Abstract
The application of immunotherapy for cancer treatment is rapidly becoming more widespread. Immunotherapeutic agents are frequently combined with various types of treatments to obtain a more durable antitumor clinical response in patients who have developed resistance to monotherapy. Chemotherapeutic drugs that induce DNA damage and trigger DNA damage response (DDR) frequently induce an increase in the expression of the programmed death ligand-1 (PD-L1) that can be employed by cancer cells to avoid immune surveillance. PD-L1 exposed on cancer cells can in turn be targeted to re-establish the immune-reactive tumor microenvironment, which ultimately increases the tumor’s susceptibility to combined therapies. Here we review the recent advances in how the DDR regulates PD-L1 expression and point out the effect of etoposide, irinotecan, and platinum compounds on the anti-tumor immune response.
1. Introduction
Surgery, chemotherapy, and radiation therapy constitute the main approaches to cancer treatment. One alternative way of targeting cancer concentrates on boosting the patient’s immune system so that it can act against the tumor. The term “cancer immunotherapy” refers to a set of treatment methods that involve either the modification of the immune system of the host or the employment of immune system components to combat cancer. Cytokines, immune cells, and monoclonal antibodies are a few examples of immune system components that have been utilized in the treatment of cancer [1]. Due to the fact that cancer cells are derived from normal cells, they may not express antigens against which an immune response can be developed [2].
Although there are several cancer antigens distinguished, they may not trigger a significant level of the immune response. Moreover, the antigen may not be efficiently uptaken, processed, and presented, leading to a diminished and insufficient immunological response. Afterwards, activation of specific T and B cells in the lymph nodes is necessary for the immune cells to migrate to the tumor site, where they can act. In case of the successful elimination of tumor cells, the second wave of antigen presentation may occur and the phenomenon known as “epitope spreading” could expand the immune repertoire that is directed against the tumor [2].
The death of cancer cells in response to the drug may be sufficient to trigger an immune response [3]. The release of antigens can activate the T-cells [4] that detect the dying cancer cells exhibiting calreticulin (CRT) and phosphatidylserine (PS) on their surface. These molecules are recognized by the immune cells such as macrophages enabling the elimination of cancer cells [5,6]. Immature dendritic cells (DCs) and macrophages express the transmembrane receptor CD91 (also known as LRP1) on their surface that interacts with CRT on tumor cells. Moreover, the autophagy-dependent release of ATP work as a recognition signal for DCs exhibiting P2X purinoceptor 7 (P2RX7) on the surface and leads to the inflammasome-mediated secretion of interleukin 1β (IL-1β). Moreover, the secretion of high-mobility group protein 1 (HMGB1)—a ligand for toll-like receptor (TLR-4)—is required for DCs activation and effective antigen presentation [7]. These molecules, called damage-associated molecular pattern molecules (DAMPS), stimulate the activation of the immune system [8,9].
All these components work in concert to bring about the sequential events of tumor cell recognition and recruitment of DCs, phagocytosis, antigen processing, maturation, and presentation to T lymphocytes. Last but not least, the cascade produces an interferon (IFN)-mediated immune response involving γδ T cells and cytotoxic CD8+ T lymphocytes (Figure 1) [10].
As described above, the immune response can identify cancer cells and eliminate them through a variety of processes that involve cooperation between the innate and adaptive branches of the immune system. T-cells play a significant part in this process. The activation of these cells helps to activate an immune response that attacks cancer cells. A specific peptide epitope of the antigen has to be displayed on the major histocompatibility complex (MHC) of an antigen-presenting cell (APC), and it has to form a complex with the T-cell receptor on T cells to become activated. The second signal resulting from the interaction of co-stimulatory molecules is essential for T-cell activation. T-cells will enter the unresponsive condition of clonal anergy if they are not given any co-stimulatory molecules to work with [11]. There are examples of both positive and negative regulators of this process. While positive regulators boost anti-tumor activity, negative regulators impede the killing process and instead promote the growth of tumors. Therefore, an immunotherapy that targets the negative regulators to increase anti-tumor responses could be a viable alternative therapeutic method of treating cancer [12].
Immune checkpoint pathways, such as the programmed death receptor-1 and programmed death ligand-1 (PD-1/PD-L1) signaling pathway, are crucial for the regulation of immune self-tolerance that can be employed by cancer cells to avoid immune surveillance. Inhibition of these T cell surface receptors leads to elevated autoimmunity, which in turn generates an immune response against cancer [13,14].
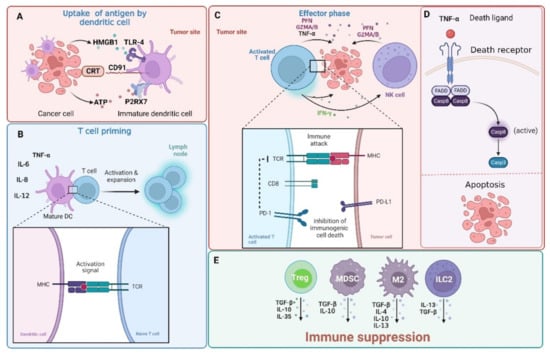
Figure 1.
The interaction between tumor cells and immune cells. Tumor cells are detected by adaptive (comprising of B and T cells) and innate immune system (mainly natural killer (NK) cells). Effector CD4+ and cytotoxic CD8+ T cells are capable of recognizing peptide antigens presented on MHC class II or MHC class I, respectively. (A) Immunogenic cell death (ICD) is associated with the release of damage-associated molecular patterns (DAMPs) from dying cells. Such molecular patterns, whether expressed on the surface of cells or released outside of the cell, can promote tumor antigen presentation and boost adaptive immunity. Calreticulin (CRT) expressed on cancer cells interacts with the CD91 receptor on dendritic cells (DCs) and high-mobility group protein 1 (HMGB1)—a ligand for the toll-like receptor (TLR-4) receptor on DCs is released by cancer cells. Additionally, ATP released from cancer cells interacts with the P2X purinoceptor 7 (P2RX7) on DSc. (B) DCs are required for cytotoxic CD8+ T cell priming. In this process, DCs uptake antigens from tumor cells undergoing apoptosis and with the support of CD4+ helper cells present them to CD8+ T cells. Moreover, DCs can secrete tumor necrosis factor α (TNF-α,) and interleukins (IL-6, IL-8, and IL-12) that help to trigger the anticancer immune response. (C) Activated CD8+ T cells can kill cancer cells through the release of granzyme A/B (GZMA/B), perforins (PFN), or interferon gamma (INFγ) and TNFα that (D) induce cell death through the activation of the extrinsic apoptosis pathway. The extrinsic pathway is triggered with the binding of certain ligands to the TNFα receptor super family. This leads to the oligomerization of the receptor and activation of procaspase-8 through the recruitment of adaptor proteins and the formation of a death-inducing signaling complex (DISC). (E) Cancer cells reshape the tumor immune microenvironment into an immunosuppressive surrounding that is rich in regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), group 2 innate lymphoid cells (ILC2s), and tumor-associated macrophages (M2) that release immunosuppressive interleukins (IL-4, IL-10, IL-13, and IL-35) or transforming growth factor β (TGF-β) and up-regulate receptors (such as programmed cell death 1 ligand 1 (PD-L1)) that “hide” tumor cells from the immune recognition (not shown here). This topic has been extensively reviewed in [15,16,17] and will not be discussed in detail here. Casp—caspase; FADD—FAS-associated death domain protein; TCR—T-cell receptor. Created with BioRender.com accessed on 1 February 2023.
Monoclonal antibodies (mAbs) targeting PD-1/PD-L1 immune checkpoint have led to a remarkable anti-tumor response in many cancer types [18]. There are now three anti-PD-1 immune checkpoint inhibitors that are licensed for use in clinical settings. These inhibitors include pembrolizumab [19,20,21,22], nivolumab [23,24,25,26], and cemiplimab [27,28]. Additionally, approval was given for three anti-PD-L1 antibodies: atezolizumab [29,30,31], avelumab [32,33,34], and durvalumab [35,36,37]. The targeting of PD-1/PD-L1 with mAbs has been reviewed by many authors [12,38,39].
2. PD-1/PD-L1 Pathway
PD-1, also known as CD279, is a transmembrane protein anchored in the cell membrane of activated T and B lymphocytes. PD-1 plays a crucial role in preventing immune-mediated tissue damage by suppressing the actions of self-reactive and inflammatory effector T lymphocytes against non-hematopoietic tissues [40,41]. The protein is built up of an extracellular IgV domain (ED), a transmembrane domain (TM), and an intracellular cytoplasmic tail (ICT). ICT comprises tyrosine-bearing signaling motifs: immunoreceptor tyrosine-based switch motif (ITSM) and immunoreceptor tyrosine-based inhibitory motif (ITIM) [42,43]. Phosphorylation of ITIMs and ITSMs leads to the recruitment of Src homology region 2 domain-containing phosphatases (SHP-1 and SHP-2) [43,44]. SHP2 is preferably recruited to the ITSM motifs, while SHP1 can bind both tyrosine motifs [45]. This event suppresses PI3K/AKT/mTOR [46] and PLCg-1/RAS/MEK/ERK1/2 [47] (for full names of the proteins see the Abbreviations section) signaling pathways responsible for the control of interleukin 2 (IL-2) expression and leads to the degradation of forkhead box protein O1 (FOXO1)—a transcription factor capable of PD-1 expression control. This results in the down-regulation of PD-1 levels [48,49]. SHP-1 and SHP-2 inhibit subsequent T-cell receptor (TCR) signaling pathways exhibiting the negative effect of T cell proliferation and the generation of cytokines including IFN-γ and IL-2, which eventually results in immune evasion by tumor cells [11]. Evidence shows that at least one of the mechanisms by which PD-1 inhibits activation of the PI3K/AKT pathway relies on phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase and the dual-specificity protein phosphatase/casein kinase II (PTEN/CK2) axis [50]. This topic was recently reviewed in [43].
PD-1 binds to particular ligands known as programmed cell death ligands PD-L1 (B7-H1) and PD-L2 (B7-DC) that belong to glycoproteins present on the surface of tumor cells. PD-L2 is thought to be expressed only in APCs, including DCs, macrophages, monocytes, and some B cells. Limited evidence suggests it may also be involved in tumor evasion in some cases [51,52,53].
In the PD-L1 structure, ED, TM, and the intracytoplasmic region can be distinguished. The ED domain of PD-L1 encompasses Ig variable distal and proximal regions. The intracytoplasmic region contains three conserved amino acid motifs: RMLDVEKC, DTSSK, and a QFEET motif. The RMLDVEKC motif is crucial for the phosphorylation of the signal transducer and activator of transcription 3 (STAT3) and the other DTSSK motif works as a suppressor of this process [54,55]. Binding of PD-L1 to PD-1 results in a reduction in the generation of cytokines as well as a suppression of the proliferation and function of T lymphocytes [11,12,54]. The expression of PD-L1 in cancer cells is controlled by several signaling pathways and proteins often mutated or up-regulated during malignant transformation, including COX2/mPGES1/PGE2 [56], hypoxia-inducible factor alpha (HIF1α) [57,58,59], nuclear factor NF-kappa-B p105 subunit (NF-κB) [60], PI3K/AKT/mTOR [61,62], RAF/MEK/ERK/MAPK [63,64,65,66] pathway, and STATs [67,68].
3. Canonical DNA Damage Response
DNA damage response (DDR) plays a central role in the control of the genomic stability of cells. DNA damaging agents trigger complex signaling pathways that direct the fate of damaged cells. Depending on the type of insult, different pathways are triggered to finally provide output reliant on the abundance of the trigger and the metabolic state of the cell. These signaling events may result in (a) damage tolerance, (b) DNA repair, (c) cell death, or (d) cell-cycle arrest. Despite the large number of DNA damage types that can be introduced in the DNA structure by a variety of DNA-damaging factors, the DDR signaling shares common features [69]. Here we focus on the cellular response to the DNA strand breaks as it is the prevailing type of DNA damage introduced by anti-cancer agents.
The DDR pathway is always initiated with the detection of damage by specific proteins. Double-strand breaks (DSBs) are sensed by MRN complexes composed of MRE11-RAD50-NBS1 proteins. This event leads to the recruitment and activation of serine-protein kinases, including ataxia telangiectasia mutated (ATM). Furthermore, the mediator of DNA damage checkpoint protein 1 (MDC-1) is recruited at the damage sites and works as a platform for ATM recruitment, leading to its retention at the chromatin close to the damage location. ATM undergoes autophosphorylation and works as a master regulator of response to DSBs as it phosphorylates a multitude of targets in the DDR including MDC1, nibrin (NBS1), TP53-binding protein 1 (53BP1), breast cancer type 1 susceptibility protein (BRCA1), and histone protein H2AX. The phosphorylation of H2AX leads to the formation of γH2AX foci (H2AX protein phosphorylated on Ser139) that recruits the MDC-1 platform protein and provides a positive feedback loop that amplifies the damage response. MDC-1 also supports the formation of repair complexes through the recruitment of E3 ubiquitin-protein ligases including RNF4, RNF8, and RNF168 that are necessary for the recruitment of other factors, including 53BP1 and BRCA1 to the sites of damage [70,71,72].
In contrast, single-stranded DNA (ssDNA) that occurs at stalled replication forks as a result of minichromosome maintenance (MCM2-7) complex helicase activity and as an intermediate in DNA repair pathways is sensed by serine/threonine-protein kinase ATR. Similar to ATM, ATR kinase exists as an inactive dimer that undergoes autophosphorylation and dissociation in response to DNA damage. ATR is recruited to the replication protein A/ATR-interacting protein (RPA-ATRIP) complexes formed on the ssDNA. Moreover, full activation requires the presence of other factors including topoisomerase 2-binding protein 1 (TOPBP1) and a cell cycle checkpoint control protein complex 9-1-1 (composed of RAD9-RAD1-HUS1) [69,71].
In the downstream events, ATR and ATM kinases activate serine/threonine-protein kinases CHK1 and CHK2 responsible for the inhibition and degradation of M-phase inducer phosphatases CDC25A and CDC25C, which normally contribute to the progression of the cell cycle through the dephosphorylation of cyclin–cyclin-dependent kinase (CDK) complexes. Furthermore, the activation of cellular tumor antigen p53 (TP53) protein up-regulates the expression of pro-apoptotic proteins and cell cycle inhibitors that ultimately results in cell cycle arrest or cell death [71,73,74]. This topic has been reviewed by multiple authors and will not be discussed here in detail [75,76,77].
4. DNA Strand Break Repair
Poly [ADP-ribose] polymerase 1 (PARP-1) is one of the earliest responders to strand breaks that catalyze the transfer of polymer ADP-ribose residues (PAR) to many downstream targets including histones, DNA repair proteins, transcription factors, as well as PARP-1 itself. This event allows the relaxation of DNA molecules near the damage site and recruitment of other DDR proteins containing PAR-binding modules that are involved in multiple pathways of DNA repair, including base excision repair (BER) and nucleotide excision repair (NER), mismatch repair (MMR), single-strand break (SSBR), and double-strand break (DSBR) repair proteins [70,72,78].
DSBs can be repaired in one of two major ways: homologous recombination (HR) [79] or non-homologous end joining (NHEJ) [80]. The pathway that is used to repair DSBs is influenced by the phase of the cell cycle and the status of the chromatin. PARP-1 can detect breaks in the DNA, and its activity plays a role in the early recruitment of various proteins such as damage sensors MRE11 and NBS1. The early recruitment of MRE11 nuclease may also promote DNA repair via HR. Moreover, the interaction of ATM with PARs can stimulate kinase activity in vitro. PARP1 is also essential for the early and prompt mobilization of BRCA1 to DSBs. In contrast, PARP1 may restrict the activity of HR through PAR-ylation of BRCA1 allowing its association with receptor-associated protein 80 (RAP80) to inhibit this type of DNA repair [78].
HR repair represents a high-fidelity mechanism of DSB repair. It is initialized with the recruitment of MRE11 that exhibits endo- and exo-nucleolytic activity that allows the formation of ssDNA fragments. Moreover, it interacts with CtBP-interacting protein (CtlP) and BRCA1 which help to control the resection process. Furthermore, CDKs and ATM phosphorylate CtBP-interacting protein (CtIP) which promotes end resection and HR through the recruitment of DNA helicase BLM and exonuclease 1 (EXO1) to the sites of DNA damage. In the next step, ssDNA is covered with RPA protein followed by recruitment of platform protein RAD52 and RAD51 recombinase that controls DNA strand invasion together with BRCA2 protein [71,72,79,81].
In contrast, the NHEJ pathway is activated in response to the DSBs that arise throughout the cell cycle. It is initiated with the binding of X-ray repair cross-complementing protein 5/6 (KU70/80) and DNA-dependent protein kinase catalytic subunit (DNA-PKcs) that form nucleoprotein complexes often described as synapses. Other NHEJ components include X-ray repair cross-complementing protein 4 (XRCC4), non-homologous end-joining factor 1 (NHEJ1/XLF), and DNA ligase IV that form complexes and allow completion of the repair process. Similarly to HR, the NHEJ repair pathway is controlled by PARP-1. PARP-1 binds and stimulates the catalytic activity of DNA-PKcs. Moreover, it may promote the recruitment of chromatin modeling enzymes to sites of DSBs for efficient repair [71,82,83].
5. DNA Damage Response and PD-1/PD-L1
Radiotherapy and chemotherapy induce DNA damage in cancer cells, which ultimately lead to their elimination. Recent research has provided evidence to support the hypothesis that DDR is a significant element impacting the effectiveness of cancer immunotherapy. The effect of conventional chemotherapy on the immune system is beginning to emerge. This is due to the fact that such agents are usually tested for their anticancer activity in cell cultures in vitro and immunodeficient mice and do not include any immunological follow-ups [84]. Accumulating evidence suggests that (re)activation of tumor-targeted immune responses is essential to the success of both conventional and targeted anticancer treatments (in addition to their direct cytostatic/cytotoxic effects). Through the enhancement of the immunogenicity of malignant cells and blockage of the immunosuppressive systems developed by cancers, the combination of chemotherapy with immune checkpoint inhibitors can provide significant benefits for cancer patients [84].
For example, it was found that exposure of cancer cells to physical DNA damaging factors including ionizing radiation [85,86] and chemical factors that induce DNA damage such as topoisomerase inhibitors (camptothecin [87], doxorubicin [88,89,90], and irinotecan [91]), alkylating agents (carboplatin [92,93], cisplatin [94,95,96,97,98], oxaliplatin [99], and mitomycin C [100]), or antimetabolites such as decitabine [101,102,103] and 5-fluorouracil (5-FU) [104,105,106] can lead to up-regulation of PD-L1 expression in the treated cells. Moreover, a growing body of evidence indicates that the effectiveness of PD-1/PD-L1 therapy may be connected to genomic instability (particularly microsatellite instability (MSI)) [107,108,109,110,111,112,113]. This profound response of tumors exhibiting high genomic instability may be partially explained by excessive production and release of peptide neoantigens that favor the recruitment or activation of tumor-infiltrating lymphocytes (TILs) and result in up-regulation of PD-1/PD-L1 in immune and cancer cells [114]. The up-regulation of PD-L1 in cancer cells might also increase the availability of epitopes to which anti-PD-L1 agents can bind and might further explain the therapeutic efficacy of the combined treatments. Indeed, tumors expressing high levels of PD-L1 respond well to anti-PD-1/PD-L1 treatments [115,116]. Multiple studies have shown that increased expression of PD-L1 also occurs following CDK2/4/6 [117,118] and serine/threonine-protein kinase mTOR [119] inhibition and that targeting of the above-mentioned kinases and simultaneous treatment with anti-PD-1/PD-L1 agents may enhance the therapeutic effect exerted by these agents alone.
The up-regulation of PD-L1 expression in response to DSBs-inducing agents such as etoposide and camptothecin or ionizing radiation is dependent on the ATM/ATR/CHK1 pathway. Moreover, the cells that survive the exposure to ionizing radiation revert to normal expression levels of PD-L1, indicating only transient up-regulation of this immune checkpoint molecule in response to DNA damage. Moreover, cells lacking crucial DSBR pathway components including BRCA2 or Ku70/80 exhibit up-regulated levels of PD-L1, and this enhanced expression of the protein is mainly due to CHK1 activation following DNA end resection by EXO1 rather than the result of DSB occurrence. This was confirmed with an experiment using the CHK1 inhibitor in BRCA2-defective cells, where up-regulation of PD-L1 was not observed. Furthermore, PD-L1 up-regulation in the absence of KU-80 and DNA damage is probably due to the build-up of replication-associated DSBs to which KU80 may bind. In summary, tumors defective in BRCA2 or KU70/80 exhibit higher expression of PD-L1 following ionizing radiation treatment due to the excessive rates of DNA end resection in the absence of KU70/80 that promotes ATR-CHK1 signaling and PD-L1 up-regulation [100].
Furthermore, in reaction to DSBs, both STAT1/3 and interferon regulatory factor 1 (IRF1) become active. It is important to note that the overexpression of PD-L1 requires IRF1, which suggests that the DSB-dependent up-regulation of PD-L1 is driven by the classical JAK1/2-STAT1/2/3-IRF1 pathway [100,106,120,121,122]. This is consistent with the early findings on the mechanism of INFγ-induced up-regulation of PD-L1 [123].
6. Cytotoxic Agents, DDR, and Immunotherapy
6.1. Irinotecan
Irinotecan is one of the best-studied topoisomerase I inhibitors. It has been employed in the treatment of various types of malignancies such as pulmonary, pancreatic, gastric, ovarian, cervical, and colorectal cancer. Irinotecan prevents the relegation of strand breaks introduced during the physiological activity of the enzyme through covalent binding with topoisomerase. The formed ternary complex composed of the drug, DNA, and topoisomerases work as an obstacle for replication and transcription machinery. Its collision with the machinery results in the formation of DSBs and activation of the ATM-CHK2-TP53 signaling pathway which eventually leads to DNA repair or apoptosis, depending on the severity and abundance of damage [124].
According to the findings of McKenzie et al., patient-derived melanoma cell lines are more vulnerable to T-cell-mediated cytotoxicity after being treated with topoisomerase I inhibitors, such as topotecan, camptothecin, and irinotecan. This effect is highly dependent on the TP53 mutational status. The T-cell response is highly proficient when the TP53 is wild-type or possesses a mutation that does not affect the TP53 activity [125]. Irinotecan treatment in murine models of cancer have provided evidence that the drug may affect the tumor immune environment in several ways, including (a) increased proliferation of tumor-specific CD8+ T cell, (b) increase in immunostimulatory IFNγ production, (c) decreased amount of FOXP3+ regulatory T cells [91], and (d) myeloid-derived suppressor cells (MDSCs) that are normally responsible for immunosuppressive effects with (e) an accompanying overall increase in MHC class I and PD-L1 expression in tumor cells. Moreover, irinotecan exhibited a synergic effect when given anti-PD-L1 antibodies [84,91,126]. In 2015, the liposomal version of irinotecan known as Nal-IRI received approval from regulatory authorities around the world for the treatment of metastatic pancreatic adenocarcinoma in conjunction with 5-FU. It has also been demonstrated that Nal-IRI can increase the T-cell-mediated cytotoxicity that is directed toward tumor cells in vivo. As a result, when paired with an anti-PD-1 mAb, it exhibited a greatly improved anticancer activity. This combined treatment increased the infiltration of CD8+ T lymphocytes into the tumor, capable of producing cytotoxic effects [124,125,127,128]. The impact of irinotecan on the DDR and the tumor microenvironment is shown in Figure 2.
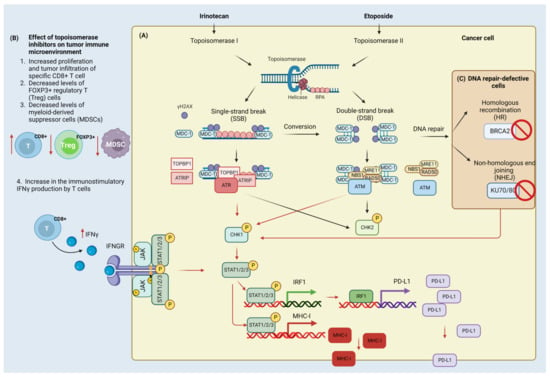
Figure 2.
Effect of irinotecan and etoposide on the DNA damage (DDR) and immune response. Cancer cell (A) Irinotecan inhibits DNA topoisomerase I which leads to the formation of single-strand breaks (SSBs) and double-strand breaks (DSBs). Single-stranded DNA (ssDNA) in the replication fork that arises due to helicase activity is coated with replication protein A (RPA) protein and a mediator of DNA damage checkpoint protein 1 (MDC-1). MDC-1 serves as a platform protein for topoisomerase 2-binding protein 1 (TOPBP1), ATR-interacting protein (ATRIP), and serine/threonine-protein kinase (ATR). In contrast, DSBs that arise through the conversion of SSBs and activity of topoisomerase II inhibitors (here etoposide) are detected by the MRN complex composed of double-strand break repair protein MRE11 (MRE11), nibrin (NBS1), and DNA repair protein (RAD50). The MRN complex is involved in the recruitment and activation of ATM kinase. Activated ATR and ATM phosphorylate have multiple targets including checkpoint kinases (CHK1/2). The phosphorylation of H2AX and accumulation of MDC-1 contribute to further recruitment of ATR/ATM kinases and amplification of damage signaling. CHK1 activation leads to the phosphorylation of signal transducers and activator of transcription (STAT) proteins that control (up-regulate) the expression of interferon regulatory factor (IRF1) and the major histocompatibility complex class I molecule (MHC-I). IRF-1 works as a transcription factor for programmed death ligand-1 (PD-L1) protein. Tumor immune microenvironment (B) Topoisomerase inhibitors stimulate the anti-tumor immune response through the increased proliferation and infiltration of CD8+ cells and the production of immunostimulatory interferon gamma (INFγ). INFγ binds to and activates the interferon-gamma receptor (INFGR) that contributes to the activation of tyrosine-protein kinase JAK (JAK)/STAT signaling to confer the increased PD-L1 expression. Moreover, it also reduces the regulatory FOXP3+ T-cells and myeloid-derived suppressor cells (MDSC) that normally attenuate the anti-tumor immune response. Cells defective in double-strand break repair components (C): Inactivation of the breast cancer type 2 susceptibility protein (BRCA2) of homologous recombination (HR) pathway and X-ray repair cross-complementing protein 5/6 (KU70/80) of the non-homologous end joining (NHEJ) pathway contributes to the activation of CHK1/STAT/IRF/PD-L1 signaling. Created with BioRender.com accessed on 1 February 2023.
6.2. Etoposide
About two-thirds of small-cell lung cancer (SCLC) patients have the advanced-stage disease at diagnosis that is associated with a poor prognosis and a 5-year survival rate of 7%. This is attributed to the high rates of tumor cell proliferation, metastasis, and resistance to currently available therapeutic options. The tumor is characterized by a high tumor mutational burden (TMB) in many genes, which suggests that DNA errors brought on by cell replication and mutations continue to build up and the immune system is unable to recognize and destroy cancer cells [129]. For more than 20 years, the gold standard for SCLC has been chemotherapy with platinum (either carboplatin or cisplatin) combined with etoposide. In conjunction with carboplatin and etoposide, atezolizumab was the first immune checkpoint inhibitor to be introduced as a first-line therapeutic option for extensive-stage small-cell lung cancer (ES-SCLC) [130]. Recent studies have shown that immune checkpoint inhibitors can improve the prognosis of patients with ES-SCLC [131,132].
Etoposide (epipodophyllotoxin) is another example of topoisomerase II poisons that introduce DSBs [133]. As a consequence of DSBs occurrence, the ATM signaling pathway is triggered which in turn results in the activation of CHK2 kinase [134,135]. A hypersensitivity to etoposide and an increased rate of chromosomal abnormalities constitute the consequences of mutations in the ATM kinase, which occurs in cells derived from patients with ataxia telangiectasia [136,137]. The activation of ATM in reaction to etoposide treatment entails the generation of the MRN complex foci [138,139,140]. Etoposide simultaneously activates ATR-mediated pathway and contributes to the recruitment of the 9–1–1 complex and activation of CHK1 kinase due to the accumulation of ssDNA coated with RPA protein [134,135,141,142]. The presence of these RPA foci following etoposide treatment may reflect an attempt by the cells to repair DSBs introduced by the agent through the HR pathway [143]. Moreover, inhibition of DNA replication relies heavily on ATR activation [135].
The majority of etoposide-induced topoisomerase-II-mediated DNA damage can be repaired by NHEJ. This is consistent with the results of Malik et al. that have established the key role of yeast KU70 of the NHEJ pathway for the survival of Saccharomyces cerevisiae upon etoposide treatment [144,145,146]. Additionally, cells deficient in KU70 and KU80 but not those with defective DNA-PKcs exhibit sensitivity to the etoposide treatment [147]. In contrast, Palmitelli et al. suggest that DNA-PKcs inhibition significantly improves DNA damaging effects of etoposide with a twofold higher rate of chromatid breaks and exchanges [148]. However, HR seems to be the primary pathway of etoposide-induced damage repair. The formation of strand breaks associated with etoposide treatment is associated with a reduction in the levels of KU70, KU86, and DNA-PKcs in addition to an increase in the concentration of RAD51 protein, suggesting HR-mediated repair [149,150].
Cancer stem cells (CSCs) are a subpopulation of cells that have both the ability to self-renew and the capability to develop into other cell types. It was discovered that cancer cells can go through the epithelial-to-mesenchymal transition (EMT) in response to stimuli from the cells that are present in the microenvironment of the tumor [151]. In turn, this results in the production of cells that have characteristics that are comparable with those of CSCs. In addition, the release of IL-6 into the milieu of the tumor by CSCs is the primary factor responsible for the transformation of non-stem cancer cells into CSCs [152,153]. Additionally, IL-6 induces the expression of other cytokines that are advantageous for the formation of CSCs. This topic was recently reviewed by our group [154]. Elevated expression of PD-L1 as a consequence of the EMT/β-catenin/STAT3 pathway activation improves the immune evasion of CSCs. Etoposide was shown to suppress the EMT/β-catenin/STAT3 pathway and down-regulate PD-L1 expression in cancer cells [155]. It has been demonstrated that etoposide can stimulate tumor-specific immunity, in which CD8+ cytotoxic T cells play an important role [156,157,158].
Contrasting evidence comes from the in vitro studies of etoposide treatment in breast cancer cells and bone marrow stromal cells, where the drug induces up-regulation of PD-L1 on the cancer cell surface [159,160]. Moreover, teniposide (etoposide derivative) induces DNA damage in tumor cells, which is associated with the activation of the NF-κB pathway and stimulation of interferon genes protein (STING)-dependent type I interferon signaling. Both of these pathways contribute to the activation of DCs, which leads to the activation of T cells. In addition, teniposide enhances the anticancer activity of anti-PD1 treatment in a variety of mouse tumor models [161]. The DDR induction by etoposide and its influence on the tumor microenvironment is presented in Figure 2.
6.3. Cisplatin and Other Platinum Compounds
Many types of human solid tumors are successfully treated with chemotherapeutic drugs that crosslink DNA [162]. Two types of DNA crosslinks can be distinguished. The intrastrand crosslink covalently connects two nucleotides of the same DNA molecule, while the interstrand crosslinks (ICLs) develop when two different DNA strands are linked. These latter crosslinks require bifunctional alkylating agents that have two reactive groups that can form covalent bonds with DNA molecules [163]. ICLs constitute one of the most difficult-to-repair lesions that cells encounter during their lifetime. The formation of ICLs triggers DDR in cancer cells. The repair is carried out with the cooperative aid of at least five repair pathways i.e., the Fanconi anemia (FA) pathway, HR, NER, MMR, and translesion DNA synthesis (TLS) [164,165]. Unrepaired ICLs prevent strand separation of DNA during DNA replication and transcription. As a consequence, cells die from mitotic catastrophe or apoptosis [166].
Cisplatin has been widely used in the treatment of many cancer types. However, the fundamental restrictions of cisplatin’s therapeutic use are the side effects and resistance mechanisms that commonly accompany cisplatin-based therapy. Soon after the introduction of cisplatin into the clinic, other platinum compounds were developed to mitigate the negative effects of cisplatin. These include two of the most comprehensively studied analogs of the drug: oxaliplatin and carboplatin [10,167].
Despite the belief that platinum-based treatments lack cell cycle selectivity, cytotoxicity is boosted when the drug is delivered to cells during the S phase of the cell cycle. Cisplatin induces a G2 cell cycle arrest and subsequent accumulation of cells in the G2/M phase through the inhibition of the CDK2-cyclin A or B kinase, which is followed by apoptosis [168]. Only a minuscule fraction of the intracellular platinum can form a complex with the DNA. Platinum compounds can interact with RNA [169,170] or various proteins which may confer the platinum-based compound’s side effects and toxicity [171,172]. Although physically different, cisplatin and oxaliplatin produce similar adducts at analogous locations in the DNA molecule [173]. The cisplatin-induced DNA damage is detected by more than 20 different proteins from different DNA repair pathways including hMSH2 or hMUTSa (MMR), nonhistone chromosomal high-mobility group 1 and 2 (HMG1/2) proteins, the human RNA polymerase I transcription ‘upstream binding factor’ (hUBF), and the transcriptional factor ‘TATA-binding protein’ (TBP). Therefore, it is plausible to postulate that DNA damage triggers many seemingly unrelated biological consequences, each of which can be initiated by a different recognition protein [174].
Multiple molecular mechanisms of action were proposed for platinum compounds such as cisplatin (Figure 3). For example, cisplatin was found to induce oxidative stress through the excessive generation of reactive oxygen species (ROS). The concentration of cisplatin and the length of exposure time are two of the most important factors that determine the generation of ROS in cancer cells [175]. ROS cause both nuclear (nDNA) and mitochondrial (mtDNA) DNA damage at rates of approximately 104 DNA lesions per cell of an organism every day. These lesions include modified bases, interstrand and DNA-protein crosslinks, SSBs, and DSBs [176,177]. The Jun amino-terminal kinase (JNK) signaling plays a crucial role in cellular responses to oxidative stress. JNK triggers several responses, including DNA repair, antioxidant synthesis, and cell death, depending on the strength and length of the damage signal [178]. P73 is a homolog of the TP53 protein that is involved in cell cycle regulation and apoptosis. Following treatment of cells with cisplatin and transplatin, P73 establishes a complex with JNK leading to apoptotic cell death [179]. Two factors are necessary for cisplatin to induce P73-dependent apoptosis: ABL tyrosine kinase activation and the cellular proficiency of the MMR repair pathway that connects damage sensing to apoptotic signaling [174].
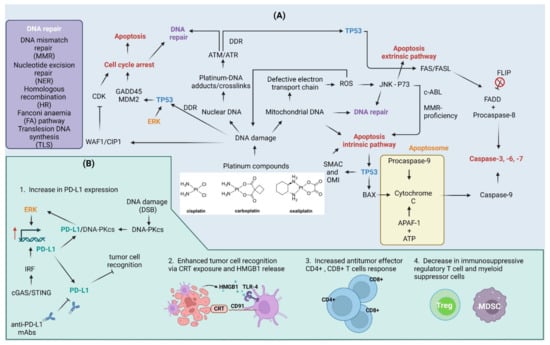
Figure 3.
Cellular and immune response to platinum compounds. (A) Cellular response to platinum compounds: Platinum compounds induce DNA damage to both nuclear and mitochondrial DNA. Mitochondrial DNA damage can lead to defects in the electron transport chain that contribute to electron leakage and the formation of reactive oxygen species (ROS). ROS contribute to DNA damage in both nuclear and mitochondrial DNA in a feedback mechanism. ROS also act as molecular messengers and contribute to the activation of the Jun amino-terminal kinase (JNK) pathway that together with the P73 protein trigger apoptotic cell death. C-ABL kinase activity and mismatch repair (MMR)-proficiency are required for cell death to occur. DNA damage activates DNA damage response (DDR), as indicated by the activation of serine/threonine kinases ATM and ATR. The outcome of DDR relies heavily on cellular tumor antigen p53 (TP53) protein: (a) activation of TP53 in response to DNA damage triggers cell cycle arrest through up-regulation of cyclin-dependent kinase inhibitor 1 (WAF1/CIP1), 45kd-growth arrest, and DNA damage (GADD45) and mouse double minute 2 homolog (MDM2) or via WAF1/CIP1-mediated inhibition of cyclin-dependent kinases (CDKs); (b) subsequent DNA repair via co-operation of nucleotide excision repair (NER), MMR, homologous recombination (HR), Fanconi anemia (FA) pathway, or translesion synthesis (TLS); or (c) apoptosis induction through intrinsic apoptosis pathway followed by ROS-damage accumulation, TP53-dependent up-regulation of Bcl2-associated agonist of cell death (BAD), Diablo IAP-binding mitochondrial protein (SMAC) and serine protease HTRA2 (OMI), or through activation of FASL/FAS extrinsic apoptosis pathway. Cisplatin induces flice-like inhibitory protein (FLIP) ubiquitination and degradation, which further activate the extrinsic pathway. (B) Effect of platinum compounds on the antitumor immune response: Cyclic GMP-AMP synthase (cGAS)/stimulator of interferon genes protein (STING) pathway and extracellular signal-regulated kinase (ERK) signaling up-regulate programmed death ligand-1 (PD-L1) expression. The DNA-dependent protein kinase catalytic subunit (DNA-PKcs) up-regulated in response to double-strand breaks (DSBs) associates with PD-L1 conferring to the activation of the ERK pathway and further up-regulation of PD-L1. This, on the one hand, contributes to immune evasion, but also increase the therapeutic efficiency of anti-PD-L1 monoclonal antibodies (mAbs). Platinum compounds also enhance tumor recognition through calreticulin (CRT) exposure on cell surface and high mobility group protein 1 (HMGB1) release, induce CD4+/CD8+ T cell response, and suppress regulatory T cells (Treg) and myeloid-derived suppressor cells (MDSC). APAF-1—apoptotic protease-activating factor 1; FADD—FAS-associated death domain protein. Created with BioRender.com accessed on 1 February 2023.
ATM, ATR, and DNA-PKcs are all rapidly activated in response to oxidative DNA damage [180,181]. Nuclear foci composed of ATR and H2AX are formed at the location of DNA damage in cells treated with cisplatin. ATR blockade using a dominant-negative mutant prevents cisplatin-induced TP53 activation and renal cell death. In ATR-deficient fibroblasts, cisplatin-induced TP53 activation and apoptosis are attenuated. Both CHK1 and CHK2 are phosphorylated in response to cisplatin treatment; however, CHK1 phosphorylation leads to the proteasomal degradation of the kinase. Therefore, ATR-CHK2 activation plays a crucial role in TP53-mediated apoptosis response [182,183]. In U87-MG glioma cells, cisplatin treatment results in apoptosis with concomitant up-regulation of cyclin-dependent kinase inhibitor 1 (WAF1/CIP1) which works as a suppressor of CDKs activity [184]. Cisplatin causes apoptosis of ovarian cancer cells by increasing the levels of TP53 in mitochondria and inducing the release of Diablo IAP-binding mitochondrial protein (SMAC), cytochrome c, and serine protease HTRA2 (OMI). Apoptosis mediated by cisplatin is dependent on TP53 and necessitates the release of SMAC. The release of SMAC is directly triggered by TP53 in mitochondria [185]. There is also evidence that TP53 can directly bind to cisplatin-damaged DNA complexes [167,186].
The apoptotic response triggered in response to platinum compounds is highly dependent on TP53 phosphorylation by a multitude of activated signaling pathways including extracellular signal-regulated kinase (ERK) [187,188], RAC-alpha serine/threonine-protein kinase (AKT) [189], and protein kinase C (PKC) [190]. ERK phosphorylates TP53 leading to up-regulation of WAF1/CIP1, 45kD-growth arrest and DNA damage (GADD45), and mouse double minute 2 homolog (MDM2), providing the time necessary to repair induced DNA damage [191]. Notably, phosphorylation of Bcl2-associated agonist of cell death (BAD) on Ser-112 is mediated through the ERK pathway, while Ser-136 phosphorylation is dependent on the PI3K/AKT pathway. Moreover, inhibition of the above-mentioned pathways sensitizes cancer cells to cisplatin [189]. Similarly, inhibition of AKT results in enhanced accumulation of TP53 in mitochondria and the release of SMAC conferring the greater efficacy of cisplatin [185]. Furthermore, cisplatin also induces the extrinsic apoptosis pathway as indicated by the activation of the FAS receptor, caspase 8, and effector caspases 3, -6, and -7 activation [192]. Moreover, cisplatin triggers apoptosis and flice-like inhibitory protein (FLIP) ubiquitination and degradation in a TP53-dependent manner in vitro [193].
Platinum compounds exhibit a variety of effects on the anti-cancer immune response (Figure 3). The up-regulation of PD-L1 in response to platinum compounds [9,93] is potentially induced through the activation of the ERK1/2 pathway in vitro and in vivo [97,98]. Moreover, PD-L1 associates with DNA-PKcs that contribute to the activation of the ERK pathway and as a feedback mechanism for the up-regulation of PD-L1. The inhibition of PD-L1 with monoclonal antibody (H1A) sensitizes human triple-negative breast cancer cells to cisplatin in vitro and in vivo [99], suggesting that the combination of the drug with anti-PD-L1 treatments may enhance the therapeutic efficacy of chemotherapeutic drug alone [9,194,195], at least in mouse tumor models. Moreover, cisplatin and oxaliplatin may have different effects on CD8+ T cell fraction and function. Oxaliplatin seems to act as a stronger activator of CD8+ T cells than cisplatin in preclinical models of head and neck cancer [9]. Moreover, oxaliplatin was found to promote PERK/eIF2α/caspase 8/BAP31 signaling (see abbreviations section) to facilitate CRT exposure on the cancer cell surface and HMGB1 release in a mouse model of colorectal cancer [196].
As mentioned above, the tumor microenvironment can be shifted from an immunosuppressive to an immune-supportive state following the use of platinum compounds. This is accompanied by the activation of dendritic cells (CD80+ and CD86+), increased antitumor effector CD4+, CD8+ T cells response, and decreased immunosuppressive regulatory T and myeloid suppressor cells ratios as observed in mice models of ovarian, lung, and colon cancer [92,194,195]. An increase in CRT, MHC class I, antigen presentation, and T-cell infiltration are some of the mechanisms by which cisplatin improves tumor immunogenicity after both short- and long-term exposure. The cyclic GMP-AMP synthase (cGAS)/STING pathway is triggered in response to cisplatin treatment. With the increased expression of PD-L1, MHC-I, and CRT in tumor cells, cGAS/STING overexpression changes tumor immunogenicity and improves anti-PD-L1 treatments in in vitro and in vivo mice models [95,197]. Conflicting results were obtained in clinical studies of platinum-based chemotherapy on PD-L1 expression, where the treatment resulted in a decrease in the immune checkpoint molecule in tumors from lung cancer patients [198]. However, both the additive and synergic effects of platinum compounds and anti-PD-L1 antibodies have been described in the literature [94,95,197,199].
7. Current Challenges and Future Prospects
The field of immunotherapy is constantly expanding. Immune checkpoint inhibitors are a promising new treatment option for cancer treatment. Multiple clinical trials have shown that immunotherapy can extend both progression-free survival (PFS) and overall survival (OS) in cancer patients. The abundance of mAbs that have been granted FDA approval and the number of cancer types for which they are used continues to grow, which is reflective of the enthusiasm for PD-1/PD-L1 inhibition and its potential application in clinical practice [200]. Still, mAbs possess many drawbacks, such as a lack of oral bioavailability or poor permeability of tumor tissues, which leads to the overall low response rate of PD-1/PD-L1 inhibition that limits their clinical effectiveness. Additionally, mAbs therapy may drive immunogenicity issues and cause serious immune-related adverse effects (irAEs), with possible deadly outcomes [201,202,203]. Alternatively, many small molecule inhibitors may have certain advantages in dealing with these problems (Figure 4). Small molecule inhibitors are more suitable for oral administration and are less prone to the occurrence of serious irAEs. Moreover, they are less expensive, have better tissue permeability, and possess many more other important characteristics, which make them more favorable for potential clinical use compared with mAbs [202].
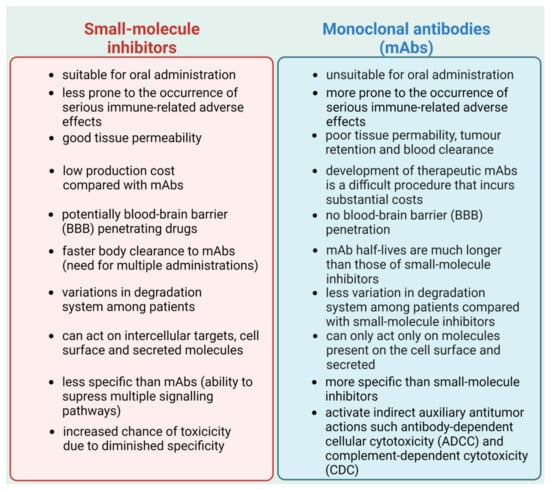
Figure 4.
Comparison of small-molecule inhibitors and monoclonal antibodies (mAbs). Based on [202]. Created with BioRender.com accessed on 1 February 2023.
Many crystal structures were published after the FDA approved the use of monoclonal antibodies targeting the PD-1/PD-L1 axis, demonstrating the interaction mechanisms of antibodies as well as small-molecule compounds with PD-1 and PD-L1. These studies provided information on hot spots that can be employed for the design of new, more specific compounds targeting this pathway [11]. Moreover, they showed that the PD-1/PD-L1 protein interaction surface is large and hydrophobic, with no profound binding pocket making it difficult to target with small-molecule inhibitors. The hydrophobic nature of this hot spot also necessitates the use of hydrophobic molecules with significant adverse properties including toxicity and low water solubility [204]. Nonetheless, multiple compounds from different chemical classes, including biphenyls [205,206,207], terphenyls [208,209], biphenyls with a ring fusion [210], elongated biphenyls [211], and symmetric biphenyls [212,213], were tested for anti-PD-1/PD-L1 interaction; however, they relied heavily on binding assay without their examination in biological systems. Moreover, the number of false-positive hits is still too high as in the case of salicylates NCI 211717 and NCI 211845. Moreover, when screening drugs with in vivo mouse models, structural variation between mouse and human PD-1/PD-L1 should be considered as it can greatly affect druggability profiles [204]. Humanized knock-in animals could help to circumvent this obstacle [214].
Currently, immunotherapy is used with different forms of treatment to obtain a long-lasting antitumor clinical response in patients who are resistant to monotherapy. In many cases, PD-L1 is up-regulated in response to chemotherapeutic agents. Besides their cytotoxic effect, chemotherapeutic drugs may enhance the tumor infiltration of CD8+ T cells and NK cells, the maturation of APCs (DCs or tumor macrophages), and in some circumstances, the activity of MDSCs as evidenced by animal model studies. In this manner, primary cytostatic and cytotoxic drugs re-establish an immune-reactive tumor microenvironment, which increases the tumor’s sensitivity to PD-L1-targeted monoclonal antibodies. However, the challenge is to combine the chemotherapeutic drugs with anti-PD-L1 agents to surpass the effectiveness of agents alone and alleviate the side effects of the drugs. A given cytotoxic drug or regimen needs to be selected not only based on its ability to effectively kill cancer and inhibit the expansion of cancer cells but also based on its tendency to modulate the activity of immune-active cells and to sustain the activity of immunotherapy. Moreover, many currently used drugs have not been examined in the context of immune activation against the tumor cells or their utility in combinations with checkpoint inhibitors [8].
However, one of the most significant drawbacks of chemotherapy is still a lack of specificity. Chemotherapy will target both the highly proliferating tumor cells as well as normal cells including lymphocytes. Therefore, lymphopenia is one of the most common side effects associated with the use of DNA-damaging agents. This can hamper the utility of immune–chemotherapy drug combinations for the treatment of cancer. This limitation could be overcome with the use of targeted agents, which exhibit lower toxic effects for normal cells compared with chemotherapy. However, the effect of such agents and their combinations, not only on PD-L1 expression but also on the alterations in the tumor immune microenvironment, need to be assessed before their use in the clinic [8,215].
Cancer chemotherapy and radiotherapy are intended to eliminate cancer cells largely by generating DNA damage. In a typical situation, the body’s inbuilt DNA damage response machinery will detect and fix any affected DNA. The cells have a chance of survival if the damaged lesions can be fixed. To precisely and effectively kill cancer cells with therapies that generate DNA damage, it is crucial to take advantage of certain defects in the DDR machinery that are present in cancer cells but not in normal cells [216]. By personalizing treatment to patients whose tumors lack specific DDR functions, targeted therapy offers the promise of a larger therapeutic window. The majority of cancers, if not all of them, will have lost at least one or more crucial DDR pathway resulting in an increased dependence on the compensatory pathways that are still functional and capable of removing arising DNA damage and thereby preventing cell death. This feature can be exploited in the design of specific DDR inhibitors that target crucial components of the above-mentioned—non-defective DNA repair pathway that is used by cells for survival. This phenomenon known as synthetic lethality may help to selectively eliminate cancer cells leaving the normal cells unharmed (such cells still have the first pathway intact and can repair the DNA damage) [217,218,219]. This strategy has been successful in the past, as evidenced by the use of PARP inhibitors in the treatment of BRCA-mutated cancers [220,221]. More recently, the expansion of effective and selective agents that target DDR signaling components has emerged as a promising therapeutic option, and this trend is expected to continue [222]. There are also several different examples of DDR-targeted drugs coupled with radiation and chemotherapeutic drugs that have shown evidence of success in preclinical and clinical testing [216,223]. On the other hand, there are a number of crucially important questions such as what dose of DDR inhibitor will serve as an efficient radiosensitizer, how much benefit will be derived from prolonged exposure to the DDR inhibitor following delivery of radiation-induced damage, or even whether radiosensitization by the DDR inhibitor is distinctive in particular DDR-deficient genetic characteristics. To determine whether enhanced antitumor cell effectiveness is paired with increases in healthy tissues toxicity, or if there is evidence that suggests an improved therapeutic index, is another matter of concern when combining different approaches. Although anticancer activity and normal tissue toxicity can be evaluated using distinct models, it is preferable to make use of a syngeneic or orthotopic immune-competent rodent model, in which antitumor activity and normal tissue toxicity can be evaluated concurrently with the effect of such a combination on the antitumor immune response. The conjunction of DDR therapeutics with chemotherapies that induce DNA damage also has its challenges. There are two primary reasons for this: the first is that chemotherapies are administered systemically, and the second is that they have a tendency to have intersecting toxicities as DDR inhibitors, specifically gastrointestinal and bone marrow toxicity. As a consequence of this, a significant number of clinical studies have been stopped since the risks involved are intolerable. This topic was thoroughly reviewed by O’Connor in an excellent paper [224] and the toxicity of DDR inhibitors was discussed recently by Martorana et al. [225]. Moreover, the resistance to DDR inhibitors is a major concern that influences the success of the therapy [226].
Large-scale functional genomic screens have identified a growing number of genetic vulnerabilities throughout the DDR landscape beyond PARPi. This has led to a variety of novel synthetic lethal targets and inhibitors, which may expand DDR inhibitor use beyond HR and address PARPi resistance. For example, berzosertib (VX-970) is a first-in-class ATR inhibitor that has been examined as monotherapy or in combination with cytotoxic chemotherapy drugs (topotecan, carboplatin, cisplatin, and gemcitabine) in phase 1 clinical studies and showed overall safety and clinical benefits in patients. These findings prompted berzosertib–cytotoxic chemotherapy research. For example, berzosertib plus gemcitabine improved PFS in a randomized phase 2 trial of platinum-resistant ovarian cancer. BAY 1895344 constitutes another oral selective and potent ATR inhibitor developed and tested in vivo. Preclinical investigations of this agent as monotherapy in DDR deficit tumors showed promising synergistic anticancer activity with DNA-damage-inducing chemotherapy or radiotherapy. The anticancer activity of BAY 1995344 as a single-agent therapy has been demonstrated to be quite promising; hence, more research is currently being conducted in combination with a PARPi (niraparib) or an immune checkpoint inhibitor (pembrolizumab). The effectiveness and safety of combining PARPi with anti-PD1/PD-L1 antibodies in treating various cancers have been the subject of multiple clinical investigations. There were no unexpected adverse events or additive toxicities seen in the MEDIOLA trial (NCT02734004), a phase 1/2 study examining the combination of olaparib and durvalumab (anti-PD-L1 antibody) in four different types of cancer: germline BRCA-mutated metastatic breast cancer, germline BRCA-mutated platinum-sensitive relapsed ovarian cancer, relapsed gastric cancer, and SCLC. A promising anticancer activity was also revealed in a phase 2 trial of melanoma patients treated with the combination of ATR inhibitor ceralasertib with durvalumab. This topic was extensively covered in other recent reviews [227,228].
The tumor immune microenvironment is profoundly impacted by tumor-promoting inflammation, which in turn affects the success of cancer immunotherapy. Evidence is mounting that inhibiting DDR in conjunction with an immune checkpoint inhibition may increase inflammation in the tumor microenvironment, hence improving immune detection and the killing of cancer cells. Micronucleus and cytosolic chromatin fragments generation are both increased in cancer cells treated with DDR inhibitors due to the DSBs occurrence and genomic instability induction. Pattern recognition receptors (PRRs), which are part of the innate immune system and identify pathogenic nucleic acids such as virus DNA or RNA, recognize the cytosolic DNA produced from DSBs and MNs. The detection of cytosolic DNA by PRRs initiates IFNγ signaling and accompanying innate and adaptive immune responses, similarly as in the case of DAMPs, facilitating the anti-tumor immune response [228]. Several genotoxic treatments, both in the clinic and in animal models, have been found to up-regulate the cGAS-STING pathway and enhance the efficacy of cancer immunotherapy. Cancer immunotherapy can be improved by activating the cGAS-STING pathway, which has been linked to the inhibition of ATM, ATR, CHK1, and PARP [229]. This topic was reviewed by Huang et al. and Wang et al. who summarized recent clinical trials involving combinations of immune checkpoint inhibitors with DDR-directed therapies [228,230].
Despite the significant response of melanoma, non-small-cell lung cancer, and lymphoma patients to PD-1/PD-L1-directed treatments, fewer benefits were observed in patients suffering from other cancer types. This could be a result of a scarcity of tumor antigens, poor antigen presentation, and the composition of the tumor microenvironment. The establishment of clinical biomarkers for anti-PD-1/PD-L1 treatments is therefore an urgent need. Most of the research focused on PD-L1 as a prognostic marker of treatment response. However, the inducible nature of PD-L1 expression and non-uniform expression of PD-L1 even in a single tumor adds another layer of complexity to the problem [11,200,231]. Furthermore, this therapeutic approach may be especially useful for a subset of patients who share certain DDR and immune biomarkers (e.g., HR-deficiency [232], TMB-high [233], MSI-high [234,235], deficiency in MMR [236,237,238], and profound expression of immune checkpoint molecules and their variation [239,240]). For example, TMB is associated with the production of abnormal proteins that are degraded proteolytically, leading to the production of neoantigens recognized by T-cells that release IFNs and lead to the stimulation of IRF and PD-L1 up-regulation. Furthermore, both BER and MMR-deficient cells will produce more neoantigens, leading to the up-regulation of PD-L1. Additionally, cells defective in HR and NHEJ pathways may respond better to the anti-PD-L1 therapies due to the release of DAMPs in response to chemotherapy or radiotherapy [241].
Moreover, drug resistance has considerably reduced the clinical benefits of immunotherapy [200]. Among the resistant mechanisms involving the DDR in response to immune checkpoint therapy, several factors can be distinguished including low neoantigen burden, low PD-L1 expression in certain types of tumors, down-regulated tumor MHC expression, recruitment of immunosuppressive cells, and the release of cytokines in the tumor milieu. Nevertheless, by increasing DNA damage with DDR pathway inhibitors, tumor microenvironment inflammation can be altered, immunogenic cancer cell death can be triggered, and anticancer immune responses can be stimulated [242]. For example, inhibiting ATR increases the inflammatory IFN response and cytokine gene expression that is triggered by radiation, both in vivo (especially CCL2, CCL5, and CXCL10) and in vitro (CCL3, CCL5, and CXCL10) [243]. Combining an ATR inhibitor with an immune checkpoint blocker is justified since, in addition to increasing DNA damage, the former also decreases PD-L1 levels and boosts anti-tumor immune responses [244].
Furthermore, various in vitro and in vivo studies have shown that classical chemotherapeutic drugs such as platinum compounds including cisplatin [245,246,247,248,249,250] and lobaplatin [251,252,253], antimetabolites including 5-FU [254] and decitabine [255], or topoisomerase inhibitors such as doxorubicin [256,257,258] may trigger cancer cell pyroptosis [259]. It appears that the expression of gasdermines (GSDMs) in target cancer cells determines whether chemotherapeutic agents and cytotoxic lymphocytes promote pyroptosis or apoptosis. This tumor suppressor gene is frequently suppressed in cancer cells, most likely as a result of promoter methylation. Therefore, DNA methyltransferase inhibitors such as decitabine may sensitize cancer cells to pyroptosis by re-establishing gasdermin E (GSDME) expression. By releasing inflammatory chemicals into the extracellular environment, pyroptotic cells trigger an inflammatory and immunological response. Cells undergoing pyroptosis produce molecules that contribute to inflammation and therefore may boost anti-PD-L1 therapies. These inflammatory factors include pro-inflammatory cytokines IL-1β, IL-18, and DAMPs such as ATP, DNA, or HMGB1, and in doing so they regulate the proportions of tumor-infiltrating immune cells such as T cells, NK cells, DCs, monocytes, and MDSCs [260,261,262,263]. Pyroptosis, which is a highly immunogenic form of cell death, generates local inflammation and draws inflammatory cell infiltration. This presents a significant potential to alleviate the immunosuppression of tumor microenvironments and elicit a systemic immune response in the treatment of solid tumors [262]. Indeed, increased levels of GSDME in cancer cells resulted in a substantial rise in the number of antigen-specific CD8+ T cells and intra-tumoral NK cells. It also resulted in a boost in the expression of granzyme B, perforins, IFN-γ, and TNF-α in TILs, as well as an increase in the phagocytosis of tumor cells by tumor-associated macrophages in a melanoma xenograft model treated with serine/threonine-protein kinase B-raf (BRAF) and mitogen-activated protein kinase kinase (MEK) inhibitors [264]. It has been, however, hypothesized that a persistent inflammatory state raises the likelihood of cancer development. Because of the local inflammatory milieu, angiogenesis, invasion, and metastasis are all more likely to occur. It seems that the level of equilibrium that exists in the microenvironment between pro-tumorigenic factors and ones that are anti-tumorigenic is what controls the formation of tumors. Moreover, the induction of pyroptosis may lead to damage to normal tissues. Nevertheless, the precise control of inflammasome activation and pyroptosis could present an opportunity to significantly enhance the efficacy of immune treatment in the near future [262,265,266]. This is evidenced by the number of clinical drugs and pre-clinical compounds that activate pyroptosis in cancer cells and their combinations with immune checkpoint inhibitors as reviewed recently by Wang et al. [263]. The existing links between DDR induced by chemotherapeutic drugs, DDR-inhibitors, and immunotherapy are presented in Figure 5.
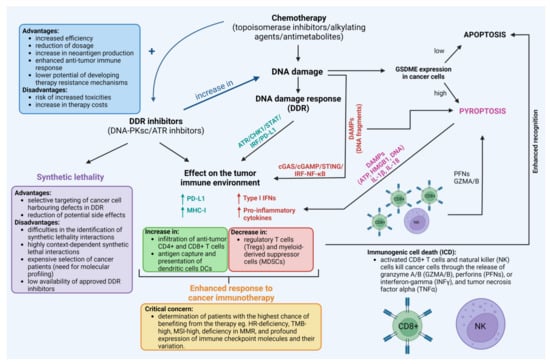
Figure 5.
The existing links between the use of chemotherapeutic drugs, DNA damage response (DDR) inhibitors, and immunotherapy with the crucial questions, advantages, and disadvantages of their use. For reference see Section 7. Created with BioRender.com accessed on 1 February 2023.
8. Conclusions
The understanding of the influence of the combinations of chemotherapeutic drugs and DDR inhibitors on the tumor immune microenvironment may help to increase the effectiveness of cancer treatment and limit the detrimental influence of therapies on normal cells, alleviating potentially life-threatening side effects. Despite the promise it holds for cancer patients, still many concerns remain. One of them is the appropriate selection of patients that could benefit the most from the combinations of drugs. The advances in the comprehension of the molecular biology of cancer and its response to potentially complementary therapies now help to increase the likelihood of therapy success.
Author Contributions
Conceptualization, M.K. and I.C.; writing—original draft preparation, M.K., D.K., M.G., R.K. and Ż.K.-K.; writing—review and editing, M.K., D.K., M.G., R.D. and Ż.K.-K.; supervision, I.C., R.D. and R.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| 53BP1 | TP53-binding protein 1 |
| 5-FU | 5-fluorouracil |
| AKT | RAC-alpha serine/threonine-protein kinase |
| APC | Antigen-presenting cell |
| APAF-1 | Apoptotic protease-activating factor 1 |
| ATM | Serine-protein kinases ataxia telangiectasia mutated |
| ATRIP | ATR-interacting protein |
| BAD | Bcl2-associated agonist of cell death |
| BAP31 | B-cell receptor-associated protein 31 |
| BER | Base excision repair |
| BRAF | Serine/threonine-protein kinase B-raf |
| BRCA1/2 | Breast cancer type 1 susceptibility protein 1/2 |
| CDC25A/C | M-phase inducer phosphatases |
| CDK | Cyclin-dependent kinase |
| cGAS | Cyclic GMP-AMP synthase |
| CHK1/2 | Serine/threonine-protein kinase CHK1/2 |
| CK2 | Casein kinase II |
| COX2 | Cyclooxygenase-2 |
| CRT | Calreticulin |
| CSC | Cancer stem cells |
| CtIP | CtBP-interacting protein |
| DAMPS | Damage-associated molecular pattern molecules |
| DCs | Dendritic cells |
| DDR | DNA damage response |
| DISC | Death-inducing signaling complex |
| DNA-PKcs | DNA-dependent protein kinase catalytic subunit |
| DSBR | Double-strand break repair |
| DSBs | Double-strand breaks |
| ED | Extracellular domain |
| eIF2α | Eukaryotic translation initiation factor 2A |
| EMT | Epithelial-to-mesenchymal transition |
| ERK | Extracellular signal-regulated kinase |
| ES-SCLC | Extensive-stage small cell lung cancer |
| EXO1 | Exonuclease 1 |
| FA | Fanconi anemia |
| FADD | FAS-associated death domain protein |
| FdUMP | 5-fluoro-2′-deoxyuridine 5′-monophosphate |
| FdUTP | Fluorodeoxyuridine triphosphate |
| FLIP | Flice-like inhibitory protein |
| FOXO1 | Forkhead box protein O1 |
| GADD45 | 45kd-growth arrest and DNA damage |
| GDSM | Gasdermin |
| GZMA/B | Granzyme A/B |
| HIF-1α | Hypoxia-inducible factor alpha |
| HMG1/2 | Nonhistone chromosomal high-mobility group 1 and 2 |
| HMGB1 | High mobility group protein 1 |
| HR | Homologous recombination |
| hUBF | Highly similar to Human upstream binding factor |
| ICD | Immunogenic cell death |
| ICLs | Interstrand crosslinks |
| ICT | Intracellular cytoplasmic tail |
| IFN | Interferon |
| IL | Interleukin |
| ILC2s | Group 2 innate lymphoid cells |
| irAEs | Immune-related adverse effects |
| IRF | Interferon regulatory factor |
| ITIM | Immunoreceptor tyrosine-based inhibitory motif |
| ITSM | Immunoreceptor tyrosine-based switch motif |
| JAK | Tyrosine-protein kinase |
| JNK | Jun amino-terminal kinase |
| KU70/80 | X-ray repair cross-complementing protein 5/6 |
| M2 | Tumor-associated macrophages M2 |
| mAbs | Monoclonal antibodies |
| MCM2/7 | Minichromosome maintenance complex |
| MDC-1 | Mediator of DNA damage checkpoint protein 1 |
| MDM2 | Mouse double minute 2 homolog |
| MDSC | Myeloid-derived suppressor cells |
| MDSCs | Myeloid-derived suppressor cells |
| MEK | Dual specificity mitogen-activated protein kinase kinase |
| MHC | Major histocompatibility complex |
| MLH1 | DNA mismatch repair protein MLH1 |
| MMR | Mismatch repair |
| mPGES1 | Prostaglandin E synthase |
| MRE11 | Double-strand break repair protein MRE11 |
| MSH2/6 | DNA mismatch repair protein MSH2/6 |
| MSI | Microsatellite instability |
| mtDNA | Mitochondrial DNA |
| mTOR | Serine/threonine-protein kinase mTOR |
| Nal-IRI | Liposomal irinotecan |
| NBS1 | Nibrin |
| nDNA | Nuclear DNA |
| NER | Nucleotide excision repair |
| NF-Kβ | Nuclear factor NF-kappa-B p105 subunit |
| NHEJ | Non-homologous end joining |
| NK | Natural killer cells |
| OMI | Serine protease HTRA2 |
| OS | Overall survival |
| P2RX7 | P2X purinoceptor 7 |
| PAR | Polymer ADP-ribose residues |
| PARP-1 | Poly [ADP-ribose] polymerase 1 |
| PARPi | PARP inhibitor |
| PD-1/PD-L1 | Programmed death receptor-1/programmed death ligand-1 |
| PERK | Eukaryotic translation initiation factor 2-alpha kinase 3 |
| PFN | Perforins |
| PFS | Progression-free survival |
| PGE2 | Prostaglandin E2 |
| PI3K | Phosphatidylinositol-4,5-bisphosphate 3-kinase |
| PKC | Protein kinase C |
| PLCg-1 | 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase gamma-1 |
| PLK | Serine/threonine-protein kinase PLK |
| PRR | Pattern recognition receptors |
| PS | Phosphatidylserine |
| PTEN | Phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase |
| RAD50 | DNA repair protein RAD50 |
| RAD51 | DNA repair protein RAD51 homolog 1 |
| RAD52 | DNA repair protein RAD52 homolog |
| RAP80 | Receptor-associated protein 80 |
| RNF4, -8, -168 | E3 ubiquitin-protein ligases |
| ROS | Reactive oxygen species |
| RPA | Replication protein A |
| SCLC | Small-cell lung cancer |
| SHP1/2 | Src homology region 2 domain containing phosphatases |
| SMACK | Diablo IAP-binding mitochondrial protein |
| SSBR | Single-strand break repair |
| ssDNA | Single-stranded DNA |
| STAT | Signal transducer and activator of transcription |
| STING | Stimulator of interferon genes protein |
| TBP | TATA binding protein |
| TCR | T-cell receptor |
| TGF-β | Transforming growth factor β |
| TICs | Tumor-initiating cells |
| TILs | Tumor infiltrating lymphocytes |
| TLR | Toll-like receptor |
| TLS | Translesion DNA synthesis |
| TM | Transmembrane domain |
| TMB | Tumor mutational burden |
| TNF-α | Tumor necrosis factor α |
| TOPBP1 | Topoisomerase 2-binding protein 1 |
| TP53 | Cellular tumor antigen p53 |
| Tregs | Regulatory T cells |
| WAF1/CIP1 | Cyclin-dependent kinase inhibitor 1 |
| XLF | Non-homologous end-joining factor 1 |
| XRCC4 | X-ray repair cross-complementing protein 4 |
References
- Dillman, R.O. Cancer Immunotherapy. Cancer Biother. Radiopharm. 2011, 26, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Davis, I.D. An Overview of Cancer Immunotherapy. Immunol. Cell. Biol. 2000, 78, 179–195. [Google Scholar] [CrossRef] [PubMed]
- Gamrekelashvili, J.; Krüger, C.; von Wasielewski, R.; Hoffmann, M.; Huster, K.M.; Busch, D.H.; Manns, M.P.; Korangy, F.; Greten, T.F. Necrotic Tumor Cell Death in Vivo Impairs Tumor-Specific Immune Responses. J. Immunol. 2007, 178, 1573–1580. [Google Scholar] [CrossRef]
- McDonnell, A.M.; Nowak, A.K.; Lake, R.A. Contribution of the Immune System to the Chemotherapeutic Response. Semin. Immunopathol. 2011, 33, 353–367. [Google Scholar] [CrossRef]
- Gardai, S.J.; McPhillips, K.A.; Frasch, S.C.; Janssen, W.J.; Starefeldt, A.; Murphy-Ullrich, J.E.; Bratton, D.L.; Oldenborg, P.-A.; Michalak, M.; Henson, P.M. Cell-Surface Calreticulin Initiates Clearance of Viable or Apoptotic Cells through Trans-Activation of LRP on the Phagocyte. Cell 2005, 123, 321–334. [Google Scholar] [CrossRef]
- Obeid, M.; Tesniere, A.; Ghiringhelli, F.; Fimia, G.M.; Apetoh, L.; Perfettini, J.-L.; Castedo, M.; Mignot, G.; Panaretakis, T.; Casares, N.; et al. Calreticulin Exposure Dictates the Immunogenicity of Cancer Cell Death. Nat. Med. 2007, 13, 54–61. [Google Scholar] [CrossRef]
- Apetoh, L.; Ghiringhelli, F.; Tesniere, A.; Obeid, M.; Ortiz, C.; Criollo, A.; Mignot, G.; Maiuri, M.C.; Ullrich, E.; Saulnier, P.; et al. Toll-like Receptor 4-Dependent Contribution of the Immune System to Anticancer Chemotherapy and Radiotherapy. Nat. Med. 2007, 13, 1050–1059. [Google Scholar] [CrossRef]
- Bailly, C.; Thuru, X.; Quesnel, B. Combined Cytotoxic Chemotherapy and Immunotherapy of Cancer: Modern Times. NAR Cancer 2020, 2, zcaa002. [Google Scholar] [CrossRef]
- Park, S.-J.; Ye, W.; Xiao, R.; Silvin, C.; Padget, M.; Hodge, J.W.; Van Waes, C.; Schmitt, N.C. Cisplatin and Oxaliplatin Induce Similar Immunogenic Changes in Preclinical Models of Head and Neck Cancer. Oral Oncol. 2019, 95, 127–135. [Google Scholar] [CrossRef]
- Terenzi, A.; Pirker, C.; Keppler, B.K.; Berger, W. Anticancer Metal Drugs and Immunogenic Cell Death. J. Inorg. Biochem. 2016, 165, 71–79. [Google Scholar] [CrossRef]
- Konstantinidou, M.; Zarganes-Tzitzikas, T.; Magiera-Mularz, K.; Holak, T.A.; Dömling, A. Immune Checkpoint PD-1/PD-L1: Is There Life Beyond Antibodies? Angew. Chem. Int. Ed. Engl. 2018, 57, 4840–4848. [Google Scholar] [CrossRef] [PubMed]
- Inthagard, J.; Edwards, J.; Roseweir, A.K. Immunotherapy: Enhancing the Efficacy of This Promising Therapeutic in Multiple Cancers. Clin. Sci. 2019, 133, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, M.; Nie, H.; Yuan, Y. PD-1 and PD-L1 in Cancer Immunotherapy: Clinical Implications and Future Considerations. Hum. Vaccines Immunother. 2019, 15, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Salmaninejad, A.; Valilou, S.F.; Shabgah, A.G.; Aslani, S.; Alimardani, M.; Pasdar, A.; Sahebkar, A. PD-1/PD-L1 Pathway: Basic Biology and Role in Cancer Immunotherapy. J. Cell Physiol. 2019, 234, 16824–16837. [Google Scholar] [CrossRef] [PubMed]
- LV, B.; Wang, Y.; Ma, D.; Cheng, W.; Liu, J.; Yong, T.; Chen, H.; Wang, C. Immunotherapy: Reshape the Tumor Immune Microenvironment. Front. Immunol. 2022, 13, 844142. [Google Scholar] [CrossRef]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the Tumor Immune Microenvironment (TIME) for Effective Therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Liu, Q.; Xiang, Y.; Gou, X.; Li, W. Role of the Tumor Immune Microenvironment in Tumor Immunotherapy. Oncol. Lett. 2022, 23, 53. [Google Scholar] [CrossRef]
- Lee, H.T.; Lee, S.H.; Heo, Y.-S. Molecular Interactions of Antibody Drugs Targeting PD-1, PD-L1, and CTLA-4 in Immuno-Oncology. Molecules 2019, 24, 1190. [Google Scholar] [CrossRef]
- Descourt, R.; Greillier, L.; Perol, M.; Ricordel, C.; Auliac, J.-B.; Falchero, L.; Gervais, R.; Veillon, R.; Vieillot, S.; Guisier, F.; et al. First-Line Single-Agent Pembrolizumab for PD-L1-Positive (Tumor Proportion Score ≥ 50%) Advanced Non-Small Cell Lung Cancer in the Real World: Impact in Brain Metastasis: A National French Multicentric Cohort (ESCKEYP GFPC Study). Cancer Immunol. Immunother. 2022, 72, 91–99. [Google Scholar] [CrossRef]
- Duan, H.; Shao, C.; Pan, M.; Liu, H.; Dong, X.; Zhang, Y.; Tong, L.; Feng, Y.; Wang, Y.; Wang, L.; et al. Neoadjuvant Pembrolizumab and Chemotherapy in Resectable Esophageal Cancer: An Open-Label, Single-Arm Study (PEN-ICE). Front. Immunol. 2022, 13, 849984. [Google Scholar] [CrossRef]
- Papadatos-Pastos, D.; Yuan, W.; Pal, A.; Crespo, M.; Ferreira, A.; Gurel, B.; Prout, T.; Ameratunga, M.; Chénard-Poirier, M.; Curcean, A.; et al. Phase 1, Dose-Escalation Study of Guadecitabine (SGI-110) in Combination with Pembrolizumab in Patients with Solid Tumors. J. Immunother. Cancer 2022, 10, e004495. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Qin, S.; Wang, L.; Tan, C.; Peng, Y.; Zeng, X.; Luo, X.; Yi, L.; Wan, X. Cost-Effectiveness of Pembrolizumab Plus Chemotherapy as First-Line Therapy for Advanced Oesophageal Cancer. Front. Pharmacol. 2022, 13, 881787. [Google Scholar] [CrossRef] [PubMed]
- Miron, B.; Geynisman, D.M. Bempegaldesleukin/Nivolumab and Challenges in First-Line Treatment of Metastatic Urothelial Carcinoma. Eur. Urol. 2022, 82, 374–376. [Google Scholar] [CrossRef] [PubMed]
- Bennani, N.N.; Kim, H.J.; Pederson, L.D.; Atherton, P.J.; Micallef, I.N.; Thanarajasingam, G.; Nowakowski, G.S.; Witzig, T.; Feldman, A.L.; Ansell, S.M. Nivolumab in Patients with Relapsed or Refractory Peripheral T-Cell Lymphoma: Modest Activity and Cases of Hyperprogression. J. Immunother. Cancer 2022, 10, e004984. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M.; Yasumatsu, R.; Masuda, M.; Yamauchi, M.; Wakasaki, T.; Hashimoto, K.; Jiromaru, R.; Manako, T.; Nakagawa, T. Five-Year Follow-up of Patients With Head and Neck Cancer Treated With Nivolumab and Long-Term Responders for Over Two Years. In Vivo 2022, 36, 1881–1886. [Google Scholar] [CrossRef]
- Stein, A.; Paschold, L.; Tintelnot, J.; Goekkurt, E.; Henkes, S.-S.; Simnica, D.; Schultheiss, C.; Willscher, E.; Bauer, M.; Wickenhauser, C.; et al. Efficacy of Ipilimumab vs FOLFOX in Combination With Nivolumab and Trastuzumab in Patients With Previously Untreated ERBB2-Positive Esophagogastric Adenocarcinoma: The AIO INTEGA Randomized Clinical Trial. JAMA Oncol. 2022, 8, 1150–1158. [Google Scholar] [CrossRef]
- Xiang, Z.; Li, J.; Zhang, Z.; Cen, C.; Chen, W.; Jiang, B.; Meng, Y.; Wang, Y.; Berglund, B.; Zhai, G.; et al. Comprehensive Evaluation of Anti-PD-1, Anti-PD-L1, Anti-CTLA-4 and Their Combined Immunotherapy in Clinical Trials: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2022, 13, 883655. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, C.; Ding, D.; Tian, H.; Yu, C. Comparative Efficacy and Safety of Anti-PD-1/PD-L1 for the Treatment of Non-Small Cell Lung Cancer: A Network Meta-Analysis of 13 Randomized Controlled Studies. Front. Oncol. 2022, 12, 827050. [Google Scholar] [CrossRef]
- Imai, H.; Nagai, Y.; Minemura, H.; Tsuda, T.; Yamada, Y.; Wasamoto, S.; Kishikawa, T.; Shiono, A.; Shiihara, J.; Yamaguchi, O.; et al. Efficacy and Safety of Amrubicin Monotherapy after Atezolizumab plus Carboplatin and Etoposide in Patients with Relapsed Small-Cell Lung Cancer. Investig. New Drugs 2022, 40, 1066–1079. [Google Scholar] [CrossRef]
- Kuwano, A.; Yada, M.; Narutomi, F.; Nagasawa, S.; Tanaka, K.; Kurosaka, K.; Ohishi, Y.; Masumoto, A.; Motomura, K. Therapeutic Efficacy of Atezolizumab plus Bevacizumab for Hepatocellular Carcinoma with WNT/β-Catenin Signal Activation. Oncol. Lett. 2022, 24, 216. [Google Scholar] [CrossRef]
- Gürbüz, M.; Kutlu, Y.; Akkuş, E.; Köksoy, E.B.; Köse, N.; Öven, B.B.; Uluç, B.O.; Demiray, A.G.; Erdem, D.; Demir, B.; et al. Atezolizumab Combined with Chemotherapy in the First-Line Treatment of Extensive-Stage Small Cell Lung Cancer: A Real-Life Data of the Turkish Oncology Group. J. Cancer Res. Clin. Oncol. 2022, 148, 3547–3555. [Google Scholar] [CrossRef] [PubMed]
- Bunse, L.; Rupp, A.-K.; Poschke, I.; Bunse, T.; Lindner, K.; Wick, A.; Blobner, J.; Misch, M.; Tabatabai, G.; Glas, M.; et al. AMPLIFY-NEOVAC: A Randomized, 3-Arm Multicenter Phase I Trial to Assess Safety, Tolerability and Immunogenicity of IDH1-Vac Combined with an Immune Checkpoint Inhibitor Targeting Programmed Death-Ligand 1 in Isocitrate Dehydrogenase 1 Mutant Gliomas. Neurol. Res. Pract. 2022, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.L.; Rodriguez-Freixinos, V.; Doherty, M.; Wasson, K.; Iscoe, N.; Raskin, W.; Hallet, J.; Myrehaug, S.; Law, C.; Thawer, A.; et al. Avelumab in Unresectable/Metastatic, Progressive, Grade 2-3 Neuroendocrine Neoplasms (NENs): Combined Results from NET-001 and NET-002 Trials. Eur. J. Cancer 2022, 169, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S. Avelumab: First Global Approval. Drugs 2017, 77, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.E.; Park, S.H.; Kozlov, V.; Dao, T.V.; Castellano, D.; Li, J.-R.; Mukherjee, S.D.; Howells, K.; Dry, H.; Lanasa, M.C.; et al. Durvalumab Plus Olaparib in Previously Untreated, Platinum-Ineligible Patients With Metastatic Urothelial Carcinoma: A Multicenter, Randomized, Phase II Trial (BAYOU). J. Clin. Oncol. 2022, 41, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Kawanaka, Y.; Yasuda, Y.; Tanizaki, J.; Iwashima, D.; Nonagase, Y.; Uemasu, K.; Hirayama, Y.; Ogura, M.; Ozaki, T.; Takahashi, K.-I. The Safety and Efficacy of Durvalumab Consolidation Therapy in the Management of Patients with Stage III Non-Small-Cell Lung Cancer and Preexisting Interstitial Lung Disease. Respir. Investig. 2022, 60, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Uchimiak, K.; Badowska-Kozakiewicz, A.M.; Sobiborowicz-Sadowska, A.; Deptała, A. Current State of Knowledge on the Immune Checkpoint Inhibitors in Triple-Negative Breast Cancer Treatment: Approaches, Efficacy, and Challenges. Clin. Med. Insights Oncol. 2022, 16, 11795549221099868. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, L.; Zuo, Y.; Qian, H.; Liu, C. Immune Checkpoint Blockade in Cancer Immunotherapy: Mechanisms, Clinical Outcomes, and Safety Profiles of PD-1/PD-L1 Inhibitors. Arch. Immunol. Ther. Exp. 2020, 68, 36. [Google Scholar] [CrossRef]
- Xin Yu, J.; Hodge, J.P.; Oliva, C.; Neftelinov, S.T.; Hubbard-Lucey, V.M.; Tang, J. Trends in Clinical Development for PD-1/PD-L1 Inhibitors. Nat. Rev. Drug Discov. 2020, 19, 163–164. [Google Scholar] [CrossRef]
- Keir, M.E.; Liang, S.C.; Guleria, I.; Latchman, Y.E.; Qipo, A.; Albacker, L.A.; Koulmanda, M.; Freeman, G.J.; Sayegh, M.H.; Sharpe, A.H. Tissue Expression of PD-L1 Mediates Peripheral T Cell Tolerance. J. Exp. Med. 2006, 203, 883–895. [Google Scholar] [CrossRef]
- Reynoso, E.D.; Elpek, K.G.; Francisco, L.; Bronson, R.; Bellemare-Pelletier, A.; Sharpe, A.H.; Freeman, G.J.; Turley, S.J. Intestinal Tolerance Is Converted to Autoimmune Enteritis upon PD-1 Ligand Blockade. J. Immunol. 2009, 182, 2102–2112. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Sun, Q.; Zhang, X. PD-1 and Its Ligands Are Important Immune Checkpoints in Cancer. Oncotarget 2016, 8, 2171–2186. [Google Scholar] [CrossRef]
- Boussiotis, V.A.; Chatterjee, P.; Li, L. Biochemical Signaling of PD-1 on T Cells and Its Functional Implications. Cancer J. 2014, 20, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Neel, B.G.; Gu, H.; Pao, L. The ’Shp’ing News: SH2 Domain-Containing Tyrosine Phosphatases in Cell Signaling. Trends Biochem. Sci. 2003, 28, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, K.-A.; Fitz, L.J.; Lee, J.M.; Benander, C.; George, J.A.; Wooters, J.; Qiu, Y.; Jussif, J.M.; Carter, L.L.; Wood, C.R.; et al. PD-1 Inhibits T-Cell Receptor Induced Phosphorylation of the ZAP70/CD3zeta Signalosome and Downstream Signaling to PKCtheta. FEBS Lett. 2004, 574, 37–41. [Google Scholar] [CrossRef]
- Parry, R.V.; Chemnitz, J.M.; Frauwirth, K.A.; Lanfranco, A.R.; Braunstein, I.; Kobayashi, S.V.; Linsley, P.S.; Thompson, C.B.; Riley, J.L. CTLA-4 and PD-1 Receptors Inhibit T-Cell Activation by Distinct Mechanisms. Mol. Cell Biol. 2005, 25, 9543–9553. [Google Scholar] [CrossRef]
- Okazaki, T.; Maeda, A.; Nishimura, H.; Kurosaki, T.; Honjo, T. PD-1 Immunoreceptor Inhibits B Cell Receptor-Mediated Signaling by Recruiting Src Homology 2-Domain-Containing Tyrosine Phosphatase 2 to Phosphotyrosine. Proc. Natl. Acad. Sci. USA 2001, 98, 13866–13871. [Google Scholar] [CrossRef]
- Ai, L.; Xu, A.; Xu, J. Roles of PD-1/PD-L1 Pathway: Signaling, Cancer, and Beyond. Adv. Exp. Med. Biol. 2020, 1248, 33–59. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 Pathway: Current Researches in Cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar]
- Patsoukis, N.; Li, L.; Sari, D.; Petkova, V.; Boussiotis, V.A. PD-1 Increases PTEN Phosphatase Activity While Decreasing PTEN Protein Stability by Inhibiting Casein Kinase 2. Mol. Cell Biol. 2013, 33, 3091–3098. [Google Scholar] [CrossRef]
- Latchman, Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y.; Long, A.J.; Brown, J.A.; Nunes, R.; et al. PD-L2 Is a Second Ligand for PD-1 and Inhibits T Cell Activation. Nat. Immunol. 2001, 2, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Ohashi, P.S. Clinical Blockade of PD1 and LAG3--Potential Mechanisms of Action. Nat. Rev. Immunol. 2015, 15, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Philips, E.A.; Garcia-España, A.; Tocheva, A.S.; Ahearn, I.M.; Adam, K.R.; Pan, R.; Mor, A.; Kong, X.-P. The Structural Features That Distinguish PD-L2 from PD-L1 Emerged in Placental Mammals. J. Biol. Chem. 2020, 295, 4372–4380. [Google Scholar] [CrossRef]
- Cheng, X.; Veverka, V.; Radhakrishnan, A.; Waters, L.C.; Muskett, F.W.; Morgan, S.H.; Huo, J.; Yu, C.; Evans, E.J.; Leslie, A.J.; et al. Structure and Interactions of the Human Programmed Cell Death 1 Receptor. J. Biol. Chem. 2013, 288, 11771–11785. [Google Scholar] [CrossRef] [PubMed]
- Escors, D.; Gato-Cañas, M.; Zuazo, M.; Arasanz, H.; García-Granda, M.J.; Vera, R.; Kochan, G. The Intracellular Signalosome of PD-L1 in Cancer Cells. Signal. Transduct. Target. Ther. 2018, 3, 26. [Google Scholar] [CrossRef]
- Prima, V.; Kaliberova, L.N.; Kaliberov, S.; Curiel, D.T.; Kusmartsev, S. COX2/MPGES1/PGE2 Pathway Regulates PD-L1 Expression in Tumor-Associated Macrophages and Myeloid-Derived Suppressor Cells. Proc. Natl. Acad. Sci. USA 2017, 114, 1117–1122. [Google Scholar] [CrossRef]
- Noman, M.Z.; Desantis, G.; Janji, B.; Hasmim, M.; Karray, S.; Dessen, P.; Bronte, V.; Chouaib, S. PD-L1 Is a Novel Direct Target of HIF-1α, and Its Blockade under Hypoxia Enhanced MDSC-Mediated T Cell Activation. J. Exp. Med. 2014, 211, 781–790. [Google Scholar] [CrossRef]
- Van Duijn, A.; Willemsen, K.J.; van Uden, N.O.P.; Hoyng, L.; Erades, S.; Koster, J.; Luiten, R.M.; Bakker, W.J. A Secondary Role for Hypoxia and HIF1 in the Regulation of (IFNγ-Induced) PD-L1 Expression in Melanoma. Cancer Immunol. Immunother. 2022, 71, 529–540. [Google Scholar] [CrossRef]
- Zhou, L.; Cha, G.; Chen, L.; Yang, C.; Xu, D.; Ge, M. HIF1α/PD-L1 Axis Mediates Hypoxia-Induced Cell Apoptosis and Tumor Progression in Follicular Thyroid Carcinoma. OncoTargets Ther. 2019, 12, 6461–6470. [Google Scholar] [CrossRef]
- Antonangeli, F.; Natalini, A.; Garassino, M.C.; Sica, A.; Santoni, A.; Di Rosa, F. Regulation of PD-L1 Expression by NF-ΚB in Cancer. Front. Immunol. 2020, 11, 584626. [Google Scholar] [CrossRef]
- Lastwika, K.J.; Wilson, W.; Li, Q.K.; Norris, J.; Xu, H.; Ghazarian, S.R.; Kitagawa, H.; Kawabata, S.; Taube, J.M.; Yao, S.; et al. Control of PD-L1 Expression by Oncogenic Activation of the AKT-MTOR Pathway in Non-Small Cell Lung Cancer. Cancer Res. 2016, 76, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Taghiloo, S.; Norozi, S.; Asgarian-Omran, H. The Effects of PI3K/Akt/MTOR Signaling Pathway Inhibitors on the Expression of Immune Checkpoint Ligands in Acute Myeloid Leukemia Cell Line. Iran. J. Allergy Asthma Immunol. 2022, 21, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Fan, S.; Li, J.; Zhou, X.; Liao, Q.; Tang, F.; Liu, W. Programmed Death-Ligand 1 Signaling and Expression Are Reversible by Lycopene via PI3K/AKT and Raf/MEK/ERK Pathways in Tongue Squamous Cell Carcinoma. Genes Nutr. 2022, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Stutvoet, T.S.; Kol, A.; de Vries, E.G.; de Bruyn, M.; Fehrmann, R.S.; Terwisscha van Scheltinga, A.G.; de Jong, S. MAPK Pathway Activity Plays a Key Role in PD-L1 Expression of Lung Adenocarcinoma Cells. J. Pathol. 2019, 249, 52–64. [Google Scholar] [CrossRef]
- Sumimoto, H.; Takano, A.; Teramoto, K.; Daigo, Y. RAS-Mitogen-Activated Protein Kinase Signal Is Required for Enhanced PD-L1 Expression in Human Lung Cancers. PLoS ONE 2016, 11, e0166626. [Google Scholar] [CrossRef]
- Xing, S.; Chen, S.; Yang, X.; Huang, W. Role of MAPK Activity in PD-L1 Expression in Hepatocellular Carcinoma Cells. J. BUON 2020, 25, 1875–1882. [Google Scholar]
- Bu, L.L.; Yu, G.T.; Wu, L.; Mao, L.; Deng, W.W.; Liu, J.F.; Kulkarni, A.B.; Zhang, W.F.; Zhang, L.; Sun, Z.J. STAT3 Induces Immunosuppression by Upregulating PD-1/PD-L1 in HNSCC. J. Dent. Res. 2017, 96, 1027–1034. [Google Scholar] [CrossRef]
- Zerdes, I.; Wallerius, M.; Sifakis, E.G.; Wallmann, T.; Betts, S.; Bartish, M.; Tsesmetzis, N.; Tobin, N.P.; Coucoravas, C.; Bergh, J.; et al. STAT3 Activity Promotes Programmed-Death Ligand 1 Expression and Suppresses Immune Responses in Breast Cancer. Cancers 2019, 11, 1479. [Google Scholar] [CrossRef]
- Jackson, S.P.; Bartek, J. The DNA-Damage Response in Human Biology and Disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Kciuk, M.; Gielecińska, A.; Kołat, D.; Kałuzińska, Ż.; Kontek, R. Cancer-Associated Transcription Factors in DNA Damage Response. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188757. [Google Scholar] [CrossRef] [PubMed]
- Kciuk, M.; Marciniak, B.; Mojzych, M.; Kontek, R. Focus on UV-Induced DNA Damage and Repair-Disease Relevance and Protective Strategies. Int. J. Mol. Sci. 2020, 21, 7264. [Google Scholar] [CrossRef] [PubMed]
- Kciuk, M.; Gielecińska, A.; Mujwar, S.; Mojzych, M.; Kontek, R. Cyclin-Dependent Kinases in DNA Damage Response. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188716. [Google Scholar] [CrossRef] [PubMed]
- Zannini, L.; Delia, D.; Buscemi, G. CHK2 Kinase in the DNA Damage Response and Beyond. J. Mol. Cell Biol. 2014, 6, 442–457. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Huang, S. The Role of Cdc25A in the Regulation of Cell Proliferation and Apoptosis. Anticancer Agents Med. Chem. 2012, 12, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Carusillo, A.; Mussolino, C. DNA Damage: From Threat to Treatment. Cells 2020, 9, 1665. [Google Scholar] [CrossRef]
- Nussenzweig, A. Causes and Consequences of the DNA Damage Response. Cell Cycle 2007, 6, 2339–2340. [Google Scholar] [CrossRef]
- Harper, J.W.; Elledge, S.J. The DNA Damage Response: Ten Years After. Mol. Cell 2007, 28, 739–745. [Google Scholar] [CrossRef]
- Ray Chaudhuri, A.; Nussenzweig, A. The Multifaceted Roles of PARP1 in DNA Repair and Chromatin Remodelling. Nat. Rev. Mol. Cell Biol. 2017, 18, 610–621. [Google Scholar] [CrossRef]
- Wright, W.D.; Shah, S.S.; Heyer, W.-D. Homologous Recombination and the Repair of DNA Double-Strand Breaks. J. Biol. Chem. 2018, 293, 10524–10535. [Google Scholar] [CrossRef]
- Lieber, M.R. The Mechanism of Double-Strand DNA Break Repair by the Nonhomologous DNA End-Joining Pathway. Annu. Rev. Biochem. 2010, 79, 181–211. [Google Scholar] [CrossRef]
- Li, X.; Heyer, W.-D. Homologous Recombination in DNA Repair and DNA Damage Tolerance. Cell Res. 2008, 18, 99–113. [Google Scholar] [CrossRef]
- Chang, H.H.Y.; Pannunzio, N.R.; Adachi, N.; Lieber, M.R. Non-Homologous DNA End Joining and Alternative Pathways to Double-Strand Break Repair. Nat. Rev. Mol. Cell Biol. 2017, 18, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Burma, S.; Chen, B.P.C.; Chen, D.J. Role of Non-Homologous End Joining (NHEJ) in Maintaining Genomic Integrity. DNA Repair 2006, 5, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Buqué, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell 2015, 28, 690–714. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Liang, H.; Burnette, B.; Beckett, M.; Darga, T.; Weichselbaum, R.R.; Fu, Y.-X. Irradiation and Anti-PD-L1 Treatment Synergistically Promote Antitumor Immunity in Mice. J. Clin. Investig. 2014, 124, 687–695. [Google Scholar] [CrossRef]
- Wu, C.-T.; Chen, W.-C.; Chang, Y.-H.; Lin, W.-Y.; Chen, M.-F. The Role of PD-L1 in the Radiation Response and Clinical Outcome for Bladder Cancer. Sci. Rep. 2016, 6, 19740. [Google Scholar] [CrossRef]
- Bedi, D.; Henderson, H.J.; Manne, U.; Samuel, T. Camptothecin Induces PD-L1 and Immunomodulatory Cytokines in Colon Cancer Cells. Medicines 2019, 6, 51. [Google Scholar] [CrossRef]
- Gilad, Y.; Eliaz, Y.; Yu, Y.; Han, S.J.; O’Malley, B.W.; Lonard, D.M. Drug-Induced PD-L1 Expression and Cell Stress Response in Breast Cancer Cells Can Be Balanced by Drug Combination. Sci. Rep. 2019, 9, 15099. [Google Scholar] [CrossRef]
- Voorwerk, L.; Slagter, M.; Horlings, H.M.; Sikorska, K.; van de Vijver, K.K.; de Maaker, M.; Nederlof, I.; Kluin, R.J.C.; Warren, S.; Ong, S.; et al. Immune Induction Strategies in Metastatic Triple-Negative Breast Cancer to Enhance the Sensitivity to PD-1 Blockade: The TONIC Trial. Nat. Med. 2019, 25, 920–928. [Google Scholar] [CrossRef]
- Wang, J.; Hu, C.; Wang, J.; Shen, Y.; Bao, Q.; He, F.; Wang, H.; Gong, L.; Liu, Z.; Hu, F.; et al. Checkpoint Blockade in Combination With Doxorubicin Augments Tumor Cell Apoptosis in Osteosarcoma. J. Immunother. 2019, 42, 321–330. [Google Scholar] [CrossRef]
- Iwai, T.; Sugimoto, M.; Wakita, D.; Yorozu, K.; Kurasawa, M.; Yamamoto, K. Topoisomerase I Inhibitor, Irinotecan, Depletes Regulatory T Cells and up-Regulates MHC Class I and PD-L1 Expression, Resulting in a Supra-Additive Antitumor Effect When Combined with Anti-PD-L1 Antibodies. Oncotarget 2018, 9, 31411–31421. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Xu, J.; Cai, H.; Lang, J. Carboplatin and Programmed Death-Ligand 1 Blockade Synergistically Produce a Similar Antitumor Effect to Carboplatin Alone in Murine ID8 Ovarian Cancer Model. J. Obstet. Gynaecol. Res. 2018, 44, 303–311. [Google Scholar] [CrossRef]
- Wahba, J.; Natoli, M.; Whilding, L.M.; Parente-Pereira, A.C.; Jung, Y.; Zona, S.; Lam, E.W.-F.; Smith, J.R.; Maher, J.; Ghaem-Maghami, S. Chemotherapy-Induced Apoptosis, Autophagy and Cell Cycle Arrest Are Key Drivers of Synergy in Chemo-Immunotherapy of Epithelial Ovarian Cancer. Cancer Immunol. Immunother. 2018, 67, 1753–1765. [Google Scholar] [CrossRef] [PubMed]
- Fournel, L.; Wu, Z.; Stadler, N.; Damotte, D.; Lococo, F.; Boulle, G.; Ségal-Bendirdjian, E.; Bobbio, A.; Icard, P.; Trédaniel, J.; et al. Cisplatin Increases PD-L1 Expression and Optimizes Immune Check-Point Blockade in Non-Small Cell Lung Cancer. Cancer Lett. 2019, 464, 5–14. [Google Scholar] [CrossRef]
- Tran, L.; Allen, C.T.; Xiao, R.; Moore, E.; Davis, R.; Park, S.-J.; Spielbauer, K.; Van Waes, C.; Schmitt, N.C. Cisplatin Alters Antitumor Immunity and Synergizes with PD-1/PD-L1 Inhibition in Head and Neck Squamous Cell Carcinoma. Cancer Immunol. Res. 2017, 5, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Ock, C.-Y.; Kim, S.; Keam, B.; Kim, S.; Ahn, Y.-O.; Chung, E.-J.; Kim, J.-H.; Kim, T.M.; Kwon, S.K.; Jeon, Y.K.; et al. Changes in Programmed Death-Ligand 1 Expression during Cisplatin Treatment in Patients with Head and Neck Squamous Cell Carcinoma. Oncotarget 2017, 8, 97920–97927. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.-F.; Lin, J.-F.; Lin, Y.-C.; Chou, K.-Y.; Chen, H.-E.; Ho, C.-Y.; Chen, P.-C.; Hwang, T.I.-S. Cisplatin Contributes to Programmed Death-Ligand 1 Expression in Bladder Cancer through ERK1/2-AP-1 Signaling Pathway. Biosci. Rep. 2019, 39, BSR20190362. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Liu, C.; Zhou, Y.; Wang, G. Cisplatin Induces Programmed Death-1-Ligand 1(PD-L1) over-Expression in Hepatoma H22 Cells via Erk /MAPK Signaling Pathway. Cell. Mol. Biol. 2010, 56, 1366–1372. [Google Scholar]
- Wu, X.; Li, Y.; Liu, X.; Chen, C.; Harrington, S.M.; Cao, S.; Xie, T.; Pham, T.; Mansfield, A.S.; Yan, Y.; et al. Targeting B7-H1 (PD-L1) Sensitizes Cancer Cells to Chemotherapy. Heliyon 2018, 4, e01039. [Google Scholar] [CrossRef]
- Sato, H.; Niimi, A.; Yasuhara, T.; Permata, T.B.M.; Hagiwara, Y.; Isono, M.; Nuryadi, E.; Sekine, R.; Oike, T.; Kakoti, S.; et al. DNA Double-Strand Break Repair Pathway Regulates PD-L1 Expression in Cancer Cells. Nat. Commun. 2017, 8, 1751. [Google Scholar] [CrossRef]
- Chatterjee, A.; Rodger, E.J.; Ahn, A.; Stockwell, P.A.; Parry, M.; Motwani, J.; Gallagher, S.J.; Shklovskaya, E.; Tiffen, J.; Eccles, M.R.; et al. Marked Global DNA Hypomethylation Is Associated with Constitutive PD-L1 Expression in Melanoma. iScience 2018, 4, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Wang, H.; Li, A.; Xu, Y.; Tang, L.; Chen, Q.; Zhang, C.; Gao, Y.; Song, J.; Du, Z. Decitibine Improve the Efficiency of Anti-PD-1 Therapy via Activating the Response to IFN/PD-L1 Signal of Lung Cancer Cells. Oncogene 2018, 37, 2302–2312. [Google Scholar] [CrossRef] [PubMed]
- Bensaid, D.; Blondy, T.; Deshayes, S.; Dehame, V.; Bertrand, P.; Grégoire, M.; Errami, M.; Blanquart, C. Assessment of New HDAC Inhibitors for Immunotherapy of Malignant Pleural Mesothelioma. Clin. Epigenet. 2018, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Lailler, C.; Lamuraglia, M.; Racine, F.; Louandre, C.; Godin, C.; Chauffert, B.; Galmiche, A.; Saidak, Z. DNA Damage Response- and JAK-Dependent Regulation of PD-L1 Expression in Head and Neck Squamous Cell Carcinoma (HNSCC) Cells Exposed to 5-Fluorouracil (5-FU). Transl. Oncol. 2021, 14, 101110. [Google Scholar] [CrossRef]
- Van Der Kraak, L.; Goel, G.; Ramanan, K.; Kaltenmeier, C.; Zhang, L.; Normolle, D.P.; Freeman, G.J.; Tang, D.; Nason, K.S.; Davison, J.M.; et al. 5-Fluorouracil Upregulates Cell Surface B7-H1 (PD-L1) Expression in Gastrointestinal Cancers. J. Immunother. Cancer 2016, 4, 65. [Google Scholar] [CrossRef]
- Doi, T.; Ishikawa, T.; Okayama, T.; Oka, K.; Mizushima, K.; Yasuda, T.; Sakamoto, N.; Katada, K.; Kamada, K.; Uchiyama, K.; et al. The JAK/STAT Pathway Is Involved in the Upregulation of PD-L1 Expression in Pancreatic Cancer Cell Lines. Oncol. Rep. 2017, 37, 1545–1554. [Google Scholar] [CrossRef]
- Rosenbaum, M.W.; Bledsoe, J.R.; Morales-Oyarvide, V.; Huynh, T.G.; Mino-Kenudson, M. PD-L1 Expression in Colorectal Cancer Is Associated with Microsatellite Instability, BRAF Mutation, Medullary Morphology and Cytotoxic Tumor-Infiltrating Lymphocytes. Mod. Pathol. 2016, 29, 1104–1112. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Roberts, J.; Salaria, S.N.; Cates, J.; Wang, Y.; Vnencak-Jones, C.; Berlin, J.; Shi, C. PD-L1 Expression Patterns in Microsatellite Instability-High Intestinal Adenocarcinoma Subtypes. Am. J. Clin. Pathol. 2019, 152, 384–391. [Google Scholar] [CrossRef]
- Morihiro, T.; Kuroda, S.; Kanaya, N.; Kakiuchi, Y.; Kubota, T.; Aoyama, K.; Tanaka, T.; Kikuchi, S.; Nagasaka, T.; Nishizaki, M.; et al. PD-L1 Expression Combined with Microsatellite Instability/CD8+ Tumor Infiltrating Lymphocytes as a Useful Prognostic Biomarker in Gastric Cancer. Sci. Rep. 2019, 9, 4633. [Google Scholar] [CrossRef]
- Cho, Y.A.; Lee, H.; Kim, D.G.; Kim, H.; Ha, S.Y.; Choi, Y.-L.; Jang, K.-T.; Kim, K.-M. PD-L1 Expression Is Significantly Associated with Tumor Mutation Burden and Microsatellite Instability Score. Cancers 2021, 13, 4659. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.F.; Bretes, L.; Furtado, I. Review of PD-1/PD-L1 Inhibitors in Metastatic DMMR/MSI-H Colorectal Cancer. Front. Oncol. 2019, 9, 396. [Google Scholar] [CrossRef]
- Xiao, Y.; Freeman, G.J. The Microsatellite Instable Subset of Colorectal Cancer Is a Particularly Good Candidate for Checkpoint Blockade Immunotherapy. Cancer Discov. 2015, 5, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Strickland, K.C.; Howitt, B.E.; Shukla, S.A.; Rodig, S.; Ritterhouse, L.L.; Liu, J.F.; Garber, J.E.; Chowdhury, D.; Wu, C.J.; D’Andrea, A.D.; et al. Association and Prognostic Significance of BRCA1/2-Mutation Status with Neoantigen Load, Number of Tumor-Infiltrating Lymphocytes and Expression of PD-1/PD-L1 in High Grade Serous Ovarian Cancer. Oncotarget 2016, 7, 13587–13598. [Google Scholar] [CrossRef] [PubMed]
- Seetharamu, N.; Preeshagul, I.R.; Sullivan, K.M. New PD-L1 Inhibitors in Non-Small Cell Lung Cancer—Impact of Atezolizumab. Lung Cancer 2017, 8, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Guo, C.-Y.; Tou, F.-F.; Wen, X.-M.; Kuang, Y.-K.; Zhu, Q.; Hu, H. Association of PD-L1 Expression Status with the Efficacy of PD-1/PD-L1 Inhibitors and Overall Survival in Solid Tumours: A Systematic Review and Meta-Analysis. Int. J. Cancer 2020, 147, 116–127. [Google Scholar] [CrossRef]
- Zhang, J.; Bu, X.; Wang, H.; Zhu, Y.; Geng, Y.; Nihira, N.T.; Tan, Y.; Ci, Y.; Wu, F.; Dai, X.; et al. Cyclin D-CDK4 Kinase Destabilizes PD-L1 via Cullin 3-SPOP to Control Cancer Immune Surveillance. Nature 2018, 553, 91–95. [Google Scholar] [CrossRef]
- Cheung, A.; Chenoweth, A.M.; Quist, J.; Sow, H.S.; Malaktou, C.; Ferro, R.; Hoffmann, R.M.; Osborn, G.; Sachouli, E.; French, E.; et al. CDK Inhibition Primes for Anti-PD-L1 Treatment in Triple-Negative Breast Cancer Models. Cancers 2022, 14, 3361. [Google Scholar] [CrossRef]
- Sun, S.-Y. Searching for the Real Function of MTOR Signaling in the Regulation of PD-L1 Expression. Transl. Oncol. 2020, 13, 100847. [Google Scholar] [CrossRef]
- Chen, B.; Hu, J.; Hu, X.; Chen, H.; Bao, R.; Zhou, Y.; Ye, Y.; Zhan, M.; Cai, W.; Li, H.; et al. DENR Controls JAK2 Translation to Induce PD-L1 Expression for Tumor Immune Evasion. Nat. Commun. 2022, 13, 2059. [Google Scholar] [CrossRef]
- Zhao, T.; Li, Y.; Zhang, J.; Zhang, B. PD-L1 Expression Increased by IFN-γ via JAK2-STAT1 Signaling and Predicts a Poor Survival in Colorectal Cancer. Oncol. Lett. 2020, 20, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, Y.; Lv, X. IL4I1 Enhances PD-L1 Expression through JAK/STAT Signaling Pathway in Lung Adenocarcinoma. Immunogenetics 2023, 75, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Diaz, A.; Shin, D.S.; Moreno, B.H.; Saco, J.; Escuin-Ordinas, H.; Rodriguez, G.A.; Zaretsky, J.M.; Sun, L.; Hugo, W.; Wang, X.; et al. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep. 2017, 19, 1189–1201. [Google Scholar] [CrossRef]
- Kciuk, M.; Marciniak, B.; Kontek, R. Irinotecan—Still an Important Player in Cancer Chemotherapy: A Comprehensive Overview. Int. J. Mol. Sci. 2020, 21, 4919. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, J.A.; Mbofung, R.M.; Malu, S.; Zhang, M.; Ashkin, E.; Devi, S.; Williams, L.; Tieu, T.; Peng, W.; Pradeep, S.; et al. The Effect of Topoisomerase I Inhibitors on the Efficacy of T-Cell-Based Cancer Immunotherapy. J. Natl. Cancer Inst. 2018, 110, 777–786. [Google Scholar] [CrossRef]
- Koyama, S.; Nagatomo, I.; Kijima, T.; Kumanogoh, A. Selecting Suitable Chemotherapies for PD-1/PD-L1 Blockade to Optimize the Tumor Immune Microenvironment. Oncotarget 2018, 9, 32552–32553. [Google Scholar] [CrossRef]
- Bailly, C. Irinotecan: 25 Years of Cancer Treatment. Pharmacol. Res. 2019, 148, 104398. [Google Scholar] [CrossRef]
- Ko, A.H. Nanomedicine Developments in the Treatment of Metastatic Pancreatic Cancer: Focus on Nanoliposomal Irinotecan. Int. J. Nanomed. 2016, 11, 1225–1235. [Google Scholar] [CrossRef]
- Xue, L.; Chen, B.; Lin, J.; Peng, J. Anti-PD-L1 Immune Checkpoint Inhibitors in Combination with Etoposide and Platinum for Extensive-Stage Small Cell Lung Cancer: A Case Report. Ann. Palliat Med. 2021, 10, 828–835. [Google Scholar] [CrossRef]
- Kataoka, N.; Kunimatsu, Y.; Tachibana, Y.; Sugimoto, T.; Sato, I.; Tani, N.; Ogura, Y.; Hirose, K.; Takeda, T. Atezolizumab in Combination with Carboplatin and Etoposide for Heavily Treated Small Cell Lung Cancer. Thorac. Cancer 2020, 11, 2740–2742. [Google Scholar] [CrossRef]
- Liu, S.V.; Reck, M.; Mansfield, A.S.; Mok, T.; Scherpereel, A.; Reinmuth, N.; Garassino, M.C.; De Castro Carpeno, J.; Califano, R.; Nishio, M.; et al. Updated Overall Survival and PD-L1 Subgroup Analysis of Patients With Extensive-Stage Small-Cell Lung Cancer Treated With Atezolizumab, Carboplatin, and Etoposide (IMpower133). J. Clin. Oncol. 2021, 39, 619–630. [Google Scholar] [CrossRef]
- Facchinetti, F.; Di Maio, M.; Tiseo, M. Adding PD-1/PD-L1 Inhibitors to Chemotherapy for the First-Line Treatment of Extensive Stage Small Cell Lung Cancer (SCLC): A Meta-Analysis of Randomized Trials. Cancers 2020, 12, 2645. [Google Scholar] [CrossRef] [PubMed]
- Montecucco, A.; Biamonti, G. Cellular Response to Etoposide Treatment. Cancer Lett. 2007, 252, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Théard, D.; Coisy, M.; Ducommun, B.; Concannon, P.; Darbon, J.M. Etoposide and Adriamycin but Not Genistein Can Activate the Checkpoint Kinase Chk2 Independently of ATM/ATR. Biochem. Biophys. Res. Commun. 2001, 289, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Lidonnici, M.R.; Soza, S.; Biamonti, G.; Montecucco, A. The Dispersal of Replication Proteins after Etoposide Treatment Requires the Cooperation of Nbs1 with the Ataxia Telangiectasia Rad3-Related/Chk1 Pathway. Cancer Res. 2006, 66, 1675–1683. [Google Scholar] [CrossRef]
- Weber, A.M.; Drobnitzky, N.; Devery, A.M.; Bokobza, S.M.; Adams, R.A.; Maughan, T.S.; Ryan, A.J. Phenotypic Consequences of Somatic Mutations in the Ataxia-Telangiectasia Mutated Gene in Non-Small Cell Lung Cancer. Oncotarget 2016, 7, 60807–60822. [Google Scholar] [CrossRef]
- Caporossi, D.; Porfirio, B.; Nicoletti, B.; Palitti, F.; Degrassi, F.; De Salvia, R.; Tanzarella, C. Hypersensitivity of Lymphoblastoid Lines Derived from Ataxia Telangiectasia Patients to the Induction of Chromosomal Aberrations by Etoposide (VP-16). Mutat. Res. 1993, 290, 265–272. [Google Scholar] [CrossRef]
- Kijas, A.W.; Lim, Y.C.; Bolderson, E.; Cerosaletti, K.; Gatei, M.; Jakob, B.; Tobias, F.; Taucher-Scholz, G.; Gueven, N.; Oakley, G.; et al. ATM-Dependent Phosphorylation of MRE11 Controls Extent of Resection during Homology Directed Repair by Signalling through Exonuclease 1. Nucleic Acids Res. 2015, 43, 8352–8367. [Google Scholar] [CrossRef]
- Lavin, M.F.; Kozlov, S.; Gatei, M.; Kijas, A.W. ATM-Dependent Phosphorylation of All Three Members of the MRN Complex: From Sensor to Adaptor. Biomolecules 2015, 5, 2877–2902. [Google Scholar] [CrossRef]
- Maser, R.S.; Monsen, K.J.; Nelms, B.E.; Petrini, J.H. HMre11 and HRad50 Nuclear Foci Are Induced during the Normal Cellular Response to DNA Double-Strand Breaks. Mol. Cell. Biol. 1997, 17, 6087–6096. [Google Scholar] [CrossRef]
- Maréchal, A.; Zou, L. RPA-Coated Single-Stranded DNA as a Platform for Post-Translational Modifications in the DNA Damage Response. Cell Res. 2015, 25, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Loegering, D.; Arlander, S.J.H.; Hackbarth, J.; Vroman, B.T.; Roos-Mattjus, P.; Hopkins, K.M.; Lieberman, H.B.; Karnitz, L.M.; Kaufmann, S.H. Rad9 Protects Cells from Topoisomerase Poison-Induced Cell Death. J. Biol. Chem. 2004, 279, 18641–18647. [Google Scholar] [CrossRef] [PubMed]
- Hoeijmakers, J.H. Genome Maintenance Mechanisms for Preventing Cancer. Nature 2001, 411, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Adachi, N.; Suzuki, H.; Iiizumi, S.; Koyama, H. Hypersensitivity of Nonhomologous DNA End-Joining Mutants to VP-16 and ICRF-193: Implications for the Repair of Topoisomerase II-Mediated DNA Damage. J. Biol. Chem. 2003, 278, 35897–35902. [Google Scholar] [CrossRef]
- Malik, M.; Nitiss, K.C.; Enriquez-Rios, V.; Nitiss, J.L. Roles of Nonhomologous End-Joining Pathways in Surviving Topoisomerase II-Mediated DNA Damage. Mol. Cancer Ther. 2006, 5, 1405–1414. [Google Scholar] [CrossRef]
- Fan, Y.; Dutta, J.; Gupta, N.; Fan, G.; Gélinas, C. Regulation of Programmed Cell Death by NF-KappaB and Its Role in Tumorigenesis and Therapy. Adv. Exp. Med. Biol. 2008, 615, 223–250. [Google Scholar] [CrossRef]
- Jin, S.; Inoue, S.; Weaver, D.T. Differential Etoposide Sensitivity of Cells Deficient in the Ku and DNA-PKcs Components of the DNA-Dependent Protein Kinase. Carcinogenesis 1998, 19, 965–971. [Google Scholar] [CrossRef]
- Palmitelli, M.; de Campos-Nebel, M.; González-Cid, M. Progression of Chromosomal Damage Induced by Etoposide in G2 Phase in a DNA-PKcs-Deficient Context. Chromosome Res. 2015, 23, 719–732. [Google Scholar] [CrossRef]
- Baldwin, E.L.; Osheroff, N. Etoposide, Topoisomerase II and Cancer. Curr. Med. Chem. Anticancer Agents 2005, 5, 363–372. [Google Scholar] [CrossRef]
- Schonn, I.; Hennesen, J.; Dartsch, D.C. Cellular Responses to Etoposide: Cell Death despite Cell Cycle Arrest and Repair of DNA Damage. Apoptosis 2010, 15, 162–172. [Google Scholar] [CrossRef]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting Cancer Stem Cell Pathways for Cancer Therapy. Signal. Transduct. Target. Ther. 2020, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Korkaya, H.; Kim, G.-I.; Davis, A.; Malik, F.; Henry, N.L.; Ithimakin, S.; Quraishi, A.A.; Tawakkol, N.; D’Angelo, R.; Paulson, A.K.; et al. Activation of an IL6 Inflammatory Loop Mediates Trastuzumab Resistance in HER2+ Breast Cancer by Expanding the Cancer Stem Cell Population. Mol. Cell 2012, 47, 570–584. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, X.; Wang, T.; Guo, Q.; Xi, T.; Zheng, L. Emerging Agents That Target Signaling Pathways in Cancer Stem Cells. J. Hematol. Oncol. 2020, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Kciuk, M.; Gielecińska, A.; Budzinska, A.; Mojzych, M.; Kontek, R. Metastasis and MAPK Pathways. Int. J. Mol. Sci. 2022, 23, 3847. [Google Scholar] [CrossRef]
- Hsu, J.-M.; Xia, W.; Hsu, Y.-H.; Chan, L.-C.; Yu, W.-H.; Cha, J.-H.; Chen, C.-T.; Liao, H.-W.; Kuo, C.-W.; Khoo, K.-H.; et al. STT3-Dependent PD-L1 Accumulation on Cancer Stem Cells Promotes Immune Evasion. Nat. Commun. 2018, 9, 1908. [Google Scholar] [CrossRef] [PubMed]
- Slater, L.M.; Stupecky, M.; Sweet, P.; Osann, K.; Eklof, A.; Arquilla, E.R. Etoposide Induction of Tumor Immunity in Lewis Lung Cancer. Cancer Chemother. Pharmacol. 2001, 48, 327–332. [Google Scholar] [CrossRef]
- Slater, L.M.; Stupecky, M.; Sweet, P.; Osann, K.E. Enhancement of Leukemia Rejection by Mice Successfully Treated for L1210 Leukemia Due to Low Dose Compared to High Dose VP-16. Leuk. Res. 2002, 26, 203–206. [Google Scholar] [CrossRef]
- Slater, L.M.; Sweet, P.; Stupecky, M.; Reynolds, J.T. Cyclosporin A/VP-16 Produced Immunity to L1210 Leukemia: The Participation of Cytotoxic CD8 T-Lymphocytes. Clin. Immunol. Immunopathol. 1995, 75, 239–245. [Google Scholar] [CrossRef]
- Zhang, P.; Su, D.-M.; Liang, M.; Fu, J. Chemopreventive Agents Induce Programmed Death-1-Ligand 1 (PD-L1) Surface Expression in Breast Cancer Cells and Promote PD-L1-Mediated T Cell Apoptosis. Mol. Immunol. 2008, 45, 1470–1476. [Google Scholar] [CrossRef]
- Yang, M.; Liu, P.; Wang, K.; Glorieux, C.; Hu, Y.; Wen, S.; Jiang, W.; Huang, P. Chemotherapy Induces Tumor Immune Evasion by Upregulation of Programmed Cell Death Ligand 1 Expression in Bone Marrow Stromal Cells. Mol. Oncol. 2017, 11, 358–372. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, J.; Hu, J.; Zhang, H.; Xu, F.; He, W.; Wang, X.; Li, M.; Lu, W.; Zeng, G.; et al. CGAS/STING Axis Mediates a Topoisomerase II Inhibitor-Induced Tumor Immunogenicity. J. Clin. Investig. 2019, 129, 4850–4862. [Google Scholar] [CrossRef]
- Kelland, L. The Resurgence of Platinum-Based Cancer Chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Noll, D.M.; Mason, T.M.; Miller, P.S. Formation and Repair of Interstrand Cross-Links in DNA. Chem. Rev. 2006, 106, 277–301. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Anai, H.; Hanada, K. Mechanisms of Interstrand DNA Crosslink Repair and Human Disorders. Genes Environ. 2016, 38, 9. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Kawasoe, Y.; Williams, H.; Coates, E.; Roy, U.; Shi, Y.; Beese, L.S.; Schärer, O.D.; Yan, H.; Gottesman, M.E.; et al. Sensing and Processing of DNA Interstrand Crosslinks by the Mismatch Repair Pathway. Cell Rep. 2017, 21, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- Tanida, S.; Mizoshita, T.; Ozeki, K.; Tsukamoto, H.; Kamiya, T.; Kataoka, H.; Sakamuro, D.; Joh, T. Mechanisms of Cisplatin-Induced Apoptosis and of Cisplatin Sensitivity: Potential of BIN1 to Act as a Potent Predictor of Cisplatin Sensitivity in Gastric Cancer Treatment. Int. J. Surg. Oncol. 2012, 2012, 862879. [Google Scholar] [CrossRef]
- Ghosh, S. Cisplatin: The First Metal Based Anticancer Drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Sorenson, C.M.; Eastman, A. Influence of Cis-Diamminedichloroplatinum(II) on DNA Synthesis and Cell Cycle Progression in Excision Repair Proficient and Deficient Chinese Hamster Ovary Cells. Cancer Res. 1988, 48, 6703–6707. [Google Scholar]
- Osborn, M.F.; White, J.D.; Haley, M.M.; DeRose, V.J. Platinum-RNA Modifications Following Drug Treatment in S. Cerevisiae Identified by Click Chemistry and Enzymatic Mapping. ACS Chem. Biol. 2014, 9, 2404–2411. [Google Scholar] [CrossRef]
- Hostetter, A.A.; Osborn, M.F.; DeRose, V.J. RNA-Pt Adducts Following Cisplatin Treatment of Saccharomyces Cerevisiae. ACS Chem. Biol. 2012, 7, 218–225. [Google Scholar] [CrossRef]
- Karasawa, T.; Sibrian-Vazquez, M.; Strongin, R.M.; Steyger, P.S. Identification of Cisplatin-Binding Proteins Using Agarose Conjugates of Platinum Compounds. PLoS ONE 2013, 8, e66220. [Google Scholar] [CrossRef] [PubMed]
- Möltgen, S.; Piumatti, E.; Massafra, G.M.; Metzger, S.; Jaehde, U.; Kalayda, G.V. Cisplatin Protein Binding Partners and Their Relevance for Platinum Drug Sensitivity. Cells 2020, 9, 1322. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Dizon, D.S. New-Generation Platinum Agents for Solid Tumors. Future Oncol. 2009, 5, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Siddik, Z.H. Cisplatin: Mode of Cytotoxic Action and Molecular Basis of Resistance. Oncogene 2003, 22, 7265–7279. [Google Scholar] [CrossRef]
- Brozovic, A.; Ambriović-Ristov, A.; Osmak, M. The Relationship between Cisplatin-Induced Reactive Oxygen Species, Glutathione, and BCL-2 and Resistance to Cisplatin. Crit. Rev. Toxicol. 2010, 40, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Davalli, P.; Marverti, G.; Lauriola, A.; D’Arca, D. Targeting Oxidatively Induced DNA Damage Response in Cancer: Opportunities for Novel Cancer Therapies. Oxid. Med. Cell Longev. 2018, 2018, 2389523. [Google Scholar] [CrossRef]
- Poetsch, A.R. The Genomics of Oxidative DNA Damage, Repair, and Resulting Mutagenesis. Comput. Struct. Biotechnol. J. 2020, 18, 207–219. [Google Scholar] [CrossRef]
- Shaukat, Z.; Liu, D.; Hussain, R.; Khan, M.; Gregory, S.L. The Role of JNK Signalling in Responses to Oxidative DNA Damage. Curr. Drug Targets 2016, 17, 154–163. [Google Scholar] [CrossRef]
- Jones, E.V.; Dickman, M.J.; Whitmarsh, A.J. Regulation of P73-Mediated Apoptosis by c-Jun N-Terminal Kinase. Biochem. J. 2007, 405, 617–623. [Google Scholar] [CrossRef]
- Chen, B.P.C.; Li, M.; Asaithamby, A. New Insights into the Roles of ATM and DNA-PKcs in the Cellular Response to Oxidative Stress. Cancer Lett. 2012, 327, 103–110. [Google Scholar] [CrossRef]
- Ditch, S.; Paull, T.T. The ATM Protein Kinase and Cellular Redox Signaling: Beyond the DNA Damage Response. Trends Biochem. Sci. 2012, 37, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Pabla, N.; Huang, S.; Mi, Q.-S.; Daniel, R.; Dong, Z. ATR-Chk2 Signaling in P53 Activation and DNA Damage Response during Cisplatin-Induced Apoptosis. J. Biol. Chem. 2008, 283, 6572–6583. [Google Scholar] [CrossRef] [PubMed]
- Damia, G.; Filiberti, L.; Vikhanskaya, F.; Carrassa, L.; Taya, Y.; Dincalci, M.; Broggini, M. Cisplatinum and Taxol Induce Different Patterns of P53 Phosphorylation. Neoplasia 2001, 3, 10–16. [Google Scholar] [CrossRef]
- Kondo, S.; Barna, B.P.; Kondo, Y.; Tanaka, Y.; Casey, G.; Liu, J.; Morimura, T.; Kaakaji, R.; Peterson, J.W.; Werbel, B.; et al. WAF1/CIP1 Increases the Susceptibility of P53 Non-Functional Malignant Glioma Cells to Cisplatin-Induced Apoptosis. Oncogene 1996, 13, 1279–1285. [Google Scholar]
- Yang, X.; Fraser, M.; Moll, U.M.; Basak, A.; Tsang, B.K. Akt-Mediated Cisplatin Resistance in Ovarian Cancer: Modulation of P53 Action on Caspase-Dependent Mitochondrial Death Pathway. Cancer Res. 2006, 66, 3126–3136. [Google Scholar] [CrossRef] [PubMed]
- Wetzel, C.C.; Berberich, S.J. P53 Binds to Cisplatin-Damaged DNA. Biochim. Biophys. Acta 2001, 1517, 392–397. [Google Scholar] [CrossRef]
- Wang, X.; Martindale, J.L.; Holbrook, N.J. Requirement for ERK Activation in Cisplatin-Induced Apoptosis. J. Biol. Chem. 2000, 275, 39435–39443. [Google Scholar] [CrossRef]
- Basu, A.; Tu, H. Activation of ERK during DNA Damage-Induced Apoptosis Involves Protein Kinase Cdelta. Biochem. Biophys. Res. Commun. 2005, 334, 1068–1073. [Google Scholar] [CrossRef]
- Hayakawa, J.; Ohmichi, M.; Kurachi, H.; Kanda, Y.; Hisamoto, K.; Nishio, Y.; Adachi, K.; Tasaka, K.; Kanzaki, T.; Murata, Y. Inhibition of BAD Phosphorylation Either at Serine 112 via Extracellular Signal-Regulated Protein Kinase Cascade or at Serine 136 via Akt Cascade Sensitizes Human Ovarian Cancer Cells to Cisplatin. Cancer Res. 2000, 60, 5988–5994. [Google Scholar]
- Basu, A.; Krishnamurthy, S. Cellular Responses to Cisplatin-Induced DNA Damage. J. Nucleic Acids 2010, 2010, 201367. [Google Scholar] [CrossRef]
- DeHaan, R.D.; Yazlovitskaya, E.M.; Persons, D.L. Regulation of P53 Target Gene Expression by Cisplatin-Induced Extracellular Signal-Regulated Kinase. Cancer Chemother. Pharmacol. 2001, 48, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Rebillard, A.; Lagadic-Gossmann, D.; Dimanche-Boitrel, M.-T. Cisplatin Cytotoxicity: DNA and Plasma Membrane Targets. Curr. Med. Chem. 2008, 15, 2656–2663. [Google Scholar] [CrossRef]
- Abedini, M.R.; Muller, E.J.; Brun, J.; Bergeron, R.; Gray, D.A.; Tsang, B.K. Cisplatin Induces P53-Dependent FLICE-like Inhibitory Protein Ubiquitination in Ovarian Cancer Cells. Cancer Res. 2008, 68, 4511–4517. [Google Scholar] [CrossRef]
- Sun, F.; Cui, L.; Li, T.; Chen, S.; Song, J.; Li, D. Oxaliplatin Induces Immunogenic Cells Death and Enhances Therapeutic Efficacy of Checkpoint Inhibitor in a Model of Murine Lung Carcinoma. J. Recept. Signal. Transduct. Res. 2019, 39, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Golchin, S.; Alimohammadi, R.; Rostami Nejad, M.; Jalali, S.A. Synergistic Antitumor Effect of Anti-PD-L1 Combined with Oxaliplatin on a Mouse Tumor Model. J. Cell Physiol. 2019, 234, 19866–19874. [Google Scholar] [CrossRef]
- Tesniere, A.; Schlemmer, F.; Boige, V.; Kepp, O.; Martins, I.; Ghiringhelli, F.; Aymeric, L.; Michaud, M.; Apetoh, L.; Barault, L.; et al. Immunogenic Death of Colon Cancer Cells Treated with Oxaliplatin. Oncogene 2010, 29, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Grabosch, S.; Bulatovic, M.; Zeng, F.; Ma, T.; Zhang, L.; Ross, M.; Brozick, J.; Fang, Y.; Tseng, G.; Kim, E.; et al. Cisplatin-Induced Immune Modulation in Ovarian Cancer Mouse Models with Distinct Inflammation Profiles. Oncogene 2019, 38, 2380–2393. [Google Scholar] [CrossRef] [PubMed]
- Rojkó, L.; Reiniger, L.; Téglási, V.; Fábián, K.; Pipek, O.; Vágvölgyi, A.; Agócs, L.; Fillinger, J.; Kajdácsi, Z.; Tímár, J.; et al. Chemotherapy Treatment Is Associated with Altered PD-L1 Expression in Lung Cancer Patients. J. Cancer Res. Clin. Oncol. 2018, 144, 1219–1226. [Google Scholar] [CrossRef]
- West, H.; McCleod, M.; Hussein, M.; Morabito, A.; Rittmeyer, A.; Conter, H.J.; Kopp, H.-G.; Daniel, D.; McCune, S.; Mekhail, T.; et al. Atezolizumab in Combination with Carboplatin plus Nab-Paclitaxel Chemotherapy Compared with Chemotherapy Alone as First-Line Treatment for Metastatic Non-Squamous Non-Small-Cell Lung Cancer (IMpower130): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2019, 20, 924–937. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Qu, B.; Yang, H.; Hu, S.; Dong, X. If Small Molecules Immunotherapy Comes, Can the Prime Be Far Behind? Eur. J. Med. Chem. 2021, 218, 113356. [Google Scholar] [CrossRef]
- Castelli, M.S.; McGonigle, P.; Hornby, P.J. The Pharmacology and Therapeutic Applications of Monoclonal Antibodies. Pharmacol. Res. Perspect. 2019, 7, e00535. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Takaoka, A. Comparing Antibody and Small-Molecule Therapies for Cancer. Nat. Rev. Cancer 2006, 6, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Baxi, S.; Yang, A.; Gennarelli, R.L.; Khan, N.; Wang, Z.; Boyce, L.; Korenstein, D. Immune-Related Adverse Events for Anti-PD-1 and Anti-PD-L1 Drugs: Systematic Review and Meta-Analysis. BMJ 2018, 360, k793. [Google Scholar] [CrossRef]
- Zarganes-Tzitzikas, T.; Konstantinidou, M.; Gao, Y.; Krzemien, D.; Zak, K.; Dubin, G.; Holak, T.A.; Dömling, A. Inhibitors of Programmed Cell Death 1 (PD-1): A Patent Review (2010–2015). Expert Opin. Ther. Pat. 2016, 26, 973–977. [Google Scholar] [CrossRef]
- Skalniak, L.; Zak, K.M.; Guzik, K.; Magiera, K.; Musielak, B.; Pachota, M.; Szelazek, B.; Kocik, J.; Grudnik, P.; Tomala, M.; et al. Small-Molecule Inhibitors of PD-1/PD-L1 Immune Checkpoint Alleviate the PD-L1-Induced Exhaustion of T-Cells. Oncotarget 2017, 8, 72167–72181. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yao, Z.; Wang, S.; Xie, T.; Wu, G.; Zhang, H.; Zhang, P.; Wu, Y.; Yuan, H.; Sun, H. Syntheses, Biological Evaluations, and Mechanistic Studies of Benzo[c][1,2,5]Oxadiazole Derivatives as Potent PD-L1 Inhibitors with In Vivo Antitumor Activity. J. Med. Chem. 2021, 64, 8391–8409. [Google Scholar] [CrossRef]
- Qin, M.; Cao, Q.; Zheng, S.; Tian, Y.; Zhang, H.; Xie, J.; Xie, H.; Liu, Y.; Zhao, Y.; Gong, P. Discovery of [1,2,4]Triazolo [4,3- a]Pyridines as Potent Inhibitors Targeting the Programmed Cell Death-1/Programmed Cell Death-Ligand 1 Interaction. J. Med. Chem. 2019, 62, 4703–4715. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, Y.; Yu, C.; Du, H.; Liu, J.; Li, H.; Huang, S.; Zhu, Q.; Xu, Y.; Zou, Y. Discovery of Novel Small-Molecule Inhibitors of PD-1/PD-L1 Interaction via Structural Simplification Strategy. Molecules 2021, 26, 3347. [Google Scholar] [CrossRef]
- Muszak, D.; Surmiak, E.; Plewka, J.; Magiera-Mularz, K.; Kocik-Krol, J.; Musielak, B.; Sala, D.; Kitel, R.; Stec, M.; Weglarczyk, K.; et al. Terphenyl-Based Small-Molecule Inhibitors of Programmed Cell Death-1/Programmed Death-Ligand 1 Protein-Protein Interaction. J. Med. Chem. 2021, 64, 11614–11636. [Google Scholar] [CrossRef]
- Chen, H.; Wang, K.; Yang, Y.; Huang, X.; Dai, X.; Feng, Z. Design, Synthesis, and Structure-Activity Relationship of Programmed Cell Death-1/Programmed Cell Death-Ligand 1 Interaction Inhibitors Bearing a Benzo[d]Isothiazole Scaffold. Eur. J. Med. Chem. 2021, 217, 113377. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, K.; Chen, H.; Feng, Z. Design, Synthesis, Evaluation, and SAR of 4-Phenylindoline Derivatives, a Novel Class of Small-Molecule Inhibitors of the Programmed Cell Death-1/ Programmed Cell Death-Ligand 1 (PD-1/PD-L1) Interaction. Eur. J. Med. Chem. 2021, 211, 113001. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-J.; Thi, E.P.; Carpio, V.H.; Bi, Y.; Cole, A.G.; Dorsey, B.D.; Fan, K.; Harasym, T.; Iott, C.L.; Kadhim, S.; et al. Checkpoint Inhibition through Small Molecule-Induced Internalization of Programmed Death-Ligand 1. Nat. Commun. 2021, 12, 1222. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, Y.; Guo, Y.; Pan, Z.; Zhong, S.; Jin, X.; Zhuang, W.; Chen, S.; Gao, J.; Huang, W.; et al. Discovery of Phenyl-Linked Symmetric Small Molecules as Inhibitors of the Programmed Cell Death-1/Programmed Cell Death-Ligand 1 Interaction. Eur. J. Med. Chem. 2021, 223, 113637. [Google Scholar] [CrossRef] [PubMed]
- Surmiak, E.; Magiera-Mularz, K.; Musielak, B.; Muszak, D.; Kocik-Krol, J.; Kitel, R.; Plewka, J.; Holak, T.A.; Skalniak, L. PD-L1 Inhibitors: Different Classes, Activities, and Mechanisms of Action. Int. J. Mol. Sci. 2021, 22, 11797. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Fletcher, R.; Yu, J.; Zhang, L. Immunogenic Effects of Chemotherapy-Induced Tumor Cell Death. Genes Dis. 2018, 5, 194–203. [Google Scholar] [CrossRef]
- Hosoya, N.; Miyagawa, K. Targeting DNA Damage Response in Cancer Therapy. Cancer Sci. 2014, 105, 370–388. [Google Scholar] [CrossRef]
- Topatana, W.; Juengpanich, S.; Li, S.; Cao, J.; Hu, J.; Lee, J.; Suliyanto, K.; Ma, D.; Zhang, B.; Chen, M.; et al. Advances in Synthetic Lethality for Cancer Therapy: Cellular Mechanism and Clinical Translation. J. Hematol. Oncol. 2020, 13, 118. [Google Scholar] [CrossRef] [PubMed]
- Setton, J.; Zinda, M.; Riaz, N.; Durocher, D.; Zimmermann, M.; Koehler, M.; Reis-Filho, J.S.; Powell, S.N. Synthetic Lethality in Cancer Therapeutics: The Next Generation. Cancer Discov. 2021, 11, 1626–1635. [Google Scholar] [CrossRef]
- Myers, S.H.; Ortega, J.A.; Cavalli, A. Synthetic Lethality through the Lens of Medicinal Chemistry. J. Med. Chem. 2020, 63, 14151–14183. [Google Scholar] [CrossRef]
- Rose, M.; Burgess, J.T.; O’Byrne, K.; Richard, D.J.; Bolderson, E. PARP Inhibitors: Clinical Relevance, Mechanisms of Action and Tumor Resistance. Front. Cell Dev. Biol. 2020, 8, 564601. [Google Scholar] [CrossRef]
- Dziadkowiec, K.N.; Gąsiorowska, E.; Nowak-Markwitz, E.; Jankowska, A. PARP Inhibitors: Review of Mechanisms of Action and BRCA1/2 Mutation Targeting. Prz. Menopauzalny 2016, 15, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Pan, W.; Xing, Y.; Xiao, Y.; Chen, J.; Xu, Z. Recent Advances in DDR (DNA Damage Response) Inhibitors for Cancer Therapy. Eur. J. Med. Chem. 2022, 230, 114109. [Google Scholar] [CrossRef] [PubMed]
- Kiss, R.C.; Xia, F.; Acklin, S. Targeting DNA Damage Response and Repair to Enhance Therapeutic Index in Cisplatin-Based Cancer Treatment. Int. J. Mol. Sci. 2021, 22, 8199. [Google Scholar] [CrossRef]
- O’Connor, M.J. Targeting the DNA Damage Response in Cancer. Mol. Cell 2015, 60, 547–560. [Google Scholar] [CrossRef]
- Martorana, F.; Da Silva, L.A.; Sessa, C.; Colombo, I. Everything Comes with a Price: The Toxicity Profile of DNA-Damage Response Targeting Agents. Cancers 2022, 14, 953. [Google Scholar] [CrossRef]
- Baxter, J.S.; Zatreanu, D.; Pettitt, S.J.; Lord, C.J. Resistance to DNA Repair Inhibitors in Cancer. Mol. Oncol. 2022, 16, 3811–3827. [Google Scholar] [CrossRef]
- Choi, W.; Lee, E.S. Therapeutic Targeting of DNA Damage Response in Cancer. Int. J. Mol. Sci. 2022, 23, 1701. [Google Scholar] [CrossRef]
- Huang, J.-L.; Chang, Y.-T.; Hong, Z.-Y.; Lin, C.-S. Targeting DNA Damage Response and Immune Checkpoint for Anticancer Therapy. Int. J. Mol. Sci. 2022, 23, 3238. [Google Scholar] [CrossRef]
- Lee, E.C.Y.; Kok, J.S.T.; Teh, B.T.; Lim, K.S. Interplay between the DNA Damage Response and Immunotherapy Response in Cancer. Int. J. Mol. Sci. 2022, 23, 13356. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, M.; Peng, Z.; Fan, R.; He, Y.; Zhang, H.; Xiong, W.; Jiang, W. Advances of DNA Damage Repair-Related Drugs and Combination With Immunotherapy in Tumor Treatment. Front. Immunol. 2022, 13, 854730. [Google Scholar] [CrossRef]
- Joshi, M.; Grivas, P.; Mortazavi, A.; Monk, P.; Clinton, S.K.; Sue-Ann Woo, M.; Holder, S.L.; Drabick, J.J.; Yin, M. Alterations of DNA Damage Response Genes Correlate with Response and Overall Survival in Anti-PD-1/PD-L1-Treated Advanced Urothelial Cancer. Cancer Med 2020, 9, 9365–9372. [Google Scholar] [CrossRef] [PubMed]
- Khaddour, K.; Felipe Fernandez, M.; Khabibov, M.; Garifullin, A.; Dressler, D.; Topchu, I.; Patel, J.D.; Weinberg, F.; Boumber, Y. The Prognostic and Therapeutic Potential of DNA Damage Repair Pathway Alterations and Homologous Recombination Deficiency in Lung Cancer. Cancers 2022, 14, 5305. [Google Scholar] [CrossRef] [PubMed]
- Strickler, J.H.; Hanks, B.A.; Khasraw, M. Tumor Mutational Burden as a Predictor of Immunotherapy Response: Is More Always Better? Clin. Cancer Res. 2021, 27, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Motta, R.; Cabezas-Camarero, S.; Torres-Mattos, C.; Riquelme, A.; Calle, A.; Figueroa, A.; Sotelo, M.J. Immunotherapy in Microsatellite Instability Metastatic Colorectal Cancer: Current Status and Future Perspectives. J. Clin. Transl. Res. 2021, 7, 511–522. [Google Scholar]
- Chang, L.; Chang, M.; Chang, H.M.; Chang, F. Microsatellite Instability: A Predictive Biomarker for Cancer Immunotherapy. Appl. Immunohistochem. Mol. Morphol. 2018, 26, e15–e21. [Google Scholar] [CrossRef]
- Lizardo, D.Y.; Kuang, C.; Hao, S.; Yu, J.; Huang, Y.; Zhang, L. Immunotherapy Efficacy on Mismatch Repair-Deficient Colorectal Cancer: From Bench to Bedside. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188447. [Google Scholar] [CrossRef]
- Zhao, P.; Li, L.; Jiang, X.; Li, Q. Mismatch Repair Deficiency/Microsatellite Instability-High as a Predictor for Anti-PD-1/PD-L1 Immunotherapy Efficacy. J. Hematol. Oncol. 2019, 12, 54. [Google Scholar] [CrossRef]
- Viale, G.; Trapani, D.; Curigliano, G. Mismatch Repair Deficiency as a Predictive Biomarker for Immunotherapy Efficacy. Biomed. Res. Int. 2017, 2017, 4719194. [Google Scholar] [CrossRef]
- Doroshow, D.B.; Bhalla, S.; Beasley, M.B.; Sholl, L.M.; Kerr, K.M.; Gnjatic, S.; Wistuba, I.I.; Rimm, D.L.; Tsao, M.S.; Hirsch, F.R. PD-L1 as a Biomarker of Response to Immune-Checkpoint Inhibitors. Nat. Rev. Clin. Oncol. 2021, 18, 345–362. [Google Scholar] [CrossRef]
- Wagner, M.; Jasek, M.; Karabon, L. Immune Checkpoint Molecules-Inherited Variations as Markers for Cancer Risk. Front. Immunol. 2020, 11, 606721. [Google Scholar] [CrossRef]
- Sato, H.; Jeggo, P.A.; Shibata, A. Regulation of Programmed Death-ligand 1 Expression in Response to DNA Damage in Cancer Cells: Implications for Precision Medicine. Cancer Sci. 2019, 110, 3415–3423. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liu, J.; Chen, J.; Zhou, Q. The Developing Landscape of Combinatorial Therapies of Immune Checkpoint Blockade with DNA Damage Repair Inhibitors for the Treatment of Breast and Ovarian Cancers. J. Hematol. Oncol. 2021, 14, 206. [Google Scholar] [CrossRef] [PubMed]
- Dillon, M.T.; Bergerhoff, K.F.; Pedersen, M.; Whittock, H.; Crespo-Rodriguez, E.; Patin, E.C.; Pearson, A.; Smith, H.G.; Paget, J.T.E.; Patel, R.R.; et al. ATR Inhibition Potentiates the Radiation-Induced Inflammatory Tumor Microenvironment. Clin. Cancer Res. 2019, 25, 3392–3403. [Google Scholar] [CrossRef]
- Sun, L.-L.; Yang, R.-Y.; Li, C.-W.; Chen, M.-K.; Shao, B.; Hsu, J.-M.; Chan, L.-C.; Yang, Y.; Hsu, J.L.; Lai, Y.-J.; et al. Inhibition of ATR Downregulates PD-L1 and Sensitizes Tumor Cells to T Cell-Mediated Killing. Am. J. Cancer Res. 2018, 8, 1307–1316. [Google Scholar]
- Yan, H.; Luo, B.; Wu, X.; Guan, F.; Yu, X.; Zhao, L.; Ke, X.; Wu, J.; Yuan, J. Cisplatin Induces Pyroptosis via Activation of MEG3/NLRP3/Caspase-1/GSDMD Pathway in Triple-Negative Breast Cancer. Int. J. Biol. Sci. 2021, 17, 2606–2621. [Google Scholar] [CrossRef]
- Li, R.-Y.; Zheng, Z.-Y.; Li, Z.-M.; Heng, J.-H.; Zheng, Y.-Q.; Deng, D.-X.; Xu, X.-E.; Liao, L.-D.; Lin, W.; Xu, H.-Y.; et al. Cisplatin-Induced Pyroptosis Is Mediated via the CAPN1/CAPN2-BAK/BAX-Caspase-9-Caspase-3-GSDME Axis in Esophageal Cancer. Chem. Biol. Interact. 2022, 361, 109967. [Google Scholar] [CrossRef]
- Wu, M.; Wang, Y.; Yang, D.; Gong, Y.; Rao, F.; Liu, R.; Danna, Y.; Li, J.; Fan, J.; Chen, J.; et al. A PLK1 Kinase Inhibitor Enhances the Chemosensitivity of Cisplatin by Inducing Pyroptosis in Oesophageal Squamous Cell Carcinoma. EBioMedicine 2019, 41, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-C.; Li, C.-G.; Wang, Y.-F.; Xu, L.-H.; He, X.-H.; Zeng, Q.-Z.; Zeng, C.-Y.; Mai, F.-Y.; Hu, B.; Ouyang, D.-Y. Chemotherapeutic Paclitaxel and Cisplatin Differentially Induce Pyroptosis in A549 Lung Cancer Cells via Caspase-3/GSDME Activation. Apoptosis 2019, 24, 312–325. [Google Scholar] [CrossRef]
- Li, Y.; Xia, W.; Wu, M.; Yin, J.; Wang, Q.; Li, S.; Zhang, A.; Huang, S.; Zhang, Y.; Jia, Z. Activation of GSDMD Contributes to Acute Kidney Injury Induced by Cisplatin. Am. J. Physiol. Ren. Physiol. 2020, 318, F96–F106. [Google Scholar] [CrossRef]
- Shen, X.; Wang, H.; Weng, C.; Jiang, H. Caspase 3/GSDME-Dependent Pyroptosis Contributes to Chemotherapy Drug-Induced Nephrotoxicity. Cell Death Dis. 2021, 12, 186. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, G.; Wu, D.; Wu, S.; Gong, L.; Li, Z.; Luo, G.; Hu, J.; Chen, J.; Huang, X.; et al. Lobaplatin Induces Pyroptosis through Regulating CIAP1/2, Ripoptosome and ROS in Nasopharyngeal Carcinoma. Biochem. Pharmacol. 2020, 177, 114023. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ge, L.; Shi, X.; Liu, J.; Ruan, H.; Heng, D.; Ye, C. Lobaplatin Induces Pyroptosis in Cervical Cancer Cells via the Caspase-3/GSDME Pathway. Anticancer Agents Med. Chem. 2022, 22, 2091–2097. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Li, S.; Qi, J.; Chen, Z.; Wu, Y.; Guo, J.; Wang, K.; Sun, X.; Zheng, J. Cleavage of GSDME by Caspase-3 Determines Lobaplatin-Induced Pyroptosis in Colon Cancer Cells. Cell Death Dis. 2019, 10, 193. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, B.; Li, D.; Wang, G.; Han, X.; Sun, X. GSDME Mediates Caspase-3-Dependent Pyroptosis in Gastric Cancer. Biochem. Biophys. Res. Commun. 2018, 495, 1418–1425. [Google Scholar] [CrossRef]
- Rogers, C.; Erkes, D.A.; Nardone, A.; Aplin, A.E.; Fernandes-Alnemri, T.; Alnemri, E.S. Gasdermin Pores Permeabilize Mitochondria to Augment Caspase-3 Activation during Apoptosis and Inflammasome Activation. Nat. Commun. 2019, 10, 1689. [Google Scholar] [CrossRef] [PubMed]
- Dessouki, F.B.A.; Kukreja, R.C.; Singla, D.K. Stem Cell-Derived Exosomes Ameliorate Doxorubicin-Induced Muscle Toxicity through Counteracting Pyroptosis. Pharmaceuticals 2020, 13, 450. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, H.; Li, D.; Zhou, X.; Qin, Q.; Zhang, Q. Caspase-3-Mediated GSDME Induced Pyroptosis in Breast Cancer Cells through the ROS/JNK Signalling Pathway. J. Cell Mol. Med. 2021, 25, 8159–8168. [Google Scholar] [CrossRef]
- Yu, P.; Wang, H.-Y.; Tian, M.; Li, A.-X.; Chen, X.-S.; Wang, X.-L.; Zhang, Y.; Cheng, Y. Eukaryotic Elongation Factor-2 Kinase Regulates the Cross-Talk between Autophagy and Pyroptosis in Doxorubicin-Treated Human Melanoma Cells in Vitro. Acta Pharmacol. Sin. 2019, 40, 1237–1244. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, W.; Shi, X.; Ding, J.; Liu, W.; He, H.; Wang, K.; Shao, F. Chemotherapy Drugs Induce Pyroptosis through Caspase-3 Cleavage of a Gasdermin. Nature 2017, 547, 99–103. [Google Scholar] [CrossRef]
- Tsuchiya, K. Switching from Apoptosis to Pyroptosis: Gasdermin-Elicited Inflammation and Antitumor Immunity. Int. J. Mol. Sci. 2021, 22, 426. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Ding, J.; Wang, C.; Zhou, X.; Gao, W.; Huang, H.; Shao, F.; Liu, Z. A Bioorthogonal System Reveals Antitumour Immune Function of Pyroptosis. Nature 2020, 579, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Chen, Q.; Li, X.; Zeng, Z.; Xiong, W.; Li, G.; Li, X.; Yang, J.; Xiang, B.; Yi, M. Pyroptosis: A New Paradigm of Cell Death for Fighting against Cancer. J. Exp. Clin. Cancer Res. 2021, 40, 153. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, L.; Sun, Z. Eliciting Pyroptosis to Fuel Cancer Immunotherapy: Mechanisms and Strategies. Cancer Biol. Med. 2022, 19, 948–964. [Google Scholar] [CrossRef] [PubMed]
- Erkes, D.A.; Cai, W.; Sanchez, I.M.; Purwin, T.J.; Rogers, C.; Field, C.O.; Berger, A.C.; Hartsough, E.J.; Rodeck, U.; Alnemri, E.S.; et al. Mutant BRAF and MEK Inhibitors Regulate the Tumor Immune Microenvironment via Pyroptosis. Cancer Discov. 2020, 10, 254–269. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.; Zhu, Y.; Yang, P.; Chen, Z.; Xia, Y.; Qiao, C.; Liu, W.; Deng, H.; Li, J.; Ning, P.; et al. Pyroptosis in Inflammatory Diseases and Cancer. Theranostics 2022, 12, 4310–4329. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Wang, X.; Deng, Y.; Li, S.; Xu, X.; Qin, Y.; Peng, L. Pyroptosis Provides New Strategies for the Treatment of Cancer. J. Cancer 2023, 14, 140–151. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).