A Promising Ash Supplementation Strategy in the Cultivation of Spirodela polyrrhiza Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions and Treatments
2.2. Z Medium
2.3. Composition of Sorghum Ash
2.4. Waters Used
2.5. Cytological Tests
3. Results
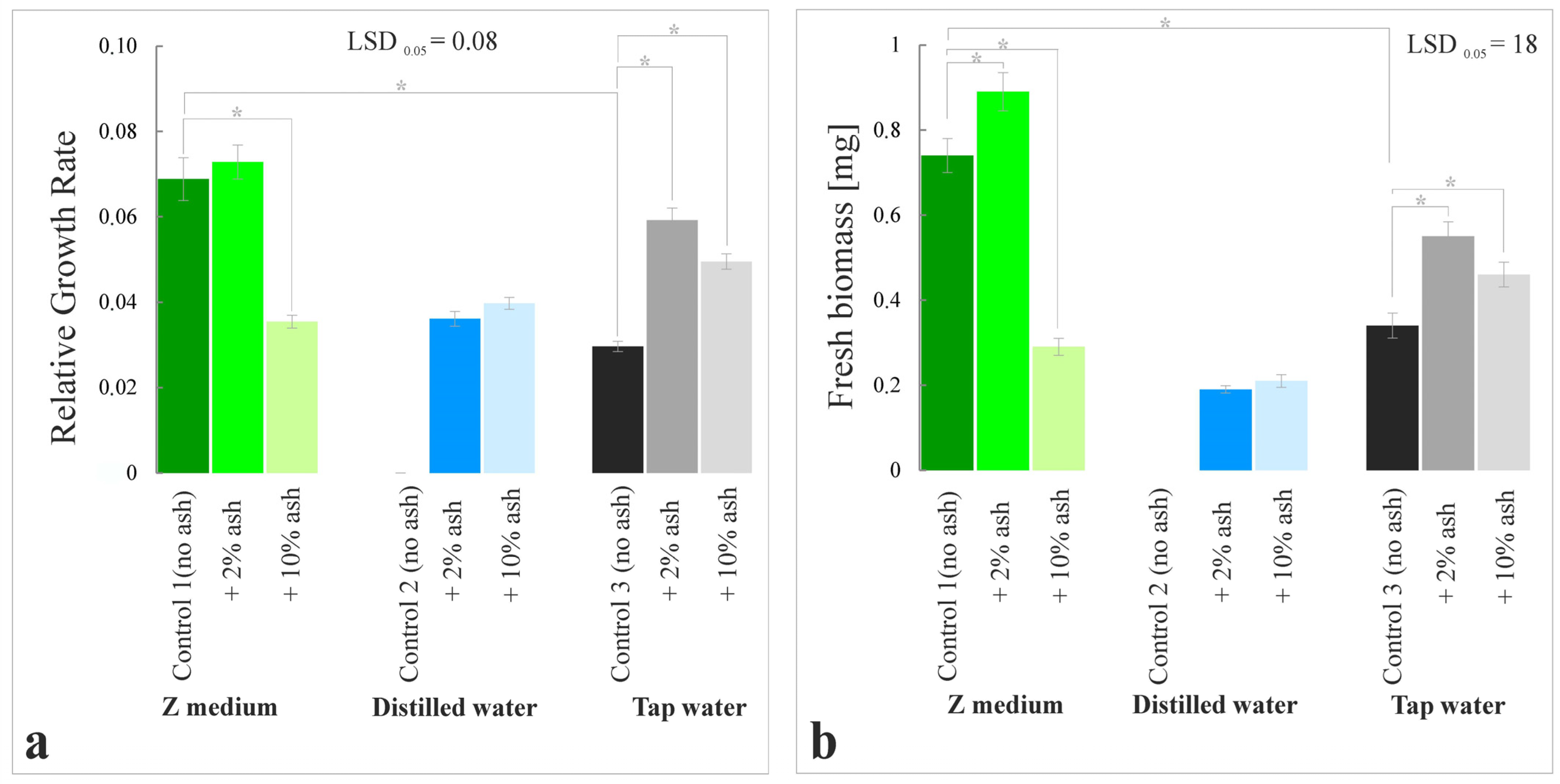
3.1. Growth Rate and Fresh Biomass
3.2. Lengths of Root Tips
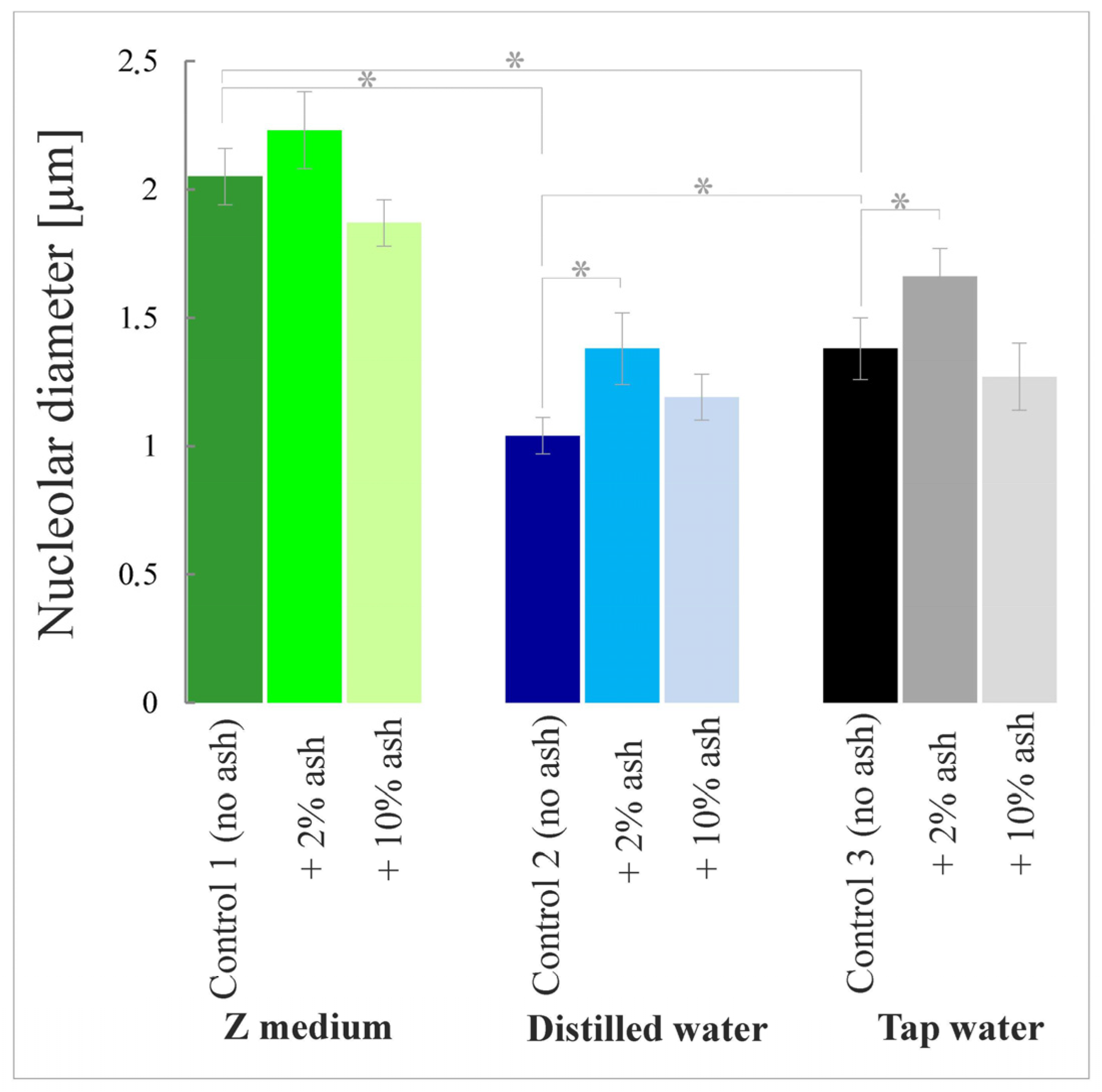
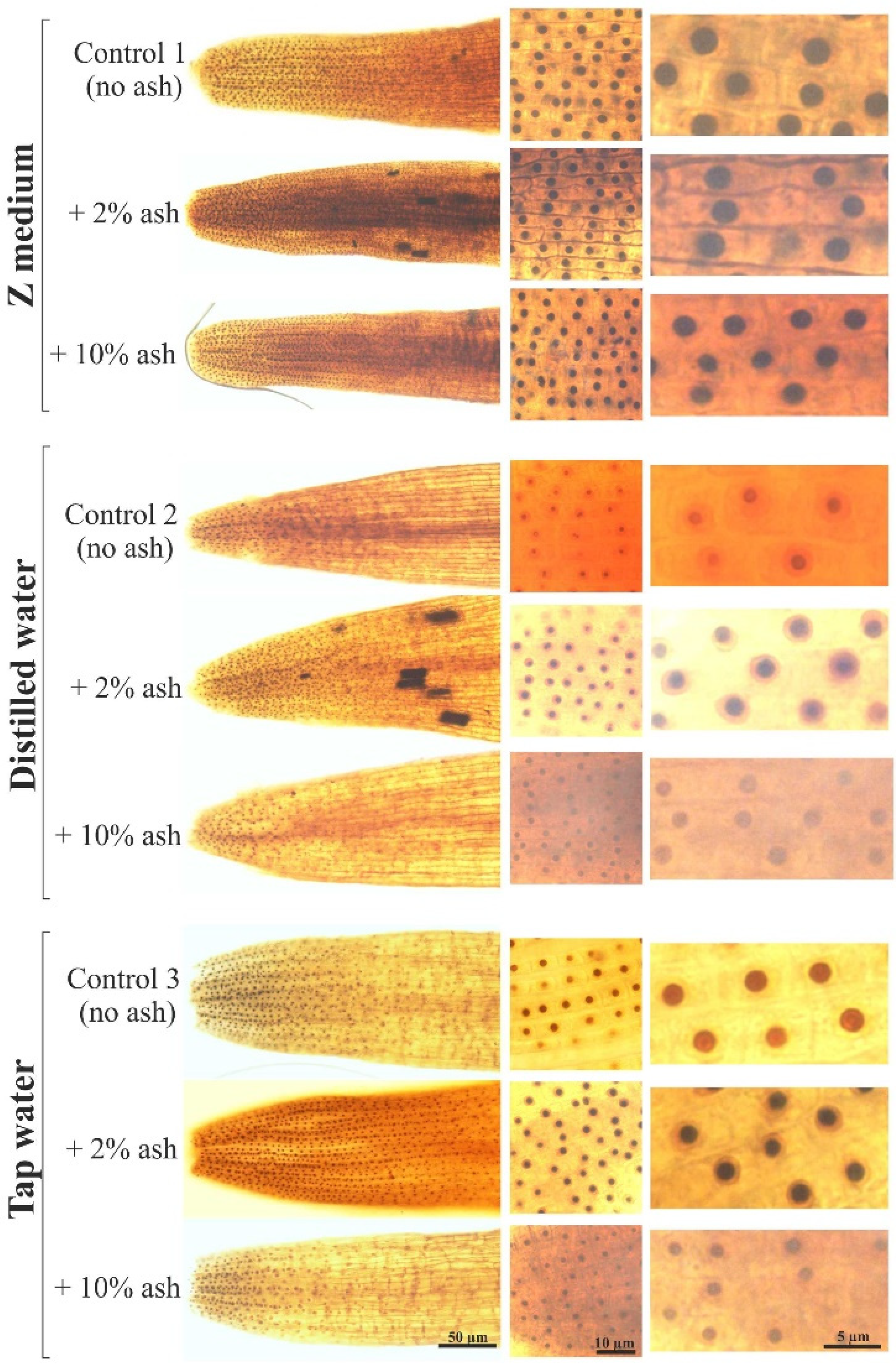
3.3. The Sizes of Nucleoli
3.4. Index of Chlorophyll Content
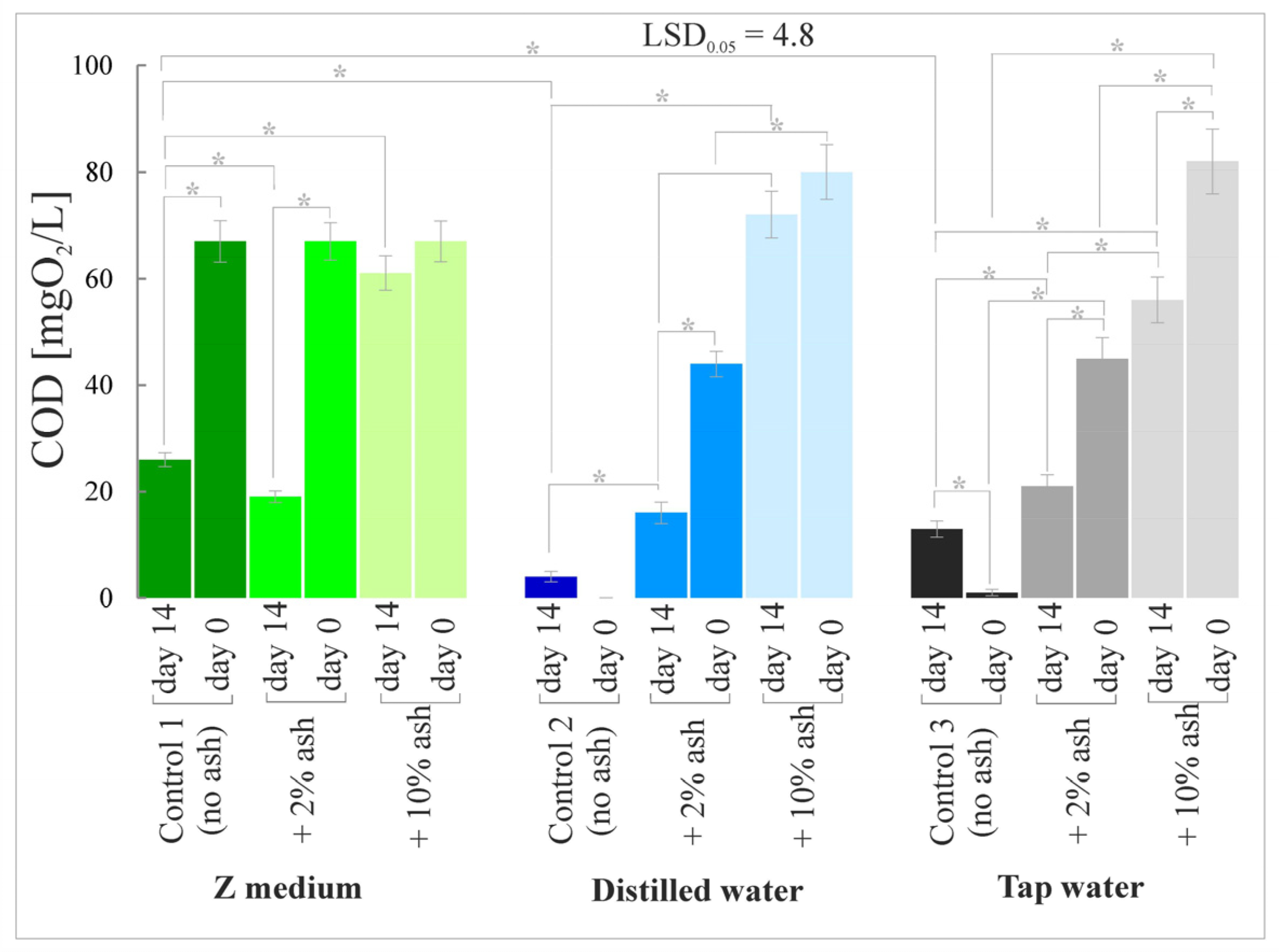
3.5. Chemical Oxygen Demand (COD)
3.6. Chlorophyll Fluorescence
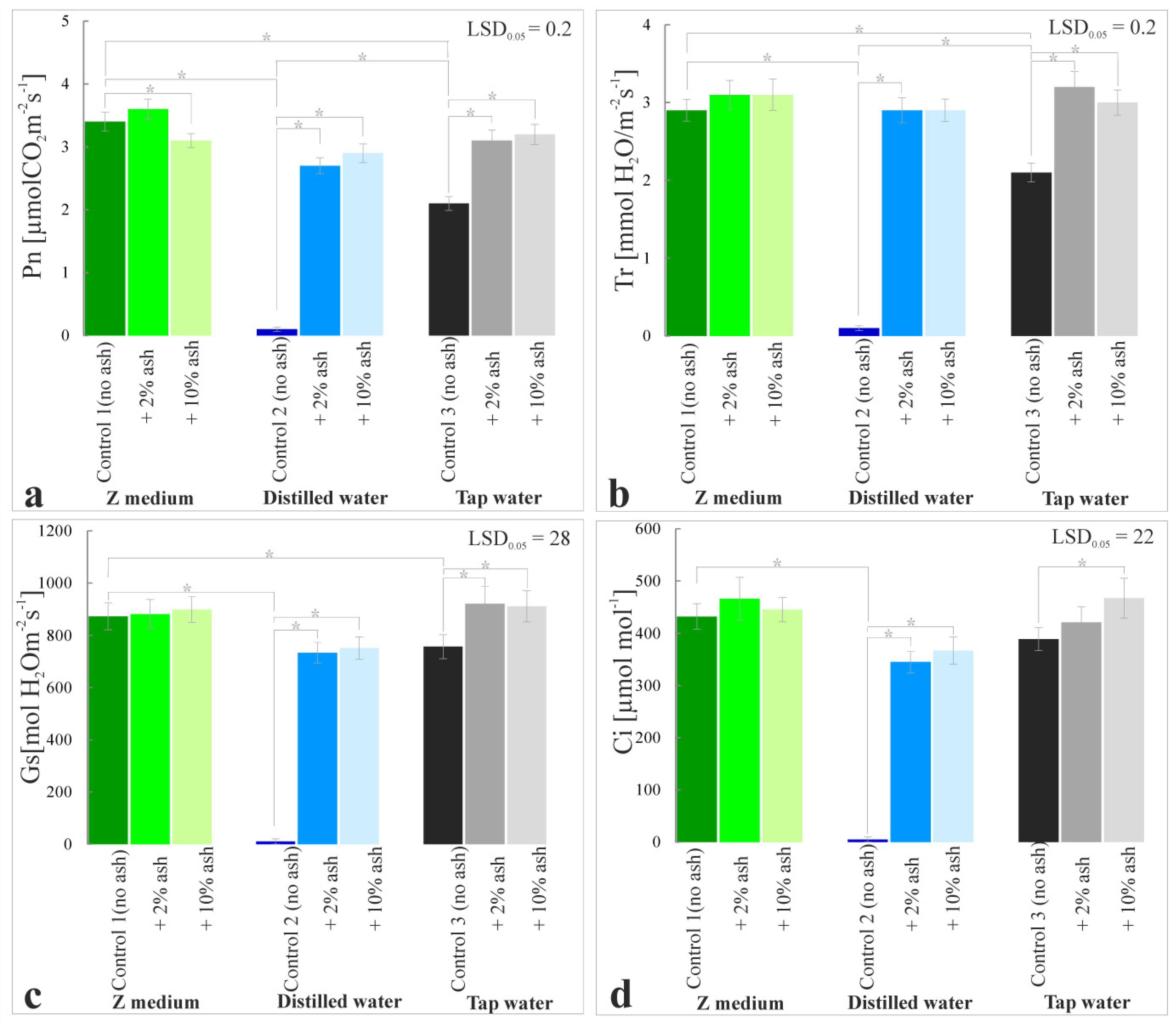
3.7. Gas Exchange Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berent-Kowalska, G.; Kacprowska, J.; Moskal, I.; Piwko, D.; Jurgaś, A.; Kacperczyk, G. Energy from Renewable Sources in 2016; Central Statistical Office: Warsaw, Poland, 2017.
- Romanowska-Duda, Z.; Grzesik, M.; Janas, R. Application of Phytotoxkit in the quick assessment of ashes suitability as fertilizers in sorghum crops. Int. Agrophys. 2019, 33, 145–152. [Google Scholar] [CrossRef]
- Uliasz-Bocheńczyk, A.; Mokrzycki, E. The elemental composition of biomass ashes as a preliminary assessment of the recovery potential. Min. Res. Managt. 2018, 34, 115–132. [Google Scholar]
- Fedler, C.B.; Duan, R. Biomass production for bioenergy using recycled wastewater in a natural waste treatment system. Environ. Sci. Res. Conserv. Recyc. 2011, 55, 793–800. [Google Scholar] [CrossRef]
- Ruekaewma, N.; Piyatiratitivorakul, S.; Powtongsook, S. Culture system for Wolffia globosa L. (Lemnaceae) for hygiene human food. J. Sci. Technol. 2015, 37, 575–580. [Google Scholar]
- Romanowska-Duda, Z.B.; Pszczółkowski, W. Lemnaceae biomass as an alternative for renewable energy. Acta Innov. 2013, 9, 1–30. [Google Scholar]
- Romanowska-Duda, Z.; Piotrowski, K.; Jagiełło, N.; Dębowski, M.; Zieliński, M. Development of new Lemnaceae breeding technology using Apol-humus and biogas plant waste. Int. Agrophys. 2019, 33, 297–302. [Google Scholar] [CrossRef]
- Romanowska-Duda, Z.; Grzesik, M.; Janas, R. Ash from Jerusalem artichoke and biopreparations enhance the growth and physiological activity of sorghum and limit environmental pollution by decreasing artificial fertilization needs. Int. Agrophys. 2020, 34, 365–379. [Google Scholar] [CrossRef]
- Gostyńska, J.; Pankiewicz, R.; Romanowska-Duda, Z.; Messyasz, B. Overview of Allelopathic Potential of Lemna minor L. Obtained from a Shallow Eutrophic Lake. Molecules 2022, 27, 3428. [Google Scholar] [CrossRef]
- Perilli, S.; Sabatini, S. Analysis of root meristem size development. Methods Mol. Biol. 2010, 655, 177–178. [Google Scholar]
- Hayashi, K.; Matsunaga, S. Heat and chilling stress induce nucleolus morphological changes. J. Plant Res. 2019, 132, 395–403. [Google Scholar] [CrossRef]
- Stępiński, D. Nucleolar chromatin organization at different activities of soybean root meristematic cell nucleoli. Protoplasma 2012, 250, 723–730. [Google Scholar] [CrossRef]
- Staub, R. Ernährungphysiologish-autökologische Untersuchung an den plank tonischen Blaualge Oscillatoria rubescens DC. Schweiz. Z. Hydrol. 1961, 23, 82–198. [Google Scholar]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dąbrowski, P.; et al. Frequently asked questions about in vivo chlorophyll fluorescence: Practical issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef] [PubMed]
- Romanowska-Duda, Z.; Grzesik, M.; Janas, R. Maximal efficiency of PSII as a marker of sorghum development fertilized with waste from a biomass biodigestion to methane. Front. Plant Sci. 2019, 9, 1920. [Google Scholar] [CrossRef]
- Howell, W.M.; Black, D.A. Controlled silver-staining of nucleolus organizer regions with a protective colloidal developer: A 1-step method. Experientia 1980, 36, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, Y. A matter of size: Developmental control of organ size in plants. Curr. Opin. Plant. Biol. 2001, 4, 533–539. [Google Scholar] [CrossRef]
- Scheres, B.; Benfey, P.; Dolan, L. Root development. Arab. Book/Am. Soc. Plant Biol. 2002, 1, e0101. [Google Scholar] [CrossRef]
- Manzano, A.I.; Larkin, O.J.; Dijkstra, C.E.; Anthony, P.; Davey, M.R.; Evans, L.; Hill, R.J.A.; Herranz, R.; Medina, F.J. Meristematic cell proliferation and ribosome biogenesis are decoupled in diamagnetically levitated Arabidopsis seedlings. BMC Plant. Biol. 2013, 13, 124. [Google Scholar] [CrossRef]
- Medina, F.J.; Cerdido, A.; de Carcer, G. The functional organization of the nucleolus in proliferating plant cells. Eur. J. Histochem. 2000, 44, 117–131. [Google Scholar]
- Biancucci, M.; Mattioli, R.; Moubayidin, L.; Sabatini, S.; Costantino, P.; Trovato, M. Proline affects the size of the root meristematic zone in Arabidopsis. BMC Plant Biol. 2015, 15, 263. [Google Scholar] [CrossRef]
- Verslues, P.E.; Longkumer, T. Size and activity of the root meristem: A key for drought resistance and a key model of drought-related signaling. Physiol. Plant. 2022, 174, e13622. [Google Scholar] [CrossRef] [PubMed]
- Desvoyes, B.; Echevarría, C.; Gutierrez, C. A perspective on cell proliferation kinetics in the root apical meristem. J. Exp. Bot. 2021, 72, 6708–6715. [Google Scholar] [CrossRef] [PubMed]
- Moubayidin, L.; Perilli, S.; Dello Ioio, R.; Di Mambro, R.; Costantino, P.; Sabatini, S. The rate of cell differentiation controls the Arabidopsis root meristem growth phase. Curr. Biol. 2010, 20, 1138–1143. [Google Scholar] [CrossRef]
- Nemeth, A.; Grummt, I. Dynamic regulation of nucleolar architecture. Curr. Opin. Cell Biol. 2018, 52, 105–111. [Google Scholar] [CrossRef]
- Stępiński, D. Organization of the nucleoli of soybean root meristematic cells at different states of their activity. Micron 2010, 41, 283–288. [Google Scholar] [CrossRef]
- Yang, K.; Yang, J.; Yi, J. Nucleolar Stress: Hallmarks, sensing mechanism and diseases. Cell Stress 2018, 2, 125–140. [Google Scholar] [CrossRef]
- Huo, C.Y.; Chang, M.L.; Cheng, H.; Ma, T.T.; Fu, Y.; Wang, Y.; Wang, Y.Y.; Kan, Y.C.; Li, D.D. Small nucleolar RNA of silkworm can translocate from the nucleolus to the cytoplasm under abiotic stress. Cell Biol. Int. 2021, 45, 1091–1097. [Google Scholar] [CrossRef]
- Weeks, S.E.; Metge, B.J.; Samant, R.S. The nucleolus: A central response hub for the stressors that drive cancer progression. Cell Mol. Life Sci. 2019, 76, 4511–4524. [Google Scholar] [CrossRef] [PubMed]
- Ohbayashi, I.; Sugiyama, M. Plant nucleolar stress response, a new face in the NAC-dependent cellular stress response. Front. Plant Sci. 2018, 8, 2247. [Google Scholar] [CrossRef]
- Picart-Picolo, A.; Picart, C.; Picault, N.; Pontvianne, F. Nucleolus-associated chromatin domains are maintained under heat stress, despite nucleolar reorganization in Arabidopsis thaliana. J. Plant Res. 2020, 133, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Matos-Perdomo, E.; Machín, F. Nucleolar and ribosomal DNA structure under stress: Yeast lessons for aging and cancer. Cells 2019, 8, 779. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.; Grummt, I. Cellular stress and nucleolar function. Cell Cycle 2005, 4, 1036–1038. [Google Scholar] [CrossRef] [PubMed]
- Derenzini, M.; Trerč, D.; Pession, A.; Govoni, M.; Sirri, V.; Chieco, P. Nucleolar size indicates the rapidity of cell proliferation in cancer tissues. J. Pathol. 2000, 191, 181–186. [Google Scholar] [CrossRef]
- Tiku, V.; Jain, C.; Raz, Y.; Nakamura, S.; Heestand, B.; Liu, W.; Spath, M.; Suchiman, H.E.D.; Muller, R.U.; Slagboom, P.E.; et al. Small nucleoli are a cellular hallmark of longevity. Nat. Comm. 2017, 8, 16083. [Google Scholar] [CrossRef]
- Kalinina, N.O.; Makarova, S.; Makhotenko, A.; Love, A.J.; Talinsky, M. The multiple functions of the nucleolus in plant development, disease and stress responses. Front. Plant Sci. 2018, 9, 132. [Google Scholar] [CrossRef]
- Kojima, H.; Suzuki, T.; Kato, T.; Enomoto, K.; Sato, S.; Kato, T.; Tabata, S.; Sáez-Vasquez, J.; Echeverría, M.; Nakagawa, T.; et al. Sugar-inducible expression of the nucleolin-1 gene of Arabidopsis thaliana and its role in ribosome synthesis, growth and development. Plant J. 2007, 49, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, X.; Li, R.; Gao, Y.; Xu, Z.; Zhang, B.; Zhou, Y. Retention of OsNMD3 in the cytoplasm disturbs protein synthesis efficiency and affects plant development in rice. J. Exp. Bot. 2014, 65, 3055–3069. [Google Scholar] [CrossRef]
- Weis, B.L.; Kovacevic, J.; Missbach, S.; Schleiff, E. Plant specific features of ribosome biogenesis. Trends Plant Sci. 2015, 20, 729–740. [Google Scholar] [CrossRef]
- Baudo, R.; Foudoulakis, M.; Arapis, G.; Perdaen, K.; Lanneau, W.; Paxinou, A.C.M.; Kouvdou, S.; Persoone, G. History and sensitivity comparison of the Spirodela polyrrhiza microbiotest and Lemna toxicity tests. Knowl. Managt. Aquat. Ecosyst. 2015, 416, 23. [Google Scholar] [CrossRef]
- Maekawa, S.; Yanagisawa, S. Nucleolar stress and sugar response in plants. Plant Signal. Behav. 2018, 13, 209–227. [Google Scholar] [CrossRef]
- Paliya, S.; Mandpe, A.; Kumar, S.; Kumar, S.M. Enhanced nodulation and higher germination using sludge ash as a carrier for biofertilizer production. J. Environ. Manag. 2019, 250, 109523. [Google Scholar] [CrossRef] [PubMed]
- Pandey, C.V. Scope of fly ash use in agriculture: Prospects and challenges. In Phytomanagement of Fly Ash; Elsevier: Amsterdam, The Netherlands, 2020; pp. 63–101. [Google Scholar] [CrossRef]
- Singh, A.; Agrawal, S.B. Response of mung bean cultivars to fly ash: Growth and yield. Ecotoxicol. Environ. Saf. 2010, 73, 1950–1958. [Google Scholar] [CrossRef] [PubMed]
- Skousen, J.; Yang, J.E.; Lee, J.; Ziemkiewicz, P. Review of fly ash as a soil amendment. Geosyst. Eng. 2013, 16, 249–256. [Google Scholar] [CrossRef]
- Ukwattage, N.L.; Ranjith, P.G.; Bouazza, M. The use of coal combustion fly ash as a soil amendment in agricultural lands (with comments on its potential to improve food security and sequester carbon). Fuel 2013, 109, 400–408. [Google Scholar] [CrossRef]
- Adriano, D.C.; Weber, J.; Bolan, N.; Paramasivam, S.; Koo, B.J.; Sajwan, K.S. Effects of high rates of coal fly ash on soil, turfgrass and groundwater quality. Water Air Soil Pollut. 2002, 139, 365–385. [Google Scholar] [CrossRef]
- Tsadilas, C.D.; Shaheen, S.M.; Samaras, V. Influence of coal fly ash application individually and mixing with sewage sludge on wheat growth and soil chemical properties under field conditions. In Proceedings of the 15th International Symposium on Environmental Pollution and its Impact on Life in the Mediterranean Region, Bari, Italy, 7–11 October 2009; p. 145. [Google Scholar]
- Rautaray, S.K.; Ghosh, B.C.; Mittra, B.N. Effect of fly ash, organic wastes and chemical fertilizers on yield, nutrient uptake, heavy metal content and residua fertility in a rice-mustard cropping sequence under acid lateritic soils. Bioresour. Technol. 2003, 90, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Sinha, S. Role of Brassica juncea (L.) Czern. (var. Vaibhav) in the phytoextraction of Ni from soil amended with fly ash: Selection of extracting for metal bioavailability. J. Hazard. Mater. 2006, 136, 371–378. [Google Scholar] [CrossRef]
- Niu, Y.; Tan, H.; Hui, S. Ash-related issues during biomass combustion: Alkali-induced slagging, silicate melt-induced slagging (ash fusion), agglomeration, corrosion, ash utilization, and related countermeasures. Prog. Energy Combust. Sci. 2016, 52, 1–61. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romanowska-Duda, Z.; Piotrowski, K.; Stępiński, D.; Popłońska, K. A Promising Ash Supplementation Strategy in the Cultivation of Spirodela polyrrhiza Plants. Cells 2023, 12, 289. https://doi.org/10.3390/cells12020289
Romanowska-Duda Z, Piotrowski K, Stępiński D, Popłońska K. A Promising Ash Supplementation Strategy in the Cultivation of Spirodela polyrrhiza Plants. Cells. 2023; 12(2):289. https://doi.org/10.3390/cells12020289
Chicago/Turabian StyleRomanowska-Duda, Zdzisława, Krzysztof Piotrowski, Dariusz Stępiński, and Katarzyna Popłońska. 2023. "A Promising Ash Supplementation Strategy in the Cultivation of Spirodela polyrrhiza Plants" Cells 12, no. 2: 289. https://doi.org/10.3390/cells12020289
APA StyleRomanowska-Duda, Z., Piotrowski, K., Stępiński, D., & Popłońska, K. (2023). A Promising Ash Supplementation Strategy in the Cultivation of Spirodela polyrrhiza Plants. Cells, 12(2), 289. https://doi.org/10.3390/cells12020289





