Multiomics Study of a Novel Naturally Derived Small Molecule, NSC772864, as a Potential Inhibitor of Proto-Oncogenes Regulating Cell Cycle Progression in Colorectal Cancer
Abstract
1. Introduction
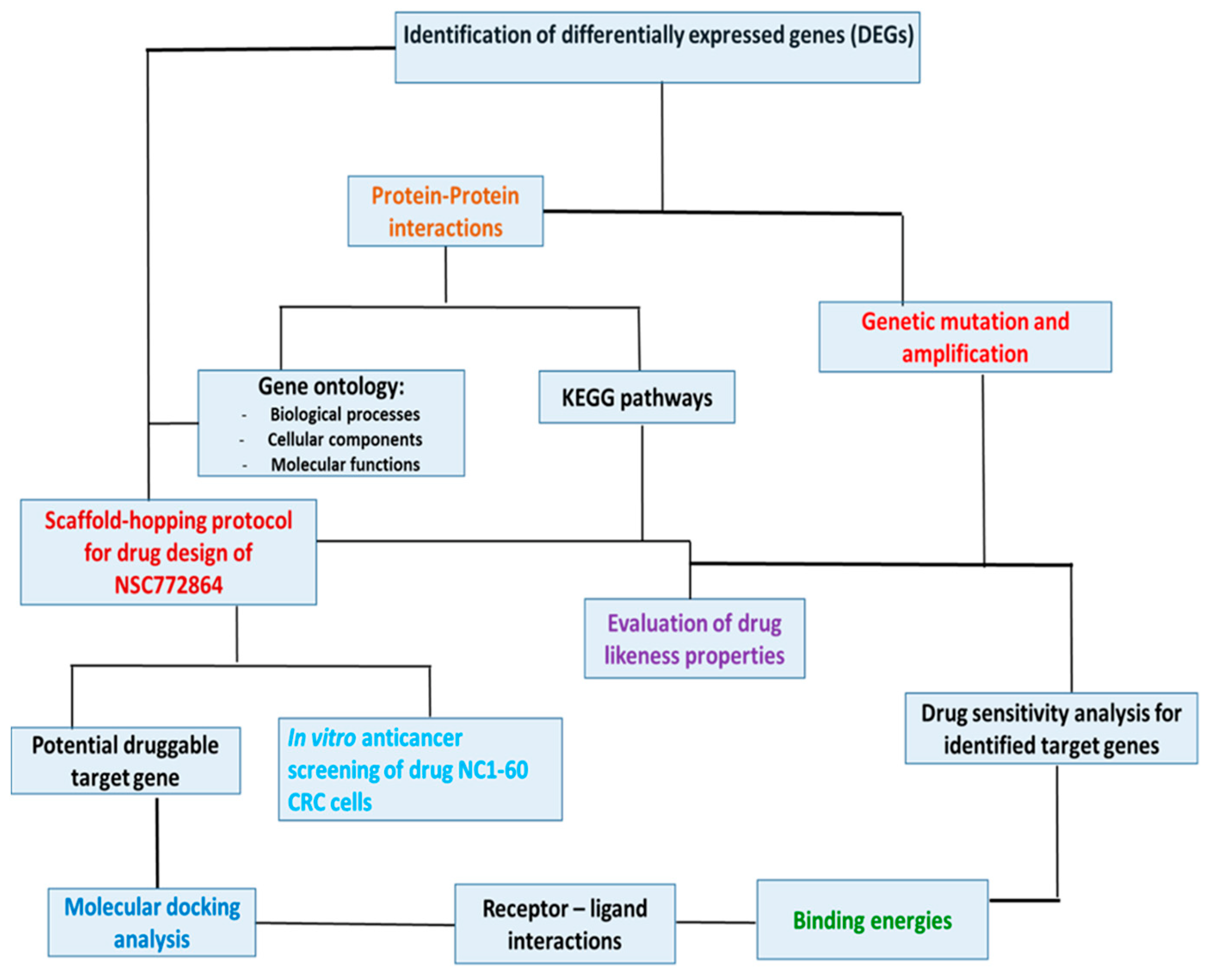
2. Material and Methods
2.1. Retrieval of the Top 25 DEGs in CRC
2.2. DEG Validation
2.3. Interacting Network Construction and Functional Enrichment Analysis
2.4. Analysis of c-Met/GSK3β/MYC/CCND1 Genetic Alterations and Mutation Analysis in CRC Tissues
2.5. Drug Sensitivity and Gene Expression Profiling for c-Met/GSK3β/MYC/CCND1 Oncogenes in Colon Tumors
2.6. c-Met, MYC, and CCND1 Are Potential Drug Targets of NSC772864
2.7. Evaluation of Drug-Likeness, Absorption, Distribution, Metabolism, and Excretion (ADME) Properties and Friendliness of NSC772864
2.8. In Vitro Anticancer Screening of NSC77286 against NC1 60 CRC Cells
2.9. Molecular Docking Analysis
2.10. Statistical Analysis
3. Results
3.1. High Expressions of c-Met/GSK3β/MYC/CCND1 Promote Colon Cancer Progression
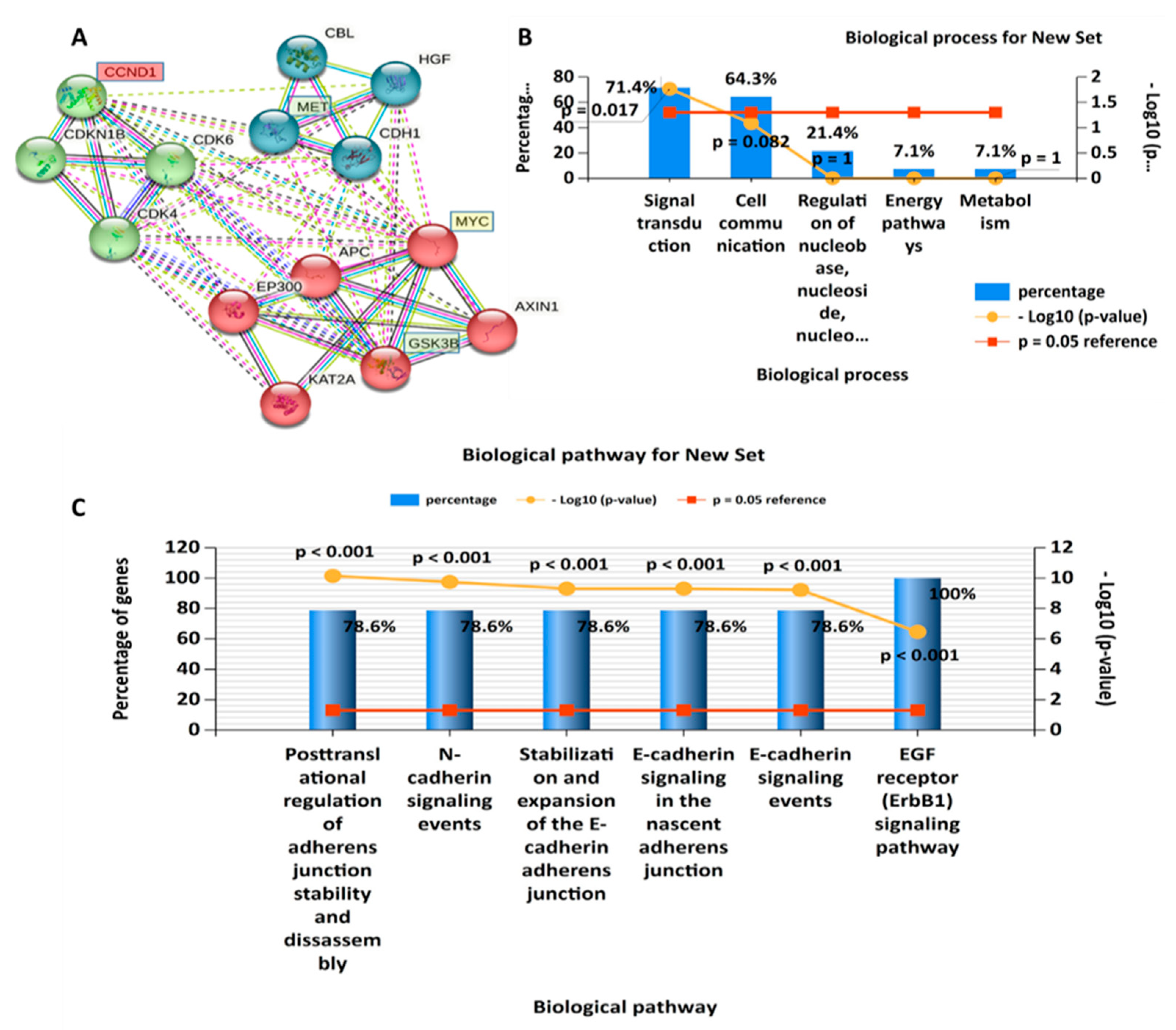
3.2. PPI Network (PIN) Construction and Enrichment Analysis
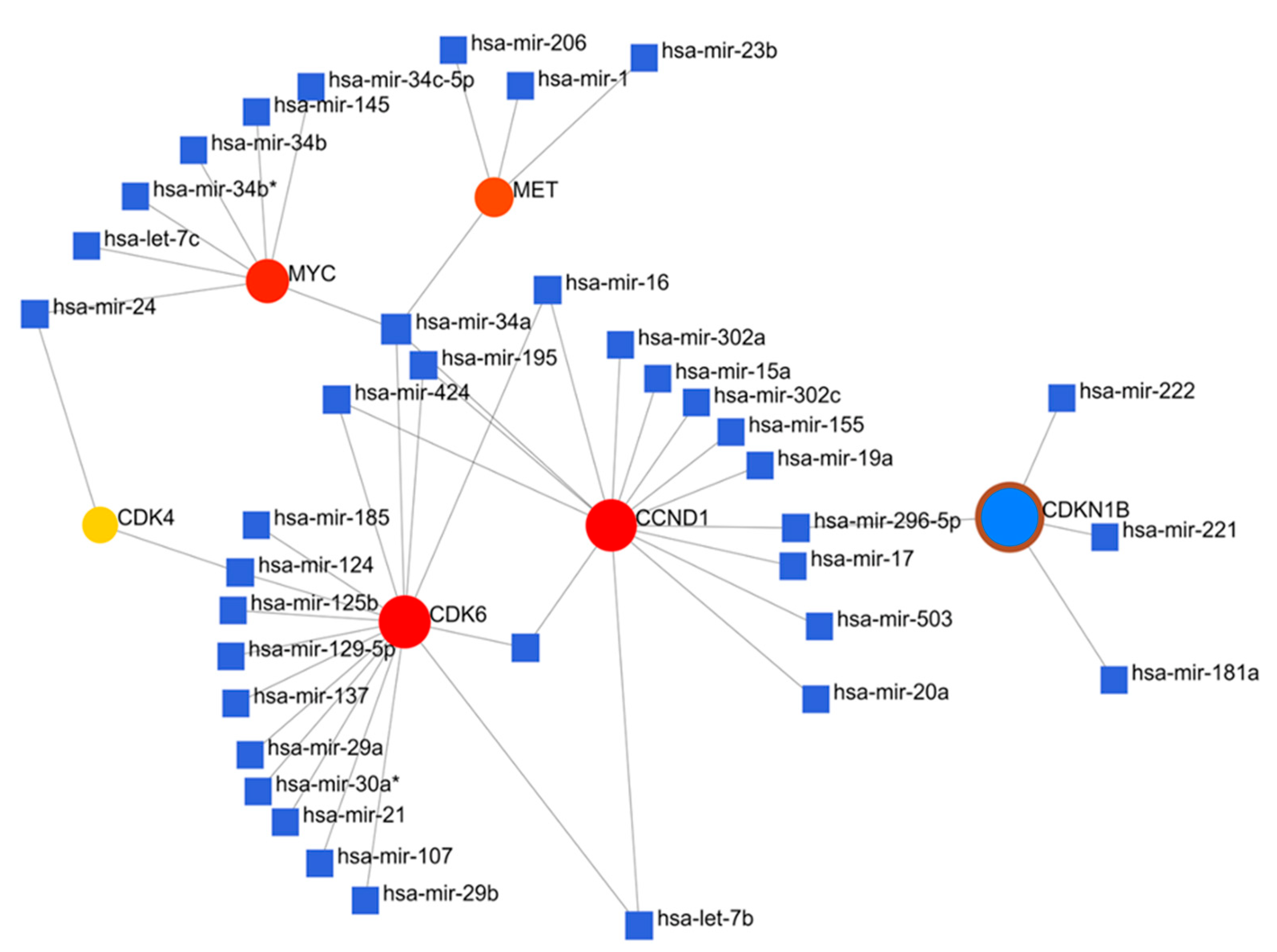
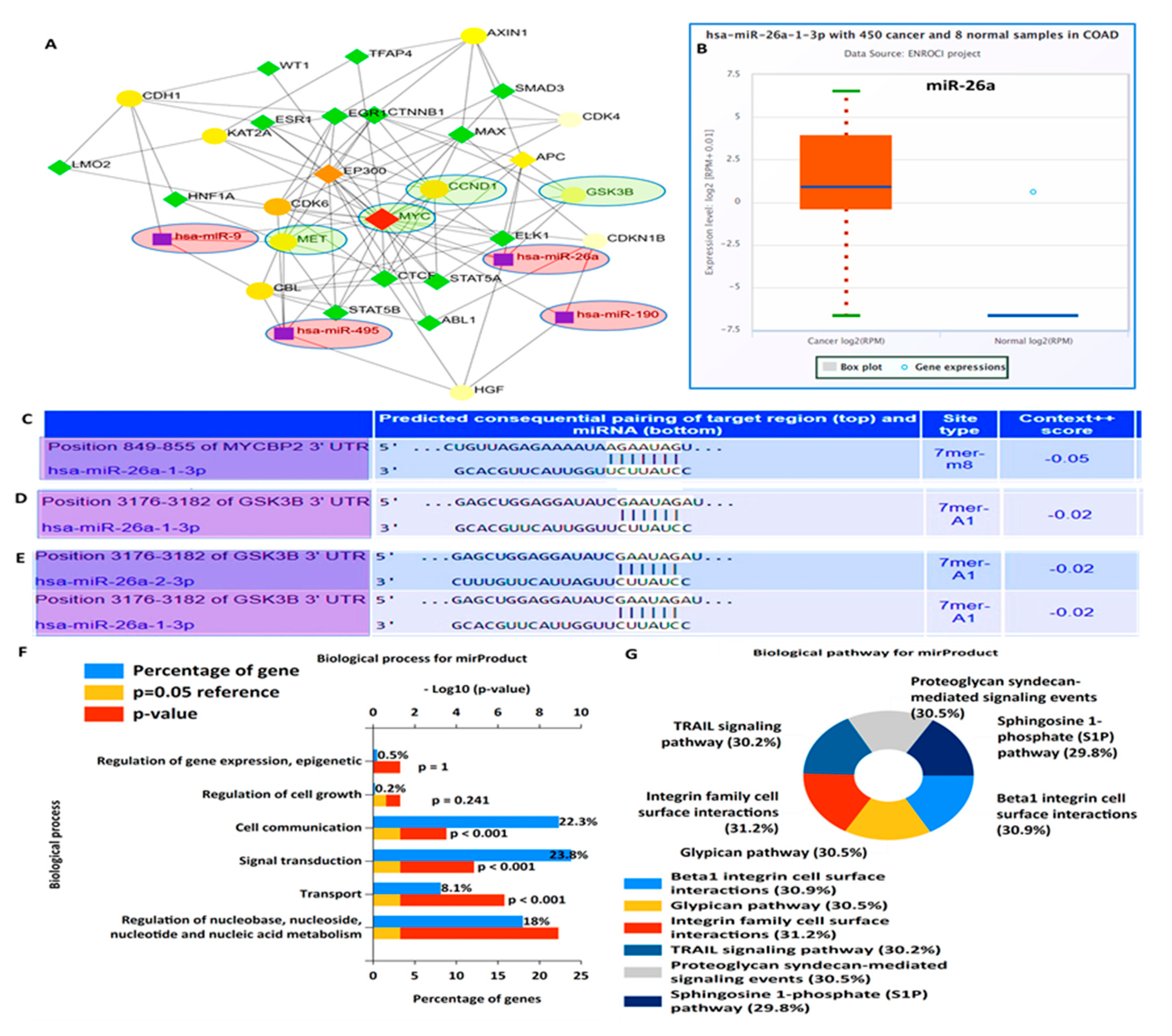
3.3. Crosstalk between Overexpression of Met/GSK3β/MYC/CCND1 Oncogenes and Upregulated miR-26a Are Associated with CRC Cancer Progression
3.4. Genomic Alterations in c-Met/GSK3β/MYC/CCND1 Signatures Are Associated with Poor Prognoses of CRC Cohorts
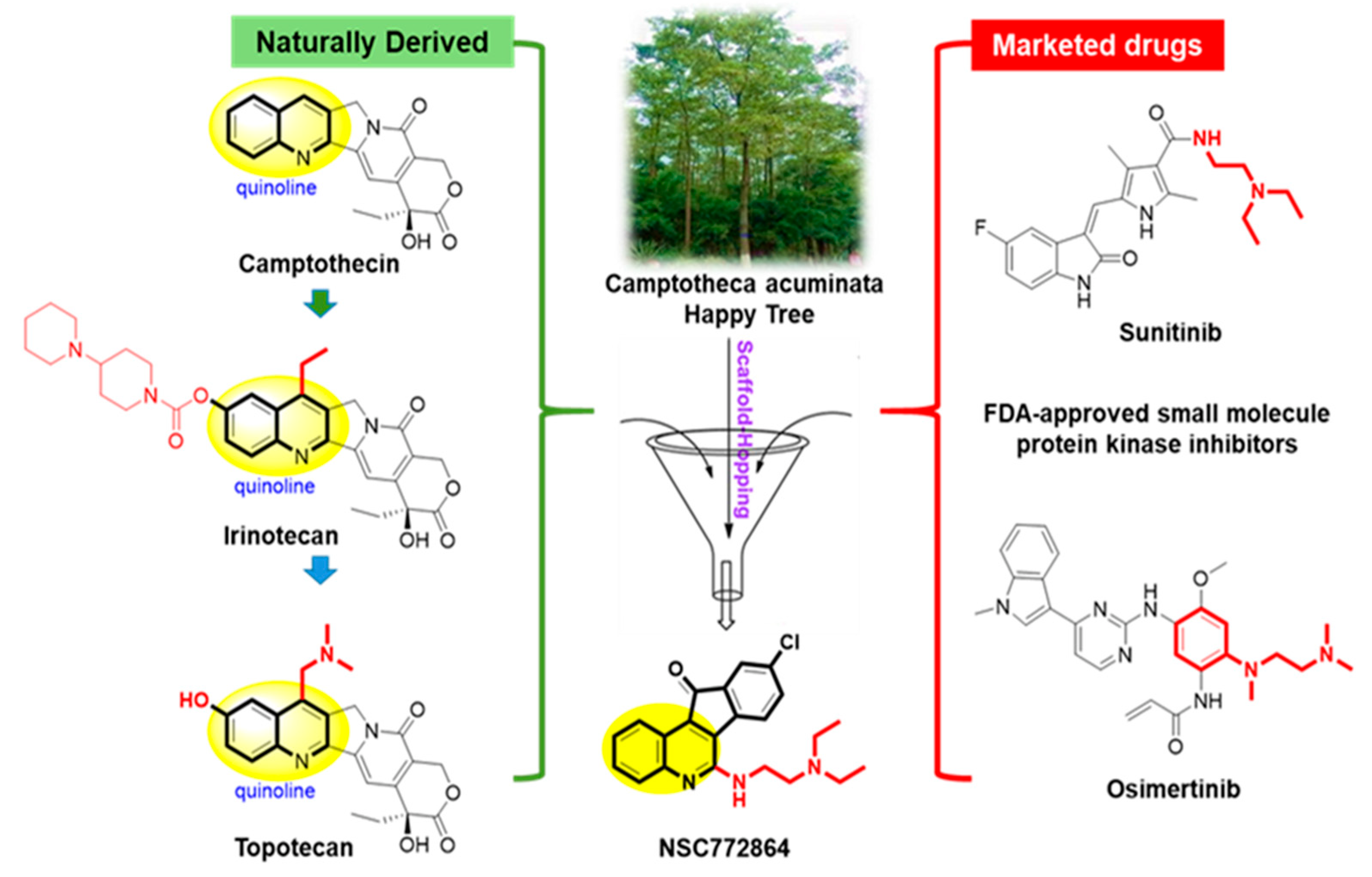
3.5. Rational Scaffold-Hopping Protocol for the Design of NSC772684
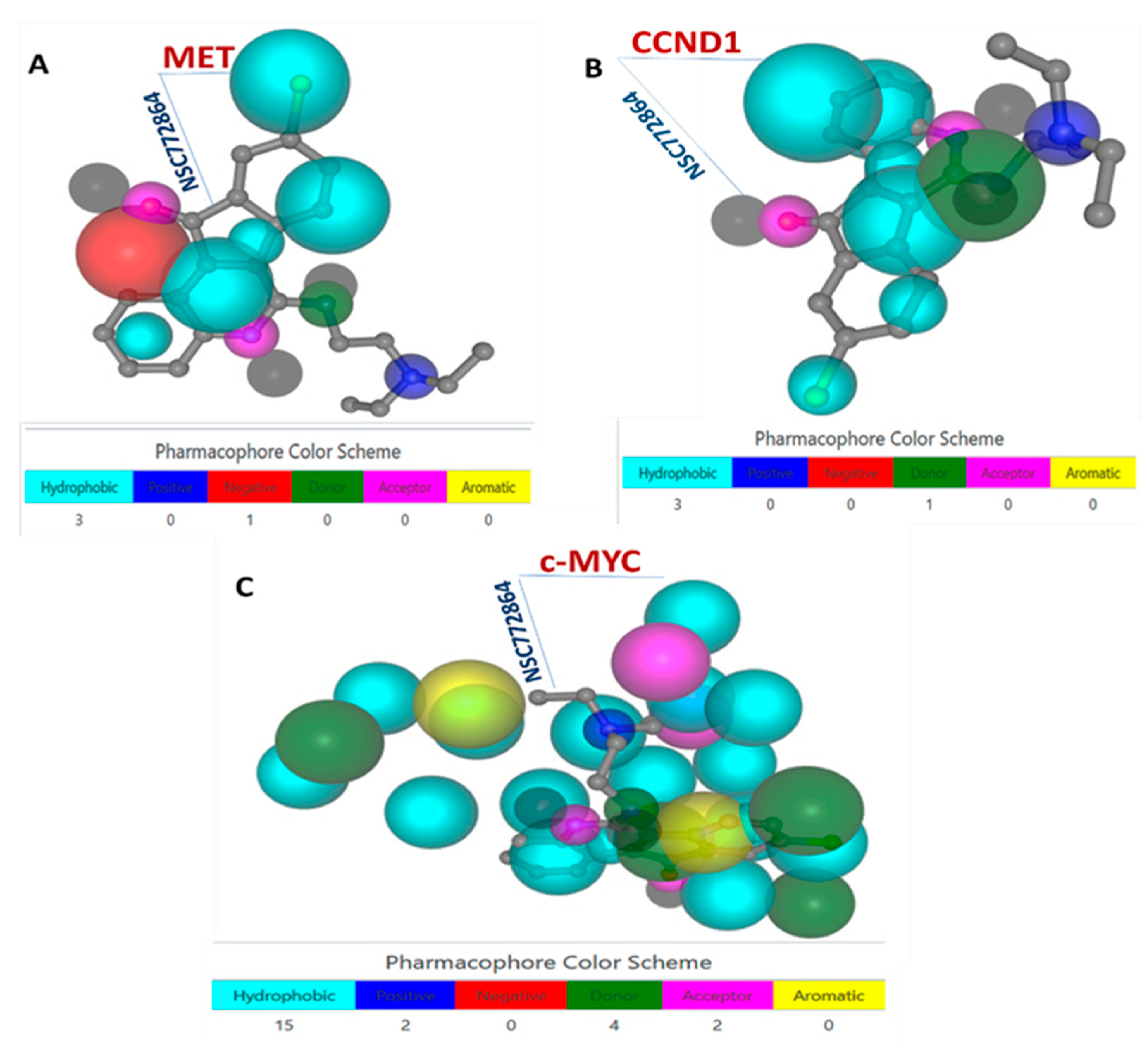
3.6. c-Met, MYC, and CCND1 Are Potential Drug Targets of NSC772864
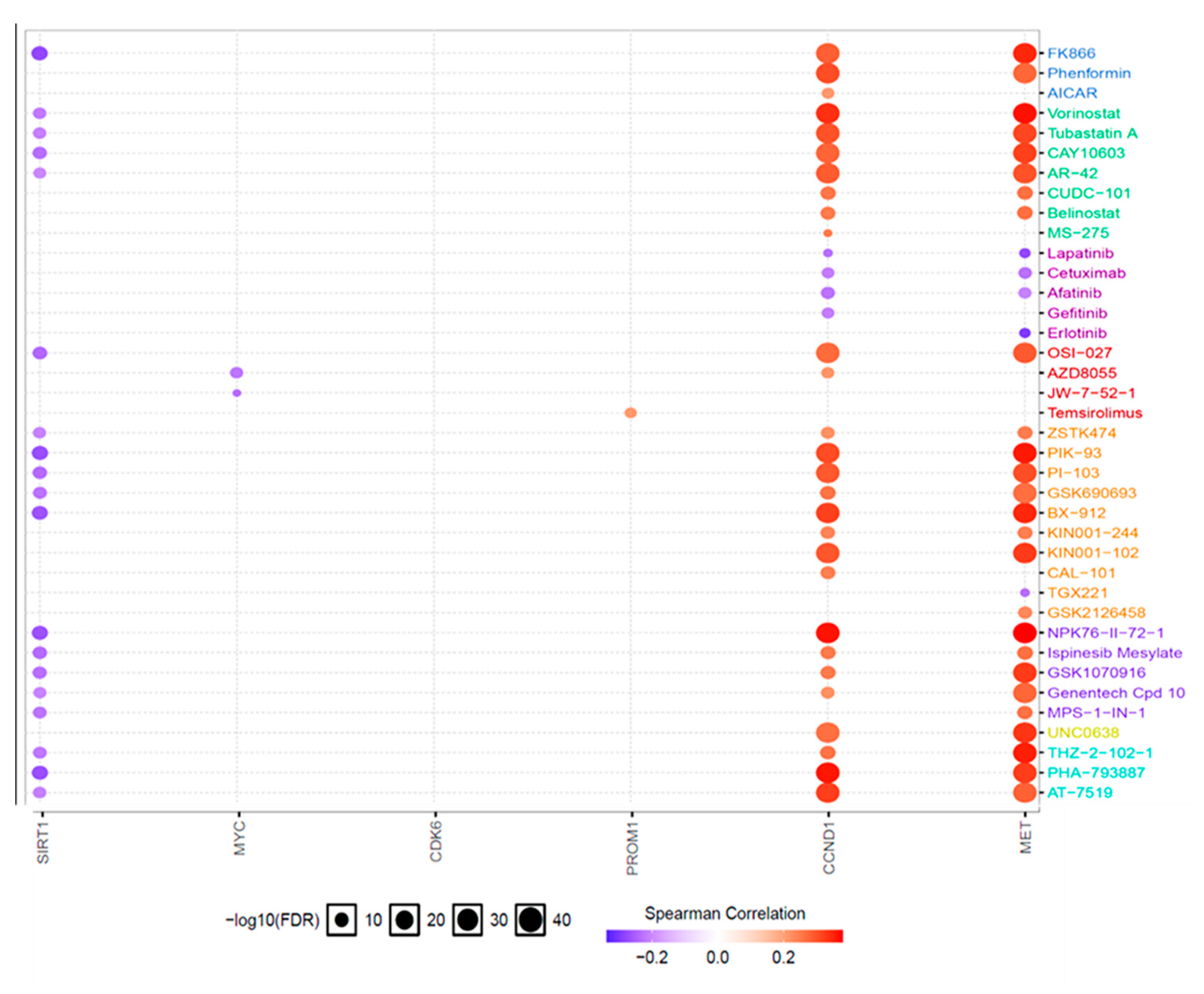
3.7. Drug Sensitivity Analysis for the c-Met/GSK3β/MYC/CCND1 Oncogenes in Colon Tumors
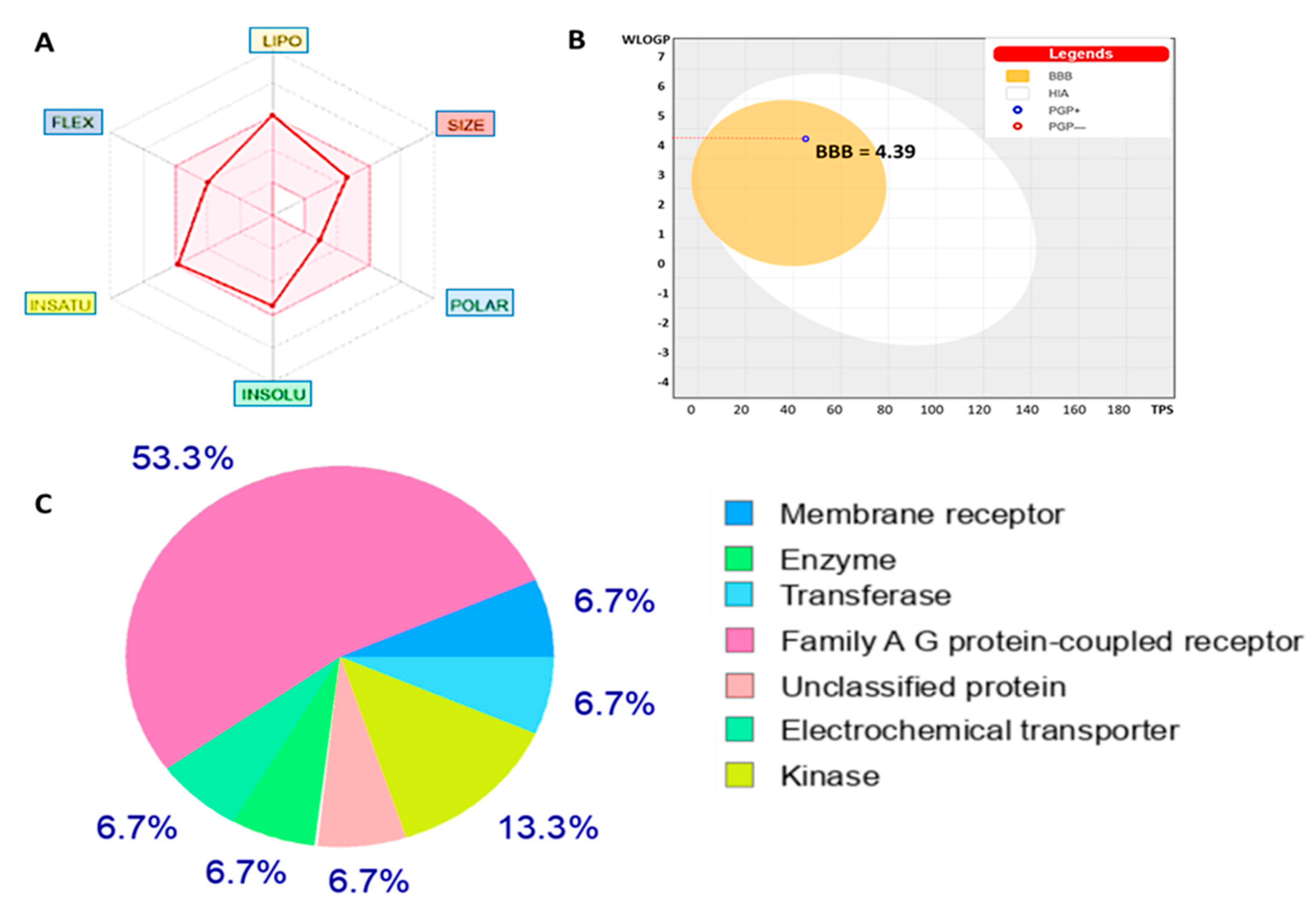
3.8. Evaluation of Drug Likeness, ADME Properties, and Friendliness of NSC772864
3.9. In Vitro Anticancer Screening of SJ3 against NC1-60 CRC Cells
3.10. Molecular Docking Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Linnekamp, J.F.; Wang, X.; Medema, J.P.; Vermeulen, L. Colorectal cancer heterogeneity and targeted therapy: A case for molecular disease subtypes. Cancer Res. 2015, 75, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Douillard, J.Y.; Oliner, K.S.; Siena, S.; Tabernero, J.; Burkes, R.; Barugel, M.; Humblet, Y.; Bodoky, G.; Cunningham, D.; Jassem, J.; et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N. Engl. J. Med. 2013, 369, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Köhne, C.H.; Láng, I.; Folprecht, G.; Nowacki, M.P.; Cascinu, S.; Shchepotin, I.; Maurel, J.; Cunningham, D.; Tejpar, S.; et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor K.R.A.S. and B.R.A.F. mutation status. J. Clin. Oncol. 2011, 29, 2011–2019. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Köhne, C.H.; Hitre, E.; Zaluski, J.; Chang Chien, C.R.; Makhson, A.; D’Haens, G.; Pintér, T.; Lim, R.; Bodoky, G.; et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N. Engl.J. Med. 2009, 360, 1408–1417. [Google Scholar] [CrossRef]
- Saltz, L.B.; Clarke, S.; Díaz-Rubio, E.; Scheithauer, W.; Figer, A.; Wong, R.; Koski, S.; Lichinitser, M.; Yang, T.S.; Rivera, F.; et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J. Clin. Oncol. 2008, 26, 2013–2019. [Google Scholar] [CrossRef]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Brenner, H.; Kloor, M.; Pox, C.P. Colorectal cancer. Lancet 2014, 383, 1490–1502. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef]
- Grady, W.M.; Markowitz, S.D. The molecular pathogenesis of colorectal cancer and its potential application to colorectal cancer screening. Dig. Dis. Sci. 2015, 60, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Sameer, A.S.; Nissar, S.; Fatima, K. Mismatch repair pathway: Molecules, functions, and role in colorectal carcinogenesis. Eur. J. Cancer Prev. 2014, 23, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Moosavi, F.; Giovannetti, E.; Saso, L.; Firuzi, O. HGF/MET pathway aberrations as diagnostic, prognostic, and predictive biomarkers in human cancers. Crit. Rev. Clin. Lab. Sci. 2019, 56, 533–566. [Google Scholar] [CrossRef] [PubMed]
- Boccaccio, C.; Comoglio, P.M. Invasive growth: A MET-driven genetic programme for cancer and stem cells. Nat. Rev. Cancer 2006, 6, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Christofori, G. New signals from the invasive front. Nature 2006, 441, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Trusolino, L.; Comoglio, P.M. Scatter-factor and semaphorin receptors: Cell signalling for invasive growth. Nat. Rev. Cancer 2002, 2, 289–300. [Google Scholar] [CrossRef]
- Ye, X.; Weinberg, R.A. Epithelial-Mesenchymal Plasticity: A Central Regulator of Cancer Progression. Trends Cell Biol. 2015, 25, 675–686. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, Q.; Shen, J.; Bindokas, V.; Xing, H.R. Pharmacologic inactivation of kinase suppressor of Ras1 sensitizes epidermal growth factor receptor and oncogenic Ras-dependent tumors to ionizing radiation treatment. Mol. Cancer Ther. 2010, 9, 2724–2736. [Google Scholar] [CrossRef]
- Kammula, U.S.; Kuntz, E.J.; Francone, T.D.; Zeng, Z.; Shia, J.; Landmann, R.G.; Paty, P.B.; Weiser, M.R. Molecular co-expression of the c-Met oncogene and hepatocyte growth factor in primary colon cancer predicts tumor stage and clinical outcome. Cancer Lett. 2007, 248, 219–228. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, J.; Park, S.H.; Park, J.O.; Lim, H.Y.; Kang, W.K.; Park, Y.S.; Kim, S.T. c-MET Overexpression in Colorectal Cancer: A Poor Prognostic Factor for Survival. Clin. Colorectal. Cancer 2018, 17, 165–169. [Google Scholar] [CrossRef]
- Lin, Y.M.; Lu, C.C.; Hsiang, Y.P.; Pi, S.C.; Chen, C.I.; Cheng, K.C.; Pan, H.L.; Chien, P.H.; Chen, Y.J. c-Met inhibition is required for the celecoxib-attenuated stemness property of human colorectal cancer cells. J. Cell Physiol. 2019, 234, 10336–10344. [Google Scholar] [CrossRef]
- Di Renzo, M.F.; Olivero, M.; Giacomini, A.; Porte, H.; Chastre, E.; Mirossay, L.; Nordlinger, B.; Bretti, S.; Bottardi, S.; Giordano, S.; et al. Overexpression and amplification of the met/HGF receptor gene during the progression of colorectal cancer. Clin. Cancer Res. 1995, 1, 147–154. [Google Scholar]
- Troiani, T.; Martinelli, E.; Napolitano, S.; Vitagliano, D.; Ciuffreda, L.P.; Costantino, S.; Morgillo, F.; Capasso, A.; Sforza, V.; Nappi, A.; et al. Increased TGF-α as a mechanism of acquired resistance to the anti-EGFR inhibitor cetuximab through EGFR-MET interaction and activation of MET signaling in colon cancer cells. Clin. Cancer Res. 2013, 19, 6751–6765. [Google Scholar] [CrossRef] [PubMed]
- Imura, Y.; Nakai, T.; Yamada, S.; Outani, H.; Takenaka, S.; Hamada, K.; Araki, N.; Itoh, K.; Yoshikawa, H.; Naka, N. Functional and therapeutic relevance of hepatocyte growth factor/c-MET signaling in synovial sarcoma. Cancer Sci. 2016, 107, 1867–1876. [Google Scholar] [CrossRef]
- Pilotto, S.; Carbognin, L.; Karachaliou, N.; Ma, P.C.; Rosell, R.; Tortora, G.; Bria, E. Tracking, MET de-addiction in lung cancer: A road towards the oncogenic target. Cancer Treat. Rev. 2017, 60, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.D.; Györffy, B.; Vogel, R.; Eckert, K.; Valenti, G.; Fang, L.; Lohneis, P.; Elezkurtaj, S.; Ziebold, U.; Birchmeier, W. Combined Wnt/β-catenin, Met, and CXCL12/CXCR4 signals characterize basal breast cancer and predict disease outcome. Cell Rep. 2013, 5, 1214–1227. [Google Scholar] [CrossRef] [PubMed]
- Tuynman, J.B.; Vermeulen, L.; Boon, E.M.; Kemper, K.; Zwinderman, A.H.; Peppelenbosch, M.P.; Richel, D.J. Cyclooxygenase-2 inhibition inhibits c-Met kinase activity and Wnt activity in colon cancer. Cancer Res. 2008, 68, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Seol, H.J.; Kim, E.H.; Rheey, J.; Jin, H.J.; Lee, Y.; Joo, K.M.; Lee, J.; Nam, D.H. Wnt/β-catenin signaling is a key downstream mediator of M.E.T. signaling in glioblastoma stem cells. Neuro Oncol. 2013, 15, 161–171. [Google Scholar] [CrossRef]
- Doble, B.W.; Woodgett, J.R. GSK-3: Tricks of the trade for a multi-tasking kinase. J. Cell Sci. 2003, 116, 1175–1186. [Google Scholar] [CrossRef]
- Polakis, P. Wnt signaling in cancer. Cold Spring Harb. Perspect. Biol. 2012, 4, 1–10. [Google Scholar] [CrossRef]
- Seow, H.F.; Yip, W.K.; Fifis, T. Advances in targeted and immunobased therapies for colorectal cancer in the genomic era. OncoTargets Ther. 2016, 9, 1899–1920. [Google Scholar] [CrossRef] [PubMed]
- Schwab, R.H.M.; Amin, N.; Flanagan, D.J.; Johanson, T.M.; Phesse, T.J.; Vincan, E. Wnt is necessary for mesenchymal to epithelial transition in colorectal cancer cells. Dev. Dyn. 2018, 247, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Boon, E.M.; van der Neut, R.; van de Wetering, M.; Clevers, H.; Pals, S.T. Wnt signaling regulates expression of the receptor tyrosine kinase met in colorectal cancer. Cancer Res. 2002, 62, 5126–5128. [Google Scholar] [PubMed]
- Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012, 487, 330–337. [CrossRef]
- He, T.C.; Sparks, A.B.; Rago, C.; Hermeking, H.; Zawel, L.; da Costa, L.T.; Morin, P.J.; Vogelstein, B.; Kinzler, K.W. Identification of c-MYC as a target of the APC pathway. Science 1998, 281, 1509–1512. [Google Scholar] [CrossRef]
- Beaulieu, M.E.; Castillo, F.; Soucek, L. Structural and Biophysical Insights into the Function of the Intrinsically Disordered Myc Oncoprotein. Cells 2020, 9, 38. [Google Scholar] [CrossRef]
- Carroll, P.A.; Freie, B.W.; Mathsyaraja, H.; Eisenman, R.N. The MYC transcription factor network: Balancing metabolism, proliferation and oncogenesis. Front. Med. 2018, 12, 412–425. [Google Scholar] [CrossRef]
- Darcy, K.M.; Brady, W.E.; Blancato, J.K.; Dickson, R.B.; Hoskins, W.J.; McGuire, W.P.; Birrer, M.J. Prognostic relevance of c-MYC gene amplification and polysomy for chromosome 8 in suboptimally-resected, advanced stage epithelial ovarian cancers: A Gynecologic Oncology Group study. Gynecol Oncol. 2009, 114, 472–479. [Google Scholar] [CrossRef]
- Fromont, G.; Godet, J.; Peyret, A.; Irani, J.; Celhay, O.; Rozet, F.; Cathelineau, X.; Cussenot, O. 8q24 amplification is associated with Myc expression and prostate cancer progression and is an independent predictor of recurrence after radical prostatectomy. Hum. Pathol. 2013, 44, 1617–1623. [Google Scholar] [CrossRef]
- Ghadimi, B.M.; Grade, M.; Liersch, T.; Langer, C.; Siemer, A.; Füzesi, L.; Becker, H. Gain of chromosome 8q23-24 is a predictive marker for lymph node positivity in colorectal cancer. Clin. Cancer Res. 2003, 9, 1808–1814. [Google Scholar]
- Kozma, L.; Kiss, I.; Szakáll, S.; Ember, I. Investigation of c-myc oncogene amplification in colorectal cancer. Cancer Lett. 1994, 81, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Kwak, Y.; Nam, K.H.; Kim, D.W.; Kang, S.B.; Choe, G.; Kim, W.H.; Lee, H.S. c-MYC Copy-Number Gain Is an Independent Prognostic Factor in Patients with Colorectal Cancer. PLoS ONE 2015, 10, e0139727. [Google Scholar] [CrossRef] [PubMed]
- Seo, A.N.; Park, K.U.; Choe, G.; Kim, W.H.; Kim, D.W.; Kang, S.B.; Lee, H.S. Clinical and prognostic value of M.E.T. gene copy number gain and chromosome 7 polysomy in primary colorectal cancer patients. Tumour. Biol. 2015, 36, 9813–9821. [Google Scholar] [CrossRef] [PubMed]
- Raghav, K.; Morris, V.; Tang, C.; Morelli, P.; Amin, H.M.; Chen, K.; Manyam, G.C.; Broom, B.; Overman, M.J.; Shaw, K.; et al. MET amplification in metastatic colorectal cancer: An acquired response to E.G.F.R. inhibition, not a de novo phenomenon. Oncotarget 2016, 7, 54627–54631. [Google Scholar] [CrossRef] [PubMed]
- Bondi, J.; Bukholm, G.; Nesland, J.M.; Bukholm, I.R. Expression of non-membranous beta-catenin and gamma-catenin, c-Myc and cyclin D1 in relation to patient outcome in human colon adenocarcinomas. Apmis 2004, 112, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Yachida, S.; Sugimoto, M.; Oshima, M.; Nakagawa, T.; Akamoto, S.; Tabata, S.; Saitoh, K.; Kato, K.; Sato, S.; et al. Global metabolic reprogramming of colorectal cancer occurs at adenoma stage and is induced by MYC. Proc. Natl. Acad. Sci. USA 2017, 114, E7697–E7706. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J. D-type cyclins. Trends Biochem. Sci. 1995, 20, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Qie, S.; Diehl, J.A. Cyclin D1, cancer progression, and opportunities in cancer treatment. J. Mol. Med. 2016, 94, 1313–1326. [Google Scholar] [CrossRef]
- Tchakarska, G.; Sola, B. The double dealing of cyclin D1. Cell Cycle 2020, 19, 163–178. [Google Scholar] [CrossRef]
- He, L.; Hannon, G.J. MicroRNAs: Small R.N.A.s with a big role in gene regulation. Nat. Rev. Genet. 2004, 5, 522–531. [Google Scholar] [CrossRef]
- Coronel-Hernández, J.; López-Urrutia, E.; Contreras-Romero, C.; Delgado-Waldo, I.; Figueroa-González, G.; Campos-Parra, A.D.; Salgado-García, R.; Martínez-Gutierrez, A.; Rodríguez-Morales, M.; Jacobo-Herrera, N.; et al. Cell migration and proliferation are regulated by miR-26a in colorectal cancer via the PTEN-AKT axis. Cancer Cell Int. 2019, 19, 80. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Henson, R.; Wehbe-Janek, H.; Ghoshal, K.; Jacob, S.T.; Patel, T. MicroRNA-21 regulates expression of the P.T.E.N. tumor suppressor gene in human hepatocellular cancer. Gastroenterology 2007, 133, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. MicroRNAs and Apoptosis in Colorectal Cancer. Int. J. Mol. Sci. 2020, 21, 5353. [Google Scholar] [CrossRef] [PubMed]
- Solomon, V.R.; Lee, H. Quinoline as a privileged scaffold in cancer drug discovery. Curr. Med. Chem. 2011, 18, 1488–1508. [Google Scholar] [CrossRef]
- Chen, J.; Deady, L.W.; Desneves, J.; Kaye, A.J.; Finlay, G.J.; Baguley, B.C.; Denny, W.A. Synthesis of substituted indeno[1,2-b]quinoline-6-carboxamides, [1]benzothieno[3,2-b]quinoline-4-carboxamides and 10H-quindoline-4-carboxamides: Evaluation of structure-activity relationships for cytotoxicity. Bioorganic Med. Chem. 2000, 8, 2461–2466. [Google Scholar] [CrossRef]
- Kerzendorfer, C.; Whibley, A.; Carpenter, G.; Outwin, E.; Chiang, S.C.; Turner, G.; Schwartz, C.; El-Khamisy, S.; Raymond, F.L.; O’Driscoll, M. Mutations in Cullin 4B result in a human syndrome associated with increased camptothecin-induced topoisomerase I-dependent D.N.A. breaks. Hum. Mol. Genet. 2010, 19, 1324–1334. [Google Scholar] [CrossRef]
- Morris, E.J.; Geller, H.M. Induction of neuronal apoptosis by camptothecin, an inhibitor of D.N.A. topoisomerase-I: Evidence for cell cycle-independent toxicity. J. Cell Biol. 1996, 134, 757–770. [Google Scholar] [CrossRef]
- Somasundaram, R.; Connelly, T.; Choi, R.; Choi, H.; Samarkina, A.; Li, L.; Gregorio, E.; Chen, Y.; Thakur, R.; Abdel-Mohsen, M.; et al. Tumor-infiltrating mast cells are associated with resistance to anti-PD-1 therapy. Nat. Commun. 2021, 12, 346. [Google Scholar] [CrossRef]
- Rini, B.I. Sunitinib. Expert Opin. Pharmacother. 2007, 8, 2359–2369. [Google Scholar] [CrossRef]
- Heinrich, M.C.; Maki, R.G.; Corless, C.L.; Antonescu, C.R.; Harlow, A.; Griffith, D.; Town, A.; McKinley, A.; Ou, W.B.; Fletcher, J.A.; et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J. Clin. Oncol. 2008, 26, 5352–5359. [Google Scholar] [CrossRef]
- Mokgautsi, N.; Kuo, Y.C.; Tang, S.L.; Liu, F.C.; Chen, S.J.; Wu, A.T.H.; Huang, H.S. Anticancer Activities of 9-chloro-6-(piperazin-1-yl)-11H-indeno[1,2-c] quinolin-11-one (SJ10) in Glioblastoma Multiforme (G.B.M.) Chemoradioresistant Cell Cycle-Related Oncogenic Signatures. Cancers 2022, 14, 262. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; Lee, Y.R.; Chen, T.C.; Chen, C.L.; Lee, C.C.; Shiau, C.Y.; Chiang, C.H.; Huang, H.S. Novel Anthra[1,2-c][1,2,5]Thiadiazole-6,11-Diones as Promising Anticancer Lead Compounds: Biological Evaluation, Characterization & Molecular Targets Determination. PLoS ONE 2016, 11, e0154278. [Google Scholar] [CrossRef]
- Lee, C.C.; Liu, F.L.; Chen, C.L.; Chen, T.C.; Chang, D.M.; Huang, H.S. Discovery of 5-(2′,4’-difluorophenyl)-salicylanilides as new inhibitors of receptor activator of NF-κB ligand (R.A.N.K.L.)-induced osteoclastogenesis. Eur. J. Med. Chem. 2015, 98, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Huang, K.F.; Lin, P.Y.; Huang, F.C.; Chen, C.L.; Chen, T.C.; Guh, J.H.; Lin, J.J.; Huang, H.S. Synthesis, anti-proliferative activities and telomerase inhibition evaluation of novel asymmetrical 1,2-disubstituted amidoanthraquinone derivatives. Eur. J. Med. Chem. 2012, 47, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.S.; Chen, T.C.; Chen, R.H.; Huang, K.F.; Huang, F.C.; Jhan, J.R.; Chen, C.L.; Lee, C.C.; Lo, Y.; Lin, J.J. Synthesis, cytotoxicity and human telomerase inhibition activities of a series of 1,2-heteroannelated anthraquinones and anthra[1,2-d]imidazole-6,11-dione homologues. Bioorganic Med. Chem. 2009, 17, 7418–7428. [Google Scholar] [CrossRef]
- Huang, H.S.; Huang, K.F.; Li, C.L.; Huang, Y.Y.; Chiang, Y.H.; Huang, F.C.; Lin, J.J. Synthesis, human telomerase inhibition and anti-proliferative studies of a series of 2,7-bis-substituted amido-anthraquinone derivatives. Bioorganic Med. Chem. 2008, 16, 6976–6986. [Google Scholar] [CrossRef]
- Matthews, H.; Hanison, J.; Nirmalan, N. “Omics”-Informed Drug and Biomarker Discovery: Opportunities, Challenges and Future Perspectives. Proteomes 2016, 4, 28. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.; Varambally, S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef]
- Bartha, Á.; Győrffy, B. TNMplot.com: A Web Tool for the Comparison of Gene Expression in Normal, Tumor and Metastatic Tissues. Int. J. Mol. Sci. 2021, 22, 2622. [Google Scholar] [CrossRef]
- Najafov, J.; Najafov, A. GECO: Gene expression correlation analysis after genetic algorithm-driven deconvolution. Bioinformatics 2019, 35, 156–159. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Tan, Q.; Collins, J.R.; Alvord, W.G.; Roayaei, J.; Stephens, R.; Baseler, M.W.; Lane, H.C.; Lempicki, R.A. The DAVID Gene Functional Classification Tool: A novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol. 2007, 8, R183. [Google Scholar] [CrossRef] [PubMed]
- Nagy, Á.; Győrffy, B. muTarget: A platform linking gene expression changes and mutation status in solid tumors. Int. J. Cancer 2021, 148, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Hu, F.F.; Xia, M.X.; Han, L.; Zhang, Q.; Guo, A.Y. GSCALite: A web server for gene set cancer analysis. Bioinformatics 2018, 34, 3771–3772. [Google Scholar] [CrossRef]
- Paull, K.D.; Shoemaker, R.H.; Hodes, L.; Monks, A.; Scudiero, D.A.; Rubinstein, L.; Plowman, J.; Boyd, M.R. Display and analysis of patterns of differential activity of drugs against human tumor cell lines: Development of mean graph and COMPARE algorithm. J. Natl. Cancer Inst. 1989, 81, 1088–1092. [Google Scholar] [CrossRef]
- Liu, X.; Ouyang, S.; Yu, B.; Liu, Y.; Huang, K.; Gong, J.; Zheng, S.; Li, Z.; Li, H.; Jiang, H. PharmMapper server: A web server for potential drug target identification using pharmacophore mapping approach. Nucleic Acids Res. 2010, 38, W609–W614. [Google Scholar] [CrossRef]
- Ritchie, T.J.; Ertl, P.; Lewis, R. The graphical representation of ADME-related molecule properties for medicinal chemists. Drug Discov. Today 2011, 16, 65–72. [Google Scholar] [CrossRef]
- Mokgautsi, N.; Wen, Y.T.; Lawal, B.; Khedkar, H.; Sumitra, M.R.; Wu, A.T.H.; Huang, H.S. An Integrated Bioinformatics Study of a Novel Niclosamide Derivative, NSC765689, a Potential GSK3β/β-Catenin/STAT3/CD44 Suppressor with Anti-Glioblastoma Properties. Int. J. Mol. Sci. 2021, 22, 2464. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Zoete, V. A BOILED-Egg To Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Martin, Y.C. A bioavailability score. J. Med. Chem. 2005, 48, 3164–3170. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, R.H. The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. Cancer 2006, 6, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Seeliger, D.; de Groot, B.L. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J. Comput. Aided Mol. Des. 2010, 24, 417–422. [Google Scholar] [CrossRef]
- Temml, V.; Kaserer, T.; Kutil, Z.; Landa, P.; Vanek, T.; Schuster, D. Pharmacophore modeling for COX-1 and -2 inhibitors with LigandScout in comparison to Discovery Studio. Future Med. Chem. 2014, 6, 1869–1881. [Google Scholar] [CrossRef]
- Mokgautsi, N.; Wang, Y.C.; Lawal, B.; Khedkar, H.; Sumitra, M.R.; Wu, A.T.H.; Huang, H.S. Network Pharmacological Analysis through a Bioinformatics Approach of Novel NSC765600 and NSC765691 Compounds as Potential Inhibitors of CCND1/CDK4/PLK1/CD44 in Cancer Types. Cancers 2021, 13, 2523. [Google Scholar] [CrossRef]
- Zhou, G.; Soufan, O.; Ewald, J.; Hancock, R.E.W.; Basu, N.; Xia, J. NetworkAnalyst 3.0: A visual analytics platform for comprehensive gene expression profiling and meta-analysis. Nucleic Acids Res 2019, 47, W234–W241. [Google Scholar] [CrossRef]
- Pathan, M.; Keerthikumar, S.; Ang, C.S.; Gangoda, L.; Quek, C.Y.; Williamson, N.A.; Mouradov, D.; Sieber, O.M.; Simpson, R.J.; Salim, A.; et al. FunRich: An open access standalone functional enrichment and interaction network analysis tool. Proteomics 2015, 15, 2597–2601. [Google Scholar] [CrossRef]
- Jasial, S.; Hu, Y.; Bajorath, J. Assessing the Growth of Bioactive Compounds and Scaffolds over Time: Implications for Lead Discovery and Scaffold Hopping. J. Chem. Inf. Model. 2016, 56, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Schneider, G.; Neidhart, W.; Giller, T.; Schmid, G. “Scaffold-Hopping” by Topological Pharmacophore Search: A Contribution to Virtual Screening. Angew. Chem. Int. Ed. Engl. 1999, 38, 2894–2896. [Google Scholar] [CrossRef]
- Shave, S.; Blackburn, E.A.; Adie, J.; Houston, D.R.; Auer, M.; Webster, S.P.; Taylor, P.; Walkinshaw, M.D. UFSRAT: Ultra-fast Shape Recognition with Atom Types—The discovery of novel bioactive small molecular scaffolds for FKBP12 and 11βHSD1. PLoS ONE 2015, 10, e0116570. [Google Scholar] [CrossRef]
- Chan-On, W.; Huyen, N.T.; Songtawee, N.; Suwanjang, W.; Prachayasittikul, S.; Prachayasittikul, V. Quinoline-based clioquinol and nitroxoline exhibit anticancer activity inducing FoxM1 inhibition in cholangiocarcinoma cells. Drug Des. Dev. Ther. 2015, 9, 2033–2047. [Google Scholar] [CrossRef]
- Diamantis, N.; Banerji, U. Antibody-drug conjugates—An emerging class of cancer treatment. Br. J. Cancer 2016, 114, 362–367. [Google Scholar] [CrossRef]
- Keri, R.S.; Patil, S.A. Quinoline: A promising antitubercular target. Biomed. Pharmacother. 2014, 68, 1161–1175. [Google Scholar] [CrossRef]
- Musiol, R. An overview of quinoline as a privileged scaffold in cancer drug discovery. Expert Opin. Drug Discov. 2017, 12, 583–597. [Google Scholar] [CrossRef]
- Huang, K.B.; Pan, Y.H.; Shu, G.N.; Yao, H.H.; Liu, X.; Zhou, M.; Wei, J.H.; Chen, Z.H.; Lu, J.; Feng, Z.H.; et al. Circular RNA circSNX6 promotes sunitinib resistance in renal cell carcinoma through the miR-1184/GPCPD1/ lysophosphatidic acid axis. Cancer Lett. 2021, 523, 121–134. [Google Scholar] [CrossRef]
- Qi, X.; Yang, M.; Ma, L.; Sauer, M.; Avella, D.; Kaifi, J.T.; Bryan, J.; Cheng, K.; Staveley-O’Carroll, K.F.; Kimchi, E.T.; et al. Synergizing sunitinib and radiofrequency ablation to treat hepatocellular cancer by triggering the antitumor immune response. J. Immunother. Cancer 2020, 8, e001038. [Google Scholar] [CrossRef]
- Ferrari, S.M.; Centanni, M.; Virili, C.; Miccoli, M.; Ferrari, P.; Ruffilli, I.; Ragusa, F.; Antonelli, A.; Fallahi, P. Sunitinib in the Treatment of Thyroid Cancer. Curr. Med. Chem. 2019, 26, 963–972. [Google Scholar] [CrossRef]
- Méjean, A.; Ravaud, A.; Thezenas, S.; Chevreau, C.; Bensalah, K.; Geoffrois, L.; Thiery-Vuillemin, A.; Cormier, L.; Lang, H.; Guy, L.; et al. Sunitinib Alone or After Nephrectomy for Patients with Metastatic Renal Cell Carcinoma: Is There Still a Role for Cytoreductive Nephrectomy? Eur. Urol. 2021, 80, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Shi, Y.; Lv, Y.; Yuan, S.; Ramirez, C.F.A.; Lieftink, C.; Wang, L.; Wang, S.; Wang, C.; Dias, M.H.; et al. EGFR activation limits the response of liver cancer to lenvatinib. Nature 2021, 595, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Peng, H.; Dara, L.; Li, T.W.; Zheng, Y.; Yang, H.; Tomasi, M.L.; Tomasi, I.; Giordano, P.; Mato, J.M.; Lu, S.C. MAT2B-GIT1 interplay activates MEK1/ERK 1 and 2 to induce growth in human liver and colon cancer. Hepatology 2013, 57, 2299–2313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kang, M.; Zhang, B.; Meng, F.; Song, J.; Kaneko, H.; Shimamoto, F.; Tang, B. m(6)A modification-mediated CBX8 induction regulates stemness and chemosensitivity of colon cancer via upregulation of LGR5. Mol. Cancer 2019, 18, 185. [Google Scholar] [CrossRef]
- Maeda, K.; Chung, Y.; Kang, S.; Ogawa, M.; Onoda, N.; Nishiguchi, Y.; Ikehara, T.; Nakata, B.; Okuno, M.; Sowa, M. Cyclin D1 overexpression and prognosis in colorectal adenocarcinoma. Oncology 1998, 55, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Romero-Moreno, R.; Curtis, K.J.; Coughlin, T.R.; Miranda-Vergara, M.C.; Dutta, S.; Natarajan, A.; Facchine, B.A.; Jackson, K.M.; Nystrom, L.; Li, J.; et al. The CXCL5/CXCR2 axis is sufficient to promote breast cancer colonization during bone metastasis. Nat. Commun. 2019, 10, 4404. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, F.; Wu, X.; Li, Z.; Wang, Z.; Ren, X.; Zhou, Y.; Song, F.; Liang, Y.; Zeng, Z.; et al. FBX8 promotes metastatic dormancy of colorectal cancer in liver. Cell Death Dis. 2020, 11, 622. [Google Scholar] [CrossRef] [PubMed]
- Coussens, N.P.; Braisted, J.C.; Peryea, T.; Sittampalam, G.S.; Simeonov, A.; Hall, M.D. Small-Molecule Screens: A Gateway to Cancer Therapeutic Agents with Case Studies of Food and Drug Administration-Approved Drugs. Pharmacol. Rev. 2017, 69, 479–496. [Google Scholar] [CrossRef]
- Schweiger, T.; Starkl, V.; Glueck, O.; Glogner, C.; Traxler, D.; Jedamzik, J.; Liebmann-Reindl, S.; Birner, P.; Streubel, B.; Klepetko, W.; et al. Clinical impact of c-MET expression and mutational status in patients with colorectal cancer lung metastases. Eur. J. Cardiothorac. Surg 2016, 49, 1103–1111, discussion 1111. [Google Scholar] [CrossRef]
- Voutsina, A.; Tzardi, M.; Kalikaki, A.; Zafeiriou, Z.; Papadimitraki, E.; Papadakis, M.; Mavroudis, D.; Georgoulias, V. Combined analysis of K.R.A.S. and PIK3CA mutations, MET and PTEN expression in primary tumors and corresponding metastases in colorectal cancer. Mod. Pathol. 2013, 26, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Trusolino, L.; Bertotti, A.; Comoglio, P.M. MET signalling: Principles and functions in development, organ regeneration and cancer. Nat. Rev. Mol. Cell Biol. 2010, 11, 834–848. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.T.; Matos, D.; Logullo, A.F.; SR, D.A.S.; Neto, R.A.; Filho, A.L.; Saad, S.S. MET Is highly expressed in advanced stages of colorectal cancer and indicates worse prognosis and mortality. Anticancer Res. 2009, 29, 4807–4811. [Google Scholar] [PubMed]
- Gu, Y.; Chen, Y.; Wei, L.; Wu, S.; Shen, K.; Liu, C.; Dong, Y.; Zhao, Y.; Zhang, Y.; Zhang, C.; et al. ABHD5 inhibits YAP-induced c-Met overexpression and colon cancer cell stemness via suppressing YAP methylation. Nat. Commun. 2021, 12, 6711. [Google Scholar] [CrossRef]
- Duffy, M.J.; O’Grady, S.; Tang, M.; Crown, J. MYC as a target for cancer treatment. Cancer Treat. Rev. 2021, 94, 102154. [Google Scholar] [CrossRef]
- Bali, A.; O’Brien, P.M.; Edwards, L.S.; Sutherland, R.L.; Hacker, N.F.; Henshall, S.M. Cyclin D1, p53, and p21Waf1/Cip1 expression is predictive of poor clinical outcome in serous epithelial ovarian cancer. Clin. Cancer Res. 2004, 10, 5168–5177. [Google Scholar] [CrossRef]
- Holland, T.A.; Elder, J.; McCloud, J.M.; Hall, C.; Deakin, M.; Fryer, A.A.; Elder, J.B.; Hoban, P.R. Subcellular localisation of cyclin D1 protein in colorectal tumours is associated with p21(WAF1/CIP1) expression and correlates with patient survival. Int. J. Cancer 2001, 95, 302–306. [Google Scholar] [CrossRef]
- Albasri, A.; Yosef, H.; Hussainy, A.S.; Sultan, S.A.; Alhujaily, A. Histopathological features of colorectal cancer in Al-Madinah region of Saudi Arabia: 8 years experience. Asian Pac. J. Cancer Prev. 2014, 15, 3133–3137. [Google Scholar] [CrossRef]
- Shen, A.; Liu, L.; Chen, H.; Qi, F.; Huang, Y.; Lin, J.; Sferra, T.J.; Sankararaman, S.; Wei, L.; Chu, J.; et al. Cell division cycle associated 5 promotes colorectal cancer progression by activating the ERK signaling pathway. Oncogenesis 2019, 8, 19. [Google Scholar] [CrossRef]
- Macmillan, J.C.; Hudson, J.W.; Bull, S.; Dennis, J.W.; Swallow, C.J. Comparative expression of the mitotic regulators SAK and PLK in colorectal cancer. Ann. Surg Oncol. 2001, 8, 729–740. [Google Scholar] [CrossRef]







| NCI Synthetic Compounds | NCI Standard Agents | ArrayCGH-Gray | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Rank | r | CCLC | Target Descriptor | r | CCLC | Target Descriptor | r | CCLC | Target Descriptor |
| 1 | 0.61 | 56 | Raloxifene | 0.46 | 55 | Tamoxifen | 0.27 | 53 | I.G.F.A. |
| 2 | 0.52 | 55 | Majoranolide | 0.37 | 46 | Menogaril | 0.23 | 57 | c-Met |
| 3 | 0.51 | 57 | Tyloxapol (usan) | 0.36 | 43 | Mitramycin | 0.12 | 55 | CCND1 |
| 4 | 0.53 | 52 | Tolonium chloride | 0.28 | 56 | Tamoxifen | 0.21 | 53 | WNT1 |
| 5 | 0.54 | 51 | Ivosidenib | 0.28 | 55 | Fluorodopan | 0.26 | 55 | CDK6 |
| 6 | 0.56 | 56 | Bafilomycin deriv | 0.42 | 59 | Thioguanine | 0.16 | 51 | AKT2 |
| 7 | 0.43 | 53 | Asbestinin-d | 0.31 | 56 | Amonafide | 0.14 | 55 | T.G.F.A. |
| 8 | 0.61 | 48 | Ml148 | 0.13 | 51 | Tetraplatin | 0.13 | 54 | MYC |
| 9 | 0.56 | 52 | Raloxifene | 0.3 | 58 | Rapamycin | 0.3 | 53 | MMP8 |
| 10 | 0.58 | 55 | Azd-1390 | 0.41 | 56 | Actinomycin D | 0.1 | 54 | PIK3CA |
| Physicochemical Properties Based on Bioavailability Radar of NSC765600 | Recommended Value | Pharmacokinetics | |||
|---|---|---|---|---|---|
| GI Absorption | High | ||||
| Formula | C21H17F2NO4 | BBB | Yes (4.39) | ||
| Molecular weight | 379.88 g/mol | 150–500 g/mol | Drug-likeness | ||
| Fraction Csp3 | 0.27 | ≤1 | Lipinski | Yes; 0 violation | |
| Num. rotatable bonds | 6 | ≤10 | Ghose | Yes | |
| Num. H-bond acceptors | 3 | ≤12 | Veber | Yes | |
| Num. H-bond donors | 1 | ≤5 | Egan | Yes | |
| Molar Refractivity | 111.86 | Muegge | Yes | ||
| TPSA | 45.23 Å2 | ≤140 Å2 | Bioavailability Score | 0.55 (55%) | |
| Log Po/w (XLOGP3) | 5.04 | −5.7 | Medical Chemistry | ||
| Log S (ESOL) | −5.41 | 0–6 | Synthetic accessibility | 3.26 | 1 (easy to make) and 10 (difficult to make) |
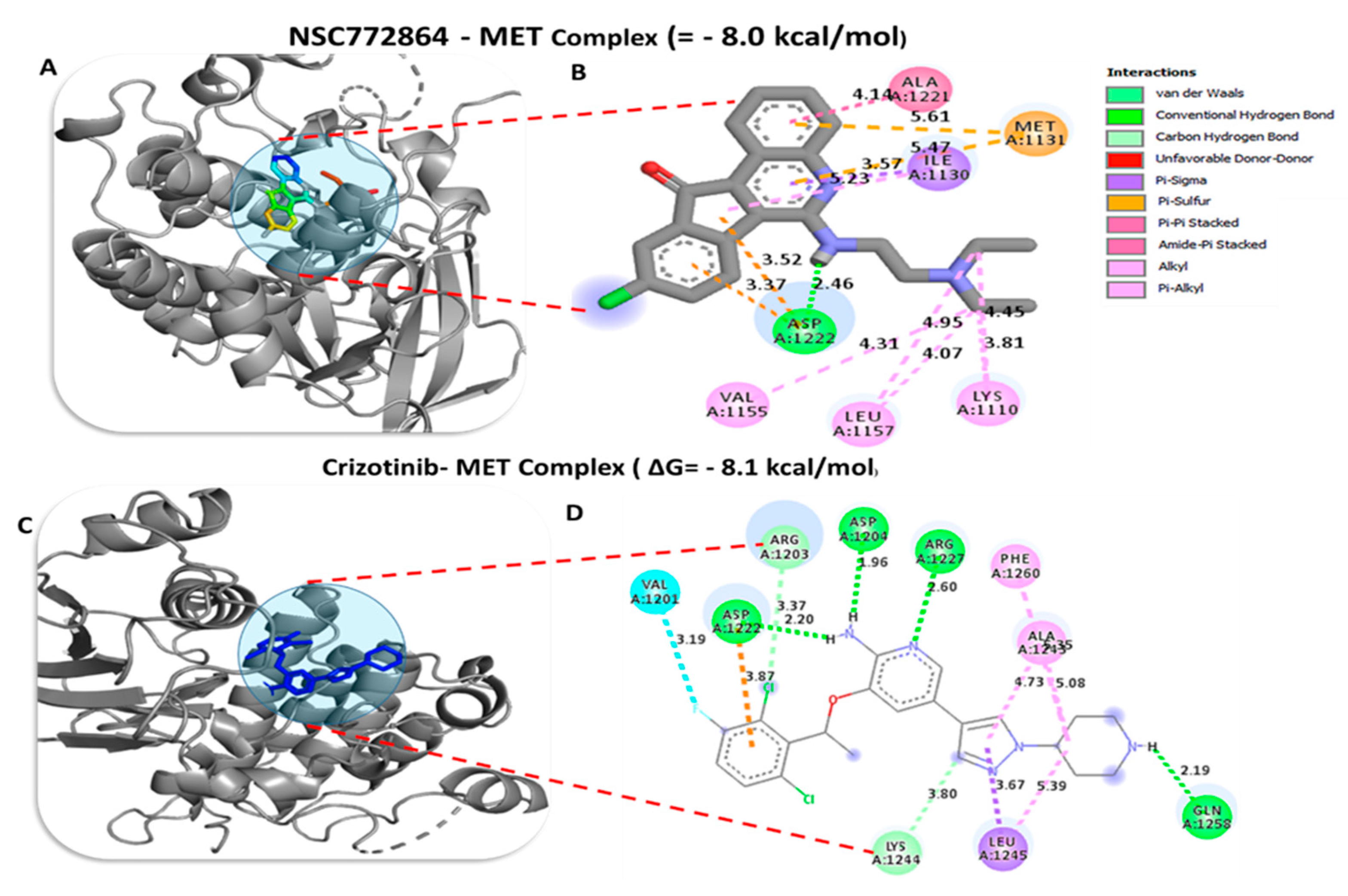
| NSC772864–c-Met Complex (ΔG = −8.0 kcal/mol) | Crizotinib–c-Met Complex (ΔG = −8.1 kcal/mol) | ||
|---|---|---|---|
| Type of interactions and number of bonds | Distance of interacting amino acids | Type of interactions and number of bonds | Distance of interacting amino acids |
| Conventional Hydrogen bond (3) | ASP1222 (2.20 Å), GLU1127 (1.85 Å), and LYS1110 (2.68 Å) | Conventional Hydrogen bond (3) | ASP1222 (2.20 Å), GLU1258 (2.19 Å), ASP1204 (1.96 Å) and ARG1227 (2.60 Å) |
| Van der Waals forces | VAL1155, GLY1128, LYS1161, GLY1163, GLY1085, LEU1140 | Carbon–Hydrogen bond | LYS1244 |
| Carbon–Hydrogen bond | PRO1158, c-Met1160 | Pi–Sigma | LEU1245 |
| Pi–Sigma | ILE1084, LEU1157, c-Met1211 | ||
| Pi–Sulfur | c-Met1131 | Alkyl | PHE1260 |
| Amide–Pi stacked | TYR1159 | Pi–Alkyl | ALA1243 |
| Pi–Pi stacked | ALA1228 | ||
| Alkyl | VAL1092, ALA1108 | ||
| Pi–Alkyl | PHE1223 | ||
| NSC772864–GSK3β Complex (=−8.6 kcal/mol) | AZD1080–GSK3β Complex (ΔG = −8.4 kcal/mol) | ||
|---|---|---|---|
| Type of interactions and number of bonds | Distance of interacting amino acids | Type of interactions and number of bonds | Distance of interacting amino acids |
| Conventional Hydrogen bond (1) | ASN64 (2.56 Å) | Conventional Hydrogen bond (2) | PHE67(2.30Å), GLN185 (2.70 Å) |
| Carbon–Hydrogen bond | SER66 | Carbon–Hydrogen bond | ASP200 |
| Pi–Anion | ASP200 | Pi–Sigma | VAL70 |
| Pi–Alkyl | VAL70 | ||
| Alkyl | LYS85 | ||
| NSC772864–MYC Complex (ΔG = −9.1 kcal/mol) | Alobresib–MYC Complex (ΔG = −7.6 kcal/mol) | ||
|---|---|---|---|
| Type of interactions and number of bonds | distance of interacting Amino acids | Type of interactions and number of bonds | distance of interacting Amino acids |
| Conventional Hydrogen bond (1) | SER221 (2.48 Å) | Pi-cation | ARG214, LYS944 |
| Van der Waals forces | SER221, THR947, GLU221, SER224, ASP220 | Pi-sigma | LEU210 |
| Carbon hydrogen bond | ARG21 | Pi-Pi stacked | HIS217 |
| Pi-sigma | LEU210 | Alkyl | VAL940, ILE218, LYS939 |
| Pi-Alkyl | PRO938, LYS213, LEU651 | Pi-alkyl | VAL941, LYS945 |
| Pi-Pi stacked | HIS217 | ||
| NSC772864–CCND1 Complex (ΔG = −8.0 kcal/mol) | Trilaciclib–CCND1 Complex (ΔG = −7.4 kcal/mol) | ||
|---|---|---|---|
| Type of interactions and number of bonds | Distance of interacting amino acids | Type of interactions and number of bonds | Distance of interacting amino acids |
| Conventional Hydrogen bond (1) | ARG26 (2.88 Å) | Conventional Hydrogen bond (2) | HIS163 (2.29 Å), ALA133 (2.04 Å) |
| Van der Waals forces | ASN131 | Carbon–Hydrogen bond | GLU162 |
| Pi–Cation | HIS65 | Pi–Sigma | VAL27 |
| Pi-Pi stacked | PHE63, PHE127 | Pi–Sulfur | c-Met155, c-Met31, |
| Alkyl | ALA130, ALA30 | Pi–Alkyl | LEU23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokgautsi, N.; Kuo, Y.-C.; Chen, C.-H.; Huang, Y.-J.; Wu, A.T.H.; Huang, H.-S. Multiomics Study of a Novel Naturally Derived Small Molecule, NSC772864, as a Potential Inhibitor of Proto-Oncogenes Regulating Cell Cycle Progression in Colorectal Cancer. Cells 2023, 12, 340. https://doi.org/10.3390/cells12020340
Mokgautsi N, Kuo Y-C, Chen C-H, Huang Y-J, Wu ATH, Huang H-S. Multiomics Study of a Novel Naturally Derived Small Molecule, NSC772864, as a Potential Inhibitor of Proto-Oncogenes Regulating Cell Cycle Progression in Colorectal Cancer. Cells. 2023; 12(2):340. https://doi.org/10.3390/cells12020340
Chicago/Turabian StyleMokgautsi, Ntlotlang, Yu-Cheng Kuo, Chien-Hsin Chen, Yan-Jiun Huang, Alexander T. H. Wu, and Hsu-Shan Huang. 2023. "Multiomics Study of a Novel Naturally Derived Small Molecule, NSC772864, as a Potential Inhibitor of Proto-Oncogenes Regulating Cell Cycle Progression in Colorectal Cancer" Cells 12, no. 2: 340. https://doi.org/10.3390/cells12020340
APA StyleMokgautsi, N., Kuo, Y.-C., Chen, C.-H., Huang, Y.-J., Wu, A. T. H., & Huang, H.-S. (2023). Multiomics Study of a Novel Naturally Derived Small Molecule, NSC772864, as a Potential Inhibitor of Proto-Oncogenes Regulating Cell Cycle Progression in Colorectal Cancer. Cells, 12(2), 340. https://doi.org/10.3390/cells12020340








