The Anti-Cancer Activity of the Naturally Occurring Dipeptide Carnosine: Potential for Breast Cancer
Abstract
:1. Introduction
2. Carnosine: History and Biological Activities
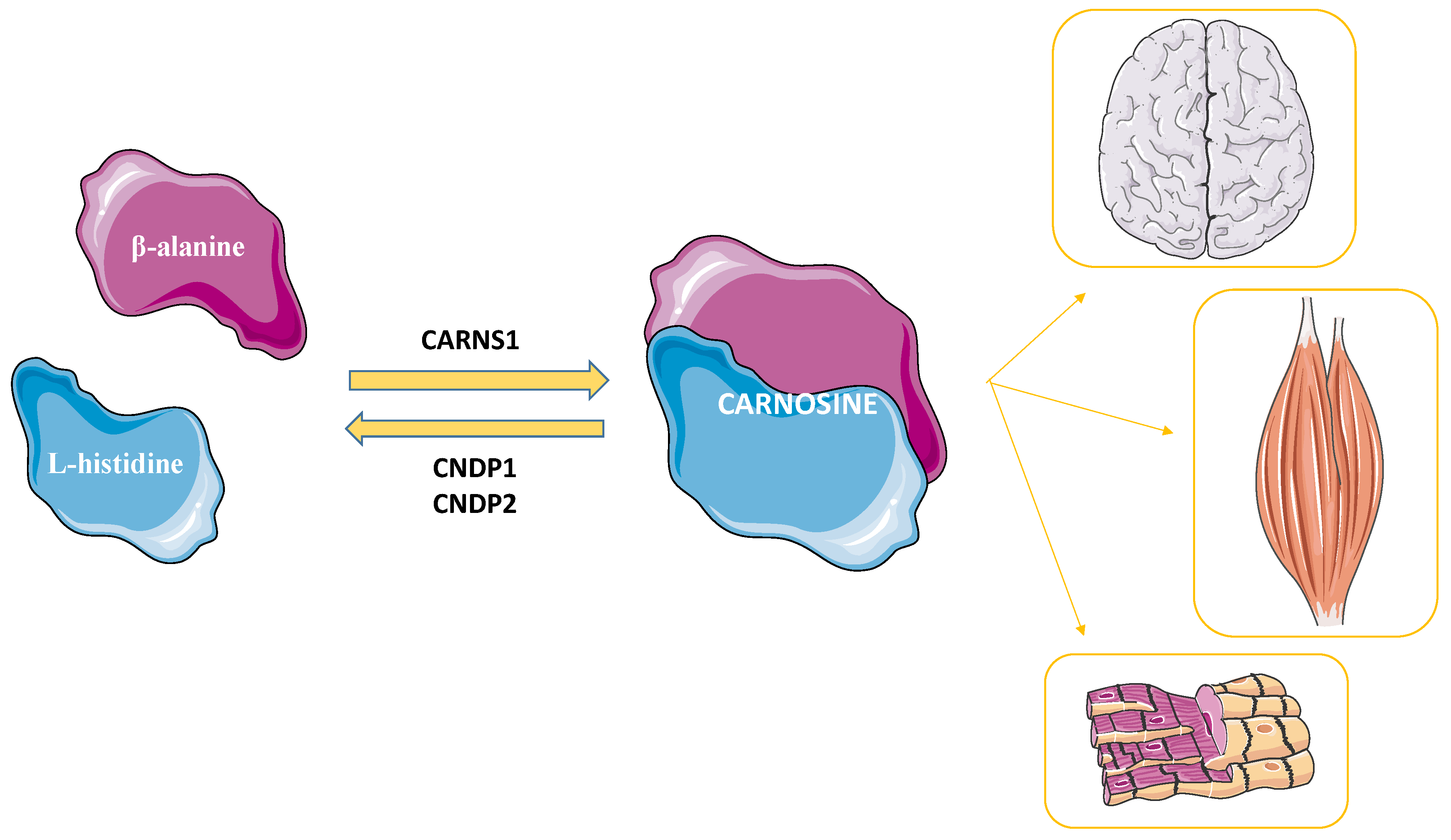
2.1. Carnosine’s Metabolism
2.2. The Multimodal Mechanism of Action of Carnosine
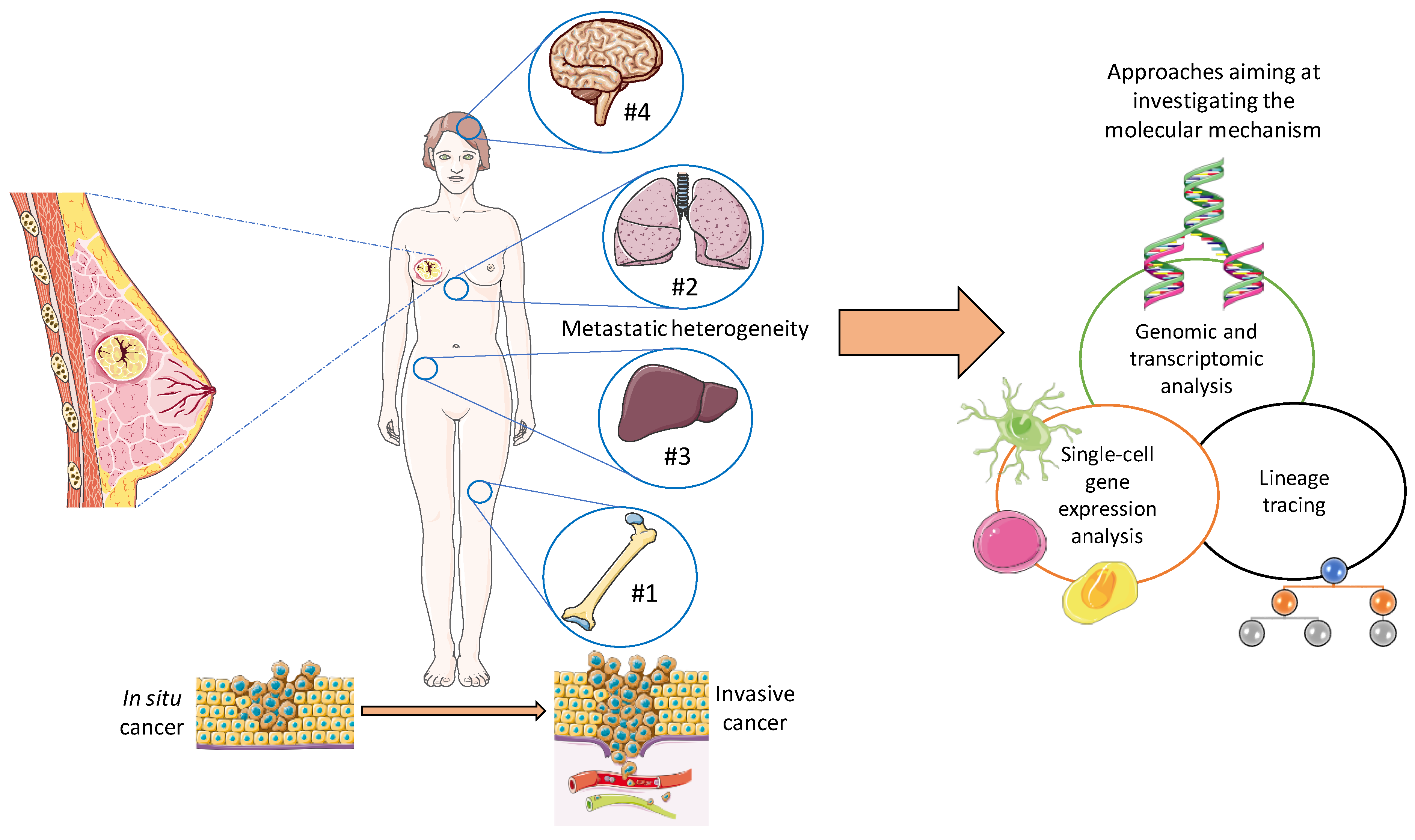
3. Pathophysiology of Breast Cancer
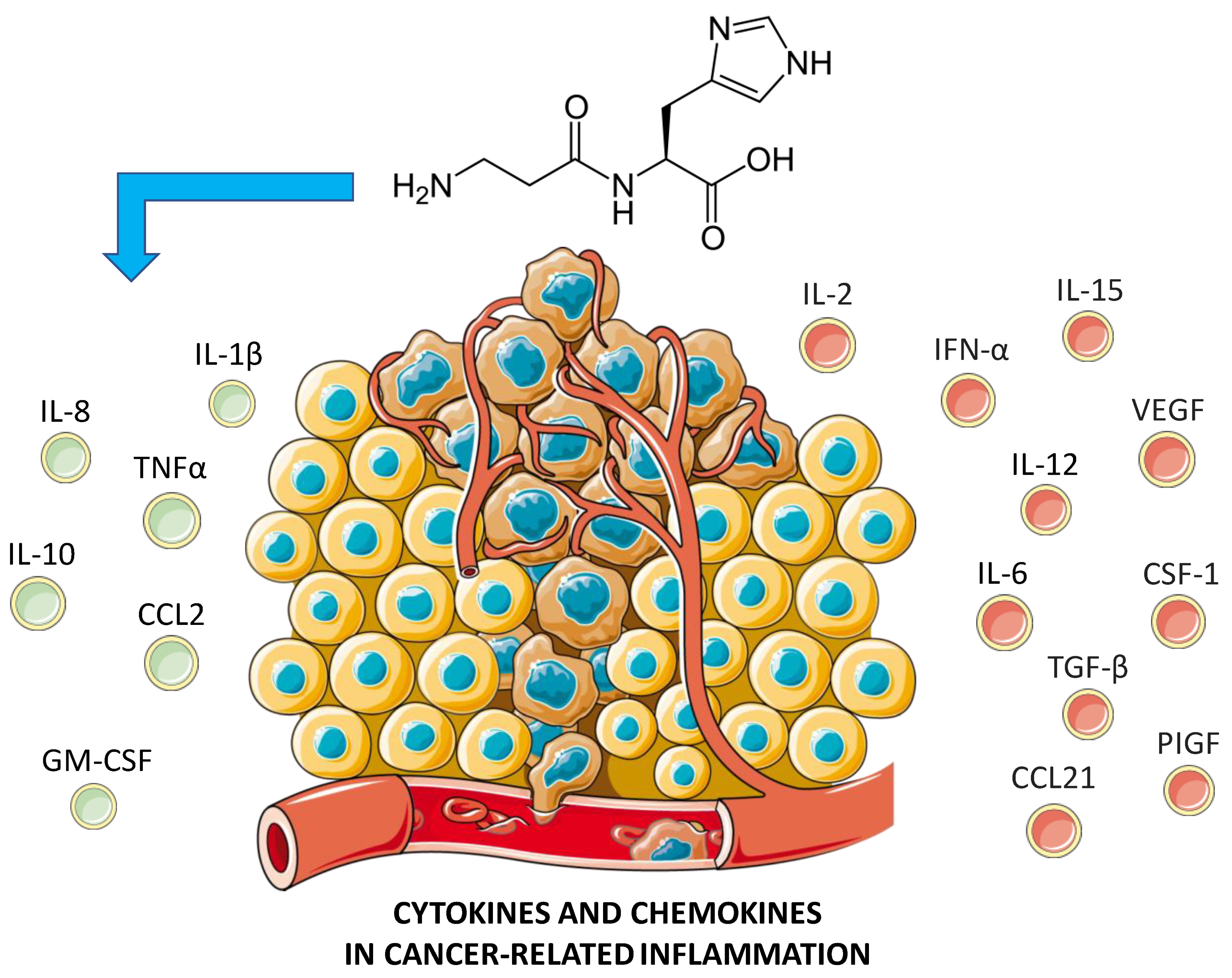
4. Anti-Tumoral Activity of Carnosine: Possible Mechanisms
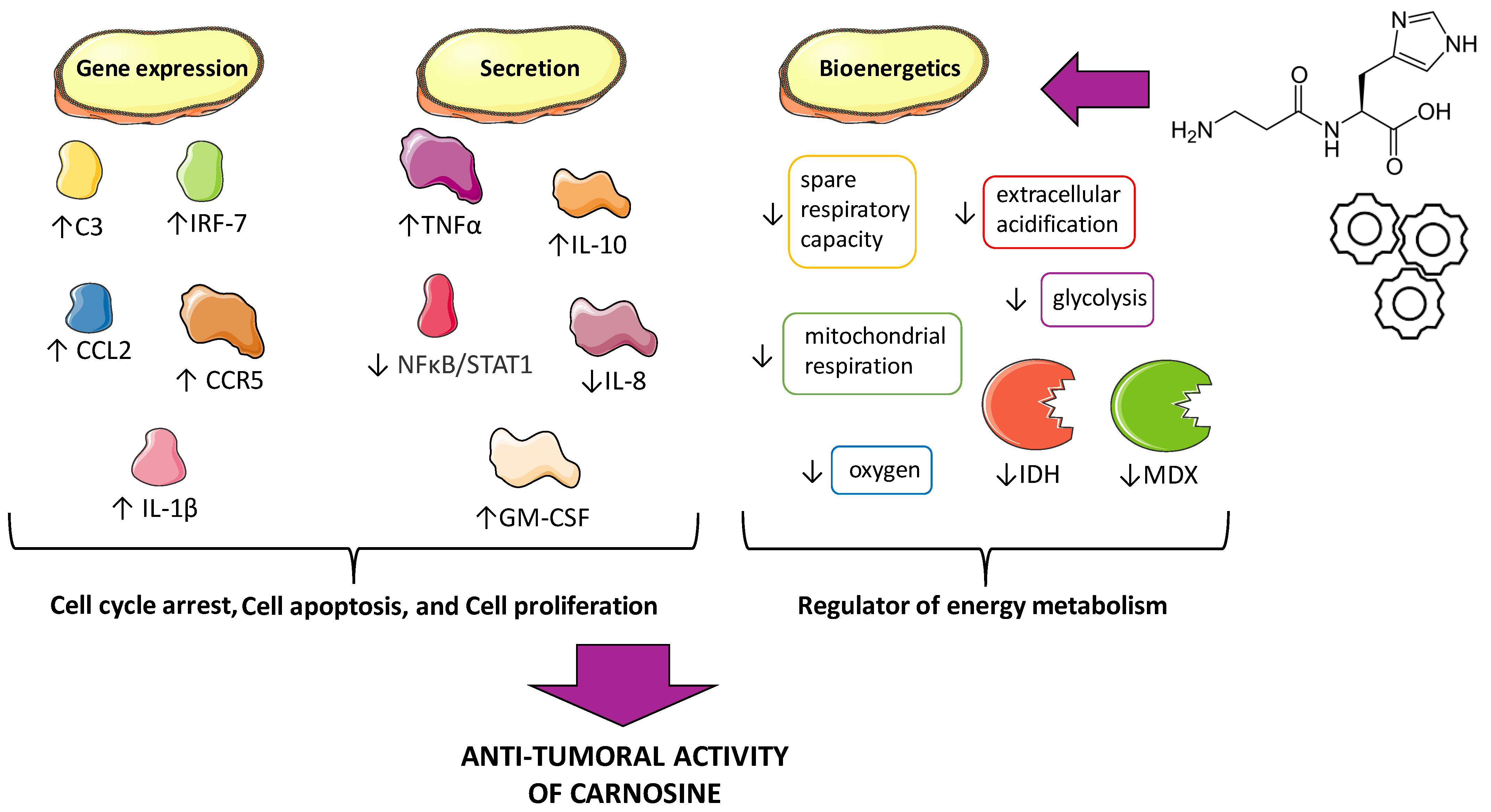
4.1. Cell Proliferation, Cell Cycle Arrest, and Apoptosis
4.2. Energy Metabolism/Bioenergetics
5. Breast Cancer: Could Carnosine Exert a Therapeutic Effect?
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roy, P.; Saikia, B. Cancer and cure: A critical analysis. Indian J. Cancer 2016, 53, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Jin, X.; Mu, P. Targeting breast cancer metastasis. Breast Cancer Basic Clin. Res. 2015, 9, S25460. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and pathophysiology of carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef] [PubMed]
- Gariballa, S.E.; Sinclair, A.J. Carnosine: Physiological properties and therapeutic potential. Age Ageing 2000, 29, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Hipkiss, A.; Preston, J.; Himsworth, D.; Worthington, V.; Keown, M.; Michaelis, J.; Lawrence, J.; Mateen, A.; Allende, L.; Eagles, P. Pluripotent protective effects of carnosine, a naturally occurring dipeptidea. Ann. N. Y. Acad. Sci. 1998, 854, 37–53. [Google Scholar] [CrossRef]
- Caruso, G.; Di Pietro, L.; Cardaci, V.; Maugeri, S.; Caraci, F. The therapeutic potential of carnosine: Focus on cellular and molecular mechanisms. Curr. Res. Pharmacol. Drug Discov. 2023, 4, 100153. [Google Scholar] [CrossRef]
- Caruso, G.; Caraci, F.; Jolivet, R.B. Pivotal role of carnosine in the modulation of brain cells activity: Multimodal mechanism of action and therapeutic potential in neurodegenerative disorders. Prog. Neurobiol. 2019, 175, 35–53. [Google Scholar] [CrossRef]
- Gulewitsch, W.; Amiradžibi, S. Ueber das Carnosin, eine neue organische Base des Fleischextractes. Berichte Dtsch. Chem. Ges. 1900, 33, 1902–1903. [Google Scholar] [CrossRef]
- Kalyankar, G.D.; Meister, A. Enzymatic synthesis of carnosine and related β-alanyl and γ-aminobutyryl peptides. J. Biol. Chem. 1959, 234, 3210–3218. [Google Scholar] [CrossRef]
- Winnick, R.; Winnick, T. Carnosine-anserine synthetase of muscle i. Preparation and properties of a soluble enyzme from chick muscle. Biochim. Biophys. Acta 1959, 31, 47–55. [Google Scholar] [CrossRef]
- Lenney, J.F.; George, R.P.; Weiss, A.M.; Kucera, C.M.; Chan, P.W.; Rinzler, G.S. Human serum carnosinase: Characterization, distinction from cellular carnosinase, and activation by cadmium. Clin. Chim. Acta 1982, 123, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Lenney, J.F.; Peppers, S.C.; Kucera-Orallo, C.M.; George, R. Characterization of human tissue carnosinase. Biochem. J. 1985, 228, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Junge, W.; McLaughlin, S. The role of fixed and mobile buffers in the kinetics of proton movement. Biochim. Biophys. Acta BBA-Bioenerg. 1987, 890, 1–5. [Google Scholar] [CrossRef]
- Caruso, G.; Privitera, A.; Antunes, B.M.; Lazzarino, G.; Lunte, S.M.; Aldini, G.; Caraci, F. The Therapeutic Potential of Carnosine as an Antidote against Drug-Induced Cardiotoxicity and Neurotoxicity: Focus on Nrf2 Pathway. Molecules 2022, 27, 4452. [Google Scholar] [CrossRef] [PubMed]
- Swietach, P.; Youm, J.B.; Saegusa, N.; Leem, C.H.; Spitzer, K.W.; Vaughan-Jones, R.D. Coupled Ca2+/H+ transport by cytoplasmic buffers regulates local Ca2+ and H+ ion signaling. Proc. Natl. Acad. Sci. USA 2013, 110, E2064–E2073. [Google Scholar] [CrossRef] [PubMed]
- Dutka, T.L.; Lamboley, C.R.; McKenna, M.J.; Murphy, R.M.; Lamb, G.D. Effects of carnosine on contractile apparatus Ca2+ sensitivity and sarcoplasmic reticulum Ca2+ release in human skeletal muscle fibers. J. Appl. Physiol. 2012, 112, 728–736. [Google Scholar] [CrossRef]
- Severin, S.E.; Kirzon, M.V.; Kaftanova, T.M. Effect of carnosine and anserine on action of isolated frog muscles. Dokl. Akad. Nauk. SSSR 1953, 91, 691–694. [Google Scholar]
- Sale, C.; Artioli, G.G.; Gualano, B.; Saunders, B.; Hobson, R.M.; Harris, R.C. Carnosine: From exercise performance to health. Amino Acids 2013, 44, 1477–1491. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Petukhov, V.B. Localization of carnosine effect on the fatigued muscle preparation. Gen. Pharmacol. 1978, 9, 17–20. [Google Scholar] [CrossRef]
- Brisola, G.M.P.; de Souza Malta, E.; Santiago, P.R.P.; Vieira, L.H.P.; Zagatto, A.M. β-Alanine Supplementation’s Improvement of High-Intensity Game Activities in Water Polo. Int. J. Sports Physiol. Perform. 2018, 13, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- de Andrade Kratz, C.; de Salles Painelli, V.; de Andrade Nemezio, K.M.; da Silva, R.P.; Franchini, E.; Zagatto, A.M.; Gualano, B.; Artioli, G.G. Beta-alanine supplementation enhances judo-related performance in highly-trained athletes. J. Sci. Med. Sport 2017, 20, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Furst, T.; Massaro, A.; Miller, C.; Williams, B.T.; LaMacchia, Z.M.; Horvath, P.J. β-Alanine supplementation increased physical performance and improved executive function following endurance exercise in middle aged individuals. J. Int. Soc. Sports. Nutr. 2018, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Glenn, J.M.; Smith, K.; Moyen, N.E.; Binns, A.; Gray, M. Effects of Acute Beta-Alanine Supplementation on Anaerobic Performance in Trained Female Cyclists. J. Nutr. Sci. Vitaminol. 2015, 61, 161–166. [Google Scholar] [CrossRef]
- Culbertson, J.Y.; Kreider, R.B.; Greenwood, M.; Cooke, M. Effects of beta-alanine on muscle carnosine and exercise performance: A review of the current literature. Nutrients 2010, 2, 75–98. [Google Scholar] [CrossRef]
- Tiedje, K.; Stevens, K.; Barnes, S.; Weaver, D. β-Alanine as a small molecule neurotransmitter. Neurochem. Int. 2010, 57, 177–188. [Google Scholar] [CrossRef]
- Fresta, C.G.; Fidilio, A.; Lazzarino, G.; Musso, N.; Grasso, M.; Merlo, S.; Amorini, A.M.; Bucolo, C.; Tavazzi, B.; Lazzarino, G.; et al. Modulation of Pro-Oxidant and Pro-Inflammatory Activities of M1 Macrophages by the Natural Dipeptide Carnosine. Int. J. Mol. Sci. 2020, 21, 776. [Google Scholar] [CrossRef]
- Caruso, G.; Fresta, C.G.; Fidilio, A.; O’Donnell, F.; Musso, N.; Lazzarino, G.; Grasso, M.; Amorini, A.M.; Tascedda, F.; Bucolo, C.; et al. Carnosine Decreases PMA-Induced Oxidative Stress and Inflammation in Murine Macrophages. Antioxidants 2019, 8, 281. [Google Scholar] [CrossRef]
- Caruso, G.; Privitera, A.; Saab, M.W.; Musso, N.; Maugeri, S.; Fidilio, A.; Privitera, A.P.; Pittalà, A.; Jolivet, R.B.; Lanzanò, L.; et al. Characterization of Carnosine Effect on Human Microglial Cells under Basal Conditions. Biomedicines 2023, 11, 474. [Google Scholar] [CrossRef]
- Privitera, A.; Cardaci, V.; Weerasekara, D.; Saab, M.W.; Diolosà, L.; Fidilio, A.; Jolivet, R.B.; Lazzarino, G.; Amorini, A.M.; Camarda, M. Microfluidic/HPLC combination to study carnosine protective activity on challenged human microglia: Focus on oxidative stress and energy metabolism. Front. Pharmacol. 2023, 14, 1161794. [Google Scholar] [CrossRef]
- Mal’tseva, V.V.; Sergienko, V.V.; Stvolinskiĭ, S.L. The effect of carnosine on hematopoietic stem cell activity in irradiated animals. Biokhimiia 1992, 57, 1378–1382. [Google Scholar] [PubMed]
- Fresta, C.G.; Chakraborty, A.; Wijesinghe, M.B.; Amorini, A.M.; Lazzarino, G.; Lazzarino, G.; Tavazzi, B.; Lunte, S.M.; Caraci, F.; Dhar, P.; et al. Non-toxic engineered carbon nanodiamond concentrations induce oxidative/nitrosative stress, imbalance of energy metabolism, and mitochondrial dysfunction in microglial and alveolar basal epithelial cells. Cell Death Dis. 2018, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Fresta, C.G.; Martinez-Becerra, F.; Antonio, L.; Johnson, R.T.; de Campos, R.P.; Siegel, J.M.; Wijesinghe, M.B.; Lazzarino, G.; Lunte, S.M. Carnosine modulates nitric oxide in stimulated murine RAW 264.7 macrophages. Mol. Cell. Biochem. 2017, 431, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Benatti, C.; Musso, N.; Fresta, C.G.; Fidilio, A.; Spampinato, G.; Brunello, N.; Bucolo, C.; Drago, F.; Lunte, S.M. Carnosine protects macrophages against the toxicity of aβ1-42 oligomers by decreasing oxidative stress. Biomedicines 2021, 9, 477. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Suda, T.; Kawasaki, K.; Mathuura, S. Action of carnosine and beta-alanine on wound healing. Surgery 1986, 100, 815–821. [Google Scholar] [PubMed]
- Abe, H. Role of histidine-related compounds as intracellular proton buffering constituents in vertebrate muscle. Biochemistry 2000, 65, 757–765. [Google Scholar] [PubMed]
- Pepper, E.D.; Farrell, M.J.; Nord, G.; Finkel, S.E. Antiglycation effects of carnosine and other compounds on the long-term survival of Escherichia coli. Appl. Environ. Microbiol. 2010, 76, 7925–7930. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Gallant, S.C.; Sukhich, G.T. Carnosine, the protective, anti-aging peptide. Biosci. Rep. 1999, 19, 581–587. [Google Scholar] [CrossRef]
- Hasanein, P.; Felegari, Z. Chelating effects of carnosine in ameliorating nickel-induced nephrotoxicity in rats. Can. J. Physiol. Pharmacol. 2017, 95, 1426–1432. [Google Scholar] [CrossRef]
- Brown, C.E.; Antholine, W.E. Chelation chemistry of carnosine. Evidence that mixed complexes may occur in vivo. J. Phys. Chem. 1979, 83, 3314–3319. [Google Scholar] [CrossRef]
- Ouyang, L.; Tian, Y.; Bao, Y.; Xu, H.; Cheng, J.; Wang, B.; Shen, Y.; Chen, Z.; Lyu, J. Carnosine decreased neuronal cell death through targeting glutamate system and astrocyte mitochondrial bioenergetics in cultured neuron/astrocyte exposed to OGD/recovery. Brain Res. Bull. 2016, 124, 76–84. [Google Scholar] [CrossRef] [PubMed]
- de Campos, R.P.; Siegel, J.M.; Fresta, C.G.; Caruso, G.; da Silva, J.A.; Lunte, S.M. Indirect detection of superoxide in RAW 264.7 macrophage cells using microchip electrophoresis coupled to laser-induced fluorescence. Anal. Bioanal. Chem. 2015, 407, 7003–7012. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Wilkinson, F.L.; Sandhu, M.A.; Lightfoot, A.P. The interplay of oxidative stress and inflammation: Mechanistic insights and therapeutic potential of antioxidants. Oxidative Med. Cell. Longev. 2021, 2021, 9851914. [Google Scholar] [CrossRef]
- Holliday, R.; McFarland, G. Inhibition of the growth of transformed and neoplastic cells by the dipeptide carnosine. Br. J. Cancer 1996, 73, 966–971. [Google Scholar] [CrossRef]
- Iovine, B.; Iannella, M.L.; Nocella, F.; Pricolo, M.R.; Bevilacqua, M.A. Carnosine inhibits KRAS-mediated HCT116 proliferation by affecting ATP and ROS production. Cancer Lett. 2012, 315, 122–128. [Google Scholar] [CrossRef]
- Shen, Y.; Yang, J.; Li, J.; Shi, X.; Ouyang, L.; Tian, Y.; Lu, J. Carnosine inhibits the proliferation of human gastric cancer SGC-7901 cells through both of the mitochondrial respiration and glycolysis pathways. PLoS ONE 2014, 9, e104632. [Google Scholar] [CrossRef]
- Mikuła-Pietrasik, J.; Książek, K. L-carnosine prevents the pro-cancerogenic activity of senescent peritoneal mesothelium towards ovarian cancer cells. Anticancer Res. 2016, 36, 665–671. [Google Scholar]
- van Denderen, B.J.; Thompson, E.W. Cancer: The to and fro of tumour spread. Nature 2013, 493, 487–488. [Google Scholar] [CrossRef]
- Weinberg, R.A. How cancer arises. Sci. Am. 1996, 275, 62–70. [Google Scholar] [CrossRef]
- Yin, W.; Wang, J.; Jiang, L.; James Kang, Y. Cancer and stem cells. Exp. Biol. Med. 2021, 246, 1791–1801. [Google Scholar] [CrossRef]
- Amatori, S.; Tavolaro, S.; Gambardella, S.; Fanelli, M. The dark side of histones: Genomic organization and role of oncohistones in cancer. Clin. Epigenet. 2021, 13, 71. [Google Scholar] [CrossRef]
- Qiu, L.; Hu, X.; Jing, Q.; Zeng, X.; Chan, K.M.; Han, J. Mechanism of cancer: Oncohistones in action. J. Genet. Genom. 2018, 45, 227–236. [Google Scholar] [CrossRef]
- Higgins, M.J.; Baselga, J. Targeted therapies for breast cancer. J. Clin. Investig. 2011, 121, 3797–3803. [Google Scholar] [CrossRef] [PubMed]
- Arteaga, C.L.; Sliwkowski, M.X.; Osborne, C.K.; Perez, E.A.; Puglisi, F.; Gianni, L. Treatment of HER2-positive breast cancer: Current status and future perspectives. Nat. Rev. Clin. Oncol. 2012, 9, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Hicks, D.G.; Kulkarni, S. HER2+ breast cancer: Review of biologic relevance and optimal use of diagnostic tools. Am. J. Clin. Pathol. 2008, 129, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, B. Targeted therapeutic options and future perspectives for HER2-positive breast cancer. Signal Transduct. Target. Ther. 2019, 4, 34. [Google Scholar] [CrossRef]
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001, 2, 127–137. [Google Scholar] [CrossRef]
- Yu, D. Mechanisms of ErbB2-mediated paclitaxel resistance and trastuzumab-mediated paclitaxel sensitization in ErbB2-overexpressing breast cancers. In Seminars in Oncology; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Garrido-Castro, A.C.; Lin, N.U.; Polyak, K. Insights into Molecular Classifications of Triple-Negative Breast Cancer: Improving Patient Selection for TreatmentHeterogeneity of Triple-Negative Breast Cancer. Cancer Discov. 2019, 9, 176–198. [Google Scholar] [CrossRef]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef]
- Christensen, R.D.; Baer, V.L.; Gordon, P.V.; Henry, E.; Whitaker, C.; Andres, R.L.; Bennett, S.T. Reference ranges for lymphocyte counts of neonates: Associations between abnormal counts and outcomes. Pediatrics 2012, 129, e1165–e1172. [Google Scholar] [CrossRef]
- Williams, E.D.; Gao, D.; Redfern, A.; Thompson, E.W. Controversies around epithelial-mesenchymal plasticity in cancer metastasis. Nat. Rev. Cancer 2019, 19, 716–732. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Kim, D.; Ko, S.; Kim, A.; Mo, K.; Yoon, H. Breast Cancer Metastasis: Mechanisms and Therapeutic Implications. Int. J. Mol. Sci. 2022, 23, 6806. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef] [PubMed]
- Valastyan, S.; Weinberg, R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef]
- Tulotta, C.; Ottewell, P. The role of IL-1B in breast cancer bone metastasis. Endocr.-Relat. Cancer 2018, 25, R421–R434. [Google Scholar] [CrossRef]
- Xiong, Z.; Deng, G.; Huang, X.; Li, X.; Xie, X.; Wang, J.; Shuang, Z.; Wang, X. Bone metastasis pattern in initial metastatic breast cancer: A population-based study. Cancer Manag. Res. 2018, 10, 287. [Google Scholar] [CrossRef]
- Smid, M.; Wang, Y.; Zhang, Y.; Sieuwerts, A.M.; Yu, J.; Klijn, J.G.; Foekens, J.A.; Martens, J.W. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008, 68, 3108–3114. [Google Scholar] [CrossRef]
- Pentheroudakis, G.; Fountzilas, G.; Bafaloukos, D.; Koutsoukou, V.; Pectasides, D.; Skarlos, D.; Samantas, E.; Kalofonos, H.P.; Gogas, H.; Pavlidis, N. Metastatic breast cancer with liver metastases: A registry analysis of clinicopathologic, management and outcome characteristics of 500 women. Breast Cancer Res. Treat. 2006, 97, 237–244. [Google Scholar] [CrossRef]
- Lin, N.U.; Bellon, J.R.; Winer, E.P. CNS metastases in breast cancer. J. Clin. Oncol. 2004, 22, 3608–3617. [Google Scholar] [CrossRef]
- Tham, Y.L.; Sexton, K.; Kramer, R.; Hilsenbeck, S.; Elledge, R. Primary breast cancer phenotypes associated with propensity for central nervous system metastases. Cancer 2006, 107, 696–704. [Google Scholar] [CrossRef]
- Quigley, M.R.; Fukui, O.; Chew, B.; Bhatia, S.; Karlovits, S. The shifting landscape of metastatic breast cancer to the CNS. Neurosurg. Rev. 2013, 36, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Curtis, C.; Shah, S.P.; Chin, S.-F.; Turashvili, G.; Rueda, O.M.; Dunning, M.J.; Speed, D.; Lynch, A.G.; Samarajiwa, S.; Yuan, Y. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 2012, 486, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Craig, D.W.; O’Shaughnessy, J.A.; Kiefer, J.A.; Aldrich, J.; Sinari, S.; Moses, T.M.; Wong, S.; Dinh, J.; Christoforides, A.; Blum, J.L. Genome and Transcriptome Sequencing in Prospective Metastatic Triple-Negative Breast Cancer Uncovers Therapeutic VulnerabilitiesGenome Sequencing in Recurrent Triple-Negative Breast Cancer. Mol. Cancer Ther. 2013, 12, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Habermann, J.K.; Doering, J.; Hautaniemi, S.; Roblick, U.J.; Bündgen, N.K.; Nicorici, D.; Kronenwett, U.; Rathnagiriswaran, S.; Mettu, R.K.; Ma, Y. The gene expression signature of genomic instability in breast cancer is an independent predictor of clinical outcome. Int. J. Cancer 2009, 124, 1552–1564. [Google Scholar] [CrossRef]
- Sims, A.H.; Ong, K.R.; Clarke, R.B.; Howell, A. High-throughput genomic technology in research and clinical management of breast cancer. Exploiting the potential of gene expression profiling: Is it ready for the clinic? Breast Cancer Res. 2006, 8, 214. [Google Scholar] [CrossRef]
- Lawson, D.A.; Bhakta, N.R.; Kessenbrock, K.; Prummel, K.D.; Yu, Y.; Takai, K.; Zhou, A.; Eyob, H.; Balakrishnan, S.; Wang, C.-Y. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature 2015, 526, 131–135. [Google Scholar] [CrossRef]
- Navin, N.E.; Hicks, J. Tracing the tumor lineage. Mol. Oncol. 2010, 4, 267–283. [Google Scholar] [CrossRef]
- Francia, G.; Cruz-Munoz, W.; Man, S.; Xu, P.; Kerbel, R.S. Mouse models of advanced spontaneous metastasis for experimental therapeutics. Nat. Rev. Cancer 2011, 11, 135–141. [Google Scholar] [CrossRef]
- Diaz-Cruz, E.S.; Cabrera, M.C.; Nakles, R.; Rutstein, B.H.; Furth, P.A. BRCA1 deficient mouse models to study pathogenesis and therapy of triple negative breast cancer. Breast Dis. 2011, 32, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Dine, J.; Deng, X.C. Mouse models of BRCA1 and their application to breast cancer research. Cancer Metastasis Rev. 2013, 32, 25–37. [Google Scholar] [CrossRef]
- Kretschmann, K.L.; Welm, A.L. Mouse models of breast cancer metastasis to bone. Cancer Metastasis Rev. 2012, 31, 579–583. [Google Scholar] [CrossRef]
- Blanco, M.A.; Kang, Y. Signaling pathways in breast cancer metastasis-novel insights from functional genomics. Breast Cancer Res. 2011, 13, 206. [Google Scholar] [CrossRef] [PubMed]
- Prakash, M.D.; Fraser, S.; Boer, J.C.; Plebanski, M.; de Courten, B.; Apostolopoulos, V. Anti-Cancer Effects of Carnosine—A Dipeptide Molecule. Molecules 2021, 26, 1644. [Google Scholar] [CrossRef] [PubMed]
- Hipkiss, A.R.; Baye, E.; de Courten, B. Carnosine and the processes of ageing. Maturitas 2016, 93, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Kohen, R.; Yamamoto, Y.; Cundy, K.C.; Ames, B.N. Antioxidant activity of carnosine, homocarnosine, and anserine present in muscle and brain. Proc. Natl. Acad. Sci. USA 1988, 85, 3175–3179. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Stvolinsky, S.L.; Fedorova, T.N.; Suslina, Z.A. Carnosine as a natural antioxidant and geroprotector: From molecular mechanisms to clinical trials. Rejuvenation Res. 2010, 13, 156–158. [Google Scholar] [CrossRef]
- Baraniuk, J.N.; El-Amin, S.; Corey, R.; Rayhan, R.; Timbol, C. Carnosine treatment for gulf war illness: A randomized controlled trial. Glob. J. Health Sci. 2013, 5, 69–81. [Google Scholar] [CrossRef]
- De Courten, B.; Jakubova, M.; De Courten, M.P.; Kukurova, I.J.; Vallova, S.; Krumpolec, P.; Valkovic, L.; Kurdiova, T.; Garzon, D.; Barbaresi, S. Effects of carnosine supplementation on glucose metabolism: Pilot clinical trial. Obesity 2016, 24, 1027–1034. [Google Scholar] [CrossRef]
- Gaunitz, F.; Hipkiss, A.R. Carnosine and Cancer: A Perspective; Springer: Berlin/Heidelberg, Germany, 2012; pp. 135–142. [Google Scholar]
- Haabeth, O.A.W.; Lorvik, K.B.; Yagita, H.; Bogen, B.; Corthay, A. Interleukin-1 is required for cancer eradication mediated by tumor-specific Th1 cells. Oncoimmunology 2016, 5, e1039763. [Google Scholar] [CrossRef]
- Li, M.; Knight, D.A.; Snyder, L.A.; Smyth, M.J.; Stewart, T.J. A role for CCL2 in both tumor progression and immunosurveillance. Oncoimmunology 2013, 2, e25474. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Nawab, O.; Patel, T.; Kossenkov, A.V.; Halama, N.; Jaeger, D.; Pestell, R.G. Recent Advances Targeting CCR5 for Cancer and Its Role in Immuno-Oncology. Cancer Res. 2019, 79, 4801–4807. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Mishra, M.K.; Eltoum, I.-E.A.; Bae, S.; Lillard, J.W., Jr.; Singh, R. CCR5/CCL5 axis interaction promotes migratory and invasiveness of pancreatic cancer cells. Sci. Rep. 2018, 8, 1323. [Google Scholar] [CrossRef] [PubMed]
- González-Martín, A.; Mira, E.; Mañes, S. CCR5 in cancer immunotherapy: More than an “attractive” receptor for T cells. Oncoimmunology 2012, 1, 106–108. [Google Scholar] [CrossRef]
- Kourtzelis, I.; Rafail, S. The dual role of complement in cancer and its implication in anti-tumor therapy. Ann. Transl. Med. 2016, 4, 265. [Google Scholar] [CrossRef]
- Bidwell, B.N.; Slaney, C.Y.; Withana, N.P.; Forster, S.; Cao, Y.; Loi, S.; Andrews, D.; Mikeska, T.; Mangan, N.E.; Samarajiwa, S.A. Silencing of Irf7 pathways in breast cancer cells promotes bone metastasis through immune escape. Nat. Med. 2012, 18, 1224–1231. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, W.; Zhu, W.; Meng, H.; Chen, J.; Zhang, J. Overexpression of Interferon Regulatory Factor 7 (IRF7) Reduces Bone Metastasis of Prostate Cancer Cells in Mice. Oncol. Res. 2017, 25, 511–522. [Google Scholar] [CrossRef]
- Zheng, L.M.; Ojcius, D.M.; Garaud, F.; Roth, C.; Maxwell, E.; Li, Z.; Rong, H.; Chen, J.; Wang, X.Y.; Catino, J.J.; et al. Interleukin-10 inhibits tumor metastasis through an NK cell-dependent mechanism. J. Exp. Med. 1996, 184, 579–584. [Google Scholar] [CrossRef]
- Sun, H.; Jackson, M.J.; Kundu, N.; Fulton, A.M. Interleukin-10 gene transfer activates interferon-γ and the interferon-γ-inducible genes Gbp-1/Mag-1 and Mig-1 in mammary tumors. Int. J. Cancer 1999, 80, 624–629. [Google Scholar] [CrossRef]
- Groux, H.; Cottrez, F.; Rouleau, M.; Mauze, S.; Antonenko, S.; Hurst, S.; McNeil, T.; Bigler, M.; Roncarolo, M.-G.; Coffman, R.L. A transgenic model to analyze the immunoregulatory role of IL-10 secreted by antigen-presenting cells. J. Immunol. 1999, 162, 1723–1729. [Google Scholar] [CrossRef]
- Mumm, J.B.; Emmerich, J.; Zhang, X.; Chan, I.; Wu, L.; Mauze, S.; Blaisdell, S.; Basham, B.; Dai, J.; Grein, J. IL-10 elicits IFNγ-dependent tumor immune surveillance. Cancer Cell 2011, 20, 781–796. [Google Scholar] [CrossRef]
- Yan, W.L.; Shen, K.Y.; Tien, C.Y.; Chen, Y.A.; Liu, S.J. Recent progress in GM-CSF-based cancer immunotherapy. Immunotherapy 2017, 9, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Josephs, S.F.; Ichim, T.E.; Prince, S.M.; Kesari, S.; Marincola, F.M.; Escobedo, A.R.; Jafri, A. Unleashing endogenous TNF-alpha as a cancer immunotherapeutic. J. Transl. Med. 2018, 16, 242. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Chen, J.J.; Yao, P.L.; Yang, P.C. The role of interleukin-8 in cancer cells and microenvironment interaction. Front. Biosci. 2005, 10, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Chen, L.; Wei, X. Inflammatory Cytokines in Cancer: Comprehensive Understanding and Clinical Progress in Gene Therapy. Cells 2021, 10, 100. [Google Scholar] [CrossRef]
- Morris, R.M.; Mortimer, T.O.; O’Neill, K.L. Cytokines: Can Cancer Get the Message? Cancers 2022, 14, 2178. [Google Scholar] [CrossRef]
- Baye, E.; Ukropcova, B.; Ukropec, J.; Hipkiss, A.; Aldini, G.; De Courten, B. Physiological and therapeutic effects of carnosine on cardiometabolic risk and disease. Amino Acids 2016, 48, 1131–1149. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free. Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Son, D.O.; Satsu, H.; Kiso, Y.; Totsuka, M.; Shimizu, M. Inhibitory effect of carnosine on interleukin-8 production in intestinal epithelial cells through translational regulation. Cytokine 2008, 42, 265–276. [Google Scholar] [CrossRef]
- Hwang, B.; Shin, S.-S.; Song, J.-H.; Choi, Y.H.; Kim, W.-J.; Moon, S.-K. Carnosine exerts antitumor activity against bladder cancers in vitro and in vivo via suppression of angiogenesis. J. Nutr. Biochem. 2019, 74, 108230. [Google Scholar] [CrossRef]
- Lee, J.; Park, J.R.; Lee, H.; Jang, S.; Ryu, S.M.; Kim, H.; Kim, D.; Jang, A.; Yang, S.R. L-carnosine induces apoptosis/cell cycle arrest via suppression of NF-κB/STAT1 pathway in HCT116 colorectal cancer cells. Vitr. Cell Dev. Biol. Anim. 2018, 54, 505–512. [Google Scholar] [CrossRef]
- Diers, A.R.; Katarzyna Broniowska, A.; Chang, C.-F.; Hogg, N. Pyruvate fuels mitochondrial respiration and proliferation of breast cancer cells: Effect of monocarboxylate transporter inhibition. Biochem. J. 2012, 444, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Barbi de Moura, M.; Vincent, G.; Fayewicz, S.L.; Bateman, N.W.; Hood, B.L.; Sun, M.; Suhan, J.; Duensing, S.; Yin, Y.; Sander, C.; et al. Mitochondrial Respiration—An Important Therapeutic Target in Melanoma. PLoS ONE 2012, 7, e40690. [Google Scholar] [CrossRef] [PubMed]
- Marx, J.L. Human Papilloma Virus and Cervical Cancer. Science 1986, 231, 920. [Google Scholar] [CrossRef]
- Tzenov, Y.R.; Andrews, P.G.; Voisey, K.; Popadiuk, P.; Xiong, J.; Popadiuk, C.; Kao, K.R. Human Papilloma Virus (HPV) E7-Mediated Attenuation of Retinoblastoma (Rb) Induces hPygopus2 Expression via Elf-1 in Cervical Cancer. Mol. Cancer Res. 2013, 11, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Bubber, P.; Hartounian, V.; Gibson, G.E.; Blass, J.P. Abnormalities in the tricarboxylic acid (TCA) cycle in the brains of schizophrenia patients. Eur. Neuropsychopharmacol. 2011, 21, 254–260. [Google Scholar] [CrossRef]
- Bao, Y.; Ding, S.; Cheng, J.; Liu, Y.; Wang, B.; Xu, H.; Shen, Y.; Lyu, J. Carnosine Inhibits the Proliferation of Human Cervical Gland Carcinoma Cells Through Inhibiting Both Mitochondrial Bioenergetics and Glycolysis Pathways and Retarding Cell Cycle Progression. Integr. Cancer Ther. 2018, 17, 80–91. [Google Scholar] [CrossRef]
- Farid, R.M.; Gaafar, P.M.E.; Hazzah, H.A.; Helmy, M.W.; Abdallah, O.Y. Chemotherapeutic potential of L-carnosine from stimuli-responsive magnetic nanoparticles against breast cancer model. Nanomedicine 2020, 15, 891–911. [Google Scholar] [CrossRef]
- Gaafar, P.M.E.; El-Salamouni, N.S.; Farid, R.M.; Hazzah, H.A.; Helmy, M.W.; Abdallah, O.Y. Pegylated liquisomes: A novel combined passive targeting nanoplatform of L-carnosine for breast cancer. Int. J. Pharm. 2021, 602, 120666. [Google Scholar] [CrossRef]
- Lei, L.; Nan, B.; Yang, F.; Xu, L.; Guan, G.; Xu, J.; Yue, R.; Wang, Y.; Huan, S.; Yin, X.; et al. Zinc-Carnosine Metallodrug Network as Dual Metabolism Inhibitor Overcoming Metabolic Reprogramming for Efficient Cancer Therapy. Nano Lett. 2023, 23, 2659–2668. [Google Scholar] [CrossRef]
- Fisusi, F.A.; Akala, E.O. Drug combinations in breast cancer therapy. Pharm. Nanotechnol. 2019, 7, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Trayes, K.P.; Cokenakes, S.E.H. Breast Cancer Treatment. Am. Fam. Physician 2021, 104, 171–178. [Google Scholar] [PubMed]
- Yuan, T.Z.; Zhan, Z.J.; Qian, C.N. New frontiers in proton therapy: Applications in cancers. Cancer Commun. 2019, 39, 61. [Google Scholar] [CrossRef]
- Ferraro, E.; Walsh, E.M.; Tao, J.J.; Chandarlapaty, S.; Jhaveri, K. Accelerating drug development in breast cancer: New frontiers for ER inhibition. Cancer Treat. Rev. 2022, 109, 102432. [Google Scholar] [CrossRef] [PubMed]
- Sudhakaran, M.; Sardesai, S.; Doseff, A.I. Flavonoids: New Frontier for Immuno-Regulation and Breast Cancer Control. Antioxidants 2019, 8, 103. [Google Scholar] [CrossRef]
- Fenga, C.; Costa, C.; Caruso, E.; Raffa, L.; Alibrando, C.; Gangemi, S.; Docea, A.O.; Tsatsakis, A.M. Current evidence on the protective effect of dietary polyphenols on breast cancer. Farmacia 2016, 64, 1–12. [Google Scholar]
- Aydemir, S. The potential anti cancer effects of L-Carnosine in MCF7 breast cancer cells. In Proceedings of the Stem Cell 2020: 4th World Congress and Expo on Cell & Stem Cell Research, Paris, France, 21–22 February 2020. [Google Scholar]
- Lee, A.V.; Oesterreich, S.; Davidson, N.E. MCF-7 Cells—Changing the Course of Breast Cancer Research and Care for 45 Years. JNCI J. Natl. Cancer Inst. 2015, 107, djv073. [Google Scholar] [CrossRef]
- Saldi, S.; Perrucci, E.; Fulcheri, C.P.L.; Mariucci, C.; Chierchini, S.; Ingrosso, G.; Falcinelli, L.; Podlesko, A.M.; Merluzzi, M.; Bini, V.; et al. Zinc-L-carnosine prevented dysphagia in breast cancer patients undergoing adjuvant radiotherapy: Results of a phase III randomized trial. Breast J. 2020, 26, 1882–1884. [Google Scholar] [CrossRef]
- Leggio, L.; L’Episcopo, F.; Magrì, A.; Ulloa-Navas, M.J.; Paternò, G.; Vivarelli, S.; Bastos, C.A.P.; Tirolo, C.; Testa, N.; Caniglia, S.; et al. Small Extracellular Vesicles Secreted by Nigrostriatal Astrocytes Rescue Cell Death and Preserve Mitochondrial Function in Parkinson’s Disease. Adv. Health Mater. 2022, 11, e2201203. [Google Scholar] [CrossRef]
- Sugihara, Y.; Onoue, S.; Tashiro, K.; Sato, M.; Hasegawa, T.; Katakura, Y. Carnosine induces intestinal cells to secrete exosomes that activate neuronal cells. PLoS ONE 2019, 14, e0217394. [Google Scholar] [CrossRef]
- Xu, Y.; Feng, K.; Zhao, H.; Di, L.; Wang, L.; Wang, R. Tumor-derived extracellular vesicles as messengers of natural products in cancer treatment. Theranostics 2022, 12, 1683–1714. [Google Scholar] [CrossRef] [PubMed]
- Leggio, L.; Arrabito, G.; Ferrara, V.; Vivarelli, S.; Paternò, G.; Marchetti, B.; Pignataro, B.; Iraci, N. Mastering the Tools: Natural versus Artificial Vesicles in Nanomedicine. Adv. Health Mater. 2020, 9, e2000731. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.W. Drugs and pharmaceuticals: Management of intoxication and antidotes. EXS 2010, 100, 397–460. [Google Scholar] [PubMed]
- Devarajan, N.; Manjunathan, R.; Ganesan, S.K. Tumor hypoxia: The major culprit behind cisplatin resistance in cancer patients. Crit. Rev. Oncol. Hematol. 2021, 162, 103327. [Google Scholar] [CrossRef]
- Wang, H.; Guo, S.; Kim, S.J.; Shao, F.; Ho, J.W.K.; Wong, K.U.; Miao, Z.; Hao, D.; Zhao, M.; Xu, J.; et al. Cisplatin prevents breast cancer metastasis through blocking early EMT and retards cancer growth together with paclitaxel. Theranostics 2021, 11, 2442–2459. [Google Scholar] [CrossRef]
- Scripture, C.D.; Figg, W.D.; Sparreboom, A. Peripheral neuropathy induced by paclitaxel: Recent insights and future perspectives. Curr. Neuropharmacol. 2006, 4, 165–172. [Google Scholar] [CrossRef]
- Tsutsumi, K.; Kaname, T.; Shiraishi, H.; Kawashiri, T.; Egashira, N. Polaprezinc reduces paclitaxel-induced peripheral neuropathy in rats without affecting anti-tumor activity. J. Pharmacol. Sci. 2016, 131, 146–149. [Google Scholar] [CrossRef]
- Noori, S.; Mahboob, T. Antioxidant effect of carnosine pretreatment on cisplatin-induced renal oxidative stress in rats. Indian J. Clin. Biochem. 2010, 25, 86–91. [Google Scholar] [CrossRef]
- Ibrahim, N.; El Said, H.; Choukair, A. Zinc carnosine-based modified bismuth quadruple therapy vs standard triple therapy for Helicobacter pylori eradication: A randomized controlled study. World J. Clin. Cases 2022, 10, 227–235. [Google Scholar] [CrossRef]
- Boldyrev, A.A. Carnosine and Oxidative Stress in Cells and Tissues; Nova Publishers: Hauppauge, NY, USA, 2007. [Google Scholar]
- Gardner, M.L.; Illingworth, K.M.; Kelleher, J.; Wood, D. Intestinal absorption of the intact peptide carnosine in man, and comparison with intestinal permeability to lactulose. J. Physiol. 1991, 439, 411–422. [Google Scholar] [CrossRef]
- Goto, K.; Maemura, H.; Takamatsu, K.; Ishii, N. Hormonal responses to resistance exercise after ingestion of carnosine and anserine. J. Strength Cond. Res. 2011, 25, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Dolu, N.; Acer, H.; Kara, A.Y. Investigation of dose-related effects of carnosine on anxiety with sympathetic skin response and T-maze. Acta Medica 2014, 57, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Décombaz, J.; Beaumont, M.; Vuichoud, J.; Bouisset, F.; Stellingwerff, T. Effect of slow-release β-alanine tablets on absorption kinetics and paresthesia. Amino Acids 2012, 43, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Salatto, R.W.; McGinnis, G.R.; Davis, D.W.; Carrier, B.; Manning, J.W.; DeBeliso, M.; Navalta, J.W. Effects of Acute Beta-Alanine Ingestion and Immersion-Plus-Exercise on Connectedness to Nature and Perceived Pain. Int. J. Environ. Res. Public Health 2021, 18, 8134. [Google Scholar] [CrossRef]
| Authors and Year | Cancer Cell Type/In Vivo Model | Effective Carnosine Dose | Time of Exposure | Outcomes | Ref. |
|---|---|---|---|---|---|
| Iovine et al., 2012 | HCT116 (colon) | 50–100 mM | 24 h | Reduction in ROS levels and induction of cell cycle arrest in G1 phase; decreased ERK1/2 phosphorylation and increased p21waf1 protein; and reduction in ATP production via glycolysis. | [45] |
| Shen et al., 2014 | SGC-7901 (gastric) | 5–20 mM | 24–48 h | Reduction in cell proliferation; decrease in mitochondrial ATP-linked respiration; reduction in maximal oxygen consumption and spare respiratory capacity; and reduction in the extracellular acidification rate and glycolysis. | [46] |
| Mikula-Pietrasik and Książek 2016 | Patient-derived primary HPMCs (human peritoneal mesothelial cells) | 20 mM | 63 days | Delay of replicative senescence and inhibition of the development of two markers of senescence (i.e., SA-β-Gal and phosphorylated γ-H2AX); suppression of mitochondria-related oxidative stress; inhibition of adhesion, migration, invasion, and proliferation induced via down-regulated secretion of different molecules (i.e., fibronectin, IL-6, IL-8, GRO1, PAI1, and TGF-β1). | [47] |
| A2780 (ovarian) | 50 mM | 24 h | Reduction in cell viability and increased percentage of apoptotic cells. | ||
| OVCAR-3 (ovarian) | |||||
| SKOV-3 (ovarian) | 100 mM | ||||
| Lee et al., 2018 | HCT116 (colon) | 100 mM or 200 mM | 24 h | Reduction in cell proliferation by inducing the arrest in the G0/G1 phase; decreased mRNA levels of cell cycle-related genes; increased levels of the cyclin D1, BAX/Bcl-2, cleaved caspase-3, p21, and p53 proteins; and inhibited phosphorylation of STAT1 on Tyr701 and NF-κB p65 on Ser276 and Ser536. | [113] |
| Bao et al., 2018 | HeLa (cervical gland) | 20 mM | 48 h | Inhibition of proliferation by inducing the arrest in the G1 phase; inhibition of both mitochondrial respiration and glycolysis, resulting in the reduction of ATP production; and decreased expression of ClpP protease. | [119] |
| Hwang et al., 2019 | EJ cells (MGH-U1) (bladder) | ≥20 mM | 24 h | Inhibition of proliferation by blocking the G1 cell cycle phase; reduced levels of cyclin D1 and CDK4; dose-dependent increase in p21WAF protein expression; increased phosphorylation of ERK and dose-dependent reduction of p38; and inhibition of the migratory and invasive potential. | [112] |
| Balb/C nude mice inoculated with EJ bladder cancer cells | 5 mg/kg and 10 mg/kg | 10 days | Reduction in the volume and weight of the tumor, without any loss of body weight as a side effect; and inhibition of angiogenesis. | ||
| Farid et al., 2020 | MCF-7 (breast) | 1–100 mM used alone or conjugated with magnetic nanoparticles (CCMNPs) | 24–48 h | CCMNPs displayed higher cytotoxic activity compared to the carnosine-free solution. | [120] |
| Female BALB/C mice implanted with Ehrlich ascites carcinoma (EAC) | 200 mg/kg/day (equivalent to 50 mM of carnosine) | 21 days | Higher reductions in the volume and weight of the tumor in CCMNPs (both subjected or not to an external magnet placed on the tumor area) compared to the carnosine-free solution; and reduction in VEGF and cyclin D1 levels and increase in caspase 3 levels (increased apoptosis). | ||
| Gaafar et al., 2021 | MCF-7 (breast) | ≥20 mM when incorporated into P-Liquisomes, phytosomes, and PEGylated liquid crystalline nanoparticles | 24–48 h | Higher cytotoxic effect compared to carnosine alone, especially for the P-Liquisomes formulation. | [121] |
| Female BALB/C mice implanted with Ehrlich ascites carcinoma (EAC) | 3 weeks | P-Liquisomes induced: (i) the highest reduction in tumor volume, (ii) the major increase in the survival rate compared to the tumor-positive control; (iii) the most significant reduction in VEGF and cyclin D1 levels; and (iv) the highest level of caspase-3 activation. | |||
| Prakash et al., 2021 | U937 (myeloid leukemia) | ≥100 mM | 5–6 days | Inhibition of cell proliferation; up-regulation of pro-inflammatory molecule (IL-8, CCL2, CD86, IL-1β, CCR5, Ly96, IRF-7, C3, and TNF) expression; increased secretion of cytokines (IL-10, GM-CSF, and TNF-α); and stimulation of cell differentiation towards a macrophage phenotype. | [85] |
| HT29 (colon) | Inhibition of cell proliferation. | ||||
| LIM2045 (colon) | |||||
| SKOV-3 (ovarian) | |||||
| ZR-75-1 (breast) | |||||
| Lei et al., 2023 | 4T1 (breast) | ≥20 µg/mL in the formulation Zn−carnosine metallodrug network nanoparticles (Zn–Car MNs) | 24 h | Reduction of cell viability accompanied by the inhibition of both OXPHOS and glycolysis, resulting in a reduced ATP production; and increased ROS production and induction of apoptosis. | [122] |
| Mice bearing 4T1 tumors | 1 mg/mL in the formulation Zn−carnosine metallodrug network nanoparticles (Zn–Car MNs) | 0, 2, and 4 days | Reduction in the volume and weight of the tumor, without body weight loss. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maugeri, S.; Sibbitts, J.; Privitera, A.; Cardaci, V.; Di Pietro, L.; Leggio, L.; Iraci, N.; Lunte, S.M.; Caruso, G. The Anti-Cancer Activity of the Naturally Occurring Dipeptide Carnosine: Potential for Breast Cancer. Cells 2023, 12, 2592. https://doi.org/10.3390/cells12222592
Maugeri S, Sibbitts J, Privitera A, Cardaci V, Di Pietro L, Leggio L, Iraci N, Lunte SM, Caruso G. The Anti-Cancer Activity of the Naturally Occurring Dipeptide Carnosine: Potential for Breast Cancer. Cells. 2023; 12(22):2592. https://doi.org/10.3390/cells12222592
Chicago/Turabian StyleMaugeri, Salvatore, Jay Sibbitts, Anna Privitera, Vincenzo Cardaci, Lucia Di Pietro, Loredana Leggio, Nunzio Iraci, Susan M. Lunte, and Giuseppe Caruso. 2023. "The Anti-Cancer Activity of the Naturally Occurring Dipeptide Carnosine: Potential for Breast Cancer" Cells 12, no. 22: 2592. https://doi.org/10.3390/cells12222592
APA StyleMaugeri, S., Sibbitts, J., Privitera, A., Cardaci, V., Di Pietro, L., Leggio, L., Iraci, N., Lunte, S. M., & Caruso, G. (2023). The Anti-Cancer Activity of the Naturally Occurring Dipeptide Carnosine: Potential for Breast Cancer. Cells, 12(22), 2592. https://doi.org/10.3390/cells12222592












