Placental Galectins in Cancer: Why We Should Pay More Attention
Abstract
1. Introduction
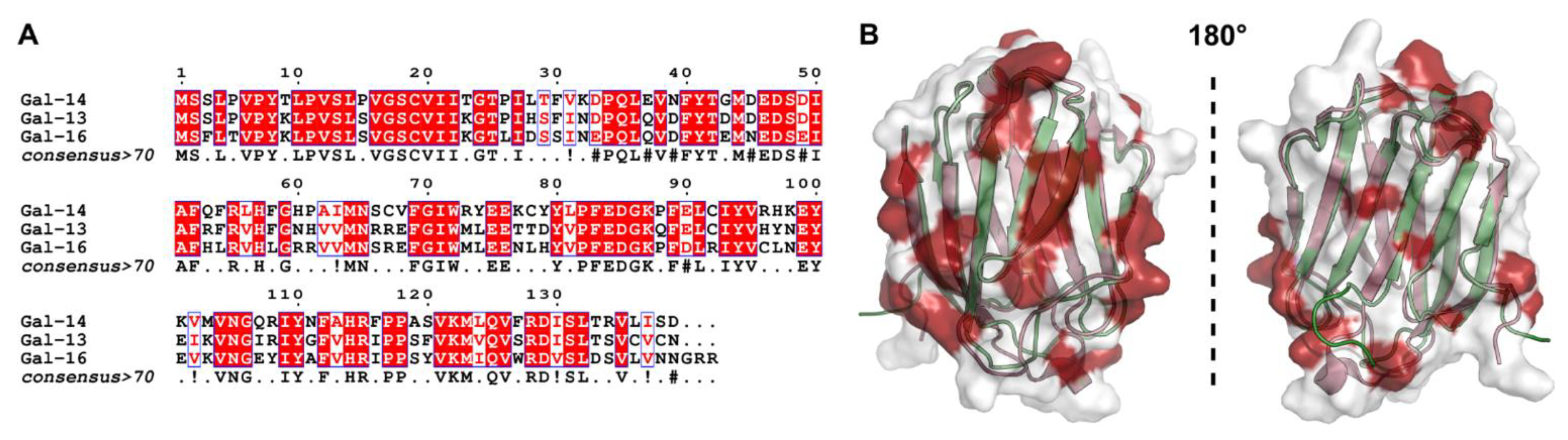
2. Placental Galectins and the Hallmarks of Cancer
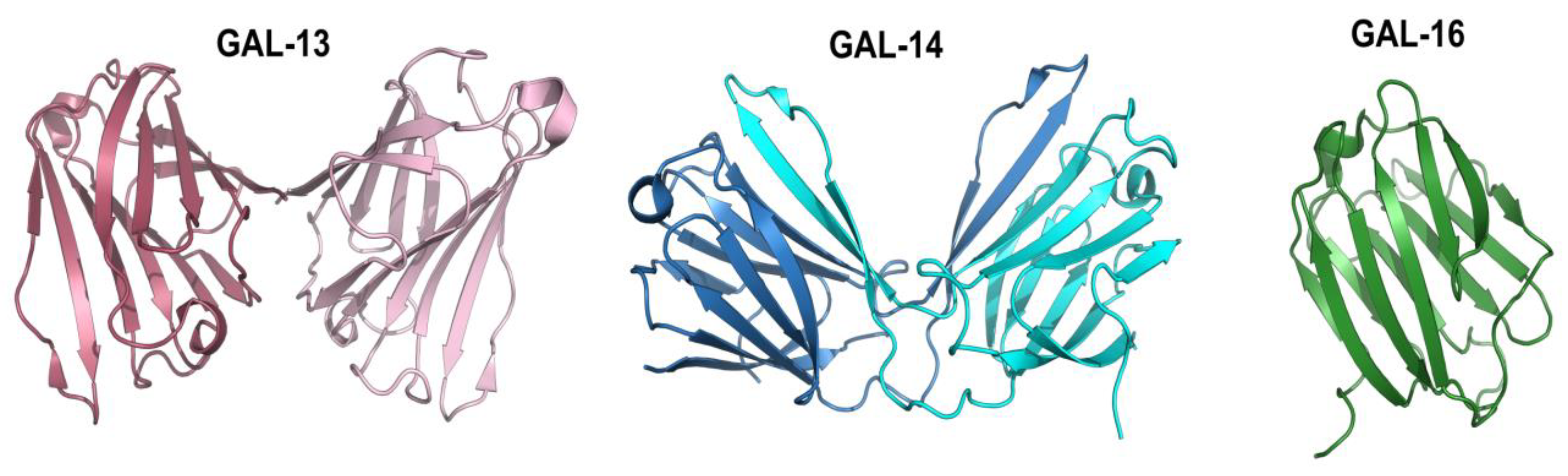
3. Expression of Placental Galectins in Cancer Cells
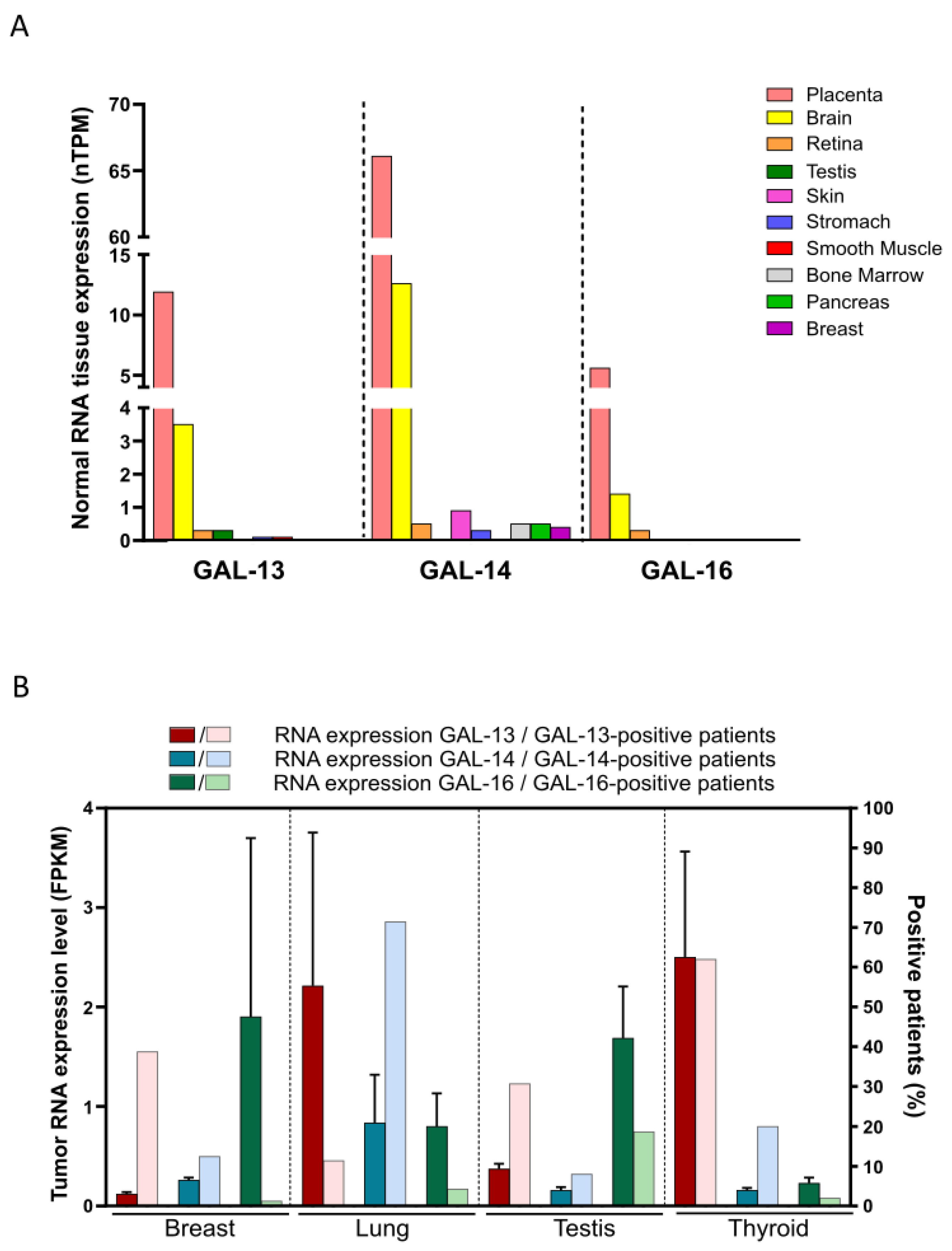
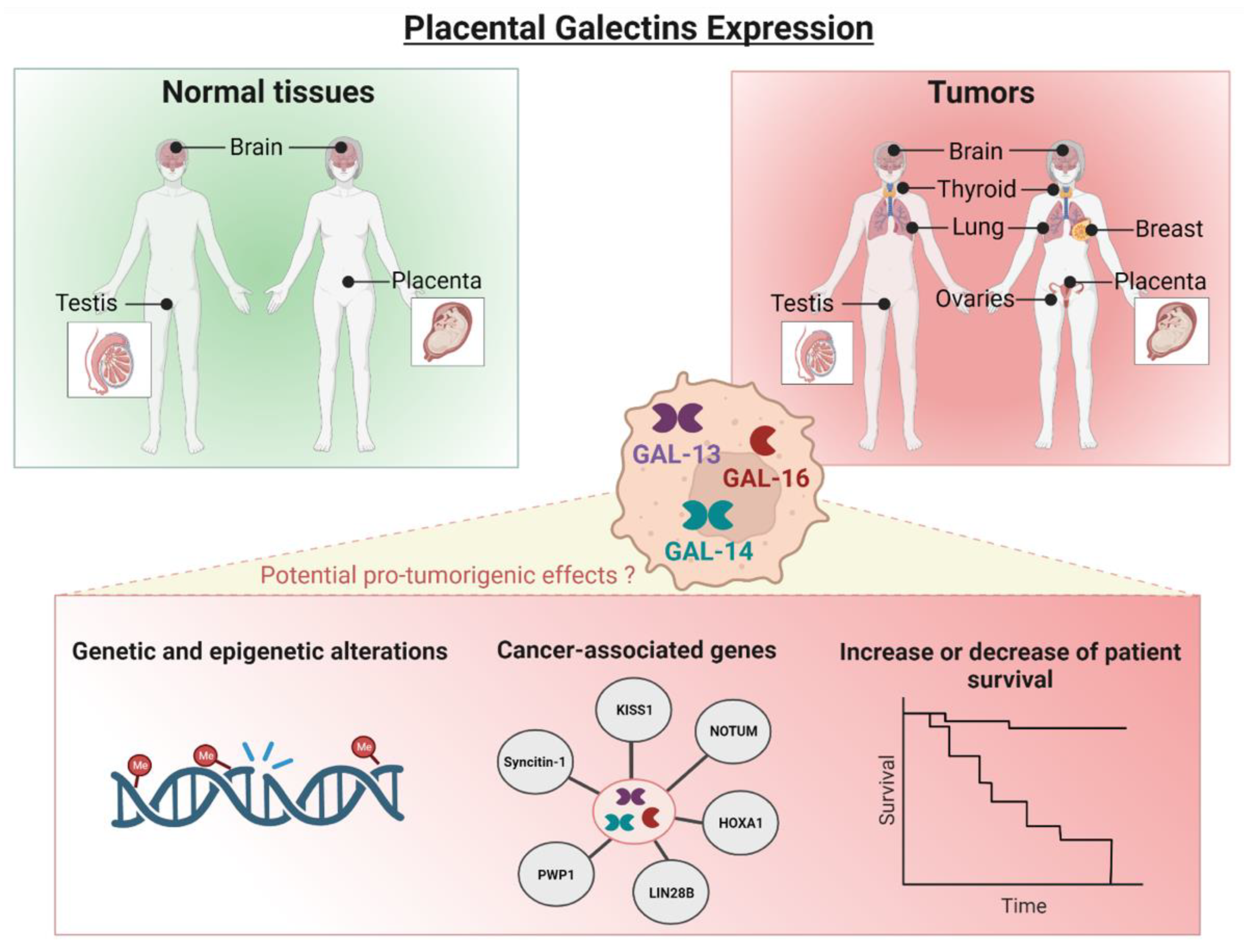
4. Identification of Cancer Tissues Expressing Placental Galectins Using Public Databases
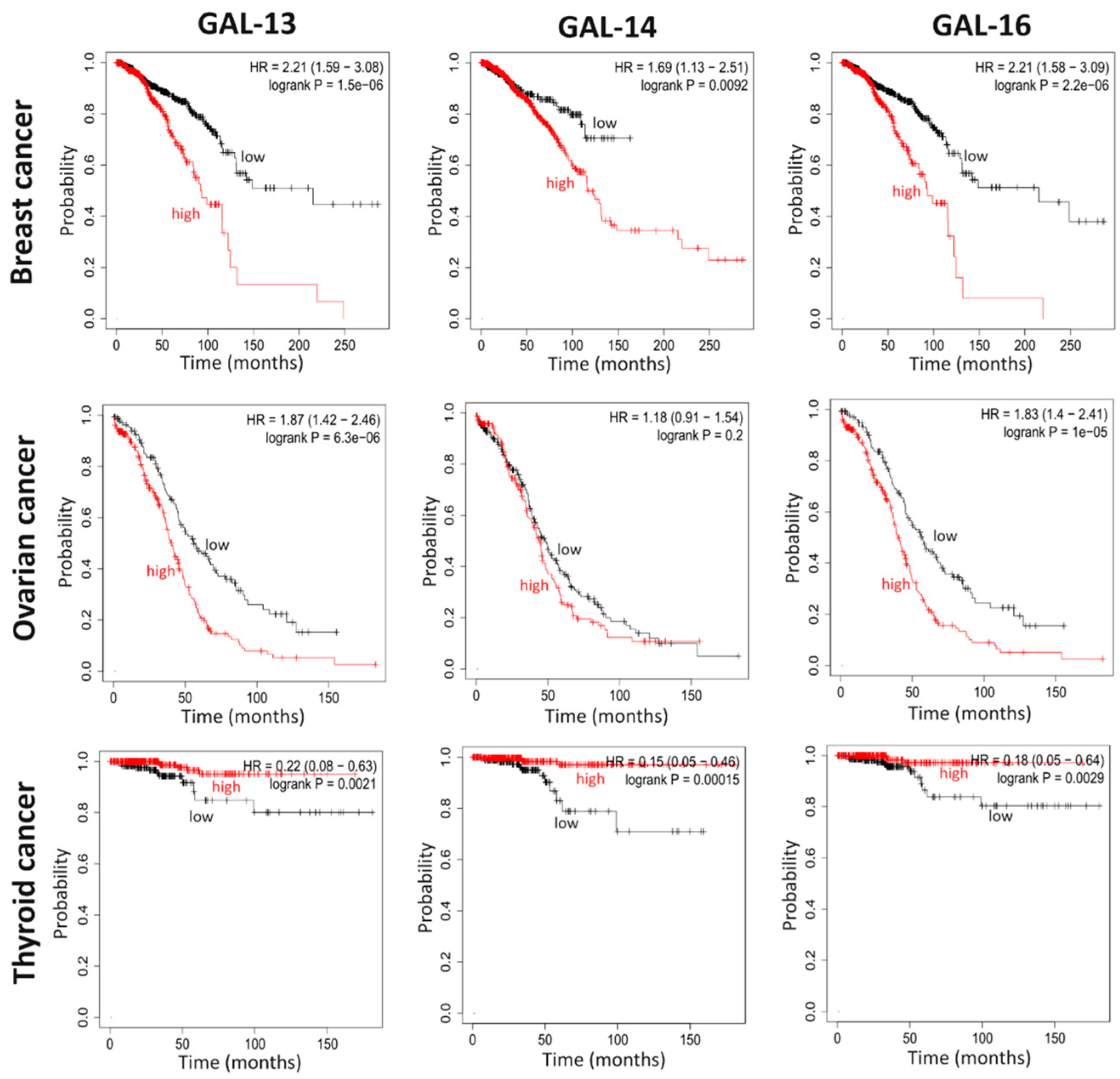
5. Placental Galectin Expression Correlates with Cancer Progression
6. A Role for Placental Galectins in Cancer Progression?
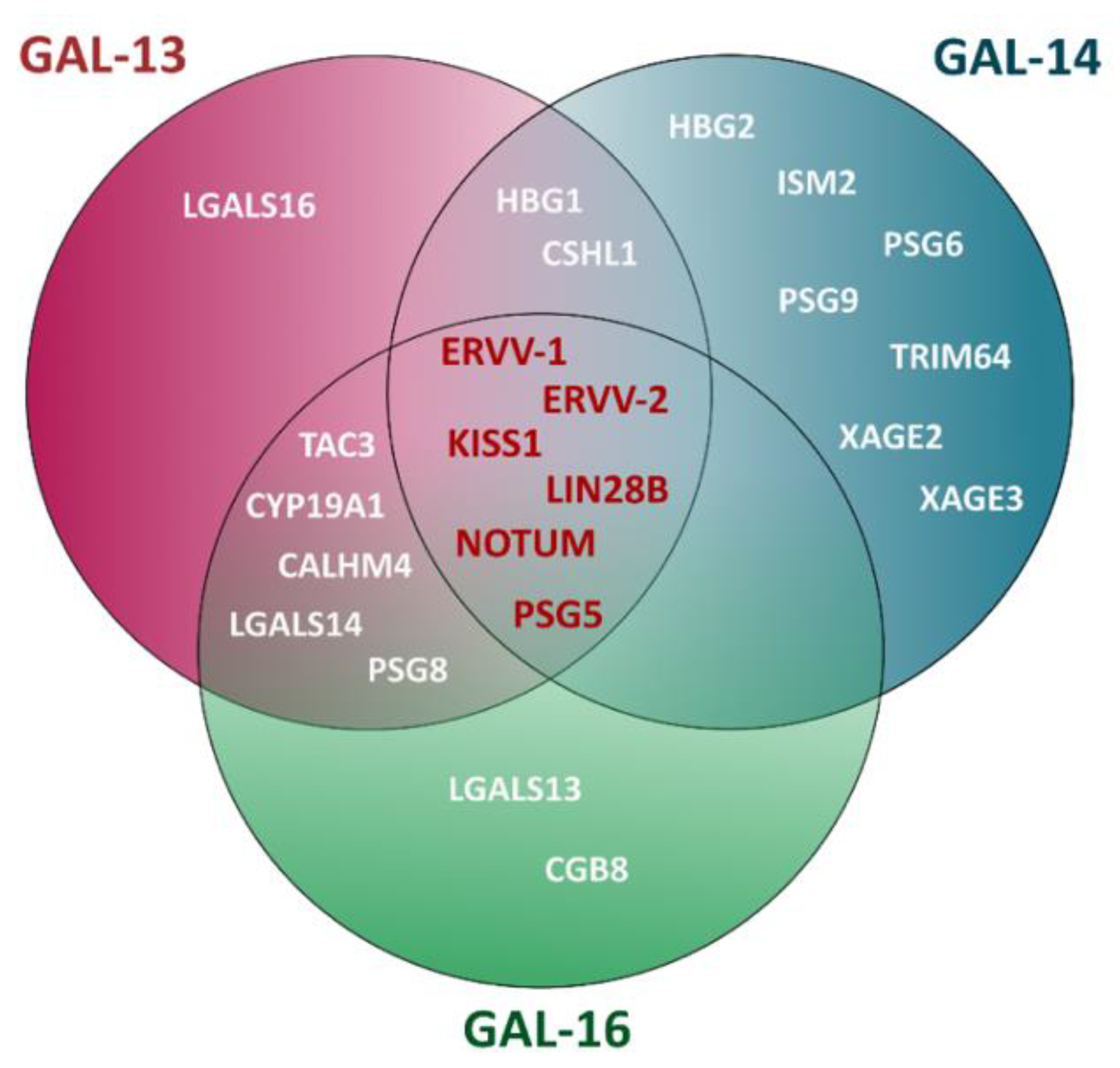
7. Candidate Gene Pathways
8. Cautionary Notes
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Barondes, S.H.; Castronovo, V.; Cooper, D.N.; Cummings, R.D.; Drickamer, K.; Feizi, T.; Gitt, M.A.; Hirabayashi, J.; Hughes, C.; Kasai, K. Galectins: A Family of Animal Beta-Galactoside-Binding Lectins. Cell 1994, 76, 597–598. [Google Scholar] [CrossRef] [PubMed]
- Partridge, E.A.; Le Roy, C.; Di Guglielmo, G.M.; Pawling, J.; Cheung, P.; Granovsky, M.; Nabi, I.R.; Wrana, J.L.; Dennis, J.W. Regulation of Cytokine Receptors by Golgi N-Glycan Processing and Endocytosis. Science 2004, 306, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.S.; Reis, C.A. Glycosylation in Cancer: Mechanisms and Clinical Implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef]
- Farhadi, S.A.; Hudalla, G.A. Engineering Galectin–Glycan Interactions for Immunotherapy and Immunomodulation. Exp. Biol. Med. 2016, 241, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, H.-C.; Zhao, J.; Wu, M.-H.; Shih, T.-C. Immunosuppressive Roles of Galectin-1 in the Tumor Microenvironment. Biomolecules 2021, 11, 1398. [Google Scholar] [CrossRef] [PubMed]
- Gordon-Alonso, M.; Bruger, A.M.; van der Bruggen, P. Extracellular Galectins as Controllers of Cytokines in Hematological Cancer. Blood 2018, 132, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Gordon-Alonso, M.; Hirsch, T.; Wildmann, C.; van der Bruggen, P. Galectin-3 Captures Interferon-Gamma in the Tumor Matrix Reducing Chemokine Gradient Production and T-Cell Tumor Infiltration. Nat. Commun. 2017, 8, 793. [Google Scholar] [CrossRef]
- Chou, F.-C.; Chen, H.-Y.; Kuo, C.-C.; Sytwu, H.-K. Role of Galectins in Tumors and in Clinical Immunotherapy. Int. J. Mol. Sci. 2018, 19, 430. [Google Scholar] [CrossRef]
- Curti, B.D.; Koguchi, Y.; Leidner, R.S.; Rolig, A.S.; Sturgill, E.R.; Sun, Z.; Wu, Y.; Rajamanickam, V.; Bernard, B.; Hilgart-Martiszus, I.; et al. Enhancing Clinical and Immunological Effects of Anti-PD-1 with Belapectin, a Galectin-3 Inhibitor. J Immunother. Cancer 2021, 9, e002371. [Google Scholar] [CrossRef]
- Nambiar, D.K.; Aguilera, T.; Cao, H.; Kwok, S.; Kong, C.; Bloomstein, J.; Wang, Z.; Rangan, V.S.; Jiang, D.; von Eyben, R.; et al. Galectin-1–Driven T Cell Exclusion in the Tumor Endothelium Promotes Immunotherapy Resistance. J. Clin. Investig. 2019, 129, 5553–5567. [Google Scholar] [CrossRef]
- Yang, R.; Sun, L.; Li, C.-F.; Wang, Y.-H.; Yao, J.; Li, H.; Yan, M.; Chang, W.-C.; Hsu, J.-M.; Cha, J.-H.; et al. Galectin-9 Interacts with PD-1 and TIM-3 to Regulate T Cell Death and Is a Target for Cancer Immunotherapy. Nat. Commun. 2021, 12, 832. [Google Scholar] [CrossRef] [PubMed]
- Girotti, M.R.; Salatino, M.; Dalotto-Moreno, T.; Rabinovich, G.A. Sweetening the Hallmarks of Cancer: Galectins as Multifunctional Mediators of Tumor Progression. J. Exp. Med. 2019, 217, e20182041. [Google Scholar] [CrossRef] [PubMed]
- Croci, D.O.; Cerliani, J.P.; Dalotto-Moreno, T.; Méndez-Huergo, S.P.; Mascanfroni, I.D.; Dergan-Dylon, S.; Toscano, M.A.; Caramelo, J.J.; García-Vallejo, J.J.; Ouyang, J.; et al. Glycosylation-Dependent Lectin-Receptor Interactions Preserve Angiogenesis in Anti-VEGF Refractory Tumors. Cell 2014, 156, 744–758. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-S.; Chang, C.-W.; Tsay, Y.-G.; Huang, L.-Y.; Wu, Y.-C.; Cheng, L.-H.; Yang, C.-C.; Wu, C.-H.; Teo, W.-H.; Hung, K.-F.; et al. HSP40 Co-Chaperone Protein Tid1 Suppresses Metastasis of Head and Neck Cancer by Inhibiting Galectin-7-TCF3-MMP9 Axis Signaling. Theranostics 2018, 8, 3841–3855. [Google Scholar] [CrossRef] [PubMed]
- Demers, M.; Rose, A.A.N.; Grosset, A.-A.; Biron-Pain, K.; Gaboury, L.; Siegel, P.M.; St-Pierre, Y. Overexpression of Galectin-7, A Myoepithelial Cell Marker, Enhances Spontaneous Metastasis of Breast Cancer Cells. Am. J. Pathol. 2010, 176, 3023–3031. [Google Scholar] [CrossRef]
- Kandel, S.; Adhikary, P.; Li, G.; Cheng, K. The TIM3/Gal9 Signaling Pathway: An Emerging Target for Cancer Immunotherapy. Cancer Lett. 2021, 510, 67–78. [Google Scholar] [CrossRef]
- Li, C.-H.; Chang, Y.-C.; Chan, M.-H.; Yang, Y.-F.; Liang, S.-M.; Hsiao, M. Galectins in Cancer and the Microenvironment: Functional Roles, Therapeutic Developments, and Perspectives. Biomedicines 2021, 9, 1159. [Google Scholar] [CrossRef]
- Vladoiu, M.C.; Labrie, M.; St-Pierre, Y. Intracellular Galectins in Cancer Cells: Potential New Targets for Therapy (Review). Int. J. Oncol. 2014, 44, 1001–1014. [Google Scholar] [CrossRef]
- Cummings, R.D.; Liu, F.-T.; Rabinovich, G.A.; Stowell, S.R.; Vasta, G.R. Galectins; Cold Spring Harbor Laboratory Press: Woodbury, NY, USA, 2022; ISBN 978-1-62182-421-3. [Google Scholar]
- Leonidas, D.D.; Elbert, B.L.; Zhou, Z.; Leffler, H.; Ackerman, S.J.; Acharya, K.R. Crystal Structure of Human Charcot-Leyden Crystal Protein, an Eosinophil Lysophospholipase, Identifies It as a New Member of the Carbohydrate-Binding Family of Galectins. Structure 1995, 3, 1379–1393. [Google Scholar] [CrossRef]
- Si, Y.; Yao, Y.; Jaramillo Ayala, G.; Li, X.; Han, Q.; Zhang, W.; Xu, X.; Tai, G.; Mayo, K.H.; Zhou, Y.; et al. Human Galectin-16 Has a Pseudo Ligand Binding Site and Plays a Role in Regulating c-Rel-Mediated Lymphocyte Activity. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129755. [Google Scholar] [CrossRef]
- Grozdanovic, M.M.; Doyle, C.B.; Liu, L.; Maybruck, B.T.; Kwatia, M.A.; Thiyagarajan, N.; Acharya, K.R.; Ackerman, S.J. Charcot-Leyden Crystal Protein/Galectin-10 Interacts with Cationic Ribonucleases and Is Required for Eosinophil Granulogenesis. J. Allergy Clin. Immunol. 2020, 146, 377–389.e10. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.E.; Leslie, J.; Perkins, N.D. C-Rel and Its Many Roles in Cancer: An Old Story with New Twists. Br. J. Cancer 2016, 114, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wells, V.; Mallucci, L. Identification of an Autocrine Negative Growth Factor: Mouse Beta-Galactoside-Binding Protein Is a Cytostatic Factor and Cell Growth Regulator. Cell 1991, 64, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Elantak, L.; Espeli, M.; Boned, A.; Bornet, O.; Bonzi, J.; Gauthier, L.; Feracci, M.; Roche, P.; Guerlesquin, F.; Schiff, C. Structural Basis for Galectin-1-Dependent Pre-B Cell Receptor (Pre-BCR) Activation. J. Biol. Chem. 2012, 287, 44703–44713. [Google Scholar] [CrossRef]
- Villeneuve, C.; Baricault, L.; Canelle, L.; Barboule, N.; Racca, C.; Monsarrat, B.; Magnaldo, T.; Larminat, F. Mitochondrial Proteomic Approach Reveals Galectin-7 as a Novel BCL-2 Binding Protein in Human Cells. Mol. Biol. Cell 2011, 22, 999–1013. [Google Scholar] [CrossRef]
- Advedissian, T.; Proux-Gillardeaux, V.; Nkosi, R.; Peyret, G.; Nguyen, T.; Poirier, F.; Viguier, M.; Deshayes, F. E-Cadherin Dynamics Is Regulated by Galectin-7 at Epithelial Cell Surface. Sci. Rep. 2017, 7, 17086. [Google Scholar] [CrossRef]
- Than, N.G.; Romero, R.; Goodman, M.; Weckle, A.; Xing, J.; Dong, Z.; Xu, Y.; Tarquini, F.; Szilagyi, A.; Gal, P.; et al. A Primate Subfamily of Galectins Expressed at the Maternal-Fetal Interface That Promote Immune Cell Death. Proc. Natl. Acad. Sci. USA 2009, 106, 9731–9736. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Wang, Y.; Si, Y.; Gao, J.; Song, C.; Cui, L.; Wu, R.; Tai, G.; Zhou, Y. Galectin-13, a Different Prototype Galectin, Does Not Bind β-Galacto-Sides and Forms Dimers via Intermolecular Disulfide Bridges between Cys-136 and Cys-138. Sci. Rep. 2018, 8, 980. [Google Scholar] [CrossRef]
- Si, Y.; Li, Y.; Yang, T.; Li, X.; Ayala, G.J.; Mayo, K.H.; Tai, G.; Su, J.; Zhou, Y. Structure-Function Studies of Galectin-14, an Important Effector Molecule in Embryology. FEBS J. 2021, 288, 1041–1055. [Google Scholar] [CrossRef]
- Szabolcsi, Z.; Demeter, A.; Kiraly, P.; Balogh, A.; Wilson, M.L.; King, J.R.; Hetey, S.; Gelencser, Z.; Matsuo, K.; Hargitai, B.; et al. Epigenetic Dysregulation of Trophoblastic Gene Expression in Gestational Trophoblastic Disease. Biomedicines 2021, 9, 1935. [Google Scholar] [CrossRef]
- Than, N.G.; Romero, R.; Xu, Y.; Erez, O.; Xu, Z.; Bhatti, G.; Leavitt, R.; Chung, T.H.; El-Azzamy, H.; LaJeunesse, C.; et al. Evolutionary Origins of the Placental Expression of Chromosome 19 Cluster Galectins and Their Complex Dysregulation in Preeclampsia. Placenta 2014, 35, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Than, N.G.; Balogh, A.; Romero, R.; Kárpáti, E.; Erez, O.; Szilágyi, A.; Kovalszky, I.; Sammar, M.; Gizurarson, S.; Matkó, J.; et al. Placental Protein 13 (PP13)—A Placental Immunoregulatory Galectin Protecting Pregnancy. Front. Immunol. 2014, 5, 348. [Google Scholar] [CrossRef] [PubMed]
- Balogh, A.; Toth, E.; Romero, R.; Parej, K.; Csala, D.; Szenasi, N.L.; Hajdu, I.; Juhasz, K.; Kovacs, A.F.; Meiri, H.; et al. Placental Galectins Are Key Players in Regulating the Maternal Adaptive Immune Response. Front. Immunol. 2019, 10, 1240. [Google Scholar] [CrossRef]
- Menkhorst, E.; Than, N.G.; Jeschke, U.; Barrientos, G.; Szereday, L.; Dveksler, G.; Blois, S.M. Medawar’s PostEra: Galectins Emerged as Key Players During Fetal-Maternal Glycoimmune Adaptation. Front. Immunol. 2021, 12, 784473. [Google Scholar] [CrossRef]
- Ferretti, C.; Bruni, L.; Dangles-Marie, V.; Pecking, A.P.; Bellet, D. Molecular Circuits Shared by Placental and Cancer Cells, and Their Implications in the Proliferative, Invasive and Migratory Capacities of Trophoblasts. Hum. Reprod. Update 2007, 13, 121–141. [Google Scholar] [CrossRef]
- Holtan, S.G.; Creedon, D.J.; Haluska, P.; Markovic, S.N. Cancer and Pregnancy: Parallels in Growth, Invasion, and Immune Modulation and Implications for Cancer Therapeutic Agents. Mayo Clin. Proc. 2009, 84, 985–1000. [Google Scholar] [CrossRef]
- Lala, P.K.; Nandi, P.; Hadi, A.; Halari, C. A Crossroad between Placental and Tumor Biology: What Have We Learnt? Placenta 2021, 116, 12–30. [Google Scholar] [CrossRef]
- Mor, G.; Aldo, P.; Alvero, A.B. The Unique Immunological and Microbial Aspects of Pregnancy. Nat. Rev. Immunol. 2017, 17, 469–482. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- St-Pierre, Y. Towards a Better Understanding of the Relationships between Galectin-7, P53 and MMP-9 during Cancer Progression. Biomolecules 2021, 11, 879. [Google Scholar] [CrossRef]
- Vokalova, L.; Balogh, A.; Toth, E.; Van Breda, S.V.; Schäfer, G.; Hoesli, I.; Lapaire, O.; Hahn, S.; Than, N.G.; Rossi, S.W. Placental Protein 13 (Galectin-13) Polarizes Neutrophils Toward an Immune Regulatory Phenotype. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Wang, M.; Xu, Y.; Wang, P.; Xu, Y.; Jin, P.; Wu, Z.; Qian, Y.; Bai, L.; Dong, M. Galectin-14 Promotes Trophoblast Migration and Invasion by Upregulating the Expression of MMP-9 and N-Cadherin. Front. Cell Dev. Biol. 2021, 9, 645658. [Google Scholar] [CrossRef]
- Blois, S.M.; Barrientos, G. Galectin Signature in Normal Pregnancy and Preeclampsia. J. Reprod. Immunol. 2014, 101–102, 127–134. [Google Scholar] [CrossRef]
- Folkman, J. Tumor Angiogenesis: Therapeutic Implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Funasaka, T.; Raz, A.; Nangia-Makker, P. Galectin-3 in Angiogenesis and Metastasis. Glycobiology 2014, 24, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Griffioen, A.W.; Thijssen, V.L. Galectins in Tumor Angiogenesis. Ann. Transl. Med. 2014, 2, 90. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-T.; Rabinovich, G.A. Galectins as Modulators of Tumour Progression. Nat. Rev. Cancer 2005, 5, 29–41. [Google Scholar] [CrossRef]
- Ribatti, D. Judah Folkman, a Pioneer in the Study of Angiogenesis. Angiogenesis 2008, 11, 3–10. [Google Scholar] [CrossRef]
- Thijssen, V.L. Galectins in Endothelial Cell Biology and Angiogenesis: The Basics. Biomolecules 2021, 11, 1386. [Google Scholar] [CrossRef]
- Baluk, P.; Hashizume, H.; McDonald, D.M. Cellular Abnormalities of Blood Vessels as Targets in Cancer. Curr. Opin. Genet. Dev. 2005, 15, 102–111. [Google Scholar] [CrossRef]
- Ziyad, S.; Iruela-Arispe, M.L. Molecular Mechanisms of Tumor Angiogenesis. Genes Cancer 2011, 2, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Nagaki, Y.; Motoyama, S.; Kuze, Y.; Hoshizaki, M.; Kemuriyama, K.; Yamaguchi, T.; Ebihara, T.; Minamiya, Y.; Suzuki, Y.; et al. Identification of Galectin-7 as a Crucial Metastatic Enhancer of Squamous Cell Carcinoma Associated with Immunosuppression. Oncogene 2022. [Google Scholar] [CrossRef] [PubMed]
- Than, N.G.; Sumegi, B.; Than, G.N.; Berente, Z.; Bohn, H. Isolation and Sequence Analysis of a CDNA Encoding Human Placental Tissue Protein 13 (PP13), a New Lysophospholipase, Homologue of Human Eosinophil Charcot-Leyden Crystal Protein. Placenta 1999, 20, 703–710. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, L.O.F.; St-Pierre, Y. Galectin-14 Expression in Ovarian Cancer. bioRxiv 2019, 717793. [Google Scholar]
- Kaminker, J.D.; Timoshenko, A.V. Expression, Regulation, and Functions of the Galectin-16 Gene in Human Cells and Tissues. Biomolecules 2021, 11, 1909. [Google Scholar] [CrossRef] [PubMed]
- Duffy, Á.; Verbanck, M.; Dobbyn, A.; Won, H.-H.; Rein, J.L.; Forrest, I.S.; Nadkarni, G.; Rocheleau, G.; Do, R. Tissue-Specific Genetic Features Inform Prediction of Drug Side Effects in Clinical Trials. Sci. Adv. 2020, 6, eabb6242. [Google Scholar] [CrossRef]
- Califice, S.; Castronovo, V.; Bracke, M.; van den Brûle, F. Dual Activities of Galectin-3 in Human Prostate Cancer: Tumor Suppression of Nuclear Galectin-3 vs Tumor Promotion of Cytoplasmic Galectin-3. Oncogene 2004, 23, 7527–7536. [Google Scholar] [CrossRef]
- Grosset, A.-A.; Labrie, M.; Vladoiu, M.C.; Yousef, E.M.; Gaboury, L.; St-Pierre, Y. Galectin Signatuares Contribute to the Heterogeneity of Breast Cancer and Provide New Prognostic Information and Therapeutic Targets. Oncotarget 2016, 7, 18183–18203. [Google Scholar] [CrossRef]
- Satelli, A.; Rao, P.S.; Thirumala, S.; Rao, U.S. Galectin-4 Functions as a Tumor Suppressor of Human Colorectal Cancer. Int. J. Cancer 2011, 129, 799–809. [Google Scholar] [CrossRef]
- Ueda, S.; Kuwabara, I.; Liu, F.-T. Suppression of Tumor Growth by Galectin-7 Gene Transfer. Cancer Res. 2004, 64, 5672–5676. [Google Scholar] [CrossRef]
- Nagy, Á.; Munkácsy, G.; Győrffy, B. Pancancer Survival Analysis of Cancer Hallmark Genes. Sci. Rep. 2021, 11, 6047. [Google Scholar] [CrossRef] [PubMed]
- Bozic, I.; Antal, T.; Ohtsuki, H.; Carter, H.; Kim, D.; Chen, S.; Karchin, R.; Kinzler, K.W.; Vogelstein, B.; Nowak, M.A. Accumulation of Driver and Passenger Mutations during Tumor Progression. Proc. Natl. Acad. Sci. USA 2010, 107, 18545–18550. [Google Scholar] [CrossRef] [PubMed]
- Magnaldo, T.; Fowlis, D.; Darmon, M. Galectin-7, a Marker of All Types of Stratified Epithelia. Differentiation 1998, 63, 159–168. [Google Scholar] [CrossRef]
- Magnaldo, T.; Bernerd, F.; Darmon, M. Galectin-7, a Human 14-KDa S-Lectin, Specifically Expressed in Keratinocytes and Sensitive to Retinoic Acid. Dev. Biol. 1995, 168, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Moisan, S.; Demers, M.; Mercier, J.; Magnaldo, T.; Potworowski, E.F.; St-Pierre, Y. Upregulation of Galectin-7 in Murine Lymphoma Cells Is Associated with Progression toward an Aggressive Phenotype. Leukemia 2003, 17, 751–759. [Google Scholar] [CrossRef]
- Demers, M.; Biron-Pain, K.; Hébert, J.; Lamarre, A.; Magnaldo, T.; St-Pierre, Y. Galectin-7 in Lymphoma: Elevated Expression in Human Lymphoid Malignancies and Decreased Lymphoma Dissemination by Antisense Strategies in Experimental Model. Cancer Res. 2007, 67, 2824–2829. [Google Scholar] [CrossRef]
- Demers, M.; Magnaldo, T.; St-Pierre, Y. A Novel Function for Galectin-7: Promoting Tumorigenesis by Up-Regulating MMP-9 Gene Expression. Cancer Res. 2005, 65, 5205–5210. [Google Scholar] [CrossRef]
- Demers, M.; Couillard, J.; Giglia-Mari, G.; Magnaldo, T.; St-Pierre, Y. Increased Galectin-7 Gene Expression in Lymphoma Cells Is under the Control of DNA Methylation. Biochem. Biophys. Res. Commun. 2009, 387, 425–429. [Google Scholar] [CrossRef]
- Kim, S.-J.; Hwang, J.-A.; Ro, J.Y.; Lee, Y.-S.; Chun, K.-H. Galectin-7 Is Epigenetically-Regulated Tumor Suppressor in Gastric Cancer. Oncotarget 2013, 4, 1461–1471. [Google Scholar] [CrossRef]
- Campion, C.G.; Labrie, M.; Grosset, A.-A.; St-Pierre, Y. The CCAAT/Enhancer-Binding Protein Beta-2 Isoform (CEBPβ-2) Upregulates Galectin-7 Expression in Human Breast Cancer Cells. PLoS ONE 2014, 9, e95087. [Google Scholar] [CrossRef]
- Campion, C.G.; Labrie, M.; Lavoie, G.; St-Pierre, Y. Expression of Galectin-7 Is Induced in Breast Cancer Cells by Mutant P53. PLoS ONE 2013, 8, e72468. [Google Scholar] [CrossRef] [PubMed]
- Dall’Olio, F.; Trinchera, M. Epigenetic Bases of Aberrant Glycosylation in Cancer. Int. J. Mol. Sci. 2017, 18, 998. [Google Scholar] [CrossRef] [PubMed]
- Katzenmaier, E.-M.; Kloor, M.; Gabius, H.-J.; Gebert, J.; Kopitz, J. Analyzing Epigenetic Control of Galectin Expression Indicates Silencing of Galectin-12 by Promoter Methylation in Colorectal Cancer. IUBMB Life 2017, 69, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Benvenuto, G.; Carpentieri, M.L.; Salvatore, P.; Cindolo, L.; Bruni, C.B.; Chiariotti, L. Cell-Specific Transcriptional Regulation and Reactivation of Galectin-1 Gene Expression Are Controlled by DNA Methylation of the Promoter Region. Mol. Cell. Biol. 1996, 16, 2736–2743. [Google Scholar] [CrossRef]
- Salvatore, P.; Benvenuto, G.; Caporaso, M.; Bruni, C.B.; Chiariotti, L. High Resolution Methylation Analysis of the Galectin-1 Gene Promoter Region in Expressing and Nonexpressing Tissues. FEBS Lett. 1998, 421, 152–158. [Google Scholar] [CrossRef]
- Timp, W.; Feinberg, A.P. Cancer as a Dysregulated Epigenome Allowing Cellular Growth Advantage at the Expense of the Host. Nat. Rev. Cancer 2013, 13, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.M.; Ezashi, T.; Schulz, L.C.; Sugimoto, J.; Schust, D.J.; Khan, T.; Zhou, J. Syncytins Expressed in Human Placental Trophoblast. Placenta 2021, 113, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Chen, H.; Li, J.; Oliver, M.; Ma, X.; Byck, D.; Gao, Y.; Jiang, S.-W. Epigenetic and Non-Epigenetic Regulation of Syncytin-1 Expression in Human Placenta and Cancer Tissues. Cell. Signal. 2014, 26, 648–656. [Google Scholar] [CrossRef]
- Liu, C.; Xu, J.; Wen, F.; Yang, F.; Li, X.; Geng, D.; Li, L.; Chen, J.; Zheng, J. Upregulation of Syncytin-1 Promotes Invasion and Metastasis by Activating Epithelial-Mesenchymal Transition-Related Pathway in Endometrial Carcinoma. Onco. Targets Ther. 2018, 12, 31–40. [Google Scholar] [CrossRef]
- Benešová, M.; Trejbalová, K.; Kovářová, D.; Vernerová, Z.; Hron, T.; Kučerová, D.; Hejnar, J. DNA Hypomethylation and Aberrant Expression of the Human Endogenous Retrovirus ERVWE1/Syncytin-1 in Seminomas. Retrovirology 2017, 14, 20. [Google Scholar] [CrossRef]
- Díaz-Carballo, D.; Acikelli, A.H.; Klein, J.; Jastrow, H.; Dammann, P.; Wyganowski, T.; Guemues, C.; Gustmann, S.; Bardenheuer, W.; Malak, S.; et al. Therapeutic Potential of Antiviral Drugs Targeting Chemorefractory Colorectal Adenocarcinoma Cells Overexpressing Endogenous Retroviral Elements. J. Exp. Clin. Cancer Res. 2015, 34, 81. [Google Scholar] [CrossRef] [PubMed]
- Rasoulzadeh, Z.; Ghods, R.; Kazemi, T.; Mirzadegan, E.; Ghaffari-Tabrizi-Wizsy, N.; Rezania, S.; Kazemnejad, S.; Arefi, S.; Ghasemi, J.; Vafaei, S.; et al. Placental Kisspeptins Differentially Modulate Vital Parameters of Estrogen Receptor-Positive and -Negative Breast Cancer Cells. PLoS ONE 2016, 11, e0153684. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Parhar, I.S. Biological Significance of Kisspeptin–Kiss 1 Receptor Signaling in the Habenula of Teleost Species. Front. Endocrinol. 2018, 9, 222. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Miele, M.E.; Hicks, D.J.; Phillips, K.K.; Trent, J.M.; Weissman, B.E.; Welch, D.R. KiSS-1, a Novel Human Malignant Melanoma Metastasis-Suppressor Gene. J. Natl. Cancer Inst. 1996, 88, 1731–1737. [Google Scholar] [CrossRef]
- Kim, T.-H.; Yoon, J.H.; Cho, S.-G. Kisspeptin Promotes Glioblastoma Cell Invasiveness Via the Gq-PLC-PKC Pathway. Anticancer. Res. 2020, 40, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Takino, T.; Koshikawa, N.; Miyamori, H.; Tanaka, M.; Sasaki, T.; Okada, Y.; Seiki, M.; Sato, H. Cleavage of Metastasis Suppressor Gene Product KiSS-1 Protein/Metastin by Matrix Metalloproteinases. Oncogene 2003, 22, 4617–4626. [Google Scholar] [CrossRef] [PubMed]
- Torisu, Y.; Watanabe, A.; Nonaka, A.; Midorikawa, Y.; Makuuchi, M.; Shimamura, T.; Sugimura, H.; Niida, A.; Akiyama, T.; Iwanari, H.; et al. Human Homolog of NOTUM, Overexpressed in Hepatocellular Carcinoma, Is Regulated Transcriptionally by β-Catenin/TCF. Cancer Sci. 2008, 99, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, T.; Yin, Q.; Luo, H. Development and Validation of Genomic and Epigenomic Signatures Associated with Tumor Immune Microenvironment in Hepatoblastoma. BMC Cancer 2021, 21, 1156. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Niu, Q.; Zhou, Y.; Wang, Y.-X.; Xu, X.-F.; Hou, K.-Z. Notum Palmitoleoyl-Protein Carboxylesterase Regulates Fas Cell Surface Death Receptor-Mediated Apoptosis via the Wnt Signaling Pathway in Colon Adenocarcinoma. Bioengineered 2021, 12, 5241–5252. [Google Scholar] [CrossRef]
- Yoon, J.H.; Kim, D.; Kim, J.; Lee, H.; Ghim, J.; Kang, B.J.; Song, P.; Suh, P.-G.; Ryu, S.H.; Lee, T.G. NOTUM Is Involved in the Progression of Colorectal Cancer. Cancer Genom. Proteom. 2018, 15, 485–497. [Google Scholar] [CrossRef]
- Thornton, J.E.; Gregory, R.I. How Does Lin28 Let-7 Control Development and Disease? Trends Cell Biol. 2012, 22, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Franses, J.W.; Philipp, J.; Missios, P.; Bhan, I.; Liu, A.; Yashaswini, C.; Tai, E.; Zhu, H.; Ligorio, M.; Nicholson, B.; et al. Pancreatic Circulating Tumor Cell Profiling Identifies LIN28B as a Metastasis Driver and Drug Target. Nat. Commun. 2020, 11, 3303. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Hu, Z.; Bao, Y.; Li, Y.; Li, S.; Zheng, Q.; Lyu, D.; Chen, D.; Yu, T.; Li, Y.; et al. A LIN28B Tumor-Specific Transcript in Cancer. Cell Rep. 2018, 22, 2016–2025. [Google Scholar] [CrossRef] [PubMed]
- Lovnicki, J.; Gan, Y.; Feng, T.; Li, Y.; Xie, N.; Ho, C.-H.; Lee, A.R.; Chen, X.; Nappi, L.; Han, B.; et al. LIN28B Promotes the Development of Neuroendocrine Prostate Cancer. J. Clin. Investig. 2020, 130, 5338–5348. [Google Scholar] [CrossRef]
- Wang, T.; Wang, G.; Hao, D.; Liu, X.; Wang, D.; Ning, N.; Li, X. Aberrant Regulation of the LIN28A/LIN28B and Let-7 Loop in Human Malignant Tumors and Its Effects on the Hallmarks of Cancer. Mol. Cancer 2015, 14, 125. [Google Scholar] [CrossRef]
- Tischfield, M.A.; Bosley, T.M.; Salih, M.A.M.; Alorainy, I.A.; Sener, E.C.; Nester, M.J.; Oystreck, D.T.; Chan, W.-M.; Andrews, C.; Erickson, R.P.; et al. Homozygous HOXA1 Mutations Disrupt Human Brainstem, Inner Ear, Cardiovascular and Cognitive Development. Nat. Genet. 2005, 37, 1035–1037. [Google Scholar] [CrossRef]
- Yang, T.; Yao, Y.; Wang, X.; Li, Y.; Si, Y.; Li, X.; Ayala, G.J.; Wang, Y.; Mayo, K.H.; Tai, G.; et al. Galectin-13/Placental Protein 13: Redox-Active Disulfides as Switches for Regulating Structure, Function and Cellular Distribution. Glycobiology 2020, 30, 120–129. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Lu, X. HOXA1 Upregulation Is Associated with Poor Prognosis and Tumor Progression in Breast Cancer. Exp. Ther. Med. 2019, 17, 1896–1902. [Google Scholar] [CrossRef]
- Zha, T.-Z.; Hu, B.-S.; Yu, H.-F.; Tan, Y.-F.; Zhang, Y.; Zhang, K. Overexpression of HOXA1 Correlates with Poor Prognosis in Patients with Hepatocellular Carcinoma. Tumor Biol. 2012, 33, 2125–2134. [Google Scholar] [CrossRef]
- Warde-Farley, D.; Donaldson, S.L.; Comes, O.; Zuberi, K.; Badrawi, R.; Chao, P.; Franz, M.; Grouios, C.; Kazi, F.; Lopes, C.T.; et al. The GeneMANIA Prediction Server: Biological Network Integration for Gene Prioritization and Predicting Gene Function. Nucleic Acids Res. 2010, 38, W214–W220. [Google Scholar] [CrossRef]
- Honoré, B.; Baandrup, U.; Nielsen, S.; Vorum, H. Endonuclein Is a Cell Cycle Regulated WD-Repeat Protein That Is up-Regulated in Adenocarcinoma of the Pancreas. Oncogene 2002, 21, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Li, P.; Luo, Y.; Zhang, M.; Yan, T.; Yang, Y.; Han, Y.; Liu, S.; Wang, E. PWP1 Promotes the Malignant Phenotypes of Lung Cancer Cells by Interacting with DVL2 and Merlin. Onco. Targets Ther. 2020, 13, 10025–10037. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuselier, C.; Dumoulin, A.; Paré, A.; Nehmé, R.; Ajarrag, S.; Granger Joly de Boissel, P.; Chatenet, D.; Doucet, N.; St-Pierre, Y. Placental Galectins in Cancer: Why We Should Pay More Attention. Cells 2023, 12, 437. https://doi.org/10.3390/cells12030437
Fuselier C, Dumoulin A, Paré A, Nehmé R, Ajarrag S, Granger Joly de Boissel P, Chatenet D, Doucet N, St-Pierre Y. Placental Galectins in Cancer: Why We Should Pay More Attention. Cells. 2023; 12(3):437. https://doi.org/10.3390/cells12030437
Chicago/Turabian StyleFuselier, Camille, Alyssa Dumoulin, Alex Paré, Rita Nehmé, Samy Ajarrag, Philippine Granger Joly de Boissel, David Chatenet, Nicolas Doucet, and Yves St-Pierre. 2023. "Placental Galectins in Cancer: Why We Should Pay More Attention" Cells 12, no. 3: 437. https://doi.org/10.3390/cells12030437
APA StyleFuselier, C., Dumoulin, A., Paré, A., Nehmé, R., Ajarrag, S., Granger Joly de Boissel, P., Chatenet, D., Doucet, N., & St-Pierre, Y. (2023). Placental Galectins in Cancer: Why We Should Pay More Attention. Cells, 12(3), 437. https://doi.org/10.3390/cells12030437









