The G Protein-Coupled Estrogen Receptor (GPER): A Critical Therapeutic Target for Cancer
Abstract
1. Introduction
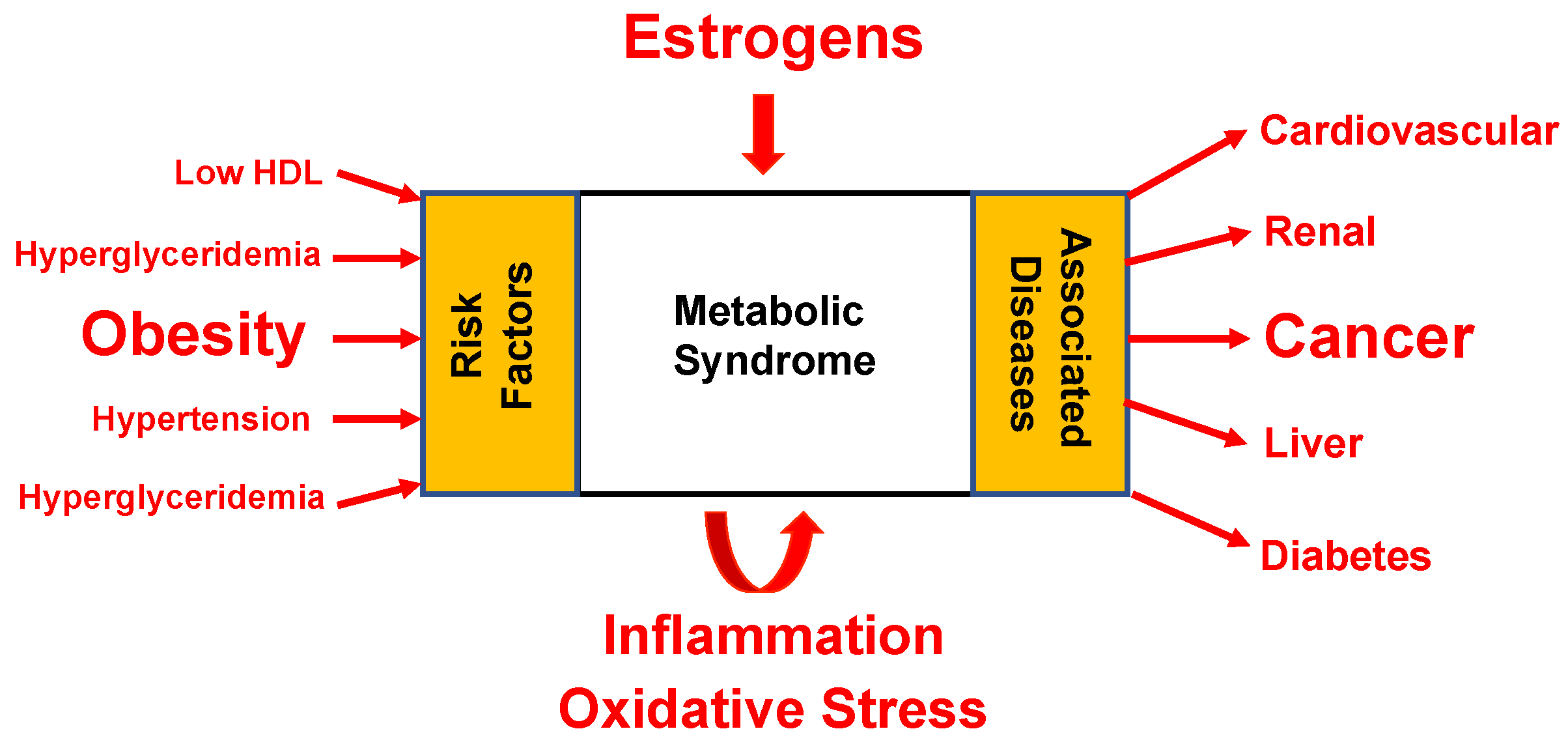
2. Estrogens and Their Implications in Carcinogenesis
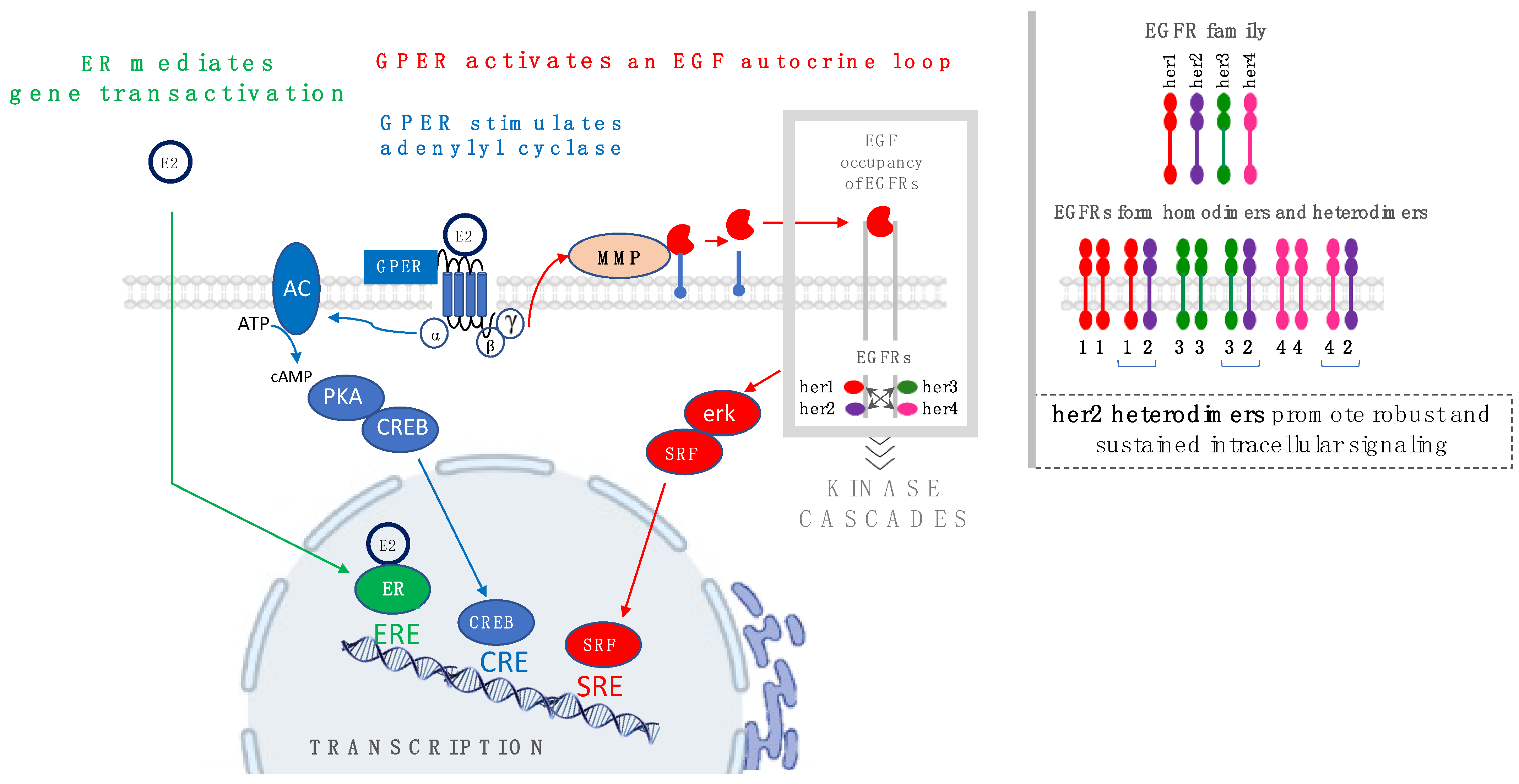
3. Estrogen Promotes Biochemical Actions That Are Manifested through Two Distinct Receptor Mechanisms
4. Promotion of Carcinogenesis via Interplay between Estrogens and Estrogen Receptors, ER and GPER
5. Human Exposure to Environmental Estrogens and Implications for Estrogen-Induced Carcinogenesis in the Context of Multiple Cancer-Involved Estrogen Receptors
6. GPER as a Therapeutic Target in Metabolic Disease and Cancer
7. GPER-Targeted Drugs for Metaboregulation and Cancer
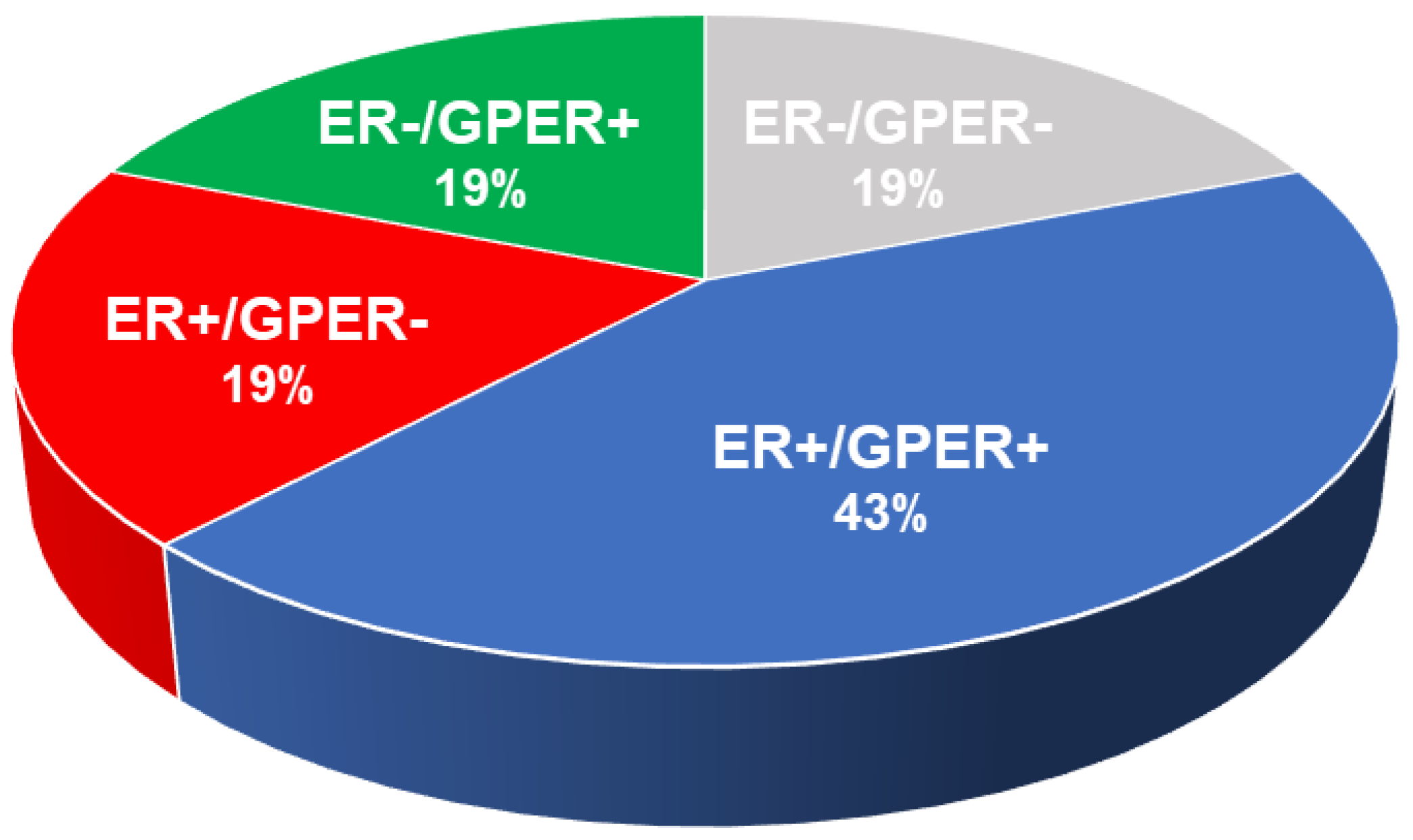
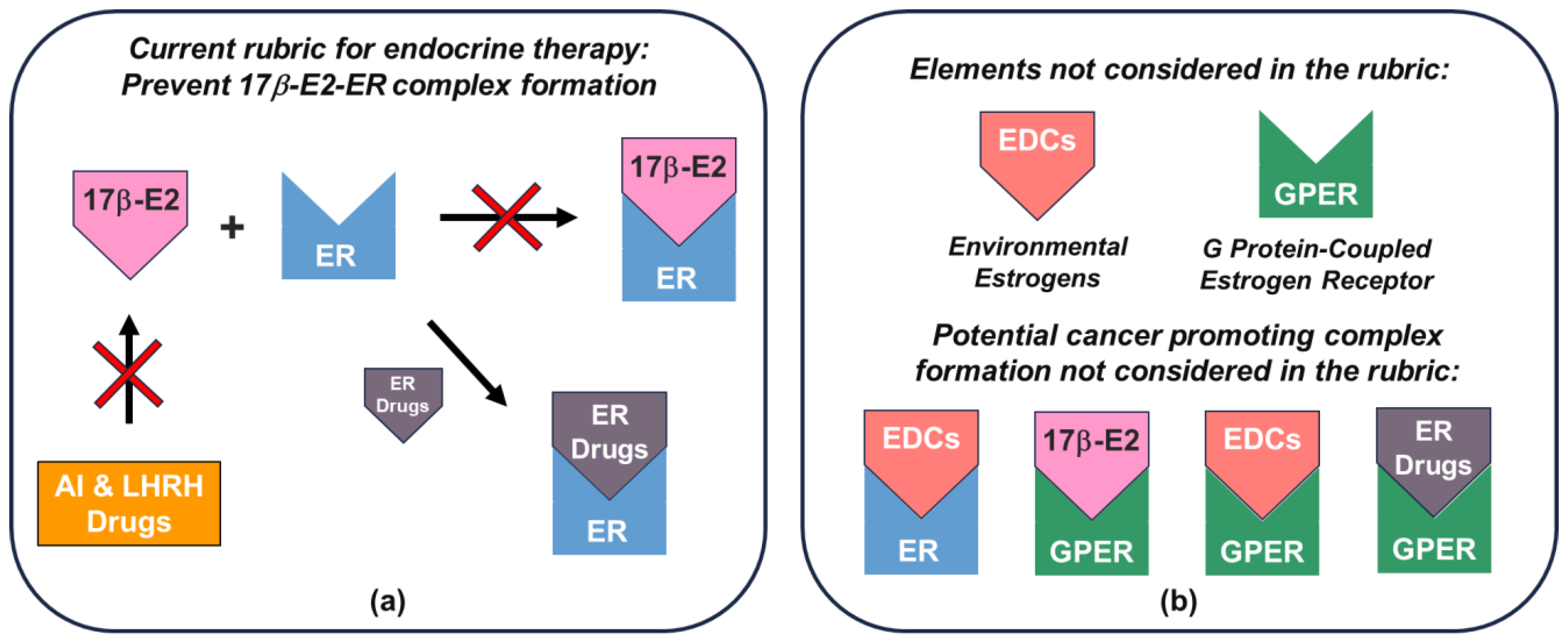
8. Rationale for Assignment of Endocrine Therapy for Breast Cancer
9. Evaluation of FDA-Approved Endocrine Therapies for the Treatment of Breast Cancer
9.1. Aromatase Inhibitors (AIs)
9.2. Luteinizing Hormone-Releasing Hormone (LHRH) Analogs
9.3. Selective Estrogen Receptor Modulators (SERMs)
9.4. Selective Estrogen Receptor Degraders (SERDs)
10. Assessment of Approved Endocrine Therapies in View of the Current Rubric and the Effect of Elements Not Considered
11. New Approaches to Targeted Protein Degradation and Future Considerations
12. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, P.; Pang, Y.; Filardo, E.J.; Dong, J. Identity of an Estrogen Membrane Receptor Coupled to a G Protein in Human Breast Cancer Cells. Endocrinology 2005, 146, 624–632. [Google Scholar] [CrossRef]
- Revankar, C.M.; Cimino, D.F.; Sklar, L.A.; Arterburn, J.B.; Prossnitz, E.R. A Transmembrane Intracellular Estrogen Receptor Mediates Rapid Cell Signaling. Science (1979) 2005, 307, 1625–1630. [Google Scholar] [CrossRef]
- Filardo, E.J.; Quinn, J.A.; Bland, K.I.; Frackelton, J. Estrogen-Induced Activation of Erk-1 and Erk-2 Requires the G Protein-Coupled Receptor Homolog, GPR30, and Occurs via Trans-Activation of the Epidermal Growth Factor Receptor through Release of HB-EGF. Mol. Endocrinol. 2000, 14, 1649–1660. [Google Scholar] [CrossRef]
- Filardo, E.J.; Quinn, J.A.; Raymond Frackelton, A.; Bland, K.I. Estrogen Action via the G Protein-Coupled Receptor, GPR30: Stimulation of Adenylyl Cyclase and CAMP-Mediated Attenuation of the Epidermal Growth Factor Receptor-to-MAPK Signaling Axis. Mol. Endocrinol. 2002, 16, 70–84. [Google Scholar] [CrossRef]
- Maggiolini, M.; Vivacqua, A.; Fasanella, G.; Recchia, A.G.; Sisci, D.; Pezzi, V.; Montanaro, D.; Musti, A.M.; Picard, D.; Andò, S. The G Protein-Coupled Receptor GPR30 Mediates c-Fos up-Regulation by 17β-Estradiol and Phytoestrogens in Breast Cancer Cells. J. Biol. Chem. 2004, 279, 27008–27016. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Arterburn, J.B. International Union of Basic and Clinical Pharmacology. XCVII. G Protein–Coupled Estrogen Receptor and Its Pharmacologic Modulators. Pharmacol. Rev. 2015, 67, 505–540. [Google Scholar] [CrossRef]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Abancens, M.; Bustos, V.; Harvey, H.; McBryan, J.; Harvey, B.J. Sexual Dimorphism in Colon Cancer. Front. Oncol. 2020, 10, 607909. [Google Scholar] [CrossRef]
- White, A.; Ironmonger, L.; Steele, R.J.C.; Ormiston-Smith, N.; Crawford, C.; Seims, A. A Review of Sex-Related Differences in Colorectal Cancer Incidence, Screening Uptake, Routes to Diagnosis, Cancer Stage and Survival in the UK. BMC Cancer 2018, 18, 906. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef]
- Sinicrope, F.A. Increasing Incidence of Early-Onset Colorectal Cancer. N. Engl. J. Med. 2022, 386, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Newbold, R.R.; Padilla-Banks, E.; Jefferson, W.N. Environmental Estrogens and Obesity. Mol. Cell. Endocrinol. 2009, 304, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Arterburn, J.B.; Prossnitz, E.R. G Protein-Coupled Estrogen Receptor GPER: Molecular Pharmacology and Therapeutic Applications. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 295–320. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Hathaway, H.J. What have we learned about GPER function in physiology and disease from knockout mice? J. Steroid Biochem. Mol. Biol. 2015, 153, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Zhang, Y.; Cheng, S.; Xiao, T. Environmental Estrogens Shape Disease Susceptibility. Int. J. Hyg. Environ. Health 2023, 249, 114125. [Google Scholar] [CrossRef]
- Agnoli, C.; Berrino, F.; Abagnato, C.A.; Muti, P.; Panico, S.; Crosignani, P.; Krogh, V. Metabolic Syndrome and Postmenopausal Breast Cancer in the ORDET Cohort: A Nested Case-Control Study. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 41–48. [Google Scholar] [CrossRef]
- Chan, D.S.M.; Vieira, A.R.; Aune, D.; Bandera, E.V.; Greenwood, D.C.; McTiernan, A.; Navarro Rosenblatt, D.; Thune, I.; Vieira, R.; Norat, T. Body mass index and survival in women with breast cancer—Systematic literature review and meta-analysis of 82 follow-up studies. Ann. Oncol. 2014, 25, 1901–1914. [Google Scholar] [CrossRef]
- Esposito, K.; Chiodini, P.; Colao, A.; Lenzi, A.; Giugliano, D. Metabolic Syndrome and Risk of Cancer: A Systematic Review and Meta-Analysis. Diabetes Care 2012, 35, 2402–2411. [Google Scholar] [CrossRef]
- Miyamoto, T.; Shiozawa, T. Two-sided role of estrogen on endometrial carcinogenesis: Stimulator or suppressor? Gynecol. Endocrinol. 2019, 35, 370–375. [Google Scholar] [CrossRef]
- Johansson, Å.; Schmitz, D.; Höglund, J.; Hadizadeh, F.; Karlsson, T.; Ek, W.E. Investigating the Effect of Estradiol Levels on the Risk of Breast, Endometrial, and Ovarian Cancer. J. Endocr. Soc. 2022, 6, bvac100. [Google Scholar] [CrossRef]
- Chakraborty, S.; Ganti, A.K.; Marr, A.; Batra, S.K. Lung Cancer in Women: Role of Estrogens. Expert Rev. Respir. Med. 2010, 4, 509–518. [Google Scholar] [CrossRef]
- Hsu, L.H.; Chu, N.M.; Kao, S.H. Estrogen, Estrogen Receptor and Lung Cancer. Int. J. Mol. Sci. 2017, 18, 1713. [Google Scholar] [CrossRef]
- Rodriguez-Lara, V.; Hernandez-Martinez, J.M.; Arrieta, O. Influence of estrogen in non-small cell lung cancer and its clinical implications. J. Thorac. Dis. 2018, 10, 482–497. [Google Scholar] [CrossRef]
- Shi, L.; Feng, Y.; Lin, H.; Ma, R.; Cai, X. Role of estrogen in hepatocellular carcinoma: Is inflammation the key? J. Transl. Med. 2014, 12, 93. [Google Scholar] [CrossRef]
- Sukocheva, O.A. Estrogen, estrogen receptors, and hepatocellular carcinoma: Are we there yet? World J. Gastroenterol. 2018, 24, 1–4. [Google Scholar] [CrossRef]
- Carruba, G. Estrogens in Hepatocellular Carcinoma: Friends or Foes? Cancers 2021, 13, 2085. [Google Scholar] [CrossRef]
- Mori, N.; Keski-Rahkonen, P.; Gicquiau, A.; Rinaldi, S.; Dimou, N.; Harlid, S.; Harbs, J.; Van Guelpen, B.; Aune, D.; Cross, A.J.; et al. Endogenous Circulating Sex Hormone Concentrations and Colon Cancer Risk in Postmenopausal Women: A Prospective Study and Meta-Analysis. JNCI Cancer Spectr. 2021, 5, pkab084. [Google Scholar] [CrossRef]
- Sun, L.; Chao, F.; Luo, B.; Ye, D.; Zhao, J.; Zhang, Q.; Ma, X.; Zhang, G. Impact of Estrogen on the Relationship between Obesity and Renal Cell Carcinoma Risk in Women. EBioMedicine 2018, 34, 108–112. [Google Scholar] [CrossRef]
- Schouten, L.J.; van de Pol, J.; Kviatkovsky, M.J.; van den Brandt, P.A. Reproductive and External Hormonal Factors and the Risk of Renal Cell Cancer in the Netherlands Cohort Study. Cancer Epidemiol. 2022, 79, 102171. [Google Scholar] [CrossRef]
- Filardo, E.J. A Role for G-Protein Coupled Estrogen Receptor (GPER) in Estrogen-Induced Carcinogenesis: Dysregulated Glandular Homeostasis, Survival and Metastasis. J. Steroid Biochem. Mol. Biol. 2018, 176, 38–48. [Google Scholar] [CrossRef]
- Yager, J.D.; Davidson, N.E. Estrogen Carcinogenesis in Breast Cancer. N. Engl. J. Med. 2006, 354, 270–282. [Google Scholar] [CrossRef]
- Bhat, H.K.; Calaf, G.; Hei, T.K.; Loya, T.; Vadgama, J. V Critical Role of Oxidative Stress in Estrogen-Induced Carcinogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 3913–3918. [Google Scholar] [CrossRef]
- Calaf, G.M.; Urzua, U.; Termini, L.; Aguayo, F. Oxidative Stress in Female Cancers. Oncotarget 2018, 9, 23824–23842. [Google Scholar] [CrossRef]
- Filardo, E.J. Epidermal Growth Factor Receptor (EGFR) Transactivation by Estrogen via the G-Protein-Coupled Receptor, GPR30: A Novel Signaling Pathway with Potential Significance for Breast Cancer. J. Steroid Biochem. Mol. Biol. 2002, 80, 231–238. [Google Scholar] [CrossRef]
- Meyer, M.R.; Barton, M. ERα, ERβ, and GpER: Novel Aspects of Oestrogen Receptor Signalling in Atherosclerosis. Cardiovasc. Res. 2009, 83, 605–610. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Barton, M. Estrogen Biology: New Insights into GPER Function and Clinical Opportunities. Mol. Cell. Endocrinol. 2014, 389, 71–83. [Google Scholar] [CrossRef]
- Eyster, K.M. The Estrogen Receptors: An Overview from Different Perspectives. Methods Mol. Biol. 2016, 1366, 1–10. [Google Scholar] [CrossRef]
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170. [Google Scholar] [CrossRef]
- Blair, R.M.; Fang, H.; Branham, W.S.; Hass, B.S.; Dial, S.L.; Moland, C.L.; Tong, W.; Shi, L.; Perkins, R.; Sheehan, D.M. The Estrogen Receptor Relative Binding Affinities of 188 Natural and Xenochemicals: Structural Diversity of Ligands. Toxicol. Sci. 2000, 54, 138–153. [Google Scholar] [CrossRef]
- Möcklinghoff , S.; Rose, R.; Carraz , M.; Visser, A.; Ottmann, C.; Brunsveld , L. Synthesis and Crystal Structure of a Phosphorylated Estrogen Receptor Ligand Binding Domain. ChemBioChem 2010, 11, 2251–2254. [Google Scholar] [CrossRef]
- Tanenbaum, D.M.; Wang, Y.; Williams, S.P.; Sigler, P.B. Crystallographic Comparison of the Estrogen and Progesterone Receptor’s Ligand Binding Domains. Proc. Natl. Acad. Sci. USA 1998, 95, 5998–6003. [Google Scholar] [CrossRef]
- Kumar, R.; Zakharov, M.N.; Khan, S.H.; Miki, R.; Jang, H.; Toraldo, G.; Singh, R.; Bhasin, S.; Jasuja, R. The Dynamic Structure of the Estrogen Receptor. J. Amino Acids 2011, 2011, 812540. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, H.; Yao, J. ERα, a Key Target for Cancer Therapy: A Review. Onco Targets Ther. 2020, 13, 2183–2191. [Google Scholar] [CrossRef]
- Quinn, J.A.; Graeber, C.T.; Frackelton, A.R.; Kim, M.; Schwarzbauer, J.E.; Filardo, E.J. Coordinate Regulation of Estrogen-Mediated Fibronectin Matrix Assembly and Epidermal Growth Factor Receptor Transactivation by the G Protein-Coupled Receptor, GPR30. Mol. Endocrinol. 2009, 23, 1052–1064. [Google Scholar] [CrossRef]
- Filardo, E.J.; Thomas, P. GPR30: A Seven-Transmembrane-Spanning Estrogen Receptor That Triggers EGF Release. Trends Endocrinol. Metab. 2005, 16, 362–367. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Maggiolini, M. Mechanisms of Estrogen Signaling and Gene Expression via GPR30. Mol. Cell. Endocrinol. 2009, 308, 32–38. [Google Scholar] [CrossRef]
- Aronica, S.M.; Kraus, W.L.; Katzenellenbogen, B.S. Estrogen Action via the cAMP signaling pathway: Stimulation of adenylate cyclase and cAMP-regulated gene transcription. Proc. Natl. Acad. Sci. USA 1994, 91, 8517–8521. [Google Scholar] [CrossRef]
- Albanito, L.; Madeo, A.; Lappano, R.; Vivacqua, A.; Rago, V.; Carpino, A.; Oprea, T.I.; Prossnitz, E.R.; Musti, A.M.; Andò, S.; et al. G Protein-Coupled Receptor 30 (GPR30) Mediates Gene Expression Changes and Growth Response to 17β-Estradiol and Selective GPR30 Ligand G-1 in Ovarian Cancer Cells. Cancer Res. 2007, 67, 1859–1866. [Google Scholar] [CrossRef]
- Talia, M.; De Francesco, E.M.; Rigiracciolo, D.C.; Muoio, M.G.; Muglia, L.; Belfiore, A.; Maggiolini, M.; Sims, A.H.; Lappano, R. The G Protein-Coupled Estrogen Receptor (GPER) Expression Correlates with pro-Metastatic Pathways in ER-Negative Breast Cancer: A Bioinformatics Analysis. Cells 2020, 9, 622. [Google Scholar] [CrossRef]
- Xu, T.; Ma, D.; Chen, S.; Tang, R.; Yang, J.; Meng, C.; Feng, Y.; Liu, L.; Wang, J.; Luo, H.; et al. High GPER Expression in Triple-Negative Breast Cancer Is Linked to pro-Metastatic Pathways and Predicts Poor Patient Outcomes. NPJ Breast Cancer 2022, 8, 100. [Google Scholar] [CrossRef]
- Phadke, S.; Mott, S.; Bashir, A.; Bellizzi, A.; Resnick, M.; Sturtevant, A.; Filardo, E. Abstract P2-11-16: Distribution of G-Protein Coupled Estrogen Receptor in Treatment-Naïve Triple Negative Breast Cancer and Association with Clinicopathologic Characteristics. Cancer Res. 2020, 80, P2-11-16. [Google Scholar] [CrossRef]
- Steiman, J.; Peralta, E.A.; Louis, S.; Kamel, O. Biology of the Estrogen Receptor, GPR30, in Triple Negative Breast Cancer. Am. J. Surg. 2013, 206, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Pal, U.; Ghosh, S.; Limaye, A.M. DNA Methylation in the Upstream CpG Island of the GPER Locus and Its Relationship with GPER Expression in Colon Cancer Cell Lines. Mol. Biol. Rep. 2020, 47, 7547–7555. [Google Scholar] [CrossRef]
- Weißenborn, C.; Ignatov, T.; Costa, S.; Zenclussen, A.; Ignatov, A. GPER Is Inactivated by Promoter Methylation and Potentially Functions as a Tumor Suppressor in Breast Cancer. Geburtshilfe Frauenheilkd 2014, 74, PO_Onko03_14. [Google Scholar] [CrossRef]
- Ottaviano, Y.L.; Issa, J.-P.; Pan, F.F.; Smith, H.S.; Baylin, S.B.; Davidson, N.E. Methylation of the estrogen receptor gene CpG island marks loss of estrogen receptor expression in human breast cancer cells. Cancer Res. 1994, 54, 2552–2555. [Google Scholar]
- Filardo, E.J.; Graeber, C.T.; Quinn, J.A.; Resnick, M.B.; Giri, D.; DeLellis, R.A.; Steinhoff, M.M.; Sabo, E. Distribution of GPR30, a Seven Membrane-Spanning Estrogen Receptor, in Primary Breast Cancer and Its Association with Clinicopathologic Determinants of Tumor Progression. Clin. Cancer Res. 2006, 12, 6359–6366. [Google Scholar] [CrossRef]
- Sjöström, M.; Hartman, L.; Grabau, D.; Fornander, T.; Malmström, P.; Nordenskjöld, B.; Sgroi, D.C.; Skoog, L.; Stål, O.; Leeb-Lundberg, L.M.F.; et al. Lack of G Protein-Coupled Estrogen Receptor (GPER) in the Plasma Membrane Is Associated with Excellent Long-Term Prognosis in Breast Cancer. Breast Cancer Res. Treat. 2014, 145, 61–71. [Google Scholar] [CrossRef]
- Smith, H.O.; Leslie, K.K.; Singh, M.; Qualls, C.R.; Revankar, C.M.; Joste, N.E.; Prossnitz, E.R. GPR30: A Novel Indicator of Poor Survival for Endometrial Carcinoma. Am. J. Obstet. Gynecol. 2007, 196, 386.e1–386.e11. [Google Scholar] [CrossRef]
- Smith, H.O.; Arias-Pulido, H.; Kuo, D.Y.; Howard, T.; Qualls, C.R.; Lee, S.J.; Verschraegen, C.F.; Hathaway, H.J.; Joste, N.E.; Prossnitz, E.R. GPR30 Predicts Poor Survival for Ovarian Cancer. Gynecol. Oncol. 2009, 114, 465–471. [Google Scholar] [CrossRef]
- Chan, Y.T.; Lai, A.C.Y.; Lin, R.J.; Wang, Y.H.; Wang, Y.T.; Chang, W.W.; Wu, H.Y.; Lin, Y.J.; Chang, W.Y.; Wu, J.C.; et al. GPER-Induced Signaling Is Essential for the Survival of Breast Cancer Stem Cells. Int. J. Cancer 2020, 146, 1674–1685. [Google Scholar] [CrossRef]
- Wang, C.; Lv, X.; He, C.; Hua, G.; Tsai, M.Y.; Davis, J.S. The G-Protein-Coupled Estrogen Receptor Agonist G-1 Suppresses Proliferation of Ovarian Cancer Cells by Blocking Tubulin Polymerization. Cell Death Dis. 2013, 4, e869. [Google Scholar] [CrossRef] [PubMed]
- Hirtz, A.; Lebourdais, N.; Rech, F.; Bailly, Y.; Vaginay, A.; Smaïl-Tabbone, M.; Dubois-Pot-schneider, H.; Dumond, H. Gper Agonist G-1 Disrupts Tubulin Dynamics and Potentiates Temozolomide to Impair Glioblastoma Cell Proliferation. Cells 2021, 10, 3438. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, G.; Natale, C.A.; Musi, E.; Garyantes, T.; Schwartz, G.K. The GPER Agonist LNS8801 Induces Mitotic Arrest and Apoptosis in Uveal Melanoma Cells. Cancer Res. Commun. 2023, 3, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Natale, C.A.; Li, J.; Zhang, J.; Dahal, A.; Dentchev, T.; Stanger, B.Z.; Ridky, T.W. Activation of G Protein-Coupled Estrogen Receptor Signaling Inhibits Melanoma and Improves Response to Immune Checkpoint Blockade. Elife 2018, 7, e31770. [Google Scholar] [CrossRef] [PubMed]
- Petrie, W.K.; Dennis, M.K.; Hu, C.; Dai, D.; Arterburn, J.B.; Smith, H.O.; Hathaway, H.J.; Prossnitz, E.R. G Protein-Coupled Estrogen Receptor-Selective Ligands Modulate Endometrial Tumor Growth. Obstet. Gynecol. Int. 2013, 2013, 472720. [Google Scholar] [CrossRef]
- Liu, C.; Liao, Y.; Fan, S.; Fu, X.; Xiong, J.; Zhou, S.; Zou, M.; Wang, J. G-Protein-Coupled Estrogen Receptor Antagonist G15 Decreases Estrogen-Induced Development of Non-Small Cell Lung Cancer. Oncol. Res. 2019, 27, 283–292. [Google Scholar] [CrossRef]
- Das, P.K.; Saha, J.; Pillai, S.; Lam, A.K.-Y.; Gopalan, V.; Islam, F. Implications of Estrogen and Its Receptors in Colorectal Carcinoma. Cancer Med. 2023, 12, 4367–4379. [Google Scholar] [CrossRef]
- Hogan, A.M.; Collins, D.; Baird, A.W.; Winter, D.C. Estrogen and Gastrointestinal Malignancy. Mol. Cell. Endocrinol. 2009, 307, 19–24. [Google Scholar] [CrossRef]
- Kennelly, R.; Kavanagh, D.O.; Hogan, A.M.; Winter, D.C. Oestrogen and the Colon: Potential Mechanisms for Cancer Prevention. Lancet Oncol. 2008, 9, 385–391. [Google Scholar] [CrossRef]
- Marino, M. Xenoestrogens Challenge 17β-Estradiol Protective Effects in Colon Cancer. World J. Gastrointest. Oncol. 2014, 6, 67–73. [Google Scholar] [CrossRef][Green Version]
- Kim, Y.S.; Kim, N. Sex-Gender Differences in Irritable Bowel Syndrome. J. Neurogastroenterol. Motil. 2018, 24, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Preedy, J.R.K.; Aitken, E.H. The Effect of Estrogen on Water and Electrolyte Metabolism. I. The Normal. J. Clin. Investig. 1956, 35, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, K. Structure, Genetics, and Mode of Disease of Cholera Toxin. In Biological Toxins and Bioterrorism; Gopalakrishnakone, P., Balali-Mood, M., Llewellyn, L., Singh, B.R., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 3–27. ISBN 978-94-007-5869-8. [Google Scholar]
- Caiazza, F.; Ryan, E.J.; Doherty, G.; Winter, D.C.; Sheahan, K. Estrogen Receptors and Their Implications in Colorectal Carcinogenesis. Front. Oncol. 2015, 5, 19. [Google Scholar] [CrossRef]
- Bustos, V.; Nolan, Á.M.; Nijhuis, A.; Harvey, H.; Parker, A.; Poulsom, R.; Mcbryan, J.; Thomas, W.; Silver, A.; Harvey, B.J. GPER Mediates Differential Effects of Estrogen on Colon Cancer Cell Proliferation and Migration under Normoxic and Hypoxic Conditions. Oncotarget 2017, 8, 84258–84275. [Google Scholar] [CrossRef]
- Sun, M.; Xie, H.; Tang, Y.; Lin, S.; Li, J.; Sun, S.; Hu, X.; Huang, Y.; Shi, W.; Jian, D. G Protein-Coupled Estrogen Receptor Enhances Melanogenesis via CAMP-Protein Kinase (PKA) by Upregulating Microphthalmia-Related Transcription Factor-Tyrosinase in Melanoma. J. Steroid Biochem. Mol. Biol. 2017, 165, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Natale, C.A.; Li, J.; Pitarresi, J.R.; Norgard, R.J.; Dentchev, T.; Capell, B.C.; Seykora, J.T.; Stanger, B.Z.; Ridky, T.W. Pharmacologic Activation of the G Protein–Coupled Estrogen Receptor Inhibits Pancreatic Ductal Adenocarcinoma. CMGH 2020, 10, 868–880.e1. [Google Scholar] [CrossRef]
- Lu, A.S.; Rouhimoghadam, M.; Arnatt, C.K.; Filardo, E.J.; Salem, A.K. Proteolytic Targeting Chimeras with Specificity for Plasma Membrane and Intracellular Estrogen Receptors. Mol. Pharm. 2021, 18, 1455–1469. [Google Scholar] [CrossRef]
- Lóránd, T.; Vigh, E.; Garai, J. hormonal action of plant derived and anthropogenic non-steroidal estrogenic compounds: Phytoestrogens and xenoestrogens. Curr. Medicial Chem. 2010, 17, 3542–3574. [Google Scholar] [CrossRef]
- Jiang, Y.; Gong, P.; Madak-Erdogan, Z.; Martin, T.; Jeyakumar, M.; Carlson, K.; Khan, I.; Smillie, T.J.; Chittiboyina, A.G.; Rotte, S.C.K.; et al. Mechanisms Enforcing the Estrogen Receptor β Selectivity of Botanical Estrogens. FASEB J. 2013, 27, 4406–4418. [Google Scholar] [CrossRef]
- Kuiper, G.G.J.M.; Lemmen, J.G.; Carlsson, B.O.; Corton, J.C.; Safe, S.H.; Van Der Saag, P.T.; Van Der Burg, B.; Gustafsson, J.-Å. Interaction of Estrogenic Chemicals and Phytoestrogens with Estrogen Receptor. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef]
- Gonzalez, T.L.; Rae, J.M.; Colacino, J.A. Implication of Environmental Estrogens on Breast Cancer Treatment and Progression. Toxicology 2019, 421, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.; Deoraj, A.; Felty, Q.; Roy, D. Environmental Estrogen-like Endocrine Disrupting Chemicals and Breast Cancer. Mol. Cell. Endocrinol. 2017, 457, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Rouhimoghadam, M.; Lu, A.S.; Salem, A.K.; Filardo, E.J. Therapeutic Perspectives on the Modulation of G-Protein Coupled Estrogen Receptor, GPER, Function. Front. Endocrinol. 2020, 11, 591217. [Google Scholar] [CrossRef] [PubMed]
- King, R.A.; Bursill, D.B. Plasma and Urinary Kinetics of the Isoflavones Daidzein and Genistein after a Single Soy Meal in Humans. Am. J. Clin. Nutr. 1998, 67, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Ariyani, W.; Miyazaki, W.; Amano, I.; Hanamura, K.; Shirao, T.; Koibuchi, N. Soy Isoflavones Accelerate Glial Cell Migration via GPER-Mediated Signal Transduction Pathway. Front. Endocrinol. 2020, 11, 554941. [Google Scholar] [CrossRef]
- Rowlands, D.J.; Chapple, S.; Siow, R.C.M.; Mann, G.E. Equol-Stimulated Mitochondrial Reactive Oxygen Species Activate Endothelial Nitric Oxide Synthase and Redox Signaling in Endothelial Cells: Roles for F-Actin and GPR30. Hypertension 2011, 57, 833–840. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Clerici, C.; Lephart, E.D.; Cole, S.J.; Heenan, C.; Castellani, D.; Wolfe, B.E.; Nechemias-Zimmer, L.; Brown, N.M.; Lund, T.D.; et al. S-Equol, a Potent Ligand for Estrogen Receptor β, Is the Exclusive Enantiomeric Form of the Soy Isoflavone Metabolite Produced by Human Intestinal Bacterial Flora. Am. J. Clin. Nutr. 2005, 81, 1072–1079. [Google Scholar] [CrossRef]
- Hod, R.; Maniam, S.; Nor, N.H.M. A Systematic Review of the Effects of Equol (Soy Metabolite) on Breast Cancer. Molecules 2021, 26, 1105. [Google Scholar] [CrossRef]
- Boszkiewicz, K.; Sawicka, E.; Piwowar, A. The impact of xenoestrogens on effectiveness of treatment for hormone-dependent breast cancer–current state of knowledge and perspectives for research. Ann. Agric. Environ. Med. 2020, 27, 526–534. [Google Scholar] [CrossRef]
- Warth, B.; Raffeiner, P.; Granados, A.; Huan, T.; Fang, M.; Forsberg, E.M.; Benton, H.P.; Goetz, L.; Johnson, C.H.; Siuzdak, G. Metabolomics Reveals That Dietary Xenoestrogens Alter Cellular Metabolism Induced by Palbociclib/Letrozole Combination Cancer Therapy. Cell Chem. Biol. 2018, 25, 291–300.e3. [Google Scholar] [CrossRef]
- López-Carrillo, L.; Hernández-Ramírez, R.U.; Calafat, A.M.; Torres-Sánchez, L.; Galván-Portillo, M.; Needham, L.L.; Ruiz-Ramos, R.; Cebrián, M.E. Exposure to Phthalates and Breast Cancer Risk in Northern Mexico. Environ. Health Perspect. 2010, 118, 539–544. [Google Scholar] [CrossRef]
- Cohn, B.A.; La Merrill, M.; Krigbaum, N.Y.; Yeh, G.; Park, J.S.; Zimmermann, L.; Cirillo, P.M. DDT Exposure in Utero and Breast Cancer. J. Clin. Endocrinol. Metab. 2015, 100, 2865–2872. [Google Scholar] [CrossRef]
- Leng, L.; Li, J.; Luo, X.; Kim, J.; Li, Y.; Guo, X.; Chen, X.; Yang, Q.; Li, G.; Tang, N. Polychlorinated Biphenyls and Breast Cancer: A Congener-Specific Meta-Analysis. Environ. Int. 2016, 88, 133–141. [Google Scholar] [CrossRef]
- Parada, H.; Sun, X.; Tse, C.K.; Engel, L.S.; Hoh, E.; Olshan, A.F.; Troester, M.A. Plasma levels of polychlorinated biphenyls (PCBs) and breast cancer mortality: The Carolina Breast Cancer Study. Int. J. Hyg. Environ. Health 2020, 227, 113522. [Google Scholar] [CrossRef]
- Seachrist, D.D.; Bonk, K.W.; Ho, S.M.; Prins, G.S.; Soto, A.M.; Keri, R.A. A review of the carcinogenic potential of bisphenol A. Reprod. Toxicol. 2016, 59, 167–182. [Google Scholar] [CrossRef]
- Brody, J.G.; Moysich, K.B.; Humblet, O.; Attfield, K.R.; Beehler, G.P.; Rudel, R.A. Environmental Pollutants and Breast Cancer. Cancer 2007, 109, 2667–2711. [Google Scholar] [CrossRef]
- Maffini, M.V.; Rubin, B.S.; Sonnenschein, C.; Soto, A.M. Endocrine Disruptors and Reproductive Health: The Case of Bisphenol-A. Mol. Cell. Endocrinol. 2006, 254–255, 179–186. [Google Scholar] [CrossRef]
- Thomas, P.; Dong, J. Binding and Activation of the Seven-Transmembrane Estrogen Receptor GPR30 by Environmental Estrogens: A Potential Novel Mechanism of Endocrine Disruption. J. Steroid Biochem. Mol. Biol. 2006, 102, 175–179. [Google Scholar] [CrossRef]
- Cao, L.Y.; Ren, X.M.; Li, C.H.; Zhang, J.; Qin, W.P.; Yang, Y.; Wan, B.; Guo, L.H. Bisphenol AF and Bisphenol B Exert Higher Estrogenic Effects than Bisphenol A via G Protein-Coupled Estrogen Receptor Pathway. Environ. Sci. Technol. 2017, 51, 19. [Google Scholar] [CrossRef]
- Porras, S.P.; Heinälä, M.; Santonen, T. Bisphenol A Exposure via Thermal Paper Receipts. Toxicol. Lett. 2014, 230, 413–420. [Google Scholar] [CrossRef]
- Russo, G.; Barbato, F.; Grumetto, L. Monitoring of Bisphenol A and Bisphenol S in Thermal Paper Receipts from the Italian Market and Estimated Transdermal Human Intake: A Pilot Study. Sci. Total Environ. 2017, 599–600, 68–75. [Google Scholar] [CrossRef]
- Corrales, J.; Kristofco, L.A.; Baylor Steele, W.; Yates, B.S.; Breed, C.S.; Spencer Williams, E.; Brooks, B.W. Global Assessment of Bisphenol a in the Environment: Review and Analysis of Its Occurrence and Bioaccumulation. Dose-Response 2015, 13, 1559325815598308. [Google Scholar] [CrossRef]
- Calafat, A.M.; Kuklenyik, Z.; Reidy, J.A.; Caudill, S.P.; Ekong, J.; Needham, L.L. Urinary Concentrations of Bisphenol A and 4-Nonylphenol in a Human Reference Population. Environ. Health Perspect. 2005, 113, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Lehmler, H.J.; Liu, B.; Gadogbe, M.; Bao, W. Exposure to Bisphenol A, Bisphenol F, and Bisphenol S in U.S. Adults and Children: The National Health and Nutrition Examination Survey 2013–2014. ACS Omega 2018, 3, 6523–6532. [Google Scholar] [CrossRef] [PubMed]
- Erickson, B.E. US FDA Urged to Limit Bisphenol A in Food Packaging Again. C&E News. 31 January 2022. Available online: https://cen.acs.org/safety/consumer-safety/US-FDA-urged-limit-bisphenol-A-in-food-packaging-again/100/web/2022/01 (accessed on 3 October 2023).
- Vandenberg, L.N. Non-Monotonic Dose Responses in Studies of Endocrine Disrupting Chemicals: Bisphenol a as a Case Study. Dose-Response 2014, 12, 259–276. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, H.; Liu, S. Low-Dose Bisphenol A Exposure: A Seemingly Instigating Carcinogenic Effect on Breast Cancer. Adv. Sci. 2017, 4, 1600248. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Hu, C.; Staquicini, D.I.; Brigman, J.L.; Liu, M.; Mauvais-Jarvis, F.; Pasqualini, R.; Arap, W.; Arterburn, J.B.; Hathaway, H.J.; et al. Preclinical efficacy of the GPER-selective agonist G-1 in mouse models of obesity and diabetes. Sci. Transl. Med. 2020, 12, eaau5956. [Google Scholar] [CrossRef]
- Boyle, P.; Boniol, M.; Koechlin, A.; Robertson, C.; Valentini, F.; Coppens, K.; Fairley, L.L.; Boniol, M.; Zheng, T.; Zhang, Y.; et al. Diabetes and Breast Cancer Risk: A Meta-Analysis. Br. J. Cancer 2012, 107, 1608–1617. [Google Scholar] [CrossRef]
- Varma, V. Metabolic Syndrome: Toxicology’s Next Patient. Available online: https://toxchange.toxicology.org/browse/blogs/blogviewer?BlogKey=8642a0f6-eb48-4a37-8a2d-ce7ac700de77 (accessed on 1 August 2023).
- Magueresse-Battistoni, B.L.; Labaronne, E.; Vidal, H.; Naville, D. Endocrine Disrupting Chemicals in Mixture and Obesity, Diabetes and Related Metabolic Disorders. World J. Biol. Chem. 2017, 8, 108. [Google Scholar] [CrossRef]
- Dvorak, H.F. Tumors: Wounds That Do Not Heal. N. Engl. J. Med. 1986, 315, 1650–1659. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, M.; Yang, L.; Tu, G.; Zhu, Q.; Chen, M.; Cheng, H.; Luo, H.; Fu, W.; Li, Z.; et al. Acquisition of Epithelial-Mesenchymal Transition Phenotype in the Tamoxifen-Resistant Breast Cancer Cell: A New Role for G Protein-Coupled Estrogen Receptor in Mediating Tamoxifen Resistance through Cancer-Associated Fibroblast-Derived Fibronectin and Β1-Integrin Signaling Pathway in Tumor Cells. Breast Cancer Res. 2015, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- He, Y.-Y.; Du, G.-Q.; Cai, B.; Yan, Q.; Zhou, L.; Chen, X.-Y.; Lu, W.; Yang, Y.-X.; Wan, X.-P. Estrogenic Transmembrane Receptor of GPR30 Mediates Invasion and Carcinogenesis by Endometrial Cancer Cell Line RL95-2. J. Cancer Res. Clin. Oncol. 2012, 138, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Cao, Y.; Tang, L.-D. Transmembrane Estrogen Receptor GPR30 Is More Frequently Expressed in Malignant Than Benign Ovarian Endometriotic Cysts and Correlates with MMP-9 Expression. Int. J. Gynecol. Cancer 2012, 22, 539. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, H.; Wen, H.; Jiang, X.; Cao, X.; Zhang, G.; Liu, G. The Novel Estrogen Receptor GPER Regulates the Migration and Invasion of Ovarian Cancer Cells. Mol. Cell. Biochem. 2013, 378, 1–7. [Google Scholar] [CrossRef]
- Kanda, N.; Watanabe, S. 17β-Estradiol Inhibits Oxidative Stress-Induced Apoptosis in Keratinocytes by Promoting Bcl-2 Expression. J. Investig. Dermatol. 2003, 121, 1500–1509. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Mizukami, Y.; Miura, T.; Fujimoto, K.; Kobayashi, S.; Matsuzaki, M. Orphan G Protein-Coupled Receptor, GPR41, Induces Apoptosis via a P53/Bax Pathway during Ischemic Hypoxia and Reoxygenation. J. Biol. Chem. 2001, 276, 26453–26460. [Google Scholar] [CrossRef]
- Magruder, H.T.; Quinn, J.A.; Schwartzbauer, J.E.; Reichner, J.; Huang, A.; Filardo, E.J. The G Protein-Coupled Estrogen Receptor-1, GPER-1, Promotes Fibrillogenesis via a Shc-Dependent Pathway Resulting in Anchorage-Independent Growth. Horm. Cancer 2014, 5, 390–404. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brunsing, R.L.; Owens, K.S.; Prossnitz, E.R. The G Protein-Coupled Estrogen Receptor (GPER) Agonist G-1 Expands the Regulatory T-Cell Population under TH17-Polarizing Conditions. J. Immunother. 2013, 36, 190–196. [Google Scholar] [CrossRef]
- De Francesco, E.M.; Lappano, R.; Santolla, M.F.; Marsico, S.; Caruso, A.; Maggiolini, M. HIF-1α/GPER Signaling Mediates the Expression of VEGF Induced by Hypoxia in Breast Cancer Associated Fibroblasts (CAFs). Breast Cancer Res. 2013, 15, R64. [Google Scholar] [CrossRef]
- De Francesco, E.M.; Sims, A.H.; Maggiolini, M.; Sotgia, F.; Lisanti, M.P.; Clarke, R.B. GPER Mediates the Angiocrine Actions Induced by IGF1 through the HIF-1α/VEGF Pathway in the Breast Tumor Microenvironment. Breast Cancer Res. 2017, 19, 129. [Google Scholar] [CrossRef]
- Lappano, R.; Maggiolini, M. GPER Is Involved in the Functional Liaison between Breast Tumor Cells and Cancer-Associated Fibroblasts (CAFs). J. Steroid Biochem. Mol. Biol. 2018, 176, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Chen, Z.; Jiang, G.; Zhou, Y.; Liu, Q.; Su, Q.; Wei, W.; Du, J.; Wang, H. Activation of GPER Suppresses Migration and Angiogenesis of Triple Negative Breast Cancer via Inhibition of NF-ΚB/IL-6 Signals. Cancer Lett. 2017, 386, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Bouskine, A.; Nebout, M.; Brücker-Davis, F.; Banahmed, M.; Fenichel, P. Low Doses of Bisphenol A Promote Human Seminoma Cell Proliferation by Activating PKA and PKG via a Membrane G-Protein-Coupled Estrogen Receptor. Environ. Health Perspect. 2009, 117, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, N.; Bouskine, A.; Fenichel, P. Bisphenol A Promotes Testicular Seminoma Cell Proliferation through GPER/GPR30. Int. J. Cancer 2012, 130, 241–242. [Google Scholar] [CrossRef]
- Babiloni-Chust, I.; dos Santos, R.S.; Medina-Gali, R.M.; Perez-Serna, A.A.; Encinar, J.A.; Martinez-Pinna, J.; Gustafsson, J.A.; Marroqui, L.; Nadal, A. G Protein-Coupled Estrogen Receptor Activation by Bisphenol-A Disrupts the Protection from Apoptosis Conferred by the Estrogen Receptors ERα and ERβ in Pancreatic Beta Cells. Environ. Int. 2022, 164, 107250. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Magdalena, P.; Morimoto, S.; Ripoll, C.; Fuentes, E.; Nadal, A. The Estrogenic Effect of Bisphenol a Disrupts Pancreatic β-Cell Function in Vivo and Induces Insulin Resistance. Environ. Health Perspect. 2006, 114, 106–112. [Google Scholar] [CrossRef]
- Stahlhut, R.W.; Myers, J.P.; Taylor, J.A.; Nadal, A.; Dyer, J.A.; vom Saal, F.S. Experimental BPA Exposure and Glucose-Stimulated Insulin Response in Adult Men and Women. J. Endocr. Soc. 2018, 2, 1173–1187. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of Chemotherapy and Hormonal Therapy for Early Breast Cancer on Recurrence and 15-Year Survival: An Overview of the Randomised Trials. Lancet 2005, 365, 1687–1717. [Google Scholar] [CrossRef]
- Filardo, E.J. GPER and ER: Estrogen receptors with distinct biological roles in breast cancer. Inflamm. Allergy Drug Targets 2012, 11, 243–254. [Google Scholar] [CrossRef]
- Marjon, N.A.; Hu, C.; Hathaway, H.J.; Prossnitz, E.R. G Protein-Coupled Estrogen Receptor Regulates Mammary Tumorigenesis and Metastasis. Mol. Cancer Res. 2014, 12, 1644–1654. [Google Scholar] [CrossRef]
- Catalano, S.; Giordano, C.; Panza, S.; Chemi, F.; Bonofiglio, D.; Lanzino, M.; Rizza, P.; Romeo, F.; Fuqua, S.A.W.; Maggiolini, M.; et al. Tamoxifen through GPER Upregulates Aromatase Expression: A Novel Mechanism Sustaining Tamoxifen-Resistant Breast Cancer Cell Growth. Breast Cancer Res. Treat. 2014, 146, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Filardo, E.J.; Quinn, J.A.; Sabo, E. Association of the Membrane Estrogen Receptor, GPR30, with Breast Tumor Metastasis and Transactivation of the Epidermal Growth Factor Receptor. Steroids 2008, 73, 870–873. [Google Scholar] [CrossRef] [PubMed]
- Sriram, K.; Insel, P.A. G Protein-Coupled Receptors as Targets for Approved Drugs: How Many Targets and How Many Drugs? Mol. Pharmacol. 2018, 93, 251–258. [Google Scholar] [CrossRef]
- Chaudhary, P.K.; Kim, S. An Insight into GPCR and G-Proteins as Cancer Drivers. Cells 2021, 10, 3288. [Google Scholar] [CrossRef] [PubMed]
- Bar-Shavit, R.; Maoz, M.; Kancharla, A.; Nag, J.K.; Agranovich, D.; Grisaru-Granovsky, S.; Uziely, B. G Protein-Coupled Receptors in Cancer. Int. J. Mol. Sci. 2016, 17, 1320. [Google Scholar] [CrossRef]
- Arang, N.; Gutkind, J.S. G Protein-Coupled receptors and heterotrimeric G proteins as cancer drivers. FEBS Lett. 2020, 594, 4201–4232. [Google Scholar] [CrossRef]
- Perez Almeria, C.V.; Setiawan, I.M.; Siderius, M.; Smit, M.J. G Protein-Coupled Receptors as Promising Targets in Cancer. Curr. Opin. Endocr. Metab. Res. 2021, 16, 119–127. [Google Scholar] [CrossRef]
- Lappano, R.; Maggiolini, M. GPCRs and Cancer. Acta Pharmacol. Sin. 2012, 33, 351–362. [Google Scholar] [CrossRef]
- Abe, Y.; Tanaka, N. Roles of the Hedgehog Signaling Pathway in Epidermal and Hair Follicle Development, Homeostasis, and Cancer. J. Dev. Biol. 2017, 5, 12. [Google Scholar] [CrossRef]
- Duchartre, Y.; Kim, Y.M.; Kahn, M. The Wnt Signaling Pathway in Cancer. Crit. Rev. Oncol. Hematol. 2016, 99, 141–149. [Google Scholar] [CrossRef]
- Nicheperovich, A.; Townsend-Nicholson, A. Towards Precision Oncology: The Role of Smoothened and Its Variants in Cancer. J. Pers. Med. 2022, 12, 1648. [Google Scholar] [CrossRef] [PubMed]
- Sari, I.N.; Phi, L.T.H.; Jun, N.; Wijaya, Y.T.; Lee, S.; Kwon, H.Y. Hedgehog Signaling in Cancer: A Prospective Therapeutic Target for Eradicating Cancer Stem Cells. Cells 2018, 7, 208. [Google Scholar] [CrossRef] [PubMed]
- Francis, P.A.; Pagani, O.; Fleming, G.F.; Walley, B.A.; Colleoni, M.; Láng, I.; Gómez, H.L.; Tondini, C.; Ciruelos, E.; Burstein, H.J.; et al. Tailoring Adjuvant Endocrine Therapy for Premenopausal Breast Cancer. N. Engl. J. Med. 2018, 379, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Abdulkareem, N.M.; Bhat, R.; Powell, R.T.; Chikermane, S.; Yande, S.; Trinh, L.; Abdelnasser, H.Y.; Tabassum, M.; Ruiz, A.; Sobieski, M.; et al. Screening of GPCR Drugs for Repurposing in Breast Cancer. Front. Pharmacol. 2022, 13, 1049640. [Google Scholar] [CrossRef]
- Usman, S.; Khawer, M.; Rafique, S.; Naz, Z.; Saleem, K. The Current Status of Anti-GPCR drugs against different cancers. J. Pharm. Anal. 2020, 10, 517–521. [Google Scholar] [CrossRef]
- Barreca, M.; Spanò, V.; Raimondi, M.V.; Bivacqua, R.; Giuffrida, S.; Montalbano, A.; Cavalli, A.; Bertoni, F.; Barraja, P. GPCR Inhibition in Treating Lymphoma. ACS Med. Chem. Lett. 2022, 13, 358–364. [Google Scholar] [CrossRef]
- Zhao, J.; DiGiacomo, V.; Ferreras-Gutierrez, M.; Dastjerdi, S.; Ibáñez de, A.; Park, J.-C.; Luebbers, A.; Chen, Q.; Beeler, A.; Blanco, F.J.; et al. Small-Molecule Targeting of GPCR-Independent Non-Canonical G-Protein 1 Signaling in Cancer. Proc. Natl. Acad. Sci. USA 2023, 120, e2213140120. [Google Scholar] [CrossRef]
- Bologa, C.G.; Revankar, C.M.; Young, S.M.; Edwards, B.S.; Arterburn, J.B.; Kiselyov, A.S.; Parker, M.A.; Tkachenko, S.E.; Savchuck, N.P.; Sklar, L.A.; et al. Virtual and Biomolecular Screening Converge on a Selective Agonist for GPR30. Nat. Chem. Biol. 2006, 2, 207–212. [Google Scholar] [CrossRef]
- Yates, M.A.; Li, Y.; Chlebeck, P.J.; Offner, H. GPR30, but Not Estrogen Receptor-α, Is Crucial in the Treatment of Experimental Autoimmune Encephalomyelitis by Oral Ethinyl Estradiol. BMC Immunol. 2010, 11, 20. [Google Scholar] [CrossRef]
- Meyer, M.R.; Prossnitz, E.R.; Barton, M. GPER/GPR30 and Regulation of Vascular Tone and Blood Pressure. Inflamm. Allergy Drug Targets 2012, 11, 255–261. [Google Scholar] [CrossRef]
- Meyer, M.R.; Fredette, N.C.; Howard, T.A.; Hu, C.; Ramesh, C.; Daniel, C.; Amann, K.; Arterburn, J.B.; Barton, M.; Prossnitz, E.R. G Protein-Coupled Estrogen Receptor Protects from Atherosclerosis. Sci. Rep. 2014, 4, 7564. [Google Scholar] [CrossRef] [PubMed]
- Amodio, N.; Leotta, M.; Morelli, E.; Calimeri, T.; Gullà, A.M.; Stamato, M.A.; Romeo, E.; Foresta, U.; Neri, A.; Di Martino, M.T.; et al. Selective G-Protein Estrogen Receptor (GPER) Activation Triggers Anti-Multiple Myeloma Activity and Synergizes with MiR-29b-Inducing Drugs. Blood 2014, 124, 4725. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Z.; Liu, K.; Liu, J.; Chai, S.; Chen, G.; Wen, S.; Ming, T.; Wang, J.; Ma, Y.; et al. Activation of the G Protein-Coupled Estrogen Receptor Prevented the Development of Acute Colitis by Protecting the Crypt Cell. J. Pharmacol. Exp. Ther. 2021, 376, 281–293. [Google Scholar] [CrossRef] [PubMed]
- da Silva, F.E.R.; Cordeiro, R.C.; de Carvalho Lima, C.N.; Cardozo, P.L.; Vasconcelos, G.S.; Monte, A.S.; Sanders, L.L.O.; Vasconcelos, S.M.M.; de Lucena, D.F.; Cruz, B.F.; et al. Sex and the Estrous-Cycle Phase Influence the Expression of G Protein-Coupled Estrogen Receptor 1 (GPER) in Schizophrenia: Translational Evidence for a New Target. Mol. Neurobiol. 2023, 60, 3650–3663. [Google Scholar] [CrossRef]
- Shoushtari, A.N.; Chaney, M.F.; Cohen, J.V.; Garyantes, T.; Lin, J.J.; Ishizuka, J.J.; Mita, A.C.; Mita, M.M.; Muller, C.; Natale, C.; et al. The Effect of LNS8801 Alone and in Combination with Pembrolizumab in Patients with Metastatic Uveal Melanoma. J. Clin. Oncol. 2023, 41, 9543. [Google Scholar] [CrossRef]
- Linnaeus Therapeutics Study Assessing MTD, Safety, Tolerability, PK and Anti-Tumor Effects of LNS8801alone and with Pembrolizumab. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04130516 (accessed on 23 July 2023).
- DeLeon, C.; Wang, H.H.; Gunn, J.; Wilhelm, M.; Cole, A.; Arnett, S.; Wang, D.Q.H.; Arnatt, C.K. A Novel GPER Antagonist Protects against the Formation of Estrogen-Induced Cholesterol Gallstones in Female Mice. J. Lipid Res. 2020, 61, 767–777. [Google Scholar] [CrossRef]
- Dennis, M.K.; Burai, R.; Ramesh, C.; Petrie, W.K.; Alcon, S.N.; Nayak, T.K.; Bologa, C.G.; Leitao, A.; Brailoiu, E.; Deliu, E.; et al. In Vivo Effects of a GPR30 Antagonist. Nat. Chem. Biol. 2009, 5, 421–427. [Google Scholar] [CrossRef]
- Lappano, R.; Rosano, C.; Pisano, A.; Santolla, M.F.; De Francesco, E.M.; De Marco, P.; Dolce, V.; Ponassi, M.; Felli, L.; Cafeo, G.; et al. A Calixpyrrole Derivative Acts as an Antagonist to GPER, a G-Protein Coupled Receptor: Mechanisms and Models. DMM Dis. Models Mech. 2015, 8, 1237–1246. [Google Scholar] [CrossRef]
- Lappano, R.; Santolla, M.F.; Pupo, M.; Sinicropi, M.S.; Caruso, A.; Rosano, C.; Maggiolini, M. MIBE Acts as Antagonist Ligand of Both Estrogen Receptor α and GPER in Breast Cancer Cells. Breast Cancer Res. 2012, 14, R12. [Google Scholar] [CrossRef]
- Dennis, M.K.; Field, A.S.; Burai, R.; Ramesh, C.; Petrie, W.K.; Bologa, C.G.; Oprea, T.I.; Yamaguchi, Y.; Hayashi, S.I.; Sklar, L.A.; et al. Identification of a GPER/GPR30 Antagonist with Improved Estrogen Receptor Counterselectivity. J. Steroid Biochem. Mol. Biol. 2011, 127, 358–366. [Google Scholar] [CrossRef]
- Yu, K.D.; Cai, Y.W.; Wu, S.Y.; Shui, R.H.; Shao, Z.M. Estrogen Receptor-Low Breast Cancer: Biology Chaos and Treatment Paradox. Cancer Commun. 2021, 41, 968–980. [Google Scholar] [CrossRef]
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Guideline Update. Arch. Pathol. Lab. Med. 2020, 144, 545–563. [Google Scholar] [CrossRef] [PubMed]
- Arias-Pulido, H.; Royce, M.; Gong, Y.; Joste, N.; Lomo, L.; Lee, S.J.; Chaher, N.; Verschraegen, C.; Lara, J.; Prossnitz, E.R.; et al. GPR30 and Estrogen Receptor Expression: New Insights into Hormone Dependence of Inflammatory Breast Cancer. Breast Cancer Res. Treat. 2010, 123, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Ignatov, A.; Ignatov, T.; Weienborn, C.; Eggemann, H.; Bischoff, J.; Semczuk, A.; Roessner, A.; Costa, S.D.; Kalinski, T. G-Protein-Coupled Estrogen Receptor GPR30 and Tamoxifen Resistance in Breast Cancer. Breast Cancer Res. Treat. 2011, 128, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Brueggemeier, R.W. Aromatase Inhibitors—Mechanisms of Steroidal Inhibitors. Breast Cancer Res. Treat. 1994, 30, 31–42. [Google Scholar] [CrossRef]
- Chen, S.-H.; Cheung, C.H.A. Challenges in Treating Estrogen Receptor-Positive Breast Cancer. In Estrogen; Khan, W.A., Ed.; IntechOpen: Rijeka, Yugoslavia, 2018; p. Ch. 1. ISBN 978-1-83880-867-9. [Google Scholar]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast Cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef]
- NIH: Inxight Drugs Formestane. Available online: https://drugs.ncats.io/substances?facet=Originator%2FLisboa,%20B.P (accessed on 22 July 2023).
- Cardoso, F.; Costa, A.; Senkus, E.; Aapro, M.; André, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.; Biganzoli, L.; Cardoso, M.J.; et al. 3rd ESO–ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 3). Breast 2017, 31, 244–259. [Google Scholar] [CrossRef]
- Lloyd, M.R.; Wander, S.A.; Hamilton, E.; Razavi, P.; Bardia, A. Next-Generation Selective Estrogen Receptor Degraders and Other Novel Endocrine Therapies for Management of Metastatic Hormone Receptor-Positive Breast Cancer: Current and Emerging Role. Ther. Adv. Med. Oncol. 2022, 14, 17588359221113694. [Google Scholar] [CrossRef]
- American Cancer Society Hormone Therapy for Breast Cancer. Available online: https://www.cancer.org/cancer/types/breast-cancer/treatment/hormone-therapy-for-breast-cancer.html (accessed on 22 July 2023).
- Yao, S.; Xu, B.; Li, Q.; Zhang, P.; Yuan, P.; Wang, J.; Ma, F.; Fan, Y. Goserelin plus Letrozole as First- or Second-Line Hormonal Treatment in Premenopausal Patients with Advanced Breast Cancer. Endocr. J. 2011, 58, 509–516. [Google Scholar] [CrossRef]
- Carlson, R.W.; Theriault, R.; Schurman, C.M.; Rivera, E.; Chung, C.T.; Phan, S.C.; Arun, B.; Dice, K.; Chiv, V.Y.; Green, M.; et al. Phase II Trial of Anastrozole plus Goserelin in the Treatment of Hormone Receptor-Positive, Metastatic Carcinoma of the Breast in Premenopausal Women. J. Clin. Oncol. 2010, 28, 3917–3921. [Google Scholar] [CrossRef]
- Bradley, R.; Braybrooke, J.; Gray, R.; Hills, R.K.; Liu, Z.; Pan, H.; Peto, R.; Dodwell, D.; McGale, P.; Taylor, C.; et al. Aromatase Inhibitors versus Tamoxifen in Premenopausal Women with Oestrogen Receptor-Positive Early-Stage Breast Cancer Treated with Ovarian Suppression: A Patient-Level Meta-Analysis of 7030 Women from Four Randomised Trials. Lancet Oncol. 2022, 23, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Nourmoussavi, M.; Pansegrau, G.; Popesku, J.; Hammond, G.L.; Kwon, J.S.; Carey, M.S. Ovarian Ablation for Premenopausal Breast Cancer: A Review of Treatment Considerations and the Impact of Premature Menopause. Cancer Treat. Rev. 2017, 55, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Jonat, W.; Kaufmann, M.; Blamey, R.W.; Howell, A.; Collins, J.P.; Coates, A.; Eiermann, W.; Jänicke, F.; Njordenskold, B.; Forbes, J.F.; et al. A Randomised Study to Compare the Effect of the Luteinising Hormone Releasing Hormone (LHRH) Analogue Goserelin with or without Tamoxifen in Pre- and Perimenopausal Patients with Advanced Breast Cancer. Eur. J. Cancer 1995, 31, 137–142. [Google Scholar] [CrossRef]
- Tamoxifen Citrate—Drug Usage Statistics. Available online: https://clincalc.com/DrugStats/Drugs/Tamoxifen (accessed on 23 July 2023).
- NIH NCI Tamoxifen Citrate. Available online: https://www.cancer.gov/about-cancer/treatment/drugs/tamoxifencitrate (accessed on 23 July 2023).
- Drugs.com Tamoxifen (Monograph). Available online: https://www.drugs.com/monograph/tamoxifen.html (accessed on 23 July 2023).
- Davies, C.; Pan, H.; Godwin, J.; Gray, R.; Arriagada, R.; Raina, V.; Abraham, M.; Medeiros Alencar, V.H.; Badran, A.; Bonfill, X.; et al. Long-Term Effects of Continuing Adjuvant Tamoxifen to 10 Years versus Stopping at 5 Years after Diagnosis of Oestrogen Receptor-Positive Breast Cancer: ATLAS, a Randomised Trial. Lancet 2013, 381, 805–816. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Burstein, H.J.; Chew, H.; Dang, C.; et al. Breast Cancer, Version 3.2022. JNCCN J. Natl. Compr. Cancer Netw. 2022, 20, 691–722. [Google Scholar] [CrossRef]
- Cosman, F.; Lindsay, R. Selective estrogen receptor modulators: Clinical spectrum. Endocr. Rev. 1999, 20, 418–434. [Google Scholar] [CrossRef]
- Sieber, P.R. Treatment of Bicalutamide-Induced Breast Events. Expert Rev. Anticancer Ther. 2007, 7, 1773–1779. [Google Scholar] [CrossRef]
- Vogel, V.G.; Costantino, J.P.; Wickerham, D.L.; Cronin, W.M.; Cecchini, R.S.; Atkins, J.N.; Bevers, T.B.; Fehrenbacher, L.; Pajon, E.R.; Wade, J.L.; et al. Effects of Tamoxifen vs Raloxifene on the Risk of Developing Invasive Breast Cancer and Other Disease OutcomesThe NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 Trial. JAMA 2006, 295, 2727–2741. [Google Scholar] [CrossRef]
- Nilsson, S.; Koehler, K.F. Oestrogen Receptors and Selective Oestrogen Receptor Modulators: Molecular and Cellular Pharmacology. Basic Clin. Pharmacol. Toxicol. 2005, 96, 15–25. [Google Scholar] [CrossRef]
- Hanker, A.B.; Sudhan, D.R.; Arteaga, C.L. Overcoming Endocrine Resistance in Breast Cancer. Cancer Cell 2020, 37, 496–513. [Google Scholar] [CrossRef]
- Fan, P.; Craig Jordan, V. Acquired Resistance to Selective Estrogen Receptor Modulators (SERMs) in Clinical Practice (Tamoxifen & Raloxifene) by Selection Pressure in Breast Cancer Cell Populations. Steroids 2014, 90, 44–52. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for Early Breast Cancer: An Overview of the Randomised Trials. Lancet 1998, 351, 1451–1467. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, S.C. The Race to Develop Oral SERDs and Other Novel Estrogen Receptor Inhibitors: Recent Clinical Trial Results and Impact on Treatment Options. Cancer Metastasis Rev. 2022, 41, 975–990. [Google Scholar] [CrossRef]
- Hartkopf, A.D.; Grischke, E.M.; Brucker, S.Y. Endocrine-Resistant Breast Cancer: Mechanisms and Treatment. Breast Care 2020, 15, 347–354. [Google Scholar] [CrossRef]
- Patel, H.K.; Bihani, T. Selective Estrogen Receptor Modulators (SERMs) and Selective Estrogen Receptor Degraders (SERDs) in Cancer Treatment. Pharmacol. Ther. 2018, 186, 1–24. [Google Scholar] [CrossRef]
- Hernando, C.; Ortega-Morillo, B.; Tapia, M.; Moragón, S.; Martínez, M.T.; Eroles, P.; Garrido-Cano, I.; Adam-Artigues, A.; Lluch, A.; Bermejo, B.; et al. Oral Selective Estrogen Receptor Degraders (SERDs) as a Novel Breast Cancer Therapy: Present and Future from a Clinical Perspective. Int. J. Mol. Sci. 2021, 22, 7812. [Google Scholar] [CrossRef]
- Burke, M.R.; Smith, A.R.; Zheng, G. Overcoming Cancer Drug Resistance Utilizing PROTAC Technology. Front. Cell Dev. Biol. 2022, 10, 872729. [Google Scholar] [CrossRef]
- Wardell, S.E.; Marks, J.R.; McDonnell, D.P. The Turnover of Estrogen Receptor α by the Selective Estrogen Receptor Degrader (SERD) Fulvestrant Is a Saturable Process That Is Not Required for Antagonist Efficacy. Biochem. Pharmacol. 2011, 82, 122–130. [Google Scholar] [CrossRef]
- Wittmann, B.M.; Sherk, A.; McDonnell, D.P. Definition of Functionally Important Mechanistic Differences among Selective Estrogen Receptor Down-Regulators. Cancer Res. 2007, 67, 9549–9560. [Google Scholar] [CrossRef]
- Lai, A.C.; Crews, C.M. Induced Protein Degradation: An Emerging Drug Discovery Paradigm. Nat. Rev. Drug Discov. 2017, 16, 101–114. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, W. Selective Estrogen Receptor Degraders (SERDs): A Promising Strategy for Estrogen Receptor Positive Endocrine-Resistant Breast Cancer. J. Med. Chem. 2020, 63, 15094–15114. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.I.; Goodwin, A.; Wilcken, N. Fulvestrant for Hormone-sensitive Metastatic Breast Cancer. Cochrane Database Syst. Rev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Dodwell, D.; Wardley, A.; Johnston, S. Postmenopausal Advanced Breast Cancer: Options for Therapy after Tamoxifen and Aromatase Inhibitors. Breast 2006, 15, 584–594. [Google Scholar] [CrossRef] [PubMed]
- De Santo, I.; McCartney, A.; Migliaccio, I.; Di Leo, A.; Malorni, L. The Emerging Role of ESR1 Mutations in Luminal Breast Cancer as a Prognostic and Predictive Biomarker of Response to Endocrine Therapy. Cancers 2019, 11, 1894. [Google Scholar] [CrossRef]
- Wang, L.; Guillen, V.S.; Sharma, N.; Flessa, K.; Min, J.; Carlson, K.E.; Toy, W.; Braqi, S.; Katzenellenbogen, B.S.; Katzenellenbogen, J.A.; et al. New Class of Selective Estrogen Receptor Degraders (SERDs): Expanding the Toolbox of PROTAC Degrons. ACS Med. Chem. Lett. 2018, 9, 803–808. [Google Scholar] [CrossRef]
- FDA Approves Elacestrant for ER-Positive, HER2-Negative, ESR1-Mutated Advanced or Metastatic Breast Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-elacestrant-er-positive-her2-negative-esr1-mutated-advanced-or-metastatic-breast-cancer (accessed on 23 July 2023).
- Bidard, F.-C.; Kaklamani, V.G.; Neven, P.; Streich, G.; Montero, A.J.; Forget, F.; Mouret-Reynier, M.-A.; Sohn, J.H.; Taylor, D.; Harnden, K.K.; et al. Elacestrant (Oral Selective Estrogen Receptor Degrader) Versus Standard Endocrine Therapy for Estrogen Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Advanced Breast Cancer: Results From the Randomized Phase III EMERALD Trial. J. Clin. Oncol. 2022, 40, 3246–3256. [Google Scholar] [CrossRef]
- AACR Camizestrant May Be Superior to Fulvestrant in Patients with Hormone Receptor-Positive, HER2-Negative Breast Cancer. Available online: https://www.aacr.org/about-the-aacr/newsroom/news-releases/camizestrant-may-be-superior-to-fulvestrant-in-patients-with-hormone-receptor-positive-her2-negative-breast-cancer/ (accessed on 23 July 2023).
- Caroline Helwick SERENA-2 Trial: Camizestrant Improves Progression-Free Survival in Advanced Breast Cancer. Available online: https://ascopost.com/issues/january-25-2023/camizestrant-improves-progression-free-survival-in-advanced-breast-cancer/ (accessed on 23 July 2023).
- Lin, J.; Jin, J.; Shen, Y.; Zhang, L.; Gong, G.; Bian, H.; Chen, H.; Nagle, D.G.; Wu, Y.; Zhang, W.; et al. Emerging protein degradation strategies: Expanding the scope to extracellular and membrane proteins. Theranostics 2021, 11, 8337–8349. [Google Scholar] [CrossRef]
- Sakamoto, K.M.; Kim, K.B.; Kumagai, A.; Mercurio, F.; Crews, C.M.; Deshaies, R.J. Protacs: Chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. Proc. Natl. Acad. Sci. USA 2001, 98, 8554–8559. [Google Scholar] [CrossRef]
- Kostic, M.; Jones, L.H. Critical Assessment of Targeted Protein Degradation as a Research Tool and Pharmacological Modality. Trends Pharmacol. Sci. 2020, 41, 305–317. [Google Scholar] [CrossRef]
- Yang, G.; Nowsheen, S.; Aziz, K.; Georgakilas, A.G. Toxicity and Adverse Effects of Tamoxifen and Other Anti-Estrogen Drugs. Pharmacol. Ther. 2013, 139, 392–404. [Google Scholar] [CrossRef]
| Estrogen | Structure | % Relative Binding Affinity: AffinityEstrogen/Affinity17β-E2 * 100% | ||
|---|---|---|---|---|
| ERα | ERβ | GPER 5 | ||
| 17β-E2 | 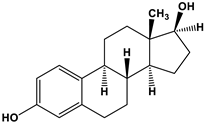 | 100 | 100 | 100 |
| Bisphenol A | 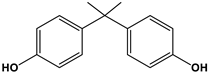 | 0.008 2 | 0.008 2 | 0.5–1 |
| Genistein | 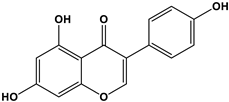 | 0.021 3 | 6.8 3 | 2–5 |
| Daidzein | 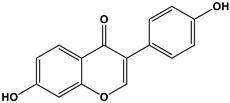 | 0.003 3 | 0.051 3 | N/D |
| 4-Hydroxytamoxifen | 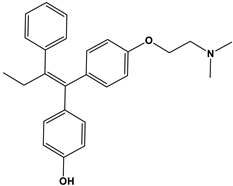 | 257 4 | 232 4 | 0.3–6 |
| Therapy/Attributes | AI | LHRH Analog | SERM | SERD |
|---|---|---|---|---|
| Class | Inhibitor | Inhibitor | Inhibitor | Degrader (noncatalytic) |
| Receptor Target | Aromatase | GnRH Receptor | ER | ER |
| Downregulation MOA | Inhibition of estrogen biosynthesis | Ovarian suppression | ER-mediated gene transactivation | ER degradation and signaling |
| Examples | Exemestane, Anastrozole, Letrozole | Leuprolide, Goserelin, Triptorelin | Tamoxifen, Toremifene, Raloxifene | Fulvestrant, Elacestrant |
| Treatment | First line | First line | First line | Second line |
| ER+/GPER+ | Yes | Yes | Yes a | Yes a |
| ER+/GPER− | Yes | Yes | Yes | Yes |
| ER−/GPER+ | No | No | No | No |
| ER−/GPER− | No | No | No | No |
| TNBC | No | No | No | No |
| Drug Resistance | Yes | Yes | Yes | Yes |
| Endogenous Estrogen | Yes | Yes | No | No |
| Environmental Estrogen | No | No | No | No |
| Premenopausal | Yes b | Yes c | Yes d | No |
| Postmenopausal | Yes | Yes | Yes | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hall, K.A.; Filardo, E.J. The G Protein-Coupled Estrogen Receptor (GPER): A Critical Therapeutic Target for Cancer. Cells 2023, 12, 2460. https://doi.org/10.3390/cells12202460
Hall KA, Filardo EJ. The G Protein-Coupled Estrogen Receptor (GPER): A Critical Therapeutic Target for Cancer. Cells. 2023; 12(20):2460. https://doi.org/10.3390/cells12202460
Chicago/Turabian StyleHall, Keith A., and Edward J. Filardo. 2023. "The G Protein-Coupled Estrogen Receptor (GPER): A Critical Therapeutic Target for Cancer" Cells 12, no. 20: 2460. https://doi.org/10.3390/cells12202460
APA StyleHall, K. A., & Filardo, E. J. (2023). The G Protein-Coupled Estrogen Receptor (GPER): A Critical Therapeutic Target for Cancer. Cells, 12(20), 2460. https://doi.org/10.3390/cells12202460




