Identification and Quantification of Proliferating Cells in Skeletal Muscle of Glutamine Supplemented Low- and Normal-Birth-Weight Piglets
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sampling
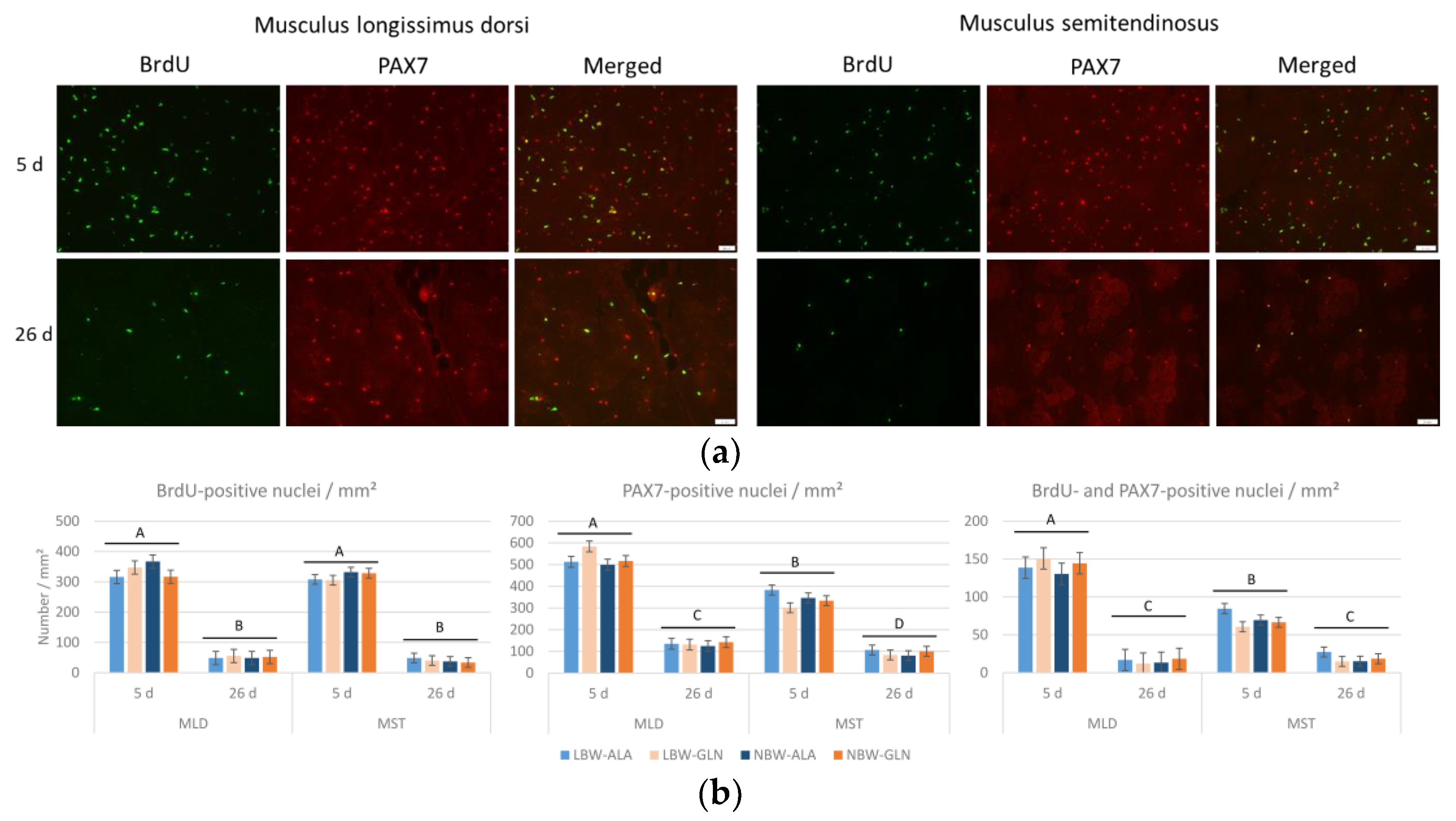
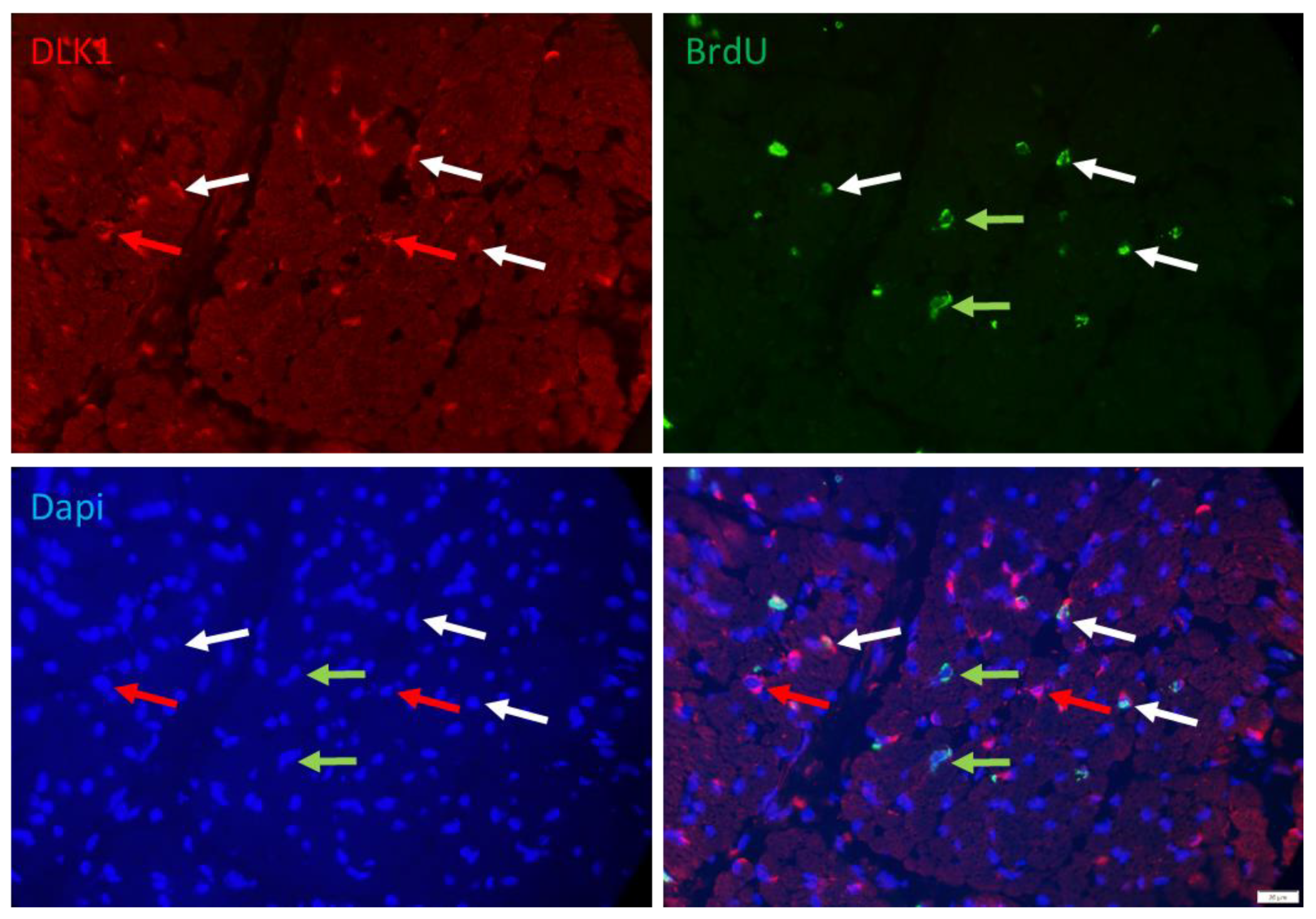
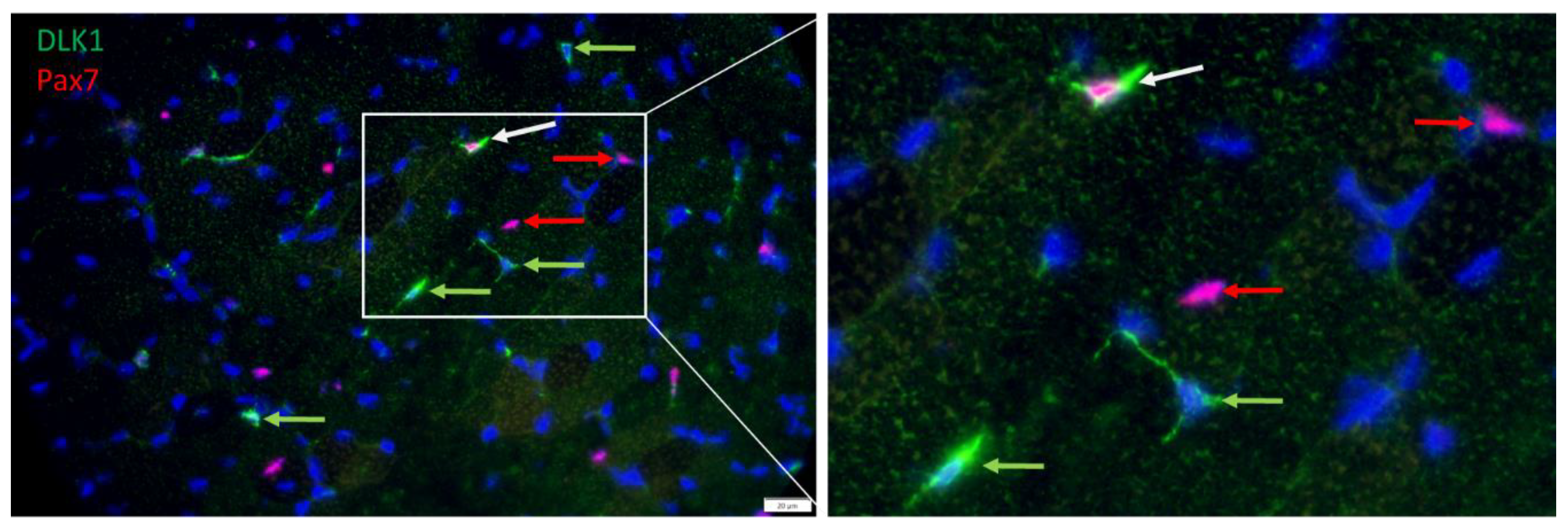
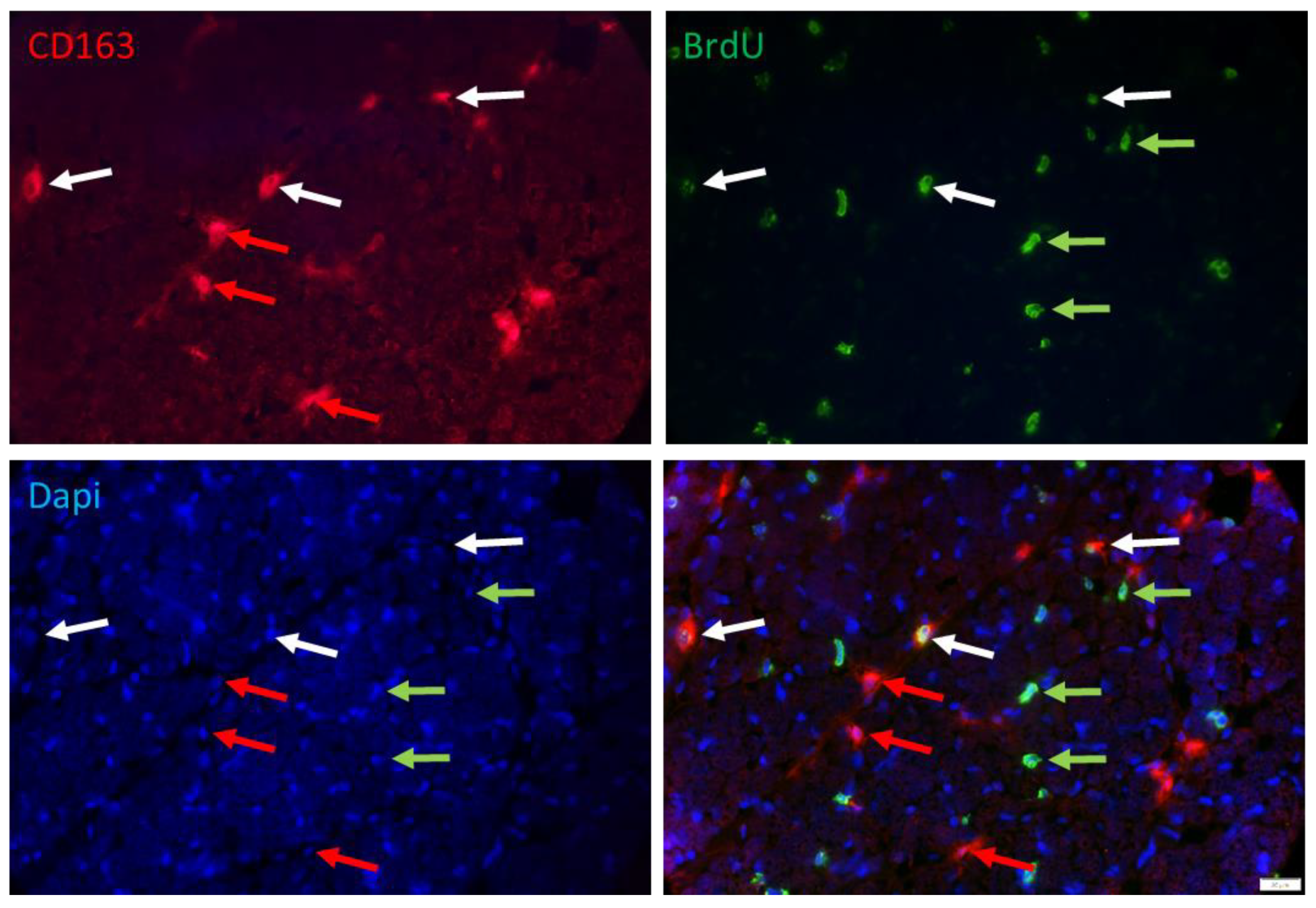
2.2. Immunohistochemistry
2.3. Microscopy and Image Analysis
2.4. Statistical Analysis
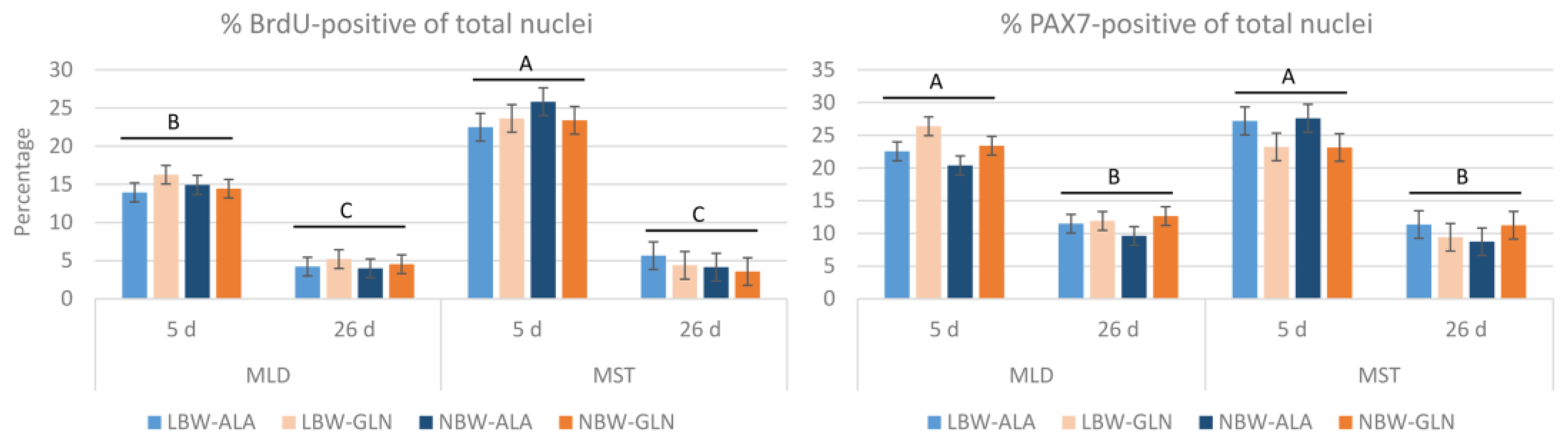
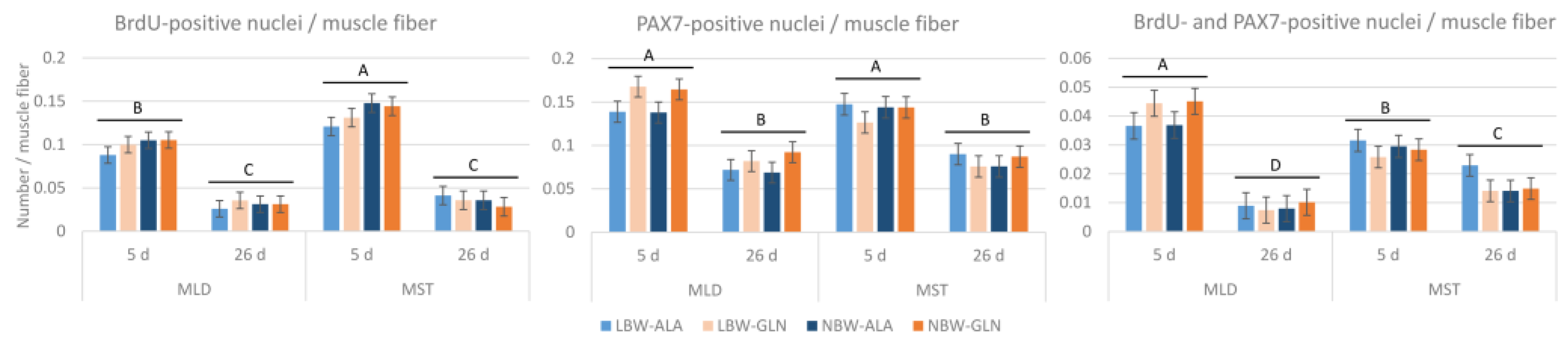
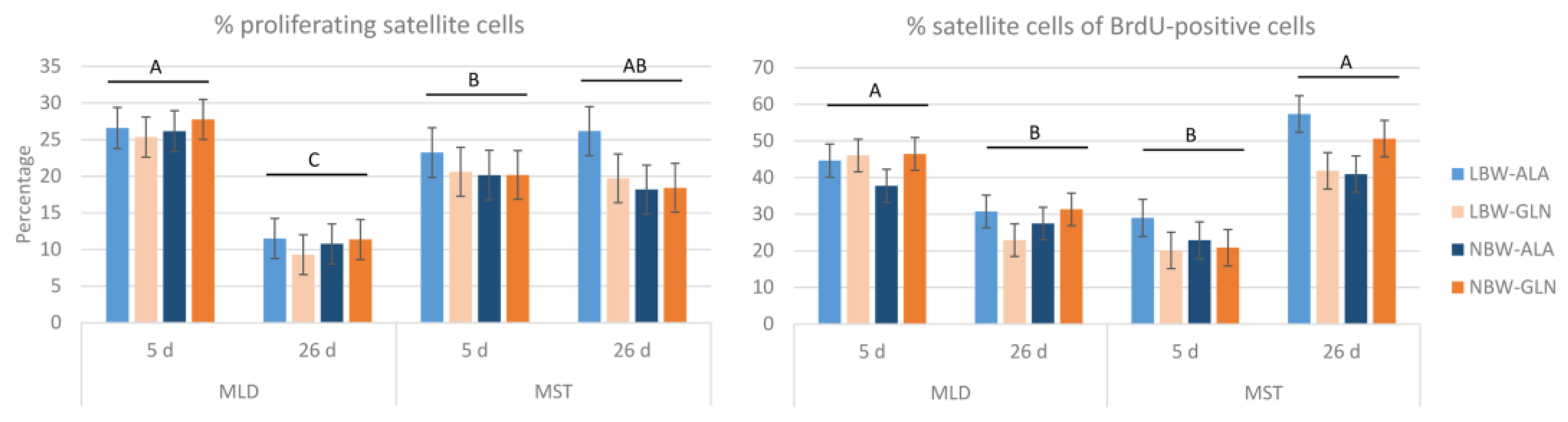
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jamin, A.; Seve, B.; Thibault, J.N.; Floc’h, N. Accelerated growth rate induced by neonatal high-protein milk formula is not supported by increased tissue protein synthesis in low-birth-weight piglets. J. Nutr. Metab. 2012, 2012, 545341. [Google Scholar] [CrossRef]
- Wan, H.; Zhu, J.; Su, G.; Liu, Y.; Hua, L.; Hu, L.; Wu, C.; Zhang, R.; Che, L.; Wu, D.; et al. Dietary supplementation with β-hydroxy-β-methylbutyrate calcium during the early postnatal period accelerates skeletal muscle fibre growth and maturity in intra-uterine growth-retarded and normal-birth-weight piglets. Br. J. Nutr. 2016, 115, 1360–1369. [Google Scholar] [CrossRef]
- Madsen, J.G.; Seoni, E.; Kreuzer, M.; Silacci, P.; Bee, G. Influence of l-carnitine and l-arginine on protein synthesis and maturation of the semitendinosus muscle of lightweight piglets. J. Anim. Physiol. Anim. Nutr. 2018, 102, 440–451. [Google Scholar] [CrossRef]
- Zhao, Y.; Albrecht, E.; Sciascia, Q.L.; Li, Z.; Görs, S.; Schregel, J.; Metges, C.C.; Maak, S. Effects of oral glutamine supplementation on early postnatal muscle morphology in low and normal birth weight piglets. Animals 2020, 10, 1976. [Google Scholar] [CrossRef]
- Rudar, M.; Fiorotto, M.L.; Davis, T.A. Regulation of muscle growth in early postnatal life in a swine model. Annu. Rev. Anim. Biosci. 2019, 7, 309–335. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.A.; Fiorotto, M.L. Regulation of muscle growth in neonates. Curr. Opin. Clin. Nutr. Metab. Care. 2009, 12, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.A.; Suryawan, A.; Orellana, R.A.; Nguyen, H.V.; Fiorotto, M.L. Postnatal ontogeny of skeletal muscle protein synthesis in pigs. J. Anim. Sci. 2008, 86, E13–E18. [Google Scholar] [CrossRef]
- Zhao, Y.; Albrecht, E.; Stange, K.; Li, Z.; Schregel, J.; Sciascia, Q.L.; Metges, C.C.; Maak, S. Glutamine supplementation stimulates cell proliferation in skeletal muscle and cultivated myogenic cells of low birth weight piglets. Sci. Rep. 2021, 11, 13432. [Google Scholar] [CrossRef] [PubMed]
- Burrin, D.G.; Reeds, P.J. Alternative fuels in the gastrointestinal tract. Curr. Opin. Gastroenterol. 1997, 13, 165–170. [Google Scholar] [CrossRef]
- Cory, J.G.; Cory, A.H. Critical roles of glutamine as nitrogen donors in purine and pyrimidine nucleotide synthesis: Asparaginase treatment in childhood acute lymphoblastic leukemia. In Vivo 2006, 20, 587–589. [Google Scholar]
- Flynn, N.E.; Wu, G. An important role for endogenous synthesis of arginine in maintaining arginine homeostasis in neonatal pigs. Am. J. Physiol. 1996, 271, R1149–R1155. [Google Scholar] [CrossRef]
- Zammit, P.S.; Relaix, F.; Nagata, Y.; Ruiz, A.P.; Collins, C.A.; Partridge, T.A.; Beauchamp, J.R. Pax7 and myogenic progression in skeletal muscle satellite cells. J. Cell Sci. 2006, 119, 1824–1832. [Google Scholar] [CrossRef]
- Patruno, M.; Caliaro, F.; Martinello, T.; Mascarello, F. Expression of the paired box domain Pax7 protein in myogenic cells isolated from the porcine semitendinosus muscle after birth. Tissue Cell. 2008, 40, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Seale, P.; Sabourin, L.A.; Girgis-Gabardo, A.; Mansouri, A.; Gruss, P.; Rudnicki, M.A. Pax7 is required for the specification of myogenic satellite cells. Cell 2000, 102, 777–786. [Google Scholar] [CrossRef]
- Rudnicki, M.A.; Schnegelsberg, P.N.J.; Stead, R.H.; Braun, T.; Arnold, H.-H.; Jaenisch, R. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell 1993, 75, 1351–1359. [Google Scholar] [CrossRef]
- Zammit, P.S. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell Dev. Biol. 2017, 72, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.H.; Teisner, B.; Højrup, P.; Rasmussen, H.B.; Madsen, O.D.; Nielsen, B.; Skjødt, K. Studies on the isolation, structural analysis and tissue localization of fetal antigen 1 and its relation to a human adrenal-specific cDNA, pG2. Hum. Reprod. 1993, 8, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, E.; Kuzinski, J.; Komolka, K.; Gotoh, T.; Maak, S. Localization and abundance of early markers of fat cell differentiation in skeletal muscle of cattle during growth—Are DLK1-positive cells the origin of marbling flecks? Meat Sci. 2015, 100, 237–245. [Google Scholar] [CrossRef]
- Sul, H.S. Minireview: Pref-1: Role in adipogenesis and mesenchymal cell fate. Mol. Endocrinol. 2009, 23, 1717–1725. [Google Scholar] [CrossRef]
- Schering, L.; Albrecht, E.; Komolka, K.; Kühn, C.; Maak, S. Increased expression of thyroid hormone responsive protein (THRSP) is the result but not the cause of higher intramuscular fat content in cattle. Int. J. Biol. Sci. 2017, 13, 532–544. [Google Scholar] [CrossRef]
- Miller, A.J.; Cole, S.E. Multiple Dlk1 splice variants are expressed during early mouse embryogenesis. Int. J. Dev. Biol. 2014, 58, 65–70. [Google Scholar] [CrossRef]
- Andersen, D.C.; Petersson, S.J.; Jørgensen, L.H.; Bollen, P.; Jensen, P.B.; Teisner, B.; Schroeder, H.D.; Jensen, C.H. Characterization of DLK1+ cells emerging during skeletal muscle remodeling in response to myositis, myopathies, and acute injury. Stem Cells. 2009, 27, 898–908. [Google Scholar] [CrossRef]
- Andersen, D.C.; Laborda, J.; Baladron, V.; Kassem, M.; Sheikh, S.P.; Jensen, C.H. Dual role of delta-like 1 homolog (DLK1) in skeletal muscle development and adult muscle regeneration. Development 2013, 140, 3743–3753. [Google Scholar] [CrossRef]
- Fu, Y.; Hao, X.; Shang, P.; Chamba, Y.; Zhang, B.; Zhang, H. Functional identification of porcine dlk1 during muscle development. Animals 2022, 12, 1523. [Google Scholar] [CrossRef]
- Zhang, L.; Uezumi, A.; Kaji, T.; Tsujikawa, K.; Andersen, D.C.; Jensen, C.H.; Fukada, S.I. Expression and functional analyses of dlk1 in muscle stem cells and mesenchymal progenitors during muscle regeneration. Int. J. Mol. Sci. 2019, 20, 3269. [Google Scholar] [CrossRef]
- Albrecht, E.; Schering, L.; Liu, Y.; Komolka, K.; Kühn, C.; Wimmers, K.; Gotoh, T.; Maak, S. Factors influencing bovine intramuscular adipose tissue development and cellularity. J. Anim. Sci. 2017, 95, 2244–2254. [Google Scholar] [CrossRef]
- Schregel, J.; Schulze Holthausen, J.; Sciascia, Q.L.; Li, Z.; Görs, S.; Eggert, A.; Tuchscherer, A.; Zentek, J.; Metges, C.C. Effects of oral glutamine supplementation on jejunal morphology, development, and amino acid profiles in male low birth weight suckling piglets. PLoS ONE 2022, 17, e0267357. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sciascia, Q.; Görs, S.; Nguyen, N.; Rayatdoost Baghal, F.; Schregel, J.; Tuchscherer, A.; Zentek, J.; Metges, C.C. Glutamine supplementation moderately affects growth, plasma metabolite and free amino acid patterns in neonatal low birth weight piglets. Br. J. Nutr. 2022, 11, 1–11. [Google Scholar] [CrossRef]
- Domeneghini, C.; Di Giancamillo, A.; Savoini, G.; Paratte, R.; Bontempo, V.; Dell’Orto, V. Structural patterns of swine ileal mucosa following L-glutamine and nucleotide administration during the weaning period. An histochemical and histometrical study. Histol. Histopathol. 2004, 19, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Blachier, F.; Boutry, C.; Bos, C.; Tomé, D. Metabolism and functions of L-glutamate in the epithelial cells of the small and large intestines. Am. J. Clin. Nutr. 2009, 90, 814S–821S. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.D.; Deng, C.X.; Wang, Z.R.; Ye, Y.L.; You, J.M. Dietary alanyl-glutamine improves growth performance of weaned piglets through maintaining intestinal morphology and digestion-absorption function. Animal 2019, 13, 1826–1833. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Johnson, G.A.; Knabe, D.A.; Burghardt, R.C.; Spencer, T.E.; Li, X.L.; Wang, J.J. Triennial Growth Symposium: Important roles for L-glutamine in swine nutrition and production. J. Anim. Sci. 2011, 89, 2017–2030. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.J.; Wang, L.X.; Yang, H.S.; Hu, A.; Yin, Y.L. Review: The roles and functions of glutamine on intestinal health and performance of weaning pigs. Animal 2019, 13, 2727–2735. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, R.A.; Usry, J.L.; Arrellano, C.; Nogueira, E.T.; Kutschenko, M.; Moeser, A.J.; Odle, J. Effects of creep feeding and supplemental glutamine or glutamine plus glutamate (Aminogut) on pre- and post-weaning growth performance and intestinal health of piglets. J. Anim. Sci. Biotechn. 2013, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Snijders, T.; Nederveen, J.P.; McKay, B.R.; Joanisse, S.; Verdijk, L.B.; vanLoon, L.J.C.; Parise, G. Satellite cells in human skeletal muscle plasticity. Front. Physiol. 2015, 6, 283. [Google Scholar] [CrossRef]
- Bérard, J.; Kalbe, C.; Lösel, D.; Tuchscherer, A.; Rehfeldt, C. Potential sources of early-postnatal increase in myofibre number in pig skeletal muscle. Histochem. Cell Biol. 2011, 136, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Oczkowicz, M.; Piestrzyska-Kajtoch, A.; Piórkowska, K.; Rejduch, B.; Rózycki, M. Expression of DLK1 and MEG3 genes in porcine tissues during postnatal development. Genet. Mol. Biol. 2010, 33, 790–794. [Google Scholar] [CrossRef]
- Olguin, H.C.; Olwin, B.B. Pax-7 up-regulation inhibits myogenesis and cell cycle progression in satellite cells: A potential mechanism for self-renewal. Dev. Biol. 2004, 275, 375–388. [Google Scholar] [CrossRef]
- Ziemkiewicz, N.; Hilliard, G.; Pullen, N.A.; Garg, K. The role of innate and adaptive immune cells in skeletal muscle regeneration. Int. J. Mol. Sci. 2021, 22, 3265. [Google Scholar] [CrossRef]
- Arnold, L.; Henry, A.; Poron, F.; Baba-Amer, Y.; van Rooijen, N.; Plonquet, A.; Gherardi, R.K.; Chazaud, B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007, 204, 1057–1069. [Google Scholar] [CrossRef]
- Nawaz, A.; Aminuddin, A.; Kado, T.; Takikawa, A.; Yamamoto, S.; Tsuneyama, K.; Igarashi, Y.; Ikutani, M.; Nishida, Y.; Nagai, Y.; et al. CD206+ M2-like macrophages regulate systemic glucose metabolism by inhibiting proliferation of adipocyte progenitors. Nat. Commun. 2017, 8, 286. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albrecht, E.; Zhao, Y.; Sciascia, Q.L.; Metges, C.C.; Maak, S. Identification and Quantification of Proliferating Cells in Skeletal Muscle of Glutamine Supplemented Low- and Normal-Birth-Weight Piglets. Cells 2023, 12, 580. https://doi.org/10.3390/cells12040580
Albrecht E, Zhao Y, Sciascia QL, Metges CC, Maak S. Identification and Quantification of Proliferating Cells in Skeletal Muscle of Glutamine Supplemented Low- and Normal-Birth-Weight Piglets. Cells. 2023; 12(4):580. https://doi.org/10.3390/cells12040580
Chicago/Turabian StyleAlbrecht, Elke, Yaolu Zhao, Quentin L. Sciascia, Cornelia C. Metges, and Steffen Maak. 2023. "Identification and Quantification of Proliferating Cells in Skeletal Muscle of Glutamine Supplemented Low- and Normal-Birth-Weight Piglets" Cells 12, no. 4: 580. https://doi.org/10.3390/cells12040580
APA StyleAlbrecht, E., Zhao, Y., Sciascia, Q. L., Metges, C. C., & Maak, S. (2023). Identification and Quantification of Proliferating Cells in Skeletal Muscle of Glutamine Supplemented Low- and Normal-Birth-Weight Piglets. Cells, 12(4), 580. https://doi.org/10.3390/cells12040580





