, 11, 2242. [Google Scholar] [CrossRef]
- Guo, Q.; Furuta, K.; Islam, S.; Caporarello, N.; Kostallari, E.; Dielis, K.; Tschumperlin, D.J.; Hirsova, P.; Ibrahim, S.H. Liver sinusoidal endothelial cell expressed vascular cell adhesion molecule 1 promotes liver fibrosis. Front. Immunol. 2022, 13, 4826. [Google Scholar] [CrossRef]
- Cao, D.; Ge, J.-Y.; Wang, Y.; Oda, T.; Zheng, Y.-W. Hepatitis B virus infection modeling using multi-cellular organoids derived from human induced pluripotent stem cells. World J. Gastroenterol. 2021, 27, 4784–4801. [Google Scholar] [CrossRef] [PubMed]
- Brovold, M.; Keller, D.; Soker, S. Differential fibrotic phenotypes of hepatic stellate cells within 3D liver organoids. Biotechnol. Bioeng. 2020, 117, 2516–2526. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, R.; Togo, S.; Kimura, M.; Shinozawa, T.; Koido, M.; Koike, H.; Thompson, W.; Karns, R.A.; Mayhew, C.; McGrath, P.S.; et al. Modeling Steatohepatitis in Humans with Pluripotent Stem Cell-Derived Organoids. Cell Metab. 2019, 30, 374–384.e6. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, T.; Shimokawa, Y.; Koike, H.; Kimura, M.; Kawano, Y.; Okuma, N.; Kawamura, R.; Yoneyama, Y.; Furuichi, Y.; Hakuno, F.; et al. Mechanical guidance of self-condensation patterns of differentiating progeny. iScience 2022, 25, 105109. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, G.; Chi, K.Y.; Kim, H.; Jang, Y.J.; Jo, S.; Lee, J.; Lee, Y.; Woo, D.-H.; Han, C.; et al. Generation of multilineage liver organoids with luminal vasculature and bile ducts from human pluripotent stem cells via modulation of Notch signaling. Stem Cell Res. Ther. 2023, 14, 19. [Google Scholar] [CrossRef]
| References | Protocol’s Growth Factors |
|---|---|
| Agarwal et al. [53] | ActA/FGF4, HGF/BSA, FGF4, HGF/FGF4, HGF, OSM, DEX |
| Song et al. [54] | ActA /BMP2, FGF4/HGF, KGF/OSM, DEX/OSM, DEX, N2, B27 |
| Touboul et al. [55] | ActA, FGF2/LY-294002, ActA, BMP4, FGF2/FGF10/FGF10, RA, SB431542/FGF4, HGF, EGF |
| Si-Tayeb et al. [9] | ActA/BMP4, FGF2/HGF/OSM |
| Vosough et al. [55] | 3D differentiation: ActA, Rapa/FGF4, HGF/OSM, DEX |
| Ogawa et al. [56] | Mix of 3D aggregation and cAMP signaling/Act A, Wnt3a, BMP4/B27, FGF10, FGF2, BMP4/HGF, OSM, DEX |
| Gieseck et al. [57] | ActA, FGF2, BMP4, LY-294002, CHIR99021/Hepatozyme-SFM, HGF, OSM |
| Pettinato et al. [8] | 3D differentiation: ActA, bFGF, TGFb-1/FGF4, BMP4/DKK-1, WIF-1/OSM, HGF |
| Pettinato et al. [58] | 3D differentiation: ActA, bFGF, TGFb-1, MK-4101/FGF4, BMP4, LY-41575/DKK-1, WIF-1/OSM, HGF, DEX |
| Cell Type | Pros | Cons | 2D Culture | 3D Culture |
|---|---|---|---|---|
| Primary hepatocytes isolated from liver | Fully mature and ideal for self-transplantation. | Loss of function in vitro after isolation. Hard to maintain in culture. | Biomatrices, Type IV collagen, laminin, matrigel, soft collagen. | Fiber bonding, freeze drying, gas foaming, melt molding, particulate leaching, and phase separation. |
| Human embryonic stem cells (hESCs) | Pluripotent capabilities to obtain any type of cells. | Ethical debates, possible generation of teratomas. | Biomatrices, collagen, matrigel, vitronectin. | Biodegradable polymers, hollow fiber, rotating bioreactor, 3D spheroid culture systems. |
| Human-induced pluripotent stem cells (hiPSCs) | Exclude ethical debates, patient’s autologous generation prevent immune suppression/rejection. | Epigenetic memory that might impair differentiation abilities. | Biomatrices, collagen, matrigel, vitronectin. | Hollow fiber/organoids, micro-cavitary hydrogel (MCG) system, Swiss 3T3 cell sheets, 3D spheroid culture systems. |
| Hepatic progenitor cells | Able to differentiate into mature hepatocyte. | Challenging to isolate. | Type IV collagen or laminin. | Bioartificial liver systems, biomatrix scaffolds, fibroblast feeder layers, nanofiber and alginate scaffolds, 3D collagen gel matrix, 3D matrixes of poly (ethylene glycol)-bpoly-(L-alanine) thermogel. |
| Mesenchymal stem cells: Adipose tissue, bone marrow, placental cells, umbilical cord amniotic cells. | Possibility to be isolated from the same patient avoiding immune rejection | Difficult to differentiate because of epigenetic memory and need of an initial dedifferentiation step. | Biomatrices, Type IV collagen, laminin, matrigel, soft collagen | Bioartificial liver systems, nanofibers and alginate scaffolds 3D matrixes of poly (ethylene glycol)-b-poly-(L-alanine) thermogel. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ietto, G.; Iori, V.; Gritti, M.; Inversini, D.; Costantino, A.; Izunza Barba, S.; Jiang, Z.G.; Carcano, G.; Dalla Gasperina, D.; Pettinato, G. Multicellular Liver Organoids: Generation and Importance of Diverse Specialized Cellular Components. Cells 2023, 12, 1429. https://doi.org/10.3390/cells12101429
Ietto G, Iori V, Gritti M, Inversini D, Costantino A, Izunza Barba S, Jiang ZG, Carcano G, Dalla Gasperina D, Pettinato G. Multicellular Liver Organoids: Generation and Importance of Diverse Specialized Cellular Components. Cells. 2023; 12(10):1429. https://doi.org/10.3390/cells12101429
Chicago/Turabian StyleIetto, Giuseppe, Valentina Iori, Mattia Gritti, Davide Inversini, Angelita Costantino, Sofia Izunza Barba, Z. Gordon Jiang, Giulio Carcano, Daniela Dalla Gasperina, and Giuseppe Pettinato. 2023. "Multicellular Liver Organoids: Generation and Importance of Diverse Specialized Cellular Components" Cells 12, no. 10: 1429. https://doi.org/10.3390/cells12101429
APA StyleIetto, G., Iori, V., Gritti, M., Inversini, D., Costantino, A., Izunza Barba, S., Jiang, Z. G., Carcano, G., Dalla Gasperina, D., & Pettinato, G. (2023). Multicellular Liver Organoids: Generation and Importance of Diverse Specialized Cellular Components. Cells, 12(10), 1429. https://doi.org/10.3390/cells12101429







