Early Age- and Sex-Dependent Regulation of Astrocyte-Mediated Glutamatergic Synapse Elimination in the Rat Prefrontal Cortex: Establishing an Organotypic Brain Slice Culture Investigating Tool
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Developing Pups
2.2.1. Protein Isolation and Immunoblotting
2.2.2. Immunofluorescent Immunohistochemistry to Examine Synaptogenesis and Astrocyte Phagocytosis in Developing Pup Brains
2.2.3. Confocal Imaging and Analysis
2.3. Organotypic Brain Slice Cultures (OBSCs)
2.3.1. Organotypic Brain Slice Preparation
2.3.2. Organotypic Brain Slice Culture
2.3.3. Cell Viability Assay
2.3.4. Protein Isolation and Immunoblotting
2.3.5. Immunofluorescent Immunohistochemistry to Examine Astrocyte Phagocytosis in OBSCs
2.3.6. Confocal Imaging and Analysis
2.4. Statistical Analysis
3. Results
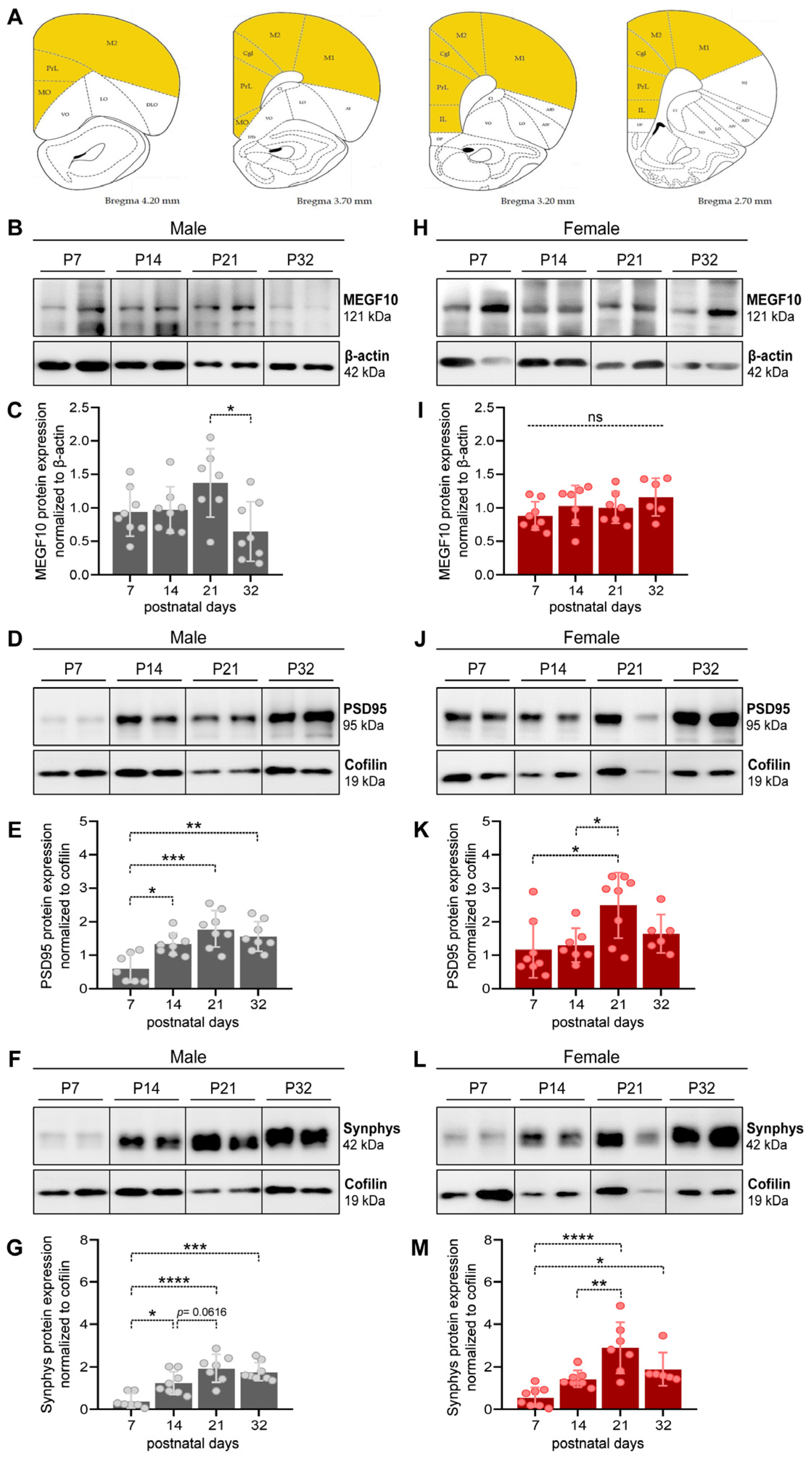
3.1. MEGF10, PSD95 and Synaptophysin Expression in the PFC of Male and Female Developing Rat Pups
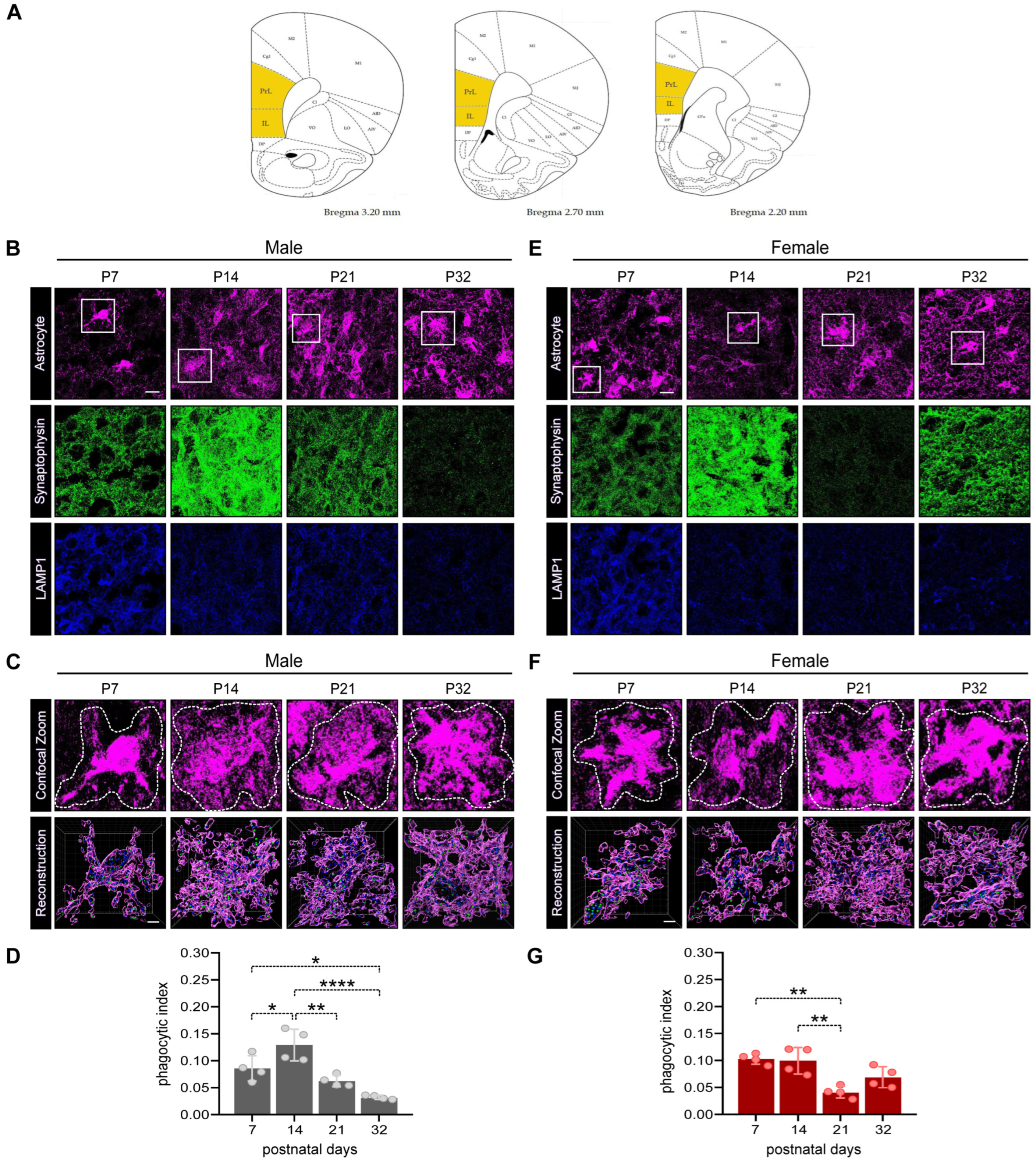
3.2. Astrocyte-Dependent Synapse Elimination in the PFC of Male and Female Developing Rat Pups
3.3. Establishing Organotypic Brain Slice Cultures (OBSCs) to Examine Synapse Elimination in the PFC during Early Postnatal Developmental Stages
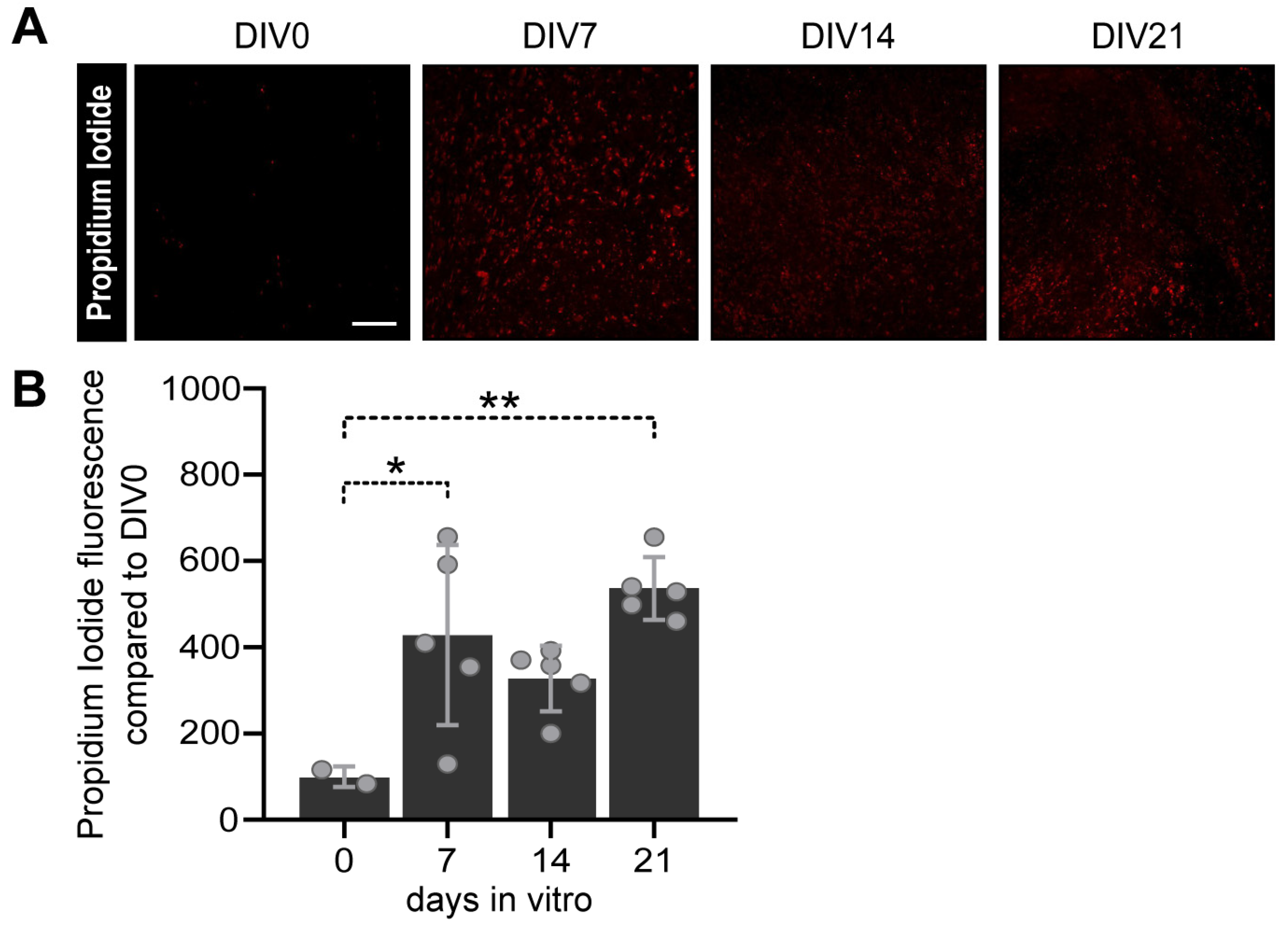
3.3.1. Viability Assay
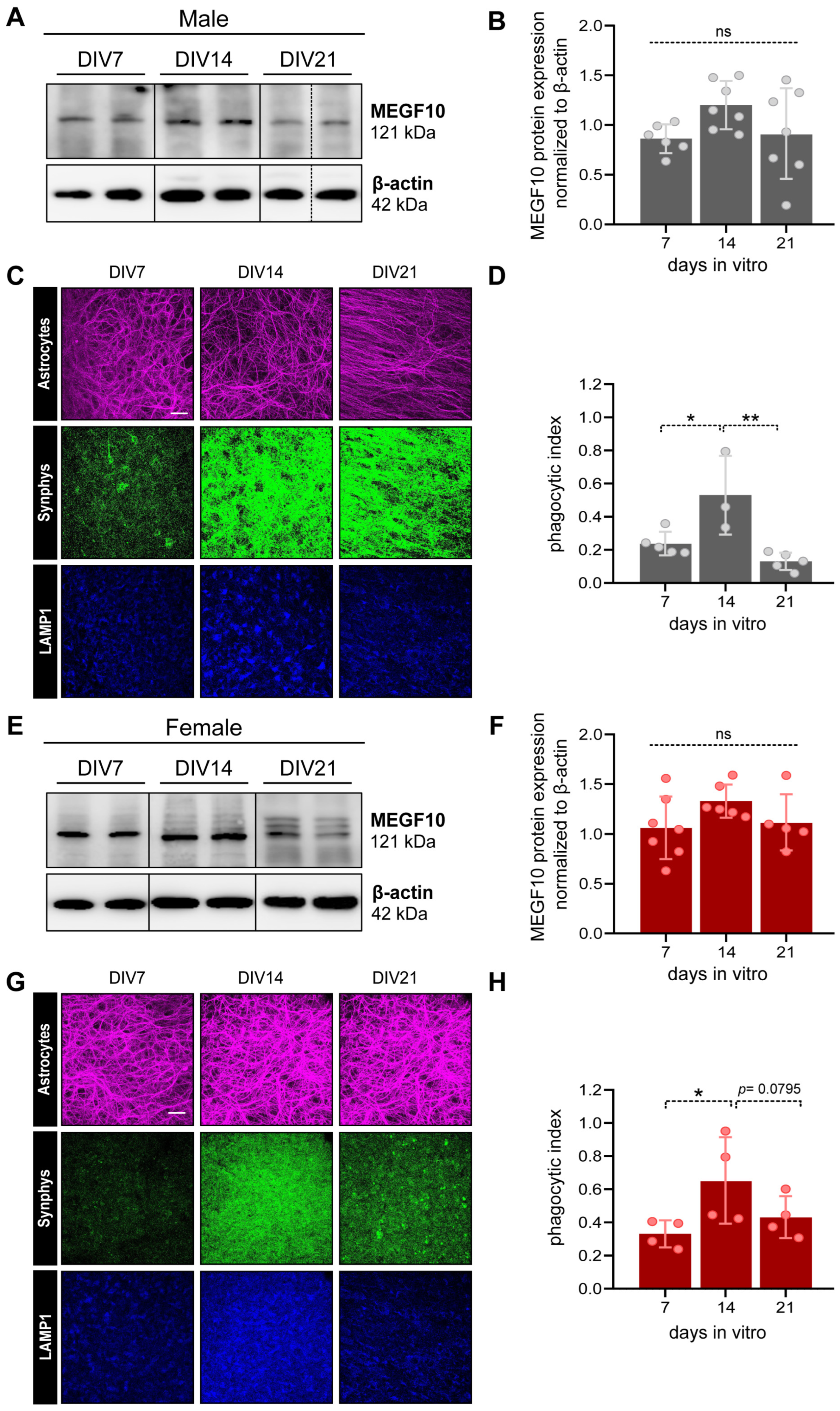
3.3.2. MEGF10 Expression and Astrocyte-Dependent Synapse Elimination in the PFC of OBSCs Derived from Male and Female Rat Pups
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magistretti, P.J.; Allaman, I. Lactate in the Brain: From Metabolic End-Product to Signalling Molecule. Nat. Rev. Neurosci. 2018, 19, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Greene, C.; Munnich, A.; Campbell, M. The CLDN5 Gene at the Blood-Brain Barrier in Health and Disease. Fluids Barriers CNS 2023, 20, 22. [Google Scholar] [CrossRef] [PubMed]
- Bosworth, A.P.; Allen, N.J. The Diverse Actions of Astrocytes during Synaptic Development. Curr. Opin. Neurobiol. 2017, 47, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Untiet, V.; Rose, C.R. Ionic Signalling in Astroglia beyond Calcium. J. Physiol. 2020, 598, 1655–1670. [Google Scholar] [CrossRef] [PubMed]
- Stogsdill, J.A.; Eroglu, C. The Interplay between Neurons and Glia in Synapse Development and Plasticity. Curr. Opin. Neurobiol. 2017, 42, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rajkowska, G.; Hughes, J.; Stockmeier, C.A.; Javier Miguel-Hidalgo, J.; Maciag, D. Coverage of Blood Vessels by Astrocytic Endfeet Is Reduced in Major Depressive Disorder. Biol. Psychiatry 2013, 73, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Rajkowska, G.; Miguel-Hidalgo, J. Gliogenesis and Glial Pathology in Depression. CNSNDDT 2007, 6, 219–233. [Google Scholar] [CrossRef]
- Miguel-Hidalgo, J.J. Astroglia in the Vulnerability to and Maintenance of Stress-Mediated Neuropathology and Depression. Front. Cell. Neurosci. 2022, 16, 869779. [Google Scholar] [CrossRef]
- De Oliveira Figueiredo, E.C.; Calì, C.; Petrelli, F.; Bezzi, P. Emerging Evidence for Astrocyte Dysfunction in Schizophrenia. Glia 2022, 70, 1585–1604. [Google Scholar] [CrossRef]
- Russo, F.B.; Freitas, B.C.; Pignatari, G.C.; Fernandes, I.R.; Sebat, J.; Muotri, A.R.; Beltrão-Braga, P.C.B. Modeling the Interplay between Neurons and Astrocytes in Autism Using Human Induced Pluripotent Stem Cells. Biol. Psychiatry 2018, 83, 569–578. [Google Scholar] [CrossRef]
- Vakilzadeh, G.; Martinez-Cerdeño, V. Pathology and Astrocytes in Autism. Neuropsychiatr. Dis. Treat. 2023, 19, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Gould, T.D.; Gottesman, I.I. Psychiatric Endophenotypes and the Development of Valid Animal Models. Genes. Brain Behav. 2006, 5, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Neumann, I.D.; Wegener, G.; Homberg, J.R.; Cohen, H.; Slattery, D.A.; Zohar, J.; Olivier, J.D.A.; Mathé, A.A. Animal Models of Depression and Anxiety: What Do They Tell Us about Human Condition? Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 1357–1375. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, B.; Malik, V.A.; Begum, S.; Jablonowski, L.; Gómez-González, G.B.; Neumann, I.D.; Rupprecht, R. Fluoxetine Requires the Endfeet Protein Aquaporin-4 to Enhance Plasticity of Astrocyte Processes. Front. Cell. Neurosci. 2016, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.A.; Zajicek, F.; Mittmann, L.A.; Klaus, J.; Unterseer, S.; Rajkumar, S.; Pütz, B.; Deussing, J.M.; Neumann, I.D.; Rupprecht, R.; et al. GDF15 Promotes Simultaneous Astrocyte Remodeling and Tight Junction Strengthening at the Blood–Brain Barrier. J. Neurosci. Res. 2020, 98, 1433–1456. [Google Scholar] [CrossRef] [PubMed]
- Akdemir, E.S.; Huang, A.Y.-S.; Deneen, B. Astrocytogenesis: Where, When, and How. F1000Res 2020, 9, 233. [Google Scholar] [CrossRef] [PubMed]
- Raponi, E.; Agenes, F.; Delphin, C.; Assard, N.; Baudier, J.; Legraverend, C.; Deloulme, J.-C. S100B Expression Defines a State in Which GFAP-Expressing Cells Lose Their Neural Stem Cell Potential and Acquire a More Mature Developmental Stage. Glia 2007, 55, 165–177. [Google Scholar] [CrossRef]
- Bandeira, F.; Lent, R.; Herculano-Houzel, S. Changing Numbers of Neuronal and Non-Neuronal Cells Underlie Postnatal Brain Growth in the Rat. Proc. Natl. Acad. Sci. USA 2009, 106, 14108–14113. [Google Scholar] [CrossRef]
- Milbocker, K.A.; Campbell, T.S.; Collins, N.; Kim, S.; Smith, I.F.; Roth, T.L.; Klintsova, A.Y. Glia-Driven Brain Circuit Refinement Is Altered by Early-Life Adversity: Behavioral Outcomes. Front. Behav. Neurosci. 2021, 15, 786234. [Google Scholar] [CrossRef]
- Chugani, H.T. A Critical Period of Brain Development: Studies of Cerebral Glucose Utilization with PET. Prev. Med. 1998, 27, 184–188. [Google Scholar] [CrossRef]
- Juraska, J.M.; Willing, J. Pubertal Onset as a Critical Transition for Neural Development and Cognition. Brain Res. 2017, 1654, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Bushong, E.A.; Martone, M.E.; Ellisman, M.H. Maturation of Astrocyte Morphology and the Establishment of Astrocyte Domains during Postnatal Hippocampal Development. Int. J. Dev. Neurosci. 2004, 22, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.J.; Eroglu, C. Cell Biology of Astrocyte-Synapse Interactions. Neuron 2017, 96, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Perez-Catalan, N.A.; Doe, C.Q.; Ackerman, S.D. The Role of Astrocyte-Mediated Plasticity in Neural Circuit Development and Function. Neural Dev. 2021, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Cahoy, J.D.; Emery, B.; Kaushal, A.; Foo, L.C.; Zamanian, J.L.; Christopherson, K.S.; Xing, Y.; Lubischer, J.L.; Krieg, P.A.; Krupenko, S.A.; et al. A Transcriptome Database for Astrocytes, Neurons, and Oligodendrocytes: A New Resource for Understanding Brain Development and Function. J. Neurosci. 2008, 28, 264–278. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.-S.; Clarke, L.E.; Wang, G.X.; Stafford, B.K.; Sher, A.; Chakraborty, C.; Joung, J.; Foo, L.C.; Thompson, A.; Chen, C.; et al. Astrocytes Mediate Synapse Elimination through MEGF10 and MERTK Pathways. Nature 2013, 504, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Iram, T.; Ramirez-Ortiz, Z.; Byrne, M.H.; Coleman, U.A.; Kingery, N.D.; Means, T.K.; Frenkel, D.; El Khoury, J. Megf10 Is a Receptor for C1Q That Mediates Clearance of Apoptotic Cells by Astrocytes. J. Neurosci. 2016, 36, 5185–5192. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kim, J.; Noh, S.; Lee, H.; Lee, S.Y.; Mun, J.Y.; Park, H.; Chung, W.-S. Astrocytes Phagocytose Adult Hippocampal Synapses for Circuit Homeostasis. Nature 2021, 590, 612–617. [Google Scholar] [CrossRef]
- Roeper, J. Closing Gaps in Brain Disease—From Overlapping Genetic Architecture to Common Motifs of Synapse Dysfunction. Curr. Opin. Neurobiol. 2018, 48, 45–51. [Google Scholar] [CrossRef]
- Blokland, G.A.M.; Grove, J.; Chen, C.-Y.; Cotsapas, C.; Tobet, S.; Handa, R.; St Clair, D.; Lencz, T.; Mowry, B.J.; Periyasamy, S.; et al. Sex-Dependent Shared and Nonshared Genetic Architecture Across Mood and Psychotic Disorders. Biol. Psychiatry 2022, 91, 102–117. [Google Scholar] [CrossRef]
- Seedat, S.; Scott, K.M.; Angermeyer, M.C.; Berglund, P.; Bromet, E.J.; Brugha, T.S.; Demyttenaere, K.; de Girolamo, G.; Haro, J.M.; Jin, R.; et al. Cross-National Associations Between Gender and Mental Disorders in the World Health Organization World Mental Health Surveys. Arch. Gen. Psychiatry 2009, 66, 785. [Google Scholar] [CrossRef] [PubMed]
- Drzewiecki, C.M.; Juraska, J.M. The Structural Reorganization of the Prefrontal Cortex during Adolescence as a Framework for Vulnerability to the Environment. Pharmacol. Biochem. Behav. 2020, 199, 173044. [Google Scholar] [CrossRef] [PubMed]
- Glantz, L.A.; Gilmore, J.H.; Hamer, R.M.; Lieberman, J.A.; Jarskog, L.F. Synaptophysin and Postsynaptic Density Protein 95 in the Human Prefrontal Cortex from Mid-Gestation into Early Adulthood. Neuroscience 2007, 149, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Markham, J.A.; Morris, J.R.; Juraska, J.M. Neuron Number Decreases in the Rat Ventral, but Not Dorsal, Medial Prefrontal Cortex between Adolescence and Adulthood. Neuroscience 2007, 144, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Stoppini, L.; Buchs, P.-A.; Muller, D. A Simple Method for Organotypic Cultures of Nervous Tissue. J. Neurosci. Methods 1991, 37, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Stoppini, L.; Buchs, P.-A.; Brun, R.; Muller, D.; Duport, S.; Parisi, L.; Seebeck, T. Infection of Organotypic Slice Cultures from Rat Central Nervous Tissue with Trypanosoma Brucei Brucei. Int. J. Med. Microbiol. 2000, 290, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.; Buchs, P.-A.; Stoppini, L. Time Course of Synaptic Development in Hippocampal Organotypic Cultures. Dev. Brain Res. 1993, 71, 93–100. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Generation of Cerebral Organoids from Human Pluripotent Stem Cells. Nat. Protoc. 2014, 9, 2329–2340. [Google Scholar] [CrossRef]
- Kyrousi, C.; Cappello, S. Using Brain Organoids to Study Human Neurodevelopment, Evolution and Disease. WIREs Dev. Biol. 2020, 9, e347. [Google Scholar] [CrossRef]
- Humpel, C. Organotypic Brain Slice Cultures: A Review. Neuroscience 2015, 305, 86–98. [Google Scholar] [CrossRef]
- Kamikubo, Y.; Jin, H.; Zhou, Y.; Niisato, K.; Hashimoto, Y.; Takasugi, N.; Sakurai, T. Ex Vivo Analysis Platforms for Monitoring Amyloid Precursor Protein Cleavage. Front. Mol. Neurosci. 2023, 15, 1068990. [Google Scholar] [CrossRef]
- Humpel, C. Organotypic Brain Slice Cultures. Curr. Protoc. Immunol. 2018, 123, e59. [Google Scholar] [CrossRef]
- Hezel, M.; Ebrahimi, F.; Koch, M.; Dehghani, F. Propidium Iodide Staining: A New Application in Fluorescence Microscopy for Analysis of Cytoarchitecture in Adult and Developing Rodent Brain. Micron 2012, 43, 1031–1038. [Google Scholar] [CrossRef]
- Staal, J.A.; Alexander, S.R.; Liu, Y.; Dickson, T.D.; Vickers, J.C. Characterization of Cortical Neuronal and Glial Alterations during Culture of Organotypic Whole Brain Slices from Neonatal and Mature Mice. PLoS ONE 2011, 6, e22040. [Google Scholar] [CrossRef]
- Huttenlocher, P.R.; Dabholkar, A.S. Regional Differences in Synaptogenesis in Human Cerebral Cortex. J. Comp. Neurol. 1997, 387, 167–178. [Google Scholar] [CrossRef]
- Petanjek, Z.; Judaš, M.; Šimić, G.; Rašin, M.R.; Uylings, H.B.M.; Rakic, P.; Kostović, I. Extraordinary Neoteny of Synaptic Spines in the Human Prefrontal Cortex. Proc. Natl. Acad. Sci. USA 2011, 108, 13281–13286. [Google Scholar] [CrossRef]
- Peter, R.H. Synaptic Density in Human Frontal Cortex—Developmental Changes and Effects of Aging. Brain Res. 1979, 163, 195–205. [Google Scholar] [CrossRef]
- Knickmeyer, R.C.; Styner, M.; Short, S.J.; Lubach, G.R.; Kang, C.; Hamer, R.; Coe, C.L.; Gilmore, J.H. Maturational Trajectories of Cortical Brain Development through the Pubertal Transition: Unique Species and Sex Differences in the Monkey Revealed through Structural Magnetic Resonance Imaging. Cereb. Cortex 2010, 20, 1053–1063. [Google Scholar] [CrossRef]
- Whitford, T.J.; Rennie, C.J.; Grieve, S.M.; Clark, C.R.; Gordon, E.; Williams, L.M. Brain Maturation in Adolescence: Concurrent Changes in Neuroanatomy and Neurophysiology. Hum. Brain Mapp. 2007, 28, 228–237. [Google Scholar] [CrossRef]
- Dosenbach, N.U.F.; Nardos, B.; Cohen, A.L.; Fair, D.A.; Power, J.D.; Church, J.A.; Nelson, S.M.; Wig, G.S.; Vogel, A.C.; Lessov-Schlaggar, C.N.; et al. Prediction of Individual Brain Maturity Using fMRI. Science 2010, 329, 1358–1361. [Google Scholar] [CrossRef]
- Doussau, F.; Dupont, J.-L.; Neel, D.; Schneider, A.; Poulain, B.; Bossu, J.L. Organotypic Cultures of Cerebellar Slices as a Model to Investigate Demyelinating Disorders. Expert. Opin. Drug Discov. 2017, 12, 1011–1022. [Google Scholar] [CrossRef]
- Hurtado De Mendoza, T.; Balana, B.; Slesinger, P.A.; Verma, I.M. Organotypic Cerebellar Cultures: Apoptotic Challenges and Detection. JoVE (Journal of Visualized Experiments) 2011, 51, e2564. [Google Scholar] [CrossRef]
- Kapfhammer, J.P. Cerebellar Slice Cultures. In Protocols for Neural Cell Culture; Doering, L.C., Ed.; Springer Protocols Handbooks; Humana Press: Totowa, NJ, USA, 2009; pp. 285–298. ISBN 978-1-60761-291-9. [Google Scholar]
- Michaelson, S.D.; Müller, T.M.; Bompolaki, M.; Miranda Tapia, A.P.; Villarroel, H.S.; Mackay, J.P.; Balogun, P.J.; Urban, J.H.; Colmers, W.F. Long-Lived Organotypic Slice Culture Model of the Rat Basolateral Amygdala. Curr. Protoc. 2021, 1, e267. [Google Scholar] [CrossRef]
- Sengpiel, F. The Critical Period. Curr. Biol. 2007, 17, R742–R743. [Google Scholar] [CrossRef]
- Singh, T.D.; Park, S.-Y.; Bae, J.; Yun, Y.; Bae, Y.-C.; Park, R.-W.; Kim, I.-S. MEGF10 Functions as a Receptor for the Uptake of Amyloid-β. FEBS Lett. 2010, 584, 3936–3942. [Google Scholar] [CrossRef]
- Guirado, R.; Perez-Rando, M.; Ferragud, A.; Gutierrez-Castellanos, N.; Umemori, J.; Carceller, H.; Nacher, J.; Castillo-Gómez, E. A Critical Period for Prefrontal Network Configurations Underlying Psychiatric Disorders and Addiction. Front. Behav. Neurosci. 2020, 14, 51. [Google Scholar] [CrossRef]
- Benediktsson, A.M.; Schachtele, S.J.; Green, S.H.; Dailey, M.E. Ballistic Labeling and Dynamic Imaging of Astrocytes in Organotypic Hippocampal Slice Cultures. J. Neurosci. Methods 2005, 141, 41–53. [Google Scholar] [CrossRef]
- Rooney, B.; Leng, K.; McCarthy, F.; Rose, I.V.L.; Herrington, K.A.; Bax, S.; Chin, M.Y.; Fathi, S.; Leonetti, M.; Kao, A.W.; et al. mTOR Controls Neurotoxic Lysosome Exocytosis in Inflammatory Reactive Astrocytes. bioRxiv 2021, 459904. [Google Scholar] [CrossRef]
- Arora, T.; Mehta, A.K.; Joshi, V.; Mehta, K.D.; Rathor, N.; Mediratta, P.K.; Sharma, K.K. Substitute of Animals in Drug Research: An Approach Towards Fulfillment of 4R’s. Indian. J. Pharm. Sci. 2011, 73, 1–6. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vivi, E.; Seeholzer, L.R.; Nagumanova, A.; Di Benedetto, B. Early Age- and Sex-Dependent Regulation of Astrocyte-Mediated Glutamatergic Synapse Elimination in the Rat Prefrontal Cortex: Establishing an Organotypic Brain Slice Culture Investigating Tool. Cells 2023, 12, 2761. https://doi.org/10.3390/cells12232761
Vivi E, Seeholzer LR, Nagumanova A, Di Benedetto B. Early Age- and Sex-Dependent Regulation of Astrocyte-Mediated Glutamatergic Synapse Elimination in the Rat Prefrontal Cortex: Establishing an Organotypic Brain Slice Culture Investigating Tool. Cells. 2023; 12(23):2761. https://doi.org/10.3390/cells12232761
Chicago/Turabian StyleVivi, Eugenia, Lea R. Seeholzer, Anastasiia Nagumanova, and Barbara Di Benedetto. 2023. "Early Age- and Sex-Dependent Regulation of Astrocyte-Mediated Glutamatergic Synapse Elimination in the Rat Prefrontal Cortex: Establishing an Organotypic Brain Slice Culture Investigating Tool" Cells 12, no. 23: 2761. https://doi.org/10.3390/cells12232761
APA StyleVivi, E., Seeholzer, L. R., Nagumanova, A., & Di Benedetto, B. (2023). Early Age- and Sex-Dependent Regulation of Astrocyte-Mediated Glutamatergic Synapse Elimination in the Rat Prefrontal Cortex: Establishing an Organotypic Brain Slice Culture Investigating Tool. Cells, 12(23), 2761. https://doi.org/10.3390/cells12232761





