The miR-100-5p Targets SMARCA5 to Regulate the Apoptosis and Intracellular Survival of BCG in Infected THP-1 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Growth and Cell Culture
2.2. Cell Infection with BCG
2.3. Total RNA Extraction and Sequencing
2.4. Cell Transfection
2.5. Quantitative Real-Time Polymerase Chain Reaction
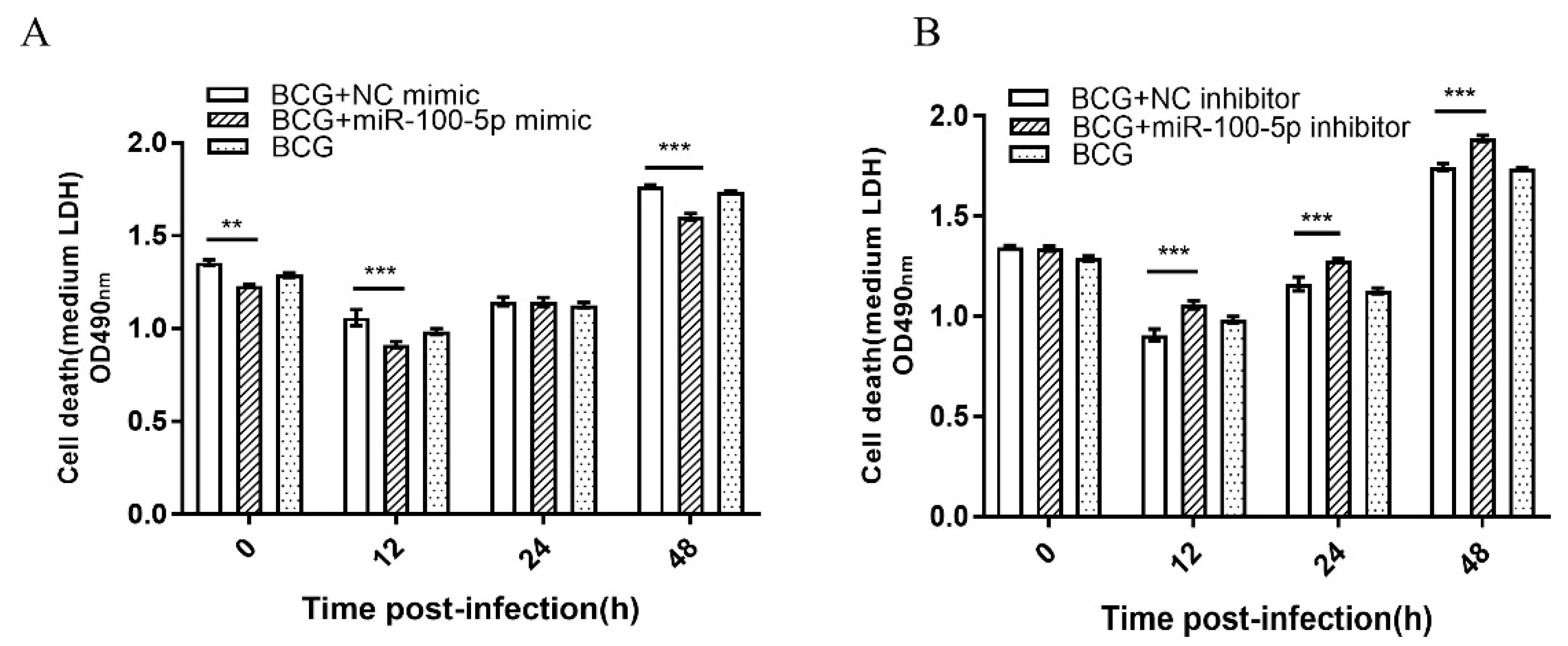
2.6. Cell Death Assay
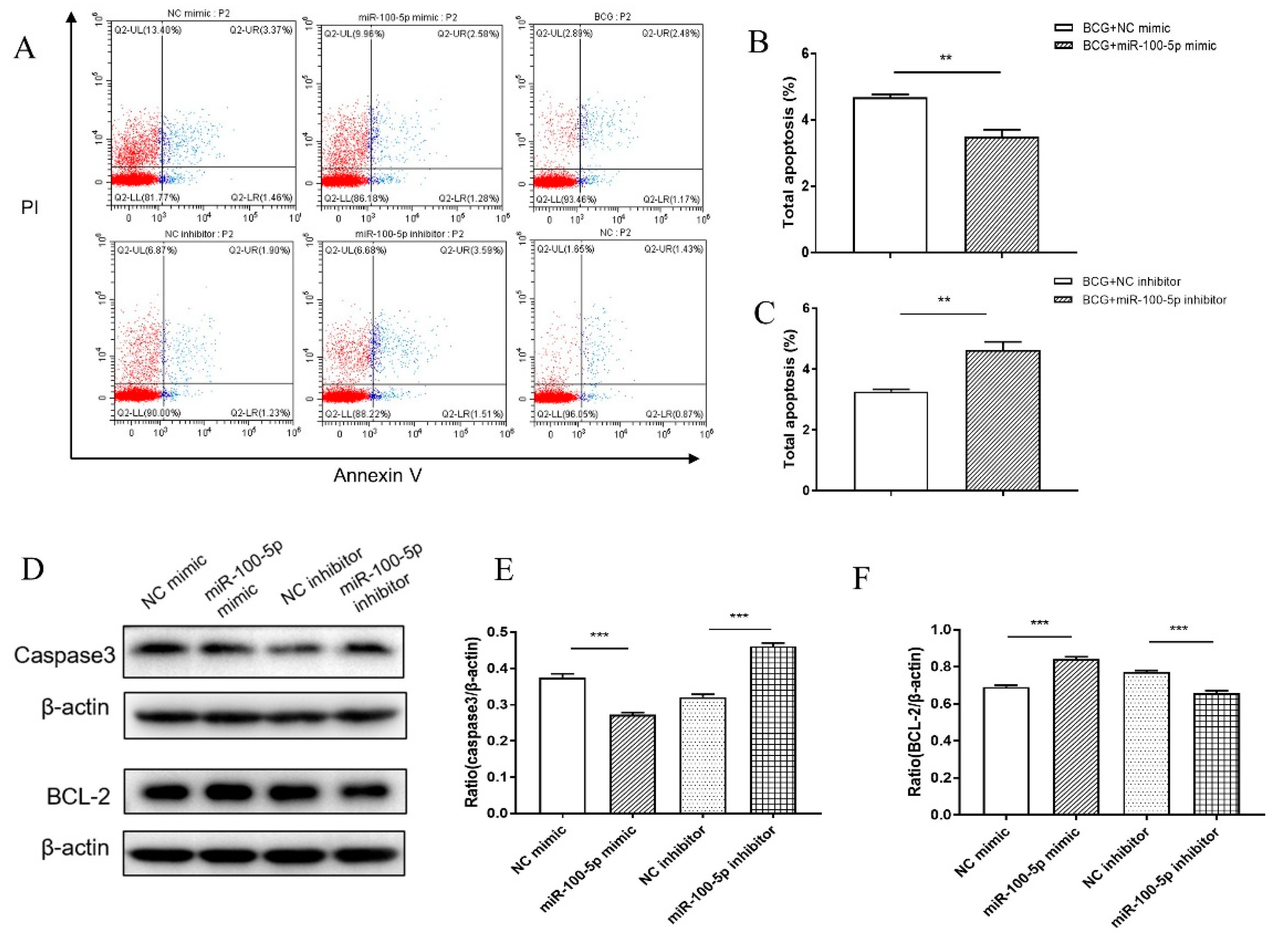
2.7. Apoptosis Assay
2.8. Western Blotting Assay
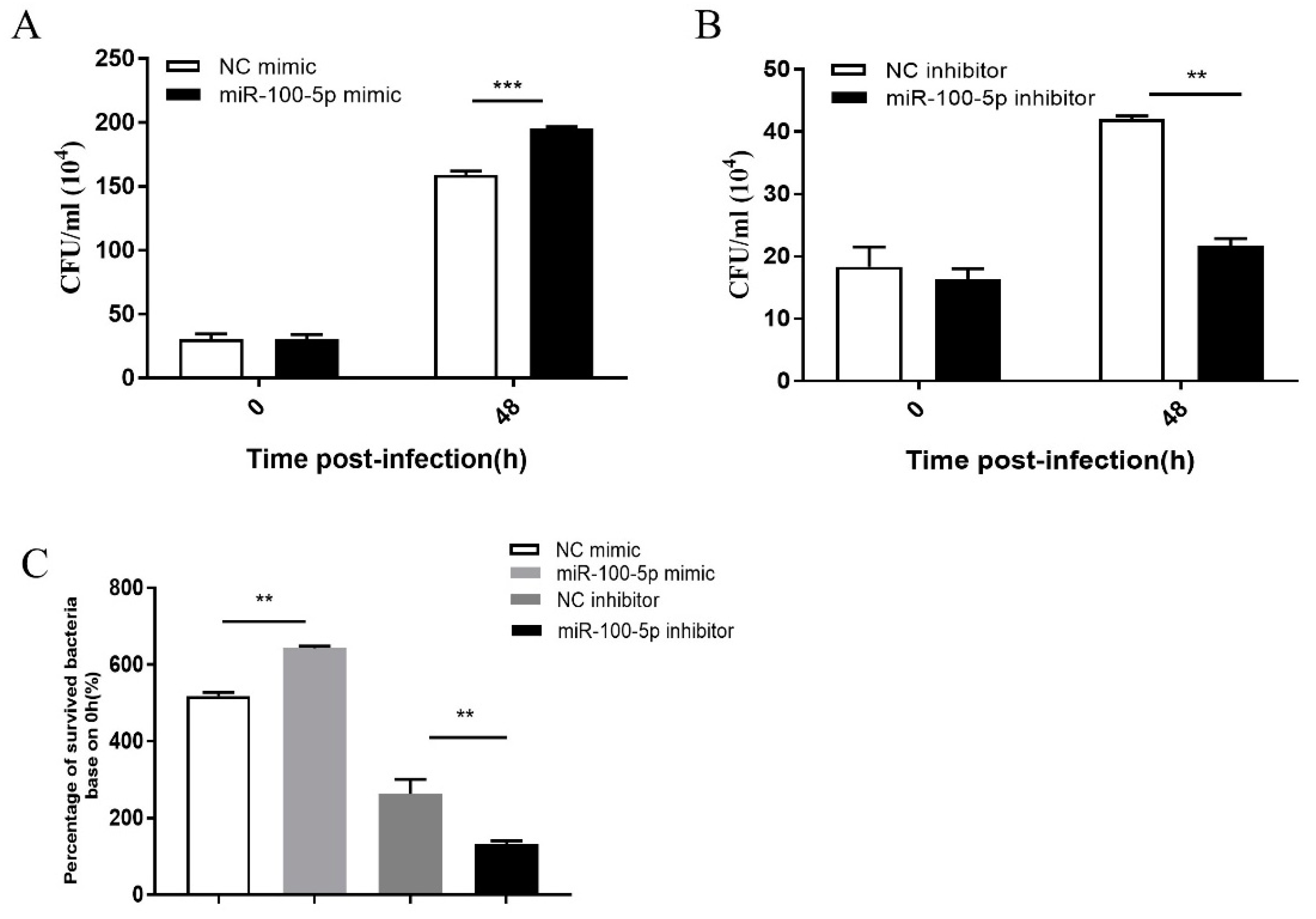
2.9. Assay on Intracellular Bacterial Number
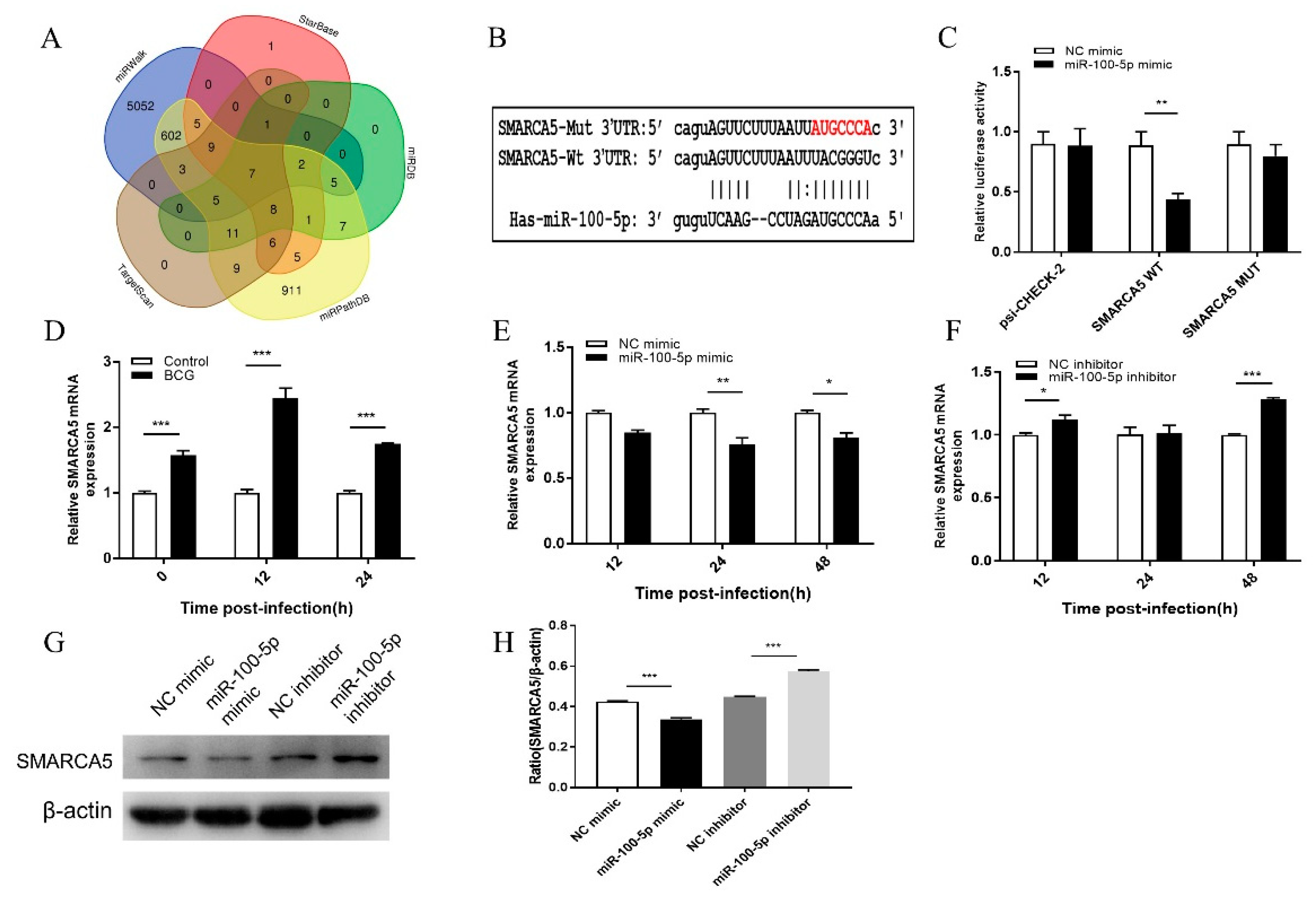
2.10. Target Gene Prediction and Analysis
2.11. Dual-Luciferase Reporter Assay
2.12. Statistical Analysis
3. Results
3.1. BCG Infection Reduced miR-100-5p Expression in THP-1 Cells
3.2. miR-100-5p Inhibited Apoptosis of BCG Infected THP-1 Cells
3.3. miR-100-5p Regulates the PI3K/AKT Signalling Pathway
3.4. miR-100-5p Promoted BCG Intracellular Survival via Suppression of Apoptosis
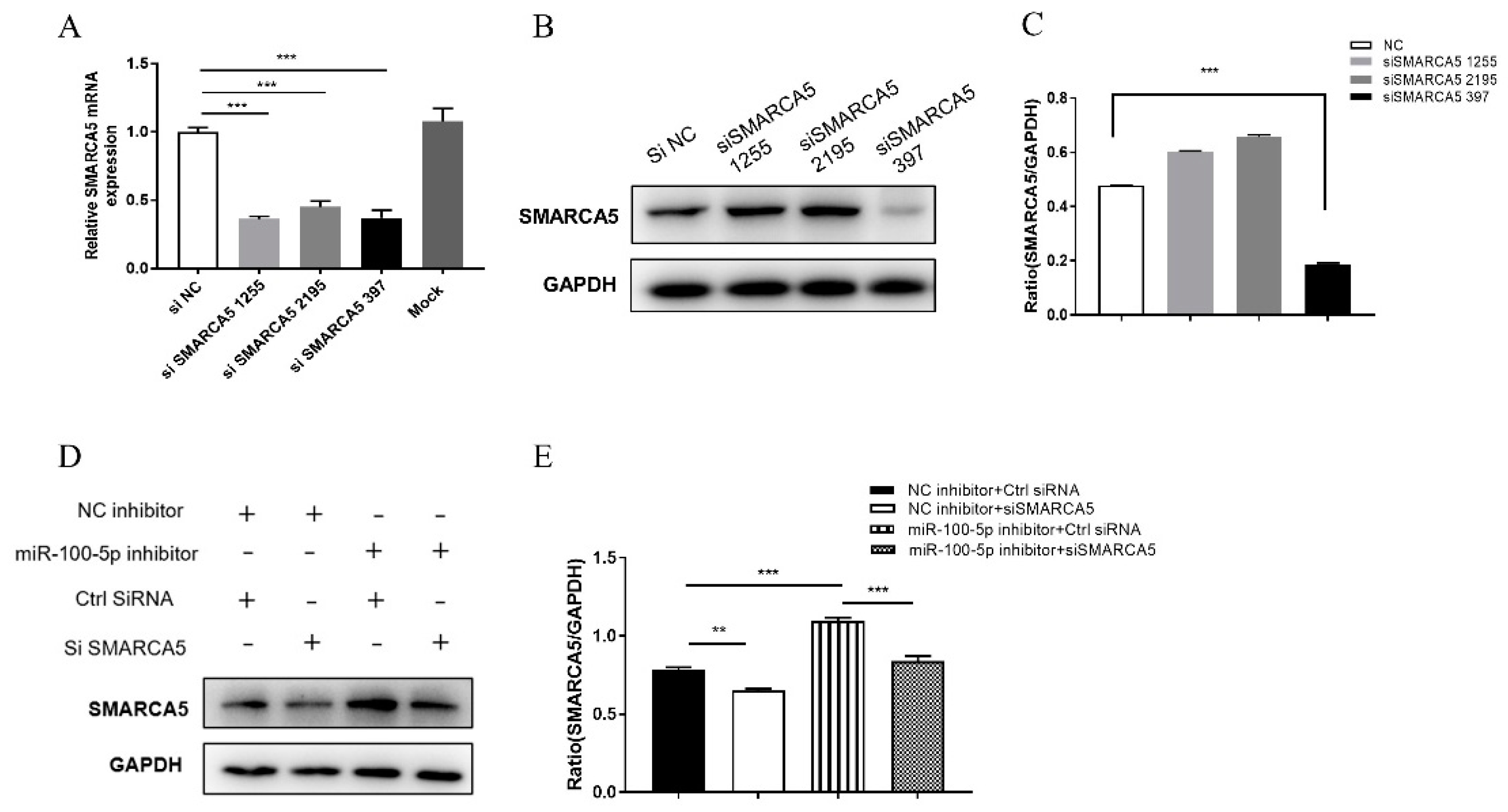
3.5. miR-100-5p Directly Targets SMARCA5
3.6. miR-100-5p Regulates Apoptosis and BCG Survival in THP-1 via SMARCA5
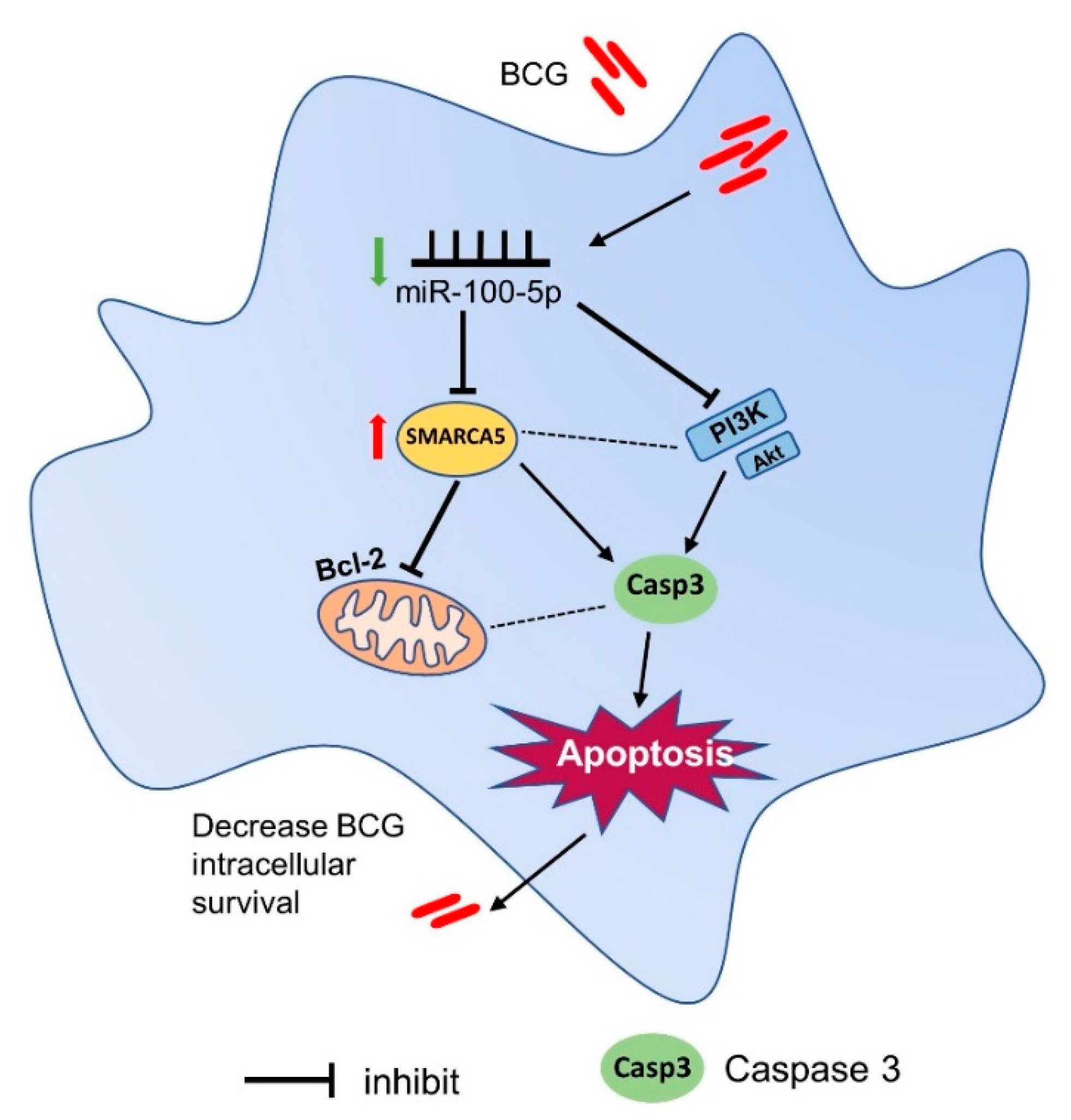
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Global Tuberculosis Report 2020. 2021. Available online: https://www.who.int/publications/i/item/9789240013131 (accessed on 13 May 2021).
- Zuniga, E.S.; Early, J.; Parish, T. The future for early-stage tuberculosis drug discovery. Future Microbiol. 2015, 10, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.X.; Song, Z.G.; Wu, Y.Y.; Gao, Y.P.; Gao, M.Q.; Liu, F.Y.; Wang, F.Y.; Zhang, Y. MicroRNA-27b Modulates Inflammatory Response and Apoptosis during Mycobacterium tuberculosis Infection. J. Immunol. 2018, 200, 3506–3518. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.Y.; Abudu, Y.P.; Claude-Taupin, A.; Gu, Y.X.; Kumar, S.; Choi, S.W.; Peters, R.; Mudd, M.H.; Allers, L.; Salemi, M.; et al. Galectins Control mTOR in Response to Endomembrane Damage. Mol. Cell 2018, 70, 120. [Google Scholar] [CrossRef]
- Behar, S.M.; Briken, V. Apoptosis inhibition by intracellular bacteria and its consequence on host immunity. Curr. Opin. Immunol. 2019, 60, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Zhai, W.J.; Wu, F.J.; Zhang, Y.Y.; Fu, Y.R.; Liu, Z.J. The Immune Escape Mechanisms of Mycobacterium Tuberculosis. Int. J. Mol. Sci. 2019, 20, 340. [Google Scholar] [CrossRef] [PubMed]
- Oddo, M.; Renno, T.; Attinger, A.; Bakker, T.; MacDonald, H.R.; Meylan, P.R.A. Fas ligand-induced apoptosis of infected human macrophages reduces the viability of intracellular Mycobacterium tuberculosis. J. Immunol. 1998, 160, 5448–5454. [Google Scholar] [CrossRef] [PubMed]
- Keane, J.; Remold, H.G.; Kornfeld, H. Virulent Mycobacterium tuberculosis strains evade apoptosis of infected alveolar macrophages. J. Immunol. 2000, 164, 2016–2020. [Google Scholar] [CrossRef]
- Riendeau, C.J.; Kornfeld, H. THP-1 cell apoptosis in response to mycobacterial infection. Infect Immun. 2003, 71, 254–259. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Ouimet, M.; Koster, S.; Sakowski, E.; Ramkhelawon, B.; van Solingen, C.; Oldebeken, S.; Karunakaran, D.; Portal-Celhay, C.; Sheedy, F.J.; Ray, T.D.; et al. Mycobacterium tuberculosis induces the miR-33 locus to reprogram autophagy and host lipid metabolism. Nat. Immunol. 2016, 17, 677–686. [Google Scholar] [CrossRef]
- Huang, J.; Jiao, J.H.; Xu, W.H.; Zhao, H.Y.; Zhang, C.X.; Shi, Y.; Xiao, Z.J. miR-155 is upregulated in patients with active tuberculosis and inhibits apoptosis of monocytes by targeting FOXO3. Mol. Med. Rep. 2015, 12, 7102–7108. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yue, Y.; Xu, W.; Xiong, S. MicroRNA-146a represses mycobacteria-induced inflammatory response and facilitates bacterial replication via targeting IRAK-1 and TRAF-6. PLoS ONE 2013, 8, e81438. [Google Scholar] [CrossRef]
- Zhao, Z.; Hao, J.; Li, X.; Chen, Y.; Qi, X. MiR-21-5p regulates mycobacterial survival and inflammatory responses by targeting Bcl-2 and TLR4 in Mycobacterium tuberculosis-infected macrophages. FEBS Lett. 2019, 593, 1326–1335. [Google Scholar] [CrossRef] [PubMed]
- Wallach, T.; Mossmann, Z.J.; Szczepek, M.; Wetzel, M.; Machado, R.; Raden, M.; Miladi, M.; Kleinau, G.; Kruger, C.; Dembny, P.; et al. MicroRNA-100-5p and microRNA-298-5p released from apoptotic cortical neurons are endogenous Toll-like receptor 7/8 ligands that contribute to neurodegeneration. Mol. Neurodegener. 2021, 16, 80. [Google Scholar] [CrossRef]
- Li, X.; Ren, Y.; Liu, D.; Yu, X.; Chen, K. Role of miR-100-5p and CDC25A in breast carcinoma cells. PeerJ 2022, 9, e12263. [Google Scholar] [CrossRef]
- Ye, Y.; Li, S.L.; Wang, J.J. miR-100-5p Downregulates mTOR to Suppress the Proliferation, Migration, and Invasion of Prostate Cancer Cells. Front Oncol. 2020, 10, 578948. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, X.; Wang, Y.; Yang, S.; Chen, Y.; Yuan, T. miR-100 inhibits the migration and invasion of nasopharyngeal carcinoma by targeting IGF1R. Oncol. Lett. 2018, 15, 8333–8338. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Wang, L.; Zhao, J.; Zheng, Z.; Peng, J.; Zhang, W.; Wen, T.; Nie, J.; Ding, L.; Yi, D. MiR-100-5p regulates cardiac hypertrophy through activation of autophagy by targeting mTOR. Hum. Cell 2021, 34, 1388–1397. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Xiao, Y.; Kong, D.L.; Liu, H.; Chen, X.; Chen, Y.Y.; Zhu, T.T.; Peng, Y.C.; Zhai, W.J.; Hu, C.M.; et al. Down-Regulation of miR-378d Increased Rab10 Expression to Help Clearance of Mycobacterium tuberculosis in Macrophages. Front Cell Infect Mi. 2020, 10, 108. [Google Scholar] [CrossRef]
- Zhu, T.; Liu, H.; Su, L.; Xiong, X.; Wang, J.; Xiao, Y.; Zhu, Y.; Peng, Y.; Dawood, A.; Hu, C.; et al. MicroRNA-18b-5p Downregulation Favors Mycobacterium tuberculosis Clearance in Macrophages via HIF-1alpha by Promoting an Inflammatory Response. ACS Infect Dis. 2021, 7, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Sahu, S.K.; Kumar, R.; Subuddhi, A.; Maji, R.K.; Jana, K.; Gupta, P.; Raffetseder, J.; Lerm, M.; Ghosh, Z.; et al. MicroRNA let-7 modulates the immune response to Mycobacterium tuberculosis infection via control of A20, an inhibitor of the NF-kappaB pathway. Cell Host Microbe 2015, 17, 345–356. [Google Scholar] [CrossRef]
- Zhu, T.; Liu, H.; Su, L.; Dawood, A.; Hu, C.; Chen, X.; Chen, H.; Chen, Y.; Guo, A. Identification of Unique Key miRNAs, TFs, and mRNAs in Virulent MTB Infection Macrophages by Network Analysis. Int. J. Mol. Sci. 2021, 23, 382. [Google Scholar] [CrossRef]
- Mohareer, K.; Asalla, S.; Banerjee, S. Cell death at the cross roads of host-pathogen interaction in Mycobacterium tuberculosis infection. Tuberculosis 2018, 113, 99–121. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.; Prabhu, R.; Gross, C.M.; Riesenberg, L.A.; Singh, V.; Aggarwal, S. Role of apoptosis and autophagy in tuberculosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 313, L218–L229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Yang, K.; Ren, T.T.; Huang, Y.; Liang, X.; Yu, Y.Y.; Wang, W.; Niu, J.F.; Lou, J.B.; Tang, X.D.; et al. miR-100-5p Inhibits Malignant Behavior of Chordoma Cells by Targeting IGF1R. Cancer Manag. Res. 2020, 12, 4129–4137. [Google Scholar] [CrossRef]
- Chen, P.; Lin, C.; Quan, J.; Lai, Y.; He, T.; Zhou, L.; Pan, X.; Wu, X.; Wang, Y.; Ni, L.; et al. Oncogenic miR-100-5p is associated with cellular viability, migration and apoptosis in renal cell carcinoma. Mol. Med. Rep. 2017, 16, 5023–5030. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhang, M.; Cao, F.; Han, J.; Han, P.; Wu, Y.; Deng, R.; Zhang, G.; An, X.; Zhang, L.; et al. Effect of MiR-100-5p on proliferation and apoptosis of goat endometrial stromal cell in vitro and embryo implantation in vivo. J. Cell Mol. Med. 2022, 26, 2543–2556. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhu, W.; Yang, Y.; Guo, M.; Qian, H.; Jiang, W.; Chen, Y.; Lian, C.; Xu, Z.; Bai, H.; et al. Exosomal miR-100-5p inhibits osteogenesis of hBMSCs and angiogenesis of HUVECs by suppressing the BMPR2/Smad1/5/9 signalling pathway. Stem Cell Res. 2021, 12, 390. [Google Scholar] [CrossRef] [PubMed]
- Alrfaei, B.M.; Clark, P.; Vemuganti, R.; Kuo, J.S. MicroRNA miR-100 Decreases Glioblastoma Growth by Targeting SMARCA5 and ErbB3 in Tumor-Initiating Cells. Technol. Cancer Res. Treat. 2020, 19, 1533033820960748. [Google Scholar] [CrossRef]
- Zikmund, T.; Kokavec, J.; Turkova, T.; Savvulidi, F.; Paszekova, H.; Vodenkova, S.; Sedlacek, R.; Skoultchi, A.I.; Stopka, T. ISWI ATPase Smarca5 Regulates Differentiation of Thymocytes Undergoing beta-Selection. J. Immunol. 2019, 202, 3434–3446. [Google Scholar] [CrossRef]
- Ai, C.; Ma, G.; Deng, Y.; Zheng, Q.; Gen, Y.; Li, W.; Li, Y.; Zu, L.; Zhou, Q. Nm23-H1 inhibits lung cancer bone-specific metastasis by upregulating miR-660-5p targeted SMARCA5. Thorac. Cancer 2020, 11, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Ma, Y.R.; Lin, Y.F.; Chen, R.; Xu, B.; Deng, J.L. SHU00238 Promotes Colorectal Cancer Cell Apoptosis Through miR-4701-3p and miR-4793-3p. Front. Genet. 2020, 10, 1320. [Google Scholar] [CrossRef]
- Mueller, A.C.; Sun, D.; Dutta, A. The miR-99 family regulates the DNA damage response through its target SNF2H. Oncogene 2013, 32, 1164–1172. [Google Scholar] [CrossRef]
- Finn, K.; Lowndes, N.F.; Grenon, M. Eukaryotic DNA damage checkpoint activation in response to double-strand breaks. Cell Mol. Life Sci. 2012, 69, 1447–1473. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.Y.; Zhang, B.; Ling, X.; Zhu, B.; Mei, S.H.; Yang, H.; Zhang, D.J.; Huo, J.P.; Zhao, Z.G. CTLA-4 Facilitates DNA Damage-Induced Apoptosis by Interacting With PP2A. Front Cell Dev. Biol. 2022, 10, 728771. [Google Scholar] [CrossRef]
- Liu, F.; Chen, J.; Wang, P.; Li, H.; Zhou, Y.; Liu, H.; Liu, Z.; Zheng, R.; Wang, L.; Yang, H.; et al. MicroRNA-27a controls the intracellular survival of Mycobacterium tuberculosis by regulating calcium-associated autophagy. Nat. Commun. 2018, 9, 4295. [Google Scholar] [CrossRef]
- Wendell, L.; Mayer, B.; Pawson, T. Cell Signaling: Principles and Mechanisms; Garland Science, Taylor and Francis Group: New York, NY, USA, 2015. [Google Scholar]
- Song, H.P.; Chu, Z.G.; Zhang, D.X.; Dang, Y.M.; Zhang, Q. PI3K-AKT Pathway Protects Cardiomyocytes Against Hypoxia-Induced Apoptosis by MitoKATP-Mediated Mitochondrial Translocation of pAKT. Cell Physiol. Biochem. 2018, 49, 717–727. [Google Scholar] [CrossRef]
- Fan, Q.; Wu, M.; Li, C.; Li, J. MiR-107 Aggravates Oxygen-Glucose Deprivation/Reoxygenation (OGD/R)-Induced Injury Through Inactivating PI3K-AKT Signalling Pathway by Targeting FGF9/FGF12 in PC12 Cells. J. Stroke Cerebrovasc. Dis. 2022, 31, 106295. [Google Scholar] [PubMed]
- Liu, Y.; Zhu, S.T.; Wang, X.; Deng, J.; Li, W.H.; Zhang, P.; Liu, B.S. MiR-100 Inhibits Osteosarcoma Cell Proliferation, Migration, and Invasion and Enhances Chemosensitivity by Targeting IGFIR. Technol. Cancer Res. Treat 2016, 15, NP40–NP48. [Google Scholar] [CrossRef]



| Sequence Name | Sequence 5′-3′ |
|---|---|
| Has-miR-100-5p mimic negative control | UUCUCCGAACGUGUCACGUTT ACGUGACACGUUCGGAGAATT |
| Has-miR-100-5p mimic | AACCCGUAGAUCCGAACUUGUG CAAGUUCGGAUCUACGGGUUUU |
| Has-miR-100-5p inhibitor negative control | CAGUACUUUUGUGUAGUACAA |
| Has-miR-100-5p inhibitor | CACAAGUUCGGAUCUACGGGUU |
| siSMARCA5 negative control | UUCUCCGAACGUGUCACGUTT ACGUGACACGUUCGGAGAATT |
| siSMARCA5 | AGGAAAUAUUUGAUGAUGCTT GCAUCAUCAAAUAUUUCCUCC |
| Control | BCG-Infected | M. tb-Infected | |
|---|---|---|---|
| 6 h | 4933.81 | 3146.33 | 2703.04 |
| 24 h | 6258.37 | 2833.35 | 3251.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, L.; Zhu, T.; Liu, H.; Zhu, Y.; Peng, Y.; Tang, T.; Zhou, S.; Hu, C.; Chen, H.; Guo, A.; et al. The miR-100-5p Targets SMARCA5 to Regulate the Apoptosis and Intracellular Survival of BCG in Infected THP-1 Cells. Cells 2023, 12, 476. https://doi.org/10.3390/cells12030476
Su L, Zhu T, Liu H, Zhu Y, Peng Y, Tang T, Zhou S, Hu C, Chen H, Guo A, et al. The miR-100-5p Targets SMARCA5 to Regulate the Apoptosis and Intracellular Survival of BCG in Infected THP-1 Cells. Cells. 2023; 12(3):476. https://doi.org/10.3390/cells12030476
Chicago/Turabian StyleSu, Li, Tingting Zhu, Han Liu, Yifan Zhu, Yongchong Peng, Tian Tang, Shiying Zhou, Changmin Hu, Huanchun Chen, Aizhen Guo, and et al. 2023. "The miR-100-5p Targets SMARCA5 to Regulate the Apoptosis and Intracellular Survival of BCG in Infected THP-1 Cells" Cells 12, no. 3: 476. https://doi.org/10.3390/cells12030476
APA StyleSu, L., Zhu, T., Liu, H., Zhu, Y., Peng, Y., Tang, T., Zhou, S., Hu, C., Chen, H., Guo, A., & Chen, Y. (2023). The miR-100-5p Targets SMARCA5 to Regulate the Apoptosis and Intracellular Survival of BCG in Infected THP-1 Cells. Cells, 12(3), 476. https://doi.org/10.3390/cells12030476





