High-Performance Polarization Microscopy Reveals Structural Remodeling in Rat Calcaneal Tendons Cultivated In Vitro
Abstract
1. Introduction
2. Materials and Methods
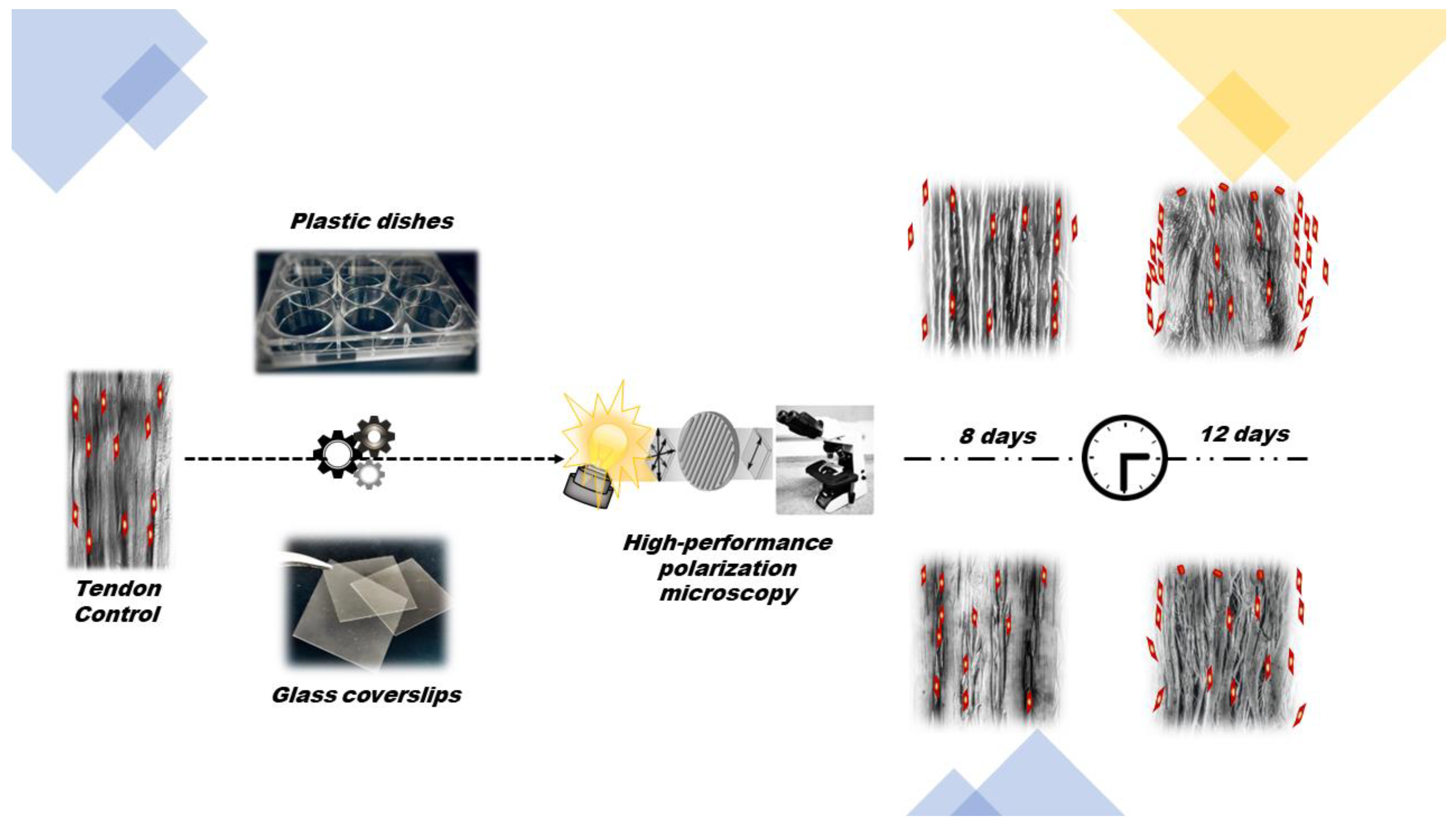
2.1. Animals and Sample Preparations
2.2. Birefringence
2.3. Differential Interference Contrast Microscopy (DIC-PLM)
2.4. Linear Dichroism (LD)
2.5. Tenocyte Migration
2.6. Statistics
3. Results
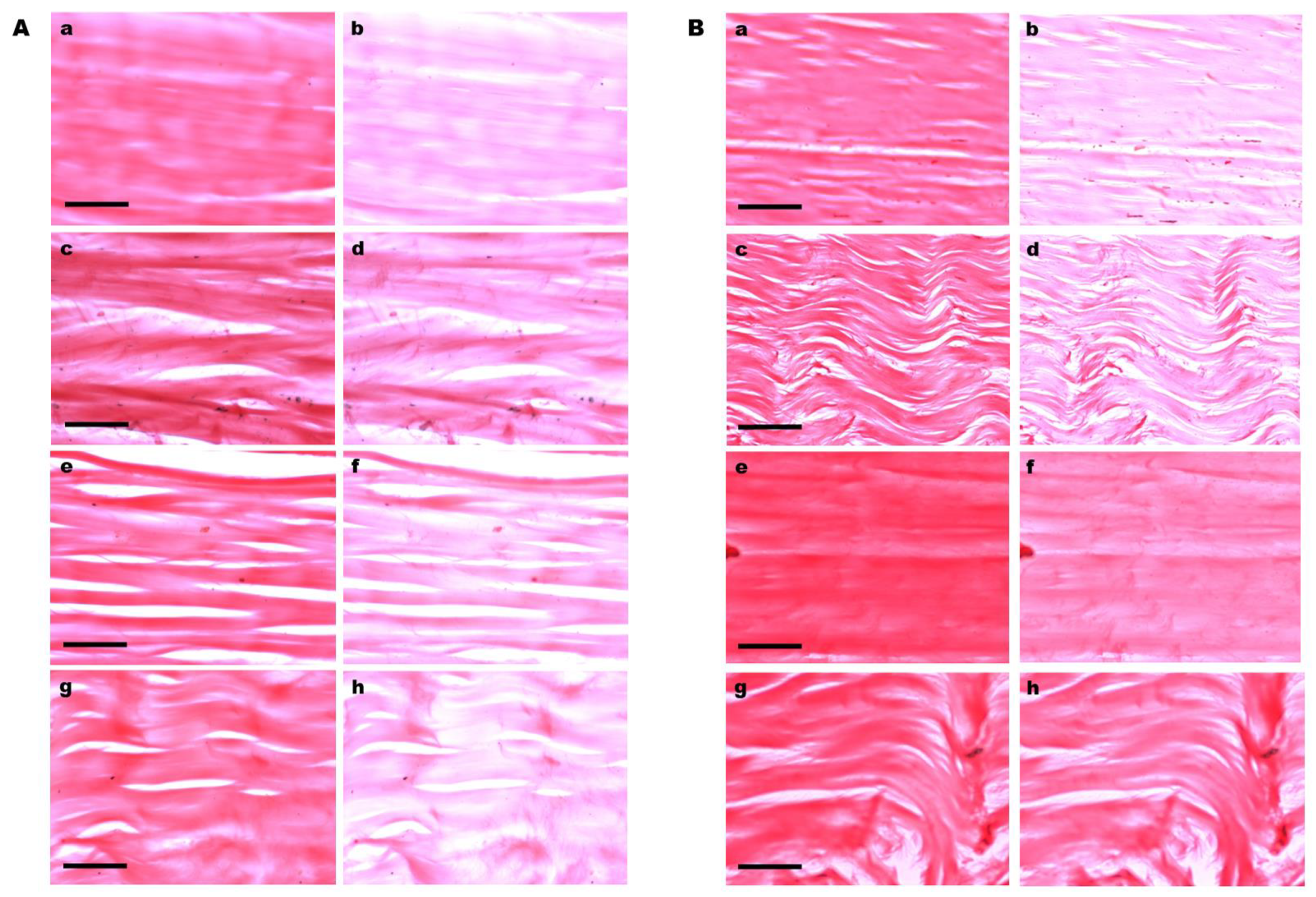
3.1. Birefringence
3.2. Linear Dichroism (LD)
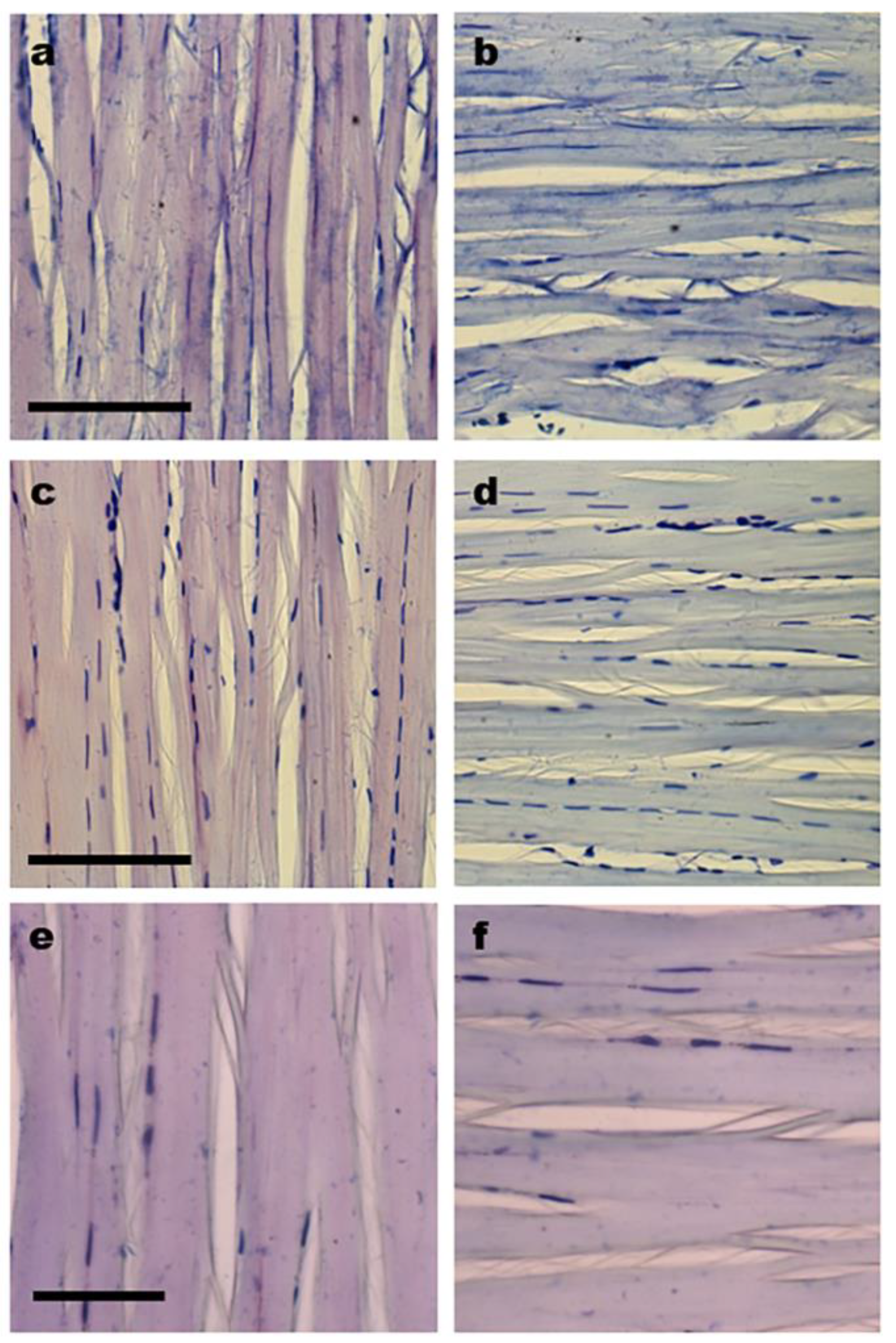
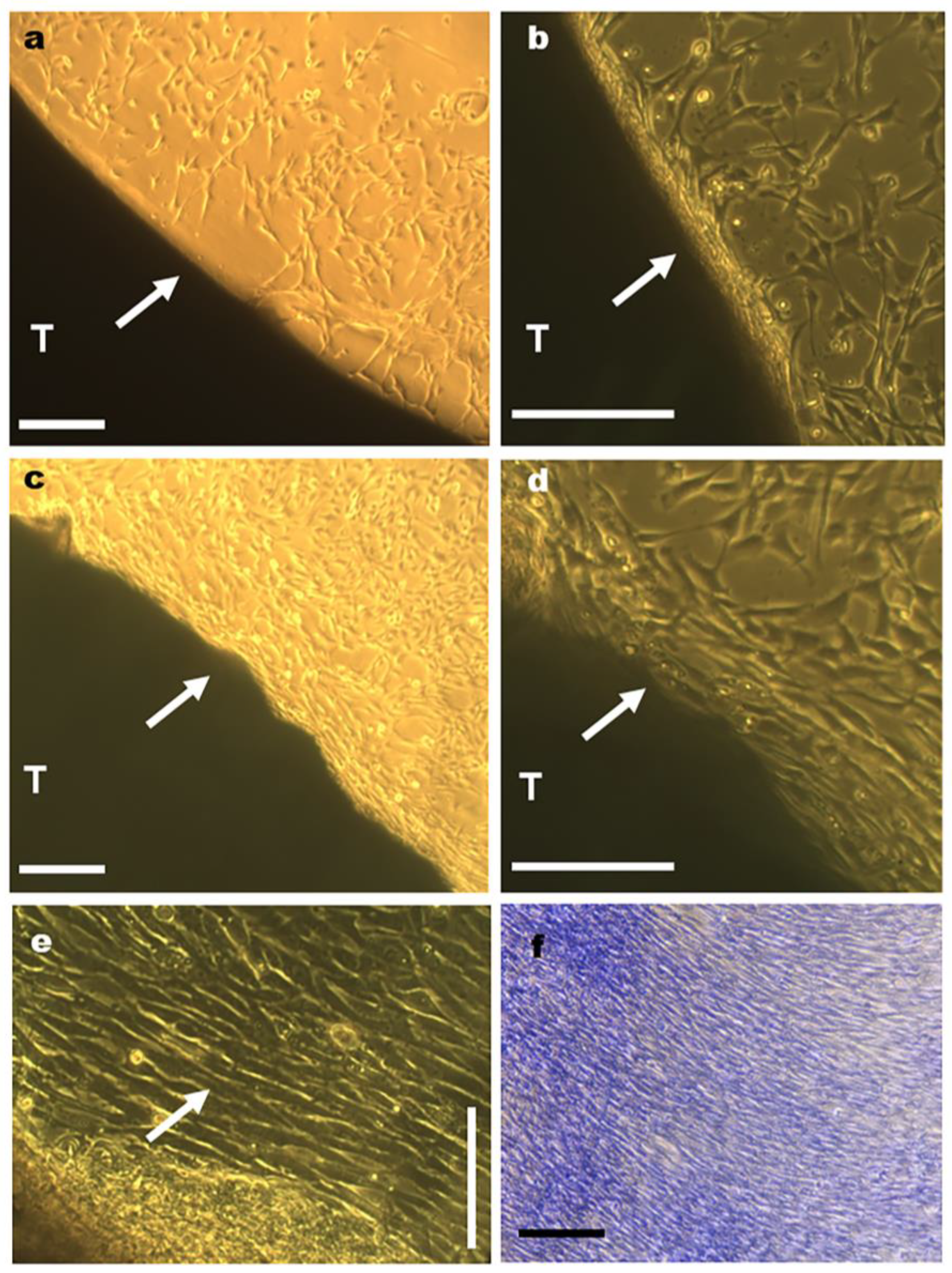
3.3. Cell Distribution
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Brien, M. Anatomy of Tendons. In Tendon Injuries; Maffulli, N., Renström, P., Leadbetter, W.B., Eds.; Springer: London, UK, 2005; pp. 3–13. [Google Scholar]
- de Aro, A.A.; Vidal, B.D.C.; Pimentel, E.R. Biochemical and anisotropical properties of tendons. Micron 2012, 43, 205–214. [Google Scholar] [CrossRef]
- Wang, J.H.-C.; Thampatty, B.P. Advances in Tendon Mechanobiology. In Mechanobiology in Health and Disease; Verbruggen, S., Ed.; Academic Press: Cambridge, MA, USA, 2008; pp. 127–155. [Google Scholar]
- Wunderli, S.L.; Blache, U.; Snedeker, J.G. Tendon explant models for physiologically relevant in vitro study of tissue biology—A perspective. Connect. Tissue Res. 2020, 61, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Izu, Y.; Adams, S.M.; Connizzo, B.K.; Beason, D.P.; Soslowsky, L.J.; Koch, M.; Birk, D.E. Collagen XII mediated cellular and extracellular mechanisms regulate establishment of tendon structure and function. Matrix Biol. 2021, 95, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Athenstaedt, H. Pyroelectric and piezoelectric properties of vertebrates. Ann. N. Y. Acad. Sci. 1974, 238, 68–94. [Google Scholar] [CrossRef]
- Akagi, K.; Piao, G.; Kaneko, S.; Sakamaki, K.; Shirakawa, H.; Kyotani, M. Helical Polyacetylene Synthesized with a Chiral Nematic Reaction Field. Science 1998, 282, 1683–1686. [Google Scholar] [CrossRef]
- Vidal, B. Form birefringence as applied to biopolymer and inorganic material supraorganization. Biotech. Histochem. 2010, 85, 365–378. [Google Scholar] [CrossRef]
- Jaiswal, D.; Yousman, L.; Neary, M.; Fernschild, E.; Zolnoski, B.; Katebifar, S.; Rudraiah, S.; Mazzocca, A.D.; Kumbar, S.G. Tendon tissue engineering: Biomechanical considerations. Biomed. Mater. 2020, 15, 052001. [Google Scholar] [CrossRef] [PubMed]
- Vidal, B.D.C. Pleochroism in tendon and its bearing to acid mucopolysaccharides. Protoplasma 1963, 56, 529–536. [Google Scholar] [CrossRef]
- Vidal, B.D.C. The part played by the mucopolysaccharides in the form birefringence of the collagen. Protoplasma 1965, 59, 472–479. [Google Scholar] [CrossRef]
- Vidal, B.D.C. The part played by proteoglycans and structural glycoproteins in the macromolecular orientation of collagen bundles. Cell. Mol. Biol. 1980, 26, 415–421. [Google Scholar]
- Mello, M.L.; Vidal, B.C. Anisotropic properties of toluidine blue-stained collagen. Ann. Histochim. 1973, 18, 103–122. [Google Scholar]
- Vidal, B.C.; Mello, M.L. Proteoglycan arrangement in tendon collagen bundles. Cell. Mol. Biol. 1984, 30, 195–204. [Google Scholar] [PubMed]
- Vidal, B.C.; Mello, M.L.S. Optical anisotropy of collagen fibers of rat calcaneal tendons: An approach to spatially resolved supramolecular organization. Acta Histochem. 2010, 112, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Bennet, S. The Microscopical Investigation of Biological Materials with Polarized Light. In McClung’s Handbook of Microscopical Technique; Jones, R.M., Ed.; Hafner: New York, NY, USA, 1967; pp. 591–677. [Google Scholar]
- Vidal, B.D.C. Evaluation of the carbohydrate role in the molecular order of collagen bundles: Microphotometric measurements of textural birefringence. Cell. Mol. Biol. 1986, 32, 527–535. [Google Scholar] [PubMed]
- Vidal, B.C. Using the FT-IR linear dichroism method for molecular order determination of tendon collagen bundles and nylon 6. Acta Histochem. 2013, 115, 686–691. [Google Scholar] [CrossRef]
- Vidal, B.D.C. Dichroism in collagen bundles stained with Xylidine-Ponceau 2R. Ann. Histochim. 1970, 15, 289–296. [Google Scholar]
- Vidal, B.C.; Mello, M.L.S. Supramolecular order following binding of the dichroic birefringent sulfonic dye Ponceau SS to collagen fibers. Biopolymers 2005, 78, 121–128. [Google Scholar] [CrossRef]
- Vidal, B.D.C.; Mello, M.L.S. Toluidine blue staining for cell and tissue biology applications. Acta Histochem. 2019, 121, 101–112. [Google Scholar] [CrossRef]
- Vidal, B.D.C. Macromolecular disorientation in detached tendons. Protoplasma 1966, 62, 121–132. [Google Scholar] [CrossRef]
- Mello, M.L.S.; Godo, C.; Vidal, B.C.; Abujadi, J.M. Changes in macromolecular orientation on collagen fibers during the process of tendon repair in the rat. Ann. Histochim. 1975, 20, 145–152. [Google Scholar]
- da Cunha, A.; Parizotto, N.; Vidal, B.D.C. The effect of therapeutic ultrasound on repair of the Achilles tendon (tendo calcaneus) of the rat. Ultrasound Med. Biol. 2001, 27, 1691–1696. [Google Scholar] [CrossRef] [PubMed]
- Carrinho, P.M.; Renno, A.C.M.; Koeke, P.; Salate, A.C.B.; Parizotto, N.A.; Vidal, B.C. Comparative Study Using 685-nm and 830-nm Lasers in the Tissue Repair of Tenotomized Tendons in the Mouse. Photomed. Laser Surg. 2006, 24, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Aro, A.A.; Vidal, B.C.; Tomiosso, T.C.; Gomes, L.; Matiello-Rosa, S.M.G.; Pimentel, E.R. Structural and Biochemical Analysis of the Effect of Immobilization Followed by Stretching on the Calcaneal Tendon of Rats. Connect. Tissue Res. 2008, 49, 443–454. [Google Scholar] [CrossRef]
- Aro, A.; Freitas, K.; Foglio, M.; Carvalho, J.; Dolder, H.; Gomes, L.; Vidal, B.; Pimentel, E. Effect of the Arrabidaea chica extract on collagen fiber organization during healing of partially transected tendon. Life Sci. 2013, 92, 799–807. [Google Scholar] [CrossRef]
- De Aro, A.A.; Carneiro, G.D.; Teodoro, L.F.R.; Da Veiga, F.C.; Ferrucci, D.L.; Simões, G.F.; Simões, P.W.; Alvares, L.E.; De Oliveira, A.L.R.; Vicente, C.P.; et al. Injured Achilles Tendons Treated with Adipose-Derived Stem Cells Transplantation and GDF-5. Cells 2018, 7, 127. [Google Scholar] [CrossRef]
- Frauz, K.; Teodoro, L.F.R.; Carneiro, G.D.; da Veiga, F.C.; Ferrucci, D.L.; Bombeiro, A.L.; Simões, P.W.; Alvares, L.E.; de Oliveira, A.L.R.; Vicente, C.P.; et al. Transected Tendon Treated with a New Fibrin Sealant Alone or Associated with Adipose-Derived Stem Cells. Cells 2019, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Mello, M.L.S.; Vidal, B.; De Carvalho, A.; Caseiro-Filho, A. Change with Age of Anisotropic Properties of Collagen Bundles. Gerontology 1979, 25, 2–8. [Google Scholar] [CrossRef]
- Vilarta, R.; Vidal, B.D.C. Anisotropic and Biomechanical Properties of Tendons Modified by Exercise and Denervation: Aggregation and Macromolecular Order in Collagen Bundles. Matrix 1989, 9, 55–61. [Google Scholar] [CrossRef]
- Vidal, B.C. Acid glycosaminoglycans and endochondral ossification: Microspectrophotometry evaluation and macromolecular orientation. Cell. Mol. Biol. 1977, 22, 45–64. [Google Scholar]
- Braga-Vilela, A.S.; Pimentel, E.R.; Marangoni, S.; Toyama, M.H.; Vidal, B.D.C. Extracellular Matrix of Porcine Pericardium: Biochemistry and Collagen Architecture. J. Membr. Biol. 2008, 221, 15–25. [Google Scholar] [CrossRef]
- Vidal, B.D.C.; Mello, M.L.S. Structural organization of collagen fibers in chordae tendineae as assessed by optical anisotropic properties and Fast Fourier transform. J. Struct. Biol. 2009, 167, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.D.F.T.D.; Vidal, B.D.C.; Zezell, D.M.; Zorn, T.M.T.; Ribeiro, M.S.; Núñez, S.C. Collagen birefringence in skin repair in response to red polarized-laser therapy. J. Biomed. Opt. 2006, 11, 024002. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.F.; dos Anjos, E.H.M.; Mello, M.L.S.; Vidal, B.D.C. Skin Collagen Fiber Molecular Order: A Pattern of Distributional Fiber Orientation as Assessed by Optical Anisotropy and Image Analysis. PLoS ONE 2013, 8, e54724. [Google Scholar] [CrossRef] [PubMed]
- Vidal, B.C.; dos Anjos, E.H.M.; Mello, M.L.S. Optical anisotropy reveals molecular order in a mouse enthesis. Cell Tissue Res. 2015, 362, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Youngstrom, D.; Barrett, J.G. Engineering Tendon: Scaffolds, Bioreactors, and Models of Regeneration. Stem Cells Int. 2016, 2016, 3919030. [Google Scholar] [CrossRef]
- Vidal, B.C. Cell and extracellular matrix interaction: A feedback theory based on molecular order and recognition-adhesion events. Rev. Fac. Ciênc. Med. Unicamp 1993, 4, 11–14. [Google Scholar]
- Vidal, B.D.C. Image analysis of tendon helical superstructure using interference and polarized light microscopy. Micron 2003, 34, 423–432. [Google Scholar] [CrossRef]
- Buschmann, J.; Bürgisser, G.M. (Eds.) Structure and Function of Tendon and Ligament Tissues. In Biomechanics of Tendons and Ligaments; Woodhead Publishing: Sawston, UK, 2017; pp. 3–29. [Google Scholar] [CrossRef]
- Vermeulen, S.; Vasilevich, A.; Tsiapalis, D.; Roumans, N.; Vroemen, P.; Beijer, N.R.; Eren, A.D.; Zeugolis, D.; de Boer, J. Identification of topographical architectures supporting the phenotype of rat tenocytes. Acta Biomater. 2018, 83, 277–290. [Google Scholar] [CrossRef]
- Zhang, T.; Day, J.H.; Su, X.; Guadarrama, A.G.; Sandbo, N.K.; Esnault, S.; Denlinger, L.C.; Berthier, E.; Theberge, A.B. Investigating Fibroblast-Induced Collagen Gel Contraction Using a Dynamic Microscale Platform. Front. Bioeng. Biotechnol. 2019, 7, 196. [Google Scholar] [CrossRef]
- Clegg, P.D.; Strassburg, S.; Smith, R.K. Cell phenotypic variation in normal and damaged tendons. Int. J. Exp. Pathol. 2007, 88, 227–235. [Google Scholar] [CrossRef]
- Patterson-Kane, J.C.; Firth, E.C. Tendon, Ligament, Bone, and Cartilage: Anatomy, Physiology, and Adaptations to Exercise and Training. In The Athletic Horse, 2nd ed.; Hodgson, D.R., McKeever, K.H., McGowan, C.M., Eds.; Saunders: Philadelphia, PA, USA, 2014; pp. 202–242. [Google Scholar] [CrossRef]
- Dos Anjos, E.H.M.; Mello, M.L.S.; Vidal, B.D.C. Birefringence optical retardations in sections of rat calcaneal tendons cultured in vitro. Repository of Research Data of Unicamp (REDU). 2021. [CrossRef]
- Valdetaro, G.P.; Aldrovani, M.; Padua, I.R.M.; Cristovam, P.C.; Gomes, J.A.P.; Laus, J.L. Supra-organization and optical anisotropies of the extracellular matrix in the amniotic membrane and limbal stroma before and after explant culture. Biomed. Opt. Express 2016, 7, 4982–4994. [Google Scholar] [CrossRef] [PubMed]
- Lucke, L.D.; Bortolazzo, F.O.; Theodoro, V.; Fujii, L.; Bombeiro, A.L.; Felonato, M.; Dalia, R.A.; Carneiro, G.D.; Cartarozzi, L.P.; Vicente, C.P.; et al. Low-level laser and adipose-derived stem cells altered remodelling genes expression and improved collagen reorganization during tendon repair. Cell Prolif. 2019, 52, e12580. [Google Scholar] [CrossRef]
- García, J.R.; Garcia, A.J. Sensing rigidity. Nat. Mater. 2014, 13, 539–540. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Ohno, K.; Hayashi, K.; Kuriyama, H.; Yasuda, K.; Kaneda, K. Effects of Stress Shielding on the Mechanical Properties of Rabbit Patellar Tendon. J. Biomech. Eng. 1993, 115, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Kim, W.; Kremen, T.J.J. In Vitro Cellular Strain Models of Tendon Biology and Tenogenic Differentiation. Front. Bioeng. Biotechnol. 2022, 10, 826748. [Google Scholar] [CrossRef] [PubMed]
- Cadby, J.A.; Buehler, E.; Godbout, C.; Van Weeren, P.R.; Snedeker, J.G. Differences between the Cell Populations from the Peritenon and the Tendon Core with Regard to Their Potential Implication in Tendon Repair. PLoS ONE 2014, 9, e92474. [Google Scholar] [CrossRef]
- Schwarz, S.; Gögel, C.; Ondruschka, B.; Hammer, N.; Kohl, B.; Schulze-Tanzil, G. Migrating myofibroblastic iliotibial band-derived fibroblasts represent a promising cell source for ligament reconstruction. Int. J. Mol. Sci. 2019, 20, 1972. [Google Scholar] [CrossRef]
- Wall, M.E.; Banes, A.J. Early responses to mechanical load in tendon: Role for calcium signaling, gap junctions and intercellular communication. J. Musculoskelet. Neuronal Interact. 2005, 5, 70–84. [Google Scholar]
- Wall, M.E.; Dyment, N.A.; Bodle, J.; Volmer, J.; Loboa, E.; Cederlund, A.; Fox, A.M.; Banes, A.J. Cell Signaling in Tenocytes: Response to Load and Ligands in Health and Disease. Adv. Exp. Med. Biol. 2016, 920, 79–95. [Google Scholar] [CrossRef]
- Schulze-Tanzil, G.; Mobasheri, A.; Clegg, P.D.; Sendzik, J.; John, T.; Shakibaei, M. Cultivation of human tenocytes in high-density culture. Histochem. Cell Biol. 2004, 122, 219–228. [Google Scholar] [CrossRef]
- Girke, G.; Kohl, B.; Busch, C.; John, T.; Godkin, O.; Ertel, W.; Schulze-Tanzil, G. Tenocyte activation and regulation of complement factors in response to in vitro cell injury. Mol. Immunol. 2014, 60, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Frisch, S.M.; Vuori, K.; Ruoslahti, E.; Chan-Hui, P.Y. Control of adhesion-dependent cell survival by focal adhesion kinase. J. Cell Biol. 1996, 134, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Ruoslahti, E. Stretching Is Good for a Cell. Science 1997, 276, 1345. [Google Scholar] [CrossRef]
- Chicurel, M.E.; Chen, C.S.; Ingber, D.E. Cellular control lies in the balance of forces. Curr. Opin. Cell Biol. 1998, 10, 232–239. [Google Scholar] [CrossRef]
- Tibbles, L.A.; Woodgett, J.R. The stress-activated protein kinase pathways. Cell. Mol. Life Sci. 1999, 55, 1230–1254. [Google Scholar] [CrossRef]
- Ramirez, F.; Rifkin, D.B. Cell signaling events: A view from the matrix. Matrix Biol. 2003, 22, 101–107. [Google Scholar] [CrossRef]
- Kjaer, M. Role of Extracellular Matrix in Adaptation of Tendon and Skeletal Muscle to Mechanical Loading. Physiol. Rev. 2004, 84, 649–698. [Google Scholar] [CrossRef]
- Jelinsky, S.A.; Li, L.; Ellis, D.; Archambault, J.; Li, J.; Andre, M.S.; Morris, C.; Seeherman, H. Treatment with rhBMP12 or rhBMP13 increase the rate and the quality of rat Achilles tendon repair. J. Orthop. Res. 2011, 29, 1604–1612. [Google Scholar] [CrossRef]
- Chang, H.-N.; Pang, J.-H.S.; Chen, C.P.; Ko, P.-C.; Lin, M.-S.; Tsai, W.-C.; Yang, Y.-M. The effect of aging on migration, proliferation, and collagen expression of tenocytes in response to ciprofloxacin. J. Orthop. Res. 2011, 30, 764–768. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, T.; Shukunami, C.; Okamoto, N.; Taniwaki, T.; Oka, K.; Sakamoto, H.; Ide, J.; Mizuta, H.; Hiraki, Y. FGF-2 Stimulates the Growth of Tenogenic Progenitor Cells to Facilitate the Generation of Tenomodulin-Positive Tenocytes in a Rat Rotator Cuff Healing Model. Am. J. Sports Med. 2015, 43, 2411–2422. [Google Scholar] [CrossRef]
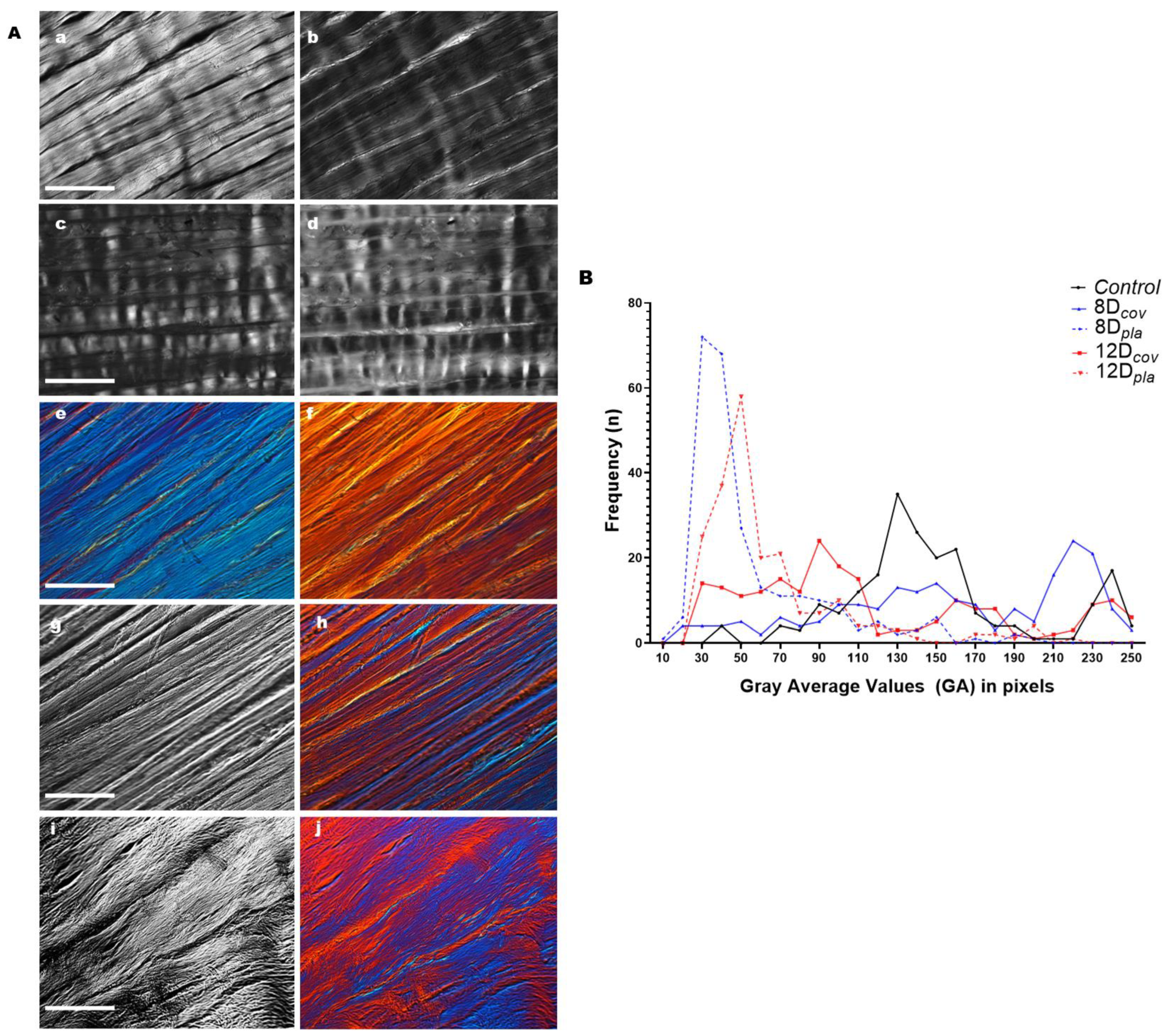
| Items | Average Gray Values in Pixels (GA) | ||||
|---|---|---|---|---|---|
| Control | 8Dcov | 8Dpla | 12Dcov | 12Dpla | |
| No. of measurements | 206 | 206 | 251 | 206 | 207 |
| Minimum GA | 40.28 | 22.57 | 14.15 | 27.26 | 28.90 |
| Maximum GA | 247.91 | 247.90 | 199.50 | 247.00 | 218.40 |
| Median | 139.95 | 157.57 | 41.21 | 96.60 | 52.84 |
| Mean | 148.32 | 158.27 | 54.86 | 114.98 | 64.96 |
| Standard Deviation | 46.57 | 61.92 | 34.56 | 64.62 | 36.55 |
| Parameter | Comparison | Test | p | Decision |
|---|---|---|---|---|
| GA (pixels) | 8Dcov vs. 12Dcov vs. Control | KW | <0.0001 | SS |
| 8Dcov vs. Control | MW | 0.0460 | S | |
| 12Dcov vs. Control | MW | <0.0001 | SS | |
| 8Dcov vs. 12Dcov | MW | <0.0001 | SS | |
| 8Dpla vs. 12Dpla vs. Control | KW | <0.0001 | SS | |
| 8Dpla vs. Control | MW | <0.0001 | SS | |
| 12Dpla vs. Control | MW | <0.0001 | SS | |
| 8Dpla vs. 12Dpla | MW | <0.0001 | SS | |
| 8Dcov vs. 8Dpla vs. Control | KW | <0.0001 | SS | |
| 8Dcov vs. 8Dpla | MW | <0.0001 | SS | |
| 8Dcov vs. 12Dpla | MW | <0.0001 | SS | |
| 12Dcov vs. 12Dpla vs. Control | KW | <0.0001 | SS | |
| 12Dcov vs. 12Dpla | MW | <0.0001 | SS | |
| 12Dcov vs. 8Dpla | MW | <0.0001 | SS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Anjos, E.H.M.; Mello, M.L.S.; Vidal, B.d.C. High-Performance Polarization Microscopy Reveals Structural Remodeling in Rat Calcaneal Tendons Cultivated In Vitro. Cells 2023, 12, 566. https://doi.org/10.3390/cells12040566
dos Anjos EHM, Mello MLS, Vidal BdC. High-Performance Polarization Microscopy Reveals Structural Remodeling in Rat Calcaneal Tendons Cultivated In Vitro. Cells. 2023; 12(4):566. https://doi.org/10.3390/cells12040566
Chicago/Turabian Styledos Anjos, Eli Heber Martins, Maria Luiza Silveira Mello, and Benedicto de Campos Vidal. 2023. "High-Performance Polarization Microscopy Reveals Structural Remodeling in Rat Calcaneal Tendons Cultivated In Vitro" Cells 12, no. 4: 566. https://doi.org/10.3390/cells12040566
APA Styledos Anjos, E. H. M., Mello, M. L. S., & Vidal, B. d. C. (2023). High-Performance Polarization Microscopy Reveals Structural Remodeling in Rat Calcaneal Tendons Cultivated In Vitro. Cells, 12(4), 566. https://doi.org/10.3390/cells12040566







