Caveolae Mechanotransduction at the Interface between Cytoskeleton and Extracellular Matrix
Abstract
1. Introduction
2. Caveolae: Composition, Organization, and Function
2.1. Caveolae Composition and Organization
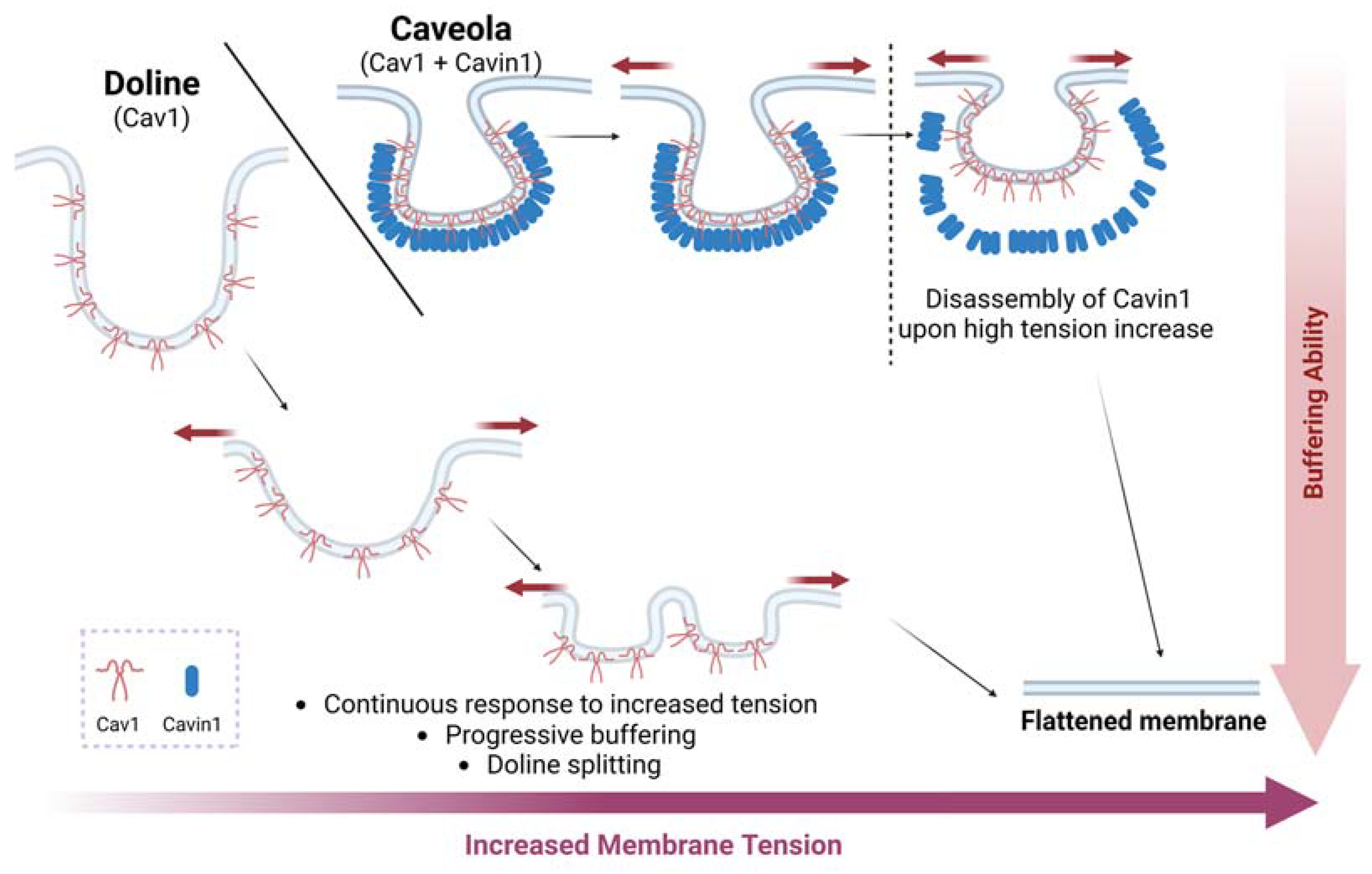
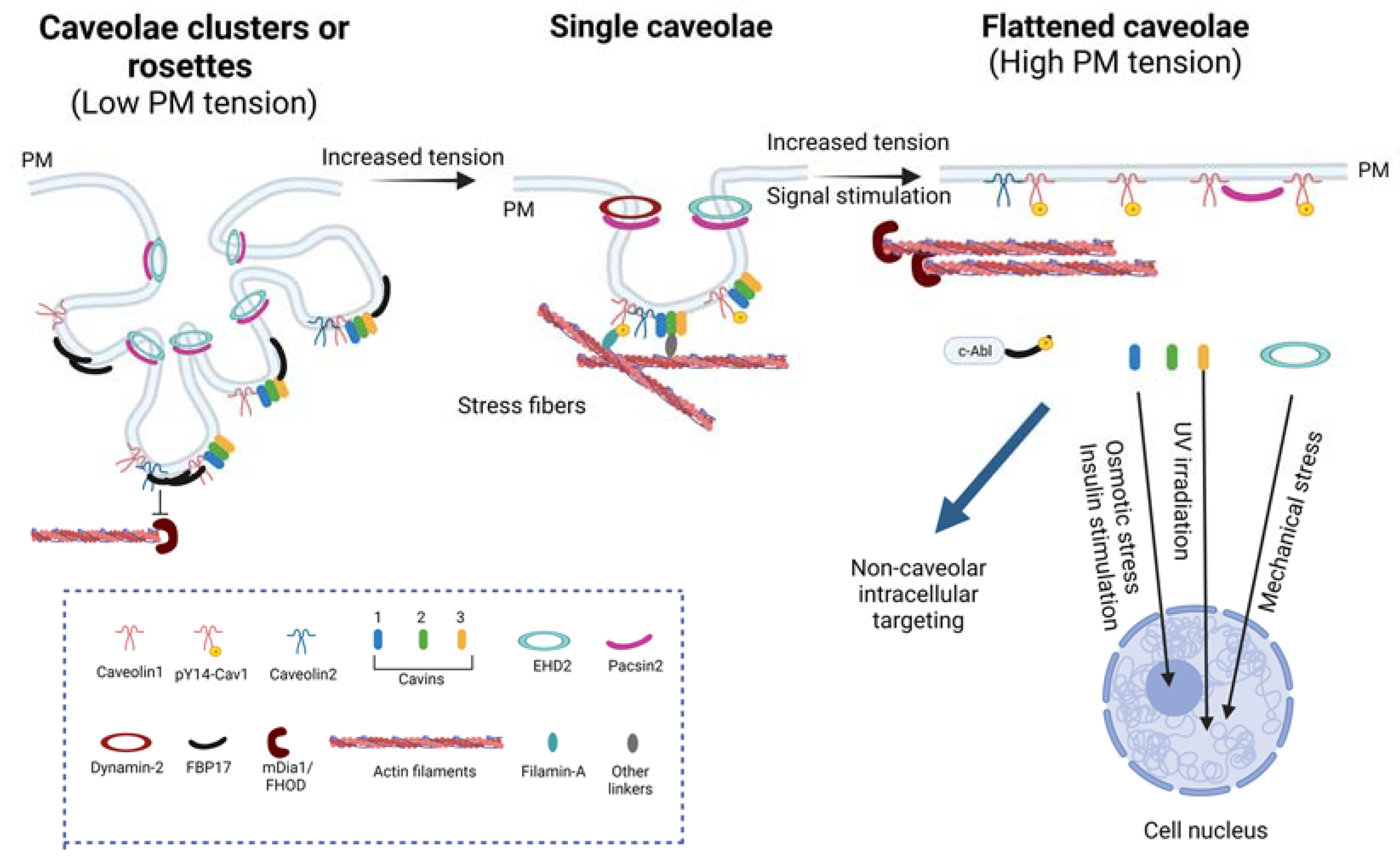
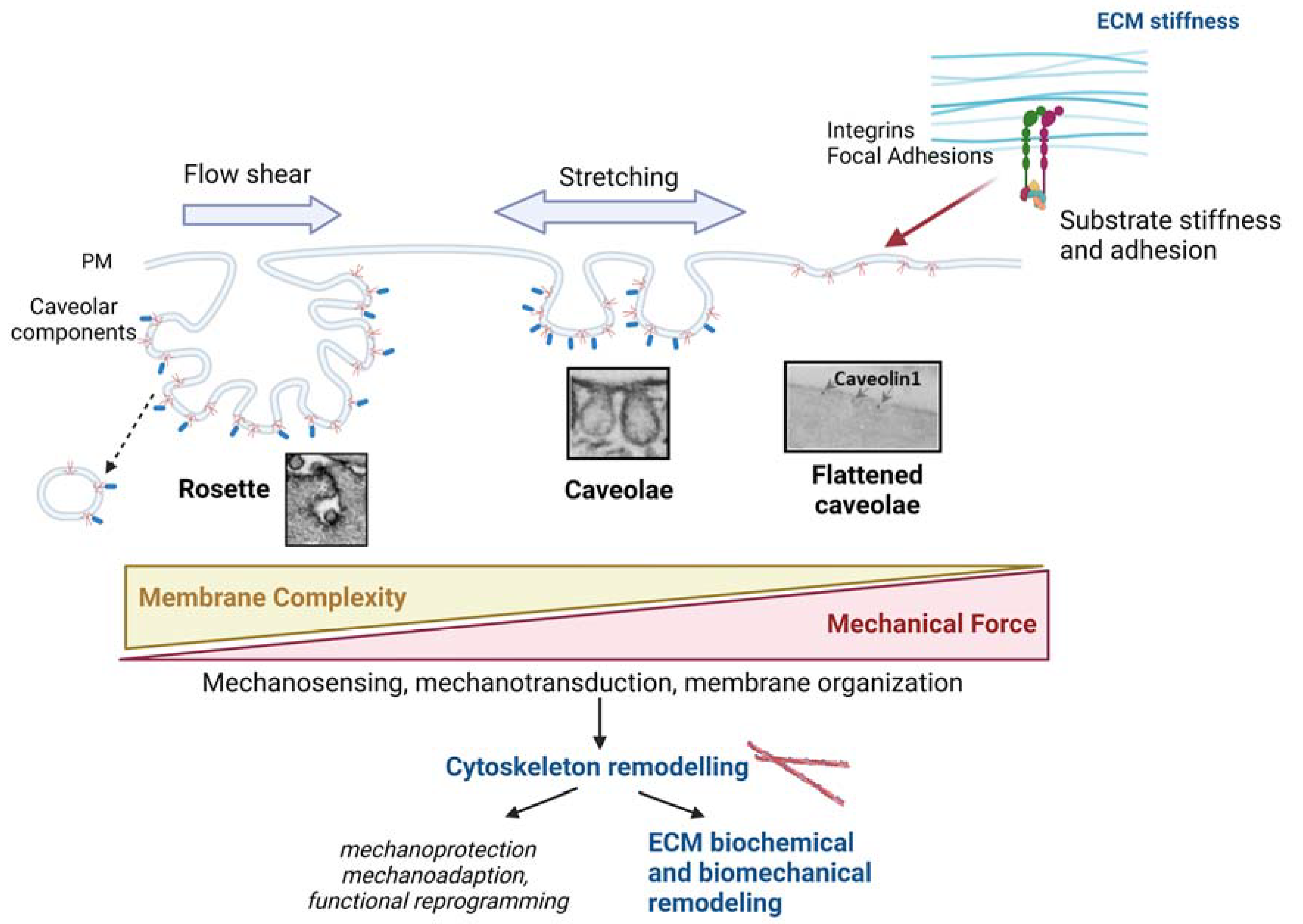
2.2. Caveolae as Mechanosensors and Mechanotransducers
2.3. Caveolae in Disease
3. Interaction between Caveolae and Cytoskeleton
3.1. Actin Cytoskeleton and Caveolae
3.1.1. Regulation of Caveolae by the Actin Cytoskeleton
3.1.2. Converse Regulation of Actin Filaments by Caveolar Components
3.2. Microtubules and Caveolae
3.3. Intermediate Filaments (IFs) and Caveolae
4. Caveolae/Cav1 as Regulators of ECM Composition and Architecture
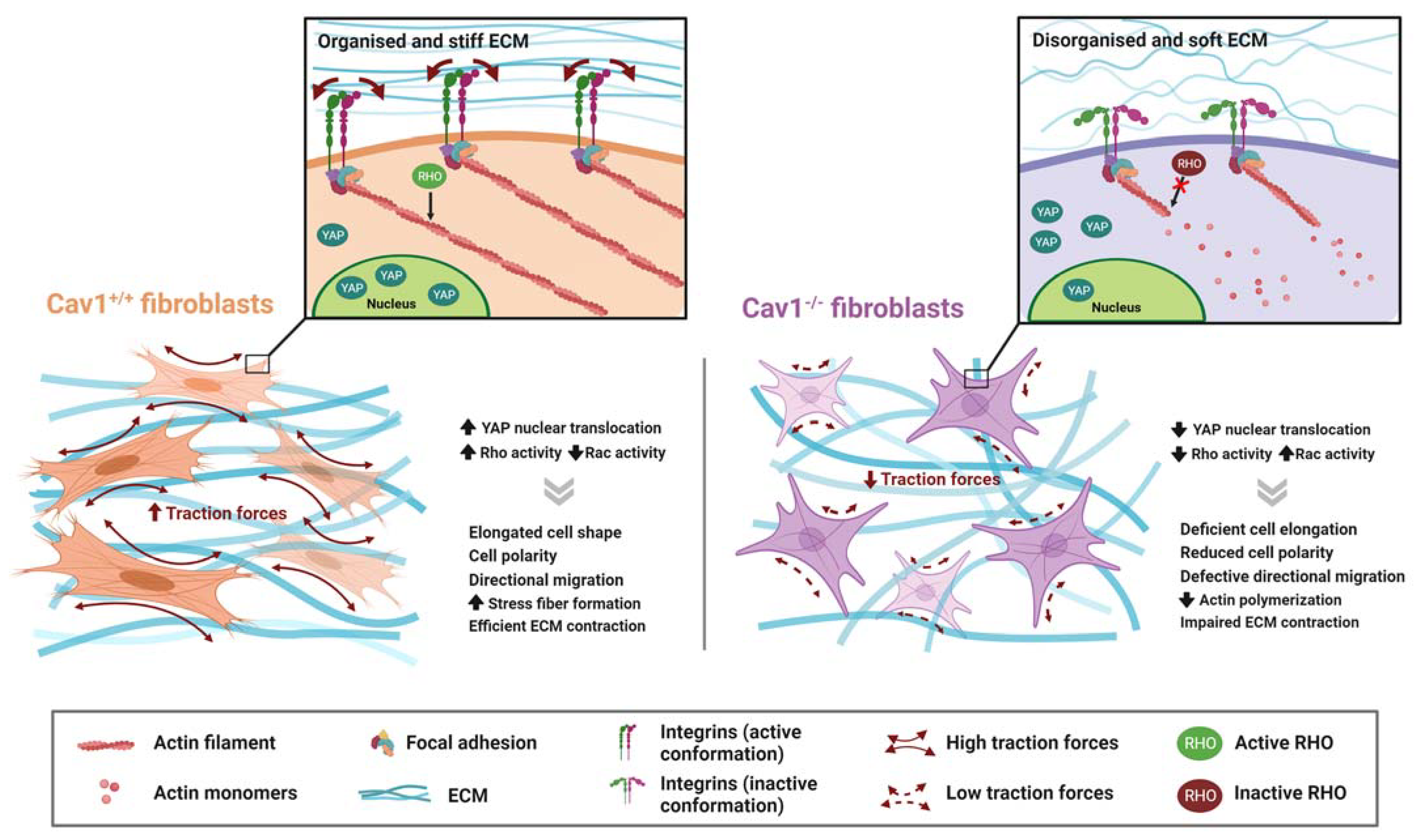
4.1. Physical Remodeling of ECM
4.1.1. Rho-Mediated Actomyosin Contraction
4.1.2. YAP
4.2. Chemical Remodeling of ECM
4.2.1. Regulation of TGFB Pathway
4.2.2. Secretion of Exosomes
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abl | Tyrosine-protein kinase ABL1 |
| AMPK | AMP-activated protein kinase |
| BioID | Proximity-dependent biotin identification |
| CAFs | Cancer-associated fibroblasts |
| Cav1 | Caveolin1 |
| Cav2 | Caveolin2 |
| Cav3 | Caveolin3 |
| CSK | Cytoskeleton |
| ECM | Extracellular Matrix |
| EMT | Epithelial-to-mesenchymal transition |
| eNOS | Endothelial nitric oxide synthase |
| EVs | Extracellular Vesicles |
| FA | Focal adhesion |
| FBP17 | Formin-binding protein 17 |
| FN | Fibronectin |
| IF | Intermediate Filaments |
| LSS | Laminar shear stress |
| MEFs | Mouse embryonic fibroblasts |
| MVBs | Multivesicular bodies |
| OSS | Oscillatory shear stress |
| PM | Plasma membrane |
| PTRF | Polymerase I and Transcription Release Factor |
| pY14Cav1 | Phosphorylated Cav1 at tyrosine residue 14 |
| Rac1 | Ras-related C3 botulinum toxin substrate 1 |
| ROCK | Rho-associated protein kinase 1 |
| TAZ | Transcriptional coactivator with PDZ-binding motif |
| TEAD | Transcriptional Enhanced Associate Domain |
| TGF-β | Transforming growth factor-β |
| TnC | Tenascin C |
| TβRs | TGF-β receptors |
| YAP | Yes-associated protein |
References
- Gauthier, N.C.; Masters, T.A.; Sheetz, M.P. Mechanical Feedback between Membrane Tension and Dynamics. Trends Cell Biol. 2012, 22, 527–535. [Google Scholar] [CrossRef]
- le Roux, A.L.; Quiroga, X.; Walani, N.; Arroyo, M.; Roca-Cusachs, P. The Plasma Membrane as a Mechanochemical Transducer. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180221. [Google Scholar] [CrossRef] [PubMed]
- Grecco, H.E.; Schmick, M.; Bastiaens, P.I.H. Signaling from the Living Plasma Membrane. Cell 2011, 144, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Parton, R.G.; del Pozo, M.A. Caveolae as Plasma Membrane Sensors, Protectors and Organizers. Nat. Rev. Mol. Cell Biol. 2013, 14, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Parton, R.G. Caveolae: Structure, Function, and Relationship to Disease. Annu. Rev. Cell Dev. Biol. 2018, 6, 34–111. [Google Scholar] [CrossRef]
- Discher, D.E.; Janmey, P.; Wang, Y.L. Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef]
- Fletcher, D.A.; Mullins, R.D. Cell Mechanics and the Cytoskeleton. Nature 2010, 463, 485–492. [Google Scholar] [CrossRef]
- Pegoraro, A.F.; Janmey, P.; Weitz, D.A. Mechanical Properties of the Cytoskeleton and Cells. Cold Spring Harb. Perspect Biol. 2017, 9, a022038. [Google Scholar] [CrossRef] [PubMed]
- Geiger, B.; Bershadsky, A.; Pankov, R.; Yamada, K.M. Transmembrane Crosstalk between the Extracellular Matrix--Cytoskeleton Crosstalk. Nat. Rev. Mol. Cell Biol. 2001, 2, 793–805. [Google Scholar] [CrossRef]
- Grande-García, A.; Echarri, A.; de Rooij, J.; Alderson, N.B.; Waterman-Storer, C.M.; Valdivielso, J.M.; del Pozo, M.A. Caveolin-1 Regulates Cell Polarization and Directional Migration through Src Kinase and Rho GTPases. J. Cell Biol. 2007, 177, 683–694. [Google Scholar] [CrossRef]
- Goetz, J.G.; Minguet, S.; Navarro-Lérida, I.; Lazcano, J.J.; Samaniego, R.; Calvo, E.; Tello, M.; Osteso-Ibáñez, T.; Pellinen, T.; Echarri, A.; et al. Biomechanical Remodeling of the Microenvironment by Stromal Caveolin-1 Favors Tumor Invasion and Metastasis. Cell 2011, 146, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Vicente, R.; Pavón, D.M.; Martín-Padura, I.; Català-Montoro, M.; Díez-Sánchez, A.; Quílez-Álvarez, A.; López, J.A.; Sánchez-Álvarez, M.; Vázquez, J.; Strippoli, R.; et al. Caveolin-1 Modulates Mechanotransduction Responses to Substrate Stiffness through Actin-Dependent Control of YAP. Cell Rep. 2018, 25, 1622–1635.e6. [Google Scholar] [CrossRef]
- del Pozo, M.A.; Lolo, F.N.; Echarri, A. Caveolae: Mechanosensing and Mechanotransduction Devices Linking Membrane Trafficking to Mechanoadaptation. Curr. Opin. Cell Biol. 2021, 68, 113–123. [Google Scholar] [CrossRef]
- Drab, M.; Verkade, P.; Elger, M.; Kasper, M.; Lohn, M.; Lauterbach, B.; Menne, J.; Lindschau, C.; Mende, F.; Luft, F.C.; et al. Loss of Caveolae, Vascular Dysfunction, and Pulmonary Defects in Caveolin-1 Gene-Disrupted Mice. Science 2001, 293, 2449–2452. [Google Scholar] [CrossRef] [PubMed]
- Galbiati, F.; Engelman, J.A.; Volonte, D.; Zhang, X.L.; Minetti, C.; Li, M.; Hou, H.; Kneitz, B.; Edelmann, W.; Lisanti, M.P. Caveolin-3 Null Mice Show a Loss of Caveolae, Changes in the Microdomain Distribution of the Dystrophin-Glycoprotein Complex, and T-Tubule Abnormalities. J. Biol. Chem. 2001, 276, 21425–21433. [Google Scholar] [CrossRef]
- Razani, B.; Combs, T.P.; Wang, X.B.; Frank, P.G.; Park, D.S.; Russell, R.G.; Li, M.; Tang, B.; Jelicks, L.A.; Scherer, P.E.; et al. Caveolin-1-Deficient Mice Are Lean, Resistant to Diet-Induced Obesity, and Show Hypertriglyceridemia with Adipocyte Abnormalities. J. Biol. Chem. 2002, 277, 8635–8647. [Google Scholar] [CrossRef]
- Razani, B.; Wang, X.B.; Engelman, J.A.; Battista, M.; Lagaud, G.; Zhang, X.L.; Kneitz, B.; Hou, H.; Christ, G.J.; Edelmann, W.; et al. Caveolin-2-Deficient Mice Show Evidence of Severe Pulmonary Dysfunction without Disruption of Caveolae. Mol. Cell Biol. 2002, 22, 2329–2344. [Google Scholar] [CrossRef]
- Dietzen, D.J.; Hastings, W.R.; Lublin, D.M. Caveolin Is Palmitoylated on Multiple Cysteine Residues. Palmitoylation Is Not Necessary for Localization of Caveolin to Caveolae. J. Biol. Chem. 1995, 270, 6838–6842. [Google Scholar] [CrossRef] [PubMed]
- Tonn Eisinger, K.R.; Woolfrey, K.M.; Swanson, S.P.; Schnell, S.A.; Meitzen, J.; Dell’Acqua, M.; Mermelstein, P.G. Palmitoylation of Caveolin-1 Is Regulated by the Same DHHC Acyltransferases That Modify Steroid Hormone Receptors. J. Biol. Chem. 2018, 293, 15901–15911. [Google Scholar] [CrossRef]
- Krishna, A.; Sengupta, D. Interplay between Membrane Curvature and Cholesterol: Role of Palmitoylated Caveolin-1. Biophys. J. 2019, 116, 69–78. [Google Scholar] [CrossRef]
- Hayer, A.; Stoeber, M.; Ritz, D.; Engel, S.; Meyer, H.H.; Helenius, A. Caveolin-1 Is Ubiquitinated and Targeted to Intralumenal Vesicles in Endolysosomes for Degradation. J. Cell Biol. 2010, 191, 615–629. [Google Scholar] [CrossRef] [PubMed]
- Ritz, D.; Vuk, M.; Kirchner, P.; Bug, M.; Schütz, S.; Hayer, A.; Bremer, S.; Lusk, C.; Baloh, R.H.; Lee, H.; et al. Endolysosomal Sorting of Ubiquitylated Caveolin-1 Is Regulated by VCP and UBXD1 and Impaired by VCP Disease Mutations. Nat. Cell Biol. 2011, 13, 1116–1124. [Google Scholar] [CrossRef]
- Lee, C.Y.; Lai, T.Y.; Tsai, M.K.; Chang, Y.C.; Ho, Y.H.; Yu, I.S.; Yeh, T.W.; Chou, C.C.; Lin, Y.S.; Lawrence, T.; et al. The Ubiquitin Ligase ZNRF1 Promotes Caveolin-1 Ubiquitination and Degradation to Modulate Inflammation. Nat. Commun. 2017, 8, 15502. [Google Scholar] [CrossRef]
- Cao, H.; Courchesne, W.E.; Mastick, C.C. A Phosphotyrosine-Dependent Protein Interaction Screen Reveals a Role for Phosphorylation of Caveolin-1 on Tyrosine 14. Recruitment of C-Terminal Src Kinase. J. Biol. Chem. 2002, 277, 8771–8774. [Google Scholar] [CrossRef] [PubMed]
- Sanguinetti, A.R.; Mastick, C.C. C-Abl Is Required for Oxidative Stress-Induced Phosphorylation of Caveolin-1 on Tyrosine 14. Cell. Signal. 2003, 15, 289–298. [Google Scholar] [CrossRef]
- Sanguinetti, A.R.; Cao, H.; Mastick, C.C. Fyn Is Required for Oxidative-and Hyperosmotic-Stress-Induced Tyrosine Phosphorylation of Caveolin-1. Biochem. J. 2003, 376, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Parton, R.G.; Joggerst, B.; Simons, K. Regulated Internalization of Caveolae. J. Cell Biol. 1994, 127, 1199–1215. [Google Scholar] [CrossRef]
- del Pozo, M.A.; Alderson, N.B.; Grande-García, A.; Balasubramanian, N.; Schwartz, M.A.; Kiosses, W.B.; Anderson, R.G.W. Phospho-Caveolin-1 Mediates Integrin-Regulated Membrane Domain Internalisation. Nat. Cell Biol. 2005, 7, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Zimnicka, A.M.; Husain, Y.S.; Shajahan, A.N.; Sverdlov, M.; Chaga, O.; Chen, Z.; Toth, P.T.; Klomp, J.; Karginov, A.V.; Tiruppathi, C.; et al. Src-Dependent Phosphorylation of Caveolin-1 Tyr-14 Promotes Swelling and Release of Caveolae. Mol. Biol. Cell 2016, 27, 2090–2106. [Google Scholar] [CrossRef]
- Joshi, B.; Bastiani, M.; Strugnell, S.S.; Boscher, C.; Parton, R.G.; Nabi, I.R. Phosphocaveolin-1 Is a Mechanotransducer That Induces Caveola Biogenesis via Egr1 Transcriptional Regulation. J. Cell Biol. 2012, 199, 425–435. [Google Scholar] [CrossRef]
- Lamaze, C.; Tardif, N.; Dewulf, M.; Vassilopoulos, S.; Blouin, C.M. The Caveolae Dress Code: Structure and Signaling. Curr. Opin. Cell Biol. 2017, 47, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Minguet, S.; Kläsener, K.; Schaffer, A.M.; Fiala, G.J.; Osteso-Ibánez, T.; Raute, K.; Navarro-Lérida, I.; Hartl, F.A.; Seidl, M.; Reth, M.; et al. Caveolin-1-Dependent Nanoscale Organization of the BCR Regulates B Cell Tolerance. Nat. Immunol. 2017, 18, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Peng, F.; Wu, D.; Ingram, A.J.; Gao, B.; Krepinsky, J.C. Caveolin-1 Phosphorylation Is Required for Stretch-Induced EGFR and Akt Activation in Mesangial Cells. Cell. Signal. 2007, 19, 1690–1700. [Google Scholar] [CrossRef] [PubMed]
- Radel, C.; Rizzo, V. Integrin Mechanotransduction Stimulates Caveolin-1 Phosphorylation and Recruitment of Csk to Mediate Actin Reorganization. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, 936–945. [Google Scholar] [CrossRef]
- Joshi, B.; Strugnell, S.S.; Goetz, J.G.; Kojic, L.D.; Cox, M.E.; Griffith, O.L.; Chan, S.K.; Jones, S.J.; Leung, S.P.; Masoudi, H.; et al. Phosphorylated Caveolin-1 Regulates Rho/ROCK-Dependent Focal Adhesion Dynamics and Tumor Cell Migration and Invasion. Cancer Res. 2008, 68, 8210–8220. [Google Scholar] [CrossRef]
- Goetz, J.G.; Joshi, B.; Lajoie, P.; Strugnell, S.S.; Scudamore, T.; Kojic, L.D.; Nabi, I.R. Concerted Regulation of Focal Adhesion Dynamics by Galectin-3 and Tyrosine-Phosphorylated Caveolin-1. J. Cell Biol. 2008, 180, 1261–1275. [Google Scholar] [CrossRef]
- Buwa, N.; Mazumdar, D.; Balasubramanian, N. Caveolin1 Tyrosine-14 Phosphorylation: Role in Cellular Responsiveness to Mechanical Cues. J. Membr. Biol. 2020, 253, 509–534. [Google Scholar] [CrossRef]
- Wong, T.H.; Dickson, F.H.; Timmins, L.R.; Nabi, I.R. Tyrosine Phosphorylation of Tumor Cell Caveolin-1: Impact on Cancer Progression. Cancer Metastasis Rev. 2020, 39, 455–469. [Google Scholar] [CrossRef]
- Schlegel, A.; Arvan, P.; Lisanti, M.P. Caveolin-1 Binding to Endoplasmic Reticulum Membranes and Entry into the Regulated Secretory Pathway Are Regulated by Serine Phosphorylation. Protein Sorting at the Level of the Endoplasmic Reticulum. J. Biol. Chem. 2001, 276, 4398–4408. [Google Scholar] [CrossRef]
- Hansen, C.G.; Nichols, B.J. Exploring the Caves: Cavins, Caveolins and Caveolae. Trends Cell Biol. 2010, 20, 177–186. [Google Scholar] [CrossRef]
- Stoeber, M.; Schellenberger, P.; Siebert, C.A.; Leyrat, C.; Grünewald, K.; Helenius, A. Model for the Architecture of Caveolae Based on a Flexible, Net-like Assembly of Cavin1 and Caveolin Discs. Proc. Natl. Acad. Sci. USA 2016, 113, E8069–E8078. [Google Scholar] [CrossRef] [PubMed]
- Parton, R.G.; McMahon, K.A.; Wu, Y. Caveolae: Formation, Dynamics, and Function. Curr. Opin. Cell Biol. 2020, 65, 8–16. [Google Scholar] [CrossRef]
- Hill, M.M.; Bastiani, M.; Luetterforst, R.; Kirkham, M.; Kirkham, A.; Nixon, S.J.; Walser, P.; Abankwa, D.; Oorschot, V.M.J.; Martin, S.; et al. PTRF-Cavin, a Conserved Cytoplasmic Protein Required for Caveola Formation and Function. Cell 2008, 132, 113–124. [Google Scholar] [CrossRef]
- Liu, L.; Brown, D.; McKee, M.; LeBrasseur, N.K.; Yang, D.; Albrecht, K.H.; Ravid, K.; Pilch, P.F. Deletion of Cavin/PTRF Causes Global Loss of Caveolae, Dyslipidemia, and Glucose Intolerance. Cell Metab. 2008, 8, 310–317. [Google Scholar] [CrossRef]
- Hansen, C.G.; Bright, N.A.; Howard, G.; Nichols, B.J. SDPR Induces Membrane Curvature and Functions in the Formation of Caveolae. Nat. Cell Biol. 2009, 11, 807–814. [Google Scholar] [CrossRef]
- McMahon, K.A.; Zajicek, H.; Li, W.P.; Peyton, M.J.; Minna, J.D.; Hernandez, V.J.; Luby-Phelps, K.; Anderson, R.G.W. SRBC/Cavin-3 Is a Caveolin Adapter Protein That Regulates Caveolae Function. EMBO J. 2009, 28, 1001–1015. [Google Scholar] [CrossRef]
- Bastiani, M.; Liu, L.; Hill, M.M.; Jedrychowski, M.P.; Nixon, S.J.; Lo, H.P.; Abankwa, D.; Luetterforst, R.; Fernandez-Rojo, M.; Breen, M.R.; et al. MURC/Cavin-4 and Cavin Family Members Form Tissue-Specific Caveolar Complexes. J. Cell Biol. 2009, 185, 1259–1273. [Google Scholar] [CrossRef]
- Lolo, F.-N.; Walani, N.; Seemann, E.; Zalvidea, D.; Pavón, D.M.; Cojoc, G.; Zamai, M.; Viaris de Lesegno, C.; Martínez de Benito, F.; Sánchez-Álvarez, M.; et al. Caveolin-1 Dolines Form a Distinct and Rapid Caveolae-Independent Mechanoadaptation System. Nat. Cell Biol. 2022, 25, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.G.; Howard, G.; Nichols, B.J. Pacsin 2 Is Recruited to Caveolae and Functions in Caveolar Biogenesis. J. Cell Sci. 2011, 124, 2777–2785. [Google Scholar] [CrossRef]
- Senju, Y.; Itoh, Y.; Takano, K.; Hamada, S.; Suetsugu, S. Essential Role of PACSIN2/Syndapin-II in Caveolae Membrane Sculpting. J. Cell Sci. 2011, 124, 2032–2040. [Google Scholar] [CrossRef] [PubMed]
- Seemann, E.; Sun, M.; Krueger, S.; Trö ger, J.; Hou, W.; Haag, N.; Schü ler, S.; Westermann, M.; Huebner, C.A.; Romeike, B.; et al. Deciphering Caveolar Functions by Syndapin III KO-Mediated Impairment of Caveolar Invagination. eLife 2017, 6, e29854. [Google Scholar] [CrossRef]
- Senju, Y.; Rosenbaum, E.; Shah, C.; Hamada-Nakahara, S.; Itoh, Y.; Yamamoto, K.; Hanawa-Suetsugu, K.; Daumke, O.; Suetsugu, S. Phosphorylation of PACSIN2 by Protein Kinase C Triggers the Removal of Caveolae from the Plasma Membrane. J. Cell Sci. 2015, 128, 2766–2780. [Google Scholar] [CrossRef]
- Daumke, O.; Lundmark, R.; Vallis, Y.; Martens, S.; Butler, P.J.G.; McMahon, H.T. Architectural and Mechanistic Insights into an EHD ATPase Involved in Membrane Remodelling. Nature 2007, 449, 923–927. [Google Scholar] [CrossRef]
- Morén, B.; Shah, C.; Howes, M.T.; Schieber, N.L.; McMahon, H.T.; Parton, R.G.; Daumke, O.; Lundmark, R. EHD2 Regulates Caveolar Dynamics via ATP-Driven Targeting and Oligomerization. Mol. Biol. Cell 2012, 23, 1316–1329. [Google Scholar] [CrossRef] [PubMed]
- Stoeber, M.; Stoeck, I.K.; HéCurrency Signnni, C.; Bleck, C.K.E.; Balistreri, G.; Helenius, A. Oligomers of the ATPase EHD2 Confine Caveolae to the Plasma Membrane through Association with Actin. EMBO J. 2012, 31, 2350–2364. [Google Scholar] [CrossRef] [PubMed]
- Morén, B.; Hansson, B.; Negoita, F.; Fryklund, C.; Lundmark, R.; Göransson, O.; Stenkula, K.G. EHD2 Regulates Adipocyte Function and Is Enriched at Cell Surface-Associated Lipid Droplets in Primary Human Adipocytes. Mol. Biol. Cell 2019, 30, 1147–1159. [Google Scholar] [CrossRef]
- Matthaeus, C.; Lahmann, I.; Kunz, S.; Jonas, W.; Alves Melo, A.; Lehmann, M.; Larsson, E.; Lundmark, R.; Kern, M.; Blüher, M.; et al. EHD2-Mediated Restriction of Caveolar Dynamics Regulates Cellular Fatty Acid Uptake. Proc. Natl. Acad. Sci. USA 2020, 117, 7471–7481. [Google Scholar] [CrossRef]
- Yeow, I.; Howard, G.; Chadwick, J.; Mendoza-Topaz, C.; Hansen, C.G.; Nichols, B.J.; Shvets, E. EHD Proteins Cooperate to Generate Caveolar Clusters and to Maintain Caveolae during Repeated Mechanical Stress. Curr. Biol. 2017, 27, 2951–2962.e5. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Lu, C.; Ida, L.; Yanagisawa, K.; Usukura, J.; Cheng, J.; Hotta, N.; Shimada, Y.; Isomura, H.; Suzuki, M.; et al. ROR1 Sustains Caveolae and Survival Signalling as a Scaffold of Cavin-1 and Caveolin-1. Nat. Commun. 2016, 7, 10060. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Hayashi, M.; Ida, L.; Yamamoto, M.; Lu, C.; Kajino, T.; Cheng, J.; Nakatochi, M.; Isomura, H.; Yamazaki, M.; et al. ROR1-CAVIN3 Interaction Required for Caveolae-Dependent Endocytosis and pro-Survival Signaling in Lung Adenocarcinoma. Oncogene 2019, 38, 5142–5157. [Google Scholar] [CrossRef]
- Echarri, A.; Pavón, D.M.; Sánchez, S.; García-García, M.; Calvo, E.; Huerta-López, C.; Velázquez-Carreras, D.; Viaris de Lesegno, C.; Ariotti, N.; Lázaro-Carrillo, A.; et al. An Abl-FBP17 Mechanosensing System Couples Local Plasma Membrane Curvature and Stress Fiber Remodeling during Mechanoadaptation. Nat. Commun. 2019, 10, 5828. [Google Scholar] [CrossRef] [PubMed]
- Pelkmans, L.; Zerial, M. Kinase-Regulated Quantal Assemblies and Kiss-and-Run Recycling of Caveolae. Nature 2005, 436, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Hayer, A.; Stoeber, M.; Bissig, C.; Helenius, A. Biogenesis of Caveolae: Stepwise Assembly of Large Caveolin and Cavin Complexes. Traffic 2010, 11, 361–382. [Google Scholar] [CrossRef]
- Gambin, Y.; Ariotti, N.; McMahon, K.-A.; Bastiani, M.; Sierecki, E.; Kovtun, O.; Polinkovsky, M.E.; Magenau, A.; Jung, W.; Okano, S.; et al. Single-Molecule Analysis Reveals Self Assembly and Nanoscale Segregation of Two Distinct Cavin Subcomplexes on Caveolae. eLife 2014, 3, e01434. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A.; Nichols, B.J.; Sandin, S. Architecture of the Caveolar Coat Complex. J. Cell Sci. 2016, 129, 3077–3083. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Peranen, J.; Schreinert, R.; Wielandt, F.; Kurzchalia, T.V.; Simons, K. VIP21/Caveolin Is a Cholesterol-Binding Protein. Proc. Natl. Acad. Sci. USA 1995, 92, 10339–10343. [Google Scholar] [CrossRef]
- Bosch, M.; Marí, M.; Gross, S.P.; Fernández-Checa, J.C.; Pol, A. Mitochondrial Cholesterol: A Connection between Caveolin, Metabolism, and Disease. Traffic 2011, 12, 1483–1489. [Google Scholar] [CrossRef]
- Pol, A.; Martin, S.; Fernandez, M.A.; Ferguson, C.; Carozzi, A.; Luetterforst, R.; Enrich, C.; Parton, R.G. Dynamic and Regulated Association of Caveolin with Lipid Bodies: Modulation of Lipid Body Motility and Function by a Dominant Negative Mutant. Mol. Biol. Cell 2004, 15, 99–110. [Google Scholar] [CrossRef]
- Bosch, M.; Marí, M.; Herms, A.; Fernández, A.; Fajardo, A.; Kassan, A.; Giralt, A.; Colell, A.; Balgoma, D.; Barbero, E.; et al. Caveolin-1 Deficiency Causes Cholesterol-Dependent Mitochondrial Dysfunction and Apoptotic Susceptibility. Curr. Biol. 2011, 21, 681–686. [Google Scholar] [CrossRef]
- Sala-Vila, A.; Navarro-Lérida, I.; Sánchez-Alvarez, M.; Bosch, M.; Calvo, C.; López, J.A.; Calvo, E.; Ferguson, C.; Giacomello, M.; Serafini, A.; et al. Interplay between Hepatic Mitochondria-Associated Membranes, Lipid Metabolism and Caveolin-1 in Mice. Sci. Rep. 2016, 6, 27351. [Google Scholar] [CrossRef]
- Hubert, M.; Larsson, E.; Vegesna, N.V.G.; Ahnlund, M.; Johansson, A.I.; Moodie, L.W.K.; Lundmark, R. Lipid Accumulation Controls the Balance between Surface Connection and Scission of Caveolae. eLife 2020, 9, e55038. [Google Scholar] [CrossRef]
- Breen, M.R.; Camps, M.; Carvalho-Simoes, F.; Zorzano, A.; Pilch, P.F. Cholesterol Depletion in Adipocytes Causes Caveolae Collapse Concomitant with Proteosomal Degradation of Cavin-2 in a Switch-like Fashion. PLoS ONE 2012, 7, e34516. [Google Scholar] [CrossRef] [PubMed]
- Fujita, A.; Cheng, J.; Tauchi-Sato, K.; Takenawa, T.; Fujimoto, T. A Distinct Pool of Phosphatidylinositol 4,5-Bisphosphate in Caveolae Revealed by a Nanoscale Labeling Technique. Proc. Natl. Acad. Sci. USA 2009, 106, 9256–9261. [Google Scholar] [CrossRef] [PubMed]
- Suetsugu, S.; Kurisu, S.; Takenawa, T. Phospholipids and Membrane-Deforming Proteins. Physiol. Rev. 2014, 94, 1219–1248. [Google Scholar] [CrossRef] [PubMed]
- Hirama, T.; Das, R.; Yang, Y.; Ferguson, C.; Won, A.; Yip, C.M.; Kay, J.G.; Grinstein, S.; Parton, R.G.; Fairn, G.D. Phosphatidylserine Dictates the Assembly and Dynamics of Caveolae in the Plasma Membrane. J. Biol. Chem. 2017, 292, 14292–14307. [Google Scholar] [CrossRef]
- Echarri, A.; Muriel, O.; Pavón, D.M.; Azegrouz, H.; Escolar, F.; Terrón, M.C.; Sanchez-Cabo, F.; Martínez, F.; Montoya, M.C.; Llorca, O.; et al. Caveolar Domain Organization and Trafficking Is Regulated by Abl Kinases and MDia1. J. Cell Sci. 2012, 125, 4413. [Google Scholar] [CrossRef]
- Golani, G.; Ariotti, N.; Parton, R.G.; Kozlov, M.M. Membrane Curvature and Tension Control the Formation and Collapse of Caveolar Superstructures. Dev. Cell 2019, 48, 523–538.e4. [Google Scholar] [CrossRef] [PubMed]
- Harder, T.; Simonst, K. Caveolae, DIGs, and the Dynamics of Sphingolipid-Cholesterol Microdomains. Curr. Opin. Cell Biol. 1997, 9, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Parton, R.G.; Kozlov, M.M.; Ariotti, N. Caveolae and Lipid Sorting: Shaping the Cellular Response to Stress. J. Cell Biol. 2020, 219. [Google Scholar] [CrossRef]
- Ikonen, E.; Parton, R.G. Caveolins and Cellular Cholesterol Balance. Traffic 2000, 1, 212–217. [Google Scholar] [CrossRef]
- Frank, P.G.; Lee, H.; Park, D.S.; Tandon, N.N.; Scherer, P.E.; Lisanti, M.P. Genetic Ablation of Caveolin-1 Confers Protection Against Atherosclerosis. Arter. Thromb. Vasc. Biol. 2004, 24, 98–105. [Google Scholar] [CrossRef]
- Galbiati, F.; Volonte’, D.; Liu, J.; Capozza, F.; Frank, P.G.; Zhu, L.; Pestell, R.G.; Lisanti, M.P. Caveolin-1 Expression Negatively Regulates Cell Cycle Progression by Inducing G0/G1 Arrest via a P53/P21WAF1/Cip1-Dependent Mechanism. Mol. Biol. Cell 2001, 12, 2229–2244. [Google Scholar] [CrossRef]
- del Pozo, M.A.; Schwartz, M.A. Rac, Membrane Heterogeneity, Caveolin and Regulation of Growth by Integrins. Trends Cell Biol. 2007, 17, 246–250. [Google Scholar] [CrossRef]
- Simons, K.; Toomre, D. Lipid Rafts and Signal Transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Razani, B.; Zhang, X.L.; Bitzer, M.; von Gersdorff, G.; Böttinger, E.P.; Lisanti, M.P. Caveolin-1 Regulates Transforming Growth Factor (TGF)-β/SMAD Signaling through an Interaction with the TGF-β Type I Receptor. J. Biol. Chem. 2001, 276, 6727–6738. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, E.A.; Reaven, E.; Topper, J.N.; Tsao, P.S. Transforming Growth Factor-β Receptors Localize to Caveolae and Regulate Endothelial Nitric Oxide Synthase in Normal Human Endothelial Cells. Biochem. J. 2005, 390, 199–206. [Google Scholar] [CrossRef]
- Santibanez, J.F.; Blanco, F.J.; Garrido-Martin, E.M.; Sanz-Rodriguez, F.; del Pozo, M.A.; Bernabeu, C. Caveolin-1 Interacts and Cooperates with the Transforming Growth Factor-β Type I Receptor ALK1 in Endothelial Caveolae. Cardiovasc. Res. 2008, 77, 791–799. [Google Scholar] [CrossRef]
- Yamamoto, M.; Toya, Y.; Schwencke, C.; Lisanti, M.P.; Myers, M.G.; Ishikawa, Y. Caveolin Is an Activator of Insulin Receptor Signaling. J. Biol. Chem. 1998, 273, 26962–26968. [Google Scholar] [CrossRef] [PubMed]
- Couet, J.; Sargiacomo, M.; Lisanti, M.P. Interaction of a Receptor Tyrosine Kinase, EGF-R, with Caveolins. Caveolin Binding Negatively Regulates Tyrosine and Serine/Threonine Kinase Activities. J. Biol. Chem. 1997, 272, 30429–30438. [Google Scholar] [CrossRef] [PubMed]
- di Guglielmo, G.M.; le Roy, C.; Goodfellow, A.F.; Wrana, J.L. Distinct Endocytic Pathways Regulate TGF-β Receptor Signalling and Turnover. Nat. Cell Biol. 2003, 5, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Fagerholm, S.; Örtegren, U.; Karlsson, M.; Ruishalme, I.; Strålfors, P. Rapid Insulin-Dependent Endocytosis of the Insulin Receptor by Caveolae in Primary Adipocytes. PLoS ONE 2009, 4, e5985. [Google Scholar] [CrossRef]
- Pelkmans, L.; Kartenbeck, J.; Helenius, A. Caveolar Endocytosis of Simian Virus 40 Reveals a New Two-Step Vesicular-Transport Pathway to the ER. Nat. Cell Biol. 2001, 3, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Damm, E.M.; Pelkmans, L.; Kartenbeck, J.; Mezzacasa, A.; Kurzchalia, T.; Helenius, A. Clathrin- and Caveolin-1-Independent Endocytosis: Entry of Simian Virus 40 into Cells Devoid of Caveolae. J. Cell Biol. 2005, 168, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Sinha, B.; Köster, D.; Ruez, R.; Gonnord, P.; Bastiani, M.; Abankwa, D.; Stan, R.V.; Butler-Browne, G.; Vedie, B.; Johannes, L.; et al. Cells Respond to Mechanical Stress by Rapid Disassembly of Caveolae. Cell 2011, 144, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Echarri, A.; del Pozo, M.A. Caveolae—Mechanosensitive Membrane Invaginations Linked to Actin Filaments. J. Cell Sci. 2015, 128, 2747–2758. [Google Scholar] [CrossRef]
- Orlichenko, L.; Huang, B.; Krueger, E.; McNiven, M.A. Epithelial Growth Factor-Induced Phosphorylation of Caveolin 1 at Tyrosine 14 Stimulates Caveolae Formation in Epithelial Cells. J. Biol. Chem. 2006, 281, 4570–4579. [Google Scholar] [CrossRef]
- Muriel, O.; Sánchez-Álvarez, M.; Strippoli, R.; del Pozo, M.A. Role of the Endocytosis of Caveolae in Intracellular Signaling and Metabolism. Prog. Mol. Subcell Biol 2018, 57, 203–234. [Google Scholar] [CrossRef] [PubMed]
- Parton, R.G.; Tillu, V.A.; Collins, B.M. Caveolae. Curr. Biol. 2018, 28, R402–R405. [Google Scholar] [CrossRef]
- Liu, L.; Pilch, P.F. PTRF/Cavin-1 Promotes Efficient Ribosomal RNA Transcription in Response to Metabolic Challenges. eLife 2016, 5, e17508. [Google Scholar] [CrossRef]
- Qifti, A.; Balaji, S.; Scarlata, S. Deformation of Caveolae Impacts Global Transcription and Translation Processes through Relocalization of Cavin-1. J. Biol. Chem. 2022, 298, 102005. [Google Scholar] [CrossRef]
- McMahon, K.A.; Wu, Y.; Gambin, Y.; Sierecki, E.; Tillu, V.A.; Hall, T.; Martel, N.; Okano, S.; Moradi, S.V.; Ruelcke, J.E.; et al. Identification of Intracellular Cavin Target Proteins Reveals Cavin-PP1alpha Interactions Regulate Apoptosis. Nat. Commun. 2019, 10, 3279. [Google Scholar] [CrossRef] [PubMed]
- McMahon, K.A.; Stroud, D.A.; Gambin, Y.; Tillu, V.A.; Bastiani, M.; Sierecki, E.; Polinkovsky, M.; Hall, T.E.; Gomez, G.A.; Wu, Y.; et al. Cavin3 Released from Caveolae Interacts with BRCA1 to Regulate the Cellular Stress Response. eLife 2021, 10, e61407. [Google Scholar] [CrossRef] [PubMed]
- Torrino, S.; Shen, W.; Blouin, C.M.; Mani, S.K.; de Lesegno, C.V.; Bost, P.; Grassart, A.; Köster, D.; Valades-Cruz, C.A.; Chambon, V.; et al. EHD2 Is a Mechanotransducer Connecting Caveolae Dynamics with Gene Transcription. J. Cell Biol. 2018, 217, 4092–4105. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, V.; McIntosh, D.P.; Oh, P.; Schnitzer, J.E. In Situ Flow Activates Endothelial Nitric Oxide Synthase in Luminal Caveolae of Endothelium with Rapid Caveolin Dissociation and Calmodulin Association. J. Biol. Chem. 1998, 273, 34724–34729. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Muller, S.; Stoltz, J.; Wang, X. Shear Stress Induces Caveolin-1 Translocation in Cultured Endothelial Cells. Eur. Biophys. J. 2002, 30, 605–611. [Google Scholar] [CrossRef]
- Boyd, N.L.; Park, H.; Yi, H.; Boo, Y.C.; Sorescu, G.P.; Sykes, M.; Jo, H. Chronic Shear Induces Caveolae Formation and Alters ERK and Akt Responses in Endothelial Cells. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H1113. [Google Scholar] [CrossRef]
- Shihata, W.A.; Michell, D.L.; Andrews, K.L.; Chin-Dusting, J.P.F. Caveolae: A Role in Endothelial Inflammation and Mechanotransduction? Front. Physiol. 2016, 7, 628. [Google Scholar] [CrossRef]
- Traub, O.; Berk, B.C. Laminar Shear Stress Mechanisms by Which Endothelial Cells Transduce an Atheroprotective Force Brief Review. Arter. Thromb. Vasc. Biol. 1998, 18, 677–685. [Google Scholar] [CrossRef]
- Yu, J.; Bergaya, S.; Murata, T.; Alp, I.F.; Bauer, M.P.; Lin, M.I.; Drab, M.; Kurzchalia, T.V.; Stan, R.V.; Sessa, W.C. Direct Evidence for the Role of Caveolin-1 and Caveolae in Mechanotransduction and Remodeling of Blood Vessels. J. Clin. Investig. 2006, 116, 1284–1291. [Google Scholar] [CrossRef]
- Harding, I.C.; Mitra, R.; Mensah, S.A.; Herman, I.M.; Ebong, E.E. Pro-Atherosclerotic Disturbed Flow Disrupts Caveolin-1 Expression, Localization, and Function via Glycocalyx Degradation. J. Transl. Med. 2018, 16, 364. [Google Scholar] [CrossRef]
- Fernández-Hernando, C.; Yu, J.; Suárez, Y.; Rahner, C.; Dávalos, A.; Lasunción, M.A.; Sessa, W.C. Genetic Evidence Supporting a Critical Role of Endothelial Caveolin-1 during the Progression of Atherosclerosis. Cell Metab. 2009, 10, 48–54. [Google Scholar] [CrossRef]
- Ramirez, C.M.; Zhang, X.; Bandyopadhyay, C.; Rotllan, N.; Sugiyama, M.G.; Aryal, B.; Liu, X.; He, S.; Kraehling, J.R.; Ulrich, V.; et al. Caveolin-1 Regulates Atherogenesis by Attenuating Low-Density Lipoprotein Transcytosis and Vascular Inflammation Independently of Endothelial Nitric Oxide Synthase Activation. Circulation 2019, 140, 225–239. [Google Scholar] [CrossRef]
- Ramírez, C.M.; Torrecilla-Parra, M.; Pardo-Marqués, V.; de-Frutos, M.F.; Pérez-García, A.; Tabraue, C.; de la Rosa, J.V.; Martín-Rodriguez, P.; Díaz-Sarmiento, M.; Nuñez, U.; et al. Crosstalk Between LXR and Caveolin-1 Signaling Supports Cholesterol Efflux and Anti-Inflammatory Pathways in Macrophages. Front. Endocrinol. 2021, 12, 635923. [Google Scholar] [CrossRef] [PubMed]
- Rausch, V.; Bostrom, J.R.; Park, J.; Bravo, I.R.; Feng, Y.; Hay, D.C.; Link, B.A.; Hansen, C.G. The Hippo Pathway Regulates Caveolae Expression and Mediates Flow Response via Caveolae. Curr. Biol. 2019, 29, 242–255.e6. [Google Scholar] [CrossRef] [PubMed]
- Strippoli, R.; Sandoval, P.; Moreno-Vicente, R.; Rossi, L.; Battistelli, C.; Terri, M.; Pascual-Antón, L.; Loureiro, M.; Matteini, F.; Calvo, E.; et al. Caveolin1 and YAP Drive Mechanically Induced Mesothelial to Mesenchymal Transition and Fibrosis. Cell Death Dis. 2020, 11, 647. [Google Scholar] [CrossRef]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in Mechanotransduction. Nature 2011, 474, 179–184. [Google Scholar] [CrossRef]
- Ma, S.; Meng, Z.; Chen, R.; Guan, K.-L.; ARjatscls, G. The Hippo Pathway: Biology and Pathophysiology. Annu. Rev. Biochem. 2019, 20, 577–604. [Google Scholar] [CrossRef]
- Zheng, Y.; Pan, D. The Hippo Signaling Pathway in Development and Disease. Dev. Cell 2019, 50, 264–282. [Google Scholar] [CrossRef] [PubMed]
- Echarri, A.; del Pozo, M.A. Caveolae Internalization Regulates Integrin-Dependent Signaling Pathways. Cell Cycle 2006, 5, 2179–2182. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.C.; Ling, J.Y.; Chen, W.C.; Lin, H.H.; Tang, M.J. Mechanotransduction of Matrix Stiffness in Regulation of Focal Adhesion Size and Number: Reciprocal Regulation of Caveolin-1 and Β1 Integrin. Sci. Rep. 2017, 7, 15008. [Google Scholar] [CrossRef]
- Razani, B.; Engelman, J.A.; Wang, X.B.; Schubert, W.; Zhang, X.L.; Marks, C.B.; Macalusol, F.; Russell, R.G.; Li, M.; Pestell, R.G.; et al. Caveolin-1 Null Mice Are Viable but Show Evidence of Hyperproliferative and Vascular Abnormalities. J. Biol. Chem. 2001, 276, 38121–38138. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-Y.; Liu, Y.; Stan, R.-V.; Fan, L.; Gu, Y.; Dalton, N.; Chu, P.-H.; Peterson, K.; Ross, J.; Chien, K.R. Defects in Caveolin-1 Cause Dilated Cardiomyopathy and Pulmonary Hypertension in Knockout Mice. Proc. Natl. Acad. Sci. USA 2002, 99, 11375–11380. [Google Scholar] [CrossRef] [PubMed]
- Minetti, C.; Sotgial, F.; Brunoi, C.; Scartezzini, P.; Broda, P.; Bado, M.; Masetti, E.; Mazzocco, M.; Egeo, A.; Alice DonatiS, M.; et al. Mutations in the Caveolin-3 Gene Cause Autosomal Dominant Limb-Girdle Muscular Dystrophy. Nat. Genet. 1998, 18, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Alston, L.; Ruschman, J.; Hegele, R.A. Heterozygous CAV1 Frameshift Mutations (MIM 601047) in Patients with Atypical Partial Lipodystrophy and Hypertriglyceridemia. Lipids Health Dis. 2008, 7, 3. [Google Scholar] [CrossRef]
- Kim, C.A.; Delépine, M.; Boutet, E.; el Mourabit, H.; le Lay, S.; Meier, M.; Nemani, M.; Bridel, E.; Leite, C.C.; Bertola, D.R.; et al. Association of a Homozygous Nonsense Caveolin-1 Mutation with Berardinelli-Seip Congenital Lipodystrophy. J. Clin. Endocrinol. Metab. 2008, 93, 1129–1134. [Google Scholar] [CrossRef]
- Schrauwen, I.; Szelinger, S.; Siniard, A.L.; Kurdoglu, A.; Corneveaux, J.J.; Malenica, I.; Richholt, R.; van Camp, G.; de Both, M.; Swaminathan, S.; et al. A Frame-Shift Mutation in CAV1 Is Associated with a Severe Neonatal Progeroid and Lipodystrophy Syndrome. PLoS ONE 2015, 10, e0131797. [Google Scholar] [CrossRef]
- Hayashi, Y.K.; Matsuda, C.; Ogawa, M.; Goto, K.; Tominaga, K.; Mitsuhashi, S.; Park, Y.E.; Nonaka, I.; Hino-Fukuyo, N.; Haginoya, K.; et al. Human PTRF Mutations Cause Secondary Deficiency of Caveolins Resulting in Muscular Dystrophy with Generalized Lipodystrophy. J. Clin. Investig. 2009, 119, 2623–2633. [Google Scholar] [CrossRef]
- Ardissone, A.; Bragato, C.; Caffi, L.; Blasevich, F.; Maestrini, S.; Bianchi, M.L.; Morandi, L.; Moroni, I.; Mora, M. Novel PTRF Mutation in a Child with Mild Myopathy and Very Mild Congenital Lipodystrophy. BMC Med. Genet. 2013, 14, 89. [Google Scholar] [CrossRef]
- Austin, E.D.; Ma, L.; LeDuc, C.; Rosenzweig, E.B.; Borczuk, A.; Phillips, J.A.; Palomero, T.; Sumazin, P.; Kim, H.R.; Talati, M.H.; et al. Whole Exome Sequencing to Identify a Novel Gene (Caveolin-1) Associated with Human Pulmonary Arterial Hypertension. Circ. Cardiovasc. Genet. 2012, 5, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Kircher, M.; del Campo, M.; Amato, R.S.; Agarwal, A.K. Whole Exome Sequencing Identifies de Novo Heterozygous CAV1 Mutations Associated with a Novel Neonatal Onset Lipodystrophy Syndrome. Am. J. Med. Genet. A 2015, 167, 1796–1806. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Copeland, C.A.; Kawano, Y.; Rosenzweig, E.B.; Austin, E.D.; Shahmirzadi, L.; Tang, S.; Raghunathan, K.; Chung, W.K.; Kenworthy, A.K. Characterization of a Caveolin-1 Mutation Associated with Both Pulmonary Arterial Hypertension and Congenital Generalized Lipodystrophy. Traffic 2016, 17, 1297–1312. [Google Scholar] [CrossRef] [PubMed]
- Copeland, C.A.; Han, B.; Tiwari, A.; Austin, E.D.; Loyd, J.E.; West, J.D.; Kenworthy, A.K. A Disease-Associated Frameshift Mutation in Caveolin-1 Disrupts Caveolae Formation and Function through Introduction of a de Novo ER Retention Signal. Mol. Biol. Cell 2017, 28, 3095–3111. [Google Scholar] [CrossRef] [PubMed]
- Marsboom, G.; Chen, Z.; Yuan, Y.; Zhang, Y.; Tiruppathi, C.; Loyd, J.E.; Austin, E.D.; Machado, R.F.; Minshall, R.D.; Rehman, J.; et al. Aberrant Caveolin-1-Mediated Smad Signaling and Proliferation Identified by Analysis of Adenine 474 Deletion Mutation (c.474delA) in Patient Fibroblasts: A New Perspective in the Mechanism of Pulmonary Hypertension. Mol. Biol. Cell 2017, 28, 1161–1283. Available online: https://www.molbiolcell.org/doi/full/10.1091/mbc.e16-11-0790 (accessed on 12 August 2022).
- Gazzerro, E.; Sotgia, F.; Bruno, C.; Lisanti, M.P.; Minetti, C. Caveolinopathies: From the Biology of Caveolin-3 to Human Diseases. Eur. J. Hum. Genet. 2010, 18, 137–145. [Google Scholar] [CrossRef]
- Dewulf, M.; Köster, D.V.; Sinha, B.; Viaris de Lesegno, C.; Chambon, V.; Bigot, A.; Bensalah, M.; Negroni, E.; Tardif, N.; Podkalicka, J.; et al. Dystrophy-Associated Caveolin-3 Mutations Reveal That Caveolae Couple IL6/STAT3 Signaling with Mechanosensing in Human Muscle Cells. Nat. Commun. 2019, 10, 1974. [Google Scholar] [CrossRef]
- Rajab, A.; Straub, V.; McCann, L.J.; Seelow, D.; Varon, R.; Barresi, R.; Schulze, A.; Lucke, B.; Lützkendorf, S.; Karbasiyan, M.; et al. Fatal Cardiac Arrhythmia and Long-QT Syndrome in a New Form of Congenital Generalized Lipodystrophy with Muscle Rippling (CGL4) Due to PTRF-CAVIN Mutations. PLoS Genet. 2010, 6, e1000874. [Google Scholar] [CrossRef]
- Rodriguez, G.; Ueyama, T.; Ogata, T.; Czernuszewicz, G.; Tan, Y.; Dorn, G.W.; Bogaev, R.; Amano, K.; Oh, H.; Matsubara, H.; et al. Molecular Genetic and Functional Characterization Implicate Muscle-Restricted Coiled-Coil Gene (MURC) as a Causal Gene for Familial Dilated Cardiomyopathy. Circ. Cardiovasc. Genet. 2011, 4, 349–358. [Google Scholar] [CrossRef]
- Goetz, J.G.; Lajoie, P.; Wiseman, S.M.; Nabi, I.R. Caveolin-1 in Tumor Progression: The Good, the Bad and the Ugly. Cancer Metastasis Rev. 2008, 27, 715–735. [Google Scholar] [CrossRef]
- Lolo, F.N.; Jiménez-Jiménez, V.; Sánchez-Álvarez, M.; del Pozo, M.Á. Tumor-Stroma Biomechanical Crosstalk: A Perspective on the Role of Caveolin-1 in Tumor Progression. Cancer Metastasis Rev. 2020, 39, 485–503. [Google Scholar] [CrossRef]
- Gvaramia, D.; Blaauboer, M.E.; Hanemaaijer, R.; Everts, V. Role of Caveolin-1 in Fibrotic Diseases. Matrix Biol. 2013, 32, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Tourkina, E.; Richard, M.; Gööz, P.; Bonner, M.; Pannu, J.; Harley, R.; Bernatchez, P.N.; Sessa, W.C.; Silver, R.M.; Hoffman, S.; et al. Antifibrotic Properties of Caveolin-1 Scaffolding Domain in Vitro and in Vivo. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 294, 843–861. [Google Scholar] [CrossRef]
- del Galdo, F.; Lisanti, M.R.; Jimenez, S.A. Caveolin-1, Transforming Growth Factor-β Receptor Internalization, and the Pathogenesis of Systemic Sclerosis. Curr. Opin. Rheumatol. 2008, 20, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Zhang, Y.; Kim, H.P.; Zhou, Z.; Feghali-Bostwick, C.A.; Liu, F.; Ifedigbo, E.; Xu, X.; Oury, T.D.; Kaminski, N.; et al. Caveolin-1: A Critical Regulator of Lung Fibrosis in Idiopathic Pulmonary Fibrosis. J. Exp. Med. 2006, 203, 2895–2906. [Google Scholar] [CrossRef] [PubMed]
- Wickström, S.A.; Lange, A.; Hess, M.W.; Polleux, J.; Spatz, J.P.; Krüger, M.; Pfaller, K.; Lambacher, A.; Bloch, W.; Mann, M.; et al. Integrin-Linked Kinase Controls Microtubule Dynamics Required for Plasma Membrane Targeting of Caveolae. Dev. Cell 2010, 19, 574–588. [Google Scholar] [CrossRef] [PubMed]
- Nixon, S.J.; Carter, A.; Wegner, J.; Ferguson, C.; Floetenmeyer, M.; Riches, J.; Key, B.; Westerfield, M.; Parton, R.G. Caveolin-1 Is Required for Lateral Line Neuromast and Notochord Development. J. Cell Sci. 2007, 120, 2151–2161. [Google Scholar] [CrossRef]
- Rohlich, P.; Allison, A.C. Oriented Pattern of Membrane-Associated Vesicles in Fibroblasts. J. Ultrastruct Res. 1976, 57, 94–103. [Google Scholar] [CrossRef]
- Rothberg, K.G.; Heuser, J.E.; Donzell, W.C.; Ying, Y.-S.; Glenney, J.R.; Anderson, R.G.W. Caveolin, a Protein Component of Caveolae Membrane Coats. Cell 1992, 68, 673–682. [Google Scholar] [CrossRef]
- Singer, I.I. Microfilament Bundles and the Control of Pinocytotic Vesicle Distribution at the Surfaces of Normal and Transformed Fibroblasts. Exp. Cell Res. 1979, 122, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Valentich, J.D.; Popov, V.; Saada, J.I.; Powell, D.W. Phenotypic Characterization of an Intestinal Subepithelial Myofibroblast Cell Line. Am. J. Physiol. 1997, 272, C1513–C1524. [Google Scholar] [CrossRef]
- Richter, T.; Floetenmeyer, M.; Ferguson, C.; Galea, J.; Goh, J.; Lindsay, M.R.; Morgan, G.P.; Marsh, B.J.; Parton, R.G. High-Resolution 3D Quantitative Analysis of Caveolar Ultrastructure and Caveola-Cytoskeleton Interactions. Traffic 2008, 9, 893–909. [Google Scholar] [CrossRef]
- Shi, X.; Wen, Z.; Wang, Y.; Liu, Y.J.; Shi, K.; Jiu, Y. Feedback-Driven Mechanisms between Phosphorylated Caveolin-1 and Contractile Actin Assemblies Instruct Persistent Cell Migration. Front. Cell Dev. Biol. 2021, 9, 665919. [Google Scholar] [CrossRef]
- McMahon, K.A.; Zhu, M.; Kwon, S.W.; Liu, P.; Zhao, Y.; Anderson, R.G.W. Detergent-Free Caveolae Proteome Suggests an Interaction with ER and Mitochondria. Proteomics 2006, 6, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Muriel, O.; Echarri, A.; Hellriegel, C.; Pavón, D.M.; Beccari, L.; del Pozo, M.A. Phosphorylated Filamin A Regulates Actin-Linked Caveolae Dynamics. J. Cell Sci. 2011, 124, 2763–2776. [Google Scholar] [CrossRef]
- Stahlhut, M.; van Deurs, B. Identification of Filamin as a Novel Ligand for Caveolin-1: Evidence for the Organization of Caveolin-1-Associated Membrane Domains by the Actin Cytoskeleton. Mol. Biol. Cell 2000, 11, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Kostan, J.; Salzer, U.; Orlova, A.; Törö, I.; Hodnik, V.; Senju, Y.; Zou, J.; Schreiner, C.; Steiner, J.; Meriläinen, J.; et al. Direct Interaction of Actin Filaments with F-BAR Protein Pacsin2. EMBO Rep. 2014, 15, 1154–1162. [Google Scholar] [CrossRef]
- Kessels, M.M.; Qualmann, B. The Syndapin Protein Family: Linking Membrane Trafficking with the Cytoskeleton. J. Cell Sci. 2004, 117, 3077–3086. [Google Scholar] [CrossRef] [PubMed]
- de Kreuk, B.J.; Nethe, M.; Fernandez-Borja, M.; Anthony, E.C.; Hensbergen, P.J.; Deelder, A.M.; Plomann, M.; Hordijk, P.L. The F-BAR Domain Protein PACSIN2 Associates with Rac1 and Regulates Cell Spreading and Migration. J. Cell Sci. 2011, 124, 2375–2388. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, J.; Ma, L.; Li, Y.; Diao, A.; Li, Y. Evidence for a Link of SDPR and Cytoskeleton. Lect. Notes Electr. Eng. 2015, 332, 165–172. [Google Scholar] [CrossRef]
- Hernandez, V.J.; Weng, J.; Ly, P.; Pompey, S.; Dong, H.; Mishra, L.; Schwarz, M.; Anderson, R.G.W.; Michaely, P. Cavin-3 Dictates the Balance between ERK and Akt Signaling. eLife 2013, 2013, e00905. [Google Scholar] [CrossRef]
- Mohan, J.; Morén, B.; Larsson, E.; Holst, M.R.; Lundmark, R. Cavin3 Interacts with Cavin1 and Caveolin1 to Increase Surface Dynamics of Caveolae. J. Cell Sci. 2015, 128, 979–991. [Google Scholar] [CrossRef]
- Roux, K.J.; Kim, D.I.; Raida, M.; Burke, B. A Promiscuous Biotin Ligase Fusion Protein Identifies Proximal and Interacting Proteins in Mammalian Cells. J. Cell Biol. 2012, 196, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Topaz, C.; Yeow, I.; Riento, K.; Nichols, B.J. BioID Identifies Proteins Involved in the Cell Biology of Caveolae. PLoS ONE 2018, 13, e0209856. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Lo, H.P.; McMahon, K.A.; Martel, N.; Jones, A.; Hill, M.M.; Parton, R.G.; Hall, T.E. In Vivo Proteomic Mapping through Gfp-Directed Proximity-Dependent Biotin Labelling in Zebrafish. eLife 2021, 10, e64631. [Google Scholar] [CrossRef] [PubMed]
- Sotgia, F.; Lee, J.K.; Das, K.; Bedford, M.; Petrucci, T.C.; Macioce, P.; Sargiacomo, M.; Bricarelli, F.D.; Minetti, C.; Sudol, M.; et al. Caveolin-3 Directly Interacts with the C-Terminal Tail of β-Dystroglycan. Identification of a Central WW-like Domain within Caveolin Family Members. J. Biol. Chem. 2000, 275, 38048–38058. [Google Scholar] [CrossRef]
- Thompson, T.G.; Chan, Y.-M.; Hack, A.A.; Brosius, M.; Rajala, M.; Lidov, H.G.W.; Mcnally, E.M.; Watkins, S.; Kunkel, L.M. Filamin 2 (FLN2): A Muscle-Specific Sarcoglycan Interacting Protein. J. Cell Biol. 2000, 148, 115–126. [Google Scholar] [CrossRef]
- Parton, R.G.; Way, M.; Zorzi, N.; Stang, E. Caveolin-3 Associates with Developing T-Tubules during Muscle Differentiation. J. Cell Biol. 1997, 136, 137–154. [Google Scholar] [CrossRef]
- Lo, H.P.; Lim, Y.W.; Xiong, Z.; Martel, N.; Ferguson, C.; Ariotti, N.; Giacomotto, J.; Rae, J.; Floetenmeyer, M.; Moradi, S.V.; et al. Cavin4 Interacts with Bin1 to Promote T-Tubule Formation and Stability in Developing Skeletal Muscle. J. Cell Biol. 2021, 220, e201905065. [Google Scholar] [CrossRef]
- Caldwell, J.L.; Smith, C.E.R.; Taylor, R.F.; Kitmitto, A.; Eisner, D.A.; Dibb, K.M.; Trafford, A.W. Dependence of Cardiac Transverse Tubules on the BAR Domain Protein Amphiphysin II (BIN-1). Circ. Res. 2014, 115, 986–996. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Yang, H.; Zhang, S.S.; Cho, H.C.; Kalashnikova, M.; Sun, B.; Zhang, H.; Bhargava, A.; Grabe, M.; Olgin, J.; et al. Cardiac BIN1 Folds T-Tubule Membrane, Controlling Ion Flux and Limiting Arrhythmia. Nat. Med. 2014, 20, 624–632. [Google Scholar] [CrossRef]
- Pradhan, B.S.; Prószyński, T.J. A Role for Caveolin-3 in the Pathogenesis of Muscular Dystrophies. Int. J. Mol. Sci. 2020, 21, 8736. [Google Scholar] [CrossRef] [PubMed]
- Peper, J.; Kownatzki-Danger, D.; Weninger, G.; Seibertz, F.; Pronto, J.R.D.; Sutanto, H.; Pacheu-Grau, D.; Hindmarsh, R.; Brandenburg, S.; Kohl, T.; et al. Caveolin3 Stabilizes McT1-Mediated Lactate/Proton Transport in Cardiomyocytes. Circ. Res. 2021, 128, E102–E120. [Google Scholar] [CrossRef]
- Fujimoto, T.; Miyawaki, A.; Mikoshiba, K. Inositol 1,4,5-Trisphosphate Receptor-like Protein in Plasmalemmal Caveolae Is Linked to Actin Filaments. J. Cell Sci. 1995, 108, 7–15. [Google Scholar] [CrossRef]
- Mundy, D.I.; Machleidt, T.; Ying, Y.S.; Anderson, R.G.W.; Bloom, G.S. Dual Control of Caveolar Membrane Traffic by Microtubules and the Actin Cytoskeleton. J. Cell Sci. 2002, 115, 4327–4339. [Google Scholar] [CrossRef]
- Matthaeus, C.; Taraska, J.W. Energy and Dynamics of Caveolae Trafficking. Front. Cell Dev. Biol. 2021, 8, 614472. [Google Scholar] [CrossRef] [PubMed]
- Courtemanche, N. Mechanisms of Formin-Mediated Actin Assembly and Dynamics. Biophys. Rev. 2018, 10, 1553–1569. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Tang, D.; Xing, Y.; Zhao, S.; Fan, C.; Zhong, J.; Cui, Y.; Shi, K.; Jiu, Y. Actin Nucleator Formins Regulate the Tension-Buffering Function of Caveolin-1. J. Mol. Cell Biol. 2022, 13, 876–888. [Google Scholar] [CrossRef]
- Liu, L.; Pilch, P.F. A Critical Role of Cavin (Polymerase I and Transcript Release Factor) in Caveolae Formation and Organization. J. Biol. Chem. 2008, 283, 4314–4322. [Google Scholar] [CrossRef]
- Kwon, H.; Lee, J.; Jeong, K.; Jang, D.; Pak, Y. A Novel Actin Cytoskeleton-Dependent Noncaveolar Microdomain Composed of Homo-Oligomeric Caveolin-2 for Activation of Insulin Signaling. Biochim. Biophys. Acta Mol. Cell Res. 2013, 1833, 2176–2189. [Google Scholar] [CrossRef]
- Xiong, N.; Li, S.; Tang, K.; Bai, H.; Peng, Y.; Yang, H.; Wu, C.; Liu, Y. Involvement of Caveolin-1 in Low Shear Stress-Induced Breast Cancer Cell Motility and Adhesion: Roles of FAK/Src and ROCK/p-MLC Pathways. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 12–22. [Google Scholar] [CrossRef]
- Ilha, M.; Moraes, K.d.S.; Rohden, F.; Martins, L.A.M.; Borojevic, R.; Lenz, G.; Barbé-Tuana, F.; Guma, F.C.R. Exogenous Expression of Caveolin-1 Is Sufficient for Hepatic Stellate Cell Activation. J. Cell Biochem. 2019, 120, 19031–19043. [Google Scholar] [CrossRef]
- Hall, A. Rho GTPases and the Actin Cytoskeleton. Science (1979) 1998, 279, 509–514. [Google Scholar] [CrossRef]
- Heasman, S.J.; Ridley, A.J. Mammalian Rho GTPases: New Insights into Their Functions from in Vivo Studies. Nat. Rev. Mol. Cell Biol. 2008, 9, 690–701. [Google Scholar] [CrossRef]
- Buwa, N.; Balasubramanian, N. Extracellular Matrix–Dependent Mechanosensing and Mechanotransduction: Role in Cell Migration. Cell Mov. Health Dis. 2022, 101–127. [Google Scholar] [CrossRef]
- Salani, B.; Maffioli, S.; Hamoudane, M.; Parodi, A.; Ravera, S.; Passalacqua, M.; Alama, A.; Nhiri, M.; Cordera, R.; Maggi, D. Caveolin-1 Is Essential for Metformin Inhibitory Effect on IGF1 Action in Non-small-cell Lung Cancer Cells. FASEB J. 2012, 26, 788–798. [Google Scholar] [CrossRef]
- Thomas, S.; Overdevest, J.B.; Nitz, M.D.; Williams, P.D.; Owens, C.R.; Sanchez-Carbayo, M.; Frierson, H.F.; Schwartz, M.A.; Theodorescu, D. Src and Caveolin-1 Reciprocally Regulate Metastasis via a Common Downstream Signaling Pathway in Bladder Cancer. Cancer Res. 2011, 71, 832–841. [Google Scholar] [CrossRef]
- Fecchi, K.; Travaglione, S.; Spadaro, F.; Quattrini, A.; Parolini, I.; Piccaro, G.; Raggi, C.; Fabbri, A.; Felicetti, F.; Carè, A.; et al. Human Melanoma Cells Express FGFR/Src/Rho Signaling That Entails an Adhesion-Independent Caveolin-1 Membrane Association. Int. J. Cancer 2012, 130, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Zhang, Y.; Mei, J.; Zhao, J.; Miao, C.; Jiu, Y. Interactive Mechanisms between Caveolin-1 and Actin Filaments or Vimentin Intermediate Filaments Instruct Cell Mechanosensing and Migration. J. Mol. Cell Biol. 2022, mjac066. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Radel, C.; Hughes, D.; Kelemen, S.; Rizzo, V. P190 RhoGTPase-Activating Protein Links the Β1 Integrin/Caveolin-1 Mechanosignaling Complex to RhoA and Actin Remodeling. Arter. Thromb. Vasc. Biol 2011, 31, 376–383. [Google Scholar] [CrossRef]
- Kawamura, S.; Miyamoto, S.; Brown, J.H. Initiation and Transduction of Stretch-Induced RhoA and Rac1 Activation through Caveolae. Cytoskeletal Regulation of ERK Translocation. J. Biol. Chem. 2003, 278, 31111–31117. [Google Scholar] [CrossRef]
- Ogata, T.; Ueyama, T.; Isodono, K.; Tagawa, M.; Takehara, N.; Kawashima, T.; Harada, K.; Takahashi, T.; Shioi, T.; Matsubara, H.; et al. MURC, a Muscle-Restricted Coiled-Coil Protein That Modulates the Rho/ROCK Pathway, Induces Cardiac Dysfunction and Conduction Disturbance. Mol. Cell Biol. 2008, 28, 3424–3436. [Google Scholar] [CrossRef]
- Rangel, L.; Bernabé-Rubio, M.; Fernández-Barrera, J.; Casares-Arias, J.; Millán, J.; Alonso, M.A.; Correas, I. Caveolin-1α Regulates Primary Cilium Length by Controlling RhoA GTPase Activity. Sci. Rep. 2019, 9, 1116. [Google Scholar] [CrossRef]
- Teo, J.L.; Gomez, G.A.; Weeratunga, S.; Davies, E.M.; Noordstra, I.; Budnar, S.; Katsuno-Kambe, H.; McGrath, M.J.; Verma, S.; Tomatis, V.; et al. Caveolae Control Contractile Tension for Epithelia to Eliminate Tumor Cells. Dev. Cell 2020, 54, 75–91.e7. [Google Scholar] [CrossRef] [PubMed]
- Katsuno-Kambe, H.; Parton, R.G.; Yap, A.S.; Teo, J.L. Caveolin-1 Influences Epithelial Collective Cell Migration via FMNL2 Formin. Biol. Cell 2021, 113, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T.; Tsaprailis, G.; Gerner, E.W. GSTΠ Stimulates Caveolin-1-Regulated Polyamine Uptake via Actin Remodeling. Oncotarget 2019, 10, 5713–5723. [Google Scholar] [CrossRef]
- Ying, Y.-S.; Con-Rad, P.A.; Anderson, R.G.W.; Conrad, P.A.; Smart, E.J.; Ying, Y.S.; Anderson, R.G.; Bloom, G.S. Caveolin Cycles between Plasma Membrane Caveolae and the Golgi Complex by LVlicrotubule-Dependent and Microtubule-Independent Steps. J. Cell Biol. 1994, 131, 1421–1433. [Google Scholar] [CrossRef]
- Hertzog, M.; Monteiro, P.; le Dez, G.; Chavrier, P. Exo70 Subunit of the Exocyst Complex Is Involved in Adhesion-Dependent Trafficking of Caveolin-1. PLoS ONE 2012, 7, e52627. [Google Scholar] [CrossRef] [PubMed]
- Hedman, A.C.; Smith, J.M.; Sacks, D.B. The Biology of IQGAP Proteins: Beyond the Cytoskeleton. EMBO Rep. 2015, 16, 427–446. [Google Scholar] [CrossRef]
- Sotgia, F.; Williams, T.M.; Cohen, A.W.; Minetti, C.; Pestell, R.G.; Lisanti, M.P. Caveolin-1-Deficient Mice Have an Increased Mammary Stem Cell Population with Upregulation of Wnt/β-Catenin Signaling. Cell Cycle 2005, 4, 1808–1816. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Watanabe, Y.; Watanabe, T.; Komitsu, N.; Aihara, M. Decreased Expression of Caveolin-1 Contributes to the Pathogenesis of Psoriasiform Dermatitis in Mice. J. Investig. Dermatol. 2015, 135, 2764–2774. [Google Scholar] [CrossRef] [PubMed]
- Osmani, N.; Pontabry, J.; Comelles, J.; Fekonja, N.; Goetz, J.G.; Riveline, D.; Georges-Labouesse, E.; Labouessea, M. An Arf6- and Caveolae-Dependent Pathway Links Hemidesmosome Remodeling and Mechanoresponse. Mol. Biol. Cell 2018, 29, 435–451. [Google Scholar] [CrossRef]
- Jiu, Y. Vimentin Intermediate Filaments Function as a Physical Barrier during Intracellular Trafficking of Caveolin-1. Biochem. Biophys. Res. Commun. 2018, 507, 161–167. [Google Scholar] [CrossRef]
- Chen, H.; Cai, J.; Wang, J.; Qiu, Y.; Jiang, C.; Wang, Y.; Wang, Y.; Yi, C.; Pan, L.; Guan, Y.; et al. Targeting Nestin+ Hepatic Stellate Cells Ameliorates Liver Fibrosis by Facilitating TβRI Degradation. J. Hepatol. 2021, 74, 1176–1187. [Google Scholar] [CrossRef]
- Sun, J.; Lu, Y.; Yu, C.; Xu, T.; Nie, G.; Miao, B.; Zhang, X. Involvement of the TGF-Β1 Pathway in Caveolin-1-Associated Regulation of Head and Neck Tumor Cell Metastasis. Oncol. Lett. 2020, 19, 1298–1304. [Google Scholar] [CrossRef]
- Kamibeppu, T.; Yamasaki, K.; Nakahara, K.; Nagai, T.; Terada, N.; Tsukino, H.; Mukai, S.; Kamoto, T. Caveolin-1 and -2 Regulate Cell Motility in Castration-Resistant Prostate Cancer. Res. Rep. Urol. 2018, 10, 135–144. [Google Scholar] [CrossRef]
- Shi, X.; Fan, C.; Jiu, Y. Unidirectional Regulation of Vimentin Intermediate Filaments to Caveolin-1. Int. J. Mol. Sci. 2020, 21, 7436. [Google Scholar] [CrossRef]
- Santilman, V.; Baran, J.A.; Anand-Apte, B.; Evans, R.M.; Parat, M.O. Caveolin-1 Polarization in Transmigrating Endothelial Cells Requires Binding to Intermediate Filaments. Angiogenesis 2007, 10, 297–305. [Google Scholar] [CrossRef]
- Jeong, K.; Kwon, H.; Lee, J.; Jang, D.; Pak, Y. Insulin-Response Epigenetic Activation of Egr-1 and JunB Genes at the Nuclear Periphery by A-Type Lamin-Associated PY19-Caveolin-2 in the Inner Nuclear Membrane. Nucleic. Acids Res. 2015, 43, 3114–3127. [Google Scholar] [CrossRef]
- Kwon, H.; Lee, J.; Jeong, K.; Jang, D.; Choi, M.; Pak, Y. A-Type Lamin-Dependent Homo-Oligomerization for PY19-Caveolin-2 to Function as an Insulin-Response Epigenetic Regulator. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2681–2689. [Google Scholar] [CrossRef]
- Choi, M.; Kwon, H.; Jeong, K.; Pak, Y. Epigenetic Regulation of Cebpb Activation by PY19-Caveolin-2 at the Nuclear Periphery in Association with the Nuclear Lamina. Biochim. Biophys. Acta Mol. Cell Res. 2022, 1869, 119363. [Google Scholar] [CrossRef]
- Muncie, J.M.; Weaver, V.M. The Physical and Biochemical Properties of the Extracellular Matrix Regulate Cell Fate. Curr. Top. Dev. Biol. 2018, 130, 1–37. [Google Scholar] [CrossRef]
- Malik, R.; Lelkes, P.I.; Cukierman, E. Biomechanical and Biochemical Remodeling of Stromal Extracellular Matrix in Cancer. Trends Biotechnol. 2015, 33, 230–236. [Google Scholar] [CrossRef]
- Midwood, K.S.; Orend, G. The Role of Tenascin-C in Tissue Injury and Tumorigenesis. J. Cell Commun. Signal. 2009, 3, 287–310. [Google Scholar] [CrossRef]
- Tan, Y.; Zhao, L.; Yang, Y.G.; Liu, W. The Role of Osteopontin in Tumor Progression Through Tumor-Associated Macrophages. Front. Oncol. 2022, 12, 953283. [Google Scholar] [CrossRef]
- Jaalouk, D.E.; Lammerding, J. Mechanotransduction Gone Awry. Nat. Rev. Mol. Cell Biol. 2009, 10, 63–73. [Google Scholar] [CrossRef]
- Humphrey, J.D.; Dufresne, E.R.; Schwartz, M.A. Mechanotransduction and Extracellular Matrix Homeostasis. Nat. Rev. Mol. Cell Biol. 2014, 15, 802–812. [Google Scholar] [CrossRef]
- Parat, M.O.; Anand-Apte, B.; Fox, P.L. Differential Caveolin-1 Polarization in Endothelial Cells during Migration in Two and Three Dimensions. Mol. Biol. Cell 2003, 14, 3156–3168. [Google Scholar] [CrossRef]
- Beardsley, A.; Fang, K.; Mertz, H.; Castranova, V.; Friend, S.; Liu, J. Loss of Caveolin-1 Polarity Impedes Endothelial Cell Polarization and Directional Movement. J. Biol. Chem. 2005, 280, 3541–3547. [Google Scholar] [CrossRef]
- Panciera, T.; Azzolin, L.; Cordenonsi, M.; Piccolo, S. Mechanobiology of YAP and TAZ in Physiology and Disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 758–770. [Google Scholar] [CrossRef]
- Rausch, V.; Hansen, C.G. The Hippo Pathway, YAP/TAZ, and the Plasma Membrane. Trends Cell Biol. 2020, 30, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Calvo, F.; Ege, N.; Grande-Garcia, A.; Hooper, S.; Jenkins, R.P.; Chaudhry, S.I.; Harrington, K.; Williamson, P.; Moeendarbary, E.; Charras, G.; et al. Mechanotransduction and YAP-Dependent Matrix Remodelling Is Required for the Generation and Maintenance of Cancer-Associated Fibroblasts. Nat. Cell Biol. 2013, 15, 637–646. [Google Scholar] [CrossRef]
- Albacete-Albacete, L.; Navarro-Lérida, I.; López, J.A.; Martín-Padura, I.; Astudillo, A.M.; Ferrarini, A.; Van-Der-Heyden, M.; Balsinde, J.; Orend, G.; Vázquez, J.; et al. ECM Deposition Is Driven by Caveolin-1–Dependent Regulation of Exosomal Biogenesis and Cargo Sorting. J. Cell Biol. 2020, 219, 2202006178. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.G. Endocytic Regulation of TGF-β Signaling. Cell Res. 2009, 19, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Sobierajska, K.; Wawro, M.E.; Ciszewski, W.M.; Niewiarowska, J. Transforming Growth Factor-β Receptor Internalization via Caveolae Is Regulated by Tubulin-Β2 and Tubulin-Β3 during Endothelial-Mesenchymal Transition. Am. J. Pathol. 2019, 189, 2531–2546. [Google Scholar] [CrossRef]
- Strippoli, R.; Loureiro, J.; Moreno, V.; Benedicto, I.; Pérez Lozano, M.L.; Barreiro, O.; Pellinen, T.; Minguet, S.; Foronda, M.; Osteso, M.T.; et al. Caveolin-1 Deficiency Induces a MEK - ERK 1/2-Snail-1-dependent Epithelial–Mesenchymal Transition and Fibrosis during Peritoneal Dialysis. EMBO Mol. Med. 2015, 7, 102–123. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Sottile, J.; Chandler, J. Fibronectin Matrix Turnover Occurs through a Caveolin-1-Dependent Process. Mol. Biol. Cell 2005, 16, 757–768. [Google Scholar] [CrossRef]
- Lowy, C.M.; Oskarsson, T. Tenascin C in Metastasis: A View from the Invasive Front. Cell Adhes. Migr. 2015, 9, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Inder, K.L.; Ruelcke, J.E.; Petelin, L.; Moon, H.; Choi, E.; Rae, J.; Blumenthal, A.; Hutmacher, D.; Saunders, N.A.; Stow, J.L.; et al. Cavin-1/PTRF Alters Prostate Cancer Cell-Derived Extracellular Vesicle Content and Internalization to Attenuate Extracellular Vesicle-Mediated Osteoclastogenesis and Osteoblast Proliferation. J. Extracell. Vesicles 2014, 3, 23784. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sotodosos-Alonso, L.; Pulgarín-Alfaro, M.; del Pozo, M.A. Caveolae Mechanotransduction at the Interface between Cytoskeleton and Extracellular Matrix. Cells 2023, 12, 942. https://doi.org/10.3390/cells12060942
Sotodosos-Alonso L, Pulgarín-Alfaro M, del Pozo MA. Caveolae Mechanotransduction at the Interface between Cytoskeleton and Extracellular Matrix. Cells. 2023; 12(6):942. https://doi.org/10.3390/cells12060942
Chicago/Turabian StyleSotodosos-Alonso, Laura, Marta Pulgarín-Alfaro, and Miguel A. del Pozo. 2023. "Caveolae Mechanotransduction at the Interface between Cytoskeleton and Extracellular Matrix" Cells 12, no. 6: 942. https://doi.org/10.3390/cells12060942
APA StyleSotodosos-Alonso, L., Pulgarín-Alfaro, M., & del Pozo, M. A. (2023). Caveolae Mechanotransduction at the Interface between Cytoskeleton and Extracellular Matrix. Cells, 12(6), 942. https://doi.org/10.3390/cells12060942





