The Neuropharmacological Evaluation of Seaweed: A Potential Therapeutic Source
Abstract
:1. Introduction
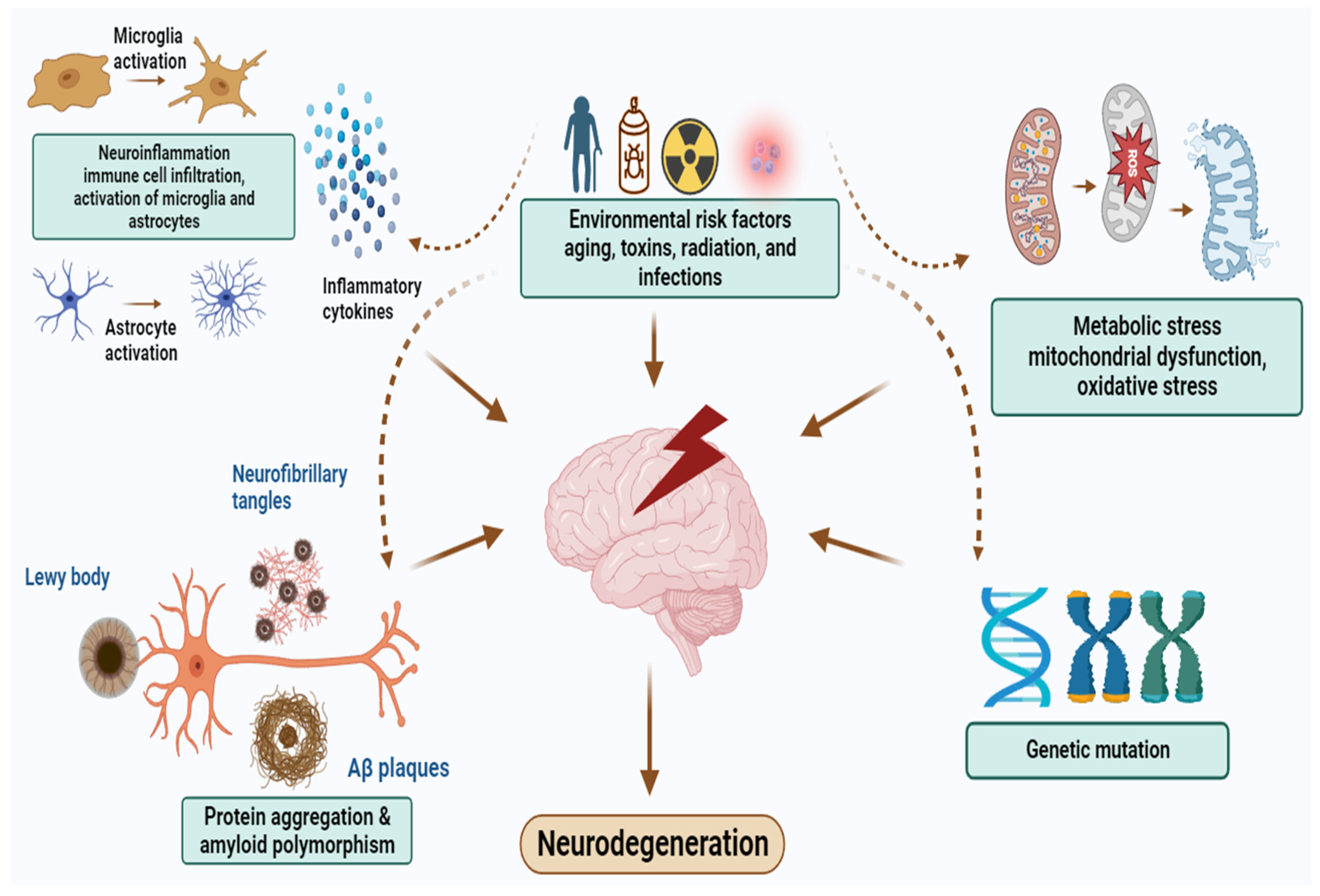
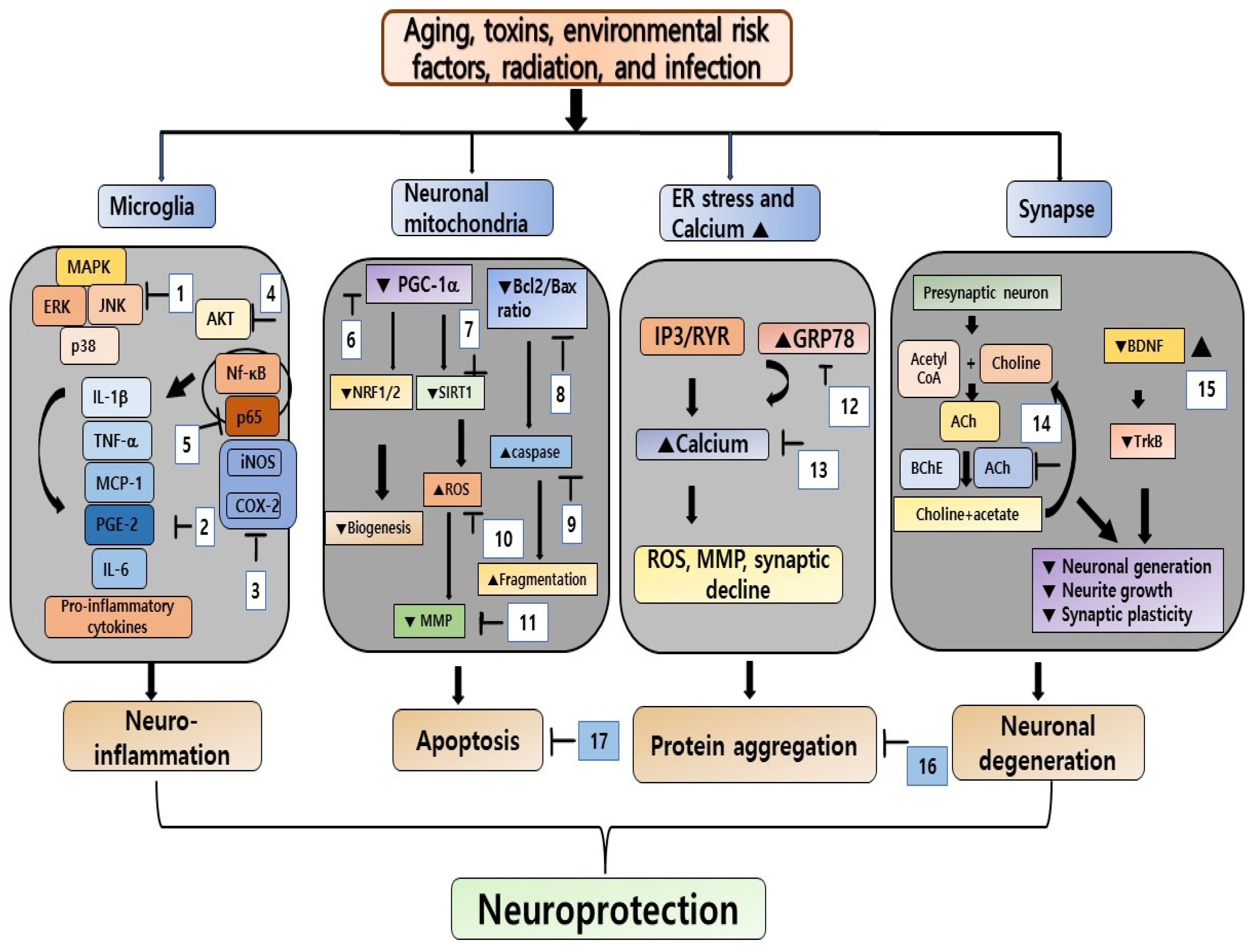
2. Pathophysiology of NDDs
3. Pharmacology and Chemistry of Seaweed and Their Bioactive Compounds
4. Neuroprotective Effects of Seaweeds and Their Bioactive Compounds in the Context of NDDs
4.1. Neuroprotective Effects of Seaweed Extracts for NDDs
4.1.1. In Vitro Studies
4.1.2. In Vivo Studies
4.2. Neuroprotective Effects of Bioactive Compounds in Seaweeds and Their Application in the Treatment of NDDs
4.2.1. Polysaccharides
4.2.2. Fatty Acids
4.2.3. Phlorotannins
4.2.4. Carotenoids
4.2.5. Amino Acids
4.2.6. Sterols
| Class of Compound | Name of Compound | Model | Dose | Effect | References |
|---|---|---|---|---|---|
| Polysaccharide | Polymannuronic Alginate-derived oligosaccharide | Aβ and LPS-stimulated BV-2 microglia cells | 500 µg/mL | Ameliorated neuroinflammation by inhibition of activation of the TLR4-NF-κB signaling pathway | [120] |
| κ-Carrageenan | 6-OHDA-induced SH-SY5Y cells and LPS-stimulated BV-2 microglia cells | 0.3 to 1.0 mg/mL | Improved mitochondrial function, inhibited caspase-3 activity, and anti-inflammatory activity | [121,126] | |
| Fucoidan | PC-12 cells (treated with fucoidan for 7 days) | 5–100 μg/mL | Enhanced neurite outgrowth | [69] | |
| Rotenone-induced PD rats | 35, 70, and 140 mg/kg/b.w. | Reduced oxidative stress, enhanced dopamine content, and increased PGC-1α and NRF2 protein expressions | [129] | ||
| Streptozotocin-induced AD rats | 100 and 200 mg/kg/b.w. | Ameliorated behavioral deficits and reduced oxidative damage by increasing antioxidant enzyme activity | [130] | ||
| MPTP-induced PD in male C57BL/6 mice | 25 mg/kg/b.w. | Increased peripheral and central movement, reduced lipid peroxidation in the corpus striatum and midbrain, and increased TH and DAT protein expressions in the SNpc | [64] | ||
| Fatty acids | Omega-3 PUFA | LPS-induced AD in Rat | 400 mg/kg/b.w. | Increased CaMKII-α gene expression and anti-inflammatory activity | [142] |
| High-fat diet-induced rats | 1 g/kg/b.w. | Reduced neuroinflammation by decreasing proinflammatory cytokines and inflammatory mediators | [143] | ||
| Eicosapentaenoic acid and docosahexaenoic acid | LPS-stimulated MG6 cells and BV-2 microglia cells | 200 µM | Reduced inflammation and increased SIRT1 mRNA levels | [145] | |
| IL-1β-induced AD in rodent | 25 and 30 mg/kg/b.w. | Upregulated BDNF and its receptor TrKB expression | [146] | ||
| MPTP-induced PD in rat | 75 mg/kg/b.w. | Elevated GAP-43 and BDNF expression and TH-positive neurons and downregulated overexpression of p-GSK3β and p-Tau | [152] | ||
| Omega-3 PUFA-rich oil supplement | Haloperidol-induced PD rat | 300 mg/kg/b.w. | Regained locomotor activity and alleviated the D2 receptor protein level | [148] | |
| n-3 long-chain PUFA | aged C57BL/6 mice | 6.2, 7, 31, 40, and 50 mg/kg/b.w. | Improved spatial learning and object recognition memory and increased the NMDA subunits of mGluR5 and GluN2B of the glutamatergic receptors | [149] | |
| docosahexaenoic acid | IFN-α-induced BV-2 microglia cells | 3 to 30 μM | Reduced neuroinflammation by regulating phosphorylation of PI3K/Akt and ERK signaling pathways | [151] | |
| Phlorotannins | Eckol, dieckol, and 8,8′-bieckol | Aβ25–35-induced PC-12 cells | 1, 10, and 50 µM | Antiapoptotic, antioxidative, and anti-neuroinflammatory properties | [154] |
| Dieckol | APPswe N2a cells and SweAPP N2a cells | 1, 10, and 50 mM | Regulated the APP processing enzymes and the reduction of Aβ levels | [156] | |
| Carotenoid | Fucoxanthin | Aβ1–42 treated BV-2 microglia cells | 5, 10, and 50 µM | Anti-neuroinflammation | [159] |
| Glutamate-induced SH-SY5Y cells | 8.25 µg/mL | Inhibited AChE and BChE | [160] | ||
| H2O2-induced SH-SY5Y cells | 0.3, 1, and 3 µM | Upregulated expression of GSK3β, antiapoptotic, and anti-neuroinflammatory activities | [161] | ||
| Aβ1–42-induced PC-12 cells | 2 µM | Increased neural outgrowth | [162,163] | ||
| Aβ1–42-treated AD mice | 100–200 mg/kg/b.w. | Increased BDNF expression, spatial learning, and memory function | [75] | ||
| MPTP-induced PD model | 10 mg/kg/b.w. | Reduced neuroinflammation and improved motor function | [167] | ||
| Amino acid | Taurine | 5xFAD transgenic mice | 1000 mg/kg/b.w. | Enhanced brain uptake of mGluR5 and increased blood flow in the cerebra | [171] |
| Aβ oligomer-induced AD mice | 250 mg/kg/b.w. | Ameliorated special learning dysfunction and memory deficits | [172] | ||
| MnCl2-treated Sprague–Dawley mice | 200 mg/kg/b.w. | Enhanced the activity of ChAT and reduced AChE activity | [173] | ||
| 6-OHDA and apomorphine-induced PD rat | 8 mg/kg/b.w. | Improved rotational behavior and partially replenished DA levels | [181] | ||
| Paraquat and maneb-induced PD mouse | 150 mg/kg/b.w. | Restored TH-positive neurons and inhibited activation of the STAT1/3 signaling pathway | [182] | ||
| Sterol | 24(S)-saringosterol | APPswe/PS1△E9 mice | 0.5 mg/kg/b.w. | Prevented cognitive decline and markedly reduced the expression of microglia activation | [183] |
| Transfected HEK293T and HepG2 cells | 0.5–40 µM | Selective agonist for LXRβ | [185] | ||
| Fucosterol | Aβ-induced SH-SY5Y cells | 10 and 20 µM | Increased levels of neuroglobin and reduced mRNA levels of APP | [186] | |
| LPS-induced C8-B4 microglial cells | 12–192 µM | Decreased neuroinflammation and inhibited AChE and BChE activity | [187] |
4.3. Toxicology of Seaweed and Its Bioactive Compounds
4.4. Recent Progressive Studies of Bioactive Compounds in Seaweeds
5. Current Limitations and Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wyss-Coray, T. Ageing, Neurodegeneration and Brain Rejuvenation. Nature 2016, 539, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.G.; Budka, H. Current Concepts of Neuropathological Diagnostics in Practice: Neurodegenerative Diseases. Clin. Neuropathol. 2010, 29, 271–288. [Google Scholar] [CrossRef] [PubMed]
- Peden, A.H.; Ironside, J.W. Molecular Pathology in Neurodegenerative Diseases. Curr. Drug Targets 2012, 13, 1548–1559. [Google Scholar] [CrossRef]
- Tycko, R. Amyloid Polymorphism: Structural Basis and Neurobiological Relevance. Neuron 2015, 86, 632–645. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Cause and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Mortada, I.; Farah, R.; Nabha, S.; Ojcius, D.M.; Fares, Y.; Almawi, W.Y.; Sadier, N.S. Immunotherapies for Neurodegenrative Diseases. Front. Neurol. 2021, 12, 654739. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, R.; Kim, Y.-S.; Kim, G.-W.; Kim, W.-J.; Hong, S.-M.; Kim, C.-G.; Choi, D.-K. Standardized Extract of Glehnia Littoralis Abrogates Memory Impairment and Neuroinflammation by Regulation of CREB/BDNF and NF-κB/MAPK Signaling in Scopolamine-Induced Amnesic Mice Model. Biomed. Pharmacother. 2023, 165, 115106. [Google Scholar] [CrossRef]
- Van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Eng. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Lowe, S.L.; Willis, B.A.; Hawdon, A.; Natanegara, F.; Chua, L.; Foster, J.; Shcherbinin, S.; Ardayfio, P.; Sims, J.R. Donanemab (LY3002813) Dose-escalation Study in Alzheimer’s Disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2021, 7, e12112. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Chi, Y.; Zhang, Q.; Ma, Y. Safety and Efficacy of Lecanemab for Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Front. Aging Neurosci. 2023, 15, 1169499. [Google Scholar] [CrossRef]
- Rashad, A.; Rasool, A.; Shaheryar, M.; Sarfraz, A.; Sarfraz, Z.; Robles-Velasco, K.; Cherrez-Ojeda, I. Donanemab for Alzheimer’s Disease: A Systematic Review of Clinical Trials. Healthcare 2022, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, R.; Park, J.-Y.; Cho, D.-Y.; Ahn, J.-Y.; Yoo, D.-S.; Seol, S.-H.; Yoon, S.-H.; Choi, D.-K. AD−1 Small Molecule Improves Learning and Memory Function in Scopolamine-Induced Amnesic Mice Model through Regulation of CREB/BDNF and NF-κB/MAPK Signaling Pathway. Antioxidants 2023, 12, 648. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative Stress, Inflammation, and Cancer: How Are They Linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Hald, A.; Lotharius, J. Oxidative Stress and Inflammation in Parkinson’s Disease: Is There a Causal Link? Exp. Neurol. 2005, 193, 279–290. [Google Scholar] [CrossRef]
- Sánchez-López, F.; Tasset, I.; Agüera, E.; Feijóo, M.; Fernández-Bolaños, R.; Sánchez, F.M.; Ruiz, M.C.; Cruz, A.H.; Gascón, F.; Túnez, I. Oxidative Stress and Inflammation Biomarkers in the Blood of Patients with Huntington’s Disease. Neurol. Res. 2012, 34, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Gutierrez, E.; Muñoz-Arenas, G.; Treviño, S.; Espinosa, B.; Chavez, R.; Rojas, K.; Flores, G.; Díaz, A.; Guevara, J. Alzheimer’s Disease and Metabolic Syndrome: A Link from Oxidative Stress and Inflammation to Neurodegeneration. Synapse 2017, 71, e21990. [Google Scholar] [CrossRef]
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A Review of the Common Neurodegenerative Disorders: Current Therapeutic Approaches and the Potential Role of Nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Oguntibeju, O.O. Type 2 Diabetes Mellitus, Oxidative Stress and Inflammation: Examining the Links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar]
- Murai, U.; Yamagishi, K.; Kishida, R.; Iso, H. Impact of Seaweed Intake on Health. Eur. J. Clin. Nutr. 2021, 75, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Liu, Z.; Wang, X.; Qin, S. The Seaweed Holobiont: From Microecology to Biotechnological Applications. Microb. Biotechnol. 2022, 15, 738–754. [Google Scholar] [CrossRef]
- Baghel, R.S.; Reddy, C.R.K.; Singh, R.P. Seaweed-Based Cellulose: Applications, and Future Perspectives. Carbohydr. Polym. 2021, 267, 118241. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.G. Current Concepts of Neurodegenerative Diseases. EMJ Neurol. 2014, 1, 10–11. [Google Scholar] [CrossRef]
- Balakrishnan, R.; Cho, D.-Y.; Kim, I.-S.; Seol, S.-H.; Choi, D.-K. Molecular Mechanisms and Therapeutic Potential of α- and β-Asarone in the Treatment of Neurological Disorders. Antioxidants 2022, 11, 281. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Halliwell, B. Oxidative Stress, Dysfunctional Glucose Metabolism and Alzheimer Disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef]
- Dalleau, S.; Baradat, M.; Guéraud, F.; Huc, L. Cell Death and Diseases Related to Oxidative Stress:4-Hydroxynonenal (HNE) in the Balance. Cell Death Differ. 2013, 20, 1615–1630. [Google Scholar] [CrossRef]
- Gella, A.; Durany, N. Oxidative Stress in Alzheimer Disease. Cell Adhes. Migr. 2009, 3, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, L.; Fernandez, F.; Johnson, J.B.; Naiker, M.; Owoola, A.G.; Broszczak, D.A. Oxidative Stress in Alzheimer’s Disease: A Review on Emergent Natural Polyphenolic Therapeutics. Complement. Ther. Med. 2020, 49, 102294. [Google Scholar] [CrossRef]
- Bhat, A.H.; Dar, K.B.; Anees, S.; Zargar, M.A.; Masood, A.; Sofi, M.A.; Ganie, S.A. Oxidative Stress, Mitochondrial Dysfunction and Neurodegenerative Diseases; a Mechanistic Insight. Biomed. Pharmacother. 2015, 74, 101–110. [Google Scholar] [CrossRef]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative Stress, Mitochondrial Damage and Neurodegenerative Diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar] [CrossRef]
- Kim, T.Y.; Leem, E.; Lee, J.M.; Kim, S.R. Control of Reactive Oxygen Species for the Prevention of Parkinson’s Disease: The Possible Application of Flavonoids. Antioxidants 2020, 9, 583. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, V.; Sharma, S. Role of Mitochondrial Dysfunction, Oxidative Stress and Autophagy in Progression of Alzheimer’s Disease. J. Neurol. Sci. 2021, 421, 117253. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.M.; Barnes, K.; De Marco, M.; Shaw, P.J.; Ferraiuolo, L.; Blackburn, D.J.; Venneri, A.; Mortiboys, H. Mitochondrial Dysfunction in Alzheimer’s Disease: A Biomarker of the Future? Biomedicines 2021, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, U.; Kayed, R. Amyloid β, Tau, and α-Synuclein Aggregates in the Pathogenesis, Prognosis, and Therapeutics for Neurodegenerative Diseases. Prog. Neurobiol. 2022, 214, 102270. [Google Scholar] [CrossRef] [PubMed]
- Pîrşcoveanu, D.F.V.; Pirici, I.; Tudorică, V.; Bălşeanu, T.A.; Albu, V.C.; Bondari, S.; Bumbea, A.M.; Pîrşcoveanu, M. Tau Protein in Neurodegenerative Diseases—A Review. Rom. J. Morphol. Embryol. 2017, 58, 1141–1150. [Google Scholar]
- Ashrafian, H.; Zadeh, E.H.; Khan, R.H. Review on Alzheimer’s Disease: Inhibition of Amyloid Beta and Tau Tangle Formation. Int. J. Biol. Macromol. 2021, 167, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Mehra, S.; Sahay, S.; Maji, S.K. α-Synuclein Misfolding and Aggregation: Implications in Parkinson’s Disease Pathogenesis. Biochim. Biophys. Acta Proteins Proteom. 2019, 1867, 890–908. [Google Scholar] [CrossRef] [PubMed]
- Puzzo, D.; Piacentini, R.; Fá, M.; Gulisano, W.; Li Puma, D.D.; Staniszewski, A.; Zhang, H.; Tropea, M.R.; Cocco, S.; Palmeri, A.; et al. LTP and Memory Impairment Caused by Extracellular Aβ and Tau Oligomers Is APP-Dependent. eLife 2017, 6, e26991. [Google Scholar] [CrossRef]
- Wang, Y.; Balaji, V.; Kaniyappan, S.; Krüger, L.; Irsen, S.; Tepper, K.; Chandupatla, R.; Maetzler, W.; Schneider, A.; Mandelkow, E.; et al. The Release and Trans-Synaptic Transmission of Tau via Exosomes. Mol. Neurodegener. 2017, 12, 5. [Google Scholar] [CrossRef]
- Fá, M.; Puzzo, D.; Piacentini, R.; Staniszewski, A.; Zhang, H.; Baltrons, M.A.; Li Puma, D.D.; Chatterjee, I.; Li, J.; Saeed, F.; et al. Extracellular Tau Oligomers Produce An Immediate Impairment of LTP and Memory. Sci. Rep. 2016, 6, 19393. [Google Scholar] [CrossRef]
- Vasili, E.; Dominguez-Meijide, A.; Outeiro, T.F. Spreading of α-Synuclein and Tau: A Systematic Comparison of the Mechanisms Involved. Front. Mol. Neurosci. 2019, 12, 107. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.W.; Iturria-Medina, Y.; Strandberg, O.T.; Smith, R.; Levitis, E.; Evans, A.C.; Hansson, O.; Weiner, M.; Aisen, P.; Petersen, R.; et al. Spread of Pathological Tau Proteins through Communicating Neurons in Human Alzheimer’s Disease. Nat. Commun. 2020, 11, 2612. [Google Scholar] [CrossRef] [PubMed]
- Buell, A.K.; Galvagnion, C.; Gaspar, R.; Sparr, E.; Vendruscolo, M.; Knowles, T.P.J.; Linse, S.; Dobson, C.M. Solution Conditions Determine the Relative Importance of Nucleation and Growth Processes in α-Synuclein Aggregation. Proc. Natl. Acad. Sci. USA 2014, 111, 7671–7676. [Google Scholar] [CrossRef]
- Du, X.; Xie, X.; Liu, R. The Role of α-Synuclein Oligomers in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 8645. [Google Scholar] [CrossRef] [PubMed]
- Calsolaro, V.; Edison, P. Neuroinflammation in Alzheimer’s Disease: Current Evidence and Future Directions. Alzheimer’s Dement. 2016, 12, 719–732. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and Microglial Activation in Alzheimer Disease: Where Do We Go from Here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in Neurodegenerative Disorders: The Roles of Microglia and Astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Zhang, J.; Yang, G. Mechanisms of NLRP3 Inflammasome Activation: Its Role in the Treatment of Alzheimer’s Disease. Neurochem. Res. 2020, 45, 2560–2572. [Google Scholar] [CrossRef]
- Liao, Y.; Xing, Q.; Li, Q.; Zhang, J.; Pan, R.; Yuan, Z. Astrocytes in Depression and Alzheimer’s Disease. Front. Med. 2021, 15, 829–841. [Google Scholar] [CrossRef]
- Gelders, G.; Baekelandt, V.; Van der Perren, A. Linking Neuroinflammation and Neurodegeneration in Parkinson’s Disease. J. Immunol. Res. 2018, 2018, 4784268. [Google Scholar] [CrossRef]
- Badanjak, K.; Fixemer, S.; Smajić, S.; Skupin, A.; Grünewald, A. The Contribution of Microglia to Neuroinflammation in Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 4676. [Google Scholar] [CrossRef]
- Belarbi, K.; Cuvelier, E.; Bonte, M.-A.; Desplanque, M.; Gressier, B.; Devos, D.; Chartier-Harlin, M.-C. Glycosphingolipids and Neuroinflammation in Parkinson’s Disease. Mol. Neurodegener. 2020, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.; Lopes, G.; Andrade, P.B.; Valentão, P. Bioprospecting of Brown Seaweeds for Biotechnological Applications: Phlorotannin Actions in Inflammation and Allergy Network. Trends Food Sci. Technol. 2019, 86, 153–171. [Google Scholar] [CrossRef]
- Schepers, M.; Martens, N.; Tiane, A.; Vanbrabant, K.; Liu, H.-B.; Lütjohann, D.; Mulder, M.; Vanmierlo, T. Edible Seaweed-Derived Constituents: An Undisclosed Source of Neuroprotective Compounds. Neural Regen. Res. 2020, 15, 790–795. [Google Scholar] [CrossRef]
- Patel, S. Seaweed-Derived Sulfated Polysaccharides. Bioactive Seaweeds for Food Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 71–93. [Google Scholar] [CrossRef]
- Sohn, S.-I.; Rathinapriya, P.; Balaji, S.; Jaya Balan, D.; Swetha, T.K.; Durgadevi, R.; Alagulakshmi, S.; Singaraj, P.; Pandian, S. Phytosterols in Seaweeds: An Overview on Biosynthesis to Biomedical Applications. Int. J. Mol. Sci. 2021, 22, 12691. [Google Scholar] [CrossRef]
- Rengasamy, K.R.R.; Mahomoodally, M.F.; Aumeeruddy, M.Z.; Zengin, G.; Xiao, J.; Kim, D.H. Bioactive Compounds in Seaweeds: An Overview of Their Biological Properties. Food Chem. Toxicol. 2020, 135, 111013. [Google Scholar] [CrossRef] [PubMed]
- Meinita, M.D.N.; Harwanto, D.; Sohn, J.-H.; Kim, J.-S.; Choi, J.-S. Hizikia fusiformis: Pharmacological and Nutritional Properties. Foods 2021, 10, 1660. [Google Scholar] [CrossRef] [PubMed]
- Rushdi, M.I.; Abdel-Rahman, I.A.M.; Saber, H.; Attia, E.Z.; Abdelraheem, W.M.; MAdkaur, H.A.; Hassan, H.M.; Elmaidomy, H.A.; Abdelmohsen, U.R. Pharmacological and Natural Products Diversity of the Brown Algae Genus Sargassum. RSC Adv. 2020, 10, 24951. [Google Scholar] [CrossRef]
- Shobier, A.H.; El Ashry, E.S. Pharmacological Applications of the Green Seaweed Ulva lactuca. Russ. J. Mar. Biol. 2021, 47, 425–439. [Google Scholar] [CrossRef]
- Khotimchenko, M.; Tiasto, V.; Kalitnik, A.; Begum, M.; Khotimchenko, R.; Leonteva, E.; Bryukhovetskiy, I.; Khotimchenko, Y. Antitumor Potential of Carrageenans from Marine Red Algae. Carbohydr. Polym. 2020, 246, 116568. [Google Scholar] [CrossRef]
- Sun, H.; Xu, L.; Wang, K.; Li, Y.; Bai, T.; Dong, S.; Wu, H.; Yao, Z. κ-Carrageenan Oligosaccharides Protect Nerves by Regulating Microglial Autophagy in Alzheimer’s Disease. ACS Chem. Neurosci. 2023, 14, 3540–3550. [Google Scholar] [CrossRef]
- Severino, P.; Da Silva, C.F.; Andrade, L.N.; De Lima Oliveira, D.; Campos, J.; Souto, E.B. Alginate Nanoparticles for Drug Delivery and Targeting. Curr. Pharm. Des. 2019, 25, 1312–1334. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.R.; Wang, X.; Cao, X.; Liu, X.; Zhou, S.N.; Zhang, H.; Yang, R.L.; Wong, K.H.; Tang, Q.J.; Dong, X.L. Alginate and its Two Components Acted Differently Against Dopaminergic Neuronal Loss in Parkinson’s Disease Mice Model. Mol. Nutr. Food Res. 2021, 66, 2100739. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Nikolova, M.; Iliev, I.; Peychev, L.; Trica, B.; Oancea, F.; Delattre, C.; et al. Immunomodulatory and Anti-Inflammatory Effects of Fucoidan: A Review. Polymers 2020, 12, 2338. [Google Scholar] [CrossRef]
- Maruyama, H.; Tamauchi, H.; Kawakami, F.; Yoshinaga, K.; Nakano, T. Suppressive Effect of Dietary Fucoidan on Proinflammatory Immune Response and MMP-1 Expression in UVB-Irradiated Mouse Skin. Planta Med. 2015, 81, 1370–1374. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Nagaoka, M.; Hara, T.; Kimura-Tagaki, I.; Mistuyama, K.; Ueyama, S. Fucoidan derived from Cladosiphon okamuranus Tokida ameliorates murine chronic colitis through the down-regulation of interleukin-6 production on colonic epithelial cells. Clin. Exp. Immunol. 2004, 136, 432–439. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Shikov, A.N.; Faustova, N.M.; Obluchinskaya, E.D.; Kosman, V.M.; Vuorela, H.; Makarov, V.G. Pharmacokinetic and Tissue Distribution of Fucoidan from Fucus vesiculosus after Oral Administration to Rats. Mar. Drugs 2018, 16, 132. [Google Scholar] [CrossRef]
- Mamangam, S.; Krishnan, D.A.; Hillary, V.E.; Raja, T.R.W.; Mathew, P.; Kumar, S.R.; Paulraj, M.G.; Ignacimuthu, S. Fucoidan Serves a Neuroprotective Effect in an Alzheimer’s Disease Model. Front. Biosci. 2020, 12, 855. [Google Scholar] [CrossRef]
- Kumar, L.R.G.; Paul, P.T.; Anas, K.K.; Tejpal, C.S.; Chatterjee, N.S.; Anupama, T.K.; Mathew, S.; Ravishankar, C.N. Phlorotannins-bioactivity and extraction perspectives. J. Appl. Phycol. 2022, 34, 2173–2185. [Google Scholar] [CrossRef]
- Bogolitsyn, K.; Druzhinina, A.; Kaplitsin, P.; Ovchinnikov, D.; Parshina, A.; Kuznetsova, M. Relationship between radical scavenging activity and polymolecular properties of brown algae polyphenols. Chem. Pap. 2019, 73, 2377–2385. [Google Scholar] [CrossRef]
- Paudel, P.; Seong, S.H.; Wu, S.; Park, S.; Jung, H.A.; Choi, J.S. Eckol as a Potential Therapeutic against Neurodegenerative Diseases Targeting Dopamine D₃/D₄ Receptors. Mar. Drugs 2019, 17, 108. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.; Valentão, P.; Andrade, P.B. Polyphenols from Brown Seaweeds (Ochrophyta, Phaeophyceae): Phlorotannins in the Pursuit of Natural Alternatives to Tackle Neurodegeneration. Mar. Drugs 2020, 18, 654. [Google Scholar] [CrossRef] [PubMed]
- Martins, B.; Vieira, M.; Delerue-Matos, C.; Grosso, C.; Soares, C. Biological Potential, Gastrointestinal Digestion, Absorption, and Bioavailability of Algae-Derived Compounds with Neuroprotective Activity: A Comprehensive Review. Mar. Drugs 2022, 20, 362. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Liu, F.; Lin, J.; Chen, H.; Huang, C.; Chen, L.; Zhou, Y.; Ye, L.; Zhang, K.; Jin, J.; et al. Fucoxanthin Inhibits β-Amyloid Assembly and Attenuates β-Amyloid Oligomer-Induced Cognitive Impairments. J. Agric. Food Chem. 2017, 65, 4092–4102. [Google Scholar] [CrossRef]
- Meinita, M.D.N.; Harwanto, D.; Tirtawijaya, G.; Negara, B.F.S.P.; Sohn, J.-H.; Kim, J.-S.; Choi, J.-S. Fucosterol of Marine Macroalgae: Bioactivity, Safety, and Toxicity on Organism. Mar. Drug 2021, 19, 545. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, J.; Zhan, N.; Yang, S.; Yu, M.; Liu, H. The pharmacokinetic characteristics and excretion studies of fucosterol from Sargassum fusiforme in rats. Biomed. Chromatogr. 2022, 36, e5309. [Google Scholar] [CrossRef]
- Bauer, S.; Jin, W.; Zhang, F.; Linhardt, R.J. The Application of Seaweed Polysaccharides and Their Derived Products with Potential for the Treatment of Alzheimer’s Disease. Mar. Drugs 2021, 19, 89. [Google Scholar] [CrossRef]
- Alghazwi, M.; Charoensiddhi, S.; Smid, S.; Zhang, W. Impact of Ecklonia Radiata Extracts on the Neuroprotective Activities against Amyloid Beta (Aβ1-42) Toxicity and Aggregation. J. Funct. Foods 2020, 68, 103893. [Google Scholar] [CrossRef]
- Nordberg, A.; Ballard, C.; Bullock, R.; Darreh-Shori, T.; Somogyi, M. A Review of Butyrylcholinesterase as a Therapeutic Target in the Treatment of Alzheimer’s Disease. Prim. Care Companion CNS Disord. 2013, 15. [Google Scholar] [CrossRef]
- Spehlmann, R.; Stahl, S.M. Dopamine Acetylcholine Imbalance in Parkinson’s Disease: Possible Regenerative of Overgrowth of Cholinergic Axon Terminal. Lancet 1976, 307, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Nho, J.A.; Shin, Y.S.; Jeong, H.-R.; Cho, S.; Heo, H.J.; Kim, G.H.; Kim, D.-O. Neuroprotective Effects of Phlorotannin-Rich Extract from Brown Seaweed Ecklonia Cava on Neuronal PC-12 and SH-SY5Y Cells with Oxidative Stress. J. Microbiol. Biotechnol. 2020, 30, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Kang, J.Y.; Kim, J.M.; Park, S.H.; Kwon, B.S.; Kim, G.-H.; Heo, H.J. Protective Effect of Fucoidan Extract from Ecklonia Cava on Hydrogen Peroxide-Induced Neurotoxicity. J. Microbiol. Biotechnol. 2018, 28, 40–49. [Google Scholar] [CrossRef]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial Membrane Potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef]
- Silva, J.; Alves, C.; Pinteus, S.; Mendes, S.; Pedrosa, R. Neuroprotective Effects of Seaweeds against 6-Hydroxidopamine-Induced Cell Death on an in Vitro Human Neuroblastoma Model. BMC Complement. Altern. Med. 2018, 18, 58. [Google Scholar] [CrossRef]
- Silva, J.; Alves, C.; Pinteus, S.; Mendes, S.; Pedrosa, R. Seaweeds’ neuroprotective potential set in vitro on a human cellular stress model. Mol. Cell Biochem. 2020, 473, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Kuo, C.-H.; Chen, P.-W. Compressional-Puffing Pretreatment Enhances Neuroprotective Effects of Fucoidans from the Brown Seaweed Sargassum Hemiphyllum on 6-Hydroxydopamine-Induced Apoptosis in SH-SY5Y Cells. Molecules 2017, 23, 78. [Google Scholar] [CrossRef] [PubMed]
- Olasehinde, T.A.; Olaniran, A.O.; Okoh, A.I. Aqueous-Ethanol Extracts of Some South African Seaweeds Inhibit Beta-Amyloid Aggregation, Cholinesterases, and Beta-Secretase Activities in Vitro. J. Food Biochem. 2019, 43, e12870. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, Y.; Li, Y.; Nie, Y.; Liang, J.; Liu, Y.; Liu, J.; Zhang, Y.; Song, C.; Qian, Z.; et al. Chemical Composition and Anti-Alzheimer’s Disease-Related Activities of a Functional Oil from the Edible Seaweed Hizikia Fusiforme. Chem. Biodivers. 2020, 17, e2000055. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.; Lee, H.; Kim, N.; Jo, H.G.; Woo, E.-R.; Lee, K.; Han, Y.S.; Park, S.R.; Ahn, G.; Cheong, S.H.; et al. The Anti-Oxidative and Anti-Neuroinflammatory Effects of Sargassum horneri by Heme Oxygenase-1 Induction in BV2 and HT22 Cells. Antioxidants 2021, 10, 859. [Google Scholar] [CrossRef]
- Fitton, J.; Dell’Acqua, G.; Gardiner, V.-A.; Karpiniec, S.; Stringer, D.; Davis, E. Topical Benefits of Two Fucoidan-Rich Extracts from Marine Macroalgae. Cosmetics 2015, 2, 66–81. [Google Scholar] [CrossRef]
- Nishibori, N.; Sagara, T.; Hiroi, T.; Sawaguchi, M.; Itoh, M.; Her, S.; Mortika, K. Protective effect of Undaria pinnatifida sporophyll extract on iron-induced cytotoxicity and oxidative stress in PC12 neuronal cells. Phytopharmacology 2012, 2, 271–284. [Google Scholar]
- Caruana, M.; Camilleri, A.; Farrugia, M.Y.; Ghio, S.; Jakubíčková, M.; Cauchi, R.J.; Vassallo, N. Extract from the Marine Seaweed Padina Pavonica Protects Mitochondrial Biomembranes from Damage by Amyloidogenic Peptides. Molecules 2021, 26, 1444. [Google Scholar] [CrossRef]
- Shanmuganathan, B.; Sheeja Malar, D.; Sathya, S.; Pandima Devi, K. Antiaggregation Potential of Padina Gymnospora against the Toxic Alzheimer’s Beta-Amyloid Peptide 25-35 and Cholinesterase Inhibitory Property of Its Bioactive Compounds. PLoS ONE 2015, 10, e0141708. [Google Scholar] [CrossRef] [PubMed]
- Machado, L.P.; Carvalho, L.R.; Young, M.C.M.; Cardoso-Lopes, E.M.; Centeno, D.C.; Zambotti-Villela, L.; Colepicolo, P.; Yokoya, N.S. Evaluation of Acetylcholinesterase Inhibitory Activity of Brazilian Red Macroalgae Organic Extracts. Rev. Bras. Farmacogn. 2015, 25, 657–662. [Google Scholar] [CrossRef]
- Sun, M.; Sheng, Y.; Zhu, Y. Ginkolide B Alleviates the Inflammatory Response and Attenuates the Activation of LPS-Induced BV-2 Cells In Vitro and In Vivo. Exp. Ther. Med. 2021, 21, 586. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.; Paíga, P.; Marques, M.; Neto, T.; Carvalho, A.P.; Paiva, A.; Simões, P.; Costa, L.; Bernardo, A.; Fernández, N.; et al. Multi-Step Subcritical Water Extracts of Fucus vesiculosus L. and Codium tomentosum Stackhouse: Composition, Health-Benefits and Safety. Processes 2021, 9, 893. [Google Scholar] [CrossRef]
- Milner, J. Cellular Regulation of SIRT1. Curr. Pharm. Des. 2009, 15, 39–44. [Google Scholar] [CrossRef]
- Herskovits, A.J.; Guarente, L. SIRT1 in Neurodevelopment and Brain Senescence. Neuron 2014, 81, 471–483. [Google Scholar] [CrossRef]
- Godoy, J.A.; Zolezzi, J.M.; Braidy, N.; Inestrosa, N.C. Role of SIRT1 during the Aging Process: Relevance to Protection of Synapses in the Brain. Mol. Neurobiol. 2014, 50, 744–756. [Google Scholar] [CrossRef] [PubMed]
- Ghemrawi, R.; Pooya, S.; Lorentz, S.; Gauchotte, G.; Arnold, C.; Gueant, J.L.; Battaglia-Hsu, S.-F. Decreased Vitamin B12 Availability Induces ER Stress Through Impaired SIRT1- Deacetylation of HSF1. Cell Death Dis. 2013, 4, e553. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, S.; Giles, A.; Nakamura, K.; Lee, J.W.; Hou, X.; Donmez, G.; Li, J.; Lou, Z.; Walsh, K.; et al. Hepatic Overexpression of SIRT1 in Mice Attenuates Endoplasmic Reticulum Stress and Insulin Resistance in the Liver. FASEB J. 2011, 25, 1664–1679. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.W.; Lee, K.T.; Lee, M.W.; Ka, K.H. SIRT1 Attenuates Palmitate-Induced Endoplasmic Reticulum Stress and Insulin Resistance in HepG2 Cells Via Induction of Oxygen-Regulated Protein 150. Biochem. Biophys. Res. Commun. 2012, 422, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Tirtawijaya, G.; Mohibbullah, M.; Meinita, M.D.N.; Moon, I.S.; Hong, Y.K. The Tropical Carrageenophyte Kappaphycus alvarezii Extract Promotes Axodendritic Maturation of Hippocampal Neurons in Primary Culture. J. Appl. Phycol. 2018, 30, 3233–3241. [Google Scholar] [CrossRef]
- Tirtawijaya, G.; Mohibbullah, M.; Meinita, M.D.N.; Moon, I.S.; Hong, Y.-K. The Ethanol Extract of the Rhodophyte Kappaphycus alvarezii Promotes Neurite Outgrowth in Hippocampal Neurons. J. Appl. Phycol. 2016, 28, 2515–2522. [Google Scholar] [CrossRef]
- Lauretti, E.; Dincer, O.; Patrico, D. Glycogen Synthase Kinase-3 Signaling in Alzheimer’s Disease. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118664. [Google Scholar] [CrossRef]
- Park, S.K.; Kang, J.Y.; Kim, J.M.; Yoo, S.K.; Han, H.J.; Chung, D.H.; Kim, D.-O.; Kim, G.-H.; Heo, H.J. Fucoidan-Rich Substances from Ecklonia Cava Improve Trimethyltin-Induced Cognitive Dysfunction via Down-Regulation of Amyloid β Production/Tau Hyperphosphorylation. Mar. Drugs 2019, 17, 591. [Google Scholar] [CrossRef]
- Balendra, V.; Singh, S.K. Therapeutic Potential of Astaxanthin and Super Oxide Dismutase in Alzheimer’s Disease. Open Biol. 2021, 11, 210013. [Google Scholar] [CrossRef] [PubMed]
- Haddad, M.; Herve, V.; Khedher, M.R.B.; Rabanel, J.M.; Ramassamy, C. Glutathione: An Old and Small Molecule with Great Functions and New Applications in the Brain and in Alzheimer’s Disease. Antioxid. Redox Signal. 2021, 35, 270–292. [Google Scholar] [CrossRef]
- Um, M.Y.; Lim, D.W.; Son, H.J.; Cho, S.; Lee, C. Phlorotannin-Rich Fraction from Ishige Foliacea Brown Seaweed Prevents the Scopolamine-Induced Memory Impairment via Regulation of ERK-CREB-BDNF Pathway. J. Funct. Foods 2018, 40, 110–116. [Google Scholar] [CrossRef]
- Ye, F.; Wu, A. The Protective Mechanism of SIRT1 in the Regulation of Mitochondrial Biogenesis and Mitochondrial autophagy in Alzheimer’s Disease. J. Alzheimer’s Dis. 2021, 82, 149–157. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, Y.; Sterling, K.; Song, W. Brain-derived Neurotropic Factor in Alzheimer’s Disease and its Pharmaceutical Potential. Transl. Neurodegener. 2022, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, D.; Lin, G.; Wu, Y.; Gao, L.; Ai, C.; Huang, Y.; Wang, M.; El-Seedi, H.R.; Chen, X.; et al. Anti-Ageing and Antioxidant Effects of Sulfate Oligosaccharides from Green Algae Ulva Lactuca and Enteromorpha prolifera in SAMP8 Mice. Int. J. Biol. Macromol. 2019, 139, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.Y.; Li, F.Y.; Kim, D.H.; Kim, S.J.; Kim, M.R. Enteromorpha prolifera Extract Improves Memory in Scopolamine-Treated Mice via Downregulating Amyloid-β Expression and Upregulating BDNF/TrkB Pathway. Antioxidants 2020, 9, 620. [Google Scholar] [CrossRef]
- Briffa, M.; Ghio, S.; Neuner, J.; Gauci, A.J.; Cacciottolo, R.; Marchal, C.; Caruana, M.; Cullin, C.; Vassallo, N.; Cauchi, R.J. Extracts from Two Ubiquitous Mediterranean Plants Ameliorate Cellular and Animal Models of Neurodegenerative Proteinopathies. Neurosci. Lett. 2017, 638, 12–20. [Google Scholar] [CrossRef]
- Chudasama, N.A.; Sequeira, R.A.; Moradiya, K.; Prasad, K. Seaweed Polysaccharide Based Products and Materials: An As-sessment on Their Production from a Sustainability Point of View. Molecules 2021, 26, 2608. [Google Scholar] [CrossRef]
- Venkatesan, J.; Lowe, B.; Anil, S.; Manivasagan, P.; Al Kheraif, A.A.; Kang, K.-H.; Kim, S.-K. Seaweed Polysaccharides and Their Potential Biomedical Applications. Starch Stärke 2015, 67, 381–390. [Google Scholar] [CrossRef]
- Pereira, L. Biological and Therapeutic Properties of the Seaweed Polysaccharides. Int. Biol. Rev. 2018, 2. [Google Scholar] [CrossRef]
- Pereira, L.; Valado, A. The Seaweed Diet in Prevention and Treatment of the Neurodegenerative Diseases. Mar. Drugs 2021, 19, 128. [Google Scholar] [CrossRef]
- Zhou, R.; Shi, X.-Y.; Bi, D.-C.; Fang, W.-S.; Wei, G.-B.; Xu, X. Alginate-Derived Oligosaccharide Inhibits Neuroinflammation and Promotes Microglial Phagocytosis of β-Amyloid. Mar. Drugs 2015, 13, 5828–5846. [Google Scholar] [CrossRef]
- Souza, R.B.; Frota, A.F.; Silva, J.; Alves, C.; Neugebauer, A.Z.; Pinteus, S.; Rodrigues, J.A.G.; Cordeiro, E.M.S.; De Almeida, R.R.; Pedrosa, R.; et al. In Vitro Activities of Kappa-Carrageenan Isolated from Red Marine Alga Hypnea musciformis: Antimicrobial, Anticancer and Neuroprotective Potential. Int. J. Biol. Macromol. 2018, 112, 1248–1256. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Kosturakis, A.K.; Cassidy, R.M.; Zhanh, H.; Kennamer-Chapman, R.M.; Jawad, A.B.; Colomand, C.M.; Harrison, D.S.; Dougherty, P.M. MAPK Signaling Downstream to TLR4 Contributes to Paclitaxel-induced Peripheral Neuropathy. Brain Behav. Immun. 2015, 49, 255–266. [Google Scholar] [CrossRef]
- Sakai, J.; Cammarota, E.; Wright, J.A.; Cicuta, P.; Gottschak, R.A.; Li, N.; Fraser, I.D.C.; Bryant, C.E. Lipopolysaccharide-induced NF-κB nuclear translocation is primarily dependent on MyD88, but TNFα expression requires TRIF and MyD88. Sci. Rep. 2017, 7, 1428. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Signaling to NF-kB by Toll Like Receptor. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, B.; Gozdz, A.; Zawazdka, M.; Ellert-Miklaszewska, A.; Lipko, M. MAPK Signal Trunsduction Underlying Brain Inflammation and Gliosis as Therapeutic Target. Anat. Rec. 2009, 292, 1902–1913. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Xu, L.; Jin, L.; Wang, B.; Fu, C.; Bai, Y.; Wu, H. κ-Carrageenan Oligosaccharides Inhibit the Inflammation of Lipopolysaccharide-Activated Microglia Via TLR4/NF-ΚB and P38/JNK MAPKs Pathways. Neurochem. Res. 2022, 47, 295–304. [Google Scholar] [CrossRef]
- Austin, S.; St-Pierre, J. PGC1-α and Mitochondrial Metabolism-Enmerging Concept and Relevance in Ageing and Neurodegenerative Disorders. J. Cell Sci. 2012, 125, 4963–4971. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Abramov, A.Y. The Emerging Role of Nrf2 in Mitochondrial Fucntion. Free Radic. Biol. Med. 2015, 88, 179–188. [Google Scholar] [CrossRef]
- Zhang, L.; Hao, J.; Zheng, Y.; Su, R.; Liao, Y.; Gong, X.; Liu, L.; Wang, X. Fucoidan Protects Dopaminergic Neurons by Enhancing the Mitochondrial Function in a Rotenone-Induced Rat Model of Parkinson’s Disease. Aging Dis. 2018, 9, 590–604. [Google Scholar] [CrossRef]
- Ramu, S.; Anbu, J.; Ammunje, D.N.; Krishnaraj, K. Fucoidan Isolated from Sargassum wightii Greville Ameliorates Intracerebro-Ventricular Streptozotocin Induced Cognitive Deficits, Oxidative Stress and Amyloidosis in Wistar Rats. Bioact. Carbohydr. Diet. Fibre 2022, 27, 100309. [Google Scholar] [CrossRef]
- Rocha, C.P.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Seaweeds as Valuable Sources of Essential Fatty Acids for Human Nutrition. Int. J. Environ. Res. Public Health 2021, 18, 4968. [Google Scholar] [CrossRef]
- Lorenzo, J.; Agregán, R.; Munekata, P.; Franco, D.; Carballo, J.; Şahin, S.; Lacomba, R.; Barba, F. Proximate Composition and Nutritional Value of Three Macroalgae: Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata. Mar. Drugs 2017, 15, 360. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.; Lopes, D.; Da Costa, E.; Conde, T.; Rego, A.; Ribeiro, A.I.; Abreu, M.H.; Domingues, M.R. Seaweed Blends as a Valuable Source of Polyunsaturated and Healthy Fats for Nutritional and Food Applications. Mar. Drugs 2021, 19, 684. [Google Scholar] [CrossRef]
- Susanto, E.; Fahmi, A.S.; Abe, M.; Hosokawa, M.; Miyashita, K. Lipids, Fatty Acids, and Fucoxanthin Content from Temperate and Tropical Brown Seaweeds. Aquat. Procedia 2016, 7, 66–75. [Google Scholar] [CrossRef]
- Marinho, G.; Holdt, S.; Jacobsen, C.; Angelidaki, I. Lipids and Composition of Fatty Acids of Saccharina latissima Cultivated Year-Round in Integrated Multi-Trophic Aquaculture. Mar. Drugs 2015, 13, 4357–4374. [Google Scholar] [CrossRef]
- Dyall, S.C. Long-Chain Omega-3 Fatty Acids and the Brain: A Review of the Independent and Shared Effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015, 7, 52. [Google Scholar] [CrossRef]
- Bentsen, H. Dietary Polyunsaturated Fatty Acids, Brain Function and Mental Health. Microb. Ecol. Health Dis. 2017, 28, 1281916. [Google Scholar] [CrossRef]
- Beltz, B.S.; Tlusty, M.F.; Benton, J.L.; Sandeman, D.C. Omega-3 Fatty Acids Upregulate Adult Neurogenesis. Neurosci. Lett. 2007, 415, 154–158. [Google Scholar] [CrossRef]
- Qin, L.; He, J.; Hanes, R.N.; Pluzarev, O.; Hong, J.-S.; Crews, F.T. Increased Systemic and Brain Cytokine Production and Neuroinflammation by Endotoxin Following Ethanol Treatment. J. Neuroinflamm. 2008, 5, 10. [Google Scholar] [CrossRef]
- Hashimoto, M.; Hossain, S.; Tanabe, Y.; Kawashima, A.; Harada, T.; Yano, T.; Mizuguchi, K.; Shido, O. The Protective Effect of Dietary Eicosapentaenoic Acid against Impairment of Spatial Cognition Learning Ability in Rats Infused with Amyloid β(1–40). J. Nutr. Biochem. 2009, 20, 965–973. [Google Scholar] [CrossRef]
- Dyall, S.C.; Michael-Titus, A.T. Neurological Benefits of Omega-3 Fatty Acids. Neuromol. Med. 2008, 10, 219–235. [Google Scholar] [CrossRef]
- Dehkordi, N.G.; Noorbakhshnia, M.; Ghaedi, K.; Esmaeili, A.; Dabaghi, M. Omega-3 Fatty Acids Prevent LPS-Induced Passive Avoidance Learning and Memory and CaMKII-α Gene Expression Impairments in Hippocampus of Rat. Pharmacol. Rep. 2015, 67, 370–375. [Google Scholar] [CrossRef]
- De Andrade, A.M.; Da Cruz Fernandes, M.; De Fraga, L.S.; Porawski, M.; Giovenardi, M.; Guedes, R.P. Omega-3 Fatty Acids Revert High-Fat Diet-Induced Neuroinflammation but Not Recognition Memory Impairment in Rats. Metab. Brain Dis. 2017, 32, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Yoshimori, T. How to Interpret LC3 Immunoblotting. Autophagy 2007, 3, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Tanaka, M.; Masuda, S.; Ohue-Kitano, R.; Yamakage, H.; Muranaka, K.; Wada, H.; Kusakabe, T.; Shimatsu, A.; Hasegawa, K.; et al. Omega-3 Polyunsaturated Fatty Acids Suppress the Inflammatory Responses of Lipopolysaccharide-Stimulated Mouse Microglia by Activating SIRT1 Pathways. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2017, 1862, 552–560. [Google Scholar] [CrossRef]
- Dong, Y.; Xu, M.; Kalueff, A.V.; Song, C. Dietary Eicosapentaenoic Acid Normalizes Hippocampal Omega-3 and 6 Polyunsaturated Fatty Acid Profile, Attenuates Glial Activation and Regulates BDNF Function in a Rodent Model of Neuroinflammation Induced by Central Interleukin-1β Administration. Eur. J. Nutr. 2018, 57, 1781–1791. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Pu, K.; Duan, W.; Chen, H.; Chen, L.; Wang, Y. Involvement of Akt/CREB Signaling Pathways in the Protective Effect of EPA against Interleukin-1β-Induced Cytotoxicity and BDNF down-Regulation in Cultured Rat Hippocampal Neurons. BMC Neurosci. 2018, 19, 52. [Google Scholar] [CrossRef]
- Barroso-Hernández, A.; Ramírez-Higuera, A.; Peña-Montes, C.; Cortés-Ramírez, S.A.; Rodríguez-Dorantes, M.; López-Franco, Ó.; Oliart-Ros, R.M. Beneficial Effects of an Algal Oil Rich in ω-3 Polyunsaturated Fatty Acids on Locomotor Function and D2 Dopamine Receptor in Haloperidol-Induced Parkinsonism. Nutr. Neurosci. 2022, 25, 519–529. [Google Scholar] [CrossRef]
- Taoro-González, L.; Pereda, D.; Valdés-Baizabal, C.; González-Gómez, M.; Pérez, J.A.; Mesa-Herrera, F.; Canerina-Amaro, A.; Pérez-González, H.; Rodríguez, C.; Díaz, M.; et al. Effects of Dietary N-3 LCPUFA Supplementation on the Hippocampus of Aging Female Mice: Impact on Memory, Lipid Raft-Associated Glutamatergic Receptors and Neuroinflammation. Int. J. Mol. Sci. 2022, 23, 7430. [Google Scholar] [CrossRef]
- Luchtman, D.W.; Meng, Q.; Song, C. Ethyl-Eicosapentaenoate (E-EPA) Attenuates Motor Impairments and Inflammation in the MPTP-Probenecid Mouse Model of Parkinson’s Disease. Behav. Brain Res. 2012, 226, 386–396. [Google Scholar] [CrossRef]
- Lu, D.-Y.; Tsao, Y.-Y.; Leung, Y.-M.; Su, K.-P. Docosahexaenoic Acid Suppresses Neuroinflammatory Responses and Induces Heme Oxygenase-1 Expression in BV-2 Microglia: Implications of Antidepressant Effects for Omega-3 Fatty Acids. Neuropsychopharmacology 2010, 35, 2238–2248. [Google Scholar] [CrossRef]
- Wu, F.; Wang, D.; Shi, H.; Wang, C.; Xue, C.; Wang, Y.; Zhang, T. N-3 PUFA-Deficiency in Early Life Exhibits Aggravated MPTP-Induced Neurotoxicity in Old Age While Supplementation with DHA/EPA-Enriched Phospholipids Exerts a Neuroprotective Effect. Mol. Nutr. Food Res. 2021, 65, 2100339. [Google Scholar] [CrossRef]
- Khan, F.; Jeong, G.-J.; Khan, M.S.A.; Tabassum, N.; Kim, Y.-M. Seaweed-Derived Phlorotannins: A Review of Multiple Biological Roles and Action Mechanisms. Mar. Drugs 2022, 20, 384. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Youn, K.; Kim, D.; Ahn, M.-R.; Yoon, E.; Kim, O.-Y.; Jun, M. Anti-Neuroinflammatory Property of Phlorotannins from Ecklonia cava on Aβ25–35-Induced Damage in PC12 Cells. Mar. Drugs 2018, 17, 7. [Google Scholar] [CrossRef]
- Lee, J.; Jun, M. Dual BACE1 and Cholinesterase Inhibitory Effects of Phlorotannins from Ecklonia cava—An In Vitro and in Silico Study. Mar. Drugs 2019, 17, 91. [Google Scholar] [CrossRef]
- Yoon, J.-H.; Lee, N.; Youn, K.; Jo, M.R.; Kim, H.-R.; Lee, D.-S.; Ho, C.-T.; Jun, M. Dieckol Ameliorates Aβ Production via PI3K/Akt/GSK-3β Regulated APP Processing in SweAPP N2a Cell. Mar. Drugs 2021, 19, 152. [Google Scholar] [CrossRef]
- Peng, J.; Yuan, J.-P.; Wu, C.-F.; Wang, J.-H. Fucoxanthin, a Marine Carotenoid Present in Brown Seaweeds and Diatoms: Metabolism and Bioactivities Relevant to Human Health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, K.; Beppu, F.; Hosokawa, M.; Liu, X.; Wang, S. Nutraceutical Characteristics of the Brown Seaweed Carotenoid Fucoxanthin. Arch. Biochem. Biophys. 2020, 686, 108364. [Google Scholar] [CrossRef]
- Pangestuti, R.; Vo, T.-S.; Ngo, D.-H.; Kim, S.-K. Fucoxanthin Ameliorates Inflammation and Oxidative Reponses in Microglia. J. Agric. Food Chem. 2013, 61, 3876–3883. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.; Barbosa, M.; Pereira, D.M.; Sousa-Pinto, I.; Valentão, P.; Azevedo, I.C.; Andrade, P.B. Chemical Profiling of Edible Seaweed (Ochrophyta) Extracts and Assessment of Their in Vitro Effects on Cell-Free Enzyme Systems and on the Viability of Glutamate-Injured SH-SY5Y Cells. Food Chem. Toxicol. 2018, 116, 196–206. [Google Scholar] [CrossRef]
- Yu, J.; Lin, J.-J.; Yu, R.; He, S.; Wang, Q.-W.; Cui, W.; Zhang, J.-R. Fucoxanthin prevents H2O2-induced neuronal apoptosis via concurrently activating the PI3-K/Akt cascade and inhibiting the ERK pathway. Food Nutr. Res. 2017, 61, 1304678. [Google Scholar] [CrossRef]
- Alghazwi, M.; Smid, S.; Musgrave, I.; Zhang, W. In Vitro Studies of the Neuroprotective Activities of Astaxanthin and Fucoxanthin against Amyloid Beta (Aβ1-42) Toxicity and Aggregation. Neurochem. Int. 2019, 124, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.T.; Plowey, E.D.; Dagda, R.K.; Hickey, R.W.; Cherra, S.J.; Clark, R.S.B. Autophagy in Neurite Injury and Neurodegeneration. Methods Enzymol. 2009, 453, 217–249. [Google Scholar] [CrossRef]
- Carnicella, S.; Drui, G.; Boulet, S.; Carcenac, C.; Favier, M.; Duran, T.; Savasta, M. Implication of Dopamine D3 Receptor Activation in the Reversion of Parkinson Disease-Related Motivational Deficits. Transl. Psychiatry 2014, 4, 401. [Google Scholar] [CrossRef]
- Cho, H.U.; Kim, S.; Kim, J.; Yang, S.; An, H.; Nam, M.H.; Jang, D.P.; Lee, C.J. Redefining Differential Role of MAO-A in Dopamine Degradation and MAO-B in Tonic GABA Synthesis. Exp. Mol. Med. 2021, 53, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Paudel, P.; Seong, S.H.; Jung, H.A.; Choi, J.S. Characterizing Fucoxanthin as a Selective Dopamine D3/D4 Receptor Agonist: Relevance to Parkinson’s Disease. Chem. Biol. Interact. 2019, 310, 108757. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Xin, T.; Zhang, R.; Liu, C.; Pang, Q. Fucoxanthin Attenuates Behavior Deficits and Neuroinflammatory Response in 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Parkinson’s Disease in Mice. Pharmacogn. Mag. 2020, 16, 51. [Google Scholar] [CrossRef]
- Kawasaki, A.; Ono, A.; Mizuta, S.; Kamiya, M.; Takenaga, T.; Murakami, S. The Taurine Content of Japanese Seaweed. Adv. Exp. Med. Biol. 2017, 975, 1105–1112. [Google Scholar] [CrossRef]
- Jakaria, M.; Azam, S.; Haque, M.E.; Jo, S.-H.; Uddin, M.S.; Kim, I.-S.; Choi, D.-K. Taurine and Its Analogs in Neurological Disorders: Focus on Therapeutic Potential and Molecular Mechanisms. Redox Biol. 2019, 24, 101223. [Google Scholar] [CrossRef]
- Chung, M.; Malatesta, P.; Bosquesi, P.; Yamasaki, P.; Dos Santos, J.L.; Vizioli, E. Advances in Drug Design Based on the Amino Acid Approach: Taurine Analogues for the Treatment of CNS Diseases. Pharmaceuticals 2012, 5, 1128–1146. [Google Scholar] [CrossRef]
- Oh, S.J.; Lee, H.-J.; Jeong, Y.J.; Nam, K.R.; Kang, K.J.; Han, S.J.; Lee, K.C.; Lee, Y.J.; Choi, J.Y. Evaluation of the Neuroprotective Effect of Taurine in Alzheimer’s Disease Using Functional Molecular Imaging. Sci. Rep. 2020, 10, 15551. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Lee, S.; Choi, S.L.; Kim, H.Y.; Baek, S.; Kim, Y. Taurine Directly Binds to Oligomeric Amyloid-β and Recovers Cognitive Deficits in Alzheimer Model Mice. Adv. Exp. Med. Biol. 2017, 975, 233–241. [Google Scholar] [CrossRef]
- Lu, C.-L.; Tang, S.; Meng, Z.-J.; He, Y.-Y.; Song, L.-Y.; Liu, Y.-P.; Ma, N.; Li, X.-Y.; Guo, S.-C. Taurine Improves the Spatial Learning and Memory Ability Impaired by Sub-Chronic Manganese Exposure. J. Biomed. Sci. 2014, 21, 51. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, H.V.; Yoon, J.H.; Kang, B.R.; Cho, S.M.; Lee, S.; Kim, J.Y.; Kim, J.W.; Cho, Y.; Woo, J.; et al. Taurine in Drinking Water Recovers Learning and Memory in the Adult APP/PS1 Mouse Model of Alzheimer’s Disease. Sci. Rep. 2014, 4, 7467. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Feng, Y.; Wang, X.-X.; Truong, D.; Wu, Y.-C. The Critical Role of SIRT1 in Parkinson’s Disease: Mechanism and Therapeutic Considerations. Aging Dis. 2020, 11, 1608–1622. [Google Scholar] [CrossRef]
- Terriente-Palacios, C.; Rubiño, S.; Hortós, M.; Peteiro, C.; Castellari, M. Taurine, Homotaurine, GABA and Hydrophobic Amino Acids Content Influences “in Vitro” Antioxidant and SIRT1 Modulation Activities of Enzymatic Protein Hydrolysates from Algae. Sci. Rep. 2022, 12, 20832. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, B.Y.; Wang, K.D.; Zhang, B.F.; Huang, M. Protective Effects of Taurine on Neurons and Microglia in Parkinson’s Disease-like Mouse Model Induced by Paraquat. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2020, 38, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, B.; Tian, T.; Zhang, B.; Shi, G.; Zhang, C.; Li, G.; Huang, M. Taurine Protects Dopaminergic Neurons in Paraquat-Induced Parkinson’s Disease Mouse Model through PI3K/Akt Signaling Pathways. Amino Acids 2022, 54, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Shi, Y.; Liu, W.; Liu, S.; Sun, M.-Z. Taurine Improves Neuron Injuries and Cognitive Impairment in a Mouse Parkinson’s Disease Model through Inhibition of Microglial Activation. Neurotoxicology 2021, 83, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Che, Y.; Hou, L.; Sun, F.; Zhang, C.; Liu, X.; Piao, F.; Zhang, D.; Li, H.; Wang, Q. Taurine Protects Dopaminergic Neurons in a Mouse Parkinson’s Disease Model through Inhibition of Microglial M1 Polarization. Cell Death Dis. 2018, 9, 435. [Google Scholar] [CrossRef] [PubMed]
- Abuirmeileh, A.N.; Abuhamdah, S.M.; Ashraf, A.; Alzoubi, K.H. Protective Effect of Caffeine and/or Taurine on the 6-Hydroxydopamine-Induced Rat Model of Parkinson’s Disease: Behavioral and Neurochemical Evidence. Restor. Neurol. Neurosci. 2021, 39, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Che, Y.; Sun, F.; Wang, Q. Taurine Protects Noradrenergic Locus Coeruleus Neurons in a Mouse Parkinson’s Disease Model by Inhibiting Microglial M1 Polarization. Amino Acids 2018, 50, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Martens, N.; Schepers, M.; Zhan, N.; Leijten, F.; Voortman, G.; Tiane, A.; Rombaut, B.; Poisquet, J.; Sande, N.v.d.; Kerksiek, A.; et al. 24(S)-Saringosterol Prevents Cognitive Decline in a Mouse Model for Alzheimer’s Disease. Mar. Drugs 2021, 19, 190. [Google Scholar] [CrossRef]
- Dai, Y.; Tan, X.; Wu, W.; Warner, M.; Gustafsson, J.-Å. Liver X Receptor β Protects Dopaminergic Neurons in a Mouse Model of Parkinson Disease. Proc. Natl. Acad. Sci. USA 2012, 109, 13112–13117. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, J.; Fu, Z.; Ye, C.; Zhang, R.; Song, Y.; Zhang, Y.; Li, H.; Ying, H.; Liu, H. 24(S)-Saringosterol from Edible Marine Seaweed Sargassum fusiforme Is a Novel Selective LXRβ Agonist. J. Agric. Food Chem. 2014, 62, 6130–6137. [Google Scholar] [CrossRef]
- Gan, S.Y.; Wong, L.Z.; Wong, J.W.; Tan, E.L. Fucosterol Exerts Protection against Amyloid β-Induced Neurotoxicity, Reduces Intracellular Levels of Amyloid β and Enhances the MRNA Expression of Neuroglobin in Amyloid β-Induced SH-SY5Y Cells. Int. J. Biol. Macromol. 2019, 121, 207–213. [Google Scholar] [CrossRef]
- Wong, C.H.; Gan, S.Y.; Tan, S.C.; Gany, S.A.; Ying, T.; Gray, A.I.; Igoli, J.; Chan, E.W.L.; Phang, S.M. Fucosterol Inhibits the Cholinesterase Activities and Reduces the Release of Pro-Inflammatory Mediators in Lipopolysaccharide and Amyloid-Induced Microglial Cells. J. Appl. Phycol. 2018, 30, 3261–3270. [Google Scholar] [CrossRef]
- Filippini, M.; Baldisserotto, A.; Menotta, S.; Fedrizzi, G.; Rubini, S.; Gigliotti, D.; Valpiani, G.; Buzzi, R.; Manfredini, S.; Vertuani, S. Heavy Metals and Potential Risks in Edible Seaweed on the Market in Italy. Chemosphere 2021, 263, 127983. [Google Scholar] [CrossRef]
- Chen, Q.; Pan, X.-D.; Huang, B.-F.; Han, J.-L. Distribution of Metals and Metalloids in Dried Seaweeds and Health Risk to Population in Southeastern China. Sci. Rep. 2018, 8, 3578. [Google Scholar] [CrossRef]
- Ramu, S.; Murali, A.; Jayaraman, A. Phytochemical Screening and Toxicological Evaluation of Sargassum Wightii Greville in Wistar Rats. Turk. J. Pharm. Sci. 2019, 16, 466–475. [Google Scholar] [CrossRef]
- Tapia-Martínez, J.; Cano-Europa, E.; Casas-Valdez, M.; Blas-Valdivia, V.; Franco-Colin, M. Toxicological and Therapeutic Evaluation of the Algae Macrocystis pyrifera (Phaeophyceae) in Rodents. Rev. Biol. Mar. Oceanogr. 2020, 55, 119–127. [Google Scholar] [CrossRef]
- Taylor, V.F.; Li, Z.; Sayarath, V.; Palys, T.J.; Morse, K.R.; Scholz-Bright, R.A.; Karagas, M.R. Distinct Arsenic Metabolites Following Seaweed Consumption in Humans. Sci. Rep. 2017, 7, 3920. [Google Scholar] [CrossRef]
- Lee, D.H.; Park, M.Y.; Shim, B.J.; Youn, H.J.; Hwang, H.J.; Shin, H.C.; Jeon, H.K. Effects of Ecklonia cava Polyphenol in Individuals with Hypercholesterolemia: A Pilot Study. J. Med. Food 2012, 15, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-C.; Kim, S.H.; Park, Y.; Lee, B.H.; Hwang, H.J. Effects of 12-Week Oral Supplementation of Ecklonia cava Polyphenols on Anthropometric and Blood Lipid Parameters in Overweight Korean Individuals: A Double-Blind Randomized Clinical Trial. Phytother. Res. 2012, 26, 363–368. [Google Scholar] [CrossRef]
- Choi, E.-K.; Park, S.-H.; Ha, K.-C.; Noh, S.-O.; Jung, S.-J.; Chae, H.-J.; Chae, S.-W.; Park, T.-S. Clinical Trial of the Hypolipidemic Effects of a Brown Alga Ecklonia cava Extract in Patients with Hypercholesterolemia. Int. J. Pharmacol. 2015, 11, 798–805. [Google Scholar] [CrossRef]
- Yun, J.-W.; Kim, S.-H.; Kim, Y.-S.; You, J.-R.; Cho, E.-Y.; Yoon, J.-H.; Kwon, E.; Yun, I.-J.; Oh, J.-H.; Jang, J.-J.; et al. Enzymatic Extract from Ecklonia cava: Acute and Subchronic Oral Toxicity and Genotoxicity Studies. Regul. Toxicol. Pharmacol. 2018, 92, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.-A.; Yan, M.-D.; Lin, H.-T.; Li, K.-L.; Lin, Y.-C. Toxicological Evaluation of Low Molecular Weight Fucoidan in Vitro and in Vivo. Mar. Drugs 2016, 14, 121. [Google Scholar] [CrossRef] [PubMed]
- Song, M.Y.; Ku, S.K.; Han, J.S. Genotoxicity Testing of Low Molecular Weight Fucoidan from Brown Seaweeds. Food Chem. Toxicol. 2012, 50, 790–796. [Google Scholar] [CrossRef]
- Bae, M.; Kim, M.B.; Park, Y.K.; Lee, J.Y. Health benefits of fucoxanthin in the prevention of chronic diseases. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158618. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA); Turck, D.; Bresson, J.-L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; et al. Safety of Ecklonia cava phlorotannins as a novel food pursuant to Regulation (EC) No 258/97. EFSA J. 2017, 15, e05003. [Google Scholar] [CrossRef]
- Li, Y.; Qian, Z.J.; Kim, M.M.; Kim, S.K. Cytotoxic activities of phlorethol and fucophlorethol derivatives isolated from Laminariaceae Ecklonia cava. J. Food Biochem. 2011, 35, 357–369. [Google Scholar] [CrossRef]
- Kang, M.C.; Kang, S.M.; Ahn, G.; Kim, K.N.; Kang, N.; Samarakoon, K.W.; Oh, M.C.; Lee, J.S.; Jeon, Y.J. Protective effect of a marine polyphenol, dieckol against carbon tetrachloride-induced acute liver damage in mouse. Environ. Toxicol. Pharmacol. 2013, 35, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Rajan, D.K.; Mohan, K.; Zhang, S.; Ganesan, A.R. Dieckol: A brown algal phlorotannin with biological potential. Biomed. Pharmacother. 2021, 142, 111988. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Feng, T.; Zhang, J.; Huang, X.; Wang, T.; Xie, Z.; Chu, X.; Yang, J.; Wang, H.; et al. Sodium Oligomannate Therapeutically Remodels Gut Microbiota and Suppresses Gut Bacterial Amino Acids-Shaped Neuroinflammation to Inhibit Alzheimer’s Disease Progression. Cell Res. 2019, 29, 787–803. [Google Scholar] [CrossRef]
- Wang, T.; Kuang, W.; Chen, W.; Xu, W.; Zhang, L.; Li, Y.; Li, H.; Peng, Y.; Chen, Y.; Wang, B.; et al. A Phase II Randomized Trial of Sodium Oligomannate in Alzheimer’s Dementia. Alzheimer’s Res. Ther. 2020, 12, 110. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Chan, P.; Wang, T.; Hong, Z.; Wang, S.; Kuang, W.; He, J.; Pan, X.; Zhou, Y.; Ji, Y.; et al. A 36-Week Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Phase 3 Clinical Trial of Sodium Oligomannate for Mild-to-Moderate Alzheimer’s Dementia. Alzheimer’s Res. Ther. 2021, 13, 62. [Google Scholar] [CrossRef]
- Syed, Y.Y. Sodium Oligomannate: First Approval. Drugs 2020, 80, 441–444. [Google Scholar] [CrossRef]
- Tolar, M.; Abushakra, S.; Hey, J.A.; Porsteinsson, A.; Sabbagh, M. Aducanumab, gantenerumab, BAN2401, and ALZ-801-the first wave of amyloid-targeting drugs for Alzheimer’s disease with potential for near term approval. Alzheimer’s Res. Ther. 2020, 12, 95. [Google Scholar] [CrossRef]
- Bolea, I.; Gella, A.; Unzeta, M. Propargylamine-derived multitarget-directed ligands: Fighting Alzheimer’s disease with monoamine oxidase inhibitors. J. Neural Transm. 2013, 120, 893–902. [Google Scholar] [CrossRef]
- Generalić Mekinić, I.; Skroza, D.; Šimat, V.; Hamed, I.; Čagalj, M.; Popović Perković, Z. Phenolic Content of Brown Algae (Pheophyceae) Species: Extraction, Identification, and Quantification. Biomolecules 2019, 9, 244. [Google Scholar] [CrossRef]
- Wang, S.-H.; Huang, C.-Y.; Chen, C.-Y.; Chang, C.-C.; Huang, C.-Y.; Dong, C.-D.; Chang, J.-S. Isolation and purification of brown algae fucoidan from Sargassum siliquosum and the analysis of anti-lipogenesis activity. Biochem. Eng. J. 2021, 165, 107798. [Google Scholar] [CrossRef]
- Maghraby, Y.R.; Farag, M.A.; Kontominas, M.G.; Shakour, Z.T.; Ramadan, A.R. Nanoencapsulated Extract of a Red Seaweed (Rhodophyta) Species as a Promising Source of Natural Antioxidants. ACS Omega 2022, 7, 6539–6548. [Google Scholar] [CrossRef] [PubMed]
- Ramos-de-la-Peña, A.M.; Contreras-Esquivel, J.C.; Aguilar, O.; González-Valdez, J. Structural and Bioactive Roles of Fucoidan in Nanogel Delivery Systems. A Review. Carbohydr. Polym. Technol. Appl. 2022, 4, 100235. [Google Scholar] [CrossRef]
- Zhang, S.; Qamar, S.A.; Junaid, M.; Munir, B.; Badar, Q.; Bilal, M. Algal Polysaccharides-Based Nanoparticles for Targeted Drug Delivery Applications. Starch Stärke 2022, 74, 2200014. [Google Scholar] [CrossRef]

| Name | Extraction Method | Model | Dose | Effect | References |
|---|---|---|---|---|---|
| Ecklonia cava | Polysaccharide, phlorotannin-rich extract | Aβ1–42-induced PC-12 | 100 µg/mL | Exhibited antiapoptotic and neurite outgrowth-enhancing properties | [79] |
| K. alvarezii | Ethanolic extract | Fetal rat hippocampal neuron | 1 µg/mL | Promotes neural outgrowth | [80,81] |
| Ecklonia cava | Phlorotannin-rich extract | H2O2 and AAPH-induced PC-12, SH-SY5Y | 62.5 and 30 µg/mL | Inhibition of AChE and BChE activities and antioxidant-enhancing properties | [82] |
| Fucoidan-rich extract | H2O2-induced PC-12 and MC-IXC cells | 50 and 100 µg/mL | Regulation of mitochondrial function | [83] | |
| E. maxima and G. gracilis | Aqueous extract | In vitro assay (Aβ1–42 was incubated with extract for 0–96 h) | 3.09 mg/mL | Inhibition of Aβ1–42 accumulation and antioxidant activity | [87] |
| Hizikia fusiforme | Functional oil | LPS-stimulated BV-2 microglia cells | 1.00 ± 0.03 mg/mL, 20 mg/mL | Exhibited AChE and BChE inhibition, and antioxidant and anti-inflammation activities | [88] |
| S. muticum and S. polyschides | Methanolic extract | Dopamine and 6-OHDA-induced SH-SY5Y | 1000 µg/mL | Repolarization of the mitochondrial membrane potential | [89,91] |
| Sargassum hemiphyllum | Fucoidan-rich extract | 6-OHDA-induced SH-SY5Y | 500 µg/mL | Exhibited antioxidant and antiapoptotic properties | [94] |
| P. pavonica | Acetone extract | Aβ-induced SH-SY5Y cell | 50 µg/mL | Maintained mitochondrial function and inhibition of protein aggregation | [95] |
| S. horneri | 70% EtOH extract, CH2Cl2 soluble fraction, and water-soluble fraction | LPS-induced BV-2 microglia cells | 100 µg/mL, 100 µg/mL, and 200 µg/mL | Exhibited anti-inflammatory potential by preventing the activation of NF-κB/p-65 signaling | [97] |
| U. pinnatifida, F. vesciculosus | Whole-plant extract | MiaPaCa-2 cells (Human pancreatic epithelial cells pretreated with extract) | 4.9 and 14.8 µg/mL | Enhanced SIRT1 expression and antioxidant activity | [104] |
| U. pinnatifida | Whole-plant extract | Iron-induced PC-12 cells | 200 µg/mL | Reduces oxidative damage by reducing lipid peroxidation and restoring antioxidant enzyme activities | [105] |
| E. cava | Fucoidan and polyphenol extract | Trimethyltin-induced ICR mice | A mixture of fucoidan and polyphenol in a 4:6 ratio | Improves the spatial learning and memory function, restoration of mitochondrial membrane potential, AChE inhibition, and upregulation of Akt/GSK-3β expression | [107] |
| I. foliacea | Phlorotannin-rich extract | Scopolamine-induced AD mouse | 50 and 100 mg/kg/b.w. | Improved spatial learning and cognitive function and upregulated ERK-CREB-BDNF signaling | [110] |
| Ulva lactuca and Enteromorpha prolifera | Aqueous extract | Senescence-accelerated prone (SAMP8) mice | 150 mg/kg/b.w. | Exhibited anti-inflammatory activity, increased expression in Sirt1, and elevated BDNF and ChAT levels | [111] |
| Enteromorpha prolifera | Ethyl acetate extract | Scopolamine-induced AD ICR mice | 50 and 100 mg/kg/b.w. | Improved spatial learning and memory function, increased BDNF expression, and inhibited Aβ and tau expression | [115] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jannat, K.; Balakrishnan, R.; Han, J.-H.; Yu, Y.-J.; Kim, G.-W.; Choi, D.-K. The Neuropharmacological Evaluation of Seaweed: A Potential Therapeutic Source. Cells 2023, 12, 2652. https://doi.org/10.3390/cells12222652
Jannat K, Balakrishnan R, Han J-H, Yu Y-J, Kim G-W, Choi D-K. The Neuropharmacological Evaluation of Seaweed: A Potential Therapeutic Source. Cells. 2023; 12(22):2652. https://doi.org/10.3390/cells12222652
Chicago/Turabian StyleJannat, Khoshnur, Rengasamy Balakrishnan, Jun-Hyuk Han, Ye-Ji Yu, Ga-Won Kim, and Dong-Kug Choi. 2023. "The Neuropharmacological Evaluation of Seaweed: A Potential Therapeutic Source" Cells 12, no. 22: 2652. https://doi.org/10.3390/cells12222652
APA StyleJannat, K., Balakrishnan, R., Han, J.-H., Yu, Y.-J., Kim, G.-W., & Choi, D.-K. (2023). The Neuropharmacological Evaluation of Seaweed: A Potential Therapeutic Source. Cells, 12(22), 2652. https://doi.org/10.3390/cells12222652









