Microglial Senescence and Activation in Healthy Aging and Alzheimer’s Disease: Systematic Review and Neuropathological Scoring
Abstract
1. Introduction and Historical Hints
2. Materials and Methods
2.1. Literature Search, Data Sources, and Studies Selection
2.2. Data Analysis, Visualization, and Data Availability
3. Results
3.1. Literature Search
3.2. Data Extraction, Synthesis, and Quality Assessment
3.3. Population of Included Studies
4. Discussion
4.1. Human Microglial “Senescence” and “Dystrophy” in Normal Aging and AD
4.2. Microglial Morphology, Nomenclatures, and States: Past and Future
4.2.1. Microglial Morphological Hallmarks
4.2.2. Microglial States in Mice Models of Neurodevelopment (PAM/ATM)
4.2.3. Microglial States in Disease Models of AD (DAM/MGnD/ARM)
4.2.4. Human Microglial Reactive States (M1/M2 Paradigm and WAM/HAM/LDAM)
4.3. Homeostatic Microglia: Responses to External/Internal Perturbations and Aging
4.4. Interactions of Human Microglia with Aβ and pTAU
4.5. Resident Microglia: Differences with Circulating Monocytes, Transcription Factors, and Interleukins in Healthy Controls and Alzheimer’s Disease
4.6. Human Microglia, Ferritin, Lysosomal Storage, and Mitochondrial Dysfunction
4.7. Human Microglia Characterization: IHC and Related Phenotypes/Transcriptomic Profiles
4.8. Microglia Scoring System: A Proposal for Neuropathological Assessment
5. Concluding Remarks
| Study | Population | Age (Years Old; Mean ± SD) | Sex (M/F) | Microglial Phenotype | Cell-Surface Markers or Gene/Transcription Factors | Characteristics (e.g., Activity, Morphology, Interactions) | Method of Analysis | Interleukines/ Cytokines/ Chemokines (e.g., Stimulation, Production) |
|---|---|---|---|---|---|---|---|---|
| DiPatre and Gelman (1997) [144] | Younger Healthy Controls: 8 | 38 | N/R | Primed | Ferritin | Young controls: sparse, not ramified Old controls: elongated bipolar microglia ADs: ramified processes, increased in number | Immunohistochemistry | N/A |
| Older Healthy Controls: 9 | 73 | N/R | ||||||
| ADs: 9 | 72 | N/R | ||||||
| Sheng et al. (1998) [25] | Healthy Controls (<60 y): 19 | range: 1–57 | 7 M | Primed | N/A | Phagocytic, enlarged, extensive cytoplasm, rod shaped, ramified | Immunohistochemistry Western blot | IL-1α |
| Healthy Aging (>60 y): 15 | range: 61–93 | 12 M | ||||||
| Walker et al. (2001) [213] | Healthy Controls: 5 | N/R | N/R | DAM/WAM | CD11a, CD11b, CD11c, IL-2 R | Vacuolization after interaction with β-amyloid, activation and proliferation | Immunohistochemistry/RNA-seq | IL-1β, TNF- α, IL-6, IL-12β, IFN- γ |
| Streit et al. (2004) [26] | Young Healthy Controls: 1 | 38 | 1 M | Senescent | HLA-DR | Deramification, spheroid formation, gnarling, fragmentation of processes | Immunohistochemistry | N/A |
| Old Healthy Controls: 1 | 68 | 1 M | ||||||
| Flanary et al. (2007) [66] | Healthy Controls: 1 | 86 | 1 M | Senescent | Iba1, CD11b | Dystrophic | Immunohistochemistry | N/A |
| ADs: 4 | range: 82–89 | 3 M | ||||||
| Lopes et al. (2008) [36] | Younger, non- demented individuals: 3 | 36.66 ± 2.08 | 4 M | Senescent | HLA-DR, Ferritin | HLA-DR+: ramified Ferritin+: dystrophic, deramified, fine processes tortuous and coiled | Immunohistochemistry Immunofluorescence | N/A |
| Aged, non-demented and amyloid-free individuals: 7 | 79.86 ± 8.05 | 6 M | ||||||
| Aged, non-demented and high amyloid-β burden: 7 | 83.43 ± 5.19 | 5 M | ||||||
| ADs: 7 | 80.29 ± 11.64 | 4 M | ||||||
| Streit et al. (2009) [27] | Healthy Controls: 4 | range 22- 77 | 1 M | Senescent | Iba1 | Dystrophic, cytoplasmic fragmentation, cytorrhexis | Immunohistochemistry | N/A |
| Minimal tau pathology: 4 | range 21–88 | 0 M | ||||||
| Maximal tau pathology without concurrent amyloid: 1 | 92 | 1 M | ||||||
| ADs: 4 | range 62–85 | 2 M | ||||||
| Dhawan et al. (2012) [152] | Healthy Controls: N/R | N/R | N/R | Primed | HLA-DR | Proliferation, microgliosis | Immunohistochemistry and Western blot | N/A |
| ADs: N/R | N/R | N/R | ||||||
| Smith et al. (2013) [214] | Autopsy brain tissue: N/A | N/A | N/A | Primed | HLA-DP,-DQ,-DR, CD45, PU.1, CX3CR1 | Activation | Immunohistochemistry Western blot | N/A |
| Post-mortem brain tissue: N/A | N/A | N/A | ||||||
| Griciuc et al. (2013) [223] | Healthy Controls: 15 | N/R | N/R | Primed | Iba1, CD33 | Activation | Western blot qT-PCR | N/A |
| ADs: 25 | N/R | N/R | ||||||
| Bachstetter et al. (2015) [224] | Healthy Controls: 9 | 86 | 6 M | Primed—Senescent | Iba 1, CD68 | Ramified, hypertrophic, dystrophic, rod shaped, amoeboid | Immunohistochemistry | N/A |
| HS of aging: 6 | 87 | 3 M | ||||||
| ADs: 7 | 77 | 4 M | ||||||
| ADs + HSs: 4 | 91 | 0 M | ||||||
| MCIs: 414 | 71.82 (7.45) | 244 M | ||||||
| ADs: 73 | 74.17 (8.37) | 38 M | ||||||
| Zeineh et al. (2015) [209] | Healthy Controls: 8 | 72.8 | 3 M | Primed—M1 | CD163 | Reactive microgliosis | Immunoblotting | N/A |
| ADs: 5 | 85.8 | 2 M | ||||||
| Tischer et al. (2016) [225] | Healthy Controls: 5 | 71.9 ± 6.8 | 11 M | Senescent | Iba1, CD68, MHCII | Dystrophic Younger controls: ramified branches Older controls: loss of branches and ramification ADs: fragmented, spheroid, shortening of branches | Immunohistochemistry Immunofluorescence | N/A |
| Prodromal ADs: 11 | 80.0 ± 7.7 | |||||||
| Progressive ADs: 10 | 26–30/78–83 | |||||||
| Satoh et al. (2016) [226] | Sporadic ADs: 10 | 70 ± 8 | 5 M | Primed | TMEM119, TREM2, Iba1, CD68 | Ramified and amoeboid morphologies | Immunohistochemistry Western blot Real-Time PCR | N/A |
| Non-ADs: 11 (Healthy Controls: 4) | 75 ± 8 | 6 M | ||||||
| Hendrickx et al. (2017) [212] | Healthy Controls: 6 | 64.8 | 2 M | Primed | Iba1, CD68, HLA-DR | Ramified microglia, rounded amoeboid microglia, foamy macrophages | Immunohistochemistry | N/A |
| ADs: 4 | 72.3 | 2 M | ||||||
| Raj et al. (2017) [227] | Healthy Controls: 20 | range: 30–85 | N/R | Primed | CD68, Iba1, HLA-DR | Activation, proliferation | Immunohistochemistry | N/A |
| ADs (EOAD + LOAD): 12 | range: 20–80 | N/R | ||||||
| Bachstetter et al. (2017) [81] | Healthy Controls: 118 | range: 20–70 | 107 M | M2 | Iba1 | Rod-shaped microglia | Immunohistochemistry | N/A |
| ADs: 50 | range: 70–89 | |||||||
| Sims et al. (2017) [228] | Healthy Controls + ADs: 508 | 76.3 (Healthy Controls) 75.9 (ADs) | N/R N/R | DAM/WAM | TREM2, ABI3, PLCG2 | N/A | Genotyping | N/A |
| Davies et al. (2017) [68] | Younger Healthy Controls: 3 | 55 ± 4 | 2 M | Senescent | Iba1 | Dystrophic, ramified, deramified, discontinuous, punctate | Immunohistochemistry Immunofluorescence | N/A |
| Older Healthy Controls: 5 | 82 ± 10 | 2 M | ||||||
| ADs: 7 | 84 ± 11 | 3 M | ||||||
| Krasemann et al. (2017) [87] | ADs (TREM2 variants): 5 | 80.6 | 3 M | M0 homeostatic microglia | Iba1, TREM2, TMEM119, P2RY12, APOE | Homeostatic microglia; Proliferation and clustering around β-amyloid plaques | Immunohistochemistry Immunofluorescence | N/A |
| ADs (TREM2 wild-type): 6 | 81 | 4 M | ||||||
| Olah et al. (2018) [153] | Healthy Controls: 4 | N/R | N/R | DAM/WAM | CD11, CD45, P2RY12, TMEM119, TREM2, GPR34, CX3CR1 | Activation, dystrophic | RNA-seq | N/A |
| Kaneshwaran et al. (2019) [229] | Healthy Controls: 420 | 86.6–93.3 | 233 M | Primed | HLA-DP, DQ, and DR | Dystrophic Stage I: thin ramified processes Stage II: plump cytoplasm and thicker processes Stage III: appearance of macrophages | Immunohistochemistry | N/A |
| ADs: 265 | 89.4 (86.3–92.9) | |||||||
| Mukherhjee et al. (2019) [230] | ADs + Healthy Controls: 637 | range: 18–106 | 319 M | DAM/WAM | FCER1G, ITGB2/CD18, MYO1F, PTPRC/CD45, TYROBP/DAP12 | N/A | WGCNA meta-analysis RNA seq | N/A |
| Parhizkar et al. (2019) [231] | Non-AD Dementia: 3 | 80.7 | 8 M | Primed—Homeostatic | Iba1, TREM2 | Clustering behavior around β-amyloid plaques and reduced clustering in patients with TREM2 loss of function variants | Immunohistochemistry Immunoblotting/Genotyping | N/A |
| ADs: 7 | 72.4 | 1 M | ||||||
| Barroeta-Espar et al. (2019) [181] | Healthy Controls: 28 | 86.1 ± 10.1/86.6 ± 10.2 | 18 M | Primed | CD68 | Activation, proliferation | Immunohistochemistry | Associated with resilience to AD: upregulation of IL-1β, IL-6, IL-13, IL-4, IL-6, IL-10, IP-10, PDGF-bb, FGF; GM-CSF, IL-17, IL-7 Associated with AD: upregulation of IL-1α and TNF-α, IL-5, IL-8, IL-12p70, MCP-1, MIP-1 α, eotaxin, IL-1ra |
| Non-Demented (Int-med risk): 33 | 83.1 ± 11.6 | 18 M | ||||||
| ADs: 29 | 82.9 ± 11.6 | 18 M | ||||||
| Felsky et al. (2019) [84] | ADs: 71 | 78 ± 8.7 | N/R | Primed DAM | N/A | Activation, dystrophic Stage I: thin ramified processes Stage II: plump cytoplasm and thicker processes Stage III: appearance of macrophages | Immunohistochemistry | N/A |
| Postmortem ADs: 90 | N/R | N/R | ||||||
| Bonham et al. (2019) [215] | Healthy Controls: 6 | N/R | N/R | Senescent | TMEM119 | Dystrophic | Gene expression mapping | N/A |
| ADs: 584 | N/R | N/R | ||||||
| Study | Population | Age (Years Old; Mean ± SD) | Sex (M/F) | Microglial Phenotype | Cell Surface Antigens/Biomarkers (e.g., Transcription Factors) | Morphological Characteristics | Methods | Interleukines/ Cytokines/ Chemokines (e.g., Stimulation, Production) |
| Li et al. (2020) [232] | Healthy Controls: 10 | 83 ± 2 | 6 M | Senescent | Iba1, TPSO, TREM2, MPO, BIN 1 | Dystrophic | Immunohistochemistry Genotyping | N/A |
| ADs: 27 | 82 ± 2 | 13 M | ||||||
| Walker et al. (2020) [166] | Low plaque non-demented: 12 | 85.9 ± 8.9 | 6 M | Primed—Senescent | CD68 P2RY12 | Ramified, dystrophic, “tufted” | Immunohistochemistry Western blot RNA expressing profile | Stimulation with: IL-4, IL-6, IFN- γ |
| High plaque non-demented: 12 | 88 ± 8 | 4 M | ||||||
| Srinivasan et al. (2020) [106] | Healthy Controls: 21 | 79 | 10 M | HAM | CD11b | Activation, proliferation | RNA-seq | N/A |
| ADs: 21 | 80 | 13 M | ||||||
| Molina-Martinez et al. (2020) [49] | Healthy Controls: 8 | 54 ± 2/65 ± 1.8 | 11 M | Primed | N/A | Activation, proliferation | Genotyping | N/A |
| ADs: 17 | 51 ± 1.8 | 7 M | ||||||
| Olah et al. (2020) [182] | Healthy Controls: 11 | N/A | N/A | DAM/WAM | Iba1, ISG15+, CD83+, and PCNA+, CD74, AIF2, APOE. TREM2 | Ramified, ameboid | Immunohistochemistry RNA-seq | IL-10, IL-4, IL-13, IFN- γ |
| MCI: 4 | 95 | 1 M | ||||||
| ADs: 10 | 91 | 2 M | ||||||
| Friedberg et al. (2020) [183] | ADs APOE ε4 negative: 34 | 87.9 ± 0.843 | 15 M | Primed—M1 | Iba1, CD68 | Activation | Immunohistochemistry | IL-1α, IL-4, IL-13 |
| ADs APOE ε4 positive: 21 | 85.9 ± 1.63 | 10 M | ||||||
| Fadul et al. (2020) [233] | Healthy Controls: 97 | 85.6 | N/R | DAM/WAM | CD 68, MHCII, NDRG2 (Astrocyte marker) | N/A | Immunohistochemistry | N/A |
| Marschallinger et al. (2020) [107] | Young Healthy Controls = 3 | <30 | N/R | LDAM | BODIPY, Iba1, CD68, TMEM119 | Pro-inflammatory phenotype Phagocytic deficits | Immunohistochemistry RNA-seq | IL-10, CCL3, CCL4, IL-6, CCL5, TNF-α, IL-1β, IL-1α, CXCL1, CXCL10 |
| Older Healthy Controls = 5 | >60 | N/R | ||||||
| Pascoal et al. (2021) [145] | Healthy Controls: 86 | 23 ± 2.4 (young)/ 72 ± 5.5 (older) | 22 M | Primed | TREM2 | Proliferation | Multiplex immunoassay analysis | TRAIL, CXCL1, CX3CL1, TGF- α, CCL3, CCL23, IL-8 |
| ADs: 16 | 70 ± 7.7 | 6 M | ||||||
| MCIs: 28 | 73 ± 8.6 | 17 M | ||||||
| March-Diaz et al. (2021) [165] | Healthy Controls: N/R | 49.5 ± 5.9 | N/R | Primed DAM | Iba1 | Reduced clustering around β-amyloid plaques | Western blot | N/A |
| ADs: N/R | 78 ± 8.5/78.3 ± 14.0/79 ± 10.0 | N/R | ||||||
| ADs: N/R | N/R | N/R | ||||||
| Kloske et al. (2021) [234] | Healthy Controls APOE ε3/3: 9 | 81 (73–90) | 4 M | Primed | P2RY12 | Activation | Immunohistochemistry | N/A |
| ADs APOE ε3/3: 9 | 81 (72–87) | 6 M | ||||||
| ADs APOE ε3/3: 10 | 85 (75–95) | 2 M | ||||||
| Hu et al. (2021) [57] | Healthy Controls: 7 | 74.28 | 4 M | Senescent/SASP | Iba1, PAI1, P19, P16, P21, CASPASE-8 (CASP8) | Distrophyc | Immunohistochemistry | IL-1β, IL-6 |
| ADs: 7 | 70.57 | 5 M | ||||||
| Cohn et al. (2021) [184] | Low-Mild ADs: 4 | 85 | 3 M | Primed DAM | CD11b, TMEM119, P2RY12, TREM2, FTH1 | Activation, proliferation | Immunoblotting/Transcriptomics | TNF-α |
| Moderate-Severe ADs: 4 | 91 | 1 M | ||||||
| Shahidehpour et al. (2021) [67] | Healthy Controls: 34 | range: 65–93 | 13 M | Senescent | Iba1, FTL | Hypertrophic, ramified, dystrophic | Immunohistochemistry | N/A |
| ADs: 8 | range: 65–85 | 5 M | ||||||
| LATEs: 9 | range: 65–93 | 4 M | ||||||
| LBDs: 11 | range: 65–97 | 9 M | ||||||
| Jiang et al. (2022) [235] | Healthy Controls: 11 | 89.8 | 4 M | Senescent | Iba1 | Dystrophic activation, proliferation | Immunohistochemistry | sST2/IL-33 |
| ADs: 102 | 80 | 47 M | ||||||
| Xie et al. (2022) [236] | Healthy Controls: 4 | Mean age cases: 84.25 | 3 M | Primed | Iba1, CatE | Activation, proliferation | Immunoblotting | N/A |
| LOADs: 4 | Mean age controls: 85 | 2 M | ||||||
| An et al. (2022) [58] | Human microglial cells HMC3 ATCC (#CRL0314) Human Brain Bank derived | N/A | N/A | Senescent—SASP | β-galactosidase (SA-β-gal), SIRT1/NRF2 pathway | Dystrophic | Western blot Real-Time PCR | TNF-α, IL-1β, IL-6 |
| Muñoz-Castro et al. (2022) [237] | Healthy Controls: 7 | 86.0 ± 2.5 | 4 M | DAM/WAM | Iba1, CD68, ferritin, MHCII, TMEM119, TSPO | Homeostatic, intermediate, reactive | Immunohistochemistry Immunofluorescence Machine learning | N/A |
| ADs: 7 | 76.7 ± 11.2 | 3 M | ||||||
| Neumann et al. (2023) [64] | Healthy Controls: 14 | 80.4 | 6 M | Senescent | Iba1, y-H2AX, 8-OHdG, HO-1, lamin B1, ferritin | Dystrophic, ramified | Immunohistochemistry | N/A |
| Aghaizu et al. (2023) [155] | Healthy Controls: 5 | 81.6 | 2 M | Primed | Iba1 | Proliferation and clustering around β-amyloid plaques | Immunohistochemistry | N/A |
| ADs: 6 | 64.5 | 2 M | ||||||
| non-AD Dementia: 2 | 92 | 0 M |
| Marker | Specificity | Labeled States | Staining Patterns | Main Applications | Reference |
|---|---|---|---|---|---|
| Iba1 | macrophages including microglia | homeostatic conditions and disease associated | visualization of microglial cell body and processes, distal extremities. diffuses throughout the cytoplasm | categorization into morphological states, microglial density distribution | Aghaizu et al. (2023) [155] March-Diaz et al. (2021) [165] Tischer et al. (2016) [225] Bachstetter et al. (2017) [81] Bachstetter et al. (2015) [224] Raj et al. (2017) [227] Jiang et al. (2022) [235] Parhizkar et al. (2019) [231] Streit et al. (2009) [27] Shahidehpour et al. (2021) [67] Hu et al. (2021) [57] Flanary et al. (2007) [66] Li et al. (2020) [232] Griciuc et al. (2013) [223] Xie et al. (2022) [236] Olah et al. (2020) [182] Friedberg et al. (2020) [183] Davies et al. (2017) [68] Muñoz-Castro et al. (2022) [237] Marschallinger et al. (2020) [107] Zhao et al. (2022) [238] |
| CD11b/c | macrophages including microglia | homeostatic conditions and disease associated | low basal expression in adult microglia, Staining is mainly restricted to the plasma membrane | categorization into morphological states, microglial density distribution, morphology ultrastructural studies of subsets downregulating IBA1 | Cohn et al. (2021) [184] Walker et al. (2001) [213] Srinivasan et al. (2020) [106] Flanary et al. (2007) [66] Olah et al. (2018) [153] |
| P2RY12 | microglia specific, state dependent | homeostatic marker, strongly downregulated in disease associated and reactive states | staining can localize the plasma membrane or diffuse throughout the cytoplasm | analysis of microglial density, distribution, and morphology ultrastructural studies | Cohn et al. (2021) [184] Walker et al. (2020) [166] Kloske et al. (2021) [234] Olah et al. (2018) [153] |
| TMEM119 | largely microglia specific, state dependent | homeostatic conditions and disease associated | microglial cell bodies and staining of their processes | categorization into morphological states, microglial density distribution, morphology ultrastructural studies in combination with IBA1+-CD68+ cells (ramified and amoeboid morphologies) | Satoh et al. (2016) [226] Cohn et al. (2021) [184] Bonham et al. (2019) [215] Olah et al. (2018) [153] |
| TREM2 | macrophages including microglia, state dependent | aging and disease conditions (e.g., amyloid plaques in AD pathology) | visualization of microglial cell body and processes, distal extremities. diffuses throughout the cytoplasm | categorization into morphological states, microglial density distribution, morphology ultrastructural studies of subsets downregulating IBA1+ cells | Fahrenhold et al. (2018) [105] Krasemann et al. (2017) [87] Satoh et al. (2016) [226] Parhizkar et al. (2019) [231] Li et al. (2020) [232] Olah et al. (2020) [182] Sims et al. (2017) [228] Cohn et al. (2021) [184] Parhizkar et al. (2019) [231] Pascoal et al. (2021) [145] |
| HLA-DR | macrophages including microglia | homeostatic conditions and disease associated | visualization of microglial cell body and processes (ramification and deramification), distal extremities. diffuses throughout the cytoplasm | categorization into morphological states, microglial density distribution, morphology ultrastructural studies | Dhawan et al. (2012) [152] Raj et al. (2017) [227] Smith et al. (2013) [214] Lopes et al. (2008) [36] Kaneshwaran et al. (2019) [229] Streit et al. (2004) [26] |
| CD68 | macrophages including microglia predominantly expressed by lysosomes | reactive states, disease associated | because lysosomes are mostly found near the nucleus in ramified and amoeboid microglia, the characteristic extrusions cannot be seen with CD68 labeling. | categorization into morphological states, microglial density distribution | Hendrickx et al. (2017) [212] |
| MHCII | macrophages including microglia | homeostatic conditions and disease associated | visualization of microglial cell body and processes, distal extremities diffuses throughout the cytoplasm | categorization into morphological states | Muñoz-Castro et al. (2022) [237] Tischer et al. (2016) [225] Fadul et al. (2020) [233] |
| CX3CR1 | macrophages including microglia | homeostatic conditions and disease associated | visualization of microglial cell body and processes | microglial density, distribution, and categorization into morphological states | Keren-Shaul et al. (2017) [91] Krasemann et al. (2017) [87] Olah et al. (2018) [153] Smith et al. (2013) [214] |
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Ginhoux, F.; Lim, S.; Hoeffel, G.; Low, D.; Huber, T. Origin and differentiation of microglia. Front. Cell. Neurosci. 2013, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Prinz, M.; Priller, J. Microglia and brain macrophages in the molecular age: From origin to neuropsychiatric disease. Nat. Rev. Neurosci. 2014, 15, 300–312. [Google Scholar] [CrossRef]
- Greenwood, E.K.; Brown, D.R. Senescent Microglia: The Key to the Ageing Brain? Int. J. Mol. Sci. 2021, 22, 4402. [Google Scholar] [CrossRef] [PubMed]
- Priller, J.; Flügel, A.; Wehner, T.; Boentert, M.; Haas, C.A.; Prinz, M.; Fernández-Klett, F.; Prass, K.; Bechmann, I.; de Boer, B.A.; et al. Targeting gene-modified hematopoietic cells to the central nervous system: Use of green fluorescent protein uncovers microglial engraftment. Nat. Med. 2001, 7, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Kent, S.A.; Miron, V.E. Microglia regulation of central nervous system myelin health and regeneration. Nat. Rev. Immunol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, U.-K.; Kettenmann, H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007, 10, 1387–1394. [Google Scholar] [CrossRef]
- Masuda, T.; Sankowski, R.; Staszewski, O.; Prinz, M. Microglia Heterogeneity in the Single-Cell Era. Cell Rep. 2020, 30, 1271–1281. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Sierra, A.; Stevens, B.; Tremblay, M.-E.; Aguzzi, A.; Ajami, B.; Amit, I.; Audinat, E.; Bechmann, I.; Bennett, M.; et al. Microglia states and nomenclature: A field at its crossroads. Neuron 2022, 110, 3458–3483. [Google Scholar] [CrossRef]
- Buttgereit, A.; Lelios, I.; Yu, X.; Vrohlings, M.; Krakoski, N.R.; Gautier, E.L.; Nishinakamura, R.; Becher, B.; Greter, M. Sall1 is a transcriptional regulator defining microglia identity and function. Nat. Immunol. 2016, 17, 1397–1406. [Google Scholar] [CrossRef]
- Matejuk, A.; Ransohoff, R.M. Crosstalk Between Astrocytes and Microglia: An Overview. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Liu, L.-R.; Liu, J.-C.; Bao, J.-S.; Bai, Q.-Q.; Wang, G.-Q. Interaction of Microglia and Astrocytes in the Neurovascular Unit. Front. Immunol. 2020, 11, 1024. [Google Scholar] [CrossRef] [PubMed]
- Kalafatakis, I.; Karagogeos, D. Oligodendrocytes and Microglia: Key Players in Myelin Development, Damage and Repair. Biomolecules 2021, 11, 1058. [Google Scholar] [CrossRef] [PubMed]
- De Biase, L.M.; Schuebel, K.E.; Fusfeld, Z.H.; Jair, K.; Hawes, I.A.; Cimbro, R.; Zhang, H.-Y.; Liu, Q.-R.; Shen, H.; Xi, Z.-X.; et al. Local Cues Establish and Maintain Region-Specific Phenotypes of Basal Ganglia Microglia. Neuron 2017, 95, 341–356.e6. [Google Scholar] [CrossRef] [PubMed]
- Lawson, L.J.; Perry, V.H.; Dri, P.; Gordon, S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 1990, 39, 151–170. [Google Scholar] [CrossRef]
- Schmid, C.D.; Sautkulis, L.N.; Danielson, P.E.; Cooper, J.; Hasel, K.W.; Hilbush, B.S.; Sutcliffe, J.G.; Carson, M.J. Heterogeneous expression of the triggering receptor expressed on myeloid cells-2 on adult murine microglia. J. Neurochem. 2002, 83, 1309–1320. [Google Scholar] [CrossRef] [PubMed]
- Minett, T.; Classey, J.; Matthews, F.E.; Fahrenhold, M.; Taga, M.; Brayne, C.; Ince, P.G.; Nicoll, J.A.R.; Boche, D. MRC CFAS Microglial immunophenotype in dementia with Alzheimer’s pathology. J. Neuroinflamm. 2016, 13, 135. [Google Scholar] [CrossRef]
- Hopperton, K.E.; Mohammad, D.; Trépanier, M.O.; Giuliano, V.; Bazinet, R.P. Markers of microglia in post-mortem brain samples from patients with Alzheimer’s disease: A systematic review. Mol. Psychiatry 2018, 23, 177–198. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Walker, D.G.; Lue, L.-F. Immune phenotypes of microglia in human neurodegenerative disease: Challenges to detecting microglial polarization in human brains. Alzheimers Res. Ther. 2015, 7, 56. [Google Scholar] [CrossRef]
- Kim, C.C.; Nakamura, M.C.; Hsieh, C.L. Brain trauma elicits non-canonical macrophage activation states. J. Neuroinflamm. 2016, 13, 117. [Google Scholar] [CrossRef]
- Gomez-Nicola, D.; Perry, V.H. Microglial dynamics and role in the healthy and diseased brain: A paradigm of functional plasticity. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2015, 21, 169–184. [Google Scholar] [CrossRef]
- Zia, S.; Rawji, K.S.; Michaels, N.J.; Burr, M.; Kerr, B.J.; Healy, L.M.; Plemel, J.R. Microglia Diversity in Health and Multiple Sclerosis. Front. Immunol. 2020, 11, 588021. [Google Scholar] [CrossRef]
- Stöberl, N.; Maguire, E.; Salis, E.; Shaw, B.; Hall-Roberts, H. Human iPSC-derived glia models for the study of neuroinflammation. J. Neuroinflamm. 2023, 20, 231. [Google Scholar] [CrossRef]
- McGeer, P.L.; Itagaki, S.; Tago, H.; McGeer, E.G. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci. Lett. 1987, 79, 195–200. [Google Scholar] [CrossRef]
- Sheng, J.G.; Mrak, R.E.; Griffin, W.S. Enlarged and phagocytic, but not primed, interleukin-1 alpha-immunoreactive microglia increase with age in normal human brain. Acta Neuropathol. 1998, 95, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Streit, W.J. Microglia and Alzheimer’s disease pathogenesis. J. Neurosci. Res. 2004, 77, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Streit, W.J.; Braak, H.; Xue, Q.-S.; Bechmann, I. Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer’s disease. Acta Neuropathol. 2009, 118, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Spittau, B. Aging Microglia-Phenotypes, Functions and Implications for Age-Related Neurodegenerative Diseases. Front. Aging Neurosci. 2017, 9, 194. [Google Scholar] [CrossRef]
- Holtzman, D.; Ulrich, J. Senescent glia spell trouble in Alzheimer’s disease. Nat. Neurosci. 2019, 22, 683–684. [Google Scholar] [CrossRef]
- Cohen, J.; Torres, C. Astrocyte senescence: Evidence and significance. Aging Cell 2019, 18, e12937. [Google Scholar] [CrossRef]
- Barzilai, N.; Cuervo, A.M.; Austad, S. Aging as a Biological Target for Prevention and Therapy. JAMA 2018, 320, 1321–1322. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Damoiseaux, J.S. Effects of aging on functional and structural brain connectivity. NeuroImage 2017, 160, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Pierce, A.L.; Kawas, C.H. Dementia in the oldest old: Beyond Alzheimer disease. PLoS Med. 2017, 14, e1002263. [Google Scholar] [CrossRef] [PubMed]
- Streit, W.J.; Xue, Q.-S. Human CNS immune senescence and neurodegeneration. Curr. Opin. Immunol. 2014, 29, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Lopes, K.O.; Sparks, D.L.; Streit, W.J. Microglial dystrophy in the aged and Alzheimer’s disease brain is associated with ferritin immunoreactivity. Glia 2008, 56, 1048–1060. [Google Scholar] [CrossRef] [PubMed]
- Coppé, J.-P.; Patil, C.K.; Rodier, F.; Sun, Y.; Muñoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.-Y.; Campisi, J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008, 6, e301. [Google Scholar] [CrossRef] [PubMed]
- Chinta, S.J.; Woods, G.; Rane, A.; Demaria, M.; Campisi, J.; Andersen, J.K. Cellular senescence and the aging brain. Exp. Gerontol. 2015, 68, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Angelova, D.M.; Brown, D.R. Microglia and the aging brain: Are senescent microglia the key to neurodegeneration? J. Neurochem. 2019, 151, 676–688. [Google Scholar] [CrossRef]
- Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef]
- Río-Hortega, P. El tercer elemento de los centros nerviosos. III. Naturaleza probable de la microglía. Bol. Soc. Esp. Biol 1919, 9, 108–120. [Google Scholar]
- Sierra, A.; de Castro, F.; Del Río-Hortega, J.; Rafael Iglesias-Rozas, J.; Garrosa, M.; Kettenmann, H. The “Big-Bang” for modern glial biology: Translation and comments on Pío del Río-Hortega 1919 series of papers on microglia. Glia 2016, 64, 1801–1840. [Google Scholar] [CrossRef] [PubMed]
- Sierra, A.; Paolicelli, R.C.; Kettenmann, H. Cien Años de Microglía: Milestones in a Century of Microglial Research. Trends Neurosci. 2019, 42, 778–792. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [PubMed]
- Varnum, M.M.; Ikezu, T. The classification of microglial activation phenotypes on neurodegeneration and regeneration in Alzheimer’s disease brain. Arch. Immunol. Ther. Exp. 2012, 60, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M. A polarizing question: Do M1 and M2 microglia exist? Nat. Neurosci. 2016, 19, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Song, G.J.; Suk, K. Pharmacological Modulation of Functional Phenotypes of Microglia in Neurodegenerative Diseases. Front. Aging Neurosci. 2017, 9, 139. [Google Scholar] [CrossRef] [PubMed]
- García-Revilla, J.; Alonso-Bellido, I.M.; Burguillos, M.A.; Herrera, A.J.; Espinosa-Oliva, A.M.; Ruiz, R.; Cruz-Hernández, L.; García-Domínguez, I.; Roca-Ceballos, M.A.; Santiago, M.; et al. Reformulating Pro-Oxidant Microglia in Neurodegeneration. J. Clin. Med. 2019, 8, 1719. [Google Scholar] [CrossRef]
- Molina-Martínez, P.; Corpas, R.; García-Lara, E.; Cosín-Tomás, M.; Cristòfol, R.; Kaliman, P.; Solà, C.; Molinuevo, J.L.; Sánchez-Valle, R.; Antonell, A.; et al. Microglial Hyperreactivity Evolved to Immunosuppression in the Hippocampus of a Mouse Model of Accelerated Aging and Alzheimer’s Disease Traits. Front. Aging Neurosci. 2020, 12, 622360. [Google Scholar] [CrossRef]
- Michelucci, A.; Heurtaux, T.; Grandbarbe, L.; Morga, E.; Heuschling, P. Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: Effects of oligomeric and fibrillar amyloid-beta. J. Neuroimmunol. 2009, 210, 3–12. [Google Scholar] [CrossRef]
- Colton, C.A.; Mott, R.T.; Sharpe, H.; Xu, Q.; Van Nostrand, W.E.; Vitek, M.P. Expression profiles for macrophage alternative activation genes in AD and in mouse models of AD. J. Neuroinflamm. 2006, 3, 27. [Google Scholar] [CrossRef][Green Version]
- Masuda, T.; Sankowski, R.; Staszewski, O.; Böttcher, C.; Amann, L.; Sagar; Scheiwe, C.; Nessler, S.; Kunz, P.; van Loo, G.; et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 2019, 566, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Butovsky, O.; Jedrychowski, M.P.; Moore, C.S.; Cialic, R.; Lanser, A.J.; Gabriely, G.; Koeglsperger, T.; Dake, B.; Wu, P.M.; Doykan, C.E.; et al. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat. Neurosci. 2014, 17, 131–143. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Coppé, J.-P.; Desprez, P.-Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Fryatt, G.L.; Ghorbani, M.; Obst, J.; Menassa, D.A.; Martin-Estebane, M.; Muntslag, T.A.O.; Olmos-Alonso, A.; Guerrero-Carrasco, M.; Thomas, D.; et al. Replicative senescence dictates the emergence of disease-associated microglia and contributes to Aβ pathology. Cell Rep. 2021, 35, 109228. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Li, Y.; Hou, Y.; Huang, S.; Pei, G. Alzheimer’s Amyloid-β Accelerates Cell Senescence and Suppresses the SIRT1/NRF2 Pathway in Human Microglial Cells. Oxid. Med. Cell. Longev. 2022, 2022, 3086010. [Google Scholar] [CrossRef]
- Sierra, A.; Gottfried-Blackmore, A.C.; McEwen, B.S.; Bulloch, K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia 2007, 55, 412–424. [Google Scholar] [CrossRef]
- Ziegler, D.V.; Wiley, C.D.; Velarde, M.C. Mitochondrial effectors of cellular senescence: Beyond the free radical theory of aging. Aging Cell 2015, 14, 1–7. [Google Scholar] [CrossRef]
- Correia-Melo, C.; Passos, J.F. Mitochondria: Are they causal players in cellular senescence? Biochim. Biophys. Acta 2015, 1847, 1373–1379. [Google Scholar] [CrossRef]
- Correia-Melo, C.; Marques, F.D.M.; Anderson, R.; Hewitt, G.; Hewitt, R.; Cole, J.; Carroll, B.M.; Miwa, S.; Birch, J.; Merz, A.; et al. Mitochondria are required for pro-ageing features of the senescent phenotype. EMBO J. 2016, 35, 724–742. [Google Scholar] [CrossRef] [PubMed]
- Njie, E.G.; Boelen, E.; Stassen, F.R.; Steinbusch, H.W.M.; Borchelt, D.R.; Streit, W.J. Ex vivo cultures of microglia from young and aged rodent brain reveal age-related changes in microglial function. Neurobiol. Aging 2012, 33, 195.e1–195.e12. [Google Scholar] [CrossRef] [PubMed]
- Neumann, P.; Lenz, D.E.; Streit, W.J.; Bechmann, I. Is microglial dystrophy a form of cellular senescence? An analysis of senescence markers in the aged human brain. Glia 2023, 71, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Flanary, B.E.; Streit, W.J. Progressive telomere shortening occurs in cultured rat microglia, but not astrocytes. Glia 2004, 45, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Flanary, B.E.; Sammons, N.W.; Nguyen, C.; Walker, D.; Streit, W.J. Evidence that aging and amyloid promote microglial cell senescence. Rejuvenation Res. 2007, 10, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Shahidehpour, R.K.; Higdon, R.E.; Crawford, N.G.; Neltner, J.H.; Ighodaro, E.T.; Patel, E.; Price, D.; Nelson, P.T.; Bachstetter, A.D. Dystrophic microglia are associated with neurodegenerative disease and not healthy aging in the human brain. Neurobiol. Aging 2021, 99, 19–27. [Google Scholar] [CrossRef]
- Davies, D.S.; Ma, J.; Jegathees, T.; Goldsbury, C. Microglia show altered morphology and reduced arborization in human brain during aging and Alzheimer’s disease. Brain Pathol. Zurich Switz. 2017, 27, 795–808. [Google Scholar] [CrossRef]
- Baik, S.H.; Kang, S.; Lee, W.; Choi, H.; Chung, S.; Kim, J.-I.; Mook-Jung, I. A Breakdown in Metabolic Reprogramming Causes Microglia Dysfunction in Alzheimer’s Disease. Cell Metab. 2019, 30, 493–507.e6. [Google Scholar] [CrossRef]
- Sierra, A.; Encinas, J.M.; Deudero, J.J.P.; Chancey, J.H.; Enikolopov, G.; Overstreet-Wadiche, L.S.; Tsirka, S.E.; Maletic-Savatic, M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 2010, 7, 483–495. [Google Scholar] [CrossRef]
- VanRyzin, J.W.; Marquardt, A.E.; Argue, K.J.; Vecchiarelli, H.A.; Ashton, S.E.; Arambula, S.E.; Hill, M.N.; McCarthy, M.M. Microglial Phagocytosis of Newborn Cells Is Induced by Endocannabinoids and Sculpts Sex Differences in Juvenile Rat Social Play. Neuron 2019, 102, 435–449.e6. [Google Scholar] [CrossRef]
- Norden, D.M.; Godbout, J.P. Review: Microglia of the aged brain: Primed to be activated and resistant to regulation. Neuropathol. Appl. Neurobiol. 2013, 39, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Niraula, A.; Sheridan, J.F.; Godbout, J.P. Microglia Priming with Aging and Stress. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2017, 42, 318–333. [Google Scholar] [CrossRef]
- Perry, V.H.; Holmes, C. Microglial priming in neurodegenerative disease. Nat. Rev. Neurol. 2014, 10, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.C.; Ruiz, C.R.; Lebson, L.; Selenica, M.-L.B.; Rizer, J.; Hunt, J.B.; Rojiani, R.; Reid, P.; Kammath, S.; Nash, K.; et al. Aging enhances classical activation but mitigates alternative activation in the central nervous system. Neurobiol. Aging 2013, 34, 1610–1620. [Google Scholar] [CrossRef] [PubMed]
- Spencer, N.G.; Schilling, T.; Miralles, F.; Eder, C. Mechanisms Underlying Interferon-γ-Induced Priming of Microglial Reactive Oxygen Species Production. PLoS ONE 2016, 11, e0162497. [Google Scholar] [CrossRef] [PubMed]
- Facci, L.; Barbierato, M.; Marinelli, C.; Argentini, C.; Skaper, S.D.; Giusti, P. Toll-like receptors 2, -3 and -4 prime microglia but not astrocytes across central nervous system regions for ATP-dependent interleukin-1β release. Sci. Rep. 2014, 4, 6824. [Google Scholar] [CrossRef]
- Hickman, S.E.; Allison, E.K.; El Khoury, J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 8354–8360. [Google Scholar] [CrossRef]
- Madry, C.; Kyrargyri, V.; Arancibia-Cárcamo, I.L.; Jolivet, R.; Kohsaka, S.; Bryan, R.M.; Attwell, D. Microglial Ramification, Surveillance, and Interleukin-1β Release Are Regulated by the Two-Pore Domain K+ Channel THIK-1. Neuron 2018, 97, 299–312.e6. [Google Scholar] [CrossRef]
- Abiega, O.; Beccari, S.; Diaz-Aparicio, I.; Nadjar, A.; Layé, S.; Leyrolle, Q.; Gómez-Nicola, D.; Domercq, M.; Pérez-Samartín, A.; Sánchez-Zafra, V.; et al. Neuronal Hyperactivity Disturbs ATP Microgradients, Impairs Microglial Motility, and Reduces Phagocytic Receptor Expression Triggering Apoptosis/Microglial Phagocytosis Uncoupling. PLoS Biol. 2016, 14, e1002466. [Google Scholar] [CrossRef]
- Bachstetter, A.D.; Ighodaro, E.T.; Hassoun, Y.; Aldeiri, D.; Neltner, J.H.; Patel, E.; Abner, E.L.; Nelson, P.T. Rod-shaped microglia morphology is associated with aging in 2 human autopsy series. Neurobiol. Aging 2017, 52, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Safaiyan, S.; Besson-Girard, S.; Kaya, T.; Cantuti-Castelvetri, L.; Liu, L.; Ji, H.; Schifferer, M.; Gouna, G.; Usifo, F.; Kannaiyan, N.; et al. White matter aging drives microglial diversity. Neuron 2021, 109, 1100–1117.e10. [Google Scholar] [CrossRef] [PubMed]
- Hammond, T.R.; Dufort, C.; Dissing-Olesen, L.; Giera, S.; Young, A.; Wysoker, A.; Walker, A.J.; Gergits, F.; Segel, M.; Nemesh, J.; et al. Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes. Immunity 2019, 50, 253–271.e6. [Google Scholar] [CrossRef] [PubMed]
- Felsky, D.; Roostaei, T.; Nho, K.; Risacher, S.L.; Bradshaw, E.M.; Petyuk, V.; Schneider, J.A.; Saykin, A.; Bennett, D.A.; De Jager, P.L. Neuropathological correlates and genetic architecture of microglial activation in elderly human brain. Nat. Commun. 2019, 10, 409. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cheng, Z.; Zhou, L.; Darmanis, S.; Neff, N.F.; Okamoto, J.; Gulati, G.; Bennett, M.L.; Sun, L.O.; Clarke, L.E.; et al. Developmental Heterogeneity of Microglia and Brain Myeloid Cells Revealed by Deep Single-Cell RNA Sequencing. Neuron 2019, 101, 207–223.e10. [Google Scholar] [CrossRef]
- Deczkowska, A.; Keren-Shaul, H.; Weiner, A.; Colonna, M.; Schwartz, M.; Amit, I. Disease-Associated Microglia: A Universal Immune Sensor of Neurodegeneration. Cell 2018, 173, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Krasemann, S.; Madore, C.; Cialic, R.; Baufeld, C.; Calcagno, N.; El Fatimy, R.; Beckers, L.; O’Loughlin, E.; Xu, Y.; Fanek, Z.; et al. The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity 2017, 47, 566–581.e9. [Google Scholar] [CrossRef] [PubMed]
- Ajami, B.; Samusik, N.; Wieghofer, P.; Ho, P.P.; Crotti, A.; Bjornson, Z.; Prinz, M.; Fantl, W.J.; Nolan, G.P.; Steinman, L. Single-cell mass cytometry reveals distinct populations of brain myeloid cells in mouse neuroinflammation and neurodegeneration models. Nat. Neurosci. 2018, 21, 541–551. [Google Scholar] [CrossRef]
- Song, W.M.; Colonna, M. The identity and function of microglia in neurodegeneration. Nat. Immunol. 2018, 19, 1048–1058. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Ferretti, M.T. Function and Dysfunction of Microglia during Brain Development: Consequences for Synapses and Neural Circuits. Front. Synaptic Neurosci. 2017, 9, 9. [Google Scholar] [CrossRef]
- Keren-Shaul, H.; Spinrad, A.; Weiner, A.; Matcovitch-Natan, O.; Dvir-Szternfeld, R.; Ulland, T.K.; David, E.; Baruch, K.; Lara-Astaiso, D.; Toth, B.; et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 2017, 169, 1276–1290.e17. [Google Scholar] [CrossRef]
- Friedman, B.A.; Srinivasan, K.; Ayalon, G.; Meilandt, W.J.; Lin, H.; Huntley, M.A.; Cao, Y.; Lee, S.-H.; Haddick, P.C.G.; Ngu, H.; et al. Diverse Brain Myeloid Expression Profiles Reveal Distinct Microglial Activation States and Aspects of Alzheimer’s Disease Not Evident in Mouse Models. Cell Rep. 2018, 22, 832–847. [Google Scholar] [CrossRef]
- Silvin, A.; Uderhardt, S.; Piot, C.; Da Mesquita, S.; Yang, K.; Geirsdottir, L.; Mulder, K.; Eyal, D.; Liu, Z.; Bridlance, C.; et al. Dual ontogeny of disease-associated microglia and disease inflammatory macrophages in aging and neurodegeneration. Immunity 2022, 55, 1448–1465.e6. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Rochford, C.D.P.; Neumann, H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J. Exp. Med. 2005, 201, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Hamerman, J.A.; Jarjoura, J.R.; Humphrey, M.B.; Nakamura, M.C.; Seaman, W.E.; Lanier, L.L. Cutting edge: Inhibition of TLR and FcR responses in macrophages by triggering receptor expressed on myeloid cells (TREM)-2 and DAP12. J. Immunol. 2006, 177, 2051–2055. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Chen, X.-F.; Wang, T.; Wang, Z.; Liao, C.; Wang, Z.; Huang, R.; Wang, D.; Li, X.; Wu, L.; et al. Soluble TREM2 induces inflammatory responses and enhances microglial survival. J. Exp. Med. 2017, 214, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, J.D.; Ulland, T.K.; Colonna, M.; Holtzman, D.M. Elucidating the Role of TREM2 in Alzheimer’s Disease. Neuron 2017, 94, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Yeh, F.L.; Hansen, D.V.; Sheng, M. TREM2, Microglia, and Neurodegenerative Diseases. Trends Mol. Med. 2017, 23, 512–533. [Google Scholar] [CrossRef]
- Carmona, S.; Zahs, K.; Wu, E.; Dakin, K.; Bras, J.; Guerreiro, R. The role of TREM2 in Alzheimer’s disease and other neurodegenerative disorders. Lancet Neurol. 2018, 17, 721–730. [Google Scholar] [CrossRef]
- Jurga, A.M.; Paleczna, M.; Kuter, K.Z. Overview of General and Discriminating Markers of Differential Microglia Phenotypes. Front. Cell. Neurosci. 2020, 14, 198. [Google Scholar] [CrossRef]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed. J. Neuroinflamm. 2014, 11, 98. [Google Scholar] [CrossRef]
- Edwards, J.P.; Zhang, X.; Frauwirth, K.A.; Mosser, D.M. Biochemical and functional characterization of three activated macrophage populations. J. Leukoc. Biol. 2006, 80, 1298–1307. [Google Scholar] [CrossRef]
- Knudsen, N.H.; Lee, C.-H. Identity Crisis: CD301b(+) Mononuclear Phagocytes Blur the M1-M2 Macrophage Line. Immunity 2016, 45, 461–463. [Google Scholar] [CrossRef]
- Hickman, S.E.; Kingery, N.D.; Ohsumi, T.K.; Borowsky, M.L.; Wang, L.; Means, T.K.; El Khoury, J. The microglial sensome revealed by direct RNA sequencing. Nat. Neurosci. 2013, 16, 1896–1905. [Google Scholar] [CrossRef]
- Fahrenhold, M.; Rakic, S.; Classey, J.; Brayne, C.; Ince, P.G.; Nicoll, J.A.R.; Boche, D. Mrc-Cfas TREM2 expression in the human brain: A marker of monocyte recruitment? Brain Pathol. 2018, 28, 595–602. [Google Scholar] [CrossRef]
- Srinivasan, K.; Friedman, B.A.; Etxeberria, A.; Huntley, M.A.; van der Brug, M.P.; Foreman, O.; Paw, J.S.; Modrusan, Z.; Beach, T.G.; Serrano, G.E.; et al. Alzheimer’s Patient Microglia Exhibit Enhanced Aging and Unique Transcriptional Activation. Cell Rep. 2020, 31, 107843. [Google Scholar] [CrossRef] [PubMed]
- Marschallinger, J.; Iram, T.; Zardeneta, M.; Lee, S.E.; Lehallier, B.; Haney, M.S.; Pluvinage, J.V.; Mathur, V.; Hahn, O.; Morgens, D.W.; et al. Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat. Neurosci. 2020, 23, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.; Medici, V.; Malagoli, D.; Chiariello, A.; Cirrincione, A.; Davin, A.; Chikhladze, M.; Vasuri, F.; Legname, G.; Ferrer, I.; et al. Expression pattern of perilipins in human brain during aging and in Alzheimer’s disease. Neuropathol. Appl. Neurobiol. 2022, 48, e12756. [Google Scholar] [CrossRef]
- Gosselin, D.; Skola, D.; Coufal, N.G.; Holtman, I.R.; Schlachetzki, J.C.M.; Sajti, E.; Jaeger, B.N.; O’Connor, C.; Fitzpatrick, C.; Pasillas, M.P.; et al. An environment-dependent transcriptional network specifies human microglia identity. Science 2017, 356, eaal3222. [Google Scholar] [CrossRef] [PubMed]
- Galatro, T.F.; Holtman, I.R.; Lerario, A.M.; Vainchtein, I.D.; Brouwer, N.; Sola, P.R.; Veras, M.M.; Pereira, T.F.; Leite, R.E.P.; Möller, T.; et al. Transcriptomic analysis of purified human cortical microglia reveals age-associated changes. Nat. Neurosci. 2017, 20, 1162–1171. [Google Scholar] [CrossRef]
- Böttcher, C.; Schlickeiser, S.; Sneeboer, M.A.M.; Kunkel, D.; Knop, A.; Paza, E.; Fidzinski, P.; Kraus, L.; Snijders, G.J.L.; Kahn, R.S.; et al. Human microglia regional heterogeneity and phenotypes determined by multiplexed single-cell mass cytometry. Nat. Neurosci. 2019, 22, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Grabert, K.; Michoel, T.; Karavolos, M.H.; Clohisey, S.; Baillie, J.K.; Stevens, M.P.; Freeman, T.C.; Summers, K.M.; McColl, B.W. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat. Neurosci. 2016, 19, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L. Complexity of danger: The diverse nature of damage-associated molecular patterns. J. Biol. Chem. 2014, 289, 35237–35245. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Coyne, C.B.; Zeh, H.J.; Lotze, M.T. PAMPs and DAMPs: Signal 0s that spur autophagy and immunity. Immunol. Rev. 2012, 249, 158–175. [Google Scholar] [CrossRef]
- Bianchi, M.E. DAMPs, PAMPs and alarmins: All we need to know about danger. J. Leukoc. Biol. 2007, 81, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R. Cytokine, Sickness Behavior, and Depression. Immunol. Allergy Clin. N. Am. 2009, 29, 247–264. [Google Scholar] [CrossRef]
- Cunningham, C.; Wilcockson, D.C.; Campion, S.; Lunnon, K.; Perry, V.H. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 9275–9284. [Google Scholar] [CrossRef]
- Sousa, C.; Golebiewska, A.; Poovathingal, S.K.; Kaoma, T.; Pires-Afonso, Y.; Martina, S.; Coowar, D.; Azuaje, F.; Skupin, A.; Balling, R.; et al. Single-cell transcriptomics reveals distinct inflammation-induced microglia signatures. EMBO Rep. 2018, 19, e46171. [Google Scholar] [CrossRef]
- Shemer, A.; Scheyltjens, I.; Frumer, G.R.; Kim, J.-S.; Grozovski, J.; Ayanaw, S.; Dassa, B.; Van Hove, H.; Chappell-Maor, L.; Boura-Halfon, S.; et al. Interleukin-10 Prevents Pathological Microglia Hyperactivation following Peripheral Endotoxin Challenge. Immunity 2020, 53, 1033–1049.e7. [Google Scholar] [CrossRef]
- Poloni, T.E.; Medici, V.; Moretti, M.; Visonà, S.D.; Cirrincione, A.; Carlos, A.F.; Davin, A.; Gagliardi, S.; Pansarasa, O.; Cereda, C.; et al. COVID-19-related neuropathology and microglial activation in elderly with and without dementia. Brain Pathol. Zurich Switz. 2021, 31, e12997. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.C.; Kern, F.; Losada, P.M.; Agam, M.R.; Maat, C.A.; Schmartz, G.P.; Fehlmann, T.; Stein, J.A.; Schaum, N.; Lee, D.P.; et al. Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature 2021, 595, 565–571. [Google Scholar] [CrossRef]
- Abdel-Haq, R.; Schlachetzki, J.C.M.; Glass, C.K.; Mazmanian, S.K. Microbiome-microglia connections via the gut-brain axis. J. Exp. Med. 2019, 216, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Gelosa, P.; Castiglioni, L.; Cimino, M.; Rizzi, N.; Pepe, G.; Lolli, F.; Marcello, E.; Sironi, L.; Vegeto, E.; et al. Sex-Specific Features of Microglia from Adult Mice. Cell Rep. 2018, 23, 3501–3511. [Google Scholar] [CrossRef]
- Lund, H.; Pieber, M.; Harris, R.A. Lessons Learned about Neurodegeneration from Microglia and Monocyte Depletion Studies. Front. Aging Neurosci. 2017, 9, 234. [Google Scholar] [CrossRef] [PubMed]
- Chitu, V.; Gökhan, Ş.; Stanley, E.R. Modeling CSF-1 receptor deficiency diseases—how close are we? FEBS J. 2022, 289, 5049–5073. [Google Scholar] [CrossRef]
- Herskovits, A.Z.; Guarente, L. SIRT1 in neurodevelopment and brain senescence. Neuron 2014, 81, 471–483. [Google Scholar] [CrossRef]
- Xu, C.; Wang, L.; Fozouni, P.; Evjen, G.; Chandra, V.; Jiang, J.; Lu, C.; Nicastri, M.; Bretz, C.; Winkler, J.D.; et al. SIRT1 is downregulated by autophagy in senescence and ageing. Nat. Cell Biol. 2020, 22, 1170–1179. [Google Scholar] [CrossRef]
- Imperatore, F.; Maurizio, J.; Vargas Aguilar, S.; Busch, C.J.; Favret, J.; Kowenz-Leutz, E.; Cathou, W.; Gentek, R.; Perrin, P.; Leutz, A.; et al. SIRT1 regulates macrophage self-renewal. EMBO J. 2017, 36, 2353–2372. [Google Scholar] [CrossRef]
- Radak, Z.; Suzuki, K.; Posa, A.; Petrovszky, Z.; Koltai, E.; Boldogh, I. The systemic role of SIRT1 in exercise mediated adaptation. Redox Biol. 2020, 35, 101467. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Wang, X.; Yang, D.; Lu, Y.; Wei, G.; Yu, W.; Liu, X.; Zheng, Q.; Ying, J.; Hua, F. Relieving Cellular Energy Stress in Aging, Neurodegenerative, and Metabolic Diseases, SIRT1 as a Therapeutic and Promising Node. Front. Aging Neurosci. 2021, 13, 738686. [Google Scholar] [CrossRef] [PubMed]
- Jiao, F.; Gong, Z. The Beneficial Roles of SIRT1 in Neuroinflammation-Related Diseases. Oxid. Med. Cell. Longev. 2020, 2020, 6782872. [Google Scholar] [CrossRef]
- Mishra, P.; Mittal, A.K.; Kalonia, H.; Madan, S.; Ghosh, S.; Sinha, J.K.; Rajput, S.K. SIRT1 Promotes Neuronal Fortification in Neurodegenerative Diseases through Attenuation of Pathological Hallmarks and Enhancement of Cellular Lifespan. Curr. Neuropharmacol. 2021, 19, 1019–1037. [Google Scholar] [CrossRef]
- Cao, K.; Dong, Y.-T.; Xiang, J.; Xu, Y.; Hong, W.; Song, H.; Guan, Z.-Z. Reduced expression of SIRT1 and SOD-1 and the correlation between these levels in various regions of the brains of patients with Alzheimer’s disease. J. Clin. Pathol. 2018, 71, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Julien, C.; Tremblay, C.; Emond, V.; Lebbadi, M.; Salem, N.; Bennett, D.A.; Calon, F. Sirtuin 1 reduction parallels the accumulation of tau in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2009, 68, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Yang, T.; Ho, L.; Zhao, Z.; Wang, J.; Chen, L.; Zhao, W.; Thiyagarajan, M.; MacGrogan, D.; Rodgers, J.T.; et al. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J. Biol. Chem. 2006, 281, 21745–21754. [Google Scholar] [CrossRef]
- Hou, Y.; Chen, H.; He, Q.; Jiang, W.; Luo, T.; Duan, J.; Mu, N.; He, Y.; Wang, H. Changes in methylation patterns of multiple genes from peripheral blood leucocytes of Alzheimer’s disease patients. Acta Neuropsychiatr. 2013, 25, 66–76. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Y.; Mueller-Steiner, S.; Chen, L.-F.; Kwon, H.; Yi, S.; Mucke, L.; Gan, L. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J. Biol. Chem. 2005, 280, 40364–40374. [Google Scholar] [CrossRef]
- Arioz, B.I.; Tastan, B.; Tarakcioglu, E.; Tufekci, K.U.; Olcum, M.; Ersoy, N.; Bagriyanik, A.; Genc, K.; Genc, S. Melatonin Attenuates LPS-Induced Acute Depressive-Like Behaviors and Microglial NLRP3 Inflammasome Activation Through the SIRT1/Nrf2 Pathway. Front. Immunol. 2019, 10, 1511. [Google Scholar] [CrossRef]
- Zhang, X.-S.; Lu, Y.; Li, W.; Tao, T.; Peng, L.; Wang, W.-H.; Gao, S.; Liu, C.; Zhuang, Z.; Xia, D.-Y.; et al. Astaxanthin ameliorates oxidative stress and neuronal apoptosis via SIRT1/NRF2/Prx2/ASK1/p38 after traumatic brain injury in mice. Br. J. Pharmacol. 2021, 178, 1114–1132. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Zhang, H.; Huang, S.; Pei, G. PL201, a Reported Rhamnoside Against Alzheimer’s Disease Pathology, Alleviates Neuroinflammation and Stimulates Nrf2 Signaling. Front. Immunol. 2020, 11, 162. [Google Scholar] [CrossRef]
- Paolini Paoletti, F.; Simoni, S.; Parnetti, L.; Gaetani, L. The Contribution of Small Vessel Disease to Neurodegeneration: Focus on Alzheimer’s Disease, Parkinson’s Disease and Multiple Sclerosis. Int. J. Mol. Sci. 2021, 22, 4958. [Google Scholar] [CrossRef]
- DiPatre, P.L.; Gelman, B.B. Microglial cell activation in aging and Alzheimer disease: Partial linkage with neurofibrillary tangle burden in the hippocampus. J. Neuropathol. Exp. Neurol. 1997, 56, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Pascoal, T.A.; Benedet, A.L.; Ashton, N.J.; Kang, M.S.; Therriault, J.; Chamoun, M.; Savard, M.; Lussier, F.Z.; Tissot, C.; Karikari, T.K.; et al. Microglial activation and tau propagate jointly across Braak stages. Nat. Med. 2021, 27, 1592–1599. [Google Scholar] [CrossRef]
- Takeda, S.; Commins, C.; DeVos, S.L.; Nobuhara, C.K.; Wegmann, S.; Roe, A.D.; Costantino, I.; Fan, Z.; Nicholls, S.B.; Sherman, A.E.; et al. Seed-competent high-molecular-weight tau species accumulates in the cerebrospinal fluid of Alzheimer’s disease mouse model and human patients. Ann. Neurol. 2016, 80, 355–367. [Google Scholar] [CrossRef] [PubMed]
- DeVos, S.L.; Miller, R.L.; Schoch, K.M.; Holmes, B.B.; Kebodeaux, C.S.; Wegener, A.J.; Chen, G.; Shen, T.; Tran, H.; Nichols, B.; et al. Tau reduction prevents neuronal loss and reverses pathological tau deposition and seeding in mice with tauopathy. Sci. Transl. Med. 2017, 9, eaag0481. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Allen, D.; Fracassi, A.; Tumurbaatar, B.; Natarajan, C.; Scaduto, P.; Woltjer, R.; Kayed, R.; Limon, A.; Krishnan, B.; et al. Functional Integrity of Synapses in the Central Nervous System of Cognitively Intact Individuals with High Alzheimer’s Disease Neuropathology Is Associated with Absence of Synaptic Tau Oligomers. J. Alzheimers Dis. JAD 2020, 78, 1661–1678. [Google Scholar] [CrossRef]
- Perez-Nievas, B.G.; Stein, T.D.; Tai, H.-C.; Dols-Icardo, O.; Scotton, T.C.; Barroeta-Espar, I.; Fernandez-Carballo, L.; de Munain, E.L.; Perez, J.; Marquie, M.; et al. Dissecting phenotypic traits linked to human resilience to Alzheimer’s pathology. Brain J. Neurol. 2013, 136, 2510–2526. [Google Scholar] [CrossRef]
- von Bartheld, C.S.; Bahney, J.; Herculano-Houzel, S. The Search for True Numbers of Neurons and Glial Cells in the Human Brain: A Review of 150 Years of Cell Counting. J. Comp. Neurol. 2016, 524, 3865–3895. [Google Scholar] [CrossRef]
- Taddei, R.N.; Perbet, R.; Mate de Gerando, A.; Wiedmer, A.E.; Sanchez-Mico, M.; Connors Stewart, T.; Gaona, A.; Melloni, A.; Amaral, A.C.; Duff, K.; et al. Tau Oligomer–Containing Synapse Elimination by Microglia and Astrocytes in Alzheimer Disease. JAMA Neurol. 2023, 80, 1209–1221. [Google Scholar] [CrossRef]
- Dhawan, G.; Floden, A.M.; Combs, C.K. Amyloid-β oligomers stimulate microglia through a tyrosine kinase dependent mechanism. Neurobiol. Aging 2012, 33, 2247–2261. [Google Scholar] [CrossRef] [PubMed]
- Olah, M.; Patrick, E.; Villani, A.-C.; Xu, J.; White, C.C.; Ryan, K.J.; Piehowski, P.; Kapasi, A.; Nejad, P.; Cimpean, M.; et al. A transcriptomic atlas of aged human microglia. Nat. Commun. 2018, 9, 539. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sudan, R.; Peng, V.; Zhou, Y.; Du, S.; Yuede, C.M.; Lei, T.; Hou, J.; Cai, Z.; Cella, M.; et al. TREM2 drives microglia response to amyloid-β via SYK-dependent and -independent pathways. Cell 2022, 185, 4153–4169.e19. [Google Scholar] [CrossRef] [PubMed]
- Aghaizu, N.D.; Jolly, S.; Samra, S.K.; Kalmar, B.; Craessaerts, K.; Greensmith, L.; Salinas, P.C.; De Strooper, B.; Whiting, P.J. Microglial Expression of the Wnt Signaling Modulator DKK2 Differs between Human Alzheimer’s Disease Brains and Mouse Neurodegeneration Models. eNeuro 2023, 10. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, D.; Link, V.M.; Romanoski, C.E.; Fonseca, G.J.; Eichenfield, D.Z.; Spann, N.J.; Stender, J.D.; Chun, H.B.; Garner, H.; Geissmann, F.; et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell 2014, 159, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Toledo, J.B.; Da, X.; Weiner, M.W.; Wolk, D.A.; Xie, S.X.; Arnold, S.E.; Davatzikos, C.; Shaw, L.M.; Trojanowski, J.Q. CSF Apo-E levels associate with cognitive decline and MRI changes. Acta Neuropathol. 2014, 127, 621–632. [Google Scholar] [CrossRef]
- Tcw, J.; Qian, L.; Pipalia, N.H.; Chao, M.J.; Liang, S.A.; Shi, Y.; Jain, B.R.; Bertelsen, S.E.; Kapoor, M.; Marcora, E.; et al. Cholesterol and matrisome pathways dysregulated in astrocytes and microglia. Cell 2022, 185, 2213–2233.e25. [Google Scholar] [CrossRef]
- Lee, S.-I.; Jeong, W.; Lim, H.; Cho, S.; Lee, H.; Jang, Y.; Cho, J.; Bae, S.; Lin, Y.-T.; Tsai, L.-H.; et al. APOE4-carrying human astrocytes oversupply cholesterol to promote neuronal lipid raft expansion and Aβ generation. Stem Cell Rep. 2021, 16, 2128–2137. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Seo, J.; Gao, F.; Feldman, H.M.; Wen, H.-L.; Penney, J.; Cam, H.P.; Gjoneska, E.; Raja, W.K.; Cheng, J.; et al. APOE4 Causes Widespread Molecular and Cellular Alterations Associated with Alzheimer’s Disease Phenotypes in Human iPSC-Derived Brain Cell Types. Neuron 2018, 98, 1294. [Google Scholar] [CrossRef]
- Liu, C.-C.; Wang, N.; Chen, Y.; Inoue, Y.; Shue, F.; Ren, Y.; Wang, M.; Qiao, W.; Ikezu, T.C.; Li, Z.; et al. Cell-autonomous effects of APOE4 in restricting microglial response in brain homeostasis and Alzheimer’s disease. Nat. Immunol. 2023, 24, 1854–1866. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Rosenzweig, N.; Kleemann, K.L.; Zhang, X.; Brandão, W.; Margeta, M.A.; Schroeder, C.; Sivanathan, K.N.; Silveira, S.; Gauthier, C.; et al. APOE4 impairs the microglial response in Alzheimer’s disease by inducing TGFβ-mediated checkpoints. Nat. Immunol. 2023, 24, 1839–1853. [Google Scholar] [CrossRef]
- Litvinchuk, A.; Suh, J.H.; Guo, J.L.; Lin, K.; Davis, S.S.; Bien-Ly, N.; Tycksen, E.; Tabor, G.T.; Remolina Serrano, J.; Manis, M.; et al. Amelioration of Tau and ApoE4-linked glial lipid accumulation and neurodegeneration with an LXR agonist. Neuron 2023. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.R.; Liu, P.; Agrawal, A.; Yip, O.; Blumenfeld, J.; Traglia, M.; Kim, M.J.; Koutsodendris, N.; Rao, A.; Grone, B.; et al. The APOE-R136S mutation protects against APOE4-driven Tau pathology, neurodegeneration and neuroinflammation. Nat. Neurosci. 2023, 26, 2104–2121. [Google Scholar] [CrossRef] [PubMed]
- March-Diaz, R.; Lara-Ureña, N.; Romero-Molina, C.; Heras-Garvin, A.; Ortega-de San Luis, C.; Alvarez-Vergara, M.I.; Sanchez-Garcia, M.A.; Sanchez-Mejias, E.; Davila, J.C.; Rosales-Nieves, A.E.; et al. Hypoxia compromises the mitochondrial metabolism of Alzheimer’s disease microglia via HIF1. Nat. Aging 2021, 1, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.G.; Tang, T.M.; Mendsaikhan, A.; Tooyama, I.; Serrano, G.E.; Sue, L.I.; Beach, T.G.; Lue, L.-F. Patterns of Expression of Purinergic Receptor P2RY12, a Putative Marker for Non-Activated Microglia, in Aged and Alzheimer’s Disease Brains. Int. J. Mol. Sci. 2020, 21, 678. [Google Scholar] [CrossRef]
- Perry, V.H.; Hume, D.A.; Gordon, S. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience 1985, 15, 313–326. [Google Scholar] [CrossRef]
- Lavin, Y.; Winter, D.; Blecher-Gonen, R.; David, E.; Keren-Shaul, H.; Merad, M.; Jung, S.; Amit, I. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 2014, 159, 1312–1326. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, L.; He, S.; Wu, Y.; Jin, W.; Yu, T.; Qu, J.Y.; Wen, Z. Temporal-Spatial Resolution Fate Mapping Reveals Distinct Origins for Embryonic and Adult Microglia in Zebrafish. Dev. Cell 2015, 34, 632–641. [Google Scholar] [CrossRef]
- Ferrero, G.; Mahony, C.B.; Dupuis, E.; Yvernogeau, L.; Di Ruggiero, E.; Miserocchi, M.; Caron, M.; Robin, C.; Traver, D.; Bertrand, J.Y.; et al. Embryonic Microglia Derive from Primitive Macrophages and Are Replaced by cmyb-Dependent Definitive Microglia in Zebrafish. Cell Rep. 2018, 24, 130–141. [Google Scholar] [CrossRef]
- Andjelkovic, A.V.; Nikolic, B.; Pachter, J.S.; Zecevic, N. Macrophages/microglial cells in human central nervous system during development: An immunohistochemical study. Brain Res. 1998, 814, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.; Gomez Perdiguero, E.; Chorro, L.; Szabo-Rogers, H.; Cagnard, N.; Kierdorf, K.; Prinz, M.; Wu, B.; Jacobsen, S.E.W.; Pollard, J.W.; et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 2012, 336, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Kierdorf, K.; Erny, D.; Goldmann, T.; Sander, V.; Schulz, C.; Perdiguero, E.G.; Wieghofer, P.; Heinrich, A.; Riemke, P.; Hölscher, C.; et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci. 2013, 16, 273–280. [Google Scholar] [CrossRef]
- Stremmel, C.; Schuchert, R.; Wagner, F.; Thaler, R.; Weinberger, T.; Pick, R.; Mass, E.; Ishikawa-Ankerhold, H.C.; Margraf, A.; Hutter, S.; et al. Yolk sac macrophage progenitors traffic to the embryo during defined stages of development. Nat. Commun. 2018, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef]
- Prinz, M.; Jung, S.; Priller, J. Microglia Biology: One Century of Evolving Concepts. Cell 2019, 179, 292–311. [Google Scholar] [CrossRef]
- Hawkes, C.A.; McLaurin, J. Selective targeting of perivascular macrophages for clearance of beta-amyloid in cerebral amyloid angiopathy. Proc. Natl. Acad. Sci. USA 2009, 106, 1261–1266. [Google Scholar] [CrossRef]
- McKercher, S.R.; Torbett, B.E.; Anderson, K.L.; Henkel, G.W.; Vestal, D.J.; Baribault, H.; Klemsz, M.; Feeney, A.J.; Wu, G.E.; Paige, C.J.; et al. Targeted disruption of the PU.1 gene results in multiple hematopoietic abnormalities. EMBO J. 1996, 15, 5647–5658. [Google Scholar] [CrossRef]
- Beers, D.R.; Henkel, J.S.; Xiao, Q.; Zhao, W.; Wang, J.; Yen, A.A.; Siklos, L.; McKercher, S.R.; Appel, S.H. Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA 2006, 103, 16021–16026. [Google Scholar] [CrossRef]
- Varvel, N.H.; Grathwohl, S.A.; Baumann, F.; Liebig, C.; Bosch, A.; Brawek, B.; Thal, D.R.; Charo, I.F.; Heppner, F.L.; Aguzzi, A.; et al. Microglial repopulation model reveals a robust homeostatic process for replacing CNS myeloid cells. Proc. Natl. Acad. Sci. USA 2012, 109, 18150–18155. [Google Scholar] [CrossRef]
- Barroeta-Espar, I.; Weinstock, L.D.; Perez-Nievas, B.G.; Meltzer, A.C.; Siao Tick Chong, M.; Amaral, A.C.; Murray, M.E.; Moulder, K.L.; Morris, J.C.; Cairns, N.J.; et al. Distinct cytokine profiles in human brains resilient to Alzheimer’s pathology. Neurobiol. Dis. 2019, 121, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Olah, M.; Menon, V.; Habib, N.; Taga, M.F.; Ma, Y.; Yung, C.J.; Cimpean, M.; Khairallah, A.; Coronas-Samano, G.; Sankowski, R.; et al. Single cell RNA sequencing of human microglia uncovers a subset associated with Alzheimer’s disease. Nat. Commun. 2020, 11, 6129. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, J.S.; Aytan, N.; Cherry, J.D.; Xia, W.; Standring, O.J.; Alvarez, V.E.; Nicks, R.; Svirsky, S.; Meng, G.; Jun, G.; et al. Associations between brain inflammatory profiles and human neuropathology are altered based on apolipoprotein E ε4 genotype. Sci. Rep. 2020, 10, 2924. [Google Scholar] [CrossRef]
- Cohn, W.; Melnik, M.; Huang, C.; Teter, B.; Chandra, S.; Zhu, C.; McIntire, L.B.; John, V.; Gylys, K.H.; Bilousova, T. Multi-Omics Analysis of Microglial Extracellular Vesicles From Human Alzheimer’s Disease Brain Tissue Reveals Disease-Associated Signatures. Front. Pharmacol. 2021, 12, 766082. [Google Scholar] [CrossRef]
- Xiong, T.; Wang, X.; Zha, Y.; Wang, Y. Interleukin-33 regulates the functional state of microglia. Front. Cell. Neurosci. 2022, 16, 1012968. [Google Scholar] [CrossRef]
- Chou, V.; Pearse, R.V.; Aylward, A.J.; Ashour, N.; Taga, M.; Terzioglu, G.; Fujita, M.; Fancher, S.B.; Sigalov, A.; Benoit, C.R.; et al. INPP5D regulates inflammasome activation in human microglia. Nat. Commun. 2023, 14, 7552. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, E.L.; Iwasaki, K.; Tsuji, Y. Intracellular iron transport and storage: From molecular mechanisms to health implications. Antioxid. Redox Signal. 2008, 10, 997–1030. [Google Scholar] [CrossRef]
- Burns, J.C.; Cotleur, B.; Walther, D.M.; Bajrami, B.; Rubino, S.J.; Wei, R.; Franchimont, N.; Cotman, S.L.; Ransohoff, R.M.; Mingueneau, M. Differential accumulation of storage bodies with aging defines discrete subsets of microglia in the healthy brain. eLife 2020, 9, e57495. [Google Scholar] [CrossRef]
- Honda, K.; Casadesus, G.; Petersen, R.B.; Perry, G.; Smith, M.A. Oxidative stress and redox-active iron in Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2004, 1012, 179–182. [Google Scholar] [CrossRef]
- Zecca, L.; Youdim, M.B.H.; Riederer, P.; Connor, J.R.; Crichton, R.R. Iron, brain ageing and neurodegenerative disorders. Nat. Rev. Neurosci. 2004, 5, 863–873. [Google Scholar] [CrossRef]
- Castellani, R.J.; Moreira, P.I.; Perry, G.; Zhu, X. The role of iron as a mediator of oxidative stress in Alzheimer disease. BioFactors Oxf. Engl. 2012, 38, 133–138. [Google Scholar] [CrossRef]
- Streit, W.J.; Xue, Q.-S. Microglia in dementia with Lewy bodies. Brain. Behav. Immun. 2016, 55, 191–201. [Google Scholar] [CrossRef]
- Streit, W.J.; Braak, H.; Del Tredici, K.; Leyh, J.; Lier, J.; Khoshbouei, H.; Eisenlöffel, C.; Müller, W.; Bechmann, I. Microglial activation occurs late during preclinical Alzheimer’s disease. Glia 2018, 66, 2550–2562. [Google Scholar] [CrossRef]
- Streit, W.J.; Khoshbouei, H.; Bechmann, I. Dystrophic microglia in late-onset Alzheimer’s disease. Glia 2020, 68, 845–854. [Google Scholar] [CrossRef]
- Lin, G.; Li, X.; Cheng, X.; Zhao, N.; Zheng, W. Manganese Exposure Aggravates β-Amyloid Pathology by Microglial Activation. Front. Aging Neurosci. 2020, 12, 556008. [Google Scholar] [CrossRef]
- Angelova, D.M.; Brown, D.R. Model Senescent Microglia Induce Disease Related Changes in α-Synuclein Expression and Activity. Biomolecules 2018, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Angelova, D.M.; Brown, D.R. Altered Processing of β-Amyloid in SH-SY5Y Cells Induced by Model Senescent Microglia. ACS Chem. Neurosci. 2018, 9, 3137–3152. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, A.; Clark, M.; So, P.-W. The Aging of Iron Man. Front. Aging Neurosci. 2018, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Bishop, G.M.; Dang, T.N.; Dringen, R.; Robinson, S.R. Accumulation of non-transferrin-bound iron by neurons, astrocytes, and microglia. Neurotox. Res. 2011, 19, 443–451. [Google Scholar] [CrossRef]
- Fernández-Mendívil, C.; Luengo, E.; Trigo-Alonso, P.; García-Magro, N.; Negredo, P.; López, M.G. Protective role of microglial HO-1 blockade in aging: Implication of iron metabolism. Redox Biol. 2020, 38, 101789. [Google Scholar] [CrossRef]
- Lill, R.; Hoffmann, B.; Molik, S.; Pierik, A.J.; Rietzschel, N.; Stehling, O.; Uzarska, M.A.; Webert, H.; Wilbrecht, C.; Mühlenhoff, U. The role of mitochondria in cellular iron-sulfur protein biogenesis and iron metabolism. Biochim. Biophys. Acta 2012, 1823, 1491–1508. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, R.C.; Sosa, J.C.; Gardeck, A.M.; Baez, A.S.; Lee, C.-H.; Wessling-Resnick, M. Inflammation-induced iron transport and metabolism by brain microglia. J. Biol. Chem. 2018, 293, 7853–7863. [Google Scholar] [CrossRef]
- Friedman, A.; Arosio, P.; Finazzi, D.; Koziorowski, D.; Galazka-Friedman, J. Ferritin as an important player in neurodegeneration. Parkinsonism Relat. Disord. 2011, 17, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Ayton, S.; Faux, N.G.; Bush, A.I. Alzheimer’s Disease Neuroimaging Initiative Ferritin levels in the cerebrospinal fluid predict Alzheimer’s disease outcomes and are regulated by APOE. Nat. Commun. 2015, 6, 6760. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.A.; Casale, M.; Alcon, B.; Pham, N.; Narayan, N.; Lynch, G. Ferritin accumulation in dystrophic microglia is an early event in the development of Huntington’s disease. Glia 2007, 55, 1074–1084. [Google Scholar] [CrossRef]
- Streit, W.J.; Xue, Q.-S.; Tischer, J.; Bechmann, I. Microglial pathology. Acta Neuropathol. Commun. 2014, 2, 142. [Google Scholar] [CrossRef]
- Carden, T.R.; Correale, J.; Pasquini, J.M.; Pérez, M.J. Transferrin Enhances Microglial Phagocytic Capacity. Mol. Neurobiol. 2019, 56, 6324–6340. [Google Scholar] [CrossRef]
- Bisht, K.; Sharma, K.P.; Lecours, C.; Sánchez, M.G.; El Hajj, H.; Milior, G.; Olmos-Alonso, A.; Gómez-Nicola, D.; Luheshi, G.; Vallières, L.; et al. Dark microglia: A new phenotype predominantly associated with pathological states. Glia 2016, 64, 826–839. [Google Scholar] [CrossRef]
- Zeineh, M.M.; Chen, Y.; Kitzler, H.H.; Hammond, R.; Vogel, H.; Rutt, B.K. Activated iron-containing microglia in the human hippocampus identified by magnetic resonance imaging in Alzheimer disease. Neurobiol. Aging 2015, 36, 2483–2500. [Google Scholar] [CrossRef]
- Hayashi, Y.; Yoshida, M.; Yamato, M.; Ide, T.; Wu, Z.; Ochi-Shindou, M.; Kanki, T.; Kang, D.; Sunagawa, K.; Tsutsui, H.; et al. Reverse of Age-Dependent Memory Impairment and Mitochondrial DNA Damage in Microglia by an Overexpression of Human Mitochondrial Transcription Factor A in Mice. J. Neurosci. 2008, 28, 8624–8634. [Google Scholar] [CrossRef]
- von Bernhardi, R.; Eugenín-von Bernhardi, L.; Eugenín, J. Microglial cell dysregulation in brain aging and neurodegeneration. Front. Aging Neurosci. 2015, 7, 124. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, D.A.E.; van Eden, C.G.; Schuurman, K.G.; Hamann, J.; Huitinga, I. Staining of HLA-DR, Iba1 and CD68 in human microglia reveals partially overlapping expression depending on cellular morphology and pathology. J. Neuroimmunol. 2017, 309, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.G.; Lue, L.F.; Beach, T.G. Gene expression profiling of amyloid beta peptide-stimulated human post-mortem brain microglia. Neurobiol. Aging 2001, 22, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Gibbons, H.M.; Oldfield, R.L.; Bergin, P.M.; Mee, E.W.; Faull, R.L.M.; Dragunow, M. The transcription factor PU.1 is critical for viability and function of human brain microglia. Glia 2013, 61, 929–942. [Google Scholar] [CrossRef]
- Bonham, L.W.; Sirkis, D.W.; Yokoyama, J.S. The Transcriptional Landscape of Microglial Genes in Aging and Neurodegenerative Disease. Front. Immunol. 2019, 10, 1170. [Google Scholar] [CrossRef]
- Ghorpade, A.; Persidsky, Y.; Swindells, S.; Borgmann, K.; Persidsky, R.; Holter, S.; Cotter, R.; Gendelman, H.E. Neuroinflammatory responses from microglia recovered from HIV-1-infected and seronegative subjects. J. Neuroimmunol. 2005, 163, 145–156. [Google Scholar] [CrossRef]
- Matschke, J.; Lütgehetmann, M.; Hagel, C.; Sperhake, J.P.; Schröder, A.S.; Edler, C.; Mushumba, H.; Fitzek, A.; Allweiss, L.; Dandri, M.; et al. Neuropathology of patients with COVID-19 in Germany: A post-mortem case series. Lancet Neurol. 2020, 19, 919–929. [Google Scholar] [CrossRef]
- Tröscher, A.R.; Wimmer, I.; Quemada-Garrido, L.; Köck, U.; Gessl, D.; Verberk, S.G.S.; Martin, B.; Lassmann, H.; Bien, C.G.; Bauer, J. Microglial nodules provide the environment for pathogenic T cells in human encephalitis. Acta Neuropathol. 2019, 137, 619–635. [Google Scholar] [CrossRef]
- Kunkle, B.W.; Schmidt, M.; Klein, H.-U.; Naj, A.C.; Hamilton-Nelson, K.L.; Larson, E.B.; Evans, D.A.; De Jager, P.L.; Crane, P.K.; Buxbaum, J.D.; et al. Novel Alzheimer Disease Risk Loci and Pathways in African American Individuals Using the African Genome Resources Panel: A Meta-analysis. JAMA Neurol. 2021, 78, 102–113. [Google Scholar] [CrossRef]
- Schaffer Aguzzoli, C.; Ferreira, P.C.L.; Povala, G.; Ferrari-Souza, J.P.; Bellaver, B.; Soares Katz, C.; Zalzale, H.; Lussier, F.Z.; Rohden, F.; Abbas, S.; et al. Neuropsychiatric Symptoms and Microglial Activation in Patients with Alzheimer Disease. JAMA Netw. Open 2023, 6, e2345175. [Google Scholar] [CrossRef]
- Spiteri, A.G.; Wishart, C.L.; Pamphlett, R.; Locatelli, G.; King, N.J.C. Microglia and monocytes in inflammatory CNS disease: Integrating phenotype and function. Acta Neuropathol. 2022, 143, 179–224. [Google Scholar] [CrossRef] [PubMed]
- Tino, E.P.; Valentina, M.; Arenn, F.C.; Annalisa, D.; Arcangelo, C.; Michela, M.; Paola, C.; Roberta, V.; Daniele, Z.; Simona, A.; et al. Antonio Guaita Abbiategrasso Brain Bank Protocol for Collecting, Processing and Characterizing Aging Brains|Protocol (Translated to Italian). Available online: https://www.jove.com/v/60296/abbiategrasso-brain-bank-protocol-for-collecting-processing?language=Italian (accessed on 31 October 2023).
- Griciuc, A.; Serrano-Pozo, A.; Parrado, A.R.; Lesinski, A.N.; Asselin, C.N.; Mullin, K.; Hooli, B.; Choi, S.H.; Hyman, B.T.; Tanzi, R.E. Alzheimer’s disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron 2013, 78, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Bachstetter, A.D.; Van Eldik, L.J.; Schmitt, F.A.; Neltner, J.H.; Ighodaro, E.T.; Webster, S.J.; Patel, E.; Abner, E.L.; Kryscio, R.J.; Nelson, P.T. Disease-related microglia heterogeneity in the hippocampus of Alzheimer’s disease, dementia with Lewy bodies, and hippocampal sclerosis of aging. Acta Neuropathol. Commun. 2015, 3, 32. [Google Scholar] [CrossRef]
- Tischer, J.; Krueger, M.; Mueller, W.; Staszewski, O.; Prinz, M.; Streit, W.J.; Bechmann, I. Inhomogeneous distribution of Iba-1 characterizes microglial pathology in Alzheimer’s disease. Glia 2016, 64, 1562–1572. [Google Scholar] [CrossRef] [PubMed]
- Satoh, J.; Kino, Y.; Asahina, N.; Takitani, M.; Miyoshi, J.; Ishida, T.; Saito, Y. TMEM119 marks a subset of microglia in the human brain. Neuropathology 2016, 36, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Raj, D.; Yin, Z.; Breur, M.; Doorduin, J.; Holtman, I.R.; Olah, M.; Mantingh-Otter, I.J.; Van Dam, D.; De Deyn, P.P.; den Dunnen, W.; et al. Increased White Matter Inflammation in Aging- and Alzheimer’s Disease Brain. Front. Mol. Neurosci. 2017, 10, 206. [Google Scholar] [CrossRef] [PubMed]
- Sims, R.; van der Lee, S.J.; Naj, A.C.; Bellenguez, C.; Badarinarayan, N.; Jakobsdottir, J.; Kunkle, B.W.; Boland, A.; Raybould, R.; Bis, J.C.; et al. Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer’s disease. Nat. Genet. 2017, 49, 1373–1384. [Google Scholar] [CrossRef]
- Kaneshwaran, K.; Olah, M.; Tasaki, S.; Yu, L.; Bradshaw, E.M.; Schneider, J.A.; Buchman, A.S.; Bennett, D.A.; De Jager, P.L.; Lim, A.S.P. Sleep fragmentation, microglial aging, and cognitive impairment in adults with and without Alzheimer’s dementia. Sci. Adv. 2019, 5, eaax7331. [Google Scholar] [CrossRef]
- Mukherjee, S.; Klaus, C.; Pricop-Jeckstadt, M.; Miller, J.A.; Struebing, F.L. A Microglial Signature Directing Human Aging and Neurodegeneration-Related Gene Networks. Front. Neurosci. 2019, 13, 2. [Google Scholar] [CrossRef]
- Parhizkar, S.; Arzberger, T.; Brendel, M.; Kleinberger, G.; Deussing, M.; Focke, C.; Nuscher, B.; Xiong, M.; Ghasemigharagoz, A.; Katzmarski, N.; et al. Loss of TREM2 function increases amyloid seeding but reduces plaque-associated ApoE. Nat. Neurosci. 2019, 22, 191–204. [Google Scholar] [CrossRef]
- Li, H.; Knight, W.C.; Yang, P.; Guo, Y.; Perlmutter, J.S.; Morris, J.C.; Bateman, R.J.; Benzinger, T.L.S.; Xu, J. Microglia Implicated in Tauopathy in the Striatum of Neurodegenerative Disease Patients from Genotype to Phenotype. Int. J. Mol. Sci. 2020, 21, 6047. [Google Scholar] [CrossRef]
- Fadul, M.M.; Garwood, C.J.; Waller, R.; Garrett, N.; Heath, P.R.; Matthews, F.E.; Brayne, C.; Wharton, S.B.; Simpson, J.E. NDRG2 Expression Correlates with Neurofibrillary Tangles and Microglial Pathology in the Ageing Brain. Int. J. Mol. Sci. 2020, 21, 340. [Google Scholar] [CrossRef] [PubMed]
- Kloske, C.M.; Dugan, A.J.; Weekman, E.M.; Winder, Z.; Patel, E.; Nelson, P.T.; Fardo, D.W.; Wilcock, D.M. Inflammatory Pathways Are Impaired in Alzheimer Disease and Differentially Associated With Apolipoprotein E Status. J. Neuropathol. Exp. Neurol. 2021, 80, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Burger, C.A.; Akhanov, V.; Liang, J.H.; Mackin, R.D.; Albrecht, N.E.; Andrade, P.; Schafer, D.P.; Samuel, M.A. Neuronal signal-regulatory protein alpha drives microglial phagocytosis by limiting microglial interaction with CD47 in the retina. Immunity 2022, 55, 2318–2335.e7. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Meng, J.; Kong, W.; Wu, Z.; Lan, F.; Narengaowa; Hayashi, Y.; Yang, Q.; Bai, Z.; Nakanishi, H.; et al. Microglial cathepsin E plays a role in neuroinflammation and amyloid β production in Alzheimer’s disease. Aging Cell 2022, 21, e13565. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Castro, C.; Noori, A.; Magdamo, C.G.; Li, Z.; Marks, J.D.; Frosch, M.P.; Das, S.; Hyman, B.T.; Serrano-Pozo, A. Cyclic multiplex fluorescent immunohistochemistry and machine learning reveal distinct states of astrocytes and microglia in normal aging and Alzheimer’s disease. J. Neuroinflamm. 2022, 19, 30. [Google Scholar] [CrossRef]
- Zhao, N.; Qiao, W.; Li, F.; Ren, Y.; Zheng, J.; Martens, Y.A.; Wang, X.; Li, L.; Liu, C.-C.; Chen, K.; et al. Elevating microglia TREM2 reduces amyloid seeding and suppresses disease-associated microglia. J. Exp. Med. 2022, 219, e20212479. [Google Scholar] [CrossRef]
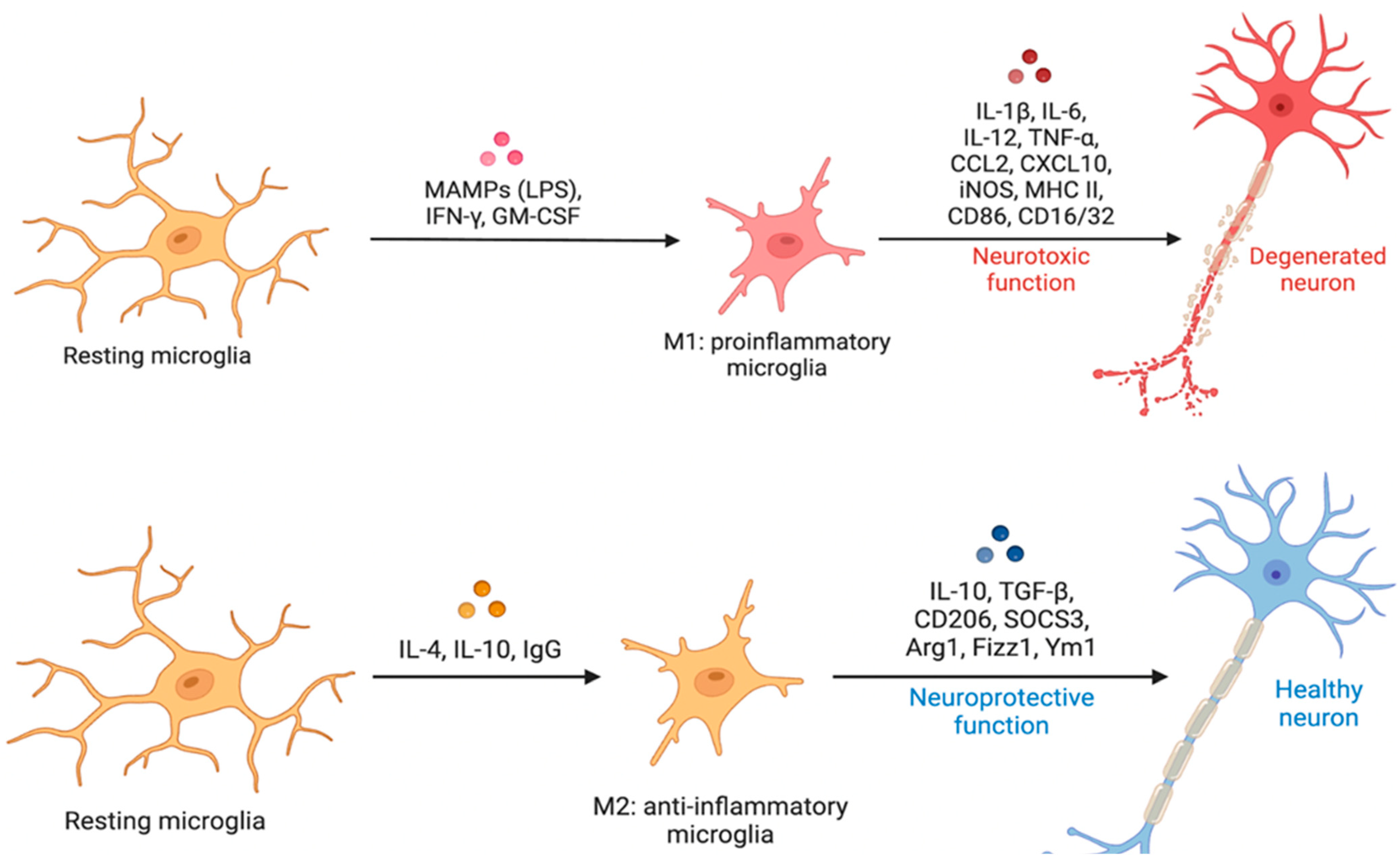
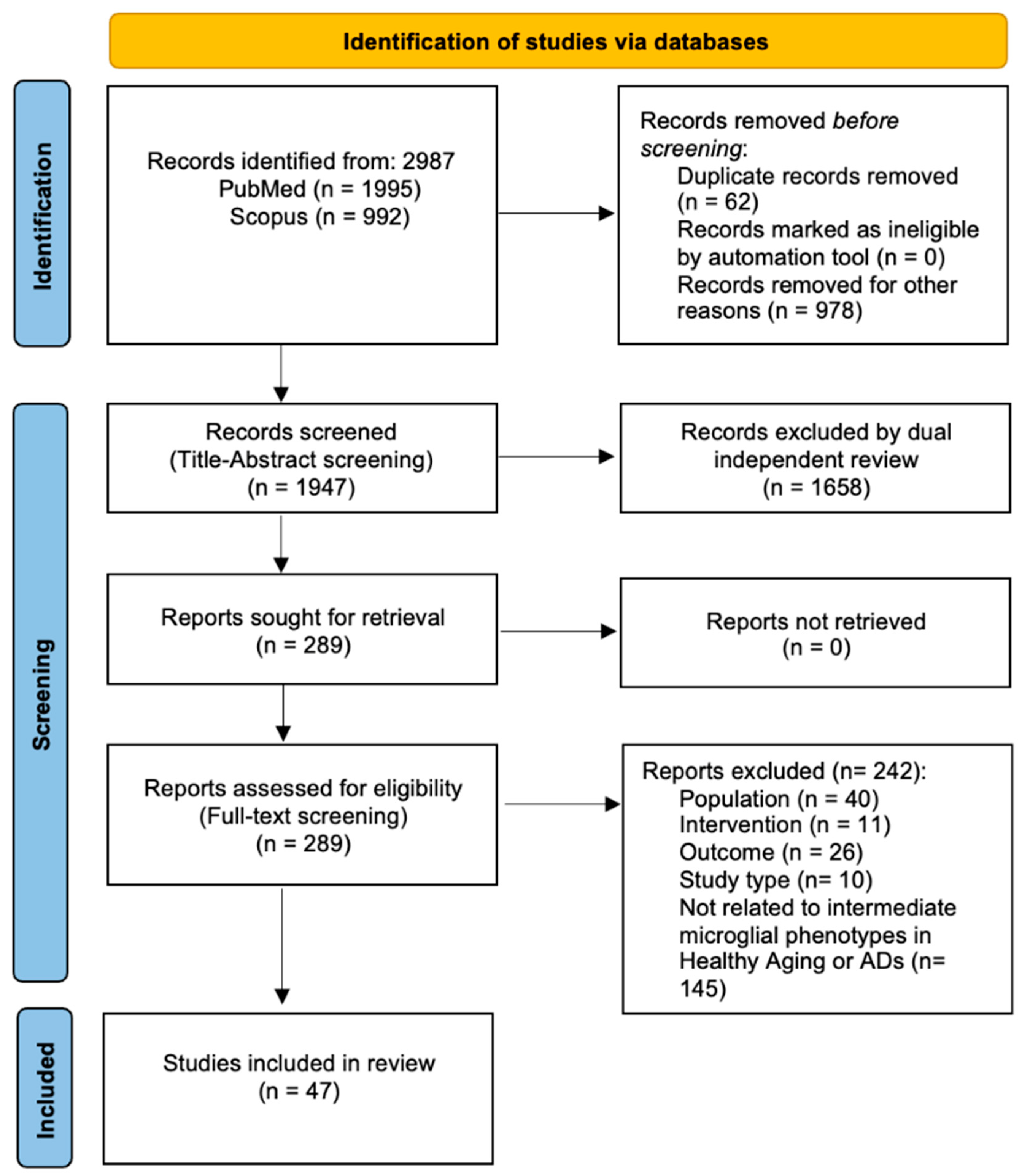

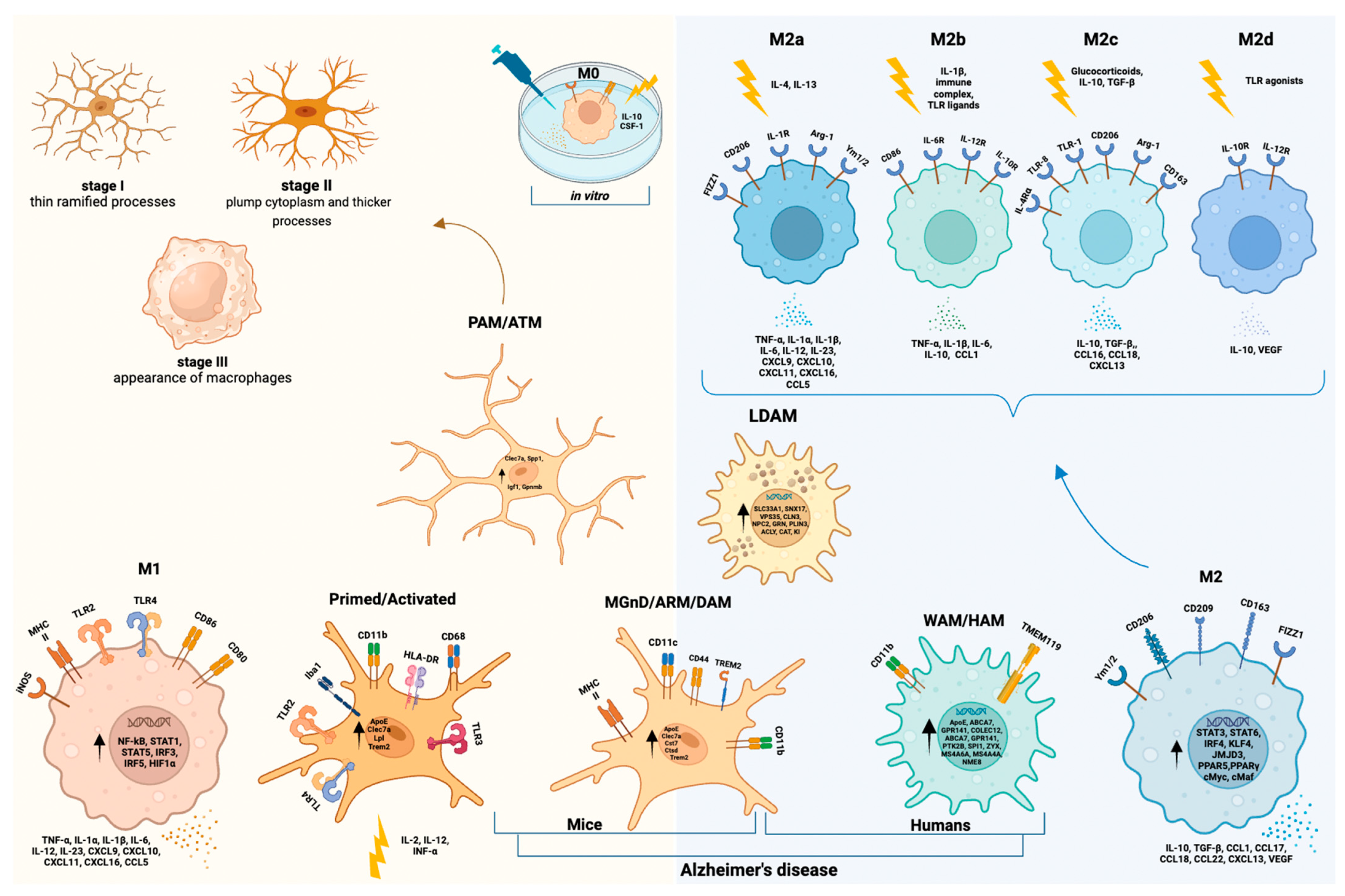
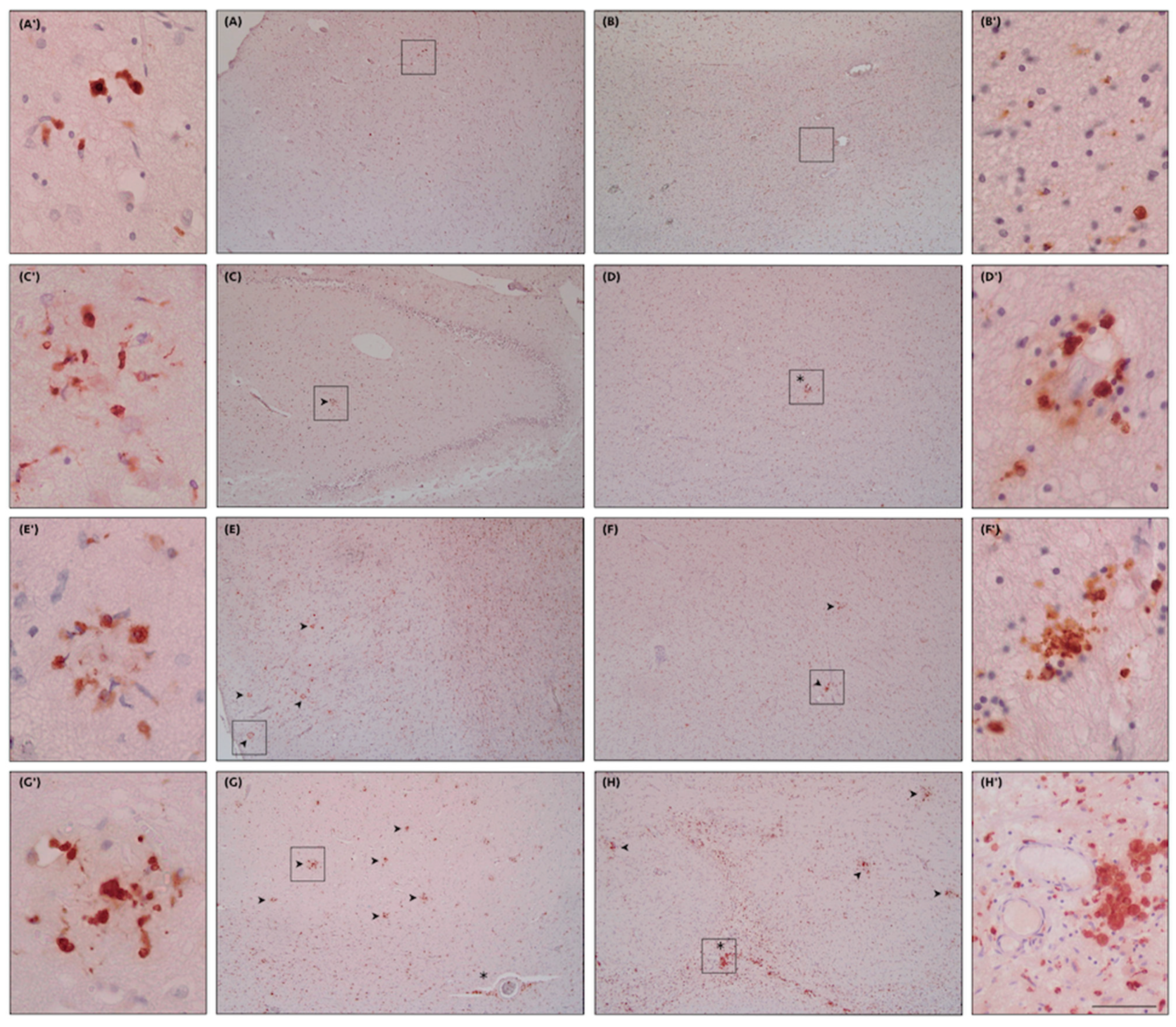
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malvaso, A.; Gatti, A.; Negro, G.; Calatozzolo, C.; Medici, V.; Poloni, T.E. Microglial Senescence and Activation in Healthy Aging and Alzheimer’s Disease: Systematic Review and Neuropathological Scoring. Cells 2023, 12, 2824. https://doi.org/10.3390/cells12242824
Malvaso A, Gatti A, Negro G, Calatozzolo C, Medici V, Poloni TE. Microglial Senescence and Activation in Healthy Aging and Alzheimer’s Disease: Systematic Review and Neuropathological Scoring. Cells. 2023; 12(24):2824. https://doi.org/10.3390/cells12242824
Chicago/Turabian StyleMalvaso, Antonio, Alberto Gatti, Giulia Negro, Chiara Calatozzolo, Valentina Medici, and Tino Emanuele Poloni. 2023. "Microglial Senescence and Activation in Healthy Aging and Alzheimer’s Disease: Systematic Review and Neuropathological Scoring" Cells 12, no. 24: 2824. https://doi.org/10.3390/cells12242824
APA StyleMalvaso, A., Gatti, A., Negro, G., Calatozzolo, C., Medici, V., & Poloni, T. E. (2023). Microglial Senescence and Activation in Healthy Aging and Alzheimer’s Disease: Systematic Review and Neuropathological Scoring. Cells, 12(24), 2824. https://doi.org/10.3390/cells12242824








