Effects of GHRH Deficiency and GHRH Antagonism on Emotional Disorders in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. In Vivo Studies
2.3. Behavioral Analysis
2.4. Hematoxylin-Eosin Staining/Light Microscopy Analysis and Immunohistochemistry
2.5. RNA Extraction, Reverse Transcription and Real-Time Reverse Transcription Polymerase Chain Reaction
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
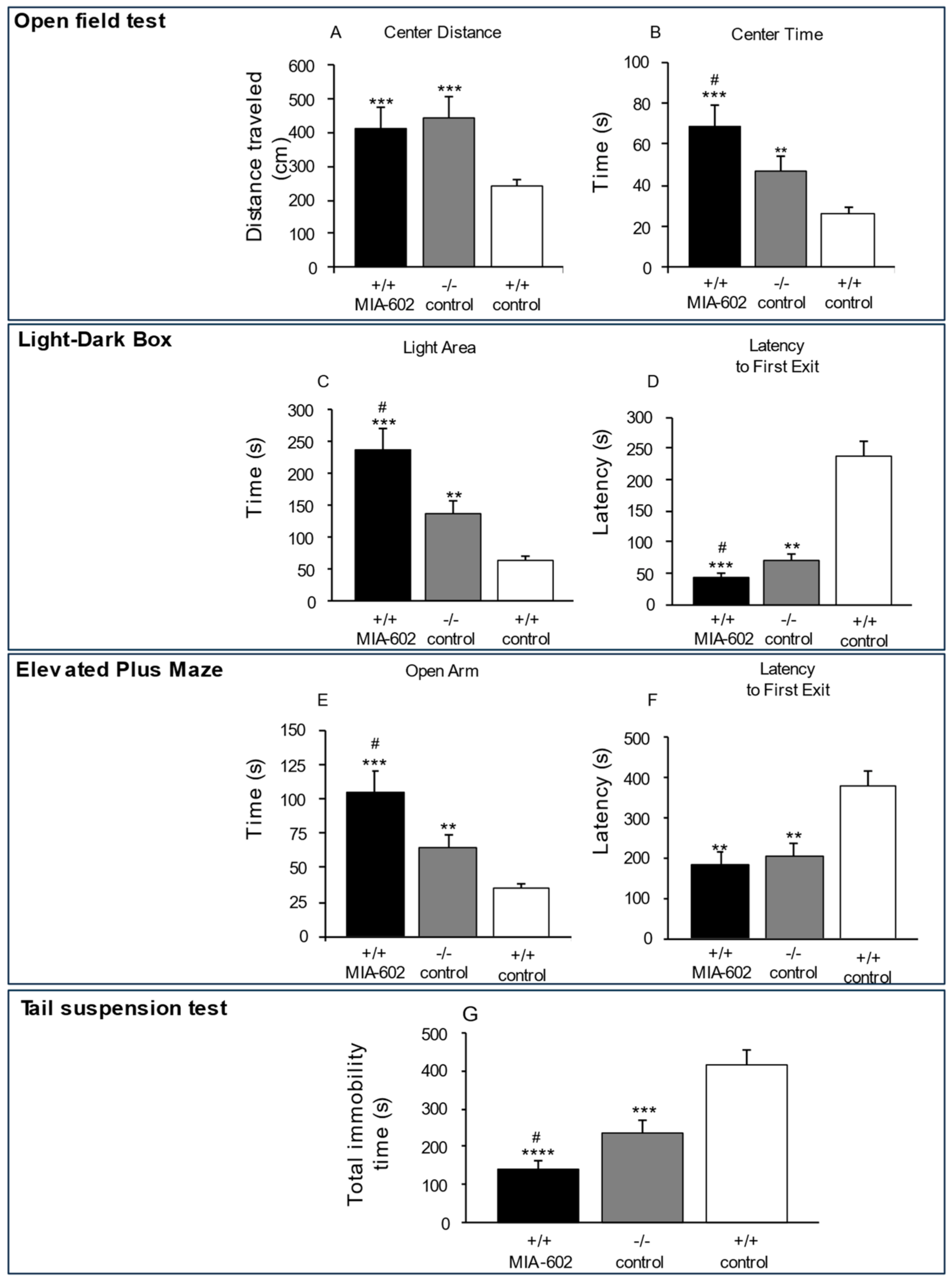
3.1. Anxiety-Related and Depression-like Behavior
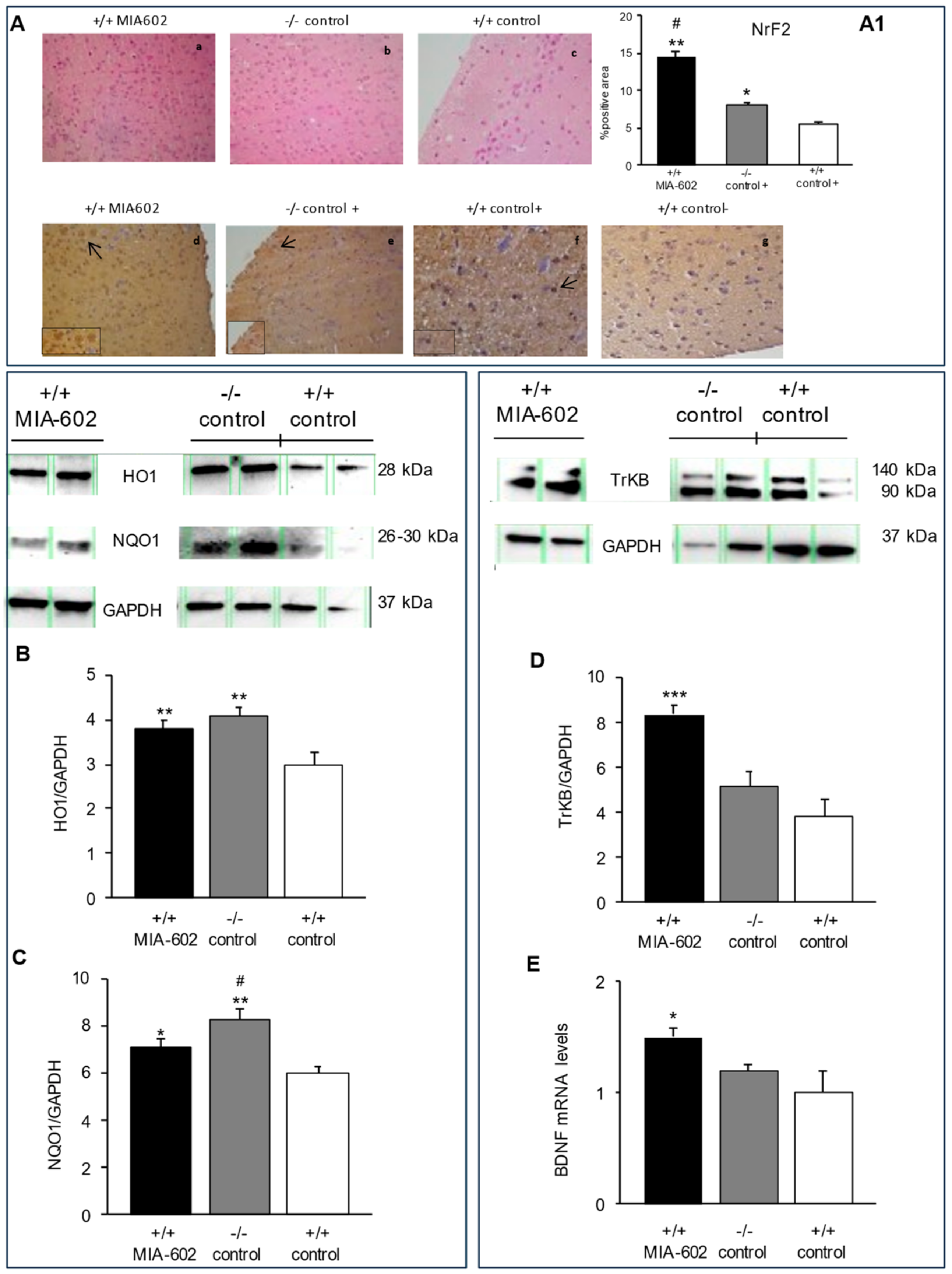
3.2. Hematoxylin-Eosin Staining and Immunohistochemical Analysis of Nrf2 in Mice Prefrontal Cortex
3.3. Heme Oxygenase-1, NAD(P)H Quinone Oxidoreductase 1, Tropomycin Receptor Kinase B, and Brain-Derived Neurotrophic Factor Evaluation in Prefrontal Cortex
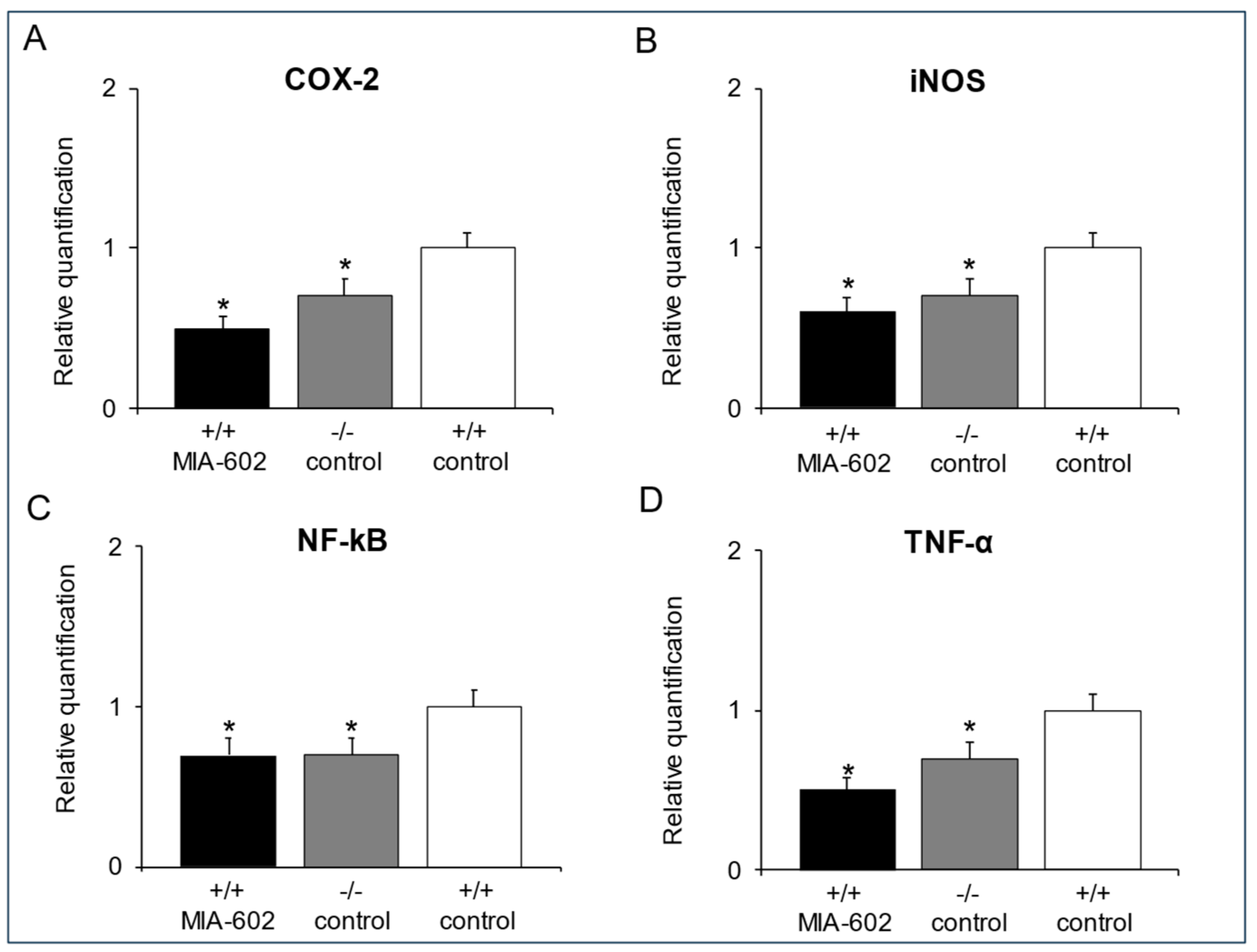
3.4. Cyclooxygenase-2, Inducible Nitric Oxide Synthase, Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells and Tumor Necrosis Factor-α Gene Expression Determination in the Prefrontal Cortex
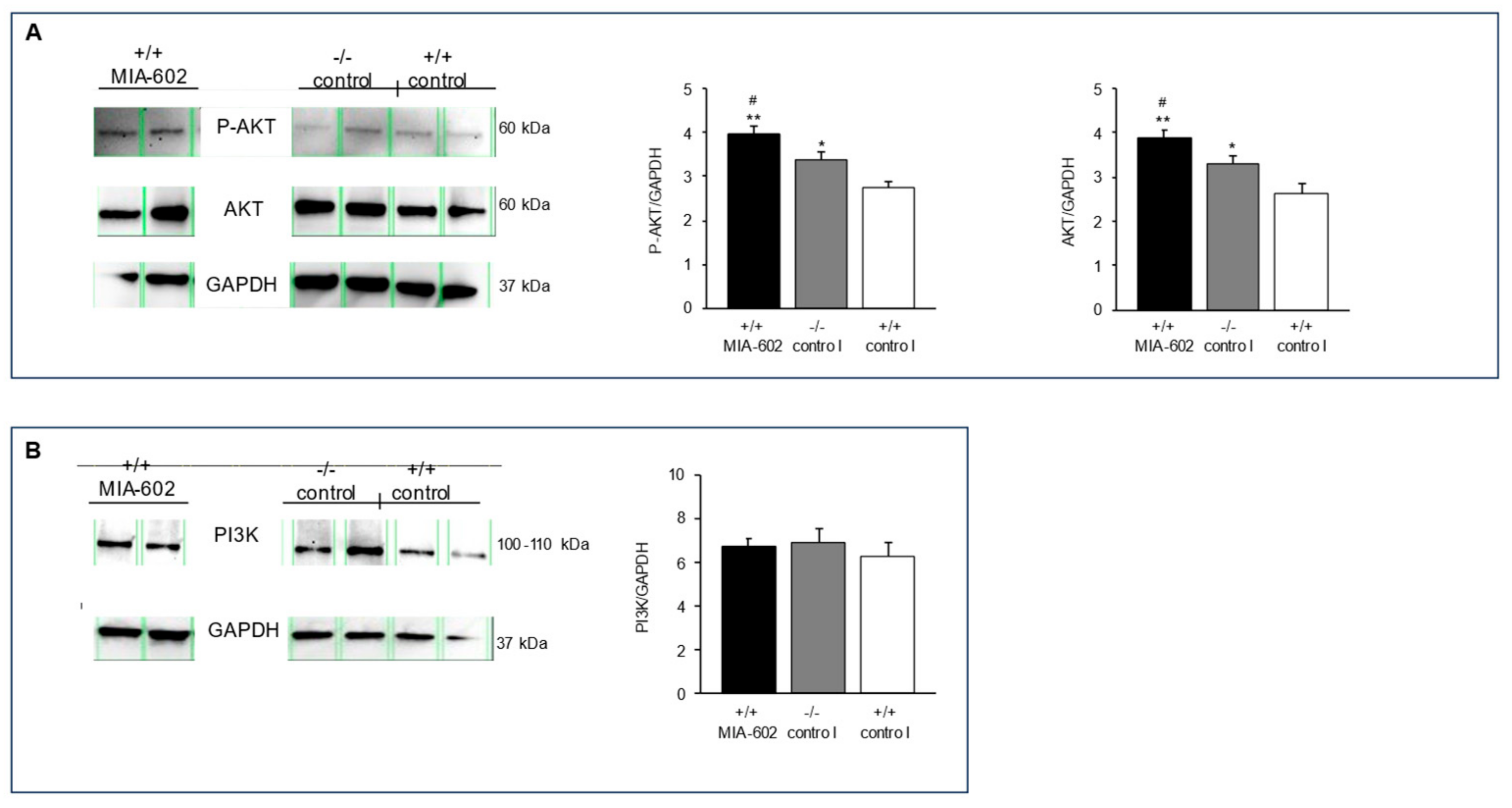
3.5. Phosphorylated AKT, Serine/Threonine Kinase, and Phosphoinositide 3-Kinase Protein Expression Levels
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kalin, N.H. The Critical Relationship Between Anxiety and Depression. Am. J. Psichiatry 2020, 177, 365–367. [Google Scholar] [CrossRef]
- Taquet, M.; Holmes, E.A.; Harrison, P.J. Depression and Anxiety Disorders during the COVID-19 Pandemic: Knowns and Unknowns. Lancet 2021, 398, 1665–1666. [Google Scholar] [CrossRef]
- Tamagno, G.; Epelbaum, J. Editorial: Neurological and Psychiatric Disorders in Endocrine Diseases. Front. Endocrinol. 2015, 6, 75. [Google Scholar]
- Kiaris, H.; Chatzistamou, I.; Papavassiliou, A.G.; Schally, A.V. Growth Hormone-Releasing Hormone: Not Only a Neurohormone. Trends Endocrinol. Metab. 2011, 22, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Prodam, F.; Caputo, M.; Belcastro, S.; Garbaccio, V.; Zavattaro, M.; Samà, M.T.; Bellone, S.; Pagano, L.; Bona, G.; Aimaretti, G. Quality of life, mood disturbances and psychological parameters in adult patients with GH deficiency. Panminerva Med. 2012, 54, 323–331. [Google Scholar] [PubMed]
- Engin, E.; Stellbrink, J.; Treit, D.; Dickson, C.T. Anxiolytic and Antidepressant Effects of Intracerebroventricularly Administered Somatostatin: Behavioral and Neurophysiological Evidence. Neuroscience 2008, 157, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Telegdy, G.; Schally, A.V. Involvement of Neurotransmitters in the Action of Growth Hormone-Releasing Hormone Antagonist on Passive Avoidance Learning. Behav. Brain Res. 2012, 233, 326–330. [Google Scholar] [CrossRef]
- Telegdy, G.; Schally, A.V. Neurotransmitter-Mediated Action of an Antagonist of Growth Hormone-Releasing Hormone on Anxiolysis in Mice. Behav. Brain Res. 2012, 233, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Leone, S.; Shohreh, R.; Manippa, F.; Recinella, L.; Ferrante, C.; Orlando, G.; Salvatori, R.; Vacca, M.; Brunetti, L. Behavioural Phenotyping of Male Growth Hormone-Releasing Hormone (GHRH) Knockout Mice. Growth Horm. IGF Res. 2014, 24, 192–197. [Google Scholar] [CrossRef]
- Leone, S.; Chiavaroli, A.; Shohreh, R.; Ferrante, C.; Ricciuti, A.; Manippa, F.; Recinella, L.; Di Nisio, C.; Orlando, G.; Salvatori, R.; et al. Increased Locomotor and Thermogenic Activity in Mice with Targeted Ablation of the GHRH Gene. Growth Horm. IGF Res. 2015, 25, 80–84. [Google Scholar] [CrossRef]
- Leone, S.; Recinella, L.; Chiavaroli, A.; Ferrante, C.; Orlando, G.; Vacca, M.; Salvatori, R.; Brunetti, L. Behavioural Phenotyping, Learning and Memory in Young and Aged Growth Hormone-Releasing Hormone-Knockout Mice. Endocr. Connect. 2018, 7, 924. [Google Scholar] [CrossRef]
- Schally, A.V.; Zhang, X.; Cai, R.; Hare, J.M.; Granata, R.; Bartoli, M. Actions and Potential Therapeutic Applications of Growth Hormone–Releasing Hormone Agonists. Endocrinology 2019, 160, 1600–1612. [Google Scholar] [CrossRef]
- Arwert, L.I.; Veltman, D.J.; Deijen, J.B.; van Dam, P.S.; Delemarre-van deWaal, H.A.; Drent, M.L. Growth Hormone Deficiency and Memory Functioning in Adults Visualized by Functional Magnetic Resonance Imaging. Neuroendocrinology 2006, 82, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Engin, E.; Treit, D. Anxiolytic and Antidepressant Actions of Somatostatin: The Role of Sst2 and Sst3 Receptors. Psychopharmacology 2009, 206, 281–289. [Google Scholar] [CrossRef]
- Recinella, L.; Chiavaroli, A.; Orlando, G.; Ferrante, C.; Marconi, G.D.; Gesmundo, I.; Granata, R.; Cai, R.; Sha, W.; Schally, A.V.; et al. Antinflammatory, Antioxidant, and Behavioral Effects Induced by Administration of Growth Hormone-Releasing Hormone Analogs in Mice. Sci. Rep. 2020, 10, 732. [Google Scholar] [CrossRef] [PubMed]
- Recinella, L.; Chiavaroli, A.; Veschi, S.; Di Valerio, V.; Lattanzio, R.; Orlando, G.; Ferrante, C.; Gesmundo, I.; Granata, R.; Cai, R.; et al. Antagonist of Growth Hormone-Releasing Hormone MIA-690 Attenuates the Progression and Inhibits Growth of Colorectal Cancer in Mice. Biomed. Pharmacother. 2022, 146, 112554. [Google Scholar] [CrossRef]
- Recinella, L.; Chiavaroli, A.; Orlando, G.; Ferrante, C.; Gesmundo, I.; Granata, R.; Cai, R.; Sha, W.; Schally, A.V.; Brunetti, L.; et al. Growth Hormone-Releasing Hormone Antagonistic Analog MIA-690 Stimulates Food Intake in Mice. Peptides 2021, 142, 170582. [Google Scholar] [CrossRef]
- Alba, M.; Salvatori, R. A mouse with targeted ablation of the growth hormone releasing hormone gene: A new model of isolated growth hormone deficiency. Endocrinology 2004, 145, 4134–4143. [Google Scholar] [CrossRef]
- Sun, L.Y.; Spong, A.; Swindell, W.R.; Fang, Y.; Hill, C.; Huber, J.A.; Boehm, J.D.; Westbrook, R.; Salvatori, R.; Bartke, A. Growth hormone-releasing hormone disruption extends lifespan and regulates response to caloric restriction in mice. Elife 2013, 2, e01098. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Capuron, L.; Miller, A.H. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol. 2006, 27, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Maletic, V.; Raison, C.L. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 2009, 65, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Pace, T.W.; Wingenfeld, K.; Schmidt, I.; Meinlschmidt, G.; Hellhammer, D.H.; Heim, C.M. Increased peripheral NF-κB pathway activity in women with childhood abuse-related posttraumatic stress disorder. Brain Behav. Immun. 2012, 26, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom. Med. 2009, 71, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Leone, S.; Chiavaroli, A.; Recinella, L.; Orlando, G.; Ferrante, C.; Marconi, G.D.; Gasparo, I.; Bitto, A.; Salvatori, R.; Brunetti, L. Increased Pain and Inflammatory Sensitivity in Growth Hormone-Releasing Hormone (GHRH) Knockout Mice. Prostaglandins Other Lipid Mediat. 2019, 144, 106362. [Google Scholar] [CrossRef]
- Leone, S.; Chiavaroli, A.; Recinella, L.; Di Valerio, V.; Veschi, S.; Gasparo, I.; Bitto, A.; Ferrante, C.; Orlando, G.; Salvatori, R.; et al. Growth Hormone-Releasing Hormone (GHRH) Deficiency Promotes Inflammation-Associated Carcinogenesis. Pharmacol. Res. 2020, 152, 104614. [Google Scholar] [CrossRef]
- Recinella, L.; Chiavaroli, A.; Orlando, G.; Ferrante, C.; Veschi, S.; Cama, A.; Marconi, G.D.; Diomede, F.; Gesmundo, I.; Granata, R.; et al. Effects of Growth Hormone-Releasing Hormone Receptor Antagonist MIA-602 in Mice with Emotional Disorders: A Potential Treatment for PTSD. Mol. Psychiatry 2021, 26, 7465–7474. [Google Scholar] [CrossRef]
- Leone, S.; Recinella, L.; Chiavaroli, A.; Martinotti, S.; Ferrante, C.; Mollica, A.; Macedonio, G.; Stefanucci, A.; Dvorácskó, S.; Tömböly, C.; et al. Emotional Disorders Induced by Hemopressin and RVD-Hemopressin(α) Administration in Rats. Pharmacol. Rep. 2017, 69, 1247–1253. [Google Scholar] [CrossRef]
- Recinella, L.; Chiavaroli, A.; Ferrante, C.; Mollica, A.; Macedonio, G.; Stefanucci, A.; Dimmito, M.P.; Dvorácskó, S.; Tömböly, C.; Brunetti, L.; et al. Effects of Central RVD-Hemopressin(α) Administration on Anxiety, Feeding Behavior and Hypothalamic Neuromodulators in the Rat. Pharmacol. Rep. 2018, 70, 650–657. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: San Diego, CA, USA, 2007. [Google Scholar]
- Di Giulio, C.; Marconi, G.D.; Zara, S.; Di Tano, A.; Porzionato, A.; Pokorski, M.; Cataldi, A.; Mazzatenta, A. Selective Expression of Galanin in Neuronal-Like Cells of the Human Carotid Body. Adv. Exp. Med. Biol. 2015, 860, 315–323. [Google Scholar] [CrossRef]
- Recinella, L.; Micheli, L.; Chiavaroli, A.; Libero, M.L.; Orlando, G.; Menghini, L.; Acquaviva, A.; Di Simone, S.; Ferrante, C.; Ghelardini, C.; et al. Anti-Inflammatory Effects Induced by a Polyphenolic Granular Complex from Olive (Olea Europaea, Mainly Cultivar Coratina): Results from In Vivo and Ex Vivo Studies in a Model of Inflammation and MIA-Induced Osteoarthritis. Nutrients 2022, 14, 1487. [Google Scholar] [CrossRef] [PubMed]
- Veschi, S.; De Lellis, L.; Florio, R.; Lanuti, P.; Massucci, A.; Tinari, N.; De Tursi, M.; di Sebastiano, P.; Marchisio, M.; Natoli, C.; et al. Effects of Repurposed Drug Candidates Nitroxoline and Nelfinavir as Single Agents or in Combination with Erlotinib in Pancreatic Cancer Cells. J. Exp. Clin. Cancer Res. 2018, 37, 236. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Charan, J.; Kantharia, N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, S.; Paterson, C.; Yang, F.; Hood, V.L.; Law, A.J. PKBβ/AKT2 Deficiency Impacts Brain mTOR Signaling, Prefrontal Cortical Physiology, Hippocampal Plasticity and Select Murine Behaviors. Mol. Psychiatry 2021, 26, 411–428. [Google Scholar] [CrossRef]
- Ackermann, T.F.; Hörtnagl, H.; Wolfer, D.P.; Colacicco, G.; Sohr, R.; Lang, F.; Hellweg, R.; Lang, U.E. Phosphatidylinositide Dependent Kinase Deficiency Increases Anxiety and Decreases GABA and Serotonin Abundance in the Amygdala. Cell. Physiol. Biochem. 2008, 22, 735–744. [Google Scholar] [CrossRef]
- Sanna, P.P.; Cammalleri, M.; Berton, F.; Simpson, C.; Lutjens, R.; Bloom, F.E.; Francesconi, W. Phosphatidylinositol 3-Kinase Is Required for the Expression but Not for the Induction or the Maintenance of Long-Term Potentiation in the Hippocampal CA1 Region. J. Neurosci. 2002, 22, 3359–3365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, R.; Zhou, R.; Li, L.; Sokabe, M.; Chen, L. Progesterone Promotes the Survival of Newborn Neurons in the Dentate Gyrus of Adult Male Mice. Hippocampus 2010, 20, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Schally, A.V.; Telegdy, G. Neurotransmission of the antidepressant-like effects of the growth hormone-releasing hormone antagonist MZ-4-71. Behav. Brain Res. 2012, 228, 388–391. [Google Scholar] [CrossRef]
- Mendez-David, I.; Tritschler, L.; Ali, Z.E.; Damiens, M.-H.; Pallardy, M.; David, D.J.; Kerdine-Römer, S.; Gardier, A.M. Nrf2-Signaling and BDNF: A New Target for the Antidepressant-like Activity of Chronic Fluoxetine Treatment in a Mouse Model of Anxiety/Depression. Neurosci. Lett. 2015, 597, 121–126. [Google Scholar] [CrossRef]
- Green, C.R.; Corsi-Travali, S.; Neumeister, A. The Role of BDNF-TrkB Signaling in the Pathogenesis of PTSD. J. Depress. Anxiety 2013, 2013, 006. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.N.; Bondy, S.C. Common Biochemical Defects Linkage between Post-Traumatic Stress Disorders, Mild Traumatic Brain Injury (TBI) and Penetrating TBI. Brain Res. 2015, 1599, 103–114. [Google Scholar] [CrossRef]
- Schinder, A.F.; Poo, M. The Neurotrophin Hypothesis for Synaptic Plasticity. Trends Neurosci. 2000, 23, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Matrisciano, F.; Bonaccorso, S.; Ricciardi, A.; Scaccianoce, S.; Panaccione, I.; Wang, L.; Ruberto, A.; Tatarelli, R.; Nicoletti, F.; Girardi, P.; et al. Changes in BDNF Serum Levels in Patients with Major Depression Disorder (MDD) after 6 Months Treatment with Sertraline, Escitalopram, or Venlafaxine. J. Psychiatr. Res. 2009, 43, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Saarelainen, T.; Hendolin, P.; Lucas, G.; Koponen, E.; Sairanen, M.; MacDonald, E.; Agerman, K.; Haapasalo, A.; Nawa, H.; Aloyz, R.; et al. Activation of the TrkB Neurotrophin Receptor Is Induced by Antidepressant Drugs and Is Required for Antidepressant-Induced Behavioral Effects. J. Neurosci. 2003, 23, 349–357. [Google Scholar] [CrossRef]
- Zhou, C.; Zhong, J.; Zou, B.; Fang, L.; Chen, J.; Deng, X.; Zhang, L.; Zhao, X.; Qu, Z.; Lei, Y.; et al. Meta-Analyses of Comparative Efficacy of Antidepressant Medications on Peripheral BDNF Concentration in Patients with Depression. PLoS ONE 2017, 12, e0172270. [Google Scholar] [CrossRef]
- Matsuda, S.; Ikeda, Y.; Murakami, M.; Nakagawa, Y.; Tsuji, A.; Kitagishi, Y. Roles of PI3K/AKT/GSK3 Pathway Involved in Psychiatric Illnesses. Diseases 2019, 7, 22. [Google Scholar] [CrossRef]
- Beaulieu, J.-M. A Role for Akt and Glycogen Synthase Kinase-3 as Integrators of Dopamine and Serotonin Neurotransmission in Mental Health. J. Psychiatry. Neurosci. 2012, 37, 7–16. [Google Scholar] [CrossRef]
- Leibrock, C.; Ackermann, T.F.; Hierlmeier, M.; Lang, F.; Borgwardt, S.; Lang, U.E. Akt2 Deficiency Is Associated with Anxiety and Depressive Behavior in Mice. Cell. Physiol. Biochem. 2013, 32, 766–777. [Google Scholar] [CrossRef]
- Karege, F.; Perroud, N.; Burkhardt, S.; Schwald, M.; Ballmann, E.; Harpe, R.L.; Malafosse, A. Alteration in Kinase Activity But Not in Protein Levels of Protein Kinase B and Glycogen Synthase Kinase-3β in Ventral Prefrontal Cortex of Depressed Suicide Victims. Biol. Psychiatry 2007, 61, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Han, M.-H.; Mazei-Robison, M.; Iñiguez, S.D.; Ables, J.L.; Vialou, V.; Berton, O.; Ghose, S.; Covington, H.E.; Wiley, M.D.; et al. AKT Signaling within the Ventral Tegmental Area Regulates Cellular and Behavioral Responses to Stressful Stimuli. Biol. Psychiatry 2008, 64, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.-S.; Zhu, W.-L.; Liu, J.-F.; Luo, Y.-X.; Si, J.-J.; Wang, S.-J.; Xue, Y.-X.; Ding, Z.-B.; Shi, J.; Lu, L. PI3K/Akt Signaling Pathway in the Basolateral Amygdala Mediates the Rapid Antidepressant-like Effects of Trefoil Factor 3. Neuropsychopharmacology 2012, 37, 2671–2683. [Google Scholar] [CrossRef]
- Wang, W.; Lu, Y.; Xue, Z.; Li, C.; Wang, C.; Zhao, X.; Zhang, J.; Wei, X.; Chen, X.; Cui, W.; et al. Rapid-acting antidepressant-like effects of acetyl-l-carnitine mediated by PI3K/AKT/BDNF/VGF signaling pathway in mice. Neuroscience 2015, 285, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Dong, Y.; Su, Q.; Wang, H.; Chen, Y.; Xue, W.; Chen, C.; Xia, B.; Duan, J.; Chen, G. Liquiritigenin Reverses Depression-like Behavior in Unpredictable Chronic Mild Stress-Induced Mice by Regulating PI3K/Akt/mTOR Mediated BDNF/TrkB Pathway. Behav. Brain Res. 2016, 308, 177–186. [Google Scholar] [CrossRef]
- Caviedes, A.; Lafourcade, C.; Soto, C.; Wyneken, U. BDNF/NF-κB Signaling in the Neurobiology of Depression. Curr. Pharm. Des. 2017, 23, 3154–3163. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. The Role of Inflammation in Depression: From Evolutionary Imperative to Modern Treatment Target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Chen, H.; Tang, X.; He, B.; Gu, L.; Feng, H. Total Saikosaponins Attenuates Depression-Like Behaviors Induced by Chronic Unpredictable Mild Stress in Rats by Regulating the PI3K/AKT/NF-κB Signaling Axis. Evid. Based Complement. Alternat. Med. 2022, 2022, 4950414. [Google Scholar] [CrossRef]
- Hoffman, J.M.; Poonawalla, A.; Icyuz, M.; Swindell, W.R.; Wilson, L.; Barnes, S.; Sun, L.Y. Transcriptomic and metabolomic profiling of long-lived growth hormone releasing hormone knock-out mice: Evidence for altered mitochondrial function and amino acid metabolism. Aging 2020, 12, 3473–3485. [Google Scholar] [CrossRef] [PubMed]
- Jaszberenyi, M.; Rick, F.G.; Szalontay, L.; Block, N.L.; Zarandi, M.; Cai, R.Z.; Schally, A.V. Beneficial effects of novel antagonists of GHRH in different models of Alzheimer’s disease. Aging 2012, 4, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Recinella, L.; Leone, S.; Ferrante, C.; Chiavaroli, A.; Shohreh, R.; Di Nisio, C.; Vacca, M.; Orlando, G.; Salvatori, R.; Brunetti, L. Effects of growth hormone-releasing hormone gene targeted ablation on ghrelin-induced feeding. Growth Horm. IGF Res. 2017, 37, 40–46. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Recinella, L.; Libero, M.L.; Veschi, S.; Piro, A.; Marconi, G.D.; Diomede, F.; Chiavaroli, A.; Orlando, G.; Ferrante, C.; Florio, R.; et al. Effects of GHRH Deficiency and GHRH Antagonism on Emotional Disorders in Mice. Cells 2023, 12, 2615. https://doi.org/10.3390/cells12222615
Recinella L, Libero ML, Veschi S, Piro A, Marconi GD, Diomede F, Chiavaroli A, Orlando G, Ferrante C, Florio R, et al. Effects of GHRH Deficiency and GHRH Antagonism on Emotional Disorders in Mice. Cells. 2023; 12(22):2615. https://doi.org/10.3390/cells12222615
Chicago/Turabian StyleRecinella, Lucia, Maria Loreta Libero, Serena Veschi, Anna Piro, Guya Diletta Marconi, Francesca Diomede, Annalisa Chiavaroli, Giustino Orlando, Claudio Ferrante, Rosalba Florio, and et al. 2023. "Effects of GHRH Deficiency and GHRH Antagonism on Emotional Disorders in Mice" Cells 12, no. 22: 2615. https://doi.org/10.3390/cells12222615
APA StyleRecinella, L., Libero, M. L., Veschi, S., Piro, A., Marconi, G. D., Diomede, F., Chiavaroli, A., Orlando, G., Ferrante, C., Florio, R., Lamolinara, A., Cai, R., Sha, W., Schally, A. V., Salvatori, R., Brunetti, L., & Leone, S. (2023). Effects of GHRH Deficiency and GHRH Antagonism on Emotional Disorders in Mice. Cells, 12(22), 2615. https://doi.org/10.3390/cells12222615













