Cytotoxic Effect Induced by Sicilian Oregano Essential Oil in Human Breast Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Harvest of Plant Material
2.3. Essential Oil Extraction
2.4. Chemicals and Reagents
2.5. Cell Cultures
2.6. Assessment of Cell Viability and Morphology
2.7. Evaluation of the Mitochondrial Membrane Potential
2.8. Intracellular Calcium Assessment
2.9. Western Blotting Analysis
2.10. Intracellular ROS
2.11. DPPH Assay
2.12. Isolation of Volatile Components
2.13. X.2. GC–MS Analysis
2.14. Statistical Analysis
3. Results
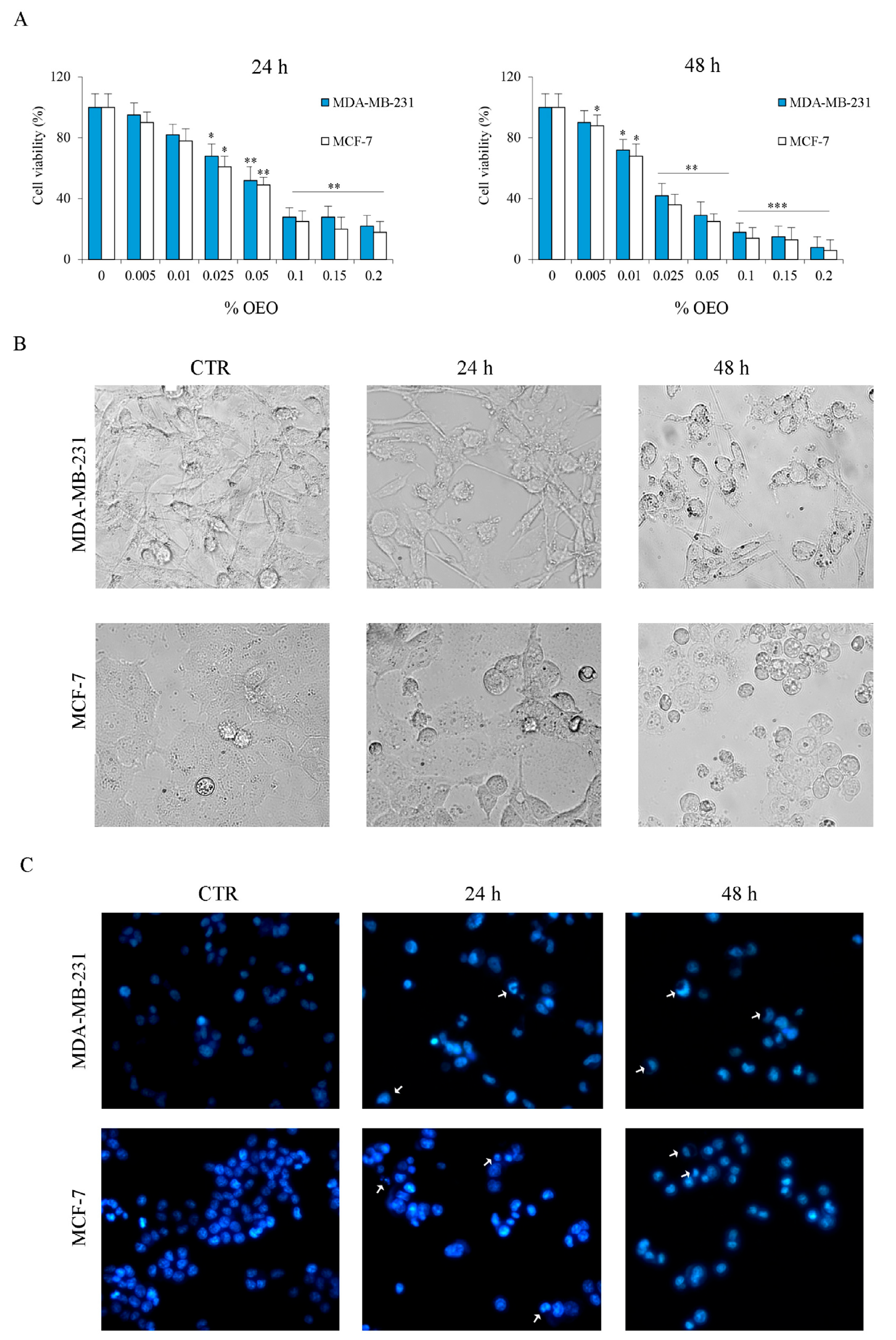
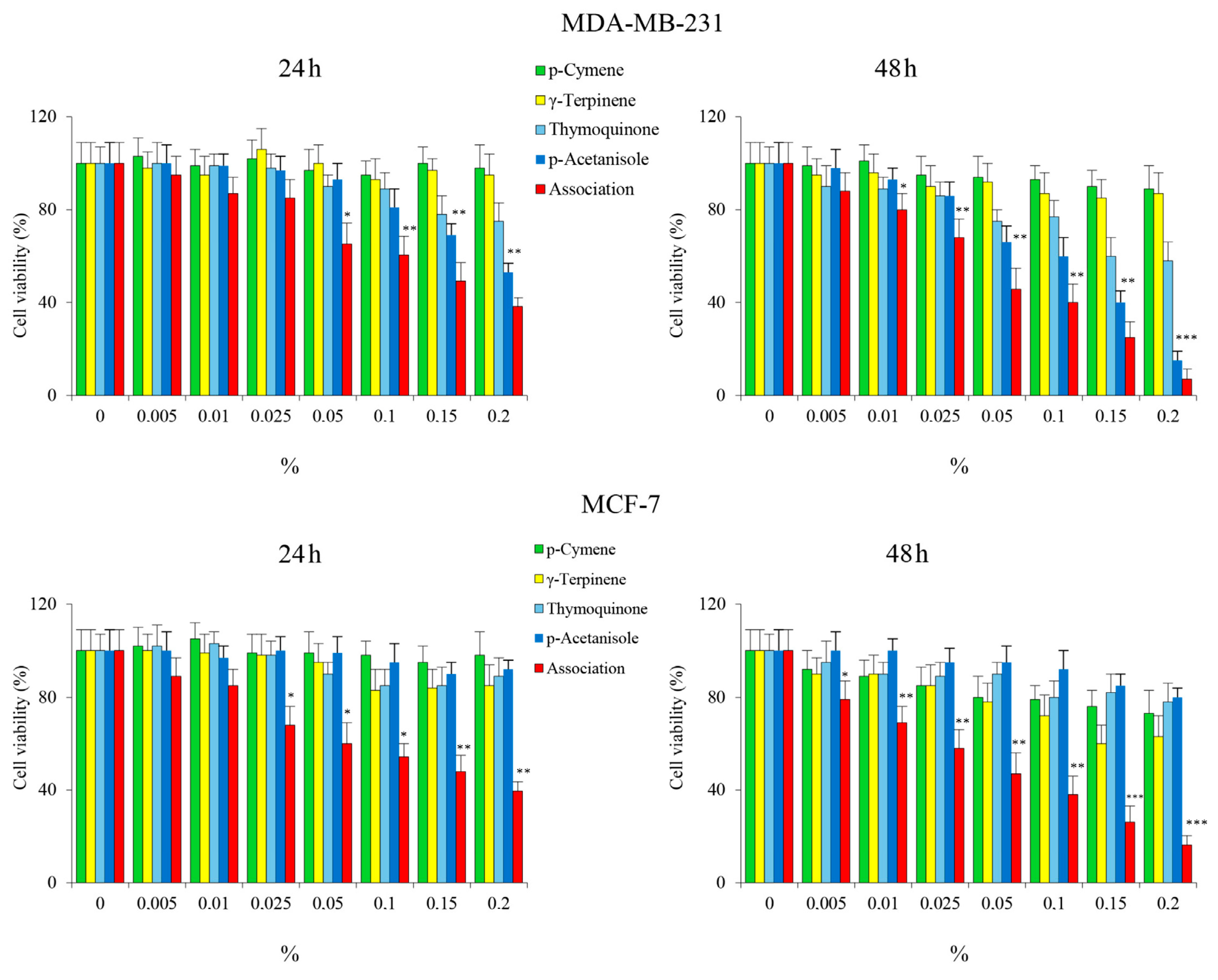
3.1. The Effects of Oregano Essential Oil (OEO) on Cell Viability
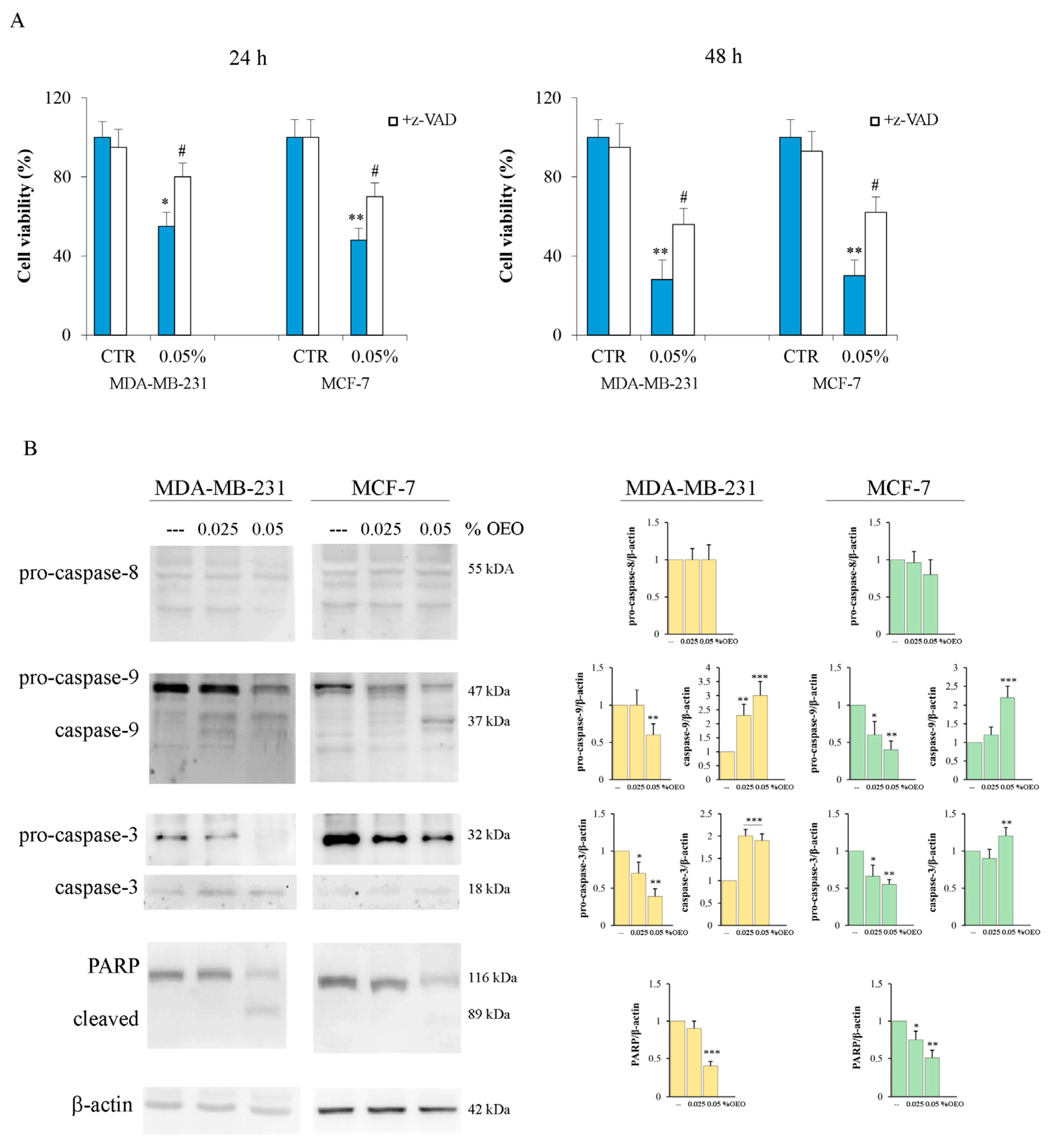
3.2. The Effects of Oregano Essential Oil (OEO) on Caspase Activation
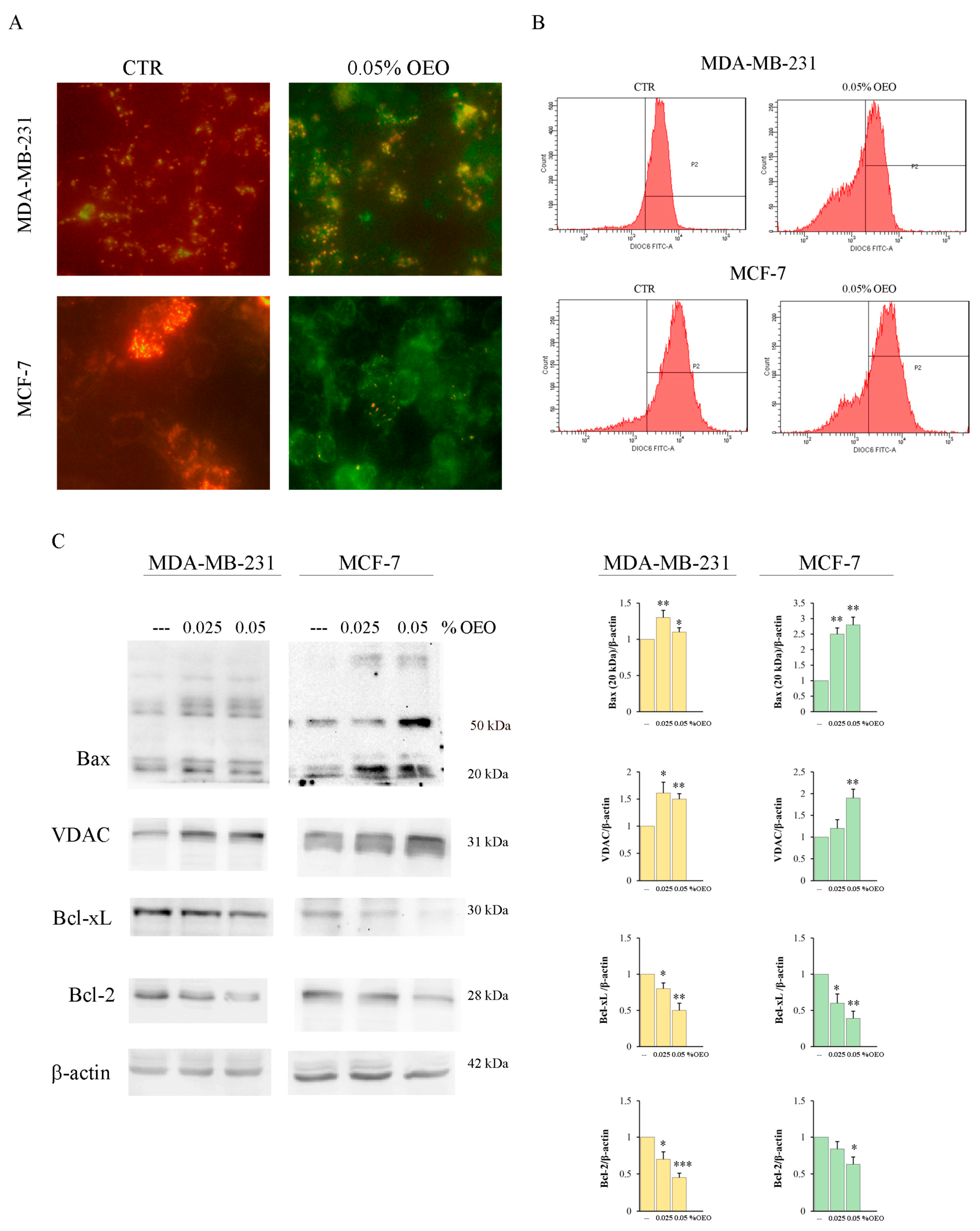
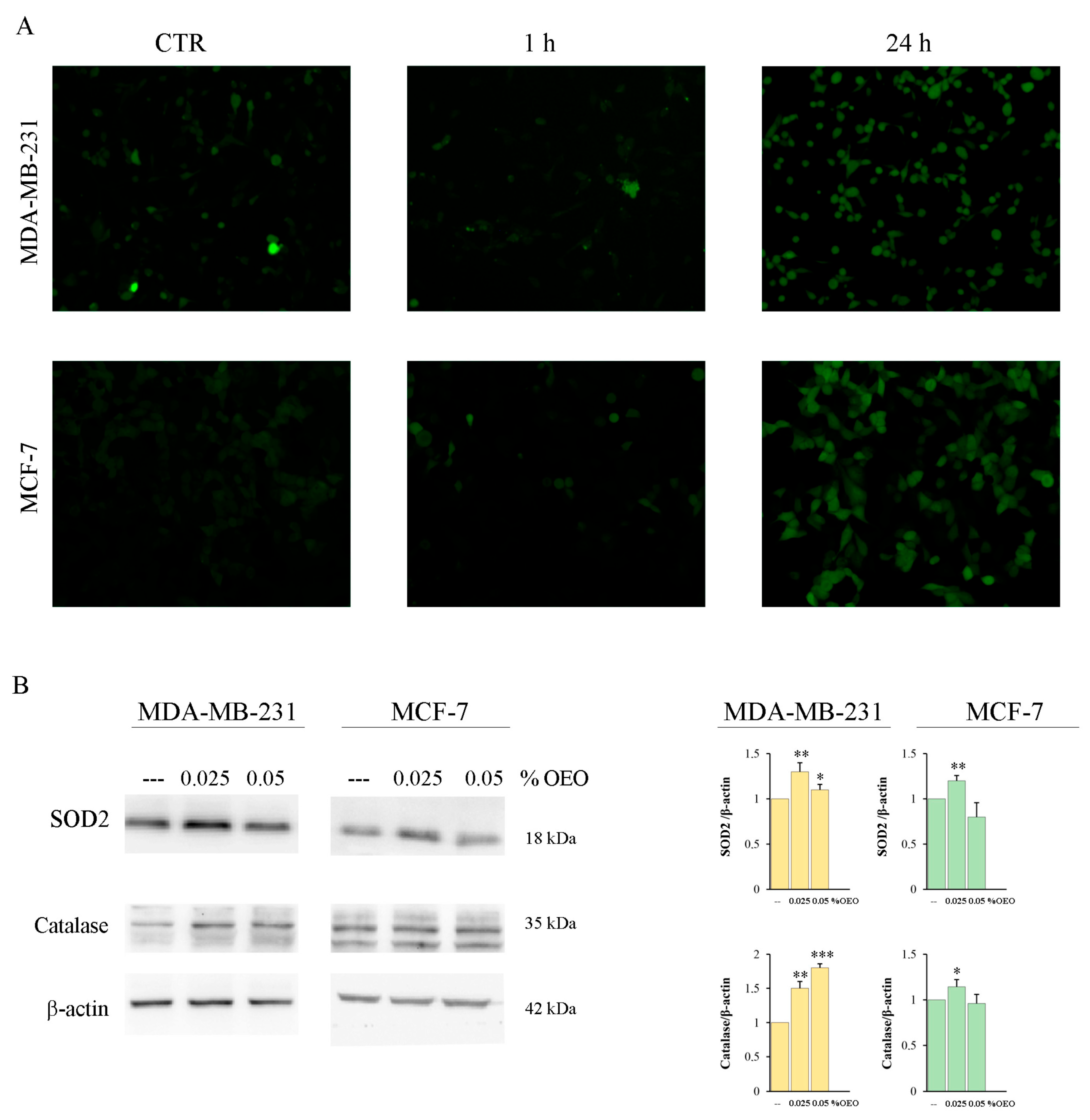
3.3. OEO Effects on Mitochondria and Bcl-2 Family Members
3.4. OEO Effect on ROS Production and Intracellular Ca2+ Level
3.5. Evaluation of OEO Composition and Effect on Cell Viability of the Main Constituents
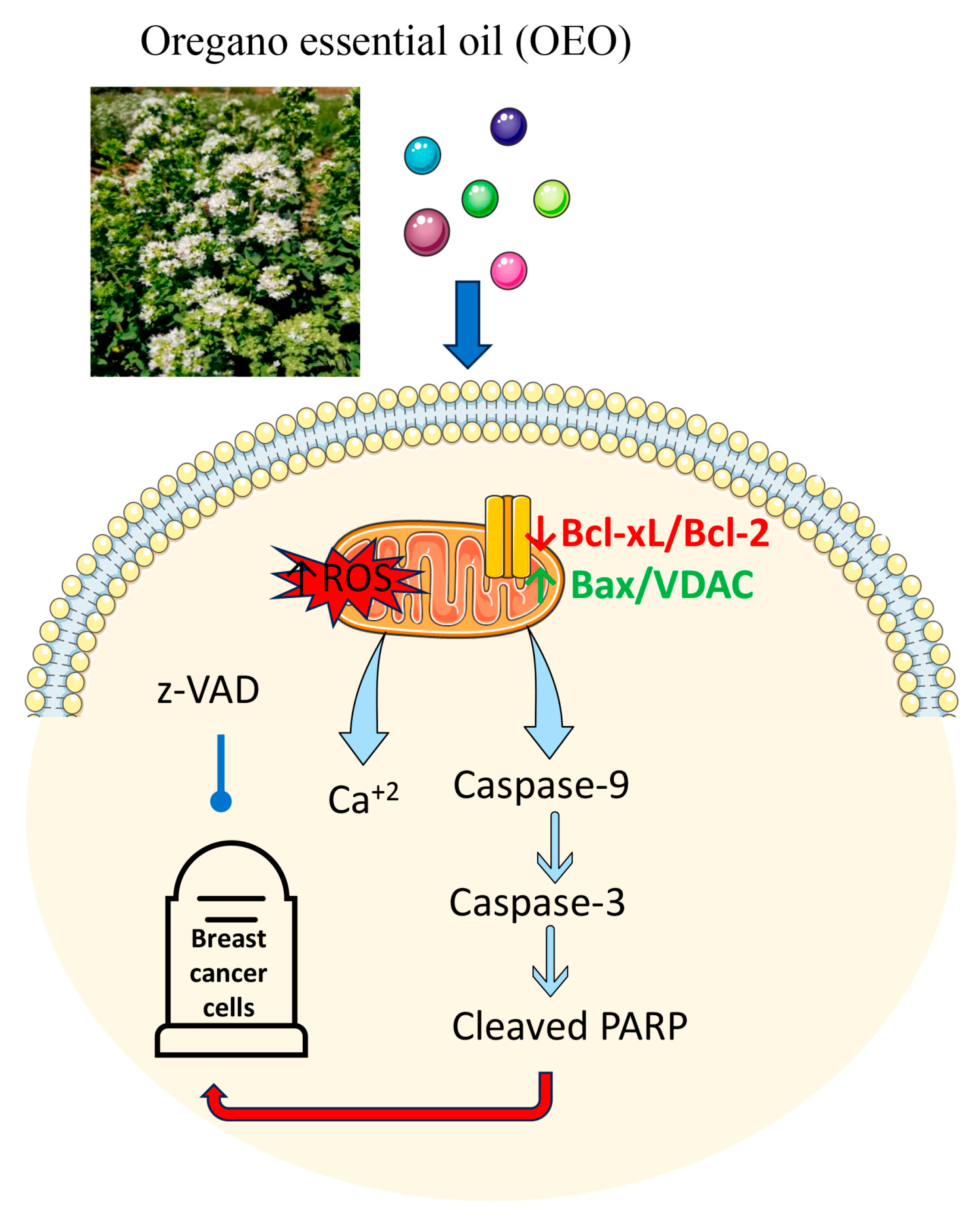
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arabaci, T.; Çelenk, S.; Özcan, T.; Martin, E.; Yazici, T.; Açar, M.; Üzel, D.; Dirmenci, T. Homoploid Hybrids of Origanum (Lamiaceae) in Turkey: Morphological and Molecular Evidence for a New Hybrid. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2021, 155, 470–482. [Google Scholar] [CrossRef]
- Harborne, M.S.; Jeffrey, B. The Taxonomy and Chemistry of Origanum. In Oregano; CRC Press: Boca Raton, FL, USA, 2002; ISBN 978-0-429-21897-2. [Google Scholar]
- Lotti, C.; Ricciardi, L.; Rainaldi, G.; Ruta, C.; Tarraf, W.; De Mastro, G. Morphological, Biochemical, and Molecular Analysis of Origanum vulgare L. Open Agric. J. 2019, 13, 116–124. [Google Scholar] [CrossRef]
- Lombrea, A.; Antal, D.; Ardelean, F.; Avram, S.; Pavel, I.Z.; Vlaia, L.; Mut, A.-M.; Diaconeasa, Z.; Dehelean, C.A.; Soica, C.; et al. A Recent Insight Regarding the Phytochemistry and Bioactivity of Origanum vulgare L. Essential Oil. Int. J. Mol. Sci. 2020, 21, 9653. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Nieto, G. Biological Activities of Three Essential Oils of the Lamiaceae Family. Medicines 2017, 4, 63. [Google Scholar] [CrossRef] [PubMed]
- Licata, M.; Tuttolomondo, T.; Dugo, G.; Ruberto, G.; Leto, C.; Napoli, E.; Rando, R.; Fede, M.; Virga, G.; Leone, R.; et al. Study of Quantitative and Qualitative Variations in Essential Oils of Sicilian Oregano Biotypes. J. Essent. Oil Res. 2015, 27, 293–306. [Google Scholar] [CrossRef]
- Tuttolomondo, T.; Dugo, G.; Ruberto, G.; Leto, C.; Napoli, E.M.; Cicero, N.; Gervasi, T.; Virga, G.; Leone, R.; Licata, M.; et al. Study of Quantitative and Qualitative Variations in Essential Oils of Sicilian Rosmarinus officinalis L. Nat. Prod. Res. 2015, 29, 1928–1934. [Google Scholar] [CrossRef]
- Virga, G.; Sabatino, L.; Licata, M.; Tuttolomondo, T.; Leto, C.; La Bella, S. Effects of Irrigation with Different Sources of Water on Growth, Yield and Essential Oil Compounds in Oregano. Plants 2020, 9, 1618. [Google Scholar] [CrossRef]
- D’Antuono, L. Variability of Essential Oil Content and Composition of Origanum vulgare L. Populations from a North Mediterranean Area (Liguria Region, Northern Italy). Ann. Bot. 2000, 86, 471–478. [Google Scholar] [CrossRef]
- Sen, T.; Samanta, S.K. Medicinal Plants, Human Health and Biodiversity: A Broad Review. In Biotechnological Applications of Biodiversity; Mukherjee, J., Ed.; Advances in Biochemical Engineering/Biotechnology; Springer: Berlin, Heidelberg, 2015; pp. 59–110. ISBN 978-3-662-45097-0. [Google Scholar]
- De Blasio, A.; D’Anneo, A.; Lauricella, M.; Emanuele, S.; Giuliano, M.; Pratelli, G.; Calvaruso, G.; Carlisi, D. The Beneficial Effects of Essential Oils in Anti-Obesity Treatment. Int. J. Mol. Sci. 2021, 22, 11832. [Google Scholar] [CrossRef]
- Ramsey, J.T.; Shropshire, B.C.; Nagy, T.R.; Chambers, K.D.; Li, Y.; Korach, K.S. Essential Oils and Health. Yale J. Biol. Med. 2020, 93, 291–305. [Google Scholar] [PubMed]
- Elshafie, H.S.; Armentano, M.F.; Carmosino, M.; Bufo, S.A.; De Feo, V.; Camele, I. Cytotoxic Activity of Origanum vulgare L. on Hepatocellular Carcinoma Cell Line HepG2 and Evaluation of Its Biological Activity. Molecules 2017, 22, 1435. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garcia, I.; Silva-Espinoza, B.A.; Ortega-Ramirez, L.A.; Leyva, J.M.; Siddiqui, M.W.; Cruz-Valenzuela, M.R.; Gonzalez-Aguilar, G.A.; Ayala-Zavala, J.F. Oregano Essential Oil as an Antimicrobial and Antioxidant Additive in Food Products. Crit. Rev. Food Sci. Nutr. 2016, 56, 1717–1727. [Google Scholar] [CrossRef] [PubMed]
- Balusamy, S.R.; Perumalsamy, H.; Huq, M.A.; Balasubramanian, B. Anti-Proliferative Activity of Origanum vulgare Inhibited Lipogenesis and Induced Mitochondrial Mediated Apoptosis in Human Stomach Cancer Cell Lines. Biomed. Pharmacother. 2018, 108, 1835–1844. [Google Scholar] [CrossRef] [PubMed]
- Bunse, M.; Daniels, R.; Gründemann, C.; Heilmann, J.; Kammerer, D.R.; Keusgen, M.; Lindequist, U.; Melzig, M.F.; Morlock, G.E.; Schulz, H.; et al. Essential Oils as Multicomponent Mixtures and Their Potential for Human Health and Well-Being. Front. Pharmacol. 2022, 13, 956541. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, H.; Papadopoulou, C. Antimicrobial Activity of Basil, Oregano, and Thyme Essential Oils. J. Microbiol. Biotechnol. 2017, 27, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, E989. [Google Scholar] [CrossRef]
- Hać-Szymańczuk, E.; Cegiełka, A.; Karkos, M.; Gniewosz, M.; Piwowarek, K. Evaluation of Antioxidant and Antimicrobial Activity of Oregano (Origanum vulgare L.) Preparations during Storage of Low-Pressure Mechanically Separated Meat (BAADER Meat) from Chickens. Food Sci. Biotechnol. 2018, 28, 449–457. [Google Scholar] [CrossRef]
- Kubatka, P.; Kello, M.; Kajo, K.; Kruzliak, P.; Výbohová, D.; Mojžiš, J.; Adamkov, M.; Fialová, S.; Veizerová, L.; Zulli, A.; et al. Oregano Demonstrates Distinct Tumour-Suppressive Effects in the Breast Carcinoma Model. Eur. J. Nutr. 2017, 56, 1303–1316. [Google Scholar] [CrossRef]
- Berrington, D.; Lall, N. Anticancer Activity of Certain Herbs and Spices on the Cervical Epithelial Carcinoma (HeLa) Cell Line. Evid. Based Complement. Altern. Med. 2012, 2012, 564927. [Google Scholar] [CrossRef]
- Altuntaş, F.Ö.; Demirtaş, İ. Real-Time Cell Analysis of the Cytotoxicity of Origanum Acutidens Essential Oil on HT-29 and HeLa Cell Lines. Turk. J. Pharm. Sci. 2017, 14, 29–33. [Google Scholar] [CrossRef]
- European Commission. European Pharmacopoeia, 5th ed.; EDQM: Strasbourg, France, 2004; Volume 5. [Google Scholar]
- Pratelli, G.; Carlisi, D.; D’Anneo, A.; Maggio, A.; Emanuele, S.; Palumbo Piccionello, A.; Giuliano, M.; De Blasio, A.; Calvaruso, G.; Lauricella, M. Bio-Waste Products of Mangifera indica L. Reduce Adipogenesis and Exert Antioxidant Effects on 3T3-L1 Cells. Antioxidants 2022, 11, 363. [Google Scholar] [CrossRef]
- Sferrazzo, G.; Palmeri, R.; Vanella, L.; Parafati, L.; Ronsisvalle, S.; Biondi, A.; Basile, F.; Li Volti, G.; Barbagallo, I. Mangifera indica L. Leaf Extract Induces Adiponectin and Regulates Adipogenesis. Int. J. Mol. Sci. 2019, 20, 3211. [Google Scholar] [CrossRef] [PubMed]
- Basile, S.; Badalamenti, N.; Riccobono, O.; Guarino, S.; Ilardi, V.; Bruno, M.; Peri, E. Chemical Composition and Evaluation of Insecticidal Activity of Calendula incana Subsp. Maritima and Laserpitium siler Subsp. Siculum Essential Oils against Stored Products Pests. Molecules 2022, 27, 588. [Google Scholar] [CrossRef] [PubMed]
- Bancheva, S.; Badalamenti, N.; Fontana, G.; Catinella, G.; Porrello, A.; Bruno, M. Chemical Composition of the Essential Oil of Cyanus adscendens (Bartl.) Soják and C. Orbelicus (Velen.) Soják Growing Wild in Bulgaria, and PCA Analysis of Genus Cyanus Mill. Nat. Prod. Res. 2023, 37, 3588–3594. [Google Scholar] [CrossRef] [PubMed]
- Timmer, J.C.; Salvesen, G.S. Caspase Substrates. Cell Death Differ. 2007, 14, 66–72. [Google Scholar] [CrossRef]
- D’Anneo, A.; Carlisi, D.; Lauricella, M.; Emanuele, S.; Di Fiore, R.; Vento, R.; Tesoriere, G. Parthenolide Induces Caspase-Independent and AIF-Mediated Cell Death in Human Osteosarcoma and Melanoma Cells. J. Cell. Physiol. 2013, 228, 952–967. [Google Scholar] [CrossRef] [PubMed]
- Kushnareva, Y.E.; Sokolove, P.M. Prooxidants Open Both the Mitochondrial Permeability Transition Pore and a Low-Conductance Channel in the Inner Mitochondrial Membrane. Arch. Biochem. Biophys. 2000, 376, 377–388. [Google Scholar] [CrossRef]
- Narita, M.; Shimizu, S.; Ito, T.; Chittenden, T.; Lutz, R.J.; Matsuda, H.; Tsujimoto, Y. Bax Interacts with the Permeability Transition Pore to Induce Permeability Transition and Cytochrome c Release in Isolated Mitochondria. Proc. Natl. Acad. Sci. USA 1998, 95, 14681–14686. [Google Scholar] [CrossRef]
- Shimizu, S.; Narita, M.; Tsujimoto, Y. Bcl-2 Family Proteins Regulate the Release of Apoptogenic Cytochrome c by the Mitochondrial Channel VDAC. Nature 1999, 399, 483–487. [Google Scholar] [CrossRef]
- Pastorino, J.G.; Tafani, M.; Rothman, R.J.; Marcineviciute, A.; Hoek, J.B.; Farber, J.L. Functional Consequences of the Sustained or Transient Activation by Bax of the Mitochondrial Permeability Transition Pore. J. Biol. Chem. 1999, 274, 31734–31739. [Google Scholar] [CrossRef]
- Ma, S.B.; Nguyen, T.N.; Tan, I.; Ninnis, R.; Iyer, S.; Stroud, D.A.; Menard, M.; Kluck, R.M.; Ryan, M.T.; Dewson, G. Bax Targets Mitochondria by Distinct Mechanisms before or during Apoptotic Cell Death: A Requirement for VDAC2 or Bak for Efficient Bax Apoptotic Function. Cell Death Differ. 2014, 21, 1925–1935. [Google Scholar] [CrossRef]
- Antonsson, B.; Montessuit, S.; Sanchez, B.; Martinou, J.-C. Bax Is Present as a High Molecular Weight Oligomer/Complex in the Mitochondrial Membrane of Apoptotic Cells *. J. Biol. Chem. 2001, 276, 11615–11623. [Google Scholar] [CrossRef] [PubMed]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Reactive Oxygen Species and Cancer Paradox: To Promote or to Suppress? Free. Radic. Biol. Med. 2017, 104, 144–164. [Google Scholar] [CrossRef] [PubMed]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Hu, P.; Tirelli, N. Scavenging ROS: Superoxide Dismutase/Catalase Mimetics by the Use of an Oxidation-Sensitive Nanocarrier/Enzyme Conjugate. Bioconjugate Chem. 2012, 23, 438–449. [Google Scholar] [CrossRef]
- Capiod, T.; Shuba, Y.; Skryma, R.; Prevarskaya, N. Calcium Signalling and Cancer Cell Growth. Subcell Biochem. 2007, 45, 405–427. [Google Scholar] [CrossRef]
- Tuttolomondo, T.; Martinelli, F.; Mariotti, L.; Leto, C.; Maggio, A.; La Bella, S. Agronomic, Metabolomic and Lipidomic Characterisation of Sicilian Origanum vulgare (L.) Ecotypes. Nat. Prod. Res. 2016, 30, 1103–1107. [Google Scholar] [CrossRef] [PubMed]
- Balahbib, A.; El Omari, N.; Hachlafi, N.E.; Lakhdar, F.; El Menyiy, N.; Salhi, N.; Mrabti, H.N.; Bakrim, S.; Zengin, G.; Bouyahya, A. Health Beneficial and Pharmacological Properties of P-Cymene. Food Chem. Toxicol. 2021, 153, 112259. [Google Scholar] [CrossRef]
- Li, J.; Liu, C.; Sato, T. Novel Antitumor Invasive Actions of p-Cymene by Decreasing MMP-9/TIMP-1 Expression Ratio in Human Fibrosarcoma HT-1080 Cells. Biol. Pharm. Bull. 2016, 39, 1247–1253. [Google Scholar] [CrossRef]
- Imran, M.; Rauf, A.; Khan, I.A.; Shahbaz, M.; Qaisrani, T.B.; Fatmawati, S.; Abu-Izneid, T.; Imran, A.; Rahman, K.U.; Gondal, T.A. Thymoquinone: A Novel Strategy to Combat Cancer: A Review. Biomed. Pharmacother. 2018, 106, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, Z.; Zhang, P.; Jiao, Q.; Gu, R.; Qu, L.; Huang, S.; Wang, X.; Liu, Y.; Lai, Y.; et al. Contribution of Constituents in Rosemary-Magnolia Compound Essential Oil to Antibacterial, Antioxidant and Cytotoxicity Bioactivities Revealed by Grey Correlation Analysis. Chem. Biodivers. 2023, 20, e202201179. [Google Scholar] [CrossRef]
- Zochedh, A.; Priya, M.; Shunmuganarayanan, A.; Thandavarayan, K.; Sultan, A.B. Investigation on Structural, Spectroscopic, DFT, Biological Activity and Molecular Docking Simulation of Essential Oil Gamma-Terpinene. J. Mol. Struct. 2022, 1268, 133651. [Google Scholar] [CrossRef]
- Morelli, M.B.; Bongiovanni, C.; Da Pra, S.; Miano, C.; Sacchi, F.; Lauriola, M.; D’Uva, G. Cardiotoxicity of Anticancer Drugs: Molecular Mechanisms and Strategies for Cardioprotection. Front. Cardiovasc. Med. 2022, 9, 847012. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef] [PubMed]
- Kamatou, G.P.P.; Makunga, N.P.; Ramogola, W.P.N.; Viljoen, A.M. South African Salvia Species: A Review of Biological Activities and Phytochemistry. J. Ethnopharmacol. 2008, 119, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Grover, M.; Behl, T.; Sanduja, M.; Rahman, M.H.; Ahmadi, A. Exploring the Potential of Aromatherapy as an Adjuvant Therapy in Cancer and Its Complications: A Comprehensive Update. Anti-Cancer Agents Med. Chem. 2022, 22, 629–653. [Google Scholar] [CrossRef]
- Aziz, Z.A.A.; Ahmad, A.; Setapar, S.H.M.; Karakucuk, A.; Azim, M.M.; Lokhat, D.; Rafatullah, M.; Ganash, M.; Kamal, M.A.; Ashraf, G.M. Essential Oils: Extraction Techniques, Pharmaceutical And Therapeutic Potential—A Review. Curr. Drug Metab. 2018, 19, 1100–1110. [Google Scholar] [CrossRef]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential Oils in Insect Control: Low-Risk Products in a High-Stakes World. Annu. Rev. Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef]
- Machado, T.Q.; da Fonseca, A.C.C.; Duarte, A.B.S.; Robbs, B.K.; de Sousa, D.P. A Narrative Review of the Antitumor Activity of Monoterpenes from Essential Oils: An Update. Biomed. Res. Int. 2022, 2022, 6317201. [Google Scholar] [CrossRef]
- Santos, M.I.S.; Marques, C.; Mota, J.; Pedroso, L.; Lima, A. Applications of Essential Oils as Antibacterial Agents in Minimally Processed Fruits and Vegetables—A Review. Microorganisms 2022, 10, 760. [Google Scholar] [CrossRef] [PubMed]
- La Bella, S.; Tuttolomondo, T.; Dugo, G.; Ruberto, G.; Leto, C.; Napoli, E.M.; Potorti, A.G.; Fede, M.R.; Virga, G.; Leone, R.; et al. Composition and Variability of the Essential Oil of the Flowers of Lavandula Stoechas from Various Geographical Sources. Nat. Prod. Commun. 2015, 10, 2001–2004. [Google Scholar] [CrossRef] [PubMed]
- Barreca, S.; La Bella, S.; Maggio, A.; Licata, M.; Buscemi, S.; Leto, C.; Pace, A.; Tuttolomondo, T. Flavouring Extra-Virgin Olive Oil with Aromatic and Medicinal Plants Essential Oils Stabilizes Oleic Acid Composition during Photo-Oxidative Stress Flavouring Extra-Virgin Olive Oil with Aromatic and Medicinal Plants Essential Oils Stabilizes Oleic Acid Composition during Photo-Oxidative. Agriculture 2021, 11, 266. [Google Scholar] [CrossRef]
- Tuttolomondo, T.; Iapichino, G.; Licata, M.; Virga, G.; Leto, C.; La Bella, S. Agronomic Evaluation and Chemical Characterization of Sicilian Salvia sclarea L. Accessions. Agronomy 2020, 10, 1114. [Google Scholar] [CrossRef]
- Mandal, R.; Barrón, J.C.; Kostova, I.; Becker, S.; Strebhardt, K. Caspase-8: The Double-Edged Sword. Biochim. Biophys. Acta (BBA) Rev. Cancer 2020, 1873, 188357. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.B.; da Silva Bortoleti, B.T.; Tomiotto-Pellissier, F.; Ganaza, A.F.M.; Gonçalves, M.D.; Carloto, A.C.M.; Rodrigues, A.C.J.; Silva, T.F.; Nakazato, G.; Kobayashi, R.K.T.; et al. Synergistic Antileishmanial Effect of Oregano Essential Oil and Silver Nanoparticles: Mechanisms of Action on Leishmania Amazonensis. Pathogens 2023, 12, 660. [Google Scholar] [CrossRef] [PubMed]
- Donovan, M.; Cotter, T.G. Control of Mitochondrial Integrity by Bcl-2 Family Members and Caspase-Independent Cell Death. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2004, 1644, 133–147. [Google Scholar] [CrossRef]
- Danese, A.; Patergnani, S.; Bonora, M.; Wieckowski, M.R.; Previati, M.; Giorgi, C.; Pinton, P. Calcium Regulates Cell Death in Cancer: Roles of the Mitochondria and Mitochondria-Associated Membranes (MAMs). Biochim. Biophys. Acta (BBA) Bioenerg. 2017, 1858, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Villani, R.M.; Wang, H.; Simpson, M.J.; Roberts, M.S.; Tang, M.; Liang, X. The Role of Cellular Reactive Oxygen Species in Cancer Chemotherapy. J. Exp. Clin. Cancer Res. 2018, 37, 266. [Google Scholar] [CrossRef]
- Nagai, K.; Fukuno, S.; Oda, A.; Konishi, H. Protective Effects of Taurine on Doxorubicin-Induced Acute Hepatotoxicity through Suppression of Oxidative Stress and Apoptotic Responses. Anti-Cancer Drugs 2016, 27, 17. [Google Scholar] [CrossRef]
- Barrera, G.; Cucci, M.A.; Grattarola, M.; Dianzani, C.; Muzio, G.; Pizzimenti, S. Control of Oxidative Stress in Cancer Chemoresistance: Spotlight on Nrf2 Role. Antioxidants 2021, 10, 510. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; An, B.; Lin, Y.; Ni, Y.; Zhao, X.; Liang, X. Molecular Mechanisms of ROS-Modulated Cancer Chemoresistance and Therapeutic Strategies. Biomed. Pharmacother. 2023, 165, 115036. [Google Scholar] [CrossRef] [PubMed]
- Pontes-Quero, G.M.; Esteban-Rubio, S.; Pérez Cano, J.; Aguilar, M.R.; Vázquez-Lasa, B. Oregano Essential Oil Micro- and Nanoencapsulation With Bioactive Properties for Biotechnological and Biomedical Applications. Front. Bioeng. Biotechnol. 2021, 9, 703684. [Google Scholar] [CrossRef] [PubMed]
- Diniz do Nascimento, L.; de Moraes, A.A.B.; da Costa, K.S.; Pereira Galúcio, J.M.; Taube, P.S.; Costa, C.M.L.; Neves Cruz, J.; de Aguiar Andrade, E.H.; Faria, L.J.G. de Bioactive Natural Compounds and Antioxidant Activity of Essential Oils from Spice Plants: New Findings and Potential Applications. Biomolecules 2020, 10, E988. [Google Scholar] [CrossRef]
- Rodriguez, S.A.; Murray, A.P. Antioxidant Activity and Chemical Composition of Essential Oil from Atriplex Undulata. Nat. Prod. Commun. 2010, 5, 1841–1844. [Google Scholar] [CrossRef]


| 24 h | 48 h | ||
|---|---|---|---|
| MDA-MB-231 | MCF-7 | MDA-MB-231 | MCF-7 |
| 0.0542% | 0.04% | 0.022% | 0.015% |
| Sample | Absorbance (517 nm) | % of Antioxidant Activity |
|---|---|---|
| DPPH | 0.41 | -- |
| 0.025% OEO | 0.30 ± 0.05 | 26.82% |
| 0.05% OEO | 0.23 ± 0.07 | 43.9% |
| a KI | b AI | Compounds | RT | BIO59 (%) | |
|---|---|---|---|---|---|
| 1 | 927 | 922 | α-Thujene | 12.853 | 1.2 |
| 2 | 932 | 927 | α-Pinene | 13.153 | 0.9 |
| 3 | 946 | 940 | Camphene | 14.053 | t |
| 4 | 974 | 969 | Sabinene | 16.002 | 0.32 |
| 5 | 995 | 994 | Myrcene | 17.652 | 2.3 |
| 6 | 1004 | 1003 | δ-3-Carene | 18.302 | 0.89 |
| 7 | 1021 | 1018 | α-Terpinene | 19.351 | 4.16 |
| 8 | 1037 | 1033 | p-Cymene | 20.451 | 8.6 |
| 9 | 1047 | 1043 | α-Phellandrene | 21.151 | 0.93 |
| 10 | 1075 | 1072 | γ-Terpinene | 23.250 | 10.5 |
| 11 | 1079 | 1076 | cis-sabinene hydrate | 23.550 | t |
| 12 | 1090 | 1089 | Terpinolene | 24.500 | 0.64 |
| 13 | 1114 | 1113 | Dehydrosabinaketone | 26.200 | 1.15 |
| 14 | 1135 | 1133 | allo-Ocimene | 27.649 | 0.51 |
| 15 | 1187 | 1185 | Terpinen-4-olo | 31.448 | 1.3 |
| 16 | 1220 | 1219 | Sabinene hydrate acetate | 33.798 | 0.36 |
| 17 | 1243 | 1241 | Carvacrol, methyl ether | 35.297 | 4.2 |
| 18 | 1255 | 1253 | Thymoquinone | 36,147 | 5.12 |
| 19 | 1348 | 1346 | p-Acetanisole | 42.395 | 21 |
| 20 | 1354 | 1366 | Thymol acetate | 43.745 | 0.27 |
| 21 | 1371 | 1369 | Carvacrol acetate | 43.945 | 0.78 |
| 22 | 1416 | 1400 | β-Longipinene | 46.894 | 3.63 |
| 23 | 1453 | 1405 | Italicene | 49.093 | 0.29 |
| 24 | 1477 | 1407 | Longifolene | 50.593 | 1.05 |
| 25 | 1510 | 1509 | Germacrene A | 52.642 | 2.65 |
| 26 | 1523 | 1522 | γ-Cadinene | 53.392 | 5.13 |
| Compounds | 24 h | 48 h | ||
|---|---|---|---|---|
| MDA-MB-231 | MCF-7 | MDA-MB-231 | MCF-7 | |
| p-Cymene | 0.4% | 0.35% | 0.28% | 0.22% |
| γ-Terpinene | 0.35% | 0.31% | 0.31% | 0.28% |
| Thymoquinone | 0.54% | 0.57% | 0.22% | 0.31% |
| p-Acetanisole | 0.04% | 0.28% | 0.025 | 0.12% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Liberto, D.; Iacuzzi, N.; Pratelli, G.; Porrello, A.; Maggio, A.; La Bella, S.; De Blasio, A.; Notaro, A.; D’Anneo, A.; Emanuele, S.; et al. Cytotoxic Effect Induced by Sicilian Oregano Essential Oil in Human Breast Cancer Cells. Cells 2023, 12, 2733. https://doi.org/10.3390/cells12232733
Di Liberto D, Iacuzzi N, Pratelli G, Porrello A, Maggio A, La Bella S, De Blasio A, Notaro A, D’Anneo A, Emanuele S, et al. Cytotoxic Effect Induced by Sicilian Oregano Essential Oil in Human Breast Cancer Cells. Cells. 2023; 12(23):2733. https://doi.org/10.3390/cells12232733
Chicago/Turabian StyleDi Liberto, Diana, Nicolò Iacuzzi, Giovanni Pratelli, Antonella Porrello, Antonella Maggio, Salvatore La Bella, Anna De Blasio, Antonietta Notaro, Antonella D’Anneo, Sonia Emanuele, and et al. 2023. "Cytotoxic Effect Induced by Sicilian Oregano Essential Oil in Human Breast Cancer Cells" Cells 12, no. 23: 2733. https://doi.org/10.3390/cells12232733
APA StyleDi Liberto, D., Iacuzzi, N., Pratelli, G., Porrello, A., Maggio, A., La Bella, S., De Blasio, A., Notaro, A., D’Anneo, A., Emanuele, S., Affranchi, F., Giuliano, M., Lauricella, M., & Carlisi, D. (2023). Cytotoxic Effect Induced by Sicilian Oregano Essential Oil in Human Breast Cancer Cells. Cells, 12(23), 2733. https://doi.org/10.3390/cells12232733