Detrimental Effects of ApoE ε4 on Blood–Brain Barrier Integrity and Their Potential Implications on the Pathogenesis of Alzheimer’s Disease
Abstract
:1. Introduction
2. Blood–Brain Barrier and ApoE—Structure and Function
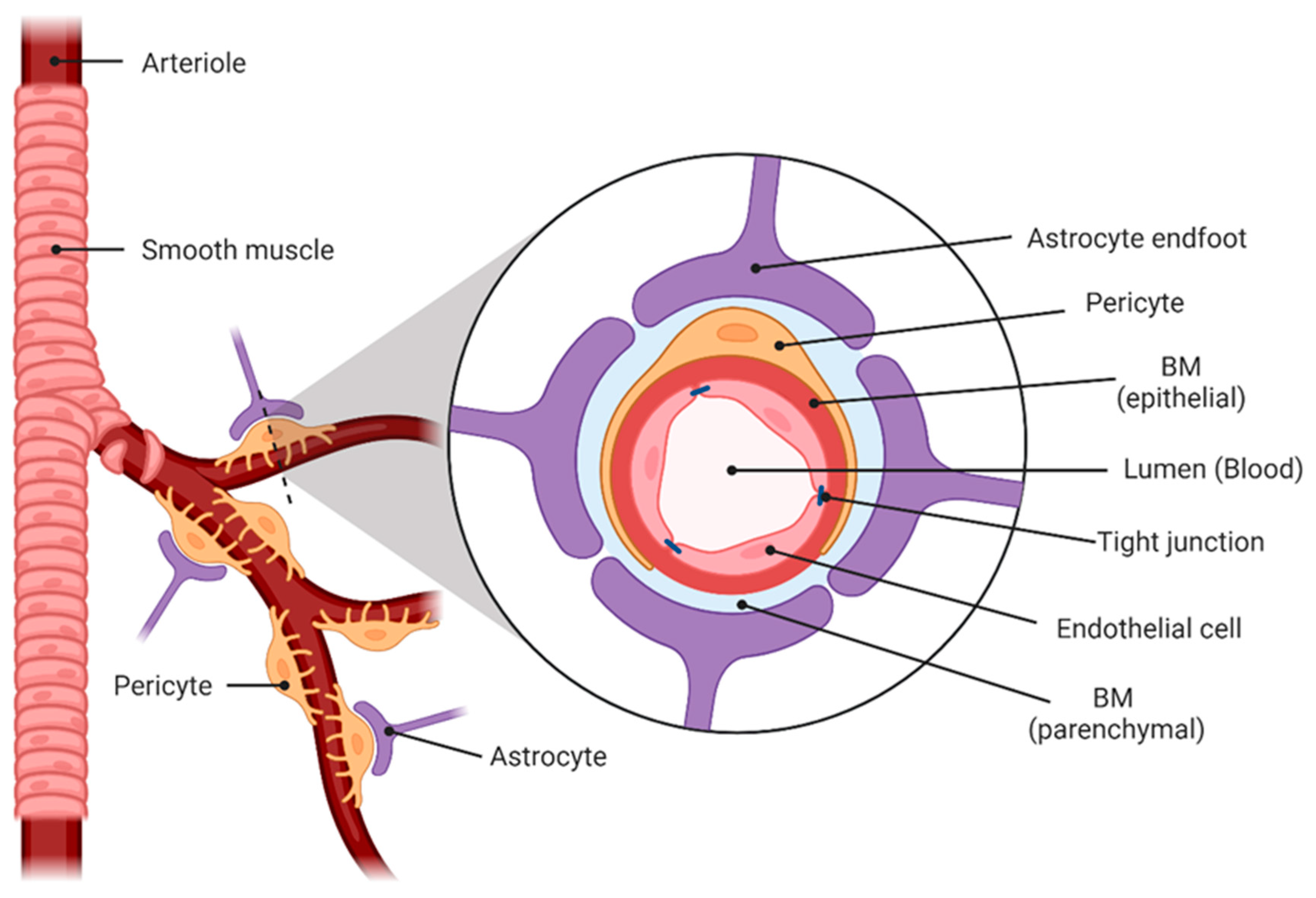
2.1. Blood–Brain Barrier
2.2. Apolipoprotein E (ApoE)
3. The Impact of ApoE on the Blood–Brain Barrier
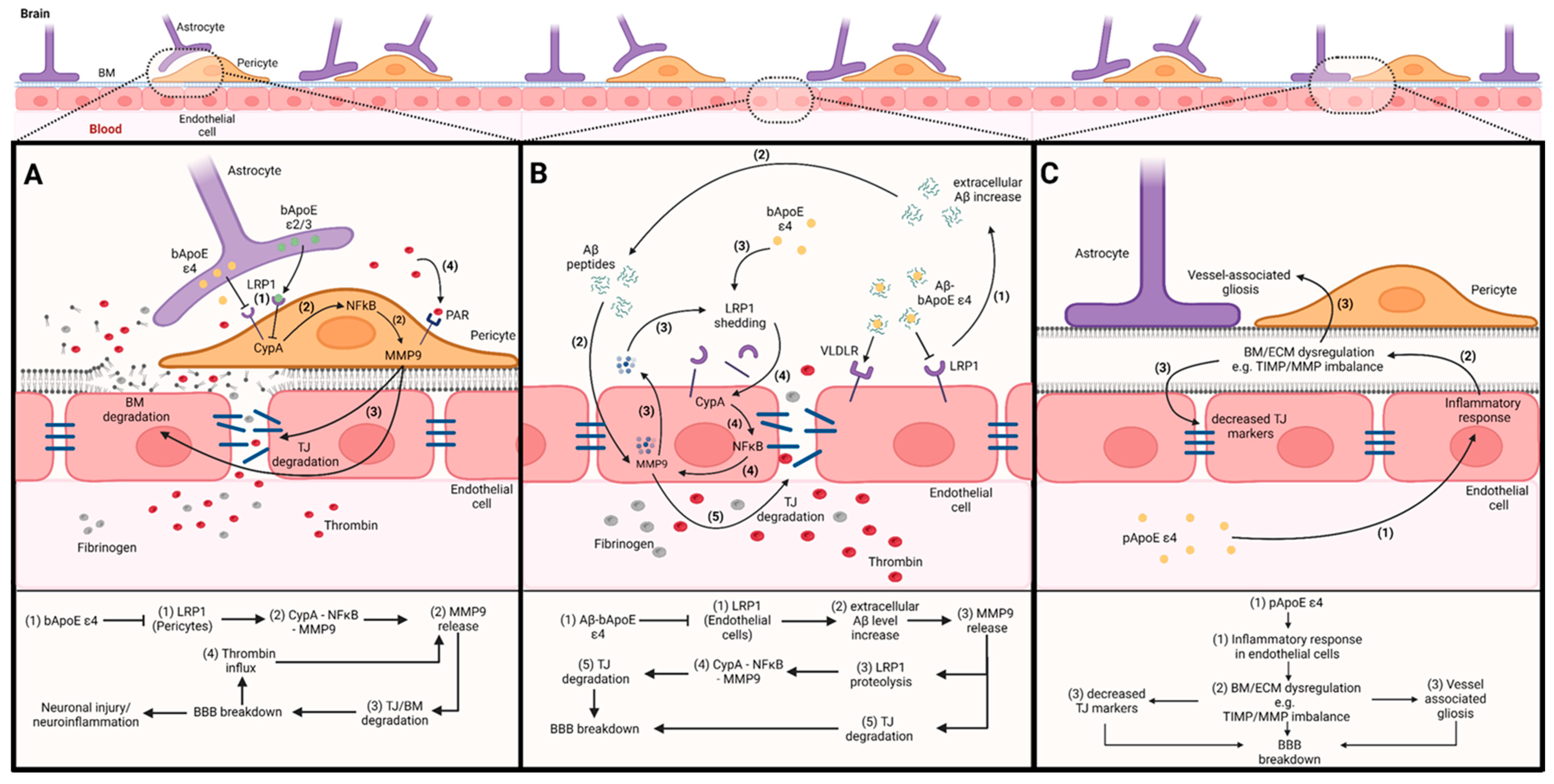
3.1. The Impact of bApoE on BBB Integrity via Pericytes
3.2. The Impact of bApoE on BBB Integrity via Endothelial Cells
3.3. The Impact of pApoE on BBB Integrity
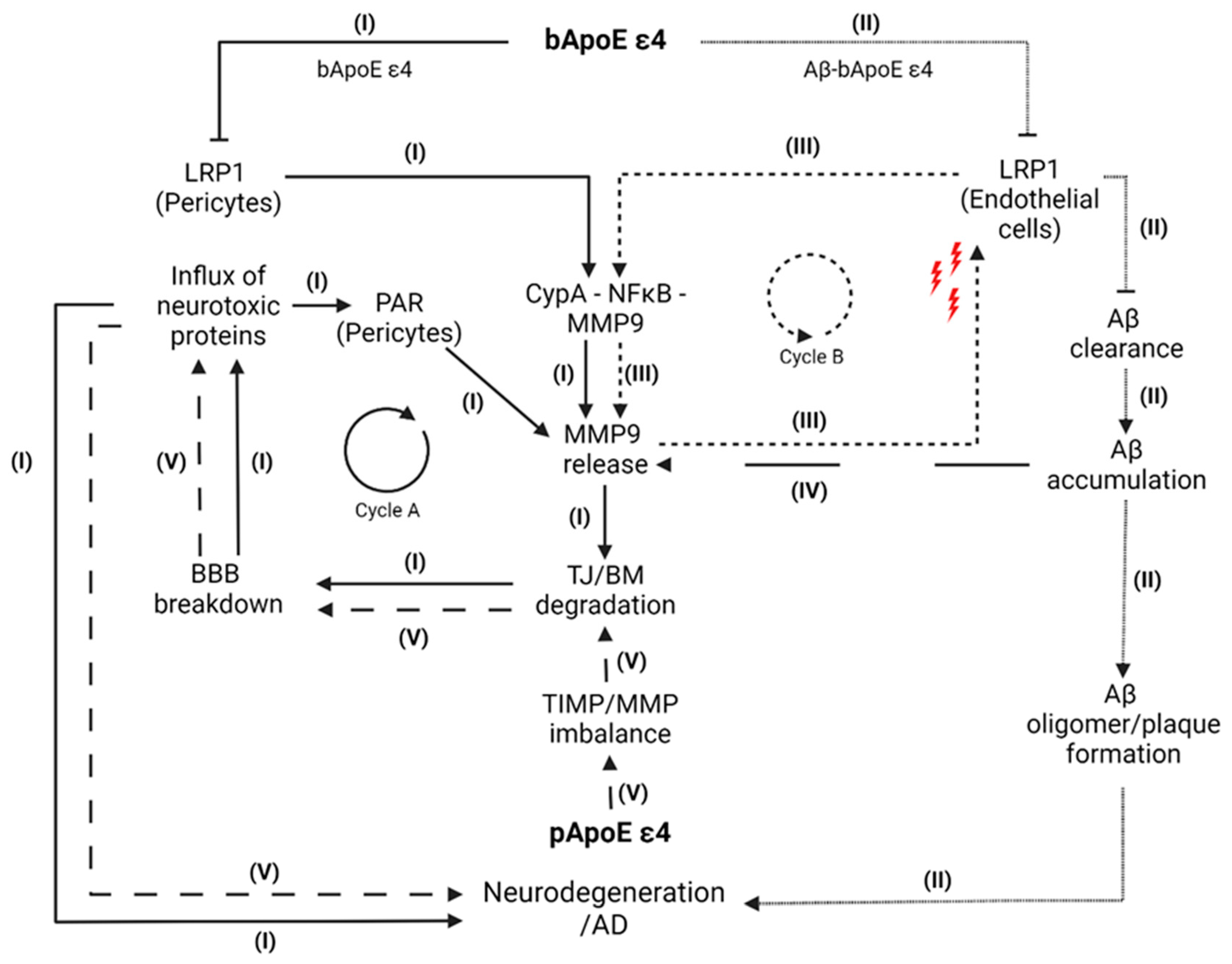
4. Pathway Model
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Passeri, E.; Elkhoury, K.; Morsink, M.; Broersen, K.; Linder, M.; Tamayol, A.; Malaplate, C.; Yen, F.T.; Arab-Tehrany, E. Alzheimer’s Disease: Treatment Strategies and Their Limitations. Int. J. Mol. Sci. 2022, 23, 13954. [Google Scholar] [CrossRef] [PubMed]
- Skaria, A.P. The economic and societal burden of Alzheimer disease: Managed care considerations. Am. J. Manag. Care 2022, 28, S188–S196. [Google Scholar] [CrossRef]
- Sengupta, U.; Nilson, A.N.; Kayed, R. The Role of Amyloid-β Oligomers in Toxicity, Propagation, and Immunotherapy. EBioMedicine 2016, 6, 42–49. [Google Scholar] [CrossRef]
- WHO. World Report on Ageing and Health; World Health Organization: Geneva, Switzerland, 2015; ISBN 9789240694811. [Google Scholar]
- Alzheimers Association. 2021 Alzheimer’s disease facts and figures. Alzheimers. Dement. 2021, 17, 327–406. [Google Scholar] [CrossRef] [PubMed]
- Kunkle, B.W.; Grenier-Boley, B.; Sims, R.; Bis, J.C.; Damotte, V.; Naj, A.C.; Boland, A.; Vronskaya, M.; van der Lee, S.J.; Amlie-Wolf, A.; et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat. Genet. 2019, 51, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V. Cerebrovascular effects of apolipoprotein E: Implications for Alzheimer disease. JAMA Neurol. 2013, 70, 440–444. [Google Scholar] [CrossRef]
- Ratan, Y.; Rajput, A.; Maleysm, S.; Pareek, A.; Jain, V.; Pareek, A.; Kaur, R.; Singh, G. An Insight into Cellular and Molecular Mechanisms Underlying the Pathogenesis of Neurodegeneration in Alzheimer’s Disease. Biomedicines 2023, 11, 1398. [Google Scholar] [CrossRef]
- Huang, Y.-W.A.; Zhou, B.; Wernig, M.; Südhof, T.C. ApoE2, ApoE3, and ApoE4 Differentially Stimulate APP Transcription and Aβ Secretion. Cell 2017, 168, 427–441.e21. [Google Scholar] [CrossRef]
- Montagne, A.; Nikolakopoulou, A.M.; Huuskonen, M.T.; Sagare, A.P.; Lawson, E.J.; Lazic, D.; Rege, S.V.; Grond, A.; Zuniga, E.; Barnes, S.R.; et al. APOE4 accelerates advanced-stage vascular and neurodegenerative disorder in old Alzheimer’s mice via cyclophilin A independently of amyloid-β. Nat. Aging 2021, 1, 506–520. [Google Scholar] [CrossRef]
- Bell, R.D.; Winkler, E.A.; Singh, I.; Sagare, A.P.; Deane, R.; Wu, Z.; Holtzman, D.M.; Betsholtz, C.; Armulik, A.; Sallstrom, J.; et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 2012, 485, 512–516. [Google Scholar] [CrossRef]
- Halliday, M.R.; Rege, S.V.; Ma, Q.; Zhao, Z.; Miller, C.A.; Winkler, E.A.; Zlokovic, B.V. Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J. Cereb. Blood Flow Metab. 2016, 36, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Montagne, A.; Nation, D.A.; Sagare, A.P.; Barisano, G.; Sweeney, M.D.; Chakhoyan, A.; Pachicano, M.; Joe, E.; Nelson, A.R.; D’Orazio, L.M.; et al. APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline. Nature 2020, 581, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Moon, W.-J.; Lim, C.; Ha, I.H.; Kim, Y.; Moon, Y.; Kim, H.-J.; Han, S.-H. Hippocampal blood-brain barrier permeability is related to the APOE4 mutation status of elderly individuals without dementia. J. Cereb. Blood Flow Metab. 2021, 41, 1351–1361. [Google Scholar] [CrossRef]
- Alata, W.; Ye, Y.; St-Amour, I.; Vandal, M.; and Calon, F. Human Apolipoprotein E ε4 Expression Impairs Cerebral Vascularization and Blood—Brain Barrier Function in Mice. J. Cereb. Blood Flow Metab. 2015, 35, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-L.; Zhang, C.-L.; Qiu, Y.-M.; Chen, A.-Q.; Li, Y.-N.; Hu, B. Dysfunction of the Blood-brain Barrier in Cerebral Microbleeds: From Bedside to Bench. Aging Dis. 2021, 12, 1898–1919. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Fan, Y.; Zhang, F.; Lin, Y. Cerebral microinfarct is emergency consequence of Alzheimer’s disease: A new insight into development of neurodegenerative diseases. Int. J. Biol. Sci. 2022, 18, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.J.; Meltzer, J.C.; Nguyen, H.; Commins, C.; Bennett, R.E.; Hudry, E.; Hyman, B.T. APOE4 derived from astrocytes leads to blood-brain barrier impairment. Brain 2022, 145, 3582–3593. [Google Scholar] [CrossRef]
- Alonso-Alonso, M.L.; Sampedro-Viana, A.; Fernández-Rodicio, S.; Bazarra-Barreiros, M.; Ouro, A.; Sobrino, T.; Campos, F.; Castillo, J.; Hervella, P.; Iglesias-Rey, R. Need for a Paradigm Shift in the Treatment of Ischemic Stroke: The Blood-Brain Barrier. Int. J. Mol. Sci. 2022, 23, 9486. [Google Scholar] [CrossRef]
- Xu, L.; Nirwane, A.; Yao, Y. Basement membrane and blood-brain barrier. Stroke Vasc. Neurol. 2019, 4, 78–82. [Google Scholar] [CrossRef]
- Huffels, C.F.M.; Middeldorp, J.; Hol, E.M. Aß Pathology and Neuron-Glia Interactions: A Synaptocentric View. Neurochem. Res. 2023, 48, 1026–1046. [Google Scholar] [CrossRef]
- Nakada-Honda, N.; Cui, D.; Matsuda, S.; Ikeda, E. Intravenous injection of cyclophilin A realizes the transient and reversible opening of barrier of neural vasculature through basigin in endothelial cells. Sci. Rep. 2021, 11, 19391. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-C.; Zhao, J.; Fu, Y.; Inoue, Y.; Ren, Y.; Chen, Y.; Doss, S.V.; Shue, F.; Jeevaratnam, S.; Bastea, L.; et al. Peripheral apoE4 enhances Alzheimer’s pathology and impairs cognition by compromising cerebrovascular function. Nat. Neurosci. 2022, 25, 1020–1033. [Google Scholar] [CrossRef] [PubMed]
- Ghebranious, N.; Ivacic, L.; Mallum, J.; Dokken, C. Detection of ApoE E2, E3 and E4 alleles using MALDI-TOF mass spectrometry and the homogeneous mass-extend technology. Nucleic Acids Res. 2005, 33, e149. [Google Scholar] [CrossRef] [PubMed]
- El Haj, M.; Antoine, P.; Amouyel, P.; Lambert, J.-C.; Pasquier, F.; Kapogiannis, D. Apolipoprotein E (APOE) ε4 and episodic memory decline in Alzheimer’s disease: A review. Ageing Res. Rev. 2016, 27, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Kepp, K.P.; Robakis, N.K.; Høilund-Carlsen, P.F.; Sensi, S.L.; Vissel, B. The amyloid cascade hypothesis: An updated critical review. Brain 2023. [CrossRef]
- Hashimoto, T.; Serrano-Pozo, A.; Hori, Y.; Adams, K.W.; Takeda, S.; Banerji, A.O.; Mitani, A.; Joyner, D.; Thyssen, D.H.; Bacskai, B.J.; et al. Apolipoprotein E, especially apolipoprotein E4, increases the oligomerization of amyloid β peptide. J. Neurosci. 2012, 32, 15181–15192. [Google Scholar] [CrossRef]
- Fontana, I.C.; Zimmer, A.R.; Rocha, A.S.; Gosmann, G.; Souza, D.O.; Lourenco, M.V.; Ferreira, S.T.; Zimmer, E.R. Amyloid-β oligomers in cellular models of Alzheimer’s disease. J. Neurochem. 2020, 155, 348–369. [Google Scholar] [CrossRef]
- Shackleton, B.; Ringland, C.; Abdullah, L.; Mullan, M.; Crawford, F.; Bachmeier, C. Influence of Matrix Metallopeptidase 9 on Beta-Amyloid Elimination Across the Blood-Brain Barrier. Mol. Neurobiol. 2019, 56, 8296–8305. [Google Scholar] [CrossRef]
- Rhea, E.M.; Banks, W.A. Interactions of Lipids, Lipoproteins, and Apolipoproteins with the Blood-Brain Barrier. Pharm. Res. 2021, 38, 1469–1475. [Google Scholar] [CrossRef]
- Nikolakopoulou, A.M.; Wang, Y.; Ma, Q.; Sagare, A.P.; Montagne, A.; Huuskonen, M.T.; Rege, S.V.; Kisler, K.; Dai, Z.; Körbelin, J.; et al. Endothelial LRP1 protects against neurodegeneration by blocking cyclophilin A. J. Exp. Med. 2021, 218, e20202207. [Google Scholar] [CrossRef]
- Ma, Q.; Zhao, Z.; Sagare, A.P.; Wu, Y.; Wang, M.; Owens, N.C.; Verghese, P.B.; Herz, J.; Holtzman, D.M.; Zlokovic, B.V. Blood-brain barrier-associated pericytes internalize and clear aggregated amyloid-β42 by LRP1-dependent apolipoprotein E isoform-specific mechanism. Mol. Neurodegener. 2018, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Machida, T.; Takata, F.; Matsumoto, J.; Takenoshita, H.; Kimura, I.; Yamauchi, A.; Dohgu, S.; Kataoka, Y. Brain pericytes are the most thrombin-sensitive matrix metalloproteinase-9-releasing cell type constituting the blood-brain barrier in vitro. Neurosci. Lett. 2015, 599, 109–114. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Shinohara, M.; Shinohara, M.; Yamazaki, A.; Murray, M.E.; Liesinger, A.M.; Heckman, M.G.; Lesser, E.R.; Parisi, J.E.; Petersen, R.C.; et al. Selective loss of cortical endothelial tight junction proteins during Alzheimer’s disease progression. Brain 2019, 142, 1077–1092. [Google Scholar] [CrossRef] [PubMed]
- Mendiola, A.S.; Yan, Z.; Dixit, K.; Johnson, J.R.; Bouhaddou, M.; Meyer-Franke, A.; Shin, M.-G.; Yong, Y.; Agrawal, A.; MacDonald, E.; et al. Defining blood-induced microglia functions in neurodegeneration through multiomic profiling. Nat. Immunol. 2023, 24, 1173–1187. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Kassiri, Z. Biology of Tissue Inhibitor of Metalloproteinase 3 (TIMP3), and Its Therapeutic Implications in Cardiovascular Pathology. Front. Physiol. 2020, 11, 661. [Google Scholar] [CrossRef]
- Jäkel, L.; Kuiperij, H.B.; Gerding, L.P.; Custers, E.E.M.; van den Berg, E.; Jolink, W.M.T.; Schreuder, F.H.B.M.; Küsters, B.; Klijn, C.J.M.; Verbeek, M.M. Disturbed balance in the expression of MMP9 and TIMP3 in cerebral amyloid angiopathy-related intracerebral haemorrhage. Acta Neuropathol. Commun. 2020, 8, 99. [Google Scholar] [CrossRef]
- Hoe, H.-S.; Cooper, M.J.; Burns, M.P.; Lewis, P.A.; van der Brug, M.; Chakraborty, G.; Cartagena, C.M.; Pak, D.T.S.; Cookson, M.R.; Rebeck, G.W. The metalloprotease inhibitor TIMP-3 regulates amyloid precursor protein and apolipoprotein E receptor proteolysis. J. Neurosci. 2007, 27, 10895–10905. [Google Scholar] [CrossRef]
- Park, J.H.; Cho, S.-J.; Jo, C.; Park, M.H.; Han, C.; Kim, E.-J.; Huh, G.Y.; Koh, Y.H. Altered TIMP-3 Levels in the Cerebrospinal Fluid and Plasma of Patients with Alzheimer’s Disease. J. Pers. Med. 2022, 12, 827. [Google Scholar] [CrossRef]
- Merlini, M.; Rafalski, V.A.; Rios Coronado, P.E.; Gill, T.M.; Ellisman, M.; Muthukumar, G.; Subramanian, K.S.; Ryu, J.K.; Syme, C.A.; Davalos, D.; et al. Fibrinogen Induces Microglia-Mediated Spine Elimination and Cognitive Impairment in an Alzheimer’s Disease Model. Neuron 2019, 101, 1099–1108.e6. [Google Scholar] [CrossRef]
- Shinohara, M.; Tachibana, M.; Kanekiyo, T.; Bu, G. Role of LRP1 in the pathogenesis of Alzheimer’s disease: Evidence from clinical and preclinical studies. J. Lipid Res. 2017, 58, 1267–1281. [Google Scholar] [CrossRef]
- Iannucci, J.; Grammas, P. Thrombin, a Key Driver of Pathological Inflammation in the Brain. Cells 2023, 12, 1222. [Google Scholar] [CrossRef]
- Rehiman, S.H.; Lim, S.M.; Lim, F.T.; Chin, A.-V.; Tan, M.P.; Kamaruzzaman, S.B.; Ramasamy, K.; Abdul Majeed, A.B. Fibrinogen isoforms as potential blood-based biomarkers of Alzheimer’s disease using a proteomics approach. Int. J. Neurosci. 2022, 132, 1014–1025. [Google Scholar] [CrossRef]
- Lazarov, O.; Hollands, C. Hippocampal neurogenesis: Learning to remember. Prog. Neurobiol. 2016, 138–140, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Régy, M.; Dugravot, A.; Sabia, S.; Fayosse, A.; Mangin, J.-F.; Chupin, M.; Fischer, C.; Bouteloup, V.; Dufouil, C.; Chêne, G.; et al. Association of APOE ε4 with cerebral gray matter volumes in non-demented older adults: The MEMENTO cohort study. Neuroimage 2022, 250, 118966. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, K.; Garvert, L.; Wittfeld, K.; Ameling, S.; Bülow, R.; Meyer Zu Schwabedissen, H.; Nauck, M.; Völzke, H.; Grabe, H.J.; van der Auwera, S. Deciphering the Effect of Different Genetic Variants on Hippocampal Subfield Volumes in the General Population. Int. J. Mol. Sci. 2023, 24, 1120. [Google Scholar] [CrossRef] [PubMed]
- Flowers, S.A.; Rebeck, G.W. APOE in the normal brain. Neurobiol. Dis. 2020, 136, 104724. [Google Scholar] [CrossRef]
- Spieth, L.; Berghoff, S.A.; Stumpf, S.K.; Winchenbach, J.; Michaelis, T.; Watanabe, T.; Gerndt, N.; Düking, T.; Hofer, S.; Ruhwedel, T.; et al. Anesthesia triggers drug delivery to experimental glioma in mice by hijacking caveolar transport. Neurooncol. Adv. 2021, 3, vdab140. [Google Scholar] [CrossRef]
- Acharya, N.K.; Goldwaser, E.L.; Forsberg, M.M.; Godsey, G.A.; Johnson, C.A.; Sarkar, A.; DeMarshall, C.; Kosciuk, M.C.; Dash, J.M.; Hale, C.P.; et al. Sevoflurane and Isoflurane induce structural changes in brain vascular endothelial cells and increase blood-brain barrier permeability: Possible link to postoperative delirium and cognitive decline. Brain Res. 2015, 1620, 29–41. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirchner, K.; Garvert, L.; Kühn, L.; Bonk, S.; Grabe, H.J.; Van der Auwera, S. Detrimental Effects of ApoE ε4 on Blood–Brain Barrier Integrity and Their Potential Implications on the Pathogenesis of Alzheimer’s Disease. Cells 2023, 12, 2512. https://doi.org/10.3390/cells12212512
Kirchner K, Garvert L, Kühn L, Bonk S, Grabe HJ, Van der Auwera S. Detrimental Effects of ApoE ε4 on Blood–Brain Barrier Integrity and Their Potential Implications on the Pathogenesis of Alzheimer’s Disease. Cells. 2023; 12(21):2512. https://doi.org/10.3390/cells12212512
Chicago/Turabian StyleKirchner, Kevin, Linda Garvert, Luise Kühn, Sarah Bonk, Hans Jörgen Grabe, and Sandra Van der Auwera. 2023. "Detrimental Effects of ApoE ε4 on Blood–Brain Barrier Integrity and Their Potential Implications on the Pathogenesis of Alzheimer’s Disease" Cells 12, no. 21: 2512. https://doi.org/10.3390/cells12212512
APA StyleKirchner, K., Garvert, L., Kühn, L., Bonk, S., Grabe, H. J., & Van der Auwera, S. (2023). Detrimental Effects of ApoE ε4 on Blood–Brain Barrier Integrity and Their Potential Implications on the Pathogenesis of Alzheimer’s Disease. Cells, 12(21), 2512. https://doi.org/10.3390/cells12212512





