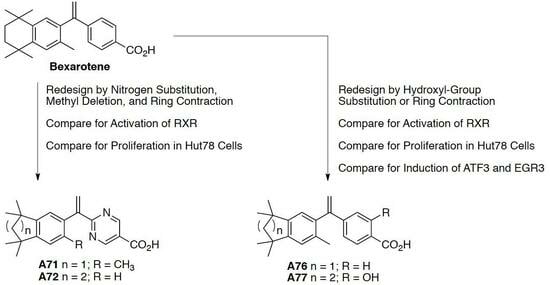
Development of Bexarotene Analogs for Treating Cutaneous T-Cell Lymphomas
Abstract
1. Introduction
2. Experimental
2.1. Mammalian Two-Hybrid Assay
2.2. RXRE-Mediated Transcription Assay
2.3. Proliferation Assay
2.4. Quantitative Real-Time PCR
2.5. Data Analysis
2.6. Cytotoxicity and Mutagenicity
2.7. HPLC, NMR, and High-Resolution Mass Spectrometry
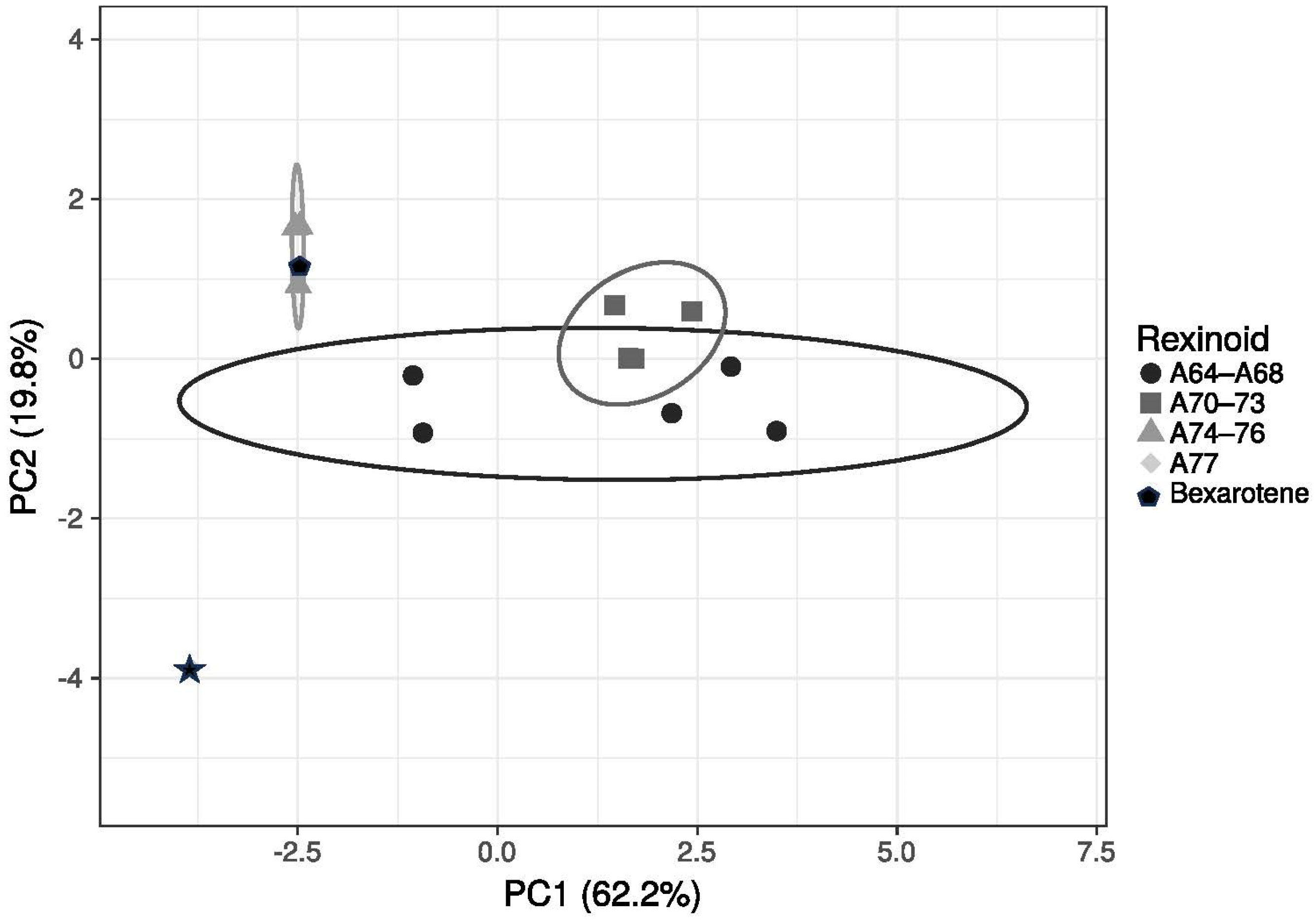
2.8. Principal Component Analysis
3. Results
3.1. Biological Evaluation of Generation 6 Analogs (A64–A68) via an M2H Luciferase-Based System
3.2. Biological Evaluation of Generation 7 Analogs (A70–A77) via an M2H Luciferase-Based System
3.3. Assessment of Generation 6 Analogs (A64–A68) via an RXRE Luciferase-Based System
3.4. Assessment of Generation 7 Analogs (A70–A77) via an RXRE Luciferase-Based System
3.5. Biological Appraisal of Generation 6 and 7 Analogs via a CTCL Cell Proliferation Assay
3.6. Cytotoxicity and Mutagenicity Assessment of Rexinoids
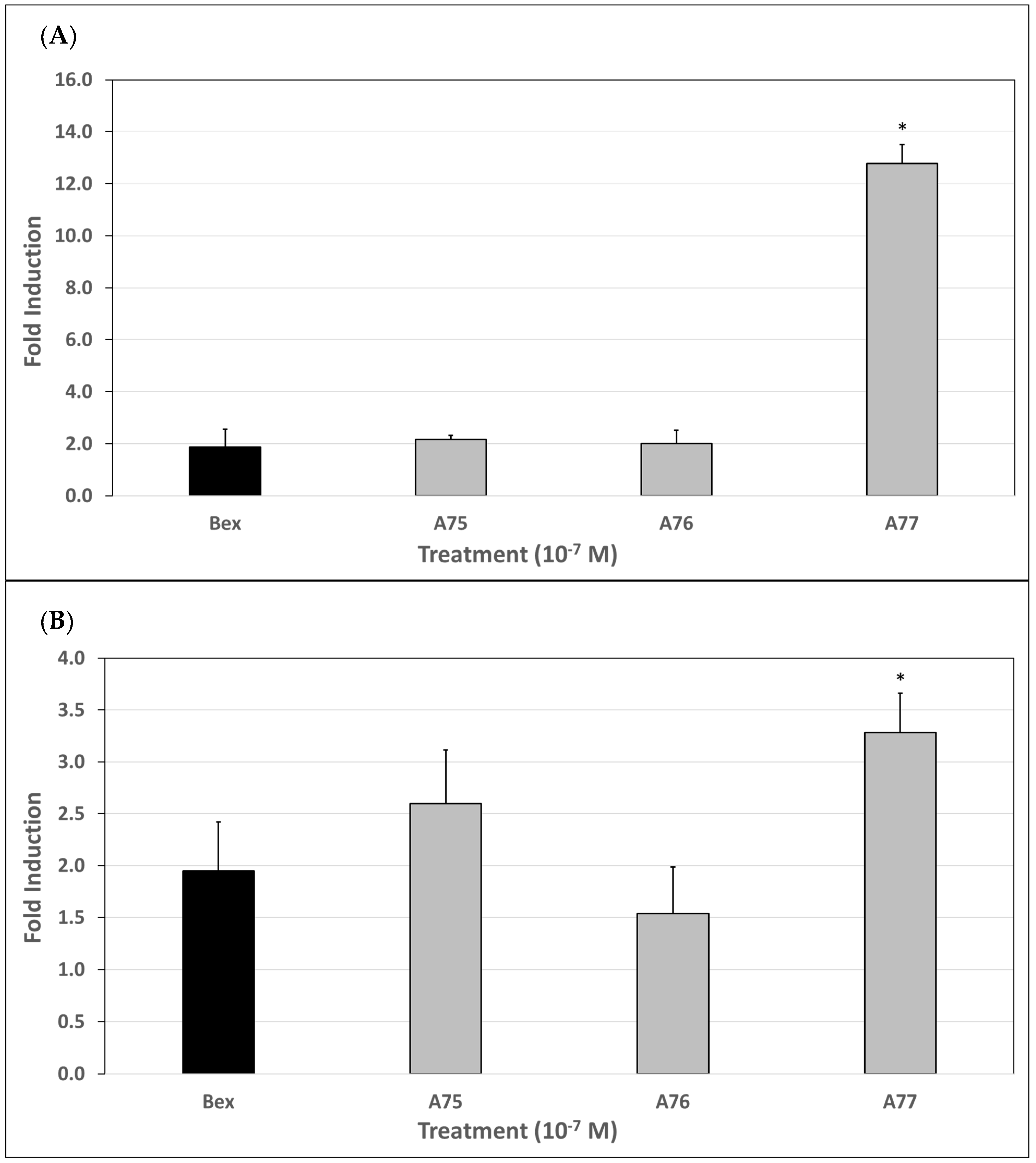
3.7. qPCR Analysis of ATF3 Gene Induction
3.8. qPCR Analysis of EGR3 Gene Induction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Willemze, R.; Jaffe, E.S.; Burg, G.; Cerroni, L.; Berti, E.; Swerdlow, S.H.; Ralfkiaer, E.; Chimenti, S.; Diaz-Perez, J.L.; Duncan, L.M.; et al. WHO-EORTC classification for cutaneous lymphomas. Blood 2005, 105, 3768–3785. [Google Scholar] [CrossRef]
- Bradford, P.T.; Devesa, S.S.; Anderson, W.F.; Toro, J.R. Cutaneous lymphoma incidence patterns in the United States: A population-based study of 3884 cases. Blood 2009, 113, 5064–5073. [Google Scholar] [CrossRef] [PubMed]
- Criscione, V.D.; Weinstock, M.A. Incidence of cutaneous T-cell lymphoma in the United States, 1973–2002. Arch. Dermatol. 2007, 143, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Jawed, S.I.; Myskowski, P.L.; Horwitz, S.; Moskowitz, A.; Querfeld, C. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sezary syndrome): Part I. Diagnosis: Clinical and histopathologic features and new molecular and biologic markers. J. Am. Acad. Dermatol. 2014, 70, 205.e1–205.e16, quiz 221–222. [Google Scholar] [CrossRef] [PubMed]
- Jonak, C.; Tittes, J.; Brunner, P.M.; Guenova, E. Mycosis fungoides and Sezary syndrome. J. Dtsch. Dermatol. Ges. 2021, 19, 1307–1334. [Google Scholar] [CrossRef] [PubMed]
- Kempf, W.; Mitteldorf, C. Cutaneous T-cell lymphomas-An update 2021. Hematol. Oncol. 2021, 39 (Suppl. S1), 46–51. [Google Scholar] [CrossRef]
- Kohnken, R.; Fabbro, S.; Hastings, J.; Porcu, P.; Mishra, A. Sezary Syndrome: Clinical and Biological Aspects. Curr. Hematol. Malig. Rep. 2016, 11, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Jawed, S.I.; Myskowski, P.L.; Horwitz, S.; Moskowitz, A.; Querfeld, C. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sezary syndrome): Part II. Prognosis, management, and future directions. J. Am. Acad. Dermatol. 2014, 70, 223.e1–223.e17, quiz 240–242. [Google Scholar] [CrossRef]
- McCusker, M.E.; Garifallou, M.; Bogen, S.A. Sezary lineage cells can be induced to proliferate via CD28-mediated costimulation. J. Immunol. 1997, 158, 4984–4991. [Google Scholar] [CrossRef]
- Yamanaka, K.; Clark, R.; Rich, B.; Dowgiert, R.; Hirahara, K.; Hurwitz, D.; Shibata, M.; Mirchandani, N.; Jones, D.A.; Goddard, D.S.; et al. Skin-derived interleukin-7 contributes to the proliferation of lymphocytes in cutaneous T-cell lymphoma. Blood 2006, 107, 2440–2445. [Google Scholar] [CrossRef]
- Schlapbach, C.; Ochsenbein, A.; Kaelin, U.; Hassan, A.S.; Hunger, R.E.; Yawalkar, N. High numbers of DC-SIGN+ dendritic cells in lesional skin of cutaneous T-cell lymphoma. J. Am. Acad. Dermatol. 2010, 62, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Vaque, J.P.; Gomez-Lopez, G.; Monsalvez, V.; Varela, I.; Martinez, N.; Perez, C.; Dominguez, O.; Grana, O.; Rodriguez-Peralto, J.L.; Rodriguez-Pinilla, S.M.; et al. PLCG1 mutations in cutaneous T-cell lymphomas. Blood 2014, 123, 2034–2043. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, R.A. Cancer-associated myeloproliferation: Old association, new therapeutic target. Mayo Clin. Proc. 2010, 85, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, R.A. Cutaneous T-cell lymphoma: 2017 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2017, 92, 1085–1102. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, P.I.; Lorber, B.; Vonderheid, E.C. Infections complicating mycosis fungoides and Sezary syndrome. JAMA 1992, 267, 1354–1358. [Google Scholar] [CrossRef]
- Epstein, E.H., Jr.; Levin, D.L.; Croft, J.D., Jr.; Lutzner, M.A. Mycosis fungoides. Survival, prognostic features, response to therapy, and autopsy findings. Medicine 1972, 51, 61–72. [Google Scholar] [CrossRef]
- Posner, L.E.; Fossieck, B.E., Jr.; Eddy, J.L.; Bunn, P.A., Jr. Septicemic complications of the cutaneous T-cell lymphomas. Am. J. Med. 1981, 71, 210–216. [Google Scholar] [CrossRef]
- Bouaziz, J.D.; Ortonne, N.; Giustiniani, J.; Schiavon, V.; Huet, D.; Bagot, M.; Bensussan, A. Circulating natural killer lymphocytes are potential cytotoxic effectors against autologous malignant cells in sezary syndrome patients. J. Investig. Dermatol. 2005, 125, 1273–1278. [Google Scholar] [CrossRef]
- Wysocka, M.; Benoit, B.M.; Newton, S.; Azzoni, L.; Montaner, L.J.; Rook, A.H. Enhancement of the host immune responses in cutaneous T-cell lymphoma by CpG oligodeoxynucleotides and IL-15. Blood 2004, 104, 4142–4149. [Google Scholar] [CrossRef]
- Wysocka, M.; Zaki, M.H.; French, L.E.; Chehimi, J.; Shapiro, M.; Everetts, S.E.; McGinnis, K.S.; Montaner, L.; Rook, A.H. Sezary syndrome patients demonstrate a defect in dendritic cell populations: Effects of CD40 ligand and treatment with GM-CSF on dendritic cell numbers and the production of cytokines. Blood 2002, 100, 3287–3294. [Google Scholar] [CrossRef]
- French, L.E.; Huard, B.; Wysocka, M.; Shane, R.; Contassot, E.; Arrighi, J.F.; Piguet, V.; Calderara, S.; Rook, A.H. Impaired CD40L signaling is a cause of defective IL-12 and TNF-alpha production in Sezary syndrome: Circumvention by hexameric soluble CD40L. Blood 2005, 105, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.N.; Duvic, M.; Tang, C.K.; Bueso-Ramos, C.; Estrov, Z.; Reuben, J.M. Dysregulated synthesis of intracellular type 1 and type 2 cytokines by T cells of patients with cutaneous T-cell lymphoma. Clin. Diagn. Lab. Immunol. 1999, 6, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Samimi, S.; Benoit, B.; Evans, K.; Wherry, E.J.; Showe, L.; Wysocka, M.; Rook, A.H. Increased programmed death-1 expression on CD4+ T cells in cutaneous T-cell lymphoma: Implications for immune suppression. Arch. Dermatol. 2010, 146, 1382–1388. [Google Scholar] [CrossRef] [PubMed]
- Yawalkar, N.; Ferenczi, K.; Jones, D.A.; Yamanaka, K.; Suh, K.Y.; Sadat, S.; Kupper, T.S. Profound loss of T-cell receptor repertoire complexity in cutaneous T-cell lymphoma. Blood 2003, 102, 4059–4066. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.M.; Evans, K.G.; Musiek, A.; Rook, A.H.; Kim, E.J. Update on treatment of cutaneous T-cell lymphoma. Curr. Opin. Oncol. 2009, 21, 131–137. [Google Scholar] [CrossRef]
- Panchal, M.R.; Scarisbrick, J.J. The utility of bexarotene in mycosis fungoides and Sezary syndrome. Onco Targets Ther. 2015, 8, 367–373. [Google Scholar]
- Budgin, J.B.; Richardson, S.K.; Newton, S.B.; Wysocka, M.; Zaki, M.H.; Benoit, B.; Rook, A.H. Biological effects of bexarotene in cutaneous T-cell lymphoma. Arch. Dermatol. 2005, 141, 315–321. [Google Scholar] [CrossRef]
- Zhang, C.; Hazarika, P.; Ni, X.; Weidner, D.A.; Duvic, M. Induction of apoptosis by bexarotene in cutaneous T-cell lymphoma cells: Relevance to mechanism of therapeutic action. Clin. Cancer Res. 2002, 8, 1234–1240. [Google Scholar]
- Richardson, S.K.; Newton, S.B.; Bach, T.L.; Budgin, J.B.; Benoit, B.M.; Lin, J.H.; Yoon, J.S.; Wysocka, M.; Abrams, C.S.; Rook, A.H. Bexarotene blunts malignant T-cell chemotaxis in Sezary syndrome: Reduction of chemokine receptor 4-positive lymphocytes and decreased chemotaxis to thymus and activation-regulated chemokine. Am. J. Hematol. 2007, 82, 792–797. [Google Scholar] [CrossRef]
- Duvic, M.; Martin, A.G.; Kim, Y.; Olsen, E.; Wood, G.S.; Crowley, C.A.; Yocum, R.C.; Worldwide Bexarotene Study Group. Phase 2 and 3 clinical trial of oral bexarotene (Targretin capsules) for the treatment of refractory or persistent early-stage cutaneous T-cell lymphoma. Arch. Dermatol. 2001, 137, 581–593. [Google Scholar]
- Heald, P.; Mehlmauer, M.; Martin, A.G.; Crowley, C.A.; Yocum, R.C.; Reich, S.D.; Worldwide Bexarotene Study Group. Topical bexarotene therapy for patients with refractory or persistent early-stage cutaneous T-cell lymphoma: Results of the phase III clinical trial. J. Am. Acad. Dermatol. 2003, 49, 801–815. [Google Scholar] [CrossRef] [PubMed]
- Forman, B.M.; Umesono, K.; Chen, J.; Evans, R.M. Unique response pathways are established by allosteric interactions among nuclear hormone receptors. Cell 1995, 81, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Marshall, P.A.; Jurutka, P.W.; Wagner, C.E.; van der Vaart, A.; Kaneko, I.; Chavez, P.I.; Ma, N.; Bhogal, J.S.; Shahani, P.; Swierski, J.C.; et al. Analysis of differential secondary effects of novel rexinoids: Select rexinoid X receptor ligands demonstrate differentiated side effect profiles. Pharmacol. Res. Perspect. 2015, 3, e00122. [Google Scholar] [CrossRef] [PubMed]
- Jurutka, P.W.; Kaneko, I.; Yang, J.; Bhogal, J.S.; Swierski, J.C.; Tabacaru, C.R.; Montano, L.A.; Huynh, C.C.; Jama, R.A.; Mahelona, R.D.; et al. Modeling, synthesis, and biological evaluation of potential retinoid X receptor (RXR) selective agonists: Novel analogues of 4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydro-2-naphthyl)ethynyl]benzoic acid (bexarotene) and (E)-3-(3-(1,2,3,4-tetrahydro-1,1,4,4,6-pentamethylnaphthalen-7-yl)-4-hydroxypheny l)acrylic acid (CD3254). J. Med. Chem. 2013, 56, 8432–8454. [Google Scholar] [PubMed]
- Jurutka, P.W.; di Martino, O.; Reshi, S.; Mallick, S.; Sabir, Z.L.; Staniszewski, L.J.P.; Warda, A.; Maiorella, E.L.; Minasian, A.; Davidson, J.; et al. Modeling, Synthesis, and Biological Evaluation of Potential Retinoid-X-Receptor (RXR) Selective Agonists: Analogs of 4-[1-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahyro-2-naphthyl)ethynyl]benzoic Acid (Bexarotene) and 6-(Ethyl(4-isobutoxy-3-isopropylphenyl)amino)nicotinic Acid (NEt-4IB). Int. J. Mol. Sci. 2021, 22, 12371. [Google Scholar] [PubMed]
- Kawata, K.; Morishita, K.; Nakayama, M.; Yamada, S.; Kobayashi, T.; Furusawa, Y.; Arimoto-Kobayashi, S.; Oohashi, T.; Makishima, M.; Naitou, H.; et al. RXR partial agonist produced by side chain repositioning of alkoxy RXR full agonist retains antitype 2 diabetes activity without the adverse effects. J. Med. Chem. 2015, 58, 912–926. [Google Scholar] [CrossRef]
- Boehm, M.F.; Zhang, L.; Badea, B.A.; White, S.K.; Mais, D.E.; Berger, E.; Suto, C.M.; Goldman, M.E.; Heyman, R.A. Synthesis and structure-activity relationships of novel retinoid X receptor-selective retinoids. J. Med. Chem. 1994, 37, 2930–2941. [Google Scholar] [CrossRef]
- Boehm, M.F.; Heyman, R.A.; Lin, Z. Compounds Having Selectivity for Retinoid X Receptors. European Patent EP0637297B1, 23 August 2000. [Google Scholar]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef]
- Doebbeling, U. A mouse model for the Sezary syndrome. J. Exp. Clin. Cancer Res. 2010, 29, 11. [Google Scholar] [CrossRef][Green Version]
- Kohnken, R.; Porcu, P.; Mishra, A. Overview of the Use of Murine Models in Leukemia and Lymphoma Research. Front. Oncol. 2017, 7, 22. [Google Scholar] [CrossRef]
- Mishra, A.; La Perle, K.; Kwiatkowski, S.; Sullivan, L.A.; Sams, G.H.; Johns, J.; Curphey, D.P.; Wen, J.; McConnell, K.; Qi, J.; et al. Mechanism, Consequences, and Therapeutic Targeting of Abnormal IL15 Signaling in Cutaneous T-cell Lymphoma. Cancer Discov. 2016, 6, 986–1005. [Google Scholar] [CrossRef] [PubMed]
- Nakahashi, K.; Nihira, K.; Suzuki, M.; Ishii, T.; Masuda, K.; Mori, K. A novel mouse model of cutaneous T-cell lymphoma revealed the combined effect of mogamulizumab with psoralen and ultraviolet a therapy. Exp. Dermatol. 2022, 31, 1693–1698. [Google Scholar] [CrossRef] [PubMed]
- van der Fits, L.; Rebel, H.G.; Out-Luiting, J.J.; Pouw, S.M.; Smit, F.; Vermeer, K.G.; van Zijl, L.; Tensen, C.P.; Weijer, K.; Vermeer, M.H. A novel mouse model for Sezary syndrome using xenotransplantation of Sezary cells into immunodeficient RAG2(-/-) gammac(-/-) mice. Exp. Dermatol. 2012, 21, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Corona, A.W.; Kodoma, N.; Casali, B.T.; Landreth, G.E. ABCA1 is Necessary for Bexarotene-Mediated Clearance of Soluble Amyloid Beta from the Hippocampus of APP/PS1 Mice. J. Neuroimmune Pharmacol. 2016, 11, 61–72. [Google Scholar] [CrossRef]
- Wang, W.; Nakashima, K.I.; Hirai, T.; Inoue, M. Anti-inflammatory effects of naturally occurring retinoid X receptor agonists isolated from Sophora tonkinensis Gagnep. via retinoid X receptor/liver X receptor heterodimers. J. Nat. Med. 2019, 73, 419–430. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, J.; Haines, C.J.; Feng, H.; Teng, X.; Han, Y. RA induces differentiation of multipotent P19 cells towards male germ cell. Vitr. Cell Dev. Biol. Anim. 2015, 51, 85–91. [Google Scholar] [CrossRef]
- Reich, L.A.; Moerland, J.A.; Leal, A.S.; Zhang, D.; Carapellucci, S.; Lockwood, B.; Jurutka, P.W.; Marshall, P.A.; Wagner, C.E.; Liby, K.T. The rexinoid V-125 reduces tumor growth in preclinical models of breast and lung cancer. Sci. Rep. 2022, 12, 293. [Google Scholar] [CrossRef]
- Wagner, C.E.; Jurutka, P.W.; Marshall, P.A.; Groy, T.L.; van der Vaart, A.; Ziller, J.W.; Furmick, J.K.; Graeber, M.E.; Matro, E.; Miguel, B.V.; et al. Modeling, synthesis and biological evaluation of potential retinoid X receptor (RXR) selective agonists: Novel analogues of 4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydro-2-naphthyl)ethynyl]benzoic acid (bexarotene). J. Med. Chem. 2009, 52, 5950–5966. [Google Scholar] [CrossRef]
- Hanish, B.J.; Price, J.F.H.; Kaneko, I.; Ma, N.; van der Vaart, A.; Wagner, C.E.; Jurutka, P.W.; Marshall, P.A. A novel gene expression analytics-based approach to structure aided design of rexinoids for development as next-generation cancer therapeutics. Steroids 2018, 135, 36–49. [Google Scholar] [CrossRef]
- Chou, C.F.; Hsieh, Y.H.; Grubbs, C.J.; Atigadda, V.R.; Mobley, J.A.; Dummer, R.; Muccio, D.D.; Eto, I.; Elmets, C.A.; Garvey, W.T.; et al. The retinoid X receptor agonist, 9-cis UAB30, inhibits cutaneous T-cell lymphoma proliferation through the SKP2-p27kip1 axis. J. Dermatol. Sci. 2018, 90, 343–356. [Google Scholar] [CrossRef]
- de Almeida, N.R.; Conda-Sheridan, M. A review of the molecular design and biological activities of RXR agonists. Med. Res. Rev. 2019, 39, 1372–1397. [Google Scholar] [CrossRef]
- McBride, P.E. Triglycerides and risk for coronary heart disease. JAMA 2007, 298, 336–338. [Google Scholar] [CrossRef]
- Sander, T.; Freyss, J.; von Korff, M.; Rufener, C. DataWarrior: An Open-Source Program For Chemistry Aware Data Visualization And Analysis. J. Chem. Info. Model. 2015, 55, 460–473. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Wager, T.T.; Hou, X.; Verhoest, P.R.; Villalobos, A. Central Nervous System Multiparameter Optimization Desirability: Application in Drug Discovery. ACS Chem. Neuro. 2016, 7, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacology. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef] [PubMed]





| Compound | Cytotoxicity |
|---|---|
| Bexarotene | None |
| A64 | 0.5 mg/mL |
| A65 | 0.5 mg/mL |
| A66 | 1 mg/mL |
| A67 | 1 mg/mL |
| A68 | 0.08 mg/mL |
| A70 | None |
| A71 | None |
| A72 | None |
| A73 | None |
| A74 | None |
| A75 | None |
| A76 | None |
| A77 | 0.08 mg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warda, A.; Staniszewski, L.J.P.; Sabir, Z.; Livingston, S.; Sausedo, M.; Reshi, S.; Ron, E.; Applegate, M.T.; Haddad, D.; Khamisi, M.; et al. Development of Bexarotene Analogs for Treating Cutaneous T-Cell Lymphomas. Cells 2023, 12, 2575. https://doi.org/10.3390/cells12212575
Warda A, Staniszewski LJP, Sabir Z, Livingston S, Sausedo M, Reshi S, Ron E, Applegate MT, Haddad D, Khamisi M, et al. Development of Bexarotene Analogs for Treating Cutaneous T-Cell Lymphomas. Cells. 2023; 12(21):2575. https://doi.org/10.3390/cells12212575
Chicago/Turabian StyleWarda, Ankedo, Lech J. P. Staniszewski, Zhela Sabir, Sarah Livingston, Michael Sausedo, Sabeeha Reshi, Eyal Ron, Michael T. Applegate, Dena Haddad, Madleen Khamisi, and et al. 2023. "Development of Bexarotene Analogs for Treating Cutaneous T-Cell Lymphomas" Cells 12, no. 21: 2575. https://doi.org/10.3390/cells12212575
APA StyleWarda, A., Staniszewski, L. J. P., Sabir, Z., Livingston, S., Sausedo, M., Reshi, S., Ron, E., Applegate, M. T., Haddad, D., Khamisi, M., Marshall, P. A., Wagner, C. E., & Jurutka, P. W. (2023). Development of Bexarotene Analogs for Treating Cutaneous T-Cell Lymphomas. Cells, 12(21), 2575. https://doi.org/10.3390/cells12212575