Validation of an ICH Q2 Compliant Flow Cytometry-Based Assay for the Assessment of the Inhibitory Potential of Mesenchymal Stromal Cells on T Cell Proliferation
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of MSCs and PBMCs
2.2. PBMC Labelling for Cell Proliferation Tracking
2.3. Stimulated Co-Culture of PBMCs and MSCs
2.4. Flow Cytometry Analysis
2.5. BrdU Assay
2.6. Statistical Evaluation and Validation of the Method
3. Results
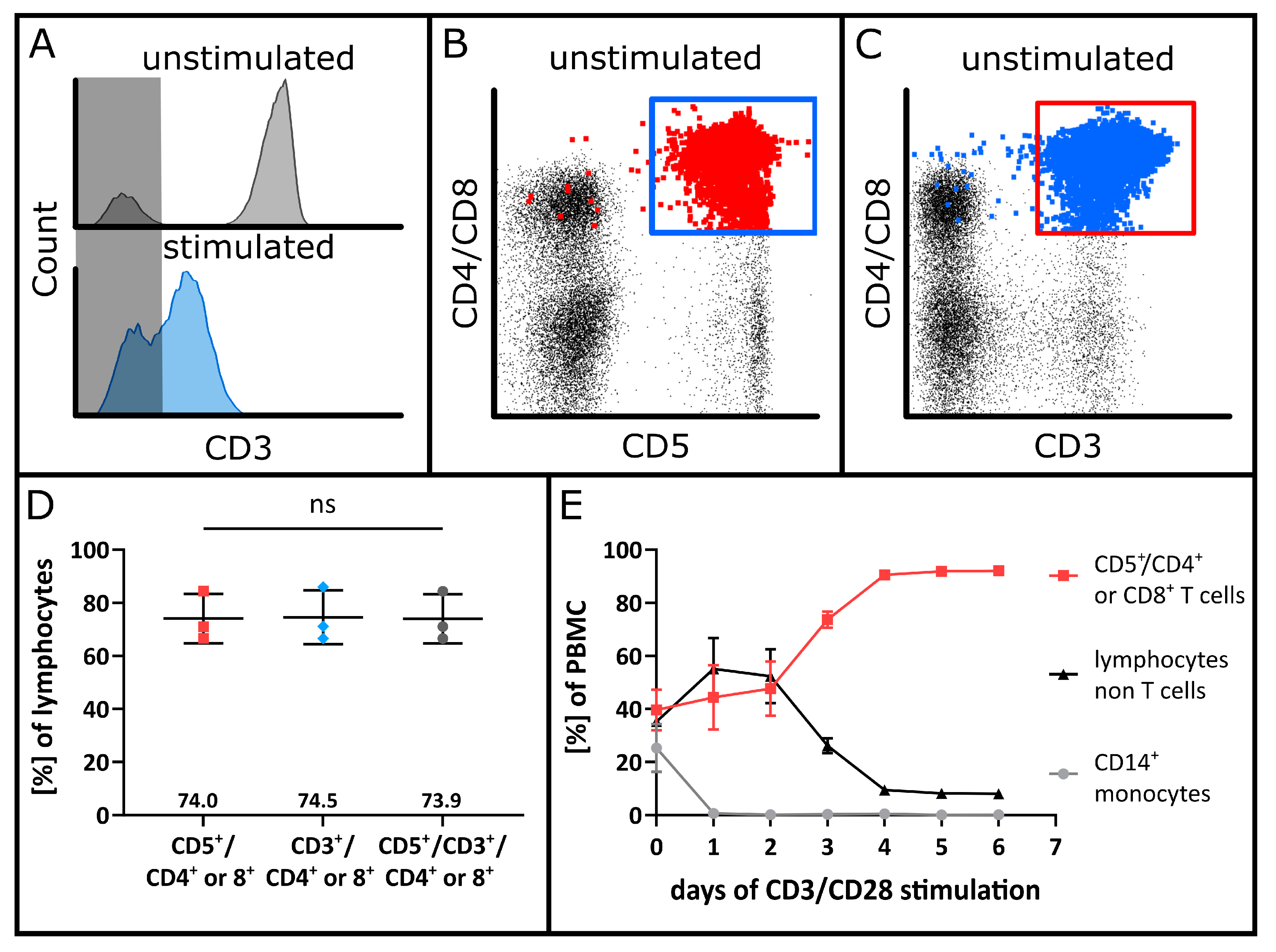
3.1. Assay Optimization Using VPD450 for Lymphocyte Proliferation Tracking
3.2. Specificity
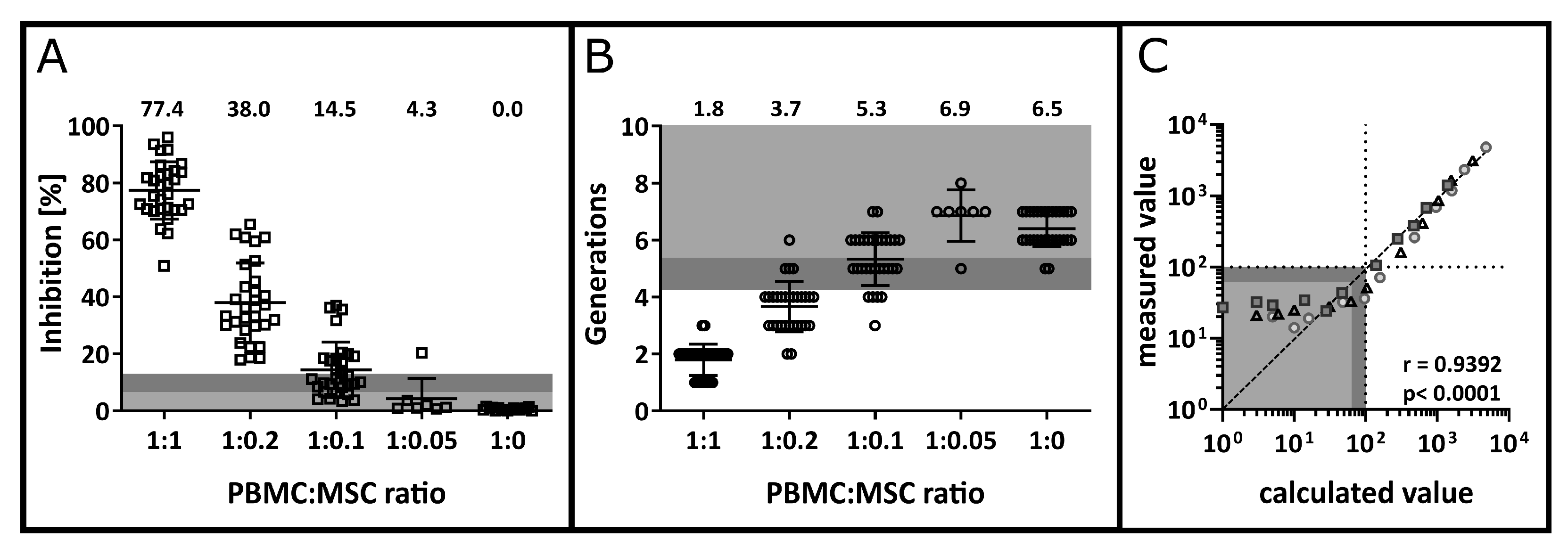
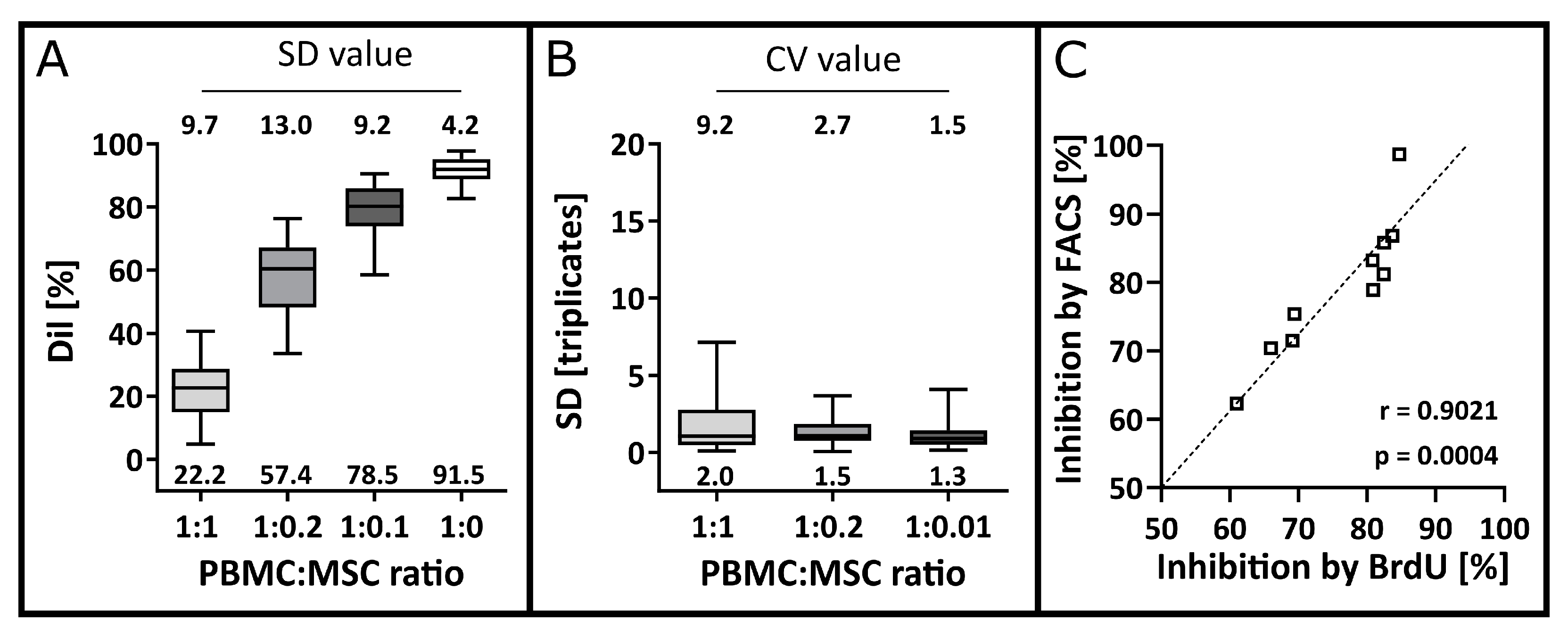
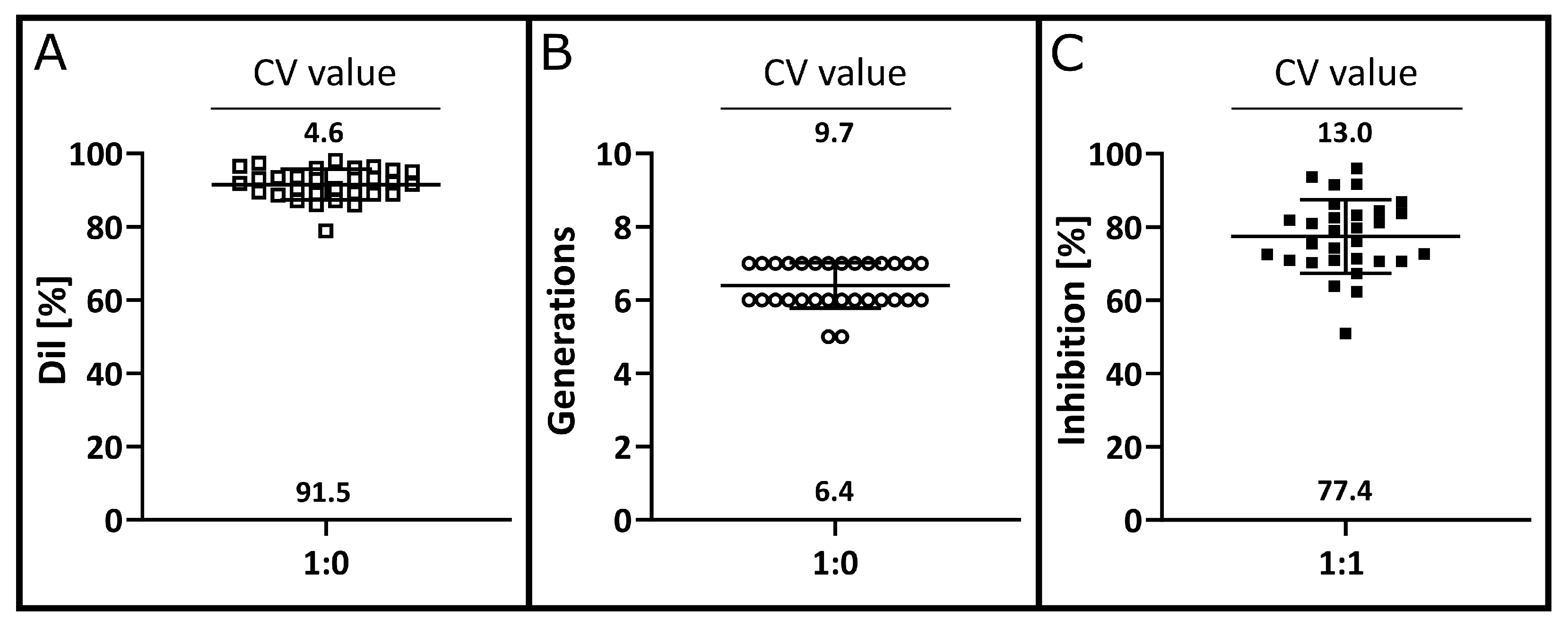
3.3. Precision and Accuracy
3.4. Detection Limit, Quantification Limit, Linearity and Range
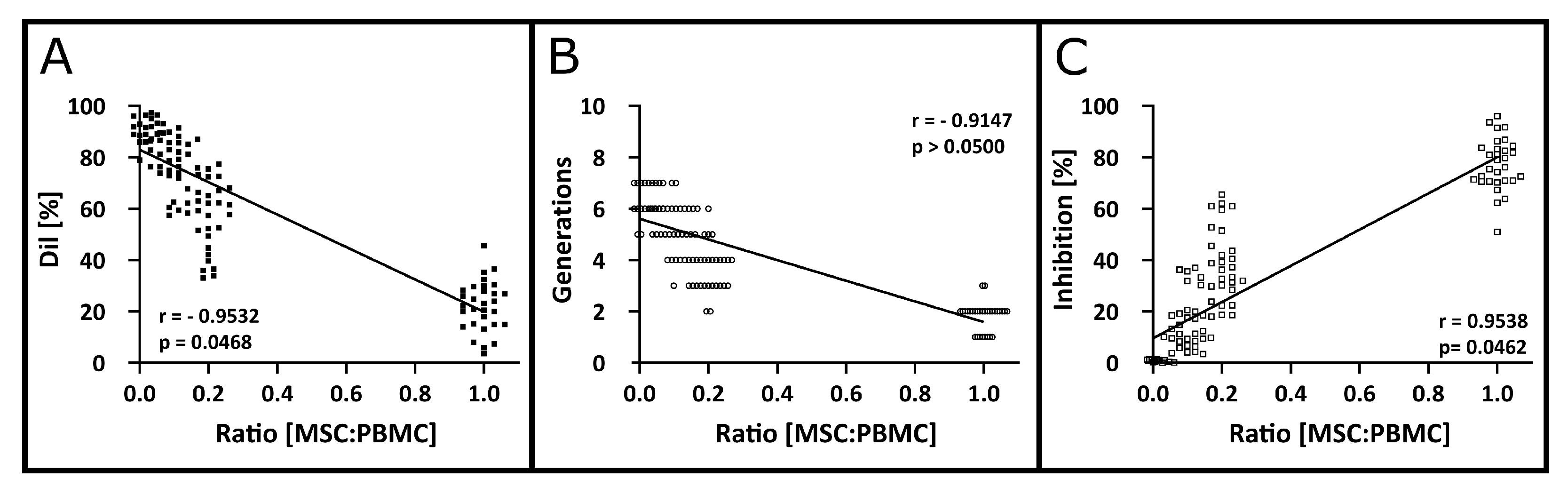
3.5. Robustness
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Locatelli, F.; Algeri, M.; Trevisan, V.; Bertaina, A. Remestemcel-L for the Treatment of Graft versus Host Disease. Expert Rev. Clin. Immunol. 2017, 13, 43–56. [Google Scholar] [CrossRef]
- Bader, P.; Kuçi, Z.; Bakhtiar, S.; Basu, O.; Bug, G.; Dennis, M.; Greil, J.; Barta, A.; Kállay, K.M.; Lang, P.; et al. Effective Treatment of Steroid and Therapy-Refractory Acute Graft-versus-Host Disease with a Novel Mesenchymal Stromal Cell Product (MSC-FFM). Bone Marrow Transplant. 2018, 53, 852–862. [Google Scholar] [CrossRef]
- Bonig, H.; Kuçi, Z.; Kuçi, S.; Bakhtiar, S.; Basu, O.; Bug, G.; Dennis, M.; Greil, J.; Barta, A.; Kállay, K.M.; et al. Children and Adults with Refractory Acute Graft-versus-Host Disease Respond to Treatment with the Mesenchymal Stromal Cell Preparation “MSC-FFM”—Outcome Report of 92 Patients. Cells 2019, 8, 1577. [Google Scholar] [CrossRef]
- Kuçi, Z.; Bönig, H.; Kreyenberg, H.; Bunos, M.; Jauch, A.; Janssen, J.W.G.; Škifić, M.; Michel, K.; Eising, B.; Lucchini, G.; et al. Mesenchymal Stromal Cells from Pooled Mononuclear Cells of Multiple Bone Marrow Donors as Rescue Therapy in Pediatric Severe Steroid-Refractory Graft-versus-Host Disease: A Multicenter Survey. Haematologica 2016, 101, 985–994. [Google Scholar] [CrossRef]
- Kurtzberg, J.; Prockop, S.; Teira, P.; Bittencourt, H.; Lewis, V.; Chan, K.W.; Horn, B.; Yu, L.; Talano, J.-A.; Nemecek, E.; et al. Allogeneic Human Mesenchymal Stem Cell Therapy (Remestemcel-L, Prochymal) as a Rescue Agent for Severe Refractory Acute Graft-versus-Host Disease in Pediatric Patients. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2014, 20, 229–235. [Google Scholar] [CrossRef]
- Lazarus, H.M.; Haynesworth, S.E.; Gerson, S.L.; Rosenthal, N.S.; Caplan, A.I. Ex Vivo Expansion and Subsequent Infusion of Human Bone Marrow-Derived Stromal Progenitor Cells (Mesenchymal Progenitor Cells): Implications for Therapeutic Use. Bone Marrow Transplant. 1995, 16, 557–564. [Google Scholar]
- Le Blanc, K.; Frassoni, F.; Ball, L.; Locatelli, F.; Roelofs, H.; Lewis, I.; Lanino, E.; Sundberg, B.; Bernardo, M.E.; Remberger, M.; et al. Mesenchymal Stem Cells for Treatment of Steroid-Resistant, Severe, Acute Graft-versus-Host Disease: A Phase II Study. Lancet 2008, 371, 1579–1586. [Google Scholar] [CrossRef]
- Le Blanc, K.; Tammik, L.; Sundberg, B.; Haynesworth, S.E.; Ringdén, O. Mesenchymal Stem Cells Inhibit and Stimulate Mixed Lymphocyte Cultures and Mitogenic Responses Independently of the Major Histocompatibility Complex. Scand. J. Immunol. 2003, 57, 11–20. [Google Scholar] [CrossRef]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal Stem Cells in Health and Disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef]
- Moll, G.; Ankrum, J.A.; Kamhieh-Milz, J.; Bieback, K.; Ringdén, O.; Volk, H.-D.; Geissler, S.; Reinke, P. Intravascular Mesenchymal Stromal/Stem Cell Therapy Product Diversification: Time for New Clinical Guidelines. Trends Mol. Med. 2019, 25, 149–163. [Google Scholar] [CrossRef]
- Cottle, C.; Porter, A.P.; Lipat, A.; Turner-Lyles, C.; Nguyen, J.; Moll, G.; Chinnadurai, R. Impact of Cryopreservation and Freeze-Thawing on Therapeutic Properties of Mesenchymal Stromal/Stem Cells and Other Common Cellular Therapeutics. Curr. Stem Cell Rep. 2022, 8, 72–92. [Google Scholar] [CrossRef]
- European Medicines Agency Q 2 (R1) Validation of Analytical Procedures: Text and Methodology (CPMP/ICH/381/95) 1995. Available online: https://www.ema.europa.eu/en/ich-q2r2-validation-analytical-procedures-scientific-guideline (accessed on 3 March 2023).
- Wood, B.; Jevremovic, D.; Béné, M.C.; Yan, M.; Jacobs, P.; Litwin, V. Validation of Cell-Based Fluorescence Assays: Practice Guidelines from the ICSH and ICCS—Part V—Assay Performance Criteria. Cytom. B Clin. Cytom. 2013, 84, 315–323. [Google Scholar] [CrossRef]
- Dekker, L.; Sanders, E.; Lindemans, C.A.; de Koning, C.; Nierkens, S. Naive T Cells in Graft Versus Host Disease and Graft Versus Leukemia: Innocent or Guilty? Front. Immunol. 2022, 13, 893545. [Google Scholar] [CrossRef]
- Bain, B.; Lowenstein, L. Genetic Studies on the Mixed Leukocyte Reaction. Science 1964, 145, 1315–1316. [Google Scholar] [CrossRef]
- Riddell, S.R.; Greenberg, P.D. The Use of Anti-CD3 and Anti-CD28 Monoclonal Antibodies to Clone and Expand Human Antigen-Specific T Cells. J. Immunol. Methods 1990, 128, 189–201. [Google Scholar] [CrossRef]
- Bach, M.A.; Bach, J.F. Activities of Immunosuppressive Agents in Vitro. II. Different Timing of Azathioprine and Methotrexate in Inhibition and Stimulation of Mixed Lymphocyte Reaction. Clin. Exp. Immunol. 1972, 11, 89–98. [Google Scholar]
- Kwon, M.; Choi, Y.J.; Sa, M.; Park, S.-H.; Shin, E.-C. Two-Round Mixed Lymphocyte Reaction for Evaluation of the Functional Activities of Anti-PD-1 and Immunomodulators. Immune Netw. 2018, 18, e45. [Google Scholar] [CrossRef]
- Bain, B. Tritiated-Thymidine Uptake in Mixed Leucocyte Cultures: Effect of Specific Activity and Exposure Time. Clin. Exp. Immunol. 1970, 6, 255–262. [Google Scholar]
- Huong, P.L.; Kolk, A.H.; Eggelte, T.A.; Verstijnen, C.P.; Gilis, H.; Hendriks, J.T. Measurement of Antigen Specific Lymphocyte Proliferation Using 5-Bromo-Deoxyuridine Incorporation. An Easy and Low Cost Alternative to Radioactive Thymidine Incorporation. J. Immunol. Methods 1991, 140, 243–248. [Google Scholar] [CrossRef]
- Ansar Ahmed, S.; Gogal, R.M.; Walsh, J.E. A New Rapid and Simple Non-Radioactive Assay to Monitor and Determine the Proliferation of Lymphocytes: An Alternative to [3H]Thymidine Incorporation Assay. J. Immunol. Methods 1994, 170, 211–224. [Google Scholar] [CrossRef]
- VanBuskirk, A.M.; Adams, P.W.; Orosz, C.G. Nonradioactive Alternative to Clinical Mixed Lymphocyte Reaction. Hum. Immunol. 1995, 43, 38–44. [Google Scholar] [CrossRef]
- Gieseke, F.; Böhringer, J.; Bussolari, R.; Dominici, M.; Handgretinger, R.; Müller, I. Human Multipotent Mesenchymal Stromal Cells Use Galectin-1 to Inhibit Immune Effector Cells. Blood 2010, 116, 3770–3779. [Google Scholar] [CrossRef]
- Ketterl, N.; Brachtl, G.; Schuh, C.; Bieback, K.; Schallmoser, K.; Reinisch, A.; Strunk, D. A Robust Potency Assay Highlights Significant Donor Variation of Human Mesenchymal Stem/Progenitor Cell Immune Modulatory Capacity and Extended Radio-Resistance. Stem Cell Res. Ther. 2015, 6, 236. [Google Scholar] [CrossRef]
- Nicotra, T.; Desnos, A.; Halimi, J.; Antonot, H.; Reppel, L.; Belmas, T.; Freton, A.; Stranieri, F.; Mebarki, M.; Larghero, J.; et al. Mesenchymal Stem/Stromal Cell Quality Control: Validation of Mixed Lymphocyte Reaction Assay Using Flow Cytometry According to ICH Q2(R1). Stem Cell Res. Ther. 2020, 11, 426. [Google Scholar] [CrossRef]
- Quah, B.J.C.; Warren, H.S.; Parish, C.R. Monitoring Lymphocyte Proliferation in Vitro and in Vivo with the Intracellular Fluorescent Dye Carboxyfluorescein Diacetate Succinimidyl Ester. Nat. Protoc. 2007, 2, 2049–2056. [Google Scholar] [CrossRef]
- Schwarting, R.; Gerdes, J.; Niehus, J.; Jaeschke, L.; Stein, H. Determination of the Growth Fraction in Cell Suspensions by Flow Cytometry Using the Monoclonal Antibody Ki-67. J. Immunol. Methods 1986, 90, 65–70. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO). Statistics—Vocabulary and Symbols, 2nd ed.; General Statistical Terms and Terms Used in Probability; ISO: Geneva, Switzerland, 2006; Volume 1. [Google Scholar]
- Roederer, M. Interpretation of Cellular Proliferation Data: Avoid the Panglossian. Cytom. Part J. Int. Soc. Anal. Cytol. 2011, 79, 95–101. [Google Scholar] [CrossRef]
- Peinelt, A.; Bremm, M.; Kreyenberg, H.; Cappel, C.; Banisharif-Dehkordi, J.; Erben, S.; Rettinger, E.; Jarisch, A.; Meisel, R.; Schlegel, P.-G.; et al. Monitoring of Circulating CAR T Cells: Validation of a Flow Cytometric Assay, Cellular Kinetics, and Phenotype Analysis Following Tisagenlecleucel. Front. Immunol. 2022, 13, 830773. [Google Scholar] [CrossRef]
- Sarikonda, G.; Mathieu, M.; Natalia, M.; Pahuja, A.; Xue, Q.; Pierog, P.L.; Trampont, P.C.; Decman, V.; Reynolds, S.; Hanafi, L.-A.; et al. Best Practices for the Development, Analytical Validation and Clinical Implementation of Flow Cytometric Methods for Chimeric Antigen Receptor T Cell Analyses. Cytom. B Clin. Cytom. 2021, 100, 79–91. [Google Scholar] [CrossRef]
- O’Hara, D.M.; Xu, Y.; Liang, Z.; Reddy, M.P.; Wu, D.Y.; Litwin, V. Recommendations for the Validation of Flow Cytometric Testing during Drug Development: II Assays. J. Immunol. Methods 2011, 363, 120–134. [Google Scholar] [CrossRef]
- Lee, J.W.; Devanarayan, V.; Barrett, Y.C.; Weiner, R.; Allinson, J.; Fountain, S.; Keller, S.; Weinryb, I.; Green, M.; Duan, L.; et al. Fit-for-Purpose Method Development and Validation for Successful Biomarker Measurement. Pharm. Res. 2006, 23, 312–328. [Google Scholar] [CrossRef]
- Van der Strate, B.; Longdin, R.; Geerlings, M.; Bachmayer, N.; Cavallin, M.; Litwin, V.; Patel, M.; Passe-Coutrin, W.; Schoelch, C.; Companjen, A.; et al. Best Practices in Performing Flow Cytometry in a Regulated Environment: Feedback from Experience within the European Bioanalysis Forum. Bioanalysis 2017, 9, 1253–1264. [Google Scholar] [CrossRef]
- Chinnadurai, R.; Rajan, D.; Qayed, M.; Arafat, D.; Garcia, M.; Liu, Y.; Kugathasan, S.; Anderson, L.J.; Gibson, G.; Galipeau, J. Potency Analysis of Mesenchymal Stromal Cells Using a Combinatorial Assay Matrix Approach. Cell Rep. 2018, 22, 2504–2517. [Google Scholar] [CrossRef]
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisanti, S.; Gianni, A.M. Human Bone Marrow Stromal Cells Suppress T-Lymphocyte Proliferation Induced by Cellular or Nonspecific Mitogenic Stimuli. Blood 2002, 99, 3838–3843. [Google Scholar] [CrossRef]
- Kim, D.S.; Jang, I.K.; Lee, M.W.; Ko, Y.J.; Lee, D.-H.; Lee, J.W.; Sung, K.W.; Koo, H.H.; Yoo, K.H. Enhanced Immunosuppressive Properties of Human Mesenchymal Stem Cells Primed by Interferon-γ. EBioMedicine 2018, 28, 261–273. [Google Scholar] [CrossRef]
- De Rosa, S.C.; Brenchley, J.M.; Roederer, M. Beyond Six Colors: A New Era in Flow Cytometry. Nat. Med. 2003, 9, 112–117. [Google Scholar] [CrossRef]
- Piekarska, K.; Urban-Wójciuk, Z.; Kurkowiak, M.; Pelikant-Małecka, I.; Schumacher, A.; Sakowska, J.; Spodnik, J.H.; Arcimowicz, Ł.; Zielińska, H.; Tymoniuk, B.; et al. Mesenchymal Stem Cells Transfer Mitochondria to Allogeneic Tregs in an HLA-Dependent Manner Improving Their Immunosuppressive Activity. Nat. Commun. 2022, 13, 856. [Google Scholar] [CrossRef]
- Müller, N.; Landwehr, K.; Langeveld, K.; Stenzel, J.; Pouwels, W.; van der Hoorn, M.A.W.G.; Seifried, E.; Bonig, H. Generation of Alloreactivity-Reduced Donor Lymphocyte Products Retaining Memory Function by Fully Automatic Depletion of CD45RA-Positive Cells. Cytotherapy 2018, 20, 532–542. [Google Scholar] [CrossRef]
- Roy, D.C.; Walker, I.; Maertens, J.; Lewalle, P.; Olavarria, E.; Selleslag, D.; Lachance, S.; Buyse, M.; Wang, K.; Rovers, J.; et al. ATIR101 Administered after T-Cell-Depleted Haploidentical HSCT Reduces NRM and Improves Overall Survival in Acute Leukemia. Leukemia 2020, 34, 1907–1923. [Google Scholar] [CrossRef]
- Banks, H.T.; Choi, A.; Huffman, T.; Nardini, J.; Poag, L.; Thompson, W.C. Quantifying CFSE Label Decay in Flow Cytometry Data. Appl. Math. Lett. 2013, 26, 571–577. [Google Scholar] [CrossRef]
- Ten Brinke, A.; Marek-Trzonkowska, N.; Mansilla, M.J.; Turksma, A.W.; Piekarska, K.; Iwaszkiewicz-Grześ, D.; Passerini, L.; Locafaro, G.; Puñet-Ortiz, J.; van Ham, S.M.; et al. Monitoring T-Cell Responses in Translational Studies: Optimization of Dye-Based Proliferation Assay for Evaluation of Antigen-Specific Responses. Front. Immunol. 2017, 8, 1870. [Google Scholar] [CrossRef]
- Le Blanc, K.; Tammik, C.; Rosendahl, K.; Zetterberg, E.; Ringdén, O. HLA Expression and Immunologic Properties of Differentiated and Undifferentiated Mesenchymal Stem Cells. Exp. Hematol. 2003, 31, 890–896. [Google Scholar] [CrossRef]
- Waterman, R.S.; Tomchuck, S.L.; Henkle, S.L.; Betancourt, A.M. A New Mesenchymal Stem Cell (MSC) Paradigm: Polarization into a pro-Inflammatory MSC1 or an Immunosuppressive MSC2 Phenotype. PLoS ONE 2010, 5, e10088. [Google Scholar] [CrossRef]
- Green, J.; Jotte, R. Interactions between T Helper Cells and Dendritic Cells during the Rat Mixed Lymphocyte Reaction. J. Exp. Med. 1985, 162, 1546–1560. [Google Scholar] [CrossRef]
- Höpken, U.E.; Lehmann, I.; Droese, J.; Lipp, M.; Schüler, T.; Rehm, A. The Ratio between Dendritic Cells and T Cells Determines the Outcome of Their Encounter: Proliferation versus Deletion. Eur. J. Immunol. 2005, 35, 2851–2863. [Google Scholar] [CrossRef]
- Steinman, R.; Gutchinov, B.; Witmer, M.; Nussenzweig, M. Dendritic Cells Are the Principal Stimulators of the Primary Mixed Leukocyte Reaction in Mice. J. Exp. Med. 1983, 157, 613–627. [Google Scholar] [CrossRef]
- Steinman, R.M.; Witmer, M.D. Lymphoid Dendritic Cells Are Potent Stimulators of the Primary Mixed Leukocyte Reaction in Mice. Proc. Natl. Acad. Sci. USA 1978, 75, 5132–5136. [Google Scholar] [CrossRef]
- Tourkova, I.L.; Yurkovetsky, Z.R.; Shurin, M.R.; Shurin, G.V. Mechanisms of Dendritic Cell-Induced T Cell Proliferation in the Primary MLR Assay. Immunol. Lett. 2001, 78, 75–82. [Google Scholar] [CrossRef]
- Greaves, M.F.; Bauminger, S.; Janossy, G. Lymphocyte Activation. III. Binding Sites for Phytomitogens on Lymphocyte Subpopulations. Clin. Exp. Immunol. 1972, 10, 537–554. [Google Scholar]
- Russo, C.; Indiveri, F.; Quaranta, V.; Molinaro, G.A.; Pellegrino, M.A.; Ferrone, S. Stimulation of Human T Lymphocytes by PHA-Activated Autologous T Lymphocytes: Analysis of the Role of Ia-like Antigens with Monoclonal Antibodies. Immunogenetics 1981, 12, 267–274. [Google Scholar] [CrossRef]
- Palacios, R. Concanavalin A Triggers T Lymphocytes by Directly Interacting with Their Receptors for Activation. J. Immunol. Baltim. Md 1950 1982, 128, 337–342. [Google Scholar] [CrossRef]
- Ishizaka, A.; Sakiyama, Y.; Takahashi, Y.; Matsumoto, S. The Activation of T Cells with Staphylococcus Aureus Cowan 1 (SAC). Immunol. Lett. 1985, 10, 13–17. [Google Scholar] [CrossRef]
- Kuiper, H.M.; de Jong, R.; Brouwer, M.; Lammers, K.; Wijdenes, J.; van Lier, R.A. Influence of CD28 Co-Stimulation on Cytokine Production Is Mainly Regulated via Interleukin-2. Immunology 1994, 83, 38–44. [Google Scholar]
- Langenhorst, D.; Haack, S.; Göb, S.; Uri, A.; Lühder, F.; Vanhove, B.; Hünig, T.; Beyersdorf, N. CD28 Costimulation of T Helper 1 Cells Enhances Cytokine Release In Vivo. Front. Immunol. 2018, 9, 1060. [Google Scholar] [CrossRef]
- Lankester, A.C.; van Schijndel, G.M.W.; Cordell, J.L.; van Noesel, C.J.M.; van Lier, R.A.W. CD5 Is Associated with the Human B Cell Antigen Receptor Complex. Eur. J. Immunol. 1994, 24, 812–816. [Google Scholar] [CrossRef]
- Osman, N.; Ley, S.C.; Crumpton, M.J. Evidence for an Association between the T Cell Receptor/CD3 Antigen Complex and the CD5 Antigen in Human T Lymphocytes. Eur. J. Immunol. 1992, 22, 2995–3000. [Google Scholar] [CrossRef]
- Laidlaw, B.J.; Craft, J.E.; Kaech, S.M. The Multifaceted Role of CD4+ T Cells in CD8+ T Cell Memory. Nat. Rev. Immunol. 2016, 16, 102–111. [Google Scholar] [CrossRef]
- Seder, R.A.; Ahmed, R. Similarities and Differences in CD4+ and CD8+ Effector and Memory T Cell Generation. Nat. Immunol. 2003, 4, 835–842. [Google Scholar] [CrossRef]
- Davies, L.C.; Heldring, N.; Kadri, N.; Le Blanc, K. Mesenchymal Stromal Cell Secretion of Programmed Death-1 Ligands Regulates T Cell Mediated Immunosuppression. Stem Cells Dayt. Ohio 2017, 35, 766–776. [Google Scholar] [CrossRef]
- Yang, S.-H.; Park, M.-J.; Yoon, I.-H.; Kim, S.-Y.; Hong, S.-H.; Shin, J.-Y.; Nam, H.-Y.; Kim, Y.-H.; Kim, B.; Park, C.-G. Soluble Mediators from Mesenchymal Stem Cells Suppress T Cell Proliferation by Inducing IL-10. Exp. Mol. Med. 2009, 41, 315–324. [Google Scholar] [CrossRef]
- Konala, V.B.R.; Mamidi, M.K.; Bhonde, R.; Das, A.K.; Pochampally, R.; Pal, R. The Current Landscape of the Mesenchymal Stromal Cell Secretome: A New Paradigm for Cell-Free Regeneration. Cytotherapy 2016, 18, 13–24. [Google Scholar] [CrossRef]
- Sompuram, S.R.; Vani, K.; Messana, E.; Bogen, S.A. A Molecular Mechanism of Formalin Fixation and Antigen Retrieval. Am. J. Clin. Pathol. 2004, 121, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Galleu, A.; Riffo-Vasquez, Y.; Trento, C.; Lomas, C.; Dolcetti, L.; Cheung, T.S.; von Bonin, M.; Barbieri, L.; Halai, K.; Ward, S.; et al. Apoptosis in Mesenchymal Stromal Cells Induces in Vivo Recipient-Mediated Immunomodulation. Sci. Transl. Med. 2017, 9, eaam7828. [Google Scholar] [CrossRef] [PubMed]
- Milone, M.C. Chapter 3 Analytical Techniques Used in Therapeutic Drug Monitoring. In Therapeutic Drug Monitoring: Newer Drugs and Biomarkers; Dasgupta, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 978-0-12-385468-1. [Google Scholar]
- U.S. Food and Drug Administration Bioanalytical Method Validation Guidance for Industry. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry (accessed on 28 November 2022).
- Galipeau, J.; Krampera, M.; Barrett, J.; Dazzi, F.; Deans, R.J.; DeBruijn, J.; Dominici, M.; Fibbe, W.E.; Gee, A.P.; Gimble, J.M.; et al. International Society for Cellular Therapy Perspective on Immune Functional Assays for Mesenchymal Stromal Cells as Potency Release Criterion for Advanced Phase Clinical Trials. Cytotherapy 2016, 18, 151–159. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piede, N.; Bremm, M.; Farken, A.; Pfeffermann, L.-M.; Cappel, C.; Bonig, H.; Fingerhut, T.; Puth, L.; Vogelsang, K.; Peinelt, A.; et al. Validation of an ICH Q2 Compliant Flow Cytometry-Based Assay for the Assessment of the Inhibitory Potential of Mesenchymal Stromal Cells on T Cell Proliferation. Cells 2023, 12, 850. https://doi.org/10.3390/cells12060850
Piede N, Bremm M, Farken A, Pfeffermann L-M, Cappel C, Bonig H, Fingerhut T, Puth L, Vogelsang K, Peinelt A, et al. Validation of an ICH Q2 Compliant Flow Cytometry-Based Assay for the Assessment of the Inhibitory Potential of Mesenchymal Stromal Cells on T Cell Proliferation. Cells. 2023; 12(6):850. https://doi.org/10.3390/cells12060850
Chicago/Turabian StylePiede, Natascha, Melanie Bremm, Anne Farken, Lisa-Marie Pfeffermann, Claudia Cappel, Halvard Bonig, Theres Fingerhut, Laura Puth, Kathrin Vogelsang, Andreas Peinelt, and et al. 2023. "Validation of an ICH Q2 Compliant Flow Cytometry-Based Assay for the Assessment of the Inhibitory Potential of Mesenchymal Stromal Cells on T Cell Proliferation" Cells 12, no. 6: 850. https://doi.org/10.3390/cells12060850
APA StylePiede, N., Bremm, M., Farken, A., Pfeffermann, L.-M., Cappel, C., Bonig, H., Fingerhut, T., Puth, L., Vogelsang, K., Peinelt, A., Marschalek, R., Müller, M., Bader, P., Kuçi, Z., Kuçi, S., & Huenecke, S. (2023). Validation of an ICH Q2 Compliant Flow Cytometry-Based Assay for the Assessment of the Inhibitory Potential of Mesenchymal Stromal Cells on T Cell Proliferation. Cells, 12(6), 850. https://doi.org/10.3390/cells12060850







