Immunotherapy: Recent Advances and Its Future as a Neoadjuvant, Adjuvant, and Primary Treatment in Colorectal Cancer
Abstract
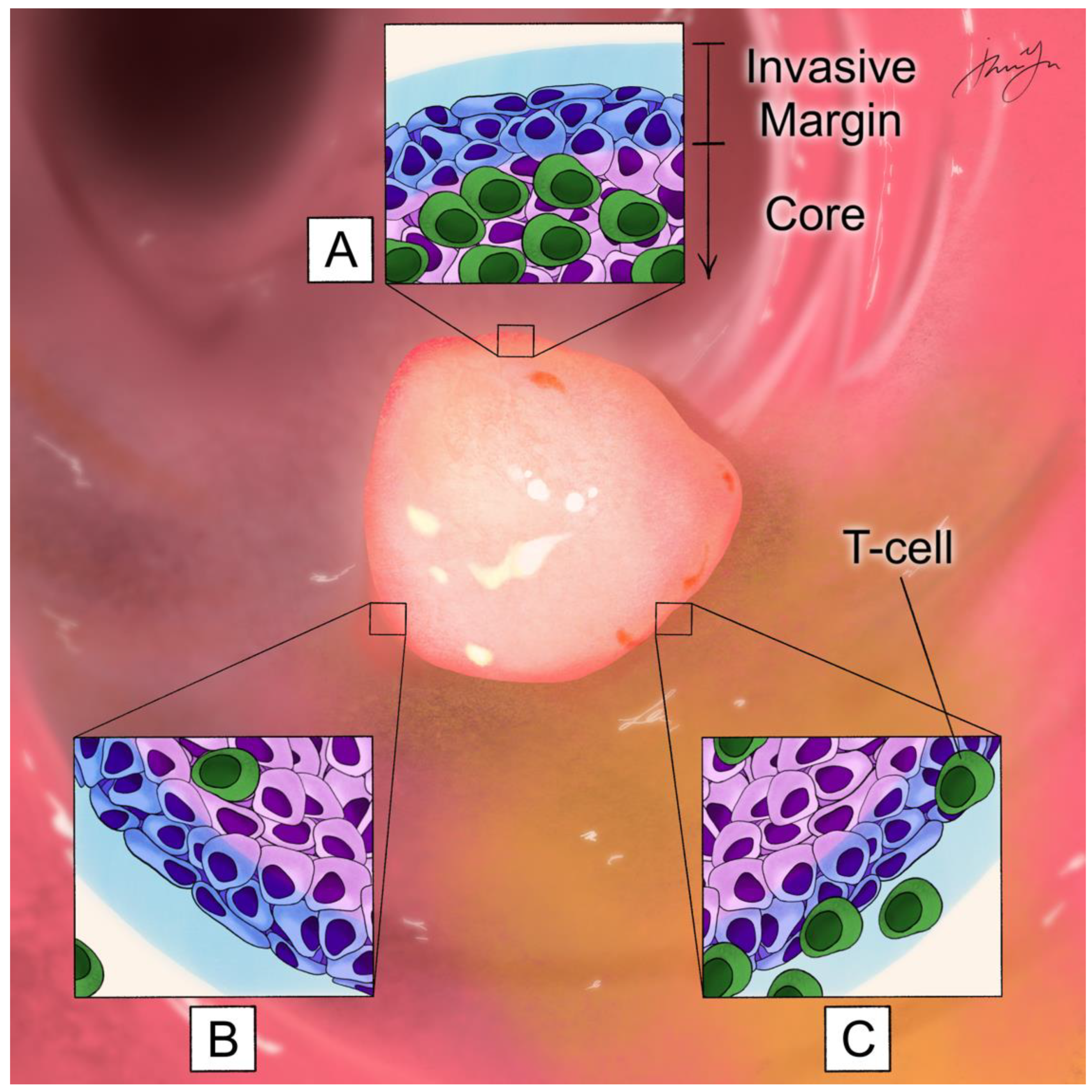
1. Introduction
2. Immune Checkpoint Inhibitors
2.1. History of Immune Checkpoint Inhibitors
2.2. Relevance of Microsatellite Status in ICI Use
2.3. ICIs in dMMR Colon Cancer
2.4. ICIs in dMMR Rectal Cancer
2.5. ICIs in pMMR Colon Cancer
2.6. Clinical Trials
2.7. Recent Advancements in Molecular Mechanisms of PD-1/PD-L1 Blockade
3. Adoptive Cell Transfer Therapy
3.1. Cytokine-Induced Killer Cells
3.2. Chimera Antigen Receptor Therapy
3.3. Combinations
4. Tumor Vaccines
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Cancer Institute. Cancer Stat Facts: Colorectal Cancer. Available online: https://seer.cancer.gov/statfacts/html/colorect.html (accessed on 2 December 2022).
- Tokumaru, Y.; Joyce, D.; Takabe, K. Current status and limitations of immunotherapy for breast cancer. Surgery 2020, 167, 628–630. [Google Scholar] [CrossRef]
- Phan, G.Q.; Yang, J.C.; Sherry, R.M.; Hwu, P.; Topalian, S.L.; Schwartzentruber, D.J.; Restifo, N.P.; Haworth, L.R.; Seipp, C.A.; Freezer, L.J.; et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc. Natl. Acad. Sci. USA 2003, 100, 8372–8377. [Google Scholar] [CrossRef]
- Hodi, F.S.; Mihm, M.C.; Soiffer, R.J.; Haluska, F.G.; Butler, M.; Seiden, M.V.; Davis, T.; Henry-Spires, R.; MacRae, S.; Willman, A.; et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc. Natl. Acad. Sci. USA 2003, 100, 4712–4717. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Oshi, M.; Asaoka, M.; Tokumaru, Y.; Angarita, F.A.; Yan, L.; Matsuyama, R.; Zsiros, E.; Ishikawa, T.; Endo, I.; Takabe, K. Abundance of Regulatory T Cell (Treg) as a Predictive Biomarker for Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer. Cancers 2020, 12, 3038. [Google Scholar] [CrossRef]
- Chan, D.V.; Gibson, H.M.; Aufiero, B.M.; Wilson, A.J.; Hafner, M.S.; Mi, Q.S.; Wong, H.K. Differential CTLA-4 expression in human CD4+ versus CD8+ T cells is associated with increased NFAT1 and inhibition of CD4+ proliferation. Genes Immun. 2014, 15, 25–32. [Google Scholar] [CrossRef]
- Tay, R.E.; Richardson, E.K.; Toh, H.C. Revisiting the role of CD4(+) T cells in cancer immunotherapy-new insights into old paradigms. Cancer Gene 2021, 28, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Seidel, J.A.; Otsuka, A.; Kabashima, K. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front. Oncol. 2018, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Q.; Ren, Y.; Ariston Gabrie, A.N.; Wang, Q.; Wang, Y.; Du, L.; Liu, X.; Wang, C.; Wang, Y.S. Advances of immune checkpoints in colorectal cancer treatment. Biomed. Pharmacother. 2020, 123, 109745. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Lipson, E.J.; Sharfman, W.H.; Drake, C.G.; Wollner, I.; Taube, J.M.; Anders, R.A.; Xu, H.; Yao, S.; Pons, A.; Chen, L.; et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin. Cancer Res. 2013, 19, 462–468. [Google Scholar] [CrossRef]
- Boland, C.R.; Thibodeau, S.N.; Hamilton, S.R.; Sidransky, D.; Eshleman, J.R.; Burt, R.W.; Meltzer, S.J.; Rodriguez-Bigas, M.A.; Fodde, R.; Ranzani, G.N.; et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: Development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998, 58, 5248–5257. [Google Scholar]
- Lee, S.Y.; Kim, D.W.; Lee, H.S.; Ihn, M.H.; Oh, H.K.; Min, B.S.; Kim, W.R.; Huh, J.W.; Yun, J.A.; Lee, K.Y.; et al. Low-Level Microsatellite Instability as a Potential Prognostic Factor in Sporadic Colorectal Cancer. Medicine 2015, 94, e2260. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Ye, Y.; Liu, X.; Zhu, G.; Xu, Y.; Sun, J.; Wu, H.; Feng, F.; Wen, Z.; Jiang, S.; et al. Analyzing microsatellite instability and gene mutation in circulating cell-free DNA to monitor colorectal cancer progression. Transl. Cancer Res. 2021, 10, 2812–2821. [Google Scholar] [CrossRef]
- Gorzo, A.; Galos, D.; Volovat, S.R.; Lungulescu, C.V.; Burz, C.; Sur, D. Landscape of Immunotherapy Options for Colorectal Cancer: Current Knowledge and Future Perspectives beyond Immune Checkpoint Blockade. Life 2022, 12, 229. [Google Scholar] [CrossRef]
- Zheng, Y.; Fu, Y.; Wang, P.P.; Ding, Z.Y. Neoantigen: A Promising Target for the Immunotherapy of Colorectal Cancer. Dis. Markers 2022, 2022, 8270305. [Google Scholar] [CrossRef] [PubMed]
- Yuza, K.; Nagahashi, M.; Watanabe, S.; Takabe, K.; Wakai, T. Hypermutation and microsatellite instability in gastrointestinal cancers. Oncotarget 2017, 8, 112103–112115. [Google Scholar] [CrossRef]
- Narayanan, S.; Kawaguchi, T.; Peng, X.; Qi, Q.; Liu, S.; Yan, L.; Takabe, K. Tumor Infiltrating Lymphocytes and Macrophages Improve Survival in Microsatellite Unstable Colorectal Cancer. Sci. Rep. 2019, 9, 13455. [Google Scholar] [CrossRef] [PubMed]
- Chalabi, M.; Fanchi, L.F.; Dijkstra, K.K.; Van den Berg, J.G.; Aalbers, A.G.; Sikorska, K.; Lopez-Yurda, M.; Grootscholten, C.; Beets, G.L.; Snaebjornsson, P.; et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat. Med. 2020, 26, 566–576. [Google Scholar] [CrossRef]
- Fessas, P.; Lee, H.; Ikemizu, S.; Janowitz, T. A molecular and preclinical comparison of the PD-1-targeted T-cell checkpoint inhibitors nivolumab and pembrolizumab. Semin. Oncol. 2017, 44, 136–140. [Google Scholar] [CrossRef]
- André, T.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- Andre, T.; Amonkar, M.; Norquist, J.M.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.J.A.; Smith, D.; Garcia-Carbonero, R.; et al. Health-related quality of life in patients with microsatellite instability-high or mismatch repair deficient metastatic colorectal cancer treated with first-line pembrolizumab versus chemotherapy (KEYNOTE-177): An open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 665–677. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Colon Cancer (Version 2.2022). Available online: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (accessed on 2 December 2022).
- Therkildsen, C.; Jensen, L.H.; Rasmussen, M.; Bernstein, I. An Update on Immune Checkpoint Therapy for the Treatment of Lynch Syndrome. Clin. Exp. Gastroenterol. 2021, 14, 181–197. [Google Scholar] [CrossRef]
- Guyot D’Asnières De Salins, A.; Tachon, G.; Cohen, R.; Karayan-Tapon, L.; Junca, A.; Frouin, E.; Godet, J.; Evrard, C.; Randrian, V.; Duval, A.; et al. Discordance between immunochemistry of mismatch repair proteins and molecular testing of microsatellite instability in colorectal cancer. ESMO Open 2021, 6, 100120. [Google Scholar] [CrossRef] [PubMed]
- Echle, A.; Grabsch, H.I.; Quirke, P.; van den Brandt, P.A.; West, N.P.; Hutchins, G.G.A.; Heij, L.R.; Tan, X.; Richman, S.D.; Krause, J.; et al. Clinical-Grade Detection of Microsatellite Instability in Colorectal Tumors by Deep Learning. Gastroenterology 2020, 159, 1406–1416.e11. [Google Scholar] [CrossRef]
- Sha, D.; Jin, Z.; Budczies, J.; Kluck, K.; Stenzinger, A.; Sinicrope, F.A. Tumor Mutational Burden as a Predictive Biomarker in Solid Tumors. Cancer Discov. 2020, 10, 1808–1825. [Google Scholar] [CrossRef]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H., Jr.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Büttner, R.; Longshore, J.W.; López-Ríos, F.; Merkelbach-Bruse, S.; Normanno, N.; Rouleau, E.; Penault-Llorca, F. Implementing TMB measurement in clinical practice: Considerations on assay requirements. ESMO Open 2019, 4, e000442. [Google Scholar] [CrossRef]
- van den Berg, I.; Coebergh van den Braak, R.R.J.; van Vugt, J.L.A.; Ijzermans, J.N.M.; Buettner, S. Actual survival after resection of primary colorectal cancer: Results from a prospective multicenter study. World J. Surg. Oncol. 2021, 19, 96. [Google Scholar] [CrossRef]
- Xu, C.; Chen, Y.P.; Du, X.J.; Liu, J.Q.; Huang, C.L.; Chen, L.; Zhou, G.Q.; Li, W.F.; Mao, Y.P.; Hsu, C.; et al. Comparative safety of immune checkpoint inhibitors in cancer: Systematic review and network meta-analysis. BMJ 2018, 363, k4226. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brahmer, J.R.; Callahan, M.K.; Flores-Chávez, A.; Keegan, N.; Khamashta, M.A.; Lambotte, O.; Mariette, X.; Prat, A.; Suárez-Almazor, M.E. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Prim. 2020, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Kunst, N.; Alarid-Escudero, F.; Aas, E.; Coupé, V.M.H.; Schrag, D.; Kuntz, K.M. Estimating Population-Based Recurrence Rates of Colorectal Cancer over Time in the United States. Cancer Epidemiol. Biomark. Prev. 2020, 29, 2710–2718. [Google Scholar] [CrossRef] [PubMed]
- Chalabi, M.; Verschoor, Y.L.; van den Berg, J.; Sikorska, K.; Beets, G.; Lent, A.V.; Grootscholten, M.C.; Aalbers, A.; Buller, N.; Marsman, H.; et al. LBA7 Neoadjuvant immune checkpoint inhibition in locally advanced MMR-deficient colon cancer: The NICHE-2 study. Ann. Oncol. 2022, 33, S1389. [Google Scholar] [CrossRef]
- André, T.; Cohen, R.; Salem, M.E. Immune Checkpoint Blockade Therapy in Patients With Colorectal Cancer Harboring Microsatellite Instability/Mismatch Repair Deficiency in 2022. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Gao, Q.; Han, A.; Zhu, H.; Yu, J. The potential mechanism, recognition and clinical significance of tumor pseudoprogression after immunotherapy. Cancer Biol. Med. 2019, 16, 655–670. [Google Scholar] [CrossRef]
- Andre, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.J.A.; Smith, D.M.; Garcia-Carbonero, R.; Alcaide, J.; Gibbs, P.; et al. Final overall survival for the phase III KN177 study: Pembrolizumab versus chemotherapy in microsatellite instability-high/mismatch repair deficient (MSI-H/dMMR) metastatic colorectal cancer (mCRC). J. Clin. Oncol. 2021, 39 (Suppl 15), 3500. [Google Scholar] [CrossRef]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litière, S.; Dancey, J.; Chen, A.; et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef]
- Hodi, F.S.; Ballinger, M.; Lyons, B.; Soria, J.-C.; Nishino, M.; Tabernero, J.; Powles, T.; Smith, D.; Hoos, A.; McKenna, C.; et al. Immune-Modified Response Evaluation Criteria In Solid Tumors (imRECIST): Refining Guidelines to Assess the Clinical Benefit of Cancer Immunotherapy. J. Clin. Oncol. 2018, 36, 850–858. [Google Scholar] [CrossRef]
- Lenz, H.J.; Van Cutsem, E.; Luisa Limon, M.; Wong, K.Y.M.; Hendlisz, A.; Aglietta, M.; García-Alfonso, P.; Neyns, B.; Luppi, G.; Cardin, D.B.; et al. First-Line Nivolumab Plus Low-Dose Ipilimumab for Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Phase II CheckMate 142 Study. J. Clin. Oncol. 2022, 40, 161–170. [Google Scholar] [CrossRef]
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M.; El Dika, I.H.; Segal, N.; Shcherba, M.; Sugarman, R.; et al. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N. Engl. J. Med. 2022, 386, 2363–2376. [Google Scholar] [CrossRef]
- Ghiringhelli, F.; Fumet, J.D. Is There a Place for Immunotherapy for Metastatic Microsatellite Stable Colorectal Cancer? Front. Immunol. 2019, 10, 1816. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.I.; Cesano, A.; Marincola, F.M. The Paradox of Cancer Immune Exclusion: Immune Oncology Next Frontier. In Tumor Microenvironment; Springer: Cham, Switzerland, 2020; Volume 180, pp. 173–195. [Google Scholar]
- Pecci, F.; Cantini, L.; Bittoni, A.; Lenci, E.; Lupi, A.; Crocetti, S.; Giglio, E.; Giampieri, R.; Berardi, R. Beyond Microsatellite Instability: Evolving Strategies Integrating Immunotherapy for Microsatellite Stable Colorectal Cancer. Curr. Treat. Options Oncol. 2021, 22, 69. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Hopkins, A.; Jaffee, E.M. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N. Engl. J. Med. 2017, 377, 2500–2501. [Google Scholar] [CrossRef] [PubMed]
- Eng, C.; Kim, T.W.; Bendell, J.; Argilés, G.; Tebbutt, N.C.; Di Bartolomeo, M.; Falcone, A.; Fakih, M.; Kozloff, M.; Segal, N.H.; et al. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2019, 20, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Grothey, A.; Van Cutsem, E.; Sobrero, A.; Siena, S.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouché, O.; Mineur, L.; Barone, C.; et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013, 381, 303–312. [Google Scholar] [CrossRef]
- Fukuoka, S.; Hara, H.; Takahashi, N.; Kojima, T.; Kawazoe, A.; Asayama, M.; Yoshii, T.; Kotani, D.; Tamura, H.; Mikamoto, Y.; et al. Regorafenib Plus Nivolumab in Patients With Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603). J. Clin. Oncol. 2020, 38, 2053–2061. [Google Scholar] [CrossRef]
- Opzoomer, J.W.; Sosnowska, D.; Anstee, J.E.; Spicer, J.F.; Arnold, J.N. Cytotoxic Chemotherapy as an Immune Stimulus: A Molecular Perspective on Turning Up the Immunological Heat on Cancer. Front. Immunol. 2019, 10, 1654. [Google Scholar] [CrossRef]
- Hecht, M.; Büttner-Herold, M.; Erlenbach-Wünsch, K.; Haderlein, M.; Croner, R.; Grützmann, R.; Hartmann, A.; Fietkau, R.; Distel, L.V. PD-L1 is upregulated by radiochemotherapy in rectal adenocarcinoma patients and associated with a favourable prognosis. Eur. J. Cancer 2016, 65, 52–60. [Google Scholar] [CrossRef]
- Chen, T.W.; Huang, K.C.; Chiang, S.F.; Chen, W.T.; Ke, T.W.; Chao, K.S.C. Prognostic relevance of programmed cell death-ligand 1 expression and CD8+ TILs in rectal cancer patients before and after neoadjuvant chemoradiotherapy. J. Cancer Res. Clin. Oncol. 2019, 145, 1043–1053. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, H.; Lv, X.; Hu, L.; Li, W.; Zi, M.; He, Y. Impact of Diets on Response to Immune Checkpoint Inhibitors (ICIs) Therapy against Tumors. Life 2022, 12, 409. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.; Simnica, D.; Schultheiß, C.; Scholz, R.; Tintelnot, J.; Gökkurt, E.; von Wenserski, L.; Willscher, E.; Paschold, L.; Sauer, M.; et al. PD-L1 targeting and subclonal immune escape mediated by PD-L1 mutations in metastatic colorectal cancer. J. Immunother. Cancer 2021, 9, e002844. [Google Scholar] [CrossRef] [PubMed]
- Segal, N.H.; Kemeny, N.E.; Cercek, A.; Reidy, D.L.; Raasch, P.J.; Warren, P.; Hrabovsky, A.E.; Campbell, N.; Shia, J.; Goodman, K.A.; et al. Non-randomized phase II study to assess the efficacy of pembrolizumab (Pem) plus radiotherapy (RT) or ablation in mismatch repair proficient (pMMR) metastatic colorectal cancer (mCRC) patients. J. Clin. Oncol. 2016, 34 (Suppl. 15), 3539. [Google Scholar] [CrossRef]
- Segal, N.H.; Cercek, A.; Ku, G.; Wu, A.J.; Rimner, A.; Khalil, D.N.; Reidy-Lagunes, D.; Cuaron, J.; Yang, T.J.; Weiser, M.R.; et al. Phase II Single-arm Study of Durvalumab and Tremelimumab with Concurrent Radiotherapy in Patients with Mismatch Repair-proficient Metastatic Colorectal Cancer. Clin. Cancer Res. 2021, 27, 2200–2208. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gao, T.; Tan, Z.; Li, S.; Xu, J.; Bai, C.; Xue, R.; Xie, L.; Zhang, L.; Fan, Z.; et al. Phase II Study of TQB2450, a Novel PD-L1 Antibody, in Combination with Anlotinib in Patients with Locally Advanced or Metastatic Soft Tissue Sarcoma. Clin. Cancer Res. 2022, 28, 3473–3479. [Google Scholar] [CrossRef]
- Dammeijer, F.; van Gulijk, M.; Mulder, E.E.; Lukkes, M.; Klaase, L.; van den Bosch, T.; van Nimwegen, M.; Lau, S.P.; Latupeirissa, K.; Schetters, S.; et al. The PD-1/PD-L1-Checkpoint Restrains T cell Immunity in Tumor-Draining Lymph Nodes. Cancer Cell 2020, 38, 685–700.e8. [Google Scholar] [CrossRef]
- Kleinovink, J.W.; Marijt, K.A.; Schoonderwoerd, M.J.A.; van Hall, T.; Ossendorp, F.; Fransen, M.F. PD-L1 expression on malignant cells is no prerequisite for checkpoint therapy. Oncoimmunology 2017, 6, e1294299. [Google Scholar] [CrossRef]
- Dong, W.; Wu, X.; Ma, S.; Wang, Y.; Nalin, A.P.; Zhu, Z.; Zhang, J.; Benson, D.M.; He, K.; Caligiuri, M.A.; et al. The Mechanism of Anti-PD-L1 Antibody Efficacy against PD-L1-Negative Tumors Identifies NK Cells Expressing PD-L1 as a Cytolytic Effector. Cancer Discov. 2019, 9, 1422–1437. [Google Scholar] [CrossRef]
- Hartley, G.P.; Chow, L.; Ammons, D.T.; Wheat, W.H.; Dow, S.W. Programmed Cell Death Ligand 1 (PD-L1) Signaling Regulates Macrophage Proliferation and Activation. Cancer Immunol. Res. 2018, 6, 1260–1273. [Google Scholar] [CrossRef]
- Mayoux, M.; Roller, A.; Pulko, V.; Sammicheli, S.; Chen, S.; Sum, E.; Jost, C.; Fransen, M.F.; Buser, R.B.; Kowanetz, M.; et al. Dendritic cells dictate responses to PD-L1 blockade cancer immunotherapy. Sci. Transl. Med. 2020, 12, eaav7431. [Google Scholar] [CrossRef] [PubMed]
- Maier, B.; Leader, A.M.; Chen, S.T.; Tung, N.; Chang, C.; LeBerichel, J.; Chudnovskiy, A.; Maskey, S.; Walker, L.; Finnigan, J.P.; et al. A conserved dendritic-cell regulatory program limits antitumour immunity. Nature 2020, 580, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.A.; Wu, D.-C.; Cheung, J.; Navarro, A.; Xiong, H.; Cubas, R.; Totpal, K.; Chiu, H.; Wu, Y.; Comps-Agrar, L.; et al. PD-L1 expression by dendritic cells is a key regulator of T-cell immunity in cancer. Nat. Cancer 2020, 1, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Diniz, M.O.; Schurich, A.; Chinnakannan, S.K.; Duriez, M.; Stegmann, K.A.; Davies, J.; Kucykowicz, S.; Suveizdyte, K.; Amin, O.E.; Alcock, F.; et al. NK cells limit therapeutic vaccine-induced CD8(+)T cell immunity in a PD-L1-dependent manner. Sci. Transl. Med. 2022, 14, eabi4670. [Google Scholar] [CrossRef]
- Morrissey, S.M.; Yan, J. Exosomal PD-L1: Roles in Tumor Progression and Immunotherapy. Trends Cancer 2020, 6, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.; Qin, B.; Zhang, Y.; Zhang, C.; Kahila, M.; Nowsheen, S.; Yin, P.; Yuan, J.; Pei, H.; Li, H.; et al. PD-L1 (B7-H1) Competes with the RNA Exosome to Regulate the DNA Damage Response and Can Be Targeted to Sensitize to Radiation or Chemotherapy. Mol. Cell 2019, 74, 1215–1226.e4. [Google Scholar] [CrossRef]
- Winifred Nompumelelo Simelane, N.; Abrahamse, H. Nanoparticle-Mediated Delivery Systems in Photodynamic Therapy of Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 12405. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Z.; Hou, J.; Xiong, W.; Kim, H.; Chen, J.; Zheng, C.; Jiang, X.; Yoon, J.; Shen, J. Tumor Selective Metabolic Reprogramming as a Prospective PD-L1 Depression Strategy to Reactivate Immunotherapy. Adv. Mater. 2022, 34, 2206121. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Y.; Song, W.; Jiang, X.; Deng, Z.; Xiong, W.; Shen, J. Metabolic reprogramming mediated PD-L1 depression and hypoxia reversion to reactivate tumor therapy. J. Control. Release 2022, 352, 793–812. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, J.; Liu, Y.; Zheng, C.; Luo, W.; Chen, L.; Zhou, S.; Li, Z.; Shen, J. Cascade two-stage tumor re-oxygenation and immune re-sensitization mediated by self-assembled albumin-sorafenib nanoparticles for enhanced photodynamic immunotherapy. Acta Pharm. Sin. B 2022, 12, 4204–4223. [Google Scholar] [CrossRef]
- Sumransub, N.; Vantanasiri, K.; Prakash, A.; Lou, E. Advances and new frontiers for immunotherapy in colorectal cancer: Setting the stage for neoadjuvant success? Mol. Ther. Oncolytics 2021, 22, 1–12. [Google Scholar] [CrossRef]
- Schmidt-Wolf, G.D.; Negrin, R.S.; Schmidt-Wolf, I.G. Activated T cells and cytokine-induced CD3+CD56+ killer cells. Ann. Hematol. 1997, 74, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Fayyaz, F.; Yazdanpanah, N.; Rezaei, N. Cytokine-induced killer cells mediated pathways in the treatment of colorectal cancer. Cell Commun. Signal. 2022, 20, 41. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Ren, Y.; Zhang, T.; Yang, X.; Zhu, W.; Zhu, H.; Li, J.; Li, J.; Pang, Y. Retrospective comparative study of the effects of dendritic cell vaccine and cytokine-induced killer cell immunotherapy with that of chemotherapy alone and in combination for colorectal cancer. Biomed. Res. Int. 2014, 2014, 214727. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yang, X.; Li, J.; Ren, Y.; Zhang, T.; Zhang, C.; Zhang, J.; Li, J.; Pang, Y. Immune response, safety, and survival and quality of life outcomes for advanced colorectal cancer patients treated with dendritic cell vaccine and cytokine-induced killer cell therapy. Biomed. Res. Int. 2014, 2014, 603871. [Google Scholar] [CrossRef]
- Schmeel, L.C.; Schmeel, F.C.; Coch, C.; Schmidt-Wolf, I.G. Cytokine-induced killer (CIK) cells in cancer immunotherapy: Report of the international registry on CIK cells (IRCC). J. Cancer Res. Clin. Oncol. 2015, 141, 839–849. [Google Scholar] [CrossRef]
- Du, C.; Liu, Z.; Ding, Z.; Guo, F.; Ma, D.; Xie, X. Autologous cytokine-induced killer cells combined with chemotherapy in the treatment of advanced colorectal cancer: A randomized control study. Chin. Ger. J. Clin. Oncol. 2013, 12, 487–491. [Google Scholar] [CrossRef]
- Qian, S.; Villarejo-Campos, P.; Guijo, I.; Hernández-Villafranca, S.; García-Olmo, D.; González-Soares, S.; Guadalajara, H.; Jiménez-Galanes, S.; Qian, C. Update for Advance CAR-T Therapy in Solid Tumors, Clinical Application in Peritoneal Carcinomatosis From Colorectal Cancer and Future Prospects. Front. Immunol. 2022, 13, 841425. [Google Scholar] [CrossRef]
- Adkins, S. CAR T-Cell Therapy: Adverse Events and Management. J. Adv. Pract. Oncol. 2019, 10 (Suppl 3), 21–28. [Google Scholar]
- Aparicio, C.; Belver, M.; Enríquez, L.; Espeso, F.; Núñez, L.; Sánchez, A.; de la Fuente, M.; González-Vallinas, M. Cell Therapy for Colorectal Cancer: The Promise of Chimeric Antigen Receptor (CAR)-T Cells. Int. J. Mol. Sci. 2021, 22, 11781. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Z.; Yang, Z.; Wang, M.; Li, S.; Li, Y.; Zhang, R.; Xiong, Z.; Wei, Z.; Shen, J.; et al. Phase I Escalating-Dose Trial of CAR-T Therapy Targeting CEA(+) Metastatic Colorectal Cancers. Mol. Ther. 2017, 25, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Zong, S.; Shi, Q.; Li, H.; Liu, S.; Yang, W.; Li, W.; Hou, F. Is Ep-CAM Expression a Diagnostic and Prognostic Biomarker for Colorectal Cancer? A Systematic Meta-Analysis. EBioMedicine 2017, 20, 61–69. [Google Scholar] [CrossRef]
- Yong, C.S.M.; Dardalhon, V.; Devaud, C.; Taylor, N.; Darcy, P.K.; Kershaw, M.H. CAR T-cell therapy of solid tumors. Immunol. Cell Biol. 2017, 95, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Newick, K.; O’Brien, S.; Moon, E.; Albelda, S.M. CAR T Cell Therapy for Solid Tumors. Ann. Rev. Med. 2017, 68, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.E.; Badie, B.; Barish, M.E.; Weng, L.; Ostberg, J.R.; Chang, W.C.; Naranjo, A.; Starr, R.; Wagner, J.; Wright, C.; et al. Bioactivity and Safety of IL13Rα2-Redirected Chimeric Antigen Receptor CD8+ T Cells in Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2015, 21, 4062–4072. [Google Scholar] [CrossRef]
- García-Olmo, D.; Villarejo Campos, P.; Barambio, J.; Gomez-Heras, S.G.; Vega-Clemente, L.; Olmedillas-Lopez, S.; Guadalajara, H.; Garcia-Arranz, M. Intraperitoneal collagenase as a novel therapeutic approach in an experimental model of colorectal peritoneal carcinomatosis. Sci. Rep. 2021, 11, 503. [Google Scholar] [CrossRef]
- Gao, D.; Li, C.; Xie, X.; Zhao, P.; Wei, X.; Sun, W.; Liu, H.C.; Alexandrou, A.T.; Jones, J.; Zhao, R.; et al. Autologous tumor lysate-pulsed dendritic cell immunotherapy with cytokine-induced killer cells improves survival in gastric and colorectal cancer patients. PLoS ONE 2014, 9, e93886. [Google Scholar] [CrossRef]
- Fritah, H.; Rovelli, R.; Chiang, C.L.; Kandalaft, L.E. The current clinical landscape of personalized cancer vaccines. Cancer Treat. Rev. 2022, 106, 102383. [Google Scholar] [CrossRef]
- Nagasaka, M.; Potugari, B.; Nguyen, A.; Sukari, A.; Azmi, A.S.; Ou, S.I. KRAS Inhibitors- yes but what next? Direct targeting of KRAS- vaccines, adoptive T cell therapy and beyond. Cancer Treat. Rev. 2021, 101, 102309. [Google Scholar] [CrossRef]
- Wooster, A.L.; Girgis, L.H.; Brazeale, H.; Anderson, T.S.; Wood, L.M.; Lowe, D.B. Dendritic cell vaccine therapy for colorectal cancer. Pharm. Res 2021, 164, 105374. [Google Scholar] [CrossRef]
- Itoh, T.; Ueda, Y.; Kawashima, I.; Nukaya, I.; Fujiwara, H.; Fuji, N.; Yamashita, T.; Yoshimura, T.; Okugawa, K.; Iwasaki, T.; et al. Immunotherapy of solid cancer using dendritic cells pulsed with the HLA-A24-restricted peptide of carcinoembryonic antigen. Cancer Immunol. Immunother. CII 2002, 51, 99–106. [Google Scholar] [CrossRef]
- Fong, L.; Hou, Y.; Rivas, A.; Benike, C.; Yuen, A.; Fisher, G.A.; Davis, M.M.; Engleman, E.G. Altered peptide ligand vaccination with Flt3 ligand expanded dendritic cells for tumor immunotherapy. Proc. Natl. Acad. Sci. USA 2001, 98, 8809–8814. [Google Scholar] [CrossRef] [PubMed]
- Hunyadi, J.; András, C.; Szabó, I.; Szántó, J.; Szluha, K.; Sipka, S.; Kovács, P.; Kiss, A.; Szegedi, G.; Altorjay, I.; et al. Autologous dendritic cell based adoptive immunotherapy of patients with colorectal cancer-A phase I-II study. Pathol. Oncol. Res. 2014, 20, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Gujar, S.; Pol, J.G.; Kroemer, G. Heating it up: Oncolytic viruses make tumors ‘hot’ and suitable for checkpoint blockade immunotherapies. Oncoimmunology 2018, 7, e1442169. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Wang, H.; Ren, J.; Liu, W.; Chen, L.; Chen, H.; Ye, J.; Dai, E.; Ma, C.; Ju, S.; et al. Oncolytic vaccinia virus delivering tethered IL-12 enhances antitumor effects with improved safety. J. Immunother. Cancer 2020, 8, e000710. [Google Scholar] [CrossRef]
- Yarchoan, M.; Huang, C.Y.; Zhu, Q.; Ferguson, A.K.; Durham, J.N.; Anders, R.A.; Thompson, E.D.; Rozich, N.S.; Thomas, D.L., 2nd; Nauroth, J.M.; et al. A phase 2 study of GVAX colon vaccine with cyclophosphamide and pembrolizumab in patients with mismatch repair proficient advanced colorectal cancer. Cancer Med. 2020, 9, 1485–1494. [Google Scholar] [CrossRef]
| Clinical Trial | Intervention | Target Population | Primary Objectives |
|---|---|---|---|
| NCT03626922 | Pembrolizumab Oxaliplatin Pemetrexed | Chemotherapy-resistant and metastatic microsatellite stable (MSS) CRC | Establish dose Determine efficacy |
| NCT04262687 | Pembrolizumab Xelox Bevacizumab | MSS CRC with high immune filtrate | Progression-free survival (PFS) at 10 months |
| NCT04044430 | Nivolumab Encorafenoib Binietinib | Metastatic MSS, BRAFV600E gene-mutated CRC | Overall response rate (ORR) Determine safety and tolerability |
| NCT03711058 | Nivolumab Copanlisib | Refractory/relapsed MSS CRC | Maximum tolerated dose (MTD) of Copanlisib ORR |
| NCT03442569 | Nivolumab Panitumumab Ipilimumab | Unresectable, refractory metastatic KRAS/NRAS/BRAF wild-type MSS CRC | ORR |
| NCT02873195 | Atezolizumab Capecitabine Bevacizumab | Metastatic, recurrent, and/or refractory CRC | PFS up to 20 months |
| NCT03608046 | Avelumab Cetuximab Irinotecan | Metastatic MSS CRC | ORR |
| NCT05645315 | TQB2450 (PD-L1 monoclonal antibody) TQB2618 (TIM-3 receptor monoclonal antibody) | Advanced malignant solid tumors | Dose-limiting toxicity (DLT) Phase II recommended dose (RP2D) ORR |
| NCT04611711 | TQB2450 Decitabine Anlotinib | PD-1 monoclonal antibody-resistant digestive system tumors | ORR |
| NCT05139082 | TQB2450 TQB3616 (CKD4/6 inhibitor) | PD-1/PD-L1 monoclonal antibody-resistant digestive system tumors | ORR |
| NCT03897283 | TQB2450 Anlotinib | Advanced solid tumors | DLT MTD RP2D |
| NCT05287399 | ASC61 (orally bioavailable small-molecule PD-L1 inhibitor) | Advanced solid tumors | Proportion of patients experiencing DLT RP2D |
| NCT04109755 | Neoadjuvant 5Gy x 5 doses + Pembrolizumab | MSS rectal cancer | Tumor regression rate |
| NCT04017455 | Neoadjuvant radiation + Bevacizumab and Atezolizumab | Resectable rectal cancer | Clinical complete and near-complete response rate |
| NCT03104439 | Radiation + Nivolumab and Ipilimumab | MSS CRC, high microsatellite instability (MSI-H) CRC | Disease control rate |
| NCT05187338 | Ipilimumab Pembrolizumab Durvalumab | Advanced solid tumors including CRC | Safety PFS at 5 years Disease control rate Duration of remission |
| NCT03539822 | Cabozantinib Durvalumab Tremelimumab | Gastrointestinal tumors including CRC | MTDORR |
| NCT04606472 | SI-B003 (bispecific anti-PD-1/CTLA-4 antibody) | Advanced solid tumors including CRC | DLT MTD Maximum administered dose Treatment-related AEs RP2D |
| NCT05426005 | AK104 (bispecific anti-PD-1/CTLA-4 antibody) | PD-1/PD-L1 blockade-refractory MSI-H or mismatch repair deficient (dMMR) advanced CRC | ORR |
| NCT04556253 | AK104 | Locally advanced MSI-H/dMMR gastric carcinoma and CRC during perioperative period | Complete pathologic response (pCR) rates |
| NCT00571644 | AK104 mFOLFOXIRI | Locally advanced CRC | pCR rates |
| NCT00571293 | Neoadjuvant Balstilimab with Botensilimab | Resectable CRC | Pathological overall response Number of adverse events (AEs) Number of serious AEs Number of complications leading to operative delay |
| NCT05627635 | Balstilimab Botensilimab FOLFOX Bevacizumab | MSS metastatic CRC | Safety and tolerability RP2DORR |
| NCT05608044 | Balstilimab Botensilimab | Metastatic CRC | ORR |
| NCT03860272 | Balstilimab Botensilimab | Refractory solid tumors including CRC without hepatic metastases | Incidence of AEs DLT of Botensilimab RP2D of Botensilimab |
| NCT04140526 | ONC-392 (humanized anti-CTLA-4 IgG1 monoclonal antibody) Pembrolizumab | Advanced or metastatic solid tumors including CRC | DLT in monotherapy MTD in monotherapy RP2D Rate of AEs |
| NCT05620134 | JK08 (IL-15 antibody fusion protein targeting CTLA-4) | Unresectable locally advanced or metastatic CRC | DLT RP2D Safety and tolerability including nature of AEs |
| Clinical Trial | Intervention | Target Population | Primary Objectives |
|---|---|---|---|
| NCT04476641 | Dendritic cell (DC) activated cytokine-induced killer cell (CIK) | Solid tumors including CRC | OS and PFS |
| NCT04282044 | Autologous CIK | Advanced solid tumors including CRC | AEs DLT |
| NCT04513431 | Carcinoembryonic antigen (CEA) chimera antigen receptor (CAR) T cells | CEA-positive stage III CRC CRC liver metastases | Treatment-related AEs |
| NCT05415475 | CEA CAR T cells | CEA-positive advanced solid tumors including CRC | Treatment-related AEs MTD of CEA CAR T cells |
| NCT05396300 | CEA CAR T cells | CEA-positive advanced solid tumors including CRC | Treatment-related AEs MTD of CEA CAR T cells |
| NCT04348643 | CEA CAR T cells | Refractory/relapsed CEA-positive CRC | Treatment-related AEs |
| NCT05240950 | CEA CAR T cells | Metastatic CRC after adjuvant chemotherapy | Treatment-related AEs Recurrence Recurrence-free survival |
| NCT05028933 | EpCAM CAR T cell | Advanced digestive system malignancies including CRC | Evaluate safety and tolerability |
| NCT03740256 | HER2 CAR T-cell Oncolytic adenovirus | HER2-positive solid tumors including CRC | Incidence of DLT |
| NCT03638206 | c-met CAR T cell | Various malignancies including CRC | Treatment-related AEs |
| NCT03950154 | PD-1-T lymphocytes Xelox Bevacizumab | Recurrent and metastatic CRC | PFS |
| NCT03904537 | Anti-PD-1 tumor-infiltrating lymphocytes (TILs) Xelox | Stage III CRC undergoing adjuvant chemotherapy | Disease-free survival |
| NCT03950154 | PD-1-T lymphocytes Bevacizumab Xelox | CRC | PFS |
| NCT02487992 | CIK plus S-1 Bevacizumab | Advanced CRC | OS |
| NCT04426669 | Cytokine inducible SH2 containing protein (CISH)-inactivated TILs Cyclophosphamide Fludarabine Aldesleukin | Gastrointestinal cancers including CRC | MTD Changes in tumor dimension Treatment-related AEs |
| NCT04842812 | Anti-PD-1 and anti-CTLA-4 CAR-TILs | Advanced solid tumors including CRC | Safety of treatment |
| NCT05213195 | Natural killer group 2 member D (NKG2D) CAR natural killer (NK) cell therapy | Refractory metastatic CRC | DLT MTD |
| NCT05248048 | NKG2D CAR-T cell therapy | Previously treated CRC with liver metastases | DLT MTD |
| NCT05194709 | Anti-oncofetal trophoblast glycoprotein (5T4) CAR-NK cells | Advanced solid tumors | Incidence of AEs |
| NCT05631886 | TP53-EphA-2-CAR-DC PD-1 antibodies Abraxane Cyclophosphamide | Adult malignant solid tumors | Incidence of AEs Clinical response Immune response |
| NCT05631899 | KRAS-EphA-2-CAR-DC PD-1 antibodies Abraxane Cyclophosphamide | Locally advanced or metastatic solid tumors | Incidence of AEs Clinical response Immune response |
| Clinical Trial | Intervention | Target Population | Primary Objectives |
|---|---|---|---|
| NCT05141721 | Patient-specific neoantigen vaccine Atezolizumab Ipilimumab Fluoropyrimidine Bevacizumab | Metastatic CRC | Molecular response |
| NCT05243862 | PolyPEPI1018 vaccine Atezolizumab | Relapsed/refractory MSS CRC | Treatment-related adverse events Administration safety |
| NCT05350501 | EO2040 vaccine Nivolumab | Minimal residual disease in Stage II, III, or IV CRC after curative therapy | Response to treatment at 6 months |
| NCT02600949 | Personalized peptide vaccine Pembrolizumab Sotigalimab | Advanced or metastatic CRC or pancreatic cancer | Demonstrate vaccine feasibility Establish vaccine safety |
| NCT04117087 | KRAS peptide vaccine Nivolumab Ipilimumab | Resected mismatch-repair proficient (pMMR) CRC and pancreatic cancer | Number of drug-related toxicities Fold change in interferon-producing mutant-KRAS-specific CD8 and CD4 T cells |
| NCT05130060 | PolyPEPI1018 vaccine TAS-102 | Metastatic CRC | Evaluate safety and tolerability |
| NCT03953235 | GRT-C903/GRT-R904 vaccine Nivolumab Ipilimumab | Solid organ tumors Including MSS CRC | Treatment-related adverse events ORR Establish Phase II dose |
| NCT04799431 | Neoantigen vaccine and Poly-ICLC Retifanlimab | Stage IV MSS CRC and pancreatic ductal adenocarcinoma | Percentage receiving vaccine Treatment-related adverse events |
| NCT04912765 | Dendritic cell (DC) vaccine Nivolumab | Liver metastases from CRC and HCC | Relapse-free survival at 24 months Induced immune response |
| NCT02919644 | DC vaccine IL2 | Stage IV CRC after curative resection | Treatment-related adverse events Immunological efficacy |
| NCT03730948 | mDC3 vaccine Cyclophosphamide | Resected hypermutated CRC | Change in number of peptide-specific CD8+ T cells Treatment-related adverse events |
| NCT04591379 | Neoadjuvant intratumoral influenza vaccine | CRC | Evaluate safety |
| NCT04491955 | Pox-virus vaccine against CEA & mucin-1 | MSS CRC, small bowel cancer | ORR |
| NCT01952730 | GVAX | Stage IV CRC | Treatment-limiting toxicity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, I.; Dakwar, A.; Takabe, K. Immunotherapy: Recent Advances and Its Future as a Neoadjuvant, Adjuvant, and Primary Treatment in Colorectal Cancer. Cells 2023, 12, 258. https://doi.org/10.3390/cells12020258
Yu I, Dakwar A, Takabe K. Immunotherapy: Recent Advances and Its Future as a Neoadjuvant, Adjuvant, and Primary Treatment in Colorectal Cancer. Cells. 2023; 12(2):258. https://doi.org/10.3390/cells12020258
Chicago/Turabian StyleYu, Irene, Anthony Dakwar, and Kazuaki Takabe. 2023. "Immunotherapy: Recent Advances and Its Future as a Neoadjuvant, Adjuvant, and Primary Treatment in Colorectal Cancer" Cells 12, no. 2: 258. https://doi.org/10.3390/cells12020258
APA StyleYu, I., Dakwar, A., & Takabe, K. (2023). Immunotherapy: Recent Advances and Its Future as a Neoadjuvant, Adjuvant, and Primary Treatment in Colorectal Cancer. Cells, 12(2), 258. https://doi.org/10.3390/cells12020258







