Repurposing Metabolic Inhibitors in the Treatment of Colon Adenocarcinoma Patient-Derived Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
2.2. Organoid Culture
2.3. Protein Expression Analysis
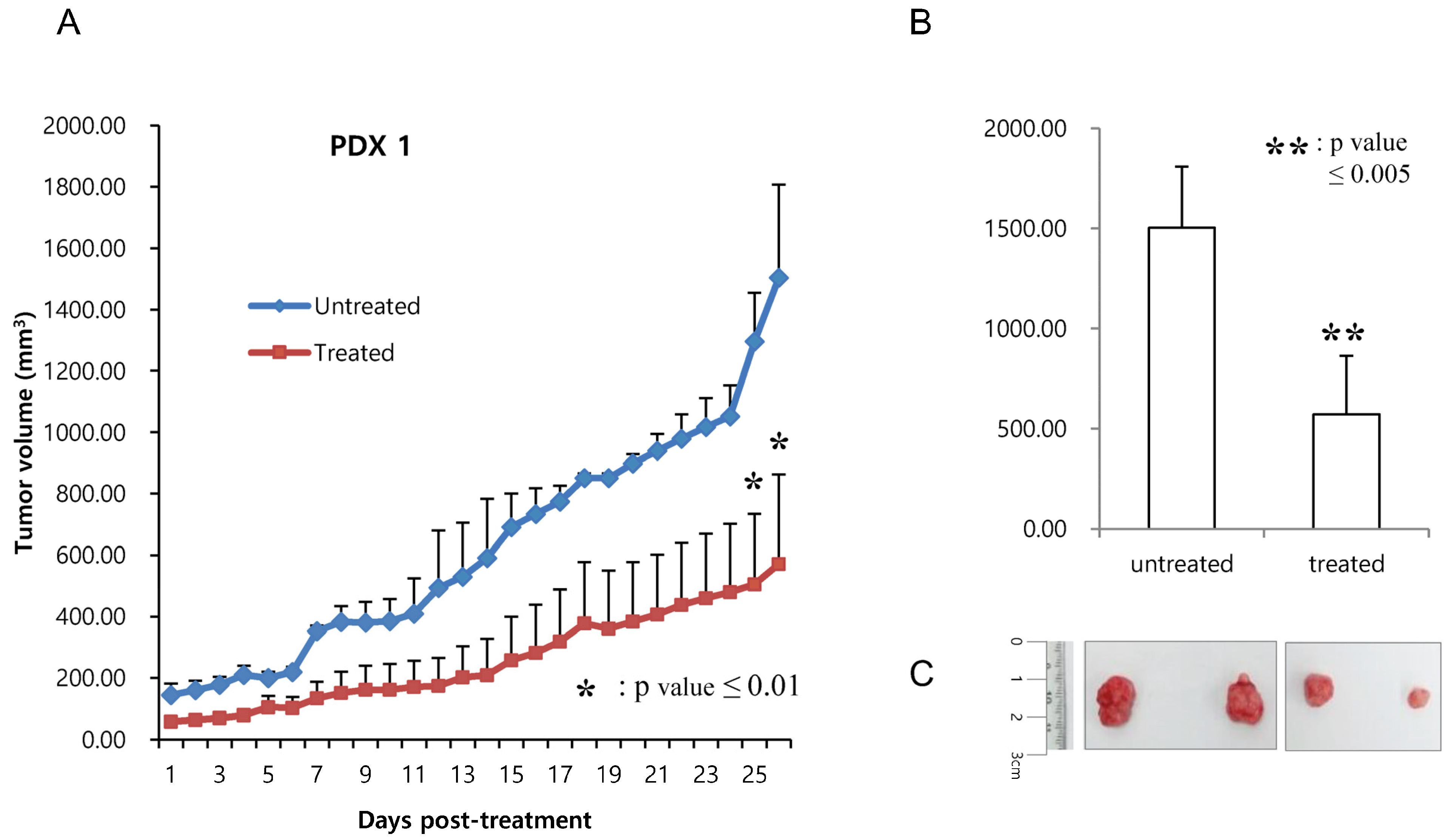
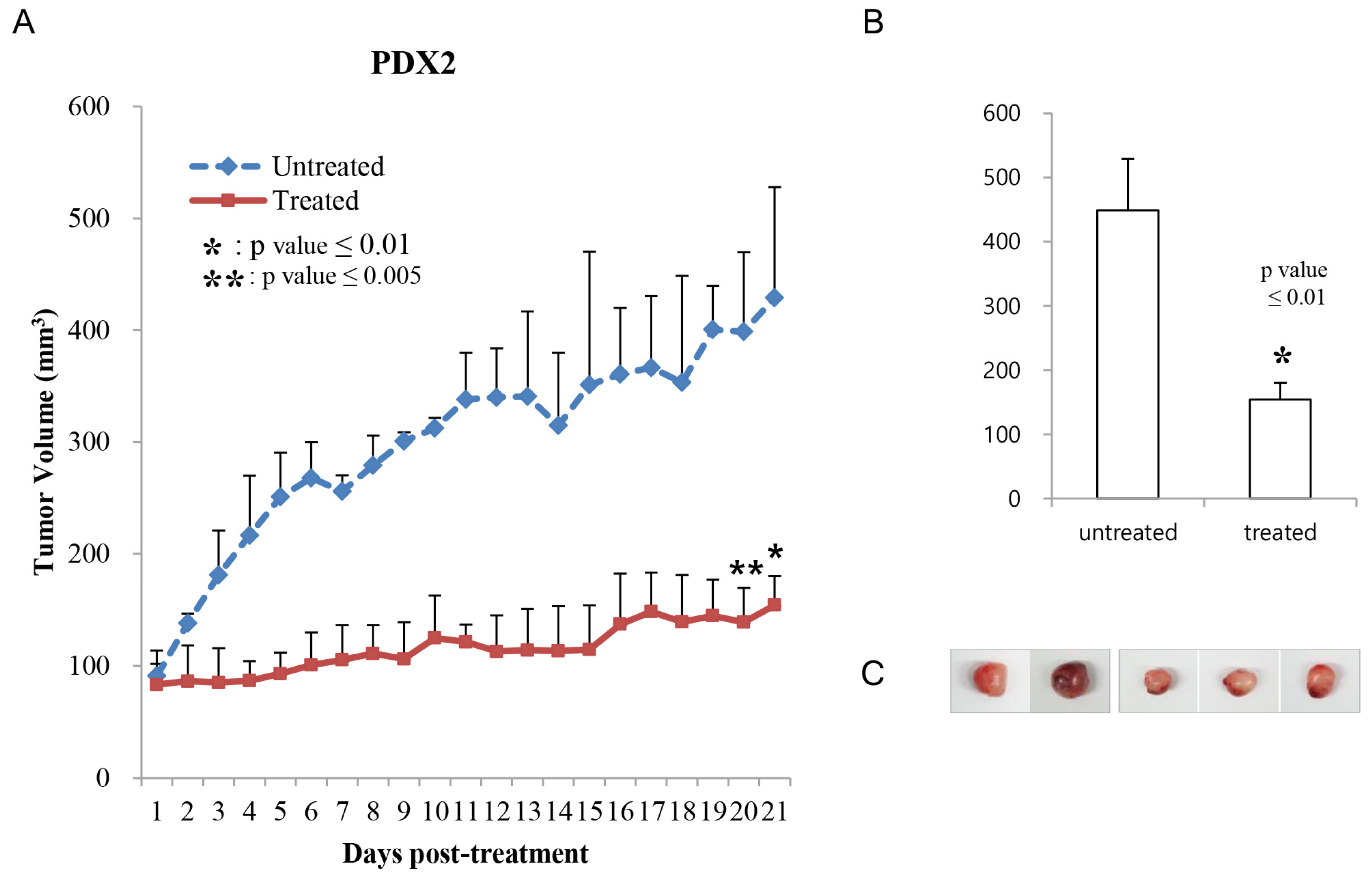
2.4. Generation of Patient-Derived (PDX) Models
2.5. Assessment of Tumor Growth Inhibition In Vivo
2.6. Cell Growth Inhibition
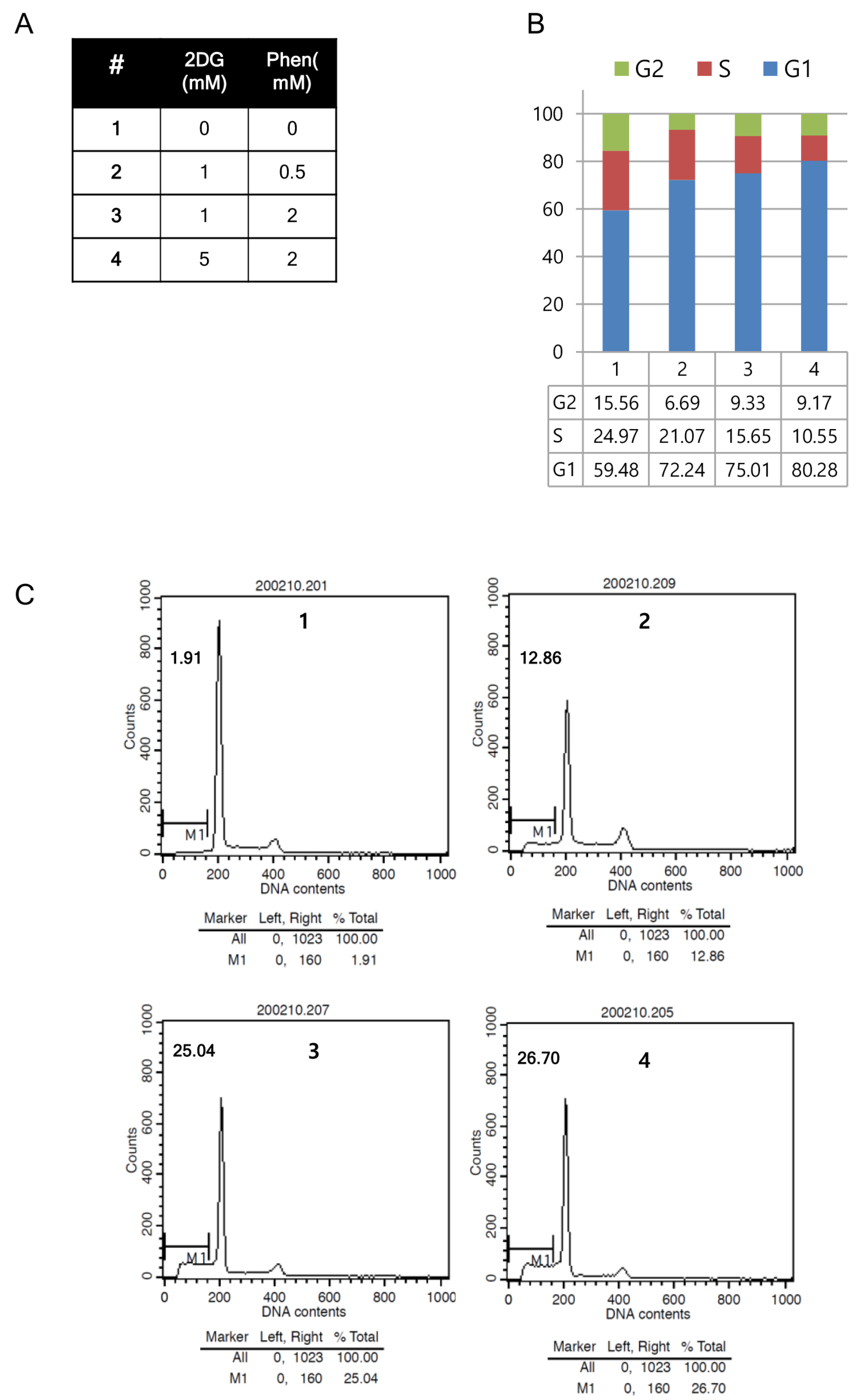
2.7. Flow Cytometric Analysis through Annexin V and Propidium Iodide Double Staining
2.8. Statistical Analysis
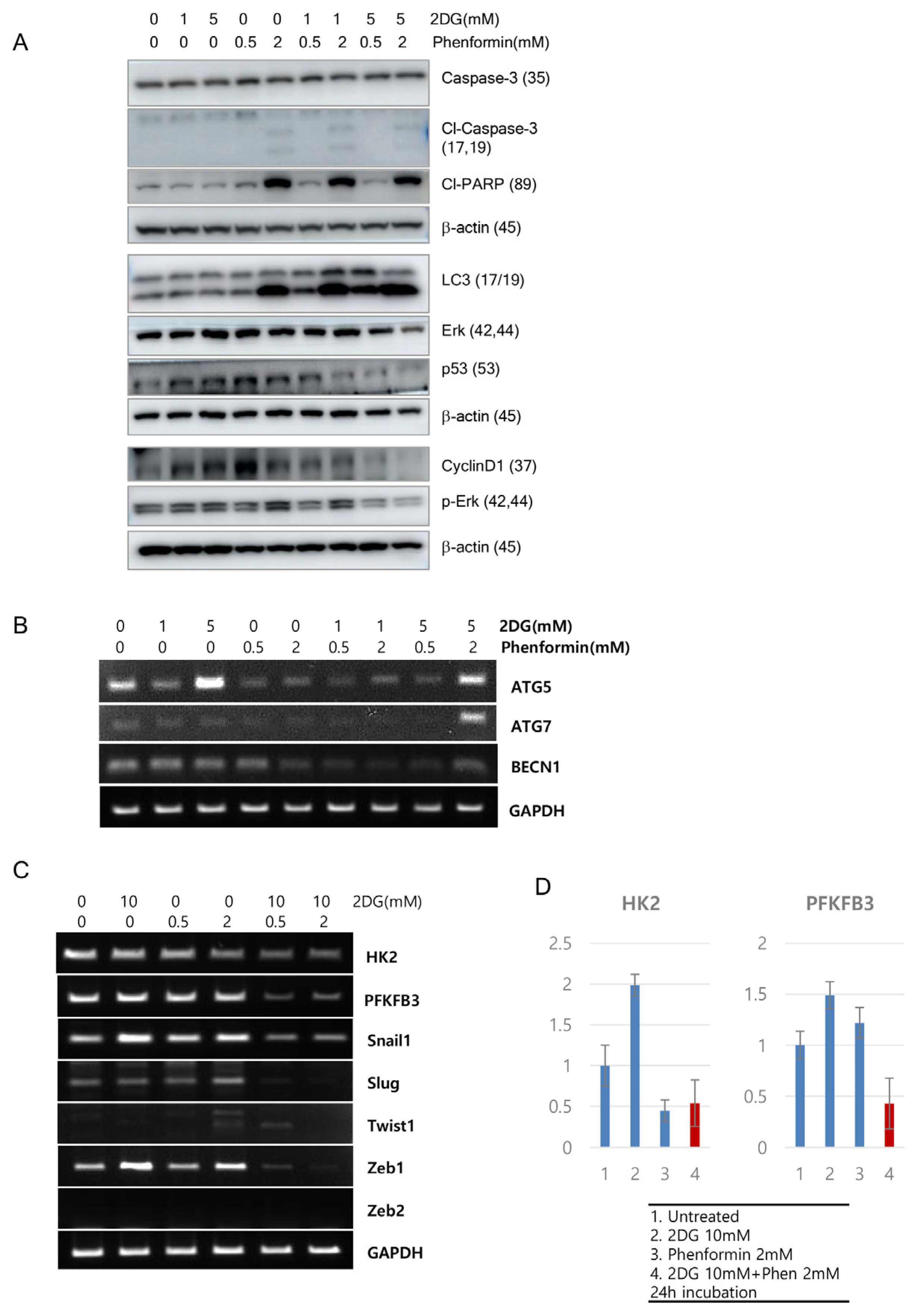
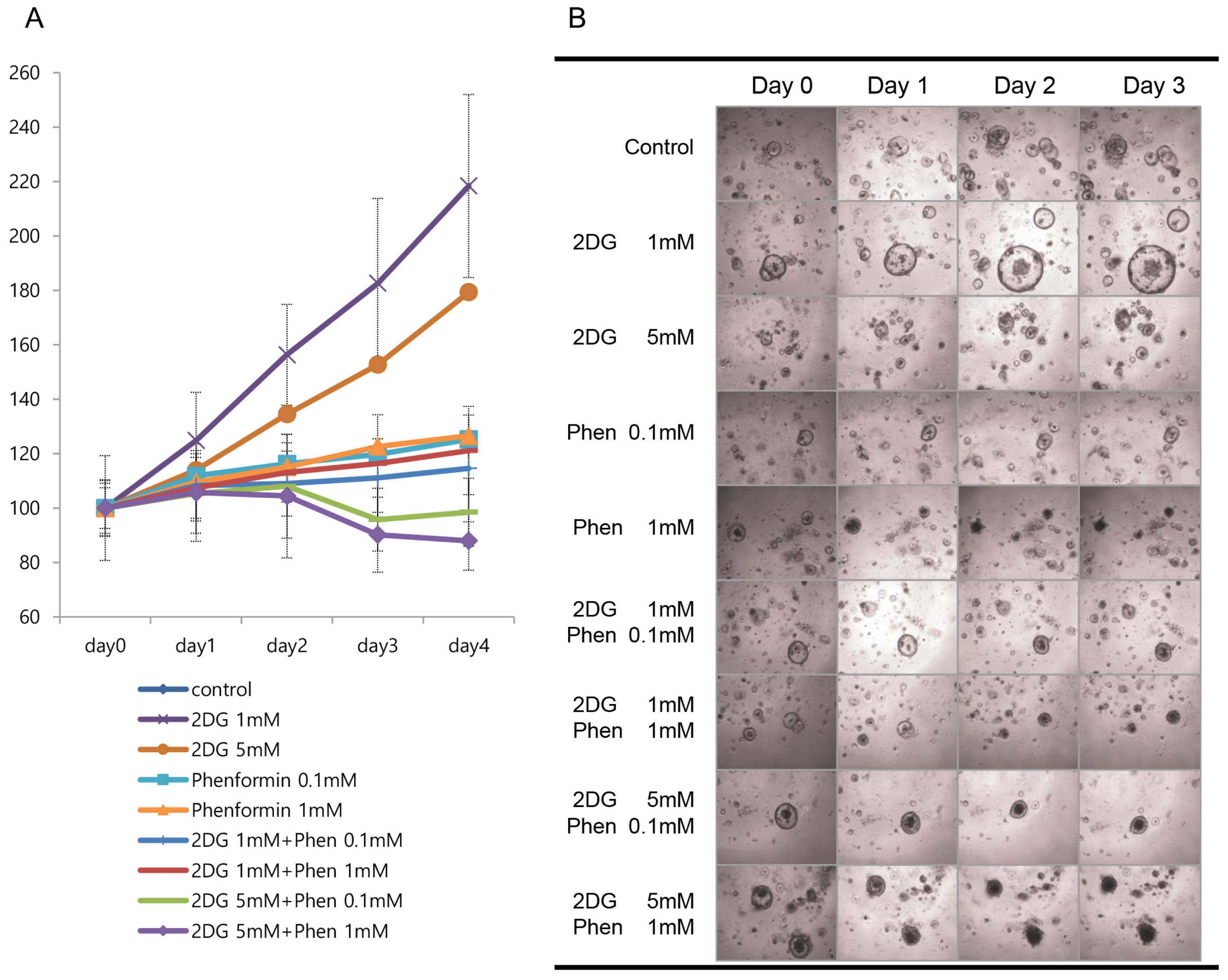
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Garcia Rubino, M.E.; Carrillo, E.; Ruiz Alcala, G.; Dominguez-Martin, A.; Marchal, J.A.; Boulaiz, H. Phenformin as an Anticancer Agent: Challenges and Prospects. Int. J. Mol. Sci. 2019, 20, 3316. [Google Scholar] [CrossRef] [PubMed]
- Kourelis, T.V.; Siegel, R.D. Metformin and cancer: New applications for an old drug. Med. Oncol. 2012, 29, 1314–1327. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ren, L.; Liu, C.; Xia, T.; Zha, X.; Wang, S. Phenformin Induces Cell Cycle Change, Apoptosis, and Mesenchymal-Epithelial Transition and Regulates the AMPK/mTOR/p70s6k and MAPK/ERK Pathways in Breast Cancer Cells. PLoS ONE 2015, 10, e0131207. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.S.; Kim, T.H.; Lim, H.; Oh, J.S.; You, J.S.; Lee, G.J.; Yu, S.K.; Kim, D.K.; Kim, H.J.; Kim, C.S.; et al. Phenformin Induces Caspase-dependent Apoptosis of FaDu Head and Neck Squamous Cell Carcinoma Cells. Anticancer Res. 2019, 39, 3499–3506. [Google Scholar] [CrossRef] [PubMed]
- Vara-Ciruelos, D.; Dandapani, M.; Russell, F.M.; Grzes, K.M.; Atrih, A.; Foretz, M.; Viollet, B.; Lamont, D.J.; Cantrell, D.A.; Hardie, D.G. Phenformin, But Not Metformin, Delays Development of T Cell Acute Lymphoblastic Leukemia/Lymphoma via Cell-Autonomous AMPK Activation. Cell Rep. 2019, 27, 690–698.e694. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, N.V.; Yabuuchi, S.; Pai, S.G.; De Oliveira, E.; Kamphorst, J.J.; Rabinowitz, J.D.; Tejero, H.; Al-Shahrour, F.; Hidalgo, M.; Maitra, A.; et al. Treatment of Pancreatic Cancer Patient-Derived Xenograft Panel with Metabolic Inhibitors Reveals Efficacy of Phenformin. Clin. Cancer Res. 2017, 23, 5639–5647. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.D.; Wei, S.Q.; Wang, Q.Y. Targeting oncogenic KRAS in non-small cell lung cancer cells by phenformin inhibits growth and angiogenesis. Am. J. Cancer Res. 2015, 5, 3339–3349. [Google Scholar]
- Yuan, P.; Ito, K.; Perez-Lorenzo, R.; Del Guzzo, C.; Lee, J.H.; Shen, C.H.; Bosenberg, M.W.; McMahon, M.; Cantley, L.C.; Zheng, B. Phenformin enhances the therapeutic benefit of BRAF(V600E) inhibition in melanoma. Proc. Natl. Acad. Sci. USA 2013, 110, 18226–18231. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, Y.H.; Park, E.H.; Lee, S.J.; Kim, H.; Kim, A.; Lee, S.B.; Shim, S.; Jang, H.; Myung, J.K.; et al. Effects of metformin and phenformin on apoptosis and epithelial-mesenchymal transition in chemoresistant rectal cancer. Cancer Sci. 2019, 110, 2834–2845. [Google Scholar] [CrossRef]
- Takahashi, A.; Kimura, F.; Yamanaka, A.; Takebayashi, A.; Kita, N.; Takahashi, K.; Murakami, T. Metformin impairs growth of endometrial cancer cells via cell cycle arrest and concomitant autophagy and apoptosis. Cancer Cell Int. 2014, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Saraei, P.; Asadi, I.; Kakar, M.A.; Moradi-Kor, N. The beneficial effects of metformin on cancer prevention and therapy: A comprehensive review of recent advances. Cancer Manag. Res. 2019, 11, 3295–3313. [Google Scholar] [CrossRef] [PubMed]
- Christou, N.; Bergen, E.S.; Canton, C.; Le Malicot, K.; Di Bartolomeo, M.; Galli, F.; Galli, F.; Labianca, R.; Shi, Q.; Alberts, S.R.; et al. Impact of diabetes and metformin use on recurrence and outcome in stage II-III colon cancer patients-A pooled analysis of three adjuvant trials. Eur. J. Cancer 2022, 166, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xia, S.; Zhu, Z. Synergistic effect of phenformin in non-small cell lung cancer (NSCLC) ionizing radiation treatment. Cell Biochem. Biophys. 2015, 71, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.K.; Kim, M.S.; Lee, J.Y.; Kim, E.H.; Ha, H. Metformin Radiosensitizes p53-Deficient Colorectal Cancer Cells through Induction of G2/M Arrest and Inhibition of DNA Repair Proteins. PLoS ONE 2015, 10, e0143596. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Nannapaneni, S.; Wang, D.; Liu, F.; Wang, X.; Jin, R.; Liu, X.; Rahman, M.A.; Peng, X.; Qian, G.; et al. Phenformin enhances the therapeutic effect of selumetinib in KRAS-mutant non-small cell lung cancer irrespective of LKB1 status. Oncotarget 2017, 8, 59008–59022. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fatehi, R.; Rashedinia, M.; Akbarizadeh, A.R.; Zamani, M.; Firouzabadi, N. Metformin enhances anti-cancer properties of resveratrol in MCF-7 breast cancer cells via induction of apoptosis, autophagy and alteration in cell cycle distribution. Biochem. Biophys. Res. Commun. 2023, 644, 130–139. [Google Scholar] [CrossRef]
- Yip, K.L.; Tsai, T.N.; Yang, I.P.; Miao, Z.F.; Chen, Y.C.; Li, C.C.; Su, W.C.; Chang, T.K.; Huang, C.W.; Tsai, H.L.; et al. Metformin Enhancement of Therapeutic Effects of 5-Fluorouracil and Oxaliplatin in Colon Cancer Cells and Nude Mice. Biomedicines 2022, 10, 955. [Google Scholar] [CrossRef]
- O’Neill, S.; Porter, R.K.; McNamee, N.; Martinez, V.G.; O’Driscoll, L. 2-Deoxy-D-Glucose inhibits aggressive triple-negative breast cancer cells by targeting glycolysis and the cancer stem cell phenotype. Sci. Rep. 2019, 9, 3788. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Xiong, H.; Wu, F.; Lan, T.; Zhang, Y.; Guo, X.; Wang, H.; Saleem, M.; Jiang, C.; et al. Co-targeting hexokinase 2-mediated Warburg effect and ULK1-dependent autophagy suppresses tumor growth of PTEN- and TP53-deficiency-driven castration-resistant prostate cancer. EBioMedicine 2016, 7, 50–61. [Google Scholar] [CrossRef]
- Lea, M.A.; Chacko, J.; Bolikal, S.; Hong, J.Y.; Chung, R.; Ortega, A.; Desbordes, C. Addition of 2-deoxyglucose enhances growth inhibition but reverses acidification in colon cancer cells treated with phenformin. Anticancer Res. 2011, 31, 421–426. [Google Scholar] [PubMed]
- van de Wetering, M.; Francies, H.E.; Francis, J.M.; Bounova, G.; Iorio, F.; Pronk, A.; van Houdt, W.; van Gorp, J.; Taylor-Weiner, A.; Kester, L.; et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 2015, 161, 933–945. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.L.; Mun, H.; Jo, S.Y.; Oh, J.H.; Lee, C.; Choi, E.K.; Jang, S.J.; Suh, Y.A. Suppression of gain-of-function mutant p53 with metabolic inhibitors reduces tumor growth in vivo. Oncotarget 2016, 7, 77664–77682. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.J.; Kim, M.; Jo, D.; Kim, J.; Oh, J.H.; Chung, H.C.; Lee, S.H.; Kim, D.; Chun, S.M.; Kim, J.; et al. Immuno-genomic classification of colorectal cancer organoids reveals cancer cells with intrinsic immunogenic properties associated with patient survival. J. Exp. Clin. Cancer Res. 2021, 40, 230. [Google Scholar] [CrossRef] [PubMed]
- Seol, H.S.; Suh, Y.A.; Ryu, Y.J.; Kim, H.J.; Chun, S.M.; Na, D.C.; Fukamachi, H.; Jeong, S.Y.; Choi, E.K.; Jang, S.J. A patient-derived xenograft mouse model generated from primary cultured cells recapitulates patient tumors phenotypically and genetically. J. Cancer Res. Clin. Oncol. 2013, 139, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Seol, H.S.; Kang, H.J.; Lee, S.I.; Kim, N.E.; Kim, T.I.; Chun, S.M.; Kim, T.W.; Yu, C.S.; Suh, Y.A.; Singh, S.R.; et al. Development and characterization of a colon PDX model that reproduces drug responsiveness and the mutation profiles of its original tumor. Cancer Lett. 2014, 345, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Park, S.S.; Ju, E.J.; Park, J.; Ko, E.J.; Hwang, J.J.; Suh, Y.A.; Jang, S.J.; Lee, J.S.; Ko, B.K.; et al. Establishment of a Patient-derived Xenograft for Development of Personalized HER2-targeting Therapy in Gastric Cancer. Anticancer Res. 2018, 38, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Abu El Maaty, M.A.; Strassburger, W.; Qaiser, T.; Dabiri, Y.; Wolfl, S. Differences in p53 status significantly influence the cellular response and cell survival to 1,25-dihydroxyvitamin D3-metformin cotreatment in colorectal cancer cells. Mol. Carcinog. 2017, 56, 2486–2498. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Zhang, Y.; Wang, J.; Deng, Y.; Lin, D. Inhibition of glycolytic enzyme hexokinase II (HK2) suppresses lung tumor growth. Cancer Cell Int. 2016, 16, 9. [Google Scholar] [CrossRef]
- Atsumi, T.; Chesney, J.; Metz, C.; Leng, L.; Donnelly, S.; Makita, Z.; Mitchell, R.; Bucala, R. High expression of inducible 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (iPFK-2; PFKFB3) in human cancers. Cancer Res. 2002, 62, 5881–5887. [Google Scholar]
- Hu, S.; Ouyang, Q.; Cheng, Q.; Wang, J.; Feng, F.; Qiao, L.; Gan, W.; Shi, Y.; Wu, D.; Jiang, X. Phenformin inhibits cell proliferation and induces cell apoptosis and autophagy in cholangiocarcinoma. Mol. Med. Rep. 2018, 17, 6028–6032. [Google Scholar] [CrossRef] [PubMed]
- Pistoni, M.; Tondelli, G.; Gallo, C.; Torricelli, F.; Maresca, A.; Carelli, V.; Ciarrocchi, A.; Dallaglio, K. Exploring metabolic reprogramming in melanoma via acquired resistance to the oxidative phosphorylation inhibitor phenformin. Melanoma Res. 2019, 30, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Veiga, S.R.; Ge, X.; Mercer, C.A.; Hernandez-Alvarez, M.I.; Thomas, H.E.; Hernandez-Losa, J.; Ramon, Y.C.S.; Zorzano, A.; Thomas, G.; Kozma, S.C. Phenformin-Induced Mitochondrial Dysfunction Sensitizes Hepatocellular Carcinoma for Dual Inhibition of mTOR. Clin. Cancer Res. 2018, 24, 3767–3780. [Google Scholar] [CrossRef] [PubMed]
- Driehuis, E.; van Hoeck, A.; Moore, K.; Kolders, S.; Francies, H.E.; Gulersonmez, M.C.; Stigter, E.C.A.; Burgering, B.; Geurts, V.; Gracanin, A.; et al. Pancreatic cancer organoids recapitulate disease and allow personalized drug screening. Proc. Natl. Acad. Sci. USA 2019, 116, 26580–26590. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Mun, H.; Sung, C.O.; Cho, E.J.; Jeon, H.J.; Chun, S.M.; Jung, D.J.; Shin, T.H.; Jeong, G.S.; Kim, D.K.; et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat. Commun. 2019, 10, 3991. [Google Scholar] [CrossRef] [PubMed]
- Tuveson, D.; Clevers, H. Cancer modeling meets human organoid technology. Science 2019, 364, 952–955. [Google Scholar] [CrossRef] [PubMed]
- Date, S.; Sato, T. Mini-gut organoids: Reconstitution of the stem cell niche. Annu. Rev. Cell Dev. Biol. 2015, 31, 269–289. [Google Scholar] [CrossRef]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef]



| IHC | Mutation Analysis | Chemo-Therapy | Follow-Up | |||||
|---|---|---|---|---|---|---|---|---|
| Sex Age | Stage | Met (1) | p53 | EGFR | Kras | FOLFOX +Ava | 2 Years | |
| C67 | M 58 | pT4aN2b | LN (2) | - | +++ | WT (4) | Treated | NA |
| C70 | F 39 | pT1N0Mx | +++ | NA (3) | WT | NT (5) | Normal | |
| C73 | M 60 | pT2N0 | +++ | NA | WT | NT | Adenoma | |
| C80 | M 47 | pT3N2b | Liver | - | ++ | WT | Treated | lung met |
| C81 | F 53 | pT3N2bM1b | LN | - | +++ | G12D | Treated | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, B.; Lee, C.; Moon, H.-M.; Jo, S.-Y.; Jang, S.J.; Suh, Y.-A. Repurposing Metabolic Inhibitors in the Treatment of Colon Adenocarcinoma Patient-Derived Models. Cells 2023, 12, 2859. https://doi.org/10.3390/cells12242859
Lee B, Lee C, Moon H-M, Jo S-Y, Jang SJ, Suh Y-A. Repurposing Metabolic Inhibitors in the Treatment of Colon Adenocarcinoma Patient-Derived Models. Cells. 2023; 12(24):2859. https://doi.org/10.3390/cells12242859
Chicago/Turabian StyleLee, Bora, ChuHee Lee, Hae-Min Moon, Se-Young Jo, Se Jin Jang, and Young-Ah Suh. 2023. "Repurposing Metabolic Inhibitors in the Treatment of Colon Adenocarcinoma Patient-Derived Models" Cells 12, no. 24: 2859. https://doi.org/10.3390/cells12242859
APA StyleLee, B., Lee, C., Moon, H.-M., Jo, S.-Y., Jang, S. J., & Suh, Y.-A. (2023). Repurposing Metabolic Inhibitors in the Treatment of Colon Adenocarcinoma Patient-Derived Models. Cells, 12(24), 2859. https://doi.org/10.3390/cells12242859







