Antibody Properties Associate with Clinical Phenotype in LGI1 Encephalitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Autoantibody Screening and Purification
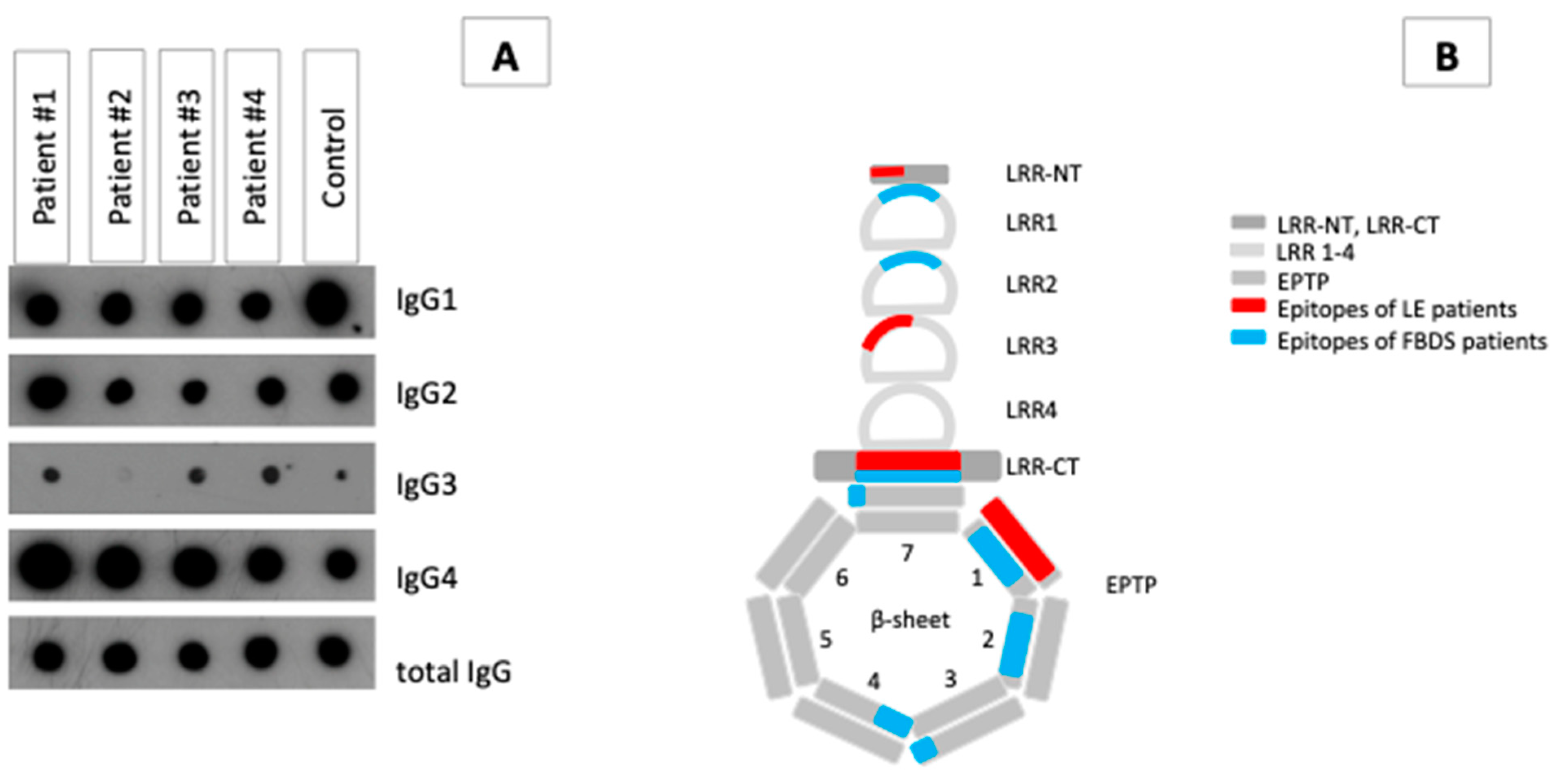
2.2. Epitope Determination
2.3. Determination of IgG Subclasses of Anti-LGI1 ab Fractions and Purification
2.4. HLA-Mapping
2.5. Isolation of IgG Fractions
2.6. Electrophysiology
2.7. Slice Preparation
2.8. Imaging and Analysis of Spine Density in Hippocampal Slices
2.9. Immunohistochemistry/Cluster Differentiation
2.10. Data Analysis and Statistics
3. Results
3.1. Patients Description
3.2. IgG Subclass Analysis and Epitope Mapping Reveal Differences between Patients with LE and FBDS
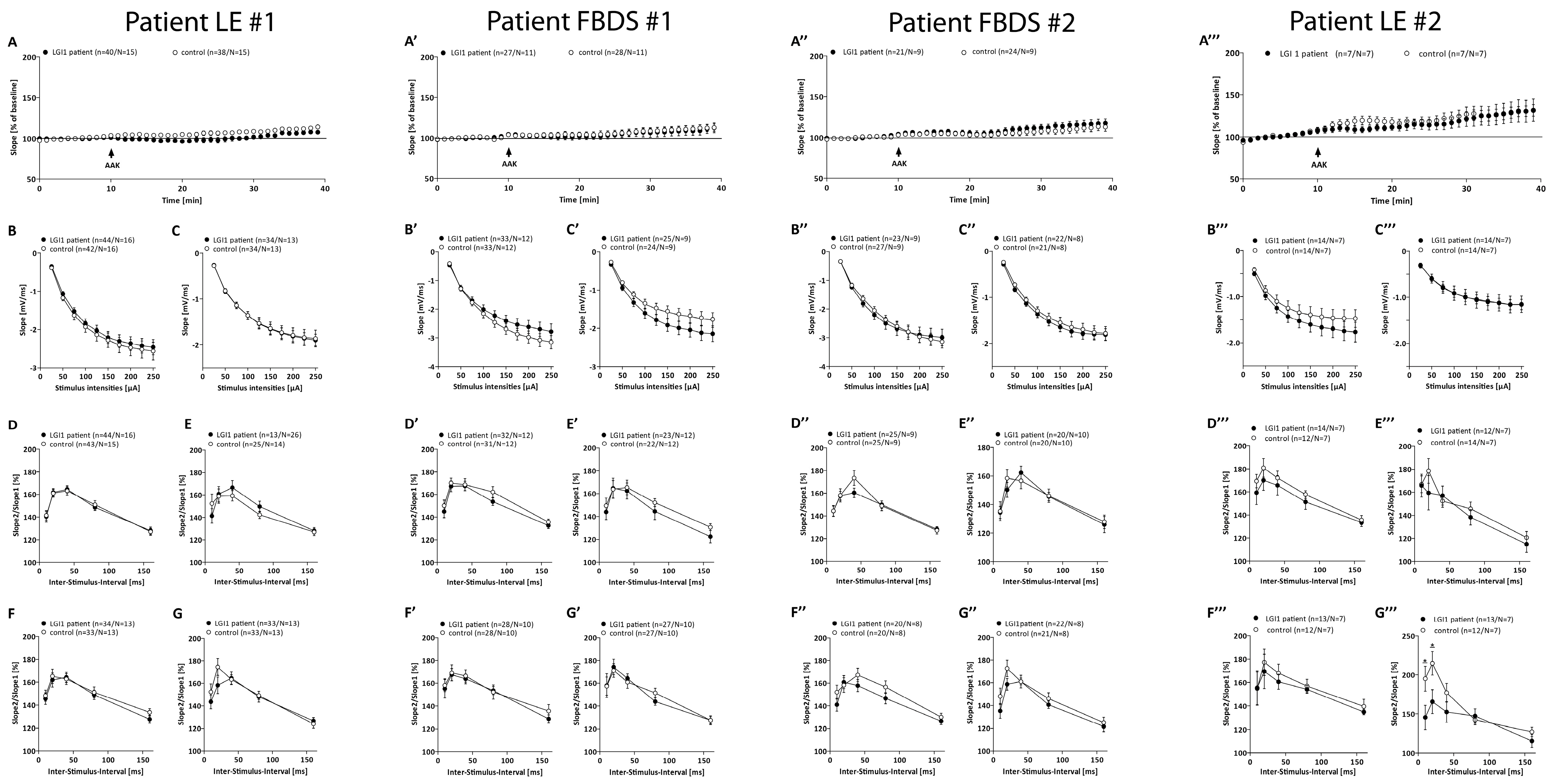
3.3. Electrophysiological Effects of LGI1 abs Differ between Patients with FBDS and the Patients with LE
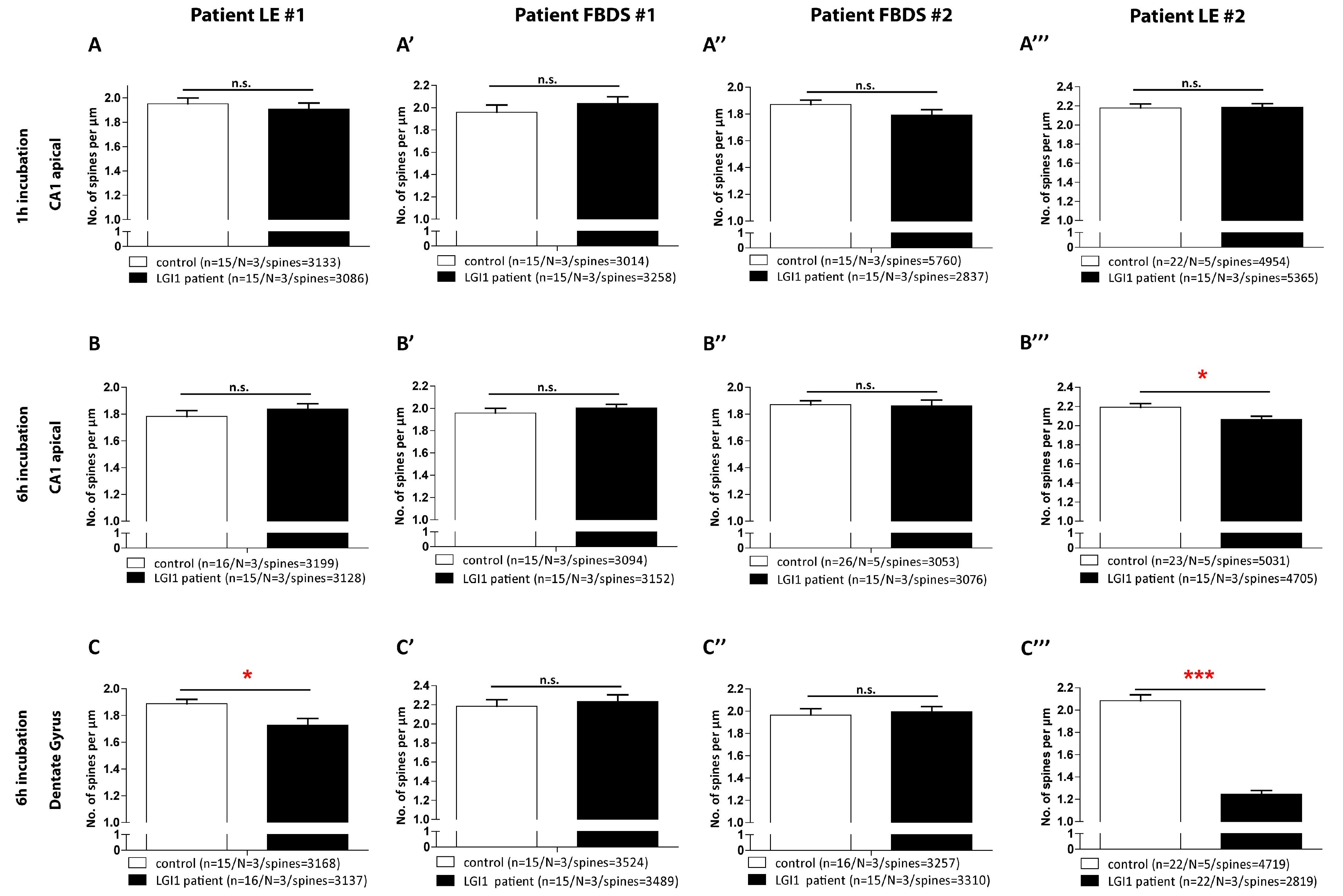
3.4. Hippocampal Spine Density Is Reduced by IgG from the Patients with LE Compared to IgG from Patients with FBDS
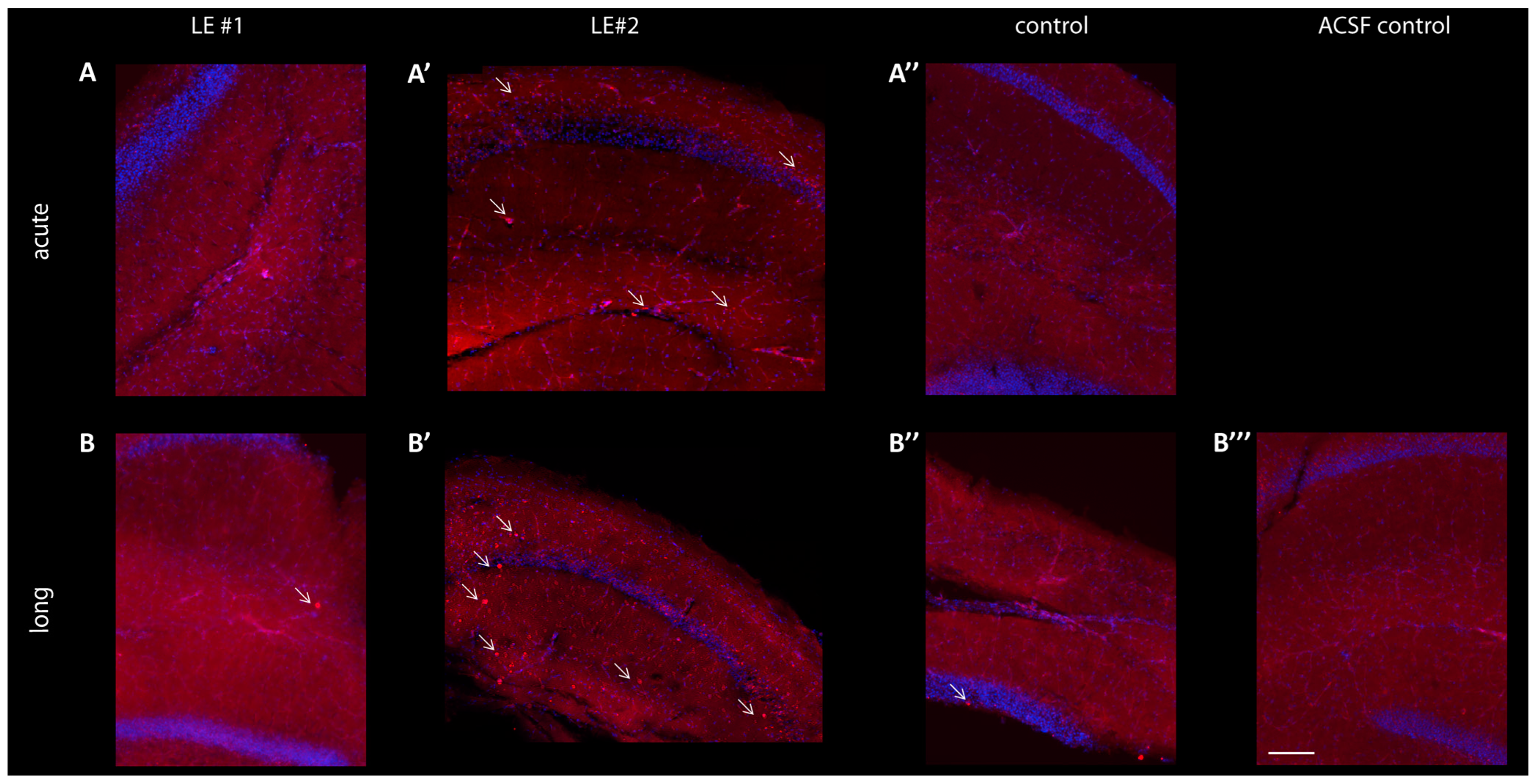
3.5. No Significant Changes of Postsynaptic AMPA-R Density Observed in Slices after Treatment with LGI1 ab from Patient LE#1, but Increased Cell Apoptosis after LGI1 ab Treatment from Patient LE#2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Sonderen, A.; Thijs, R.D.; Coenders, E.C.; Jiskoot, L.C.; Sanchez, E.; de Bruijn, M.A.A.M.; van Coevorden-Hameete, M.H.; Wirtz, P.W.; Schreurs, M.W.J.; Sillevis Smitt, P.A.E.; et al. Anti-LGI1 encephalitis: Clinical syndrome and long-term follow-up. Neurology 2016, 87, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Ariño, H.; Armangué, T.; Petit-Pedrol, M.; Sabater, L.; Martinez-Hernandez, E.; Hara, M.; Lancaster, E.; Saiz, A.; Dalmau, J.; Graus, F. Anti-LGI1—Associated cognitive impairment Presentation and long-term outcome. Neurology 2016, 87, 1–8. [Google Scholar] [CrossRef]
- Thompson, J.; Bi, M.; Murchison, A.G.; Makuch, M.; Bien, C.G.; Chu, K.; Farooque, P.; Gelfand, J.M.; Geschwind, M.D.; Hirsch, L.J.; et al. The importance of early immunotherapy in patients with faciobrachial dystonic seizures. Brain 2018, 141, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Herranz-Pérez, V.; Olucha-Bordonau, F.E.; Morante-Redolat, J.M. Regional distribution of the leucine-rich glioma inactivated (LGI) gene family transcripts in the adult mouse brain. Brain Res. 2010, 1307, 177–194. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Franco, J.; Debreux, K.; Extremet, J.; Maulet, Y.; Belghazi, M.; Villard, C.; Sangiardi, M.; Youssouf, F.; El Far, L.; Lévêque, C.; et al. Patient-derived antibodies reveal the subcellular distribution and heterogeneous interactome of LGI1. Brain 2022, 145, 3843–3858. [Google Scholar] [CrossRef] [PubMed]
- Fukata, Y.; Adesnik, H.; Iwanaga, T.; Bredt, D.S.; Nicoll, R.A.; Fukata, M. Epilepsy-Related Ligand/Receptor Complex LGI1 and ADAM22 Regulate Synpatic Transmission. Science 2006, 313, 1792–1795. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, A.; Miyazaki, Y.; Yokoi, N.; Shigematsu, H.; Sato, Y.; Goto-Ito, S.; Maeda, A.; Goto, T.; Sanbo, M.; Hirabayashi, M.; et al. Structural basis of epilepsy-related ligand-receptor complex LGI1-ADAM22. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fukata, Y.; Yokoi, N.; Miyazaki, Y.; Fukata, M. The LGI1–ADAM22 protein complex in synaptic transmission and synaptic disorders. Neurosci. Res. 2017, 116, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Fukata, Y.; Lovero, K.L.; Iwanaga, T.; Watanabe, A.; Yokoi, N.; Tabuchi, K.; Shigemoto, R.; Nicoll, R.A.; Fukata, M. Disruption of LGI1-linked synaptic complex causes abnormal synaptic transmission and epilepsy. Proc. Natl. Acad. Sci. USA 2010, 107, 3799–3804. [Google Scholar] [CrossRef]
- Seagar, M.; Russier, M.; Caillard, O.; Maulet, Y.; Fronzaroli-Molinieres, L.; De San Feliciano, M.; Boumedine-Guignon, N.; Rodriguez, L.; Zbili, M.; Usseglio, F.; et al. LGI1 tunes intrinsic excitability by regulating the density of axonal Kv1 channels. Proc. Natl. Acad. Sci. USA 2017, 114, 7719–7724. [Google Scholar] [CrossRef]
- Ohkawa, T.; Fukata, Y.; Yamasaki, M.; Miyazaki, T.; Yokoi, N.; Takashima, H.; Watanabe, M.; Watanabe, O.; Fukata, M. Autoantibodies to Epilepsy-Related LGI1 in Limbic Encephalitis Neutralize LGI1-ADAM22 Interaction and Reduce Synaptic AMPA Receptors. J. Neurosci. 2013, 33, 18161–18174. [Google Scholar] [CrossRef] [PubMed]
- Fels, E.; Mayeur, M.E.; Wayere, E.; Vincent, C.; Malleval, C.; Honnorat, J.; Pascual, O. Dysregulation of the hippocampal neuronal network by LGI1 auto-antibodies. PLoS ONE 2022, 17, e0272277. [Google Scholar] [CrossRef] [PubMed]
- Petit-Pedrol, M.; Sell, J.; Planagumà, J.; Mannara, F.; Radosevic, M.; Haselmann, H.; Ceanga, M.; Sabater, L.; Spatola, M.; Soto, D.; et al. LGI1 antibodies alter K v 1.1 and AMPA receptors changing synaptic excitability, plasticity and memory. Brain 2018, 141, 3144–3159. [Google Scholar] [PubMed]
- Ramberger, M.; Berretta, A.; Tan, J.M.M.; Sun, B.; Michael, S.; Yeo, T.; Theorell, J.; Bashford-Rogers, R.; Paneva, S.; O’Dowd, V.; et al. Distinctive binding properties of human monoclonal LGI1 autoantibodies determine pathogenic mechanisms. Brain 2020, 143, 1731–1745. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.C.; Ludewig, S.; Winschel, A.; Abel, T.; Bold, C.; Salzburger, L.R.; Klein, S.; Han, K.; Weyer, S.W.; Fritz, A.K.; et al. Distinct in vivo roles of secreted APP ectodomain variants APPsα and APPsβ in regulation of spine density, synaptic plasticity, and cognition. EMBO J. 2018, 37, e98335. [Google Scholar] [CrossRef] [PubMed]
- Hick, M.; Herrmann, U.; Weyer, S.W.; Mallm, J.P.; Tschäpe, J.A.; Borgers, M.; Mercken, M.; Roth, F.C.; Draguhn, A.; Slomianka, L.; et al. Acute function of secreted amyloid precursor protein fragment APPsα in synaptic plasticity. Acta Neuropathol. 2015, 129, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Ayşit-Altuncu, N.; Ulusoy, C.; Öztürk, G.; Tüzün, E. Effect of LGI1 antibody-positive IgG on hippocampal neuron survival: A preliminary study. Neuroreport 2018, 29, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Lüscher, C.; Malenka, R.C. NMDA Receptor-Dependent Long-Term Potentiation and Long-Term Depression (LTP/LTD). Cold Spring Harb. Perspect. Biol. 2012, 4, a005710. [Google Scholar] [CrossRef] [PubMed]
- Vanderlugt, C.L.; Miller, S.D. Epitope spreading in immune-mediated diseases: Implications for immunotherapy. Nat. Rev. Immunol. 2002, 2, 85–95. [Google Scholar] [CrossRef] [PubMed]


| Recorded data | Variable | LE #1 | LE #2 | FBDS #1 | FBDS #2 |
|---|---|---|---|---|---|
| Symptoms | Limbic encephalitis | Limbic encephalitis | FBDS, complex partial sz. | FBDS | |
| LGI1 titre | initial | 1:100 | 1:20 | 1:1000 | 1:160 |
| after treament | 1:30 | none | 1:100 | 1:10 | |
| Treatment | MP, PLEX, Rit. | MP, Rit. | MP, PLEX, Rit. | MP | |
| Epitopes | KKPAK, HTFR, CEGPP, | GTSVVC, AQPFTGKCIF, VEKTFRNYDN | NDEYV, VEYL | ISEGSF, ISDO, CIITE, LND, PIVIET, VEY | |
| HLA Type | DRB1*04:02[DR4]; DQA1*03:01;DQB1*03:02[DQ8(3);DPA1*01:03;DPA1*02:01,DPB1*01:01[DPw1],DPB1*04:01[DPw4] | DRB1*07:01[DR7],DQA1*02:01,DQB1*02:02[DQ2],DRB1*08:01[DR8], DQA1*04:01, DQB1*04:02[DQ4 | DRB1*04[DR4],DQA1*03,DRB1*07[DR7],DQB1*02:02[DQ2],DQB1*03:02[DQ8(3),DPA1*01:03,DPA1*02:01,DPB1*02:01[DPw2],DPB1*04:01[DPw4] | DRB1*04:07[DR4],DQA1*02:01; DQB1*02:02[DQ2],DQB1*03:01[DQ7(3)],DRB1*01[DR7 | |
| Electrophysiology | LTP mean of averaged potentiation values (%) | Patient 1: 134.32% ± 3.92; Control: 128.06% ± 3.31 | Patient 2: 118.88% ± 3.35; Control: 120.81% ± 3.63 | Patient 3: 123.36% ± 3.37; Control: 124.80% ± 4.80 | Patient 4: 132.9% ± 3.6; Control: 134.4% ± 8.4 |
| 1 h incubation | N = 13, n = 27 | N = 6, n = 12 | N = 11, n = 19 | N = 10, n = 18 | |
| p = 0.419 | p = 0.876 | p = 0.706 | p = 0.810 | ||
| 6 h incubation | Patient 1: 111.67% ± 4.07; Control: 126.29% ± 3.94 | Patient 2: 118.30% ± 3.92; Control: 115.34% ± 2.75 | Patient 3: 115.38% ± 2.33; Control: 115.4% 8 ± 2.45 | Patient 4: 120.3% ± 7.2; Control: 125.6% ± 6.9 | |
| N = 13, n = 31 | N = 6, n = 10 | N = 10, n = 23 | N = 8, n = 21 | ||
| p = 0.015 | p = 0.601 | p = 0.451 | p = 0.978 | ||
| Spine density | spine density | Patient 1: 1.91 ± 0.05; Control: 1.95 ± 0.05 spines/µm dendrite | Patient 2: 2.04 ± 0.06; Control: 1.96 ± 0.06 spines/µm dendrite | Patient 3: 1.86 ± 0.04; Control: 1.87 ± 0.03 spines/µm dendrite | Patient 4: 2.19 ± 0.04; Control: 2.18 ± 0.04 spines/µm dendrite |
| CA1 apical | 1 h incubation | N = 3, n = 15 | N = 3, n = 15 | N = 3, n = 15 | N = 3, n = 15 |
| 3086 spines | 5365 spines | 3258 spines | 2738 spines | ||
| p = 0.548 | p = 0.9013 | p = 0.393 | p = 0.202 | ||
| 6 h incubation | Patient 1: 1.84 ± 0.04; Control: 1.78 ± 0.04 spines/µm dendrite | Patient 2: 2.00 ± 0.03; Control: 1.96 ± 0.04 spines/µm dendrite | Patient 3: 1.80 ± 0.04; Control: 1.87 ± 0.03 spines/µm dendrite | Patient 4: 2.07 ± 0.03; Control: 2.19 ± 0.03 spines/µm dendrite | |
| N = 3, n = 15 | N = 3, n = 15 | N = 3, n = 15 | N = 3, n = 15 | ||
| 3128 spines | 4705 spines | 3152 spines | 3067 spines | ||
| p = 0.345 | p = 0.0152 | p = 0.414 | p = 0.147 | ||
| Dentate Gyrus | 6 h incubation | Patient 1: 1.73 ± 0.05; Control: 1.89 ± 0.03 spines/µm dendrite | Patient 2: 2.23 ± 0.07; Control: 2.19 ± 0.07 spines/µm dendrite | Patient 3: 2.00 ± 0.05; Control: 1.96 ± 0.06 spines/µm dendrite | Patient 4: 2.08 ± 0.05; Control: 1.25 ± 0.03 spines/µm dendrite |
| N = 3, n = 15 | N = 3, n = 15 | N = 3, n = 15 | N= 3, n = 15 | ||
| 3137 spines | 2819 | 3489 | 3310 | ||
| p= 0.014 | p < 0.0001 | p = 0.630 | p = 0.705 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ludewig, S.; Salzburger, L.; Goihl, A.; Rohne, J.; Leypoldt, F.; Bittner, D.; Düzel, E.; Schraven, B.; Reinhold, D.; Korte, M.; et al. Antibody Properties Associate with Clinical Phenotype in LGI1 Encephalitis. Cells 2023, 12, 282. https://doi.org/10.3390/cells12020282
Ludewig S, Salzburger L, Goihl A, Rohne J, Leypoldt F, Bittner D, Düzel E, Schraven B, Reinhold D, Korte M, et al. Antibody Properties Associate with Clinical Phenotype in LGI1 Encephalitis. Cells. 2023; 12(2):282. https://doi.org/10.3390/cells12020282
Chicago/Turabian StyleLudewig, Susann, Leonie Salzburger, Alexander Goihl, Jana Rohne, Frank Leypoldt, Daniel Bittner, Emrah Düzel, Burkhart Schraven, Dirk Reinhold, Martin Korte, and et al. 2023. "Antibody Properties Associate with Clinical Phenotype in LGI1 Encephalitis" Cells 12, no. 2: 282. https://doi.org/10.3390/cells12020282
APA StyleLudewig, S., Salzburger, L., Goihl, A., Rohne, J., Leypoldt, F., Bittner, D., Düzel, E., Schraven, B., Reinhold, D., Korte, M., & Körtvélyessy, P. (2023). Antibody Properties Associate with Clinical Phenotype in LGI1 Encephalitis. Cells, 12(2), 282. https://doi.org/10.3390/cells12020282






