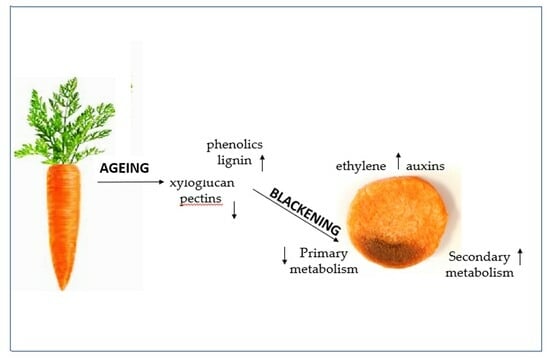
Restraining Quiescence Release-Related Ageing in Plant Cells: A Case Study in Carrot
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Sample Preparation
2.2. Cell Wall Analyses
2.3. Immunolabeling and Fluorescence Imaging and Processing
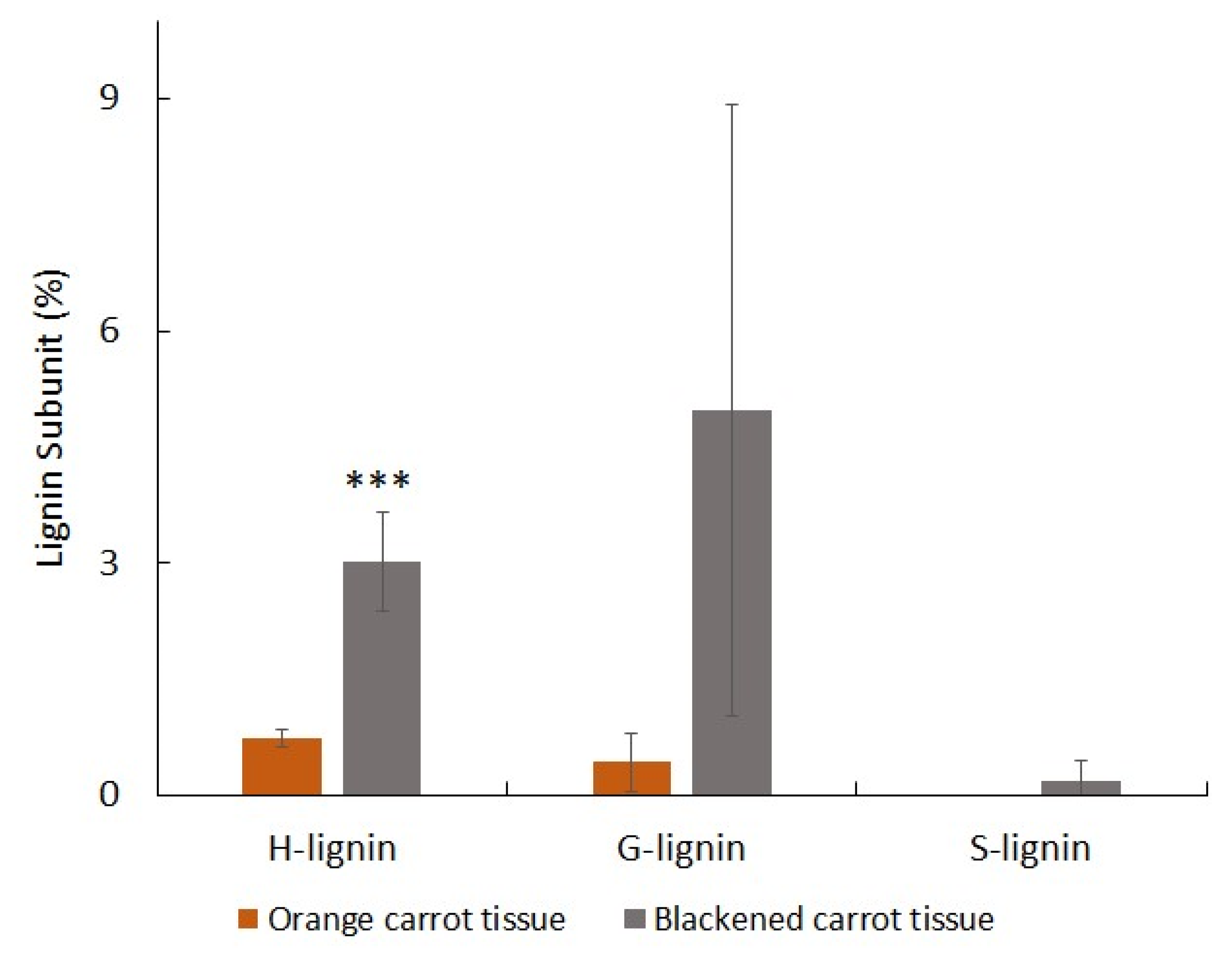
2.4. Measurement of Lignin
2.5. Non-Cellulosic Carbohydrate Content
2.6. Lignin Pyrolysis-GC-MS
2.7. Metabolite Profiling
2.8. RNA Sequencing (RNA-seq)
2.9. RNA-Seq Data Analysis
3. Results
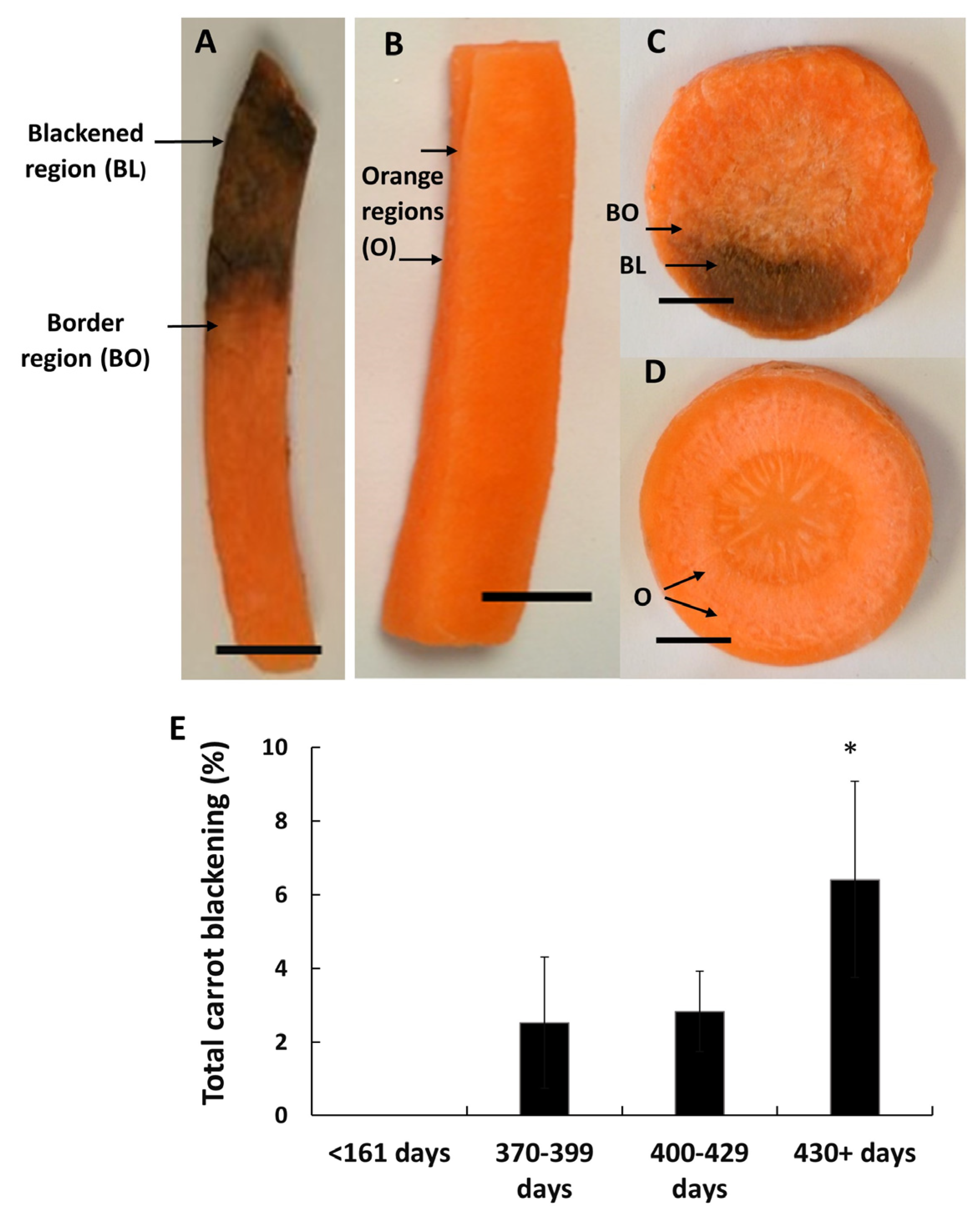
3.1. Susceptibility to Blackening Increases with the Time of Storage Prior to Harvest
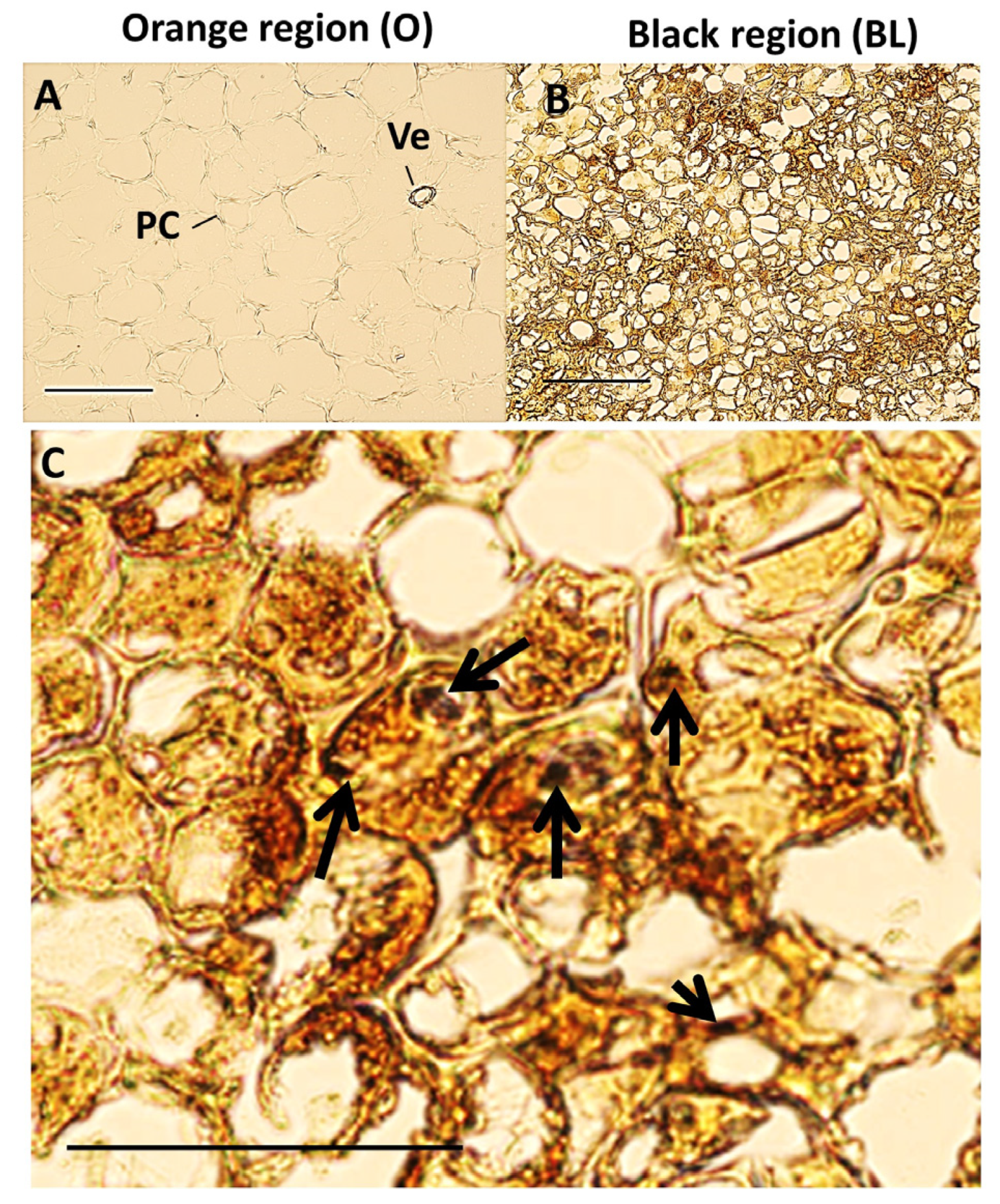
3.2. Black Region Cells Are Distinct from Orange Region Cells under Bright Field Microscopy
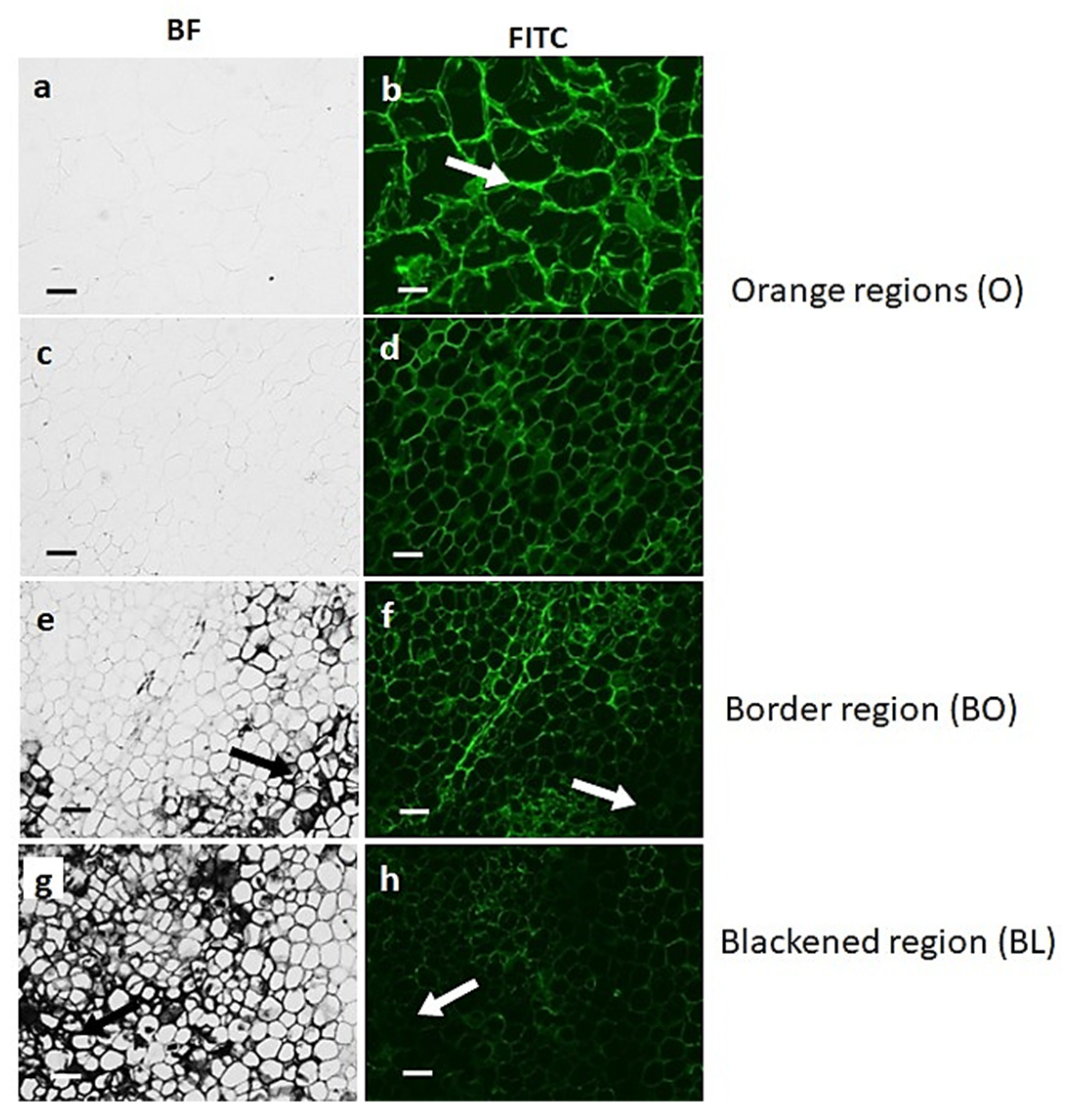
3.3. Orange and Black Tissues of Carrot Batons Have Differences in Cell Wall Polysaccharides
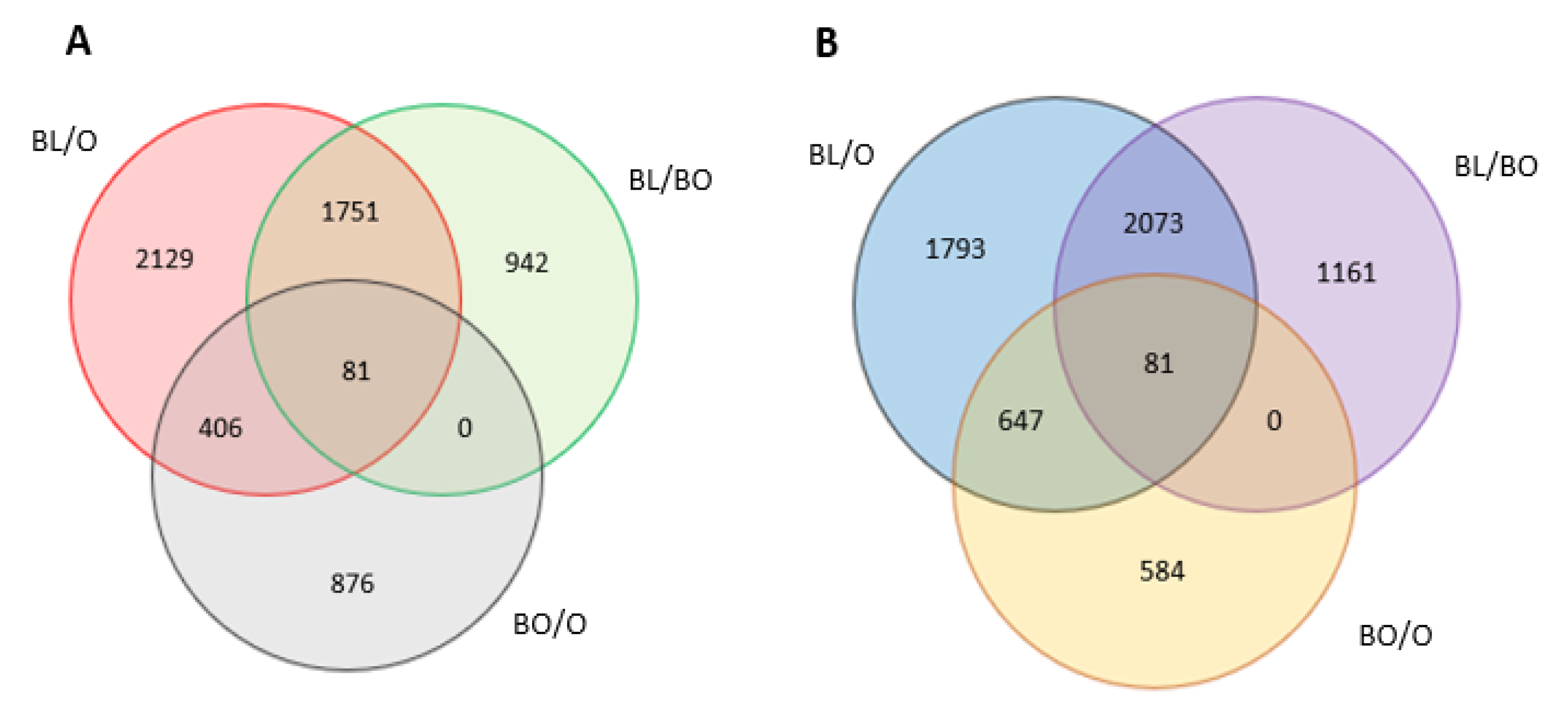
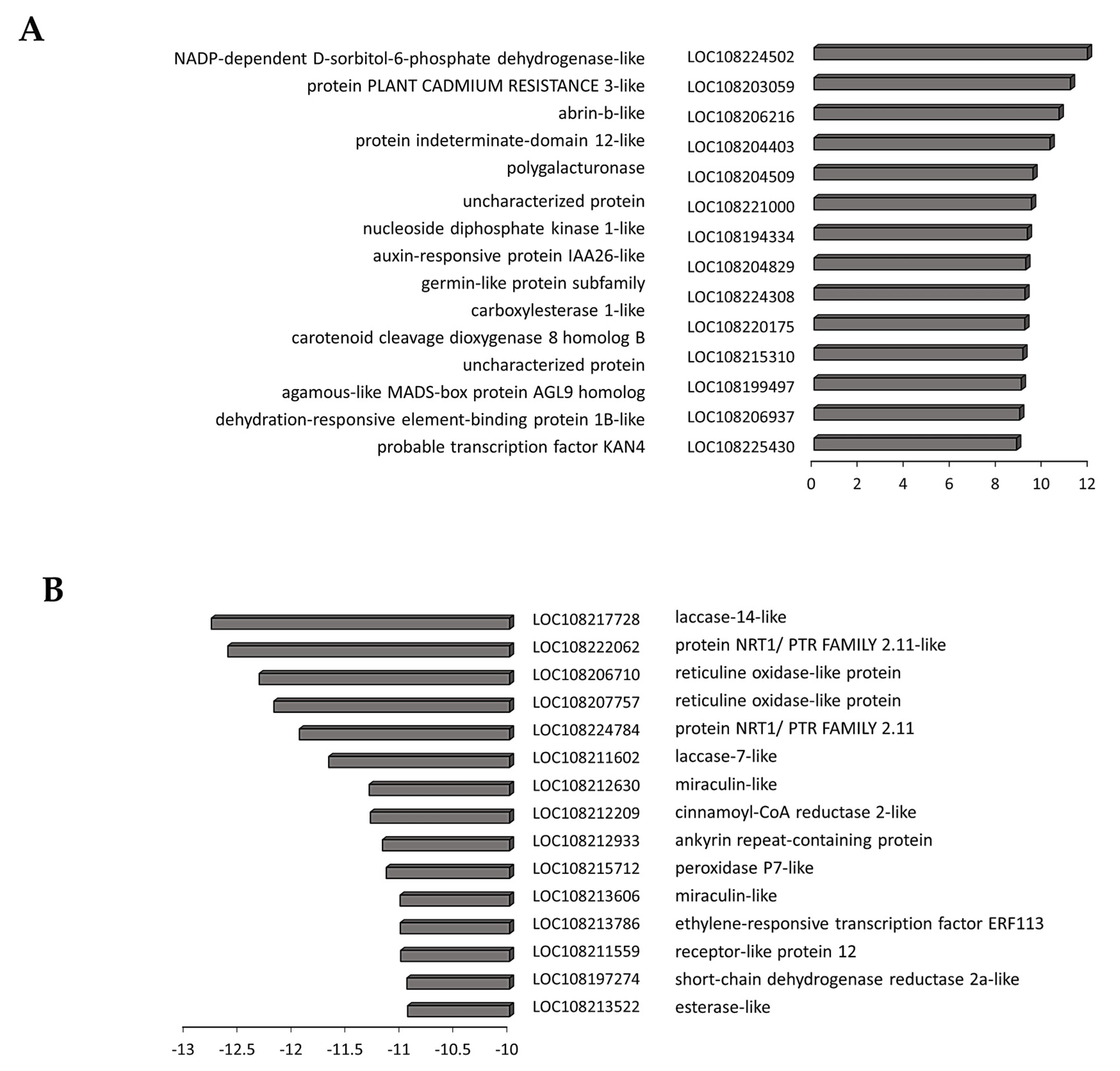
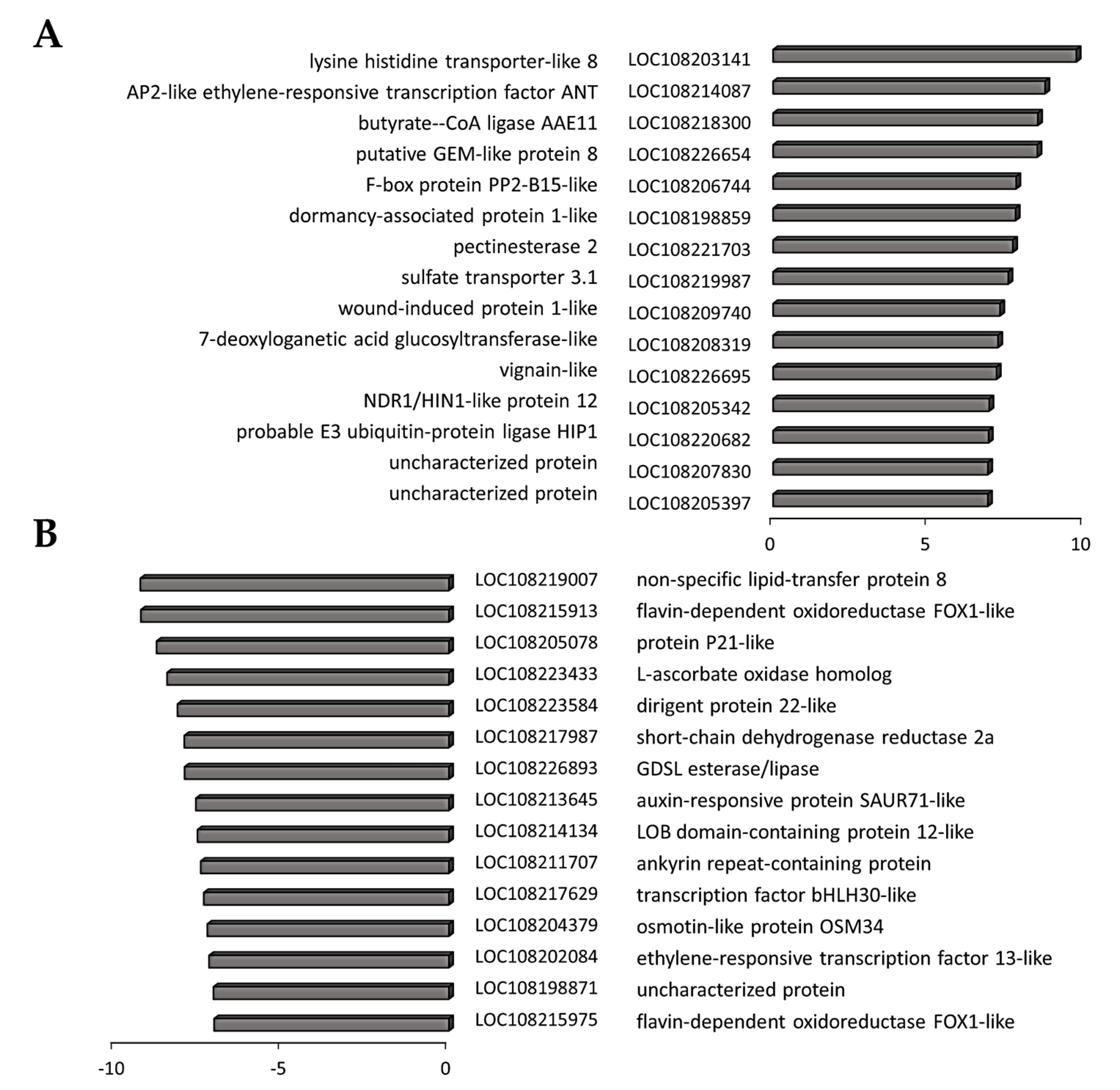
3.4. Transcriptome Reprogramming Occurs in Black Regions
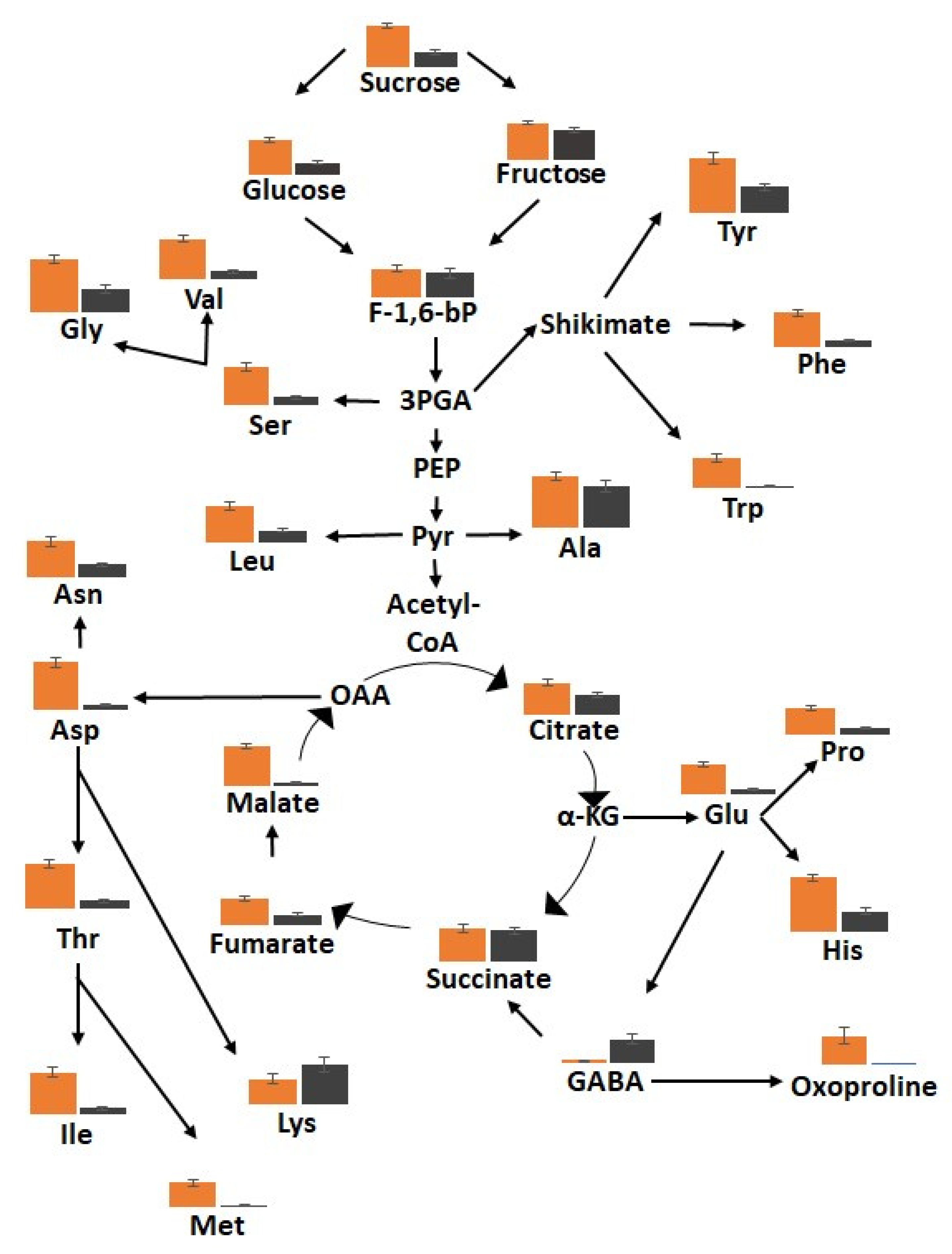
3.5. The Metabolic Profiles Are Significantly Changed in Orange and Black Carrot Regions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tulik, M.; Jura-Morawiec, J.; Bieniasz, A.; Marciszewska, K. How long do wood parenchyma cells live in the stem of a Scots Pine (Pinus sylvestris L.)? Studies on cell nuclei status along the radial and longitudinal stem axes. Forests 2019, 10, 977. [Google Scholar] [CrossRef]
- Zhou, Y.; Tian, Y.; Yang, B. Root vegetable side streams as sources of functional ingredients for food, nutraceutical and pharmaceutical applications: The current status and future prospects. Trends Food Sci. Technol. 2023, 137, 1–16. [Google Scholar] [CrossRef]
- Queiroz, C.; Lopes, M.L.M.; Fialho, E.; Valente-Mesquita, V.L. Polyphenol oxidase: Characteristics and mechanisms of browning control. Food Rev. Int. 2008, 24, 361–375. [Google Scholar] [CrossRef]
- Moon, K.M.; Kwon, E.-B.; Lee, B.; Kim, C.Y. Recent trends in controlling the enzymatic browning of fruit and vegetable products. Molecules 2020, 25, 2754. [Google Scholar] [CrossRef] [PubMed]
- Glagoleva, A.Y.; Shoeva, O.Y.; Khlestkina, E.K. Melanin pigment in plants: Current knowledge and future perspectives. Front. Plant Sci. 2020, 11, 770. [Google Scholar] [CrossRef]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Bou Daher, F.; Braybrook, S.A. How to let go: Pectin and plant cell adhesion. Front. Plant Sci. 2015, 6, 523. [Google Scholar]
- Fry, S.C. Ripening. In Encyclopedia of Applied Plant Sciences; Thomas, B., Murray, B.G., Murphy, D.J., Eds.; Academic Press: Waltham, MA, USA, 2017; Volume 1, pp. 323–334. [Google Scholar]
- Anderson, C.T. We be jammin’: An update on pectin biosynthesis, trafficking and dynamics. J. Exp. Bot. 2016, 67, 495–502. [Google Scholar] [CrossRef]
- Fries, M.; Ihrig, J.; Brocklehurst, K.; Shevchik, V.E.; Pickersgill, R.W. Molecular basis of the activity of the phytopathogen pectin methylesterase. EMBO J. 2007, 26, 3879–3887. [Google Scholar] [CrossRef]
- Zheng, L.; Xu, Y.; Li, Q.; Zhu, B. Pectinolytic lyases: A comprehensive review of sources, category, property, structure, and catalytic mechanism of pectate lyases and pectin lyases. Bioresour. Bioprocess. 2021, 8, 79. [Google Scholar] [CrossRef]
- Goulao, L.F.; Santos, J.; de Sousa, I.; Oliveira, C.M. Patterns of enzymatic activity of cell wall-modifying enzymes during the growth and ripening of apples. Postharvest Biol. Technol. 2007, 43, 307–318. [Google Scholar] [CrossRef]
- De Lorenzo, G.; Ferrari, S.; Giovannoni, M.; Mattei, B.; Cervone, F. Cell wall traits that influence plant development, immunity, and bioconversion. Plant J. 2019, 97, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Barros, J.; Serk, H.; Granlund, I.; Pesquet, E. The cell biology of lignification in higher plants. Ann. Bot. 2015, 115, 1053–1074. [Google Scholar] [CrossRef] [PubMed]
- Chezem, W.R.; Memon, A.; Li, F.-S.; Weng, J.-K.; Clay, N.K. SG2-Type R2R3-MYB transcription factor MYB15 controls defense-induced lignification and basal immunity in Arabidopsis. Plant Cell 2017, 29, 1907–1926. [Google Scholar] [CrossRef]
- Degenhardt, B.; Gimmler, H. Cell wall adaptations to multiple environmental stresses in maize roots. J. Exp. Bot. 2000, 51, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Denness, L.; McKenna, J.F.; Segonzac, C.; Wormit, A.; Madhou, P.; Bennett, M.; Mansfield, J.; Zipfel, C.; Hamann, T. Cell wall damage-induced lignin biosynthesis is regulated by a reactive oxygen species- and jasmonic acid-dependent process in Arabidopsis. Plant Physiol. 2011, 156, 1364–1374. [Google Scholar] [CrossRef] [PubMed]
- Mellidou, I.; Buts, K.; Hatoum, D.; Ho, Q.T.; Johnston, J.W.; Watkins, C.B.; Schaffer, R.J.; Gapper, N.E.; Giovannoni, J.J.; Rudell, D.R.; et al. Transcriptomic events associated with internal browning of apple during postharvest storage. BMC Plant Biol. 2014, 14, 328. [Google Scholar] [CrossRef]
- Pedersen, H.L.; Fangel, J.U.; McCleary, B.; Ruzanski, C.; Rydahl, M.G.; Ralet, M.-C.; Farkas, V.; von Schantz, L.; Marcus, S.E.; Andersen, M.C.F.; et al. Versatile high-resolution oligosaccharide microarrays for plant glycobiology and cell wall research. J. Biol. Chem. 2012, 287, 39429–39438. [Google Scholar] [CrossRef]
- Andersen, M.C.F.; Boos, I.; Marcus, S.E.; Kračun, S.K.; Rydahl, M.G.; Willats, W.G.T.; Knox, J.P.; Clausen, M.H. Characterization of the LM5 pectic galactan epitope with synthetic analogues of β-1,4-d-galactotetraose. Carbohydr. Res. 2016, 436, 36–40. [Google Scholar] [CrossRef]
- Cornuault, V.; Buffetto, F.; Marcus, S.E.; Crépeau, M.-J.; Guillon, F.; Ralet, M.-C.; Knox, J.P. LM6-M: A high avidity rat monoclonal antibody to pectic α-1,5-L-arabinan. BioRxiv 2017, 161604. [Google Scholar] [CrossRef]
- Verhertbruggen, Y.; Marcus, S.E.; Haeger, A.; Ordaz-Ortiz, J.J.; Knox, J.P. An extended set of monoclonal antibodies to pectic homogalacturonan. Carbohydr. Res. Pectin Struct. Funct. 2009, 344, 1858–1862. [Google Scholar] [CrossRef]
- Hatfield, R.D.; Grabber, J.; Ralph, J.; Brei, K. Using the acetyl bromide assay to determine lignin concentrations in herbaceous plants: Some cautionary notes. J. Agric. Food Chem. 1999, 47, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Kärkönen, A.; Tapanila, T.; Laakso, T.; Seppänen, M.; Isolahti, M.; Hyrkäs, M.; Virkajärvi, P.; Saranpää, P. Effect of lignin content and subunit composition on digestibility in clones of timothy (Phleum pratense L.). J. Agric. Food Chem. 2014, 62, 6091–6099. [Google Scholar] [CrossRef] [PubMed]
- Sundberg, A.; Sundberg, K.; Lillandt, C.; Holmhom, B. Determination of hemicelluloses and pectins in wood and pulp fibres by acid methanolysis and gas chromatography. Nord. Pulp Pap. Res. J. 1996, 11, 216–219. [Google Scholar]
- Viljamaa, S.; Dikareva, E.; Tolonen, J.; Edesi, J.; Nickolov, K.; Laitinen, T.; Laakso, T.; Korpinen, R.; Saranpää, P.; Jokipii-Lukkari, S.; et al. Cryopreservation of the Norway spruce tissue culture line able to produce extracellular lignin. Plant Cell Tissue Organ Cult. 2018, 133, 225–235. [Google Scholar] [CrossRef]
- Chang, S.; Puryear, J.; Cairney, J. A simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Rep. 1993, 11, 113–116. [Google Scholar] [CrossRef]
- Iorizzo, M.; Ellison, S.; Senalik, D.; Zeng, P.; Satapoomin, P.; Huang, J.; Bowman, M.; Iovene, M.; Sanseverino, W.; Cavagnaro, P.; et al. A high-quality carrot genome assembly provides new insights into carotenoid accumulation and asterid genome evolution. Nat. Gen. 2016, 48, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Machaj, G.; Grzebelus, D. Characteristics of the AT-Hook motif containing nuclear localized (AHL) genes in carrot provides insight into their role in plant growth and storage root development. Genes 2021, 12, 764. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Okonechnikov, K.; Conesa, A.; García-Alcalde, F. Qualimap 2: Advanced multi-sample quality control for high-throughput sequencing data. Bioinformatics 2016, 32, 292–294. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. The R package Rsubread is easier, faster, cheaper and better for alignment and quantification of RNA sequencing reads. Nucleic Acids Res. 2019, 47, e47. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.-H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Schulz, K.E. The Biology of the Blackening Phenomenon in Cut Carrots. Ph.D Thesis, The University of Leeds, Leeds, UK, 2021. [Google Scholar]
- Miryeganeh, M. Senescence: The compromised time of death that plants may call on themselves. Genes 2021, 12, 143. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, J.; Trierweiler, B.; Bunzel, M. Maturation-related changes of carrot lignins: Maturation-related changes of carrot lignins. J. Sci. Food Agric. 2018, 98, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Caldana, C.; Degenkolbe, T.; Cuadros-Inostroza, A.; Klie, S.; Sulpice, R.; Leisse, A.; Steinhauser, D.; Fernie, A.R.; Willmitzer, L.; Hannah, M.A. High-density kinetic analysis of the metabolomic and transcriptomic response of Arabidopsis to eight environmental conditions. Plant J. 2011, 67, 869–884. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Tomás-Barberán, F.A.; Saltveit, M.E. Wound-induced phenolic accumulation and browning in lettuce (Lactuca sativa L.) leaf tissue is reduced by exposure to n-alcohols. Postharvest Biol. Technol. 2005, 37, 47–55. [Google Scholar] [CrossRef]
- Xu, Q.; Truong, T.T.; Barrero, J.M.; Jacobsen, J.V.; Hocart, C.H.; Gubler, F. A role for jasmonates in the release of dormancy by cold stratification in wheat. J. Exp. Bot. 2016, 67, 3497–3508. [Google Scholar] [CrossRef]
- Rolling, W.R.; Senalik, D.; Iorizzo, M.; Ellison, S.; Van Deynze, A.; Simon, P.W. CarrotOmics: A genetics and comparative genomics database for carrot (Daucus carota). Database 2022, 2022, baac079. [Google Scholar] [CrossRef]
- Pérez-Alonso, M.M.; Ortiz-García, P.; Moya-Cuevas, J.; Lehmann, T.; Sánchez-Parra, B.; Björk, R.G.; Karim, S.; Amirjani, M.R.; Aronsson, H.; Wilkinson, M.D.; et al. Endogenous indole-3-acetamide levels contribute to the crosstalk between auxin and abscisic acid, and trigger plant stress responses in Arabidopsis. J. Exp. Bot. 2020, 72, 459–475. [Google Scholar] [CrossRef]
- Shin, Y.; Chane, A.; Jung, M.; Lee, Y. Recent advances in understanding the roles of pectin as an active participant in plant signaling networks. Plants 2021, 10, 1712. [Google Scholar] [CrossRef] [PubMed]
- Bethke, G.; Thao, A.; Xiong, G.; Li, B.; Soltis, N.E.; Hatsugai, N.; Hillmer, R.A.; Katagiri, F.; Kliebenstein, D.J.; Pauly, M.; et al. Pectin biosynthesis is critical for cell wall integrity and immunity in Arabidopsis thaliana. Plant Cell 2016, 28, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, M.; Pontiggia, D.; Raggi, S.; Cheng, Z.; Scaloni, F.; Ferrari, S.; Ausubel, F.M.; Cervone, F.; De Lorenzo, G. Plant immunity triggered by engineered in vivo release of oligogalacturonides, damage-associated molecular patterns. Proc. Natl. Acad. Sci. USA 2015, 112, 5533–5538. [Google Scholar] [CrossRef] [PubMed]
- Gramegna, G.; Modesti, V.; Savatin, D.V.; Sicilia, F.; Cervone, F.; De Lorenzo, G. GRP-3 and KAPP, encoding interactors of WAK1, negatively affect defense responses induced by oligogalacturonides and local response to wounding. J. Exp. Bot. 2016, 67, 1715–1729. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, T.; Tao, N.; Yan, T.; Wang, Q.; Li, Q. Nitric oxide is essential to keep the postharvest quality of fruits and vegetables. Horticulturae 2023, 9, 135. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulz, K.; Machaj, G.; Knox, P.; Hancock, R.D.; Verrall, S.R.; Korpinen, R.; Saranpää, P.; Kärkönen, A.; Karpinska, B.; Foyer, C.H. Restraining Quiescence Release-Related Ageing in Plant Cells: A Case Study in Carrot. Cells 2023, 12, 2465. https://doi.org/10.3390/cells12202465
Schulz K, Machaj G, Knox P, Hancock RD, Verrall SR, Korpinen R, Saranpää P, Kärkönen A, Karpinska B, Foyer CH. Restraining Quiescence Release-Related Ageing in Plant Cells: A Case Study in Carrot. Cells. 2023; 12(20):2465. https://doi.org/10.3390/cells12202465
Chicago/Turabian StyleSchulz, Katie, Gabriela Machaj, Paul Knox, Robert D. Hancock, Susan R. Verrall, Risto Korpinen, Pekka Saranpää, Anna Kärkönen, Barbara Karpinska, and Christine H. Foyer. 2023. "Restraining Quiescence Release-Related Ageing in Plant Cells: A Case Study in Carrot" Cells 12, no. 20: 2465. https://doi.org/10.3390/cells12202465
APA StyleSchulz, K., Machaj, G., Knox, P., Hancock, R. D., Verrall, S. R., Korpinen, R., Saranpää, P., Kärkönen, A., Karpinska, B., & Foyer, C. H. (2023). Restraining Quiescence Release-Related Ageing in Plant Cells: A Case Study in Carrot. Cells, 12(20), 2465. https://doi.org/10.3390/cells12202465