A New Synthetic Curcuminoid Displays Antitumor Activities in Metastasized Melanoma
Abstract
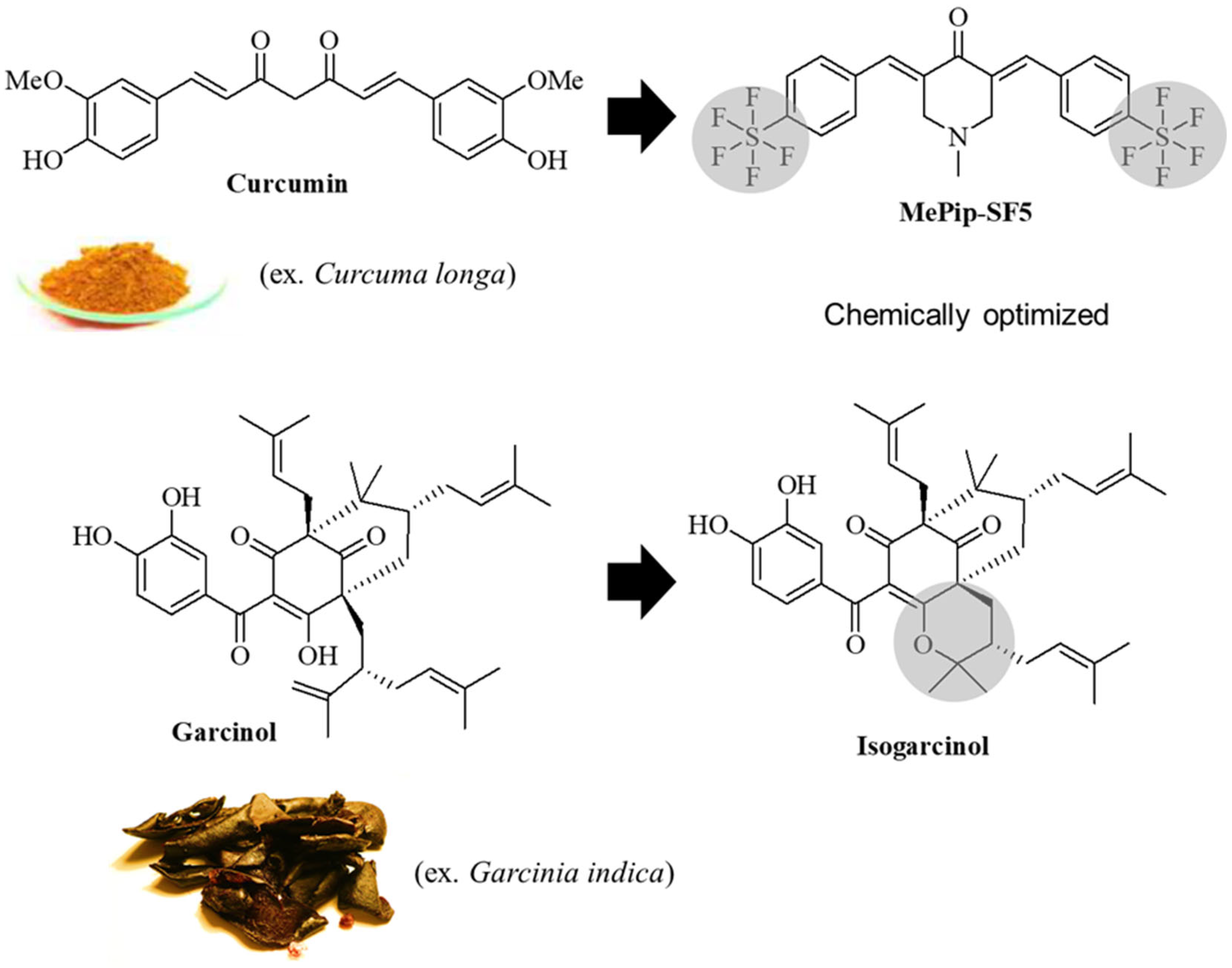
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cytotoxicity Assay
2.3. Bone-Marrow-Derived Macrophages (BMDM)
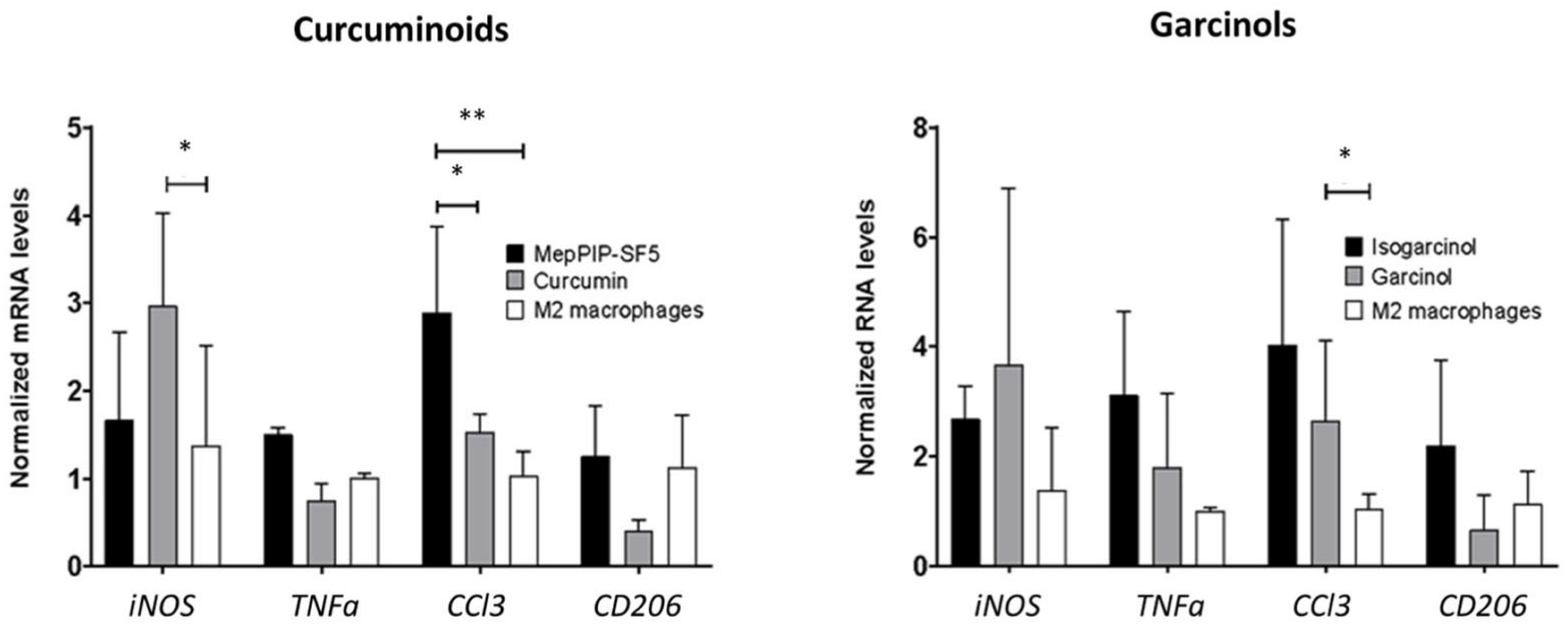
2.4. Phenotype Modulation of BMDM with MePiP-SF5 and Isogarcinol
2.5. Quantitative Reverse Transcription PCR (RT-qPCR)
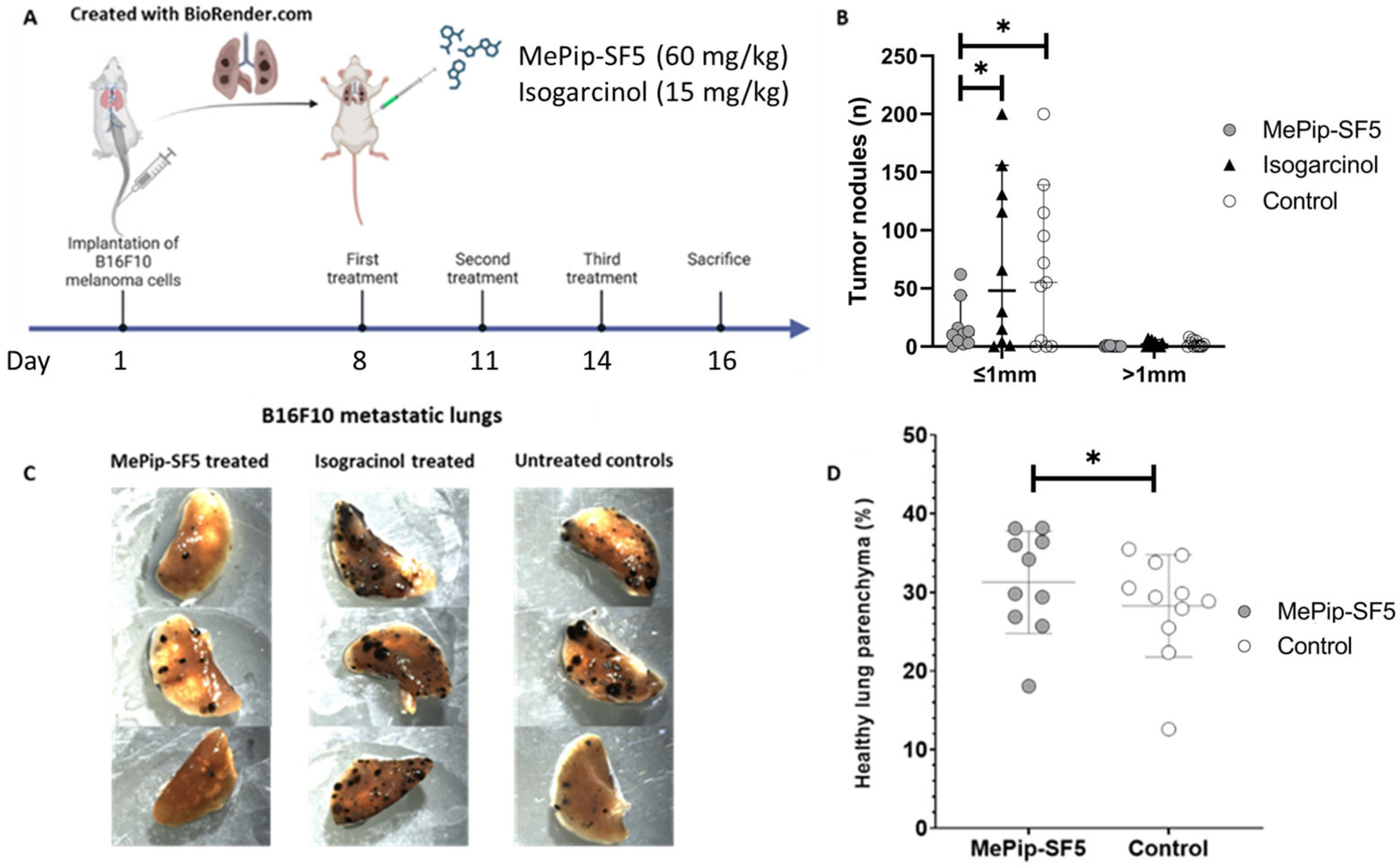
2.6. Generation of Lung Metastases
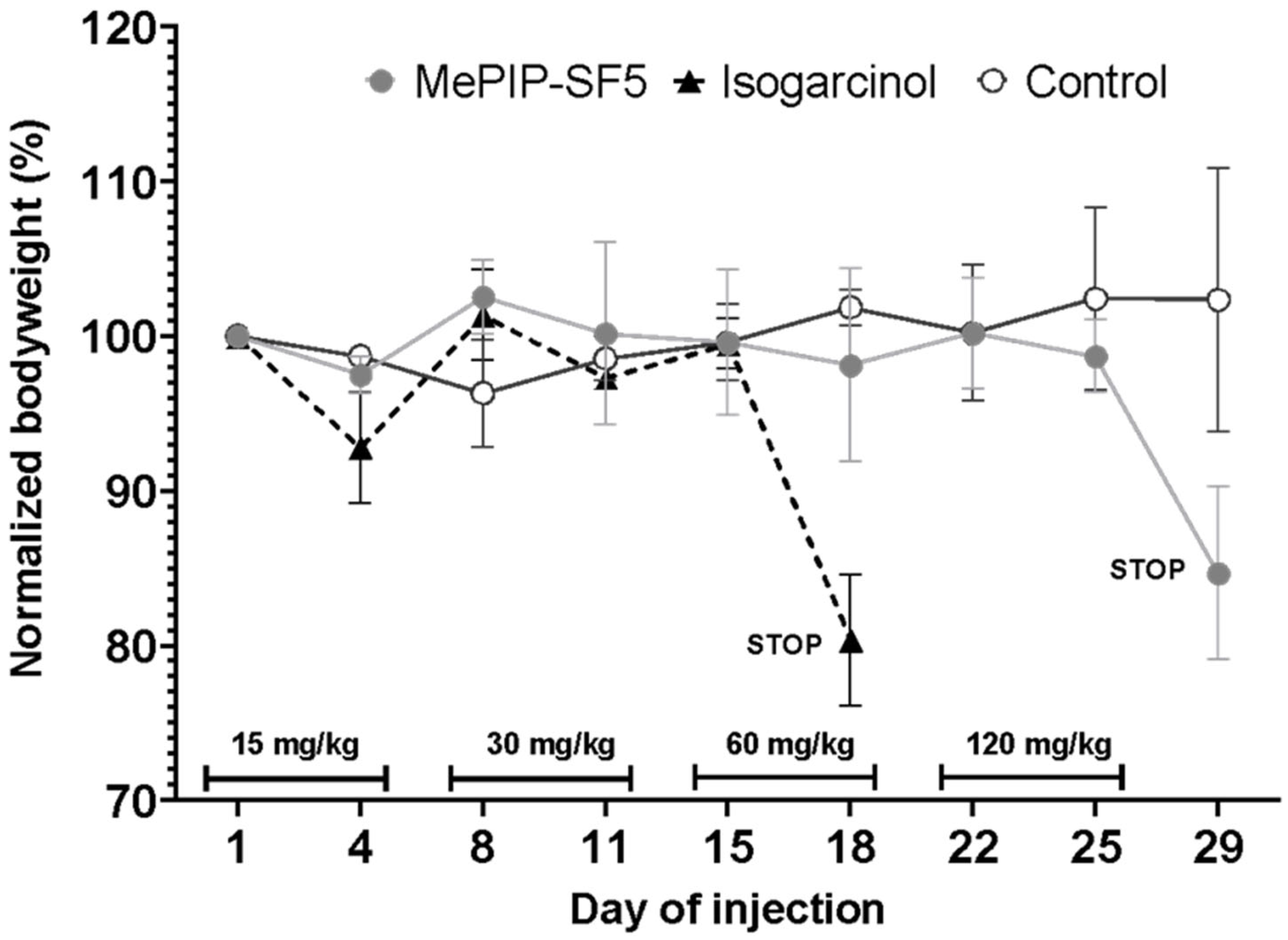
2.7. In Vivo Toxicity and Dose Finding
2.8. In Vivo Antitumor Therapy
2.9. Quantification of Tumor Burden in the Lungs
2.10. Statistics
3. Results
3.1. In Vitro Toxicity
3.2. In Vivo Dose Finding
3.3. Antimetastatic Effect of MePip-SF5 and Isogarcinol
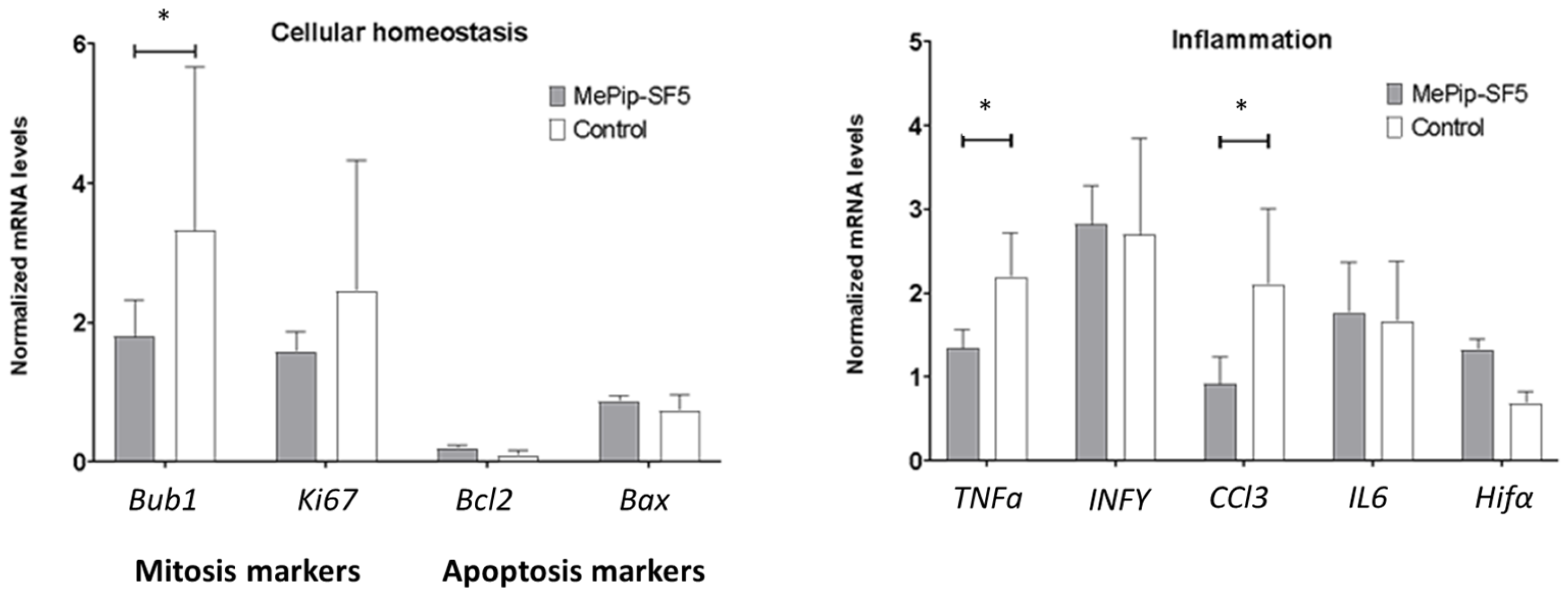
3.4. Anti-Inflammatory and Antimitotic Effect of MePip-SF5
3.5. Immunomodulatory Effect of MePip-SF5 on Primary Macrophages
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Gandalovičová, A.; Rosel, D.; Fernandes, M.; Veselý, P.; Heneberg, P.; Čermák, V.; Petruželka, L.; Kumar, S.; Sanz-Moreno, V.; Brábek, J. Migrastatics—Anti-metastatic and Anti-invasion Drugs: Promises and Challenges. Trends Cancer 2017, 3, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Sundararajan, S.; Thida, A.M.; Yadlapati, S.; Koya, S. Metastatic Melanoma; StatPearls: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Saikat, A.S.M.; Al-Khafaji, K.; Akter, H.; Choi, J.-G.; Hasan, M.; Lee, S.-S. Nature-Derived Compounds as Potential Bioactive Leads against CDK9-Induced Cancer: Computational and Network Pharmacology Approaches. Processes 2022, 10, 2512. [Google Scholar] [CrossRef]
- Jităreanu, A.; Trifan, A.; Vieriu, M.; Caba, I.-C.; Mârțu, I.; Agoroaei, L. Current Trends in Toxicity Assessment of Herbal Medicines: A Narrative Review. Processes 2023, 11, 83. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: The Indian solid gold. Adv. Exp. Med. Biol. 2007, 595, 1–75. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II Trial of Curcumin in Patients with Advanced Pancreatic Cancer. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef]
- Kanai, M.; Yoshimura, K.; Asada, M.; Imaizumi, A.; Suzuki, C.; Matsumoto, S.; Nishimura, T.; Mori, Y.; Masui, T.; Kawaguchi, Y.; et al. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother. Pharmacol. 2011, 68, 157–164. [Google Scholar] [CrossRef]
- Panknin, T.M.; Howe, C.L.; Hauer, M.; Bucchireddigari, B.; Rossi, A.M.; Funk, J.L. Curcumin Supplementation and Human Disease: A Scoping Review of Clinical Trials. Int. J. Mol. Sci. 2023, 24, 4476. [Google Scholar] [CrossRef]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase I Clinical Trial of Oral Curcumin. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef]
- Hao, M.; Chu, Y.; Lei, J.; Yao, Z.; Wang, P.; Chen, Z.; Wang, K.; Sang, X.; Han, X.; Wang, L.; et al. Pharmacological Mechanisms and Clinical Applications of Curcumin: Update. Aging Dis. 2023, 14, 716–749. [Google Scholar] [CrossRef]
- Garcea, G.; Berry, D.P.; Jones, D.J.L.; Singh, R.; Dennison, A.R.; Farmer, P.B.; Sharma, R.A.; Steward, W.P.; Gescher, A.J. Consumption of the putative chemopreventive agent curcumin by cancer patients: Assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol. Prev. Biomark. 2005, 14, 120–125. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Hegde, M.; Parama, D.; Girisa, S.; Kumar, A.; Daimary, U.D.; Garodia, P.; Yenisetti, S.C.; Oommen, O.V.; Aggarwal, B.B. Role of Turmeric and Curcumin in Prevention and Treatment of Chronic Diseases: Lessons Learned from Clinical Trials. ACS Pharmacol. Transl. Sci. 2023, 6, 447–518. [Google Scholar] [CrossRef]
- Schaffer, M.; Schaffer, P.M.; Zidan, J.; Bar Sela, G. Curcuma as a functional food in the control of cancer and inflammation. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Bhaumik, S.; Anjum, R.; Rangaraj, N.; Pardhasaradhi, B.; Khar, A. Curcumin mediated apoptosis in AK-5 tumor cells involves the production of reactive oxygen intermediates. FEBS Lett. 1999, 456, 311–314. [Google Scholar] [CrossRef]
- Altomonte, S.; Zanda, M. Synthetic chemistry and biological activity of pentafluorosulphanyl (SF5) organic molecules. J. Fluor. Chem. 2012, 143, 57–93. [Google Scholar] [CrossRef]
- Beier, P.; Pastýříková, T. Synthesis of SF5-containing benzisoxazoles, quinolines, and quinazolines by the Davis reaction of nitro-(pentafluorosulfanyl)benzenes. Beilstein J. Org. Chem. 2013, 9, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Linder, B.; Köhler, L.H.F.; Reisbeck, L.; Menger, D.; Subramaniam, D.; Herold-Mende, C.; Anant, S.; Schobert, R.; Biersack, B.; Kögel, D. A New Pentafluorothio-Substituted Curcuminoid with Superior Antitumor Activity. Biomolecules 2021, 11, 947. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, F.; Gold, M.; Begemann, G.; Andronache, I.; Biersack, B.; Schobert, R. Fluoro and pentafluorothio analogs of the antitumoral curcuminoid EF24 with superior antiangiogenic and vascular-disruptive effects. Bioorganic Med. Chem. 2017, 25, 4894–4903. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.-H.; Chang, W.-L.; Lin-Shiau, S.-Y.; Ho, C.-T.; Lin, J.-K. Induction of Apoptosis by Garcinol and Curcumin through Cytochrome c Release and Activation of Caspases in Human Leukemia HL-60 Cells. J. Agric. Food Chem. 2001, 49, 1464–1474. [Google Scholar] [CrossRef] [PubMed]
- Schobert, R.; Biersack, B. Chemical and Biological Aspects of Garcinol and Isogarcinol: Recent Developments. Chem. Biodivers. 2019, 16, e1900366. [Google Scholar] [CrossRef]
- Kaps, L.; Leber, N.; Klefenz, A.; Choteschovsky, N.; Zentel, R.; Nuhn, L.; Schuppan, D. In Vivo siRNA Delivery to Immunosuppressive Liver Macrophages by α-Mannosyl-Functionalized Cationic Nanohydrogel Particles. Cells 2020, 9, 1905. [Google Scholar] [CrossRef]
- Schindelin, J.; Rueden, C.T.; Hiner, M.C.; Eliceiri, K.W. The ImageJ ecosystem: An open platform for biomedical image analysis. Mol. Reprod. Dev. 2015, 82, 518–529. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Kerneur, C.; Cano, C.E.; Olive, D. Major pathways involved in macrophage polarization in cancer. Front. Immunol. 2022, 13, 1026954. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Lin, H.; Li, M.; Lin, B. Tumor−associated macrophage polarization in the inflammatory tumor microenvironment. Front. Oncol. 2023, 13, 1103149. [Google Scholar] [CrossRef] [PubMed]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS−) vs. Alternatively Activated Macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef] [PubMed]
- Gschwandtner, M.; Derler, R.; Midwood, K.S. More Than Just Attractive: How CCL2 Influences Myeloid Cell Behavior Beyond Chemotaxis. Front. Immunol. 2019, 10, 2759. [Google Scholar] [CrossRef]
- Kopytko, P.; Piotrowska, K.; Janisiak, J.; Tarnowski, M. Garcinol—A Natural Histone Acetyltransferase Inhibitor and New Anti-Cancer Epigenetic Drug. Int. J. Mol. Sci. 2021, 22, 2828. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.S.; Kaur, J.; Aggarwal, D.; Parashar, G.; Parashar, N.C.; Kulkarni, S.; Kaur, G.; Sak, K.; Kumar, M.; et al. Garcinol Exhibits Anti-Neoplastic Effects by Targeting Diverse Oncogenic Factors in Tumor Cells. Biomedicines 2020, 8, 103. [Google Scholar] [CrossRef]
- Pouliquen, D.L.; Trošelj, K.G.; Anto, R.J. Curcuminoids as Anticancer Drugs: Pleiotropic Effects, Potential for Metabolic Reprogramming and Prospects for the Future. Pharmaceutics 2023, 15, 1612. [Google Scholar] [CrossRef]
- Mirzaei, H.; Naseri, G.; Rezaee, R.; Mohammadi, M.; Banikazemi, Z.; Mirzaei, H.R.; Salehi, H.; Peyvandi, M.; Pawelek, J.M.; Sahebkar, A. Curcumin: A new candidate for melanoma therapy? Int. J. Cancer 2016, 139, 1683–1695. [Google Scholar] [CrossRef]
- Cragg, G.M.; Pezzuto, J.M. Natural Products as a Vital Source for the Discovery of Cancer Chemotherapeutic and Chemopreventive Agents. Med. Princ. Pract. 2016, 25, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Blesso, C.N.; Barreto, G.E.; Banach, M.; Majeed, M.; Sahebkar, A. Macrophage plasticity, polarization and function in response to curcumin, a diet-derived polyphenol, as an immunomodulatory agent. J. Nutr. Biochem. 2019, 66, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hulsart-Billström, G.; Dawson, J.; Hofmann, S.; Müller, R.; Stoddart, M.; Alini, M.; Redl, R.; El Haj, A.; Brown, R.; Salih, V.; et al. A surprisingly poor correlation between in vitro and in vivo testing of biomaterials for bone regeneration: Results of a multicentre analysis. Eur. Cells Mater. 2016, 31, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, K.G. Effect of blood protein concentrations on drug-dosing regimes: Practical guidance. Theor. Biol. Med. Model. 2013, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, W.; Hu, G.; Sun, H.; Kong, Q. Bioactivities of EF24, a Novel Curcumin Analog: A Review. Front. Oncol. 2018, 8, 614. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, D.; Nicholes, N.D.; Dhar, A.; Umar, S.; Awasthi, V.; Welch, D.R.; Jensen, R.A.; Anant, S. 3,5-Bis(2,4-Difluorobenzylidene)-4-piperidone, a Novel Compound That Affects Pancreatic Cancer Growth and Angiogenesis. Mol. Cancer Ther. 2011, 10, 2146–2156. [Google Scholar] [CrossRef] [PubMed]
- Standing, D.; Arnold, L.; Dandawate, P.; Ottemann, B.; Snyder, V.; Ponnurangam, S.; Sayed, A.; Subramaniam, D.; Srinivasan, P.; Choudhury, S.; et al. Doublecortin-like kinase 1 is a therapeutic target in squamous cell carcinoma. Mol. Carcinog. 2023, 62, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Beyer, A.; Aebersold, R. On the Dependency of Cellular Protein Levels on mRNA Abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef]
| Forward Primer | Reverse Primer | |
|---|---|---|
| Tnf-a | CCTGTAGCCCACGTCGTAG | GGGAGTAGACAAGGTACAACCC |
| iNos | GTTCTCAGCCCAACAATACAAGA | GTGGACGGGTCGATGTCAC |
| Ccl3 | TTCTCTGTACCATGACACTCTGC | CGTGGAATCTTCCGGCTGTAG |
| Mrc1 | AAGGCTATCCTGGTGGAAGAA | AGGGAAGGGTCAGTCTGTGTT |
| Bcl2 | TACCGTCGTGACTTCGCAGAG | GGCAGGCTGAGCAGGGTCTT |
| Bax | AGACAGGGGCCTTTTTGCTAC | AATTCGCCGGAGACACTCG |
| Ki67 | ATCATTGACCGCTCCTTTAGGT | GCTCGCCTTGATGGTTCCT |
| Bub1 | ATGCAAAGCTACACGGGTAATG | GGTCACTGTTGTACTCAGCAAA |
| Hif1-α | CGACACCATCATCTCTCTGG | TGATTCAGTGCAGGATCAGC |
| IL-6 | CTGCAAGAGACTTCCATCCAG | AGTGGTATAGACAGGTCTGTTGG |
| INF-γ | CGGCACAGTCATTGAAAGCC | TGCATCCTTTTTCGCCTTGC |
| β-Actin | GGCATTGTTACCAACTGGGACGAC | CCAGAGGCATACAGGGACAGCACAG |
| Cell Lines | Curcumin | MePip-SF5 | p-Value (Curcumin vs. MePip-SF5) | Garcinol | Isogarcinol | p-Value (Garcinol vs. Isogarcinol) | p-Value (MePip-SF5 vs. Isogarcinol) |
|---|---|---|---|---|---|---|---|
| µM (95% Confidence Interval) | |||||||
| B16F10 melanoma cells | 13.8 | 2.8 | p < 0.0001 | 3.1 | 2.1 | p < 0.0001 | p < 0.0001 |
| (12.31–15.5) | (2.087–3.661) | (2.664–3.694) | (1.824–2.369) | ||||
| 3T3 fibroblasts | 6.8 | 0.7 | p < 0.0001 | 8.7 | 3.2 | p < 0.0001 | p < 0.0001 |
| (6.246–7.423) | (0.711–0.804) | (7.112–10.79) | (2.959–3.555) | ||||
| AML12 hepatocytes | 10.8 | 2.3 | p < 0.0001 | 10.1 | 4.3 | p < 0.0001 | p < 0.0001 |
| (9.421–12.46) | (1.506–3.51) | (9.023–11.51) | (3.326–5.572) | ||||
| RAW macrophages | 5.5 | 1.3 | p < 0.0001 | 3.8 | 2.3 | p < 0.0001 | p < 0.0001 |
| (5.017–6.065) | (1.024–1.517) | (3.275–4.368) | (2.058–2.489) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaps, L.; Klefenz, A.; Traenckner, H.; Schneider, P.; Andronache, I.; Schobert, R.; Biersack, B.; Schuppan, D. A New Synthetic Curcuminoid Displays Antitumor Activities in Metastasized Melanoma. Cells 2023, 12, 2619. https://doi.org/10.3390/cells12222619
Kaps L, Klefenz A, Traenckner H, Schneider P, Andronache I, Schobert R, Biersack B, Schuppan D. A New Synthetic Curcuminoid Displays Antitumor Activities in Metastasized Melanoma. Cells. 2023; 12(22):2619. https://doi.org/10.3390/cells12222619
Chicago/Turabian StyleKaps, Leonard, Adrian Klefenz, Henry Traenckner, Paul Schneider, Ion Andronache, Rainer Schobert, Bernhard Biersack, and Detlef Schuppan. 2023. "A New Synthetic Curcuminoid Displays Antitumor Activities in Metastasized Melanoma" Cells 12, no. 22: 2619. https://doi.org/10.3390/cells12222619
APA StyleKaps, L., Klefenz, A., Traenckner, H., Schneider, P., Andronache, I., Schobert, R., Biersack, B., & Schuppan, D. (2023). A New Synthetic Curcuminoid Displays Antitumor Activities in Metastasized Melanoma. Cells, 12(22), 2619. https://doi.org/10.3390/cells12222619








