Effects of Dietary Components on Mast Cells: Possible Use as Nutraceuticals for Allergies?
Abstract
:1. Introduction
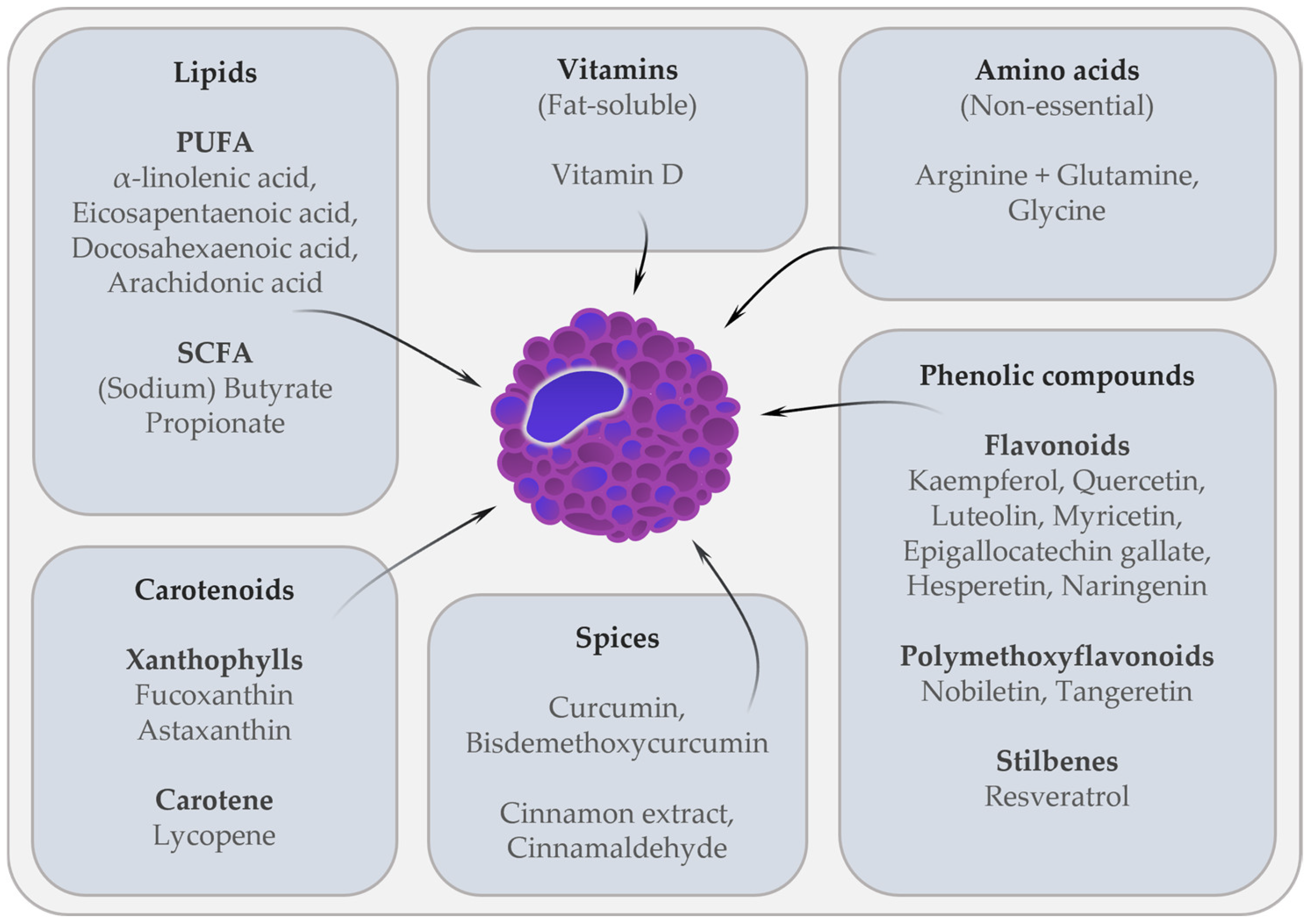
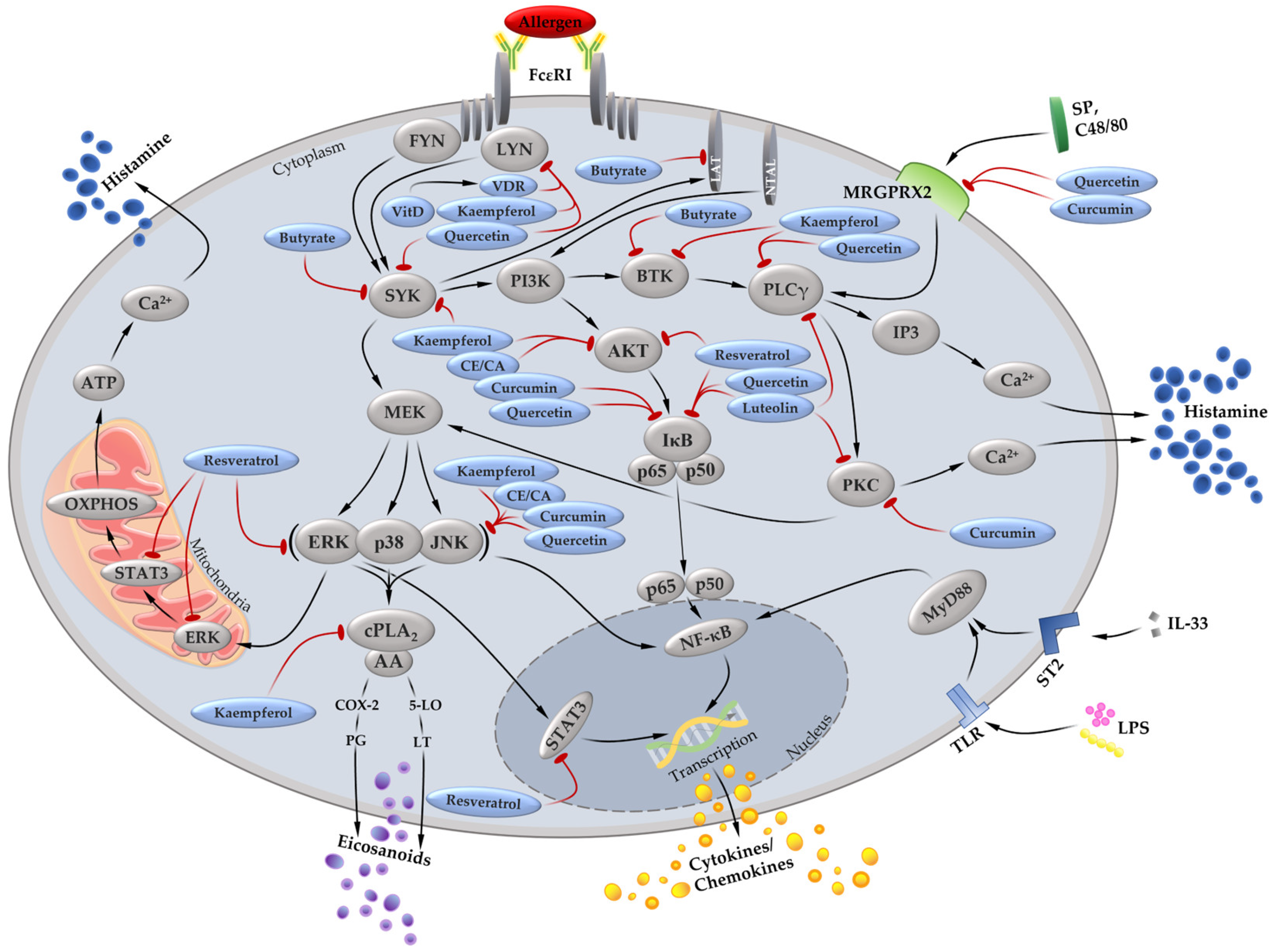
2. Effects of Dietary Components on MC In Vitro and In Vivo
2.1. Fatty Acids
2.2. Amino Acids
2.3. Vitamin D
2.4. Carotenoids
2.5. Flavonoids
2.6. Polymethoxyflavonoids
2.7. Resveratrol
2.8. Spices
2.8.1. Curcumin
2.8.2. Cinnamon Extract
3. Effect of Dietary Components on Allergic Diseases in Randomized Controlled Trials
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Akt | Protein kinase B |
| AR | Allergic rhinitis |
| BMMC | Bone marrow-derived mast cell |
| Btk | Bruton’s tyrosine kinase |
| C48/80 | Compound 48/80 |
| CBMC | Cord blood-derived mast cell |
| CCL | CC chemokine ligand |
| CXCL | C-X-C motif chemokine ligand |
| cys-LT | Cysteinyl-leukotriene |
| EGCG | Epigallocatechin gallate |
| ERK | Extracellular signal-regulated kinase |
| FcεRI | High affinity IgE receptor |
| hiMC | Human intestinal mast cell |
| HMC-1 | Human mast cell line 1 |
| hsMC | Human skin mast cell |
| JNK | c-Jun N-terminal kinase |
| LAD2 | Laboratory of Allergic Disease 2 |
| LTC4 | Leukotriene C4 |
| Lyn | Lck/Yes-related novel protein tyrosine kinase |
| MAPK | Mitogen-activated protein kinase |
| MCP-1/CCL2 | Monocyte chemoattractant protein-1 |
| mMCP-1 | Mucosal mast cell protease-1 |
| NF-κB | Nuclear factor kappa B |
| OVA | Ovalbumin |
| PBMCMC | Peripheral blood mononuclear cell-derived mast cell |
| RBL-2H3 | Rat basophilic leukemia 2H3 |
| RCT | Randomized controlled trial |
| Syk | Spleen tyrosine kinase |
References
- Roger, A.; Basagana, M.; Teniente-Serra, A.; Depreux, N.; Jurgens, Y.; Padro, C.; Miquel, S.; Elduque, C.; Martinez-Caceres, E.M. Immunotheraphy in Allergic Diseases. Curr. Pharm. Des. 2018, 24, 1174–1194. [Google Scholar] [CrossRef] [PubMed]
- Shamji, M.H.; Boyle, R.J. Allergic diseases and novel targets in allergen immunotherapy. Clin. Exp. Allergy 2021, 51, 1526–1528. [Google Scholar] [CrossRef] [PubMed]
- Poncet, P.; Sénéchal, H.; Charpin, D. Update on pollen-food allergy syndrome. Expert Rev. Clin. Immunol. 2020, 16, 561–578. [Google Scholar] [CrossRef] [PubMed]
- Orban, N.T.; Jacobson, M.R.; Nouri-Aria, K.T.; Durham, S.R.; Eifan, A.O. Repetitive nasal allergen challenge in allergic rhinitis: Priming and Th2-type inflammation but no evidence of remodelling. Clin. Exp. Allergy 2021, 51, 329–338. [Google Scholar] [CrossRef]
- Forster, F.; Heumann, C.; Schaub, B.; Böck, A.; Nowak, D.; Vogelberg, C.; Radon, K. Parental occupational exposures prior to conception and offspring wheeze and eczema during first year of life. Ann. Epidemiol. 2023, 77, 90–97. [Google Scholar] [CrossRef]
- Solarz, K.; Obuchowicz, A.; Asman, M.; Nowak, W.; Witecka, J.; Pietrzak, J.; Marek, M.; Łonak, A.; Stadnicka, I.; Hajduga-Staśko, B. Abundance of domestic mites in dwellings of children and adolescents with asthma in relation to environmental factors and allergy symptoms. Sci. Rep. 2021, 11, 18453. [Google Scholar] [CrossRef]
- Forster, F.; Kreißl, S.; Wengenroth, L.; Vogelberg, C.; von Mutius, E.; Schaub, B.; Nowak, D.; Weinmann, T.; Radon, K.; Gerlich, J. Third Follow-Up of the Study on Occupational Allergy Risks (SOLAR III) in Germany: Design, Methods, and Initial Data Analysis. Front. Public Health 2021, 9, 591717. [Google Scholar] [CrossRef]
- Dimitrov, I.; Bangov, I.; Flower, D.R.; Doytchinova, I. AllerTOP v.2—A server for in silico prediction of allergens. J. Mol. Model. 2014, 20, 2278. [Google Scholar] [CrossRef]
- Blank, U.; Falcone, F.H.; Nilsson, G. The history of mast cell and basophil research—Some lessons learnt from the last century. Allergy 2013, 68, 1093–1101. [Google Scholar] [CrossRef]
- Amin, K. The role of mast cells in allergic inflammation. Respir. Med. 2012, 106, 9–14. [Google Scholar] [CrossRef]
- Che, D.; Wang, J.; Ding, Y.; Liu, R.; Cao, J.; Zhang, Y.; Hou, Y.; An, H.; Gao, Z.; Zhang, T. Mivacurium induce mast cell activation and pseudo-allergic reactions via MAS-related G protein coupled receptor-X2. Cell. Immunol. 2018, 332, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Lansu, K.; Karpiak, J.; Liu, J.; Huang, X.-P.; McCorvy, J.D.; Kroeze, W.K.; Che, T.; Nagase, H.; Carroll, F.I.; Jin, J.; et al. In silico design of novel probes for the atypical opioid receptor MRGPRX2. Nat. Chem. Biol. 2017, 13, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Reber, L.L.; Sibilano, R.; Mukai, K.; Galli, S.J. Potential effector and immunoregulatory functions of mast cells in mucosal immunity. Mucosal Immunol. 2015, 8, 444–463. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Akin, C.; Hartmann, K.; Nilsson, G.; Reiter, A.; Hermine, O.; Sotlar, K.; Sperr, W.R.; Escribano, L.; George, T.I.; et al. Mast cells as a unique hematopoietic lineage and cell system: From Paul Ehrlich’s visions to precision medicine concepts. Theranostics 2020, 10, 10743–10768. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Shabnam, B.; Girisa, S.; Harsha, C.; Banik, K.; Devi, T.B.; Choudhury, R.; Sahu, H.; Parama, D.; Sailo, B.L.; et al. Inflammation, NF-κB, and Chronic Diseases: How are They Linked? Crit. Rev. Immunol. 2020, 40, 1–39. [Google Scholar] [CrossRef]
- Bilotta, S.; Paruchuru, L.B.; Feilhauer, K.; Köninger, J.; Lorentz, A. Resveratrol Is a Natural Inhibitor of Human Intestinal Mast Cell Activation and Phosphorylation of Mitochondrial ERK1/2 and STAT3. Int. J. Mol. Sci. 2021, 22, 7640. [Google Scholar] [CrossRef]
- Dave, N.D.; Xiang, L.; Rehm, K.E.; Marshall, G.D. Stress and allergic diseases. Immunol. Allergy Clin. N. Am. 2011, 31, 55–68. [Google Scholar] [CrossRef]
- DeFelice, S.L. The nutraceutical revolution: Its impact on food industry R&D. Trends Food Sci. Technol. 1995, 6, 59–61. [Google Scholar] [CrossRef]
- Bergamin, A.; Mantzioris, E.; Cross, G.; Deo, P.; Garg, S.; Hill, A.M. Nutraceuticals: Reviewing their Role in Chronic Disease Prevention and Management. Pharmaceut. Med. 2019, 33, 291–309. [Google Scholar] [CrossRef]
- Das, L.; Bhaumik, E.; Raychaudhuri, U.; Chakraborty, R. Role of nutraceuticals in human health. J. Food Sci. Technol. 2012, 49, 173–183. [Google Scholar] [CrossRef]
- Hagenlocher, Y.; Lorentz, A. Immunomodulation of mast cells by nutrients. Mol. Immunol. 2015, 63, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Alam, S.B.; Kulka, M. The effects of age, origin, and biological sex on rodent mast cell (BMMC and MC/9) and basophil (RBL-2H3) phenotype and function. Cell. Immunol. 2023, 391–392, 104751. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, G.; Blom, T.; Kusche-Gullberg, M.; Kjellén, L.; Butterfield, J.H.; Sundström, C.; Nilsson, K.; Hellman, L. Phenotypic characterization of the human mast-cell line HMC-1. Scand. J. Immunol. 1994, 39, 489–498. [Google Scholar] [CrossRef]
- Sundström, M.; Vliagoftis, H.; Karlberg, P.; Butterfield, J.H.; Nilsson, K.; Metcalfe, D.D.; Nilsson, G. Functional and phenotypic studies of two variants of a human mast cell line with a distinct set of mutations in the c-kit proto-oncogene. Immunology 2003, 108, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Kirshenbaum, A.S.; Akin, C.; Wu, Y.; Rottem, M.; Goff, J.P.; Beaven, M.A.; Rao, V.; Metcalfe, D.D. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcεRI or FcγRI. Leuk. Res. 2003, 27, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Meurer, S.K.; Neß, M.; Weiskirchen, S.; Kim, P.; Tag, C.G.; Kauffmann, M.; Huber, M.; Weiskirchen, R. Isolation of Mature (Peritoneum-Derived) Mast Cells and Immature (Bone Marrow-Derived) Mast Cell Precursors from Mice. PLoS ONE 2016, 11, e0158104. [Google Scholar] [CrossRef]
- Yip, K.-H.; Kolesnikoff, N.; Yu, C.; Hauschild, N.; Taing, H.; Biggs, L.; Goltzman, D.; Gregory, P.A.; Anderson, P.H.; Samuel, M.S.; et al. Mechanisms of vitamin D3 metabolite repression of IgE-dependent mast cell activation. J. Allergy Clin. Immunol. 2014, 133, 1356–1364. [Google Scholar] [CrossRef]
- Benyon, R.C.; Lowman, M.A.; Church, M.K. Human skin mast cells: Their dispersion, purification, and secretory characterization. J. Immunol. 1987, 138, 861–867. [Google Scholar] [CrossRef]
- Lorentz, A.; Sellge, G.; Bischoff, S.C. Isolation and Characterization of Human Intestinal Mast Cells. Mast Cells; Humana Press: New York, NY, USA, 2015; pp. 163–177. [Google Scholar]
- Zhang, Q.; Zhu, W.; Zou, Z.; Yu, W.; Gao, P.; Wang, Y.; Chen, J. A Preliminary Study in Immune Response of BALB/c and C57BL/6 Mice with a Locally Allergic Rhinitis Model. Am. J. Rhinol. Allergy 2023, 37, 410–418. [Google Scholar] [CrossRef]
- Helm, R.M.; Burks, A.W. Animal models of food allergy. Curr. Opin. Allergy Clin. Immunol. 2002, 2, 541. [Google Scholar] [CrossRef]
- Nishino, R.; Fukuyama, T.; Watanabe, Y.; Kurosawa, Y.; Ueda, H.; Kosaka, T. Effect of Mouse Strain in a Model of Chemical-induced Respiratory Allergy. Exp. Anim. 2014, 63, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, Y.; Li, C.; Zhang, Y.; Hu, S.; Gao, J.; Liu, R.; An, H. α-Linolenic acid attenuates pseudo-allergic reactions by inhibiting Lyn kinase activity. Phytomedicine 2021, 80, 153391. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ilarraza, R.; Tancowny, B.P.; Alam, S.B.; Kulka, M. Disrupted Lipid Raft Shuttling of FcεRI by n-3 Polyunsaturated Fatty Acid Is Associated With Ligation of G Protein-Coupled Receptor 120 (GPR120) in Human Mast Cell Line LAD2. Front. Nutr. 2020, 7, 597809. [Google Scholar] [CrossRef] [PubMed]
- Park, B.-K.; Park, S.; Park, J.-B.; Park, M.C.; Min, T.S.; Jin, M. Omega-3 fatty acids suppress Th2-associated cytokine gene expressions and GATA transcription factors in mast cells. J. Nutr. Biochem. 2013, 24, 868–876. [Google Scholar] [CrossRef]
- Van den Elsen, L.W.J.; Nusse, Y.; Balvers, M.; Redegeld, F.A.; Knol, E.F.; Garssen, J.; Willemsen, L.E.M. n-3 Long-chain PUFA reduce allergy-related mediator release by human mast cells in vitro via inhibition of reactive oxygen species. Br. J. Nutr. 2013, 109, 1821–1831. [Google Scholar] [CrossRef]
- Zhang, H.; Du, M.; Yang, Q.; Zhu, M.-J. Butyrate suppresses murine mast cell proliferation and cytokine production through inhibiting histone deacetylase. J. Nutr. Biochem. 2016, 27, 299–306. [Google Scholar] [CrossRef]
- Folkerts, J.; Redegeld, F.; Folkerts, G.; Blokhuis, B.; van den Berg, M.P.M.; de Bruijn, M.J.W.; van IJcken, W.F.J.; Junt, T.; Tam, S.-Y.; Galli, S.J.; et al. Butyrate inhibits human mast cell activation via epigenetic regulation of FcεRI-mediated signaling. Allergy 2020, 75, 1966–1978. [Google Scholar] [CrossRef]
- Lechowski, S.; Feilhauer, K.; Staib, L.; Coëffier, M.; Bischoff, S.C.; Lorentz, A. Combined arginine and glutamine decrease release of de novo synthesized leukotrienes and expression of proinflammatory cytokines in activated human intestinal mast cells. Eur. J. Nutr. 2013, 52, 505–512. [Google Scholar] [CrossRef]
- van Bergenhenegouwen, J.; Braber, S.; Loonstra, R.; Buurman, N.; Rutten, L.; Knipping, K.; Savelkoul, P.J.; Harthoorn, L.F.; Jahnsen, F.L.; Garssen, J.; et al. Oral exposure to the free amino acid glycine inhibits the acute allergic response in a model of cow’s milk allergy in mice. Nutr. Res. 2018, 58, 95–105. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Li, X.-X.; Qiu, S.-Q.; Yu, Y.; Li, M.-G.; Yang, L.-T.; Li, L.-J.; Wang, S.; Zheng, P.-Y.; Liu, Z.-G.; et al. Vitamin D contributes to mast cell stabilization. Allergy 2017, 72, 1184–1192. [Google Scholar] [CrossRef]
- Zhao, J.-W.; Ping, J.-D.; Wang, Y.-F.; Liu, X.-N.; Li, N.; Hu, Z.-L.; Ming, L. Vitamin D suppress the production of vascular endothelial growth factor in mast cell by inhibiting PI3K/Akt/p38 MAPK/HIF-1α pathway in chronic spontaneous urticaria. Clin. Immunol. 2020, 215, 108444. [Google Scholar] [CrossRef] [PubMed]
- Manabe, Y.; Hirata, T.; Sugawara, T. Suppressive effects of carotenoids on the antigen-induced degranulation in RBL-2H3 rat basophilic leukemia cells. J. Oleo Sci. 2014, 63, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Ahn, Y.-T.; Lee, G.S.; Cho, S.I.; Kim, J.-M.; Lee, C.; Lim, B.K.; Ju, S.-A.; An, W.G. Effects of astaxanthin on dinitrofluorobenzene-induced contact dermatitis in mice. Mol. Med. Rep. 2015, 12, 3632–3638. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wang, Y.; Hu, S.; Ding, Y.; Jia, Q.; Zhu, J.; An, H. Kaempferol ameliorates secretagogue-induced pseudo-allergic reactions via inhibiting intracellular calcium fluctuation. J. Pharm. Pharmacol. 2020, 72, 1221–1231. [Google Scholar] [CrossRef]
- Cao, J.; Li, C.; Ma, P.; Ding, Y.; Gao, J.; Jia, Q.; Zhu, J.; Zhang, T. Effect of kaempferol on IgE-mediated anaphylaxis in C57BL/6 mice and LAD2 cells. Phytomedicine 2020, 79, 153346. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Park, S.-H.; Choi, Y.-J.; Kim, Y.-H.; Antika, L.D.; Habibah, N.U.; Kang, M.-K.; Kang, Y.-H. Dietary Compound Kaempferol Inhibits Airway Thickening Induced by Allergic Reaction in a Bovine Serum Albumin-Induced Model of Asthma. Int. J. Mol. Sci. 2015, 16, 29980–29995. [Google Scholar] [CrossRef]
- Nagata, K.; Araumi, S.; Ando, D.; Ito, N.; Ando, M.; Ikeda, Y.; Takahashi, M.; Noguchi, S.; Yasuda, Y.; Nakano, N.; et al. Kaempferol Suppresses the Activation of Mast Cells by Modulating the Expression of FcεRI and SHIP1. Int. J. Mol. Sci. 2023, 24, 5997. [Google Scholar] [CrossRef]
- Ding, Y.; Li, C.; Zhang, Y.; Ma, P.; Zhao, T.; Che, D.; Cao, J.; Wang, J.; Liu, R.; Zhang, T.; et al. Quercetin as a Lyn kinase inhibitor inhibits IgE-mediated allergic conjunctivitis. Food Chem. Toxicol. 2020, 135, 110924. [Google Scholar] [CrossRef]
- Ding, Y.; Che, D.; Li, C.; Cao, J.; Wang, J.; Ma, P.; Zhao, T.; An, H.; Zhang, T. Quercetin inhibits Mrgprx2-induced pseudo-allergic reaction via PLCγ-IP3R related Ca2+ fluctuations. Int. Immunopharmacol. 2019, 66, 185–197. [Google Scholar] [CrossRef]
- Kim, M.; Lim, S.J.; Kang, S.W.; Um, B.-H.; Nho, C.W. Aceriphyllum rossii extract and its active compounds, quercetin and kaempferol inhibit IgE-mediated mast cell activation and passive cutaneous anaphylaxis. J. Agric. Food Chem. 2014, 62, 3750–3758. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, Y.; Dang, B.; Wang, Y.; Zheng, G.; Zhang, T.; An, H. Myricetin alleviated immunologic contact urticaria and mast cell degranulation via the PI3K/Akt/NF-κB pathway. Phytother. Res. 2023, 37, 2024–2035. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.S.; Le, T.T.; Kim, S.-Y.; Ngo, D.-H. The role of myricetin from Rhodomyrtus tomentosa (Aiton) Hassk fruits on downregulation of FcɛRI-mediated mast cell activation. J. Food Biochem. 2020, 44, e13143. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Che, D.; Yu, Y.; Liu, L.; Mi, S.; Zhang, Y.; Hao, J.; Li, W.; Ji, M.; Geng, S.; et al. Luteolin inhibits FcεRΙ- and MRGPRX2-mediated mast cell activation by regulating calcium signaling pathways. Phytother. Res. 2022, 36, 2197–2206. [Google Scholar] [CrossRef] [PubMed]
- Jeon, I.H.; Kim, H.S.; Kang, H.J.; Lee, H.-S.; Jeong, S.I.; Kim, S.J.; Jang, S.I. Anti-inflammatory and antipruritic effects of luteolin from Perilla (P. frutescens L.) leaves. Molecules 2014, 19, 6941–6951. [Google Scholar] [CrossRef] [PubMed]
- Che, D.N.; Shin, J.Y.; Kang, H.J.; Cho, B.O.; Kim, Y.-S.; Jang, S.I. Luteolin suppresses IL-31 production in IL-33-stimulated mast cells through MAPK and NF-κB signaling pathways. Int. Immunopharmacol. 2020, 83, 106403. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y.; Kang, H.-G.; Nam, S.-Y.; Kim, H.-M.; Jeong, H.-J. Blockade of RANKL/RANK signaling pathway by epigallocatechin gallate alleviates mast cell-mediated inflammatory reactions. Int. Immunopharmacol. 2020, 88, 106872. [Google Scholar] [CrossRef]
- Fujimura, Y.; Yoshimoto, T.; Fujino, K.; Nezu, A.; Marugame, Y.; Bae, J.; Kumazoe, M.; Tachibana, H. Bioactivity-boosting strategy based on combination of anti-allergic O-methylated catechin with a Citrus flavanone, hesperetin. J. Nat. Med. 2023, 77, 363–369. [Google Scholar] [CrossRef]
- Fujimura, Y.; Fujino, K.; Yoshimoto, T.; Nezu, A.; Marugame, Y.; Bae, J.; Kumazoe, M.; Tachibana, H. Eriodictyol-Amplified 67-kDa Laminin Receptor Signaling Potentiates the Antiallergic Effect of O-Methylated Catechin. J. Nat. Prod. 2021, 84, 1823–1830. [Google Scholar] [CrossRef]
- Han, N.-R.; Moon, P.-D.; Ryu, K.-J.; Kim, N.-R.; Kim, H.-M.; Jeong, H.-J. Inhibitory effect of naringenin via IL-13 level regulation on thymic stromal lymphopoietin-induced inflammatory reactions. Clin. Exp. Pharmacol. Physiol. 2018, 45, 362–369. [Google Scholar] [CrossRef]
- Hagenlocher, Y.; Feilhauer, K.; Schäffer, M.; Bischoff, S.C.; Lorentz, A. Citrus peel polymethoxyflavones nobiletin and tangeretin suppress LPS- and IgE-mediated activation of human intestinal mast cells. Eur. J. Nutr. 2017, 56, 1609–1620. [Google Scholar] [CrossRef]
- Jang, S.-E.; Ryu, K.-R.; Park, S.-H.; Chung, S.; Teruya, Y.; Han, M.J.; Woo, J.-T.; Kim, D.-H. Nobiletin and tangeretin ameliorate scratching behavior in mice by inhibiting the action of histamine and the activation of NF-κB, AP-1 and p38. Int. Immunopharmacol. 2013, 17, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Shirley, D.; McHale, C.; Gomez, G. Resveratrol preferentially inhibits IgE-dependent PGD2 biosynthesis but enhances TNF production from human skin mast cells. Biochim. Biophys. Acta 2016, 1860, 678–685. [Google Scholar] [CrossRef]
- Nakajima, S.; Ishimaru, K.; Kobayashi, A.; Yu, G.; Nakamura, Y.; Oh-Oka, K.; Suzuki-Inoue, K.; Kono, K.; Nakao, A. Resveratrol inhibits IL-33-mediated mast cell activation by targeting the MK2/3-PI3K/Akt axis. Sci. Rep. 2019, 9, 18423. [Google Scholar] [CrossRef]
- Li, X.; Lee, Y.J.; Jin, F.; Park, Y.N.; Deng, Y.; Kang, Y.; Yang, J.H.; Chang, J.-H.; Kim, D.-Y.; Kim, J.-A.; et al. Sirt1 negatively regulates FcεRI-mediated mast cell activation through AMPK- and PTP1B-dependent processes. Sci. Rep. 2017, 7, 6444. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, Q.; Guo, X.; Xiang, L.; Zhao, G. Resveratrol attenuates IL-33-induced mast cell inflammation associated with inhibition of NF-κB activation and the P38 signaling pathway. Mol. Med. Rep. 2020, 21, 1658–1666. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-Y.; Choi, Y.-J.; Kang, M.-K.; Park, J.H.Y.; Kang, Y.-H. Resveratrol Suppresses Cytokine Production Linked to FcεRI-MAPK Activation in IgE-Antigen Complex-Exposed Basophilic Mast Cells and Mice. Am. J. Chin. Med. 2015, 43, 1605–1623. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Hu, S.; Ge, S.; Jia, M.; Wang, N. Resveratrol inhibits MRGPRX2-mediated mast cell activation via Nrf2 pathway. Int. Immunopharmacol. 2021, 93, 107426. [Google Scholar] [CrossRef]
- Moon, P.-D.; Han, N.-R.; Lee, J.S.; Jee, H.-W.; Kim, J.-H.; Kim, H.-M.; Jeong, H.-J. Effects of Resveratrol on Thymic Stromal Lymphopoietin Expression in Mast Cells. Medicina 2020, 57, 21. [Google Scholar] [CrossRef]
- Kong, Z.-L.; Sudirman, S.; Lin, H.-J.; Chen, W.-N. In vitro anti-inflammatory effects of curcumin on mast cell-mediated allergic responses via inhibiting FcεRI protein expression and protein kinase C delta translocation. Cytotechnology 2020, 72, 81–95. [Google Scholar] [CrossRef]
- Li, X.; Lu, Y.; Jin, Y.; Son, J.-K.; Lee, S.H.; Chang, H.W. Curcumin inhibits the activation of immunoglobulin e-mediated mast cells and passive systemic anaphylaxis in mice by reducing serum eicosanoid and histamine levels. Biomol. Ther. 2014, 22, 27–34. [Google Scholar] [CrossRef]
- Zhang, N.; Li, H.; Jia, J.; He, M. Anti-inflammatory effect of curcumin on mast cell-mediated allergic responses in ovalbumin-induced allergic rhinitis mouse. Cell. Immunol. 2015, 298, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Kong, R.; Kang, O.-H.; Seo, Y.-S.; Zhou, T.; Kim, S.-A.; Shin, D.-W.; Kwon, D.-Y. MAPKs and NF-κB pathway inhibitory effect of bisdemethoxycurcumin on phorbol-12-myristate-13-acetate and A23187-induced inflammation in human mast cells. Mol. Med. Rep. 2018, 17, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Hagenlocher, Y.; Bergheim, I.; Zacheja, S.; Schäffer, M.; Bischoff, S.C.; Lorentz, A. Cinnamon extract inhibits degranulation and de novo synthesis of inflammatory mediators in mast cells. Allergy 2013, 68, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Hagenlocher, Y.; Kiessling, K.; Schäffer, M.; Bischoff, S.C.; Lorentz, A. Cinnamaldehyde is the main mediator of cinnamon extract in mast cell inhibition. Eur. J. Nutr. 2015, 54, 1297–1309. [Google Scholar] [CrossRef]
- Ahn, S.; Kim, E.; Lee, K.; Lee, D.-C. Cinnamaldehyde derivatives inhibit degranulation and inflammatory mediator production in rat basophilic leukemia cells. Int. Immunopharmacol. 2016, 38, 342–348. [Google Scholar] [CrossRef]
- Wang, C.C.; Wu, H.; Lin, F.H.; Gong, R.; Xie, F.; Peng, Y.; Feng, J.; Hu, C.H. Sodium butyrate enhances intestinal integrity, inhibits mast cell activation, inflammatory mediator production and JNK signaling pathway in weaned pigs. Innate Immun. 2018, 24, 40–46. [Google Scholar] [CrossRef]
- He, Y.; Qu, J.; Yang, Q.; Wu, Z.; Liu, M.; Tso, P. Effect of L-Glutamine on Chylomicron Formation and Fat-Induced Activation of Intestinal Mucosal Mast Cells in Sprague-Dawley Rats. Nutrients 2022, 14, 1777. [Google Scholar] [CrossRef]
- Sugiura, Y.; Kinoshita, Y.; Usui, M.; Tanaka, R.; Matsushita, T.; Miyata, M. The Suppressive Effect of a Marine Carotenoid, Fucoxanthin, on Mouse Ear Swelling through Regulation of Activities and mRNA Expression of Inflammation-associated Enzymes. Food Sci. Technol. Res. 2016, 22, 227–234. [Google Scholar] [CrossRef]
- Park, J.W.; Song, H.-S. Effect of Astaxanthin on Anti-Inflammatory and Anti-Oxidative Effects of Astaxanthin Treatment for Atopic Dermatitis-induced Mice. J. Acupunct. Res. 2021, 38, 293–299. [Google Scholar] [CrossRef]
- Park, J.H.; Yeo, I.J.; Han, J.H.; Suh, J.W.; Lee, H.P.; Hong, J.T. Anti-inflammatory effect of astaxanthin in phthalic anhydride-induced atopic dermatitis animal model. Exp. Dermatol. 2018, 27, 378–385. [Google Scholar] [CrossRef]
- Lee, Y.S.; Jeon, S.H.; Ham, H.J.; Lee, H.P.; Song, M.J.; Hong, J.T. Improved Anti-Inflammatory Effects of Liposomal Astaxanthin on a Phthalic Anhydride-Induced Atopic Dermatitis Model. Front. Immunol. 2020, 11, 565285. [Google Scholar] [CrossRef] [PubMed]
- Yoshihisa, Y.; Andoh, T.; Matsunaga, K.; Rehman, M.U.; Maoka, T.; Shimizu, T. Efficacy of Astaxanthin for the Treatment of Atopic Dermatitis in a Murine Model. PLoS ONE 2016, 11, e0152288. [Google Scholar] [CrossRef] [PubMed]
- Ushiroda, C.; Takagi, T.; Fuke, N.; Mizushima, K.; Hirai, Y.; Higashimura, Y.; Harusato, A.; Kamada, K.; Uchiyama, K.; Ishikawa, T.; et al. Lycopene intake induces colonic regulatory T cells in mice and suppresses food allergy symptoms. Pediatr. Allergy Immunol. 2022, 33, e13691. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Fu, S.; Ni, S.; Zou, L.; Liu, Y.; Hong, T. Anti-inflammatory effect of epigallocatechin gallate in a mouse model of ovalbumin-induced allergic rhinitis. Int. Immunopharmacol. 2017, 49, 102–108. [Google Scholar] [CrossRef]
- Şahin, A.; Sakat, M.S.; Kılıç, K.; Aktan, B.; Yildirim, S.; Kandemir, F.M.; Dortbudak, M.B.; Kucukler, S. The protective effect of Naringenin against ovalbumin-induced allergic rhinitis in rats. Eur. Arch. Otorhinolaryngol. 2021, 278, 4839–4846. [Google Scholar] [CrossRef]
- Kim, T.-H.; Kim, G.-D.; Ahn, H.-J.; Cho, J.-J.; Park, Y.S.; Park, C.-S. The inhibitory effect of naringenin on atopic dermatitis induced by DNFB in NC/Nga mice. Life Sci. 2013, 93, 516–524. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Liu, Q.-M.; Gao, Y.-Y.; Liu, B.; Liu, H.; Cao, M.-J.; Yang, X.-W.; Liu, G.-M. Attenuation of allergic responses following treatment with resveratrol in anaphylactic models and IgE-mediated mast cells. Food Funct. 2019, 10, 2030–2039. [Google Scholar] [CrossRef]
- Bilotta, S.; Arbogast, J.; Schart, N.; Frei, M.; Lorentz, A. Resveratrol Treatment Prevents Increase of Mast Cells in Both Murine OVA Enteritis and IL-10−/− Colitis. Int. J. Mol. Sci. 2022, 23, 1213. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, X.; Han, X.; Hu, D.; Hu, X.; Li, Y.; Huang, P.; Yao, W. Resveratrol Suppresses Gut-Derived NLRP3 Inflammasome Partly through Stabilizing Mast Cells in a Rat Model. Mediat. Inflamm. 2018, 2018, 6158671. [Google Scholar] [CrossRef]
- Carlucci, C.D.; Hui, Y.; Chumanevich, A.P.; Robida, P.A.; Fuseler, J.W.; Sajish, M.; Nagarkatti, P.; Nagarkatti, M.; Oskeritzian, C.A. Resveratrol Protects against Skin Inflammation through Inhibition of Mast Cell, Sphingosine Kinase-1, Stat3 and NF-κB p65 Signaling Activation in Mice. Int. J. Mol. Sci. 2023, 24, 6707. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, P.; Zhang, J.; Hong, T. Bisdemethoxycurcumin attenuates OVA-induced food allergy by inhibiting the MAPK and NF-κB signaling pathways. Exp. Ther. Med. 2022, 23, 401. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Fu, S.; Ni, S.; Wang, D.; Hong, T. Inhibitory effects of bisdemethoxycurcumin on mast cell-mediated allergic diseases. Int. Immunopharmacol. 2018, 65, 182–189. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, S.K.; Psota, T.L.; Harris, W.S.; Kris-Etherton, P.M. n-3 fatty acid dietary recommendations and food sources to achieve essentiality and cardiovascular benefits. Am. J. Clin. Nutr. 2006, 83, 1526S–1535S. [Google Scholar] [CrossRef] [PubMed]
- Hogenkamp, A.; Ehlers, A.; Garssen, J.; Willemsen, L.E.M. Allergy Modulation by N-3 Long Chain Polyunsaturated Fatty Acids and Fat Soluble Nutrients of the Mediterranean Diet. Front. Pharmacol. 2020, 11, 1244. [Google Scholar] [CrossRef]
- Migliaccio, A.R.; Rana, R.A.; Sanchez, M.; Lorenzini, R.; Centurione, L.; Bianchi, L.; Vannucchi, A.M.; Migliaccio, G.; Orkin, S.H. GATA-1 as a regulator of mast cell differentiation revealed by the phenotype of the GATA-1low mouse mutant. J. Exp. Med. 2003, 197, 281–296. [Google Scholar] [CrossRef]
- Tsai, F.Y.; Orkin, S.H. Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cell formation, but not for erythroid and myeloid terminal differentiation. Blood 1997, 89, 3636–3643. [Google Scholar] [CrossRef]
- Calton, E.K.; Keane, K.N.; Newsholme, P.; Soares, M.J. The Impact of Vitamin D Levels on Inflammatory Status: A Systematic Review of Immune Cell Studies. PLoS ONE 2015, 10, e0141770. [Google Scholar] [CrossRef]
- Aguilar-Shea, A.L. Vitamin D, the natural way. Clin. Nutr. ESPEN 2021, 41, 10–12. [Google Scholar] [CrossRef]
- Passeron, T.; Bouillon, R.; Callender, V.; Cestari, T.; Diepgen, T.L.; Green, A.C.; van der Pols, J.C.; Bernard, B.A.; Ly, F.; Bernerd, F.; et al. Sunscreen photoprotection and vitamin D status. Br. J. Dermatol. 2019, 181, 916–931. [Google Scholar] [CrossRef]
- Kim, B.-G.; Lee, P.-H.; Lee, S.-H.; Hong, J.; Jang, A.-S. Claudins, VEGF, Nrf2, Keap1, and Nonspecific Airway Hyper-Reactivity Are Increased in Mice Co-Exposed to Allergen and Acrolein. Chem. Res. Toxicol. 2019, 32, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J.; Mandić, A.I.; Bantis, F.; Böhm, V.; Borge, G.I.A.; Brnčić, M.; Bysted, A.; Cano, M.P.; Dias, M.G.; Elgersma, A.; et al. A comprehensive review on carotenoids in foods and feeds: Status quo, applications, patents, and research needs. Crit. Rev. Food Sci. Nutr. 2022, 62, 1999–2049. [Google Scholar] [CrossRef]
- Deng, J.-J.; Mao, H.-H.; Fang, W.; Li, Z.-Q.; Shi, D.; Li, Z.-W.; Zhou, T.; Luo, X.-C. Enzymatic conversion and recovery of protein, chitin, and astaxanthin from shrimp shell waste. J. Clean. Prod. 2020, 271, 122655. [Google Scholar] [CrossRef]
- Saini, R.K.; Mahomoodally, M.F.; Sadeer, N.B.; Keum, Y.-S.; Rr Rengasamy, K. Characterization of nutritionally important lipophilic constituents from brown kelp Ecklonia radiata (C. Ag.). J. Agardh. Food Chem. 2021, 340, 127897. [Google Scholar] [CrossRef] [PubMed]
- Viuda-Martos, M.; Sanchez-Zapata, E.; Sayas-Barberá, E.; Sendra, E.; Pérez-Álvarez, J.A.; Fernández-López, J. Tomato and tomato byproducts. Human health benefits of lycopene and its application to meat products: A review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1032–1049. [Google Scholar] [CrossRef]
- Sugawara, T.; Baskaran, V.; Tsuzuki, W.; Nagao, A. Brown algae fucoxanthin is hydrolyzed to fucoxanthinol during absorption by Caco-2 human intestinal cells and mice. J. Nutr. 2002, 132, 946–951. [Google Scholar] [CrossRef]
- Shik, D.; Tomar, S.; Lee, J.-B.; Chen, C.-Y.; Smith, A.; Wang, Y.-H. IL-9-producing cells in the development of IgE-mediated food allergy. Semin. Immunopathol. 2017, 39, 69–77. [Google Scholar] [CrossRef]
- Thilakarathna, S.H.; Rupasinghe, H.P.V. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Park, H.-H.; Lee, S.; Son, H.-Y.; Park, S.-B.; Kim, M.-S.; Choi, E.-J.; Singh, T.S.K.; Ha, J.-H.; Lee, M.-G.; Kim, J.-E.; et al. Flavonoids inhibit histamine release and expression of proinflammatory cytokines in mast cells. Arch. Pharm. Res. 2008, 31, 1303–1311. [Google Scholar] [CrossRef]
- Kubatka, P.; Mazurakova, A.; Samec, M.; Koklesova, L.; Zhai, K.; Al-Ishaq, R.; Kajo, K.; Biringer, K.; Vybohova, D.; Brockmueller, A.; et al. Flavonoids against non-physiologic inflammation attributed to cancer initiation, development, and progression-3PM pathways. EPMA J. 2021, 12, 559–587. [Google Scholar] [CrossRef] [PubMed]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.-M. Luteolin, a flavonoid with potentials for cancer prevention and therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Bhia, M.; Motallebi, M.; Abadi, B.; Zarepour, A.; Pereira-Silva, M.; Saremnejad, F.; Santos, A.C.; Zarrabi, A.; Melero, A.; Jafari, S.M.; et al. Naringenin Nano-Delivery Systems and Their Therapeutic Applications. Pharmaceutics 2021, 13, 291. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Jin, L.; Zhang, F.; Zhang, C.; Liang, W. Naringenin as a potential immunomodulator in therapeutics. Pharmacol. Res. 2018, 135, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lo, C.-Y.; Ho, C.-T. Hydroxylated polymethoxyflavones and methylated flavonoids in sweet orange (Citrus sinensis) peel. J. Agric. Food Chem. 2006, 54, 4176–4185. [Google Scholar] [CrossRef]
- Tominari, T.; Hirata, M.; Matsumoto, C.; Inada, M.; Miyaura, C. Polymethoxy flavonoids, nobiletin and tangeretin, prevent lipopolysaccharide-induced inflammatory bone loss in an experimental model for periodontitis. J. Pharmacol. Sci. 2012, 119, 390–394. [Google Scholar] [CrossRef]
- Murakami, A.; Nakamura, Y.; Torikai, K.; Tanaka, T.; Koshiba, T.; Koshimizu, K.; Kuwahara, S.; Takahashi, Y.; Ogawa, K.; Yano, M.; et al. Inhibitory effect of citrus nobiletin on phorbol ester-induced skin inflammation, oxidative stress, and tumor promotion in mice. Cancer Res. 2000, 60, 5059–5066. [Google Scholar]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Xing, C.; Wang, Y.; Dai, X.; Yang, F.; Luo, J.; Liu, P.; Zhang, C.; Cao, H.; Hu, G. The protective effects of resveratrol on antioxidant function and the mRNA expression of inflammatory cytokines in the ovaries of hens with fatty liver hemorrhagic syndrome. Poult. Sci. 2020, 99, 1019–1027. [Google Scholar] [CrossRef]
- Chen, M.; Fu, Q.; Song, X.; Muhammad, A.; Jia, R.; Zou, Y.; Yin, L.; Li, L.; He, C.; Ye, G.; et al. Preparation of resveratrol dry suspension and its immunomodulatory and anti-inflammatory activity in mice. Pharm. Biol. 2020, 58, 8–15. [Google Scholar] [CrossRef]
- Singh, C.K.; Liu, X.; Ahmad, N. Resveratrol, in its natural combination in whole grape, for health promotion and disease management. Ann. N. Y. Acad. Sci. 2015, 1348, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Perrone, D.; Fuggetta, M.P.; Ardito, F.; Cottarelli, A.; de Filippis, A.; Ravagnan, G.; de Maria, S.; Lo Muzio, L. Resveratrol (3,5,4′-trihydroxystilbene) and its properties in oral diseases. Exp. Ther. Med. 2017, 14, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.J.; Crozier, A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-Y.; Bae, J.-Y.; Park, S.-H.; Kim, Y.-H.; Park, J.H.Y.; Kang, Y.-H. Resveratrol inhibits IgE-mediated basophilic mast cell degranulation and passive cutaneous anaphylaxis in mice. J. Nutr. 2013, 143, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Wedman, P.A.; Aladhami, A.; Chumanevich, A.P.; Fuseler, J.W.; Oskeritzian, C.A. Mast cells and sphingosine-1-phosphate underlie prelesional remodeling in a mouse model of eczema. Allergy 2018, 73, 405–415. [Google Scholar] [CrossRef]
- Oskeritzian, C.A.; Hait, N.C.; Wedman, P.; Chumanevich, A.; Kolawole, E.M.; Price, M.M.; Falanga, Y.T.; Harikumar, K.B.; Ryan, J.J.; Milstien, S.; et al. The sphingosine-1-phosphate/sphingosine-1-phosphate receptor 2 axis regulates early airway T-cell infiltration in murine mast cell-dependent acute allergic responses. J. Allergy Clin. Immunol. 2015, 135, 1008–1018.e1. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.W.; Ko, N.Y.; Mun, S.H.; Her, E.; Kim, B.K.; Han, J.W.; Lee, H.Y.; Beaven, M.A.; Kim, Y.M.; et al. Curcumin, a constituent of curry, suppresses IgE-mediated allergic response and mast cell activation at the level of Syk. J. Allergy Clin. Immunol. 2008, 121, 1225–1231. [Google Scholar] [CrossRef]
- Makuch, S.; Więcek, K.; Woźniak, M. The Immunomodulatory and Anti-Inflammatory Effect of Curcumin on Immune Cell Populations, Cytokines, and In Vivo Models of Rheumatoid Arthritis. Pharmaceuticals 2021, 14, 309. [Google Scholar] [CrossRef]
- Brockmueller, A.; Mueller, A.-L.; Kunnumakkara, A.B.; Aggarwal, B.B.; Shakibaei, M. Multifunctionality of Calebin A in inflammation, chronic diseases and cancer. Front. Oncol. 2022, 12, 962066. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Yuan, W.; Li, S.; Gupta, S.C. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: Identification of novel components of turmeric. Mol. Nutr. Food Res. 2013, 57, 1529–1542. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zong, Y.; Du, Y.; Zhang, M.; Ye, F.; Zhang, J.; Yang, Y.; Zhu, C.; Tang, Z. Curcumin inhibits the pruritus in mice through mast cell MrgprB2 receptor. Inflamm. Res. 2023, 72, 933–945. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Karin, M. Mammalian MAP kinase signalling cascades. Nature 2001, 410, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Vitte, J.; Vibhushan, S.; Bratti, M.; Montero-Hernández, J.E.; Blank, U. Allergy, Anaphylaxis and Non-Allergic Hypersensitivity: IgE, Mast Cells and Beyond. Med. Princ. Pract. 2022, 31, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, X.; Ren, Y.; Yang, H.; Sun, X.; Huang, H. Clinical Efficacy of Vitamin D3 Adjuvant Therapy in Allergic Rhinitis: A Randomized Controlled Trial. Iran. J. Immunol. 2020, 17, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Bakhshaee, M.; Sharifian, M.; Esmatinia, F.; Rasoulian, B.; Mohebbi, M. Therapeutic effect of vitamin D supplementation on allergic rhinitis. Eur. Arch. Otorhinolaryngol. 2019, 276, 2797–2801. [Google Scholar] [CrossRef]
- Yamada, S.; Shirai, M.; Inaba, Y.; Takara, T. Effects of repeated oral intake of a quercetin-containing supplement on allergic reaction: A randomized, placebo-controlled, double-blind parallel-group study. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 4331–4345. [Google Scholar] [CrossRef]
- Masuda, S.; Maeda-Yamamoto, M.; Usui, S.; Fujisawa, T. ‘Benifuuki’ Green Tea Containing O-Methylated Catechin Reduces Symptoms of Japanese Cedar Pollinosis: A Randomized, Double- Blind, Placebo-Controlled Trial. Allergol. Int. 2014, 63, 211–217. [Google Scholar] [CrossRef]
- Lv, C.; Zhang, Y.; Shen, L. Preliminary Clinical Effect Evaluation of Resveratrol in Adults with Allergic Rhinitis. Int. Arch. Allergy Immunol. 2018, 175, 231–236. [Google Scholar] [CrossRef]
- Del Miraglia Giudice, M.; Maiello, N.; Capristo, C.; Alterio, E.; Capasso, M.; Perrone, L.; Ciprandi, G. Resveratrol plus carboxymethyl-β-glucan reduces nasal symptoms in children with pollen-induced allergic rhinitis. Curr. Med. Res. Opin. 2014, 30, 1931–1935. [Google Scholar] [CrossRef]
- Wu, S.; Xiao, D. Effect of curcumin on nasal symptoms and airflow in patients with perennial allergic rhinitis. Ann. Allergy Asthma Immunol. 2016, 117, 697–702.e1. [Google Scholar] [CrossRef] [PubMed]
- Steels, E.; Steels, E.; Deshpande, P.; Thakurdesai, P.; Dighe, S.; Collet, T. A randomized, double-blind placebo-controlled study of intranasal standardized cinnamon bark extract for seasonal allergic rhinitis. Complement. Ther. Med. 2019, 47, 102198. [Google Scholar] [CrossRef]
- Navarro Suarez, L.; Thein, S.; Kallinich, C.; Rohn, S. Electrochemical Oxidation as a Tool for Generating Vitamin D Metabolites. Molecules 2019, 24, 2369. [Google Scholar] [CrossRef] [PubMed]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability of nutraceuticals: Role of the food matrix, processing conditions, the gastrointestinal tract, and nanodelivery systems. Compr. Rev. Food Sci. Food Saf. 2020, 19, 954–994. [Google Scholar] [CrossRef] [PubMed]
- Parada, J.; Aguilera, J.M. Food microstructure affects the bioavailability of several nutrients. J. Food Sci. 2007, 72, R21–R32. [Google Scholar] [CrossRef] [PubMed]
- Faridi Esfanjani, A.; Assadpour, E.; Jafari, S.M. Improving the bioavailability of phenolic compounds by loading them within lipid-based nanocarriers. Trends Food Sci. Technol. 2018, 76, 56–66. [Google Scholar] [CrossRef]
- Rezaei, A.; Fathi, M.; Jafari, S.M. Nanoencapsulation of hydrophobic and low-soluble food bioactive compounds within different nanocarriers. Food Hydrocoll. 2019, 88, 146–162. [Google Scholar] [CrossRef]
- Atal, N.; Bedi, K.L. Bioenhancers: Revolutionary concept to market. J. Ayurveda Integr. Med. 2010, 1, 96–99. [Google Scholar] [CrossRef]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef]
- Johnson, J.J.; Nihal, M.; Siddiqui, I.A.; Scarlett, C.O.; Bailey, H.H.; Mukhtar, H.; Ahmad, N. Enhancing the bioavailability of resveratrol by combining it with piperine. Mol. Nutr. Food Res. 2011, 55, 1169–1176. [Google Scholar] [CrossRef]
- Shaikh, J.; Ankola, D.D.; Beniwal, V.; Singh, D.; Kumar, M.N.V.R. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur. J. Pharm. Sci. 2009, 37, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.M.; McClements, D.J. Nanotechnology Approaches for Increasing Nutrient Bioavailability. Adv. Food Nutr. Res. 2017, 81, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Shin, G.H.; Kim, J.T.; Park, H.J. Recent developments in nanoformulations of lipophilic functional foods. Trends Food Sci. Technol. 2015, 46, 144–157. [Google Scholar] [CrossRef]
- Cohen, Y.; Levi, M.; Lesmes, U.; Margier, M.; Reboul, E.; Livney, Y.D. Re-assembled casein micelles improve in vitro bioavailability of vitamin D in a Caco-2 cell model. Food Funct. 2017, 8, 2133–2141. [Google Scholar] [CrossRef] [PubMed]
- Penalva, R.; Esparza, I.; Larraneta, E.; González-Navarro, C.J.; Gamazo, C.; Irache, J.M. Zein-Based Nanoparticles Improve the Oral Bioavailability of Resveratrol and Its Anti-inflammatory Effects in a Mouse Model of Endotoxic Shock. J. Agric. Food Chem. 2015, 63, 5603–5611. [Google Scholar] [CrossRef]
- Pan, L.; Wang, H.; Gu, K. Nanoliposomes as Vehicles for Astaxanthin: Characterization, In Vitro Release Evaluation and Structure. Molecules 2018, 23, 2822. [Google Scholar] [CrossRef]
- Peralta, M.F.; Guzmán, M.L.; Pérez, A.P.; Apezteguia, G.A.; Fórmica, M.L.; Romero, E.L.; Olivera, M.E.; Carrer, D.C. Liposomes can both enhance or reduce drugs penetration through the skin. Sci. Rep. 2018, 8, 13253. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.; Feng, J.; Xuan, R. Research progress of Astaxanthin nano-based drug delivery system: Applications, prospects and challenges? Front. Pharmacol. 2023, 14, 1102888. [Google Scholar] [CrossRef]
- Riva, A.; Ronchi, M.; Petrangolini, G.; Bosisio, S.; Allegrini, P. Improved Oral Absorption of Quercetin from Quercetin Phytosome®, a New Delivery System Based on Food Grade Lecithin. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 169–177. [Google Scholar] [CrossRef]
- Kaya-Celiker, H.; Mallikarjunan, K. Better Nutrients and Therapeutics Delivery in Food Through Nanotechnology. Food Eng. Rev. 2012, 4, 114–123. [Google Scholar] [CrossRef]
- Lamprecht, A.; Saumet, J.-L.; Roux, J.; Benoit, J.-P. Lipid nanocarriers as drug delivery system for ibuprofen in pain treatment. Int. J. Pharm. 2004, 278, 407–414. [Google Scholar] [CrossRef] [PubMed]
| Substance | Dosage | MC Model | Stimulation | MC Degranulation | AA Metabolites | Cytokines/Chemokines | Signaling Molecules | Ref. |
|---|---|---|---|---|---|---|---|---|
| ALA | 50, 100, 200 µM | LAD2 | C48/80, SP | ↓ β-hex (≈70%) ↓ Histamine (≈60%) with 200 µM | - | ↓ IL-8, IL-13, TNF-α (dd) | ↓ p-Lyn, p-PLCγ, p-IP3R ↓ p-38, p-IKK, NF-κB ↓ Lyn kinase activity | [33] |
| EPA/DHA | 100 µM | LAD2 | IgE-biotin/SA | ↓ β-hex (25%) | ↓ cys-LT (80%/76%) | - | ↓ FcεRI localization into lipid rafts (> 50%) ↓ p-Lyn (37%/50%), p-Syk (33%/40%) ↓ p-LAT (37%/43%) | [34] |
| ALA, EPA, DHA | 50, 75, 100 μM | BMMC, MC/9 | IONO/PMA or Anti-DNP-IgE/DNP-BSA | - | - | ↓ IL-4, IL-5, IL-13 (dd) | ↓ GATA-1 and GATA-2 | [35] |
| EPA, DHA | 100 μM | HMC-1 | IONO/PMA | ↔ β-hex | ↓ PGD2 (≈75%) | ↓ IL-4 (≈70%), IL-13 (≈65%) | ↓ p-ERK, p-JNK, p-p38 | [36] |
| Butyrate | 2 mM | BMMC | IgE/TNP-BSA | ↔ β-hex | - | ↓ TNF-α and IL-6 (≈70%) | ↓ p-ERK, p-JNK, p-p38 | [37] |
| Butyrate, Propionate | 1, 5, 25 mM | BMMC PBMCMC | IgE/DNP-HSA or C48/80, SP | ↓ β-hex (≈90% with 25 mM) | - | ↓ IL-6 and TNF-α (≈90–100% with 25 mM) | ↓ p-Btk (64%), p-Syk (43%), p-LAT (70%) | [38] |
| Glutamine/ Arginine | 10 mM/2 mM | hiMC | Human myeloma IgE/anti-human IgE | ↔ β-hex | ↓ LTC4 (≈45%) | ↓ CCL2, CCL4, CXCL8 and TNF-α (≈50%) | ↓ p-ERK1/2, p-JNK, p-p38 ↓ p-MEK1/2 (≈30–45%), p-Akt (≈25%) | [39] |
| Glycine | 250, 500 µg/mL | RBL-2H3 | IgE/DNP-BSA | ↔ | - | ↓ TNF-α (≈60%), IL-4 (≈75%), IL-13 (≈45%) with 250 µg/mL | - | [40] |
| Calcitriol | 10 nM | BMMC, HMC-1, RBL-2H3, p815 | DNP-IgE/DNP | ↓ Histamine (≈90–100%) | - | ↓ TNF-α (≈90–100%) | ↓ p-Syk, p-p38, p-NF-κB(p50/65) | [41] |
| Calcidiol | 10−8, 10−7, 10−6 M | BMMC | IgE/DNP-HSA | ↓ Histamine (dd; 23–34%) | ↓ cys-LT (dd; 34–44%) | ↓ TNF-α and IL-6 (35% with 10−8, 10−7 M) | - | [27] |
| Calcitriol | 10−8, 10−7 M | CBMC, PBMC | Human myeloma IgE/anti- human IgE | ↓ Histamine (dd; ≈20, ≈30%) | ↓ cys-LT (dd; ns, ≈20%) | ↓ TNF-α (dd; ≈50, ≈50%) ↔ IL-10 | - | [27] |
| Calcidiol | 10−8, 10−7, 10−6 M | CBMC, PBMC | Human myeloma IgE/anti- human IgE | ↓ Histamine (dd; ≈10, ≈30%) | ↓ cys-LT (dd; ≈15–30%) | ↓ TNF-α (dd; ≈10–30%) ↔ IL-10 | - | [27] |
| Calcidiol | 10−9–10−6 M | LAD2 | IgE of sera of CSU patients | - | - | - | ↓ p-Akt, p-p38, HIF-α, p-NF-κB | [42] |
| Carotenoids * | 10 µM | RBL-2H3 | Anti-DNP-IgE/DNP-BSA | ↓ β-hex (≈70–30%) | - | - | - | [43] |
| Astaxanthin | 100, 200, 400 µg/mL | RBL-2H3 | PMA + calcium ionophore A23187 | ↓ β-hex, histamine (dd; ≈10–30%) | - | - | - | [44] |
| Kaempferol | 100, 200 µM | LAD2 | IgE/DNP-HSA, C48/80 | ↓ β-hex (≈60%, ≈80%) ↓ Histamine (≈50%, ≈50%) | - | ↓ TNF-α (≈45%, ≈75%) ↓ IL-8 (≈55%, ≈80%) ↓ MCP-1 (≈20%, ≈50%) | ↓ Lyn, Syk, Btk, Akt, MAPK, NF-κB ↓ PLCγ, IP3R, PKC, Ca2+ | [45,46] |
| Kaempferol | 10, 20 µM | RBL-2H3 | Anti-DNP-IgE/DNP-BSA | ↓ β-hex (≈50, ≈70%) | ↓ PGD2 (≈20%) ↓ PGF2α (≈90%) | - | ↓ p-Syk, p-PLCγ, p-PKCµ, p-ERK ↓ p-cPLA2, COX-2 | [47] |
| Kaempferol | 25–50 µM | BMMC | Anti-TNP-IgE/TNP-BSA | - | - | ↓ TNF (≈80%), IL-6 (≈80%) and IL-13 (≈86%) with 50 µM | ↓ PLCγ, FcεRI surface expression ↑ SHIP1 | [48] |
| Quercetin | 100, 200 µM | LAD2 | IgE/DNP-HSA, C48/80 | ↓ β-hex (≈70%, ≈60%) ↓ Histamine (≈80%, ≈60%) | - | ↓ TNF-α (≈40%), IL-8 (≈70%, ≈90%) ↓ MCP-1 (≈90%, ≈50%), IL-13 (≈50%) | ↓ p-Lyn, p-PLCγ, p-IP3R, p-ERK1/2 ↓ p-IKK, NF-κB | [49,50] |
| ARE | 20 µM | RBL-2H3 | IgE/DNP-BSA | ↓ Histamine (≈40%) | ↓ PGE2 (≈90%) ↓ LTB4 (≈50%) | ↓ TNF-α (≈40%), IL-4 (≈20%) | ↓ p-Syk, p-PLCγ, p-PKCµ ↓ p-ERK, p-JNK, p-p38, COX-2, 5-LO | [51] |
| Myricetin | 100 µM | LAD2 | Streptavidin | ↓ β-hex (≈50%) | - | ↓ TNF-α (≈60%), IL-8 (≈70%) ↓ MCP-1 (≈40%) | ↓ p-PLCγ1 (≈90%), p-Akt (≈70%) ↓ p-NF-κB (≈60%), p-p38 (≈45%) | [52] |
| Myricetin | 10, 20, 40 µM | RBL-2H3 | Anti-DNP-IgE/DNP-BSA | ↓ β-hex (≈60%, ≈40%, ≈20%) | - | ↓ IL-4 and TNF-α (≈60% with 20 µM) | ↓ p-Syk, p-PLCγ ↓ IκBα, NF-κB(p65, p50) | [53] |
| Luteolin | 20 µM | LAD2 | IgE/DNP-BSA, C48/80 | ↓ β-hex (≈80%) ↓ Histamine (≈60%) | ↓ PGD2 (≈40%) | ↓ TNF-α, MCP-1, IL-8, IL-13 (dd) | ↓ PLCγ ↔ Lyn, Btk | [54] |
| Luteolin | 5, 10, 20 µM | HMC-1 RPMC | PMA + calcium ionophore A23187 or C48/80 | ↓ Histamine (≈70% with 20 µM in RPBMC) | - | ↓ IL-1β (dd; 27.3–81.2%) in HMC-1 ↓ TNF-α (dd; 31.9–76.8%) in HMC-1 | - | [55] |
| Luteolin | 5, 10, 20 µM | HMC-1.2 | IL-33 | - | - | ↓ IL-31 (dd; ≈17–70%) | ↓ p-ERK, p-JNK, p-p38 ↓ p-p65, p-PKC, p-IKK | [56] |
| EGCG | 0.1, 1, 10 µg/mL | HMC-1 | RANKL | ↓ Histamine (dd; ≈20–40%) | - | ↓ TSLP (≈45%), IL-1β (≈80%), IL-6 (≈20%), IL-8 (≈80%) with 10 µg/mL | ↓ p-PI3K, p-Akt, p-IκBα ↓ p-ERK, p-p38, p-JNK | [57] |
| EGCG″Me + eriodityol/hesperetin | 1, 5, 25 µM | RBL-2H3 | Anti-DNP-IgE/DNP-HSA | ↓ β-hex (dd) | - | - | ↓ 67LR/sGc/ASM | [58,59] |
| Naringenin | 10 µM | HMC-1 | TSLP | - | - | ↓ TNF-α (≈70%), IL-13 (≈40%) | ↓ p-STAT6 and MDM2 ↑ p53 and PARP | [60] |
| Nobiletin/ Tangeretin | 15, 45, 100 µM | hiMC | LPS/sCD14 | - | - | ↓ CXCL8, CCL3, CCL4, IL-1β (dd) | ↓ NF-κB | [61] |
| Nobiletin/ Tangeretin | 15, 45, 100 µM | hiMC | Human myeloma IgE/anti-human IgE | Nobiletin: ↓ β-hex (dd; ≈20–40%) | Nobiletin: ↓ LTC4 | ↓ CXCL8, CCL3, CCL4, TNF-α (dd) Tangeretin: ↓ IL-1β | ↓ p-ERK1/2 | [61] |
| Nobiletin/ Tangeretin | 10, 25 µM | RBL-2H3 | PMA or histamine | - | - | ↓ IL-4 (≈50%, ≈60%) and TNF-α (≈45%, ≈50%) with 25 µM | ↓ NF-κB(p65), p-c-Jun, p-p38 ↓ PKC | [62] |
| Resveratrol | 50, 100 µM | hiMC | mAb 22E7 (IgE-dependent activation) | ↓ β-hex (≈75% at 50 µM) | - | ↓ CXCL8, CCL2, CCL4, TNF-α, CCL3 (dd; ≈80–100%) | ↓ p-STAT3 and p-ERK1/2 in nuclear and mitochondrial fractions | [16] |
| Resveratrol | 100 µM | hsMC | IgE/NP-BSA | ↓ β-hex (≈80%) | ↓ PGD2 | ↓ TNF-α, IL-6 | ↓ p-Akt, p-p38, COX-2; ↔ Syk | [63] |
| Resveratrol | 1–25 µM | BMMC | IL-33 and Anti-DNP-IgE/anti-IgE | ↓ CD63 counts (≈70% with 25 µM) | - | ↓ IL-6, IL-13, TNF-α (dd; ≈20–30% with 10 µM, ≈40–50% with 25 µM) | ↓ p-Akt ↔ p-IKKα/ß, p-p65, p-p38, p-MK2 | [64] |
| Resveratrol | 10 µM | BMMC | Anti-DNP-IgE/DNP-HSA | ↓ β-hex (≈65%) | ↓ LTC4 and PGD2 (≈80%) | ↓ IL-6 and TNF-α (≈70%) | ↓ p-Akt, p-p38, p-Syk, p-PTP1B | [65] |
| Resveratrol | 10 µM | RBL-2H3 | IL-33 and IgE/DNP-HSA | - | - | ↓ IL-6, IL-13, TNF-α, MCP-1 | ↓ p-p38, IκBα, NF-κB (p65) ↔ ST2, p-ERK1/2, p-JNK | [66] |
| Resveratrol | 1–25 µM | RBL-2H3 | Anti-DNP/DNP-HSA | - | - | ↓ TNF-α, IL-4, IL-3, IL-13 (dd) | ↓ p-p38, p-ERK1/2, p-JNK, p-Src | [67] |
| Resveratrol | 50, 100, 200 µM | LAD2 | C48/80 | ↓ β-hex (dd: ≈20–90%) ↓ Histamine (dd; ≈20–80%) | ↓ PGD2 | ↓ MCP-1 (≈40%), TNF-α (≈60%) ↓ IL-1β (≈80%), TNF-α, IL-8 (dd) | ↑ Nrf2, Ho-1, Nqo-1 (≈50–100%) ↓ MRGPRX2 mRNA expression | [68] |
| Resveratrol | 0.03, 0.3, 3 µM | HMC-1 | PMA + calcium ionophore A23187 | - | - | ↓ TSLP (≈25% with 3 µM) | ↓ RIP2, caspase-1 ↓ NF-κB, p-IκBα | [69] |
| Curcumin | 5–30 µM | RBL-2H3 | Anti-DNP-IgE/DNP-BSA | ↓ β-hex (dd; ≈50–80%) ↓ Histamine (dd; ≈30–60%) | - | - | ↓ PKC-δ translocation | [70] |
| Curcumin | 10 µM | BMMC | Anti-DNP-IgE/DNP-HSA | - | ↓ LTC4 and PGD2 (≈80%) | - | ↓ p-Akt, p-IKK, p-p65, p-MAPK ↓ p-PLCγ, p-cPLA2, 5-LO | [71] |
| Curcumin | 10 µM | HMC-1 | OVA or PMA | ↓ Histamine (≈70%) with OVA | - | ↓ TNF-α (≈60%), IL-1β (≈70%), IL-6 (≈70%), IL-8 (≈70%) with PMA | ↓ p-ERK1/2, p-p38, p-JNK ↓ p-IκBα, NF-κB(p65) | [72] |
| BDMC | 25, 50 µM | HMC-1 | PMA + calcium ionophore A23187 | - | - | ↓ IL-6 (≈60%), IL-8 (≈90%), TNF-α (≈60%) with 50 µM | ↓ p-ERK, p-p38, p-JNK ↓ NF-κB, IκBα | [73] |
| CE | 0.1, 1, 10 µM | hiMC RBL-2H3 | IgE/anti-human IgE IgE/DNP | ↓ β-hex (dd; ≈50–80%) | ↓ cys-LT (dd) | ↓ CXCL8, CCL2, CCL3, CCL4, TNF-α (dd; ≈50–95%) | ↓ p-Akt, p-ERK, p-JNK, p-p38 | [74] |
| CA | 100, 250, 500 µM | hiMC RBL-2H3 | IgE/anti-human IgE IgE/DNP | ↓ β-hex (≈70–90%) | ↓ LTC4 (> 90%) | ↓ CXCL8 (dd) ↓ CCL2, CCL3, CCL4 (dd) | ↓ p-ERK ↓ p-PLCγ1 | [75] |
| 4-chloro-CA 4-trifluoro-CA | 40, 50, 60 µM | RBL-2H3 | PMA + calcium ionophore A23187 | ↓ β-hex (dd; ≈40–60%) | - | ↓ IL-4, TNF-α (dd) | ↓ p-MEK1/2, p-MKK4 ↓ p-ERK, p-p38, p-JNK, COX-2 | [76] |
| Substance | Dosage | Animal Model | Allergy Model (Trigger) | Prestored MC Mediators | De Novo Synthesized MC Mediators | Signaling Molecules | Others | Ref. |
|---|---|---|---|---|---|---|---|---|
| ALA | 1, 2, 4 mg/kg (in 0.2 mL saline) (i.v. into tail vein) | ♂ C57BL/6 mice | PCA (C48/80 or SP, i.d.) | ↓ Serum histamine (dd; ≈20–60%) | ↓ IL-8 (≈55%), IL-13 (≈45%) and TNF-α (≈70%) with 4 mg/kg | - | ↓ Hind paw thickness, vasodilation ↓ Eosinophils ↓ Degranulated MC | [33] |
| Fish oil * | 40 mg/kg (oral, daily for 6 wk) | ♂ NC/Nga mice | AD (TNCB, dorsal skin) | - | - | ↓ GATA-1 (≈40%) | ↓ Infiltration of MC, eosinophils ↓ MC count | [35] |
| Sodium butyrate | 450 mg/kg (oral, daily for 2 wk) | Weaned piglets | - | ↓ Histamine (≈40%) in jejunum mucosa | ↓ IL-6 (≈30%), TNF-α (≈45%) ↓ IL-13 (≈20%) in jejunum mucosa | ↓ p-JNK/JNK ↔ p-ERK, p-p38 | ↓ MC tryptase (≈40%), MC count (≈20%) ↓ Degranulated MC (≈50%) | [77] |
| L-Glutamine | 85 mM (duodenal infusion cannula over 2–3 min) | ♂ Sprague Dawley rats | Dietary fat | ↑ Lymphatic histamine (≈60%) | ↑ Lymphatic PGD2 (≈70%) | - | ↑ RMCPII | [78] |
| Glycine | 50, 100 mg (in 200 µL water) (oral, 1× wk for 5 wk) | C3H/HeOuJ mice | CMA (whey) | ↓ mMCP-1 in serum and jejunum (≈30%) | - | ↓ Serum whey specific IgE1 (≈10%, ≈20%) | [40] | |
| Fucoxanthin | 150 nmol by feeding needle or mouse ear | ♂ ICR mice | AA, PMA, OXA | - | - | - | ↓ Ear swelling (oral: 20–28%, percutane: >50%) | [79] |
| Astaxanthin | 1, 2 mg/mL (in 100 µL) (ears and back skin, 3× wk for 4 wk) | HR-1 mice | AD (PA, ear dorsum) | - | ↓ Serum TNF-α (≈50%, ≈60%) ↓ Serum IL-1β (≈30%, ≈50%) ↓ Serum IL-6 (≈90%, ≈80%) | ↓ p-IκBα ↓ iNOS, COX-2 | ↓ MC count (≈50%, ≈70%) ↓ Serum IgE levels, MDA ↑ GSH, GPx-1, HO-1 | [80,81] |
| Astaxanthin | 1 mg/mL (in AOO) (ear dorsum, 1× every 2 days for 8 days) | ♂ BALB/c mice | CD (DNFB, ear dorsum) | - | ↓ TNF-α (≈60%) and IFN-γ (≈70%) in ear tissue | - | ↓ Ear thickness and weight | [44] |
|
Astaxanthin (free or liposomal) | 1 mg/mL (in 200 µL AOO) (dorsal back skin, 3× wk for 4 wk) | ♂ SKH-1 mice | AD (PA, dorsal skin) | - | ↓ IL-1β, IL-6, IL-4, IL-13 in skin tissue (L-AST: 20–30% higher inhibition) | ↓ p-STAT3, p-IκBα ↓ NF-κB(p50/p65) ↓ iNOS, COX-2 | ↑ GPx-1, HO-1 ↓ Serum IgE levels, MC count in skin ↓ Epidermal thickening | [82] |
| Astaxanthin | 100 mg/kg (oral, 3× wk for 26 days) | ♂ NC/Nga mice | AD (Mites) | - | ↓ Eotaxin, MIF, IL-4, IL-5 ↔ TNF-α, IL-1β in skin tissue | - | ↓ Serum IgE, eosinophils (≈80%) ↓ Degranulated and total MC count (≈50%) ↓ Spontaneous scratching behavior | [83] |
| Lycopene | 0.1% (w/w) of standard chow diet (daily for 28 days) | ♀ BALB/c mice | FA (OVA) | - | ↓ IL-4 (≈60%), IL-13 (≈60%) ↓ IL-9 (≈70%), mMCP-1 (≈50%) ↔ IL-5 in colonic mucosa | - | ↓ MC count (≈50%) | [84] |
| Kaempferol | 5, 10, 20 mg/kg (in DMSO) (oral) | ♂ C57BL/6 mice | PCA (OVA or C48/80, i.p./i.d.) | ↓ Serum histamine (≈80% with OVA and 10 mg/kg) (≈60% with C48/80 and 20 mg/kg) | ↓ Serum TNF-α and IL-8 (dd) ↓ Serum MCP-1 (dd) | - | ↓ Hind paw swelling ↓ Primary MC activation from paw skin ↓ Rehabilitated the hypothermia | [45,46] |
| Quercetin | 1, 2, 4 mg/mL (i.v. into each mouse front paw) | C57BL/6 mice | Pseudo allergy (C48/80 or SP, i.d.) | ↓ Serum histamine (≈0%, ≈20%, ≈40%) | ↓ MCP-1 (≈40%, ≈50%, ≈60%) ↓ IL-8 (≈40%, ≈40%, ≈50%) in serum | - | ↓ Hind paw thickness, vasodilation ↓ Degranulated MC ↓ Eosinophils | [50] |
| Quercetin | 1, 2, 4 mg/kg (oral, daily for 7 days) | ♂ C57BL/6 mice | AC (OVA, i.p.) | ↓ Serum histamine (≈20%, ≈60%, ≈60%) | ↓ IL-4 (dd; ≈20–90%) ↓ TNF-α (dd; ≈10–40%) in serum | - | ↓ Serum IgE, eosinophils ↓ Degranulated MC (≈50%) ↓ Vascular permeability | [49] |
| Myricetin | 50 mg/kg (oral, daily for 4 days) | ♂ BALB/c mice | ICU (DNFB, ear and back skin) | ↓ Serum histamine (43%) | ↓ IL-4 (51%), TNF-α (43%) and MCP-1 (67%) in serum | ↓ PI3K, Akt ↓ NF-κB | ↓ Serum IgE levels (45%) ↓ Degranulated MC (42%) ↓ Scratching behavior, ear swelling | [52] |
| Myricetin | 12.5, 25, 50 mg/kg (oral) | ♂ C57BL/6 mice | PCA (OVA) | ↓ Serum histamine (75%) with 50 mg/kg | ↓ IL-4 (47%), TNF-α (42%) and MCP-1 (52%) in serum with 50 mg/kg | ↓ OVA-induced PCA reaction ↓ Degranulated MC | [52] | |
| Luteolin | 20 mg/kg (in PBS) (oral) | ♂ ICR mice | (C48/80, i.d.) | - | - | ↓ Scratching behavior (≈70%) ↓ Skin vascular permeability (≈60%) | [55] | |
| EGCG | 25, 50, 100 mg/kg (oral, 1× day for 10 days) | ♀ BALB/c mice | AR (OVA, nasal vestibule) | ↓ Serum histamine (dd; ≈10–30%) | ↓ IL-1β (≈40%), IL-4 (≈50%) and IL-6 (≈60%) in nasal mucosa with 100 mg/kg | ↓ COX-2 (≈50%) | ↓ Nasal rubbing, sneezing ↓ Serum IgE levels (dd; ≈10–30%) | [85] |
| Naringenin | 100 mg/kg (in 2 mL saline and CMC) (oral, 1× day for 7 days) | ♀ Sprague Dawley rats | AR (OVA, i.p., nostrils) | - | ↓ IL-4 (≈10%), IL-5 (≈20%) in plasma | - | ↓ Nasal scratching and sneezing (≈60%) ↓ Desquamation in the nasal epithelium ↓ Serum IgE levels (≈30%) | [86] |
| Naringenin | 50, 100 mg/kg (i.p., 1× day for 6 days) | ♂ NC/Nga mice | AD (DNFB, ear and back skin) | - | ↓ IFN-γ (≈45%, ≈35%) by activated lymph node CD4+ T cells | - | ↓ CD4+, CD8+ cells ↓ Serum IgE levels (≈50%) ↓ Degranulated MC (≈40%) ↓ Ear swelling, back skin lesions | [87] |
|
Nobiletin/ Tangeretin | 25 mg/kg (in cremophor) (oral) | ♂ ICR mice | (Histamine or C48/80, i.d.) | - | ↓ TNF-α (94%/96%) and IL-4 (84%/96%) in skin tissue | ↓ p-p65 ↓ p-c-Jun | ↓ Scratching behavior (≈70% with histamine), ≈60% with C48/80) ↓ Vascular permeability (≈50%) | [62] |
| Resveratrol | 10, 20 mg/kg (oral, 1× day for 13 days) | ♀ BALB/c mice | FA (OVA, i.p., oral) | ↓ Serum histamine (dd; ≈25% -50%) | ↓ Serum mMCP-1 (dd; ≈30–50%) | - | ↓ OVA-specific serum IgE (≈45%) | [88] |
| Resveratrol | 50 mg/kg (in drinking water for 28 days) | ♀ BALB/c mice | FA (OVA, i.p., oral) | - | - | ↓ IL-3Rα mRNA (≈80%) | ↓ MC numbers (≈60%) | [89] |
| Resveratrol | 15 mg/kg (i.p., 1x day for 5 days) | ♂ Sprague Dawley rats | IIR | ↓ Intestinal β-hex (≈50%) | ↓ TNF-α (≈50%), IL-1β (≈40%) ↓ IL-18 (≈50%) | ↓ Mucosal NLRP3 and caspase-1 p20 (≈50%), IL-1β p17 and IL-18 (≈60%) | - | [90] |
| Resveratrol | 5 mg/kg (i.p., 1× day for 7 days) | ♂ Sprague Dawley rats | (IL-33, i.p.) | - | ↓ Plasma IL-6 (≈50%), IL-13 (≈40%), TNF-α (≈60%), MCP-1 (≈50%) | - | - | [66] |
| Resveratrol | 10 mg/kg in 100 µL (oral, once) | ♂ BALB/c mice | PCA (anti-DNP-IgE/DNP-HSA, i.d./i.v.) | ↓ Plasma histamine (≈50%) | ↓ MCP-1 (≈50%), MIP-2 (≈40%) in dorsal dermis | ↓ Syk, ↓ PLCγ, PKCµ | ↓ Vascular permeability (≈75%), thickness of ears (≈50%) ↓ Degranulated MC in dorsal dermis | [67] |
| Resveratrol | 5, 10, and 20 mg/kg (i.g.) | ♂ C57BL/6 mice | Pseudo allergy (C48/80, i.v.) | ↓ Serum histamine (≈60%, ≈60%, ≈70%) | ↓ MCP-1 (≈50%, ≈70%, ≈80%) ↓ TNF-α (≈30%, ≈50%, ≈70%) ↓ IL-8 (≈20%, ≈30%, ≈50%) | - | ↓ Degranulated MC (ns; ≈50%; ≈70%) ↓ Paw thickness (≈20%; ≈60%; ≈60%) | [68] |
| Resveratrol | 2.5 µg/mL (patches on back skin, 1× day for 7 days) | ♀ C57BL/6J | AD (OVA, patches) | - | ↓ CCL2, CCL3 and CCL5 in skin tissue | ↓ p-SphK1 ↓ p-STAT3 ↓ p-NF-κB(p65) | ↓ FcεRIα mRNA expression ↓ Epidermal thickening ↓ Skin MC activation | [91] |
| Curcumin | 25, 50 mg/kg (oral) | ICR mice | PSA (IgE/DNP-HSA, i.v.) | ↓ Serum histamine (≈30%, ≈40%) | ↓ Serum LTC4 (≈30%, ≈50%) ↓ Serum PGD2 (≈20%, ≈50%) | - | - | [71] |
| Curcumin | 100, 200 mg/kg (oral, 1× day for 3 days) | ♀ BALB/c mice | AR (OVA, i.p./i.n.) | ↓ Serum histamine (≈50%, ≈70%) | ↓ Serum TNF-α (≈60%, ≈70%) | ↓ Fyn, Lyn, Syk | ↓ OVA-sIgE (≈70%, ≈80%) ↓ Nasal sneezing and rubbing | [72] |
| BDMC | 100, 200 mg/kg (oral, 1× day for 10 days) | ♀ BALB/c mice | FA (OVA, i.g., nasal vestibule) | ↓ Serum histamine (≈20%, ≈50%) | ↓ Serum IL-4, IL-5 and IL-13 (dd; < 30%) ↓ Serum mMCP-1, ↑ IFN-γ | ↓ GATA-3, ↓ NF-κB(p65) ↓ p38, JNK, ERK | ↓ OVA-sIGE (≈20%, ≈50%) ↓ OVA-sIgG1 (≈20%, ≈50%) ↓ Diarrhea, anaphylaxis symptoms | [92] |
| BDMC | 100, 200 mg/kg (oral, 1× day for 10 days) | ♂ BALB/c mice | AR (OVA, i.p.) | ↓ Serum histamine (≈30%, ≈40%) | - | - | ↓ OVA-sIgE levels (≈30%, ≈40%) ↓ Nasal rubbing | [93] |
| Cinnamon extract | 4.5 mL/kg (in tap water for 6 wk) | C57BL/6J mice | - | - | ↔ IL-4 and IL-1β | - | ↓ Carboxypeptidase A ↓ MC tryptase | [74] |
| Substance | Dosage | Study Type and Duration | Study Population | Results | Ref. |
|---|---|---|---|---|---|
| Vitamin D | Vitamin D3 1.5 × 106 IU nasal drops (1× wk) + desloratadine citrate disodium (8.8 mg/day) | RCT (1 month) | N = 60 children/adults with mild seasonal pollen AR Mean age: 27.3 | ↑ Serum 25(OH)D3 level (≈50%) ↓ AR symptoms score (≈40%) ↓ Serum IL-4 (≈20%) ↓ Peripheral blood eosinophils (≈20%) | [136] |
| Vitamin D | Vitamin D 50,000 IU (1× wk, oral) + cetirizine | RCT (2 months) | N = 68 adults with vitamin D deficiency and AR Mean age: 29.4 years | ↑ Serum 25(OH)D3 level (≈60%) ↓ AR symptom severity (≈24%) ↓ Rhinorrhea (≈26%), nasal itching (≈27%), sneezing (≈28%) and postnasal drip (≈28%) | [137] |
| Quercetin | 200 mg/day (4 × 50 mg tablets/day divided into two doses) | RCT (1 month) | N = 60 adults with AR Mean age: 46.85 years | ↓ Total AR symptom score (≈27%) ↓ Sleeping disorder (≈40%) ↓ Itching sensation other than ocular/nasal itching sensation ↓ Eosinophil count in nasal discharge | [138] |
| O- methylated EGCG | Benifuuki green tea (700 mL/day) (5.83 mg/100 mL O-methylated EGCG) | RCT (4 months) | N = 26 adults with Japanese cedar pollinosis Mean age: 39.6 years | ↓ Runny nose (≈8%), itchy eyes (≈18%) and tearing (≈17%) ↓ Total nasal symptom score (≈10%) and total ocular symptom score (≈17%) ↓ Nasal symptom-medication score (≈8%) ↓ Ocular symptom-medication score (≈12%) ↓ Peripheral eosinophil counts (≈30%) | [139] |
| Resveratrol | Resveratrol (0.1%) (2 sprays (100 µL/spray) in each nostril 3× day) | RCT (1 month) | N = 151 adults with severe persistent AR Age: 18–60 years | ↓ Nasal symptoms ↓ Blood levels of IgE (≈40%), IL-4 (≈30%), and TNF-α (≈10%) ↓ Eosinophile number in blood (≈80%) | [140] |
| Resveratrol | Resveratrol (0.05%) + carboxymethyl-β-glucan (0.33%) (2 sprays (100 µL/spray) in each nostril 3×/day) | RCT (2 months) | N = 68 children with AR Mean age: 7.9 years | ↓ Itching, sneezing, rhinorrhea, and obstruction ↓ Antihistamine use | [141] |
| Curcumin | 500 mg in capsules (1× day) | RCT (2 months) | N = 241 adults with perennial AR Mean age: 32.6 years | ↓ Nasal symptoms (≈60%) ↓ Sneezing (≈60%), itching (≈60%), rhinorrhea (≈80%), obstruction (≈50%) ↑ Nasal airflow baseline ↓ IL-4 (≈30%), TNF-α (≈40%) and ↑ IL-10 (≈20%) in activated MNC ↓ IL-8 (≈30%) and ↑ sICAM-1 (≈30%) in PMN ↔ IFN-γ and IL-17 in MNCs, PGE2 and LTC4 in PMN | [142] |
| Cinnamon bark extract | IND02 100 µg in 100 µL (2 sprays (100 µL/spray) in each nostril 2× day) | RCT (7 days) | N = 60 adults with acute symptoms of AR Mean age: 43.25 years | ↓ Nasal (47.7%) and eye (50.9%) symptoms, non-nose/eye symptoms (50.5%)↓ Activity limitation (41.8%) ↓ Sleep disorder (46%) ↓ Total white blood cells (≈20%) ↓ Neutrophils (≈20%) | [143] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaag, S.; Lorentz, A. Effects of Dietary Components on Mast Cells: Possible Use as Nutraceuticals for Allergies? Cells 2023, 12, 2602. https://doi.org/10.3390/cells12222602
Kaag S, Lorentz A. Effects of Dietary Components on Mast Cells: Possible Use as Nutraceuticals for Allergies? Cells. 2023; 12(22):2602. https://doi.org/10.3390/cells12222602
Chicago/Turabian StyleKaag, Sina, and Axel Lorentz. 2023. "Effects of Dietary Components on Mast Cells: Possible Use as Nutraceuticals for Allergies?" Cells 12, no. 22: 2602. https://doi.org/10.3390/cells12222602
APA StyleKaag, S., & Lorentz, A. (2023). Effects of Dietary Components on Mast Cells: Possible Use as Nutraceuticals for Allergies? Cells, 12(22), 2602. https://doi.org/10.3390/cells12222602







