Life-Cycle-Dependent Toxicities of Mono- and Bifunctional Alkylating Agents in the 3R-Compliant Model Organism C. elegans
Abstract
1. Introduction
2. Materials and Methods
2.1. C. elegans Maintenance
2.2. Treatment with Mustards
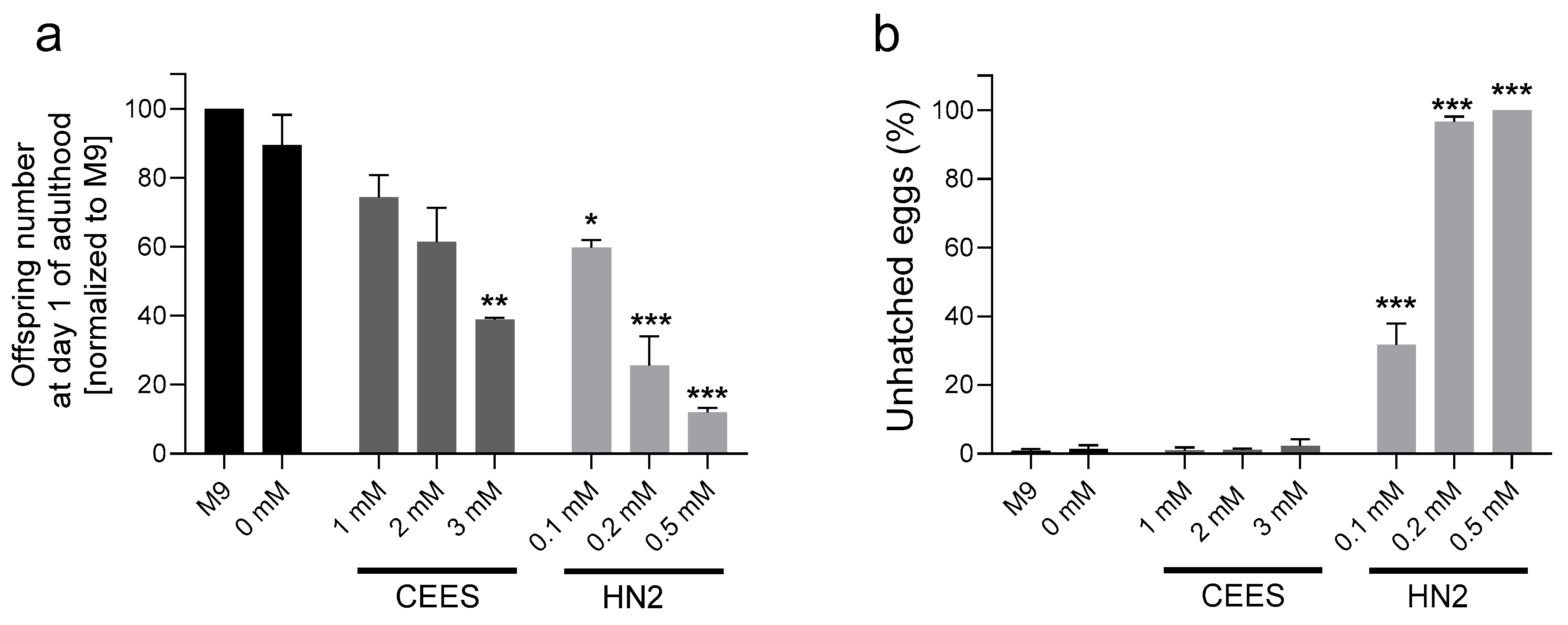
2.3. Survival and Reproduction
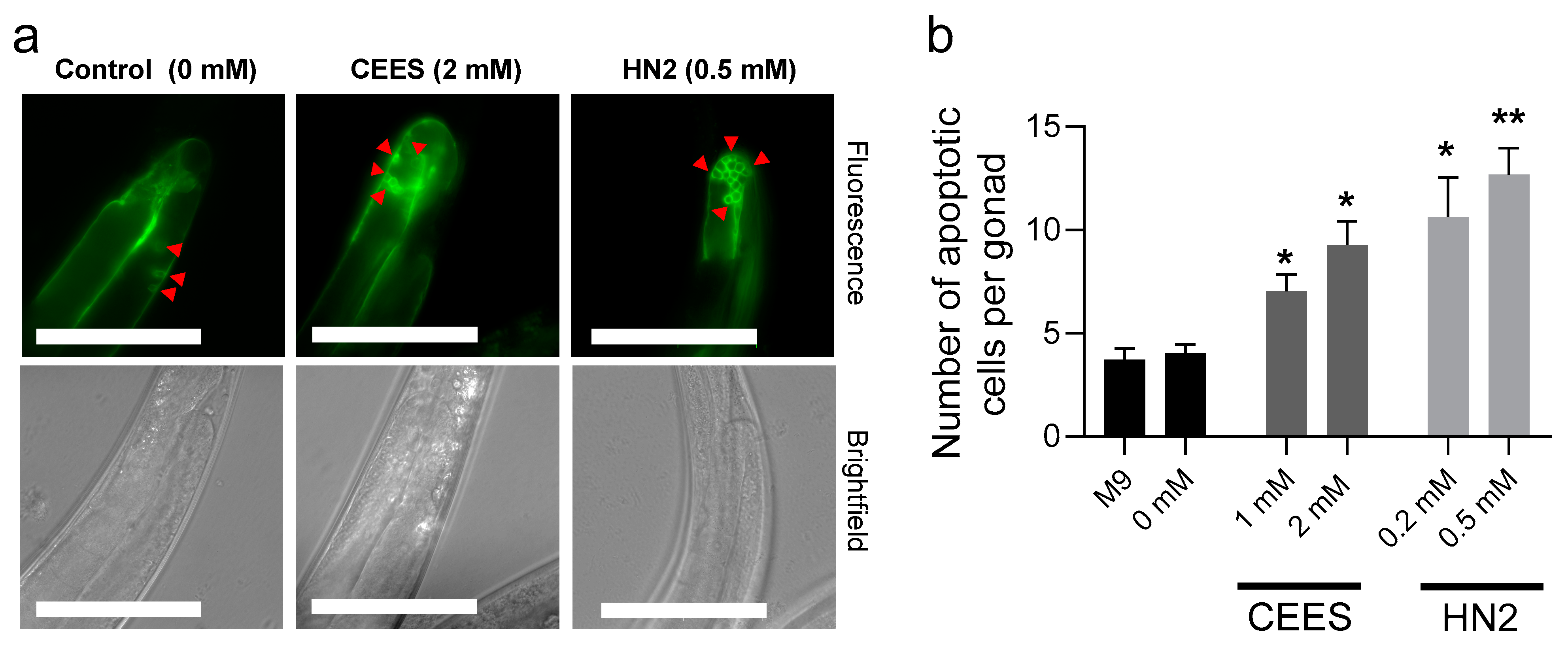
2.4. Germline Apoptosis
2.5. Neurotoxicity
2.6. NAD+ Levels
2.7. Statistical Analysis
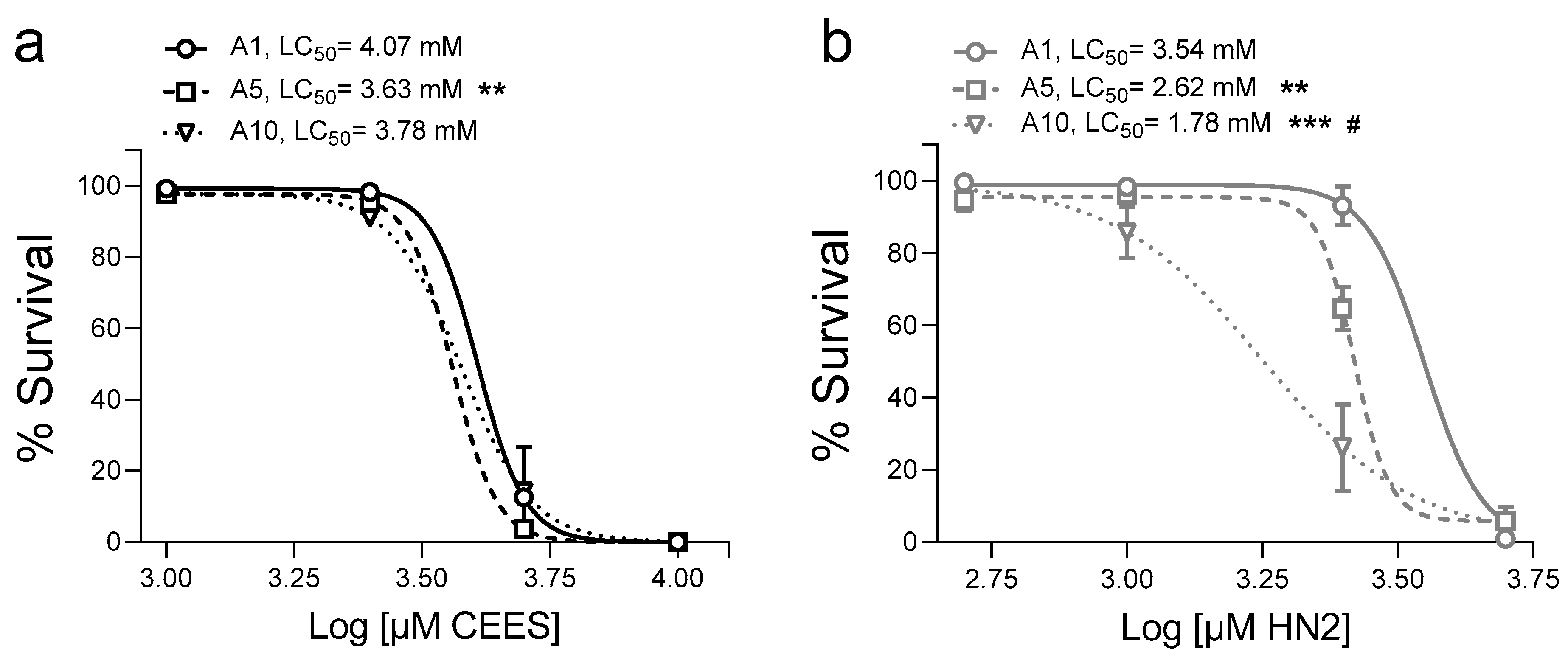
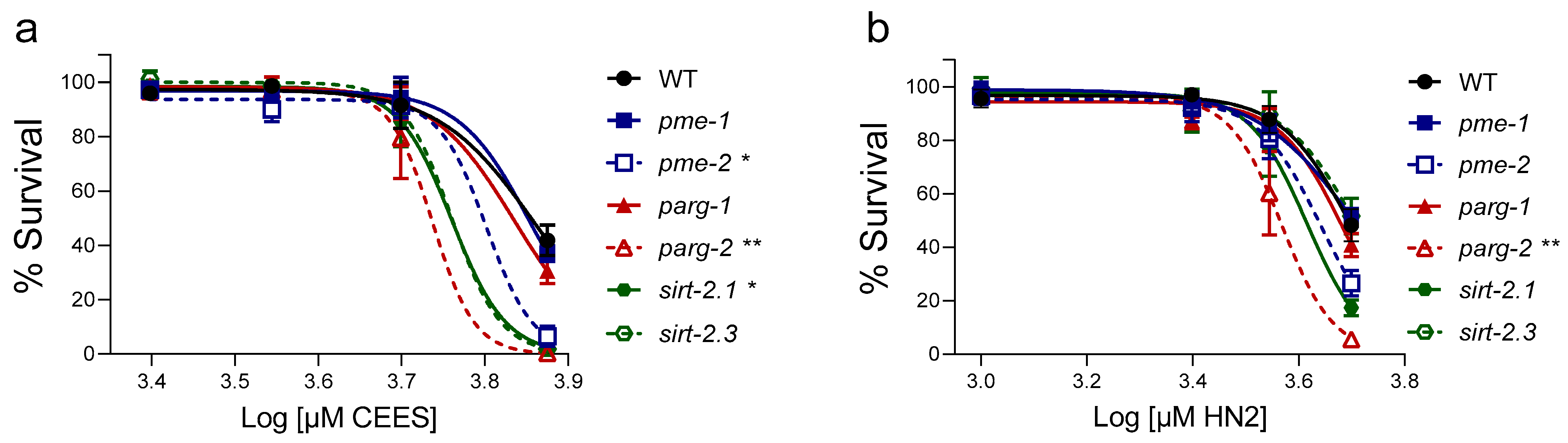
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mack, H.I.D.; Heimbucher, T.; Murphy, C.T. The nematode Caenorhabditis elegans as a model for aging research. Drug Discov. Today Dis. Models 2018, 27, 3–13. [Google Scholar] [CrossRef]
- Corsi, A.K.; Wightman, B.; Chalfie, M. A Transparent Window into Biology: A Primer on Caenorhabditis elegans. Genetics 2015, 200, 387–407. [Google Scholar] [CrossRef]
- Honnen, S. Caenorhabditis elegans as a powerful alternative model organism to promote research in genetic toxicology and biomedicine. Arch. Toxicol. 2017, 91, 2029–2044. [Google Scholar] [CrossRef]
- Hunt, P.R. The C. elegans model in toxicity testing. J. Appl. Toxicol. 2017, 37, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Tejeda-Benitez, L.; Olivero-Verbel, J. Caenorhabditis elegans, a Biological Model for Research in Toxicology. Rev. Environ. Contam. Toxicol. 2016, 237, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Long, N.P.; Kang, J.S.; Kim, H.M. Caenorhabditis elegans: A model organism in the toxicity assessment of environmental pollutants. Environ. Sci. Pollut. Res. Int. 2023, 30, 39273–39287. [Google Scholar] [CrossRef] [PubMed]
- Ruszkiewicz, J.A.; Pinkas, A.; Miah, M.R.; Weitz, R.L.; Lawes, M.J.A.; Akinyemi, A.J.; Ijomone, O.M.; Aschner, M.C. elegans as a model in developmental neurotoxicology. Toxicol. Appl. Pharmacol. 2018, 354, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Mangerich, A.; Esser, C. Chemical warfare in the First World War: Reflections 100 years later. Arch. Toxicol. 2014, 88, 1909–1911. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Abdolghaffari, A.H.; Sahebkar, A. A review on symptoms, treatments protocols, and proteomic profile in sulfur mustard-exposed victims. J. Cell. Biochem. 2018, 119, 197–206. [Google Scholar] [CrossRef]
- Etemad, L.; Moshiri, M.; Balali-Mood, M. Advances in treatment of acute sulfur mustard poisoning—A critical review. Crit. Rev. Toxicol. 2019, 49, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Neidle, S.; Thurston, D.E. Chemical approaches to the discovery and development of cancer therapies. Nat. Rev. Cancer 2005, 5, 285–296. [Google Scholar] [CrossRef]
- Singh, R.K.; Kumar, S.; Prasad, D.N.; Bhardwaj, T.R. Therapeutic journery of nitrogen mustard as alkylating anticancer agents: Historic to future perspectives. Eur. J. Med. Chem. 2018, 151, 401–433. [Google Scholar] [CrossRef]
- Ludlum, D.B.; Austin-Ritchie, P.; Hagopian, M.; Niu, T.Q.; Yu, D. Detection of sulfur mustard-induced DNA modifications. Chem. Biol. Interact. 1994, 91, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Kehe, K.; Balszuweit, F.; Steinritz, D.; Thiermann, H. Molecular toxicology of sulfur mustard-induced cutaneous inflammation and blistering. Toxicology 2009, 263, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Povirk, L.F.; Shuker, D.E. DNA damage and mutagenesis induced by nitrogen mustards. Mutat. Res. /Rev. Genet. Toxicol. 1994, 318, 205–226. [Google Scholar] [CrossRef] [PubMed]
- Zubel, T.; Hochgesand, S.; John, H.; Steinritz, D.; Schmidt, A.; Burkle, A.; Mangerich, A. A mass spectrometric platform for the quantitation of sulfur mustard-induced nucleic acid adducts as mechanistically relevant biomarkers of exposure. Arch. Toxicol. 2019, 93, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Dębiak, M.; Panas, A.; Steinritz, D.; Kehe, K.; Bürkle, A. High-throughput analysis of DNA interstrand crosslinks in human peripheral blood mononuclear cells by automated reverse FADU assay. Toxicology 2011, 280, 53–60. [Google Scholar] [CrossRef]
- Debiak, M.; Lex, K.; Ponath, V.; Burckhardt-Boer, W.; Thiermann, H.; Steinritz, D.; Schmidt, A.; Mangerich, A.; Bürkle, A. Immunochemical analysis of poly(ADP-ribosyl)ation in HaCaT keratinocytes induced by the mono-alkylating agent 2-chloroethyl ethyl sulfide (CEES): Impact of experimental conditions. Toxicol. Lett. 2016, 244, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, A.J.; Perrone, R.; Grozio, A.; Verdin, E. NAD(+) metabolism and its roles in cellular processes during ageing. Nat. Rev. Mol. Cell Biol. 2021, 22, 119–141. [Google Scholar] [CrossRef]
- Lüscher, B.; Ahel, I.; Altmeyer, M.; Ashworth, A.; Bai, P.; Chang, P.; Cohen, M.; Corda, D.; Dantzer, F.; Daugherty, M.D.; et al. ADP-ribosyltransferases, an update on function and nomenclature. FEBS J. 2022, 289, 7399–7410. [Google Scholar] [CrossRef]
- Mol, M.E.; de Vries, R.; Kluivers, A.W. Effects of nicotinamide on biochemical changes and microblistering induced by sulfur mustard in human skin organ cultures. Toxicol. Appl. Pharmacol. 1991, 107, 439–449. [Google Scholar] [CrossRef]
- Yourick, J.J.; Dawson, J.S.; Mitcheltree, L.W. Sulfur mustard-induced microvesication in hairless guinea pigs: Effect of short-term niacinamide administration. Toxicol. Appl. Pharmacol. 1992, 117, 104–109. [Google Scholar] [CrossRef]
- Yourick, J.J.; Dawson, J.S.; Benton, C.D.; Craig, M.E.; Mitcheltree, L.W. Pathogenesis of 2,2′-dichlorodiethyl sulfide in hairless guinea pigs. Toxicology 1993, 84, 185–197. [Google Scholar] [CrossRef]
- Mol, M.A.; van de Ruit, A.M.; Kluivers, A.W. NAD+ levels and glucose uptake of cultured human epidermal cells exposed to sulfur mustard. Toxicol. Appl. Pharmacol. 1989, 98, 159–165. [Google Scholar] [CrossRef]
- Smith, W.J.; Gross, C.L.; Chan, P.; Meier, H.L. The use of human epidermal keratinocytes in culture as a model for studying the biochemical mechanisms of sulfur mustard toxicity. Cell Biol. Toxicol. 1990, 6, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Ruszkiewicz, J.; Papatheodorou, Y.; Jäck, N.; Melzig, J.; Eble, F.; Pirker, A.; Thomann, M.; Haberer, A.; Rothmiller, S.; Bürkle, A.; et al. NAD+ Acts as a Protective Factor in Cellular Stress Response to DNA Alkylating Agents. Cells 2023, 12, 2396. [Google Scholar] [CrossRef]
- Papirmeister, B.; Gross, C.L.; Meier, H.L.; Petrali, J.P.; Johnson, J.B. Molecular Basis for Mustard-Induced Vesication12. Toxicol. Sci. 1985, 5, 134–149. [Google Scholar] [CrossRef]
- Ruszkiewicz, J.A.; Burkle, A.; Mangerich, A. NAD(+) in sulfur mustard toxicity. Toxicol. Lett. 2020, 324, 95–103. [Google Scholar] [CrossRef]
- Ray Chaudhuri, A.; Nussenzweig, A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat. Rev. Mol. Cell Biol. 2017, 18, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Cortes, U.; Tong, W.M.; Coyle, D.L.; Meyer-Ficca, M.L.; Meyer, R.G.; Petrilli, V.; Herceg, Z.; Jacobson, E.L.; Jacobson, M.K.; Wang, Z.Q. Depletion of the 110-kilodalton isoform of poly(ADP-ribose) glycohydrolase increases sensitivity to genotoxic and endotoxic stress in mice. Mol. Cell. Biol. 2004, 24, 7163–7178. [Google Scholar] [CrossRef]
- St-Laurent, J.F.; Desnoyers, S. Poly(ADP-ribose) metabolism analysis in the nematode Caenorhabditis elegans. Methods Mol. Biol. 2011, 780, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Jeong, H.; Park, K.H.; Kim, K.W. Effects of NAD(+) in Caenorhabditis elegans Models of Neuronal Damage. Biomolecules 2020, 10, 993. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Santelli, J.; Mitchell, D.H.; Stiles, J.W.; Sanadi, D.R. A simple method for maintaining large, aging populations of Caenorhabditis elegans. Mech. Ageing Dev. 1980, 12, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Jacques, M.T.; Bornhorst, J.; Soares, M.V.; Schwerdtle, T.; Garcia, S.; Ávila, D.S. Reprotoxicity of glyphosate-based formulation in Caenorhabditis elegans is not due to the active ingredient only. Environ Pollut. 2019, 252, 1854–1862. [Google Scholar] [CrossRef] [PubMed]
- de la Guardia, Y.; Gilliat, A.F.; Hellberg, J.; Rennert, P.; Cabreiro, F.; Gems, D. Run-on of germline apoptosis promotes gonad senescence in C. elegans. Oncotarget 2016, 7, 39082–39096. [Google Scholar] [CrossRef]
- Zhou, Z.; Hartwieg, E.; Horvitz, H.R. CED-1 is a transmembrane receptor that mediates cell corpse engulfment in C. elegans. Cell 2001, 104, 43–56. [Google Scholar] [CrossRef]
- Ijomone, O.M.; Gubert, P.; Okoh, C.O.A.; Varão, A.M.; Amara, L.O.; Aluko, O.M.; Aschner, M. Application of Fluorescence Microscopy and Behavioral Assays to Demonstrating Neuronal Connectomes and Neurotransmitter Systems in C. elegans. Neuromethods 2021, 172, 399–426. [Google Scholar]
- Ijomone, O.M.; Miah, M.R.; Peres, T.V.; Nwoha, P.U.; Aschner, M. Null allele mutants of trt-1, the catalytic subunit of telomerase in Caenorhabditis elegans, are less sensitive to Mn-induced toxicity and DAergic degeneration. NeuroToxicology 2016, 57, 54–60. [Google Scholar] [CrossRef]
- Nass, R.; Hall, D.H.; Miller, D.M., 3rd; Blakely, R.D. Neurotoxin-induced degeneration of dopamine neurons in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2002, 99, 3264–3269. [Google Scholar] [CrossRef]
- Doitsidou, M.; Flames, N.; Lee, A.C.; Boyanov, A.; Hobert, O. Automated screening for mutants affecting dopaminergic-neuron specification in C. elegans. Nat. Methods 2008, 5, 869–872. [Google Scholar] [CrossRef]
- Jacobson, E.L.; Jacobson, M.K. Pyridine nucleotide levels as a function of growth in normal and transformed 3T3 cells. Arch. Biochem. Biophys. 1976, 175, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Mangerich, A.; Debiak, M.; Birtel, M.; Ponath, V.; Balszuweit, F.; Lex, K.; Martello, R.; Burckhardt-Boer, W.; Strobelt, R.; Siegert, M.; et al. Sulfur and nitrogen mustards induce characteristic poly(ADP-ribosyl)ation responses in HaCaT keratinocytes with distinctive cellular consequences. Toxicol. Lett. 2016, 244, 56–71. [Google Scholar] [CrossRef][Green Version]
- Wang, Q.Q.; Begum, R.A.; Day, V.W.; Bowman-James, K. Sulfur, oxygen, and nitrogen mustards: Stability and reactivity. Org. Biomol. Chem. 2012, 10, 8786–8793. [Google Scholar] [CrossRef]
- Bailly, A.P.; Freeman, A.; Hall, J.; Déclais, A.C.; Alpi, A.; Lilley, D.M.; Ahmed, S.; Gartner, A. The Caenorhabditis elegans homolog of Gen1/Yen1 resolvases links DNA damage signaling to DNA double-strand break repair. PLoS Genet. 2010, 6, e1001025. [Google Scholar] [CrossRef]
- Hartman, J.H.; Widmayer, S.J.; Bergemann, C.M.; King, D.E.; Morton, K.S.; Romersi, R.F.; Jameson, L.E.; Leung, M.C.K.; Andersen, E.C.; Taubert, S.; et al. Xenobiotic metabolism and transport in Caenorhabditis elegans. J. Toxicol. Environ. Health B Crit. Rev. 2021, 24, 51–94. [Google Scholar] [CrossRef]
- Meier, B.; Cooke, S.L.; Weiss, J.; Bailly, A.P.; Alexandrov, L.B.; Marshall, J.; Raine, K.; Maddison, M.; Anderson, E.; Stratton, M.R.; et al. C. elegans whole-genome sequencing reveals mutational signatures related to carcinogens and DNA repair deficiency. Genome Res. 2014, 24, 1624–1636. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.T.; Mohideen, F.; Meyer, K.; Harper, J.W.; Colaiácovo, M.P. SLX-1 is required for maintaining genomic integrity and promoting meiotic noncrossovers in the Caenorhabditis elegans germline. PLoS Genet. 2012, 8, e1002888. [Google Scholar] [CrossRef]
- Bu, M.; Farrer, M.J.; Khoshbouei, H. Dynamic control of the dopamine transporter in neurotransmission and homeostasis. NPJ Park. Dis. 2021, 7, 22. [Google Scholar] [CrossRef]
- Jiang, N.; Zhang, P.; Shen, W.; Zhang, Y.; Zhou, W. Clinical and experimental research progress on neurotoxicity of sulfur mustard and its possible mechanisms. Toxicology 2023, 483, 153372. [Google Scholar] [CrossRef] [PubMed]
- Darchini-Maragheh, E.; Nemati-Karimooy, H.; Hasanabadi, H.; Balali-Mood, M. Delayed neurological complications of sulphur mustard and tabun poisoning in 43 Iranian veterans. Basic Clin. Pharmacol. Toxicol. 2012, 111, 426–432. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Hefazi, M.; Mahmoudi, M.; Jalali, E.; Attaran, D.; Maleki, M.; Razavi, M.E.; Zare, G.; Tabatabaee, A.; Jaafari, M.R. Long-term complications of sulphur mustard poisoning in severely intoxicated Iranian veterans. Fundam. Clin. Pharmacol. 2005, 19, 713–721. [Google Scholar] [CrossRef]
- Shoeibi, N.; Mousavi, M.N.; Balali-Mood, M.; Moshiri, M.; Darchini-Maragheh, E.; Mousavi, S.R.; Abrishami, M. Long-term complications of sulfur mustard poisoning: Retinal electrophysiological assessment in 40 severely intoxicated Iranian veterans. Int. J. Retin. Vitr. 2017, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, A.B.; Eriksen, J.; Smidt-Nielsen, K. Chronic neuropathic symptoms after exposure to mustard gas: A long-term investigation. J. Am. Acad. Dermatol. 1998, 39, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Roshan, R.; Rahnama, P.; Ghazanfari, Z.; Montazeri, A.; Soroush, M.R.; Naghizadeh, M.M.; Melyani, M.; Tavoli, A.; Ghazanfari, T. Long-term effects of sulfur mustard on civilians’ mental health 20 years after exposure (The Sardasht-Iran Cohort Study). Health Qual. Life Outcomes 2013, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Hernandez, A.; Roney, A.; Goswami, D.G.; Tewari-Singh, N.; Brown, J.M. A review of chemical warfare agents linked to respiratory and neurological effects experienced in Gulf War Illness. Inhal. Toxicol. 2022, 34, 412–432. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, T.W.; Wang, Y.; Song, Y.; Villanueva, M.; Jimenez, A. Sulphur mustard induces progressive toxicity and demyelination in brain cell aggregate culture. Neurotoxicology 2021, 84, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Kisby, G.E.; Springer, N.; Spencer, P.S. In vitro neurotoxic and DNA-damaging properties of nitrogen mustard. J. Appl. Toxicol. 2000, 20 (Suppl. S1), S35–S41. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.R.; Sunkaria, A.; Bal, A.; Bhutia, Y.D.; Vijayaraghavan, R.; Flora, S.J.S.; Gill, K.D. Neurobehavioral impairments, generation of oxidative stress and release of pro-apoptotic factors after chronic exposure to sulphur mustard in mouse brain. Toxicol. Appl. Pharmacol. 2009, 240, 208–218. [Google Scholar] [CrossRef]
- Tekiner, A.; Yucel, O.; Sargin, A.K.; Genc, O.; Can, B.; Karayilanoglu, T.; Karakaya, J.; Bayar, M.A. The effect of nitrogen mustard on the rat brain and the therapeutic value of proanthocyanidin. Turk. Neurosurg. 2009, 19, 360–366. [Google Scholar]
- Tekiner, A.; Yucel, D.; Bayar, M.A.; Yucel, O.; Erdem, Y.; Karakaya, J. The effect of Nitrogen Mustard on the enzymatic antioxidant defense of rat brain tissue and the therapeutic value of proanthocyanidin. Turk. Neurosurg. 2011, 21, 461–466. [Google Scholar] [CrossRef]
- Jafari, M. Dose- and time-dependent effects of sulfur mustard on antioxidant system in liver and brain of rat. Toxicology 2007, 231, 30–39. [Google Scholar] [CrossRef]
- Gilardoni, M.; Léonço, D.; Caffin, F.; Gros-Désormeaux, F.; Eldin, C.; Béal, D.; Ouzia, S.; Junot, C.; Fenaille, F.; Piérard, C.; et al. Evidence for the systemic diffusion of (2-chloroethyl)-ethyl-sulfide, a sulfur mustard analog, and its deleterious effects in brain. Toxicology 2021, 462, 152950. [Google Scholar] [CrossRef]
- Gadsden-Gray, J.; Mukherjee, S.; Ogunkua, O.; Das, S.K. Induction of neuronal damage in guinea pig brain by intratracheal infusion of 2-chloroethyl ethyl sulfide, a mustard gas analog. J. Biochem. Mol. Toxicol. 2012, 26, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Spanoudakis, E.; Tavernarakis, N. Age-associated anatomical and physiological alterations in Caenorhabditis elegans. Mech. Ageing Dev. 2023, 213, 111827. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, Q.; Song, Y.; He, Z.; Zhang, H.; Song, M.; Zhang, X.; Dai, Y.; Karalay, O.; Dieterich, C.; et al. Ageing induces tissue-specific transcriptomic changes in Caenorhabditis elegans. EMBO J. 2022, 41, e109633. [Google Scholar] [CrossRef]
- Copes, N.; Edwards, C.; Chaput, D.; Saifee, M.; Barjuca, I.; Nelson, D.; Paraggio, A.; Saad, P.; Lipps, D.; Stevens, S.M., Jr.; et al. Metabolome and proteome changes with aging in Caenorhabditis elegans. Exp. Gerontol. 2015, 72, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Son, H.G.; Altintas, O.; Kim, E.J.E.; Kwon, S.; Lee, S.V. Age-dependent changes and biomarkers of aging in Caenorhabditis elegans. Aging Cell 2019, 18, e12853. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.F.; Scheibye-Knudsen, M.; Brace, L.E.; Kassahun, H.; SenGupta, T.; Nilsen, H.; Mitchell, J.R.; Croteau, D.L.; Bohr, V.A. Defective mitophagy in XPA via PARP-1 hyperactivation and NAD(+)/SIRT1 reduction. Cell 2014, 157, 882–896. [Google Scholar] [CrossRef]
- Hyun, M.; Lee, J.; Lee, K.; May, A.; Bohr, V.A.; Ahn, B. Longevity and resistance to stress correlate with DNA repair capacity in Caenorhabditis elegans. Nucleic Acids Res. 2008, 36, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Marchal, L.; Hamsanathan, S.; Karthikappallil, R.; Han, S.; Shinglot, H.; Gurkar, A.U. Analysis of representative mutants for key DNA repair pathways on healthspan in Caenorhabditis elegans. Mech. Ageing Dev. 2021, 200, 111573. [Google Scholar] [CrossRef]
- Debès, C.; Papadakis, A.; Grönke, S.; Karalay, Ö.; Tain, L.S.; Mizi, A.; Nakamura, S.; Hahn, O.; Weigelt, C.; Josipovic, N.; et al. Ageing-associated changes in transcriptional elongation influence longevity. Nature 2023, 616, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Bernstein, I.A.; Vaughan, F.L. Failure to observe a relationship between bis-(beta-chloroethyl)sulfide-induced NAD depletion and cytotoxicity in the rat keratinocyte culture. J. Toxicol. Environ. Health 1994, 42, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Dequen, F.; Gagnon, S.N.; Desnoyers, S. Ionizing radiations in Caenorhabditis elegans induce poly(ADP-ribosyl)ation, a conserved DNA-damage response essential for survival. DNA Repair (Amst.) 2005, 4, 814–825. [Google Scholar] [CrossRef]
- Crone, B.; Aschner, M.; Schwerdtle, T.; Karst, U.; Bornhorst, J. Elemental bioimaging of Cisplatin in Caenorhabditis elegans by LA-ICP-MS. Metallomics 2015, 7, 1189–1195. [Google Scholar] [CrossRef]
- St-Laurent, J.F.; Gagnon, S.N.; Dequen, F.; Hardy, I.; Desnoyers, S. Altered DNA damage response in Caenorhabditis elegans with impaired poly(ADP-ribose) glycohydrolases genes expression. DNA Repair (Amst.) 2007, 6, 329–343. [Google Scholar] [CrossRef]
- Bae, W.; Park, J.H.; Lee, M.H.; Park, H.W.; Koo, H.S. Hypersensitivity to DNA double-strand breaks associated with PARG deficiency is suppressed by exo-1 and polq-1 mutations in Caenorhabditis elegans. FEBS J. 2020, 287, 1101–1115. [Google Scholar] [CrossRef]
- Janisiw, E.; Raices, M.; Balmir, F.; Paulin, L.F.; Baudrimont, A.; von Haeseler, A.; Yanowitz, J.L.; Jantsch, V.; Silva, N. Poly(ADP-ribose) glycohydrolase coordinates meiotic DNA double-strand break induction and repair independent of its catalytic activity. Nat Commun 2020, 11, 4869. [Google Scholar] [CrossRef]
- Trivedi, S.; Blazícková, J.; Silva, N. PARG and BRCA1-BARD1 cooperative function regulates DNA repair pathway choice during gametogenesis. Nucleic Acids Res. 2022, 50, 12291–12308. [Google Scholar] [CrossRef]
- Byrne, A.B.; McWhirter, R.D.; Sekine, Y.; Strittmatter, S.M.; Miller, D.M.; Hammarlund, M. Inhibiting poly(ADP-ribosylation) improves axon regeneration. eLife 2016, 5, e12734. [Google Scholar] [CrossRef] [PubMed]
- Lagunas-Rangel, F.A. Current role of mammalian sirtuins in DNA repair. DNA Repair (Amst.) 2019, 80, 85–92. [Google Scholar] [CrossRef]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef] [PubMed]
- Tissenbaum, H.A.; Guarente, L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 2001, 410, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.A.; Arango, M.; Abderrahmane, S.; Lambert, E.; Tourette, C.; Catoire, H.; Néri, C. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nat. Genet. 2005, 37, 349–350. [Google Scholar] [CrossRef] [PubMed]
- Sangaletti, R.; D’Amico, M.; Grant, J.; Della-Morte, D.; Bianchi, L. Knock-out of a mitochondrial sirtuin protects neurons from degeneration in Caenorhabditis elegans. PLoS Genet. 2017, 13, e1006965. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruszkiewicz, J.; Endig, L.; Güver, E.; Bürkle, A.; Mangerich, A. Life-Cycle-Dependent Toxicities of Mono- and Bifunctional Alkylating Agents in the 3R-Compliant Model Organism C. elegans. Cells 2023, 12, 2728. https://doi.org/10.3390/cells12232728
Ruszkiewicz J, Endig L, Güver E, Bürkle A, Mangerich A. Life-Cycle-Dependent Toxicities of Mono- and Bifunctional Alkylating Agents in the 3R-Compliant Model Organism C. elegans. Cells. 2023; 12(23):2728. https://doi.org/10.3390/cells12232728
Chicago/Turabian StyleRuszkiewicz, Joanna, Lisa Endig, Ebru Güver, Alexander Bürkle, and Aswin Mangerich. 2023. "Life-Cycle-Dependent Toxicities of Mono- and Bifunctional Alkylating Agents in the 3R-Compliant Model Organism C. elegans" Cells 12, no. 23: 2728. https://doi.org/10.3390/cells12232728
APA StyleRuszkiewicz, J., Endig, L., Güver, E., Bürkle, A., & Mangerich, A. (2023). Life-Cycle-Dependent Toxicities of Mono- and Bifunctional Alkylating Agents in the 3R-Compliant Model Organism C. elegans. Cells, 12(23), 2728. https://doi.org/10.3390/cells12232728





