Hypergravity Increases Blood–Brain Barrier Permeability to Fluorescent Dextran and Antisense Oligonucleotide in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Centrifugation
2.2. In Vivo Injection of Antisense Oligonucleotide and Dextrans
2.3. Collection of Biological Samples
2.4. Corticosterone Assay
2.5. Histology
2.6. Image Acquisition
2.7. Fluorescence Analyses
2.8. Gene Expression by RT-qPCR
2.9. Statistical Analysis
3. Results
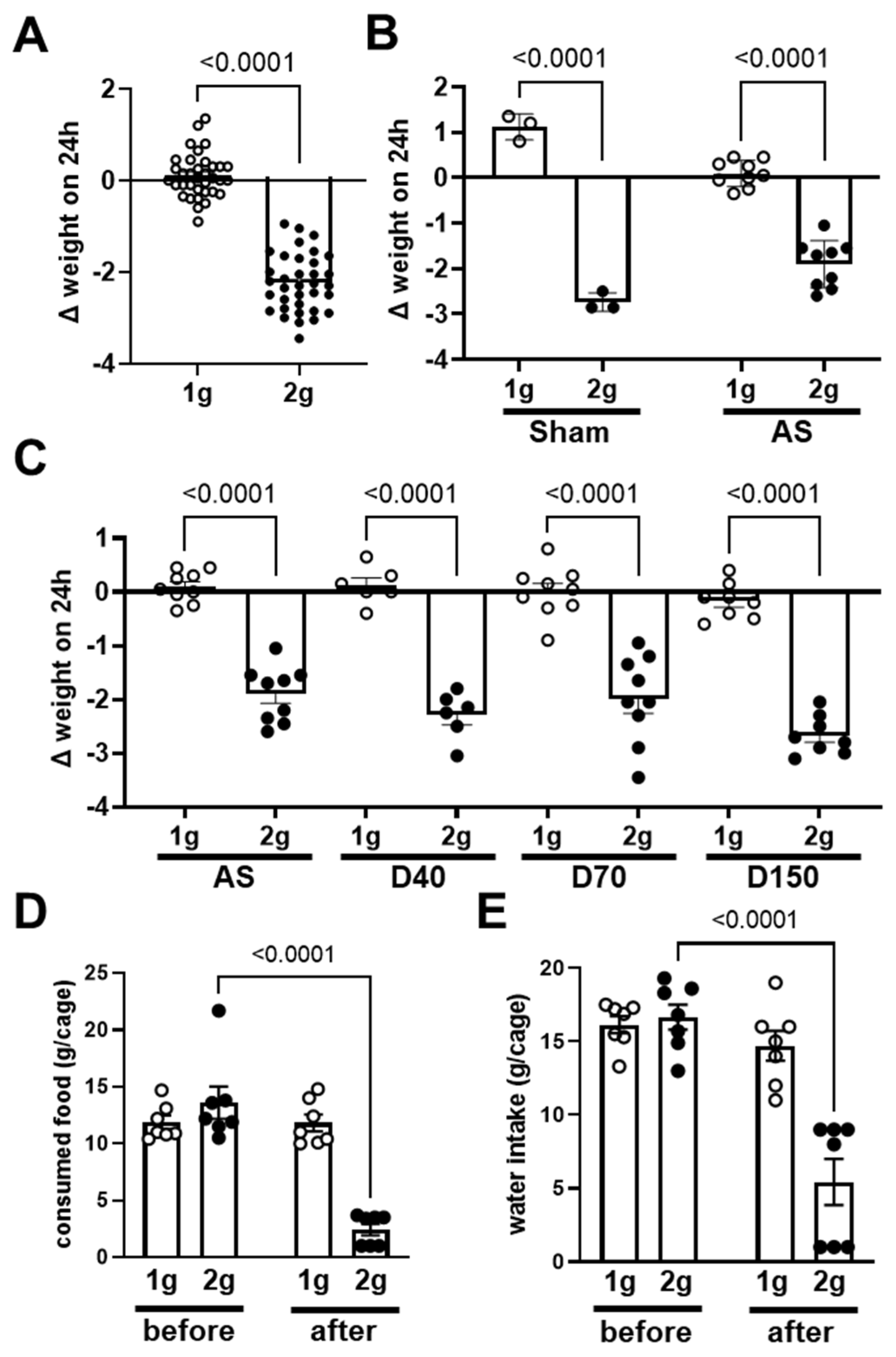
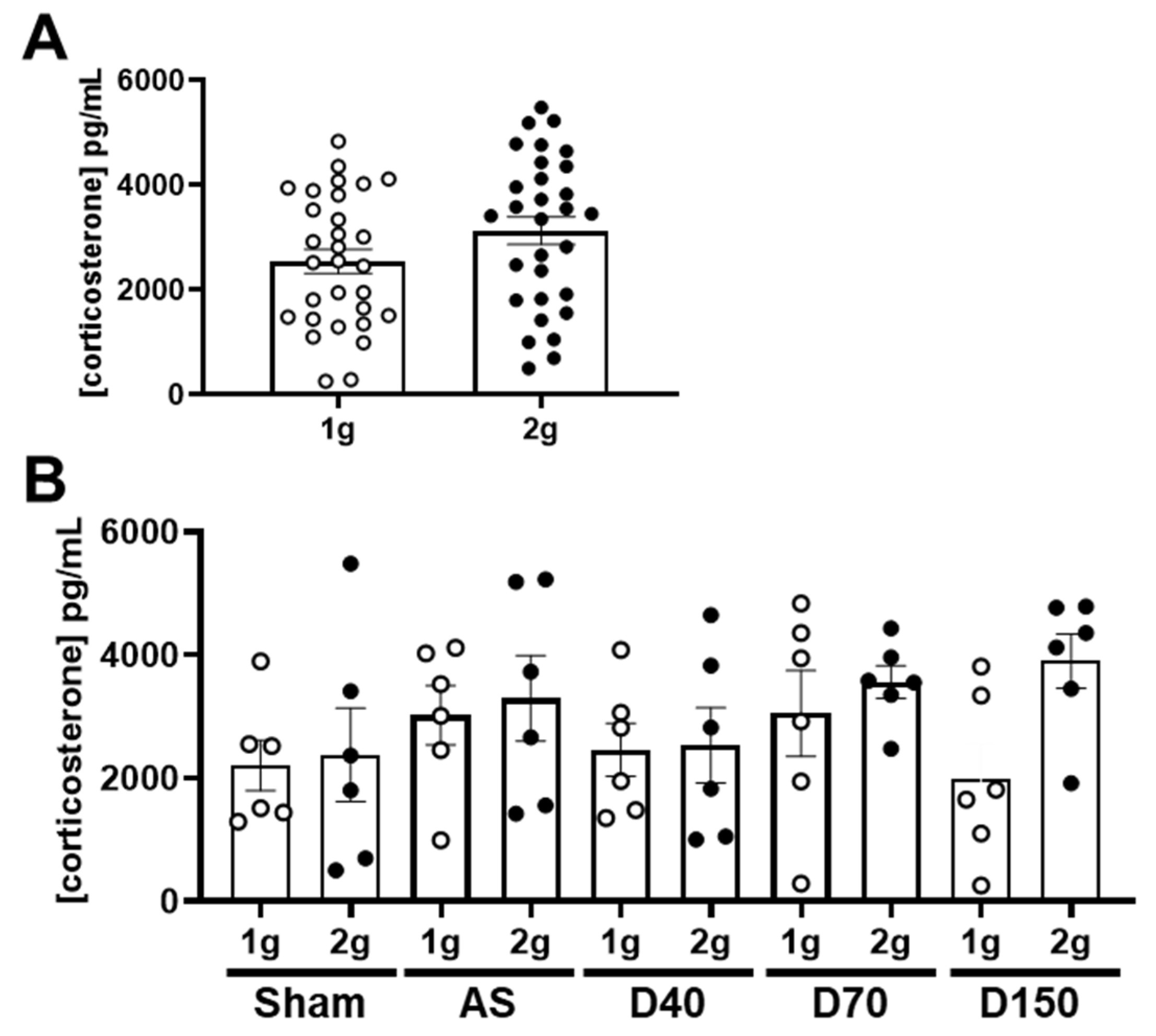
3.1. Effects of Centrifugation on Mice
3.2. Effects of Centrifugation on Extravasation of Fluorescent Dextrans in Brain
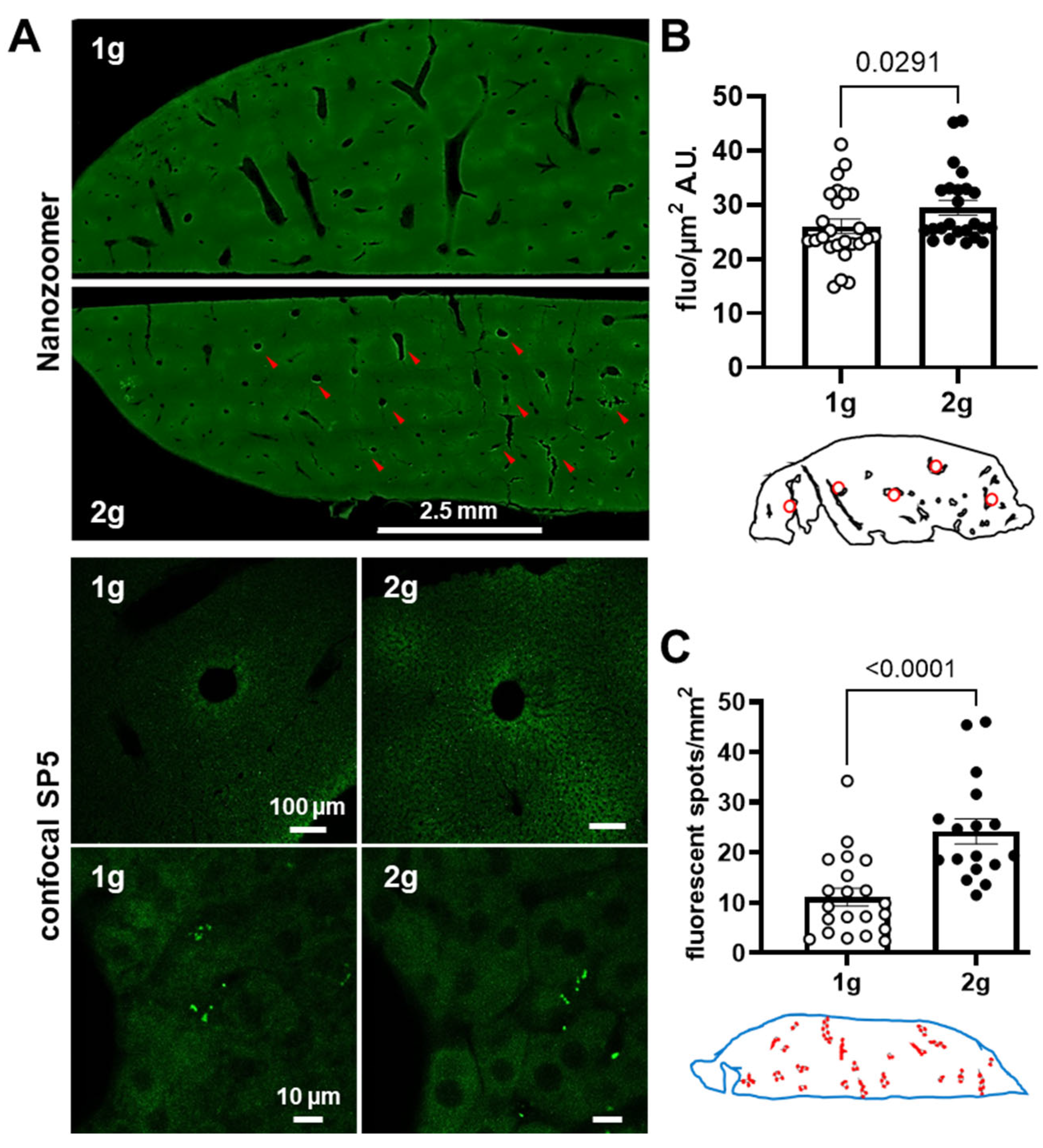
3.3. Effects of Centrifugation on Extravasation of Fluorescent AS in Liver
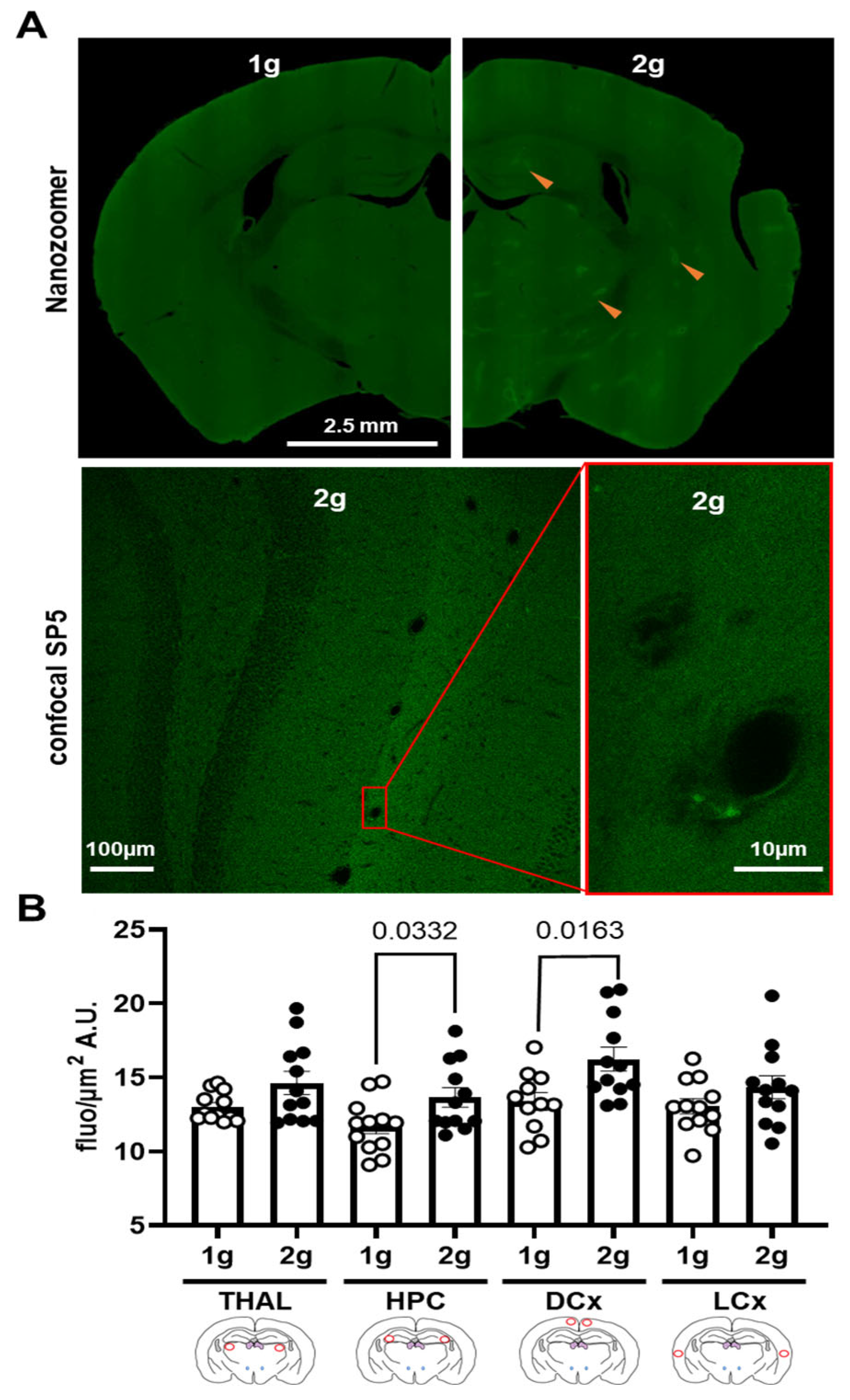
3.4. Effects of Centrifugation on Extravasation of Fluorescent AS in Brain
3.5. Effects of Centrifugation on Expression of Genes Involved in Endothelial Cells Interactions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Graybiel, A. Significance of vestibular organs in problems of weightlessness. Life Sci. Space Res. 1963, 1, 19–32. [Google Scholar] [PubMed]
- Lackner, J.R.; Dizio, P. Space motion sickness. Exp. Brain Res. 2006, 175, 377–399. [Google Scholar] [CrossRef] [PubMed]
- Gurovskiy, N.N.; Bryanov, I.I.; Yegorov, A.D. Changes in the vestibular function during space flight. Acta Astronaut. 1975, 2, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Matsnev, E.I.; Yakovleva, I.Y.; Tarasov, I.K.; Alekseev, V.N.; Kornilova, L.N.; Mateev, A.D.; Gorgiladze, G.I. Space motion sickness: Phenomenology, countermeasures, and mechanisms. Aviat. Space Environ. Med. 1983, 54, 312–317. [Google Scholar]
- Gazenko, O.G.; Schulzhenko, E.B.; Grigoriev, A.I.; Atkov, O.Y.; Egorov, A.D. Review of basic medical results of the Salyut-7--Soyuz-T 8-month manned flight. Acta Astronaut. 1988, 17, 155–160. [Google Scholar] [CrossRef]
- Reschke, M.F.; Good, E.F.; Clément, G.R. Neurovestibular Symptoms in Astronauts Immediately after Space Shuttle and International Space Station Missions. OTO Open 2017, 1, 2473974X17738767. [Google Scholar] [CrossRef]
- Davis, J.R.; Vanderploeg, J.M.; Santy, P.A.; Jennings, R.T.; Stewart, D.F. Space motion sickness during 24 flights of the space shuttle. Aviat. Space Environ. Med. 1988, 59, 1185–1189. [Google Scholar]
- von Baumgarten, R.J.; Thumler, R. A model for vestibular function in altered gravitational states. Life Sci. Space Res. 1979, 17, 161–170. [Google Scholar]
- Golding, J.F.; Paillard, A.C.; Normand, H.; Besnard, S.; Denise, P. Prevalence, Predictors, and Prevention of Motion Sickness in Zero-G Parabolic Flights. Aerosp. Med. Hum. Perform. 2017, 88, 3–9. [Google Scholar] [CrossRef]
- Jirak, P.; Mirna, M.; Rezar, R.; Motloch, L.J.; Lichtenauer, M.; Jordan, J.; Binneboessel, S.; Tank, J.; Limper, U.; Jung, C. How spaceflight challenges human cardiovascular health. Eur. J. Prev. Cardiol. 2022, 29, 1399–1411. [Google Scholar] [CrossRef]
- Kermorgant, M.; Nasr, N.; Czosnyka, M.; Arvanitis, D.N.; Hélissen, O.; Senard, J.-M.; Traon, A.P.-L. Impacts of Microgravity Analogs to Spaceflight on Cerebral Autoregulation. Front. Physiol. 2020, 11, 778. [Google Scholar] [CrossRef] [PubMed]
- Baran, R.; Marchal, S.; Campos, S.G.; Rehnberg, E.; Tabury, K.; Baselet, B.; Wehland, M.; Grimm, D.; Baatout, S. The Cardiovascular System in Space: Focus on In Vivo and In Vitro Studies. Biomedicines 2022, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Dabertrand, F.; Porte, Y.; Macrez, N.; Morel, J.-L. Spaceflight regulates ryanodine receptor subtype 1 in portal vein myocytes in the opposite way of hypertension. J. Appl. Physiol. 2012, 112, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Delp, M.D.; Charvat, J.M.; Limoli, C.L.; Globus, R.K.; Ghosh, P. Apollo Lunar Astronauts Show Higher Cardiovascular Disease Mortality: Possible Deep Space Radiation Effects on the Vascular Endothelium. Sci. Rep. 2016, 6, 29901. Available online: http://www.nature.com/articles/srep29901 (accessed on 20 June 2019). [CrossRef]
- Morel, J.-L.; Boittin, F.-X.; Halet, G.; Arnaudeau, S.; Mironneau, C.; Mironneau, J. Effect of a 14-day hindlimb suspension on cytosolic Ca2+ concentration in rat portal vein myocytes. Am. J. Physiol. Circ. Physiol. 1997, 273, H2867–H2875. [Google Scholar] [CrossRef]
- Navasiolava, N.M.; Dignat-George, F.; Sabatier, F.; Larina, I.M.; Demiot, C.; Fortrat, J.-O.; Gauquelin-Koch, G.; Kozlovskaya, I.B.; Custaud, M.-A. Enforced physical inactivity increases endothelial microparticle levels in healthy volunteers. Am. J. Physiol. Circ. Physiol. 2010, 299, H248–H256. [Google Scholar] [CrossRef]
- Sofronova, S.I.; Tarasova, O.S.; Gaynullina, D.; Borzykh, A.A.; Behnke, B.J.; Stabley, J.N.; McCullough, D.J.; Maraj, J.J.; Hanna, M.; Muller-Delp, J.M.; et al. Spaceflight on the Bion-M1 biosatellite alters cerebral artery vasomotor and mechanical properties in mice. J. Appl. Physiol. 2015, 118, 830–838. [Google Scholar] [CrossRef]
- Abe, C.; Katayama, C.; Horii, K.; Ogawa, B.; Ohbayashi, K.; Iwasaki, Y.; Nin, F.; Morita, H. Hypergravity load-induced hyperglycemia occurs due to hypothermia and increased plasma corticosterone level in mice. J. Physiol. Sci. 2022, 72, 18. [Google Scholar] [CrossRef]
- Iwasaki, K.-I.; Shiozawa, T.; Kamiya, A.; Michikami, D.; Hirayanagi, K.; Yajima, K.; Iwase, S.; Mano, T. Hypergravity exercise against bed rest induced changes in cardiac autonomic control. Eur. J. Appl. Physiol. 2005, 94, 285–291. [Google Scholar] [CrossRef]
- Iwasaki, K.I.; Sasaki, T.; Hirayanagi, K.; Yajima, K. Usefulness of daily +2Gz load as a countermeasure against physiological problems during weightlessness. Acta Astronaut. 2001, 49, 227–235. [Google Scholar] [CrossRef]
- Sasaki, T.; Iwasaki, K.I.; Hirayanagi, K.; Yamaguchi, N.; Miyamoto, A.; Yajima, K. Effects of daily 2-Gz load on human cardiovascular function during weightlessness simulation using 4-day head-down bed rest. Uchu Koku Kankyo Igaku 1999, 36, 113–123. [Google Scholar] [PubMed]
- Young, L.R.; Paloski, W.H. Short radius intermittent centrifugation as a countermeasure to bed-rest and 0-G deconditioning: IMAG pilot study summary and recommendations for research. J. Gravit. Physiol. 2007, 14, P31–P33. [Google Scholar]
- Beraneck, M.; Bojados, M.; Le Séac’H, A.; Jamon, M.; Vidal, P.-P. Ontogeny of Mouse Vestibulo-Ocular Reflex Following Genetic or Environmental Alteration of Gravity Sensing. PLoS ONE 2012, 7, e40414. [Google Scholar] [CrossRef] [PubMed]
- Idoux, E.; Tagliabue, M.; Beraneck, M. No Gain No Pain: Relations Between Vestibulo-Ocular Reflexes and Motion Sickness in Mice. Front. Neurol. 2018, 9, 918. Available online: https://www.frontiersin.org/article/10.3389/fneur.2018.00918/full (accessed on 9 November 2019). [CrossRef] [PubMed]
- Sebastian, C.; Horn, E.; Eβeling, K.; Neubert, J. Readaptation of the vestibuloocular reflex to 1g-Condition in immature lower vertebrates (Xenopus laevis) after micro- or hypergravity exposure. Acta Astronaut. 1995, 36, 487–503. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, C.E.; Pfau, K.; Horn, E.R. An age-dependent sensitivity of the roll-induced vestibuloocular reflex to hypergravity exposure of several days in an amphibian (Xenopus laevis). Acta Astronaut. 1998, 42, 419–430. [Google Scholar] [CrossRef]
- Horii, A.; Mitani, K.; Masumura, C.; Uno, A.; Imai, T.; Morita, Y.; Takahashi, K.; Kitahara, T.; Inohara, H. Hippocampal gene expression, serum cortisol level, and spatial memory in rats exposed to hypergravity. J. Vestib. Res. 2017, 27, 209–215. [Google Scholar] [CrossRef]
- Kawakami, S.; Kashiwagi, K.; Furuno, N.; Yamashita, M.; Kashiwagi, A. Effects of hypergravity environments on amphibian development, gene expression and apoptosis. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2006, 145, 65–72. [Google Scholar] [CrossRef]
- Pulga, A.; Porte, Y.; Morel, J.-L. Changes in C57BL6 Mouse Hippocampal Transcriptome Induced by Hypergravity Mimic Acute Corticosterone-Induced Stress. Front. Mol. Neurosci. 2016, 9, 153. [Google Scholar] [CrossRef]
- Guéguinou, N.; Bojados, M.; Jamon, M.; Derradji, H.; Baatout, S.; Tschirhart, E.; Frippiat, J.-P.; Legrand-Frossi, C. Stress response and humoral immune system alterations related to chronic hypergravity in mice. Psychoneuroendocrinology 2012, 37, 137–147. [Google Scholar] [CrossRef]
- Lee, J.; Jang, D.; Jeong, H.; Kim, K.S.; Yang, S. Impairment of synaptic plasticity and novel object recognition in the hypergravity-exposed rats. Sci. Rep. 2020, 10, 15813. [Google Scholar] [CrossRef]
- Porte, Y.; Morel, J.L. Learning on Jupiter, learning on the Moon: The dark side of the G-force. Effects of gravity changes on neurovascular unit and modulation of learning and memory. Front Behav. Neurosci. 2012, 6, 64. [Google Scholar] [CrossRef] [PubMed]
- Buytaert, K.I.; MacDougall, H.G.; Moore, S.T.; Clement, G.; Pattyn, N.; Migeotte, P.F.; Wuyts, F.L. Validation of centrifugation as a countermeasure for otolith deconditioning during spaceflight: Preliminary data of the ESA SPIN study. J. Vestib. Res. 2013, 23, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.M.; Knapp, C.F.; Goswami, N. Artificial Gravity as a Countermeasure to the Cardiovascular Deconditioning of Spaceflight: Gender Perspectives. Front. Physiol. 2018, 9, 716. Available online: https://www.frontiersin.org/article/10.3389/fphys.2018.00716/full (accessed on 6 June 2019). [CrossRef] [PubMed]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Benveniste, H.; Nedergaard, M.; Zlokovic, B.V.; Mestre, H.; Lee, H.; Doubal, F.N.; Brown, R.; Ramirez, J.; MacIntosh, B.J.; et al. Perivascular spaces in the brain: Anatomy, physiology and pathology. Nat. Rev. Neurol. 2020, 16, 137–153. [Google Scholar] [CrossRef]
- Cuddapah, V.A.; Zhang, S.L.; Sehgal, A. Regulation of the Blood–Brain Barrier by Circadian Rhythms and Sleep. Trends Neurosci. 2019, 42, 500–510. [Google Scholar] [CrossRef]
- Pan, W.; Kastin, A.J. The Blood-Brain Barrier: Regulatory Roles in Wakefulness and Sleep. Neuroscientist 2017, 23, 124–136. [Google Scholar] [CrossRef]
- Zhang, S.L.; Lahens, N.F.; Yue, Z.; Arnold, D.M.; Pakstis, P.P.; Schwarz, J.E.; Sehgal, A. A circadian clock regulates efflux by the blood-brain barrier in mice and human cells. Nat. Commun. 2021, 12, 617. [Google Scholar] [CrossRef]
- Zhang, S.L.; Yue, Z.; Arnold, D.M.; Artiushin, G.; Sehgal, A. A Circadian Clock in the Blood-Brain Barrier Regulates Xenobiotic Efflux. Cell 2018, 173, 130–139.e10. [Google Scholar] [CrossRef]
- Montagne, A.; Nikolakopoulou, A.M.; Zhao, Z.; Sagare, A.P.; Si, G.; Lazic, D.; Barnes, S.R.; Daianu, M.; Ramanathan, A.; Go, A.; et al. Pericyte degeneration causes white matter dysfunction in the mouse central nervous system. Nat. Med. 2018, 24, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Nation, D.A.; Sweeney, M.D.; Montagne, A.; Sagare, A.P.; D’Orazio, L.M.; Pachicano, M.; Sepehrband, F.; Nelson, A.R.; Buennagel, D.P.; Harrington, M.G.; et al. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 2019, 25, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Nikolakopoulou, A.M.; Wang, Y.; Ma, Q.; Sagare, A.P.; Montagne, A.; Huuskonen, M.T.; Rege, S.V.; Kisler, K.; Dai, Z.; Körbelin, J.; et al. Endothelial LRP1 protects against neurodegeneration by blocking cyclophilin A. J. Exp. Med. 2021, 218. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Montagne, A.; Sagare, A.P.; Nation, D.A.; Schneider, L.S.; Chui, H.C.; Harrington, M.G.; Pa, J.; Law, M.; Wang, D.J.J.; et al. Vascular dysfunction—The disregarded partner of Alzheimer’s disease. Alzheimer’s Dement. 2019, 15, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.M.; Cialdai, F.; Monici, M.; Morbidelli, L. The Impact of Microgravity and Hypergravity on Endothelial Cells. BioMed Res. Int. 2015, 2015, 434803. [Google Scholar] [CrossRef] [PubMed]
- Bors, L.A.; Erdő, F. Overcoming the Blood–Brain Barrier. Challenges and Tricks for CNS Drug Delivery. Sci. Pharm. 2019, 87, 6. [Google Scholar] [CrossRef]
- Bauer, J.; Bussen, M.; Wise, P.; Wehland, M.; Schneider, S.; Grimm, D. Searching the literature for proteins facilitates the identification of biological processes, if advanced methods of analysis are linked: A case study on microgravity-caused changes in cells. Expert Rev. Proteom. 2016, 13, 697–705. [Google Scholar] [CrossRef]
- Costa-Almeida, R.; Carvalho, D.T.O.; Ferreira, M.J.S.; Aresta, G.; Gomes, M.E.; van Loon, J.J.W.A.; Van der Heiden, K.; Granja, P.L. Effects of hypergravity on the angiogenic potential of endothelial cells. J. R. Soc. Interface 2016, 13, 20160688. [Google Scholar] [CrossRef]
- De Cesari, C.; Barravecchia, I.; Pyankova, O.V.; Vezza, M.; Germani, M.M.; Scebba, F.; van Loon, J.J.; Angeloni, D. Hypergravity Activates a Pro-Angiogenic Homeostatic Response by Human Capillary Endothelial Cells. Int. J. Mol. Sci. 2020, 21, 2354. [Google Scholar] [CrossRef]
- Spisni, E.; Bianco, M.C.; Blasi, F.; Santi, S.; Riccio, M.; Toni, M.; Griffoni, C.; Tomasi, V. Hypergravity impairs angiogenic response of in vitro cultured human primary endothelial cells. J. Gravit. Physiol. 2002, 9, P285–P286. [Google Scholar]
- Wehland, M.; Ma, X.; Braun, M.; Hauslage, J.; Hemmersbach, R.; Bauer, J.; Grosse, J.; Infanger, M.; Grimm, D. The Impact of Altered Gravity and Vibration on Endothelial Cells During a Parabolic Flight. Cell. Physiol. Biochem. 2013, 31, 432–451. [Google Scholar] [CrossRef]
- Szulcek, R.; Van Bezu, J.; Boonstra, J.; Van Loon, J.J.W.A.; van Nieuw Amerongen, G.P. Transient Intervals of Hyper-Gravity Enhance Endothelial Barrier Integrity: Impact of Mechanical and Gravitational Forces Measured Electrically. PLoS ONE 2015, 10, e0144269. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kwon, S. Mechanical load increase–induced changes in cytoskeletal structure and cellular barrier function in human cerebral endothelial cells. Biotechnol. Bioeng. 2018, 115, 2624–2631. [Google Scholar] [CrossRef]
- Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates, Compact—5th Edition. Available online: https://www.elsevier.com/books/paxinos-and-franklins-the-mouse-brain-in-stereotaxic-coordinates-compact/franklin/978-0-12-816159-3 (accessed on 7 July 2022).
- Ovadia, H.; Abramsky, O.; Feldman, S.; Weidenfeld, J. Evaluation of the effect of stress on the blood–brain barrier: Critical role of the brain perfusion time. Brain Res. 2001, 905, 21–25. [Google Scholar] [CrossRef]
- Yan, R.; Liu, H.; Lv, F.; Deng, Y.; Li, Y. Rac1/Wave2/Arp3 Pathway Mediates Rat Blood-Brain Barrier Dysfunction under Simulated Microgravity Based on Proteomics Strategy. Int. J. Mol. Sci. 2021, 22, 5165. [Google Scholar] [CrossRef] [PubMed]
- Bellone, J.A.; Gifford, P.S.; Nishiyama, N.C.; Hartman, R.E.; Mao, X.W. Long-term effects of simulated microgravity and/or chronic exposure to low-dose gamma radiation on behavior and blood-brain barrier integrity. NPJ Microgravity 2016, 2, 16019. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.W.; Nishiyama, N.C.; Byrum, S.D.; Stanbouly, S.; Jones, T.; Holley, J.; Sridharan, V.; Boerma, M.; Tackett, A.J.; Willey, J.S.; et al. Spaceflight induces oxidative damage to blood-brain barrier integrity in a mouse model. FASEB J. 2020, 34, 15516–15530. [Google Scholar] [CrossRef]
- Carriot, J.; Mackrous, I.; Cullen, K.E. Challenges to the Vestibular System in Space: How the Brain Responds and Adapts to Microgravity. Front. Neural Circuits 2021, 15, 127. [Google Scholar] [CrossRef]
- Moran, M.M.; Stein, T.P.; Wade, C.E. Hormonal modulation of food intake in response to low leptin levels induced by hypergravity. Exp. Biol. Med. 2001, 226, 740–745. [Google Scholar] [CrossRef]
- Yuwaki, K.; Okuno, M. Changes in food intake and growth rate in mice under hypergravity. Biol. Sci. Space 2003, 17, 219–220. [Google Scholar]
- Kawao, N.; Morita, H.; Obata, K.; Tamura, Y.; Okumoto, K.; Kaji, H. The vestibular system is critical for the changes in muscle and bone induced by hypergravity in mice. Physiol. Rep. 2016, 4, e12979. [Google Scholar] [CrossRef]
- Petrak, J.; Mravec, B.; Jurani, M.; Baranovska, M.; Tillinger, A.; Hapala, I.; Frollo, I.; Kvetňanský, R. Hypergravity-induced Increase in Plasma Catecholamine and Corticosterone Levels in Telemetrically Collected Blood of Rats during Centrifugation. Ann. N. Y. Acad. Sci. 2008, 1148, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Yamaguchi, A.; Shiba, D.; Shirakawa, M.; Takahashi, S. Impact of a simulated gravity load for atmospheric reentry, 10 g for 2 min, on conscious mice. J. Physiol. Sci. 2017, 67, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Dubayle, D.; Vanden-Bossche, A.; Beraneck, M.; Vico, L.; Morel, J.-L. Effects of centrifugation and whole-body vibrations on blood–brain barrier permeability in mice. npj Microgravity 2020, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Storch, K.J.; Stolfi, A.; Mohler, S.R.; Frey, M.A.; Stein, T.P. Weight Loss in Humans in Space. Aviat. Space Environ. Med. 2011, 82, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Thornton, W.E.; Bonato, F. Space Motion Sickness and Motion Sickness: Symptoms and Etiology. Aviat. Space Environ. Med. 2013, 84, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-L.; Wang, J.-Q.; Qi, R.-R.; Pan, L.-L.; Li, M.; Cai, Y.-L. Motion Sickness: Current Knowledge and Recent Advance. CNS Neurosci. Ther. 2015, 22, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Abe, C.; Tanaka, K.; Iwata, C.; Morita, H. Vestibular-mediated increase in central serotonin plays an important role in hypergravity-induced hypophagia in rats. J. Appl. Physiol. 2010, 109, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- Alauzet, C.; Cunat, L.; Wack, M.; Lozniewski, A.; Busby, H.; Agrinier, N.; Cailliez-Grimal, C.; Frippiat, J.-P. Hypergravity disrupts murine intestinal microbiota. Sci. Rep. 2019, 9, 9410. [Google Scholar] [CrossRef]
- Yoon, G.; Kim, H.-S. Gastric acid response to acute exposure to hypergravity. Oncotarget 2017, 8, 64–69. [Google Scholar] [CrossRef]
- Ishizawa, M.; Iwasaki, K.-I.; Kato, S.; Makishima, M. Hypergravity modulates vitamin D receptor target gene mRNA expression in mice. Am. J. Physiol. Metab. 2009, 297, E728–E734. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, C.; Li, H.; Ogura, R.; Yoshimura, Y.; Kudo, T.; Shirakawa, M.; Shiba, D.; Takahashi, S.; Morita, H.; Shiga, T. Effects of gravity changes on gene expression of BDNF and serotonin receptors in the mouse brain. PLoS ONE 2017, 12, e0177833. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Wang, S.; Tung, Y.-S.; Morrison, B.; Konofagou, E.E. Molecules of Various Pharmacologically-Relevant Sizes Can Cross the Ultrasound-Induced Blood-Brain Barrier Opening in vivo. Ultrasound Med. Biol. 2010, 36, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Hultström, D.; Malmgren, L.; Gilstring, D.; Olsson, Y. FITC-Dextrans as tracers for macromolecular movements in the nervous system. Acta Neuropathol. 1983, 59, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Olsson, Y.; Svensjö, E.; Arfors, K.E.; Hultström, D. Fluorescein labelled dextrans as tracers for vascular permeability studies in the nervous system. Acta Neuropathol. 1975, 33, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Yang, J.; Ronaldson, P.T.; Davis, T.P. Structure, Function, and Regulation of the Blood-Brain Barrier Tight Junction in Central Nervous System Disorders. Front. Physiol. 2020, 11, 914. [Google Scholar] [CrossRef]
- Saunders, N.R.; Dziegielewska, K.M.; Møllgård, K.; Habgood, M.D. Markers for blood-brain barrier integrity: How appropriate is Evans blue in the twenty-first century and what are the alternatives? Front Neurosci. 2015, 9, 385. [Google Scholar] [CrossRef]
- Caulfield, J.P.; Farquhar, M.G. The permeability of glomerular capillaries to graded dextrans. Identification of the basement membrane as the primary filtration barrier. J. Cell Biol. 1974, 63, 883–903. [Google Scholar] [CrossRef]
- Öztaş, B.; Akgül, S.; Arslan, F. Influence of surgical pain stress on the blood-brain barrier permeability in rats. Life Sci. 2004, 74, 1973–1979. [Google Scholar] [CrossRef]
- Harris, W.J.; Asselin, M.-C.; Hinz, R.; Parkes, L.M.; Allan, S.; Schiessl, I.; Boutin, H.; Dickie, B.R. In vivo methods for imaging blood–brain barrier function and dysfunction. Eur. J. Nucl. Med. 2022. Available online: https://doi.org/10.1007/s00259-022-05997-1 (accessed on 9 November 2019). [CrossRef] [PubMed]
- Montagne, A.; Barnes, S.R.; Sweeney, M.D.; Halliday, M.R.; Sagare, A.P.; Zhao, Z.; Toga, A.W.; Jacobs, R.E.; Liu, C.Y.; Amezcua, L.; et al. Blood-Brain Barrier Breakdown in the Aging Human Hippocampus. Neuron 2015, 85, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Sakamoto, H.; Tomimoto, H.; Akiguchi, I.; Onodera, M.; Huang, C.L.; Kanenishi, K. Blood-brain barrier is impaired in the hippocampus of young adult spontaneously hypertensive rats. Acta Neuropathol. 2004, 107, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Yuan, S.; Liu, K. Occludin regulation of blood–brain barrier and potential therapeutic target in ischemic stroke. Brain Circ. 2020, 6, 152–162. [Google Scholar]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight junctions: From simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef]
- Akwii, R.G.; Sajib, M.S.; Zahra, F.T.; Mikelis, C.M. Role of Angiopoietin-2 in Vascular Physiology and Pathophysiology. Cells 2019, 8, 471. [Google Scholar] [CrossRef]
- Csaba, Z.; Vitalis, T.; Charriaut-Marlangue, C.; Margaill, I.; Coqueran, B.; Leger, P.; Parente, I.; Jacquens, A.; Titomanlio, L.; Constans, C.; et al. A simple novel approach for detecting blood–brain barrier permeability using GPCR internalization. Neuropathol. Appl. Neurobiol. 2021, 47, 297–315. [Google Scholar] [CrossRef]
- Andreev-Andrievskiy, A.; Popova, A.; Lloret, J.-C.; Aubry, P.; Borovik, A.; Tsvirkun, D.; Vinogradova, O.; Ilyin, E.; Gauquelin-Koch, G.; Gharib, C.; et al. BION-M 1: First continuous blood pressure monitoring in mice during a 30-day spaceflight. Life Sci. Space Res. 2017, 13, 19–26. [Google Scholar] [CrossRef]
- Janigro, D.; Bailey, D.M.; Lehmann, S.; Badaut, J.; O’Flynn, R.; Hirtz, C.; Marchi, N. Peripheral Blood and Salivary Biomarkers of Blood–Brain Barrier Permeability and Neuronal Damage: Clinical and Applied Concepts. Front. Neurol. 2021, 11, 577312. Available online: https://www.frontiersin.org/articles/10.3389/fneur.2020.577312 (accessed on 6 December 2019). [CrossRef]

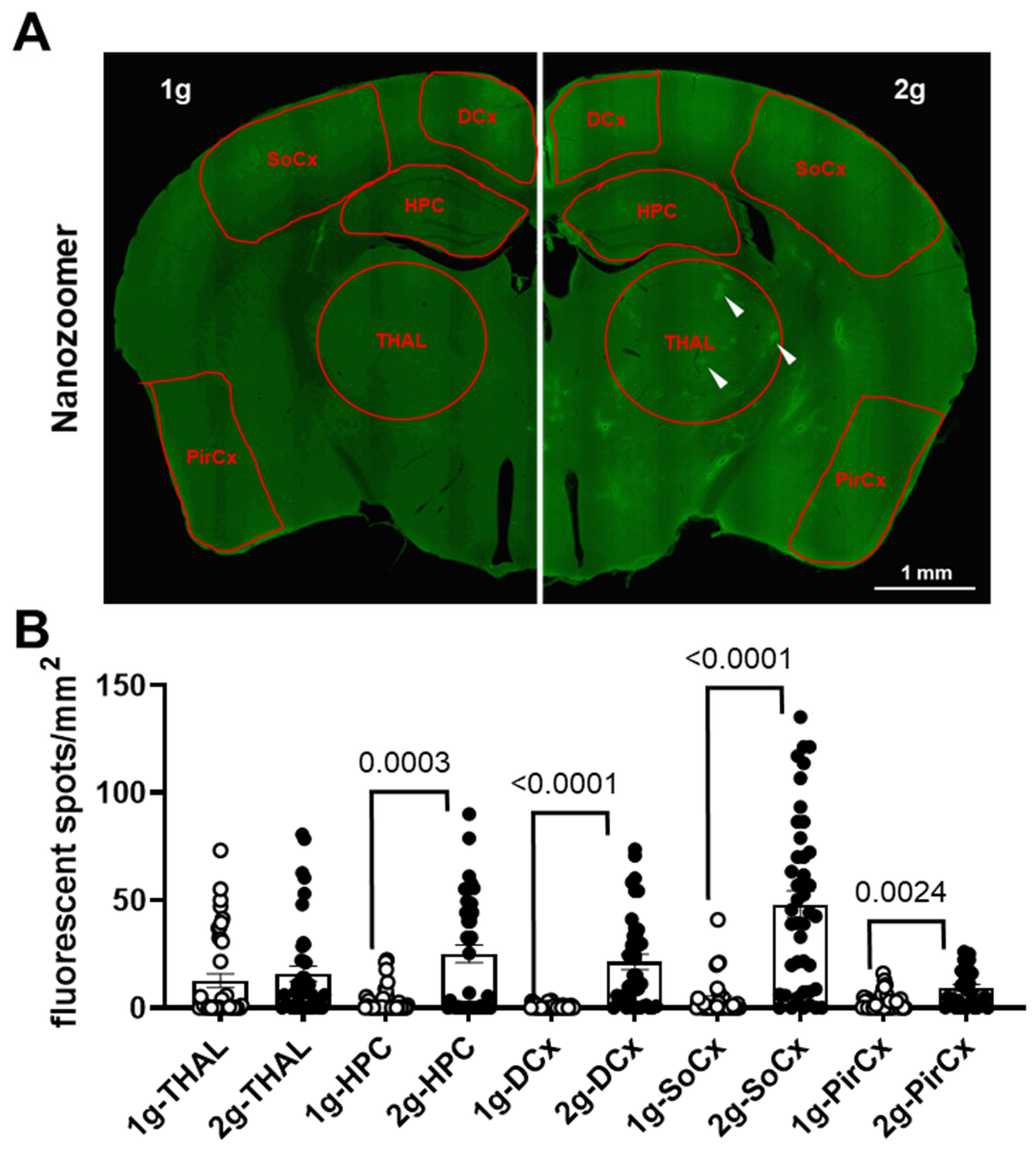
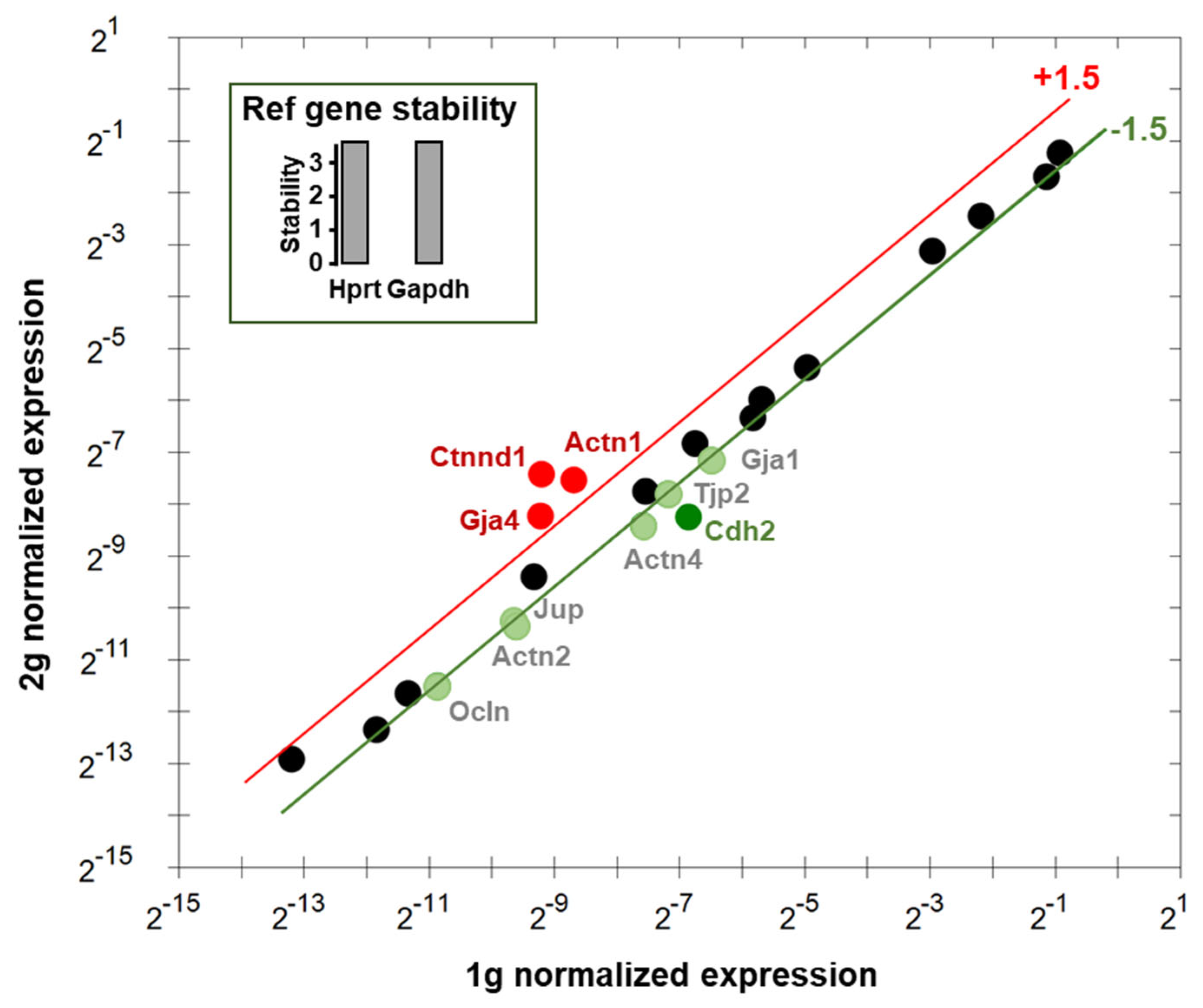
| Gene | Name | Relative exp. # | Function of the Genes |
|---|---|---|---|
| Actn1 | Actinin α1 | 2.24 |
|
| Actn2 * | Actinin α2 | −1.67 |
|
| Actn4 | Actinin α4 | −1.78 |
|
| Cdh2 | Cadherin 2 | −2.61 |
|
| Ctnnd1 | Catenin δ1 | 3.46 |
|
| Gja1 | Gap junction protein α1 | −1.58 |
|
| Gja4 | Gap junction protein α4 | 2.00 |
|
| Jup | Junction plakoglobulin | −1.52 |
|
| Ocln | Occludin | −1.54 |
|
| Tjp2 | Tight junction protein 2 | −1.52 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubayle, D.; Vanden-Bossche, A.; Peixoto, T.; Morel, J.-L. Hypergravity Increases Blood–Brain Barrier Permeability to Fluorescent Dextran and Antisense Oligonucleotide in Mice. Cells 2023, 12, 734. https://doi.org/10.3390/cells12050734
Dubayle D, Vanden-Bossche A, Peixoto T, Morel J-L. Hypergravity Increases Blood–Brain Barrier Permeability to Fluorescent Dextran and Antisense Oligonucleotide in Mice. Cells. 2023; 12(5):734. https://doi.org/10.3390/cells12050734
Chicago/Turabian StyleDubayle, David, Arnaud Vanden-Bossche, Tom Peixoto, and Jean-Luc Morel. 2023. "Hypergravity Increases Blood–Brain Barrier Permeability to Fluorescent Dextran and Antisense Oligonucleotide in Mice" Cells 12, no. 5: 734. https://doi.org/10.3390/cells12050734
APA StyleDubayle, D., Vanden-Bossche, A., Peixoto, T., & Morel, J.-L. (2023). Hypergravity Increases Blood–Brain Barrier Permeability to Fluorescent Dextran and Antisense Oligonucleotide in Mice. Cells, 12(5), 734. https://doi.org/10.3390/cells12050734






