BAP1 Malignant Pleural Mesothelioma Mutations in Caenorhabditis elegans Reveal Synthetic Lethality between ubh-4/BAP1 and the Proteasome Subunit rpn-9/PSMD13
Abstract
1. Introduction
2. Materials and Methods
2.1. Caenorhabditis elegans Strains
2.2. CRISPR Generation of Strains
2.3. Body Length
2.4. Brood Size
2.5. RNA Interference
2.6. Bortezomib Treatment and Survival Assay
2.7. Immunofluorescence with Dissected Animals
2.8. Immunohistochemical Analysis
2.9. Graph Plotting and Statistical Analysis
3. Results
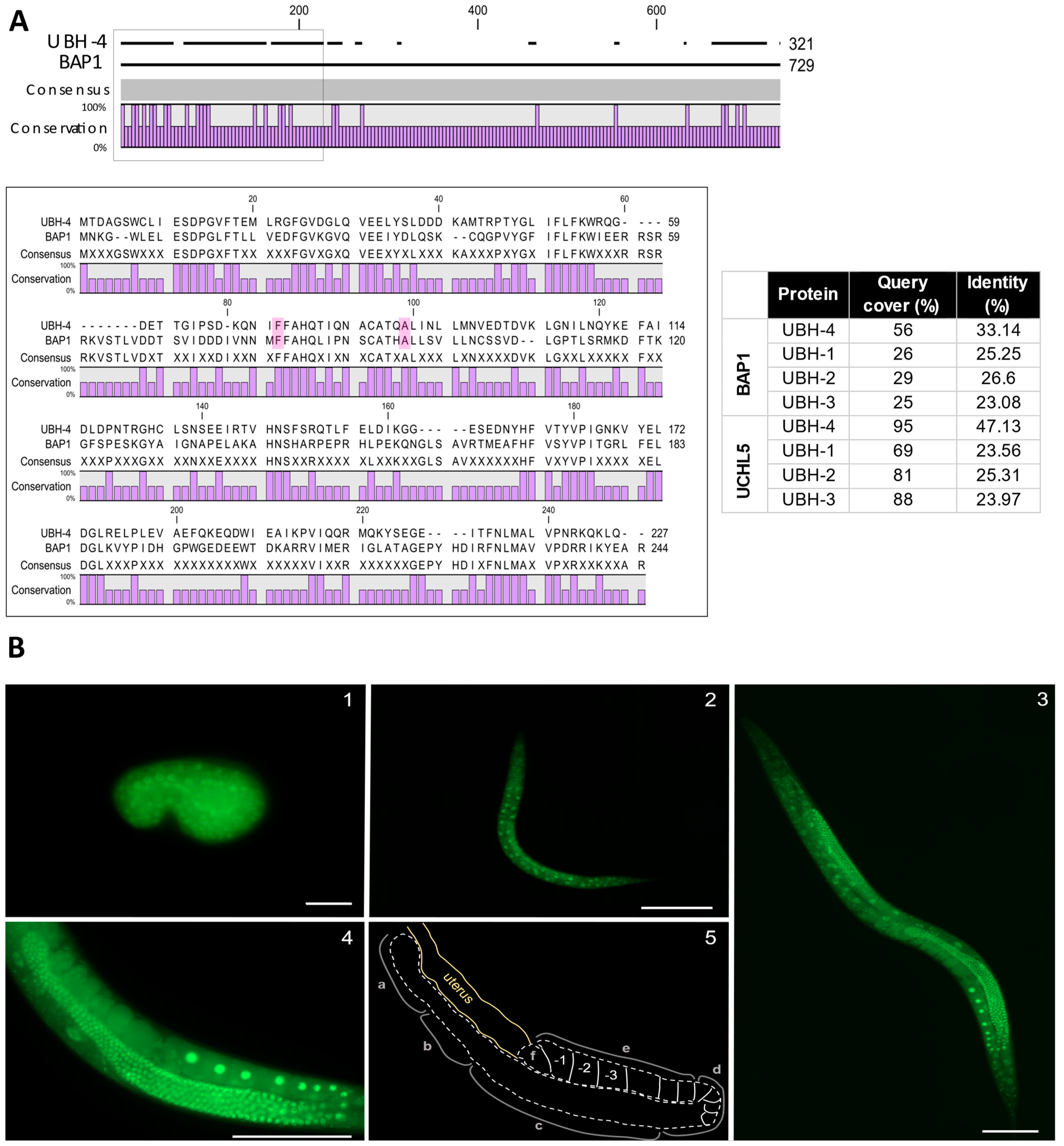
3.1. ubh-4 Is the C. elegans Ortholog of Human Proteins UCHL5 and BAP1
3.2. ubh-4 Is Ubiquitously Expressed in All Developmental Stages
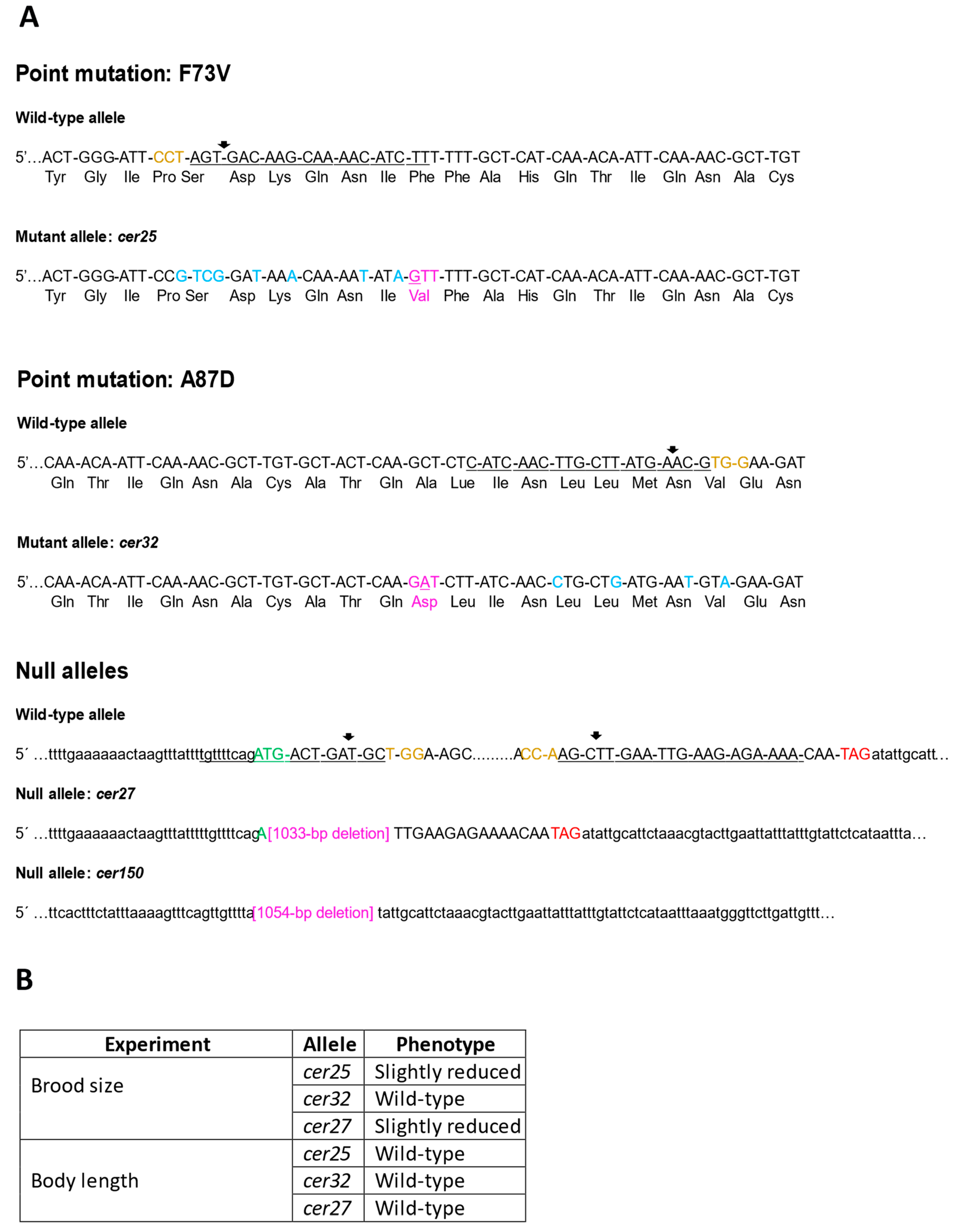
3.3. Mimicking Human BAP1 Mutations in C. elegans ubh-4
3.4. RNAi-Based Screen Reveals rpn-9 as Genetic Interactor of ubh-4
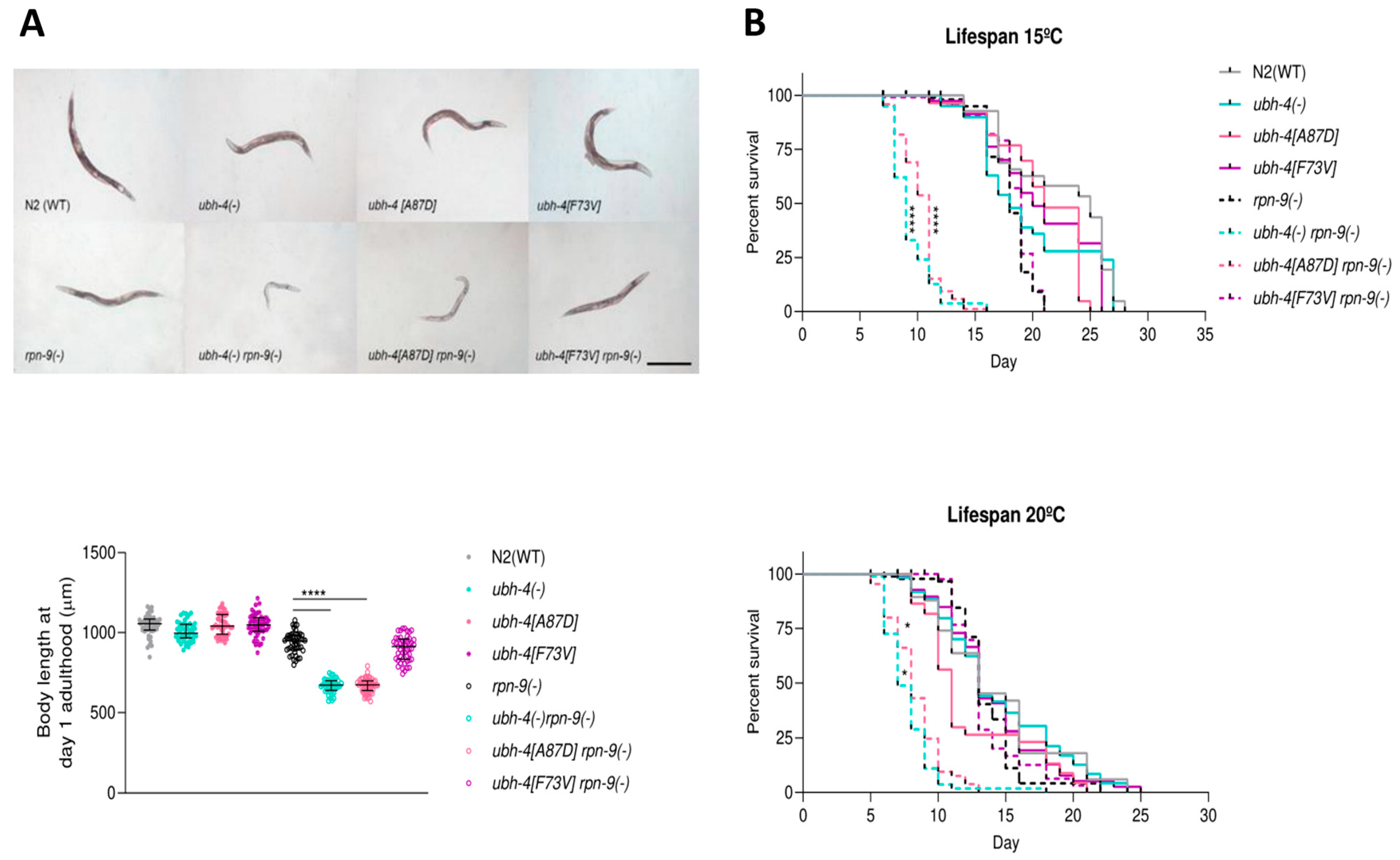
3.5. BAP1 Cancer-Related Mutation A87D Mimics ubh-4 Deletion Phenotypes in rpn-9(gk140) Background
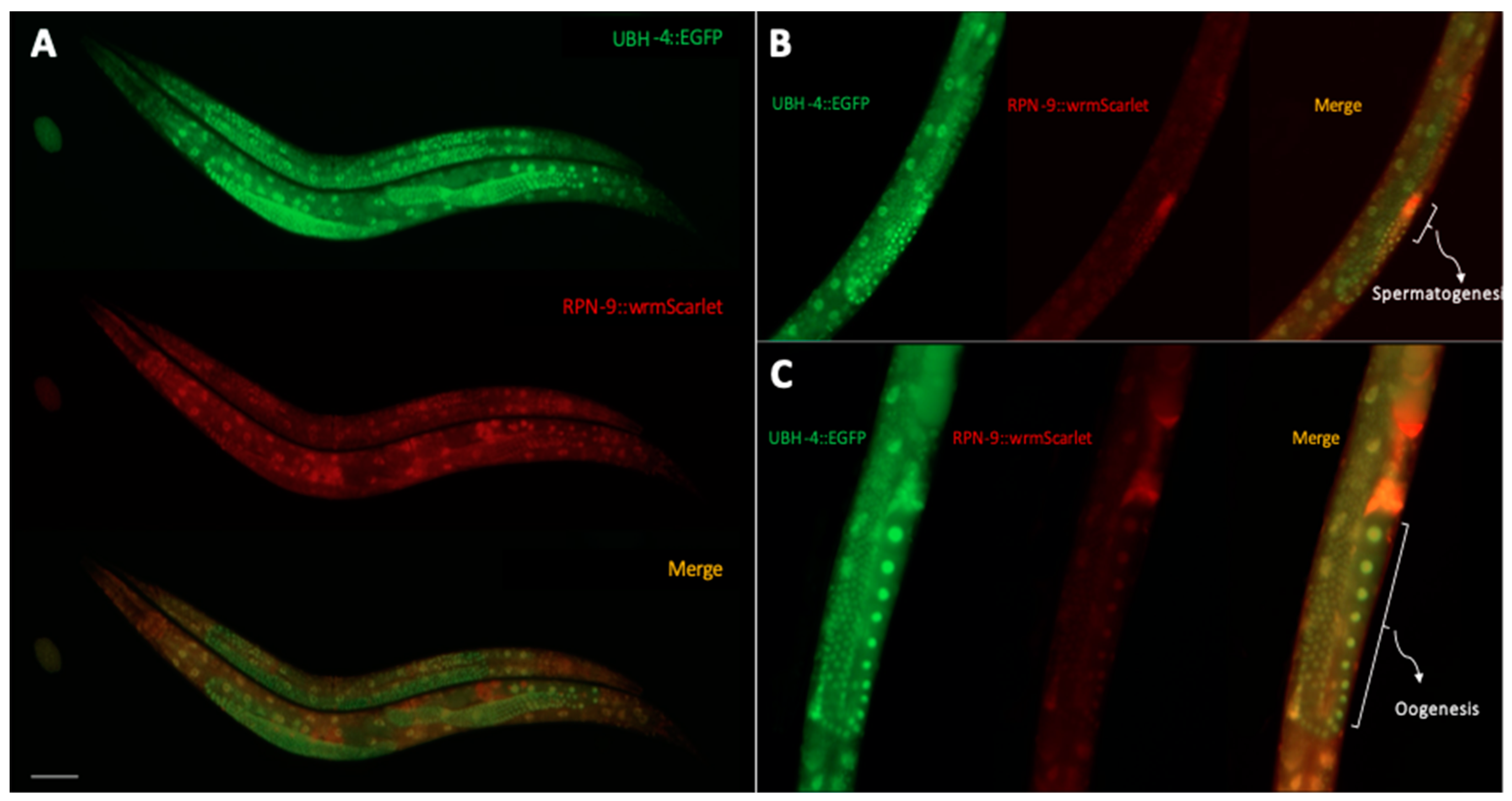
3.6. UBH-4 and RPN-9 Present Ubiquitous and Overlapping Expression Patterns
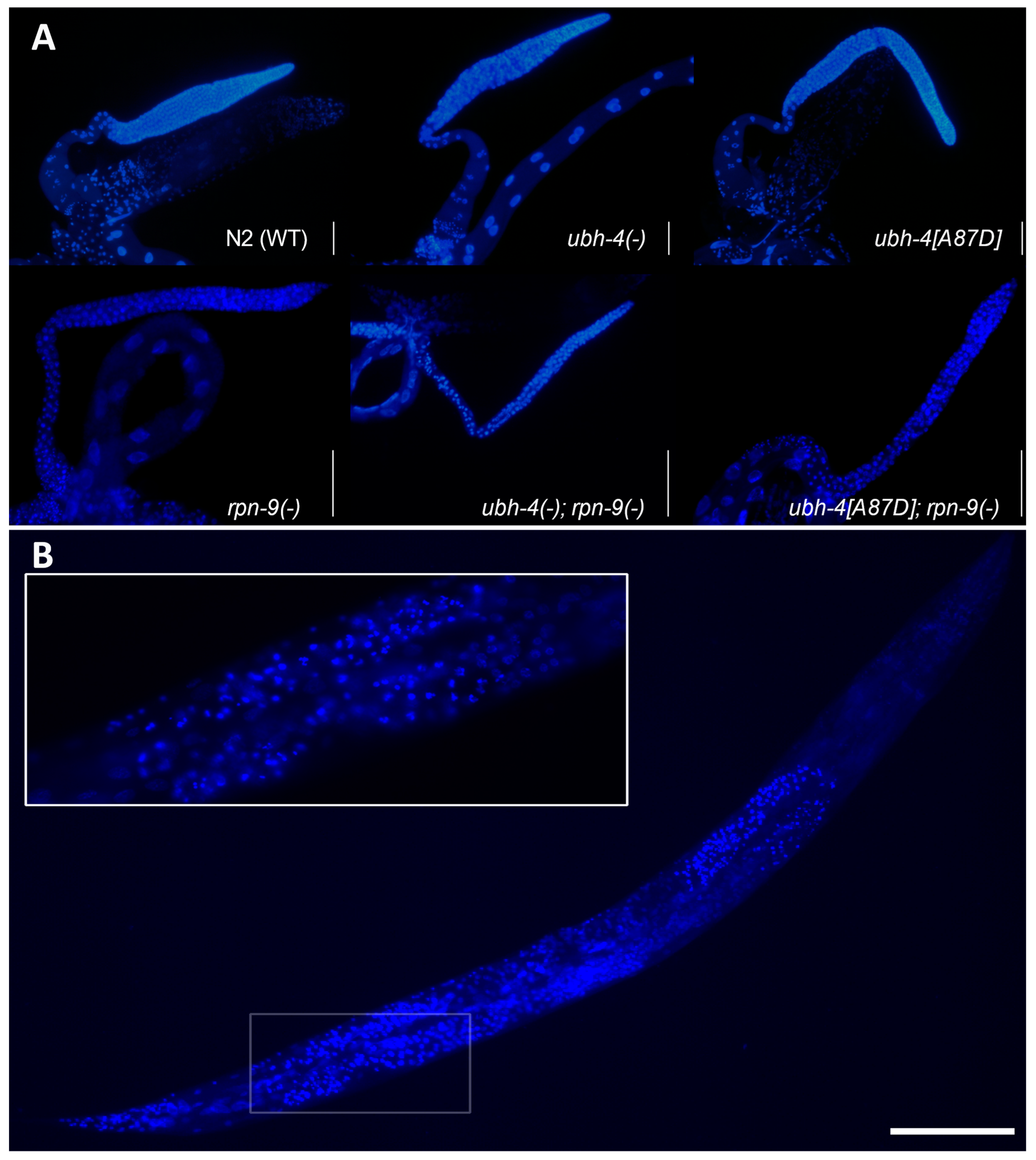
3.7. ubh-4 Deletion and A87D Missense Mutation Enhance the Germline Phenotype of the rpn-9 Deletion Mutant
3.8. Animals Defective in ubh-4 Are Sensitive to the Proteasome Inhibitor Bortezomib
4. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ventii, K.H.; Devi, N.S.; Friedrich, K.L.; Chernova, T.A.; Meir, E.G.V.; Wilkinson, K.D. BAP1 Is a Tumor Suppressor That Requires Deubiquitinating Activity and Nuclear Localization. Cancer Res. 2008, 68, 6953–6962. [Google Scholar] [CrossRef] [PubMed]
- Walpole, S.; Pritchard, A.L.; Cebulla, C.M.; Pilarski, R.; Stautberg, M.; Davidorf, F.H.; De La Fouchardière, A.; Cabaret, O.; Golmard, L.; Stoppa-Lyonnet, D.; et al. Comprehensive Study of the Clinical Phenotype of Germline BAP1 Variant-Carrying Families Worldwide. J. Natl. Cancer Inst. 2018, 110, 1328–1341. [Google Scholar] [CrossRef]
- Carbone, M.; Yang, H.; Pass, H.I.; Krausz, T.; Testa, J.R.; Gaudino, G. BAP1 and Cancer. Nat. Rev. Cancer 2013, 13, 153–159. [Google Scholar] [CrossRef]
- Bott, M.; Brevet, M.; Taylor, B.S.; Shimizu, S.; Ito, T.; Wang, L.; Creaney, J.; Lake, R.A.; Zakowski, M.F.; Reva, B. The Nuclear Deubiquitinase BAP1 Is Commonly Inactivated by Somatic Mutations and 3p21.1 Losses in Malignant Pleural Mesothelioma. Nat. Genet. 2011, 43, 668–672. [Google Scholar] [CrossRef]
- Harbour, J.W.; Onken, M.D.; Roberson, E.D.O.; Duan, S.; Cao, L.; Worley, L.A.; Council, M.L.; Matatall, K.A.; Helms, C.; Bowcock, A.M. Frequent Mutation of BAP1 in Metastasizing Uveal Melanomas. Science 2010, 330, 1410–1413. [Google Scholar] [CrossRef]
- Murali, R.; Wiesner, T.; Scolyer, R.A. Tumours Associated with BAP1 Mutations. Pathology 2013, 45, 116–126. [Google Scholar] [CrossRef]
- Wald, O.; Sugarbaker, D.J. New Concepts in the Treatment of Malignant Pleural Mesothelioma. Annu. Rev. Med. 2018, 69, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Baas, P.; Fennell, D.; Kerr, K.M.; Van Schil, P.E.; Haas, R.L.; Peters, S.; Committee, G. Malignant Pleural Mesothelioma: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-up. Ann. Oncol. 2015, 26, v31–v39. [Google Scholar] [CrossRef]
- Zalcman, G.; Mazieres, J.; Margery, J.; Greillier, L.; Audigier-Valette, C.; Moro-Sibilot, D.; Molinier, O.; Corre, R.; Monnet, I.; Gounant, V.; et al. Bevacizumab for Newly Diagnosed Pleural Mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet 2016, 387, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Scherpereel, A.; Antonia, S.; Bautista, Y.; Grossi, F.; Kowalski, D.; Zalcman, G.; Nowak, A.K.; Fujimoto, N.; Peters, S.; Tsao, A.S.; et al. First-Line Nivolumab plus Ipilimumab versus Chemotherapy for the Treatment of Unresectable Malignant Pleural Mesothelioma: Patient-Reported Outcomes in CheckMate 743. Lung Cancer 2022, 167, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Mashtalir, N.; Daou, S.; Barbour, H.; Sen, N.N.; Gagnon, J.; Hammond-Martel, I.; Dar, H.H.; Therrien, M.; Affar, E.B. Autodeubiquitination Protects the Tumor Suppressor BAP1 from Cytoplasmic Sequestration Mediated by the Atypical Ubiquitin Ligase UBE2O. Mol. Cell 2014, 54, 392–406. [Google Scholar] [CrossRef]
- Carbone, M.; Harbour, J.W.; Brugarolas, J.; Bononi, A.; Pagano, I.; Dey, A.; Krausz, T.; Pass, H.I.; Yang, H.; Gaudino, G. Biological Mechanisms and Clinical Significance of BAP1 Mutations in Human Cancer. Cancer Discov. 2020, 10, 1103–1120. [Google Scholar] [CrossRef]
- Nishikawa, H.; Wu, W.; Koike, A.; Kojima, R.; Gomi, H.; Fukuda, M.; Ohta, T. BRCA1-Associated Protein 1 Interferes with BRCA1/BARD1 RING Heterodimer Activity. Cancer Res. 2009, 69, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Balasubramani, A.; Larjo, A.; Bassein, J.A.; Chang, X.; Hastie, R.B.; Togher, S.M.; Lähdesmäki, H.; Rao, A. Cancer-Associated ASXL1 Mutations May Act as Gain-of-Function Mutations of the ASXL1-BAP1 Complex. Nat. Commun. 2015, 6, 7307. [Google Scholar] [CrossRef] [PubMed]
- Baughman, J.M.; Rose, C.M.; Kolumam, G.; Webster, J.D.; Wilkerson, E.M.; Merrill, A.E.; Rhoads, T.W.; Noubade, R.; Katavolos, P.; Lesch, J. NeuCode Proteomics Reveals Bap1 Regulation of Metabolism. Cell Rep. 2016, 16, 583–595. [Google Scholar] [CrossRef]
- Dey, A.; Seshasayee, D.; Noubade, R.; French, D.M.; Liu, J.; Chaurushiya, M.S.; Kirkpatrick, D.S.; Pham, V.C.; Lill, J.R.; Bakalarski, C.E. Loss of the Tumor Suppressor BAP1 Causes Myeloid Transformation. Science 2012, 33, 1541–1546. [Google Scholar] [CrossRef] [PubMed]
- Jensen, D.E.; Proctor, M.; Marquis, S.T.; Gardner, H.P.; Ha, S.I.; Chodosh, L.A.; Ishov, A.M.; Tommerup, N.; Vissing, H.; Sekido, Y. BAP1: A Novel Ubiquitin Hydrolase Which Binds to the BRCA1 RING Finger and Enhances BRCA1-Mediated Cell Growth Suppression. Oncogene 1998, 16, 1097–1112. [Google Scholar] [CrossRef]
- Ruan, H.B.; Han, X.; Li, M.D.; Singh, J.P.; Qian, K.; Azarhoush, S.; Zhao, L.; Bennett, A.M.; Samuel, V.T.; Wu, J. O-GlcNAc Transferase/Host Cell Factor C1 Complex Regulates Gluconeogenesis by Modulating PGC-1α Stability. Cell Metab. 2012, 16, 226–237. [Google Scholar] [CrossRef]
- Zarrizi, R.; Menard, J.A.; Belting, M.; Massoumi, R. Deubiquitination of γ-Tubulin by BAP1 Prevents Chromosome Instability in Breast Cancer Cells. Cancer Res 2014, 74, 6499–6508. [Google Scholar] [CrossRef]
- Stiernagle, T. Maintenance of C. elegans. In WormBook; OUP: Oxford, UK, 2006; pp. 1–11. [Google Scholar]
- Porta-de-la-Riva, M.; Fontrodona, L.; Villanueva, A.; Cerón, J. Basic Caenorhabditis Elegans Methods: Synchronization and Observation. J. Vis. Exp. 2012, e4019. [Google Scholar] [CrossRef]
- Kim, H.; Ishidate, T.; Ghanta, K.S.; Seth, M.; Conte, D.; Shirayama, M.; Mello, C.C. A Co-CRISPR Strategy for Efficient Genome Editing in Caenorhabditis Elegans. Genetics 2014, 197, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Arribere, J.A.; Bell, R.T.; Fu, B.X.H.; Artiles, K.L.; Hartman, P.S.; Fire, A.Z. Efficient Marker-Free Recovery of Custom Genetic Modifications with CRISPR/Cas9 in Caenorhabditis Elegans. Genetics 2014, 198, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Rual, J.F.; Ceron, J.; Koreth, J.; Hao, T.; Nicot, A.S.; Hirozane-Kishikawa, T.; Vandenhaute, J.; Orkin, S.H.; Hill, D.E.; Heuvel, S. Toward Improving Caenorhabditis Elegans Phenome Mapping with an ORFeome-Based RNAi Library. Genome Res. 2004, 14, 2162–2168. [Google Scholar] [CrossRef]
- Kamath, R.S.; Fraser, A.G.; Dong, Y.; Poulin, G.; Durbin, R.; Gotta, M.; Kanapin, A.; Le Bot, N.; Moreno, S.; Sohrmann, M. Systematic Functional Analysis of the Caenorhabditis Elegans Genome Using RNAi. Nature 2003, 421, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Mikkonen, E.; Haglund, C.; Holmberg, C.I. Immunohistochemical Analysis Reveals Variations in Proteasome Tissue Expression in C. Elegans. PLoS ONE 2017, 12, e0183403. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and Applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Pulido, L.; Kong, L.; Ponting, C.P.; Valencia, A. A Common Ancestry for BAP1 and Uch37 Regulators. Bioinforma. Discov. NOTE 2012, 28, 1953–1956. [Google Scholar] [CrossRef]
- Vicencio, J.; Martínez-Fernández, C.; Serrat, X.; Cerón, J. Efficient Generation of Endogenous Fluorescent Reporters by Nested CRISPR in Caenorhabditis Elegans. Genetics 2019, 211, 1143–1154. [Google Scholar] [CrossRef]
- Matilainen, O.; Arpalahti, L.; Rantanen, V.; Hautaniemi, S.; Holmberg, C.I. Insulin/IGF-1 Signaling Regulates Proteasome Activity through the Deubiquitinating Enzyme UBH-4. Cell Rep. 2013, 3, 1980–1995. [Google Scholar] [CrossRef]
- Kato, S.; Tomson, B.N.; Buys, T.P.H.; Elkin, S.K.; Carter, J.L.; Kurzrock, R. Genomic Landscape of Malignant Mesotheliomas. Mol. Cancer Ther. 2016, 15, 2498–2507. [Google Scholar] [CrossRef]
- De Rienzo, A.; Archer, M.A.; Yeap, B.Y.; Dao, N.; Sciaranghella, D.; Sideris, A.C.; Zheng, Y.; Holman, A.G.; Wang, Y.E.; Dal Cin, P.S.; et al. Gender-Specific Molecular and Clinical Features Underlie Malignant Pleural Mesothelioma. Cancer Res. 2016, 76, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Poulin, G.; Nandakumar, R.; Ahringer, J. Genome-Wide RNAi Screens in Caenorhabditis Elegans: Impact on Cancer Research. Oncogene 2004, 23, 8340–8345. [Google Scholar] [CrossRef] [PubMed]
- Soura, E.; Eliades, P.J.; Shannon, K.; Stratigos, A.J.; Tsao, H. Hereditary Melanoma: Update on Syndromes and Management Genetics of Familial Atypical Multiple Mole Melanoma Syndrome. J. Am. Acad. Dermatol. 2016, 74, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING V11: Protein-Protein Association Networks with Increased Coverage, Supporting Functional Discovery in Genome-Wide Experimental Datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Papaevgeniou, N.; Chondrogianni, N. The Ubiquitin Proteasome System in Caenorhabditis Elegans and Its Regulation. Redox Biol. 2014, 2, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Zhu, L.; Zeng, Z.; Jing, X.; Liang, Y.; Guo, L.; Shi, Q.; Xu, A.; Tao, E. Investigations into the Role of 26S Proteasome Non-ATPase Regulatory Subunit 13 in Neuroinflammation. Neuroimmunomodulation 2014, 21, 331–337. [Google Scholar] [CrossRef]
- Li, S.; Izumi, T.; Hu, J.; Jin, H.H.; Siddiqui, A.A.A.; Jacobson, S.G.; Bok, D.; Jin, M. Rescue of Enzymatic Function for Disease-Associated RPE65 Proteins Containing Various Missense Mutations in Non-Active Sites. J. Biol. Chem. 2014, 289, 18943–18956. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 72, 27–30. [Google Scholar] [CrossRef]
- Takeuchi, J.; Fujimuro, M.; Yokosawa, H.; Tanaka, K.; Toh-e, A. Rpn9 Is Required for Efficient Assembly of the Yeast 26S Proteasome. Mol. Cell. Biol. 1999, 19, 6575–6584. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, Y.; Li, Q.; Zhang, W.; Jin, C. Solution Structure of Yeast Rpn9: Insights into Proteasome Lid Assembly. J. Biol. Chem. 2015, 290, 6878–6889. [Google Scholar] [CrossRef]
- Wang, J.; Robida-Stubbs, S.; Tullet, J.M.A.; Rual, J.-F.; Vidal, M.; Blackwell, T.K. RNAi Screening Implicates a SKN-1–Dependent Transcriptional Response in Stress Resistance and Longevity Deriving from Translation Inhibition. PLoS Genet. 2010, 6, 1001048. [Google Scholar] [CrossRef] [PubMed]
- Fernando, L.M.; Quesada-Candela, C.; Murray, M.; Ugoaru, C.; Yanowitz, J.L.; Allen, A.K. Proteasomal Subunit Depletions Differentially Affect Germline Integrity in C. Elegans. Front. Cell Dev. Biol. 2022, 10, 901320. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Rae, R. A Functional Genomic Screen for Evolutionarily Conserved Genes Required for Lifespan and Immunity in Germline-Deficient C. Elegans. PLoS ONE 2014, 9, e101970. [Google Scholar] [CrossRef]
- Burger, J.; Merlet, J.; Tavernier, N.; Richaudeau, B.; Arnold, A.; Ciosk, R.; Bowerman, B.; Pintard, L. CRL2LRR-1 E3-Ligase Regulates Proliferation and Progression through Meiosis in the Caenorhabditis Elegans Germline. PLoS Genet. 2013, 9, e1003375. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Kanematsu, K.; Tanaka, K.; Yokosawa, H.; Kawahara, H. Proteasomal Ubiquitin Receptor RPN-10 Controls Sex Determination in Caenorhabditis Elegans. Mol. Biol. Cell 2006, 17, 5356–5371. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.M.; McDonald, K.L.; Dernburg, A.F. Cytological Analysis of Meiosis in Caenorhabditis elegans. Methods Mol. Biol. 2009, 558, 171–195. [Google Scholar]
- Sanford, C.; Perry, M.D. Asymmetrically Distributed Oligonucleotide Repeats in the Caenorhabditis Elegans Genome Sequence That Map to Regions Important for Meiotic Chromosome Segregation. Nucleic Acids Res. 2001, 29, 2920–2926. [Google Scholar] [CrossRef]
- Kumar, G.A.; Subramaniam, K. PUF-8 Facilitates Homologous Chromosome Pairing by Promoting Proteasome Activity during Meiotic Entry in C. elegans. Development 2018, 145, dev.163949. [Google Scholar] [CrossRef]
- Ahuja, J.S.; Sandhu, R.; Mainpal, R.; Lawson, C.; Henley, H.; Hunt, P.A.; Yanowitz, J.L.; Valentin Börner, G. Control of Meiotic Pairing and Recombination by Chromosomally Tethered 26S Proteasome. Science 2017, 355, 408–411. [Google Scholar] [CrossRef]
- Adamo, A.; Montemauri, P.; Silva, N.; Ward, J.D.; Boulton, S.J.; Volpe, A.L. BRC-1 Acts in the Inter-Sister Pathway of Meiotic Double-Strand Break Repair. EMBO Rep. 2008, 9, 287–292. [Google Scholar] [CrossRef]
- Boulton, S.J.; Martin, J.S.; Polanowska, J.; Hill, D.E.; Gartner, A.; Vidal, M. BRCA1/BARD1 Orthologs Required for DNA Repair in Caenorhabditis Elegans. Curr. Biol 2004, 14, 33–39. [Google Scholar] [CrossRef]
- Li, Q.; Saito, T.T.; Martinez-Garcia, M.; Deshong, A.J.; Nadarajan, S.; Lawrence, K.S.; Checchi, P.M.; Colaiacovo, M.P.; Engebrecht, J.A. The Tumor Suppressor BRCA1-BARD1 Complex Localizes to the Synaptonemal Complex and Regulates Recombination under Meiotic Dysfunction in Caenorhabditis Elegans. PLoS Genet. 2018, 14, e1007701. [Google Scholar] [CrossRef]
- Janisiw, E.; Dello Stritto, M.R.; Jantsch, V.; Silva, N. BRCA1-BARD1 Associate with the Synaptonemal Complex and pro-Crossover Factors and Influence RAD-51 Dynamics during Caenorhabditis Elegans Meiosis. PLoS Genet. 2018, 14, e1007653. [Google Scholar] [CrossRef] [PubMed]
- Caporali, S.; Butera, A.; Amelio, I. BAP1 in Cancer: Epigenetic Stability and Genome Integrity. Discov. Oncol. 2022, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, A. Recent Progress and Perspectives on the Mechanisms Underlying Asbestos Toxicity. Genes Environ. Off. J. Jpn. Environ. Mutagen. Soc. 2021, 43, 46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lv, L.; Huang, Y.; Ren, X.; Shi, Q. Chromosome Nondisjunction during Bipolar Mitoses of Binucleated Intermediates Promote Aneuploidy Formation along with Multipolar Mitoses Rather than Chromosome Loss in Micronuclei Induced by Asbestos. Oncotarget 2017, 8, 11030–11041. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, F.; Bocchini, M.; Bronte, G.; Delmonte, A.; Guidoboni, M.; Crinò, L.; Mazza, M. Malignant Pleural Mesothelioma: State-of-the-Art on Current Therapies and Promises for the Future. Front. Oncol. 2019, 9, 1519. [Google Scholar] [CrossRef] [PubMed]
- Hmeljak, J.; Sanchez-Vega, F.; Hoadley, K.A.; Shih, J.; Stewart, C.; Heiman, D.; Tarpey, P.; Danilova, L.; Drill, E.; Gibb, E.A.; et al. Integrative Molecular Characterization of Malignant Pleural Mesothelioma. Cancer Discov. 2018, 8, 1549–1565. [Google Scholar] [CrossRef]
- Sugarbaker, D.J.; Richards, W.G.; Gordon, G.J.; Dong, L.; De Rienzo, A.; Maulik, G.; Glickman, J.N.; Chirieac, L.R.; Hartman, M.L.; Taillon, B.E.; et al. Transcriptome Sequencing of Malignant Pleural Mesothelioma Tumors. Proc. Natl. Acad. Sci. USA 2008, 105, 3521–3526. [Google Scholar] [CrossRef]
- Torricelli, F.; Lococo, F.; Di Stefano, T.S.; Lorenzini, E.; Piana, S.; Valli, R.; Rena, O.; Veronesi, G.; Billè, A.; Ciarrocchi, A. Deep Sequencing Analysis Identified a Specific Subset of Mutations Distinctive of Biphasic Malignant Pleural Mesothelioma. Cancers 2020, 12, 2454. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The CBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Cerruti, F.; Jocollè, G.; Salio, C.; Oliva, L.; Paglietti, L.; Alessandria, B.; Mioletti, S.; Donati, G.; Numico, G.; Cenci, S.; et al. Proteasome Stress Sensitizes Malignant Pleural Mesothelioma Cells to Bortezomib-Induced Apoptosis. Sci. Rep. 2017, 7, 17626. [Google Scholar] [CrossRef]
- Gordon, G.J.; Mani, M.; Maulik, G.; Mukhopadhyay, L.; Yeap, B.Y.; Kindler, H.L.; Salgia, R.; Sugarbaker, D.J.; Bueno, R. Preclinical Studies of the Proteasome Inhibitor Bortezomib in Malignant Pleural Mesothelioma. Cancer Chemother. Pharmacol. 2008, 61, 549–558. [Google Scholar] [CrossRef]
- Fennell, D.A.; McDowell, C.; Busacca, S.; Webb, G.; Moulton, B.; Cakana, A.; O’Byrne, K.J.; Meerbeeck, J.V.; Donnellan, P.; McCaffrey, J.; et al. Phase II Clinical Trial of First or Second-Line Treatment with Bortezomib in Patients with Malignant Pleural Mesothelioma. J. Thorac. Oncol. 2012, 7, 1466–1470. [Google Scholar] [CrossRef]
- O’Brien, M.E.R.; Gaafar, R.M.; Popat, S.; Grossi, F.; Price, A.; Talbot, D.C.; Cufer, T.; Ottensmeier, C.; Danson, S.; Pallis, A.; et al. Phase II Study of First-Line Bortezomib and Cisplatin in Malignant Pleural Mesothelioma and Prospective Validation of Progression Free Survival Rate as a Primary End-Point for Mesothelioma Clinical Trials (European Organisation for Research and Treatment of Cancer 08052). Eur. J. Cancer 2013, 49, 2815–2822. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Fernández, C.; Jha, S.; Aliagas, E.; Holmberg, C.I.; Nadal, E.; Cerón, J. BAP1 Malignant Pleural Mesothelioma Mutations in Caenorhabditis elegans Reveal Synthetic Lethality between ubh-4/BAP1 and the Proteasome Subunit rpn-9/PSMD13. Cells 2023, 12, 929. https://doi.org/10.3390/cells12060929
Martínez-Fernández C, Jha S, Aliagas E, Holmberg CI, Nadal E, Cerón J. BAP1 Malignant Pleural Mesothelioma Mutations in Caenorhabditis elegans Reveal Synthetic Lethality between ubh-4/BAP1 and the Proteasome Subunit rpn-9/PSMD13. Cells. 2023; 12(6):929. https://doi.org/10.3390/cells12060929
Chicago/Turabian StyleMartínez-Fernández, Carmen, Sweta Jha, Elisabet Aliagas, Carina I. Holmberg, Ernest Nadal, and Julián Cerón. 2023. "BAP1 Malignant Pleural Mesothelioma Mutations in Caenorhabditis elegans Reveal Synthetic Lethality between ubh-4/BAP1 and the Proteasome Subunit rpn-9/PSMD13" Cells 12, no. 6: 929. https://doi.org/10.3390/cells12060929
APA StyleMartínez-Fernández, C., Jha, S., Aliagas, E., Holmberg, C. I., Nadal, E., & Cerón, J. (2023). BAP1 Malignant Pleural Mesothelioma Mutations in Caenorhabditis elegans Reveal Synthetic Lethality between ubh-4/BAP1 and the Proteasome Subunit rpn-9/PSMD13. Cells, 12(6), 929. https://doi.org/10.3390/cells12060929







