Prognostic Role of Soluble and Extracellular Vesicle-Associated PD-L1, B7-H3 and B7-H4 in Non-Small Cell Lung Cancer Patients Treated with Immune Checkpoint Inhibitors
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Enrolment and Sample Collection
2.2. Tumor PD-L1 Assessment
2.3. Soluble Biomarkers Evaluation
2.4. EV Isolation and Characterization
2.5. Flow Cytometry Characterization of EVs
2.6. Multiplex EV Surface Marker Analysis
2.7. Statistical Analysis
3. Results
3.1. Clinicopathologic Features of Patient Cohorts
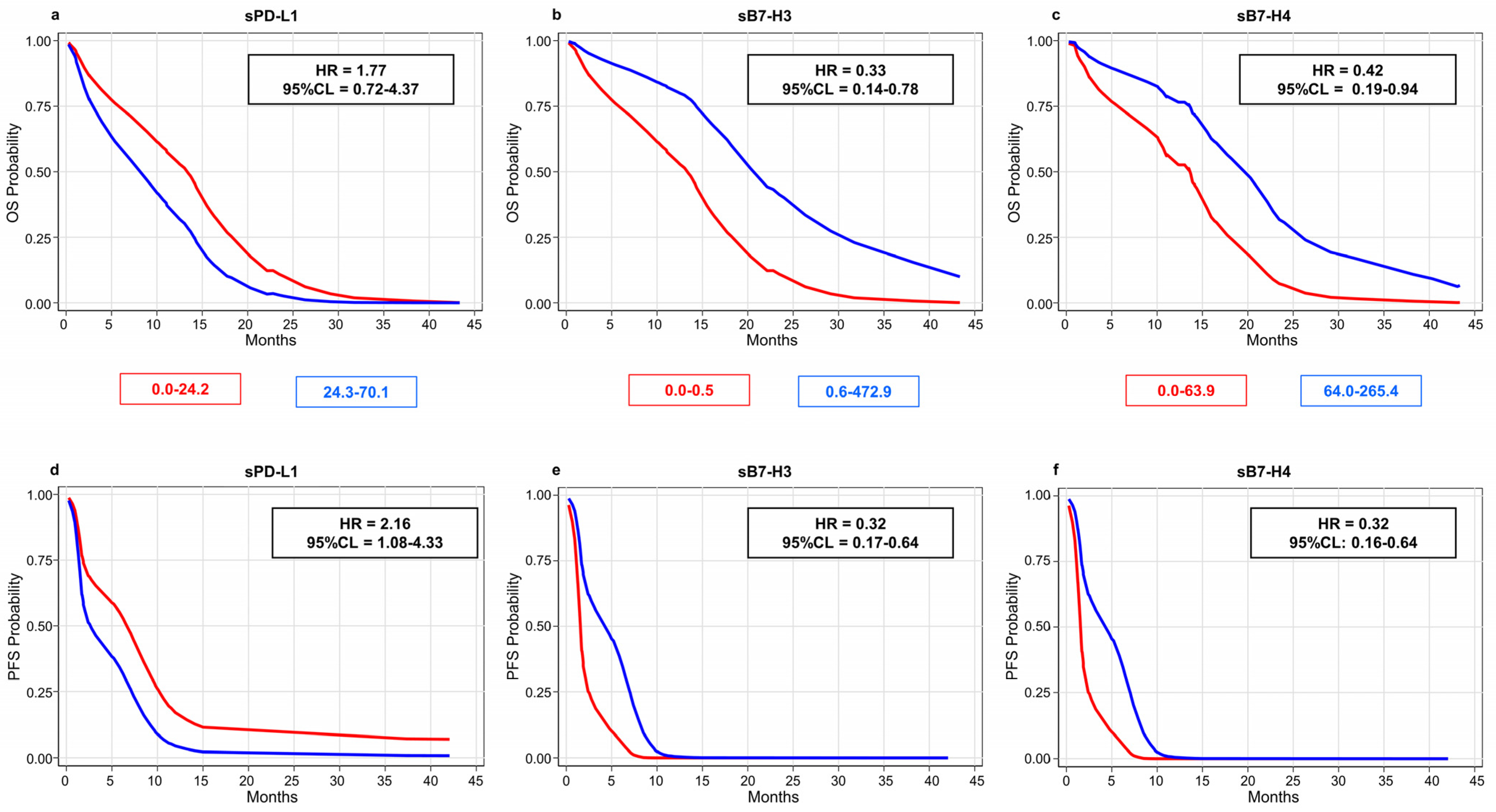
3.2. Soluble Molecules Are Prognostic Markers in Patients Treated with Pembrolizumab
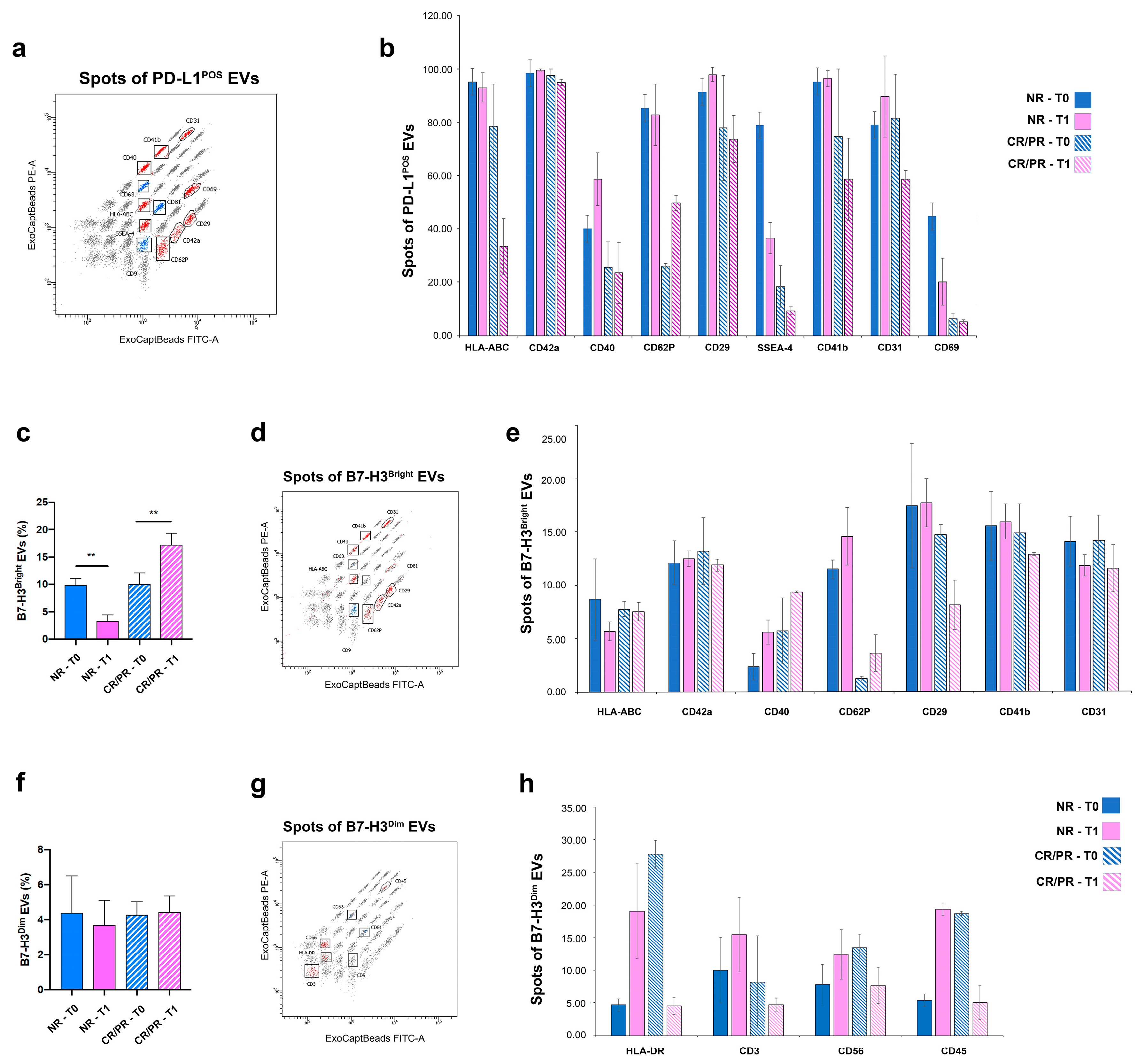
3.3. PD-L1 and B7-H3 Are Associated to Plasma EVs
3.4. EV-Associated PD-L1 and B7-H3 Predict the Response to ICI
3.5. PD-L1- and B7-H3-EVs Show Different Cell Origins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Garon, E.B.; Kim, D.W.; Cho, B.C.; Gervais, R.; Perez-Gracia, J.L.; Han, J.Y.; Majem, M.; Forster, M.D.; Monnet, I.; et al. Five Year Survival Update From KEYNOTE-010: Pembrolizumab Versus Docetaxel for Previously Treated, Programmed Death-Ligand 1-Positive Advanced NSCLC. J. Thorac. Oncol. 2021, 16, 1718–1732. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Çay Şenler, F.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Giaccone, G.; de Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Taube, J.M.; Anders, R.A.; Pardoll, D.M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer 2016, 16, 275. [Google Scholar] [CrossRef]
- Smith, J.B.; Stashwick, C.; Powell, D.J. B7-H4 as a potential target for immunotherapy for gynecologic cancers: A closer look. Gynecol. Oncol. 2014, 134, 181–189. [Google Scholar] [CrossRef]
- Altan, M.; Pelekanou, V.; Schalper, K.A.; Toki, M.; Gaule, P.; Syrigos, K.; Herbst, R.S.; Rimm, D.L. B7-H3 Expression in NSCLC and Its Association with B7-H4, PD-L1 and Tumor-Infiltrating Lymphocytes. Clin. Cancer Res. 2017, 23, 5202–5209. [Google Scholar] [CrossRef] [PubMed]
- Genova, C.; Boccardo, S.; Mora, M.; Rijavec, E.; Biello, F.; Rossi, G.; Tagliamento, M.; Dal Bello, M.G.; Coco, S.; Alama, A.; et al. Correlation between B7-H4 and Survival of Non-Small-Cell Lung Cancer Patients Treated with Nivolumab. J. Clin. Med. 2019, 8, 1566. [Google Scholar] [CrossRef] [PubMed]
- Okuma, Y.; Hosomi, Y.; Nakahara, Y.; Watanabe, K.; Sagawa, Y.; Homma, S. High plasma levels of soluble programmed cell death ligand 1 are prognostic for reduced survival in advanced lung cancer. Lung Cancer 2017, 104, 1–6. [Google Scholar] [CrossRef]
- Okuma, Y.; Wakui, H.; Utsumi, H.; Sagawa, Y.; Hosomi, Y.; Kuwano, K.; Homma, S. Soluble Programmed Cell Death Ligand 1 as a Novel Biomarker for Nivolumab Therapy for Non-Small-cell Lung Cancer. Clin. Lung Cancer 2018, 19, 410–417.e1. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, H.R.; Choi, Y.J.; Kim, S.Y.; Lee, J.E.; Sung, K.J.; Sung, Y.H.; Pack, C.G.; Jung, M.K.; Han, B.; et al. Exosomal PD-L1 promotes tumor growth through immune escape in non-small cell lung cancer. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- de Miguel-Perez, D.; Russo, A.; Gunasekaran, M.; Buemi, F.; Hester, L.; Fan, X.; Carter-Cooper, B.A.; Lapidus, R.G.; Peleg, A.; Arroyo-Hernández, M.; et al. Baseline extracellular vesicle TGF-β is a predictive biomarker for response to immune checkpoint inhibitors and survival in non-small cell lung cancer. Cancer 2023, 129, 521–530. [Google Scholar] [CrossRef]
- de Miguel-Perez, D.; Russo, A.; Arrieta, O.; Ak, M.; Barron, F.; Gunasekaran, M.; Mamindla, P.; Lara-Mejia, L.; Peterson, C.B.; Er, M.E.; et al. Extracellular vesicle PD-L1 dynamics predict durable response to immune-checkpoint inhibitors and survival in patients with non-small cell lung cancer. J. Exp. Clin. Cancer Res. 2022, 41, 186. [Google Scholar] [CrossRef]
- Alama, A.; Coco, S.; Genova, C.; Rossi, G.; Fontana, V.; Tagliamento, M.; Dal Bello, M.G.; Rosa, A.; Boccardo, S.; Rijavec, E.; et al. Prognostic Relevance of Circulating Tumor Cells and Circulating Cell-Free DNA Association in Metastatic Non-Small Cell Lung Cancer Treated with Nivolumab. J. Clin. Med. 2019, 8, 1011. [Google Scholar] [CrossRef]
- Gorgun, C.; Ceresa, D.; Lesage, R.; Villa, F.; Reverberi, D.; Balbi, C.; Santamaria, S.; Cortese, K.; Malatesta, P.; Geris, L.; et al. Dissecting the effects of preconditioning with inflammatory cytokines and hypoxia on the angiogenic potential of mesenchymal stromal cell (MSC)-derived soluble proteins and extracellular vesicles (EVs). Biomaterials 2021, 269, 120633. [Google Scholar] [CrossRef]
- Gorgun, C.; Reverberi, D.; Rotta, G.; Villa, F.; Quarto, R.; Tasso, R. Isolation and Flow Cytometry Characterization of Extracellular-Vesicle Subpopulations Derived from Human Mesenchymal Stromal Cells. Curr. Protoc. Stem Cell Biol. 2019, 48, e76. [Google Scholar] [CrossRef] [PubMed]
- McNutt, L.A.; Wu, C.; Xue, X.; Hafner, J.P. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am. J. Epidemiol. 2003, 157, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Daassi, D.; Mahoney, K.M.; Freeman, G.J. The importance of exosomal PDL1 in tumour immune evasion. Nat. Rev. Immunol. 2020, 20, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Dong, C. New B7 Family Checkpoints in Human Cancers. Mol. Cancer Ther. 2017, 16, 1203–1211. [Google Scholar] [CrossRef]
- Flies, D.B.; Chen, L. The new B7s: Playing a pivotal role in tumor immunity. J. Immunother. 2007, 30, 251–260. [Google Scholar] [CrossRef]
- Wang, L.; Kang, F.B.; Shan, B.E. B7-H3-mediated tumor immunology: Friend or foe? Int. J. Cancer 2014, 134, 2764–2771. [Google Scholar] [CrossRef]
- Chapoval, A.I.; Ni, J.; Lau, J.S.; Wilcox, R.A.; Flies, D.B.; Liu, D.; Dong, H.; Sica, G.L.; Zhu, G.; Tamada, K.; et al. B7-H3: A costimulatory molecule for T cell activation and IFN-gamma production. Nat. Immunol. 2001, 2, 269–274. [Google Scholar] [CrossRef]
- Kovaleva, O.V.; Belova, T.P.; Korotkova, E.A.; Kushlinskii, D.N.; Gratchev, A.N.; Petrikova, N.A.; Kudlay, D.A.; Kushlinskii, N.E. Soluble B7-H3 in Ovarian Cancer and Its Predictive Value. Bull. Exp. Biol. Med. 2021, 171, 472–474. [Google Scholar] [CrossRef]
- Wang, L.; Kang, F.B.; Zhang, G.C.; Wang, J.; Xie, M.F.; Zhang, Y.Z. Clinical significance of serum soluble B7-H3 in patients with osteosarcoma. Cancer Cell Int. 2018, 18, 115. [Google Scholar] [CrossRef]
- Azuma, T.; Sato, Y.; Ohno, T.; Azuma, M.; Kume, H. Serum soluble B7-H4 is a prognostic marker for patients with non-metastatic clear cell renal cell carcinoma. PLoS ONE 2018, 13, e0199719. [Google Scholar] [CrossRef]
- Azuma, T.; Zhu, G.; Xu, H.; Rietz, A.C.; Drake, C.G.; Matteson, E.L.; Chen, L. Potential role of decoy B7-H4 in the pathogenesis of rheumatoid arthritis: A mouse model informed by clinical data. PLoS Med. 2009, 6, e1000166. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, Y.; Kobori, H.; Piao, J.; Hashiguchi, M.; Matsumoto, K.; Hirose, S.; Azuma, M. Possible involvement of soluble B7-H4 in T cell-mediated inflammatory immune responses. Biochem. Biophys. Res. Commun. 2009, 389, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Gottschling, S.; Jensen, K.; Warth, A.; Herth, F.J.F.; Thomas, M.; Schnabel, P.A.; Herpel, E. Stage-specific embryonic antigen-4 is expressed in basaloid lung cancer and associated with poor prognosis. Eur. Respir. J. 2013, 41, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Hou, J.; Shi, J.; Yu, G.; Lu, B.; Zhang, X. Soluble CD276 (B7-H3) is released from monocytes, dendritic cells and activated T cells and is detectable in normal human serum. Immunology 2008, 123, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, R.J.; Freeman, G.J.; Sharpe, A.H. The B7 family revisited. Annu. Rev. Immunol. 2005, 23, 515–548. [Google Scholar] [CrossRef]
- Carrega, P.; Morandi, B.; Costa, R.; Frumento, G.; Forte, G.; Altavilla, G.; Ratto, G.B.; Mingari, M.C.; Moretta, L.; Ferlazzo, G. Natural killer cells infiltrating human nonsmall-cell lung cancer are enriched in CD56 bright CD16(-) cells and display an impaired capability to kill tumor cells. Cancer 2008, 112, 863–875. [Google Scholar] [CrossRef]
- Chen, M.; Hou, L.; Hu, L.; Tan, C.; Wang, X.; Bao, P.; Ran, Q.; Chen, L.; Li, Z. Platelet detection as a new liquid biopsy tool for human cancers. Front. Oncol. 2022, 12, 983724. [Google Scholar] [CrossRef]
- Bhuniya, A.; Sarkar, A.; Guha, A.; Choudhury, P.R.; Bera, S.; Sultana, J.; Chakravarti, M.; Dhar, S.; Das, J.; Guha, I.; et al. Tumor activated platelets induce vascular mimicry in mesenchymal stem cells and aid metastasis. Cytokine 2022, 158, 155998. [Google Scholar] [CrossRef]
- Oh, S.Y.; Kim, S.; Keam, B.; Kim, T.M.; Kim, D.W.; Heo, D.S. Soluble PD-L1 is a predictive and prognostic biomarker in advanced cancer patients who receive immune checkpoint blockade treatment. Sci. Rep. 2021, 11, 19712. [Google Scholar] [CrossRef]
- Ohkuma, R.; Ieguchi, K.; Watanabe, M.; Takayanagi, D.; Goshima, T.; Onoue, R.; Hamada, K.; Kubota, Y.; Horiike, A.; Ishiguro, T.; et al. Increased Plasma Soluble PD-1 Concentration Correlates with Disease Progression in Patients with Cancer Treated with Anti-PD-1 Antibodies. Biomedicines 2021, 9, 1929. [Google Scholar] [CrossRef]
- Balaphas, A.; Meyer, J.; Sadoul, K.; Fontana, P.; Morel, P.; Gonelle-Gispert, C.; Bühler, L.H. Platelets and Platelet-Derived Extracellular Vesicles in Liver Physiology and Disease. Hepatol. Commun. 2019, 3, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Puhm, F.; Boilard, E.; MacHlus, K.R. Platelet Extracellular Vesicles: Beyond the Blood. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Hinterleitner, C.; Strähle, J.; Malenke, E.; Hinterleitner, M.; Henning, M.; Seehawer, M.; Bilich, T.; Heitmann, J.; Lutz, M.; Mattern, S.; et al. Platelet PD-L1 reflects collective intratumoral PD-L1 expression and predicts immunotherapy response in non-small cell lung cancer. Nat. Commun. 2021, 12, 7005. [Google Scholar] [CrossRef]
- Zaidi, M.R.; Merlino, G. The two faces of interferon-γ in cancer. Clin. Cancer Res. 2011, 17, 6118–6124. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.; Cardoso, A.P.; Gonçalves, R.M.; Serre, K.; Oliveira, M.J. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018, 9, 847. [Google Scholar] [CrossRef]
- Benci, J.L.; Xu, B.; Qiu, Y.; Wu, T.J.; Dada, H.; Twyman-Saint Victor, C.; Cucolo, L.; Lee, D.S.M.; Pauken, K.E.; Huang, A.C.; et al. Tumor Interferon Signaling Regulates a Multigenic Resistance Program to Immune Checkpoint Blockade. Cell 2016, 167, 1540–1554.e12. [Google Scholar] [CrossRef]
- Wang, X.B.; Zheng, C.Y.; Giscombe, R.; Lefvert, A.K. Regulation of surface and intracellular expression of CTLA-4 on human peripheral T cells. Scand. J. Immunol. 2001, 54, 453–458. [Google Scholar] [CrossRef]
- Mimura, K.; Teh, J.L.; Okayama, H.; Shiraishi, K.; Kua, L.F.; Koh, V.; Smoot, D.T.; Ashktorab, H.; Oike, T.; Suzuki, Y.; et al. PD-L1 expression is mainly regulated by interferon gamma associated with JAK-STAT pathway in gastric cancer. Cancer Sci. 2018, 109, 43–53. [Google Scholar] [CrossRef]
- Abiko, K.; Matsumura, N.; Hamanishi, J.; Horikawa, N.; Murakami, R.; Yamaguchi, K.; Yoshioka, Y.; Baba, T.; Konishi, I.; Mandai, M. IFN-γ from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br. J. Cancer 2015, 112, 1501–1509. [Google Scholar] [CrossRef]



| Characteristics | PC | NC |
|---|---|---|
| Median age at start of therapy (Range) | 70.1 (50.5–88.8) | 70.1 (44.2–87.6) |
| Gender | N (%) | N (%) |
| Male | 43 (76.8) | 91 (72.2) |
| Female | 13 (23.2) | 35 (27.8) |
| Smoking habit | N (%) | N (%) |
| Never | 2 (3.6) | 10 (7.9) |
| Former | 31 (55.4) | 70 (55.6) |
| Current | 21 (37.5) | 44 (34.9) |
| Missing | 2 (3.6) | 2 (1.6) |
| ECOG-PS | N (%) | N (%) |
| 0 | 19 (33.9) | 29 (23.0) |
| 1 | 27 (48.2) | 82 (65.1) |
| 2 | 10 (17.9) | 14 (11.1) |
| 3 | 0 (0.0) | 1 (0.8) |
| Histotype | N (%) | N (%) |
| Adenocarcinoma | 29 (51.8) | 90 (71.4) |
| Squamous cell carcinoma | 14 (25.0) | 30 (23.8) |
| Other | 13 (23.2) | 6 (4.8) |
| Stage | N (%) | N (%) |
| IIIB | 0 (0.0) | 6 (4.8) |
| IV | 56 (100.0) | 120 (95.2) |
| Therapy line | N (%) | N (%) |
| 1st | 56 (100) | 0 (0.0) |
| 2nd | 0 (0.0) | 76 (60.3) |
| 3rd | 0 (0.0) | 29 (23.0) |
| Other | 0 (0.0) | 21 (16.7) |
| Cycles of therapy received | N (%) | N (%) |
| 1–8 | 27 (48.2) | 69 (54.8) |
| 9–123 | 29 (51.8) | 56 (44.4) |
| Missing | 0 (0.0) | 1 (0.8) |
| Total | 56 (100.0) | 126 (100.0) |
| Cohort | Biomarkers (Units) | N | Median | P25 | P75 | Range |
|---|---|---|---|---|---|---|
| Pembro | sPD-L1 (pg/mL) | 56 | 24.2 | 13.9 | 32.7 | 0.0–70.1 |
| sB7-H3 (ng/mL) | 56 | 0.5 | 0.1 | 1.9 | 0.0–472.9 | |
| sB7-H4 (pg/mL) | 56 | 63.9 | 13.3 | 141.6 | 0.0–265.4 | |
| IFNG (pg/mL) | 56 | 1 | 0.7 | 1.5 | 0.2–4.1 | |
| Nivo | sPD-L1 (pg/mL) | 126 | 24.7 | 17.5 | 36.7 | 0.0–94.2 |
| sB7-H3 (ng/mL) | 124 | 0.4 | 0 | 1.2 | 0.0–33.6 | |
| sB7-H4 (pg/mL) | 125 | 51.4 | 27.9 | 89.2 | 0.0–738.1 | |
| IFNG (pg/mL) | 126 | 1 | 0.7 | 1.9 | 0.4–16.1 |
| Cohort | Biomarker and Levels | PFS (RECIST) | OS | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CL | p-Value | HR | 95% CL | p-Value | ||
| PC | sPD-L1 | 0.030 * | 0.215 | ||||
| 0.0–24.2 | 1.00 | (Ref.) | 1.00 | (Ref.) | |||
| 24.3–70.1 | 2.16 | 1.08–4.33 | 1.77 | 0.72–4.37 | |||
| sB7-H3 | 0.001 * | 0.011 * | |||||
| 0.0–0.5 | 1.00 | (Ref.) | 1.00 | (Ref.) | |||
| 0.6–472.9 | 0.32 | 0.17–0.64 | 0.33 | 0.14–0.78 | |||
| sB7-H4 | 0.001 * | 0.036 * | |||||
| 0.0–63.9 | 1.00 | (Ref.) | 1.00 | (Ref.) | |||
| 64.0–265.4 | 0.32 | 0.16–0.64 | 0.42 | 0.19–0.94 | |||
| IFNG | 0.362 | 0.021 * | |||||
| 0.2–1.0 | 1.00 | (Ref.) | 1.00 | (Ref.) | |||
| 1.1–4.1 | 0.73 | 0.38–1.43 | 2.95 | 1.18–7.36 | |||
| NC | sPD-L1 | 0.247 | 0.399 | ||||
| 0.0–24.7 | 1.00 | (Ref.) | 1.00 | (Ref.) | |||
| 24.8–94.2 | 0.79 | 0.53–1.18 | 0.84 | 0.56–1.26 | |||
| sB7-H3 | 0.068 | 0.009 * | |||||
| 0.0–0.4 | 1.00 | (Ref.) | 1.00 | (Ref.) | |||
| 0.5–33.6 | 0.69 | 0.46–1.03 | 0.54 | 0.34–0.86 | |||
| sB7-H4 | 0.743 | 0.932 | |||||
| 0.0–51.4 | 1.00 | (Ref.) | 1.00 | (Ref.) | |||
| 51.5–738.1 | 1.07 | 0.71–1.60 | 1.02 | 0.67–1.56 | |||
| IFNG | 0.861 | 0.203 | |||||
| 0.4–1.0 | 1.00 | (Ref.) | 1.00 | (Ref.) | |||
| 1.1–16.1 | 1.04 | 0.69–1.55 | 1.32 | 0.86–2.03 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genova, C.; Tasso, R.; Rosa, A.; Rossi, G.; Reverberi, D.; Fontana, V.; Marconi, S.; Croce, M.; Dal Bello, M.G.; Dellepiane, C.; et al. Prognostic Role of Soluble and Extracellular Vesicle-Associated PD-L1, B7-H3 and B7-H4 in Non-Small Cell Lung Cancer Patients Treated with Immune Checkpoint Inhibitors. Cells 2023, 12, 832. https://doi.org/10.3390/cells12060832
Genova C, Tasso R, Rosa A, Rossi G, Reverberi D, Fontana V, Marconi S, Croce M, Dal Bello MG, Dellepiane C, et al. Prognostic Role of Soluble and Extracellular Vesicle-Associated PD-L1, B7-H3 and B7-H4 in Non-Small Cell Lung Cancer Patients Treated with Immune Checkpoint Inhibitors. Cells. 2023; 12(6):832. https://doi.org/10.3390/cells12060832
Chicago/Turabian StyleGenova, Carlo, Roberta Tasso, Alessandra Rosa, Giovanni Rossi, Daniele Reverberi, Vincenzo Fontana, Silvia Marconi, Michela Croce, Maria Giovanna Dal Bello, Chiara Dellepiane, and et al. 2023. "Prognostic Role of Soluble and Extracellular Vesicle-Associated PD-L1, B7-H3 and B7-H4 in Non-Small Cell Lung Cancer Patients Treated with Immune Checkpoint Inhibitors" Cells 12, no. 6: 832. https://doi.org/10.3390/cells12060832
APA StyleGenova, C., Tasso, R., Rosa, A., Rossi, G., Reverberi, D., Fontana, V., Marconi, S., Croce, M., Dal Bello, M. G., Dellepiane, C., Tagliamento, M., Ciferri, M. C., Zullo, L., Fedeli, A., Alama, A., Cortese, K., Gentili, C., Cella, E., Anselmi, G., ... Coco, S. (2023). Prognostic Role of Soluble and Extracellular Vesicle-Associated PD-L1, B7-H3 and B7-H4 in Non-Small Cell Lung Cancer Patients Treated with Immune Checkpoint Inhibitors. Cells, 12(6), 832. https://doi.org/10.3390/cells12060832












