Promises and Challenges of Predictive Blood Biomarkers for Locally Advanced Rectal Cancer Treated with Neoadjuvant Chemoradiotherapy
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Literature Search Results
3.2. Protein Tumor Markers
3.2.1. CEA
3.2.2. CA 19-9
3.3. Hematological Markers
3.3.1. Thrombocytes
3.3.2. Hemoglobin (Hb)
3.4. Leukocytes and Inflammatory Markers
3.4.1. Albumin and C-Reactive Protein
3.4.2. Leukocytes
3.5. Lipid Marker
Apolipoprotein A-1
3.6. Nucleic Acids Marker
3.6.1. Cell Free DNA
3.6.2. Circulating Tumor DNA
3.6.3. MicroRNA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel, C.; Cervantes, A.; Arnold, D.; Committee, E.G. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv22–iv40. [Google Scholar] [CrossRef] [PubMed]
- Araghi, M.; Soerjomataram, I.; Jenkins, M.; Brierley, J.; Morris, E.; Bray, F.; Arnold, M. Global trends in colorectal cancer mortality: Projections to the year 2035. Int. J. Cancer 2019, 144, 2992–3000. [Google Scholar] [CrossRef] [PubMed]
- Minicozzi, P.; Innos, K.; Sánchez, M.-J.; Trama, A.; Walsh, P.M.; Marcos-Gragera, R.; Dimitrova, N.; Botta, L.; Visser, O.; Rossi, S.; et al. Quality analysis of population-based information on cancer stage at diagnosis across Europe, with presentation of stage-specific cancer survival estimates: A EUROCARE-5 study. Eur. J. Cancer 2017, 84, 335–353. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Cederquist, L.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Engstrom, P.F.; et al. Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 874–901. [Google Scholar] [CrossRef]
- Macchia, G.; Gambacorta, M.A.; Masciocchi, C.; Chiloiro, G.; Mantello, G.; di Benedetto, M.; Lupattelli, M.; Palazzari, E.; Belgioia, L.; Bacigalupo, A.; et al. Time to surgery and pathologic complete response after neoadjuvant chemoradiation in rectal cancer: A population study on 2094 patients. Clin. Transl. Radiat. Oncol. 2017, 4, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Erlandsson, J.; Holm, T.; Pettersson, D.; Berglund, A.; Cedermark, B.; Radu, C.; Johansson, H.; Machado, M.; Hjern, F.; Hallböök, O.; et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): A multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. 2017, 18, 336–346. [Google Scholar] [CrossRef]
- Narang, A.K.; Meyer, J. Neoadjuvant Short-Course Radiation Therapy for Rectal Cancer: Trends and Controversies. Curr. Oncol. Rep. 2018, 20, 68. [Google Scholar] [CrossRef]
- Qiaoli, W.; Yongping, H.; Wei, X.; Guoqiang, X.; Yunhe, J.; Qiuyan, L.; Cheng, L.; Mengling, G.; Jiayi, L.; Yi, Y. Preoperative short-course radiotherapy (5 × 5 Gy) with delayed surgery versus preoperative long-course radiotherapy for locally resectable rectal cancer: A meta-analysis. Int. J. Color. Dis. 2019, 34, 2171–2183. [Google Scholar] [CrossRef] [PubMed]
- Bahadoer, R.R.; Dijkstra, E.A.; van Etten, B.; Marijnen, C.A.M.; Putter, H.; Kranenbarg, E.M.-K.; Roodvoets, A.G.H.; Nagtegaal, I.D.; Beets-Tan, R.G.H.; Blomqvist, L.K.; et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 29–42. [Google Scholar] [CrossRef]
- Conroy, T.; Bosset, J.-F.; Etienne, P.-L.; Rio, E.; François, E.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouché, O.; Gargot, D.; et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 702–715. [Google Scholar] [CrossRef]
- Oronsky, B.; Reid, T.; Larson, C.; Knox, S.J. Locally advanced rectal cancer: The past, present, and future. Semin. Oncol. 2020, 47, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Hoendervangers, S.; Couwenberg, A.M.; Intven, M.P.; van Grevenstein, W.M.; Verkooijen, H.M. Comparison of pathological complete response rates after neoadjuvant short-course radiotherapy or chemoradiation followed by delayed surgery in locally advanced rectal cancer. Eur. J. Surg. Oncol. 2018, 44, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-L.; Ji, J.-F.; Cai, Y.; Li, X.-F.; Li, Y.-H.; Wu, H.; Xu, B.; Dou, F.-Y.; Li, Z.-Y.; Bu, Z.-D.; et al. Preoperative concomitant boost intensity-modulated radiotherapy with oral capecitabine in locally advanced mid-low rectal cancer: A phase II trial. Radiother. Oncol. 2012, 102, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Trevisan, F.; Cabiddu, M.; Sgroi, G.; Bruschieri, L.; Rausa, E.; Ghidini, M.; Turati, L. Total Neoadjuvant Therapy in Rectal Cancer: A Systematic Review and Meta-analysis of Treatment Outcomes. Ann. Surg. 2020, 271, 440–448. [Google Scholar] [CrossRef]
- Chessin, D.B.; Enker, W.; Cohen, A.M.; Paty, P.B.; Weiser, M.R.; Saltz, L.; Minsky, B.D.; Wong, W.D.; Guillem, J.G. Complications after Preoperative Combined Modality Therapy and Radical Resection of Locally Advanced Rectal Cancer: A 14-Year Experience from a Specialty Service. J. Am. Coll. Surg. 2005, 200, 876–882. [Google Scholar] [CrossRef]
- Dewdney, A.; Cunningham, D. Toward the Non-surgical Management of Locally Advanced Rectal Cancer. Curr. Oncol. Rep. 2012, 14, 267–276. [Google Scholar] [CrossRef]
- Hammarström, S. The carcinoembryonic antigen (CEA) family: Structures, suggested functions and expression in normal and malignant tissues. Semin. Cancer Biol. 1999, 9, 67–81. [Google Scholar] [CrossRef]
- Tiernan, J.P.; Perry, S.L.; Verghese, E.T.; West, N.; Yeluri, S.; Jayne, D.G.; Hughes, T. Carcinoembryonic antigen is the preferred biomarker for in vivo colorectal cancer targeting. Br. J. Cancer 2013, 108, 662–667. [Google Scholar] [CrossRef]
- Saito, G.; Sadahiro, S.; Kamata, H.; Miyakita, H.; Okada, K.; Tanaka, A.; Suzuki, T. Monitoring of Serum Carcinoembryonic Antigen Levels after Curative Resection of Colon Cancer: Cutoff Values Determined according to Preoperative Levels Enhance the Diagnostic Accuracy for Recurrence. Oncology 2017, 92, 276–282. [Google Scholar] [CrossRef]
- Shen, D.; Wang, X.; Wang, H.; Xu, G.; Xie, Y.; Zhuang, Z.; Li, J.; Lin, J.; Wang, P.; Huang, M.; et al. Current Surveillance After Treatment is Not Sufficient for Patients With Rectal Cancer With Negative Baseline CEA. J. Natl. Compr. Cancer Netw. 2022, 20, 653–662.e3. [Google Scholar] [CrossRef]
- Engel, R.M.; Oliva, K.; Koulis, C.; Yap, R.; McMurrick, P.J. Predictive factors of complete pathological response in patients with locally advanced rectal cancer. Int. J. Color. Dis. 2020, 35, 1759–1767. [Google Scholar] [CrossRef] [PubMed]
- Aires, F.; Rodrigues, D.; Lamas, M.P.; Herdeiro, M.T.; Figueiras, A.; Oliveira, M.J.; Marques, M.; Pinto, A.T. C-Reactive Protein as Predictive Biomarker for Response to Chemoradiotherapy in Patients with Locally Advanced Rectal Cancer: A Retrospective Study. Cancers 2022, 14, 491. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Huang, X.; Chen, Z.; Chen, M.; Lin, Q.; Li, A.; Chen, Y.; Xu, B. Predictive value of carcinoembryonic antigen and carbohydrate antigen 19-9 related to downstaging to stage 0–I after neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Cancer Manag. Res. 2018, 10, 3101–3108. [Google Scholar] [CrossRef] [PubMed]
- Jang, N.Y.; Kang, S.-B.; Kim, D.-W.; Kim, J.H.; Lee, K.-W.; Kim, I.A.; Kim, J.-S. The Role of Carcinoembryonic Antigen After Neoadjuvant Chemoradiotherapy in Patients With Rectal Cancer. Dis. Colon Rectum 2011, 54, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Chun, M.; Noh, O.K.; Oh, Y.-T.; Suh, K.W.; Park, J.E.; Cho, O. Sustaining Blood Lymphocyte Count during Preoperative Chemoradiotherapy as a Predictive Marker for Pathologic Complete Response in Locally Advanced Rectal Cancer. Cancer Res. Treat. 2016, 48, 232–239. [Google Scholar] [CrossRef]
- Yeo, S.-G.; Kim, D.Y.; Kim, T.H.; Kim, S.Y.; Baek, J.Y.; Chang, H.J.; Park, J.W.; Oh, J.H. Carbohydrate antigen 19-9 levels associated with pathological responses to preoperative chemoradiotherapy in rectal cancer. Asian Pac. J. Cancer Prev. 2014, 15, 5383–5387. [Google Scholar] [CrossRef]
- Kitayama, J.; Yasuda, K.; Kawai, K.; Sunami, E.; Nagawa, H. Circulating lymphocyte number has a positive association with tumor response in neoadjuvant chemoradiotherapy for advanced rectal cancer. Radiat. Oncol. 2010, 5, 47. [Google Scholar] [CrossRef]
- Kim, H.J.; Choi, G.-S.; Park, J.S.; Park, S.; Kawai, K.; Watanabe, T. Clinical Significance of Thrombocytosis Before Preoperative Chemoradiotherapy in Rectal Cancer: Predicting Pathologic Tumor Response and Oncologic Outcome. Ann. Surg. Oncol. 2015, 22, 513–519. [Google Scholar] [CrossRef]
- Choi, E.; Kim, J.H.; Kim, O.B.; Kim, M.Y.; Oh, Y.K.; Baek, S.G. Predictors of pathologic complete response after preoperative concurrent chemoradiotherapy of rectal cancer: A single center experience. Radiat. Oncol. J. 2016, 34, 106–112. [Google Scholar] [CrossRef]
- Yang, J.; Ling, X.; Tang, W.; Hu, D.; Zhou, H.; Yin, G. Analyses of predictive factors for pathological complete remission in neoadjuvant therapy for locally advanced rectal cancer. J. B. U. ON. 2019, 24, 77–83. [Google Scholar]
- Cheong, C.; Shin, J.S.; Suh, K.W. Prognostic value of changes in serum carcinoembryonic antigen levels for preoperative chemoradiotherapy response in locally advanced rectal cancer. World J. Gastroenterol. 2020, 26, 7022–7035. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-M.; Huang, C.-W.; Ma, C.-J.; Yeh, Y.-S.; Su, W.-C.; Chang, T.-K.; Tsai, H.-L.; Juo, S.-H.; Huang, M.-Y.; Wang, J.-Y. Predictive Value of FOLFOX-Based Regimen, Long Interval, Hemoglobin Levels and Clinical Negative Nodal Status, and Postchemoradiotherapy CEA Levels for Pathological Complete Response in Patients with Locally Advanced Rectal Cancer after Neoadjuvant Chemoradiotherapy. J. Oncol. 2020, 2020, 9437684. [Google Scholar] [CrossRef] [PubMed]
- Gago, T.; Caldeira, P.; Cunha, C.; Campelo, P.; Guerreiro, H. Can we optimize the CEA as a response marker in rectal cancer? Rev. Esp. Enferm. Dig. 2020, 113, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.-P.; Chen, C.; Zeng, Z.-F.; Wang, Q.-X.; Jiang, W.; Gao, Y.-H.; Chang, H. Serum Apolipoprotein A-I Predicts Response of Rectal Cancer to Neoadjuvant Chemoradiotherapy. Cancer Manag. Res. 2021, 13, 2623–2631. [Google Scholar] [CrossRef]
- Zhang, Q.; Liang, J.; Chen, J.; Mei, S.; Wang, Z. Predictive Factors for Pathologic Complete Response Following Neoadjuvant Chemoradiotherapy for Rectal Cancer. Asian Pac. J. Cancer Prev. 2021, 22, 1607–1611. [Google Scholar] [CrossRef]
- Wada, Y.; Shimada, M.; Morine, Y.; Ikemoto, T.; Saito, Y.; Zhu, Z.; Wang, X.; Etxart, A.; Park, Y.; Bujanda, L.; et al. Circulating miRNA Signature Predicts Response to Preoperative Chemoradiotherapy in Locally Advanced Rectal Cancer. JCO Precis. Oncol. 2021, 5, 1788–1801. [Google Scholar] [CrossRef]
- Murahashi, S.; Akiyoshi, T.; Sano, T.; Fukunaga, Y.; Noda, T.; Ueno, M.; Zembutsu, H. Serial circulating tumour DNA analysis for locally advanced rectal cancer treated with preoperative therapy: Prediction of pathological response and postoperative recurrence. Br. J. Cancer 2020, 123, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Sawada, R.; Akiyoshi, T.; Kitagawa, Y.; Hiyoshi, Y.; Mukai, T.; Nagasaki, T.; Yamaguchi, T.; Konishi, T.; Yamamoto, N.; Ueno, M.; et al. Systemic Inflammatory Markers Combined with Tumor-Infiltrating Lymphocyte Density for the Improved Prediction of Response to Neoadjuvant Chemoradiotherapy in Rectal Cancer. Ann. Surg. Oncol. 2021, 28, 6189–6198. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Huang, L.; Chen, Y.; Xie, X.; Zou, Y.; Lan, P.; Wu, X. CEA Decline Predicts Tumor Regression and Prognosis in Locally Advanced Rectal Cancer Patients with Elevated Baseline CEA. J. Cancer 2020, 11, 6565–6570. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.-L.; Yang, S.-H.; Liang, W.-Y.; Kuo, Y.-J.; Lin, J.-K.; Lin, T.-C.; Chen, W.-S.; Jiang, J.-K.; Wang, H.-S.; Chang, S.-C.; et al. Carcinoembryonic antigen (CEA) level, CEA ratio, and treatment outcome of rectal cancer patients receiving pre-operative chemoradiation and surgery. Radiat. Oncol. 2013, 8, 43. [Google Scholar] [CrossRef]
- Tawfik, B.; Mokdad, A.A.; Patel, P.M.; Li, H.C.; Huerta, S. The neutrophil to albumin ratio as a predictor of pathological complete response in rectal cancer patients following neoadjuvant chemoradiation. Anti-Cancer Drugs 2016, 27, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Restivo, A.; Zorcolo, L.; Cocco, I.M.F.; Manunza, R.; Margiani, C.; Marongiu, L.; Casula, G. Elevated CEA Levels and Low Distance of the Tumor from the Anal Verge are Predictors of Incomplete Response to Chemoradiation in Patients with Rectal Cancer. Ann. Surg. Oncol. 2013, 20, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Huang, J.; Lan, P.; Wang, L.; Huang, M.; Wang, J.; Deng, Y. CEA clearance pattern as a predictor of tumor response to neoadjuvant treatment in rectal cancer: A post-hoc analysis of FOWARC trial. BMC Cancer 2018, 18, 1145. [Google Scholar] [CrossRef]
- Deng, Y.; Chi, P.; Lan, P.; Wang, L.; Chen, W.; Cui, L.; Chen, D.; Cao, J.; Wei, H.; Peng, X.; et al. Modified FOLFOX6 With or Without Radiation Versus Fluorouracil and Leucovorin With Radiation in Neoadjuvant Treatment of Locally Advanced Rectal Cancer: Initial Results of the Chinese FOWARC Multicenter, Open-Label, Randomized Three-Arm Phase III Trial. J. Clin. Oncol. 2016, 34, 3300–3307. [Google Scholar] [CrossRef]
- Scarà, S.; Bottoni, P.; Scatena, R. CA 19-9: Biochemical and Clinical Aspects. In Advances in Cancer Biomarkers: From Biochemistry to Clinic for a Critical Revision; Scatena, R., Ed.; Springer: Dordrecht, Netherlands, 2015; pp. 247–260. [Google Scholar]
- Lee, T.; Teng, T.Z.J.; Shelat, V.G. Carbohydrate antigen 19-9—tumor marker: Past, present, and future. World J. Gastrointest. Surg. 2020, 12, 468–490. [Google Scholar] [CrossRef]
- Goonetilleke, K.S.; Siriwardena, A.K. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur. J. Surg. Oncol. 2007, 33, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Ducreux, M.; Cuhna, A.S.; Caramella, C.; Hollebecque, A.; Burtin, P.; Goéré, D.; Seufferlein, T.; Haustermans, K.; Van Laethem, J.L.; Conroy, T.; et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. S5), v56–v68. [Google Scholar] [CrossRef] [PubMed]
- Buergy, D.; Wenz, F.; Groden, C.; Brockmann, M.A. Tumor-platelet interaction in solid tumors. Int. J. Cancer 2012, 130, 2747–2760. [Google Scholar] [CrossRef]
- Brown, K.M.; Domin, C.; Aranha, G.V.; Yong, S.; Shoup, M. Increased preoperative platelet count is associated with decreased survival after resection for adenocarcinoma of the pancreas. Am. J. Surg. 2005, 189, 278–282. [Google Scholar] [CrossRef]
- Tomita, M.; Shimizu, T.; Hara, M.; Ayabe, T.; Onitsuka, T. Prognostic impact of thrombocytosis in resectable non-small cell lung cancer. Interact. Cardiovasc. Thorac. Surg. 2008, 7, 613–615. [Google Scholar] [CrossRef]
- Krauthamer, M.; Rouvinov, K.; Ariad, S.; Man, S.; Walfish, S.; Pinsk, I.; Sztarker, I.; Charkovsky, T.; Lavrenkov, K. A Study of Inflammation-Based Predictors of Tumor Response to Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer. Oncology 2013, 85, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Robnett, T.J.; Machtay, M.; Hahn, S.M.; Shrager, J.B.; Friedberg, J.S.; Kaiser, L.R. Pathological Response to Preoperative Chemoradiation Worsens with Anemia in Non---Small Cell Lung Cancer Patients. Cancer J. 2002, 8, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Rakotomalala, A.; Escande, A.; Furlan, A.; Meignan, S.; Lartigau, E. Hypoxia in Solid Tumors: How Low Oxygenation Impacts the “Six Rs” of Radiotherapy. Front. Endocrinol. 2021, 12, 742215. [Google Scholar] [CrossRef] [PubMed]
- Pakravan, N. Tumorigenesis: Cell defense against hypoxia? Oncol. Rev. 2013, 7, e1. [Google Scholar] [CrossRef]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015, 3, 83–92. [Google Scholar] [CrossRef]
- Harrison, L.B.; Chadha, M.; Hill, R.J.; Hu, K.; Shasha, D. Impact of Tumor Hypoxia and Anemia on Radiation Therapy Outcomes. Oncologist 2002, 7, 492–508. [Google Scholar] [CrossRef]
- Berardi, R.; Braconi, C.; Mantello, G.; Scartozzi, M.; Del Prete, S.; Luppi, G.; Martinelli, R.; Fumagalli, M.; Valeri, G.; Bearzi, I.; et al. Anemia may influence the outcome of patients undergoing neo-adjuvant treatment of rectal cancer. Ann. Oncol. 2006, 17, 1661–1664. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Jung, S.W.; Park, I.J.; Oh, S.H.; Yeom, S.-S.; Lee, J.L.; Yoon, Y.S.; Kim, C.W.; Lim, S.-B.; Lee, J.B.; Yu, C.S.; et al. Association of immunologic markers from complete blood counts with the response to preoperative chemoradiotherapy and prognosis in locally advanced rectal cancer. Oncotarget 2017, 8, 59757–59765. [Google Scholar] [CrossRef]
- Dreyer, S.B.; Powell, A.G.M.T.; McSorley, S.T.; Waterston, A.; Going, J.J.; Edwards, J.; McMillan, D.C.; Horgan, P.G. The Pretreatment Systemic Inflammatory Response is an Important Determinant of Poor Pathologic Response for Patients Undergoing Neoadjuvant Therapy for Rectal Cancer. Ann. Surg. Oncol. 2017, 24, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Moman, R.N.; Gupta, N.; Varacallo, M. Physiology, Albumin; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Akirov, A.; Masri-Iraqi, H.; Atamna, A.; Shimon, I. Low Albumin Levels Are Associated with Mortality Risk in Hospitalized Patients. Am. J. Med. 2017, 130, 1465.e11–1465.e19. [Google Scholar] [CrossRef] [PubMed]
- Caraceni, P.; Tufoni, M.; Bonavita, M.E. Clinical use of albumin. Blood Transfus. 2013, 11 (Suppl. S4), s18–s25. [Google Scholar] [CrossRef] [PubMed]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Volanakis, J.E. Human C-reactive protein: Expression, structure, and function. Mol. Immunol. 2001, 38, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Wang, S.; Long, G. C-reactive protein is a significant predictor of improved survival in patients with advanced non-small cell lung cancer. Medicine 2019, 98, e16238. [Google Scholar] [CrossRef] [PubMed]
- Okugawa, Y.; Toiyama, Y.; Yamamoto, A.; Shigemori, T.; Ide, S.; Kitajima, T.; Fujikawa, H.; Yasuda, H.; Hiro, J.; Yoshiyama, S.; et al. Lymphocyte-C-reactive Protein Ratio as Promising New Marker for Predicting Surgical and Oncological Outcomes in Colorectal Cancer. Ann. Surg. 2020, 272, 342–351. [Google Scholar] [CrossRef]
- Petrelli, F.; Barni, S.; Coinu, A.; Bertocchi, P.; Borgonovo, K.; Cabiddu, M.; Ghilardi, M.; Zaniboni, A. The Modified Glasgow Prognostic Score and Survival in Colorectal Cancer: A Pooled Analysis of the Literature. Rev. Recent Clin. Trials 2015, 10, 135–141. [Google Scholar] [CrossRef]
- Choi, K.W.; Hong, S.W.; Chang, Y.G.; Lee, W.Y.; Lee, B.; Paik, I.W.; Lee, H. Inflammation-based score (Glasgow prognostic score) as an independent prognostic factor in colorectal cancer patients. Ann. Surg. Treat. Res. 2014, 86, 309–313. [Google Scholar] [CrossRef]
- Tong, T.; Guan, Y.; Xiong, H.; Wang, L.; Pang, J. A Meta-Analysis of Glasgow Prognostic Score and Modified Glasgow Prognostic Score as Biomarkers for Predicting Survival Outcome in Renal Cell Carcinoma. Front. Oncol. 2020, 10, 1541. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Yang, W.-X.; Dou, W.-C.; Shao, Y.-X.; Li, X. Modified Glasgow prognostic score as a prognostic factor for renal cell carcinomas: A systematic review and meta-analysis. Cancer Manag. Res. 2019, 11, 6163–6173. [Google Scholar] [CrossRef] [PubMed]
- Zahorec, R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl Lek Listy 2021, 122, 474–488. [Google Scholar] [CrossRef] [PubMed]
- Bowen, R.C.; Little, N.A.B.; Harmer, J.R.; Ma, J.; Mirabelli, L.G.; Roller, K.D.; Breivik, A.M.; Signor, E.; Miller, A.B.; Khong, H.T. Neutrophil-to-lymphocyte ratio as prognostic indicator in gastrointestinal cancers: A systematic review and meta-analysis. Oncotarget 2017, 8, 32171–32189. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, G.J.K.; Charles, K.A.; Roxburgh, C.S.D.; Horgan, P.G.; McMillan, D.C.; Clarke, S.J. The systemic inflammation-based neutrophil–lymphocyte ratio: Experience in patients with cancer. Crit. Rev. Oncol. Hematol. 2013, 88, 218–230. [Google Scholar] [CrossRef]
- Ferro, M.; Babă, D.-F.; de Cobelli, O.; Musi, G.; Lucarelli, G.; Terracciano, D.; Porreca, A.; Busetto, G.M.; Del Giudice, F.; Soria, F.; et al. Neutrophil percentage-to-albumin ratio predicts mortality in bladder cancer patients treated with neoadjuvant chemotherapy followed by radical cystectomy. Future Sci. OA 2021, 7, FSO709. [Google Scholar] [CrossRef]
- Li, R.; Sun, Z.; Song, S.; He, X.; Shi, X.; Li, Z.; Song, J. NARFIB: A Novel Prognostic Score Based on the Neutrophil-to-Albumin Ratio and Fibrinogen Can Predict the Prognosis of Gastrointestinal Stromal Tumors. Cancer Manag. Res. 2020, 12, 11183–11190. [Google Scholar] [CrossRef]
- Tingle, S.J.; Ma, G.R.S.; Goodfellow, M.; Moir, J.A.; White, S.A. NARCA: A novel prognostic scoring system using neutrophil-albumin ratio and Ca19-9 to predict overall survival in palliative pancreatic cancer. J. Surg. Oncol. 2018, 118, 680–686. [Google Scholar] [CrossRef]
- Goto, W.; Kashiwagi, S.; Asano, Y.; Takada, K.; Takahashi, K.; Hatano, T.; Takashima, T.; Tomita, S.; Motomura, H.; Hirakawa, K.; et al. Predictive value of lymphocyte-to-monocyte ratio in the preoperative setting for progression of patients with breast cancer. BMC Cancer 2018, 18, 1137. [Google Scholar] [CrossRef]
- Hu, P.; Shen, H.; Wang, G.; Zhang, P.; Liu, Q.; Du, J. Prognostic Significance of Systemic Inflammation-Based Lymphocyte- Monocyte Ratio in Patients with Lung Cancer: Based on a Large Cohort Study. PLoS ONE 2014, 9, e108062. [Google Scholar] [CrossRef]
- Templeton, A.J.; Ace, O.; McNamara, M.G.; Al-Mubarak, M.; Vera-Badillo, F.E.; Hermanns, T.; Šeruga, B.; Ocaña, A.; Tannock, I.F.; Amir, E. Prognostic Role of Platelet to Lymphocyte Ratio in Solid Tumors: A Systematic Review and Meta-Analysis. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1204–1212. [Google Scholar] [CrossRef]
- Takamizawa, Y.; Shida, D.; Boku, N.; Nakamura, Y.; Ahiko, Y.; Yoshida, T.; Tanabe, T.; Takashima, A.; Kanemitsu, Y. Nutritional and inflammatory measures predict survival of patients with stage IV colorectal cancer. BMC Cancer 2020, 20, 1092. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Huang, Y. Lipid Signaling in Tumorigenesis. Mol. Cell. Pharmacol. 2014, 6, 1–9. [Google Scholar] [PubMed]
- van Meeteren, L.A.; Moolenaar, W.H. Regulation and biological activities of the autotaxin–LPA axis. Prog. Lipid Res. 2007, 46, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Corn, K.C.; Windham, M.A.; Rafat, M. Lipids in the tumor microenvironment: From cancer progression to treatment. Prog. Lipid Res. 2020, 80, 101055. [Google Scholar] [CrossRef]
- Reimers, N.; Pantel, K. Liquid biopsy: Novel technologies and clinical applications. Clin. Chem. Lab. Med. 2019, 57, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Pantel, K.; Alix-Panabières, C. Circulating tumour cells in cancer patients: Challenges and perspectives. Trends Mol. Med. 2010, 16, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Alix-Panabières, C.; Pantel, K. Liquid Biopsy: From Discovery to Clinical Application. Cancer Discov. 2021, 11, 858–873. [Google Scholar] [CrossRef]
- Morais, M.; Pinto, D.M.; Machado, J.C.; Carneiro, S. ctDNA on liquid biopsy for predicting response and prognosis in locally advanced rectal cancer: A systematic review. Eur. J. Surg. Oncol. 2021, 48, 218–227. [Google Scholar] [CrossRef]
- De Palma, F.D.E.; Luglio, G.; Tropeano, F.P.; Pagano, G.; D’Armiento, M.; Kroemer, G.; Maiuri, M.C.; De Palma, G.D. The Role of Micro-RNAs and Circulating Tumor Markers as Predictors of Response to Neoadjuvant Therapy in Locally Advanced Rectal Cancer. Int. J. Mol. Sci. 2020, 21, 7040. [Google Scholar] [CrossRef]
- Mandel, P.; Metais, P. Nuclear Acids In Human Blood Plasma. C. R. Seances Soc. Biol. Fil. 1948, 142, 241–243. [Google Scholar]
- Duvvuri, B.; Lood, C. Cell-Free DNA as a Biomarker in Autoimmune Rheumatic Diseases. Front. Immunol. 2019, 10, 502. [Google Scholar] [CrossRef] [PubMed]
- Fettke, H.; Kwan, E.M.; Azad, A.A. Cell-free DNA in cancer: Current insights. Cell. Oncol. 2019, 42, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S. Apoptotic DNA Fragmentation. Exp. Cell Res. 2000, 256, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Fleischhacker, M.; Schmidt, B. Circulating nucleic acids (CNAs) and cancer—A survey. Biochim. Biophys. Acta 2007, 1775, 181–232. [Google Scholar] [CrossRef] [PubMed]
- Bronkhorst, A.J.; Wentzel, J.F.; Aucamp, J.; van Dyk, E.; du Plessis, L.; Pretorius, P.J. Characterization of the cell-free DNA released by cultured cancer cells. Biochim. Biophys. Acta 2016, 1863, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.M.D.; Zhang, J.; Leung, T.N.; Lau, T.K.; Chang, A.M.; Hjelm, N.M. Rapid Clearance of Fetal DNA from Maternal Plasma. Am. J. Hum. Genet. 1999, 64, 218–224. [Google Scholar] [CrossRef]
- Bryzgunova, O.E.; Laktionov, P.P. Extracellular Nucleic Acids in Urine: Sources, Structure, Diagnostic Potential. Acta Nat. 2015, 7, 48–54. [Google Scholar] [CrossRef]
- Zitt, M.; Müller, H.M.; Rochel, M.; Schwendinger, V.; Zitt, M.; Goebel, G.; DeVries, A.; Margreiter, R.; Oberwalder, M.; Zeillinger, R.; et al. Circulating Cell-Free DNA in Plasma of Locally Advanced Rectal Cancer Patients Undergoing Preoperative Chemoradiation: A Potential Diagnostic Tool for Therapy Monitoring. Dis. Markers 2008, 25, 159–165. [Google Scholar] [CrossRef]
- Agostini, M.; Pucciarelli, S.; Enzo, M.V.; Del Bianco, P.; Briarava, M.; Bedin, C.; Maretto, I.; Friso, M.L.; Lonardi, S.; Mescoli, C.; et al. Circulating Cell-Free DNA: A Promising Marker of Pathologic Tumor Response in Rectal Cancer Patients Receiving Preoperative Chemoradiotherapy. Ann. Surg. Oncol. 2011, 18, 2461–2468. [Google Scholar] [CrossRef]
- Sun, W.; Sun, Y.; Zhu, M.; Wang, Z.; Zhang, H.; Xin, Y.; Jiang, G.; Guo, X.; Zhang, Z.; Liu, Y. The role of plasma cell-free DNA detection in predicting preoperative chemoradiotherapy response in rectal cancer patients. Oncol. Rep. 2014, 31, 1466–1472. [Google Scholar] [CrossRef]
- Schou, J.; Larsen, F.; Sørensen, B.; Abrantes, R.; Boysen, A.; Johansen, J.; Jensen, B.; Nielsen, D.; Spindler, K. Circulating cell-free DNA as predictor of treatment failure after neoadjuvant chemo-radiotherapy before surgery in patients with locally advanced rectal cancer. Ann. Oncol. 2018, 29, 610–615. [Google Scholar] [CrossRef]
- Shalaby, S.M.; El-Shal, A.S.; Abdelaziz, L.A.; Abd-Elbary, E.; Khairy, M.M. Promoter methylation and expression of DNA repair genes MGMT and ERCC1 in tissue and blood of rectal cancer patients. Gene 2018, 644, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Huang, H.-Y.; Chen, Y.-C.; Bristow, R.E.; Kassauei, K.; Cheng, C.-C.; Roden, R.; Sokoll, L.J.; Chan, D.W.; Shih, I.-M. Increased plasma DNA integrity in cancer patients. Cancer Res 2003, 63, 3966–3968. [Google Scholar] [PubMed]
- Stroun, M.; Anker, P.; Maurice, P.; Lyautey, J.; Lederrey, C.; Beljanski, M. Neoplastic Characteristics of the DNA Found in the Plasma of Cancer Patients. Oncology 1989, 46, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Volik, S.; Alcaide, M.; Morin, R.D.; Collins, C.C. Cell-free DNA (cfDNA): Clinical Significance and Utility in Cancer Shaped By Emerging Technologies. Mol. Cancer Res. 2016, 14, 898–908. [Google Scholar] [CrossRef]
- Pazdirek, F.; Minarik, M.; Benesova, L.; Halkova, T.; Belsanova, B.; Macek, M.; Stepanek, L.; Hoch, J. Monitoring of Early Changes of Circulating Tumor DNA in the Plasma of Rectal Cancer Patients Receiving Neoadjuvant Concomitant Chemoradiotherapy: Evaluation for Prognosis and Prediction of Therapeutic Response. Front. Oncol. 2020, 10, 1028. [Google Scholar] [CrossRef]
- Carpinetti, P.; Donnard, E.; Bettoni, F.; Asprino, P.; Koyama, F.; Rozanski, A.; Sabbaga, J.; Habr-Gama, A.; Parmigiani, R.B.; Galante, P.A.; et al. The use of personalized biomarkers and liquid biopsies to monitor treatment response and disease recurrence in locally advanced rectal cancer after neoadjuvant chemoradiation. Oncotarget 2015, 6, 38360–38371. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, C.; Lin, G.; Xiao, Y.; Jia, W.; Xiao, G.; Liu, Q.; Wu, B.; Wu, A.; Qiu, H.; et al. Serial Circulating Tumor DNA in Predicting and Monitoring the Effect of Neoadjuvant Chemoradiotherapy in Patients with Rectal Cancer: A Prospective Multicenter Study. Clin. Cancer Res. 2021, 27, 301–310. [Google Scholar] [CrossRef]
- McDuff, S.G.R.; Hardiman, K.M.; Ulintz, P.J.; Parikh, A.R.; Zheng, H.; Kim, D.W.; Lennerz, J.K.; Hazar-Rethinam, M.; Van Seventer, E.E.; Fetter, I.J.; et al. Circulating Tumor DNA Predicts Pathologic and Clinical Outcomes Following Neoadjuvant Chemoradiation and Surgery for Patients With Locally Advanced Rectal Cancer. JCO Precis. Oncol. 2021, 5, 123–132. [Google Scholar] [CrossRef]
- Khakoo, S.; Carter, P.D.; Brown, G.; Valeri, N.; Picchia, S.; Bali, M.A.; Shaikh, R.; Jones, T.; Begum, R.; Rana, I.; et al. MRI Tumor Regression Grade and Circulating Tumor DNA as Complementary Tools to Assess Response and Guide Therapy Adaptation in Rectal Cancer. Clin. Cancer Res. 2020, 26, 183–192. [Google Scholar] [CrossRef]
- Sclafani, F.; Chau, I.; Cunningham, D.; Hahne, J.C.; Vlachogiannis, G.; Eltahir, Z.; Lampis, A.; Braconi, C.; Kalaitzaki, E.; De Castro, D.G.; et al. KRAS and BRAF mutations in circulating tumour DNA from locally advanced rectal cancer. Sci. Rep. 2018, 8, 1445. [Google Scholar] [CrossRef] [PubMed]
- Appelt, A.L.; Andersen, R.F.; Lindebjerg, J.; Jakobsen, A. Prognostic Value of Serum NPY Hypermethylation in Neoadjuvant Chemoradiotherapy for Rectal Cancer: Secondary Analysis of a Randomized Trial. Am. J. Clin. Oncol. 2020, 43, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Tie, J.; Cohen, J.D.; Wang, Y.; Li, L.; Christie, M.; Simons, K.; Elsaleh, H.; Kosmider, S.; Wong, R.; Yip, D.; et al. Serial circulating tumour DNA analysis during multimodality treatment of locally advanced rectal cancer: A prospective biomarker study. Gut 2019, 68, 663–671. [Google Scholar] [CrossRef]
- Ying, W.; Gao, H.; Dos Reis, F.C.G.; Bandyopadhyay, G.; Ofrecio, J.M.; Luo, Z.; Ji, Y.; Jin, Z.; Ly, C.; Olefsky, J.M. MiR-690, an exosomal-derived miRNA from M2-polarized macrophages, improves insulin sensitivity in obese mice. Cell Metab. 2021, 33, 781–790.e5. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, M.; Paone, A.; Calore, F.; Galli, R.; Gaudio, E.; Santhanam, R.; Lovat, F.; Fadda, P.; Mao, C.; Nuovo, G.J.; et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc. Natl. Acad. Sci. USA 2012, 109, E2110–E2116. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Q.; Zhang, R.; Dai, X.; Chen, W.; Xing, D. Circulating microRNAs: Biomarkers of disease. Clin. Chim. Acta 2021, 516, 46–54. [Google Scholar] [CrossRef]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef] [PubMed]
- Dilsiz, N. Role of exosomes and exosomal microRNAs in cancer. Future Sci. OA 2020, 6, FSO465. [Google Scholar] [CrossRef]
- Bhome, R.; Del Vecchio, F.; Lee, G.-H.; Bullock, M.D.; Primrose, J.N.; Sayan, A.E.; Mirnezami, A.H. Exosomal microRNAs (exomiRs): Small molecules with a big role in cancer. Cancer Lett. 2018, 420, 228–235. [Google Scholar] [CrossRef]
- Svoboda, M.; Holla, L.I.; Sefr, R.; Vrtkova, I.; Kocakova, I.; Tichy, B.; Dvorak, J. Micro-RNAs miR125b and miR137 are frequently upregulated in response to capecitabine chemoradiotherapy of rectal cancer. Int. J. Oncol. 2008, 33, 541–547. [Google Scholar] [CrossRef]
- Della Vittoria Scarpati, G.; Falcetta, F.; Carlomagno, C.; Ubezio, P.; Marchini, S.; De Stefano, A.; Singh, V.K.; D’Incalci, M.; De Placido, S.; Pepe, S. A Specific miRNA Signature Correlates With Complete Pathological Response to Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Kheirelseid, E.A.H.; Miller, N.; Chang, K.H.; Curran, C.; Hennessey, E.; Sheehan, M.; Newell, J.; Lemetre, C.; Balls, G.; Kerin, M.J. miRNA expressions in rectal cancer as predictors of response to neoadjuvant chemoradiation therapy. Int. J. Color. Dis. 2013, 28, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Dreussi, E.; Pucciarelli, S.; De Paoli, A.; Polesel, J.; Canzonieri, V.; Agostini, M.; Friso, M.L.; Belluco, C.; Buonadonna, A.; Lonardi, S.; et al. Predictive role of microRNA-related genetic polymorphisms in the pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer patients. Oncotarget 2016, 7, 19781–19793. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, E.; Fassan, M.; Maretto, I.; Pucciarelli, S.; Zanon, C.; Digito, M.; Rugge, M.; Nitti, D.; Agostini, M. Serum miR-125b is a non-invasive predictive biomarker of the pre-operative chemoradiotherapy responsiveness in patients with rectal adenocarcinoma. Oncotarget 2016, 7, 28647–28657. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Li, N.; Wang, X.; Ren, H.; Wang, W.; Wang, S.; Song, Y.; Liu, Y.; Li, Y.; Zhou, X.; et al. Circulating serum microRNA-345 correlates with unfavorable pathological response to preoperative chemoradiotherapy in locally advanced rectal cancer. Oncotarget 2016, 7, 64233–64243. [Google Scholar] [CrossRef]
- Azizian, A.; Kramer, F.; Jo, P.; Wolff, H.A.; Beißbarth, T.; Skarupke, R.; Bernhardt, M.; Grade, M.; Ghadimi, B.M.; Gaedcke, J. Preoperative Prediction of Lymph Node Status by Circulating Mir-18b and Mir-20a During Chemoradiotherapy in Patients with Rectal Cancer. World J. Surg. 2015, 39, 2329–2335. [Google Scholar] [CrossRef]
- Meltzer, S.; Bjørnetrø, T.; Lyckander, L.G.; Flatmark, K.; Dueland, S.; Samiappan, R.; Johansen, C.; Kalanxhi, E.; Ree, A.H.; Redalen, K.R. Circulating Exosomal miR-141-3p and miR-375 in Metastatic Progression of Rectal Cancer. Transl. Oncol. 2019, 12, 1038–1044. [Google Scholar] [CrossRef]
- Baek, D.W.; Kim, G.; Kang, B.W.; Kim, H.J.; Park, S.Y.; Park, J.S.; Choi, G.-S.; Kang, M.K.; Hur, K.; Kim, J.G. High expression of microRNA-199a-5p is associated with superior clinical outcomes in patients with locally advanced rectal cancer. J. Cancer Res. Clin. Oncol. 2020, 146, 105–115. [Google Scholar] [CrossRef]
- Hiyoshi, Y.; Akiyoshi, T.; Inoue, R.; Murofushi, K.; Yamamoto, N.; Fukunaga, Y.; Ueno, M.; Baba, H.; Mori, S.; Yamaguchi, T. Serum miR-143 levels predict the pathological response to neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer. Oncotarget 2017, 8, 79201–79211. [Google Scholar] [CrossRef]
- Bartkowiak, K.; Koch, C.; Gärtner, S.; Andreas, A.; Gorges, T.M.; Pantel, K. In Vitro Modeling of Reoxygenation Effects on mRNA and Protein Levels in Hypoxic Tumor Cells upon Entry into the Bloodstream. Cells 2020, 9, 1316. [Google Scholar] [CrossRef]
- Diehl, F.; Li, M.; Dressman, D.; He, Y.; Shen, D.; Szabo, S.; Diaz, L.A.; Goodman, S.N.; David, K.A.; Juhl, H.; et al. Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proc. Natl. Acad. Sci. USA 2005, 102, 16368–16373. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.A.; Binkley, M.S.; Osmundson, E.C.; Alizadeh, A.A.; Diehn, M. Predicting Radiotherapy Responses and Treatment Outcomes Through Analysis of Circulating Tumor DNA. Semin. Radiat. Oncol. 2015, 25, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Glimelius, B. On a prolonged interval between rectal cancer (chemo)radiotherapy and surgery. Upsala J. Med. Sci. 2017, 122, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Glimelius, B. What is most relevant in preoperative rectal cancer chemoradiotherapy—the chemotherapy, the radiation dose or the timing to surgery? Acta Oncol. 2016, 55, 1381–1385. [Google Scholar] [CrossRef] [PubMed]
- Nagtegaal, I.D.; Glynne-Jones, R. How to measure tumour response in rectal cancer? An explanation of discrepancies and suggestions for improvement. Cancer Treat. Rev. 2020, 84, 101964. [Google Scholar] [CrossRef]
- McMillan, D.C. The systemic inflammation-based Glasgow Prognostic Score: A decade of experience in patients with cancer. Cancer Treat. Rev. 2013, 39, 534–540. [Google Scholar] [CrossRef]
- Galamb, O.; Barták, B.K.; Kalmár, A.; Nagy, Z.B.; Szigeti, K.A.; Tulassay, Z.; Igaz, P.; Molnár, B. Diagnostic and prognostic potential of tissue and circulating long non-coding RNAs in colorectal tumors. World J. Gastroenterol. 2019, 25, 5026–5048. [Google Scholar] [CrossRef]
- Li, A.; Wang, W.C.; McAlister, V.; Zhou, Q.; Zheng, X. Circular RNA in colorectal cancer. J. Cell. Mol. Med. 2021, 25, 3667–3679. [Google Scholar] [CrossRef]
- Pulverer, W.; Kruusmaa, K.; Schönthaler, S.; Huber, J.; Bitenc, M.; Bachleitner-Hofmann, T.; Bhangu, J.S.; Oehler, R.; Egger, G.; Weinhäusel, A. Multiplexed DNA Methylation Analysis in Colorectal Cancer Using Liquid Biopsy and Its Diagnostic and Predictive Value. Curr. Issues Mol. Biol. 2021, 43, 1419–1435. [Google Scholar] [CrossRef]
- Biomarkers Definitions Working Group; Atkinson, A.J., Jr.; Colburn, W.A.; DeGruttola, V.G.; DeMets, D.L.; Downing, G.J.; Hoth, D.F.; Oates, J.A.; Peck, C.C.; Spilker, B.A.; et al. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef]
- Fratte, C.D.; Mezzalira, S.; Polesel, J.; De Mattia, E.; Palumbo, A.; Buonadonna, A.; Palazzari, E.; De Paoli, A.; Belluco, C.; Canzonieri, V.; et al. A Panel of Tumor Biomarkers to Predict Complete Pathological Response to Neoadjuvant Treatment in Locally Advanced Rectal Cancer. Oncol. Res. 2022, 28, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.; Creavin, B.; Sheahan, K. Delivery of Personalized Care for Locally Advanced Rectal Cancer: Incorporating Pathological, Molecular Genetic, and Immunological Biomarkers Into the Multimodal Paradigm. Front. Oncol. 2020, 10, 1369. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Kim, S.; Cho, M.; Hur, H.; Min, B.; Lee, K.; Lim, J.; Kim, N. The Accuracy of Mr Trg for Prediction of Tumor Response and Oncologic Outcomes in Rectal Cancer after Preop.Crt: Correlation with Pathologic Trg. Dis. Colon Rectum 2019, 62, E212. [Google Scholar] [CrossRef]
- Yi, Y.; Shen, L.; Shi, W.; Xia, F.; Zhang, H.; Wang, Y.; Zhang, J.; Wang, Y.; Sun, X.; Zhang, Z.; et al. Gut Microbiome Components Predict Response to Neoadjuvant Chemoradiotherapy in Patients with Locally Advanced Rectal Cancer: A Prospective, Longitudinal Study. Clin. Cancer Res. 2021, 27, 1329–1340. [Google Scholar] [CrossRef]
- Galon, J.; Mlecnik, B.; Bindea, G.; Angell, H.K.; Berger, A.; Lagorce, C.; Lugli, A.; Zlobec, I.; Hartmann, A.; Bifulco, C.; et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J. Pathol. 2014, 232, 199–209. [Google Scholar] [CrossRef]
- El Sissy, C.; Kirilovsky, A.; Van den Eynde, M.; Muşină, A.-M.; Anitei, M.-G.; Romero, A.M.S.; Marliot, F.; Junca, A.; Doyen, J.; Mlecnik, B.; et al. A Diagnostic Biopsy-Adapted Immunoscore Predicts Response to Neoadjuvant Treatment and Selects Patients with Rectal Cancer Eligible for a Watch-and-Wait Strategy. Clin. Cancer Res. 2020, 26, 5198–5207. [Google Scholar] [CrossRef]
- Chatila, W.K.; Kim, J.K.; Walch, H.; Marco, M.R.; Chen, C.-T.; Wu, F.; Omer, D.M.; Khalil, D.N.; Ganesh, K.; Qu, X.; et al. Genomic and transcriptomic determinants of response to neoadjuvant therapy in rectal cancer. Nat. Med. 2022, 28, 1646–1655. [Google Scholar] [CrossRef]

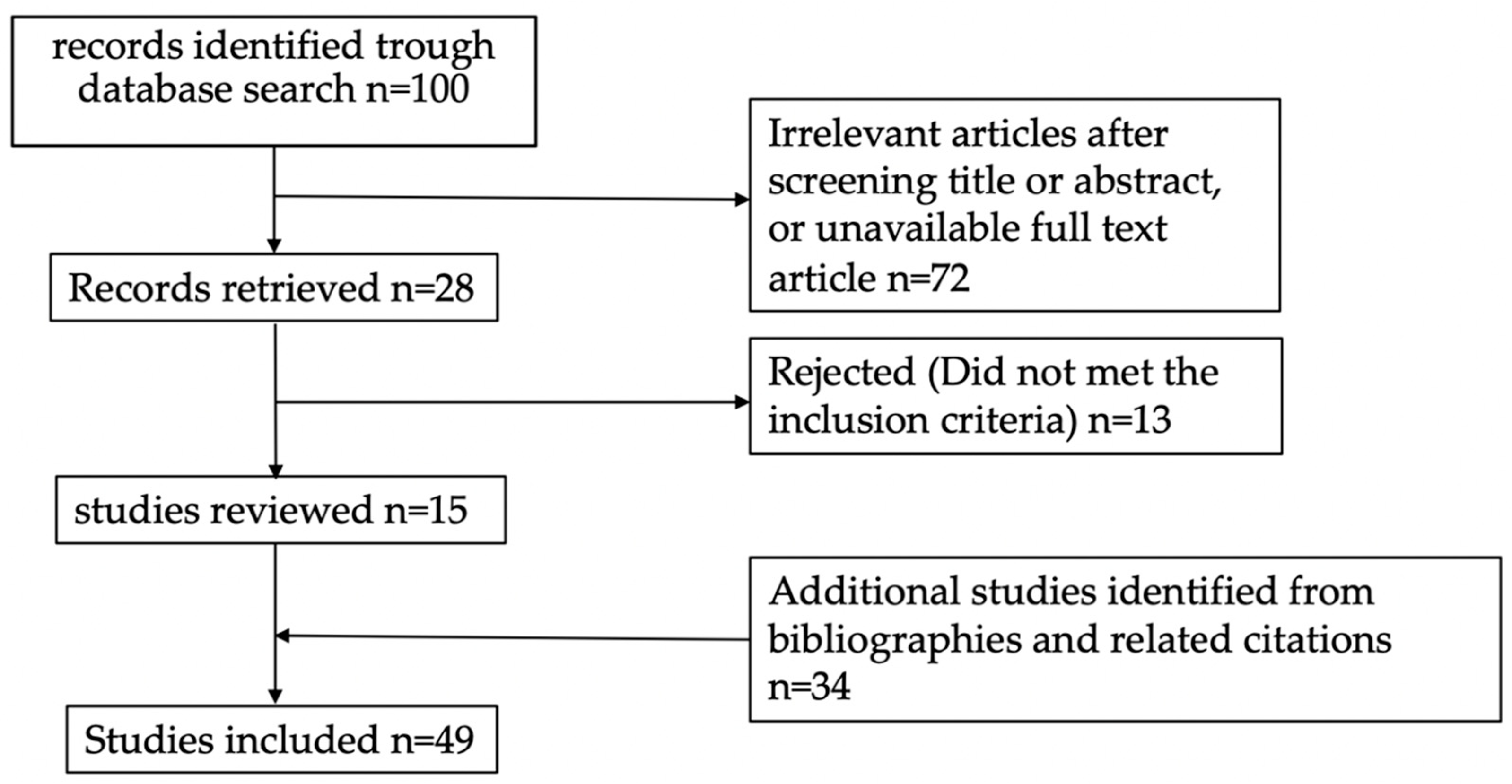
| Study | Cut-Off | N | Measured Outcome | Sn | Sp | VPP | VPN | p Value (Univariate Analysis) |
|---|---|---|---|---|---|---|---|---|
| pre-CRT | ||||||||
| Engel et al. [21] | 2.5 ng/mL | 209 | pCR | 59.5% | 65.3% | 30.1% | 86.5% | 0.004 |
| Aires et al. [22] | 2.7 ng/mL | 171 | TRG 0–1 (Rayan) | 43.6% | 75.4% | n/a | n/a | 0.0213 |
| Song et al. [23] | 2.85 ng/mL | 674 | pCR | 52.2% | 66.5% | 28.3% | 84.6% | <0.0001 |
| Jang et al. [24] | 3.5 ng/mL | 109 | TRG 3–4 (Dworak) | 66.6% | 53.1% | 50% | 69.4% | ns |
| Heo et al. [25] | 4.4 ng/mL | 52 | pCR | 14.3% | 36.8% | 7.7% | 53.8% | <0.01 * |
| Yeo et al. [26] | 5 ng/mL | 260 | Yp Stage 0–1 | 80.8% | 45.8% | 45.8% | 80.8% | <0.01 * |
| Kitayama et al. [27] | 5 ng/mL | 73 | pCR | 70% | 56.5% | 20.6% | 92.1% | ns |
| Kim et al. [28] | 5 ng/mL | 314 | TRG 3–4 (Dworak) | 81.1% | 41.8% | 16.4% | 94% | 0.007 * |
| Choi et al. [29] | 5 ng/mL | 53 | pCR | 63.6% | 45.3% | 23.3% | 86.4% | ns |
| Yang J. et al. [30] | 5 ng/mL | 531 | pCR | 66% | 50% | 23.5% | 86.4% | 0.021 * |
| Cheong et al. [31] | 5 ng/mL | 145 | pCR | 92.6% | 63% | 38.5% | 97.1% | <0.001* |
| Huang et al. [32] | 5 ng/mL | 236 | pCR | 71.4% | 42.2% | 27.8% | 82.6% | ns |
| Gago et al. [33] | 5 ng/mL | 89 | pCR | 63.2% | 43.5% | 25.5% | 79.4% | ns |
| Guo et al. [34] | 5 ng/mL | 751 | TRG 1–2 (Mandard) | 61.7% | 51.8% | 54.1% | 59.4% | 0.009 * |
| Zhang et al. [35] | 5 ng/mL | 432 | pCR | n/a | n/a | n/a | n/a | 0.001 * |
| Wada et al. [36] | 5 ng/mL | 106 | pCR | 72.2% | 30.1% | 18.3% | 83.3% | ns |
| Murahashi et al. [37] | 5 ng/mL | 85 | pCR | n/a | n/a | n/a | n/a | ns |
| Sawada et al. [38] | 5 ng/mL | 267 | TRG 3–4 (Dworak) | n/a | n/a | n/a | n/a | ns |
| Cai et al. [39] | 5 ng/mL | 284 | pCR | n/a | n/a | n/a | n/a | ns |
| Yang K.L. et al. [40] | 6 ng/mL | 138 | pCR | n/a | n/a | n/a | n/a | ns |
| Tawfik et al. [41] | n/a | 98 | pCR | n/a | n/a | n/a | n/a | 0.002 * |
| post-CRT pre-surgery | ||||||||
| Huang et al. [32] | 2 ng/mL | 236 | pCR | 16.1 % | 93.9% | 45% | 78.2% | 0.0285 * |
| Song et al. [23] | 2.45 ng/mL | 674 | pCR | 66.9% | 54.5% | 27.1% | 86.7% | <0.0001 * |
| Yang K.L. et al. [40] | 2.61 ng/mL | 138 | pCR | 76% | 58.4% | n/a | n/a | 0.026 * |
| Jang et al. [24] | 2.7 ng/mL | 109 | TRG 3–4 (Dworak) | 88.9% | 42.2% | 51.9% | 84.4% | <0.001 * |
| Choi et al. [29] | 5 ng/mL | 53 | pCR | 90.9% | 14.3% | 22.2% | 100% | ns |
| Cheong et al. [31] | 5 ng/mL | 135 | pCR | 100% | 25% | 25% | 100% | 0.008 * |
| Wada et al. [36] | 5 ng/mL | 106 | pCR | 94.7% | 4.9% | 18.8% | 80% | ns |
| Cai et al. [39] | 5 ng/mL | 284 | TRG 0–1 (NCCN) | n/a | n/a | n/a | n/a | <0.001 * |
| Restivo et al. [42] | 5 ng/mL | 260 | pCR | 95.3% | 32.3% | 21.8% | 97.2% | <0.0001 * |
| Hu et al. [43] | 5 ng/mL | 71 | pCR | 81.8% | 36.7% | 19.1% | 91.7% | ns |
| post-CRT/pre-CRT ratio | ||||||||
| Cai et al. [39] | 0.23 | 284 | TRG 0–1 (NCCN) | n/a | n/a | n/a | n/a | <0.001 * |
| pre-CRT/post-CRT ratio | ||||||||
| Song et al. [23] | 1.07 | 674 | pCR | 38.2% | 73.8% | 26.9% | 82.5% | 0.006 * |
| clearance pattern (R2) | ||||||||
| Hu et al. [43] | 0.9 | 71 | pCR | 81.8% | 63.3% | 29% | 95% | 0.008 * |
| pre-CRT/tumor size ratio | ||||||||
| Gago et al. [33] | 2.4 ng/mL per cm | 89 | pCR | 82.4% | 19.6% | 23.7% | 78.6% | 0.04 |
| Study | Cut-Off | N | Measure Outcome | Sn | Sp | VPP | VPN | p. Value (Univariate) |
|---|---|---|---|---|---|---|---|---|
| pre-CRT | ||||||||
| Kim et al. [28] | 370 G/L | 314 | pCR | 94.7% | 22.8% | 14.6% | 96.9% | 0.01 * |
| Krauthamer et al. [52] | 350 G/L | 140 | pCR | 68.2% | 42.9% | 34.9% | 75% | ns |
| Aires et al. [22] | 253.5 G/L | 171 | TRG 0–1 (Rayan) | 75.5% | 47.8% | n/a | n/a | 0.0018 |
| Tawfik et al. [41] | n/a | 98 | pCR | n/a | n/a | n/a | n/a | ns |
| post-CRT pre-Surgery | ||||||||
| Tawfik et al. [41] | n/a | 98 | pCR | n/a | n/a | n/a | n/a | ns |
| Study | Method | N | Measure Outcome | Measured Marker | Time Point | Significant Markers (p < 0.05) |
|---|---|---|---|---|---|---|
| Zitt et al. [100] | qPCR | 26 | ypDownstaging | cfDNA levels | pre-CRT post-CRT post-Surgery | post-Surgery cfDNA levels |
| Agostini et al. [101] | qPCR | 67 | TRG (Mandard) | cfDNA levels Integrity index | pre-CRT post-CRT | post-Integrity index |
| Sun et al. [102] | qPCR | 34 | TRG (Dworak) | cfDNA levels cfDNA integrity MGMT promoter methylation KRAS mutation | pre-CRT post-CRT | pre-CRT 400 bp cfDNA concentration cfDNA integrity pre-CRT MGMT promoter methylation |
| Schou et al. [103] | Direct Fluorescence | 123 | pCR | cfDNA levels | pre-CRT post-CRT | ns |
| Shalaby et al. [104] | qPCR | 93 | TRG (Dworak) | MGMT and ERCC-1 promoter methylation | pre-CRT | pre-CRT methylation of MGMT and ERCC-1 promoters |
| Study | Method | N | Measure Outcome | Measured Marker | Time Point (Detection Rate) | Significant Markers (p < 0.05) |
|---|---|---|---|---|---|---|
| Murahashi et al. [37] | Amplicon-based deep sequencing | 85 | TRG (Dworak) | ctDNA level | pre-CRT (57.6%) post-CRT (22.3%) post-Surgery | ctDNA reduction (post-CRT/pre-CRT ctDNA levels) |
| Pazdirek et al. [108] | singleplex PCR | 36 | TRG (Dworak) | ctDNA level | pre-CRT (21.2%) during-CRT | ns |
| Carpinetti et al. [109] | WGS | 4 | TRG (Dworak) | ctDNA level | pre-CRT during-CRT post-CRT | ns |
| Zhou et al. [110] | NGS | 104 | pCR TRG (CAP) | ctDNA level | pre-CRT (75%) during-CRT (15.6%) post-CRT (10.5%) post-Surgery (6.7%) | post-CRT ctDNA level |
| McDuff et al. [111] | NGS ddPCR | 29 | pCR | ctDNA level | pre-CRT post-CRT | ns |
| Khakoo et al. [112] | ddPCR | 47 | mrTRG | ctDNA level | pre-CRT (74%) during CRT (21%) post-CRT (21%) post-Surgery (13%) | post-CRT ctDNA Level |
| Sclafani et al. [113] | ddPCR | 97 | CR (RECIST 1.1) | ctDNA level KRAS/BRAF mutation | pre-CRT (50–66%) | ns |
| Appelt et al. [114] | ddPCR | 146 | TRG (Mandard) | Meth-ctDNA (NPY) | pre-CRT (20.5%) | ns |
| Tie et al. [115] | NGS | 159 | pCR | ctDNA level | pre-CRT (77%) post-CRT (8.3%) post-Surgery (12%) | ns |
| Study | Method | N | V | Measure Outcome | Measured Marker | Time Point | Significant Markers (p < 0.05) |
|---|---|---|---|---|---|---|---|
| Wada et al. [36] | qRT-PCR | 41 | 65 | TRG (Mandard) | miRNA (8-panel) | pre-CRT | All panel: miR-30e-5p miR-33a-5p miR-130a-5p miR-210-3p miR-214-3p miR-320a miR-338-3p miR-1260a |
| Dreussi et al. [125] | DNA sequencing | 265 | / | pCR | miRNA-related SNP (114-panel) | n/a | DROSHA-rs10719 SMAD3-rs17228212 SMAD3 rs744910 SMAD3-rs745103 TRBP-rs6088619 |
| D’Angelo et al. [126] | qRT-PCR | 34 | / | TRG (Mandard) | miRNA (11-panel) | pre-CRT | miR-125b |
| Yu et al. [127] | qRT-PCR | 87 | 42 | TRG (Mandard) | miRNA (16-panel) | pre-CRT | miR-345 |
| Azizian et al. [128] | Real time PCR | 42 | / | Lymph Node Negativity | miRNA (5-panel) | pre-CRT during CRT post-CRT | miR-20a miR-18b |
| Meltzer et al. [129] | n/a | 29 | 64 | TRG (CAP) | Exosomal miRNA (372 panel) | pre-CRT | ns |
| Baek et al. [130] | qRT-PCR | 89 | / | TRG (Rödel) | Exosomal miRNA (16-panel) | pre-CRT | Exosomal miR-199b-5p |
| Hiyoshi et al. [131] | RT-PCR | 94 | / | TRG (Dworak) | miRNA (18-panel) | pre-CRT | miR-43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado Carvalho, J.V.; Dutoit, V.; Corrò, C.; Koessler, T. Promises and Challenges of Predictive Blood Biomarkers for Locally Advanced Rectal Cancer Treated with Neoadjuvant Chemoradiotherapy. Cells 2023, 12, 413. https://doi.org/10.3390/cells12030413
Machado Carvalho JV, Dutoit V, Corrò C, Koessler T. Promises and Challenges of Predictive Blood Biomarkers for Locally Advanced Rectal Cancer Treated with Neoadjuvant Chemoradiotherapy. Cells. 2023; 12(3):413. https://doi.org/10.3390/cells12030413
Chicago/Turabian StyleMachado Carvalho, Joao Victor, Valérie Dutoit, Claudia Corrò, and Thibaud Koessler. 2023. "Promises and Challenges of Predictive Blood Biomarkers for Locally Advanced Rectal Cancer Treated with Neoadjuvant Chemoradiotherapy" Cells 12, no. 3: 413. https://doi.org/10.3390/cells12030413
APA StyleMachado Carvalho, J. V., Dutoit, V., Corrò, C., & Koessler, T. (2023). Promises and Challenges of Predictive Blood Biomarkers for Locally Advanced Rectal Cancer Treated with Neoadjuvant Chemoradiotherapy. Cells, 12(3), 413. https://doi.org/10.3390/cells12030413







