A Three-Dimensional Engineered Cardiac In Vitro Model: Controlled Alignment of Cardiomyocytes in 3D Microphysiological Systems
Abstract
1. Introduction
2. Materials and Methods
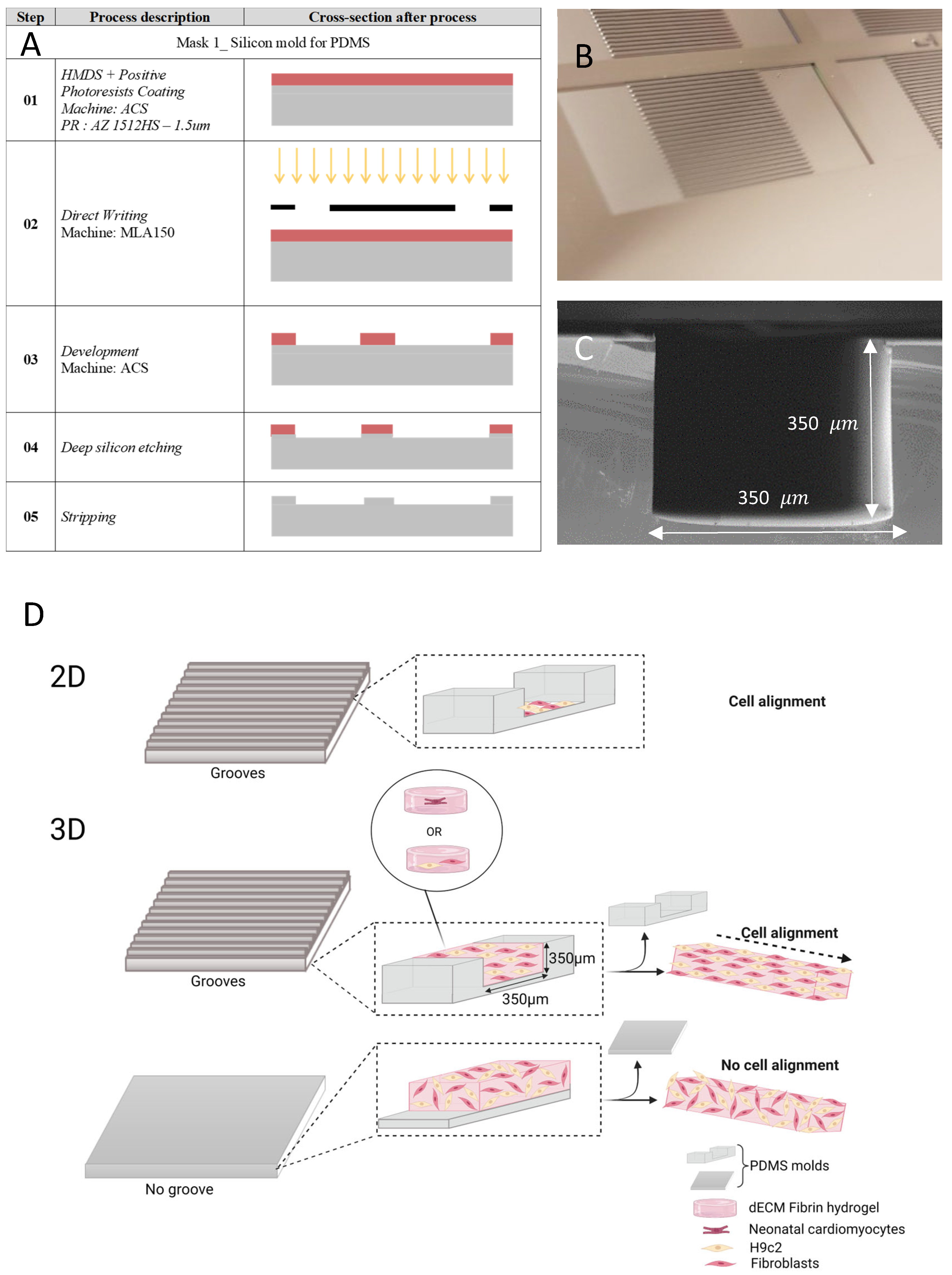
2.1. Microfabricated Grooves for Cell Alignment
2.2. Hydrogel Preparation
2.3. Cell Culture
2.4. Cell Seeding on PDMS (2D) and in 3D Hydrogel
2.5. Staining and 3D Imaging
2.6. Cell Orientation
2.7. Beating Characteristics
2.8. Statistical Analysis
3. Results
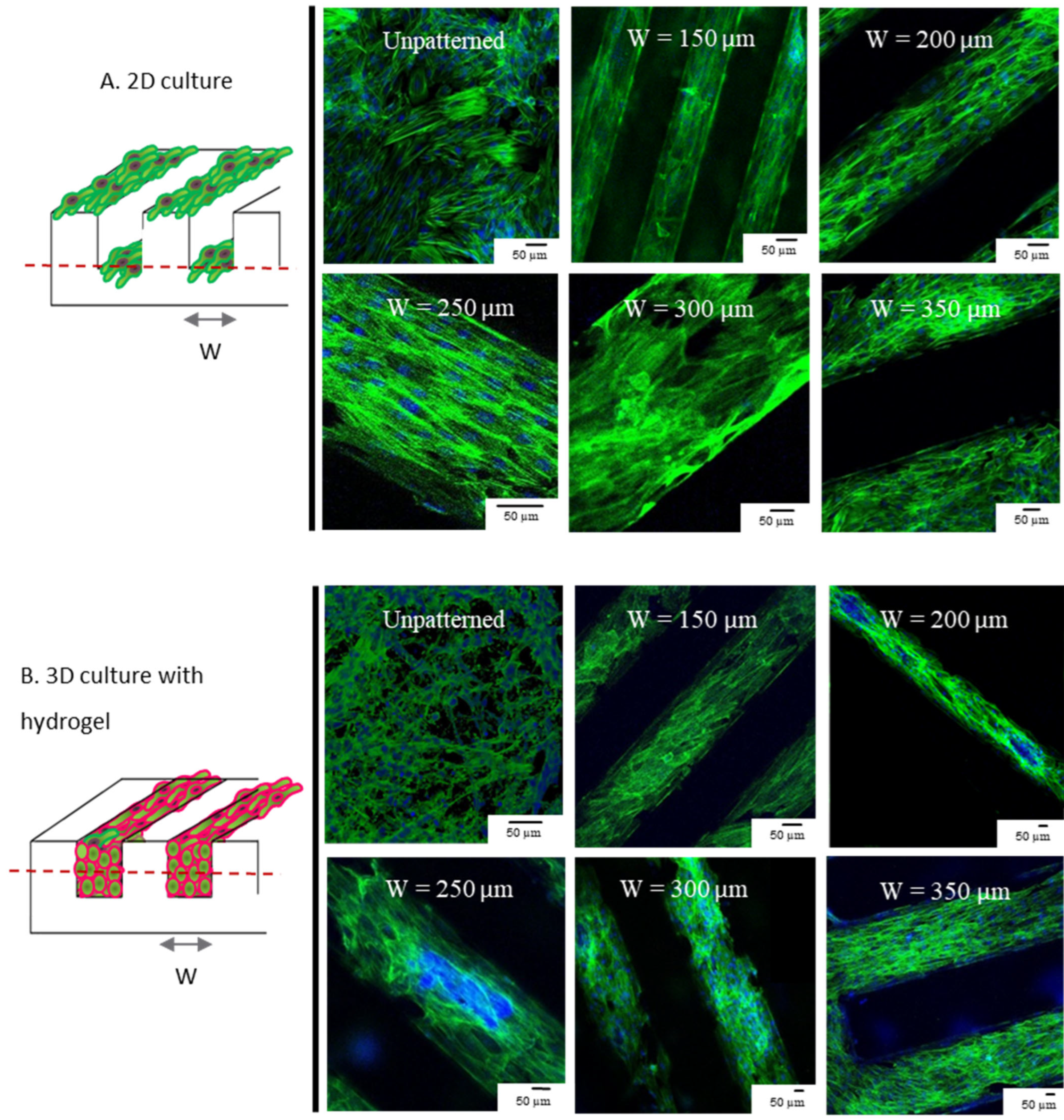
3.1. 3D and 2D Cultures Show Preferential Alignment and Present Elongated Aspect Regardless of Groove Dimension
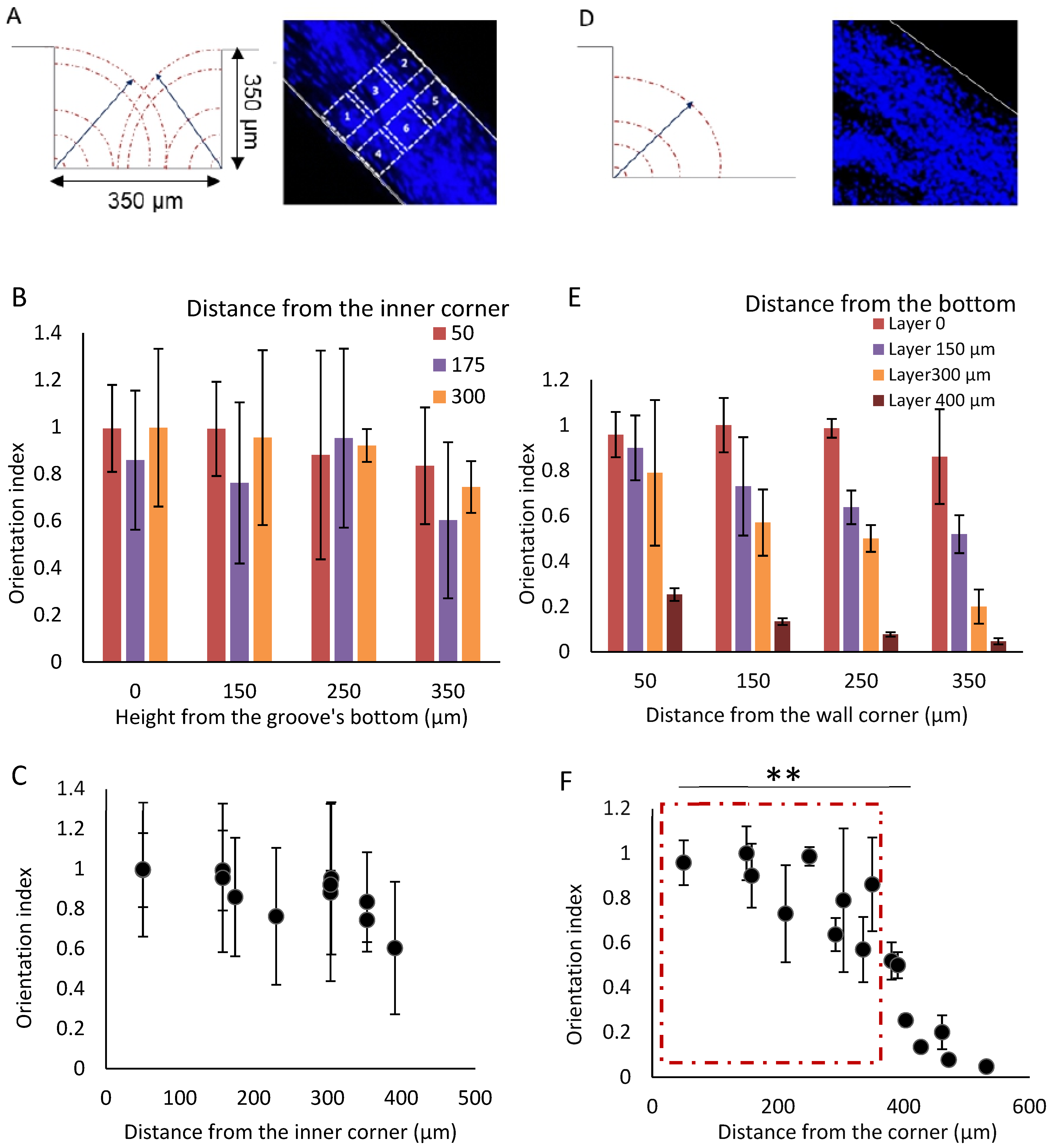
3.2. The Cell Alignment Propagates in the Grooves Thank to the Contact Guidance
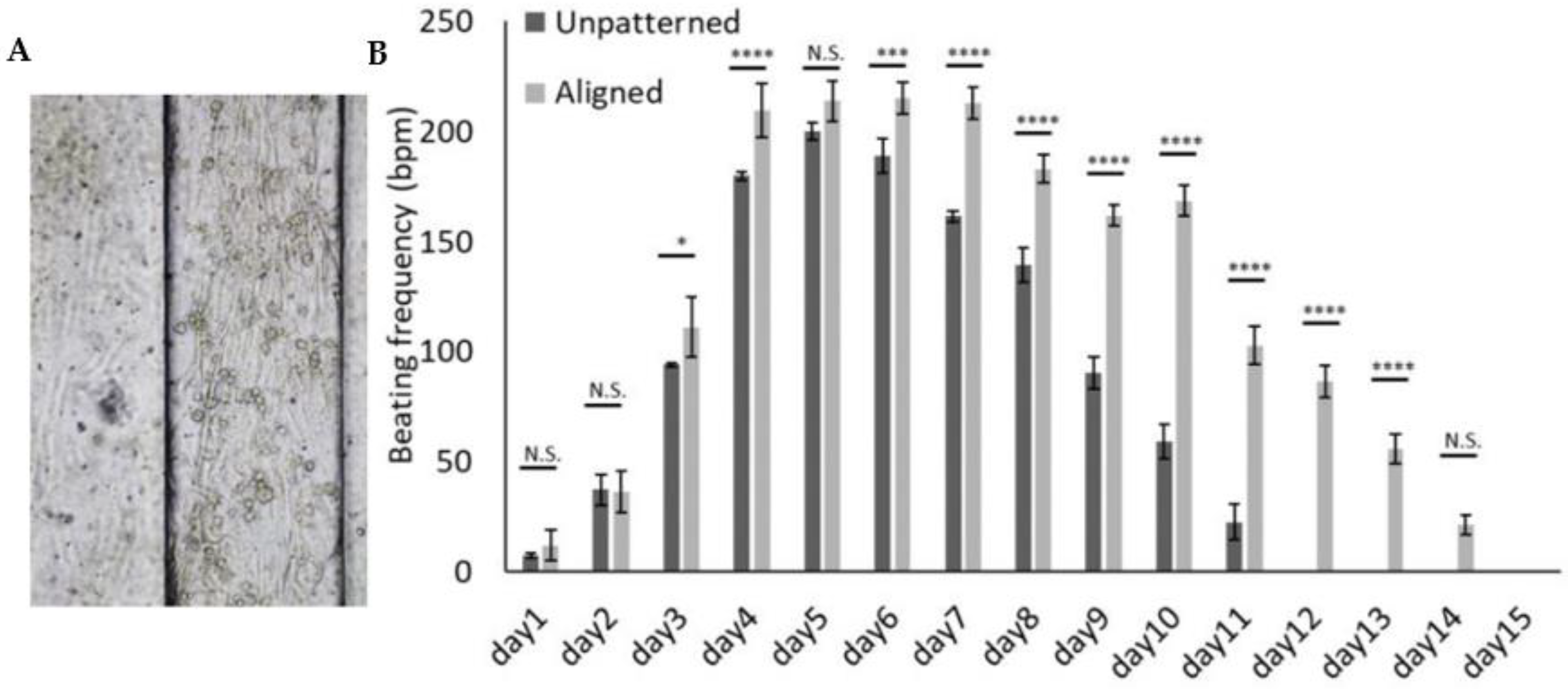
3.3. 3D Patterned Hydrogel Aids a Reliable Measurement of Beating
3.4. Successful Detachability and Stability of the Hydrogel from the PDMS Substrate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mathur, A.; Ma, Z.; Loskill, P.; Jeeawoody, S.; Healy, K.E. In vitro cardiac tissue models: Current status and future prospects. Adv. Drug Deliv. Rev. 2016, 96, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, Y.K.; George, S.C. Tissue engineering the cardiac microenvironment: Multicellular microphysiological systems for drug screening. Adv. Drug Deliv. Rev. 2016, 96, 225–233. [Google Scholar] [CrossRef]
- Aubin, H.; Nichol, J.W.; Hutson, C.B.; Bae, H.; Sieminski, A.L.; Cropek, D.M.; Akhyari, P.; Khademhosseini, A. Directed 3D cell alignment and elongation in microengineered hydrogels. Biomaterials 2010, 31, 6941–6951. [Google Scholar] [CrossRef] [PubMed]
- Helm, P.; Beg, M.F.; Miller, M.I.; Winslow, R.L. Measuring and Mapping Cardiac Fiber and Laminar Architecture Using Diffusion Tensor MR Imaging. Ann. N. Y. Acad. Sci. 2005, 1047, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Camelliti, P.; Borg, T.K.; Kohl, P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc. Res. 2005, 65, 40–51. [Google Scholar] [CrossRef]
- Vasserman, I.N.; Matveenko, V.P.; Shardakov, I.N.; Shestakov, A.P. Numerical simulation of the propagation of electrical excitation in the heart wall taking its fibrous laminar structure into account. Biophysics 2015, 60, 613–621. [Google Scholar] [CrossRef]
- Hsu, E.W.; Henriquez, C.S. Myocardial fiber orientation mapping using reduced encoding diffusion tensor imaging. J. Cardiovasc. Magn. Reson. 2001, 3, 339–347. [Google Scholar] [CrossRef] [PubMed]
- McLean, M.; Prothero, J. Determination of relative fiber orientation in heart muscle: Methodological problems. Anat. Rec. 1992, 232, 459–465. [Google Scholar] [CrossRef]
- Fatemifar, F.; Feldman, M.D.; Oglesby, M.; Han, H.-C. Comparison of Biomechanical Properties and Microstructure of Trabeculae Carneae, Papillary Muscles, and Myocardium in the Human Heart. J. Biomech. Eng. 2018, 141, 021007. [Google Scholar] [CrossRef]
- Halaney, D.L.; Sanyal, A.; Nafissi, N.A.; Escobedo, D.; Goros, M.; Michalek, J.; Acevedo, P.J.; Pérez, W.; Escobar, G.P.; Feldman, M.D.; et al. The Effect of Trabeculae Carneae on Left Ventricular Diastolic Compliance: Improvement in Compliance With Trabecular Cutting. J. Biomech. Eng. 2017, 139, 0310121–0310128. [Google Scholar] [CrossRef]
- Goo, S.; Joshi, P.; Sands, G.; Gerneke, D.; Taberner, A.; Dollie, Q.; LeGrice, I.; Loiselle, D. Trabeculae carneae as models of the ventricular walls: Implications for the delivery of oxygen. J. Gen. Physiol. 2009, 134, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-C.; Taberner, A.J.; Nielsen, P.M.F.; Loiselle, D.S. Interventricular comparison of the energetics of contraction of trabeculae carneae isolated from the rat heart. J. Physiol. 2013, 591, 701–717. [Google Scholar] [CrossRef] [PubMed]
- Sands, G.; Goo, S.; Gerneke, D.; LeGrice, I.; Loiselle, D. The collagenous microstructure of cardiac ventricular trabeculae carneae. J. Struct. Biol. 2011, 173, 110–116. [Google Scholar] [CrossRef]
- Kolewe, M.E.; Park, H.; Gray, C.; Ye, X.; Langer, R.; Freed, L.E. 3D Structural Patterns in Scalable, Elastomeric Scaffolds Guide Engineered Tissue Architecture. Adv. Mater. 2013, 25, 4459–4465. [Google Scholar] [CrossRef] [PubMed]
- Eyckmans, J.; Boudou, T.; Yu, X.; Chen, C.S. A Hitchhiker’s Guide to Mechanobiology. Dev. Cell 2011, 21, 35–47. [Google Scholar] [CrossRef]
- Nikkhah, M.; Edalat, F.; Manoucheri, S.; Khademhosseini, A. Engineering microscale topographies to control the cell–substrate interface. Biomaterials 2012, 33, 5230–5246. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.M.; Stevens, M.M.; George, J.H. Exploring and Engineering the Cell Surface Interface. Science 2005. Available online: https://www.science.org/doi/abs/10.1126/science.1106587 (accessed on 1 September 2021). [CrossRef]
- Grosberg, A.; Alford, P.W.; McCain, M.L.; Parker, K.K. Ensembles of engineered cardiac tissues for physiological and pharmacological study: Heart on a chip. Lab Chip 2011, 11, 4165–4173. [Google Scholar] [CrossRef] [PubMed]
- Bursac, N.; Parker, K.; Iravanian, S.; Tung, L. Cardiomyocyte Cultures With Controlled Macroscopic Anisotropy. Circ. Res. 2002, 91, e45–e54. [Google Scholar] [CrossRef]
- Au, H.T.H.; Cui, B.; Chu, Z.E.; Veres, T.; Radisic, M. Cell culture chips for simultaneous application of topographical and electrical cues enhance phenotype of cardiomyocytes. Lab Chip 2009, 9, 564–575. [Google Scholar] [CrossRef]
- Annabi, N.; Selimovic, S.; Cox, J.P.A.; Ribas, J.; Bakooshli, M.A.; Heintze, D.; Weiss, A.; Cropek, D.; Khademhosseini, A. Hydrogel-coated microfluidic channels for cardiomyocyte culture. Lab Chip 2013, 13, 3569–3577. [Google Scholar] [CrossRef] [PubMed]
- Mosiewicz, K.A.; Kolb, L.; Van Der Vlies, A.J.; Martino, M.; Lienemann, P.; Hubbell, J.; Ehrbar, M.; Lutolf, M.P. In situ cell manipulation through enzymatic hydrogel photopatterning. Nat. Mater. 2013, 12, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.J.; Desai, T.A. Control of Cellular Organization in Three Dimensions Using a Microfabricated Polydimethylsiloxane–Collagen Composite Tissue Scaffold. Tissue Eng. 2005, 11, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Min, J.H.; Cho, K.; Seo, I.H.; Ryu, W.; Koh, W.-G. Fabrication of microgrooved scaffolds using near-field electrospinning-assisted lithography (NFEAL). J. Ind. Eng. Chem. 2019, 80, 471–478. [Google Scholar] [CrossRef]
- Engelmayr, G.C., Jr.; Cheng, M.; Bettinger, C.J.; Borenstein, J.T.; Langer, R.; Freed, L.E. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nat. Mater. 2008, 7, 1003–1010. [Google Scholar] [CrossRef]
- Zimmermann, W.-H.; Schneiderbanger, K.; Schubert, P.; Didié, M.; Münzel, F.; Heubach, J.; Kostin, S.; Neuhuber, W.; Eschenhagen, T. Tissue Engineering of a Differentiated Cardiac Muscle Construct. Circ. Res. 2002, 90, 223–230. [Google Scholar] [CrossRef]
- Zimmermann, W.-H.; Melnychenko, I.; Wasmeier, G.H.; Didié, M.; Naito, H.; Nixdorff, U.; Hess, A.; Budinsky, L.; Brune, K.; Michaelis, B.; et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat. Med. 2006, 12, 452–458. [Google Scholar] [CrossRef]
- Li, Y.; Huang, G.; Zhang, X.; Wang, L.; Du, Y.; Lu, T.J.; Xu, F. Engineering cell alignment in vitro. Biotechnol. Adv. 2014, 32, 347–365. [Google Scholar] [CrossRef]
- Navaee, F.; Renaud, P.; Kleger, A.; Braschler, T. Highly Efficient Cardiac Differentiation and Maintenance by Thrombin-Coagulated Fibrin Hydrogels Enriched with Decellularized Porcine Heart Extracellular Matrix. Int. J. Mol. Sci. 2023, 24, 2842. [Google Scholar] [CrossRef]
- Fatemi Far, S.; Feldman, M.; Han, H.-C. Characterization of Biomechanical Properties of Human Trabeculae Carneae. In Proceedings of the Bioengineering and Biotransport Conference, Tucson, AZ, USA, 21–24 June 2017. [Google Scholar]
- de Mello, R.A.; Mountzios, G.; Tavares, Á.A. International Manual of Oncology Practice: (iMOP)-Principles of Medical Oncology; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Smith, A.S.T.; Yoo, H.; Yi, H.; Ahn, E.H.; Lee, J.H.; Shao, G.; Nagornyak, E.; Laflamme, M.A.; Murry, C.E.; Kim, D.-H. Micro- and nano-patterned conductive graphene–PEG hybrid scaffolds for cardiac tissue engineering. Chem. Commun. 2017, 53, 7412–7415. [Google Scholar] [CrossRef]
- Uttayarat, P.; Chen, M.; Li, M.; Allen, F.D.; Composto, R.J.; Lelkes, P.I. Microtopography and flow modulate the direction of endothelial cell migration. Am. J. Physiol. Circ. Physiol. 2008, 294, H1027–H1035. [Google Scholar] [CrossRef] [PubMed]
- Cortella, L.R.X.; Cestari, I.A.; Lahuerta, R.D.; Araña, M.C.; Soldera, M.; Rank, A.; Lasagni, A.F.; Cestari, I.N. Conditioning of hiPSC-derived cardiomyocytes using surface topography obtained with high throughput technology. Biomed. Mater. 2021, 16, 065007. [Google Scholar] [CrossRef]
- Litowczenko, J.; Maciejewska, B.M.; Wychowaniec, J.K.; Kościński, M.; Jurga, S.; Warowicka, A. Groove-patterned surfaces induce morphological changes in cells of neuronal origin. J. Biomed. Mater. Res. Part A 2019, 107, 2244–2256. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.; Seo, T.; Hariri, S.; Choi, C.; Varghese, S. Matrix Topographical Cue-Mediated Myogenic Differentiation of Human Embryonic Stem Cell Derivatives. Polymers 2017, 9, 580. [Google Scholar] [CrossRef]
- Prager-Khoutorsky, M.; Lichtenstein, A.; Krishnan, R.; Rajendran, K.; Mayo, A.; Kam, Z.; Geiger, B.; Bershadsky, A.D. Fibroblast polarization is a matrix-rigidity-dependent process controlled by focal adhesion mechanosensing. Nat. Cell Biol. 2011, 13, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Cabezas, M.D.; Meckes, B.; Mirkin, C.A.; Mrksich, M. Subcellular Control over Focal Adhesion Anisotropy, Independent of Cell Morphology, Dictates Stem Cell Fate. ACS Nano 2019, 13, 11144–11152. [Google Scholar] [CrossRef] [PubMed]
- Sniadecki, N.J.; Desai, R.A.; Ruiz, S.A.; Chen, C. Nanotechnology for Cell–Substrate Interactions. Ann. Biomed. Eng. 2006, 34, 59–74. [Google Scholar] [CrossRef]
- Baptista, D.; Teixeira, L.; van Blitterswijk, C.; Giselbrecht, S.; Truckenmüller, R. Overlooked? Underestimated? Effects of Substrate Curvature on Cell Behavior. Trends Biotechnol. 2019, 37, 838–854. [Google Scholar] [CrossRef]
- Tijore, A.; Irvine, S.A.; Sarig, U.; Mhaisalkar, P.; Baisane, V.; Venkatraman, S.S. Contact guidance for cardiac tissue engineering using 3D bioprinted gelatin patterned hydrogel. Biofabrication 2017, 10, 025003. [Google Scholar] [CrossRef]
- Naseer, S.M.; Manbachi, A.; Samandari, M.; Walch, P.; Gao, Y.; Zhang, Y.S.; Davoudi, F.; Wang, W.; Abrinia, K.; Cooper, J.M.; et al. Surface acoustic waves induced micropatterning of cells in gelatin methacryloyl (GelMA) hydrogels. Biofabrication 2017, 9, 015020. [Google Scholar] [CrossRef] [PubMed]
- Kankala, R.K.; Zhu, K.; Sun, X.-N.; Liu, C.-G.; Wang, S.-B.; Chen, A.-Z. Cardiac Tissue Engineering on the Nanoscale. ACS Biomater. Sci. Eng. 2018, 4, 800–818. [Google Scholar] [CrossRef] [PubMed]
- You, R.; Li, X.; Luo, Z.; Qu, J.; Li, M. Directional cell elongation through filopodia-steered lamellipodial extension on patterned silk fibroin films. Biointerphases 2015, 10, 011005. [Google Scholar] [CrossRef] [PubMed]
- Yim, E.K.; Darling, E.M.; Kulangara, K.; Guilak, F.; Leong, K.W. Nanotopography-induced changes in focal adhesions, cytoskeletal organization, and mechanical properties of human mesenchymal stem cells. Biomaterials 2010, 31, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-L.; Rivera, A.L.; Williams, V.; Welter, J.F.; Mansour, J.M.; Drazba, J.A.; Sakai, T.; Baskaran, H. Micrometer scale guidance of mesenchymal stem cells to form structurally oriented large-scale tissue engineered cartilage. Acta Biomater. 2017, 60, 210–219. [Google Scholar] [CrossRef]
- Salick, M.R.; Napiwocki, B.N.; Sha, J.; Knight, G.T.; Chindhy, S.A.; Kamp, T.J.; Ashton, R.S.; Crone, W.C. Micropattern width dependent sarcomere development in human ESC-derived cardiomyocytes. Biomaterials 2014, 35, 4454–4464. [Google Scholar] [CrossRef]
- Izu, L.T.; Kohl, P.; Boyden, P.A.; Miura, M.; Banyasz, T.; Chiamvimonvat, N.; Trayanova, N.; Bers, D.M.; Chen-Izu, Y. Mechano-electric and mechano-chemo-transduction in cardiomyocytes. J. Physiol. 2020, 598, 1285–1305. [Google Scholar] [CrossRef]
- Sartiani, L.; Bettiol, E.; Stillitano, F.; Mugelli, A.; Cerbai, E.; Jaconi, M.E. Developmental Changes in Cardiomyocytes Differentiated from Human Embryonic Stem Cells: A Molecular and Electrophysiological Approach. Stem Cells 2007, 25, 1136–1144. [Google Scholar] [CrossRef]
- van Spreeuwel, A.C.C.; Bax, N.A.M.; Bastiaens, A.J.; Foolen, J.; Loerakker, S.; Borochin, M.; van der Schaft, D.W.J.; Chen, C.S.; Baaijens, F.P.T.; Bouten, C.V.C. The influence of matrix (an)isotropy on cardiomyocyte contraction in engineered cardiac microtissues. Integr. Biol. 2014, 6, 422–429. [Google Scholar] [CrossRef]
- Ponard, J.G.; Kondratyev, A.A.; Kucera, J.P. Mechanisms of Intrinsic Beating Variability in Cardiac Cell Cultures and Model Pacemaker Networks. Biophys. J. 2007, 92, 3734–3752. [Google Scholar] [CrossRef]
- Ahearne, M. Introduction to cell–hydrogel mechanosensing. Interface Focus 2014, 4, 20130038. [Google Scholar] [CrossRef]
- van Meer, B.; de Vries, H.; Firth, K.; van Weerd, J.; Tertoolen, L.; Karperien, H.; Jonkheijm, P.; Denning, C.; Ijzerman, A.; Mummery, C. Small molecule absorption by PDMS in the context of drug response bioassays. Biochem. Biophys. Res. Commun. 2017, 482, 323–328. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navaee, F.; Khornian, N.; Longet, D.; Heub, S.; Boder-Pasche, S.; Weder, G.; Kleger, A.; Renaud, P.; Braschler, T. A Three-Dimensional Engineered Cardiac In Vitro Model: Controlled Alignment of Cardiomyocytes in 3D Microphysiological Systems. Cells 2023, 12, 576. https://doi.org/10.3390/cells12040576
Navaee F, Khornian N, Longet D, Heub S, Boder-Pasche S, Weder G, Kleger A, Renaud P, Braschler T. A Three-Dimensional Engineered Cardiac In Vitro Model: Controlled Alignment of Cardiomyocytes in 3D Microphysiological Systems. Cells. 2023; 12(4):576. https://doi.org/10.3390/cells12040576
Chicago/Turabian StyleNavaee, Fatemeh, Niloofar Khornian, David Longet, Sarah Heub, Stephanie Boder-Pasche, Gilles Weder, Alexander Kleger, Philippe Renaud, and Thomas Braschler. 2023. "A Three-Dimensional Engineered Cardiac In Vitro Model: Controlled Alignment of Cardiomyocytes in 3D Microphysiological Systems" Cells 12, no. 4: 576. https://doi.org/10.3390/cells12040576
APA StyleNavaee, F., Khornian, N., Longet, D., Heub, S., Boder-Pasche, S., Weder, G., Kleger, A., Renaud, P., & Braschler, T. (2023). A Three-Dimensional Engineered Cardiac In Vitro Model: Controlled Alignment of Cardiomyocytes in 3D Microphysiological Systems. Cells, 12(4), 576. https://doi.org/10.3390/cells12040576






