Maternal High Fat Diet Anticipates the AD-like Phenotype in 3xTg-AD Mice by Epigenetic Dysregulation of Aβ Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Diet and Housing Conditions
2.3. Behavioral Experiments
2.4. Ex Vivo Electrophysiology on Hippocampal Slices
2.5. Real-Time PCR
2.6. ELISA Assay
2.7. Western Blotting
2.8. Chromatin Immunoprecipitation
2.9. Statistical Analysis
3. Results
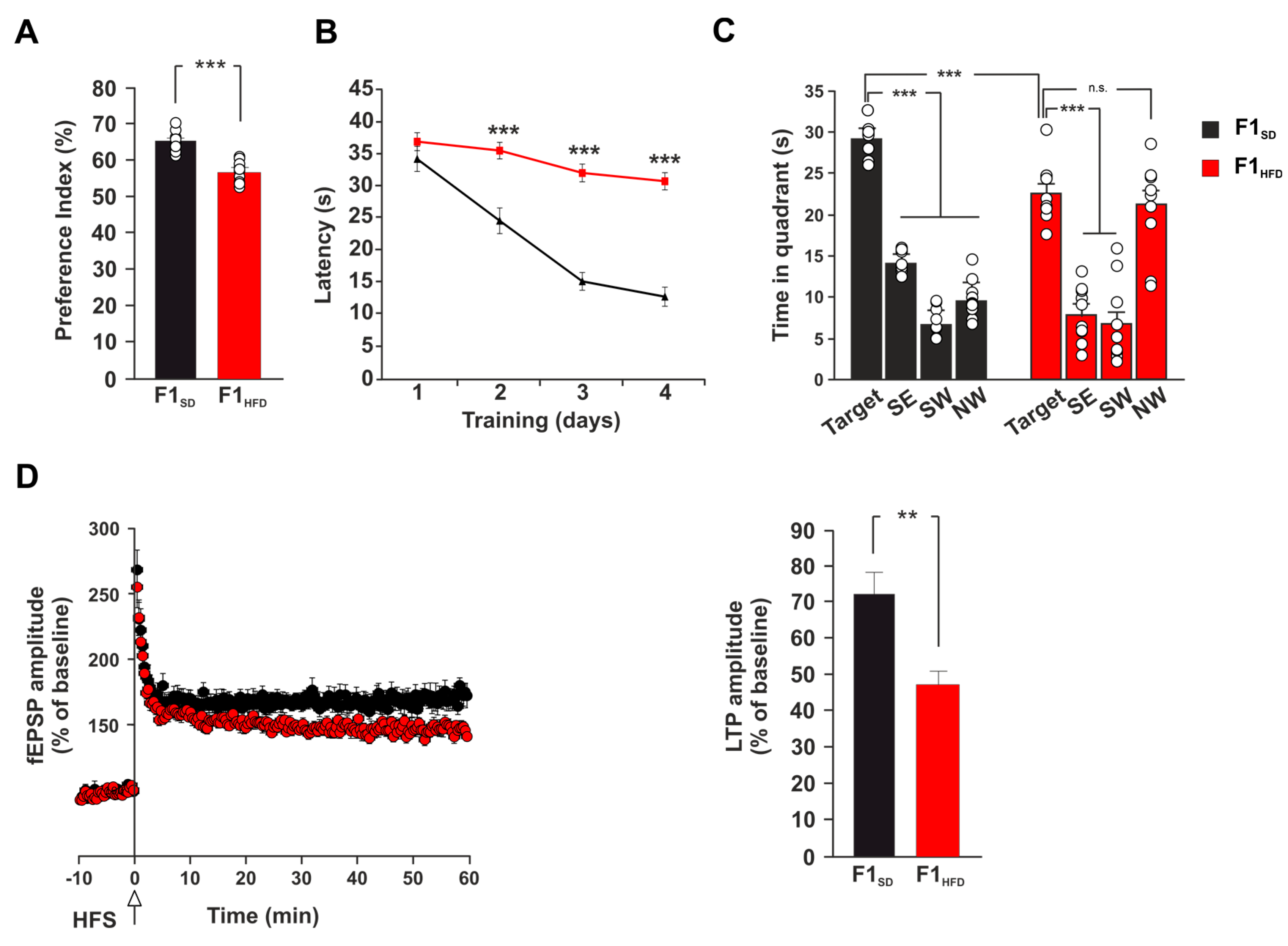
3.1. Maternal HFD Anticipates LTP Impairment and Memory Deficits in 3xTg-AD Mice
3.2. Maternal HFD Alters the Expression of AD-Related Genes and Enhances Aβ Deposition in the Hippocampus of the Offspring
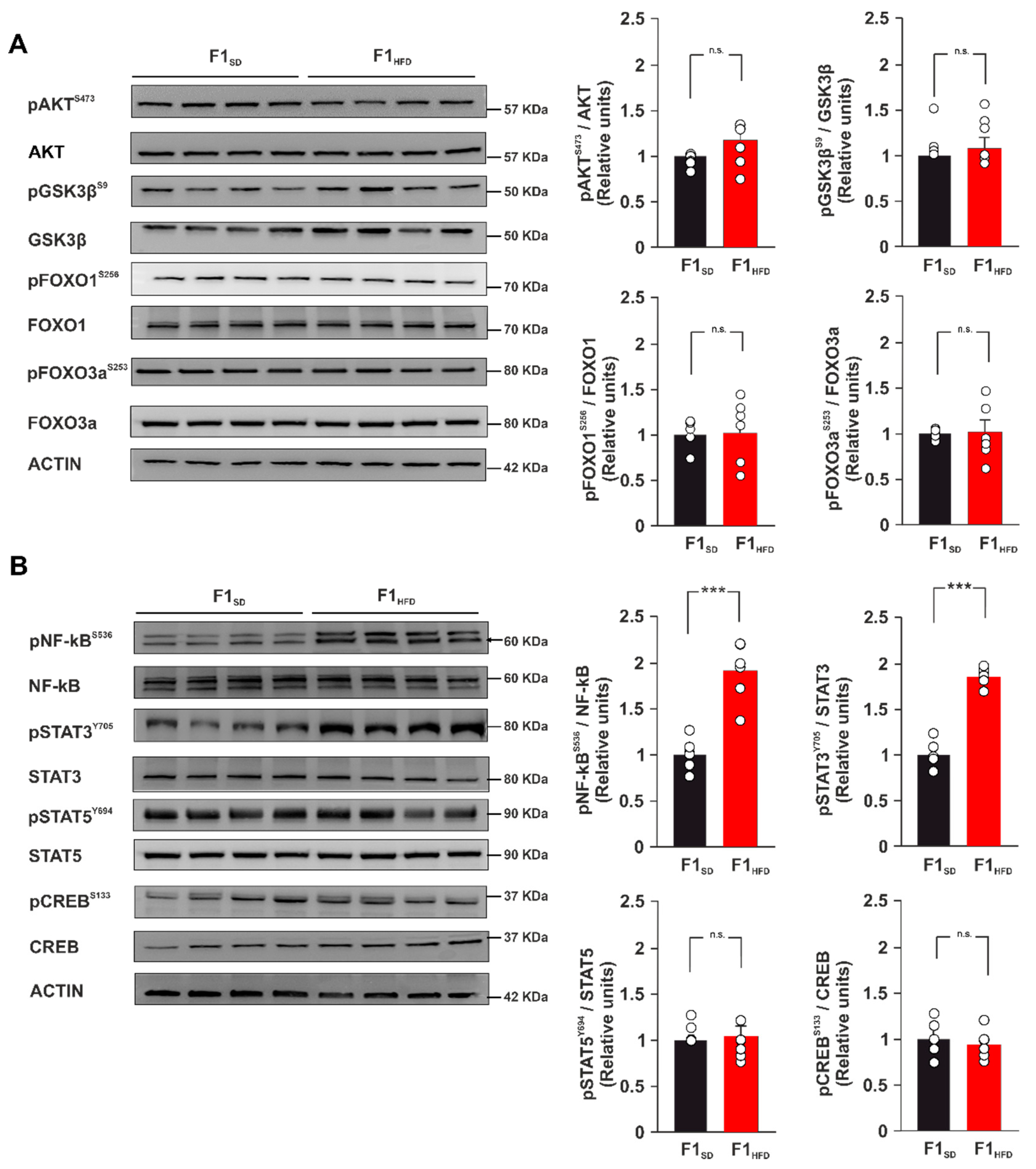
3.3. Maternal Overnutrition Induces Hyper-Activation of Transcription Factors NF-kB and STAT3
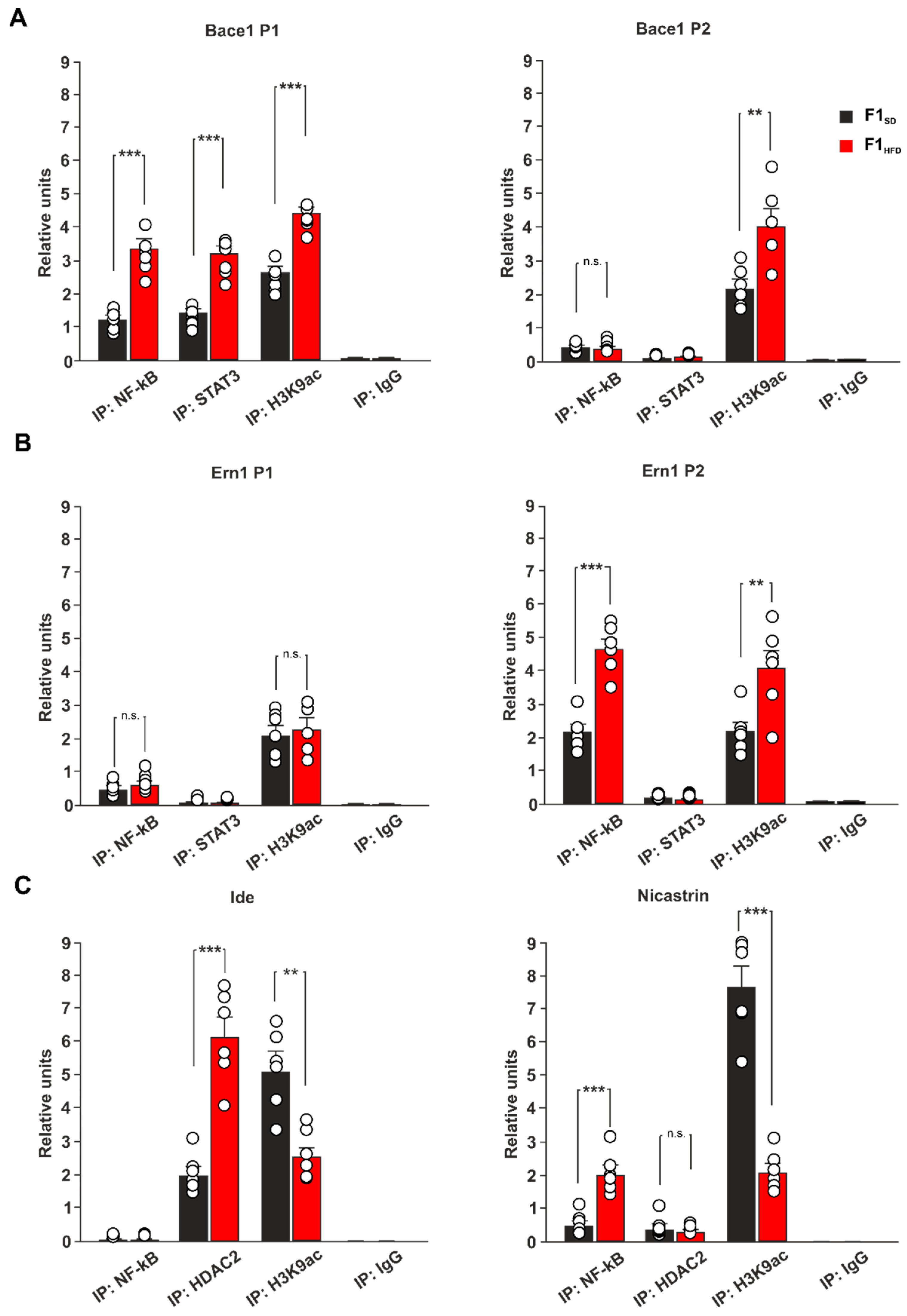
3.4. Maternal HFD Epigenetically Dysregulates the Promoters of Genes Driving APP Metabolism in the Hippocampus of 3xTg-AD Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer disease. Nat. Rev. Di.s Prim. 2021, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Tönnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef]
- Reiss, A.B.; Arain, H.A.; Stecker, M.M.; Siegart, N.M.; Kasselman, L.J. Amyloid toxicity in Alzheimer’s disease. Rev. Neurosci. 2018, 29, 613–627. [Google Scholar] [CrossRef]
- Lambert, J.-C.; Amouyel, P. Genetic heterogeneity of Alzheimer’s disease: Complexity and advances. Psychoneuroendocrinology 2007, 32 (Suppl S1), S62–S70. [Google Scholar] [CrossRef] [PubMed]
- Gold, G.; Blouin, J.-L.; Herrmann, F.; Michon, A.; Mulligan, R.; Saïl, G.D.; Bouras, C.; Giannakopoulos, P.; Antonarakis, S.E. Specific BACE1 genotypes provide additional risk for late-onset Alzheimer disease in APOE epsilon 4 carriers. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2003, 119B, 44–47. [Google Scholar] [CrossRef]
- Sato, N.; Morishita, R. Brain alterations and clinical symptoms of dementia in diabetes: Aβ/tau-dependent and independent mechanisms. Front. Endocrinol. 2014, 5, 143. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, M.; Fusco, S.; Mainardi, M.; Scala, F.; Natale, F.; Lapenta, R.; Mattera, A.; Rinaudo, M.; Puma, D.D.L.; Ripoli, C.; et al. Brain insulin resistance impairs hippocampal synaptic plasticity and memory by increasing GluA1 palmitoylation through FoxO3a. Nat. Commun. 2017, 8, 2009. [Google Scholar] [CrossRef]
- Natale, F.; Leone, L.; Rinaudo, M.; Sollazzo, R.; Barbati, S.A.; La Greca, F.; Spinelli, M.; Fusco, S.; Grassi, C. Neural Stem Cell-Derived Extracellular Vesicles Counteract Insulin Resistance-Induced Senescence of Neurogenic Niche. Stem Cells 2022, 40, 318–331. [Google Scholar] [CrossRef]
- Fusco, S.; Spinelli, M.; Cocco, S.; Ripoli, C.; Mastrodonato, A.; Natale, F.; Rinaudo, M.; Livrizzi, G.; Grassi, C. Maternal insulin resistance multigenerationally impairs synaptic plasticity and memory via gametic mechanisms. Nat. Commun. 2019, 10, 4799. [Google Scholar] [CrossRef]
- Dulac, C. Brain function and chromatin plasticity. Nature 2010, 465, 728–735. [Google Scholar] [CrossRef]
- Natale, F.; Spinelli, M.; Barbati, S.A.; Leone, L.; Fusco, S.; Grassi, C. High Fat Diet Multigenerationally Affects Hippocampal Neural Stem Cell Proliferation via Epigenetic Mechanisms. Cells 2022, 11, 2661. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M.; Natale, F.; La Greca, F.; Spinelli, M.; Farsetti, A.; Paciello, F.; Fusco, S.; Grassi, C. Hippocampal Estrogen Signaling Mediates Sex Differences in Retroactive Interference. Biomedicines 2022, 10, 1387. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, M.; Natale, F.; Rinaudo, M.; Leone, L.; Mezzogori, D.; Fusco, S.; Grassi, C. Neural Stem Cell-Derived Exosomes Revert HFD-Dependent Memory Impairment via CREB-BDNF Signalling. Int. J. Mol. Sci. 2020, 21, 8994. [Google Scholar] [CrossRef]
- Huot, R.L.; Plotsky, P.M.; Lenox, R.H.; McNamara, R.K. Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain Res. 2002, 950, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Mirescu, C.; Peters, J.D.; Gould, E. Early life experience alters response of adult neurogenesis to stress. Nat. Neurosci. 2004, 7, 841–846. [Google Scholar] [CrossRef]
- Brunson, K.L.; Kramar, E.; Lin, B.; Chen, Y.; Colgin, L.; Yanagihara, T.K.; Lynch, G.; Baram, T.Z. Mechanisms of late-onset cognitive decline after early-life stress. J. Neurosci. 2005, 25, 9328–9338. [Google Scholar] [CrossRef]
- Tozuka, Y.; Wada, E.; Wada, K. Diet-induced obesity in female mice leads to peroxidized lipid accumulations and impairment of hippocampal neurogenesis during the early life of their offspring. FASEB J. 2009, 23, 1920–1934. [Google Scholar] [CrossRef]
- White, C.L.; Pistell, P.J.; Purpera, M.N.; Gupta, S.; Fernandez-Kim, S.-O.; Hise, T.L.; Keller, J.N.; Ingram, D.K.; Morrison, C.D.; Bruce-Keller, A.J. Effects of high fat diet on Morris maze performance, oxidative stress, and inflammation in rats: Contributions of maternal diet. Neurobiol. Dis. 2009, 35, 3–13. [Google Scholar] [CrossRef]
- Curi, H.T.; Dias, C.T.; Camargo, M.L.M.d.L.; Gomez, P.D.S.; Gomes, M.F.P.; Beserra-Filho, J.I.A.; Medeiros, A.; Ribeiro, A.M.; Simabuco, F.M.; Lambertucci, R.H.; et al. Maternal high-fat diet increases anhedonic behavior and modulates hippocampal Mash1 and BDNF expression in adult offspring. Neurosci. Lett. 2021, 764, 136239. [Google Scholar] [CrossRef]
- Oddo, S.; Caccamo, A.; Shepherd, J.D.; Murphy, M.P.; Golde, T.E.; Kayed, R.; Metherate, R.; Mattson, M.P.; Akbari, Y.; LaFerla, F.M. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: Intracellular Abeta and synaptic dysfunction. Neuron 2003, 39, 409–421. [Google Scholar] [CrossRef]
- Billings, L.M.; Oddo, S.; Green, K.N.; McGaugh, J.L.; LaFerla, F.M. Intraneuronal Abeta causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron 2005, 45, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Stover, K.R.; Campbell, M.A.; Van Winssen, C.M.; Brown, R.E. Early detection of cognitive deficits in the 3xTg-AD mouse model of Alzheimer’s disease. Behav. Brain Res. 2015, 289, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Bienkowski, M.J.; Shuck, M.E.; Miao, H.; Tory, M.C.; Pauley, A.M.; Brashler, J.R.; Stratman, N.C.; Mathews, W.R.; Buhl, A.E.; et al. Membrane-anchored aspartyl protease with Alzheimer’s disease beta-secretase activity. Nature 1999, 402, 533–537. [Google Scholar] [CrossRef]
- Vassar, R.; Bennett, B.D.; Babu-Khan, S.; Kahn, S.; Mendiaz, E.A.; Denis, P.; Teplow, D.B.; Ross, S.; Amarante, P.; Loeloff, R.; et al. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 1999, 286, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Pardossi-Piquard, R.; Dunys, J.; Yu, G.; George-Hyslop, P.S.; Da Costa, C.A.; Checler, F. Neprilysin activity and expression are controlled by nicastrin. J. Neurochem. 2006, 97, 1052–1056. [Google Scholar] [CrossRef]
- Farris, W.; Mansourian, S.; Chang, Y.; Lindsley, L.; Eckman, E.; Frosch, M.P.; Eckman, C.B.; Tanzi, R.E.; Selkoe, D.J.; Guénette, S. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc. Natl. Acad. Sci. USA 2003, 100, 4162–4167. [Google Scholar] [CrossRef]
- Zuliani, I.; Lanzillotta, C.; Tramutola, A.; Barone, E.; Perluigi, M.; Rinaldo, S.; Paone, A.; Cutruzzolà, F.; Bellanti, F.; Spinelli, M.; et al. High-Fat Diet Leads to Reduced Protein O-GlcNAcylation and Mitochondrial Defects Promoting the Development of Alzheimer’s Disease Signatures. Int. J. Mol. Sci. 2021, 22, 3746. [Google Scholar] [CrossRef] [PubMed]
- Barone, E.; Di Domenico, F.; Cassano, T.; Arena, A.; Tramutola, A.; Lavecchia, M.A.; Coccia, R.; Butterfield, D.A.; Perluigi, M. Impairment of biliverdin reductase-A promotes brain insulin resistance in Alzheimer disease: A new paradigm. Free Radic. Biol. Med. 2016, 91, 127–142. [Google Scholar] [CrossRef]
- Lucassen, P.J.; Naninck, E.F.; van Goudoever, J.B.; Fitzsimons, C.; Joels, M.; Korosi, A. Perinatal programming of adult hippocampal structure and function; emerging roles of stress, nutrition and epigenetics. Trends Neurosci. 2013, 36, 621–631. [Google Scholar] [CrossRef]
- Migliore, L.; Coppedè, F. Gene-environment interactions in Alzheimer disease: The emerging role of epigenetics. Nat. Rev. Neurol. 2022, 18, 643–660. [Google Scholar] [CrossRef]
- De Strooper, B.; Karran, E. The Cellular Phase of Alzheimer’s Disease. Cell 2016, 164, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.-G.; Zhu, X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. Acta 2014, 1842, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Knowles, T.; Vendruscolo, M.; Dobson, C.M. The amyloid state and its association with protein misfolding diseases. Nat. Rev. Mol. Cell. Biol. 2014, 15, 384–396. [Google Scholar] [CrossRef]
- Burillo, J.; Marqués, P.; Jiménez, B.; González-Blanco, C.; Benito, M.; Guillén, C. Insulin Resistance and Diabetes Mellitus in Alzheimer’s Disease. Cells 2021, 10, 1236. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, M.; Fusco, S.; Grassi, C. Brain insulin resistance impairs hippocampal plasticity. Vitam. Horm. 2020, 114, 281–306. [Google Scholar] [CrossRef]
- Tammen, S.A.; Friso, S.; Choi, S.-W. Epigenetics: The link between nature and nurture. Mol. Asp. Med. 2013, 34, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Nizari, S.; Carare, R.O.; Hawkes, C.A. Increased Aβ pathology in aged Tg2576 mice born to mothers fed a high fat diet. Sci. Rep. 2016, 6, 21981. [Google Scholar] [CrossRef]
- Martin, S.A.L.; Jameson, C.H.; Allan, S.M.; Lawrence, C.B. Maternal high-fat diet worsens memory deficits in the triple-transgenic (3xTgAD) mouse model of Alzheimer’s disease. PLoS ONE 2014, 9, e99226. [Google Scholar] [CrossRef]
- Cho, H.J.; Son, S.M.; Jin, S.M.; Hong, H.S.; Shin, D.H.; Kim, S.J.; Huh, K.; Mook-Jung, I. RAGE regulates BACE1 and Abeta generation via NFAT1 activation in Alzheimer’s disease animal model. FASEB J. 2009, 23, 2639–2649. [Google Scholar] [CrossRef]
- González-Casimiro, C.M.; Cámara-Torres, P.; Merino, B.; Diez-Hermano, S.; Postigo-Casado, T.; Leissring, M.A.; Cózar-Castellano, I.; Perdomo, G. Effects of Fasting and Feeding on Transcriptional and Posttranscriptional Regulation of Insulin-Degrading Enzyme in Mice. Cells 2021, 10, 2446. [Google Scholar] [CrossRef]
- Martín-Martín, Y.; Pérez-García, A.; Torrecilla-Parra, M.; Frutos, M.F.-D.; Pardo-Marqués, V.; Casarejos, M.J.; Busto, R.; Ramírez, C.M. New Insights on the Regulation of the Insulin-Degrading Enzyme: Role of microRNAs and RBPs. Cells 2022, 11, 2538. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.J.; Hernandez, C.M.; Levenson, J.M.; Lubin, F.D.; Liou, H.-C.; Sweatt, J.D. c-Rel, an NF-kappaB family transcription factor, is required for hippocampal long-term synaptic plasticity and memory formation. Learn. Mem. 2008, 15, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Kounatidis, I.; Chtarbanova, S.; Cao, Y.; Hayne, M.; Jayanth, D.; Ganetzky, B.; Ligoxygakis, P. NF-κB Immunity in the Brain Determines Fly Lifespan in Healthy Aging and Age-Related Neurodegeneration. Cell Rep. 2017, 19, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Kaltschmidt, B.; Sparna, T.; Kaltschmidt, C. Activation of NF-kappa B by reactive oxygen intermediates in the nervous system. Antioxid. Redox Signal. 1999, 1, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Samidurai, M.; Ramasamy, V.S.; Jo, J. β-amyloid inhibits hippocampal LTP through TNFR/IKK/NF-κB pathway. Neurol. Res. 2018, 40, 268–276. [Google Scholar] [CrossRef]
- Chen, C.-H.; Zhou, W.; Liu, S.; Deng, Y.; Cai, F.; Tone, M.; Tone, Y.; Tong, Y.; Song, W. Increased NF-κB signalling up-regulates BACE1 expression and its therapeutic potential in Alzheimer’s disease. Int. J. Neuropsychopharmacol. 2012, 15, 77–90. [Google Scholar] [CrossRef]
- Reichenbach, N.; Delekate, A.; Plescher, M.; Schmitt, F.; Krauss, S.; Blank, N.; Halle, A.; Petzold, G.C. Inhibition of Stat3-mediated astrogliosis ameliorates pathology in an Alzheimer’s disease model. EMBO Mol. Med. 2019, 11, e9665. [Google Scholar] [CrossRef]
- Choi, M.; Kim, H.; Yang, E.-J.; Kim, H.-S. Inhibition of STAT3 phosphorylation attenuates impairments in learning and memory in 5XFAD mice, an animal model of Alzheimer’s disease. J. Pharmacol. Sci. 2020, 143, 290–299. [Google Scholar] [CrossRef]
- Millot, P.; San, C.; Bennana, E.; Porte, B.; Vignal, N.; Hugon, J.; Paquet, C.; Hosten, B.; Mouton-Liger, F. STAT3 inhibition protects against neuroinflammation and BACE1 upregulation induced by systemic inflammation. Immunol. Lett. 2020, 228, 129–134. [Google Scholar] [CrossRef]
- Mainardi, M.; Spinelli, M.; Scala, F.; Mattera, A.; Fusco, S.; D’Ascenzo, M.; Grassi, C. Loss of Leptin-Induced Modulation of Hippocampal Synaptic Trasmission and Signal Transduction in High-Fat Diet-Fed Mice. Front. Cell. Neurosci. 2017, 11, 225. [Google Scholar] [CrossRef]
- Zhang, K.; Schrag, M.; Crofton, A.; Trivedi, R.; Vinters, H.; Kirsch, W. Targeted proteomics for quantification of histone acetylation in Alzheimer’s disease. Proteomics 2012, 12, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Marzi, S.; Leung, S.K.; Ribarska, T.; Hannon, E.; Smith, A.R.; Pishva, E.; Poschmann, J.; Moore, K.; Troakes, C.; Al-Sarraj, S.; et al. A histone acetylome-wide association study of Alzheimer’s disease identifies disease-associated H3K27ac differences in the entorhinal cortex. Nat. Neurosci. 2018, 21, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Klein, H.-U.; McCabe, C.; Gjoneska, E.; Sullivan, S.E.; Kaskow, B.J.; Tang, A.; Smith, R.V.; Xu, J.; Pfenning, A.R.; Bernstein, B.E.; et al. Epigenome-wide study uncovers large-scale changes in histone acetylation driven by tau pathology in aging and Alzheimer’s human brains. Nat. Neurosci. 2019, 22, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Maloney, B.; Lahiri, D.K. Epigenetics of dementia: Understanding the disease as a transformation rather than a state. Lancet Neurol. 2016, 15, 760–774. [Google Scholar] [CrossRef]
- Ross, M.; Desai, M.; Khorram, O.; McKnight, R.; Lane, R.; Torday, J. Gestational programming of offspring obesity: A potential contributor to Alzheimer’s disease. Curr. Alzheimer Res. 2007, 4, 213–217. [Google Scholar] [CrossRef]
- Caudill, M.A.; Strupp, B.J.; Muscalu, L.; Nevins, J.E.H.; Canfield, R.L. Maternal choline supplementation during the third trimester of pregnancy improves infant information processing speed: A randomized, double-blind, controlled feeding study. FASEB J. 2018, 32, 2172–2180. [Google Scholar] [CrossRef]
- Rathod, R.; Khaire, A.; Kemse, N.; Kale, A.; Joshi, S. Maternal omega-3 fatty acid supplementation on vitamin B12 rich diet improves brain omega-3 fatty acids, neurotrophins and cognition in the Wistar rat offspring. Brain Dev. 2014, 36, 853–863. [Google Scholar] [CrossRef]
- Yan, Z.; Jiao, F.; Yan, X.; Ou, H. Maternal Chronic Folate Supplementation Ameliorates Behavior Disorders Induced by Prenatal High-Fat Diet Through Methylation Alteration of BDNF and Grin2b in Offspring Hippocampus. Mol. Nutr. Food Res. 2017, 61, 1700461. [Google Scholar] [CrossRef]
- Daher-Abdi, A.; Hernández, S.O.; Castro, L.A.R.; Mezo-González, C.E.; Croyal, M.; García-Santillán, J.A.; Ouguerram, K.; Zambrano, E.; Bolaños-Jiménez, F. Maternal DHA Supplementation during Pregnancy and Lactation in the Rat Protects the Offspring against High-Calorie Diet-Induced Hepatic Steatosis. Nutrients 2021, 13, 3075. [Google Scholar] [CrossRef]
- Yu, L.; Zhong, X.; He, Y.; Shi, Y. Butyrate, but not propionate, reverses maternal diet-induced neurocognitive deficits in offspring. Pharmacol. Res. 2020, 160, 105082. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Xia, B.; Jin, X.; Zou, Q.; Zeng, Z.; Zhao, W.; Yan, S.; Li, L.; Yuan, S.; et al. High-fiber diet mitigates maternal obesity-induced cognitive and social dysfunction in the offspring via gut-brain axis. Cell Metab. 2021, 33, 923–938. [Google Scholar] [CrossRef] [PubMed]
- Atlante, A.; Amadoro, G.; Bobba, A.; Latina, V. Functional Foods: An Approach to Modulate Molecular Mechanisms of Alzheimer’s Disease. Cells 2020, 9, 2347. [Google Scholar] [CrossRef] [PubMed]
- Alawdi, S.H.; El-Denshary, E.S.; Safar, M.M.; Eidi, H.; David, M.-O.; Abdel-Wahhab, M.A. Neuroprotective Effect of Nanodiamond in Alzheimer’s Disease Rat Model: A Pivotal Role for Modulating NF-κB and STAT3 Signaling. Mol. Neurobiol. 2017, 54, 1906–1918. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natale, F.; Spinelli, M.; Rinaudo, M.; Cocco, S.; Nifo Sarrapochiello, I.; Fusco, S.; Grassi, C. Maternal High Fat Diet Anticipates the AD-like Phenotype in 3xTg-AD Mice by Epigenetic Dysregulation of Aβ Metabolism. Cells 2023, 12, 220. https://doi.org/10.3390/cells12020220
Natale F, Spinelli M, Rinaudo M, Cocco S, Nifo Sarrapochiello I, Fusco S, Grassi C. Maternal High Fat Diet Anticipates the AD-like Phenotype in 3xTg-AD Mice by Epigenetic Dysregulation of Aβ Metabolism. Cells. 2023; 12(2):220. https://doi.org/10.3390/cells12020220
Chicago/Turabian StyleNatale, Francesca, Matteo Spinelli, Marco Rinaudo, Sara Cocco, Ida Nifo Sarrapochiello, Salvatore Fusco, and Claudio Grassi. 2023. "Maternal High Fat Diet Anticipates the AD-like Phenotype in 3xTg-AD Mice by Epigenetic Dysregulation of Aβ Metabolism" Cells 12, no. 2: 220. https://doi.org/10.3390/cells12020220
APA StyleNatale, F., Spinelli, M., Rinaudo, M., Cocco, S., Nifo Sarrapochiello, I., Fusco, S., & Grassi, C. (2023). Maternal High Fat Diet Anticipates the AD-like Phenotype in 3xTg-AD Mice by Epigenetic Dysregulation of Aβ Metabolism. Cells, 12(2), 220. https://doi.org/10.3390/cells12020220







