Abstract
Epithelial-mesenchymal transition (EMT) is crucial to metastasis by increasing cancer cell migration and invasion. At the cellular level, EMT-related morphological and functional changes are well established. At the molecular level, critical signaling pathways able to drive EMT have been described. Yet, the translation of EMT into efficient diagnostic methods and anti-metastatic therapies is still missing. This highlights a gap in our understanding of the precise mechanisms governing EMT. Here, we discuss evidence suggesting that overcoming this limitation requires the integration of multiple omics, a hitherto neglected strategy in the EMT field. More specifically, this work summarizes results that were independently obtained through epigenomics/transcriptomics while comprehensively reviewing the achievements of proteomics in cancer research. Additionally, we prospect gains to be obtained by applying spatio-temporal multiomics in the investigation of EMT-driven metastasis. Along with the development of more sensitive technologies, the integration of currently available omics, and a look at dynamic alterations that regulate EMT at the subcellular level will lead to a deeper understanding of this process. Further, considering the significance of EMT to cancer progression, this integrative strategy may enable the development of new and improved biomarkers and therapeutics capable of increasing the survival and quality of life of cancer patients.
1. Introduction to EMT
Epithelial-mesenchymal transition (EMT) is recognized as a critical turning point for epithelial cells to acquire a mesenchymal phenotype with new biological functionalities. EMT is a process that happens to epithelial cells with appropriate external stimuli, where downregulation of epithelial traits and upregulation of mesenchymal characteristics allow these cells to escape mechanical and biochemical constraints while facilitating cell detachment and movement [1,2,3]. This process involves alterations in cell junctions, polarity, and cytoskeletal arrangements, which are all important factors in determining the cell phenotype [1,2,4]. Due to its essential roles in both physiological and disease states, EMT gained vast interest from researchers in different biomedical fields, especially in cancer research.
During cancer progression, particularly for carcinomas, EMT-related alterations enhance the migratory and invasive potential of malignant cells, critically contributing to the early stages of metastasis. In recent years, an enormous number of epigenomics, transcriptomics, and proteomics studies have helped to characterize the regulatory mechanisms and consequences of EMT on cancer [5,6,7,8]. However, a comprehensive view of the complex alterations that occur at multiple molecular levels in malignant cells undergoing EMT is still missing.
Here, the current understanding achieved by independently using different omics to depict the EMT is reviewed. Large efforts have been made to portray the changes associated with the epithelial and mesenchymal phenotypes of carcinoma cells. Still, limitations imposed by available methods and experimental design have delayed and compromised the precise characterization of EMT drivers and the impact of their crosstalk during cancer progression. Therefore, implementing an approach focused on spatio-temporal multiomics may be the next step still required for the translation of EMT-related knowledge into clinics. This strategy may allow the development of efficient methods to detect, prevent, and treat disease progression, thus, improving the outcome of cancer patients.
1.1. Molecular Regulation of EMT
EMT is a complex event that involves multiple levels of molecular regulation that dynamically change throughout the process. Several positive and negative molecular regulators have been associated with this phenotypic alteration while several others are yet to be elucidated. Considering the multiple biological processes associated with the EMT, characterizing its regulatory mechanisms served as the basis for several in vitro and in vivo studies and the development of useful techniques for the diagnosis and treatment of different diseases [9,10,11].
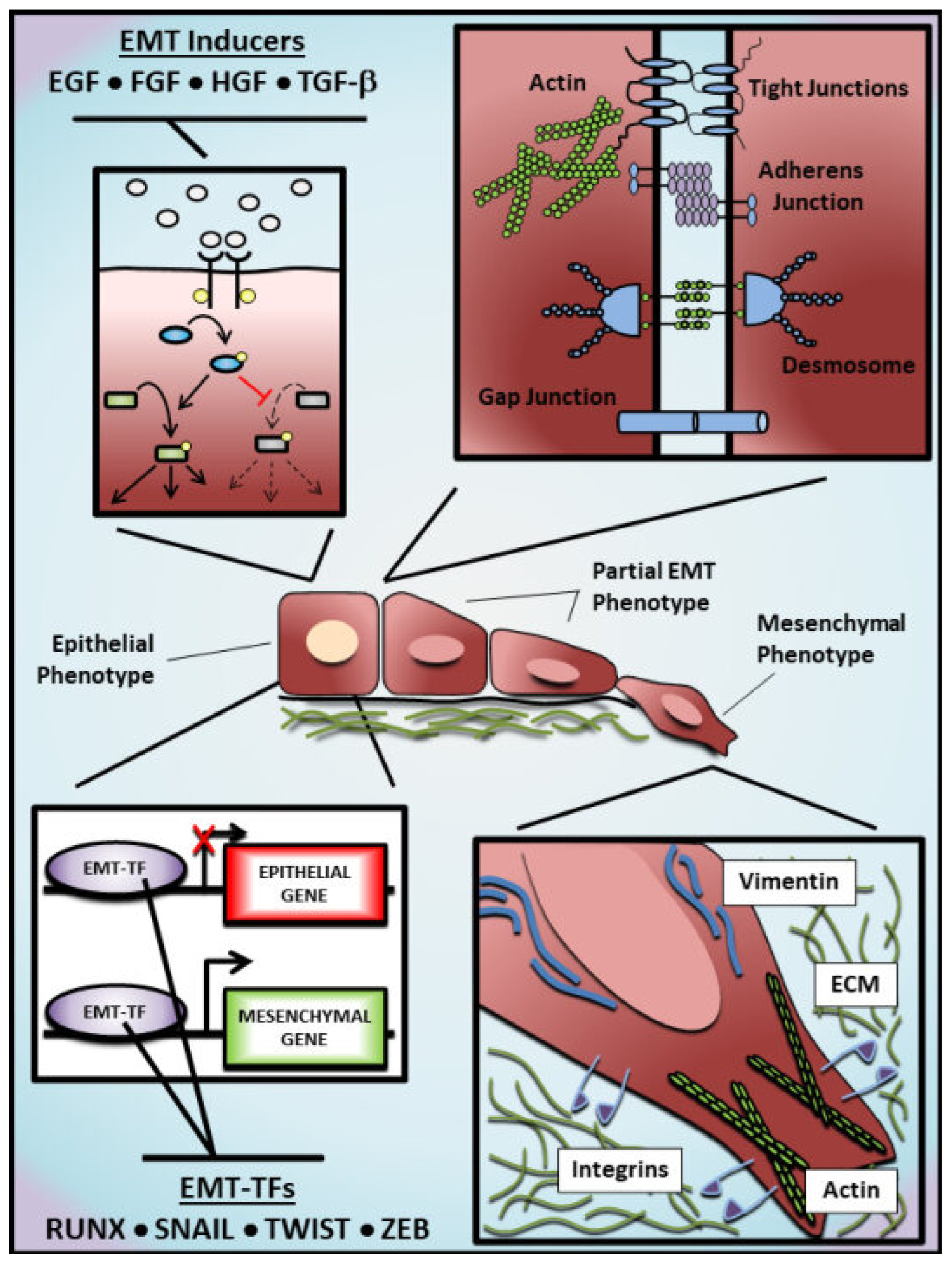
EMT begins with the activity of EMT-inducers in the tissue environment, such as Epithelial growth factor (EGF), Fibroblast growth factor (FGF) and Transforming growth factor-beta (TGF-β) [12,13,14,15,16,17]. Their interaction with corresponding receptors in the plasma membrane leads to the activation of ligand-receptor complexes and intracellular signal transduction, which consequently initiates the activation/suppression of downstream cellular effectors. Such intracellular effectors are tightly regulated by post-translational modifications critically impacting their stability, localization, and activity [18,19,20,21,22]. Additionally, alterations in intracellular effectors contribute to the expression of target genes associated with EMT, resulting in a feedback loop that could either increase the acquisition of mesenchymal characteristics or reverse this phenotypic transition. To promote EMT, these effectors downregulate epithelial proteins (e.g., E-cadherin and ZO-1) and upregulate mesenchymal proteins (e.g., N-cadherin, Vimentin), leading to an elongated phenotype and a front-rear polarity typical of cells undergoing EMT [23] (Figure 1). Importantly, as these changes (particularly those observed at the cellular level) are well established, their detection helps researchers to investigate further EMT-associated changes in vitro and in vivo.
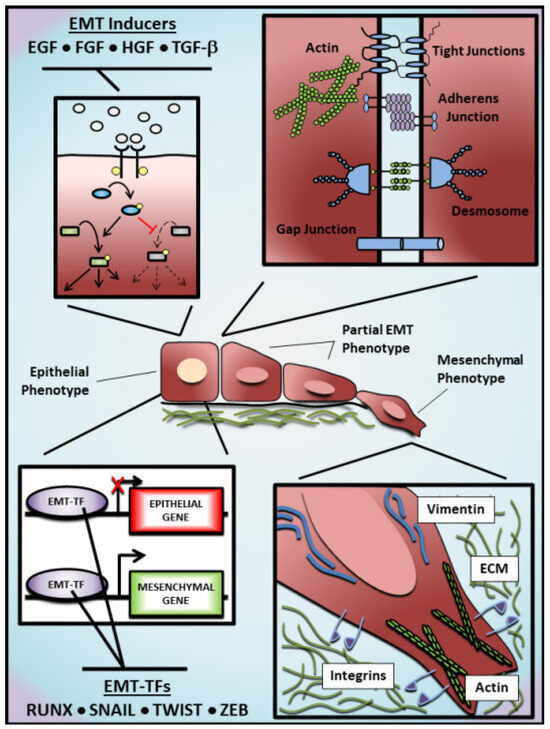
Figure 1.
Molecular regulation of epithelial-mesenchymal transition. The epithelial-mesenchymal transition (EMT) represents a continuum of alterations from an epithelial phenotype towards a mesenchymal phenotype, passing through diverse intermediate EMT states. Growth factors such as Epithelial growth factor (EGF), Fibroblast growth factor (FGF), Hepatocyte growth factor (HGF), and Transforming growth factor-beta (TGF-β) are frequently used as EMT inducers in experimental models to activate/inhibit downstream effectors and trigger an EMT program that includes alterations in the epigenome, transcriptome, and proteome of stimulated cells. Among these alterations, EMT transcription factors (EMT-TFs), such as RUNX, SNAIL, TWIST and ZEB family members, are positively regulated to inhibit the transcription of epithelial genes (e.g., CDH1, OCLN and TJP1) and promote the transcription of mesenchymal genes (e.g., CDH2, VIM and ACTA2). As an alternative to EMT inducers, EMT can also be experimentally promoted by the overexpression of EMT-TFs. Cells undergoing EMT exhibit the dissolution of protein complexes responsible for cell-cell adhesion, losing the apicobasal polarity and acquiring a back-front polarity that is characterized by the presence of actin stress fibers and an elongated morphology. In addition, these cells show increased migratory and invasive potential, which is commonly associated with basement membrane degradation and extracellular-matrix remodeling. Arrows shown in the top left represent the activation (black) or inhibition (red) of hypothetical signaling pathways by EMT inducers. Dashed arrows represent the attenuation of the activity of hypothetical effectors.
As EMT may incur drastic disturbances to the tissue structure, this phenotypic transition is tightly regulated under physiological conditions to control the extent and duration of EMT [15,24]. Negative regulatory processes may include the suppression of EMT-promoting signaling pathways through the expression of inhibitory proteins, some of which are induced by these signaling pathways themselves as a negative feedback mechanism [25,26,27,28,29]. Otherwise, extracellular autocrine or paracrine signals inducing a mesenchymal-epithelial transition (MET) can also balance this process [24,30,31]. Restoring the levels of epithelial and mesenchymal proteins during MET facilitates the re-establishment of an epithelial cell phenotype [31,32].
As EMT and MET are critical for homeostasis, deregulated alterations in the mechanisms that control these processes unequivocally contribute to disease development and progression. Indeed, modifications driving EMT and MET in cancer are not easily controlled, being amplified by the dynamic modification of the tumor microenvironment and the crosstalk between cancer cells and non-cancer cells. Such deregulated changes critically correlate with cancer metastasis and resistance to therapy, which both are major causes of death for cancer patients. Therefore, characterizing the phenotypic alterations and the precise plethora of molecular mechanisms that collectively control EMT/MET in malignant cells may help to overcome existing limitations in the diagnosis and treatment of cancer patients.
1.2. EMT as an ‘Accomplice’ to Cancer Metastasis
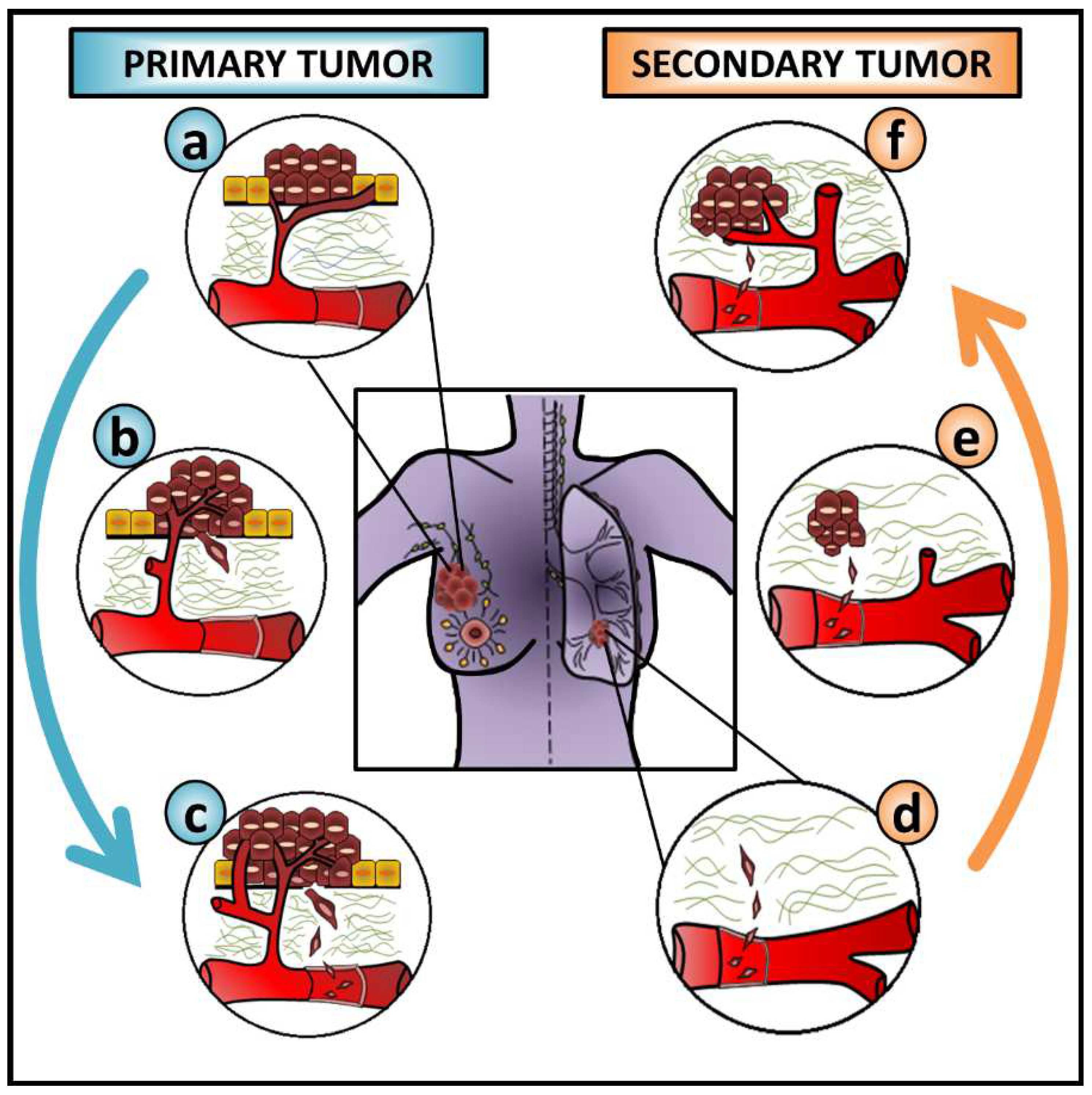
Although triggered by common inducers and characterized by similar molecular and cellular changes, EMT occurs in three contextually different situations. According to the outcomes, these are classified as type I EMT—embryogenesis, type II EMT—wound healing or fibrosis, and type III EMT—cancer progression and metastasis [1]. As metastasis is a multistep process, cancer cells must undergo several steps of change to leave the primary tumor and colonize distant sites. To successfully migrate and invade surrounding tissues, cancer cells undergo a profound change in a profile of proteins controlling their interactions with neighboring cells, the basement membrane, and the extracellular matrix (ECM). After blood/lymph vessel intravasation, cancer cells disseminate, extravasate the vasculature and seed secondary sites [33,34]. Then, cancer cells are expected to reverse their phenotype by MET to form micrometastases that may later result in macroscopic lesions [32,35,36,37,38] (Figure 2).
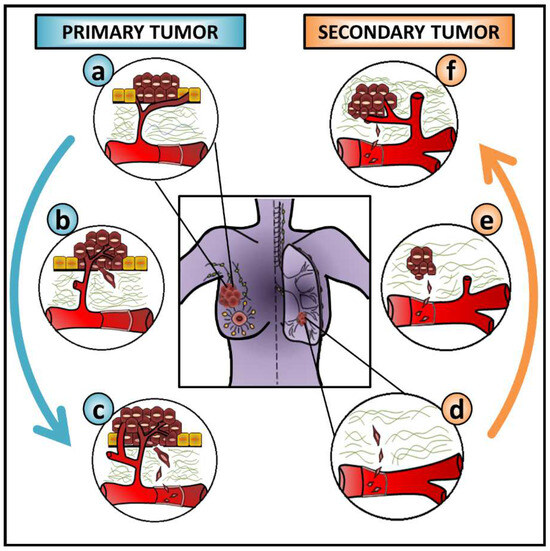
Figure 2.
The role of EMT and MET during cancer metastasis. This figure exemplifies the contribution of epithelial-mesenchymal transition (EMT) and mesenchymal-epithelial transition (MET) during cancer progression when breast cancer cells metastasize to the lung. Overall, this is expected to be common to many carcinoma cells from different cancer types metastasizing to distinct sites. Cancer cells from (a) the primary tumor (b) undergo EMT, degrade the basement membrane and the ECM, and invade surrounding tissues before (c) blood vessel intravasation. After (d) the extravasation of circulating tumor cells (CTCs) into a distant organ, their interaction with the local microenvironment stimulates cancer cells to undergo (e) MET, seeding and colonizing a secondary site, and allowing (f) the growth of a secondary tumor. The blue arrow on the left side of the figure and the orange arrow on the right side of the figure represent the EMT and MET progress, respectively.
Interestingly, cancer cells undergoing EMT may show heterogeneous degrees of epithelial and mesenchymal characteristics, including intermediate states known as ‘partial’ (p)EMT [35,36,39] (Figure 1). This is often reflected in the ratio of EMT markers in cancer cells, such as E-cadherin and N-cadherin. Whereas cancer cells that complete the EMT may benefit from single-cell migration, the epithelial traits conserved by cancer cells with pEMT may also enhance their dissemination in the circulation as a cluster, facilitating their colonization at distant organs [40,41,42,43,44]. The existence of a pEMT adds complexity and difficulties to omic-based studies. Averaged quantifications of gene/protein levels in multiple cancer cells cannot adequately represent the heterogeneous phenotypes of individual cells showing distinct EMT degrees in between themselves and over time.
Noteworthy, although many of the EMT outcomes are well described, further analyses of regulatory mechanisms are yet to be detailed. This need is associated with the complexity of this process and may be attributed to the large scale of molecular adaptations happening at different stages of EMT that are regulated by the crosstalk between multiple signaling pathways. Crucial molecular pathways and traditional transcription factors responsible for driving the molecular reprogramming that is associated with the EMT have been reasonably characterized so far, but understanding their crosstalk during EMT is still a complex task. An alternative to obtaining an unbiased portrait of changes during EMT is the simultaneous use of methods such as RNA and protein microarrays, RNA-sequencing (RNA-seq) and mass spectrometry (MS). Still, these technologies were rarely combined in EMT studies. Therefore, in the following sections, we review EMT-related changes established by studies at either DNA, RNA, or protein level, before discussing the integration of multiple platforms. As such, the limitations of the individual methods are minimized and compensated to better integrate them into mechanistic studies and clinical applications.
2. Benefits and Pitfalls of Analyzing Cancer Cell EMT at the DNA/RNA Level
In order to evaluate the gene expression profile of cancer cells undergoing EMT, it is important to successfully recapitulate this process under experimental conditions. Different models have been used to study EMT-related alterations and their impact on cancer progression. In vitro studies, as those discussed here, frequently rely on the treatment of cancer cells with known EMT inducers. Alternatively, the exogenously introduced overexpression of such molecules can also be used to investigate downstream consequences on cell transcriptomes. Moreover, while EMT is known to drive therapeutic resistance, recent studies have demonstrated that drug treatment can also induce EMT in cancer cells [45,46]. Noteworthy, because the phenotypic changes associated with EMT are very well-established, researchers can use different approaches to induce EMT that are easily verifiable, increasing the reliability of the conclusions obtained. In this section, selected studies are briefly discussed aiming at exemplifying the main findings in the field while allowing further comparison with changes observed at protein level in cancer cells undergoing EMT.
Among other results, evaluating gene expression alterations that are associated with cancer cell EMT contributed to portraying the many modifications triggered during cancer progression [47,48,49,50]. Such studies helped to determine not only the duration of EMT-related alterations at the molecular level but also to establish how different effectors cooperate to regulate EMT over time. In bladder carcinoma cells, for example, while alterations in typical EMT markers (e.g., Keratins and Vimentin) were only observed 24 h after FGF-1 treatment, changes in the expression of different transcription factors (e.g., ETS and JUNB) were reported to start and end shortly (2 h) after cell stimulation [47]. Interestingly, alterations in the gene expression of transcription factors were also observed in prostate cancer cells undergoing EMT [51]. Exogenous expression of Runx2 in these cancer cells led to SOX9 and SNAI2 upregulation, reinforcing the existence of cooperation between transcription factors notoriously associated with EMT towards the amplification of this process [51]. Indeed, the relevance of EMT-TFs for the regulation of the EMT program over time has been recently reinforced by Frey and colleagues investigating the pro-metastatic phenotype of SMAD4-defective colorectal cancer cells [52]. Although SMAD4 is a transcription factor critical to canonical TGF-β and Bone morphogenetic protein (BMP) signaling activity, overexpression of the EMT-TF SNAI1 in SMAD4-mutant colorectal cancer cells compensated for this deficiency, inducing cancer cell elongation and invasion in vitro [52]. Accordingly, SMAD4-mutant cells overexpressing SNAI1 also exhibited typical transcriptome changes expected in cells undergoing EMT, including the activation of other pro-EMT molecular pathways (e.g., Wnt signaling pathway) and increased activity of other EMT-TFs (e.g., FOXO4) [52]. These studies emphasize the complexity of the EMT program where distinct signaling pathways can cooperate to enhance this phenotypic shift or compensate each other in case of specific losses or deficiencies.
Interestingly, different studies have reported the failure to establish a good correlation between particular pairs of mRNA and proteins in the EMT context. Although protein degradation must certainly account for part of this observation, post-transcriptional modifications are also likely to contribute to this result. Post-transcriptional modifications respond to an additional layer of complexity in the regulatory mechanisms of the EMT by modifying mRNA stability. For instance, Shapiro and colleagues (2011) described little overlap between genes regulated at the expression level and at the splicing level in HMLE mammary cells undergoing EMT [53]. Yet, both regulatory mechanisms were reported to largely impact the levels of mRNA transcribed from genes associated with cytoskeleton assembling and cell-cell junctions [53]. The impact of splicing events on mRNA levels during EMT was also characterized in prostate cancer cells where 900 differential alternative splicing events were described, impacting the expression of several genes associated with EMT, migration and invasion [49]. Therefore, these and other studies in the field found a tight control of the EMT program that actively operates on RNA species and, more specifically, not only precedes but also follows transcription.
Still, whereas post-transcriptional modifications partially respond to changes in RNA levels, alterations in the epigenome are also expected to contribute to this process. For example, Taube and colleagues (2013) reported a 10-fold gain in the DNA methylation levels at the promoter of the microRNA-203 (miR-203) in Twist-overexpressing HMLE cells [54]. Interestingly, reduced levels of miR-203 were also observed in breast cancer cell lines naturally exhibiting mesenchymal traits (e.g., MDA-MB-231 breast cancer cells), whereas increased miR-203 levels were detected in epithelial counterparts (e.g., MCF-7 breast cancer cells) [54]. Also investigating epigenetic alterations, Peixoto et al. (2019) reported an association between EMT and increased methylation in histone H3 residues, such as in lysine 4 (H3K4) [55]. Moreover, 23% of all upregulated genes characterized in this study coincided with genes potentially activated by H3K4 bi-methylation (H3K4me2) [55]. In fact, increased levels of the inducer mark H3K4me2 and decreased levels of the repressive mark H3K27me3 were found at the promoter of Matrix metalloproteinase 9 (MMP9), a proteinase typically associated with ECM remodeling and cell invasion [55].
In addition to alterations previously described that can regulate epithelial and mesenchymal characteristics, the activity of non-coding RNA can also interfere with mRNA levels. Loboda et al. (2011), for example, reported a negative correlation between the expression of miR-200 family members and EMT-related genes in colorectal cancers (CRCs) [50]. In head and neck squamous cell carcinomas (HNSCCs), the expression of the long non-coding RNA (lncRNA) lnc-LCE5A-1 and lnc-KCTD6-3 was associated with reduced overall survival, while the ectopic expression of this lncRNA decreased Vimentin mRNA levels and impaired HNSCC cell migration in vitro [56]. Reinforcing the importance of characterizing alterations in non-coding RNA, Liao et al. (2017) showed that the impact of TGF-β treatment on the global levels of lncRNA is even higher than its impact on mRNA levels in MCF10A cells [57]. Moreover, the knockdown of the RP6-65G23.5 lncRNA in this model decreased E-cadherin and ZO-1 expression while increasing Vimetin, N-cadherin and Fibronectin levels, and inducing cell migration and invasion [57].
Beyond the evaluation of mRNA levels using in vitro models, transcriptome studies of human tumor samples have consolidated the association between cancer cell EMT and poor prognosis, highlighting the significance of transcriptome analysis in clinical applications. For example, Marquardt et al. (2014) reported the enrichment of an EMT signature in advanced hepatocellular carcinoma (HCC) samples which was also correlated with reduced survival and increased recurrence [58]. Interestingly, several genes differentially regulated in this molecular signature are known targets of TGF-β, Notch, and Vascular endothelial growth factor (VEGF), and are related to cell adhesion, cytoskeleton remodeling and EMT [58]. Nevertheless, it is well-known that changes in EMT-related genes may be impacted by the occurrence of non-cancer cells within tumor masses. This is particularly concerning when considering stroma-rich tumors, where cells from mesenchymal origin expressing high levels of mesenchymal-related genes may mislead the interpretation of bulk transcriptomic analyses of tumor samples with low cancer cell purity [59,60,61]. To overcome this limitation, the increasing use of single-cell (sc)RNA-seq in cancer studies has helped to better understand the significance of such modifications [62,63]. Instead of necessarily refuting previous studies, new methodologies may point to directions otherwise counterintuitive [60,64]. For instance, while cancer cell EMT and invasion are commonly associated, the invasive side of endometrioid adenocarcinomas is reported to be poorly represented by cancer cells expressing EMT-related genes. Such an EMT-related pattern, however, is observed in the endometrial side of these tumors [64]. Moreover, whereas the expression of epithelial and mesenchymal traits are often anticorrelated, increasing evidence highlights the coexistence of such phenotypes. Indeed, scRNA-seq coupled with trajectory inference analysis of HNSCCs has demonstrated that while some cancer cells show low levels of epithelial and mesenchymal markers, they can transition into a phenotype that simultaneously expresses high levels of epithelial- and mesenchymal-related genes [65]. Additionally, other HNSCC cancer cells can sustain elevated epithelial traits while dynamically regulating the expression of mesenchymal genes [65]. Noteworthy, these and other observations have driven the development of methods that enable estimating the composition of tumor samples analyzed by bulk transcriptomics through a combination of deconvolution and inference of tumor purity [66,67,68,69]. These approaches improve our comprehension of the relevance of cancer cells and stromal components during cancer progression while additionally increasing the accuracy of analyses relying on EMT signatures.
As discussed in this section, very important findings regarding cancer cell EMT originated from studies exploring alterations at the DNA/RNA level. Methodologies such as microarrays, RNA-seq, scRNA-seq, and assay for transposase-accessible chromatin with sequencing (ATAC)-seq can be used in a less biased way to reveal crucial players that must then be further investigated in more restricted conditions using additional controls. Still, however important, RNA shows limited functions and the main phenotypic changes observed during EMT come from protein activity. Thus, using similar EMT models to investigate the proteome of cancer cells is also critical to deepening our understanding of the main drivers involved in this phenotypic transition.
3. Proteomics Translated from Bench-to-Bedside
Characterizing specific protein functions has always been greatly important in cancer research due to the multiple roles these molecules can play in distinct cellular processes. Changes in protein level, localization, and activity—which are regulated by post-translational modifications and interaction with other molecules—largely affect EMT. However, similar to studying alterations in mRNA levels, focusing on one or a few proteins may be detrimental to portraying the complex scenario that involves EMT. Proteome studies help to overcome this limitation by reducing bias while analyzing this process. Moreover, if integrated into the transcriptome and epigenome analysis, proteomics can be used to identify crucial biomarkers and molecular pathways not only altered as a consequence of EMT but also responsible for driving this molecular program.
In this section, we will discuss how methodologies applied to describe the proteome of cancer cells undergoing EMT established the fundamental concepts associated with cancer cell migration and invasion (Section 3.1). Techniques such as MS and tissue microarray (TMA) have been used in different models, allowing not only the identification of EMT-related proteins, but also changes in their stability, activity, and localization in response to post-translational modifications (Figure 3). Because resistance to therapy and metastasis are closely associated with EMT, we next focus on studies that characterized the proteome of human tumor samples (Section 3.2) and biological fluids from cancer patients (Section 3.3). Understanding past studies that explored EMT-related biomarkers in vitro and in clinical samples may improve the diagnosis and treatment of cancer patients.
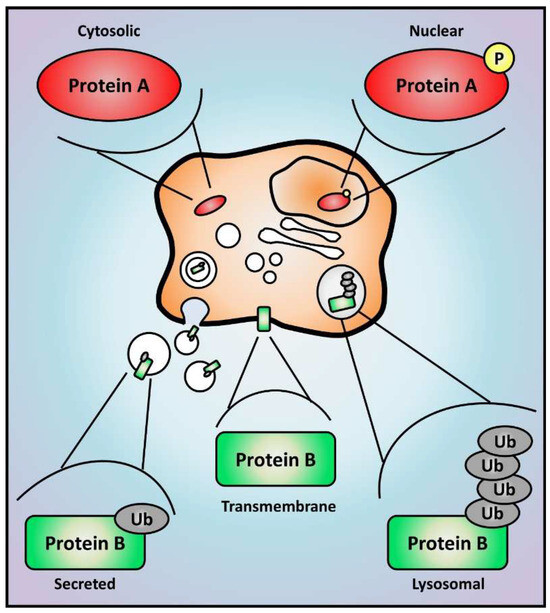
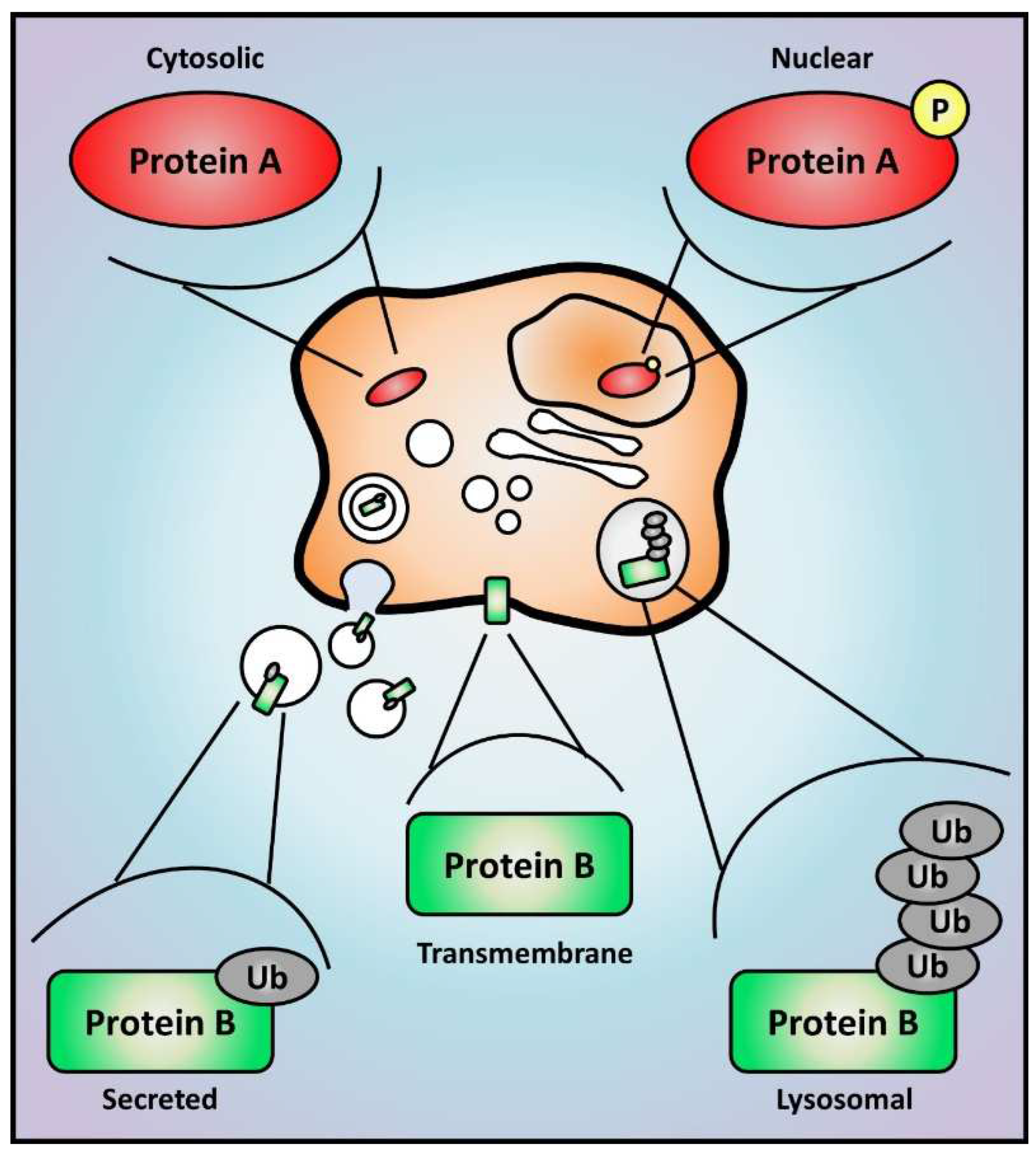
Figure 3.
Proteomic analysis determines protein expression, activation, and localization. The study of cell proteome offers an unbiased view of global protein levels, and enrichment methods can also reveal major alterations in post-translational modifications that are able to impact protein activity, stability, and localization. Hypothetical changes are represented here for proteins A and B. Protein A translocates from the cytoplasm to the nucleus if phosphorylated, which could represent its activation as a transcription factor. Protein B is a plasma membrane protein that can be secreted into extracellular vesicles if monoubiquitinated or degraded in lysosomes if polyubiquitinated. Yellow circles and grey ellipses represent protein phosphorylation (P) and ubiquitination (Ub), respectively.
3.1. Using In Vitro Models to Analyze the Proteome of Cancer Cells Undergoing EMT
As previously discussed regarding the characterization of cancer cell epigenome or transcriptome, the use of cell cultures as models helps us to understand how individual or combined signaling pathways are altered and/or alter cancer cell EMT. Again, strategies employed for this purpose mainly include the induction of cancer cell EMT by stimulation with EMT-related growth factors or ectopic expression of EMT-inducers.
For example, TGF-β treatment was shown to induce EMT by triggering global alterations in the expression of several cytoskeleton proteins, such as β-actin and Cofilin1 [70], but also by indirectly changing the localization of cell adhesion proteins (e.g., ZO-1) through ADAM metallopeptidase domain 12 (ADAM12L) upregulation [71,72]. Similarly, downregulation of proteins associated with cell adhesion (e.g., E-cadherin and ZO-1) was reported in gastric cancer (GC) cells overexpressing Ubiquitin like with PHD and ring finger domains 2 (UHRF2) [73]. In this context, UHRF2 reduces E-cadherin mRNA levels by binding to the E-cadherin promoter and repressing the transcription of this epithelial marker [73]. UHRF2 overexpression was also associated with the upregulated activity of typical EMT-transcription factors (EMT-TFs), and the interaction between UHRF2 and Transcription factor 7 like 2 (TCF7L2) was further demonstrated in this model [73]. In another example, overexpression of Mitogen-activated protein kinase kinase (MEK)5 overexpression in breast cancer cells (BCs) resistant to Tumor necrosis factor-alpha (TNF-α) upregulated Vimentin and downregulated Keratins 8 and 19 [74]. Accordingly, MEK5 overexpression also induced EMT-related morphology changes and colony formation [74].
It is interesting to note that many studies evaluating alterations in the proteome of cells undergoing EMT frequently report cytoskeleton and other structural proteins among the main molecules differentially expressed. As discussed before, while a proteome-based investigation enables the broadest identification of global changes, the sensitivity of most methods is still low if compared with techniques used to evaluate modifications at the DNA/RNA level. Therefore, improving the sensitivity of these methods is a notorious goal for most studies that attempt to reveal the proteome of cancer cells or patient samples, and the analysis of sub-proteomes can help to achieve this aim.
3.1.1. Compartmentalization and Specificity of Sub-Proteome
Although analyzing whole cell lysates can lead to a wide view of the changes that cancer cells are subjected to when transitioning towards a mesenchymal phenotype, many important mediators can be expressed at low levels. Therefore, the relevance of these less abundant proteins can be masked by highly expressed proteins. As an alternative to solve this problem, the fractionation of whole cells before protein extraction can enrich protein samples and allow the identification of otherwise overlooked EMT-related proteins. While improving the detection of poorly expressed proteins, the analysis of sub-proteomes also allows researchers to visualize the distribution of these molecules across distinct cell compartments such as the nucleus, cytoplasm, and membranes.
Chen et al. (2011), for example, used this approach to study the membrane proteome of 21D1 cells (HRAS-transformed MDCK cell clones) undergoing TGF-β-induced EMT [75]. In addition to a shift towards a pattern of integrin-mediated adhesion, researchers showed that Wnt5a was the top upregulated protein in this model and that its expression repressed the canonical Wnt signaling pathway in 21D1 cells [75]. Interestingly, a similar elevation in integrin membrane levels has also been described in EMT models of ovarian and breast cancers [76,77]. Sub-proteome analysis of ovarian adenocarcinoma cells demonstrated that EGF treatment upregulates Integrin α2 in the membrane fraction of Caov-3 cells during EMT [76]. In BC cells, Palma and collaborators (2016) demonstrated that SNAIL overexpression drives cell elongation and increases Integrin β1 expression in the membrane fraction of MCF7 cells [77]. Also evaluating the sub-proteome of breast cells, Silvestrini and colleagues (2020) described the upregulation of Ubiquitin specific peptidase (USP)47 in the cytoplasm fraction of TGF-β2-treated MCF10A cells [78]. Interestingly, treatment with a pharmacological inhibitor targeting USP47 (P5091) upregulated E-cadherin and downregulated SNAIL both in the nucleus and cytoplasm, while reducing morphological changes otherwise observed in MCF10A cells treated with TGF-β2 [78].
As observed by the results discussed here, cell fractionation is indeed a potent strategy to unmask changes in the levels of poorly expressed proteins. Still, many important proteins associated with the EMT program do not remain inside the cell. For example, proteins associated with cell-cell communication and ECM structural components have their main activity in the extracellular space. Thus, enrichment methods should be expanded to further investigate alterations in the secretome that are associated with EMT.
3.1.2. What Is on the Outside Matters: Secretome and Cell Communication
The study of sub-proteomes with a focus on secreted molecules contributes to the characterization of proteins that are critically associated with EMT by regulating, for instance, cell-cell communication and ECM remodeling. Traditionally, the global evaluation of these proteins has been referenced as secretome. Still, different routes of secretion exist, and some studies have also investigated non-canonical pathways driving protein secretion through extracellular vesicles (EVs). Although many types of EVs exist and differ in biogenesis and composition, most results converge towards a critical role for EVs in mediating cell-cell communication, as comprehensively reviewed by Greening [79], Xu [80], Raposo [81], Couch [82], and respective collaborators. Many studies have already demonstrated the contribution of EVs to different processes associated with cancer growth and progression. Here, studies investigating the profile of proteins secreted by cancer cells (mediated or not by EVs) are discussed with a focus on their relevance to EMT. Importantly, different EV populations were historically described as exosomes only considering their small diameter (30–100 nm) and without further characterization of their biogenesis. This nomenclature is no longer recommended in most circumstances [83], but the term will be used henceforward in this review respecting original publications.
Large alterations in the secretome of cells undergoing EMT have been reported. For instance, 287 differentially expressed proteins were described in the secretome of 21D1 cells compared with the secretome of parental MDCK cells, many associated with ECM remodeling [84,85]. Interestingly, 71% of these differentially expressed proteins exhibited concordant altered mRNA levels, including Collagen α-2I and Serine peptidase inhibitor Kazal type (SPINK)5, suggesting a major impact on regulatory mechanisms acting at the DNA/RNA level [84,85]. In BC cells, HMGA1 knockdown induced a cobblestone-like morphology in MDA-MB-231 cells [86], while reducing the secretion of several proteins associated with decreased cancer relapse and distant metastasis-free survival [e.g., Serpin E1 (SERPINE1) and Plasminogen activator, urokinase (PLAU)] [87]. Also, using BC models, Erin et al. (2018) compared alterations in the proteome of poorly metastatic 67NR cells and highly metastatic 4T1 cells [88]. Strikingly, whereas several proteins associated with ECM composition and remodeling were similarly expressed in whole cell lysates of both cell lines, proteins such as BMP1, Fibulin-4, MMP-3, and MMP-9 were increased in the secretome of metastatic cells [88].
Focused on specific alterations that impact non-canonical routes of secretion, Tauro and collaborators (2013) compared the proteome of exosomes secreted by epithelial MDCK cells and mesenchymal 21D1 clones to establish the contribution of these EVs to EMT [89]. Several proteins downregulated in 21D1-exosomes were associated with cell-cell adhesion [e.g., E-cadherin (CDH1) and Epithelial cell adhesion molecule (EPCAM)], while cytoskeleton proteins (e.g., Vimentin) and proteases (e.g., MMPs 1/14/19) were upregulated in these EVs [89]. Interestingly, because MMP-14 and -19 were not previously observed in the classical secretome of MDCK/21D1 cells [85], it was suggested that exosomes may represent a specific mechanism to transport these proteases to distant sites [89]. Also using MDCK cells, Gopal and colleagues established a YBX1-overexpressing clone (MDCKYBX1 cells) showing a pEMT state [35,90]. Compared with the parental cell line, MDCKYBX1-exosomes were shown to contain enriched oncogenic proteins (e.g., KRAS, HRAS, RhoC), and proteins with a known role in angiogenesis [e.g., Integrin subunit beta 1 binding protein 1 (ITGB1BP1)] and cell motility (e.g., Rac1) [35,90]. Additionally, MDCKYBX1-exosomes effectively transferred Rac1 to 2F-2B endothelial cells, inducing 2F-2B cell migration in vitro [35,90]. These results are of great relevance by showing that cells undergoing EMT can effectively use these vesicles to transfer cargo to other cell types and induce a behavior largely associated with cancer growth and progression, such as angiogenesis.
Although limited by available techniques, in vitro studies have performed a deep evaluation of the proteome of cancer cells undergoing EMT and their secretome. It is clear so far that different EMT inducers and regulators can trigger similar mechanisms, thus driving cells to a common outcome. However, further analyses are still needed to better characterize the precise extent of the crosstalk and overlap between these mechanisms. Improvements in existing methodologies will allow not only the identification of poorly expressed relevant proteins, but also determine post-translational modifications that may be critical for their stability, localization, and activity. Still, from the understanding obtained from cell culture-based studies, many EMT effectors have been revealed. As discussed here, high-resolution methods have begun to be used to interrogate changes at the sub-cellular level. Similarly, as seen for enriched fractions from cell lysates, much information has also been described by exploring the localization of proteins in EVs [91,92,93,94,95,96]. In this context, the preferential expression of proteins in the membrane or lumen of EVs could help to explain the targeting, interaction, and intracellular fate of EVs in recipient cells. Likewise, determining the source of such proteins carried by EVs may further elucidate the mechanisms of activation for specific signaling pathways and their propensity to regulate EMT in targeted cells. Evaluating the levels, localization, and orientation of such proteins in clinical samples may lead to new targeted therapies and biomarkers with enhanced potential to detect and treat early-stage metastases, as well as to predict cancer recurrence, metastatic progression, and resistance to therapy.
3.2. Looking towards New EMT Biomarkers in Primary Tumors by Using Proteomics
Although in vitro models are useful for exploring specific mechanisms that drive or block the EMT process, they usually lack natural intratumor and intertumor heterogeneity otherwise observed in real cancers. In addition, representing the interaction between cancer cells and non-cancer cells within the tumor microenvironment is a complex task. Therefore, characterizing the proteome of only one cell type may also mislead the interpretation of its real significance. In this context, the best approach to understanding the contribution of the EMT to the progression of real tumors may demand a careful analysis of the proteome of tumor samples. Still, as confounding factors might be more difficult to isolate in this scenario than in vitro, additional considerations should be kept in mind, such as the existence of distinctive molecular subtypes and clinicopathological characteristics for the patients included in the study.
Considering that most proteomic techniques are very expensive and time-consuming, studies aiming to evaluate the proteome of human cancers would hardly be designed to exclusively evaluate EMT-related effectors or biomarkers. Otherwise, such proteins may eventually emerge among the set of differentially expressed molecules, particularly considering their well-established relevance in cancer invasion and metastasis, as discussed before. For instance, Moreira et al. (2004) have observed this pattern of differentially expressed proteins when comparing the proteome of bladder specimens derived from normal tissues and transitional cell carcinomas (TCCs) [97]. Sixty percent of the tumors analyzed expressed high levels of Vimentin and PGP9.5 [also known as Ubiquitin C-terminal hydrolase L1 (UCHL1)], suggesting the presence of cancer cells undergoing EMT [97]. Additionally, invasive tumors showed lower levels of the epithelial protein 14-3-3σ (also known as Stratifin) than normal tissues and non-invasive tumors [97]. Interestingly, the evaluation of tumors exhibiting heterogeneous staining for 14-3-3σ demonstrated the progressive loss of its expression, with noteworthy negative staining in invasive areas [97]. Similarly, Sun and colleagues reported that the mesenchymal marker Vimentin was consistently overexpressed in hepatocellular carcinomas (HCCs) compared with cirrhotic and normal liver tissues, reinforcing the association between EMT and cancer progression [98].
The focus on proteins with a known significance to EMT might however mask the detection of new biomarkers contributing to EMT-related effects. Because EMT is frequently associated with cancer progression, an alternative is to group the samples according to the TNM stage, thus considering tumor size, impacts on nearby lymph nodes and metastatic occurrence. Celis and collaborators used this experimental design and reported that the downregulation of keratin-13, alpha-Fatty acid binding protein (α-FABP), Glutathione S-transferase (GST)-µ, and D-3-phosphoglycerate dehydrogenase (PDGH) was correlated with increasing grade in TCCs [99,100]. In BCs, a molecular signature including several EMT markers (e.g., CK5/6, CK8/18, E-cadherin, and P-cadherin) was associated with metastatic progression and successfully used to group cancer patients in poor- or good-prognosis clusters [101]. Also studying the proteome of BCs, Cawthorn and colleagues described 477 proteins differentially expressed between cancer samples from patients affected or not by lymph node (LN) metastasis [102]. Among these proteins, Decorin levels in cancer cells were correlated with LN metastasis in terms of both occurrence and number of LNs affected, whereas the expression of Heat shock protein (HSP)-90B1 was associated with distant metastasis [102]. In bladder cancers, high grade tumors were enriched in mesenchymal markers (e.g., Vimentin, Collagen α-1I), basal and cancer stem cell markers [e.g., Aldehyde dehydrogenase 1 family member A1 (ALDH1A1) and Bcl-2-associated X protein (BAX)], proteins associated with cell migration (e.g., Fibronectin 1 and Vitronectin), and EMT inducers (e.g., TGF-β1) [103].
As discussed before for in vitro studies, the presence of highly expressed proteins can mask the presence of less abundant molecules able to play critical roles in the biological process evaluated. Although sub-proteomes of human tumors have not been frequently evaluated, some examples highlight their relevance. The analysis of the cytosolic fraction of BCs, for example, associated higher levels of Ferritin light chain (FTL) with reduced metastasis-free survival [104,105]. Interestingly, histological analyses revealed that FTL was mostly expressed by stromal cells and its levels were correlated with the expression of CD138 (also known as Syndecan) [104]. Because CD138 is a typical mesenchymal marker, its expression in both stromal cells and cancer cells suggested the occurrence of EMT in at least part of these breast cancers [104].
Altogether, these studies confirm that many EMT-related proteins identified in vitro show potential use as biomarkers for different types of cancer, being associated with cancer recurrence, lymph node metastasis, and distant metastasis. Moreover, while important EMT-related alterations have been characterized in vitro, many proteins are regulated by the interactions between cancer cells and stromal cells. This observation reinforces the importance of integrative studies analyzing the proteome of immortalized cancer cells and human cancer samples. Still, because analyzing proteins from tumor samples demands highly invasive approaches to obtain biopsies and resected samples, this may be a problem when monitoring cancer patients before/after treatment. In the next section, studies focused on overcoming this limitation by investigating and establishing biomarkers in biological fluids are discussed.
3.3. Biological Fluids: An Easier Access to EMT-Related Biomarkers
The analysis of tumor samples, particularly their proteomes, requires invasive procedures to obtain enough tissue to detect low-abundance proteins. Otherwise, the evaluation of biomarkers in biological fluids such as blood, saliva, and urine requires less invasive methodologies that enable a closer and easier follow-up of the patients. Moreover, analysis of patient-derived data obtained over time (e.g., before, during, and after therapeutic intervention) may help to more accurately characterize the dynamic networks that regulate such a fluid process as the EMT.
Aiming to establish biomarkers for bladder cancers, research led by Celis [106] and Ostergaard [107] reported increased levels of Psoriasin (also known as S100A7) in squamous cell carcinomas (SCCs) and urine samples from cancer patients. Interestingly, Psoriasin was not observed among the serum proteins of SCC patients, indicating its specific use as a biomarker to be screened in the urine of these patients [106,107]. In another example, Sun and colleagues reported Vimentin as a sensitive and specific biomarker when analyzing the proteome of serum samples from HCC patients and non-neoplastic controls [98]. In this study, Vimentin levels were used to distinguish even patients with small HCCs (<2 cm) from non-neoplastic controls [98].
Whereas the detection of cancer proteins in biological fluids shows clear benefits, it also incurs a methodological issue associated with the presence of highly expressed proteins that may mask less abundant molecules. To improve the detection of relevant molecules, particularly from serum and plasma samples, the depletion of excessively abundant proteins is recommended. In BC patients, proteomic analysis of albumin-depleted serum samples demonstrated the association between LN metastasis and several proteins related to the cytoskeleton and ECM structure or remodeling, including collagen α4I, Serpin C1, Fibrinogen gamma chain (FGG), and Tenascin XB (TNXB) [108]. Moreover, in this study, TNXB was detected in the serum samples of all patients diagnosed with benign breast diseases and LN-negative cancer patients, but not in LN-positive patients, indicating that loss of circulating TNXB could be used as a biomarker of LN metastasis [108].
Several cancer types lack specific diagnostic or prognostic biomarkers. Even for cancers better characterized (e.g., breast, colorectal, and lung cancers), only a small number of biomarkers exist, and their use is restricted by reduced sensitivity and specificity. Moreover, many different conditions with clinical relevance cannot be determined by current biomarkers, including the progression toward resistance to anti-cancer therapies and the development of locoregional and distant recurrence. As observed by the studies discussed here, EMT-related proteins could be used as cancer biomarkers, particularly if considering their typical association with cancer progression and metastasis. Still, the reduced number of publications evaluating cohorts specifically grouped according to EMT-associated outcomes limits the generalization of the conclusions obtained. For instance, additional proteome-based studies including patients who progressed to LN metastasis or distant metastasis, may confirm the reliability of EMT-related proteins for this purpose. Investigations focused on recurrence and resistance to therapy have also been overlooked, and cancer types with lower incidence have been often ignored in this kind of evaluation. In addition to an experimental design focused on EMT-associated modifications, improved technologies may allow the characterization of cancer protein profiles, as has been currently performed for the characterization of cancer transcriptomes.
In the next section, new perspectives to integrate multiomics data from real tumors are discussed to highlight how these approaches can further increase our understanding of EMT. Also, we prospect a better comprehension of the proteome of EVs and CTCs led by emerging methods with superior sensitivity. In addition to EVs and CTCs, improved methods may enable characterizing the proteome of cancer cells and non-cancer cells in the tumor microenvironment from a spatio-temporal point of view.
4. Integration of Multiomics and Spatio-Temporal Analyses for a Comprehensive Understanding of EMT-Driven Cancer Progression
In contrast to studying the changes at the DNA/RNA level, current proteomic methods cannot satisfactorily cover the entire diversity of proteins within cancer cells or the tumor mass. Additionally, evaluating the protein profile of multiple samples is highly expensive and time-consuming. These and other reasons led to a better understanding of how modifications in the epigenome and transcriptome impact biological processes while depicting global changes in protein levels was left behind. But to what extent should we rely on a single omics when translating EMT-related findings to the clinic? Although several studies individually demonstrated global alterations either in the epigenome, the transcriptome, or the proteome of cells undergoing EMT, the direct comparison of these results is still an issue. Besides analyzing different cancer types, in vitro studies frequently induce EMT by exploring only one EMT inducer at a time. In addition to differences across studies, this strategy limits the investigation of possible crosstalk between multiple signaling pathways. Therefore, multiomics may provide a more reliable source for the comparison of the many regulatory mechanisms impacting cancer cells at multiple levels during EMT—particularly when analyzing cancer patient samples that are inherently affected by several EMT regulators.
In fact, seminal studies published throughout the last decade have already begun to adopt multiomics as an approach to analyze alterations in cancer samples that simultaneously impact DNA, RNA, and protein levels. Although most of these studies investigated different cancer types, some similarities are worth mentioning. Among them is the common divergence between transcriptome- or proteome-derived data, such as that reported in colorectal [109], breast [110,111], ovarian [112], gastric [113], and lung [114] cancers. Besides methodological parameters influencing this correlation, spatio-temporal changes in RNA species largely affect their localization and availability for translation, thus, impacting protein levels [115,116,117,118]. Importantly, this effect is reported to change in pathological conditions, being increased in cancers when compared with normal tissues, and particularly enhanced with cancer progression [115]. Moreover, copy number alterations (CNAs) and post-translational modifications are also shown to significantly impact gene expression in a way that is not necessarily translated into protein modifications [110,114]. In addition to reinforcing an important difference in the mechanisms that impact cancers at the molecular level, this analytical divergence has a profound impact on the ability to determine patient prognosis. Remarkably, depending on the method of choice, patients showing significantly different probabilities of survival cannot be distinguished by such omic analysis. For example, in CRCs, only a proteome-based clustering—but not other types of analysis—revealed a typical EMT signature correlated with poor prognosis [109]. Furthermore, the identification of a molecular subtype associated with cell invasion and poor survival rate in early-onset gastric cancers (EOGCs) required an integrative clustering using global mRNA, proteome, phosphoproteome, and N-glycoproteome [113]. This observation reinforces a critical problem as the use of single omic methods may not suffice to accurately describe the myriad of alterations within a tumor, therefore, representing an obvious issue in determining therapeutic approaches.
Although the integration of multiple omics helps to overcome limitations otherwise imposed by the individual use of each approach, spatial alterations are often masked in bulk analysis, and detecting temporal modifications remains unfeasible—especially considering a clinical context. As mentioned before, the increasing association of deconvolution strategies and transcriptomic-focused methods with single-cell resolution has been instrumental in depicting the contribution of different tumor compartments regarding EMT-related alterations. Tissue microdissection also partially helps to overcome this limitation to individually investigate molecular signatures associated with either tumor epithelium or stroma. For instance, in microdissected prostate cancers, a gradual decline in phosphorylated (p-) Mitogen-activated protein kinase (ERK) levels and concurrent increase in p-AKT levels have been associated with cancer progression [119]. Similar results were also reported in microdissected CRCs, where decreased p-ERK and p-p38 levels were observed in cancer tissues compared with uninvolved mucosa [120]. Thus, integrating tissue microdissection and tissue microarray may increase the understanding of spatial modifications otherwise overlooked by the analysis of bulk samples.
Further, a combined approach can also help to characterize alterations in rare samples that are not located within the cancer mass but have been shed by the tumor and may be scattered throughout the body, such as CTCs. For instance, in a study simulating CTCs by spiking immortalized cancer cells into blood samples, 4000 proteins were identified by one-dimensional high-resolution porous layer open tube-liquid chromatography (LC)-MS in samples spiked with 100–200 MCF7 breast cancer cells [121]. Impressive results were also described by using nano-LC-MS for the analysis of 1–5 LNCaP prostate cancer cells spiked and recovered from blood samples [122]. In HNSCC patient samples, the use of mass cytometry and unsupervised clustering allowed the identification of epithelial and EMT sub-groups of CTCs, where the latter accounted for more than 80% of all CTCs [123]. Interestingly, the expression of immune checkpoint proteins (e.g., PD-L1 and CTLA4) was lower in CTCs with an EMT phenotype when compared with epithelial counterparts [123]. Further, analysis of molecular pathway activity revealed that CTCs expressing EMT traits were also enriched in p-CREB and p-ERK proteins, but showed reduced levels of other intracellular effectors, such as p-STAT3, p-STAT5, p-PARP, and p-AKT [123]. Establishing the profile and significance of immune checkpoints and intracellular effectors in CTCs may have a profound impact on the development of therapeutic strategies focused on overcoming metastatic progression and resistance to therapy. Noteworthy, while methodological improvement is still required to analyze the proteome of patient CTCs, innovative studies have already begun to characterize the genome, transcriptome, and metabolome of these shedded cells [124,125,126,127,128,129,130,131,132,133,134]. Such analyses have not only helped to elucidate how mutations and molecular programs impact the dissemination of cancer cells but have also validated the perspective of employing a multiomic strategy to improve our knowledge of cancer progression.
As for CTCs, few studies have comprehensively investigated the protein profile of EVs isolated from cancer patient biofluids. Nevertheless, initial studies in circulating EVs have already demonstrated an association between HCC progression and increased Galectin-3-binding protein (LG3BP) levels [135]. In CRC, Transferrin receptor protein 1 (TFR1) was reported to be enriched in circulating EVs from non-metastatic patients [136]. In BC, the establishment of protein signatures for circulating EVs (including EGFR, p-cadherin, and fibronectin) enabled differentiating cancer patients from healthy subjects and was further associated with cancer progression, and relapse [137]. Moreover, while the isolation and characterization of EVs from biofluids remains challenging, the development of microfluidic devices for the isolation and enrichment of such membranous particles brings interesting possibilities for the diagnosis and monitoring of cancer patients. For example, it has been recently reported that the analysis of epithelial and mesenchymal markers on plasma EVs captured by microfluidic devices can be successfully used to establish the prognosis of patients with pancreatic cystic lesions [138]. This strategy is particularly important as it uses tumor-derived EVs to monitor EMT dynamics in pancreatic cells and further inform on whether these patients may or may not undergo surgery [138]. Similarly, quantification of the EMT markers in melanoma-EVs through microfluidic devices has been described as an innovative strategy to monitor disease progression. In this context, increased levels of mesenchymal markers (N-cadherin and ABCB5) compared to epithelial markers (E-cadherin and THBS1) characterized a shift in the serum EVs of melanoma patients that was also correlated with the development of drug resistance [139]. Overall, although limited in number, the significance of these studies is remarkable and may be increased if combined with those where DNA and RNA species transported by cancer patient EVs are analyzed and also associated with diagnostic or prognostic potential [140,141,142]. Furthermore, since EVs and CTCs can both be obtained from blood samples and separated based on physicochemical properties, new methods are emerging to optimize their sequential isolation and analysis in a parallelized multidimensional analytic framework [143,144]. Importantly, such methods must not be understood simplistically as additional strategies for the discovery of EMT-related biomarkers. Rather, innovations improving the analysis of rare samples in liquid biopsies (e.g., CTCs and EVs) are paramount to generate a holistic view of the signaling pathways underlying EMT while also providing information on its dynamic regulation during metastasis. In this scenario, biomarkers emerge from an in-depth understanding of the molecular machinery that drives disease progression. Consequently, the translation of such biomarkers into clinical practice will improve existing diagnostic and monitoring methods due to enhanced specificity and sensitivity.
Altogether, the results discussed here demonstrate the possibility of evaluating the proteogenome of small samples, but future studies are still needed before implementing such analyses in clinical routine. While in vitro experiments benefit from “unlimited” starting material, human samples are finite, restricting the number of tests and especially compromising the detection of rare proteins. In addition to increasing the sensitivity of proteomics and their integration with epigenomics/transcriptomics, current studies highlight the importance of improving methods for the isolation of scarce samples, such as EVs and CTCs that are naturally obtained at small concentrations. Overcoming these limitations may lead to novel mechanisms and biomarkers to be used for the diagnosis and treatment of cancer patients (Figure 4).
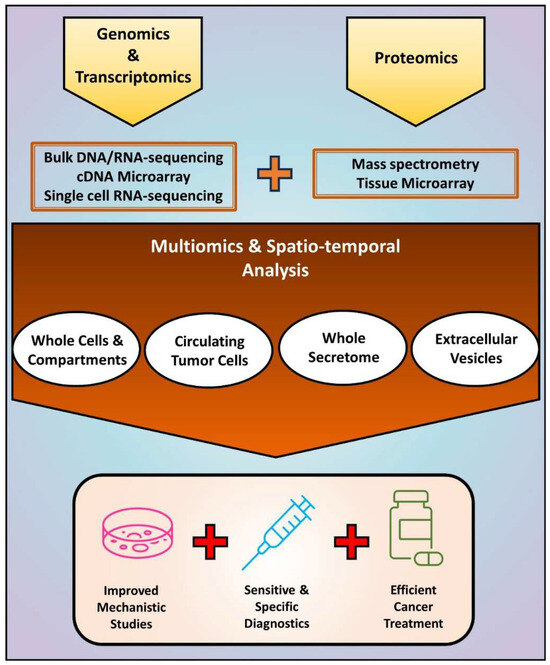
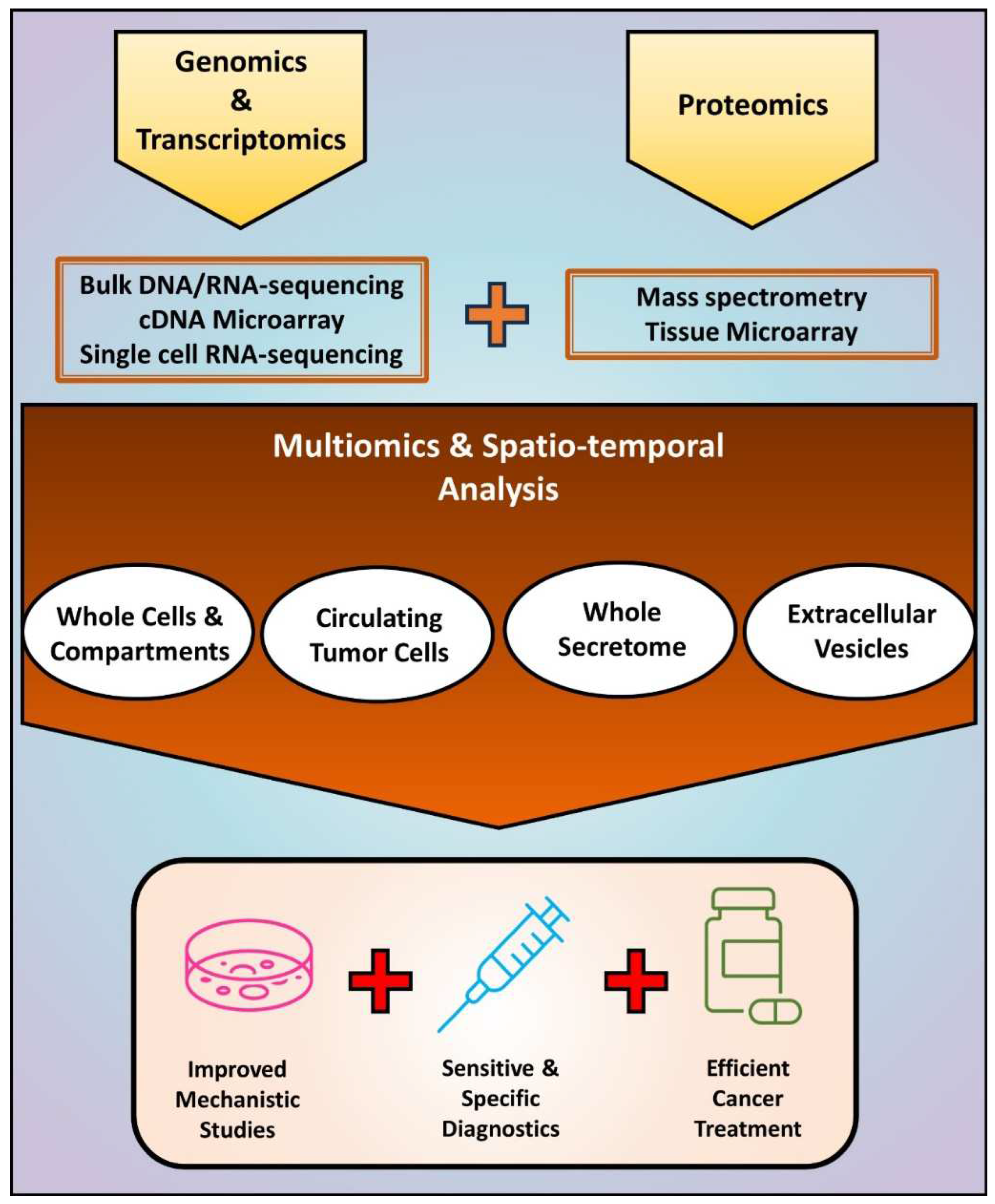
Figure 4.
An integrated approach based on multiomics and spatio-temporal analysis of EMT-driven cancer progression. Current studies evaluating EMT at DNA, RNA, and protein levels have relied on the independent use of methods such as bulk DNA/RNA-sequencing, cDNA microarray, single cell RNA-sequencing, mass spectrometry, and tissue microarray. The combination of these methodologies enables obtaining information about simultaneous alterations in cancer cells and fractions that include the intracellular and the extracellular milieu. In addition to improving mechanistic studies in vitro and in vivo, such strategy may lead to more sensitive and specific diagnostic methods, and efficient anti-cancer therapies able to prevent and treat EMT-driven metastasis and recurrence.
5. Future Directions
Although targeted therapies against some of the major EMT-inducers have been vastly used to treat cancer patients (e.g., anti-EGFR tyrosine kinase inhibitors), others have repeatedly failed in clinical trials (e.g., anti-TGF-β inhibitors) [15,145]. Consistent with this notion is the delayed development of efficient therapies against metastasis and recurrence, while major achievements have been seen over the years for drugs targeting primary tumor growth [146]. This observation highlights major gaps in our understanding of the EMT program, which consequently impair the development and the adequate use of anti-EMT therapies in the treatment of cancer patients. As discussed throughout this review, addressing this issue will likely involve the integration of multiomics and spatio-temporal approaches to precisely characterize the crosstalk between the multiple molecular pathways that regulate EMT and MET during cancer progression. Ideally, new technologies and methods will improve our understanding of the architecture of the tumor microenvironment while simultaneously allowing the investigation of alterations taking place in intracellular and extracellular compartments. For instance, structures such as endosomes, lysosomes and extracellular vesicles are highly specialized, and the trafficking of different molecules along them is tightly regulated. Precisely characterizing the spatio-temporal alterations impacting molecules that are trafficked through these compartments and used in cell-cell communication might inform about the fate of cancer cells and disease progression.
Moreover, whereas important signaling pathways able to induce EMT-related cancer progression have been described, the existing methods used for the diagnosis and monitoring of cancer patients have rarely taken advantage of such well-defined knowledge. More specifically, the poorly efficient methods currently used in clinics still fail to efficiently distinguish healthy subjects and different cancer stages because of intrinsic conceptual limitations that overlook the impact of EMT inducers and regulators. Underlying such issues is the common adoption of biomarkers that are not involved in the core molecular program that ultimately promotes cancer progression. Accordingly, these methods are typically associated with reduced sensitivity and specificity. Thus, overcoming these limitations to improve patient diagnosis and monitoring will require the establishment of sensitive methods with a focus on detecting and tracking alterations in biomarkers with real impact on cancer progression, especially EMT drivers [147,148,149].
Furthermore, it is worth noting that the clinical samples analyzed in many studies (as those discussed here) are usually collected before treatment or, at best, once following the administration of anti-cancer therapies. Such a strategy may mask several alterations with critical relevance for the progression of the disease that cannot be detected when patient samples are collected and analyzed too long before or after treatment. This may be particularly important if considering low-abundance biomarkers that are unique to a small fraction of cells (or EVs) with elevated metastatic potential. Thus, a closer monitoring of cancer patients and the analysis of multiple samples collected over time might lead to the identification of alterations in molecules associated with response to treatment, therefore, helping to guide patient care. As omic-based methods may not be appropriate in this context, they may be used in conjunction with other methods of cheaper and quicker execution (e.g., ELISA and qPCR). Moreover, as sampling fragments of the tumor may not be suitable for repeated analyses, using biological fluids would be advantageous as they require less invasive techniques to be collected, thus preserving the quality of life of cancer patients.
6. Conclusions
Whereas the past decades have been a stage for many studies focused on characterizing EMT drivers and regulators, efficient diagnostics and therapies based on these molecules are still missing. This highlights a major problem in precisely characterizing the mechanisms that promote cancer progression and our failure to use well-established mechanisms to benefit cancer patients. Thus, an innovative strategy to overcome such limitations is urgently needed.
EMT is an intricate process that involves the crosstalk between multiple signaling pathways [17,150,151,152,153,154,155]. Many signaling pathways controlling cancer cell EMT are also required for tissue homeostasis, which explains their tight regulation. Characterizing the mechanisms controlling EMT and the myriad of associated molecules is an arduous task that favors overlooking those molecules that are poorly expressed, irrespective of their potential significance in clinics. Although interrogating changes in one molecular pathway at a time has been crucial to depicting the EMT program, integrating multiple omics may help reveal the persisting blind spots that still compromise our understanding of this process. While changes at DNA and RNA levels reflect quick responses exhibited by cancer cells and non-cancer cells, alterations in protein levels and post-translational modifications impact their localization, stability, and activity. Similarly, while investigating modifications in the genome and transcriptome of these cells is favored by high sensitivity due to amplification-based methodologies, alterations in their proteome may more accurately reveal the real drivers of EMT-related phenotypic changes.
Furthermore, besides combining multiple omic techniques, improvements in current in vitro and in vivo methods will increase our understanding of the real complexity of EMT-related alterations. At the same time, the integration of pre-clinical studies with improved real-world data will lead to better options in the fight against cancer metastasis and recurrence. In the context of diagnostics and treatment, this will enable obtaining more precise and useful information about EMT-related changes to monitor disease progression in (nearly) real-time to ultimately improve patient outcomes.
Author Contributions
Conceptualization: H.-J.Z.; Supervision: H.-J.Z.; Writing—Original Draft: A.F.T. and S.W.; Writing—Review and Editing: A.F.T., S.W., R.L. and H.-J.Z.; Visualization: A.F.T. and S.W.; Funding Acquisition: H.-J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
Research was supported by the Australia’s National Health and Medical Research Council (NHMRC, #433618) (H.-J.Z.), and Friends of the Royal Melbourne Hospital Neurosciences Foundation (H.-J.Z.). A.F.T. was supported by the Melbourne Research Scholarship. S.W. was supported by the Research Training Scholarship, R.J. Fletcher Research Scholarship and Nick Christopher PhD Scholarship.
Conflicts of Interest
The authors declare no conflict of interest. A.F.T., S.W., R.L. and H.-J.Z. are members of the research team at the Huagene Institute. Huagene Institute had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Akhmetkaliyev, A.; Alibrahim, N.; Shafiee, D.; Tulchinsky, E. EMT/MET plasticity in cancer and Go-or-Grow decisions in quiescence: The two sides of the same coin? Mol. Cancer 2023, 22, 90. [Google Scholar] [CrossRef]
- Buckley, C.E.; St Johnston, D. Apical–basal polarity and the control of epithelial form and function. Nat. Rev. Mol. Cell Biol. 2022, 23, 559–577. [Google Scholar] [CrossRef]
- Lu, C.; Sidoli, S.; Kulej, K.; Ross, K.; Wu, C.H.; Garcia, B.A. Coordination between TGF-β cellular signaling and epigenetic regulation during epithelial to mesenchymal transition. Epigenetics Chromatin 2019, 12, 11. [Google Scholar] [CrossRef]
- Puram, S.V.; Tirosh, I.; Parikh, A.S.; Patel, A.P.; Yizhak, K.; Gillespie, S.; Rodman, C.; Luo, C.L.; Mroz, E.A.; Emerick, K.S.; et al. Single-Cell Transcriptomic Analysis of Primary and Metastatic Tumor Ecosystems in Head and Neck Cancer. Cell 2017, 171, 1611–1624.e1624. [Google Scholar] [CrossRef] [PubMed]
- Vasaikar, S.V.; Deshmukh, A.P.; den Hollander, P.; Addanki, S.; Kuburich, N.A.; Kudaravalli, S.; Joseph, R.; Chang, J.T.; Soundararajan, R.; Mani, S.A. EMTome: A resource for pan-cancer analysis of epithelial-mesenchymal transition genes and signatures. Br. J. Cancer 2021, 124, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.P.; Sambath, J.; Sathe, G.; George, I.A.; Pandey, A.; Thompson, E.W.; Kumar, P. Pan-cancer quantitation of epithelial-mesenchymal transition dynamics using parallel reaction monitoring-based targeted proteomics approach. J. Transl. Med. 2022, 20, 84. [Google Scholar] [CrossRef] [PubMed]
- Jonckheere, S.; Adams, J.; De Groote, D.; Campbell, K.; Berx, G.; Goossens, S. Epithelial-Mesenchymal Transition (EMT) as a Therapeutic Target. Cells Tissues Organs 2021, 211, 157–182. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hong, W.; Wei, X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J. Hematol. Oncol. 2022, 15, 129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ng, A.S.; Cai, S.; Li, Q.; Yang, L.; Kerr, D. Novel therapeutic strategies: Targeting epithelial–mesenchymal transition in colorectal cancer. Lancet Oncol. 2021, 22, e358–e368. [Google Scholar] [CrossRef] [PubMed]
- Georgakopoulos-Soares, I.; Chartoumpekis, D.V.; Kyriazopoulou, V.; Zaravinos, A. EMT Factors and Metabolic Pathways in Cancer. Front. Oncol. 2020, 10, 499. [Google Scholar] [CrossRef]
- Nam, M.W.; Kim, C.W.; Choi, K.C. Epithelial-Mesenchymal Transition-Inducing Factors Involved in the Progression of Lung Cancers. Biomol. Ther. 2022, 30, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Szymczyk, J.; Sluzalska, K.D.; Materla, I.; Opalinski, L.; Otlewski, J.; Zakrzewska, M. FGF/FGFR-Dependent Molecular Mechanisms Underlying Anti-Cancer Drug Resistance. Cancers 2021, 13, 5796. [Google Scholar] [CrossRef]
- Wu, S.; Luwor, R.B.; Zhu, H.-J. Dynamics of transforming growth factor β signaling and therapeutic efficacy. Growth Factors 2023, 41, 82–100. [Google Scholar] [CrossRef] [PubMed]
- Vallés, A.M.; Boyer, B.; Badet, J.; Tucker, G.C.; Barritault, D.; Thiery, J.P. Acidic fibroblast growth factor is a modulator of epithelial plasticity in a rat bladder carcinoma cell line. Proc. Natl. Acad. Sci. USA 1990, 87, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tian, X.J.; Xing, J. Signal Transduction Pathways of EMT Induced by TGF-β, SHH, and WNT and Their Crosstalks. J. Clin. Med. 2016, 5, 41. [Google Scholar] [CrossRef]
- Fan, C.; Wang, Q.; van der Zon, G.; Ren, J.; Agaser, C.; Slieker, R.C.; Iyengar, P.V.; Mei, H.; ten Dijke, P. OVOL1 inhibits breast cancer cell invasion by enhancing the degradation of TGF-β type I receptor. Signal Transduct. Target. Ther. 2022, 7, 126. [Google Scholar] [CrossRef]
- Lioulia, E.; Mokos, P.; Panteris, E.; Dafou, D. UBE2T promotes β-catenin nuclear translocation in hepatocellular carcinoma through MAPK/ERK-dependent activation. Mol. Oncol. 2022, 16, 1694–1713. [Google Scholar] [CrossRef]
- Mi, K.; Zeng, L.; Chen, Y.; Ning, J.; Zhang, S.; Zhao, P.; Yang, S. DHX38 enhances proliferation, metastasis, and EMT progression in NSCLC through the G3BP1-mediated MAPK pathway. Cell. Signal. 2024, 113, 110962. [Google Scholar] [CrossRef]
- Ma, X.; Ma, X.; Zhu, L.; Zhao, Y.; Chen, M.; Li, T.; Lin, Y.; Ma, D.; Sun, C.; Han, L. The E3 ubiquitin ligase MG53 inhibits hepatocellular carcinoma by targeting RAC1 signaling. Oncogenesis 2022, 11, 40. [Google Scholar] [CrossRef]
- Nie, X.; Liu, D.; Zheng, M.; Li, X.; Liu, O.; Guo, Q.; Zhu, L.; Lin, B. HERPUD1 promotes ovarian cancer cell survival by sustaining autophagy and inhibit apoptosis via PI3K/AKT/mTOR and p38 MAPK signaling pathways. BMC Cancer 2022, 22, 1338. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef]
- Zheng, H.; Kang, Y. Multilayer control of the EMT master regulators. Oncogene 2014, 33, 1755–1763. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Akhurst, R.J.; Balmain, A. TGF-β signaling in tumor suppression and cancer progression. Nat. Genet. 2001, 29, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yun, F.; Shi, L.; Li, Z.H.; Luo, N.R.; Jia, Y.F. Roles of Signaling Pathways in the Epithelial-Mesenchymal Transition in Cancer. Asian Pac. J. Cancer Prev. 2015, 16, 6201–6206. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, B.J.; Grail, D.; Nheu, T.; Najdovska, M.; Wang, B.; Waring, P.; Inglese, M.; McLoughlin, R.M.; Jones, S.A.; Topley, N.; et al. Hyperactivation of Stat3 in gp130 mutant mice promotes gastric hyperproliferation and desensitizes TGF-β signaling. Nat. Med. 2005, 11, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.J.; Iaria, J.; Sizeland, A.M. Smad7 differentially regulates transforming growth factor β-mediated signaling pathways. J. Biol. Chem. 1999, 274, 32258–32264. [Google Scholar] [CrossRef] [PubMed]
- Khatibi, S.; Zhu, H.-J.; Wagner, J.; Tan, C.W.; Manton, J.H.; Burgess, A.W. Mathematical model of TGF-β signalling: Feedback coupling is consistent with signal switching. BMC Syst. Biol. 2017, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, M.; Hervouet, E.; Baudu, T.; Herfs, M.; Parratte, C.; Feugeas, J.P.; Perez, V.; Reynders, C.; Ancion, M.; Vigneron, M.; et al. GABARAPL1 Inhibits EMT Signaling through SMAD-Tageted Negative Feedback. Biology 2021, 10, 956. [Google Scholar] [CrossRef] [PubMed]
- Pei, D.; Shu, X.; Gassama-Diagne, A.; Thiery, J.P. Mesenchymal–epithelial transition in development and reprogramming. Nat. Cell Biol. 2019, 21, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Bakir, B.; Chiarella, A.M.; Pitarresi, J.R.; Rustgi, A.K. EMT, MET, Plasticity, and Tumor Metastasis. Trends Cell Biol. 2020, 30, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.N.; Jayachandran, G.; Moore, R.G.; Cristofanilli, M.; Lang, J.E.; Khoury, J.D.; Press, M.F.; Kim, K.K.; Khazan, N.; Zhang, Q.; et al. A Multi-Center Clinical Study to Harvest and Characterize Circulating Tumor Cells from Patients with Metastatic Breast Cancer Using the Parsortix® PC1 System. Cancers 2022, 14, 5238. [Google Scholar] [CrossRef] [PubMed]
- Boya, M.; Ozkaya-Ahmadov, T.; Swain, B.E.; Chu, C.-H.; Asmare, N.; Civelekoglu, O.; Liu, R.; Lee, D.; Tobia, S.; Biliya, S.; et al. High throughput, label-free isolation of circulating tumor cell clusters in meshed microwells. Nat. Commun. 2022, 13, 3385. [Google Scholar] [CrossRef]
- Gopal, S.K.; Greening, D.W.; Mathias, R.A.; Ji, H.; Rai, A.; Chen, M.; Zhu, H.J.; Simpson, R.J. YBX1/YB-1 induces partial EMT and tumourigenicity through secretion of angiogenic factors into the extracellular microenvironment. Oncotarget 2015, 6, 13718–13730. [Google Scholar] [CrossRef] [PubMed]
- Haerinck, J.; Berx, G. Partial EMT takes the lead in cancer metastasis. Dev. Cell 2021, 56, 3174–3176. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Abdollahi, B.; Wilkins, O.M.; Lu, H.; Chakraborty, P.; Ognjenovic, N.B.; Muller, K.E.; Jolly, M.K.; Christensen, B.C.; Hassanpour, S.; et al. Phenotypic heterogeneity driven by plasticity of the intermediate EMT state governs disease progression and metastasis in breast cancer. Sci. Adv. 2022, 8, eabj8002. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Wang, Q.; Kuipers, T.B.; Cats, D.; Iyengar, P.V.; Hagenaars, S.C.; Mesker, W.E.; Devilee, P.; Tollenaar, R.A.E.M.; Mei, H.; et al. LncRNA LITATS1 suppresses TGF-β-induced EMT and cancer cell plasticity by potentiating TβRI degradation. EMBO J. 2023, 42, e112806. [Google Scholar] [CrossRef]
- Saitoh, M. Involvement of partial EMT in cancer progression. J. Biochem. 2018, 164, 257–264. [Google Scholar] [CrossRef]
- Lüönd, F.; Sugiyama, N.; Bill, R.; Bornes, L.; Hager, C.; Tang, F.; Santacroce, N.; Beisel, C.; Ivanek, R.; Bürglin, T.; et al. Distinct contributions of partial and full EMT to breast cancer malignancy. Dev. Cell 2021, 56, 3203–3221.e3211. [Google Scholar] [CrossRef]
- Gkountela, S.; Castro-Giner, F.; Szczerba, B.M.; Vetter, M.; Landin, J.; Scherrer, R.; Krol, I.; Scheidmann, M.C.; Beisel, C.; Stirnimann, C.U.; et al. Circulating Tumor Cell Clustering Shapes DNA Methylation to Enable Metastasis Seeding. Cell 2019, 176, 98–112.e114. [Google Scholar] [CrossRef] [PubMed]
- Krol, I.; Schwab, F.D.; Carbone, R.; Ritter, M.; Picocci, S.; De Marni, M.L.; Stepien, G.; Franchi, G.M.; Zanardi, A.; Rissoglio, M.D.; et al. Detection of clustered circulating tumour cells in early breast cancer. Br. J. Cancer 2021, 125, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, A.; Koppaka, D.; Anand, A.; Deb, B.; Grenci, G.; Viasnoff, V.; Thompson, E.W.; Gowda, H.; Bhat, R.; Rangarajan, A.; et al. Circulating Tumor Cell cluster phenotype allows monitoring response to treatment and predicts survival. Sci. Rep. 2019, 9, 7933. [Google Scholar] [CrossRef] [PubMed]
- Au, S.H.; Storey, B.D.; Moore, J.C.; Tang, Q.; Chen, Y.-L.; Javaid, S.; Sarioglu, A.F.; Sullivan, R.; Madden, M.W.; O’Keefe, R.; et al. Clusters of circulating tumor cells traverse capillary-sized vessels. Proc. Natl. Acad. Sci. USA 2016, 113, 4947–4952. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.-D.; Pang, K.; Wu, Z.-X.; Dong, Y.; Hao, L.; Qin, J.-X.; Wang, W.; Chen, Z.-S.; Han, C.-H. Tumor cell plasticity in targeted therapy-induced resistance: Mechanisms and new strategies. Signal Transduct. Target. Ther. 2023, 8, 113. [Google Scholar] [CrossRef]
- Kralj, J.; Pernar Kovač, M.; Dabelić, S.; Polančec, D.S.; Wachtmeister, T.; Köhrer, K.; Brozovic, A. Transcriptome analysis of newly established carboplatin-resistant ovarian cancer cell model reveals genes shared by drug resistance and drug-induced EMT. Br. J. Cancer 2023, 128, 1344–1359. [Google Scholar] [CrossRef] [PubMed]
- Billottet, C.; Tuefferd, M.; Gentien, D.; Rapinat, A.; Thiery, J.P.; Broet, P.; Jouanneau, J. Modulation of several waves of gene expression during FGF-1 induced epithelial-mesenchymal transition of carcinoma cells. J. Cell Biochem. 2008, 104, 826–839. [Google Scholar] [CrossRef]
- Lenferink, A.E.; Cantin, C.; Nantel, A.; Wang, E.; Durocher, Y.; Banville, M.; Paul-Roc, B.; Marcil, A.; Wilson, M.R.; O’Connor-McCourt, M.D. Transcriptome profiling of a TGF-β-induced epithelial-to-mesenchymal transition reveals extracellular clusterin as a target for therapeutic antibodies. Oncogene 2010, 29, 831–844. [Google Scholar] [CrossRef]
- Lu, Z.X.; Huang, Q.; Park, J.W.; Shen, S.; Lin, L.; Tokheim, C.J.; Henry, M.D.; Xing, Y. Transcriptome-wide landscape of pre-mRNA alternative splicing associated with metastatic colonization. Mol. Cancer Res. 2015, 13, 305–318. [Google Scholar] [CrossRef]
- Loboda, A.; Nebozhyn, M.V.; Watters, J.W.; Buser, C.A.; Shaw, P.M.; Huang, P.S.; Van’t Veer, L.; Tollenaar, R.A.E.M.; Jackson, D.B.; Agrawal, D.; et al. EMT is the dominant program in human colon cancer. BMC Med. Genom. 2011, 4, 9. [Google Scholar] [CrossRef]
- Baniwal, S.K.; Khalid, O.; Gabet, Y.; Shah, R.R.; Purcell, D.J.; Mav, D.; Kohn-Gabet, A.E.; Shi, Y.; Coetzee, G.A.; Frenkel, B. Runx2 transcriptome of prostate cancer cells: Insights into invasiveness and bone metastasis. Mol. Cancer 2010, 9, 258. [Google Scholar] [CrossRef]
- Frey, P.; Devisme, A.; Rose, K.; Schrempp, M.; Freihen, V.; Andrieux, G.; Boerries, M.; Hecht, A. SMAD4 mutations do not preclude epithelial–mesenchymal transition in colorectal cancer. Oncogene 2022, 41, 824–837. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, I.M.; Cheng, A.W.; Flytzanis, N.C.; Balsamo, M.; Condeelis, J.S.; Oktay, M.H.; Burge, C.B.; Gertler, F.B. An EMT-driven alternative splicing program occurs in human breast cancer and modulates cellular phenotype. PLoS Genet. 2011, 7, e1002218. [Google Scholar] [CrossRef] [PubMed]
- Taube, J.H.; Malouf, G.G.; Lu, E.; Sphyris, N.; Vijay, V.; Ramachandran, P.P.; Ueno, K.R.; Gaur, S.; Nicoloso, M.S.; Rossi, S.; et al. Epigenetic silencing of microRNA-203 is required for EMT and cancer stem cell properties. Sci. Rep. 2013, 3, 2687. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, P.; Etcheverry, A.; Aubry, M.; Missey, A.; Lachat, C.; Perrard, J.; Hendrick, E.; Delage-Mourroux, R.; Mosser, J.; Borg, C.; et al. EMT is associated with an epigenetic signature of ECM remodeling genes. Cell Death Dis. 2019, 10, 205. [Google Scholar] [CrossRef] [PubMed]
- Zou, A.E.; Ku, J.; Honda, T.K.; Yu, V.; Kuo, S.Z.; Zheng, H.; Xuan, Y.; Saad, M.A.; Hinton, A.; Brumund, K.T.; et al. Transcriptome sequencing uncovers novel long noncoding and small nucleolar RNAs dysregulated in head and neck squamous cell carcinoma. RNA 2015, 21, 1122–1134. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.Y.; Wu, J.; Wang, Y.J.; He, J.H.; Deng, W.X.; Hu, K.; Zhang, Y.C.; Zhang, Y.; Yan, H.; Wang, D.L.; et al. Deep sequencing reveals a global reprogramming of lncRNA transcriptome during EMT. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, J.U.; Seo, D.; Andersen, J.B.; Gillen, M.C.; Kim, M.S.; Conner, E.A.; Galle, P.R.; Factor, V.M.; Park, Y.N.; Thorgeirsson, S.S. Sequential transcriptome analysis of human liver cancer indicates late stage acquisition of malignant traits. J. Hepatol. 2014, 60, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Calon, A.; Lonardo, E.; Berenguer-Llergo, A.; Espinet, E.; Hernando-Momblona, X.; Iglesias, M.; Sevillano, M.; Palomo-Ponce, S.; Tauriello, D.V.; Byrom, D.; et al. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat. Genet. 2015, 47, 320–329. [Google Scholar] [CrossRef]
- Isella, C.; Terrasi, A.; Bellomo, S.E.; Petti, C.; Galatola, G.; Muratore, A.; Mellano, A.; Senetta, R.; Cassenti, A.; Sonetto, C.; et al. Stromal contribution to the colorectal cancer transcriptome. Nat. Genet. 2015, 47, 312–319. [Google Scholar] [CrossRef]
- Wang, L.; Saci, A.; Szabo, P.M.; Chasalow, S.D.; Castillo-Martin, M.; Domingo-Domenech, J.; Siefker-Radtke, A.; Sharma, P.; Sfakianos, J.P.; Gong, Y.; et al. EMT- and stroma-related gene expression and resistance to PD-1 blockade in urothelial cancer. Nat. Commun. 2018, 9, 3503. [Google Scholar] [CrossRef]
- Li, H.; Courtois, E.T.; Sengupta, D.; Tan, Y.; Chen, K.H.; Goh, J.J.L.; Kong, S.L.; Chua, C.; Hon, L.K.; Tan, W.S.; et al. Reference component analysis of single-cell transcriptomes elucidates cellular heterogeneity in human colorectal tumors. Nat. Genet. 2017, 49, 708–718. [Google Scholar] [CrossRef]
- Szabo, P.M.; Vajdi, A.; Kumar, N.; Tolstorukov, M.Y.; Chen, B.J.; Edwards, R.; Ligon, K.L.; Chasalow, S.D.; Chow, K.H.; Shetty, A.; et al. Cancer-associated fibroblasts are the main contributors to epithelial-to-mesenchymal signatures in the tumor microenvironment. Sci. Rep. 2023, 13, 3051. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Tabuchi, Y.; Yurino, H.; Hirohashi, Y.; Deshimaru, S.; Asano, T.; Mariya, T.; Oshima, K.; Takamura, Y.; Ukita, Y.; et al. Comprehensive single-cell transcriptome analysis reveals heterogeneity in endometrioid adenocarcinoma tissues. Sci. Rep. 2017, 7, 14225. [Google Scholar] [CrossRef]
- Bocci, F.; Zhou, P.; Nie, Q. Single-Cell RNA-Seq Analysis Reveals the Acquisition of Cancer Stem Cell Traits and Increase of Cell–Cell Signaling during EMT Progression. Cancers 2021, 13, 5726. [Google Scholar] [CrossRef]
- Tyler, M.; Tirosh, I. Decoupling epithelial-mesenchymal transitions from stromal profiles by integrative expression analysis. Nat. Commun. 2021, 12, 2592. [Google Scholar] [CrossRef]
- Foroutan, M.; Bhuva, D.D.; Lyu, R.; Horan, K.; Cursons, J.; Davis, M.J. Single sample scoring of molecular phenotypes. BMC Bioinform. 2018, 19, 404. [Google Scholar] [CrossRef]
- Sutton, G.J.; Poppe, D.; Simmons, R.K.; Walsh, K.; Nawaz, U.; Lister, R.; Gagnon-Bartsch, J.A.; Voineagu, I. Comprehensive evaluation of deconvolution methods for human brain gene expression. Nat. Commun. 2022, 13, 1358. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, K.; Shahmoradgoli, M.; Martínez, E.; Vegesna, R.; Kim, H.; Torres-Garcia, W.; Treviño, V.; Shen, H.; Laird, P.W.; Levine, D.A.; et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat. Commun. 2013, 4, 2612. [Google Scholar] [CrossRef] [PubMed]
- Keshamouni, V.G.; Jagtap, P.; Michailidis, G.; Strahler, J.R.; Kuick, R.; Reka, A.K.; Papoulias, P.; Krishnapuram, R.; Srirangam, A.; Standiford, T.J.; et al. Temporal quantitative proteomics by iTRAQ 2D-LC-MS/MS and corresponding mRNA expression analysis identify post-transcriptional modulation of actin-cytoskeleton regulators during TGF-β-Induced epithelial-mesenchymal transition. J. Proteome Res. 2009, 8, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Ruff, M.; Leyme, A.; Le Cann, F.; Bonnier, D.; Le Seyec, J.; Chesnel, F.; Fattet, L.; Rimokh, R.; Baffet, G.; Theret, N. The Disintegrin and Metalloprotease ADAM12 Is Associated with TGF-β-Induced Epithelial to Mesenchymal Transition. PLoS ONE 2015, 10, e0139179. [Google Scholar] [CrossRef] [PubMed]
- Dekky, B.; Ruff, M.; Bonnier, D.; Legagneux, V.; Théret, N. Proteomic screening identifies the zonula occludens protein ZO-1 as a new partner for ADAM12 in invadopodia-like structures. Oncotarget 2018, 9, 21366–21382. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lai, M.; Liang, L.; Chen, J.; Qiu, N.; Ge, S.; Ji, S.; Shi, T.; Zhen, B.; Liu, M.; Ding, C.; et al. Multidimensional Proteomics Reveals a Role of UHRF2 in the Regulation of Epithelial-Mesenchymal Transition (EMT). Mol. Cell. Proteom. 2016, 15, 2263–2278. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Nitschke, A.M.; Xiong, W.; Zhang, Q.; Tang, Y.; Bloch, M.; Elliott, S.; Zhu, Y.; Bazzone, L.; Yu, D.; et al. Proteomic analysis of tumor necrosis factor-α resistant human breast cancer cells reveals a MEK5/Erk5-mediated epithelial-mesenchymal transition phenotype. Breast Cancer Res. 2008, 10, R105. [Google Scholar] [CrossRef]
- Chen, Y.S.; Mathias, R.A.; Mathivanan, S.; Kapp, E.A.; Moritz, R.L.; Zhu, H.J.; Simpson, R.J. Proteomics profiling of Madin-Darby canine kidney plasma membranes reveals Wnt-5a involvement during oncogenic H-Ras/TGF-β-mediated epithelial-mesenchymal transition. Mol. Cell. Proteom. 2011, 10, M110.001131. [Google Scholar] [CrossRef]
- Grassi, M.L.; Palma, C.S.; Thome, C.H.; Lanfredi, G.P.; Poersch, A.; Faca, V.M. Proteomic analysis of ovarian cancer cells during epithelial-mesenchymal transition (EMT) induced by epidermal growth factor (EGF) reveals mechanisms of cell cycle control. J. Proteom. 2017, 151, 2–11. [Google Scholar] [CrossRef]
- Palma Cde, S.; Grassi, M.L.; Thome, C.H.; Ferreira, G.A.; Albuquerque, D.; Pinto, M.T.; Ferreira Melo, F.U.; Kashima, S.; Covas, D.T.; Pitteri, S.J.; et al. Proteomic Analysis of Epithelial to Mesenchymal Transition (EMT) Reveals Cross-talk between SNAIL and HDAC1 Proteins in Breast Cancer Cells. Mol. Cell. Proteom. 2016, 15, 906–917. [Google Scholar] [CrossRef]
- Silvestrini, V.C.; Thome, C.H.; Albuquerque, D.; de Souza Palma, C.; Ferreira, G.A.; Lanfredi, G.P.; Masson, A.P.; Delsin, L.E.A.; Ferreira, F.U.; de Souza, F.C.; et al. Proteomics analysis reveals the role of ubiquitin specific protease (USP47) in Epithelial to Mesenchymal Transition (EMT) induced by TGFβ2 in breast cells. J. Proteom. 2020, 219, 103734. [Google Scholar] [CrossRef]
- Greening, D.W.; Gopal, S.K.; Mathias, R.A.; Liu, L.; Sheng, J.; Zhu, H.J.; Simpson, R.J. Emerging roles of exosomes during epithelial-mesenchymal transition and cancer progression. Semin. Cell Dev. Biol. 2015, 40, 60–71. [Google Scholar] [CrossRef]
- Xu, R.; Greening, D.W.; Zhu, H.-J.; Takahashi, N.; Simpson, R.J. Extracellular vesicle isolation and characterization: Toward clinical application. J. Clin. Investig. 2016, 126, 1152–1162. [Google Scholar] [CrossRef]
- Raposo, G.; Stahl, P.D. Extracellular vesicles: A new communication paradigm? Nat. Rev. Mol. Cell Biol. 2019, 20, 509–510. [Google Scholar] [CrossRef] [PubMed]
- Couch, Y.; Buzàs, E.I.; Di Vizio, D.; Gho, Y.S.; Harrison, P.; Hill, A.F.; Lötvall, J.; Raposo, G.; Stahl, P.D.; Théry, C.; et al. A brief history of nearly EV-erything—The rise and rise of extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12144. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Mathias, R.A.; Chen, Y.S.; Wang, B.; Ji, H.; Kapp, E.A.; Moritz, R.L.; Zhu, H.J.; Simpson, R.J. Extracellular remodelling during oncogenic Ras-induced epithelial-mesenchymal transition facilitates MDCK cell migration. J. Proteome Res. 2010, 9, 1007–1019. [Google Scholar] [CrossRef]
- Mathias, R.A.; Wang, B.; Ji, H.; Kapp, E.A.; Moritz, R.L.; Zhu, H.J.; Simpson, R.J. Secretome-based proteomic profiling of Ras-transformed MDCK cells reveals extracellular modulators of epithelial-mesenchymal transition. J. Proteome Res. 2009, 8, 2827–2837. [Google Scholar] [CrossRef]
- Pegoraro, S.; Ros, G.; Piazza, S.; Sommaggio, R.; Ciani, Y.; Rosato, A.; Sgarra, R.; Del Sal, G.; Manfioletti, G. HMGA1 promotes metastatic processes in basal-like breast cancer regulating EMT and stemness. Oncotarget 2013, 4, 1293–1308. [Google Scholar] [CrossRef] [PubMed]
- Resmini, G.; Rizzo, S.; Franchin, C.; Zanin, R.; Penzo, C.; Pegoraro, S.; Ciani, Y.; Piazza, S.; Arrigoni, G.; Sgarra, R.; et al. HMGA1 regulates the Plasminogen activation system in the secretome of breast cancer cells. Sci. Rep. 2017, 7, 11768. [Google Scholar] [CrossRef]
- Erin, N.; Ogan, N.; Yerlikaya, A. Secretomes reveal several novel proteins as well as TGF-β1 as the top upstream regulator of metastatic process in breast cancer. Breast Cancer Res. Treat. 2018, 170, 235–250. [Google Scholar] [CrossRef]
- Tauro, B.J.; Mathias, R.A.; Greening, D.W.; Gopal, S.K.; Ji, H.; Kapp, E.A.; Coleman, B.M.; Hill, A.F.; Kusebauch, U.; Hallows, J.L.; et al. Oncogenic H-ras reprograms Madin-Darby canine kidney (MDCK) cell-derived exosomal proteins following epithelial-mesenchymal transition. Mol. Cell. Proteom. 2013, 12, 2148–2159. [Google Scholar] [CrossRef]
- Gopal, S.K.; Greening, D.W.; Hanssen, E.G.; Zhu, H.J.; Simpson, R.J.; Mathias, R.A. Oncogenic epithelial cell-derived exosomes containing Rac1 and PAK2 induce angiogenesis in recipient endothelial cells. Oncotarget 2016, 7, 19709–19722. [Google Scholar] [CrossRef]
- Rai, A.; Fang, H.; Claridge, B.; Simpson, R.J.; Greening, D.W. Proteomic dissection of large extracellular vesicle surfaceome unravels interactive surface platform. J. Extracell. Vesicles 2021, 10, e12164. [Google Scholar] [CrossRef]
- Cvjetkovic, A.; Jang, S.C.; Konečná, B.; Höög, J.L.; Sihlbom, C.; Lässer, C.; Lötvall, J. Detailed Analysis of Protein Topology of Extracellular Vesicles–Evidence of Unconventional Membrane Protein Orientation. Sci. Rep. 2016, 6, 36338. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Nawrocki, A.; Jensen, S.G.; Thorsen, K.; Whitehead, B.; Howard, K.A.; Dyrskjøt, L.; Ørntoft, T.F.; Larsen, M.R.; Ostenfeld, M.S. Quantitative proteomics of fractionated membrane and lumen exosome proteins from isogenic metastatic and nonmetastatic bladder cancer cells reveal differential expression of EMT factors. Proteomics 2014, 14, 699–712. [Google Scholar] [CrossRef]
- Wu, D.; Yan, J.; Shen, X.; Sun, Y.; Thulin, M.; Cai, Y.; Wik, L.; Shen, Q.; Oelrich, J.; Qian, X.; et al. Profiling surface proteins on individual exosomes using a proximity barcoding assay. Nat. Commun. 2019, 10, 3854. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Greening, D.W.; Chen, M.; Rai, A.; Ji, H.; Takahashi, N.; Simpson, R.J. Surfaceome of Exosomes Secreted from the Colorectal Cancer Cell Line SW480: Peripheral and Integral Membrane Proteins Analyzed by Proteolysis and TX114. Proteomics 2019, 19, 1700453. [Google Scholar] [CrossRef] [PubMed]
- Zaborowski, M.P.; Lee, K.; Na, Y.J.; Sammarco, A.; Zhang, X.; Iwanicki, M.; Cheah, P.S.; Lin, H.-Y.; Zinter, M.; Chou, C.-Y. Methods for systematic identification of membrane proteins for specific capture of cancer-derived extracellular vesicles. Cell Rep. 2019, 27, 255–268.e256. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.M.; Gromov, P.; Celis, J.E. Expression of the tumor suppressor protein 14-3-3 sigma is down-regulated in invasive transitional cell carcinomas of the urinary bladder undergoing epithelial-to-mesenchymal transition. Mol. Cell. Proteom. 2004, 3, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Poon, R.T.P.; Lee, N.P.; Yeung, C.; Chan, K.L.; Ng, I.O.L.; Day, P.J.R.; Luk, J.M. Proteomics of Hepatocellular Carcinoma: Serum Vimentin As a Surrogate Marker for Small Tumors (≤2 cm). J. Proteome Res. 2010, 9, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Celis, J.E.; Celis, P.; Palsdottir, H.; Ostergaard, M.; Gromov, P.; Primdahl, H.; Orntoft, T.F.; Wolf, H.; Celis, A.; Gromova, I. Proteomic strategies to reveal tumor heterogeneity among urothelial papillomas. Mol. Cell. Proteom. 2002, 1, 269–279. [Google Scholar] [CrossRef]
- Celis, J.E.; Ostergaard, M.; Basse, B.; Celis, A.; Lauridsen, J.B.; Ratz, G.P.; Andersen, I.; Hein, B.; Wolf, H.; Orntoft, T.F.; et al. Loss of adipocyte-type fatty acid binding protein and other protein biomarkers is associated with progression of human bladder transitional cell carcinomas. Cancer Res. 1996, 56, 4782–4790. [Google Scholar]
- Jacquemier, J.; Ginestier, C.; Rougemont, J.; Bardou, V.J.; Charafe-Jauffret, E.; Geneix, J.; Adélaïde, J.; Koki, A.; Houvenaeghel, G.; Hassoun, J.; et al. Protein expression profiling identifies subclasses of breast cancer and predicts prognosis. Cancer Res. 2005, 65, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Cawthorn, T.R.; Moreno, J.C.; Dharsee, M.; Tran-Thanh, D.; Ackloo, S.; Zhu, P.H.; Sardana, G.; Chen, J.; Kupchak, P.; Jacks, L.M.; et al. Proteomic analyses reveal high expression of decorin and endoplasmin (HSP90B1) are associated with breast cancer metastasis and decreased survival. PLoS ONE 2012, 7, e30992. [Google Scholar] [CrossRef] [PubMed]
- Stroggilos, R.; Mokou, M.; Latosinska, A.; Makridakis, M.; Lygirou, V.; Mavrogeorgis, E.; Drekolias, D.; Frantzi, M.; Mullen, W.; Fragkoulis, C.; et al. Proteome-based classification of Nonmuscle Invasive Bladder Cancer. Int. J. Cancer 2020, 146, 281–294. [Google Scholar] [CrossRef]
- Jézéquel, P.; Campion, L.; Spyratos, F.; Loussouarn, D.; Campone, M.; Guérin-Charbonnel, C.; Joalland, M.-P.; André, J.; Descotes, F.; Grenot, C.; et al. Validation of tumor-associated macrophage ferritin light chain as a prognostic biomarker in node-negative breast cancer tumors: A multicentric 2004 national PHRC study. Int. J. Cancer 2012, 131, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Ricolleau, G.; Charbonnel, C.; Lode, L.; Loussouarn, D.; Joalland, M.P.; Bogumil, R.; Jourdain, S.; Minvielle, S.; Campone, M.; Deporte-Fety, R.; et al. Surface-enhanced laser desorption/ionization time of flight mass spectrometry protein profiling identifies ubiquitin and ferritin light chain as prognostic biomarkers in node-negative breast cancer tumors. Proteomics 2006, 6, 1963–1975. [Google Scholar] [CrossRef]
- Celis, J.E.; Rasmussen, H.H.; Vorum, H.; Madsen, P.; Honoré, B.; Wolf, H.; Orntoft, T.F. Bladder squamous cell carcinomas express psoriasin and externalize it to the urine. J. Urol. 1996, 155, 2105–2112. [Google Scholar] [CrossRef] [PubMed]
- Ostergaard, M.; Rasmussen, H.H.; Nielsen, H.V.; Vorum, H.; Orntoft, T.F.; Wolf, H.; Celis, J.E. Proteome profiling of bladder squamous cell carcinomas: Identification of markers that define their degree of differentiation. Cancer Res. 1997, 57, 4111–4117. [Google Scholar] [PubMed]
- Hu, X.; Zhang, Y.; Zhang, A.; Li, Y.; Zhu, Z.; Shao, Z.; Zeng, R.; Xu, L.X. Comparative serum proteome analysis of human lymph node negative/positive invasive ductal carcinoma of the breast and benign breast disease controls via label-free semiquantitative shotgun technology. Omics 2009, 13, 291–300. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, J.; Wang, X.; Zhu, J.; Liu, Q.; Shi, Z.; Chambers, M.C.; Zimmerman, L.J.; Shaddox, K.F.; Kim, S.; et al. Proteogenomic characterization of human colon and rectal cancer. Nature 2014, 513, 382–387. [Google Scholar] [CrossRef]
- Mertins, P.; Mani, D.R.; Ruggles, K.V.; Gillette, M.A.; Clauser, K.R.; Wang, P.; Wang, X.; Qiao, J.W.; Cao, S.; Petralia, F.; et al. Proteogenomics connects somatic mutations to signalling in breast cancer. Nature 2016, 534, 55–62. [Google Scholar] [CrossRef]
- Johansson, H.J.; Socciarelli, F.; Vacanti, N.M.; Haugen, M.H.; Zhu, Y.; Siavelis, I.; Fernandez-Woodbridge, A.; Aure, M.R.; Sennblad, B.; Vesterlund, M.; et al. Breast cancer quantitative proteome and proteogenomic landscape. Nat. Commun. 2019, 10, 1600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, T.; Zhang, Z.; Payne, S.H.; Zhang, B.; McDermott, J.E.; Zhou, J.Y.; Petyuk, V.A.; Chen, L.; Ray, D.; et al. Integrated Proteogenomic Characterization of Human High-Grade Serous Ovarian Cancer. Cell 2016, 166, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Mun, D.G.; Bhin, J.; Kim, S.; Kim, H.; Jung, J.H.; Jung, Y.; Jang, Y.E.; Park, J.M.; Kim, H.; Jung, Y.; et al. Proteogenomic Characterization of Human Early-Onset Gastric Cancer. Cancer Cell 2019, 35, 111–124.e110. [Google Scholar] [CrossRef] [PubMed]
- Gillette, M.A.; Satpathy, S.; Cao, S.; Dhanasekaran, S.M.; Vasaikar, S.V.; Krug, K.; Petralia, F.; Li, Y.; Liang, W.W.; Reva, B.; et al. Proteogenomic Characterization Reveals Therapeutic Vulnerabilities in Lung Adenocarcinoma. Cell 2020, 182, 200–225.e235. [Google Scholar] [CrossRef] [PubMed]
- Andrieux, G.; Chakraborty, S.; Das, T.; Boerries, M. Alteration of Proteotranscriptomic Landscape Reveals the Transcriptional Regulatory Circuits Controlling Key-Signaling Pathways and Metabolic Reprogramming During Tumor Evolution. Front. Cell Dev. Biol. 2020, 8, 586479. [Google Scholar] [CrossRef]
- Maier, T.; Güell, M.; Serrano, L. Correlation of mRNA and protein in complex biological samples. FEBS Lett. 2009, 583, 3966–3973. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Beyer, A.; Aebersold, R. On the Dependency of Cellular Protein Levels on mRNA Abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef]
- Edfors, F.; Danielsson, F.; Hallström, B.M.; Käll, L.; Lundberg, E.; Pontén, F.; Forsström, B.; Uhlén, M. Gene-specific correlation of RNA and protein levels in human cells and tissues. Mol. Syst. Biol. 2016, 12, 883. [Google Scholar] [CrossRef]
- Paweletz, C.P.; Charboneau, L.; Bichsel, V.E.; Simone, N.L.; Chen, T.; Gillespie, J.W.; Emmert-Buck, M.R.; Roth, M.J.; Petricoin, I.E.; Liotta, L.A. Reverse phase protein microarrays which capture disease progression show activation of pro-survival pathways at the cancer invasion front. Oncogene 2001, 20, 1981–1989. [Google Scholar] [CrossRef]
- Gulmann, C.; Sheehan, K.M.; Conroy, R.M.; Wulfkuhle, J.D.; Espina, V.; Mullarkey, M.J.; Kay, E.W.; Liotta, L.A.; Petricoin, E.F., 3rd. Quantitative cell signalling analysis reveals down-regulation of MAPK pathway activation in colorectal cancer. J. Pathol. 2009, 218, 514–519. [Google Scholar] [CrossRef]
- Li, S.; Plouffe, B.D.; Belov, A.M.; Ray, S.; Wang, X.; Murthy, S.K.; Karger, B.L.; Ivanov, A.R. An Integrated Platform for Isolation, Processing, and Mass Spectrometry-based Proteomic Profiling of Rare Cells in Whole Blood. Mol. Cell. Proteom. 2015, 14, 1672–1683. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Podolak, J.; Zhao, R.; Shukla, A.K.; Moore, R.J.; Thomas, G.V.; Kelly, R.T. Proteome Profiling of 1 to 5 Spiked Circulating Tumor Cells Isolated from Whole Blood Using Immunodensity Enrichment, Laser Capture Microdissection, Nanodroplet Sample Processing, and Ultrasensitive nanoLC-MS. Anal. Chem. 2018, 90, 11756–11759. [Google Scholar] [CrossRef] [PubMed]
- Payne, K.; Brooks, J.; Batis, N.; Khan, N.; El-Asrag, M.; Nankivell, P.; Mehanna, H.; Taylor, G. Feasibility of mass cytometry proteomic characterisation of circulating tumour cells in head and neck squamous cell carcinoma for deep phenotyping. Br. J. Cancer 2023, 129, 1590–1598. [Google Scholar] [CrossRef] [PubMed]
- Negishi, R.; Yamakawa, H.; Kobayashi, T.; Horikawa, M.; Shimoyama, T.; Koizumi, F.; Sawada, T.; Oboki, K.; Omuro, Y.; Funasaka, C.; et al. Transcriptomic profiling of single circulating tumor cells provides insight into human metastatic gastric cancer. Commun. Biol. 2022, 5, 20. [Google Scholar] [CrossRef]
- Ring, A.; Campo, D.; Porras, T.B.; Kaur, P.; Forte, V.A.; Tripathy, D.; Lu, J.; Kang, I.; Press, M.F.; Jeong, Y.J.; et al. Circulating Tumor Cell Transcriptomics as Biopsy Surrogates in Metastatic Breast Cancer. Ann. Surg. Oncol. 2022, 29, 2882–2894. [Google Scholar] [CrossRef]
- Poonia, S.; Goel, A.; Chawla, S.; Bhattacharya, N.; Rai, P.; Lee, Y.F.; Yap, Y.S.; West, J.; Bhagat, A.A.; Tayal, J.; et al. Marker-free characterization of full-length transcriptomes of single live circulating tumor cells. Genome Res. 2023, 33, 80–95. [Google Scholar] [CrossRef]
- Thiele, J.A.; Pitule, P.; Hicks, J.; Kuhn, P. Single-Cell Analysis of Circulating Tumor Cells. Methods Mol. Biol. 2019, 1908, 243–264. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, F.; Yao, J.; Mao, C.; Zhu, M.; Qian, M.; Hu, J.; Zhong, H.; Zhou, J.; Shi, X.; et al. Single-cell metabolic fingerprints discover a cluster of circulating tumor cells with distinct metastatic potential. Nat. Commun. 2023, 14, 2485. [Google Scholar] [CrossRef]
- Lu, S.; Chang, C.J.; Guan, Y.; Szafer-Glusman, E.; Punnoose, E.; Do, A.; Suttmann, B.; Gagnon, R.; Rodriguez, A.; Landers, M.; et al. Genomic Analysis of Circulating Tumor Cells at the Single-Cell Level. J. Mol. Diagn. 2020, 22, 770–781. [Google Scholar] [CrossRef]
- Kojima, M.; Harada, T.; Fukazawa, T.; Kurihara, S.; Saeki, I.; Takahashi, S.; Hiyama, E. Single-cell DNA and RNA sequencing of circulating tumor cells. Sci. Rep. 2021, 11, 22864. [Google Scholar] [CrossRef]
- Li, M.; Wu, S.; Zhuang, C.; Shi, C.; Gu, L.; Wang, P.; Guo, F.; Wang, Y.; Liu, Z. Metabolomic analysis of circulating tumor cells derived liver metastasis of colorectal cancer. Heliyon 2023, 9, e12515. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Liu, Q.; Liang, D.; Guo, Y.; Liu, G.; Ren, J.; He, Y.; Shan, B. Circulating Tumor Cell and Metabolites as Novel Biomarkers for Early-Stage Lung Cancer Diagnosis. Front. Oncol. 2021, 11, 630672. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Yang, X.; Li, Y.; Zhao, P.; Fu, R.; Ren, T.; Hu, P.; Wu, Y.; Yang, H.; Guo, N. Clinical significance of circulating tumor cells and metabolic signatures in lung cancer after surgical removal. J. Transl. Med. 2020, 18, 243. [Google Scholar] [CrossRef] [PubMed]
- Abouleila, Y.; Onidani, K.; Ali, A.; Shoji, H.; Kawai, T.; Lim, C.T.; Kumar, V.; Okaya, S.; Kato, K.; Hiyama, E.; et al. Live single cell mass spectrometry reveals cancer-specific metabolic profiles of circulating tumor cells. Cancer Sci. 2019, 110, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Arbelaiz, A.; Azkargorta, M.; Krawczyk, M.; Santos-Laso, A.; Lapitz, A.; Perugorria, M.J.; Erice, O.; Gonzalez, E.; Jimenez-Aguero, R.; Lacasta, A.; et al. Serum extracellular vesicles contain protein biomarkers for primary sclerosing cholangitis and cholangiocarcinoma. Hepatology 2017, 66, 1125–1143. [Google Scholar] [CrossRef]
- Shiromizu, T.; Kume, H.; Ishida, M.; Adachi, J.; Kano, M.; Matsubara, H.; Tomonaga, T. Quantitation of putative colorectal cancer biomarker candidates in serum extracellular vesicles by targeted proteomics. Sci. Rep. 2017, 7, 12782. [Google Scholar] [CrossRef]
- Vinik, Y.; Ortega, F.G.; Mills, G.B.; Lu, Y.; Jurkowicz, M.; Halperin, S.; Aharoni, M.; Gutman, M.; Lev, S. Proteomic analysis of circulating extracellular vesicles identifies potential markers of breast cancer progression, recurrence, and response. Sci. Adv. 2020, 6, eaba5714. [Google Scholar] [CrossRef]
- Gurudatt, N.G.; Gwak, H.; Hyun, K.-A.; Jeong, S.-E.; Lee, K.; Park, S.; Chung, M.J.; Kim, S.-E.; Jo, J.H.; Jung, H.-I. Electrochemical detection and analysis of tumor-derived extracellular vesicles to evaluate malignancy of pancreatic cystic neoplasm using integrated microfluidic device. Biosens. Bioelectron. 2023, 226, 115124. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, J.; Zhang, Z.; Wuethrich, A.; Lobb, R.J.; Trau, M. Tracking the EMT-like phenotype switching during targeted therapy in melanoma by analyzing extracellular vesicle phenotypes. Biosens. Bioelectron. 2024, 244, 115819. [Google Scholar] [CrossRef]
- Lee, S.E.; Park, H.Y.; Hur, J.Y.; Kim, H.J.; Kim, I.A.; Kim, W.S.; Lee, K.Y. Genomic profiling of extracellular vesicle-derived DNA from bronchoalveolar lavage fluid of patients with lung adenocarcinoma. Transl. Lung Cancer Res. 2021, 10, 104–116. [Google Scholar] [CrossRef]
- Vitale, S.R.; Helmijr, J.A.; Gerritsen, M.; Coban, H.; van Dessel, L.F.; Beije, N.; van der Vlugt-Daane, M.; Vigneri, P.; Sieuwerts, A.M.; Dits, N.; et al. Detection of tumor-derived extracellular vesicles in plasma from patients with solid cancer. BMC Cancer 2021, 21, 315. [Google Scholar] [CrossRef] [PubMed]
- Shi, A.; Kasumova, G.G.; Michaud, W.A.; Cintolo-Gonzalez, J.; Díaz-Martínez, M.; Ohmura, J.; Mehta, A.; Chien, I.; Frederick, D.T.; Cohen, S.; et al. Plasma-derived extracellular vesicle analysis and deconvolution enable prediction and tracking of melanoma checkpoint blockade outcome. Sci. Adv. 2020, 6, eabb3461. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Tan, Z.; Zhang, J.; An, M.; Khaykin, V.M.; Cuneo, K.C.; Parikh, N.D.; Lubman, D.M. Sequential Method for Analysis of CTCs and Exosomes from the Same Sample of Patient Blood. ACS Omega 2022, 7, 37581–37588. [Google Scholar] [CrossRef]
- Paul, I.; Bolzan, D.; Youssef, A.; Gagnon, K.A.; Hook, H.; Karemore, G.; Oliphant, M.U.J.; Lin, W.; Liu, Q.; Phanse, S.; et al. Parallelized multidimensional analytic framework applied to mammary epithelial cells uncovers regulatory principles in EMT. Nat. Commun. 2023, 14, 688. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.F.; Ten Dijke, P.; Zhu, H.J. On-Target Anti-TGF-β Therapies Are Not Succeeding in Clinical Cancer Treatments: What Are Remaining Challenges? Front. Cell Dev. Biol. 2020, 8, 605. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.L.; Balasas, T.; Callaghan, J.; Coombes, R.C.; Evans, J.; Hall, J.A.; Kinrade, S.; Jones, D.; Jones, P.S.; Jones, R.; et al. A framework for the development of effective anti-metastatic agents. Nat. Rev. Clin. Oncol. 2019, 16, 185–204. [Google Scholar] [CrossRef]
- Fonseca Teixeira, A.; Iaria, J.; Zhu, H.J. Fast Quantitation of TGF-β Signaling Using Adenoviral Reporter. Methods Mol. Biol. 2022, 2488, 13–22. [Google Scholar] [CrossRef]
- Luwor, R.B.; Wang, B.; Nheu, T.V.; Iaria, J.; Tsantikos, E.; Hibbs, M.L.; Sieber, O.M.; Zhu, H.J. New reagents for improved in vitro and in vivo examination of TGF-β signalling. Growth Factors 2011, 29, 211–218. [Google Scholar] [CrossRef]
- Chen, H.; Ware, T.M.B.; Iaria, J.; Zhu, H.J. Live Cell Imaging of the TGF- β/Smad3 Signaling Pathway In Vitro and In Vivo Using an Adenovirus Reporter System. J. Vis. Exp. 2018, 137, e57926. [Google Scholar] [CrossRef]
- Liu, S.; Iaria, J.; Simpson, R.J.; Zhu, H.J. Ras enhances TGF-β signaling by decreasing cellular protein levels of its type II receptor negative regulator SPSB1. Cell Commun. Signal. 2018, 16, 10. [Google Scholar] [CrossRef]
- Moustakas, A.; Heldin, C.-H. Signaling networks guiding epithelial–mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci. 2007, 98, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Morgani, S.M.; David, C.J.; Wang, Q.; Er, E.E.; Huang, Y.-H.; Basnet, H.; Zou, Y.; Shu, W.; Soni, R.K.; et al. TGF-β orchestrates fibrogenic and developmental EMTs via the RAS effector RREB1. Nature 2020, 577, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Budi, E.H.; Muthusamy, B.-P.; Derynck, R. The insulin response integrates increased TGF-β signaling through Akt-induced enhancement of cell surface delivery of TGF-β receptors. Sci. Signal. 2015, 8, ra96. [Google Scholar] [CrossRef] [PubMed]
- Hua, W.; ten Dijke, P.; Kostidis, S.; Giera, M.; Hornsveld, M. TGFβ-induced metabolic reprogramming during epithelial-to-mesenchymal transition in cancer. Cell. Mol. Life Sci. 2020, 77, 2103–2123. [Google Scholar] [CrossRef]
- Hua, W.; Kostidis, S.; Mayboroda, O.; Giera, M.; Hornsveld, M.; ten Dijke, P. Metabolic Reprogramming of Mammary Epithelial Cells during TGF-β-Induced Epithelial-to-Mesenchymal Transition. Metabolites 2021, 11, 626. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).