Treatment of Naturally Occurring Tendon Disease with Allogeneic Multipotent Mesenchymal Stromal Cells: A Randomized, Controlled, Triple-Blinded Pilot Study in Horses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Aims and Study Design
2.2. MSC Recovery and Culture
2.3. Animals and Inclusion Criteria
2.4. Treatment Procedure
- If a horse was lame at a trot (score 1 or 2/5) during a scheduled examination or showed an increase in clinical signs of inflammation (increase in heat, pain, or swelling) compared with the previous examination, the exercise level was not further increased. If the horse had already been scheduled to trot or gallop, exercise was reduced to hand walking. In these cases, a clinical revaluation was performed three weeks later, and the level of exercise was determined depending on the respective clinical findings.
- If a horse was lame at a walk (score ≥ 3/5) on the day of examination, the horse received box rest and was re-evaluated 7 days later. If lameness at walk was detected on the day of re-evaluation, the standard exercise schedule was restarted from the beginning.
2.5. Clinical Follow-Up
2.6. Diagnostic Imaging
2.7. Statistical Analysis
3. Results
3.1. MSC Characterization
3.2. Animals and Tendon Lesions Included
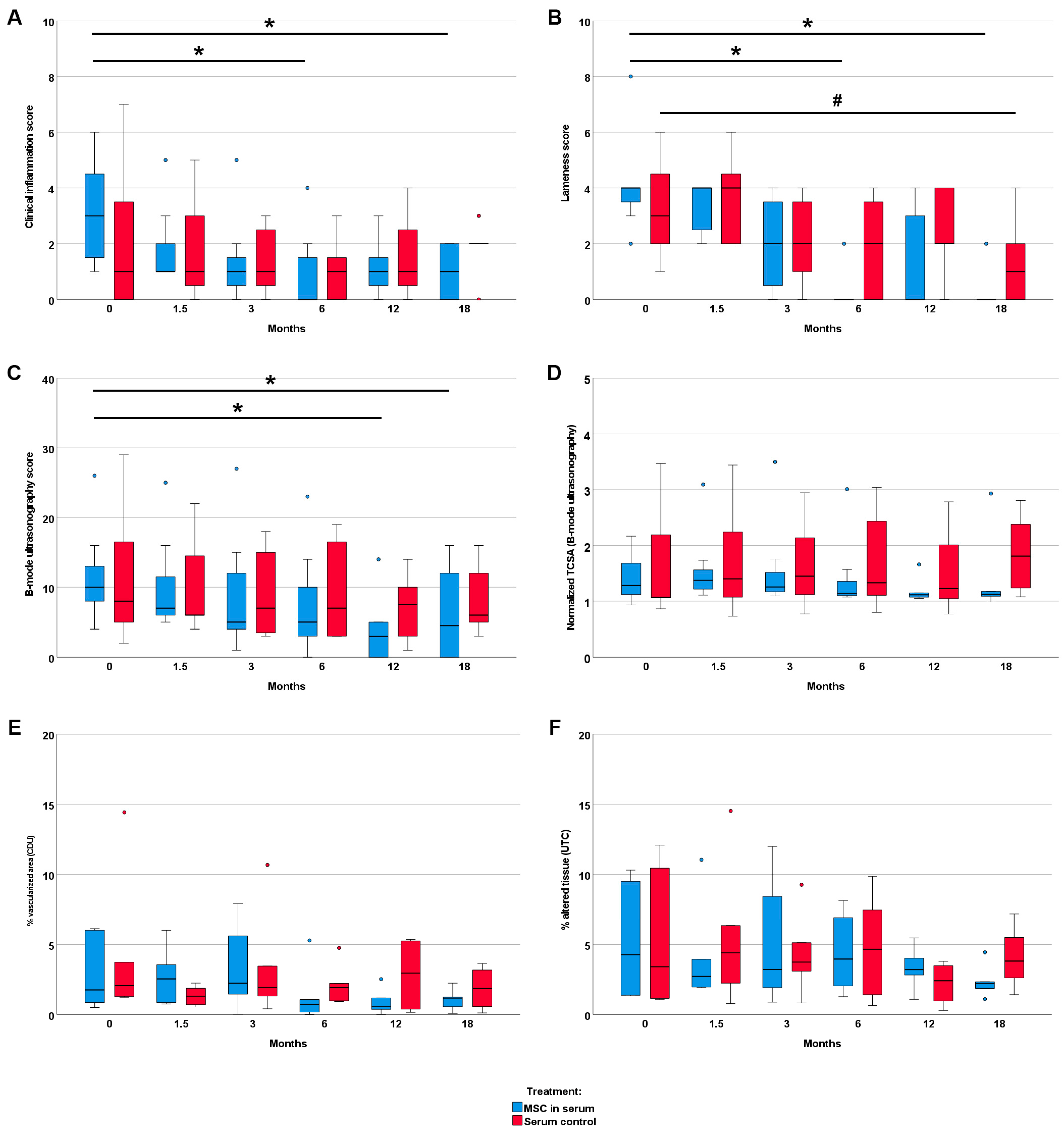
3.3. Clinical Findings
3.4. Diagnostic Imaging Findings
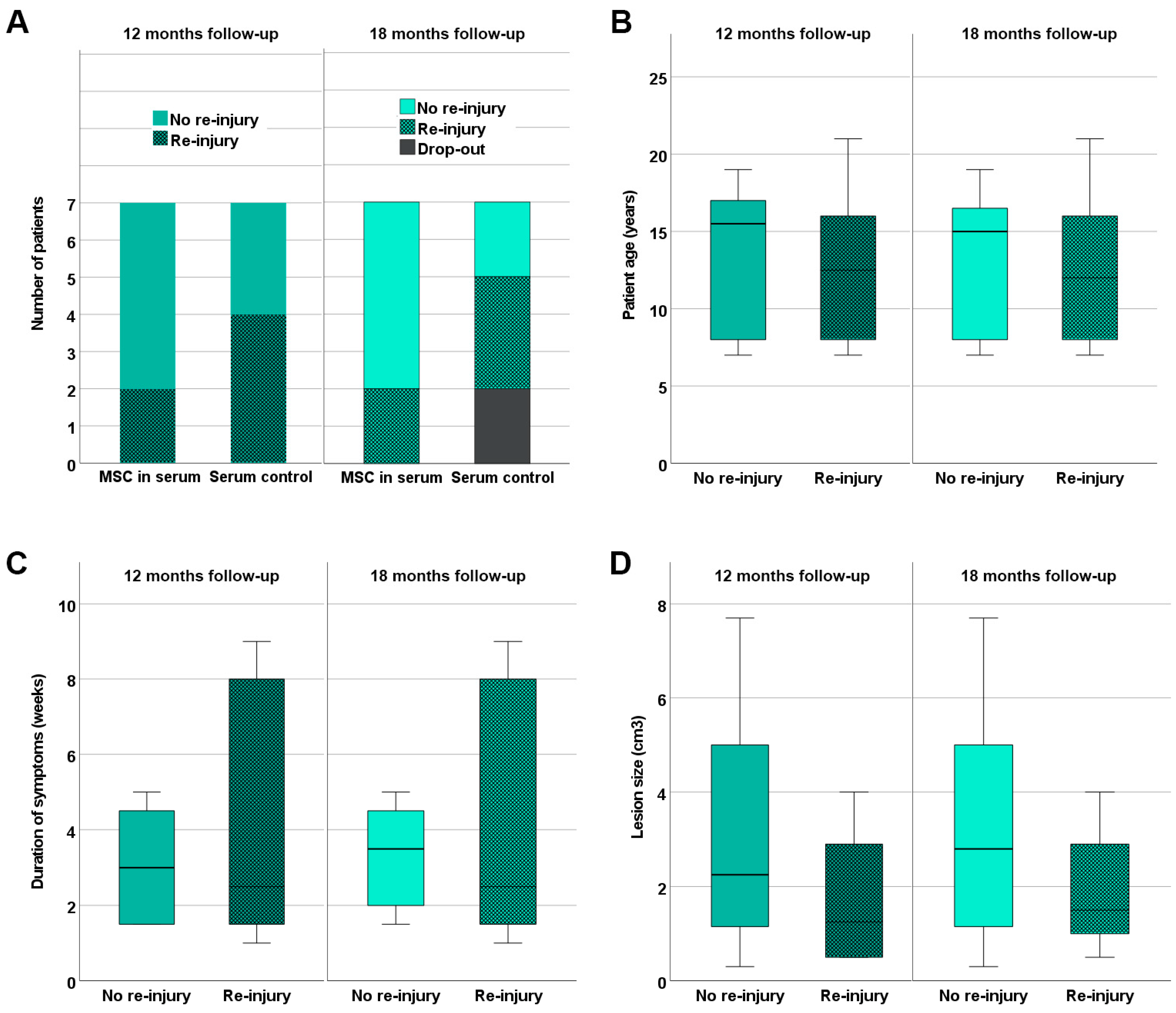
3.5. Re-Injury Rates and Return to Training
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CDU | Color Doppler ultrasonography |
| MRI | Magnetic resonance imaging |
| MSC | Multipotent mesenchymal stromal cell |
| TCSA | Total cross-sectional area |
| UTC | Ultrasound tissue characterization |
References
- Smith, R.K.; Korda, M.; Blunn, G.W.; Goodship, A.E. Isolation and implantation of autologous equine mesenchymal stem cells from bone marrow into the superficial digital flexor tendon as a potential novel treatment. Equine Vet. J. 2003, 35, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.C.; Stewart, A.A. Mesenchymal stem cells: Characteristics, sources, and mechanisms of action. Vet. Clin. N. Am. Equine Pract. 2011, 27, 243–261. [Google Scholar] [CrossRef] [PubMed]
- De Schauwer, C.; Van de Walle, G.R.; Van Soom, A.; Meyer, E. Mesenchymal stem cell therapy in horses: Useful beyond orthopedic injuries? Vet. Q. 2013, 33, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Voga, M.; Adamic, N.; Vengust, M.; Majdic, G. Stem Cells in Veterinary Medicine-Current State and Treatment Options. Front. Vet. Sci. 2020, 7, 278. [Google Scholar] [CrossRef]
- Cequier, A.; Sanz, C.; Rodellar, C.; Barrachina, L. The Usefulness of Mesenchymal Stem Cells beyond the Musculoskeletal System in Horses. Animals 2021, 11, 931. [Google Scholar] [CrossRef]
- Prządka, P.; Buczak, K.; Frejlich, E.; Gąsior, L.; Suliga, K.; Kiełbowicz, Z. The Role of Mesenchymal Stem Cells (MSCs) in Veterinary Medicine and Their Use in Musculoskeletal Disorders. Biomolecules 2021, 11, 1141. [Google Scholar] [CrossRef]
- Barrett, J.G.; MacDonald, E.S. Use of Biologics and Stem Cells in the Treatment of Other Inflammatory Diseases in the Horse. Vet. Clin. N. Am. Equine Pract. 2023; Online ahead of print. [Google Scholar]
- Watts, A.E. Use of Stem Cells for the Treatment of Musculoskeletal Injuries in Horses. Vet. Clin. N. Am. Equine Pract, 2023; Online ahead of print. [Google Scholar] [CrossRef]
- Godwin, E.E.; Young, N.J.; Dudhia, J.; Beamish, I.C.; Smith, R.K. Implantation of bone marrow-derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Vet. J. 2012, 44, 25–32. [Google Scholar] [CrossRef]
- Beerts, C.; Suls, M.; Broeckx, S.Y.; Seys, B.; Vandenberghe, A.; Declercq, J.; Duchateau, L.; Vidal, M.A.; Spaas, J.H. Tenogenically Induced Allogeneic Peripheral Blood Mesenchymal Stem Cells in Allogeneic Platelet-Rich Plasma: 2-Year Follow-up after Tendon or Ligament Treatment in Horses. Front. Vet. Sci. 2017, 4, 158. [Google Scholar] [CrossRef]
- Salz, R.O.; Elliott, C.R.B.; Zuffa, T.; Bennet, E.D.; Ahern, B.J. Treatment of racehorse superficial digital flexor tendonitis: A comparison of stem cell treatments to controlled exercise rehabilitation in 213 cases. Equine Vet. J. 2023, 55, 979–987. [Google Scholar] [CrossRef]
- Schnabel, L.V.; Lynch, M.E.; van der Meulen, M.C.; Yeager, A.E.; Kornatowski, M.A.; Nixon, A.J. Mesenchymal stem cells and insulin-like growth factor-I gene-enhanced mesenchymal stem cells improve structural aspects of healing in equine flexor digitorum superficialis tendons. J. Orthop. Res. 2009, 27, 1392–1398. [Google Scholar] [CrossRef]
- Crovace, A.; Lacitignola, L.; Rossi, G.; Francioso, E. Histological and immunohistochemical evaluation of autologous cultured bone marrow mesenchymal stem cells and bone marrow mononucleated cells in collagenase-induced tendinitis of equine superficial digital flexor tendon. Vet. Med. Int. 2010, 2010, 250978. [Google Scholar] [CrossRef] [PubMed]
- Carvalho Ade, M.; Badial, P.R.; Álvarez, L.E.; Yamada, A.L.; Borges, A.S.; Deffune, E.; Hussni, C.A.; Garcia Alves, A.L. Equine tendonitis therapy using mesenchymal stem cells and platelet concentrates: A randomized controlled trial. Stem Cell Res. Ther. 2013, 4, 85. [Google Scholar] [CrossRef] [PubMed]
- Depuydt, E.; Broeckx, S.Y.; Van Hecke, L.; Chiers, K.; Van Brantegem, L.; van Schie, H.; Beerts, C.; Spaas, J.H.; Pille, F.; Martens, A. The Evaluation of Equine Allogeneic Tenogenic Primed Mesenchymal Stem Cells in a Surgically Induced Superficial Digital Flexor Tendon Lesion Model. Front. Vet. Sci. 2021, 8, 641441. [Google Scholar] [CrossRef] [PubMed]
- Geburek, F.; Roggel, F.; van Schie, H.T.M.; Beineke, A.; Estrada, R.; Weber, K.; Hellige, M.; Rohn, K.; Jagodzinski, M.; Welke, B.; et al. Effect of single intralesional treatment of surgically induced equine superficial digital flexor tendon core lesions with adipose-derived mesenchymal stromal cells: A controlled experimental trial. Stem Cell Res. Ther. 2017, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Ahrberg, A.B.; Horstmeier, C.; Berner, D.; Brehm, W.; Gittel, C.; Hillmann, A.; Josten, C.; Rossi, G.; Schubert, S.; Winter, K.; et al. Effects of mesenchymal stromal cells versus serum on tendon healing in a controlled experimental trial in an equine model. BMC Musculoskelet. Disord. 2018, 19, 230. [Google Scholar] [CrossRef]
- Lui, P.P.Y.; Maffulli, N.; Rolf, C.; Smith, R.K.W. What are the validated animal models for tendinopathy? Scand. J. Med. Sci. Sports 2011, 21, 3–17. [Google Scholar] [CrossRef]
- Patterson-Kane, J.C.; Becker, D.L.; Rich, T. The pathogenesis of tendon microdamage in athletes: The horse as a natural model for basic cellular research. J. Comp. Pathol. 2012, 147, 227–247. [Google Scholar] [CrossRef]
- Meeremans, M.; Van de Walle, G.R.; Van Vlierberghe, S.; De Schauwer, C. The Lack of a Representative Tendinopathy Model Hampers Fundamental Mesenchymal Stem Cell Research. Front. Cell Dev. Biol. 2021, 9, 651164. [Google Scholar] [CrossRef]
- Luo, J.; Wang, Z.; Tang, C.; Yin, Z.; Huang, J.; Ruan, D.; Fei, Y.; Wang, C.; Mo, X.; Li, J.; et al. Animal model for tendinopathy. J. Orthop. Translat. 2023, 42, 43–56. [Google Scholar] [CrossRef]
- Smith, R.K.; Werling, N.J.; Dakin, S.G.; Alam, R.; Goodship, A.E.; Dudhia, J. Beneficial effects of autologous bone marrow-derived mesenchymal stem cells in naturally occurring tendinopathy. PLoS ONE 2013, 8, e75697. [Google Scholar] [CrossRef]
- Schubert, S.; Brehm, W.; Hillmann, A.; Burk, J. Serum-free human MSC medium supports consistency in human but not in equine adipose-derived multipotent mesenchymal stromal cell culture. Cytometry A 2018, 93, 60–72. [Google Scholar] [CrossRef]
- Paebst, F.; Piehler, D.; Brehm, W.; Heller, S.; Schroeck, C.; Tárnok, A.; Burk, J. Comparative immunophenotyping of equine multipotent mesenchymal stromal cells: An approach toward a standardized definition. Cytometry A 2014, 85, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Lang, H.M.; Schnabel, L.V.; Cassano, J.M.; Fortier, L.A. Effect of needle diameter on the viability of equine bone marrow derived mesenchymal stem cells. Vet. Surg. 2017, 46, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Raabe, O.; Addicks, K.; Wenisch, S.; Arnhold, S. Effects of non-steroidal anti-inflammatory drugs on proliferation, differentiation and migration in equine mesenchymal stem cells. Cell Biol. Int. 2011, 35, 235–248. [Google Scholar] [CrossRef]
- Smith, R.K.; McIlwraith, C.W. Consensus on equine tendon disease: Building on the 2007 Havemeyer symposium. Equine Vet J. 2012, 44, 2–6. [Google Scholar] [CrossRef]
- Geburek, F.; Lietzau, M.; Beineke, A.; Rohn, K.; Stadler, P.M. Effect of a single injection of autologous conditioned serum (ACS) on tendon healing in equine naturally occurring tendinopathies. Stem Cell Res. Ther. 2015, 6, 126. [Google Scholar] [CrossRef] [PubMed]
- Genovese, R.L.; Rantanen, N.W.; Hauser, M.L.; Simpson, B.S. Diagnostic ultrasonography of equine limbs. Vet. Clin. N. Am. Equine Pract. 1986, 2, 145–226. [Google Scholar] [CrossRef] [PubMed]
- Rantanen, N.W.; Jorgensen, J.S.; Genovese, R.L. Ultrasonographic evaluation of the equine limb: Technique. In Diagnosis and Management of Lameness in the Horse, 1st ed.; Ross, M.W., Dyson, S.J., Eds.; Elsevier: St. Louis, MO, USA, 2003; pp. 166–188. [Google Scholar]
- Conze, P.; van Schie, H.T.; van Weeren, R.; Staszyk, C.; Conrad, S.; Skutella, T.; Hopster, K.; Rohn, K.; Stadler, P.; Geburek, F. Effect of autologous adipose tissue-derived mesenchymal stem cells on neovascularization of artificial equine tendon lesions. Regen. Med. 2014, 9, 743–757. [Google Scholar] [CrossRef]
- Berner, D.; Brehm, W.; Gerlach, K.; Offhaus, J.; Scharner, D.; Burk, J. Variation in the MRI signal intensity of naturally occurring equine superficial digital flexor tendinopathies over a 12-month period. Vet. Rec. 2020, 187, e53. [Google Scholar] [CrossRef]
- Carrade, D.D.; Affolter, V.K.; Outerbridge, C.A.; Watson, J.L.; Galuppo, L.D.; Buerchler, S.; Kumar, V.; Walker, N.J.; Borjesson, D.L. Intradermal injections of equine allogeneic umbilical cord-derived mesenchymal stem cells are well tolerated and do not elicit immediate or delayed hypersensitivity reactions. Cytotherapy 2011, 13, 1180–1192. [Google Scholar] [CrossRef]
- Kol, A.; Wood, J.A.; Carrade Holt, D.D.; Gillette, J.A.; Bohannon-Worsley, L.K.; Puchalski, S.M.; Walker, N.J.; Clark, K.C.; Watson, J.L.; Borjesson, D.L. Multiple intravenous injections of allogeneic equine mesenchymal stem cells do not induce a systemic inflammatory response but do alter lymphocyte subsets in healthy horses. Stem Cell Res. Ther. 2015, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.B.; Co, C.; Koenig, J.B.; Tse, C.; Lindsay, E.; Koch, T.G. Response to Intravenous Allogeneic Equine Cord Blood-Derived Mesenchymal Stromal Cells Administered from Chilled or Frozen State in Serum and Protein-Free Media. Front. Vet. Sci. 2016, 3, 56. [Google Scholar] [CrossRef] [PubMed]
- Brandão, J.S.; Alvarenga, M.L.; Pfeifer, J.P.H.; Dos Santos, V.H.; Fonseca-Alves, C.E.; Rodrigues, M.; Laufer-Amorim, R.; Castillo, J.A.L.; Alves, A.L.G. Allogeneic mesenchymal stem cell transplantation in healthy equine superficial digital flexor tendon: A study of the local inflammatory response. Res. Vet. Sci. 2018, 118, 423–430. [Google Scholar] [CrossRef]
- Pezzanite, L.M.; Fortier, L.A.; Antczak, D.F.; Cassano, J.M.; Brosnahan, M.M.; Miller, D.; Schnabel, L.V. Equine allogeneic bone marrow-derived mesenchymal stromal cells elicit antibody responses in vivo. Stem Cell Res. Ther. 2015, 6, 54. [Google Scholar] [CrossRef]
- Owens, S.D.; Kol, A.; Walker, N.J.; Borjesson, D.L. Allogeneic Mesenchymal Stem Cell Treatment Induces Specific Alloantibodies in Horses. Stem Cells Int. 2016, 2016, 5830103. [Google Scholar] [CrossRef] [PubMed]
- Berglund, A.K.; Schnabel, L.V. Allogeneic major histocompatibility complex-mismatched equine bone marrow-derived mesenchymal stem cells are targeted for death by cytotoxic anti-major histocompatibility complex antibodies. Equine Vet. J. 2017, 49, 539–544. [Google Scholar] [CrossRef]
- Barrachina, L.; Cequier, A.; Romero, A.; Vitoria, A.; Zaragoza, P.; Vázquez, F.J.; Rodellar, C. Allo-antibody production after intraarticular administration of mesenchymal stem cells (MSCs) in an equine osteoarthritis model: Effect of repeated administration, MSC inflammatory stimulation, and equine leukocyte antigen (ELA) compatibility. Stem Cell Res. Ther. 2020, 11, 52. [Google Scholar] [CrossRef]
- Joswig, A.J.; Mitchell, A.; Cummings, K.J.; Levine, G.J.; Gregory, C.A.; Smith, R., 3rd; Watts, A.E. Repeated intra-articular injection of allogeneic mesenchymal stem cells causes an adverse response compared to autologous cells in the equine model. Stem Cell Res. Ther. 2017, 8, 42. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Wang, H.; Liu, X.; Zhang, T.; Wang, Y.; Hu, D. The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy. Stem Cell Res. Ther. 2015, 6, 234. [Google Scholar] [CrossRef]
- Shah, K.; Shah, N.; Ghassemi, F.; Ly, C.; George, T.; Lutz, C.; Sumer, H. Alloreactivity of Allogeneic Mesenchymal Stem/Stromal Cells and Other Cellular Therapies: A Concise Review. Stem Cells Int. 2022, 2022, 9589600. [Google Scholar] [CrossRef]
- Rowland, A.L.; Burns, M.E.; Levine, G.J.; Watts, A.E. Preparation Technique Affects Recipient Immune Targeting of Autologous Mesenchymal Stem Cells. Front. Vet. Sci. 2021, 8, 724041. [Google Scholar] [CrossRef] [PubMed]
- Naskou, M.C.; Sumner, S.M.; Chocallo, A.; Kemelmakher, H.; Thoresen, M.; Copland, I.; Galipeau, J.; Peroni, J.F. Platelet lysate as a novel serum-free media supplement for the culture of equine bone marrow-derived mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.P.; Tsuzuki, N.; Haneda, S.; Yamada, K.; Furuoka, H.; Tabata, Y.; Sasaki, N. Comparison of allogeneic platelet lysate and fetal bovine serum for in vitro expansion of equine bone marrow-derived mesenchymal stem cells. Res. Vet. Sci. 2013, 95, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.A.; Koch, T.G. Equine platelet lysate as an alternative to fetal bovine serum in equine mesenchymal stromal cell culture—Too much of a good thing? Equine Vet. J. 2016, 48, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Hendijani, F. Explant culture: An advantageous method for isolation of mesenchymal stem cells from human tissues. Cell Prolif. 2017, 50, e12334. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop Dj Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Geburek, F.; Mundle, K.; Conrad, S.; Hellige, M.; Walliser, U.; van Schie, H.T.; van Weeren, R.; Skutella, T.; Stadler, P.M. Tracking of autologous adipose tissue-derived mesenchymal stromal cells with in vivo magnetic resonance imaging and histology after intralesional treatment of artificial equine tendon lesions-a pilot study. Stem Cell Res. Ther. 2016, 7, 21. [Google Scholar] [CrossRef]
- Burk, J.; Berner, D.; Brehm, W.; Hillmann, A.; Horstmeier, C.; Josten, C.; Paebst, F.; Rossi, G.; Schubert, S.; Ahrberg, A.B. Long-Term Cell Tracking Following Local Injection of Mesenchymal Stromal Cells in the Equine Model of Induced Tendon Disease. Cell Transplant. 2016, 25, 2199–2211. [Google Scholar] [CrossRef]
- Espina, M.; Jülke, H.; Brehm, W.; Ribitsch, I.; Winter, K.; Delling, U. Evaluation of transport conditions for autologous bone marrow-derived mesenchymal stromal cells for therapeutic application in horses. PeerJ. 2016, 4, e1773. [Google Scholar] [CrossRef]
- Dyson, S.J. Medical management of superficial digital flexor tendonitis: A comparative study in 219 horses (1992–2000). Equine Vet. J. 2004, 36, 415–419. [Google Scholar] [CrossRef]
- Jiang, L.; Lu, J.; Chen, Y.; Lyu, K.; Long, L.; Wang, X.; Liu, T.; Li, S. Mesenchymal stem cells: An efficient cell therapy for tendon repair (Review). Int. J. Mol. Med. 2023, 52, 70. [Google Scholar] [CrossRef] [PubMed]
- Peroni, J.F.; Borjesson, D.L. Anti-inflammatory and immunomodulatory activities of stem cells. Vet. Clin. N. Am. Equine Pract. 2011, 27, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Song, S.U. Immunomodulatory properties of mesenchymal stem cells and their therapeutic applications. Arch. Pharm. Res. 2012, 35, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Kormpakis, I.; Havlioglu, N.; Linderman, S.W.; Sakiyama-Elbert, S.E.; Erickson, I.E.; Zarembinski, T.; Silva, M.J.; Gelberman, R.H.; Thomopoulos, S. The effect of mesenchymal stromal cell sheets on the inflammatory stage of flexor tendon healing. Stem Cell Res. Ther. 2016, 7, 144. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, Y.; Hsiao, A.W.; Xu, J.; Tong, W.; Chang, L.; Zhang, X.; Chen, Y.F.; Li, J.; Chen, W.; et al. Bioactive glass elicited stem cell-derived extracellular vesicles regulate M2 macrophage polarization and angiogenesis to improve tendon regeneration and functional recovery. Biomaterials 2023, 294, 121998. [Google Scholar] [CrossRef] [PubMed]
- Ohberg, L.; Alfredson, H. Ultrasound guided sclerosis of neovessels in painful chronic Achilles tendinosis: Pilot study of a new treatment. Br. J. Sports Med. 2002, 36, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Kristoffersen, M.; Ohberg, L.; Johnston, C.; Alfredson, H. Neovascularisation in chronic tendon injuries detected with colour Doppler ultrasound in horse and man: Implications for research and treatment. Knee Surg. Sports Traumatol. Arthrosc. 2005, 13, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Hatazoe, T.; Endo, Y.; Iwamoto, Y.; Korosue, K.; Kuroda, T.; Inoue, S.; Murata, D.; Hobo, S.; Misumi, K. A study of the distribution of color Doppler flows in the superficial digital flexor tendon of young Thoroughbreds during their training periods. J. Equine Sci. 2015, 26, 99–104. [Google Scholar] [CrossRef]
- Ceserani, V.; Ferri, A.; Berenzi, A.; Benetti, A.; Ciusani, E.; Pascucci, L.; Bazzucchi, C.; Coccè, V.; Bonomi, A.; Pessina, A.; et al. Angiogenic and anti-inflammatory properties of micro-fragmented fat tissue and its derived mesenchymal stromal cells. Vasc. Cell. 2016, 8, 3. [Google Scholar] [CrossRef]
- Siadat, S.M.; Zamboulis, D.E.; Thorpe, C.T.; Ruberti, J.W.; Connizzo, B.K. Tendon Extracellular Matrix Assembly, Maintenance and Dysregulation Throughout Life. Adv. Exp. Med. Biol. 2021, 1348, 45–103. [Google Scholar]
- Birch, H.L.; Thorpe, C.T.; Rumian, A.P. Specialisation of extracellular matrix for function in tendons and ligaments. Muscles Ligaments Tendons J. 2013, 3, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Hammer, N.; Huster, D.; Fritsch, S.; Hädrich, C.; Koch, H.; Schmidt, P.; Sichting, F.; Wagner, M.F.; Boldt, A. Do cells contribute to tendon and ligament biomechanics? PLoS ONE 2014, 9, e105037. [Google Scholar] [CrossRef] [PubMed]
- Ehrle, A.; Lilge, S.; Clegg, P.D.; Maddox, T.W. Equine flexor tendon imaging part 1: Recent developments in ultrasonography, with focus on the superficial digital flexor tendon. Vet. J. 2021, 278, 105764. [Google Scholar] [CrossRef] [PubMed]
- van Schie, H.T.; Bakker, E.M.; Jonker, A.M.; van Weeren, P.R. Computerized ultrasonographic tissue characterization of equine superficial digital flexor tendons by means of stability quantification of echo patterns in contiguous transverse ultrasonographic images. Am. J. Vet. Res. 2003, 64, 366–375. [Google Scholar] [CrossRef] [PubMed]
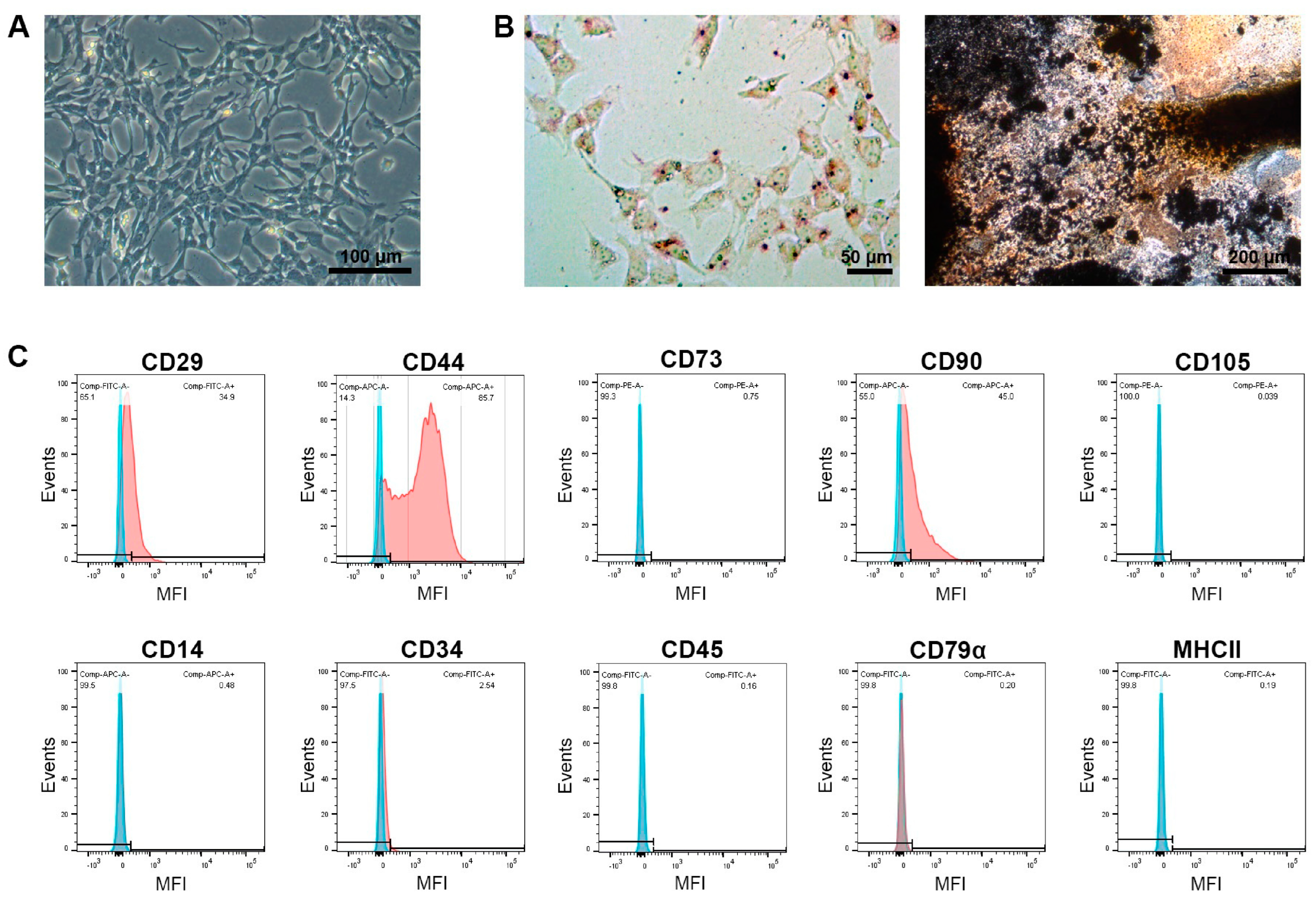

| Horse Number | Group | Age (Years) | Sex | Duration of Symptoms at First Presentation | Lesion Size (cm3) |
|---|---|---|---|---|---|
| A1 | Control | 7 | gelding | 1.5 weeks | 0.3 |
| A2 | Control | 17 | mare | 1.5 weeks | 1.7 |
| A3 | MSC | 12 | gelding | 1 week | 2.9 |
| A4 | MSC | 17 | gelding | 1.5 weeks | 1.4 |
| B1 | MSC | 16 | gelding | 5 weeks | 2.8 |
| B2 | Control | 16 | gelding | 8 weeks | 4.0 |
| B3 | MSC | 8 | mare | 9 weeks (+acute re-injury) | 1.0 |
| B4 | Control | 21 | mare | 2.5 weeks | 0.5 |
| B5 | MSC | 7 | mare | 5 weeks | 0.9 |
| B6 | Control | 7 | mare | 2.5 weeks | 1.5 |
| B7 | Control | 19 | gelding | 3.5 weeks | 7.0 |
| B8 | MSC | 15 | mare | 4 weeks | 7.7 |
| B9 | Control | 13 | gelding | 2.5 weeks | 0.5 |
| B10 | MSC | 9 | gelding | 2.5 weeks | 3.0 |
| Summary | |||||
| Median (Mean) | MSC | 12 (12) | 4 geldings, 3 mares | 4 weeks (4 weeks) | 2.8 (2.8) |
| Median (Mean) | Control | 16 (14) | 4 geldings, 3 mares | 2.5 weeks (3 weeks) | 1.5 (2.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burk, J.; Wittenberg-Voges, L.; Schubert, S.; Horstmeier, C.; Brehm, W.; Geburek, F. Treatment of Naturally Occurring Tendon Disease with Allogeneic Multipotent Mesenchymal Stromal Cells: A Randomized, Controlled, Triple-Blinded Pilot Study in Horses. Cells 2023, 12, 2513. https://doi.org/10.3390/cells12212513
Burk J, Wittenberg-Voges L, Schubert S, Horstmeier C, Brehm W, Geburek F. Treatment of Naturally Occurring Tendon Disease with Allogeneic Multipotent Mesenchymal Stromal Cells: A Randomized, Controlled, Triple-Blinded Pilot Study in Horses. Cells. 2023; 12(21):2513. https://doi.org/10.3390/cells12212513
Chicago/Turabian StyleBurk, Janina, Liza Wittenberg-Voges, Susanna Schubert, Carolin Horstmeier, Walter Brehm, and Florian Geburek. 2023. "Treatment of Naturally Occurring Tendon Disease with Allogeneic Multipotent Mesenchymal Stromal Cells: A Randomized, Controlled, Triple-Blinded Pilot Study in Horses" Cells 12, no. 21: 2513. https://doi.org/10.3390/cells12212513
APA StyleBurk, J., Wittenberg-Voges, L., Schubert, S., Horstmeier, C., Brehm, W., & Geburek, F. (2023). Treatment of Naturally Occurring Tendon Disease with Allogeneic Multipotent Mesenchymal Stromal Cells: A Randomized, Controlled, Triple-Blinded Pilot Study in Horses. Cells, 12(21), 2513. https://doi.org/10.3390/cells12212513





