In Vitro Drug Repurposing: Focus on Vasodilators
Abstract
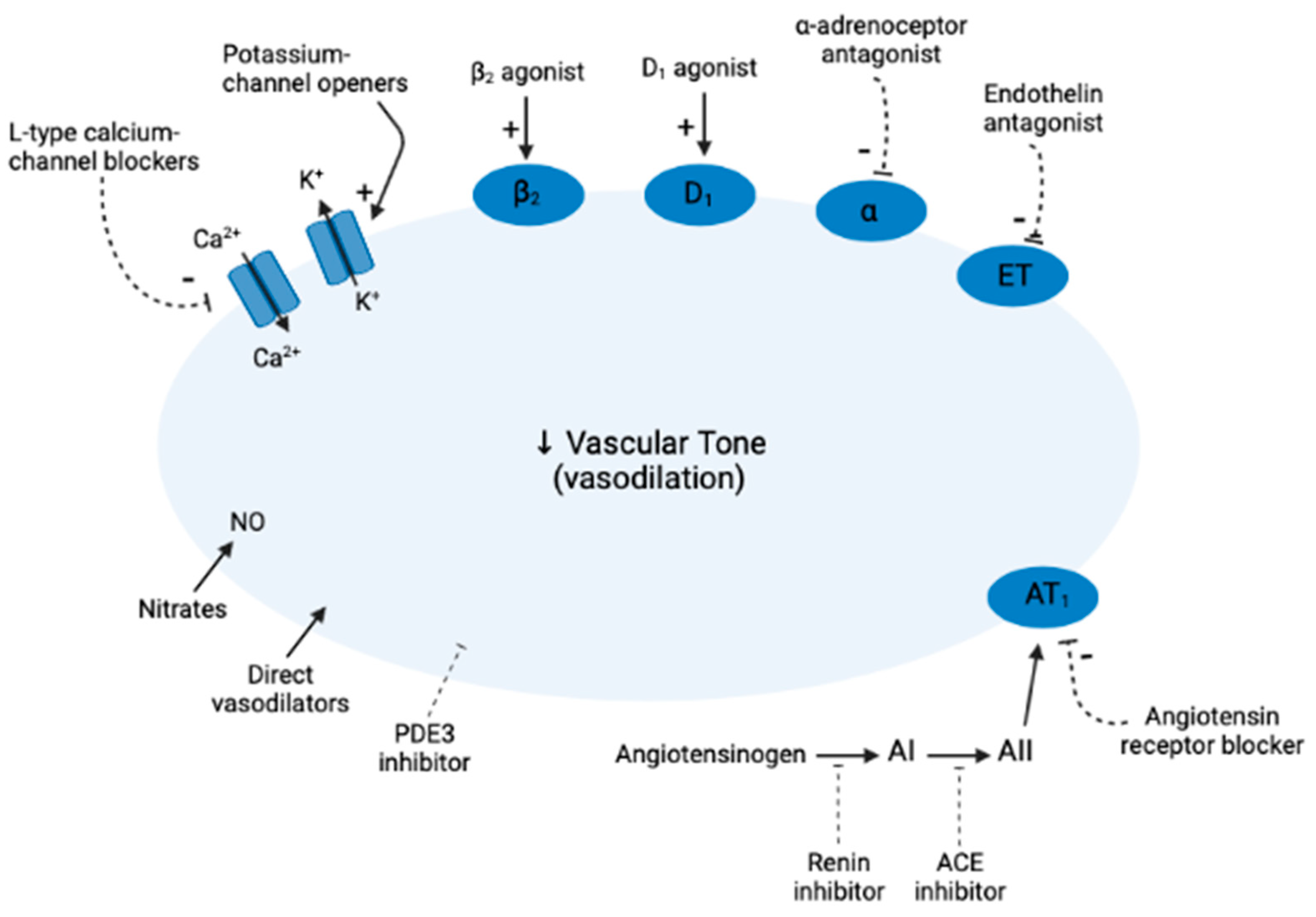
1. Introduction
2. Angiotensin-Converting Enzyme (ACE)
2.1. Enalapril
2.2. Captopril
2.3. Perindopril
2.4. Trandolapril
3. Angiotensin Receptor Blockers (ARBs)
3.1. Azilsartan
3.2. Candesartan
3.3. Irbesartan
3.4. Losartan
3.5. Olmesartan
3.6. Telmisartan
3.7. Valsartan
4. Calcium Channel Blockers (CCBs)
4.1. Amlodipine
4.2. Nicardipine
4.3. Felodipine
4.4. Nifedipine
4.5. Diltiazem
4.6. Verapamil
5. Direct Vasodilators
5.1. Hydralazine
5.2. Minoxidil
6. Nitrates
6.1. Nitroglycerin
6.2. Isosorbide Mononitrate
6.3. Sodium Nitroprusside (SNP)
7. Phosphodiesterase 5 (PDE5) Inhibitors
7.1. Sildenafil
7.2. Tadalafil
8. Calcium Sensitizers
Levosimendan
9. Critical Appraisal of the Use of Vasodilators in Cancer in the Future
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oparil, S.; Aronson, S.; Deeb, G.M.; Epstein, M.; Levy, J.H.; Luther, R.R.; Prielipp, R.; Taylor, A. Fenoldopam: A new parenteral antihypertensive: Consensus roundtable on the management of perioperative hypertension and hypertensive crises. Am. J. Hypertens. 1999, 12, 653–664. [Google Scholar] [CrossRef]
- Mac Carthy, E.P. Vasodilator Therapy in Hypertension. Cardiovasc. Drug Rev. 1990, 8, 155–171. [Google Scholar] [CrossRef]
- Na Takuathung, M.; Sakuludomkan, W.; Khatsri, R.; Dukaew, N.; Kraivisitkul, N.; Ahmadmusa, B.; Mahakkanukrauh, C.; Wangthaweesap, K.; Onin, J.; Srichai, S.; et al. Adverse Effects of Angiotensin-Converting Enzyme Inhibitors in Humans: A Systematic Review and Meta-Analysis of 378 Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2022, 19, 8373. [Google Scholar] [CrossRef] [PubMed]
- McChord, J.; Hubert, A.; Bekeredjian, R.; Ong, P. Contemporary pharmacological treatment strategies for patients with angina and unobstructed coronary arteries (ANOCA) due to coronary microvascular dysfunction. Vessel Plus 2021, 5, 49. [Google Scholar] [CrossRef]
- Brown, N.J.; Vaughan, D.E. Angiotensin-Converting Enzyme Inhibitors. Circulation 1998, 97, 1411–1420. [Google Scholar] [CrossRef]
- Tsoi, B.; Akioyamen, L.E.; Bonner, A.; Frankfurter, C.; Levine, M.; Pullenayegum, E.; Goeree, R.; O’Reilly, D. Comparative Efficacy of Angiotensin II Antagonists in Essential Hypertension: Systematic Review and Network Meta-Analysis of Randomised Controlled Trials. Heart Lung Circ. 2018, 27, 666–682. [Google Scholar] [CrossRef]
- Elliott, W.J.; Ram, C.V.S. Calcium Channel Blockers. J. Clin. Hypertens. 2011, 13, 687–689. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.M. Pharmacology and mechanisms of action of calcium-channel blockers. J. Clin. Hypertens. 1986, 2, 28S–37S. [Google Scholar]
- Frishman, W.H. Calcium channel blockers: Differences between subclasses. Am. J. Cardiovasc. Drugs 2007, 7 (Suppl. S1), 17–23. [Google Scholar] [CrossRef]
- Pavasini, R.; Camici, P.G.; Crea, F.; Danchin, N.; Fox, K.; Manolis, A.J.; Marzilli, M.; Rosano, G.M.C.; Lopez-Sendon, J.L.; Pinto, F.; et al. Anti-anginal drugs: Systematic review and clinical implications. Int. J. Cardiol. 2019, 283, 55–63. [Google Scholar] [CrossRef]
- Lin, Y.; Ma, L. Blood pressure lowering effect of calcium channel blockers on perioperative hypertension: A systematic review and meta-analysis. Medicine 2018, 97, e13152. [Google Scholar] [CrossRef] [PubMed]
- Sica, D.A.; Gehr, T.W.B. Direct Vasodilators and their Role in Hypertension Management: Minoxidil. J. Clin. Hypertens. 2001, 3, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Palmer, R.M.; Ferrige, A.G.; Moncada, S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987, 327, 524–526. [Google Scholar] [CrossRef]
- Tarkin, J.M.; Kaski, J.C. Nicorandil and Long-acting Nitrates: Vasodilator Therapies for the Management of Chronic Stable Angina Pectoris. Eur. Cardiol. Rev. 2018, 13, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Fung, H.L.; Chung, S.J.; Bauer, J.A.; Chong, S.; Kowaluk, E.A. Biochemical mechanism of organic nitrate action. Am. J. Cardiol. 1992, 70, B4–B10. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; McDonald, K.M.; Hastie, T.; Fadel, B.; Hagan, V.; Lee, B.K.; Hlatky, M.A. Meta-analysis of trials comparing beta-blockers, calcium antagonists, and nitrates for stable angina. JAMA 1999, 281, 1927–1936. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Feil, R.; Mülsch, A.; Lohmann, S.M.; Hofmann, F.; Walter, U. Physiology and pathophysiology of vascular signaling controlled by guanosine 3′,5′-cyclic monophosphate-dependent protein kinase [corrected]. Circulation 2003, 108, 2172–2183. [Google Scholar] [CrossRef]
- Perrone, S.V.; Kaplinsky, E.J. Calcium sensitizer agents: A new class of inotropic agents in the treatment of decompensated heart failure. Int. J. Cardiol. 2005, 103, 248–255. [Google Scholar] [CrossRef]
- Huang, S.A.; Lie, J.D. Phosphodiesterase-5 (PDE5) Inhibitors In the Management of Erectile Dysfunction. J. Formul. Manag. 2013, 38, 407–419. [Google Scholar]
- Duarte, D.; Rêma, A.; Amorim, I.; Vale, N. Drug Combinations: A New Strategy to Extend Drug Repurposing and Epithelial-Mesenchymal Transition in Breast and Colon Cancer Cells. Biomolecules 2022, 12, 190. [Google Scholar] [CrossRef]
- Sahragardjoonegani, B.; Beall, R.F.; Kesselheim, A.S.; Hollis, A. Repurposing existing drugs for new uses: A cohort study of the frequency of FDA-granted new indication exclusivities since 1997. J. Pharm. Policy Pract. 2021, 14, 3. [Google Scholar] [CrossRef]
- Khataniar, A.; Pathak, U.; Rajkhowa, S.; Jha, A.N. A Comprehensive Review of Drug Repurposing Strategies against Known Drug Targets of COVID-19. COVID 2022, 2, 148–167. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, L.; Xie, N.; Nice, E.C.; Zhang, T.; Cui, Y.; Huang, C. Overcoming cancer therapeutic bottleneck by drug repurposing. Signal Transduct. Target. Ther. 2020, 5, 113. [Google Scholar] [CrossRef]
- Alqahtani, S. In silico ADME-Tox modeling: Progress and prospects. Expert Opin. Drug Metab. Toxicol. 2017, 13, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Jose, J.; Mathew, B.; Parambi, D.G.T. In Silico Trial Approach for Biomedical Products: A Regulatory Perspective. Comb. Chem. High Throughput Screen. 2022, 25, 1991–2000. [Google Scholar] [CrossRef]
- Todd, P.A.; Heel, R.C. Enalapril. Drugs 1986, 31, 198–248. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, H.; Nielsen, D.; Jensen, B.V.; Eriksen, J.; Skovsgaard, T. Angiotensin converting enzyme inhibitors for cancer treatment? Acta Oncol. 2004, 43, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J. G1 cell-cycle control and cancer. Nature 2004, 432, 298–306. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, L.; Xu, Y.; Liu, Y.; Li, W.; Cai, J.; Zhang, Y. Enalapril overcomes chemoresistance and potentiates antitumor efficacy of 5-FU in colorectal cancer by suppressing proliferation, angiogenesis, and NF-κB/STAT3-regulated proteins. Cell Death Dis. 2020, 11, 477. [Google Scholar] [CrossRef]
- Mostafapour, A.; Baharara, J.; Khazaeİ, M.; Avan, A.; HassanİAn, S.M. The Angiotensin-Converting Enzyme Inhibitor ‘Enalapril’ Increases the Anti-Proliferative Activity of 5-Fluorouracil in Colorectal Cancer Cells. Eurasian J. Med. Oncol. 2021, 5, 318–326. [Google Scholar] [CrossRef]
- Bowman, T.; Garcia, R.; Turkson, J.; Jove, R. STATs in oncogenesis. Oncogene 2000, 19, 2474–2488. [Google Scholar] [CrossRef]
- Purclutepe, O.; Iskender, G.; Kiper, H.D.; Tezcanli, B.; Se-lvi, N.; Avci, C.B.; Kosova, B.; Gokbulut, A.A.; Sahin, F.; Baran, Y.; et al. Enalapril-induced apoptosis of acute promyelocytic leukaemia cells involves STAT5A. Anticancer Res. 2012, 32, 2885–2893. [Google Scholar]
- Small, W., Jr.; Molteni, A.; Kim, Y.T.; Taylor, J.M.; Ts’ao, C.H.; Ward, W.F. Mechanism of captopril toxicity to a human mammary ductal carcinoma cell line in the presence of copper. Breast Cancer Res. Treat. 1999, 55, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Bauer, H.; Lametschwandtner, A.; Steiner, M.; Bauer, H.C. Influence of angiotensin converting enzyme inhibitor (captopril) on kidney epithelial cells in vitro: Studies on potassium (86Rb) influx and cellular proliferation. Clin. Chim. Acta 1990, 187, 47–53. [Google Scholar] [CrossRef]
- Chen, L.; Re, R.N.; Prakash, O.; Mondal, D. Angiotensin-converting enzyme inhibition reduces neuroblastoma cell growth rate. Proc. Soc. Exp. Biol. Med. 1991, 196, 280–283. [Google Scholar] [CrossRef]
- Nguyen, L.; Ward, W.F.; Ts’ao, C.H.; Molteni, A. Captopril inhibits proliferation of human lung fibroblasts in culture: A potential antifibrotic mechanism. Proc. Soc. Exp. Biol. Med. 1994, 205, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.K.; Baskaran, K.; Molteni, A. Inhibitors of Angiotensin-Converting Enzyme Modulate Mitosis and Gene Expression in Pancreatic Cancer Cells. Proc. Soc. Exp. Biol. Med. 1995, 210, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Shebl, R.I. Anti-cancer Potential of Captopril and Botulinum Toxin Type-A and Associated p53 Gene Apototic Stimulating Activity. Iran. J. Pharm. Res. 2019, 18, 1967–1977. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wang, X.; Lu, J.; Salfenmoser, M.; Wirsik, N.M.; Schleussner, N.; Imle, A.; Freire Valls, A.; Radhakrishnan, P.; Liang, J.; et al. Reduction of Liver Metastasis Stiffness Improves Response to Bevacizumab in Metastatic Colorectal Cancer. Cancer Cell 2020, 37, 800–817.e7. [Google Scholar] [CrossRef]
- Riddiough, G.E.; Fifis, T.; Walsh, K.A.; Muralidharan, V.; Christophi, C.; Tran, B.M.; Vincan, E.; Perini, M.V. Captopril, a Renin-Angiotensin System Inhibitor, Attenuates Features of Tumor Invasion and Down-Regulates C-Myc Expression in a Mouse Model of Colorectal Cancer Liver Metastasis. Cancers 2021, 13, 2734. [Google Scholar] [CrossRef]
- Chen, X.; Jia, F.; Huang, Y.; Jin, Q.; Ji, J. Cancer-Associated Fibroblast-Targeted Delivery of Captopril to Overcome Penetration Obstacles for Enhanced Pancreatic Cancer Therapy. ACS Appl. Bio. Mater. 2022, 5, 3544–3553. [Google Scholar] [CrossRef]
- Pinheiro, L.; Perdomo-Pantoja, A.; Casaos, J.; Huq, S.; Paldor, I.; Vigilar, V.; Mangraviti, A.; Wang, Y.; Witham, T.F.; Brem, H.; et al. Captopril inhibits Matrix Metalloproteinase-2 and extends survival as a temozolomide adjuvant in an intracranial gliosarcoma model. Clin. Neurol. Neurosurg. 2021, 207, 106771. [Google Scholar] [CrossRef]
- Tan, X.; He, W.; Liu, Y. Combination therapy with paricalcitol and trandolapril reduces renal fibrosis in obstructive nephropathy. Kidney Int. 2009, 76, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Sarfati, M.; Mateo, V.; Baudet, S.; Rubio, M.; Fernandez, C.; Davi, F.; Binet, J.L.; Delic, J.; Merle-Beral, H. Sildenafil and vardenafil, types 5 and 6 phosphodiesterase inhibitors, induce caspase-dependent apoptosis of B-chronic lymphocytic leukemia cells. Blood 2003, 101, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Black, K.L.; Yin, D.; Ong, J.M.; Hu, J.; Konda, B.M.; Wang, X.; Ko, M.K.; Bayan, J.-A.; Sacapano, M.R.; Espinoza, A.; et al. PDE5 inhibitors enhance tumor permeability and efficacy of chemotherapy in a rat brain tumor model. Brain Res. 2008, 1230, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Durrant, D.; Mitchell, C.; Mayton, E.; Hoke, N.N.; Salloum, F.N.; Park, M.A.; Qureshi, I.; Lee, R.; Dent, P.; et al. Sildenafil increases chemotherapeutic efficacy of doxorubicin in prostate cancer and ameliorates cardiac dysfunction. Proc. Natl. Acad. Sci. USA 2010, 107, 18202–18207. [Google Scholar] [CrossRef]
- Di, X.; Gennings, C.; Bear, H.D.; Graham, L.J.; Sheth, C.M.; White, K.L., Jr.; Gewirtz, D.A. Influence of the phosphodiesterase-5 inhibitor, sildenafil, on sensitivity to chemotherapy in breast tumor cells. Breast Cancer Res. Treat. 2010, 124, 349–360. [Google Scholar] [CrossRef]
- Marques, J.G.; Gaspar, V.M.; Markl, D.; Costa, E.C.; Gallardo, E.; Correia, I.J. Co-delivery of Sildenafil (Viagra®) and Crizotinib for synergistic and improved anti-tumoral therapy. Pharm. Res. 2014, 31, 2516–2528. [Google Scholar] [CrossRef]
- de Melo-Diogo, D.; Gaspar, V.M.; Costa, E.C.; Moreira, A.F.; Oppolzer, D.; Gallardo, E.; Correia, I.J. Combinatorial delivery of Crizotinib-Palbociclib-Sildenafil using TPGS-PLA micelles for improved cancer treatment. Eur. J. Pharm. Biopharm. 2014, 88, 718–729. [Google Scholar] [CrossRef]
- Booth, L.; Roberts, J.L.; Cruickshanks, N.; Conley, A.; Durrant, D.E.; Das, A.; Fisher, P.B.; Kukreja, R.C.; Grant, S.; Poklepovic, A.; et al. Phosphodiesterase 5 inhibitors enhance chemotherapy killing in gastrointestinal/genitourinary cancer cells. Mol. Pharm. 2014, 85, 408–419. [Google Scholar] [CrossRef]
- Roberts, J.L.; Poklepovic, A.; Booth, L. Curcumin interacts with sildenafil to kill GI tumor cells via endoplasmic reticulum stress and reactive oxygen/ nitrogen species. Oncotarget 2017, 8, 99451–99469. [Google Scholar] [CrossRef]
- Mei, X.L.; Yang, Y.; Zhang, Y.J.; Li, Y.; Zhao, J.M.; Qiu, J.G.; Zhang, W.J.; Jiang, Q.W.; Xue, Y.Q.; Zheng, D.W.; et al. Sildenafil inhibits the growth of human colorectal cancer in vitro and in vivo. Am. J. Cancer Res. 2015, 5, 3311–3324. [Google Scholar]
- Liu, P.; Pei, J.J.; Li, L.; Li, J.W.; Ke, X.P. Sildenafil Inhibits the Growth and Epithelial-to-mesenchymal Transition of Cervical Cancer via the TGF-β1/Smad2/3 Pathway. Curr. Cancer Drug Targets 2023, 23, 145–158. [Google Scholar] [CrossRef] [PubMed]
- AboYoussef, A.M.; Khalaf, M.M.; Malak, M.N.; Hamzawy, M.A. Repurposing of sildenafil as antitumour; induction of cyclic guanosine monophosphate/protein kinase G pathway, caspase-dependent apoptosis and pivotal reduction of Nuclear factor kappa light chain enhancer of activated B cells in lung cancer. J. Pharm. Pharm. 2021, 73, 1080–1091. [Google Scholar] [CrossRef] [PubMed]
- Urla, C.; Stagno, M.J.; Fuchs, J.; Warmann, S.W.; Schmid, E. Combination therapy of doxorubicin and Sildenafil inhibits the growth of pediatric rhabdomyosarcoma. J. Cancer Res. Clin. Oncol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, Y.; Zhang, W.; Li, C.; Zhu, Y.; Wang, S. Tadalafil Reverses the Effect of Three-Dimensional Cell Culture System on Stem Cell Features in A549 and SK-MES-1. DNA Cell Biol. 2021, 40, 869–880. [Google Scholar] [CrossRef]
- Coward, R.M.; Carson, C.C. Tadalafil in the treatment of erectile dysfunction. Ther. Clin. Risk Manag. 2008, 4, 1315–1330. [Google Scholar] [CrossRef] [PubMed]
- Kumazoe, M.; Kim, Y.; Bae, J.; Takai, M.; Murata, M.; Suemasu, Y.; Sugihara, K.; Yamashita, S.; Tsukamoto, S.; Huang, Y.; et al. Phosphodiesterase 5 inhibitor acts as a potent agent sensitizing acute myeloid leukemia cells to 67-kDa laminin receptor-dependent apoptosis. FEBS Lett. 2013, 587, 3052–3057. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Mei, L.; Fan, X.; Tang, C.; Ji, X.; Hu, X.; Shi, W.; Qian, Y.; Hussain, M.; Wu, J.; et al. Phosphodiesterase 5/protein kinase G signal governs stemness of prostate cancer stem cells through Hippo pathway. Cancer Lett. 2016, 378, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, E.; Khosroushahi, A.Y.; Eftekhari, A.; Farajnia, S.; Babaei, H.; Eghbal, M.A. Novel angiotensin receptor blocker, azilsartan induces oxidative stress and NFkB-mediated apoptosis in hepatocellular carcinoma cell line HepG2. Biomed. Pharmacother. 2018, 99, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Martínez, V.R.; Aguirre, M.V.; Todaro, J.S.; Piro, O.E.; Echeverría, G.A.; Ferrer, E.G.; Williams, P.A.M. Azilsartan and its Zn(II) complex. Synthesis, anticancer mechanisms of action and binding to bovine serum albumin. Toxicol. Vitr. 2018, 48, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Alaaeldin, R.; Ali, F.E.M.; Bekhit, A.A.; Zhao, Q.L.; Fathy, M. Inhibition of NF-kB/IL-6/JAK2/STAT3 Pathway and Epithelial-Mesenchymal Transition in Breast Cancer Cells by Azilsartan. Molecules 2022, 27, 7825. [Google Scholar] [CrossRef] [PubMed]
- Tabatabai, E.; Khazaei, M.; Asgharzadeh, F.; Nazari, S.E.; Shakour, N.; Fiuji, H.; Ziaeemehr, A.; Mostafapour, A.; Parizadeh, M.R.; Nouri, M.; et al. Inhibition of angiotensin II type 1 receptor by candesartan reduces tumor growth and ameliorates fibrosis in colorectal cancer. Excli. J. 2021, 20, 863–878. [Google Scholar] [CrossRef]
- Tong, Y.; Liu, Y.; Zheng, H.; Zheng, L.; Liu, W.; Wu, J.; Ou, R.; Zhang, G.; Li, F.; Hu, M.; et al. Artemisinin and its derivatives can significantly inhibit lung tumorigenesis and tumor metastasis through Wnt/β-catenin signaling. Oncotarget 2016, 7, 31413–31428. [Google Scholar] [CrossRef]
- Chen, T.; Chen, Z.; Lian, X.; Wu, W.; Chu, L.; Zhang, S.; Wang, L. MUC 15 Promotes Osteosarcoma Cell Proliferation, Migration and Invasion through Livin, MMP-2/MMP-9 and Wnt/β-Catenin Signal Pathway. J. Cancer 2021, 12, 467–473. [Google Scholar] [CrossRef]
- Rasheduzzaman, M.; Park, S.Y. Antihypertensive drug-candesartan attenuates TRAIL resistance in human lung cancer via AMPK-mediated inhibition of autophagy flux. Exp. Cell Res. 2018, 368, 126–135. [Google Scholar] [CrossRef]
- Cai, X.J.; Wang, Z.; Xu, Y.Y.; Yang, G.Y.; Zhang, R.Y.; Wang, Y. Candesartan treatment enhances liposome penetration and anti-tumor effect via depletion of tumor stroma and normalization of tumor vessel. Drug Deliv. Transl. Res. 2021, 11, 1186–1197. [Google Scholar] [CrossRef] [PubMed]
- Hachiya, K.; Masuya, M.; Kuroda, N.; Yoneda, M.; Tsuboi, J.; Nagaharu, K.; Nishimura, K.; Shiotani, T.; Ohishi, K.; Tawara, I.; et al. Irbesartan, an angiotensin II type 1 receptor blocker, inhibits colitis-associated tumourigenesis by blocking the MCP-1/CCR2 pathway. Sci. Rep. 2021, 11, 19943. [Google Scholar] [CrossRef]
- Feng, L.H.; Sun, H.C.; Zhu, X.D.; Zhang, S.Z.; Li, X.L.; Li, K.S.; Liu, X.F.; Lei, M.; Li, Y.; Tang, Z.Y. Irbesartan inhibits metastasis by interrupting the adherence of tumor cell to endothelial cell induced by angiotensin II in hepatocellular carcinoma. Ann. Transl. Med. 2021, 9, 207. [Google Scholar] [CrossRef]
- Du, N.; Feng, J.; Hu, L.J.; Sun, X.; Sun, H.B.; Zhao, Y.; Yang, Y.P.; Ren, H. Angiotensin II receptor type 1 blockers suppress the cell proliferation effects of angiotensin II in breast cancer cells by inhibiting AT1R signaling. Oncol. Rep. 2012, 27, 1893–1903. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, X.; Cai, L.; Yang, Y.; Sui, G.; Wu, J. Angiotensin II suppresses adriamycin-induced apoptosis through activation of phosphatidylinositol 3-kinase/Akt signaling in human breast cancer cells. Acta Biochim. Biophys. Sin. 2008, 40, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Hashemzehi, M.; Rahmani, F.; Khoshakhlagh, M.; Avan, A.; Asgharzadeh, F.; Barneh, F.; Moradi-Marjaneh, R.; Soleimani, A.; Fiuji, H.; Ferns, G.A.; et al. Angiotensin receptor blocker Losartan inhibits tumor growth of colorectal cancer. Excli. J. 2021, 20, 506–521. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Kaji, K.; Nishimura, N.; Ishida, K.; Ogawa, H.; Takaya, H.; Kawaratani, H.; Moriya, K.; Namisaki, T.; Akahane, T.; et al. The Angiotensin II Receptor Blocker Losartan Sensitizes Human Liver Cancer Cells to Lenvatinib-Mediated Cytostatic and Angiostatic Effects. Cells 2021, 10, 575. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Wang, Z.; Li, Z.; Zhang, A.; Jin, Y.; Chen, H.; Le, X. Angiotensin II Enhances Proliferation and Inflammation through AT1/PKC/NF-κB Signaling Pathway in Hepatocellular Carcinoma Cells. Cell Physiol. Biochem. 2016, 39, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Saber, S.; Mahmoud, A.A.A.; Goda, R.; Helal, N.S.; El-Ahwany, E.; Abdelghany, R.H. Perindopril, fosinopril and losartan inhibited the progression of diethylnitrosamine-induced hepatocellular carcinoma in mice via the inactivation of nuclear transcription factor kappa-B. Toxicol. Lett. 2018, 295, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; He, X.; Qin, X.; Liu, Y.; Jiang, H.; Wang, J.; Wu, S.; Zhou, R.; Yu, C.; Liu, S.; et al. Enhanced Therapeutic Efficacy of Combining Losartan and Chemo-Immunotherapy for Triple Negative Breast Cancer. Front. Immunol. 2022, 13, 938439. [Google Scholar] [CrossRef]
- Li, W.; Li, S.; Chen, I.X.; Liu, Y.; Ramjiawan, R.R.; Leung, C.H.; Gerweck, L.E.; Fukumura, D.; Loeffler, J.S.; Jain, R.K.; et al. Combining losartan with radiotherapy increases tumor control and inhibits lung metastases from a HER2/neu-positive orthotopic breast cancer model. Radiat. Oncol. 2021, 16, 48. [Google Scholar] [CrossRef]
- Zhao, Y.; Cao, J.; Melamed, A.; Worley, M.; Gockley, A.; Jones, D.; Nia, H.T.; Zhang, Y.; Stylianopoulos, T.; Kumar, A.S.; et al. Losartan treatment enhances chemotherapy efficacy and reduces ascites in ovarian cancer models by normalizing the tumor stroma. Proc. Natl. Acad. Sci. USA 2019, 116, 2210–2219. [Google Scholar] [CrossRef]
- Kurikawa, N.; Suga, M.; Kuroda, S.; Yamada, K.; Ishikawa, H. An angiotensin II type 1 receptor antagonist, olmesartan medoxomil, improves experimental liver fibrosis by suppression of proliferation and collagen synthesis in activated hepatic stellate cells. Br. J. Pharm. 2003, 139, 1085–1094. [Google Scholar] [CrossRef]
- Masamune, A.; Hamada, S.; Kikuta, K.; Takikawa, T.; Miura, S.; Nakano, E.; Shimosegawa, T. The angiotensin II type I receptor blocker olmesartan inhibits the growth of pancreatic cancer by targeting stellate cell activities in mice. Scand. J. Gastroenterol. 2013, 48, 602–609. [Google Scholar] [CrossRef]
- Bakhtiari, E.; Hosseini, A.; Boroushaki, M.T.; Mousavi, S.H. Angiotensin II receptor antagonist olmesartan and NF-kappaB inhibitor as cytotoxic and apoptotic agents in MCF-7 human cell line. J. Chemother. 2016, 28, 314–320. [Google Scholar] [CrossRef]
- Mousavi, S.H.; Hersey, P. Role of Caspases and Reactive Oxygen Species in Rose Bengal-Induced Toxicity in Melanoma Cells. Iran. J. Basic Med. Sci. 2007, 10, 118–123. [Google Scholar] [CrossRef]
- Deavall, D.G.; Martin, E.A.; Horner, J.M.; Roberts, R. Drug-induced oxidative stress and toxicity. J. Toxicol. 2012, 2012, 645460. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiari, E.; Hosseini, A.; Mousavi, S.H. The role of ROS and NF-κB pathway in olmesartan induced-toxicity in HeLa and mcf-7 cell lines. Biomed. Pharmacother. 2017, 93, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Khorsand, M.; Mostafavi-Pour, Z.; Razban, V.; Khajeh, S.; Zare, R. Combinatorial effects of telmisartan and docetaxel on cell viability and metastatic gene expression in human prostate and breast cancer cells. Mol. Biol. Res. Commun. 2022, 11, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Kobara, H.; Fujihara, S.; Iwama, H.; Matsui, T.; Fujimori, A.; Chiyo, T.; Tingting, S.; Kobayashi, N.; Nishiyama, N.; Yachida, T.; et al. Antihypertensive drug telmisartan inhibits cell proliferation of gastrointestinal stromal tumor cells in vitro. Mol. Med. Rep. 2020, 22, 1063–1071. [Google Scholar] [CrossRef]
- Koyama, N.; Nishida, Y.; Ishii, T.; Yoshida, T.; Furukawa, Y.; Narahara, H. Telmisartan induces growth inhibition, DNA double-strand breaks and apoptosis in human endometrial cancer cells. PLoS ONE 2014, 9, e93050. [Google Scholar] [CrossRef]
- Funao, K.; Matsuyama, M.; Kawahito, Y.; Sano, H.; Chargui, J.; Touraine, J.L.; Nakatani, T.; Yoshimura, R. Telmisartan as a peroxisome proliferator-activated receptor-γ ligand is a new target in the treatment of human renal cell carcinoma. Mol. Med. Rep. 2009, 2, 193–198. [Google Scholar] [CrossRef]
- Samukawa, E.; Fujihara, S.; Oura, K.; Iwama, H.; Yamana, Y.; Tadokoro, T.; Chiyo, T.; Kobayashi, K.; Morishita, A.; Nakahara, M.; et al. Angiotensin receptor blocker telmisartan inhibits cell proliferation and tumor growth of cholangiocarcinoma through cell cycle arrest. Int. J. Oncol. 2017, 51, 1674–1684. [Google Scholar] [CrossRef]
- Matsui, T.; Chiyo, T.; Kobara, H.; Fujihara, S.; Fujita, K.; Namima, D.; Nakahara, M.; Kobayashi, N.; Nishiyama, N.; Yachida, T.; et al. Telmisartan Inhibits Cell Proliferation and Tumor Growth of Esophageal Squamous Cell Carcinoma by Inducing S-Phase Arrest In Vitro and In Vivo. Int. J. Mol. Sci. 2019, 20, 3197. [Google Scholar] [CrossRef]
- Khorsand, M.; Khajeh, S.; Eslami, M.; Nezafat, N.; Ghasemi, Y.; Razban, V.; Mostafavi-Pour, Z. Telmisartan anti-cancer activities mechanism through targeting N-cadherin by mimicking ADH-1 function. J. Cell Mol. Med. 2022, 26, 2392–2403. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, T.; Li, C.; Guo, J.; Xu, B.; Xue, L. Telmisartan attenuates human glioblastoma cells proliferation and oncogenicity by inducing the lipid oxidation. Asia Pac. J. Clin. Oncol. 2022, 18, 217–223. [Google Scholar] [CrossRef]
- Mielczarek-Puta, M.; Otto-Ślusarczyk, D.; Chrzanowska, A.; Filipek, A.; Graboń, W. Telmisartan Influences the Antiproliferative Activity of Linoleic Acid in Human Colon Cancer Cells. Nutr. Cancer 2020, 72, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Tsujiya, Y.; Yamamori, M.; Hasegawa, A.I.; Yamamoto, Y.; Yashiro, M.; Okamura, N. Telmisartan Exerts Cytotoxicity in Scirrhous Gastric Cancer Cells by Inducing G0/G1 Cell Cycle Arrest. Anticancer Res. 2021, 41, 5461–5468. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhao, W.; Wu, G. Valsartan inhibits NPC cell line CNE-2 proliferation and invasion and promotes its sensitivity to radiation. Eur. J. Cancer Prev. 2009, 18, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; He, D.; Qiu, Y.; Krawczuk, P.; Sun, X.; Xie, L. Rational discovery of dual-indication multi-target PDE/Kinase inhibitor for precision anti-cancer therapy using structural systems pharmacology. PLoS Comput. Biol. 2019, 15, e1006619. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.E.; D’Aloisio, A.A.; Sandler, D.P.; Taylor, J.A. Long-term use of calcium channel blocking drugs and breast cancer risk in a prospective cohort of US and Puerto Rican women. Breast Cancer Res. 2016, 18, 61. [Google Scholar] [CrossRef]
- Lee, A.R.; Seo, M.J.; Kim, J.; Lee, D.M.; Kim, I.Y.; Yoon, M.J.; Hoon, H.; Choi, K.S. Lercanidipine Synergistically Enhances Bortezomib Cytotoxicity in Cancer Cells via Enhanced Endoplasmic Reticulum Stress and Mitochondrial Ca2+ Overload. Int. J. Mol. Sci. 2019, 20, 6112. [Google Scholar] [CrossRef]
- Alqudah, M.A.Y.; Al-Samman, R.; Azaizeh, M.; Alzoubi, K.H. Amlodipine inhibits proliferation, invasion, and colony formation of breast cancer cells. Biomed. Rep. 2022, 16, 50. [Google Scholar] [CrossRef]
- Fu, B.; Dou, X.; Zou, M.; Lu, H.; Wang, K.; Liu, Q.; Liu, Y.; Wang, W.; Jin, M.; Kong, D. Anticancer Effects of Amlodipine Alone or in Combination With Gefitinib in Non-Small Cell Lung Cancer. Front. Pharmacol. 2022, 13, 902305. [Google Scholar] [CrossRef]
- Yoshida, J.; Ishibashi, T.; Nishio, M. G1 cell cycle arrest by amlodipine, a dihydropyridine Ca2+ channel blocker, in human epidermoid carcinoma A431 cells. Biochem. Pharm. 2007, 73, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Shiozaki, A.; Katsurahara, K.; Kudou, M.; Shimizu, H.; Kosuga, T.; Ito, H.; Arita, T.; Konishi, H.; Komatsu, S.; Kubota, T.; et al. Amlodipine and Verapamil, Voltage-Gated Ca2+ Channel Inhibitors, Suppressed the Growth of Gastric Cancer Stem Cells. Ann. Surg. Oncol. 2021, 28, 5400–5411. [Google Scholar] [CrossRef] [PubMed]
- Panneerpandian, P.; Rao, D.B.; Ganesan, K. Calcium channel blockers lercanidipine and amlodipine inhibit YY1/ERK/TGF-β mediated transcription and sensitize the gastric cancer cells to doxorubicin. Toxicol. Vitr. 2021, 74, 105152. [Google Scholar] [CrossRef]
- Alandağ, C.; Karaman, E.; Yüce, E. Amlodipine improves the outcomes of regorafenib in metastatic colorectal cancer. Anticancer Drugs 2022, 33, 389–393. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chen, J.-H.; Tsai, C.-F.; Wu, C.-T.; Wu, M.-H.; Chang, P.-C.; Yeh, W.-L. Nicardipine Inhibits Breast Cancer Migration via Nrf2/HO-1 Axis and Matrix Metalloproteinase-9 Regulation. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Arisawa, C.; Kageyama, Y.; Kawakami, S.; Kihara, K. TNP-470 combined with nicardipine suppresses in vivo growth of PC-3, a human prostate cancer cell line. Urol. Oncol. 2002, 7, 229–234. [Google Scholar] [CrossRef]
- Shi, J.; Dong, X.; Li, H.; Wang, H.; Jiang, Q.; Liu, L.; Wang, L.; Dong, J. Nicardipine sensitizes temozolomide by inhibiting autophagy and promoting cell apoptosis in glioma stem cells. Aging 2021, 13, 6820–6831. [Google Scholar] [CrossRef]
- Braconi, C.; Swenson, E.; Kogure, T.; Huang, N.; Patel, T. Targeting the IL-6 dependent phenotype can identify novel therapies for cholangiocarcinoma. PLoS ONE 2010, 5, e15195. [Google Scholar] [CrossRef]
- Jacquemet, G.; Baghirov, H.; Georgiadou, M.; Sihto, H.; Peuhu, E.; Cettour-Janet, P.; He, T.; Perälä, M.; Kronqvist, P.; Joensuu, H.; et al. L-type calcium channels regulate filopodia stability and cancer cell invasion downstream of integrin signalling. Nat. Commun. 2016, 7, 13297. [Google Scholar] [CrossRef]
- Arjonen, A.; Kaukonen, R.; Ivaska, J. Filopodia and adhesion in cancer cell motility. Cell Adh. Migr. 2011, 5, 421–430. [Google Scholar] [CrossRef]
- Wu, L.; Lin, W.; Liao, Q.; Wang, H.; Lin, C.; Tang, L.; Lian, W.; Chen, Z.; Li, K.; Xu, L.; et al. Calcium Channel Blocker Nifedipine Suppresses Colorectal Cancer Progression and Immune Escape by Preventing NFAT2 Nuclear Translocation. Cell Rep. 2020, 33, 108327. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Sayeed, M.M.; Wurster, R.D. Inhibition of cell growth and intracellular Ca2+ mobilization in human brain tumor cells by Ca2+ channel antagonists. Mol. Chem. Neuropathol. 1994, 22, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Chovancova, B.; Liskova, V.; Miklikova, S.; Hudecova, S.; Babula, P.; Penesova, A.; Sevcikova, A.; Durinikova, E.; Novakova, M.; Matuskova, M.; et al. Calcium signaling affects migration and proliferation differently in individual cancer cells due to nifedipine treatment. Biochem. Pharm. 2020, 171, 113695. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Yin, D.; Morimura, T.; Kubo, H.; Nakatsu, S.; Takeuchi, J. Combination therapy with cisplatin and nifedipine induces apoptosis in cisplatin-sensitive and cisplatin-resistant human glioblastoma cells. Br. J. Cancer 1995, 71, 282–289. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Wu, C.-T.; Chen, J.-H.; Tsai, C.-F.; Wu, C.-Y.; Chang, P.-C.; Yeh, W.-L. Diltiazem inhibits breast cancer metastasis via mediating growth differentiation factor 15 and epithelial-mesenchymal transition. Oncogenesis 2022, 11, 48. [Google Scholar] [CrossRef]
- Al-malky, H.S.; Damanhouri, Z.A.; Aama, J.Y.A.; Qahtani, A.A.A.; Ramadan, W.S.; AlKreathy, H.M.; Harthi, S.E.A.; Osman, A.-M.M. Diltiazem potentiation of doxorubicin cytotoxicity and cellular uptake in human breast cancer cells. Breast Cancer Manag. 2019, 8, BMT31. [Google Scholar] [CrossRef]
- Wong, B.S.; Chiu, L.Y.; Tu, D.G.; Sheu, G.T.; Chan, T.T. Anticancer Effects of Antihypertensive L-Type Calcium Channel Blockers on Chemoresistant Lung Cancer Cells via Autophagy and Apoptosis. Cancer Manag. Res. 2020, 12, 1913–1927. [Google Scholar] [CrossRef]
- Broxterman, H.J.; Lankelma, J.; Pinedo, H.M. How to probe clinical tumour samples for P-glycoprotein and multidrug resistance-associated protein. Eur. J. Cancer 1996, 32, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Ince, P.; Appleton, D.R.; Finney, K.J.; Sunter, J.P.; Watson, A.J. Verapamil increases the sensitivity of primary human colorectal carcinoma tissue to vincristine. Br. J. Cancer 1986, 53, 137–139. [Google Scholar] [CrossRef]
- Merry, S.; Fetherston, C.A.; Kaye, S.B.; Freshney, R.I.; Plumb, J.A. Resistance of human glioma to adriamycin in vitro: The role of membrane transport and its circumvention with verapamil. Br. J. Cancer 1986, 53, 129–135. [Google Scholar] [CrossRef]
- Morrow, M.; Wait, R.B.; Rosenthal, R.A.; Gamelli, R.L. Verapamil enhances antitumor activity without increasing myeloid toxicity. Surgery 1987, 101, 63–68. [Google Scholar]
- Tsuruo, T.; Iida, H.; Tsukagoshi, S.; Sakurai, Y. Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res. 1981, 41, 1967–1972. [Google Scholar]
- Shchepotin, I.B.; Soldatenkov, V.; Buras, R.R.; Nauta, R.J.; Shabahang, M.; Evans, S.R. Apoptosis of human primary and metastatic colon adenocarcinoma cell lines in vitro induced by 5-fluorouracil, verapamil, and hyperthermia. Anticancer Res. 1994, 14, 1027–1031. [Google Scholar] [PubMed]
- Shchepotin, I.B.; Soldatenkov, V.; Wroblewski, J.T.; Surin, A.; Shabahang, M.; Buras, R.R.; Nauta, R.J.; Pulyaeva, H.; Evans, S.R. Apoptosis induced by hyperthermia and verapamil in vitro in a human colon cancer cell line. Int. J. Hyperth. 1997, 13, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.; Barone, A.; Dziak, R. Effects of Verapamil on Bone Cancer Cells in vitro. J. Cell Biol. Cell Metab. 2016, 3, 13. [Google Scholar] [CrossRef]
- Zeng, S.; Pöttler, M.; Lan, B.; Grützmann, R.; Pilarsky, C.; Yang, H. Chemoresistance in Pancreatic Cancer. Int. J. Mol. Sci. 2019, 20, 4504. [Google Scholar] [CrossRef]
- Miranda, A.; Hamilton, P.T.; Zhang, A.W.; Pattnaik, S.; Becht, E.; Mezheyeuski, A.; Bruun, J.; Micke, P.; de Reynies, A.; Nelson, B.H. Cancer stemness, intratumoral heterogeneity, and immune response across cancers. Proc. Natl. Acad. Sci. USA 2019, 116, 9020–9029. [Google Scholar] [CrossRef]
- Sethy, C.; Kundu, C.N. 5-Fluorouracil (5-FU) resistance and the new strategy to enhance the sensitivity against cancer: Implication of DNA repair inhibition. Biomed. Pharmacother. 2021, 137, 111285. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Tang, J.N.; Xie, H.X.; Du, Y.A.; Huang, L.; Yu, P.F.; Cheng, X.D. 5-Fluorouracil chemotherapy of gastric cancer generates residual cells with properties of cancer stem cells. Int. J. Biol. Sci. 2015, 11, 284–294. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, Y.; Schwarz, B.; Mysliwietz, J.; Hartig, R.; Camaj, P.; Bao, Q.; Jauch, K.W.; Guba, M.; Ellwart, J.W.; et al. Verapamil inhibits tumor progression of chemotherapy-resistant pancreatic cancer side population cells. Int. J. Oncol. 2016, 49, 99–110. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Wang, K.; Gao, M.; Zhang, H.; Xu, X. Metabolomics analysis of multidrug resistance in colorectal cancer cell and multidrug resistance reversal effect of verapamil. Biomed. Chromatogr. 2021, 35, e4976. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, M.B.; Camilo, V.; Lopes, N.; Moreira-Silva, F.; Correia, M.P.; Henrique, R.; Jerónimo, C. Hydralazine and Panobinostat Attenuate Malignant Properties of Prostate Cancer Cell Lines. Pharmaceuticals 2021, 14, 670. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Magaña, M.J.; Martínez-Aguilar, R.; Lucendo, E.; Campillo-Davo, D.; Schulze-Osthoff, K.; Ruiz-Ruiz, C. The antihypertensive drug hydralazine activates the intrinsic pathway of apoptosis and causes DNA damage in leukemic T cells. Oncotarget 2016, 7, 21875–21886. [Google Scholar] [CrossRef] [PubMed]
- Lopes, N.; Pacheco, M.B.; Soares-Fernandes, D.; Correia, M.P.; Camilo, V.; Henrique, R.; Jerónimo, C. Hydralazine and Enzalutamide: Synergistic Partners against Prostate Cancer. Biomedicines 2021, 9, 976. [Google Scholar] [CrossRef] [PubMed]
- Karmacharya, M.B.; Sultan, L.R.; Hunt, S.J.; Sehgal, C.M. Hydralazine augmented ultrasound hyperthermia for the treatment of hepatocellular carcinoma. Sci. Rep. 2021, 11, 15553. [Google Scholar] [CrossRef] [PubMed]
- Fukushiro-Lopes, D.; Hegel, A.D.; Russo, A.; Senyuk, V.; Liotta, M.; Beeson, G.C.; Beeson, C.C.; Burdette, J.; Potkul, R.K.; Gentile, S. Repurposing Kir6/SUR2 Channel Activator Minoxidil to Arrests Growth of Gynecologic Cancers. Front. Pharmacol. 2020, 11, 577. [Google Scholar] [CrossRef]
- Qiu, S.; Fraser, S.P.; Pires, W.; Djamgoz, M.B.A. Anti-invasive effects of minoxidil on human breast cancer cells: Combination with ranolazine. Clin. Exp. Metastasis 2022, 39, 679–689. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Frederiksen, L.J.; Siemens, D.R.; Heaton, J.P.; Maxwell, L.R.; Adams, M.A.; Graham, C.H. Hypoxia induced resistance to doxorubicin in prostate cancer cells is inhibited by low concentrations of glyceryl trinitrate. J. Urol. 2003, 170, 1003–1007. [Google Scholar] [CrossRef]
- Ushmorov, A.; Ratter, F.; Lehmann, V.; Dröge, W.; Schirrmacher, V.; Umansky, V. Nitric-oxide-induced apoptosis in human leukemic lines requires mitochondrial lipid degradation and cytochrome C release. Blood 1999, 93, 2342–2352. [Google Scholar] [CrossRef]
- Nagai, H.; Yasuda, H.; Hatachi, Y.; Xue, D.; Sasaki, T.; Yamaya, M.; Sakamori, Y.; Togashi, Y.; Masago, K.; Ito, I.; et al. Nitric oxide (NO) enhances pemetrexed cytotoxicity via NO-cGMP signaling in lung adenocarcinoma cells in vitro and in vivo. Int. J. Oncol. 2012, 41, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.C.; Chen, J.C.; Tseng, P.Y.; Hsieh, J.M.; Chiang, C.S.; Liu, L.L.; Chien, C.C.; Huang, I.H.; Lin, Y.W. Nitroglycerin-induced downregulation of AKT- and ERK1/2-mediated radiation-sensitive 52 expression to enhance pemetrexed-induced cytotoxicity in human lung cancer cells. Toxicol. Res. 2022, 11, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Diao, Y.; Liu, Y.; Gao, N.; Gao, D.; Wan, Y.; Zhong, J.; Jin, G. Synergistic apoptosis-inducing effect of aspirin and isosorbide mononitrate on human colon cancer cells. Mol. Med. Rep. 2015, 12, 4750–4758. [Google Scholar] [CrossRef] [PubMed]
- Pipili-Synetos, E.; Papageorgiou, A.; Sakkoula, E.; Sotiropoulou, G.; Fotsis, T.; Karakiulakis, G.; Maragoudakis, M.E. Inhibition of angiogenesis, tumour growth and metastasis by the NO-releasing vasodilators, isosorbide mononitrate and dinitrate. Br. J. Pharm. 1995, 116, 1829–1834. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Ling, M.; Yu, K.; Yang, W.; Liu, Q.; He, L.; Cai, X.; Zhong, M.; Mai, Z.; Sun, R.; et al. Synergetic delivery of artesunate and isosorbide 5-mononitrate with reduction-sensitive polymer nanoparticles for ovarian cancer chemotherapy. J. Nanobiotechnol. 2022, 20, 471. [Google Scholar] [CrossRef]
- Yang, L.; Lan, C.; Fang, Y.; Zhang, Y.; Wang, J.; Guo, J.; Wan, S.; Yang, S.; Wang, R.; Fang, D. Sodium nitroprusside (SNP) sensitizes human gastric cancer cells to TRAIL-induced apoptosis. Int. Immunopharmacol. 2013, 17, 383–389. [Google Scholar] [CrossRef]
- Blackburn, R.V.; Galoforo, S.S.; Berns, C.M.; Motwani, N.M.; Corry, P.M.; Lee, Y.J. Differential induction of cell death in human glioma cell lines by sodium nitroprusside. Cancer 1998, 82, 1137–1145. [Google Scholar] [CrossRef]
- Ma, S.C.; Zhang, J.Q.; Yan, T.H.; Miao, M.X.; Cao, Y.M.; Cao, Y.B.; Zhang, L.C.; Li, L. Novel strategies to reverse chemoresistance in colorectal cancer. Cancer Med. 2023, 1–24. [Google Scholar] [CrossRef]
- Cushman, D.W.; Ondetti, M.A. History of the design of captopril and related inhibitors of angiotensin converting enzyme. Hypertension 1991, 17, 589–592. [Google Scholar] [CrossRef]
- Burris, J.F. The expanding role of angiotensin converting enzyme inhibitors in the management of hypertension. J. Clin. Pharmacol. 1995, 35, 337–342. [Google Scholar] [CrossRef]
- Garg, R.; Yusuf, S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Collaborative Group on ACE Inhibitor Trials. JAMA 1995, 273, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Prisant, L.M. Management of hypertension in patients with cardiac disease: Use of renin-angiotensin blocking agents. Am. J. Med. 2008, 121, S8–S15. [Google Scholar] [CrossRef] [PubMed]
- Todd, P.A.; Fitton, A. Perindopril. A review of its pharmacological properties and therapeutic use in cardiovascular disorders. Drugs 1991, 42, 90–114. [Google Scholar] [CrossRef] [PubMed]
- Ghiadoni, L. Perindopril for the treatment of hypertension. Expert Opin. Pharmacother. 2011, 12, 1633–1642. [Google Scholar] [CrossRef] [PubMed]
- Denhart, B.C.; Guidi, A.J.; Tognazzi, K.; Dvorak, H.F.; Brown, L.F. Vascular permeability factor/vascular endothelial growth factor and its receptors in oral and laryngeal squamous cell carcinoma and dysplasia. Lab. Investig. 1997, 77, 659–664. [Google Scholar]
- Yoshiji, H.; Kuriyama, S.; Kawata, M.; Yoshii, J.; Ikenaka, Y.; Noguchi, R.; Nakatani, T.; Tsujinoue, H.; Fukui, H. The angiotensin-I-converting enzyme inhibitor perindopril suppresses tumor growth and angiogenesis: Possible role of the vascular endothelial growth factor. Clin. Cancer Res. 2001, 7, 1073–1078. [Google Scholar]
- Yasumatsu, R.; Nakashima, T.; Masuda, M.; Ito, A.; Kuratomi, Y.; Nakagawa, T.; Komune, S. Effects of the angiotensin-I converting enzyme inhibitor perindopril on tumor growth and angiogenesis in head and neck squamous cell carcinoma cells. J. Cancer Res. Clin. Oncol. 2004, 130, 567–573. [Google Scholar] [CrossRef]
- Zakaria, S.; Allam, S.; El-Sisi, A.E. Perindopril sensitizes hepatocellular carcinoma to chemotherapy: A possible role of leptin/Wnt/β-catenin axis with subsequent inhibition of liver cancer stem cells. Saudi Pharm. J. 2022, 30, 1170–1180. [Google Scholar] [CrossRef]
- Campbell, D.J. A Review of Perindopril in the Reduction of Cardiovascular Events. Vasc. Health Risk Manag. 2006, 2, 117–124. [Google Scholar] [CrossRef]
- Bakris, G.L.; Sica, D.; Weber, M.; White, W.B.; Roberts, A.; Perez, A.; Cao, C.; Kupfer, S. The comparative effects of azilsartan medoxomil and olmesartan on ambulatory and clinic blood pressure. J. Clin. Hypertens. 2011, 13, 81–88. [Google Scholar] [CrossRef]
- Zaiken, K.; Cheng, J.W. Azilsartan medoxomil: A new Angiotensin receptor blocker. Clin. Ther. 2011, 33, 1577–1589. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.X.; Liu, K.K.C.; Sakya, S.M.; Flick, A.C.; O’Donnell, C.J. Synthetic approaches to the 2011 new drugs. Bioorganic Amp. Med. Chem. 2013, 21, 2795–2825. [Google Scholar] [CrossRef] [PubMed]
- McClellan, K.J.; Goa, K.L. Candesartan cilexetil. A review of its use in essential hypertension. Drugs 1998, 56, 847–869. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, K.; Naka, T.; Chatani, F.; Yoshimura, Y. Candesartan cilexetil: A review of its preclinical pharmacology. J. Hum. Hypertens. 1997, 11 (Suppl. S2), S9–S17. [Google Scholar] [PubMed]
- Pfeffer, M.A.; Swedberg, K.; Granger, C.B.; Held, P.; McMurray, J.J.; Michelson, E.L.; Olofsson, B.; Ostergren, J.; Yusuf, S.; Pocock, S. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: The CHARM-Overall programme. Lancet 2003, 362, 759–766. [Google Scholar] [CrossRef]
- Bulsara, K.G.; Makaryus, A.N. Candesartan; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Jia, S.; Qu, T.; Wang, X.; Feng, M.; Yang, Y.; Feng, X.; Ma, R.; Li, W.; Hu, Y.; Feng, Y.; et al. KIAA1199 promotes migration and invasion by Wnt/β-catenin pathway and MMPs mediated EMT progression and serves as a poor prognosis marker in gastric cancer. PLoS ONE 2017, 12, e0175058. [Google Scholar] [CrossRef]
- Ferdinand, K.C. A Review of the Antihypertensive and Renal-protective Effects of Irbesartan. US Cardiol. 2010, 7, 25–33. [Google Scholar] [CrossRef]
- Adams, M.A.; Trudeau, L. Irbesartan: Review of pharmacology and comparative properties. Can. J. Clin. Pharmacol. 2000, 7, 22–31. [Google Scholar]
- Carides, G.W. Losartan: A pharmacoeconomic review. J. Med. Econ. 2007, 10, 573–585. [Google Scholar] [CrossRef]
- Redon, J.; Fabia, M.J. Efficacy in angiotensin receptor blockade: A comparative review of data with olmesartan. J. Renin Angiotensin Aldosterone Syst. 2009, 10, 147–156. [Google Scholar] [CrossRef]
- Bakhtiari, E.; Hosseini, A.; Boroushaki, M.T.; Mousavi, S.H. Synergistic, cytotoxic and apoptotic activities of olmesartan with NF-κB inhibitor against HeLa human cell line. Toxicol. Mech. Methods 2015, 25, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Battershill, A.J.; Scott, L.J. Telmisartan: A review of its use in the management of hypertension. Drugs 2006, 66, 51–83. [Google Scholar] [CrossRef]
- Lai, M.-C.; Wu, S.-N.; Huang, C.-W. Telmisartan, an Antagonist of Angiotensin II Receptors, Accentuates Voltage-Gated Na+ Currents and Hippocampal Neuronal Excitability. Front. Neurosci. 2020, 14, 902. [Google Scholar] [CrossRef] [PubMed]
- Funao, K.; Matsuyama, M.; Kawahito, Y.; Sano, H.; Chargui, J.; Touraine, J.L.; Nakatani, T.; Yoshimura, R. Telmisartan is a potent target for prevention and treatment in human prostate cancer. Oncol. Rep. 2008, 20, 295–300. [Google Scholar] [PubMed]
- Flesch, G.; Müller, P.; Lloyd, P. Absolute bioavailability and pharmacokinetics of valsartan, an angiotensin II receptor antagonist, in man. Eur. J. Clin. Pharmacol. 1997, 52, 115–120. [Google Scholar] [CrossRef]
- Fares, H.; DiNicolantonio, J.J.; O’Keefe, J.H.; Lavie, C.J. Amlodipine in hypertension: A first-line agent with efficacy for improving blood pressure and patient outcomes. Open Heart 2016, 3, e000473. [Google Scholar] [CrossRef]
- Amenta, F.; Tomassoni, D.; Traini, E.; Mignini, F.; Veglio, F. Nicardipine: A hypotensive dihydropyridine-type calcium antagonist with a peculiar cerebrovascular profile. Clin. Exp. Hypertens. 2008, 30, 808–826. [Google Scholar] [CrossRef]
- Yedinak, K.C.; Lopez, L.M. Felodipine: A new dihydropyridine calcium-channel antagonist. DICP 1991, 25, 1193–1206. [Google Scholar] [CrossRef] [PubMed]
- Talreja, O.; Cassagnol, M. Diltiazem; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- El-Mahdy, H.A.; El-Husseiny, A.A.; Kandil, Y.I.; Gamal El-Din, A.M. Diltiazem potentiates the cytotoxicity of gemcitabine and 5-fluorouracil in PANC-1 human pancreatic cancer cells through inhibition of P-glycoprotein. Life Sci. 2020, 262, 118518. [Google Scholar] [CrossRef]
- Singh, B.N.; Ellrodt, G.; Peter, C.T. Verapamil: A review of its pharmacological properties and therapeutic use. Drugs 1978, 15, 169–197. [Google Scholar] [CrossRef]
- Vohra, J. Verapamil in cardiac arrhythmias: An overview. Clin. Exp. Pharmacol. Physiol. Suppl. 1982, 6, 129–134. [Google Scholar] [PubMed]
- Gonzalo, S. Epigenetic alterations in aging. J. Appl. Physiol. 2010, 109, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Arce, C.; Segura-Pacheco, B.; Perez-Cardenas, E.; Taja-Chayeb, L.; Candelaria, M.; Dueñnas-Gonzalez, A. Hydralazine target: From blood vessels to the epigenome. J. Transl. Med. 2006, 4, 10. [Google Scholar] [CrossRef]
- Deng, C.; Lu, Q.; Zhang, Z.; Rao, T.; Attwood, J.; Yung, R.; Richardson, B. Hydralazine may induce autoimmunity by inhibiting extracellular signal-regulated kinase pathway signaling. Arthritis Rheum 2003, 48, 746–756. [Google Scholar] [CrossRef]
- Gupta, A.K.; Talukder, M.; Venkataraman, M.; Bamimore, M.A. Minoxidil: A comprehensive review. J. Dermatol. Treat. 2022, 33, 1896–1906. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Li, Y.; Saito, T.; Nakaya, H. Minoxidil opens mitochondrial K(ATP) channels and confers cardioprotection. Br. J. Pharm. 2004, 141, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Divakaran, S.; Loscalzo, J. The Role of Nitroglycerin and Other Nitrogen Oxides in Cardiovascular Therapeutics. J. Am. Coll. Cardiol. 2017, 70, 2393–2410. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.F.; Mark, V.H.; Soloway, A.H.; Leskowitz, S. The effect of antibody to L-phenylalanine mustard conjugate on malignant cells selectively marked through "early inflammatory-like" vascular permeability. Cancer Res. 1966, 26, 1893–1904. [Google Scholar]
- Seymour, L.W.; Ulbrich, K.; Steyger, P.S.; Brereton, M.; Subr, V.; Strohalm, J.; Duncan, R. Tumour tropism and anti-cancer efficacy of polymer-based doxorubicin prodrugs in the treatment of subcutaneous murine B16F10 melanoma. Br. J. Cancer 1994, 70, 636–641. [Google Scholar] [CrossRef]
- Millet, A.; Bettaieb, A.; Renaud, F.; Prevotat, L.; Hammann, A.; Solary, E.; Mignotte, B.; Jeannin, J.F. Influence of the nitric oxide donor glyceryl trinitrate on apoptotic pathways in human colon cancer cells. Gastroenterology 2002, 123, 235–246. [Google Scholar] [CrossRef]
- Ko, J.C.; Chen, J.C.; Yen, T.C.; Chen, T.Y.; Ma, P.F.; Lin, Y.C.; Cheng, H.H.; Taso, Y.C.; Lin, Y.W. Nitroglycerin Enhances Cisplatin-Induced Cytotoxicity via AKT Inactivation and Thymidylate Synthase Downregulation in Human Lung Cancer Cells. Pharmacology 2020, 105, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Hu, J.T.; Tu, Y.J.; Wu, J.C.; Liang, J.; Gao, L.L.; Wang, Z.G.; Yang, B.F.; Dong, D.L. Effects of isosorbide mononitrate on the restoration of injured artery in mice in vivo. Eur. J. Pharm. 2010, 640, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, R.; Nunes, I.; Bolognese, J.A.; Miller, D.L.; Salotti, D.; McCarthy, J.M.; Smith, W.B.; Herman, G.A.; Feig, P.U. Single-dose effects of isosorbide mononitrate alone or in combination with losartan on central blood pressure. J. Am. Soc. Hypertens. 2010, 4, 311–318. [Google Scholar] [CrossRef]
- Abrams, M.J.; Murrer, B.A. Metal compounds in therapy and diagnosis. Science 1993, 261, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Boolell, M.; Allen, M.J.; Ballard, S.A.; Gepi-Attee, S.; Muirhead, G.J.; Naylor, A.M.; Osterloh, I.H.; Gingell, C. Sildenafil: An orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int. J. Impot. Res. 1996, 8, 47–52. [Google Scholar]
- Bender, A.T.; Beavo, J.A. Cyclic nucleotide phosphodiesterases: Molecular regulation to clinical use. Pharmacol. Rev. 2006, 58, 488–520. [Google Scholar] [CrossRef] [PubMed]
- Eggen, T.; Sager, G.; Berg, T.; Nergaard, B.; Moe, B.T.; Ørbo, A. Increased gene expression of the ABCC5 transporter without distinct changes in the expression of PDE5 in human cervical cancer cells during growth. Anticancer Res. 2012, 32, 3055–3061. [Google Scholar]
- Zhang, X.; Yan, G.; Ji, J.; Wu, J.; Sun, X.; Shen, J.; Jiang, H.; Wang, H. PDE5 inhibitor promotes melanin synthesis through the PKG pathway in B16 melanoma cells. J. Cell Biochem. 2012, 113, 2738–2743. [Google Scholar] [CrossRef]
- Karami-Tehrani, F.; Moeinifard, M.; Aghaei, M.; Atri, M. Evaluation of PDE5 and PDE9 expression in benign and malignant breast tumors. Arch. Med. Res. 2012, 43, 470–475. [Google Scholar] [CrossRef]
- Zhu, B.; Strada, S.J. The novel functions of cGMP-specific phosphodiesterase 5 and its inhibitors in carcinoma cells and pulmonary/cardiovascular vessels. Curr. Top. Med. Chem. 2007, 7, 437–454. [Google Scholar] [CrossRef]
- Tuttle, T.R.; Mierzwa, M.L.; Wells, S.I.; Fox, S.R.; Ben-Jonathan, N. The cyclic GMP/protein kinase G pathway as a therapeutic target in head and neck squamous cell carcinoma. Cancer Lett. 2016, 370, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Pashkovetsky, E.; Gupta, C.A.; Aronow, W.S. Use of levosimendan in acute and advanced heart failure: Short review on available real-world data. Ther. Clin. Risk Manag. 2019, 15, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Conti, N.; Gatti, M.; Raschi, E.; Diemberger, I.; Potena, L. Evidence and Current Use of Levosimendan in the Treatment of Heart Failure: Filling the Gap. Drug Des. Devel. Ther. 2021, 15, 3391–3409. [Google Scholar] [CrossRef]
- Mattiuzzi, C.; Lippi, G. Current Cancer Epidemiology. J. Epidemiol. Glob. Health 2019, 9, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Malik, D.; Mahendiratta, S.; Kaur, H.; Medhi, B. Futuristic approach to cancer treatment. Gene 2021, 805, 145906. [Google Scholar] [CrossRef] [PubMed]
- Hauner, K.; Maisch, P.; Retz, M. Side effects of chemotherapy. Urol. A 2017, 56, 472–479. [Google Scholar] [CrossRef]
- ROSENTHAL, T.; GAVRAS, I. Renin–Angiotensin Inhibition in Combating Malignancy: A Review. Anticancer Res. 2019, 39, 4597–4602. [Google Scholar] [CrossRef]
- Gross, S.; Mallu, P.; Joshi, H.; Schultz, B.; Go, C.; Soboloff, J. Ca2+ as a therapeutic target in cancer. Adv. Cancer Res. 2020, 148, 233–317. [Google Scholar] [CrossRef]
- Huang, Z.; Fu, J.; Zhang, Y. Nitric Oxide Donor-Based Cancer Therapy: Advances and Prospects. J. Med. Chem. 2017, 60, 7617–7635. [Google Scholar] [CrossRef]
- Jing, X.; Yang, F.; Shao, C.; Wei, K.; Xie, M.; Shen, H.; Shu, Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 2019, 18, 157. [Google Scholar] [CrossRef] [PubMed]
- Catalano, S.; Campana, A.; Giordano, C.; Győrffy, B.; Tarallo, R.; Rinaldi, A.; Bruno, G.; Ferraro, A.; Romeo, F.; Lanzino, M.; et al. Expression and Function of Phosphodiesterase Type 5 in Human Breast Cancer Cell Lines and Tissues: Implications for Targeted Therapy. Clin. Cancer Res. 2016, 22, 2271–2282. [Google Scholar] [CrossRef] [PubMed]
- Piazza, G.A.; Thompson, W.J.; Pamukcu, R.; Alila, H.W.; Whitehead, C.M.; Liu, L.; Fetter, J.R.; Gresh, W.E., Jr.; Klein-Szanto, A.J.; Farnell, D.R.; et al. Exisulind, a novel proapoptotic drug, inhibits rat urinary bladder tumorigenesis. Cancer Res. 2001, 61, 3961–3968. [Google Scholar] [PubMed]
- Pusztai, L.; Zhen, J.H.; Arun, B.; Rivera, E.; Whitehead, C.; Thompson, W.J.; Nealy, K.M.; Gibbs, A.; Symmans, W.F.; Esteva, F.J.; et al. Phase I and II study of exisulind in combination with capecitabine in patients with metastatic breast cancer. J. Clin. Oncol. 2003, 21, 3454–3461. [Google Scholar] [CrossRef]
- Whitehead, C.M.; Earle, K.A.; Fetter, J.; Xu, S.; Hartman, T.; Chan, D.C.; Zhao, T.L.; Piazza, G.; Klein-Szanto, A.J.; Pamukcu, R.; et al. Exisulind-induced apoptosis in a non-small cell lung cancer orthotopic lung tumor model augments docetaxel treatment and contributes to increased survival. Mol. Cancer Ther. 2003, 2, 479–488. [Google Scholar]
- Barone, I.; Giordano, C.; Bonofiglio, D.; Andò, S.; Catalano, S. Phosphodiesterase type 5 and cancers: Progress and challenges. Oncotarget 2017, 8, 99179–99202. [Google Scholar] [CrossRef]
- Lehár, J.; Krueger, A.S.; Avery, W.; Heilbut, A.M.; Johansen, L.M.; Price, E.R.; Rickles, R.J.; Short, G.F., 3rd; Staunton, J.E.; Jin, X.; et al. Synergistic drug combinations tend to improve therapeutically relevant selectivity. Nat. Biotechnol. 2009, 27, 659–666. [Google Scholar] [CrossRef] [PubMed]
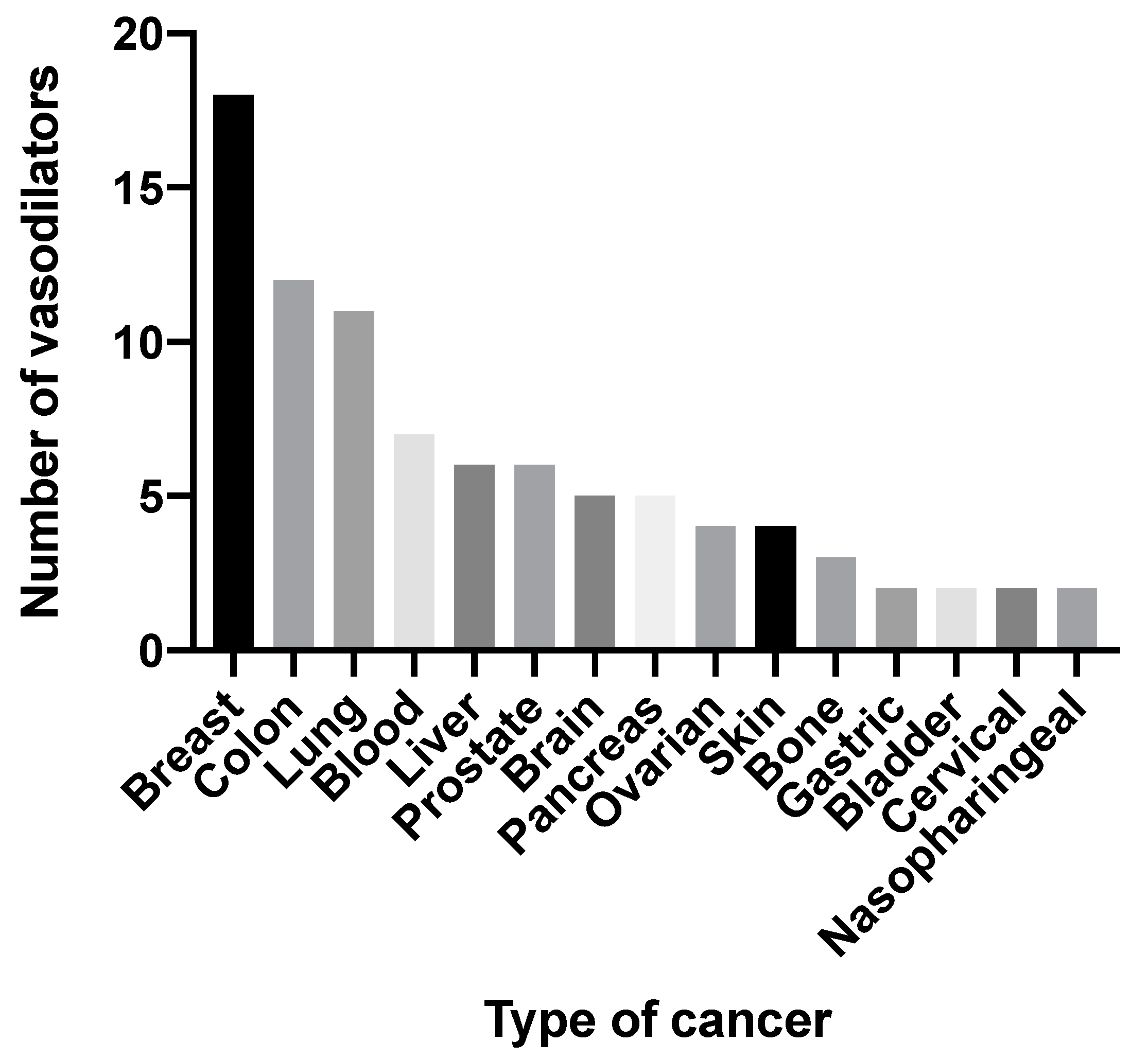
| Drug | Drug Class | Clinical Use | Cell Lines | Effects In Vitro | References |
|---|---|---|---|---|---|
| Enalapril | ACE inhibitor | Hypertension and heart failure | HCT116, SW620, CT26, HT29, SW40, and HL60 | Inhibits cell proliferation, suppresses EMT, and induces apoptosis | [26,27,28,29,30,31,32] |
| Captopril | ACE inhibitor | Hypertension and congestive heart failure | DU145, HCT116, and Hs578T | Inhibits mitosis, growth, and migration | [33,34,35,36,37,38,39,40,41,42] |
| Trandolapril | ACE inhibitor | Hypertension, congestive heart failure, and after myocardial infarction | K562, KU812, U937, and HL60 | Inhibits cell growth and induces apoptosis | [43] |
| Sildenafil | PDE5 inhibitor | Erectile dysfunction and pulmonary hypertension | HT-29, SW489, SW620, HCT116, SW116, PC-3, DU145, 4T1, MCF-7, A549, HeLa, SiHa, RD, and RH30 | Inhibits proliferation, migration, and invasion; induces apoptosis, cell-cycle arrest at G1 phase, and accumulation of ROS | [44,45,46,47,48,49,50,51,52,53,54,55] |
| Tadalafil | PDE5 inhibitor | Erectile dysfunction | PC-3, UM1, UM47, UM6, CAL27, A549, and SK-MES-1 | Inhibits proliferation | [56,57,58,59] |
| Azilsartan | ARB | Hypertension | HepG2, A549, MCF-7, and MDA-MB-231 | Induces oxidative stress, cytochrome c release, cytotoxicity, cell-cycle arrest, and suppression of NF-kB/IL-6/JAK2/STAT3 signaling pathway | [60,61,62] |
| Candesartan | ARB | Congestive heart failure and hypertension | CT-26, SW480, HOS, MG63, and U-2OS | Induces E-cadherin, downregulation of MMP3/9, inhibits Wnt/β-catenin signaling, and induces apoptosis | [63,64,65,66,67,68] |
| Irbesartan | ARB | Hypertension | HCCLM3, HMHCC97-H, HMHCC97-L, SMMC-7721, Huh-7, Hep-3B, PLC, MCF-7, T47D, ZR-75-30, MDA-MB-231, MDA-MB-435, and MDA-MB-468 | Inhibits adhesion of HCC cells to endothelial cells and suppresses cell proliferation | [69,70] |
| Losertan | ARB | Hypertension | MCF-7, CT-26, Huh-7, JHH-6, MHCC97H, HepG-2, and SMMC-7721 | Induces apoptosis and G1 cell-cycle arrest | [71,72,73,74,75,76,77,78] |
| Olmesartan | ARB | Hypertension | A549, HeLa, MCF-7, Me 4405, Sk-Mel-28, PC-3, Du145, MDA-MB-468, and HEK | Inhibits growth; induces apoptosis and ROS | [79,80,81,82,83,84,85] |
| Telmisartan | ARB | Hypertension | GIST-T1, PC-3, MDA-MB-468, and DU145 | Induces cell-cycle arrest in the G0/G1 phase and apoptosis; inhibits cell proliferation | [86,87,88,89,90,91,92,93,94] |
| Valsartan | ARB | Hypertension | CNE-2 | Inhibits growth | [95] |
| Levosimendan | Calcium sensitiser | Acute and advanced heart failure and hypertension | SU-DHL-8 | Inhibits RIOK1 | [96] |
| Amlodipine | CCB | Hypertension, angina and coronary artery disease | MDA-MB-231, MCF-7, A549, and A431 | Inhibits proliferation, invasion, colony formation, and cell-cycle arrest at G0/G1 phase | [97,98,99,100,101,102,103,104] |
| Nicardipine | CCB | Hypertension and angina | PC-3, 4T1, JC, and MDA-MB-231 | Inhibits cell migration and colony formation and increases Nrf2 expression | [105,106,107] |
| Felodipine | CCB | Hypertension, chronic stable angina pectoris and congestive heart failure | Mz-ChA-1, KMCH, CC-LP-1, and TFK-1 | Increases caspase 3/7 and decreases cell viability | [108,109,110] |
| Nifedipine | CCB | Hypertension | DLD1 and MDA-MB-231 | Suppresses cancer progression, migration, and immune escape; reduces expression of NFAT2; induces cell-cycle arrest at S phase | [111,112,113,114] |
| Diltiazem | CCB | Hypertension, angina, and congestive heart failure | MCF-7, JC, 4T1, MDA-MB-231, and A549 | Attenuates colony formation, cell migration, and EMT | [115,116,117] |
| Verapamil | CCB | Hypertension and angina | HT-29, G292, L3.6pl, AsPC-1, G-UVW, G-CCM, G-MCF, P388, and A549 | Promotes intracellular drug accumulation, fragmentation of chromatin; decreases cell viability and migration; increases cell apoptosis | [117,118,119,120,121,122,123,124,125,126,127,128,129,130,131] |
| Hydralazine | Direct vasodilator | Hypertension | Jurkat, MOLT-4, CEM-6, DU145, LNCaP, 22Rv1, PC-3, HeLa, CaSki, SiHa, A549, and H1703 | Loss of mitochondrial membrane, ROS production, and reduced colony formation, invasion, and migration capabilities | [132,133,134,135] |
| Minoxidil | Direct vasodilator | Hypertension | PC-3, LNCaP, LaPC4, HepG2, MDA-MB-231, MDA-MB-468, and OVCAR-8 | Increases ROS accumulation; induces apoptosis | [136,137] |
| Nitroglycerin | Nitrate | Angina pectoris, hypertension, congestive heart failure, and for induction of surgical hypotension | HCT116, SW480, SW620, and PC-3 | Decreases concentration of cardiolipin, downregulates respiratory chain complex activities, releases cytochrome c into the cytosol, and activates caspase-9 and caspase-3 | [138,139,140,141,142] |
| Isosorbide minitrate | Nitrate | Angina pectoris, acute myocardial infraction, and congestive heart failure | HCT116, SW620, SKOV3, and HO8910 | Inhibits cell growth and proliferation; induces apoptosis, chromatin condensation, ROS production, and mitochondrial damage | [143,144,145] |
| Sodium Nitroprusside | Nitrate | Acute hypertension and vascular surgery | SGC-7901, AGS, MKN45, MKN28, U343, U251, and LN-Z308 | Apoptosis induction and cell growth inhibition | [146,147] |
| Vasodilator | Type of Cancer | Clinical Trial | Status | Identifier Trial Number (https://clinicaltrials.gov) accessed on 7 February 2023 |
|---|---|---|---|---|
| Enalapril | Woman breast | Not applicable | Completed (2015) | NCT00895414 |
| Captopril | Lung | Phase 2 | Completed (2016) | NCT00077064 |
| Perindopril | Colorectal | Phase 2 | Completed (2018) | NCT02651415 |
| Losartan | Pancreatic Pancreatic Breast Pancreatic Pancreatic | Phase 2 Phase 2 Phase 2 Phase 2 Phase 1 | Active Active Active Recruiting Recruiting | NCT03563248 NCT01821729 NCT05097248 NCT05077800 NCT05365893 |
| Amlodipine | Breast Breast | Phases 1 and 2 Phase 2 | Completed (2021) Recruiting | NCT02834403 NCT05660083 |
| Verapamil | Brain Lymphoma | Phase 2 Phase 1 | Completed (2017) Active | NCT00706810 NCT03013933 |
| Hydralazine | Breast Ovarian Lung Rectal Cervical Breast Refractory solid tumors Cervical | Phases 1 and 2 Phase 3 Phase 1 Phases 1 and 2 Phase 3 Phase 2 Phase 2 Phase 3 | Withdrawn Completed (2009) Completed (2013) Withdrawn Completed (2010) Completed (2006) Completed (2006) Completed (2018) | NCT00575978 NCT00533299 NCT00996060 NCT00575640 NCT00532818 NCT00395655 NCT00404508 NCT02446652 |
| Minoxidil | Ovarian | Phase 2 | Recruiting | NCT05272462 |
| Nitroglycerin | Rectal Lung Prostate Lung Lung Brain | Phase 1 Phase 2 Phase 3 Phase 2 Phase 2 Phase 2 | Completed (2021) Completed (2017) Completed (2013) Completed (2020) Unknown (2009) Completed (2020) | NCT01407107 NCT01210378 NCT01704274 NCT01171170 NCT00886405 NCT04338867 |
| Sildenafil | Lung Solid tumor | Phases 2 and 3 Phase 1 | Completed (2011) Completed (2019) | NCT00752115 NCT02466802 |
| Tadalafil | Pancreatic Liver, pancreatic, and colorectal Head and neck Head and neck Pancreatic Gastric Pancreatic Myeloma Head and neck | Phase 2 Phase 2 Phases 1 and 2 Phase 2 Phase 1 Phase 2 Phase1 Phase 2 Phase 2 | Recruiting Completed (2022) Completed (2021) Active Completed (2018) Not yet recruiting Completed (2018) Completed (2014) Completed (2014) | NCT05014776 NCT03785210 NCT02544880 NCT03993353 NCT01903083 NCT05709574 NCT01342224 NCT01374217 NCT01697800 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, E.; Costa, B.; Vasques-Nóvoa, F.; Vale, N. In Vitro Drug Repurposing: Focus on Vasodilators. Cells 2023, 12, 671. https://doi.org/10.3390/cells12040671
Ribeiro E, Costa B, Vasques-Nóvoa F, Vale N. In Vitro Drug Repurposing: Focus on Vasodilators. Cells. 2023; 12(4):671. https://doi.org/10.3390/cells12040671
Chicago/Turabian StyleRibeiro, Eduarda, Bárbara Costa, Francisco Vasques-Nóvoa, and Nuno Vale. 2023. "In Vitro Drug Repurposing: Focus on Vasodilators" Cells 12, no. 4: 671. https://doi.org/10.3390/cells12040671
APA StyleRibeiro, E., Costa, B., Vasques-Nóvoa, F., & Vale, N. (2023). In Vitro Drug Repurposing: Focus on Vasodilators. Cells, 12(4), 671. https://doi.org/10.3390/cells12040671








