Unlocking Therapeutic Synergy: Tailoring Drugs for Comorbidities such as Depression and Diabetes through Identical Molecular Targets in Different Cell Types
Abstract
1. Introduction
2. Depression and Diabetes as Comorbidities
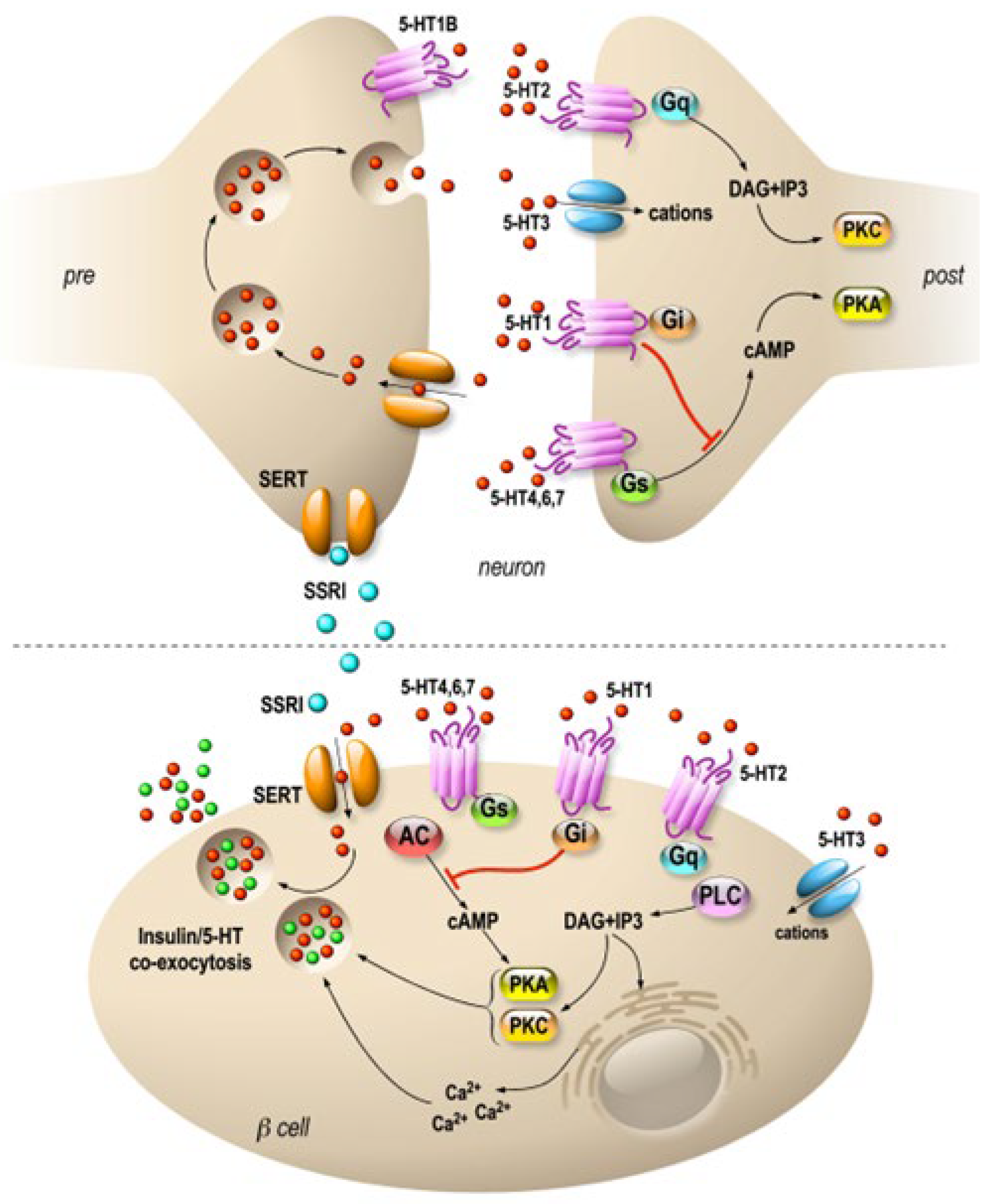
3. Fluoxetine
3.1. Fluoxetine as an Antidepressant Reference Treatment
3.2. Fluoxetine Action on Pancreatic Endocrine Function
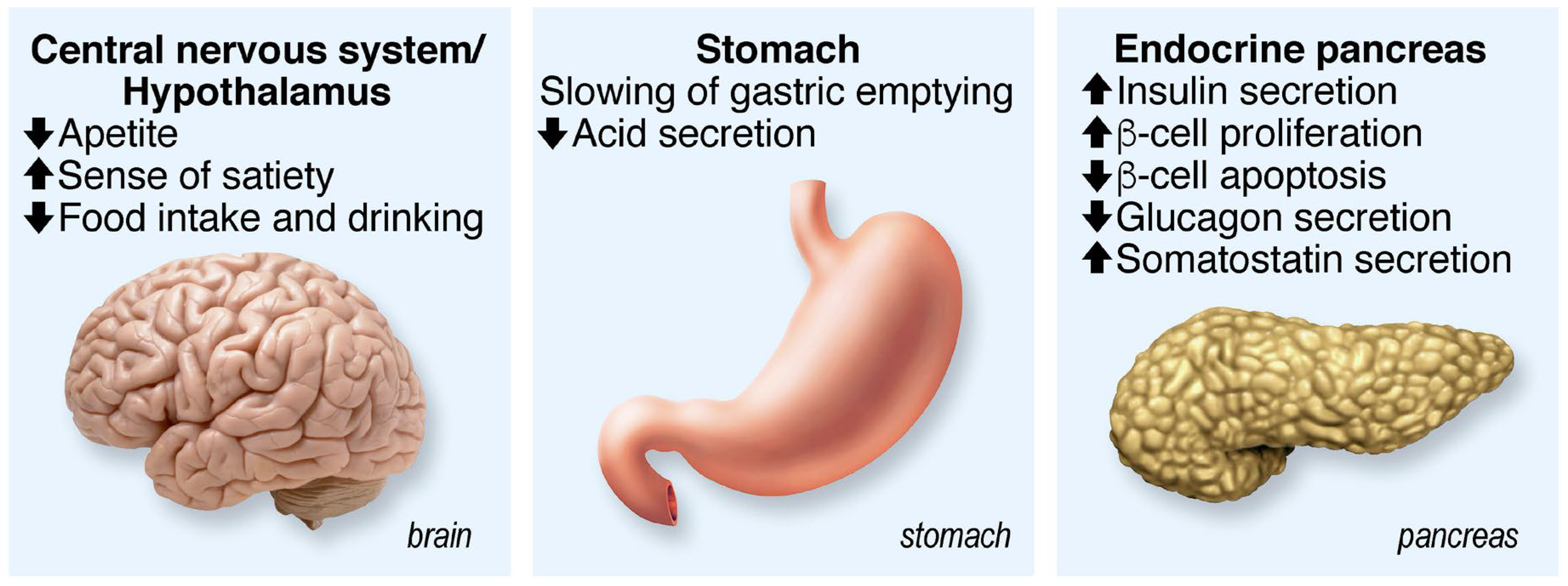
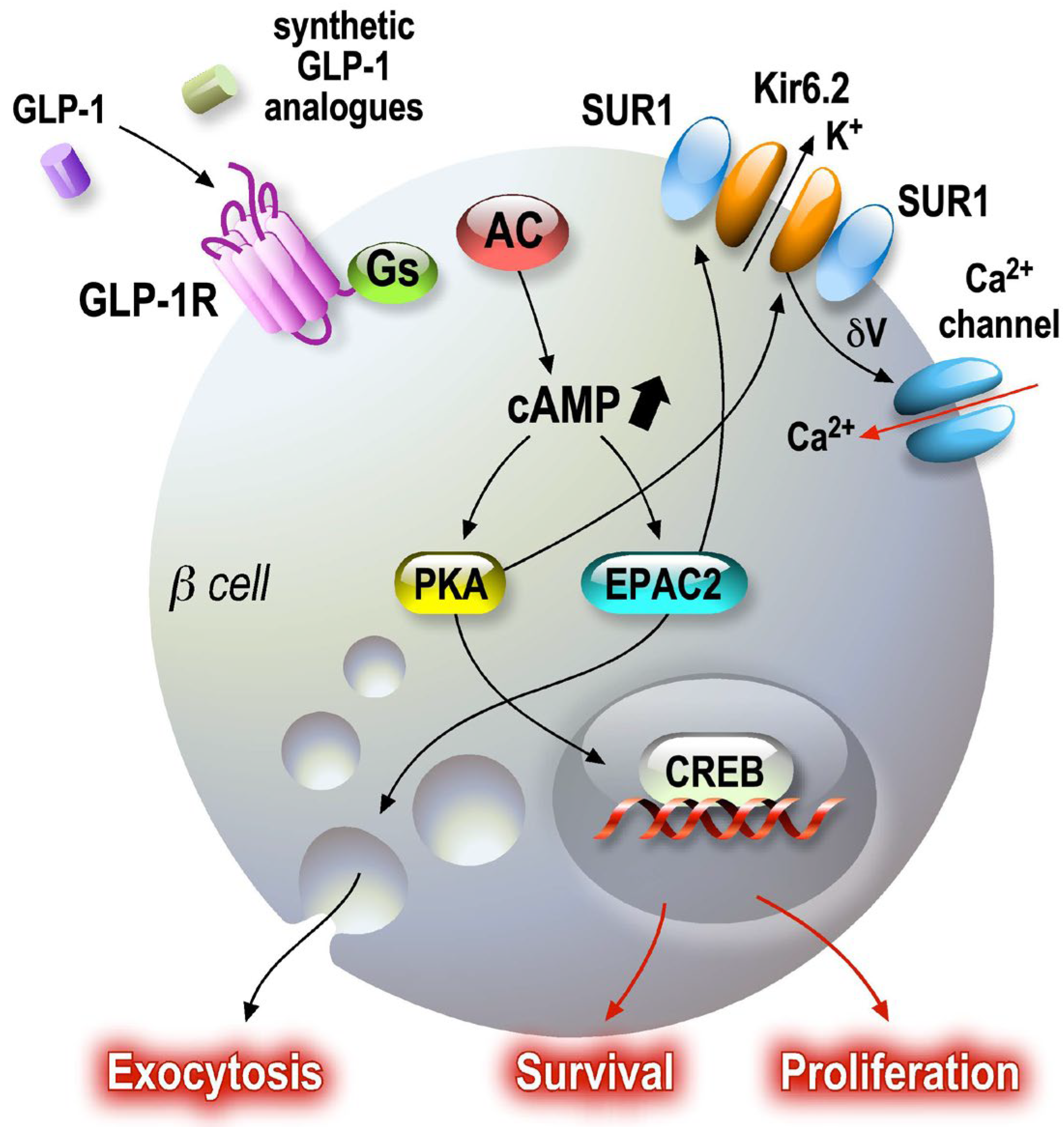
4. GLP-1
4.1. GLP-1R Agonists as Antidiabetic Drugs
4.2. GLP-1 as a Neuromodulator
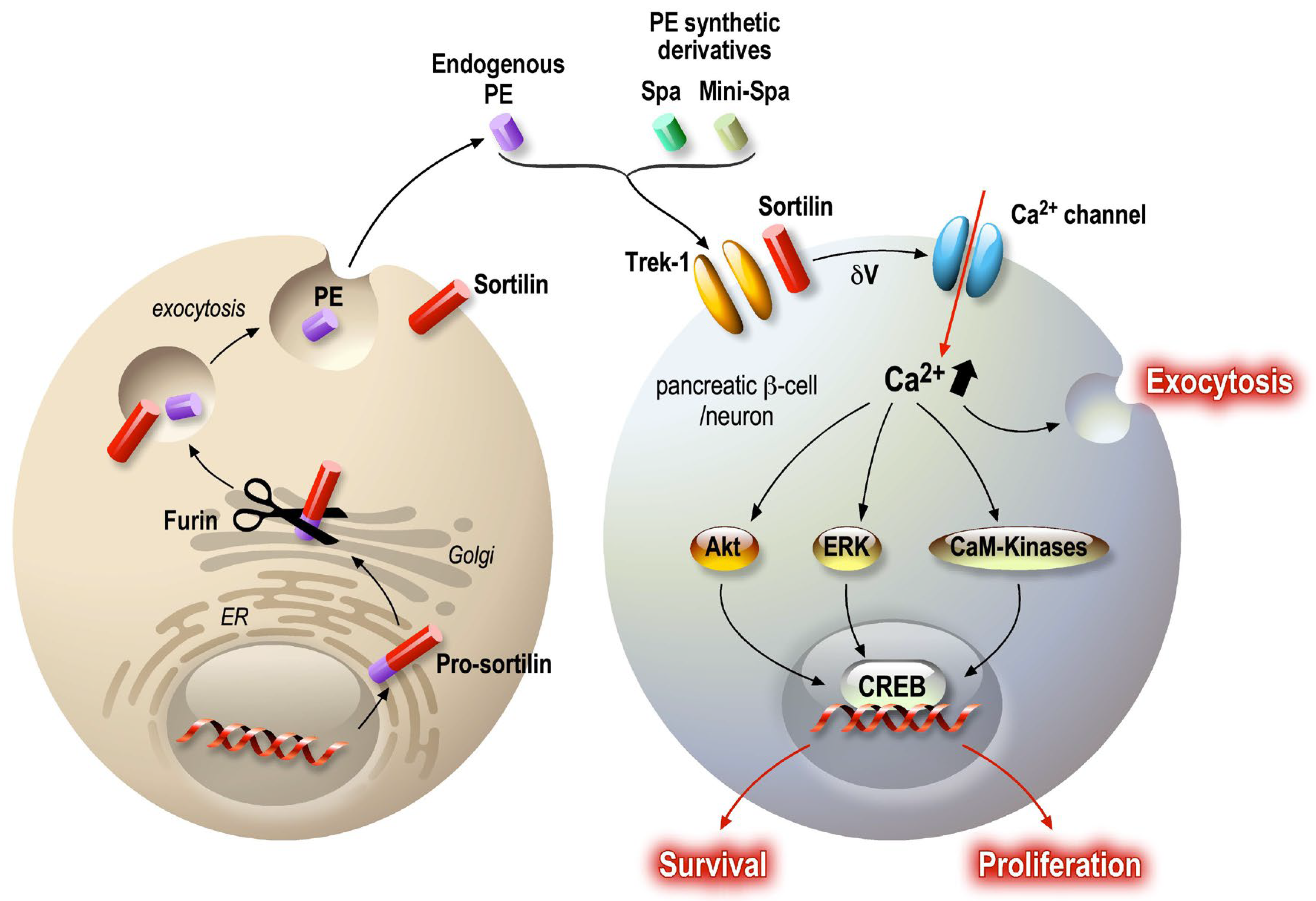
5. PE/Spadin
6. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brady, P.A.; Terzic, A. The sulfonylurea controversy: More questions from the heart. J. Am. Coll. Cardiol. 1998, 31, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Goodnick, P.J.; Henry, J.H.; Buki, V.M. Treatment of depression in patients with diabetes mellitus. J. Clin. Psychiatry 1995, 56, 128–136. [Google Scholar] [CrossRef]
- Herrman, H.; Patel, V.; Kieling, C.; Berk, M.; Buchweitz, C.; Cuijpers, P.; Furukawa, T.A.; Kessler, R.C.; Kohrt, B.A.; Maj, M.; et al. Time for united action on depression: A Lancet-World Psychiatric Association Commission. Lancet 2022, 399, 957–1022. [Google Scholar] [CrossRef] [PubMed]
- Coppen, A. The biochemistry of affective disorders. Br. J. Psychiatry 1967, 113, 1237–1264. [Google Scholar] [CrossRef] [PubMed]
- Delgado, P.L. Depression: The case for a monoamine deficiency. J. Clin. Psychiatry 2000, 61 (Suppl. S6), 7–11. [Google Scholar] [PubMed]
- Plana-Ripoll, O.; Pedersen, C.B.; Agerbo, E.; Holtz, Y.; Erlangsen, A.; Canudas-Romo, V.; Andersen, P.K.; Charlson, F.J.; Christensen, M.K.; Erskine, H.E.; et al. A comprehensive analysis of mortality-related health metrics associated with mental disorders: A nationwide, register-based cohort study. Lancet 2019, 394, 1827–1835. [Google Scholar] [CrossRef]
- Miola, A.; Meda, N.; Perini, G.; Sambataro, F. Structural and functional features of treatment-resistant depression: A systematic review and exploratory coordinate-based meta-analysis of neuroimaging studies. Psychiatry Clin. Neurosci. 2023, 77, 252–263. [Google Scholar] [CrossRef]
- de Wit, L.M.; Fokkema, M.; van Straten, A.; Lamers, F.; Cuijpers, P.; Penninx, B.W. Depressive and anxiety disorders and the association with obesity, physical, and social activities. Depress. Anxiety 2010, 27, 1057–1065. [Google Scholar] [CrossRef]
- Milaneschi, Y.; Simmons, W.K.; van Rossum, E.F.C.; Penninx, B.W. Depression and obesity: Evidence of shared biological mechanisms. Mol. Psychiatry 2019, 24, 18–33. [Google Scholar] [CrossRef]
- Delahanty, L.M.; Meigs, J.B.; Hayden, D.; Williamson, D.A.; Nathan, D.M.; Diabetes Prevenion Program Research, G. Psychological and behavioral correlates of baseline BMI in the diabetes prevention program (DPP). Diabetes Care 2002, 25, 1992–1998. [Google Scholar] [CrossRef]
- Heo, M.; Pietrobelli, A.; Fontaine, K.R.; Sirey, J.A.; Faith, M.S. Depressive mood and obesity in US adults: Comparison and moderation by sex, age, and race. Int. J. Obes. 2006, 30, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, K.H.; Ahn, J.C.; Kim, J.A.; Lee, G.; Son, J.S.; Choi, S.J.; Oh, Y.H.; Park, S.M. Prevalence, awareness, treatment, and control of diabetes mellitus by depressive symptom severity: A cross-sectional analysis of NHANES 2011-2016. BMJ Open Diabetes Res. Care 2021, 9, e002268. [Google Scholar] [CrossRef] [PubMed]
- Srisurapanont, M.; Suttajit, S.; Kosachunhanun, N.; Likhitsathian, S.; Suradom, C.; Maneeton, B. Antidepressants for depressed patients with type 2 diabetes mellitus: A systematic review and network meta-analysis of short-term randomized controlled trials. Neurosci. Biobehav. Rev. 2022, 139, 104731. [Google Scholar] [CrossRef] [PubMed]
- Grigolon, R.B.; Brietzke, E.; Mansur, R.B.; Idzikowski, M.A.; Gerchman, F.; De Felice, F.G.; McIntyre, R.S. Association between diabetes and mood disorders and the potential use of anti-hyperglycemic agents as antidepressants. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 95, 109720. [Google Scholar] [CrossRef]
- Moulton, C.D.; Pickup, J.C.; Ismail, K. The link between depression and diabetes: The search for shared mechanisms. Lancet Diabetes Endocrinol. 2015, 3, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Hryhorczuk, C.; Sharma, S.; Fulton, S.E. Metabolic disturbances connecting obesity and depression. Front. Neurosci. 2013, 7, 177. [Google Scholar] [CrossRef]
- Alshehri, T.; Boone, S.; de Mutsert, R.; Penninx, B.; Rosendaal, F.; le Cessie, S.; Milaneschi, Y.; Mook-Kanamori, D. The association between overall and abdominal adiposity and depressive mood: A cross-sectional analysis in 6459 participants. Psychoneuroendocrinology 2019, 110, 104429. [Google Scholar] [CrossRef]
- Levitan, R.D.; Davis, C.; Kaplan, A.S.; Arenovich, T.; Phillips, D.I.; Ravindran, A.V. Obesity comorbidity in unipolar major depressive disorder: Refining the core phenotype. J. Clin. Psychiatry 2012, 73, 1119–1124. [Google Scholar] [CrossRef]
- Freedman, M.R.; Castonguay, T.W.; Stern, J.S. Effect of adrenalectomy and corticosterone replacement on meal patterns of Zucker rats. Am. J. Physiol. 1985, 249, R584–R594. [Google Scholar] [CrossRef]
- Gregus, A.; Wintink, A.J.; Davis, A.C.; Kalynchuk, L.E. Effect of repeated corticosterone injections and restraint stress on anxiety and depression-like behavior in male rats. Behav. Brain Res. 2005, 156, 105–114. [Google Scholar] [CrossRef]
- Liu, Y.; Nakagawa, Y.; Wang, Y.; Li, R.; Li, X.; Ohzeki, T.; Friedman, T.C. Leptin activation of corticosterone production in hepatocytes may contribute to the reversal of obesity and hyperglycemia in leptin-deficient ob/ob mice. Diabetes 2003, 52, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; McIntyre, R.S.; Li, R.; Yang, M.; Xue, Y.; Cao, B. Genetic association between major depressive disorder and type 2 diabetes mellitus: Shared pathways and protein networks. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 111, 110339. [Google Scholar] [CrossRef] [PubMed]
- Xiu, J.; Han, R.; Liu, Z.; Li, J.; Liu, S.; Shen, Y.; Ding, Y.Q.; Xu, Q. Hijacking Dorsal Raphe to Improve Metabolism and Depression-like Behaviors via BDNF Gene Transfer in Mice. Diabetes 2021, 70, 1780–1793. [Google Scholar] [CrossRef] [PubMed]
- Barnes, N.M.; Ahern, G.P.; Becamel, C.; Bockaert, J.; Camilleri, M.; Chaumont-Dubel, S.; Claeysen, S.; Cunningham, K.A.; Fone, K.C.; Gershon, M.; et al. International Union of Basic and Clinical Pharmacology. CX. Classification of Receptors for 5-hydroxytryptamine; Pharmacology and Function. Pharmacol. Rev. 2021, 73, 310–520. [Google Scholar] [CrossRef] [PubMed]
- Celada, P.; Bortolozzi, A.; Artigas, F. Serotonin 5-HT1A receptors as targets for agents to treat psychiatric disorders: Rationale and current status of research. CNS Drugs 2013, 27, 703–716. [Google Scholar] [CrossRef]
- Celada, P.; Puig, M.; Amargos-Bosch, M.; Adell, A.; Artigas, F. The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. J. Psychiatry Neurosci. 2004, 29, 252–265. [Google Scholar]
- Wong, D.T.; Horng, J.S.; Bymaster, F.P.; Hauser, K.L.; Molloy, B.B. A selective inhibitor of serotonin uptake: Lilly 110140, 3-(p-trifluoromethylphenoxy)-N-methyl-3-phenylpropylamine. Life Sci. 1974, 15, 471–479. [Google Scholar] [CrossRef]
- Fuller, R.W.; Perry, K.W.; Wong, D.T.; Molloy, B.B. Effects of some homologues of 4-chloroamphetamine on brain serotonin metabolism. Neuropharmacology 1974, 13, 609–614. [Google Scholar] [CrossRef]
- Fuller, R.W.; Wong, D.T.; Robertson, D.W. Fluoxetine, a selective inhibitor of serotonin uptake. Med. Res. Rev. 1991, 11, 17–34. [Google Scholar] [CrossRef]
- Stokes, P.E. Fluoxetine: A five-year review. Clin. Ther. 1993, 15, 216–243, discussion 215. [Google Scholar]
- Coleman, J.A.; Green, E.M.; Gouaux, E. X-ray structures and mechanism of the human serotonin transporter. Nature 2016, 532, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Casarotto, P.C.; Girych, M.; Fred, S.M.; Kovaleva, V.; Moliner, R.; Enkavi, G.; Biojone, C.; Cannarozzo, C.; Sahu, M.P.; Kaurinkoski, K.; et al. Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell 2021, 184, 1299–1313.e1219. [Google Scholar] [CrossRef]
- Daubresse, J.C.; Kolanowski, J.; Krzentowski, G.; Kutnowski, M.; Scheen, A.; Van Gaal, L. Usefulness of fluoxetine in obese non-insulin-dependent diabetics: A multicenter study. Obes. Res. 1996, 4, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Lustman, P.J.; Anderson, R.J.; Freedland, K.E.; de Groot, M.; Carney, R.M.; Clouse, R.E. Depression and poor glycemic control: A meta-analytic review of the literature. Diabetes Care 2000, 23, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Chen, L.; Yang, Z.; Li, Q.; Huang, Y.; He, M.; Zhang, S.; Zhang, Z.; Wang, X.; Zhao, W.; et al. Metabolic effects of fluoxetine in adults with type 2 diabetes mellitus: A meta-analysis of randomized placebo-controlled trials. PLoS ONE 2011, 6, e21551. [Google Scholar] [CrossRef] [PubMed]
- Breum, L.; Bjerre, U.; Bak, J.F.; Jacobsen, S.; Astrup, A. Long-term effects of fluoxetine on glycemic control in obese patients with non-insulin-dependent diabetes mellitus or glucose intolerance: Influence on muscle glycogen synthase and insulin receptor kinase activity. Metabolism 1995, 44, 1570–1576. [Google Scholar] [CrossRef]
- Maheux, P.; Ducros, F.; Bourque, J.; Garon, J.; Chiasson, J.L. Fluoxetine improves insulin sensitivity in obese patients with non-insulin-dependent diabetes mellitus independently of weight loss. Int. J. Obes. Relat. Metab. Disord. 1997, 21, 97–102. [Google Scholar] [CrossRef]
- Dryden, S.; Frankish, H.M.; Wang, Q.; Pickavance, L.; Williams, G. The serotonergic agent fluoxetine reduces neuropeptide Y levels and neuropeptide Y secretion in the hypothalamus of lean and obese rats. Neuroscience 1996, 72, 557–566. [Google Scholar] [CrossRef]
- Gutierrez, A.; Saracibar, G.; Casis, L.; Echevarria, E.; Rodriguez, V.M.; Macarulla, M.T.; Abecia, L.C.; Portillo, M.P. Effects of fluoxetine administration on neuropeptide y and orexins in obese zucker rat hypothalamus. Obes. Res. 2002, 10, 532–540. [Google Scholar] [CrossRef]
- Bernardis, L.L.; Bellinger, L.L. The lateral hypothalamic area revisited: Neuroanatomy, body weight regulation, neuroendocrinology and metabolism. Neurosci. Biobehav. Rev. 1993, 17, 141–193. [Google Scholar] [CrossRef]
- Kupari, J.; Haring, M.; Agirre, E.; Castelo-Branco, G.; Ernfors, P. An Atlas of Vagal Sensory Neurons and Their Molecular Specialization. Cell Rep. 2019, 27, 2508–2523.e2504. [Google Scholar] [CrossRef]
- Guyot, M.; Simon, T.; Ceppo, F.; Panzolini, C.; Guyon, A.; Lavergne, J.; Murris, E.; Daoudlarian, D.; Brusini, R.; Zarif, H.; et al. Pancreatic nerve electrostimulation inhibits recent-onset autoimmune diabetes. Nat. Biotechnol. 2019, 37, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- Bennet, H.; Balhuizen, A.; Medina, A.; Dekker Nitert, M.; Ottosson Laakso, E.; Essen, S.; Spegel, P.; Storm, P.; Krus, U.; Wierup, N.; et al. Altered serotonin (5-HT) 1D and 2A receptor expression may contribute to defective insulin and glucagon secretion in human type 2 diabetes. Peptides 2015, 71, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Gylfe, E. Association between 5-hydroxytryptamine release and insulin secretion. J. Endocrinol. 1978, 78, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, L.R.; Cortes, V.A.; Mizgier, M.L.; Aranda, E.; Mezzano, D.; Olmos, P.; Galgani, J.E.; Suazo, J.; Santos, J.L. Fluoxetine impairs insulin secretion without modifying extracellular serotonin levels in MIN6 beta-cells. Exp. Clin. Endocrinol. Diabetes 2015, 123, 473–478. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cataldo, L.R.; Mizgier, M.L.; Busso, D.; Olmos, P.; Galgani, J.E.; Valenzuela, R.; Mezzano, D.; Aranda, E.; Cortes, V.A.; Santos, J.L. Serotonin- and Dopamine-Related Gene Expression in db/db Mice Islets and in MIN6 beta-Cells Treated with Palmitate and Oleate. J. Diabetes Res. 2016, 2016, 3793781. [Google Scholar] [CrossRef] [PubMed]
- Almaca, J.; Molina, J.; Menegaz, D.; Pronin, A.N.; Tamayo, A.; Slepak, V.; Berggren, P.O.; Caicedo, A. Human Beta Cells Produce and Release Serotonin to Inhibit Glucagon Secretion from Alpha Cells. Cell Rep. 2016, 17, 3281–3291. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Ruz-Maldonado, I.; Toczyska, K.; Olaniru, O.E.; Zariwala, M.G.; Hopkins, D.; Zhao, M.; Persaud, S.J. The selective serotonin reuptake inhibitor fluoxetine has direct effects on beta cells, promoting insulin secretion and increasing beta-cell mass. Diabetes Obes. Metab. 2022, 24, 2038–2050. [Google Scholar] [CrossRef]
- De Long, N.E.; Hyslop, J.R.; Raha, S.; Hardy, D.B.; Holloway, A.C. Fluoxetine-induced pancreatic beta cell dysfunction: New insight into the benefits of folic acid in the treatment of depression. J. Affect. Disord. 2014, 166, 6–13. [Google Scholar] [CrossRef]
- Chang, H.Y.; Chen, S.L.; Shen, M.R.; Kung, M.L.; Chuang, L.M.; Chen, Y.W. Selective serotonin reuptake inhibitor, fluoxetine, impairs E-cadherin-mediated cell adhesion and alters calcium homeostasis in pancreatic beta cells. Sci. Rep. 2017, 7, 3515. [Google Scholar] [CrossRef]
- Isaac, R.; Boura-Halfon, S.; Gurevitch, D.; Shainskaya, A.; Levkovitz, Y.; Zick, Y. Selective serotonin reuptake inhibitors (SSRIs) inhibit insulin secretion and action in pancreatic beta cells. J. Biol. Chem. 2013, 288, 5682–5693. [Google Scholar] [CrossRef]
- Cataldo Bascunan, L.R.; Lyons, C.; Bennet, H.; Artner, I.; Fex, M. Serotonergic regulation of insulin secretion. Acta Physiol. 2019, 225, e13101. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Choi, S.B. Does fluoxetine administration influence insulin resistance in 90% pancreatectomized rats? Metabolism 2002, 51, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J.; Holst, J.J. The expanding incretin universe: From basic biology to clinical translation. Diabetologia 2023, 66, 1765–1779. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J. Incretin hormones: Their role in health and disease. Diabetes Obes. Metab. 2018, 20 (Suppl. S1), 5–21. [Google Scholar] [CrossRef]
- Holst, J.J.; Gromada, J. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E199–E206. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.E.; Drucker, D.J. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013, 17, 819–837. [Google Scholar] [CrossRef]
- Holz, G.G. Epac: A new cAMP-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic beta-cell. Diabetes 2004, 53, 5–13. [Google Scholar] [CrossRef]
- Weir, G.C.; Mojsov, S.; Hendrick, G.K.; Habener, J.F. Glucagonlike peptide I (7-37) actions on endocrine pancreas. Diabetes 1989, 38, 338–342. [Google Scholar] [CrossRef]
- Jarrard, R.E.; Wang, Y.; Salyer, A.E.; Pratt, E.P.; Soderling, I.M.; Guerra, M.L.; Lange, A.M.; Broderick, H.J.; Hockerman, G.H. Potentiation of sulfonylurea action by an EPAC-selective cAMP analog in INS-1 cells: Comparison of tolbutamide and gliclazide and a potential role for EPAC activation of a 2-APB-sensitive Ca2+ influx. Mol. Pharmacol. 2013, 83, 191–205. [Google Scholar] [CrossRef]
- Ahren, B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat. Rev. Drug Discov. 2009, 8, 369–385. [Google Scholar] [CrossRef]
- Leiser, M.; Fleischer, N. cAMP-dependent phosphorylation of the cardiac-type alpha 1 subunit of the voltage-dependent Ca2+ channel in a murine pancreatic beta-cell line. Diabetes 1996, 45, 1412–1418. [Google Scholar] [CrossRef]
- Yang, S.N.; Berggren, P.O. The role of voltage-gated calcium channels in pancreatic beta-cell physiology and pathophysiology. Endocr. Rev. 2006, 27, 621–676. [Google Scholar] [CrossRef]
- Henquin, J.C.; Schmeer, W.; Meissner, H.P. Forskolin, an activator of adenylate cyclase, increases CA2+-dependent electrical activity induced by glucose in mouse pancreatic B cells. Endocrinology 1983, 112, 2218–2220. [Google Scholar] [CrossRef] [PubMed]
- Henquin, J.C.; Schmeer, W.; Nenquin, M.; Meissner, H.P. Forskolin suppresses the slow cyclic variations of glucose-induced electrical activity in pancreatic B cells. Biochem. Biophys. Res. Commun. 1984, 120, 797–803. [Google Scholar] [CrossRef]
- Kanno, T.; Suga, S.; Wu, J.; Kimura, M.; Wakui, M. Intracellular cAMP potentiates voltage-dependent activation of L-type Ca2+ channels in rat islet beta-cells. Pflug. Arch. 1998, 435, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Hisatomi, M.; Hidaka, H.; Niki, I. Ca2+/calmodulin and cyclic 3,5′ adenosine monophosphate control movement of secretory granules through protein phosphorylation/dephosphorylation in the pancreatic beta-cell. Endocrinology 1996, 137, 4644–4649. [Google Scholar] [CrossRef] [PubMed]
- Lonart, G.; Sudhof, T.C. Region-specific phosphorylation of rabphilin in mossy fiber nerve terminals of the hippocampus. J. Neurosci. 1998, 18, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Lonart, G.; Schoch, S.; Kaeser, P.S.; Larkin, C.J.; Sudhof, T.C.; Linden, D.J. Phosphorylation of RIM1alpha by PKA triggers presynaptic long-term potentiation at cerebellar parallel fiber synapses. Cell 2003, 115, 49–60. [Google Scholar] [CrossRef]
- Laidlaw, K.M.E.; Livingstone, R.; Al-Tobi, M.; Bryant, N.J.; Gould, G.W. SNARE phosphorylation: A control mechanism for insulin-stimulated glucose transport and other regulated exocytic events. Biochem. Soc. Trans. 2017, 45, 1271–1277. [Google Scholar] [CrossRef]
- Patel, P.; Prescott, G.R.; Burgoyne, R.D.; Lian, L.Y.; Morgan, A. Phosphorylation of Cysteine String Protein Triggers a Major Conformational Switch. Structure 2016, 24, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Chheda, M.G.; Ashery, U.; Thakur, P.; Rettig, J.; Sheng, Z.H. Phosphorylation of Snapin by PKA modulates its interaction with the SNARE complex. Nat. Cell Biol. 2001, 3, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Song, W.J.; Seshadri, M.; Ashraf, U.; Mdluli, T.; Mondal, P.; Keil, M.; Azevedo, M.; Kirschner, L.S.; Stratakis, C.A.; Hussain, M.A. Snapin mediates incretin action and augments glucose-dependent insulin secretion. Cell Metab. 2011, 13, 308–319. [Google Scholar] [CrossRef]
- Vikman, J.; Svensson, H.; Huang, Y.C.; Kang, Y.; Andersson, S.A.; Gaisano, H.Y.; Eliasson, L. Truncation of SNAP-25 reduces the stimulatory action of cAMP on rapid exocytosis in insulin-secreting cells. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E452–E461. [Google Scholar] [CrossRef] [PubMed]
- Nagy, G.; Reim, K.; Matti, U.; Brose, N.; Binz, T.; Rettig, J.; Neher, E.; Sorensen, J.B. Regulation of releasable vesicle pool sizes by protein kinase A-dependent phosphorylation of SNAP-25. Neuron 2004, 41, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.J.; Tang-Christensen, M.; Holst, J.J.; Orskov, C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 1997, 77, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Secher, A.; Jelsing, J.; Baquero, A.F.; Hecksher-Sorensen, J.; Cowley, M.A.; Dalboge, L.S.; Hansen, G.; Grove, K.L.; Pyke, C.; Raun, K.; et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J. Clin. Investig. 2014, 124, 4473–4488. [Google Scholar] [CrossRef]
- Rowlands, J.; Heng, J.; Newsholme, P.; Carlessi, R. Pleiotropic Effects of GLP-1 and Analogs on Cell Signaling, Metabolism, and Function. Front. Endocrinol. 2018, 9, 672. [Google Scholar] [CrossRef]
- Hayes, M.R.; Mietlicki-Baase, E.G.; Kanoski, S.E.; De Jonghe, B.C. Incretins and amylin: Neuroendocrine communication between the gut, pancreas, and brain in control of food intake and blood glucose. Annu. Rev. Nutr. 2014, 34, 237–260. [Google Scholar] [CrossRef]
- Muscogiuri, G.; DeFronzo, R.A.; Gastaldelli, A.; Holst, J.J. Glucagon-like Peptide-1 and the Central/Peripheral Nervous System: Crosstalk in Diabetes. Trends Endocrinol. Metab. 2017, 28, 88–103. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, E.; Martinez, M.D.; Roncero, I.; Chowen, J.A.; Garcia-Cuartero, B.; Gispert, J.D.; Sanz, C.; Vazquez, P.; Maldonado, A.; de Caceres, J.; et al. The expression of GLP-1 receptor mRNA and protein allows the effect of GLP-1 on glucose metabolism in the human hypothalamus and brainstem. J. Neurochem. 2005, 92, 798–806. [Google Scholar] [CrossRef]
- Heppner, K.M.; Kirigiti, M.; Secher, A.; Paulsen, S.J.; Buckingham, R.; Pyke, C.; Knudsen, L.B.; Vrang, N.; Grove, K.L. Expression and distribution of glucagon-like peptide-1 receptor mRNA, protein and binding in the male nonhuman primate (Macaca mulatta) brain. Endocrinology 2015, 156, 255–267. [Google Scholar] [CrossRef]
- Kamei, S.; Kaneto, H.; Tanabe, A.; Kinoshita, T.; Obata, A.; Kimura, T.; Hirukawa, H.; Tatsumi, F.; Shimoda, M.; Kohara, K.; et al. Increase in cortisol/ACTH ratio after chronic treatment with liraglutide in patients with type 2 diabetes. Diabetes Metab. 2017, 43, 398–399. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Powell, A.M.; Kaidanovich-Beilin, O.; Soczynska, J.K.; Alsuwaidan, M.; Woldeyohannes, H.O.; Kim, A.S.; Gallaugher, L.A. The neuroprotective effects of GLP-1: Possible treatments for cognitive deficits in individuals with mood disorders. Behav. Brain Res. 2013, 237, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Decarie-Spain, L.; Fisette, A.; Zhu, Z.; Yang, B.; DiMarchi, R.D.; Tschop, M.H.; Finan, B.; Fulton, S.; Clemmensen, C. GLP-1/dexamethasone inhibits food reward without inducing mood and memory deficits in mice. Neuropharmacology 2019, 151, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Kamble, M.; Gupta, R.; Rehan, H.S.; Gupta, L.K. Neurobehavioral effects of liraglutide and sitagliptin in experimental models. Eur. J. Pharmacol. 2016, 774, 64–70. [Google Scholar] [CrossRef]
- Fan, H.; Wang, L.; Guo, F.; Wei, S.; Zhao, R. Neonatal intramuscular injection of plasmid encoding glucagon-like peptide-1 affects anxiety behaviour and expression of the hippocampal glucocorticoid receptor in adolescent rats. J. Biosci. 2010, 35, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.N.; Ligade, S.S.; Sharma, J.N.; Shukla, P.; Elased, K.M.; Lucot, J.B. GLP-1 receptor agonist liraglutide reverses long-term atypical antipsychotic treatment associated behavioral depression and metabolic abnormalities in rats. Metab. Brain Dis. 2015, 30, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Komsuoglu Celikyurt, I.; Mutlu, O.; Ulak, G.; Uyar, E.; Bektas, E.; Yildiz Akar, F.; Erden, F.; Tarkun, I. Exenatide treatment exerts anxiolytic- and antidepressant-like effects and reverses neuropathy in a mouse model of type-2 diabetes. Med. Sci. Monit. Basic. Res. 2014, 20, 112–117. [Google Scholar] [CrossRef]
- Bode, B.W.; Testa, M.A.; Magwire, M.; Hale, P.M.; Hammer, M.; Blonde, L.; Garber, A.; Group, L.-S. Patient-reported outcomes following treatment with the human GLP-1 analogue liraglutide or glimepiride in monotherapy: Results from a randomized controlled trial in patients with type 2 diabetes. Diabetes Obes. Metab. 2010, 12, 604–612. [Google Scholar] [CrossRef]
- Grant, P.; Lipscomb, D.; Quin, J. Psychological and quality of life changes in patients using GLP-1 analogues. J. Diabetes Complicat. 2011, 25, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Mansur, R.B.; Ahmed, J.; Cha, D.S.; Woldeyohannes, H.O.; Subramaniapillai, M.; Lovshin, J.; Lee, J.G.; Lee, J.H.; Brietzke, E.; Reininghaus, E.Z.; et al. Liraglutide promotes improvements in objective measures of cognitive dysfunction in individuals with mood disorders: A pilot, open-label study. J. Affect. Disord. 2017, 207, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Kahal, H.; Kilpatrick, E.; Rigby, A.; Coady, A.; Atkin, S. The effects of treatment with liraglutide on quality of life and depression in young obese women with PCOS and controls. Gynecol. Endocrinol. 2019, 35, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Cantini, G.; Mannucci, E.; Luconi, M. Perspectives in GLP-1 Research: New Targets, New Receptors. Trends Endocrinol. Metab. 2016, 27, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Perlis, R.H.; Moorjani, P.; Fagerness, J.; Purcell, S.; Trivedi, M.H.; Fava, M.; Rush, A.J.; Smoller, J.W. Pharmacogenetic analysis of genes implicated in rodent models of antidepressant response: Association of TREK1 and treatment resistance in the STAR(*)D study. Neuropsychopharmacology 2008, 33, 2810–2819. [Google Scholar] [CrossRef]
- Fink, M.; Lesage, F.; Duprat, F.; Heurteaux, C.; Reyes, R.; Fosset, M.; Lazdunski, M. A neuronal two P domain K+ channel stimulated by arachidonic acid and polyunsaturated fatty acids. EMBO J. 1998, 17, 3297–3308. [Google Scholar] [CrossRef]
- Heurteaux, C.; Lucas, G.; Guy, N.; El Yacoubi, M.; Thümmler, S.; Peng, X.D.; Noble, F.; Blondeau, N.; Widmann, C.; Borsotto, M.; et al. Deletion of the background potassium channel TREK-1 results in a depression-resistant phenotype. Nat. Neurosci. 2006, 9, 1134–1141. [Google Scholar] [CrossRef]
- Maruyama, Y.; Yamada, M. [TREK-1: A potential target for novel antidepressants]. Nihon Shinkei Seishin Yakurigaku Zasshi 2007, 27, 147–151. [Google Scholar]
- Kindler, C.H.; Pietruck, C.; Yost, C.S.; Sampson, E.R.; Gray, A.T. Localization of the tandem pore domain K+ channel TASK-1 in the rat central nervous system. Brain Res. Mol. Brain Res. 2000, 80, 99–108. [Google Scholar] [CrossRef]
- Lesage, F.; Lazdunski, M. Molecular and functional properties of two-pore-domain potassium channels. Am. J. Physiol. Ren. Physiol. 2000, 279, F793–F801. [Google Scholar] [CrossRef]
- Duprat, F.; Lesage, F.; Patel, A.J.; Fink, M.; Romey, G.; Lazdunski, M. The neuroprotective agent riluzole activates the two P domain K(+) channels TREK-1 and TRAAK. Mol. Pharmacol. 2000, 57, 906–912. [Google Scholar] [PubMed]
- Patel, A.J.; Honore, E.; Lesage, F.; Fink, M.; Romey, G.; Lazdunski, M. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat. Neurosci. 1999, 2, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.J.; Honore, E.; Maingret, F.; Lesage, F.; Fink, M.; Duprat, F.; Lazdunski, M. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J. 1998, 17, 4283–4290. [Google Scholar] [CrossRef]
- Lin, D.H.; Zhang, X.R.; Ye, D.Q.; Xi, G.J.; Hui, J.J.; Liu, S.S.; Li, L.J.; Zhang, Z.J. The Role of the Two-Pore Domain Potassium Channel TREK-1 in the Therapeutic Effects of Escitalopram in a Rat Model of Poststroke Depression. CNS Neurosci. Ther. 2015, 21, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Munck Petersen, C.; Nielsen, M.S.; Jacobsen, C.; Tauris, J.; Jacobsen, L.; Gliemann, J.; Moestrup, S.K.; Madsen, P. Propeptide cleavage conditions sortilin/neurotensin receptor-3 for ligand binding. EMBO J. 1999, 18, 595–604. [Google Scholar] [CrossRef]
- Mazella, J.; Pétrault, O.; Lucas, G.; Deval, E.; Béraud-Dufour, S.; Gandin, C.; El-Yacoubi, M.; Widmann, C.; Guyon, A.; Chevet, E.; et al. Spadin, a sortilin-derived peptide, targeting rodent TREK-1 channels: A new concept in the antidepressant drug design. PLoS Biol. 2010, 8, e1000355. [Google Scholar] [CrossRef]
- Nykjaer, A.; Willnow, T.E. Sortilin: A receptor to regulate neuronal viability and function. Trends Neurosci. 2012, 35, 261–270. [Google Scholar] [CrossRef]
- Moha Ou Maati, H.; Veyssiere, J.; Labbal, F.; Coppola, T.; Gandin, C.; Widmann, C.; Mazella, J.; Heurteaux, C.; Borsotto, M. Spadin as a new antidepressant: Absence of TREK-1-related side effects. Neuropharmacology 2012, 62, 278–288. [Google Scholar] [CrossRef]
- Devader, C.; Khayachi, A.; Veyssiere, J.; Moha Ou Maati, H.; Roulot, M.; Moreno, S.; Borsotto, M.; Martin, S.; Heurteaux, C.; Mazella, J. In vitro and in vivo regulation of synaptogenesis by the novel antidepressant spadin. Br. J. Pharmacol. 2015, 172, 2604–2617. [Google Scholar] [CrossRef]
- Devader, C.; Roulot, M.; Moréno, S.; Minelli, A.; Bortolomasi, M.; Congiu, C.; Gennarelli, M.; Borsotto, M.; Heurteaux, C.; Mazella, J. Serum sortilin-derived propeptides concentrations are decreased in major depressive disorder patients. J. Affect. Disord. 2017, 208, 443–447. [Google Scholar] [CrossRef]
- Roulot, M.; Minelli, A.; Bortolomasi, M.; Maffioletti, E.; Gennarelli, M.; Borsotto, M.; Heurteaux, C.; Mazella, J. Increased serum levels of sortilin-derived propeptide after electroconvulsive therapy in treatment-resistant depressed patients. Neuropsychiatr. Dis. Treat. 2018, 14, 2307–2312. [Google Scholar] [CrossRef]
- Moreno, S.; Devader, C.M.; Pietri, M.; Borsotto, M.; Heurteaux, C.; Mazella, J. Altered Trek-1 Function in Sortilin Deficient Mice Results in Decreased Depressive-Like Behavior. Front. Pharmacol. 2018, 9, 863. [Google Scholar] [CrossRef] [PubMed]
- Arvaniti, K.; Huang, Q.; Richard, D. Effects of leptin and corticosterone on the expression of corticotropin-releasing hormone, agouti-related protein, and proopiomelanocortin in the brain of ob/ob mouse. Neuroendocrinology 2001, 73, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Bi, L.; Chiang, J.Y.; Ding, W.X.; Dunn, W.; Roberts, B.; Li, T. Saturated fatty acids activate ERK signaling to downregulate hepatic sortilin 1 in obese and diabetic mice. J. Lipid Res. 2013, 54, 2754–2762. [Google Scholar] [CrossRef]
- Li, J.; Matye, D.J.; Li, T. Insulin resistance induces posttranslational hepatic sortilin 1 degradation in mice. J. Biol. Chem. 2015, 290, 11526–11536. [Google Scholar] [CrossRef] [PubMed]
- Hivelin, C.; Mazella, J.; Coppola, T. Sortilin derived propeptide regulation during adipocyte differentiation and inflammation. Biochem. Biophys. Res. Commun. 2017, 482, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Kaddai, V.; Jager, J.; Gonzalez, T.; Najem-Lendom, R.; Bonnafous, S.; Tran, A.; Le Marchand-Brustel, Y.; Gual, P.; Tanti, J.F.; Cormont, M. Involvement of TNF-alpha in abnormal adipocyte and muscle sortilin expression in obese mice and humans. Diabetologia 2009, 52, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Coppola, T.; Beraud-Dufour, S.; Antoine, A.; Vincent, J.P.; Mazella, J. Neurotensin protects pancreatic beta cells from apoptosis. Int. J. Biochem. Cell Biol. 2008, 40, 2296–2302. [Google Scholar] [CrossRef]
- Beraud-Dufour, S.; Coppola, T.; Massa, F.; Mazella, J. Neurotensin receptor-2 and -3 are crucial for the anti-apoptotic effect of neurotensin on pancreatic beta-TC3 cells. Int. J. Biochem. Cell Biol. 2009, 41, 2398–2402. [Google Scholar] [CrossRef]
- Hivelin, C.; Béraud-Dufour, S.; Devader, C.; Abderrahmani, A.; Moreno, S.; Moha Ou Maati, H.; Djillani, A.; Heurteaux, C.; Borsotto, M.; Mazella, J.; et al. Potentiation of Calcium Influx and Insulin Secretion in Pancreatic Beta Cell by the Specific TREK-1 Blocker Spadin. J. Diabetes Res. 2016, 2016, 3142175. [Google Scholar] [CrossRef]
- Blondeau, N.; Beraud-Dufour, S.; Lebrun, P.; Hivelin, C.; Coppola, T. Sortilin in Glucose Homeostasis: From Accessory Protein to Key Player? Front. Pharmacol. 2018, 9, 1561. [Google Scholar] [CrossRef] [PubMed]
- Daziano, G.; Blondeau, N.; Beraud-Dufour, S.; Abderrahmani, A.; Rovere, C.; Heurteaux, C.; Mazella, J.; Lebrun, P.; Coppola, T. Sortilin-derived peptides promote pancreatic beta-cell survival through CREB signaling pathway. Pharmacol. Res. 2021, 167, 105539. [Google Scholar] [CrossRef] [PubMed]
- Beraud-Dufour, S.; Abderrahmani, A.; Noel, J.; Brau, F.; Waeber, G.; Mazella, J.; Coppola, T. Neurotensin is a regulator of insulin secretion in pancreatic beta-cells. Int. J. Biochem. Cell Biol. 2010, 42, 1681–1688. [Google Scholar] [CrossRef] [PubMed]
- Kennard, L.E.; Chumbley, J.R.; Ranatunga, K.M.; Armstrong, S.J.; Veale, E.L.; Mathie, A. Inhibition of the human two-pore domain potassium channel, TREK-1, by fluoxetine and its metabolite norfluoxetine. Br. J. Pharmacol. 2005, 144, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.Y.; Pike, A.C.; Mackenzie, A.; McClenaghan, C.; Aryal, P.; Dong, L.; Quigley, A.; Grieben, M.; Goubin, S.; Mukhopadhyay, S.; et al. K2P channel gating mechanisms revealed by structures of TREK-2 and a complex with Prozac. Science 2015, 347, 1256–1259. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coppola, T.; Daziano, G.; Legroux, I.; Béraud-Dufour, S.; Blondeau, N.; Lebrun, P. Unlocking Therapeutic Synergy: Tailoring Drugs for Comorbidities such as Depression and Diabetes through Identical Molecular Targets in Different Cell Types. Cells 2023, 12, 2768. https://doi.org/10.3390/cells12232768
Coppola T, Daziano G, Legroux I, Béraud-Dufour S, Blondeau N, Lebrun P. Unlocking Therapeutic Synergy: Tailoring Drugs for Comorbidities such as Depression and Diabetes through Identical Molecular Targets in Different Cell Types. Cells. 2023; 12(23):2768. https://doi.org/10.3390/cells12232768
Chicago/Turabian StyleCoppola, Thierry, Guillaume Daziano, Ilona Legroux, Sophie Béraud-Dufour, Nicolas Blondeau, and Patricia Lebrun. 2023. "Unlocking Therapeutic Synergy: Tailoring Drugs for Comorbidities such as Depression and Diabetes through Identical Molecular Targets in Different Cell Types" Cells 12, no. 23: 2768. https://doi.org/10.3390/cells12232768
APA StyleCoppola, T., Daziano, G., Legroux, I., Béraud-Dufour, S., Blondeau, N., & Lebrun, P. (2023). Unlocking Therapeutic Synergy: Tailoring Drugs for Comorbidities such as Depression and Diabetes through Identical Molecular Targets in Different Cell Types. Cells, 12(23), 2768. https://doi.org/10.3390/cells12232768





