A Systematic Review of Progress toward Unlocking the Power of Epigenetics in NSCLC: Latest Updates and Perspectives
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection and Data Extraction
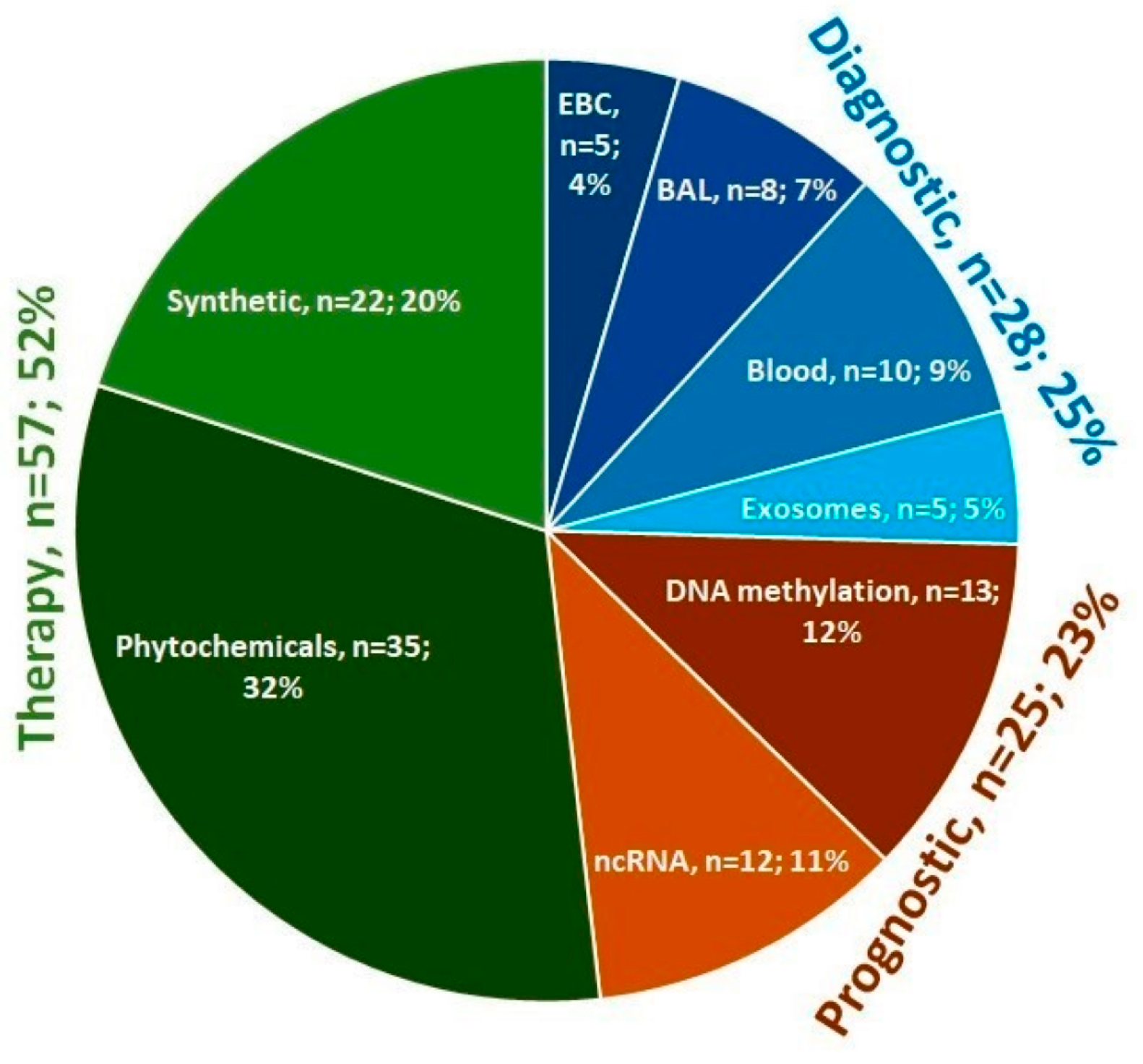
3. Results
4. Detailed Results and Discussion
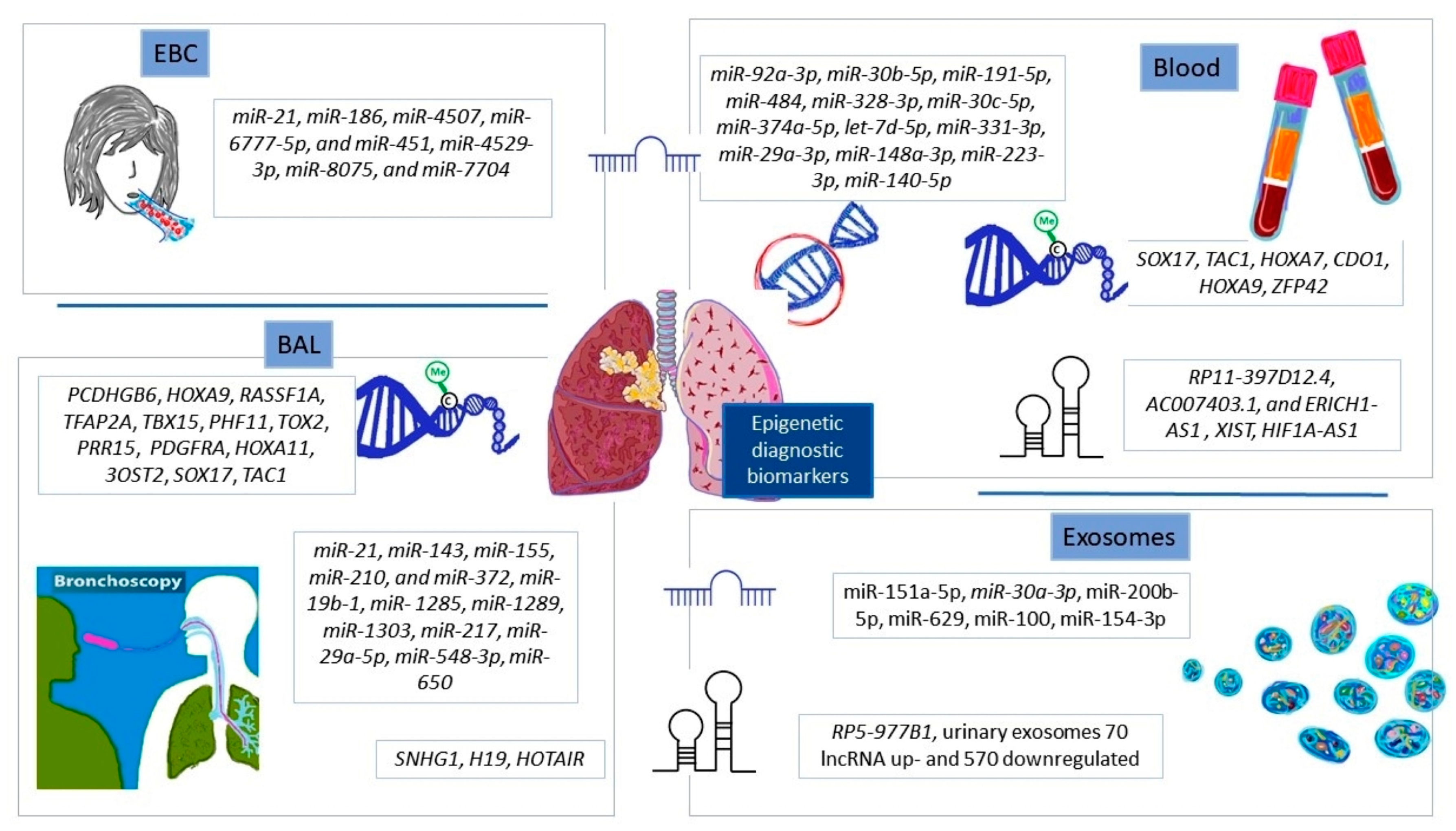
4.1. Diagnostic Epigenetic Biomarkers in NSCLC
4.1.1. Exhaled Breath Condensate
4.1.2. Bronchial Secretions
4.1.3. Peripheral Blood
4.1.4. Exosomes for Detection of NSCLC
4.2. Prognostic Epigenetic Biomarkers in NSCLC
4.2.1. From a Single Gene to Genome-Wide DNA Methylation Profiling
4.2.2. Non-Coding RNAs’ Expression Profiling
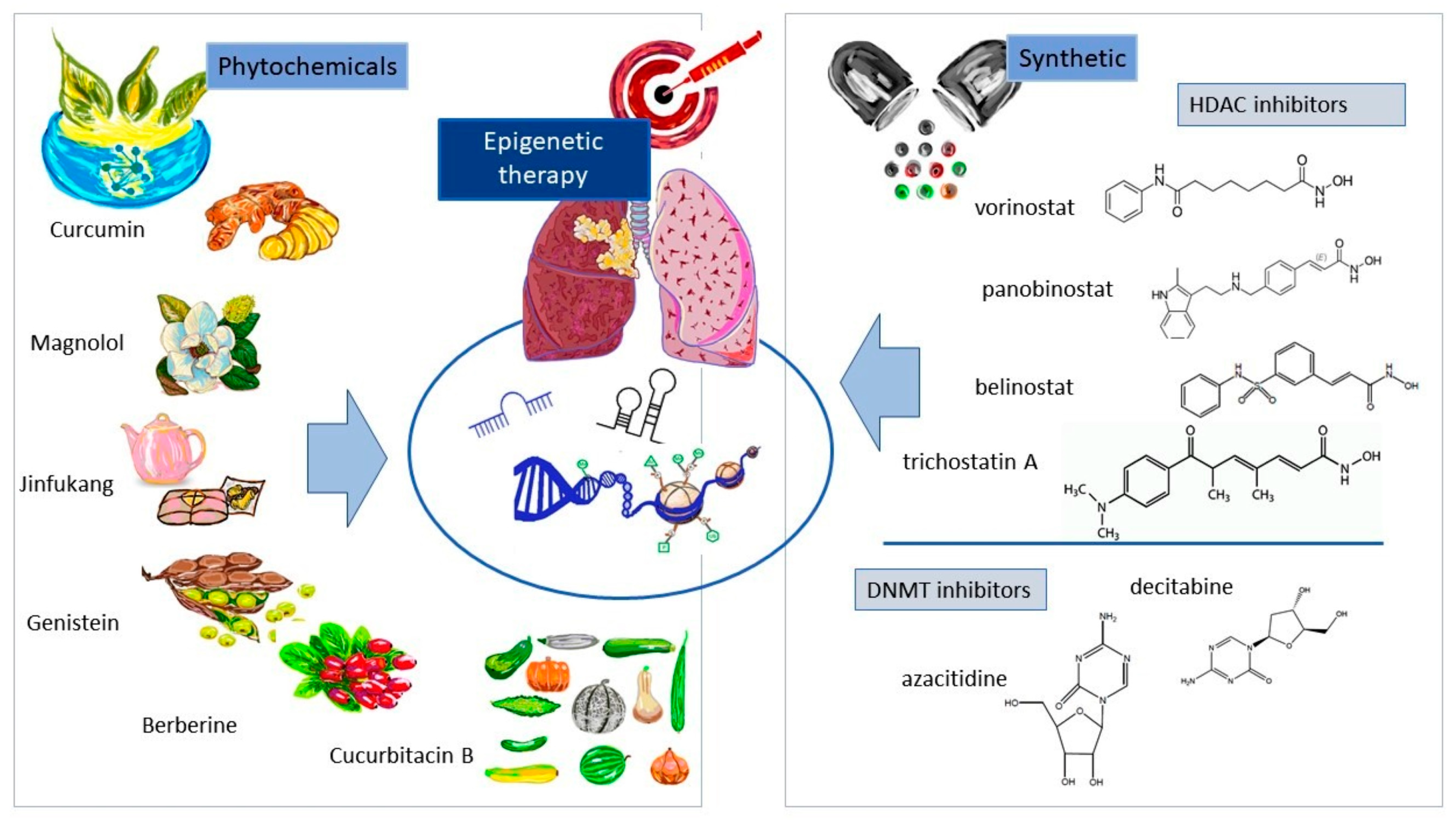
4.3. Epigenetic-Based Therapy for NSCLC
4.3.1. Natural Substances and Their Derivatives
4.3.2. Synthetic Epigenetic Modalities
4.3.3. Perspectives on Epigenetic Therapy for NSCLC
4.4. Summary and Future Strategies for Epigenetic Research on NSCLC
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mari-Alexandre, J.; Diaz-Lagares, A.; Villalba, M.; Juan, O.; Crujeiras, A.; Calvo, A.; Sandoval, J. Translating cancer epigenomics into the clinic: Focus on lung cancer. Trasl. Res. 2017, 189, 76–92. [Google Scholar] [CrossRef] [PubMed]
- Ansari, J.; Shackelford, R.; El-Osta, H. Epigenetics in non-small cell lung cancer: From basics to therapeutics. Trasl. Lung Cancer Res. 2016, 5, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Spangle, J.M.; Roberts, T.M.; Zhao, J.J. The emerging role of PI3K/AKT-mediated epigenetic regulation in cancer. Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 123–131. [Google Scholar] [CrossRef]
- Sharma, A.; Mir, R.; Galande, S. Epigenetic Regulation of the Wnt/β-Catenin Signaling Pathway in Cancer. Front. Genet. 2021, 12, 681053. [Google Scholar] [CrossRef]
- Mamdani, H.; Jalal, S.I. Histone Deacetylase Inhibition in Non-small Cell Lung Cancer: Hype or Hope? Front. Cell Dev. Biol. 2020, 8, 582370. [Google Scholar] [CrossRef]
- Ginn, L.; Shi, L.; La Montagna, M.; Garofalo, M. LncRNAs in Non-Small-Cell Lung Cancer. Non-Coding RNA 2020, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, R.; Sato, M.; Kakumu, T.; Hase, T.; Yogo, N.; Maruyama, E.; Sekido, Y.; Kondo, M.; Hasegawa, Y. Growth inhibitory effects of miR-221 and miR-222 in non-small cell lung cancer cells. Cancer Med. 2015, 4, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.L.; Lehmann, U. DNA methylation, microRNAs, and their crosstalk as potential biomarkers in hepatocellular carcinoma. World J. Gastroenterol. 2014, 20, 7894–7913. [Google Scholar] [CrossRef]
- Watanabe, K.; Takai, D. Disruption of the expression and function of microRNAs in lung cancer as a result of epigenetic changes. Front. Genet. 2013, 4, 275. [Google Scholar] [CrossRef]
- Tellez, C.; Juri, D.; Do, K.; Picchi, M.; Wang, T.; Liu, G.; Spira, A.; Belinsky, S. miR-196b Is Epigenetically Silenced during the Premalignant Stage of Lung Carcinogenesis. Cancer Res. 2016, 76, 4741–4751. [Google Scholar] [CrossRef]
- Watanabe, K.; Amano, Y.; Ishikawa, R.; Sunohara, M.; Kage, H.; Ichinose, J.; Sano, A.; Nakajima, J.; Fukayama, M.; Yatomi, Y.; et al. Histone methylation-mediated silencing of miR-139 enhances invasion of non-small-cell lung cancer. Cancer Med. 2015, 4, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.J.; Du, Y.; Fan, Y. Long noncoding RNAs in lung cancer: What we know in 2015. Clin. Transl. Oncol. 2016, 18, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, Y.; Liu, B.; Li, Y.; Fang, N.; Zu, L.; Li, X.; Zhou, Q. MicroRNA-449a inhibits cell growth in lung cancer and regulates long noncoding RNA nuclear enriched abundant transcript 1. Indian J. Cancer 2014, 51, e77–e81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-L.; Li, X.-B.; Hou, Y.-X.; Fang, N.-Z.; You, J.-C.; Zhou, Q.-H. The lncRNA XIST exhibits oncogenic properties via regulation of miR-449a and Bcl-2 in human non-small cell lung cancer. Acta Pharmacol. Sin. 2017, 38, 371–381. [Google Scholar] [CrossRef]
- Sun, M.; Liu, X.-H.; Wang, K.-M.; Nie, F.; Kong, R.; Yang, J.; Xia, R.; Xu, T.-P.; Ijn, F.-Y.; Liu, Z.-J.; et al. Downregulation of BRAF activated non-coding RNA is associated with poor prognosis for non-small cell lung cancer and promotes metastasis by affecting epithelial-mesenchymal transition. Mol. Cancer 2014, 13, 68. [Google Scholar] [CrossRef]
- Morita, S.; Horii, T.; Kimura, M.; Ochiya, T.; Tajima, S.; Hatada, I. miR-29 represses the activities of DNA methyltransferases and DNA demethylases. Int. J. Mol. Sci. 2013, 14, 14647–14658. [Google Scholar] [CrossRef]
- Yan, F.; Shen, N.; Pang, J.; Xie, D.; Deng, B.; Molina, J.R.; Yang, P.; Liu, S. Restoration of miR-101 suppresses lung tumorigenesis through inhibition of DNMT3a-dependent DNA methylation. Cell Death Dis. 2014, 5, e1413. [Google Scholar] [CrossRef]
- Amodio, N.; Rossi, M.; Raimondi, L.; Pitari, M.R.; Botta, C.; Tagliaferri, P.; Tassone, P. miR-29s: A family of epi-miRNAs with therapeutic implications in hematologic malignancies. Oncotarget 2015, 6, 12837–12861. [Google Scholar] [CrossRef]
- Liu, C.; Lv, D.; Li, M.; Zhang, X.; Sun, G.; Bai, Y.; Chang, D. Hypermethylation of miRNA-589 promoter leads to upregulation of HDAC5 which promotes malignancy in non-small cell lung cancer. Int. J. Oncol. 2017, 50, 2079–2090. [Google Scholar] [CrossRef]
- Langevin, S.M.; Kratzke, R.A.; Kelsey, K.T. Epigenetics of Lung Cancer. Transl. Res. 2015, 165, 74–90. [Google Scholar] [CrossRef]
- Kalia, M. Biomarkers for personalized oncology: Recent advances and future challenges. Metabolism 2015, 64, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Brzeziańska, E.; Dutkowska, A.; Antczak, A. The significance of epigenetic alterations in lung carcinogenesis. Mol. Biol. Rep. 2013, 40, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-X.; Sheng, D.-Q.; Cheng, L.; Song, X.-Y. Current Landscape of Epigenetics in Lung Cancer: Focus on the Mechanism and Application. J. Oncol. 2019, 2019, 8107318. [Google Scholar] [CrossRef] [PubMed]
- Su, S.-F.; Liu, C.-H.; Cheng, C.-L.; Ho, C.-C.; Yang, T.-Y.; Chen, K.-C.; Hsu, K.-H.; Tseng, J.-S.; Chen, H.-W.; Chang, G.-C.; et al. Genome-Wide Epigenetic Landscape of Lung Adenocarcinoma Links HOXB9 DNA Methylation to Intrinsic EGFR-TKI Resistance and Heterogeneous Responses. JCO Precis. Oncol. 2021, 5, 418–431. [Google Scholar] [CrossRef]
- Chao, Y.L.; Pecot, C.V. Targeting Epigenetics in Lung Cancer. Cold Spring Harb. Perspect. Med. 2021, 11, a038000. [Google Scholar] [CrossRef]
- Yang, S.; Huang, Y.; Zhao, Q. Epigenetic Alterations and Inflammation as Emerging Use for the Advancement of Treatment in Non-Small Cell Lung Cancer. Front. Immunol. 2022, 13, 878740. [Google Scholar] [CrossRef]
- Mao, Y.; Huang, P.; Wang, Y.; Wang, M.; Li, M.D.; Yang, Z. Genome-wide methylation and expression analyses reveal the epigenetic landscape of immune-related diseases for tobacco smoking. Clin. Epigenet. 2021, 13, 215. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Drilon, A.; Sugita, H.; Sima, C.S.; Zauderer, M.; Rudin, C.M.; Kris, M.G.; Rusch, V.W.; Azzoli, C.G. A prospective study of tumor suppressor gene methylation as a prognostic biomarker in surgically resected stage I to IIIA non-small-cell lung cancers. J. Thorac. Oncol. 2014, 9, 1272–1277. [Google Scholar] [CrossRef]
- Duppel, U.; Woenckhaus, M.; Schulz, C.; Merk, J.; Dietmaier, W. Quantitative detection of TUSC3 promoter methylation -a potential biomarker for prognosis in lung cancer. Oncol. Lett. 2016, 12, 3004–3012. [Google Scholar] [CrossRef][Green Version]
- Botana-Rial, M.; Chiara, L.D.; Valverde, D.; Leiro-Fernández, V.; Represas-Represas, C.; Campo-Pérez, V.; Alberto, F.-V. Prognostic value of aberrant hypermethylation in pleural effusion of lung adenocarcinoma. Cancer Biol. Ther. 2012, 13, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Heller, G.; Babinsky, V.N.; Ziegler, B.; Weinzierl, M.; Noll, C.; Altenberger, C.; Müllauer, L.; Dekan, G.; Grin, Y.; Lang, G.; et al. Genome-wide CpG island methylation analyses in non-small cell lung cancer patients. Carcinogenesis 2013, 34, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-A.; Lee, B.B.; Kim, Y.; Hong, S.-H.; Kim, Y.-H.; Han, J.; Shim, Y.M.; Yoon, C.-Y.; Lee, Y.-S.; Kim, D.-H. HOXA9 inhibits migration of lung cancer cells and its hypermethylation is associated with recurrence in non-small cell lung cancer. Mol. Carcinog. 2015, 54, 72–80. [Google Scholar] [CrossRef]
- Zhou, C.; Qin, Y.; Xie, Z.; Zhang, J.; Yang, M.; Li, S.; Chen, R. NPTX1 is a novel epigenetic regulation gene and associated with prognosis in lung cancer. Biochem. Biophys. Res. Commun. 2015, 458, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Soejima, K.; Arai, E.; Hamamoto, J.; Yasuda, H.; Arai, D.; Ishioka, K.; Ohgino, K.; Naoki, K.; Kohno, T.; et al. Prognostic implication of PTPRH hypomethylation in non-small cell lung cancer. Oncol. Rep. 2015, 34, 1137–1145. [Google Scholar] [CrossRef]
- Kuo, I.-Y.; Jen, J.; Hsu, L.-H.; Hsu, H.-S.; Lai, W.-W.; Wang, Y.-C. A prognostic predictor panel with DNA methylation biomarkers for early-stage lung adenocarcinoma in Asian and Caucasian populations. J. Biomed. Sci. 2016, 23, 58. [Google Scholar] [CrossRef]
- Youssef, O.; Sarhadi, V.K.; Armengol, G.; Piirilä, P.; Knuuttila, A.; Knuutila, S. Exhaled breath condensate as a source of biomarkers for lung carcinomas. A focus on genetic and epigenetic markers-A mini-review. Genes Chromosom. Cancer 2016, 55, 905–914. [Google Scholar] [CrossRef]
- Sulewska, A.; Niklinski, J.; Charkiewicz, R.; Karabowicz, P.; Biecek, P.; Baniecki, H.; Kowalczuk, O.; Kozlowski, M.; Modzelewska, P.; Majewski, P.; et al. A Signature of 14 Long Non-Coding RNAs (lncRNAs) as a Step towards Precision Diagnosis for NSCLC. Cancers 2022, 14, 439. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, E.Y.; Kim, T.; Chang, Y.S. Compared to plasma, bronchial washing fluid shows higher diagnostic yields for detecting EGFR-TKI sensitizing mutations by ddPCR in lung cancer. Respir. Res. 2020, 21, 142. [Google Scholar] [CrossRef]
- Balgkouranidou, I.; Liloglou, T.; Lianidou, E.S. Lung cancer epigenetics: Emerging biomarkers. Biomark. Med. 2013, 7, 49–58. [Google Scholar] [CrossRef]
- Lissa, D.; Robles, A.I. Methylation analyses in liquid biopsy. Transl. Lung Cancer Res. 2016, 5, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Palanca-Ballester, C.; Rodriguez-Casanova, A.; Torres, S.; Calabuig-Fariñas, S.; Exposito, F.; Serrano, D.; Redin, E.; Valencia, K.; Diaz-Lagares, E.J.-L.; Sandoval, L.M.J.; et al. Cancer Epigenetic Biomarkers in Liquid Biopsy for High Incidence Malignancies. Cancers 2021, 13, 3016. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-L.; Chen, J.-R.; Han, H.-N.; Zhou, F.; Lv, X.-D.; Ma, H. Clinical significance of miRNA21 in exhaled breath condensate of non-small-cell lung cancer. Int. J. Clin. Exp. Med. 2016, 9, 17232–17238. [Google Scholar]
- Xie, H.; Chen, J.; Lv, X.; Zhang, L.; Wu, J.; Ge, X.; Yang, Q.; Zhang, D.; Chen, J. Clinical Value of Serum and Exhaled Breath Condensate miR-186 and IL-1β Levels in Non-Small Cell Lung Cancer. Technol. Cancer Res. Treat. 2020, 19, 1533033820947490. [Google Scholar] [CrossRef]
- Pérez-Sánchez, C.; Barbarroja, N.; Pantaleão, L.C.; López-Sánchez, L.M.; Ozanne, S.E.; Jurado-Gámez, B.; Aranda, E.; Lopez-Pedrera, C.; Rodríguez-Ariza, A. Clinical Utility of microRNAs in Exhaled Breath Condensate as Biomarkers for Lung Cancer. J. Pers. Med. 2021, 11, 111. [Google Scholar] [CrossRef]
- Mozzoni, P.; Banda, I.; Goldoni, M.; Corradi, M.; Tiseo, M.; Acampa, O.; Balestra, V.; Ampollini, L.; Casalini, A.; Carbognani, P.; et al. Plasma and EBC microRNAs as early biomarkers of non-small-cell lung cancer. Biomarkers 2013, 18, 679–686. [Google Scholar] [CrossRef]
- Xiao, P.; Chen, J.; Zhou, F.; Lu, C.; Yang, Q.; Tao, G.; Tao, Y.; Chen, J. Methylation of P16 in exhaled breath condensate for diagnosis of non-small cell lung cancer. Lung Cancer 2014, 83, 56–60. [Google Scholar] [CrossRef]
- Millares, L.; Rosell, A.; Setó, L.; Sanz, J.; Andreo, F.; Monsó, E. Variability in the measurement of the methylation status of lung cancer-related genes in bronchial secretions. Oncol. Rep. 2014, 32, 1435–1440. [Google Scholar] [CrossRef][Green Version]
- Ma, Y.; Bai, Y.; Mao, H.; Hong, Q.; Yang, D.; Zhang, H.; Liu, F.; Wu, Z.; Jin, Q.; Zhou, H.; et al. A panel of promoter methylation markers for invasive and noninvasive early detection of NSCLC using a quantum dots-based FRET approach. Biosens. Bioelectron. 2016, 15, 641–648. [Google Scholar] [CrossRef]
- Um, S.-W.; Kim, Y.; Lee, B.-B.; Kim, D.; Lee, K.-J.; Kim, H.-K.; Han, J.; Kim, H.; Shim, Y.-M.; Kim, D.-H. Genome-wide analysis of DNA methylation in bronchial washings. Clin. Epigenet. 2018, 10, 65. [Google Scholar] [CrossRef]
- Su, Y.; Fang, H.; Jiang, F. Integrating DNA methylation and microRNA biomarkers in sputum for lung cancer detection. Clin. Epigenet. 2016, 8, 109. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Fang, H.B.; Jiang, F. An epigenetic classifier for early stage lung cancer. Clin. Epigenet. 2018, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.O.; Gazala, S.; Razzak, R.; Guo, L.; Ghosh, S.; Roa, W.H.; Bédard, E.L.R. Non-small cell lung cancer detection using microRNA expression profiling of bronchoalveolar lavage fluid and sputum. Anticancer Res. 2015, 35, 1873–1880. [Google Scholar]
- Rehbein, G.; Schmidt, B.; Fleischhacker, M. Extracellular microRNAs in bronchoalveolar lavage samples from patients with lung diseases as predictors for lung cancer. Clin. Chim. Acta 2015, 450, 78–82. [Google Scholar] [CrossRef]
- Gupta, C.; Su, J.; Zhan, M.; Stass, S.A.; Jiang, F. Sputum long non-coding RNA biomarkers for diagnosis of lung cancer. Cancer Biomark. 2019, 26, 219–227. [Google Scholar] [CrossRef]
- Montani, F.; Marzi, M.J.; Dezi, F.; Dama, E.; Carletti, R.M.; Bonizzi, G.; Bertolotti, R.; Bellomi, M.; Rampinelli, C.; Maisonneuve, P.; et al. miR-Test: A blood test for lung cancer early detection. J. Natl. Cancer Inst. 2015, 107, djv063. [Google Scholar] [CrossRef] [PubMed]
- Hulbert, A.; Jusue-Torres, I.; Stark, A.; Chen, C.; Rodgers, K.; Lee, B.; Griffin, C.; Yang, A.; Huang, P.; Wrangle, J.; et al. Early Detection of Lung Cancer Using DNA Promoter Hypermethylation in Plasma and Sputum. Clin. Cancer Res. 2017, 23, 1998–2005. [Google Scholar] [CrossRef]
- Pu, Q.; Huang, Y.; Lu, Y.; Peng, Y.; Zhang, J.; Feng, G.; Wang, C.; Liu, L.; Dai, Y. Tissue-specific and plasma microRNA profiles could be promising biomarkers of histological classification and TNM stage in non-small cell lung cancer. Thorac. Cancer 2016, 7, 348–354. [Google Scholar] [CrossRef]
- Ma, J.; Lin, Y.; Zhan, M.; Mann, D.L.; Stass, S.A.; Jiang, F. Differential miRNA expressions in peripheral blood mononuclear cells for diagnosis of lung cancer. Lab. Investig. 2015, 95, 1197–1206. [Google Scholar] [CrossRef]
- Tang, Q.; Ni, Z.; Cheng, Z.; Xu, J.; Yu, H.; Yin, P. Three circulating long non-coding RNAs act as biomarkers for predicting NSCLC. Cell. Physiol. Biochem. 2015, 37, 1002–1009. [Google Scholar] [CrossRef]
- Liang, W.; Lv, T.; Shi, X.; Liu, H.; Zhu, Q.; Zeng, J.; Yang, W.; Yin, J.; Song, Y. Circulating long noncoding RNA GAS5 is a novel biomarker for the diagnosis of nonsmall cell lung cancer. Medicine 2016, 95, e46082016. [Google Scholar] [CrossRef]
- Weber, D.G.; Johnen, G.; Casjens, S.; Bryk, O.; Pesch, B.; Jöckel, K.-H.; Kollmeier, J.; Brüning, T. Evaluation of long noncoding RNA MALAT1 as a candidate blood-based biomarker for the diagnosis of non-small cell lung cancer. BMC Res. Notes 2013, 6, 518. [Google Scholar] [CrossRef] [PubMed]
- Nadal, E.; Truini, A.; Nakata, A.; Lin, J.; Reddy, R.M.; Chang, A.C.; Ramnath, N.; Gotoh, N.; Beer, D.G.; Chen, G. A Novel Serum 4-microRNA Signature for Lung Cancer Detection. Sci. Rep. 2015, 5, 12464. [Google Scholar] [CrossRef] [PubMed]
- Powrózek, T.; Krawczyk, P.; Kowalski, D.M.B.K.-K.; Winiarczyk, K.; Olszyna-Serementa, M.; Batura-Gabryel, H.; Milanowski, J. Application of plasma circulating microRNA-448, 506, 4316, and 4478 analysis for non-invasive diagnosis of lung cancer. Tumour Biol. 2016, 37, 2049–2055. [Google Scholar] [CrossRef] [PubMed]
- Tantai, J.; Hu, D.; Yang, Y.; Geng, J. Combined identification of long non-coding RNA XIST and HIF1A-AS1 in serum as an effective screening for non-small cell lung cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 7887–7895. [Google Scholar]
- Qian, Z.; Shen, Q.; Yang, X.; Qiu, Y.; Zhang, W. The Role of Extracellular Vesicles: An Epigenetic View of the Cancer Microenvironment. Biomed Res. Int. 2015, 2015, 649161. [Google Scholar] [CrossRef]
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key players in cancer and potential therapeutic strategy. Signal Transduct. Target. Ther. 2020, 5, 145. [Google Scholar] [CrossRef]
- Jin, X.; Chen, Y.; Chen, H.; Fei, S.; Chen, D.; Cai, X.; Liu, L.; Lin, B.; Su, H.; Zhao, L.; et al. Evaluation of Tumor-Derived Exosomal miRNA as Potential Diagnostic Biomarkers for Early-Stage Non-Small Cell Lung Cancer Using Next-Generation Sequencing. Clin. Cancer Res. 2017, 23, 5311–5319. [Google Scholar] [CrossRef]
- Cazzoli, R.; Buttitta, F.; Nicola, M.D.; Malatesta, S.; Marchetti, A.; Rom, W.N.; Pass, H.I. microRNAs derived from circulating exosomes as noninvasive biomarkers for screening and diagnosing lung cancer. J. Thorac. Oncol. 2013, 8, 1156–1162. [Google Scholar] [CrossRef]
- Lin, Q.; Xie, D.; Pan, L.; Lou, Y.; Shi, M. Urinary exosomal long noncoding RNAs serve as biomarkers for early detection of non-small cell lung cancer. Biosci. Rep. 2021, 41, BSR20210908. [Google Scholar] [CrossRef]
- Zang, X.; Gu, J.; Zhang, J.; Shi, H.; Hou, S.; Xu, X.; Chen, Y.; Zhang, Y.; Mao, F.; Qian, H.; et al. Exosome-transmitted lncRNA UFC1 promotes non-small-cell lung cancer progression by EZH2-mediated epigenetic silencing of PTEN expression. Cell Death Dis. 2020, 11, 215. [Google Scholar] [CrossRef] [PubMed]
- Min, L.; Zhu, T.; Lv, B.; An, T.; Zhang, Q.; Shang, Y.; Yu, Z.; Zheng, L.; Wang, Q. Exosomal LncRNA RP5-977B1 as a novel minimally invasive biomarker for diagnosis and prognosis in non-small cell lung cancer. Int. J. Clin. Oncol. 2022, 27, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Reclusa, P.; Sirera, R.; Araujo, A.; Giallombardo, M.; Valentino, A.; Sorber, L.; Bazo, I.G.; Pauwels, P.; Rolfo, C. Exosomes genetic cargo in lung cancer: A truly Pandora’s box. Transl. Lung Cancer Res. 2016, 5, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Liloglou, T.; Bediaga, N.; Brown, B.; Field, J.; Davies, M. Epigenetic biomarkers in lung cancer. Cancer Lett. 2014, 342, 200–212. [Google Scholar] [CrossRef]
- Hong, Y.; Kim, W.J. DNA Methylation Markers in Lung Cancer. Curr. Genom. 2021, 22, 79–87. [Google Scholar] [CrossRef]
- Robles, A.I.; Arai, E.; Mathé, E.A.; Okayama, H.; Schetter, A.J.; Brown, D.; Petersen, D.; Bowman, E.D.; Noro, R.; Welsh, J.A.; et al. An Integrated Prognostic Classifier for Stage I Lung Adenocarcinoma Based on mRNA, microRNA, and DNA Methylation Biomarkers. J. Thorac. Oncol. 2015, 10, 1037–1048. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, X.; Wang, J.; Liang, T.; Gu, Y.; Yang, D. Down-regulation of PAX6 by promoter methylation is associated with poor prognosis in non small cell lung cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11452–11457. [Google Scholar]
- Liu, W.; Han, F.; Jiang, X.; Chen, H.; Zhao, H.; Liu, Y.; Li, Y.; Huang, C.; Cao, J.; Liu, J. TMEM196 acts as a novel functional tumour suppressor inactivated by DNA methylation and is a potential prognostic biomarker in lung cancer. Oncotarget 2015, 6, 21225–21239. [Google Scholar] [CrossRef]
- Xia, W.; Chen, Q.; Wang, J.; Mao, Q.; Dong, G.; Shi, R.; Zheng, Y.; Xu, L.; Jiang, F. DNA methylation mediated silencing of microRNA-145 is a potential prognostic marker in patients with lung adenocarcinoma. Sci. Rep. 2015, 5, 16901. [Google Scholar] [CrossRef]
- Dai, X.; Li, S. Advances and Trends in Omics Technology Development. Front. Med. 2022, 9, 911861. [Google Scholar] [CrossRef]
- Lokk, K.; Vooder, T.; Kolde, R.; Välk, K.; Võsa, U.; Roosipuu, R.; Milani, L.; Fischer, K.; Koltsina, M.; Urgard, E.; et al. Methylation markers of early-stage non-small cell lung cancer. PLoS ONE 2012, 7, e398132012. [Google Scholar] [CrossRef] [PubMed]
- Bjaanæs, M.M.; Fleischer, T.; Halvorsen, A.R.; Daunay, A.; Busato, F.; Solberg, S.; Jørgensen, L.; Kure, E.; Edvardsen, H.; Børresen-Dale, A.-L.; et al. Genome-wide DNA methylation analyses in lung adenocarcinomas: Association with EGFR, KRAS and TP53 mutation status, gene expression and prognosis. Mol. Oncol. 2016, 10, 330–343. [Google Scholar] [CrossRef]
- Barros-Silva, D.; Marques, J.; Henrique, R.; Jerónimo, C. Profiling DNA Methylation Based on Next-Generation Sequencing Approaches: New Insights and Clinical Applications. Genes 2018, 9, 429. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tian, H.; Yang, J.; Gong, Z. Long Noncoding RNAs Regulate Cell Growth, Proliferation, and Apoptosis. DNA Cell Biol. 2016, 35, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Bi, N.; Wu, L.; Ding, X.; Men, Y.; Zhou, W.; Li, L.; Zhang, W.; Shi, S.; Song, Y.; et al. MicroRNA-29c functions as a tumor suppressor by targeting VEGFA in lung adenocarcinoma. Mol. Cancer 2017, 16, 50. [Google Scholar] [CrossRef]
- Gao, X.; Wu, Y.; Yu, W.; Li, H. Identification of a seven-miRNA signature as prognostic biomarker for lung squamous cell carcinoma. Oncotarget 2016, 7, 81670–81679. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, Z.; Yuan, S.; Xie, W.; Li, C.; Hu, Z.; Xiang, Y.; Wu, N.; Wu, L.; Bai, L.; et al. Circulating exosomal microRNAs as prognostic biomarkers for non-small-cell lung cancer. Oncotarget 2017, 8, 13048–13058. [Google Scholar] [CrossRef]
- Guo, J.; Meng, R.; Yin, Z.; Li, P.; Zhou, R.; Zhang, S.; Dong, X.; Liu, L.; Wu, G. A serum microRNA signature as a prognostic factor for patients with advanced NSCLC and its association with tissue microRNA expression profiles. Mol. Med. Rap. 2016, 13, 4643–4653. [Google Scholar] [CrossRef]
- Mo, D.; Gu, B.; Gong, X.; Wu, L.; Wang, H.; Jiang, Y.; Zhang, B.; Zhang, M.; Zhang, Y.; Xu, J.; et al. miR-1290 is a potential prognostic biomarker in non-small cell lung cancer. J. Thorac. Dis. 2015, 7, 1570–1579. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, Z.; Fu, J.; Wang, Z.; Fan, Z.; Lei, T. Endogenous microRNA-424 predicts clinical outcome and its inhibition acts as cancer suppressor in human non-small cell lung cancer. Biomed. Pharmacother. 2017, 89, 208–214. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, H.; Shi, X.; Yao, Y.; Yang, W.; Song, Y. The long non-coding RNA HNF1A-AS1 regulates proliferation and metastasis in lung adenocarcinoma. Oncotarget 2015, 6, 9160–9172. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Yuan, Y.; Chen, X.; Zhai, H.; An, Y.; Tang, L.; Wang, J.; Zhang, D.; Zhang, L.; Cheng, W.; et al. Identification and Validation of Long Non-Coding RNA LCIIAR as a Biomarker in LUAD. Front. Oncol. 2022, 12, 933071. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Liu, H.-T.; Mei, J.; Ding, F.-B.; Xiao, H.-B.; Hu, F.-Q.; Hu, R.; Wang, M.-S. LncRNA HMlincRNA717 is down-regulated in non-small cell lung cancer and associated with poor prognosis. Int. J. Clin. Exp. Pathol. 2014, 7, 8881–8886. [Google Scholar] [PubMed]
- Han, L.; Zhang, E.; Yin, D.; Kong, R.; Xu, T.; Chen, W.; Xia, R.; Shu, Y.; De, W. Low expression of long noncoding RNA PANDAR predicts a poor prognosis of non-small cell lung cancer and affects cell apoptosis by regulating Bcl-2. Cell Death Dis. 2015, 6, e16652015. [Google Scholar] [CrossRef]
- Zhou, M.; Xu, W.; Yue, X.; Zhao, H.; Wang, Z.; Shi, H.; Cheng, L.; Sun, J. Relapse-related long non-coding RNA signature to improve prognosis prediction of lung adenocarcinoma. Oncotarget 2016, 7, 29720–29738. [Google Scholar] [CrossRef]
- Ahmad, A. Epigenetics in Personalized Management of Lung Cancer. Adv. Exp. Med. Biol. 2016, 890, 111–122. [Google Scholar] [CrossRef]
- Schiffmann, I.; Greve, G.; Jung, M.; Lübbert, M. Epigenetic therapy approaches in non-small cell lung cancer: Update and perspectives. Epigenetics. 2016, 11, 858–870. [Google Scholar] [CrossRef]
- Mehta, A.; Dobersch, S.; Romero-Olmedo, A.J.; Barreto, G. Epigenetics in lung cancer diagnosis and therapy. Cancer Metastasis Rev. 2015, 34, 229–241. [Google Scholar] [CrossRef]
- Fortunato, O.; Boeri, M.; Verri, C.; Moro, M.; Sozzi, G. Therapeutic use of microRNAs in lung cancer. Biomed Res. Int. 2014, 2014, 756975. [Google Scholar] [CrossRef]
- Jones, P.A.; Issa, J.-P.J.; Baylin, S. Targeting the cancer epigenome for therapy. Nat. Rev. Genet. 2016, 17, 630–641. [Google Scholar] [CrossRef]
- He, Y.-Z.; Yu, S.-L.; Li, X.-N.; Bai, X.-H.; Li, H.-T.; Liu, Y.-C.; Lv, B.-L.; Zhao, X.-M.; Wei, D.; Zhang, H.-L.; et al. Curcumin increases crizotinib sensitivity through the inactivation of autophagy via epigenetic modulation of the miR-142-5p/Ulk1 axis in non-small cell lung cancer. Cancer Biomark. 2022, 34, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Zhang, J.; Zhang, J.; Miao, Q.; Yao, L.; Zhang, J. Curcumin promotes apoptosis by activating the p53-miR-192-5p/215-XIAP pathway in non-small cell lung cancer. Cancer Lett. 2015, 357, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Sun, Y.; Liu, Z.; Zhang, C. miR-192-5p upregulation mediates the suppression of curcumin in human NSCLC cell proliferation, migration and invasion by targeting c-Myc and inactivating the Wnt/β-catenin signaling pathway. Mol. Med. Rap. 2022, 22, 1594–1604. [Google Scholar] [CrossRef]
- Gao, L.; Shao, T.; Zheng, W.; Ding, J. Curcumin suppresses tumor growth of gemcitabine-resistant non-small cell lung cancer by regulating lncRNA-MEG3 and PTEN signaling. Clin. Transl. Oncol. 2021, 23, 1386–1393. [Google Scholar] [CrossRef]
- Wang, W.-H.; Chen, J.; Zhang, B.-R.; Lu, S.-J.; Wang, F.; Peng, L.; Dai, J.-H.; Sun, Y.-Z. Curcumin inhibits proliferation and enhances apoptosis in A549 cells by downregulating lncRNA UCA1. Pharmazie 2018, 73, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.; Weng, Y.-S.; Wang, H.-Y.; Hsu, F.-T.; Chueh, F.-S.; Wu, J.-Y.; Chen, W.-L.; Chen, J.-H. Magnolol Induces Apoptosis Through Extrinsic/intrinsic Pathways and Attenuates NF-κB/STAT3 Signaling in Non-small-cell Lung Cancer Cells. Anticancer Res. 2022, 42, 3825–3833. [Google Scholar] [CrossRef]
- Zhao, M.; Zheng, Y.-H.; Zhao, Q.-Y.; Zheng, W.; Yang, J.-H.; Pei, H.-Y.; Liu, L.; Liu, K.-J.; Xue, L.-L.; Deng, D.-X.; et al. Synthesis and evaluation of new compounds bearing 3-(4-aminopiperidin-1-yl)methyl magnolol scaffold as anticancer agents for the treatment of non-small cell lung cancer via targeting autophagy. Eur. J. Med. Chem. 2021, 209, 112922. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zhang, Y.; Li, D.; Fu, S.; Tang, M.; Wan, L.; Chen, K.; Liu, Z.; Xue, L.; Peng, A.; et al. Discovery and synthesis of novel magnolol derivatives with potent anticancer activity in non-small cell lung cancer. Eur. J. Med. Chem. 2018, 156, 190–205. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tong, Y.; Yang, X.; Li, F.; Zheng, L.; Liu, W.; Wu, J.; Ou, R.; Zhang, G.; Hu, M.; et al. Novel histone deacetylase inhibitors derived from Magnolia officinalis significantly enhance TRAIL-induced apoptosis in non-small cell lung cancer. Pharmacol. Res. 2016, 111, 113–125. [Google Scholar] [CrossRef]
- Que, Z.; Zhou, Z.; Luo, B.; Dong, C.; Jiang, Y.; Li, H.; Tian, J. Jingfukang induces anti-cancer activity through oxidative stress-mediated DNA damage in circulating human lung cancer cells. BMC Complement. Altern. Med. 2019, 19, 204. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, X.; Shen, T.; Ma, C.; Wu, J.; Kong, H.; Tian, J.; Shao, Z.; Zhao, X.; Xu, L. Epigenetic Profiling of H3K4Me3 Reveals Herbal Medicine Jinfukang-Induced Epigenetic Alteration Is Involved in Anti-Lung Cancer Activity. Evidence-Based Complement. Altern. Med. 2016, 2016, 7276161. [Google Scholar] [CrossRef] [PubMed]
- Que, Z.-J.; Yao, J.-L.; Zhou, Z.-Y.; Yu, P.; Luo, B.; Li, H.-G.; Liu, J.-X.; Xu, H.-X.; Tian, J.-H. Jinfukang inhibits lung cancer metastasis by upregulating CX3CL1 to recruit NK cells to kill CTCs. J. Ethnopharmacol. 2021, 275, 114175. [Google Scholar] [CrossRef]
- Que, Z.-J.; Yang, Y.; Liu, H.-T.; Shang-Guan, W.-J.; Yu, P.; Zhu, L.-H.; Li, H.-G.; Liu, H.-M.; Tian, J.-H. Jinfukang regulates integrin/Src pathway and anoikis mediating circulating lung cancer cells migration. J. Ethnopharmacol. 2021, 267, 113473. [Google Scholar] [CrossRef] [PubMed]
- Que, Z.-J.; Luo, B.; Wang, C.-T.; Qian, F.-F.; Jiang, Y.; Li, Y.; Han, X.-H.; Li, H.-G.; Liu, I.-X.; Tian, J.-H. Proteomics analysis of tumor exosomes reveals vital pathways of Jinfukang inhibiting circulating tumor cells metastasis in lung cancer. J. Ethnopharmacol. 2020, 256, 112802. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Sun, J.; Sun, J. Combined Treatment with JFKD and Gefitinib Overcomes Drug Resistance in Non-Small Cell Lung Cancer. Curr. Pharm. Biotechnol. 2021, 22, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ma, H.; Zha, L.; Li, Q.; Pan, H.; Zhang, L. Genistein promotes apoptosis of lung cancer cells through the IMPDH2/AKT1 pathway. Am. J. Transl. Res. 2022, 14, 7040–7051. [Google Scholar]
- Yang, Y.; Zang, A.; Jia, Y.; Shang, Y.; Zhang, Z.; Ge, K.; Zhang, J.; Fan, W.; Wang, B. Genistein inhibits A549 human lung cancer cell proliferation via miR-27a and MET signaling. Oncol. Lett. 2016, 12, 2189–2193. [Google Scholar] [CrossRef]
- Liu, X.; Sun, C.; Liu, B.; Jin, X.; Li, P.; Zheng, X.; Zhao, T.; Li, F.; Li, Q. Genistein mediates the selective radiosensitizing effect in NSCLC A549 cells via inhibiting methylation of the keap1 gene promoter region. Oncotarget 2016, 7, 27267–27279. [Google Scholar] [CrossRef]
- Wu, T.-C.; Lin, Y.-C.; Chen, H.-L.; Huang, P.-R.; Liu, S.-Y.; Yeh, S.-L. The enhancing effect of genistein on apoptosis induced by trichostatin A in lung cancer cells with wild type p53 genes is associated with upregulation of histone acetyltransferase. Toxicol. Appl. Pharmacol. 2016, 292, 94–102. [Google Scholar] [CrossRef]
- Sacko, K.; Thangavel, K.; Shoyele, S.A. Codelivery of Genistein and miRNA-29b to A549 Cells Using Aptamer-Hybrid Nanoparticle Bioconjugates. Nanomaterials 2019, 9, 1052. [Google Scholar] [CrossRef]
- Zhang, J.; Su, H.; Li, Q.; Li, J.; Zhao, Q. Genistein decreases A549 cell viability via inhibition of the PI3K/AKT/HIF-1α/VEGF and NF-κB/COX-2 signaling pathways. Mol. Med. Rep. 2017, 15, 2296–2302. [Google Scholar] [CrossRef] [PubMed]
- Achi, I.T.; Sarbadhikary, P.; George, B.P.; Abrahamse, H. Multi-Target Potential of Berberine as an Antineoplastic and Antimetastatic Agent: A Special Focus on Lung Cancer Treatment. Cells 2022, 11, 3433. [Google Scholar] [CrossRef] [PubMed]
- Alnuqaydan, A.M.; Almutary, A.G.; Azam, M.; Manandhar, B.; Yin, G.H.S.; Yen, L.L.; Madheswaran, T.; Paudel, K.R.; Hansbro, P.M.; Chellappan, D.K.; et al. Evaluation of the Cytotoxic Activity and Anti-Migratory Effect of Berberine-Phytantriol Liquid Crystalline Nanoparticle Formulation on Non-Small-Cell Lung Cancer In Vitro. Pharmaceutics 2022, 14, 1119. [Google Scholar] [CrossRef] [PubMed]
- Kalaiarasi, A.; Anusha, C.; Sankar, R.; Rajasekaran, S.; Marshal, J.J.; Muthusamy, K.; Ravikumar, V. Plant Isoquinoline Alkaloid Berberine Exhibits Chromatin Remodeling by Modulation of Histone Deacetylase To Induce Growth Arrest and Apoptosis in the A549 Cell Line. J. Agric. Food Chem. 2016, 64, 9542–9550. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Li, J.; Ma, C.; Tang, X.; Tang, Q.; Wu, J.; Chai, X.; Xie, J.; Yang, X.-B.; Hann, S.S. Novel regulation of miR-34a-5p and HOTAIR by the combination of berberine and gefitinib leading to inhibition of EMT in human lung cancer. J. Cell. Mol. Med. 2020, 24, 5578–5592. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, X.; Tao, C.; Wang, L.; Chen, Z.; Li, X.; Zeng, Q.; Ma, M.; Zhang, R.; Wu, Z. Berberine chloride suppresses non-small cell lung cancer by deregulating Sin3A/TOP2B pathway in vitro and in vivo. Cancer Chemother. Pharmacol. 2020, 86, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Hou, Y.; Li, D.; Ding, Z.; Xu, X.; Hao, B.; Xia, Q.; Li, M.; Fan, L. Berberine induces non-small cell lung cancer apoptosis via the activation of the ROS/ASK1/JNK pathway. Ann. Transl. Med. 2022, 10, 485. [Google Scholar] [CrossRef]
- Ni, L.; Li, Z.; Ren, H.; Kong, L.; Chen, X.; Xiong, M.; Zhang, X.; Ning, B.; Li, J. Berberine inhibits non-small cell lung cancer cell growth through repressing DNA repair and replication rather than through apoptosis. Clin. Exp. Pharmacol. Physiol. 2022, 49, 134–144. [Google Scholar] [CrossRef]
- Liu, J.; Huang, X.; Liu, D.; Ji, K.; Tao, C.; Zhang, R.; Chen, J. Demethyleneberberine induces cell cycle arrest and cellular senescence of NSCLC cells via c-Myc/HIF-1α pathway. Phytomedicine 2021, 91, 153678. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Wang, C.; Zhao, X.; Ma, C.; Fu, K.; Liu, Y.; Peng, C.; Li, Y. Cucurbitacin B: A review of its pharmacology, toxicity, and pharmacokinetics. Pharmacol. Res. 2023, 187, 106587. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Khan, S.; Kumar, S.; Sinha, S.; Farhan, M.; Bora, H.K.; Maurya, R.; Meeran, S.M. Cucurbitacin B Alters the Expression of Tumor-Related Genes by Epigenetic Modifications in NSCLC and Inhibits NNK-Induced Lung Tumorigenesis. Cancer Prev. Res. 2015, 8, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-H.; Li, C.; Cao, L.; Zhang, C.-H.; Zhang, Z.-H. Cucurbitacin B regulates lung cancer cell proliferation and apoptosis via inhibiting the IL-6/STAT3 pathway through the lncRNA XIST/miR-let-7c axis. Phamaceut. Biol. 2022, 60, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zheng, L.; Tang, H.; Wang, W.; Lin, Y. Cucurbitacin B enhances apoptosis in gefitinib resistant non-small cell lung cancer by modulating the miR-17-5p/STAT3 axis. Mol. Med. Rep. 2021, 24, 710. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Fan, Q.; Liang, X.; Han, S.; He, J.; Wang, Q.-Q.; Gao, H.; Feng, Y.; Yang, S. Cucurbitacin B inhibits TGF-β1-induced epithelial-mesenchymal transition (EMT) in NSCLC through regulating ROS and PI3K/Akt/mTOR pathways. Chin. Med. 2022, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Xiang, Y.; Liu, X.; Zhang, T.; Yang, R.; Chen, S.; Xu, L.; Yu, Q.; Zhao, H.; Zhang, L.; et al. Cucurbitacin B Induces the Lysosomal Degradation of EGFR and Suppresses the CIP2A/PP2A/Akt Signaling Axis in Gefitinib-Resistant Non-Small Cell Lung Cancer. Molecules 2019, 24, 647. [Google Scholar] [CrossRef]
- Kusagawa, E.; Okuda, C.; Yamaguchi, R.; Nakano, K.; Miyake, Y.; Kataoka, T. Cucurbitacin B Down-Regulates TNF Receptor 1 Expression and Inhibits the TNF-α-Dependent Nuclear Factor κB Signaling Pathway in Human Lung Adenocarcinoma A549 Cells. Int. J. Mol. Sci. 2022, 23, 7130. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Sinha, S.; Khan, S.; Kumar, S.; Singh, K.; Mitra, K.; Maurya, R.; Meeran, S.M. Cucurbitacin B inhibits the stemness and metastatic abilities of NSCLC via downregulation of canonical Wnt/β-catenin signaling axis. Sci. Rep. 2016, 6, 21860. [Google Scholar] [CrossRef] [PubMed]
- Heng, W.S.; Kruyt, F.A.E.; Cheah, S.-C. Understanding Lung Carcinogenesis from a Morphostatic Perspective: Prevention and Therapeutic Potential of Phytochemicals for Targeting Cancer Stem Cells. Int. J. Mol. Sci. 2021, 22, 5697. [Google Scholar] [CrossRef]
- Shankar, S.; Kumar, D.; Srivastava, R.K. Epigenetic Modifications by Dietary Phytochemicals: Implications for Personalized Nutrition. Pharmacol. Ther. 2013, 138, 1–17. [Google Scholar] [CrossRef]
- Takeuchi, S.; Hase, T.; Shimizu, S.; Ando, M.; Hata, A.; Murakami, H.; Kawakami, T.; Nagase, K.; Yoshimura, K.; Fujiwara, T.; et al. Phase I study of vorinostat with gefitinib in BIM deletion polymorphism/epidermal growth factor receptor mutation double-positive lung cancer. Cancer Sci. 2020, 111, 561–570. [Google Scholar] [CrossRef]
- Wang, L.; Syn, N.L.-X.; Subhash, V.V.; Any, Y.; Thuya, W.L.; Cheow, E.S.H.; Kong, L.; Yu, F.; Peethala, P.C.; Wong, A.L.-A.; et al. Pan-HDAC inhibition by panobinostat mediates chemosensitization to carboplatin in non-small cell lung cancer via attenuation of EGFR signaling. Cancer Lett. 2018, 417, 152–160. [Google Scholar] [CrossRef] [PubMed]
- To, K.K.-W.; Tong, W.-S.; Fu, L.-W. Reversal of platinum drug resistance by the histone deacetylase inhibitor belinostat. Lung Cancer 2017, 103, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Erkin, Ö.C.; Cömertpay, B.; Göv, E. Integrative Analysis for Identification of Therapeutic Targets and Prognostic Signatures in Non-Small Cell Lung Cancer. Bioinform. Biol. Insights 2022, 16, 11779322221088796. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.N.; Hollenbach, P.W.; Richard, N.; Luna-Moran, A.; Brady, H.; Heise, C.; MacBeth, K.J. Azacitidine and decitabine have different mechanisms of action in non-small cell lung cancer cell lines. Lung Cancer Targets Ther. 2010, 1, 119–140. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.E.; Saltos, A.; Tanvetyanon, T.; Haura, E.B.; Creelan, B.; Antonia, S.J.; Shafique, M.; Zheng, H.; Dai, W.; Saller, J.J.; et al. Phase 1/1b study of pembrolizumab plus vorinostat in advanced/metastatic non-small cell lung cancer. Clin. Cancer Res. 2019, 25, 6623–6632. [Google Scholar] [CrossRef]
- Tu, B.; He, Y.; Chen, B.; Wang, Y.; Gao, Y.; Shi, M.; Liu, T.; Asrorov, A.M.; Huang, Y. Deformable liposomal codelivery of vorinostat and simvastatin promotes antitumor responses through remodeling tumor microenvironment. Biomater. Sci. 2020, 8, 7166–7176. [Google Scholar] [CrossRef]
- Takashina, T.; Kinoshita, I.; Kikuchi, J.; Shimizu, Y.; Sakakibara-Konishi, J.; Oizumi, S.; Nishimura, M.; Dosaka-Akita, H. Combined inhibition of EZH2 and histone deacetylases as a potential epigenetic therapy for non-small-cell lung cancer cells. Cancer Sci. 2016, 107, 955–962. [Google Scholar] [CrossRef]
- Liang, Y.; Zeng, J.; Jelicks, L.; Ma, S.; Liu, J.; Mei, J.; Perez-Soler, R.; Zou, Y. Pax5 Re-expression in H460 Cells Treated with the Combination of Demethylating Agent and Histone Deacetylase Inhibitor is Associated with the Enhancement of P53 Binding to Pax5 Promoter Region. Curr. Cancer Drug Targets 2017, 17, 169–176. [Google Scholar] [CrossRef]
- Wu, Y.; Lyu, H.; Liu, H.; Shi, X.; Song, Y.; Liu, B. Downregulation of the long noncoding RNA GAS5-AS1 contributes to tumor metastasis in non-small cell lung cancer. Sci. Rep. 2016, 6, 31093. [Google Scholar] [CrossRef]
- Takhar, H.S.; Singhal, N.; Gowda, R.; Penniment, M.; Takhar, P.; Brown, M.P. Phase I study evaluating the safety and efficacy of oral panobinostat in combination with radiotherapy or chemoradiotherapy in patients with inoperable stage III non-small-cell lung cancer. Anticancer Drugs 2015, 26, 1069–1077. [Google Scholar] [CrossRef]
- Ong, P.-S.; Wang, L.; Chia, D.M.-H.; Seah, J.Y.-X.; Kong, L.-R.; Thuya, W.-L.; Chinnathambi, A.; Lau, J.-Y.A.; Wong, A.L.-A.; Yong, W.-P.; et al. A novel combinatorial strategy using Seliciclib(®) and Belinostat(®) for eradication of non-small cell lung cancer via apoptosis induction and BID activation. Cancer Lett. 2016, 381, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Shindo, Y.; Arai, W.; Konno, T.; Kohno, T.; Kodera, Y.; Chiba, H.; Miyajima, M.; Sakuma, Y.; Watanabe, A.; Kojima, T. Effects of histone deacetylase inhibitors Tricostatin A and Quisinostat on tight junction proteins of human lung adenocarcinoma A549 cells and normal lung epithelial cells. Histochem. Cell Biol. 2021, 155, 637–653. [Google Scholar] [CrossRef]
- Tang, D.; Yao, R.; Zhao, D.; Zhou, L.; Wu, Y.; Yang, Y.; Sun, Y.; Lu, L.; Gao, W. Trichostatin A reverses the chemoresistance of lung cancer with high IGFBP2 expression through enhancing autophagy. Sci. Rep. 2018, 8, 3917. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Zou, Y.; Shah, C.D.; Fan, N.; Bhagat, T.D.; Gucalp, R.; Kim, M.; Verma, A.; Piperdi, B.; Spivack, S.D.; et al. First-in-human study of inhaled Azacitidine in patients with advanced non-small cell lung cancer. Lung Cancer 2021, 154, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Villalba, M.; Redin, E.; Exposito, F.; Pajares, M.J.; Sainz, C.; Hervas, D.; Guruceaga, E.; Diaz-Lagares, A.; Cirauqui, C.; Redrado, M.; et al. Identification of a novel synthetic lethal vulnerability in non-small cell lung cancer by co-targeting TMPRSS4 and DDR1. Sci. Rep. 2019, 9, 15400. [Google Scholar] [CrossRef] [PubMed]
- Nehme, E.; Rahal, Z.; Sinjab, A.; Khalil, A.; Chami, H.; Nemer, G.; Kadara, H. Epigenetic Suppression of the T-box Subfamily 2 ( TBX2) in Human Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2019, 20, 1159. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhu, J.; Qin, H.; Shen, D.; Lei, Z.; Li, W.; Ding, Z.; Huang, J.-A.; Liu, Z. Methylated +322-327 CpG site decreases hOGG1 mRNA expression in non-small cell lung cancer. Oncol. Rep. 2017, 38, 529–537. [Google Scholar] [CrossRef][Green Version]
- Zhang, X.; Rong, X.; Chen, Y.; Su, L. Methylation-mediated loss of SFRP2 enhances invasiveness of non-small cell lung cancer cells. Hum. Exp. Toxicol. 2018, 37, 155–162. [Google Scholar] [CrossRef]
- Topper, M.J.; Vaz, M.; Chiappinelli, K.B.; Shields DeStefano, C.E.; Niknafs, N.; Yen, R.-W.C.; Wenzel, A.; Hicks, J.; Ballew, M.; Stone, M.; et al. Epigenetic Therapy Ties MYC Depletion to Reversing Immune Evasion and Treating Lung Cancer. Cell 2017, 171, 1284–1300. [Google Scholar] [CrossRef]
- Xu, M.; Song, B.; Yang, X.; Li, N. The combination of decitabine and aspirin inhibits tumor growth and metastasis in non-small cell lung cancer. J. Int. Med. Res. 2022, 50, 3000605221112024. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, Y.; Wang, Y.; Zhao, M.; Tu, L.; Luo, F. Decitabine reverses TGF-β1-induced epithelial-mesenchymal transition in non-small-cell lung cancer by regulating miR-200/ZEB axis. Drug Des. Devel. Ther. 2017, 11, 969–983. [Google Scholar] [CrossRef] [PubMed]
- Drzewiecka, H.; Gałęcki, B.; Jarmołowska-Jurczyszyn, D.; Kluk, A.; Dyszkiewicz, W.; Jagodziński, P.P. Decreased expression of connective tissue growth factor in non-small cell lung cancer is associated with clinicopathological variables and can be restored by epigenetic modifiers. J. Cancer Res. Clin. Oncol. 2016, 142, 1927–1946. [Google Scholar] [CrossRef] [PubMed]
- Husso, T.; Turunen, M.P.; Parker, N.; Ylä-Herttuala, S. Epigenetherapy, a new concept. Biomol. Concepts 2011, 2, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ren, Y.; Weng, S.; Xu, H.; Li, L.; Han, X. A New Trend in Cancer Treatment: The Combination of Epigenetics and Immunotherapy. Front. Immunol. 2022, 13, 809761. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, T.; Meng, Y.; Luo, J.; Chen, J.; Xia, B.; Zhang, Z.; Cheng, Z.; Wang, X. BET inhibitors combined with chemotherapy synergistically inhibit the growth of NSCLC cells. Oncol. Rep. 2021, 45, 70. [Google Scholar] [CrossRef]
- Sun, X.; Yi, J.; Yang, J.; Han, Y.; Qian, X.; Liu, Y.; Li, J.; Lu, B.; Zhang, J.; Pan, X.; et al. An integrated epigenomic-transcriptomic landscape of lung cancer reveals novel methylation driver genes of diagnostic and therapeutic relevance. Theranostics. 2021, 11, 5346–5364. [Google Scholar] [CrossRef]
- Brasil, S.; Neves, C.J.; Rijoff, T.; Falcão, M.; Valadão, G.; Videira, P.A.; Ferreira, V.D.R.F. Artificial Intelligence in Epigenetic Studies: Shedding Light on Rare Diseases. Front. Mol. Biosci. 2021, 8, 648012. [Google Scholar] [CrossRef]
- Bahado-Singh, R.; Vlachos, K.T.; Aydas, B.; Gordevicius, J.; Radhakrishna, U.; Vishweswaraiah, S. Precision Oncology: Artificial Intelligence and DNA Methylation Analysis of Circulating Cell-Free DNA for Lung Cancer Detection. Front. Oncol. 2022, 12, 790645. [Google Scholar] [CrossRef]
- Rauschert, S.; Raubenheimer, K.; Melton, P.E.; Huang, R.C. Machine learning and clinical epigenetics: A review of challenges for diagnosis and classification. Clin. Epigenet. 2020, 12, 51. [Google Scholar] [CrossRef]
- Rozek, L.S.; Dolinoy, D.C.; Sartor, M.A.; Omenn, G.S. Epigenetics: Relevance and implications for public health. Annu. Rev. Public Health 2014, 35, 105–122. [Google Scholar] [CrossRef]
- Dyke, S.O.M.; Saulnier, K.M.; Dupras, C.; Webster, A.P.; Maschke, K.; Rothstein, M.; Siebert, R.; Walter, J.; Beck, S.; Pastinen, T.; et al. Points-to-consider on the return of results in epigenetic research. Genome Med. 2019, 11, 31. [Google Scholar] [CrossRef] [PubMed]

| Topic | No. | Item | Location Where Item Is Reported |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review. | page 1 |
| ABSTRACT | |||
| Abstract | 2 | See PRISMA 2020 for Abstracts checklist. | |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | pages 2 and 3 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | page 3 |
| METHODS | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | page 7 |
| Information sources | 6 | Specify all databases, registers, websites, organizations, reference lists, and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | page 3 |
| Search strategy | 7 | Present the full search strategies for all databases, registers, and websites, including any filters and limits used. | pages 6 and 7 |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process. | pages 7 and 8 |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools used in the process. | pages 7 and 8 |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g., for all measures, time points, analyses), and if not, the methods used to decide which results to collect. | pages 7 and 8 |
| 10b | List and define all other variables for which data were sought (e.g., participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | N/A | |
| Study risk of bias assessment | 11 | Specify the methods used to assess risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently, and if applicable, details of automation tools used in the process. | page 8 |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g., risk ratio, mean difference) used in the synthesis or presentation of results. | page 8 |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g., tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item 5)). | N/A |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics, or data conversions. | N/A | |
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses. | N/A | |
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. | N/A | |
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g., subgroup analysis, meta-regression). | N/A | |
| 13f | Describe any sensitivity analyses conducted to assess robustness of the synthesized results. | N/A | |
| Reporting bias assessment | 14 | Describe any methods used to assess risk of bias due to missing results in a synthesis (arising from reporting biases). | N/A |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | N/A |
| RESULTS | |||
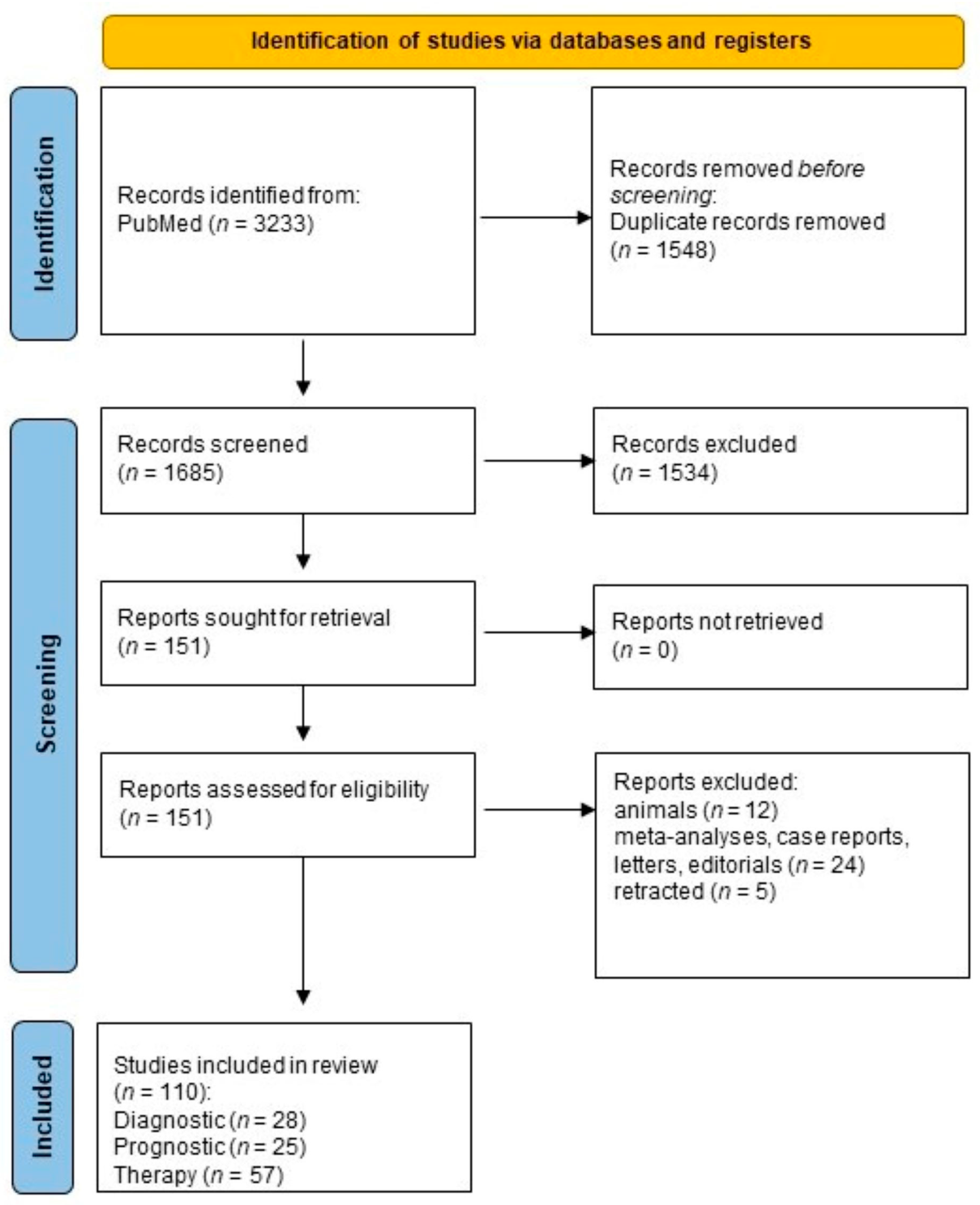
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram. | pages 9–11 |
| 16b | Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded. | N/A | |
| Study characteristics | 17 | Cite each included study and present its characteristics. | pages 12–23 |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study. | page 10 |
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g., confidence/credible interval), ideally using structured tables or plots. | pages 12–23 |
| Results of syntheses | 20a | For each synthesis, briefly summarize the characteristics and risk of bias among contributing studies. | N/A |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was conducted, present for each the summary estimate and its precision (e.g., confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | N/A | |
| 20c | Present results of all investigations of possible causes of heterogeneity among study results. | N/A | |
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results. | N/A | |
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. | N/A |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | N/A |
| DISCUSSION | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | pages 12–23 |
| 23b | Discuss any limitations of the evidence included in the review. | pages 12–23 | |
| 23c | Discuss any limitations of the review processes used. | N/A | |
| 23d | Discuss implications of the results for practice, policy, and future research. | pages 24–26 | |
| OTHER INFORMATION | |||
| Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered. | N/A |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared. | N/A | |
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | N/A | |
| Support | 25 | Describe sources of financial or non-financial support for the review, and the role of the funders or sponsors in the review. | N/A |
| Competing interests | 26 | Declare any competing interests of review authors. | N/A |
| Availability of data, code, and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review. | N/A |
| Database | PubMed |
|---|---|
| Date | from inception to 22 December 2022 |
| #1 | “lung cancer epigenetic” AND ((DNA methylation diagnosis) OR miRNA diagnosis) OR lncRNA diagnosis) OR (liquid biopsy AND lung cancer diagnosis)) OR exhaled breath condensate) OR methylation detection methods) OR DNA methylation prognosis) OR miRNA prognosis) OR lncRNA prognosis) OR epigenetic-targeted therapy) OR novel therapeutics) OR clinical trials) OR preclinical trials) OR nutriceuticals) AND full text[sb] AND Humans[MeSH] AND English[lang]) |
| #2 | (((NSCLC diagnosis) AND (DNA methylation)) OR ((miRNA) AND (NSCLC diagnosis)) OR ((lncRNA) AND (NSCLC diagnosis)) OR ((liquid biopsy) AND (NSCLC diagnosis)) OR ((exhaled breath condensate) AND (NSCLC diagnosis)) AND full text[sb] AND AND Humans[MeSH] AND English[lang]) |
| #3 | (((NSCLC prognosis) AND (DNA methylation)) OR ((miRNA) AND (NSCLC prognosis)) OR ((lncRNA) AND (NSCLC prognosis)) AND full text[sb] AND Humans[MeSH] AND English[lang]) |
| #4 | (((NSCLC therapy) AND (DNA methylation)) OR ((miRNA) AND (NSCLC therapy)) OR ((lncRNA) AND (NSCLC therapy)) OR ((epigenetic therapy) AND (lung cancer)) OR ((novel therapeutics) AND (NSCLC)) OR ((epigenetic therapy) AND (clinical trial)) OR ((epigenetic therapy) AND (preclinical trial)) OR ((epigenetic therapy) AND (nutriceuticals)) AND full text[sb] AND Humans[MeSH] AND English[lang]) |
| Search Terms Used in the Systematic Review | |
|---|---|
| Lung cancer epigenetics | DNA methylation diagnosis |
| NSCLC diagnosis | miRNA diagnosis |
| NSCLC prognosis | lncRNA diagnosis |
| NSCLC therapy | Epigenetic therapy |
| Liquid biopsy | miRNA prognosis |
| Lung cancer diagnosis | lncRNA prognosis |
| Exhaled breath condensate | Epigenetic-targeted therapy |
| Methylation detection methods | Novel therapeutics |
| DNA methylation prognosis | Nutriceuticals |
| Measures and Methods for the Studies Included in the Systematic Review | ||
|---|---|---|
| Chapter | Measures | Methods |
| Section 4.1 | Diagnostic epigenetic biomarkers in NSCLC | |
| Cancerous vs. non-cancerous tissue | Sensitivity and specificity as given in AUC measures | |
| Section 4.1.1 | Exhaled breath condensate findings | Proportion in study population |
| Section 4.1.2 | Bronchial secretions | Sensitivity and specificity as given in AUC measures |
| Section 4.1.3 | Peripheral blood | Sensitivity and specificity |
| Section 4.1.4 | Exosomes for detection of NSCLC | Size and concentration |
| Section 4.2 | Prognostic epigenetic biomarkers in NSCLC | |
| Classical survival parameters since diagnosis | Overall survival | |
| Section 4.2.1 | Single-gene/genome-wide DNA methylation profiling | Overall survival |
| Section 4.2.2 | Non-coding RNA expression profiling | Survival parameters |
| Section 4.3 | Epigenetic-based therapy for NSCLC | |
| Efficacy of/response to treatment | Measurement of tumor load | |
| Section 4.3.1 | Natural substances and their derivatives | Expression vs. tumor load/apoptosis/growth/metastasis |
| Section 4.3.2 | Synthetic epigenetic modalities | Inhibition of tumor cell growth and metastases |
| Results of Single-Gene/Genome-Wide DNA Methylation Profiling | |
|---|---|
| Methylation | Result |
| DAPK1 [29] and TUSC3 [30] in NSCLC | Improved overall survival |
| P16/INK4a and BRCA1 [31] in adenocarcinoma | Shorter overall survival |
| RARβ [31] in adenocarcinoma | Longer overall survival |
| HOXA2 and HOXA10 [32] in squamous cell carcinoma | Shorter overall survival |
| HOXA9 in NSCLC lifelong non-smokers [33] | Poor recurrence-free survival |
| NPTX1 in NSCLC [34] | Shorter overall survival |
| PTPRH in adenocarcinoma [35] | Poor prognosis (OS) |
| AGTRL, ALDH1A3, BDKRB1, CTSE, EFNA2, NFAM1, SEMA4A, and TMEM129 in adenocarcinoma [36] | Poor prognosis (OS) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulewska, A.; Pilz, L.; Manegold, C.; Ramlau, R.; Charkiewicz, R.; Niklinski, J. A Systematic Review of Progress toward Unlocking the Power of Epigenetics in NSCLC: Latest Updates and Perspectives. Cells 2023, 12, 905. https://doi.org/10.3390/cells12060905
Sulewska A, Pilz L, Manegold C, Ramlau R, Charkiewicz R, Niklinski J. A Systematic Review of Progress toward Unlocking the Power of Epigenetics in NSCLC: Latest Updates and Perspectives. Cells. 2023; 12(6):905. https://doi.org/10.3390/cells12060905
Chicago/Turabian StyleSulewska, Anetta, Lothar Pilz, Christian Manegold, Rodryg Ramlau, Radoslaw Charkiewicz, and Jacek Niklinski. 2023. "A Systematic Review of Progress toward Unlocking the Power of Epigenetics in NSCLC: Latest Updates and Perspectives" Cells 12, no. 6: 905. https://doi.org/10.3390/cells12060905
APA StyleSulewska, A., Pilz, L., Manegold, C., Ramlau, R., Charkiewicz, R., & Niklinski, J. (2023). A Systematic Review of Progress toward Unlocking the Power of Epigenetics in NSCLC: Latest Updates and Perspectives. Cells, 12(6), 905. https://doi.org/10.3390/cells12060905







