Beyond the Basics: Unraveling the Complexity of Coronary Artery Calcification
Abstract
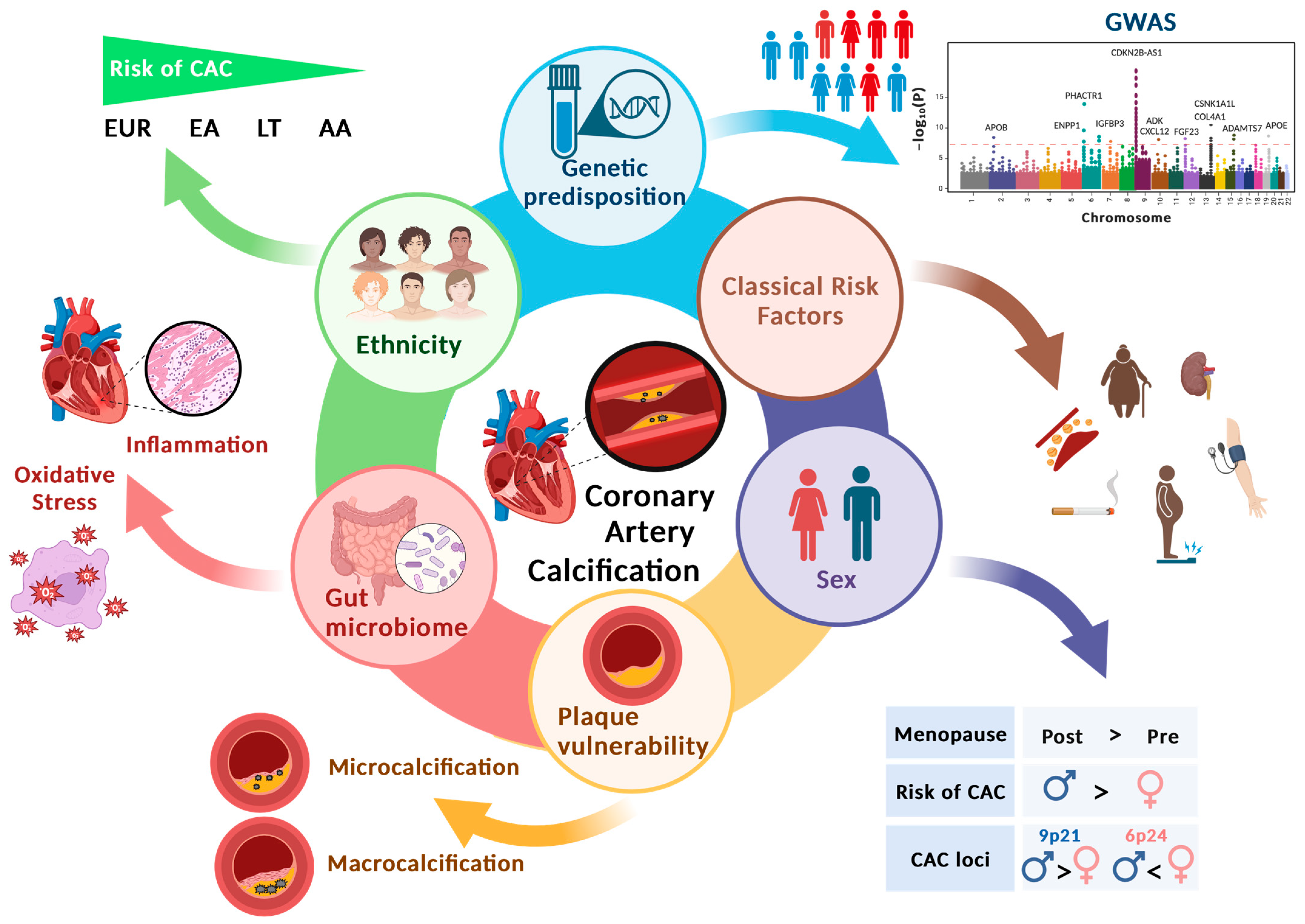
:1. Introduction
2. Pathophysiology of CAC
3. CAC and Plaque Vulnerability
4. Genetic Architecture of CAC
| Locus ID | Nearest Genes | Index rsIDs | Coordinates (hg38) | GTEx Vascular eQTL | References | ||
|---|---|---|---|---|---|---|---|
| Protein Coding | RNA Coding | Artery | Gene | ||||
| 1 | APOB | rs5742904-T | Chr 2:21006288 | - | - | [33] | |
| 2 | PHACTR1, GFOD1, TBC1D7 | RP1-257A7.4 | rs9349379-A | Chr 6:12903725 | Tibial | PHACTR1, TBC1D7, RP1-257A7.4, GFOD1 | [23,24,29,33] |
| Coronary | PHACTR1 | ||||||
| Aorta | PHACTR1 | ||||||
| 3 | PHACTR1, GFOD1, TBC1D7 | RP1-257A7.4 | rs9369640-A | Chr 6: 12901209 | Tibial | PHACTR1, RP1-257A7.4 | [23,24,29,33] |
| Aorta | PHACTR1 | ||||||
| 4 | PHACTR1, GFOD1, TBC1D7 | RP1-257A7.4 | rs10456561-A | Chr 6: 12887233 | - | - | [24] |
| 5 | ENPP3, ENPP1 | miR-548h-5 | rs3844006-C | Chr 6: 131773862 | - | - | [24] |
| 6 | IGFBP3 | rs2854746-C | Chr 6: 45921046 | Tibial | IGFBP3 | [24] | |
| Aorta | IGFBP3 | ||||||
| 7 | CDKN2A, CDKN2B | CDKN2B-AS1 | rs1333049-C | Chr 9:22125504 | - | - | [23,24,33] |
| 8 | CDKN2A, CDKN2B | CDKN2B-AS1 | rs1537370-T | Chr 9:22084311 | - | - | [24,28,29,33] |
| 9 | CDKN2A, CDKN2B | CDKN2B-AS1 | rs72652478-C | Chr 9:22102044 | - | - | [24] |
| 10 | CDKN2A, CDKN2B | CDKN2B-AS1 | rs62555371-A | Chr 9: 22107239 | - | - | [24] |
| 11 | CXCL12 | AL512640.1 | rs10899970-A | Chr 10: 44020268 | - | - | [24] |
| 12 | ARID5B | rs9633535-T | Chr 10: 62076329 | - | - | [24] | |
| 13 | ADK | rs10762577-A | Chr 10: 74157673 | Tibial | ADK | [24] | |
| Coronary | BMS1P4 | ||||||
| 14 | FGF23 | rs11063120-A | Chr 12: 4377452 | - | - | [24] | |
| 15 | COL4A1, COL4A2 | rs9515203-T | Chr 13: 110397276 | - | - | [24] | |
| 16 | CSNK1A1L | LINC01048, RPS12P24 | rs8000449-T | Chr 13:37224221 | - | - | [32] |
| 17 | ADAMTS7 | rs7182103-T | Chr 15: 78831604 | Tibial | ADAMTS7, MORF4L1, ADAMTS7P3 | [24] | |
| Coronary | ADAMTS7 | ||||||
| Aorta | ADAMTS7, MORF4L1, ADAMTS7P3, RPL21P116 | ||||||
| 18 | APOE | rs7412-T | Chr 19:44908822 | - | - | [24,33] | |
5. Race/Ethnicity and Sex in CAC
6. Association between the Gut Microbiome and CAC
7. Therapeutic Implications in CAC
8. Present Limitations and Future Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Otsuka, F.; Sakakura, K.; Yahagi, K.; Joner, M.; Virmani, R. Has Our Understanding of Calcification in Human Coronary Atherosclerosis Progressed? Arterioscler. Thromb. Vasc. Biol. 2014, 34, 724–736. [Google Scholar] [CrossRef]
- Blaha, M.J.; Mortensen, M.B.; Kianoush, S.; Tota-Maharaj, R.; Cainzos-Achirica, M. Coronary artery calcium scoring: Is it time for a change in methodology? JACC Cardiovasc. Imaging 2017, 10, 923–937. [Google Scholar] [CrossRef]
- Mori, H.; Torii, S.; Kutyna, M.; Sakamoto, A.; Finn, A.V.; Virmani, R. Coronary Artery Calcification and its Progression: What Does it Really Mean? JACC Cardiovasc. Imaging 2018, 11, 127–142. [Google Scholar] [CrossRef]
- Greenland, P.; LaBree, L.; Azen, S.P.; Doherty, T.M.; Detrano, R.C. Coronary Artery Calcium Score Combined with Framingham Score for Risk Prediction in Asymptomatic Individuals. JAMA 2004, 291, 210–215. [Google Scholar] [CrossRef]
- Criqui, M.H.; Denenberg, J.O.; Ix, J.H.; McClelland, R.L.; Wassel, C.L.; Rifkin, D.E.; Carr, J.J.; Budoff, M.J.; Allison, M.A. Calcium Density of Coronary Artery Plaque and Risk of Incident Cardiovascular Events. JAMA 2014, 311, 271–278. [Google Scholar] [CrossRef]
- Jin, H.Y.; Weir-McCall, J.R.; Leipsic, J.A.; Son, J.W.; Sellers, S.L.; Shao, M.; Blanke, P.; Ahmadi, A.; Hadamitzky, M.; Kim, Y.J.; et al. The Relationship Between Coronary Calcification and the Natural History of Coronary Artery Disease. JACC Cardiovasc. Imaging 2021, 14, 233–242. [Google Scholar] [CrossRef]
- Mehta, A.; Vasquez, N.; Ayers, C.R.; Patel, J.; Hooda, A.; Khera, A.; Blumenthal, R.S.; Shapiro, M.D.; Rodriguez, C.J.; Tsai, M.Y.; et al. Independent Association of Lipoprotein(a) and Coronary Artery Calcification with Atherosclerotic Cardiovascular Risk. J. Am. Coll Cardiol. 2022, 79, 757–768. [Google Scholar] [CrossRef]
- Ferencik, M.; Pencina, K.M.; Liu, T.; Ghemigian, K.; Baltrusaitis, K.; Massaro, J.M.; D’Agostino Sr, R.B.; O’Donnell, C.J.; Hoffmann, U. Coronary artery calcium distribution is an independent predictor of incident major coronary heart disease events: Results from the Framingham Heart Study. Circ. Cardiovasc. Imaging 2017, 10, e006592. [Google Scholar] [CrossRef]
- Mitchell, J.D.; Paisley, R.; Moon, P.; Novak, E.; Villines, T.C. Coronary Artery Calcium and Long-Term Risk of Death, Myocardial Infarction, and Stroke: The Walter Reed Cohort Study. JACC Cardiovasc. Imaging 2018, 11, 1799–1806. [Google Scholar] [CrossRef]
- Budoff, M.J.; Young, R.; Burke, G.; Jeffrey Carr, J.; Detrano, R.C.; Folsom, A.R.; Kronmal, R.; Lima, J.A.C.; Liu, K.J.; McClelland, R.L.; et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: The multi-ethnic study of atherosclerosis (MESA). Eur. Heart J. 2018, 39, 2401–2408. [Google Scholar] [CrossRef]
- Abuzaid, A.; Saad, M.; Addoumieh, A.; Ha, L.D.; Elbadawi, A.; Mahmoud, A.N.; Elgendy, A.; Abdelaziz, H.K.; Barakat, A.F.; Mentias, A.; et al. Coronary artery calcium score and risk of cardiovascular events without established coronary artery disease: A systemic review and meta-analysis. Coron. Artery Dis. 2021, 32, 317–328. [Google Scholar] [CrossRef]
- Kwok, C.S.; Bennett, S.; Lip, G.Y.H. Coronary artery calcium score and its association with stroke: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2023, 53, e13892. [Google Scholar] [CrossRef]
- Al Rifai, M.; Kanaya, A.M.; Kandula, N.R.; Patel, J.; Al-Mallah, M.H.; Budoff, M.; Cainzos-Achirica, M.; Criqui, M.H.; Virani, S.S. Association of Coronary Artery Calcium Density and Volume with Predicted Atherosclerotic Cardiovascular Disease Risk and Cardiometabolic Risk Factors in South Asians: The Mediators of Atherosclerosis in South Asians Living in America (MASALA) Study. Curr. Probl. Cardiol. 2023, 48, 101105. [Google Scholar] [CrossRef]
- Budoff, M.J.; McClelland, R.L.; Nasir, K.; Greenland, P.; Kronmal, R.A.; Kondos, G.T.; Shea, S.; Lima, J.A.; Blumenthal, R.S. Cardiovascular events with absent or minimal coronary calcification: The Multi-Ethnic Study of Atherosclerosis (MESA). Am. Heart J. 2009, 158, 554–561. [Google Scholar] [CrossRef]
- Schmermund, A.; Baumgart, D.; Mohlenkamp, S.; Kriener, P.; Pump, H.; Gronemeyer, D.; Seibel, R.; Erbel, R. Natural history and topographic pattern of progression of coronary calcification in symptomatic patients: An electron-beam CT study. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 421–426. [Google Scholar] [CrossRef]
- Detrano, R.; Guerci, A.D.; Carr, J.J.; Bild, D.E.; Burke, G.; Folsom, A.R.; Liu, K.; Shea, S.; Szklo, M.; Bluemke, D.A. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N. Engl. J. Med. 2008, 358, 1336–1345. [Google Scholar] [CrossRef]
- Shaw, L.J.; Chandrashekhar, Y.; Narula, J. Progression of coronary calcium scores: Harder gets the evidence. JACC Cardiovasc. Imaging 2012, 5, 465–466. [Google Scholar] [CrossRef]
- Greenland, P.; Blaha, M.J.; Budoff, M.J.; Erbel, R.; Watson, K.E. Coronary calcium score and cardiovascular risk. J. Am. Coll. Cardiol. 2018, 72, 434–447. [Google Scholar] [CrossRef]
- Arnett, D.; Blumenthal, R.; Albert, M.; Buroker, A.; Goldberger, Z.; Hahn, E.; Ziaeian, B. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 74, 1376–1414. [Google Scholar] [CrossRef]
- Hecht, H.; Blaha, M.J.; Berman, D.S.; Nasir, K.; Budoff, M.; Leipsic, J.; Blankstein, R.; Narula, J.; Rumberger, J.; Shaw, L.J. Clinical indications for coronary artery calcium scoring in asymptomatic patients: Expert consensus statement from the Society of Cardiovascular Computed Tomography. J. Cardiovasc. Comput. Tomogr. 2017, 11, 157–168. [Google Scholar] [CrossRef]
- Cainzos-Achirica, M.; Quispe, R.; Dudum, R.; Greenland, P.; Lloyd-Jones, D.; Rana, J.S.; Lima, J.A.; Doria de Vasconcellos, H.; Joshi, P.H.; Khera, A. CAC for risk stratification among individuals with hypertriglyceridemia free of clinical atherosclerotic cardiovascular disease. Cardiovasc. Imaging 2022, 15, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Rifai, M.A. The Utility of Coronary Artery Calcium for Guiding Treatment with Preventive Pharmacotherapy. JACC Cardiovasc. Imaging 2022, 15, 652–654. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, C.J.; Kavousi, M.; Smith, A.V.; Kardia, S.L.; Feitosa, M.F.; Hwang, S.J.; Sun, Y.V.; Province, M.A.; Aspelund, T.; Dehghan, A.; et al. Genome-wide association study for coronary artery calcification with follow-up in myocardial infarction. Circulation 2011, 124, 2855–2864. [Google Scholar] [CrossRef] [PubMed]
- Kavousi, M.; Bos, M.M.; Barnes, H.J.; Lino Cardenas, C.L.; Wong, D.; Lu, H.; Hodonsky, C.J.; Landsmeer, L.P.L.; Turner, A.W.; Kho, M.; et al. Multi-ancestry genome-wide study identifies effector genes and druggable pathways for coronary artery calcification. Nat. Genet. 2023, 55, 1651–1664. [Google Scholar] [CrossRef] [PubMed]
- Buniello, A.; MacArthur, J.A.L.; Cerezo, M.; Harris, L.W.; Hayhurst, J.; Malangone, C.; McMahon, A.; Morales, J.; Mountjoy, E.; Sollis, E.; et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019, 47, D1005–D1012. [Google Scholar] [CrossRef] [PubMed]
- Aguet, F.; Barbeira, A.N.; Bonazzola, R.; Jo, B.; Kasela, S.; Liang, Y.; Parsana, P.; Aguet, F.; Battle, A.; Brown, A.; et al. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 2020, 369, 1318–1330. [Google Scholar] [CrossRef]
- Wojczynski, M.K.; Li, M.; Bielak, L.F.; Kerr, K.F.; Reiner, A.P.; Wong, N.D.; Yanek, L.R.; Qu, L.; White, C.C.; Lange, L.A.; et al. Genetics of coronary artery calcification among African Americans, a meta-analysis. BMC Med. Genet. 2013, 14, 75. [Google Scholar] [CrossRef]
- van Setten, J.; Isgum, I.; Smolonska, J.; Ripke, S.; de Jong, P.A.; Oudkerk, M.; de Koning, H.; Lammers, J.-W.J.; Zanen, P.; Groen, H.J.M.; et al. Genome-wide association study of coronary and aortic calcification implicates risk loci for coronary artery disease and myocardial infarction. Atherosclerosis 2013, 228, 400–405. [Google Scholar] [CrossRef]
- Pechlivanis, S.; Mühleisen, T.W.; Möhlenkamp, S.; Schadendorf, D.; Erbel, R.; Jöckel, K.-H.; Hoffmann, P.; Nöthen, M.M.; Scherag, A.; Moebus, S.; et al. Risk loci for coronary artery calcification replicated at 9p21 and 6q24 in the Heinz Nixdorf Recall Study. BMC Med. Genet. 2013, 14, 23. [Google Scholar] [CrossRef]
- Divers, J.; Palmer, N.D.; Langefeld, C.D.; Brown, W.M.; Lu, L.; Hicks, P.J.; Smith, S.C.; Xu, J.; Terry, J.G.; Register, T.C.; et al. Genome-wide association study of coronary artery calcified atherosclerotic plaque in African Americans with type 2 diabetes. BMC Genet. 2017, 18, 105. [Google Scholar] [CrossRef]
- O’Donnell, C.J.; Cupples, L.A.; D’Agostino, R.B.; Fox, C.S.; Hoffmann, U.; Hwang, S.J.; Ingellson, E.; Liu, C.; Murabito, J.M.; Polak, J.F.; et al. Genome-wide association study for subclinical atherosclerosis in major arterial territories in the NHLBI’s Framingham Heart Study. BMC Med. Genet. 2007, 8 (Suppl. S1), S4. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Dimitrov, L.; Chen, S.-H.; Bielak, L.F.; Bis, J.C.; Feitosa, M.F.; Lu, L.; Kavousi, M.; Raffield, L.M.; Smith, A.V.; et al. Multiethnic Genome-Wide Association Study of Subclinical Atherosclerosis in Individuals with Type 2 Diabetes. Circ. Genom. Precis. Med. 2021, 14, e003258. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, P.; Bis, J.C.; Bielak, L.F.; Cox, A.J.; Dörr, M.; Feitosa, M.F.; Franceschini, N.; Guo, X.; Hwang, S.J.; Isaacs, A.; et al. Multiethnic Exome-Wide Association Study of Subclinical Atherosclerosis. Circ. Cardiovasc. Genet. 2016, 9, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.K.; Adams, M.; Klopfenstein, H. Estrogen modulates responses of atherosclerotic coronary arteries. Circulation 1990, 81, 1680–1687. [Google Scholar] [CrossRef] [PubMed]
- Burke, A.P.; Farb, A.; Malcom, G.; Virmani, R. Effect of menopause on plaque morphologic characteristics in coronary atherosclerosis. Am. Heart J. 2001, 141, S58–S62. [Google Scholar] [CrossRef]
- Bild, D.E.; Detrano, R.; Peterson, D.; Guerci, A.; Liu, K.; Shahar, E.; Ouyang, P.; Jackson, S.; Saad, M.F. Ethnic differences in coronary calcification: The Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2005, 111, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, G.; Iacobini, C.; Fantauzzi, C.B.; Menini, S. The dark and bright side of atherosclerotic calcification. Atherosclerosis 2015, 238, 220–230. [Google Scholar] [CrossRef]
- Huang, X.; D’Addabbo, J.; Nguyen, P.K. Coronary artery calcification: More than meets the eye. J. Nucl. Cardiol. 2021, 28, 2215–2219. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Q.; Jiang, H. Gut microbiota in atherosclerosis: Focus on trimethylamine N-oxide. Apmis 2020, 128, 353–366. [Google Scholar] [CrossRef]
- Liu, Y.H.; Peng, P.; Hung, W.C.; Wu, P.H.; Kao, C.Y.; Wu, P.Y.; Huang, J.C.; Hung, C.H.; Su, H.M.; Chen, S.C.; et al. Comparative Gut Microbiome Differences between High and Low Aortic Arch Calcification Score in Patients with Chronic Diseases. Int. J. Mol. Sci. 2023, 24, 5673. [Google Scholar] [CrossRef]
- Suwita, B.M.; Suroyo, I.; Rusdi, L.; Prihartono, J. The correlation between coronary artery, aortic, and mitral valve calcification in patients with coronary atherosclerosis. Egypt. J. Radiol. Nucl. Med. 2023, 54, 108. [Google Scholar] [CrossRef]
- Koulaouzidis, G.; Nicoll, R.; MacArthur, T.; Jenkins, P.; Henein, M.Y. Coronary artery calcification correlates with the presence and severity of valve calcification. Int. J. Cardiol. 2013, 168, 5263–5266. [Google Scholar] [CrossRef] [PubMed]
- Messika-Zeitoun, D.; Bielak, L.F.; Peyser, P.A.; Sheedy, P.F.; Turner, S.T.; Nkomo, V.T.; Breen, J.F.; Maalouf, J.; Scott, C.; Tajik, A.J. Aortic valve calcification: Determinants and progression in the population. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Lanzer, P.; Boehm, M.; Sorribas, V.; Thiriet, M.; Janzen, J.; Zeller, T.; St Hilaire, C.; Shanahan, C. Medial vascular calcification revisited: Review and perspectives. Eur. Heart J. 2014, 35, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, F.; Yahagi, K.; Sakakura, K.; Virmani, R. Why is the mammary artery so special and what protects it from atherosclerosis? Ann. Cardiothorac. Surg. 2013, 2, 519. [Google Scholar] [PubMed]
- Kraler, S.; Libby, P.; Evans, P.C.; Akhmedov, A.; Schmiady, M.O.; Reinehr, M.; Camici, G.G.; Lüscher, T.F. Resilience of the Internal Mammary Artery to Atherogenesis: Shifting from Risk to Resistance to Address Unmet Needs. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2237–2251. [Google Scholar] [CrossRef]
- Vengrenyuk, Y.; Carlier, S.; Xanthos, S.; Cardoso, L.; Ganatos, P.; Virmani, R.; Einav, S.; Gilchrist, L.; Weinbaum, S. A hypothesis for vulnerable plaque rupture due to stress-induced debonding around cellular microcalcifications in thin fibrous caps. Proc. Natl. Acad. Sci. USA 2006, 103, 14678–14683. [Google Scholar] [CrossRef]
- Kelly-Arnold, A.; Maldonado, N.; Laudier, D.; Aikawa, E.; Cardoso, L.; Weinbaum, S. Revised microcalcification hypothesis for fibrous cap rupture in human coronary arteries. Proc. Natl. Acad. Sci. USA 2013, 110, 10741–10746. [Google Scholar] [CrossRef]
- Burke, A.; Kolodgie, F.; Farb, A.; Virmani, R. The Vulnerable Atherosclerotic Plaque: Strategies for Diagnosis and Management; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Hutcheson, J.D.; Goettsch, C.; Bertazzo, S.; Maldonado, N.; Ruiz, J.L.; Goh, W.; Yabusaki, K.; Faits, T.; Bouten, C.; Franck, G.; et al. Genesis and growth of extracellular-vesicle-derived microcalcification in atherosclerotic plaques. Nat. Mater. 2016, 15, 335–343. [Google Scholar] [CrossRef]
- New, S.E.; Goettsch, C.; Aikawa, M.; Marchini, J.F.; Shibasaki, M.; Yabusaki, K.; Libby, P.; Shanahan, C.M.; Croce, K.; Aikawa, E. Macrophage-derived matrix vesicles: An alternative novel mechanism for microcalcification in atherosclerotic plaques. Circ. Res. 2013, 113, 72–77. [Google Scholar] [CrossRef]
- Kawakami, R.; Katsuki, S.; Travers, R.; Romero, D.C.; Becker-Greene, D.; Passos, L.S.A.; Higashi, H.; Blaser, M.C.; Sukhova, G.K.; Buttigieg, J.; et al. S100A9-RAGE Axis Accelerates Formation of Macrophage-Mediated Extracellular Vesicle Microcalcification in Diabetes Mellitus. Arter. Thromb. Vasc. Biol. 2020, 40, 1838–1853. [Google Scholar] [CrossRef] [PubMed]
- Goettsch, C.; Hutcheson, J.D.; Aikawa, M.; Iwata, H.; Pham, T.; Nykjaer, A.; Kjolby, M.; Rogers, M.; Michel, T.; Shibasaki, M.; et al. Sortilin mediates vascular calcification via its recruitment into extracellular vesicles. J. Clin. Investig. 2016, 126, 1323–1336. [Google Scholar] [CrossRef] [PubMed]
- Whyte, M.P. Physiological role of alkaline phosphatase explored in hypophosphatasia. Ann. N. Y. Acad. Sci. 2010, 1192, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Tanimura, A.; McGregor, D.H.; Anderson, H.C. Calcification in atherosclerosis. I. Human studies. J. Exp. Pathol. 1986, 2, 261–273. [Google Scholar] [PubMed]
- Hessle, L.; Johnson, K.A.; Anderson, H.C.; Narisawa, S.; Sali, A.; Goding, J.W.; Terkeltaub, R.; Millán, J.L. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc. Natl. Acad. Sci. USA 2002, 99, 9445–9449. [Google Scholar] [CrossRef]
- Aikawa, E.; Blaser, M.C. 2020 Jeffrey M. Hoeg Award Lecture: Calcifying Extracellular Vesicles as Building Blocks of Microcalcifications in Cardiovascular Disorders. Arter. Thromb. Vasc. Biol. 2021, 41, 117–127. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Feng, G.; Fan, T.; Jiang, H.; Wang, Z. Advances in CT Techniques in Vascular Calcification. Front. Cardiovasc. Med. 2021, 8, 716822. [Google Scholar] [CrossRef]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons from Sudden Coronary Death. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef]
- Corti, A.; De Paolis, A.; Grossman, P.; Dinh, P.A.; Aikawa, E.; Weinbaum, S.; Cardoso, L. The effect of plaque morphology, material composition and microcalcifications on the risk of cap rupture: A structural analysis of vulnerable atherosclerotic plaques. Front. Cardiovasc. Med. 2022, 9, 1019917. [Google Scholar] [CrossRef]
- Speer, M.Y.; Yang, H.-Y.; Brabb, T.; Leaf, E.; Look, A.; Lin, W.-L.; Frutkin, A.; Dichek, D.; Giachelli, C.M. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ. Res. 2009, 104, 733–741. [Google Scholar] [CrossRef]
- Steitz, S.A.; Speer, M.Y.; Curinga, G.; Yang, H.-Y.; Haynes, P.; Aebersold, R.; Schinke, T.; Karsenty, G.; Giachelli, C.M. Smooth muscle cell phenotypic transition associated with calcification: Upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ. Res. 2001, 89, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Dhore, C.R.; Cleutjens, J.P.M.; Lutgens, E.; Cleutjens, K.B.J.M.; Geusens, P.P.M.; Kitslaar, P.J.E.H.M.; Tordoir, J.H.M.; Spronk, H.M.H.; Vermeer, C.; Daemen, M.J.A.P. Differential Expression of Bone Matrix Regulatory Proteins in Human Atherosclerotic Plaques. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1998–2003. [Google Scholar] [CrossRef] [PubMed]
- Hortells, L.; Sur, S.; Hilaire, C.S. Cell phenotype transitions in cardiovascular calcification. Front. Cardiovasc. Med. 2018, 5, 27. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Feng, W.; Chen, R.; Chen, J.; Touyz, R.M.; Wang, J.; Huang, H. Interleukin-18 enhances vascular calcification and osteogenic differentiation of vascular smooth muscle cells through TRPM7 activation. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1933–1943. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, L.C.; Schrader, J.; Niebergall, U.; Viereck, V.; Burchert, A.; Hörsch, D.; Preissner, K.T.; Schoppet, M. Interleukin-4 differentially regulates osteoprotegerin expression and induces calcification in vascular smooth muscle cells. Thromb. Haemost. 2006, 95, 708–714. [Google Scholar] [PubMed]
- Kanno, Y.; Into, T.; Lowenstein, C.J.; Matsushita, K. Nitric oxide regulates vascular calcification by interfering with TGF-β signalling. Cardiovasc. Res. 2008, 77, 221–230. [Google Scholar] [CrossRef]
- Sun, Y.; Byon, C.H.; Yuan, K.; Chen, J.; Mao, X.; Heath, J.M.; Javed, A.; Zhang, K.; Anderson, P.G.; Chen, Y. Smooth muscle cell–specific Runx2 deficiency inhibits vascular calcification. Circ. Res. 2012, 111, 543–552. [Google Scholar] [CrossRef]
- Byon, C.H.; Sun, Y.; Chen, J.; Yuan, K.; Mao, X.; Heath, J.M.; Anderson, P.G.; Tintut, Y.; Demer, L.L.; Wang, D. Runx2-upregulated receptor activator of nuclear factor κB ligand in calcifying smooth muscle cells promotes migration and osteoclastic differentiation of macrophages. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1387–1396. [Google Scholar] [CrossRef]
- Vieceli Dalla Sega, F.; Fortini, F.; Severi, P.; Rizzo, P.; Gardi, I.; Cimaglia, P.; Rapezzi, C.; Tavazzi, L.; Ferrari, R. Cardiac Calcifications: Phenotypes, Mechanisms, Clinical and Prognostic Implications. Biology 2022, 11, 414. [Google Scholar] [CrossRef]
- Zhou, X.; Cui, Y.; Zhou, X.; Han, J. Phosphate/pyrophosphate and MV-related proteins in mineralisation: Discoveries from mouse models. Int. J. Biol. Sci. 2012, 8, 778. [Google Scholar] [CrossRef]
- Yahagi, K.; Kolodgie, F.D.; Lutter, C.; Mori, H.; Romero, M.E.; Finn, A.V.; Virmani, R. Pathology of Human Coronary and Carotid Artery Atherosclerosis and Vascular Calcification in Diabetes Mellitus. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Alfrey, A.C.; Ibels, L.S. Role of phosphate and pyrophosphate in soft tissue calcification. Homeost. Phosphate Other Miner. 1978, 187–193. [Google Scholar]
- Jono, S.; McKee, M.D.; Murry, C.E.; Shioi, A.; Nishizawa, Y.; Mori, K.; Morii, H.; Giachelli, C.M. Phosphate regulation of vascular smooth muscle cell calcification. Circ. Res. 2000, 87, e10–e17. [Google Scholar] [CrossRef]
- Reynolds, J.L.; Joannides, A.J.; Skepper, J.N.; McNair, R.; Schurgers, L.J.; Proudfoot, D.; Jahnen-Dechent, W.; Weissberg, P.L.; Shanahan, C.M. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: A potential mechanism for accelerated vascular calcification in ESRD. J. Am. Soc. Nephrol. 2004, 15, 2857–2867. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Xu, M.-J.; Zhao, M.-M.; Dai, X.-Y.; Kong, W.; Wilson, G.M.; Guan, Y.; Wang, C.-Y.; Wang, X. Activation of nuclear factor-kappa B accelerates vascular calcification by inhibiting ankylosis protein homolog expression. Kidney Int. 2012, 82, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Souma, Y.; Akakabe, Y.; Kitamura, Y.; Matsuo, K.; Shimoda, Y.; Ueyama, T.; Matoba, S.; Yamada, H.; Okigaki, M.; et al. Macrophages play a unique role in the plaque calcification by enhancing the osteogenic signals exerted by vascular smooth muscle cells. Biochem. Biophys Res. Commun. 2012, 425, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Ceneri, N.; Zhao, L.; Young, B.D.; Healy, A.; Coskun, S.; Vasavada, H.; Yarovinsky, T.O.; Ike, K.; Pardi, R.; Qin, L.; et al. Rac2 Modulates Atherosclerotic Calcification by Regulating Macrophage Interleukin-1β Production. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 328–340. [Google Scholar] [CrossRef]
- Crossman, D.; Rothman, A. The Canakinumab Antiinflammatory Thrombosis Outcome Study trial-the starting gun has fired. J. Thorac. Dis. 2017, 9, 4922–4925. [Google Scholar] [CrossRef]
- Al-Huseini, I.; Ashida, N.; Kimura, T. Deletion of IκB-Kinase β in Smooth Muscle Cells Induces Vascular Calcification through β-Catenin-Runt-Related Transcription Factor 2 Signaling. J. Am. Heart Assoc. 2018, 7, e007405. [Google Scholar] [CrossRef]
- Chinetti-Gbaguidi, G.; Daoudi, M.; Rosa, M.; Vinod, M.; Louvet, L.; Copin, C.; Fanchon, M.; Vanhoutte, J.; Derudas, B.; Belloy, L.; et al. Human Alternative Macrophages Populate Calcified Areas of Atherosclerotic Lesions and Display Impaired RANKL-Induced Osteoclastic Bone Resorption Activity. Circ. Res. 2017, 121, 19–30. [Google Scholar] [CrossRef]
- Kay, A.M.; Simpson, C.L.; Stewart, J.A. The role of AGE/RAGE signaling in diabetes-mediated vascular calcification. J. Diabetes Res. 2016, 2016, 6809703. [Google Scholar] [CrossRef] [PubMed]
- Yahagi, K.; Kolodgie, F.D.; Otsuka, F.; Finn, A.V.; Davis, H.R.; Joner, M.; Virmani, R. Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat. Rev. Cardiol. 2016, 13, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Uemura, S.; Watanabe, M.; Sugawara, Y.; Soeda, T.; Okayama, S.; Takeda, Y.; Kawata, H.; Kawakami, R.; Saito, Y. Colocalization of thin-cap fibroatheroma and spotty calcification is a powerful predictor of procedure-related myocardial injury after elective coronary stent implantation. Coron Artery Dis. 2014, 25, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, L.; Weinbaum, S. Microcalcifications, Their Genesis, Growth, and Biomechanical Stability in Fibrous Cap Rupture. Adv. Exp. Med. Biol. 2018, 1097, 129–155. [Google Scholar] [CrossRef] [PubMed]
- van Rosendael, A.R.; Narula, J.; Lin, F.Y.; van den Hoogen, I.J.; Gianni, U.; Al Hussein Alawamlh, O.; Dunham, P.C.; Peña, J.M.; Lee, S.-E.; Andreini, D.; et al. Association of High-Density Calcified 1K Plaque with Risk of Acute Coronary Syndrome. JAMA Cardiol. 2020, 5, 282–290. [Google Scholar] [CrossRef]
- Torii, S.; Sato, Y.; Otsuka, F.; Kolodgie, F.D.; Jinnouchi, H.; Sakamoto, A.; Park, J.; Yahagi, K.; Sakakura, K.; Cornelissen, A.; et al. Eruptive Calcified Nodules as a Potential Mechanism of Acute Coronary Thrombosis and Sudden Death. J. Am. Coll. Cardiol. 2021, 77, 1599–1611. [Google Scholar] [CrossRef] [PubMed]
- Mintz, G.S.; Pichard, A.D.; Popma, J.J.; Kent, K.M.; Satler, L.F.; Bucher, T.A.; Leon, M.B. Determinants and correlates of target lesion calcium in coronary artery disease: A clinical, angiographic and intravascular ultrasound study. J. Am. Coll. Cardiol. 1997, 29, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Rutsch, F.; Nitschke, Y.; Terkeltaub, R. Genetics in arterial calcification: Pieces of a puzzle and cogs in a wheel. Circ. Res. 2011, 109, 578–592. [Google Scholar] [CrossRef]
- Assimes, T.L.; Knowles, J.W.; Basu, A.; Iribarren, C.; Southwick, A.; Tang, H.; Absher, D.; Li, J.; Fair, J.M.; Rubin, G.D.; et al. Susceptibility locus for clinical and subclinical coronary artery disease at chromosome 9p21 in the multi-ethnic ADVANCE study. Hum. Mol. Genet. 2008, 17, 2320–2328. [Google Scholar] [CrossRef]
- Lange, L.A.; Lange, E.M.; Bielak, L.F.; Langefeld, C.D.; Kardia, S.L.; Royston, P.; Turner, S.T.; Sheedy, P.F., 2nd; Boerwinkle, E.; Peyser, P.A. Autosomal genome-wide scan for coronary artery calcification loci in sibships at high risk for hypertension. Arter. Thromb. Vasc. Biol. 2002, 22, 418–423. [Google Scholar] [CrossRef]
- Aherrahrou, R.; Aherrahrou, Z.; Schunkert, H.; Erdmann, J. Coronary artery disease associated gene Phactr1 modulates severity of vascular calcification in vitro. Biochem. Biophys Res. Commun. 2017, 491, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Trillhaase, A.; Schmidt, B.; Märtens, M.; Haferkamp, U.; Erdmann, J.; Aherrahrou, Z. The CAD risk locus 9p21 increases the risk of vascular calcification in an iPSC-derived VSMC model. Stem. Cell Res. Ther. 2021, 12, 166. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; Lloyd-Jones, D.M.; Guo, X.; Rajamannan, N.M.; Lin, S.; Du, P.; Huang, Q.; Hou, L.; Liu, K. Gene expression variation between African Americans and whites is associated with coronary artery calcification: The multiethnic study of atherosclerosis. Physiol. Genom. 2011, 43, 836–843. [Google Scholar] [CrossRef]
- Lee, T.C.; O’Malley, P.G.; Feuerstein, I.; Taylor, A.J. The prevalence and severity of coronary artery calcification on coronary artery computed tomography in black and white subjects. J. Am. Coll Cardiol. 2003, 41, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Henry, Y.; Eastell, R. Ethnic and gender differences in bone mineral density and bone turnover in young adults: Effect of bone size. Osteoporos. Int. 2000, 11, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Volgman, A.S.; Palaniappan, L.S.; Aggarwal, N.T.; Gupta, M.; Khandelwal, A.; Krishnan, A.V.; Lichtman, J.H.; Mehta, L.S.; Patel, H.N.; Shah, K.S.; et al. Atherosclerotic Cardiovascular Disease in South Asians in the United States: Epidemiology, Risk Factors, and Treatments: A Scientific Statement from the American Heart Association. Circulation 2018, 138, e1–e34. [Google Scholar] [CrossRef] [PubMed]
- Pursnani, S.; Merchant, M. South Asian ethnicity as a risk factor for coronary heart disease. Atherosclerosis 2020, 315, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Kanaya, A.M.; Vittinghoff, E.; Lin, F.; Kandula, N.R.; Herrington, D.; Liu, K.; Blaha, M.; Budoff, M.J. Incidence and progression of coronary artery calcium in South Asians compared with 4 race/ethnic groups. J. Am. Heart Assoc. 2019, 8, e011053. [Google Scholar] [CrossRef]
- Agarwala, A.; Patel, J.; Blaha, M.; Cainzos-Achirica, M.; Nasir, K.; Budoff, M. Leveling the playing field: The utility of coronary artery calcium scoring in cardiovascular risk stratification in South Asians. Am. J. Prev. Cardiol. 2023, 13, 100455. [Google Scholar] [CrossRef]
- Hatwalkar, A.; Agrawal, N.; Reiss, D.S.; Budoff, M.J. Comparison of prevalence and severity of coronary calcium determined by electron beam tomography among various ethnic groups. Am. J. Cardiol. 2003, 91, 1225–1227. [Google Scholar] [CrossRef]
- Fornage, M.; Boerwinkle, E.; Doris, P.A.; Jacobs, D.; Liu, K.; Wong, N.D. Polymorphism of the soluble epoxide hydrolase is associated with coronary artery calcification in African-American subjects: The Coronary Artery Risk Development in Young Adults (CARDIA) study. Circulation 2004, 109, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Poornima, I.G.; Mackey, R.H.; Allison, M.A.; Manson, J.E.; Carr, J.J.; LaMonte, M.J.; Chang, Y.; Kuller, L.H. Coronary Artery Calcification (CAC) and Post-Trial Cardiovascular Events and Mortality within the Women’s Health Initiative (WHI) Estrogen-Alone Trial. J. Am. Heart Assoc. 2017, 6, e006887. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.H.; Yang, W.L.; Su, C.Y.; Lu, X.H.; He, L.; Zhang, A.H. Relationship between gut microbiota and vascular calcification in hemodialysis patients. Ren. Fail. 2023, 45, 2148538. [Google Scholar] [CrossRef] [PubMed]
- Seldin, M.M.; Meng, Y.; Qi, H.; Zhu, W.; Wang, Z.; Hazen, S.L.; Lusis, A.J.; Shih, D.M. Trimethylamine N-Oxide Promotes Vascular Inflammation through Signaling of Mitogen-Activated Protein Kinase and Nuclear Factor-κB. J. Am. Heart Assoc. 2016, 5, e002767. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jing, L.; Zhai, C.; Xiang, Q.; Tian, H.; Hu, H. Intestinal Flora Metabolite Trimethylamine Oxide Is Inextricably Linked to Coronary Heart Disease. J. Cardiovasc. Pharmacol. 2023, 81, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Yang, P.; Liu, X.; Lu, L.; Chen, Y.; Zhong, X.; Li, Z.; Liu, H.; Ou, C.; et al. Trimethylamine-N-Oxide Promotes Vascular Calcification through Activation of NLRP3 (Nucleotide-Binding Domain, Leucine-Rich-Containing Family, Pyrin Domain-Containing-3) Inflammasome and NF-κB (Nuclear Factor κB) Signals. Arter. Thromb. Vasc. Biol. 2020, 40, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Liu, T.; Li, X.; Gao, X.; Wu, T.; Li, P. The role of gut microbiota metabolite trimethylamine N-oxide in functional impairment of bone marrow mesenchymal stem cells in osteoporosis disease. Ann. Transl. Med. 2020, 8, 1009. [Google Scholar] [CrossRef]
- Toya, T.; Ozcan, I.; Corban, M.T.; Sara, J.D.; Marietta, E.V.; Ahmad, A.; Horwath, I.E.; Loeffler, D.L.; Murray, J.A.; Lerman, L.O.; et al. Compositional change of gut microbiome and osteocalcin expressing endothelial progenitor cells in patients with coronary artery disease. PLoS ONE 2021, 16, e0249187. [Google Scholar] [CrossRef]
- Okami, Y.; Arima, H.; Kondo, K.; Hexun, Z.; Yano, Y.; Kadota, A.; Torii, S.; Hisamatsu, T.; Fujiyoshi, A.; Kadowaki, S.; et al. The gut microbiota and coronary artery calcification in Japanese men. Am. Heart J. 2023, 267, 12–21. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; Simes, J.; et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [CrossRef]
- Davignon, J. Advances in lipid-lowering therapy in atherosclerosis. Adv. Exp. Med. Biol. 2001, 498, 49–58. [Google Scholar] [CrossRef]
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Bairey Merz, C.N.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.M.; et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2889–2934. [Google Scholar] [CrossRef]
- Mahmood, D.; Jahan, K.; Habibullah, K. Primary prevention with statins in cardiovascular diseases: A Saudi Arabian perspective. J. Saudi. Heart Assoc. 2015, 27, 179–191. [Google Scholar] [CrossRef]
- Feingold, K.R. Utility of Advanced Lipoprotein Testing in Clinical Practice. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. J. Prev. Cardiol. 2022, 29, 5–115. [Google Scholar] [CrossRef]
- Henein, M.; Granåsen, G.; Wiklund, U.; Schmermund, A.; Guerci, A.; Erbel, R.; Raggi, P. High dose and long-term statin therapy accelerate coronary artery calcification. Int. J. Cardiol. 2015, 184, 581–586. [Google Scholar] [CrossRef]
- Dykun, I.; Lehmann, N.; Kälsch, H.; Möhlenkamp, S.; Moebus, S.; Budde, T.; Seibel, R.; Grönemeyer, D.; Jöckel, K.-H.; Erbel, R. Statin medication enhances progression of coronary artery calcification: The Heinz Nixdorf Recall Study. J. Am. Coll. Cardiol. 2016, 68, 2123–2125. [Google Scholar] [CrossRef]
- Xian, J.Z.; Lu, M.; Fong, F.; Qiao, R.; Patel, N.R.; Abeydeera, D.; Iriana, S.; Demer, L.L.; Tintut, Y. Statin Effects on Vascular Calcification. Arterioscler. Thromb. Vasc. Biol. 2021, 41, e185–e192. [Google Scholar] [CrossRef]
- Xinyu, Z.; Dongxia, M.; Yue, H.; Xiao, J.; Wang, L.; Xiaoping, J. Statins Accelerate Coronary Calcification and Reduce the Risk of Cardiovascular Events. Cardiol. Rev. 2023, 31, 293–298. [Google Scholar] [CrossRef]
- Healy, A.; Berus, J.M.; Christensen, J.L.; Lee, C.; Mantsounga, C.; Dong, W.; Watts, J.P., Jr.; Assali, M.; Ceneri, N.; Nilson, R. Statins disrupt macrophage Rac1 regulation leading to increased atherosclerotic plaque calcification. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 714–732. [Google Scholar] [CrossRef]
- Sutton, N.R.; Malhotra, R.; St Hilaire, C.; Aikawa, E.; Blumenthal, R.S.; Gackenbach, G.; Goyal, P.; Johnson, A.; Nigwekar, S.U.; Shanahan, C.M.; et al. Molecular Mechanisms of Vascular Health: Insights from Vascular Aging and Calcification. Arter. Thromb. Vasc. Biol. 2023, 43, 15–29. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; De Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, 3168–3209. [Google Scholar] [CrossRef]
- Lee, S.-E.; Chang, H.-J.; Sung, J.M.; Park, H.-B.; Heo, R.; Rizvi, A.; Lin, F.Y.; Kumar, A.; Hadamitzky, M.; Kim, Y.J. Effects of statins on coronary atherosclerotic plaques: The PARADIGM study. JACC Cardiovasc. Imaging 2018, 11, 1475–1484. [Google Scholar] [CrossRef]
- Osei, A.D.; Mirbolouk, M.; Berman, D.; Budoff, M.J.; Miedema, M.D.; Rozanski, A.; Rumberger, J.A.; Shaw, L.; Al Rifai, M.; Dzaye, O.; et al. Prognostic value of coronary artery calcium score, area, and density among individuals on statin therapy vs. non-users: The coronary artery calcium consortium. Atherosclerosis 2021, 316, 79–83. [Google Scholar] [CrossRef]
- Cui, L.; Li, Z.; Chang, X.; Cong, G.; Hao, L. Quercetin attenuates vascular calcification by inhibiting oxidative stress and mitochondrial fission. Vasc. Pharmacol. 2017, 88, 21–29. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Li, T.; Wang, Y.; Tu, W.P. Vitamin K supplementation and vascular calcification: A systematic review and meta-analysis of randomized controlled trials. Front. Nutr. 2023, 10, 1115069. [Google Scholar] [CrossRef]
- Boström, K.I.; Jumabay, M.; Matveyenko, A.; Nicholas, S.B.; Yao, Y. Activation of vascular bone morphogenetic protein signaling in diabetes mellitus. Circ. Res. 2011, 108, 446–457. [Google Scholar] [CrossRef]
- Shanahan, C.M.; Crouthamel, M.H.; Kapustin, A.; Giachelli, C.M. Arterial calcification in chronic kidney disease: Key roles for calcium and phosphate. Circ. Res. 2011, 109, 697–711. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Weng, T.; Chen, Z.; Sun, X.; Wei, J.; Cai, Z.; Xiang, M. Association between metformin use and coronary artery calcification in type 2 diabetic patients. J. Diabetes Res. 2019, 2019, 9484717. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.B.; Aroda, V.R.; Bluemke, D.A.; Barrett-Connor, E.; Budoff, M.; Crandall, J.P.; Dabelea, D.; Horton, E.S.; Mather, K.J.; Orchard, T.J. Effect of long-term metformin and lifestyle in the diabetes prevention program and its outcome study on coronary artery calcium. Circulation 2017, 136, 52–64. [Google Scholar] [CrossRef]
- Cao, X.; Li, H.; Tao, H.; Wu, N.; Yu, L.; Zhang, D.; Lu, X.; Zhu, J.; Lu, Z.; Zhu, Q. Metformin inhibits vascular calcification in female rat aortic smooth muscle cells via the AMPK-eNOS-NO pathway. Endocrinology 2013, 154, 3680–3689. [Google Scholar] [CrossRef]
- Schinzari, F.; Tesauro, M.; Bertoli, A.; Valentini, A.; Veneziani, A.; Campia, U.; Cardillo, C. Calcification biomarkers and vascular dysfunction in obesity and type 2 diabetes: Influence of oral hypoglycemic agents. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E658–E666. [Google Scholar] [CrossRef]
- Siew, W.S.; Tang, Y.Q.; Kong, C.K.; Goh, B.H.; Zacchigna, S.; Dua, K.; Chellappan, D.K.; Duangjai, A.; Saokaew, S.; Phisalprapa, P.; et al. Harnessing the Potential of CRISPR/Cas in Atherosclerosis: Disease Modeling and Therapeutic Applications. Int. J. Mol. Sci. 2021, 22, 8422. [Google Scholar] [CrossRef]
- Cao, G.; Xuan, X.; Zhang, R.; Hu, J.; Dong, H. Gene Therapy for Cardiovascular Disease: Basic Research and Clinical Prospects. Front. Cardiovasc. Med. 2021, 8, 8422. [Google Scholar] [CrossRef]
- Gupta, R.M.; Schnitzler, G.R.; Fang, S.; Lee-Kim, V.S.; Barry, A. Multiomic Analysis and CRISPR Perturbation Screens Identify Endothelial Cell Programs and Novel Therapeutic Targets for Coronary Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 600–608. [Google Scholar] [CrossRef]
- Mujwara, D.; Kintzle, J.; Di Domenico, P.; Busby, G.B.; Bottà, G. Cost-effectiveness analysis of implementing polygenic risk score in a workplace cardiovascular disease prevention program. Front. Public Health 2023, 11, 1139496. [Google Scholar] [CrossRef]
- Cornelissen, A.; Gadhoke, N.V.; Ryan, K.; Hodonsky, C.J.; Mitchell, R.; Bihlmeyer, N.; Duong, T.; Chen, Z.; Dikongue, A.; Sakamoto, A.; et al. Polygenic Risk Score Associates with Atherosclerotic Plaque Characteristics at Autopsy. bioRxiv 2023. [Google Scholar] [CrossRef]
- Patel, A.P.; Wang, M.; Ruan, Y.; Koyama, S.; Clarke, S.L.; Yang, X.; Tcheandjieu, C.; Agrawal, S.; Fahed, A.C.; Ellinor, P.T.; et al. A multi-ancestry polygenic risk score improves risk prediction for coronary artery disease. Nat. Med. 2023, 29, 1793–1803. [Google Scholar] [CrossRef] [PubMed]
- Aherrahrou, Z.; Schunkert, H. Genetics of atherosclerosis and vascular calcification go hand-in-hand. Atherosclerosis 2013, 228, 325–326. [Google Scholar] [CrossRef] [PubMed]
- Massy, Z.A.; Ivanovski, O.; Nguyen-Khoa, T.; Angulo, J.; Szumilak, D.; Mothu, N.; Phan, O.; Daudon, M.; Lacour, B.; Drüeke, T.B.; et al. Uremia accelerates both atherosclerosis and arterial calcification in apolipoprotein E knockout mice. J. Am. Soc. Nephrol. 2005, 16, 109–116. [Google Scholar] [CrossRef]
- Davies, M.R.; Lund, R.J.; Hruska, K.A. BMP-7 is an efficacious treatment of vascular calcification in a murine model of atherosclerosis and chronic renal failure. J. Am. Soc. Nephrol. 2003, 14, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Babic, M.; Tölle, M.; van der Giet, M.; Schuchardt, M. Research Models for Studying Vascular Calcification. Int. J. Mol. Sci. 2020, 21, 2204. [Google Scholar] [CrossRef]
- Herrmann, J.; Gummi, M.R.; Xia, M.; van der Giet, M.; Tölle, M.; Schuchardt, M. Vascular Calcification in Rodent Models-Keeping Track with an Extented Method Assortment. Biology 2021, 10, 459. [Google Scholar] [CrossRef]
- DeFina, L.F.; Radford, N.B.; Barlow, C.E.; Willis, B.L.; Leonard, D.; Haskell, W.L.; Farrell, S.W.; Pavlovic, A.; Abel, K.; Berry, J.D. Association of all-cause and cardiovascular mortality with high levels of physical activity and concurrent coronary artery calcification. JAMA Cardiol. 2019, 4, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.J.; Fong, F.; Patel, R.; Qiao, R.; Lo, K.; Soundia, A.; Chang, C.-C.; Le, V.; Tseng, C.-H.; Demer, L.L. Changes in microarchitecture of atherosclerotic calcification assessed by 18 F-NaF PET and CT after a progressive exercise regimen in hyperlipidemic mice. J. Nucl. Cardiol. 2021, 28, 2207–2214. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Osborne, M.T.; Tung, B.; Li, M.; Li, Y. Imaging Cardiovascular Calcification. J. Am. Heart Assoc. 2018, 7, e008564. [Google Scholar] [CrossRef] [PubMed]
- Kwiecinski, J. Imaging coronary and aortic microcalcification activity with 18F-sodium fluoride. J. Nucl. Cardiol. 2022, 29, 3366–3368. [Google Scholar] [CrossRef]
- Kwiecinski, J.; Slomka, P.J.; Dweck, M.R.; Newby, D.E.; Berman, D.S. Vulnerable plaque imaging using 18F-sodium fluoride positron emission tomography. Br. J. Radiol. 2020, 93, 20190797. [Google Scholar] [CrossRef]
- Joshi, N.V.; Vesey, A.T.; Williams, M.C.; Shah, A.S.; Calvert, P.A.; Craighead, F.H.; Yeoh, S.E.; Wallace, W.; Salter, D.; Fletcher, A.M.; et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: A prospective clinical trial. Lancet 2014, 383, 705–713. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashmi, S.; Shah, P.W.; Aherrahrou, Z.; Aikawa, E.; Aherrahrou, R. Beyond the Basics: Unraveling the Complexity of Coronary Artery Calcification. Cells 2023, 12, 2822. https://doi.org/10.3390/cells12242822
Hashmi S, Shah PW, Aherrahrou Z, Aikawa E, Aherrahrou R. Beyond the Basics: Unraveling the Complexity of Coronary Artery Calcification. Cells. 2023; 12(24):2822. https://doi.org/10.3390/cells12242822
Chicago/Turabian StyleHashmi, Satwat, Pashmina Wiqar Shah, Zouhair Aherrahrou, Elena Aikawa, and Rédouane Aherrahrou. 2023. "Beyond the Basics: Unraveling the Complexity of Coronary Artery Calcification" Cells 12, no. 24: 2822. https://doi.org/10.3390/cells12242822
APA StyleHashmi, S., Shah, P. W., Aherrahrou, Z., Aikawa, E., & Aherrahrou, R. (2023). Beyond the Basics: Unraveling the Complexity of Coronary Artery Calcification. Cells, 12(24), 2822. https://doi.org/10.3390/cells12242822






