High-Intensity Focused Ultrasound Increases Collagen and Elastin Fiber Synthesis by Modulating Caveolin-1 in Aging Skin
Abstract
:1. Introduction
2. Materials and Methods
2.1. HIFU System
2.2. Temperature Measurements in the Dermal Layer after HIFU Treatment
2.3. Thermal Pattern Confirmation after HIFU Treatment
2.4. In Vitro Model for Testing the Effects of HIFU Treatment
2.5. Cav-1 Silencing in Senescent Fibroblasts
2.6. Application of Hyperthermia to Senescent Fibroblasts
2.7. Aging Mouse Model to Test the Effects of HIFU Treatment on Skin Rejuvenation
2.8. Protein Isolation
2.9. Western Blot
2.10. Co-Immunoprecipitation (Co-IP)
2.11. Enzyme-Linked Immunosorbent Assay (ELISA)
2.12. Hydroxyproline Assay
2.13. Paraffin-Embedded Skin Tissue Block Preparation and Sectioning
2.14. 3,3′-Diaminobenzidine (DAB) Staining
2.15. RNA Extraction and cDNA Synthesis
2.16. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.17. Masson’s Trichrome Staining
2.18. Herovici’s Collagen Staining
2.19. Verhoeff’s Elastic Staining
2.20. Measurement of Epidermal Thickness
2.21. Statistical Analysis
3. Results
3.1. HIFU Increases Local Skin Temperatures
3.2. HIFU Decreases p16 and Increases HSP70 Expression
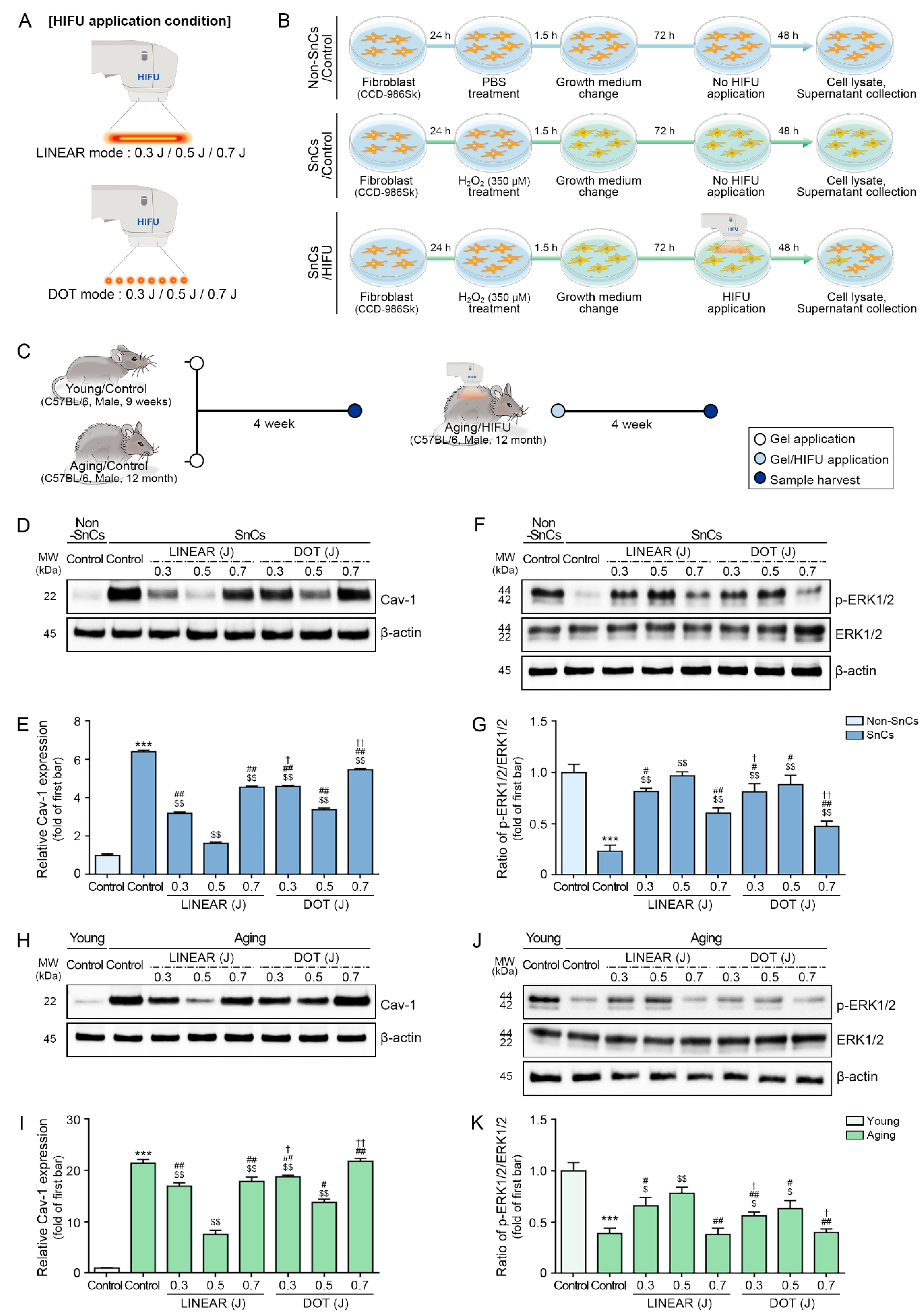
3.3. HIFU Decreases Cav-1 and Increases ERK1/2 Expression
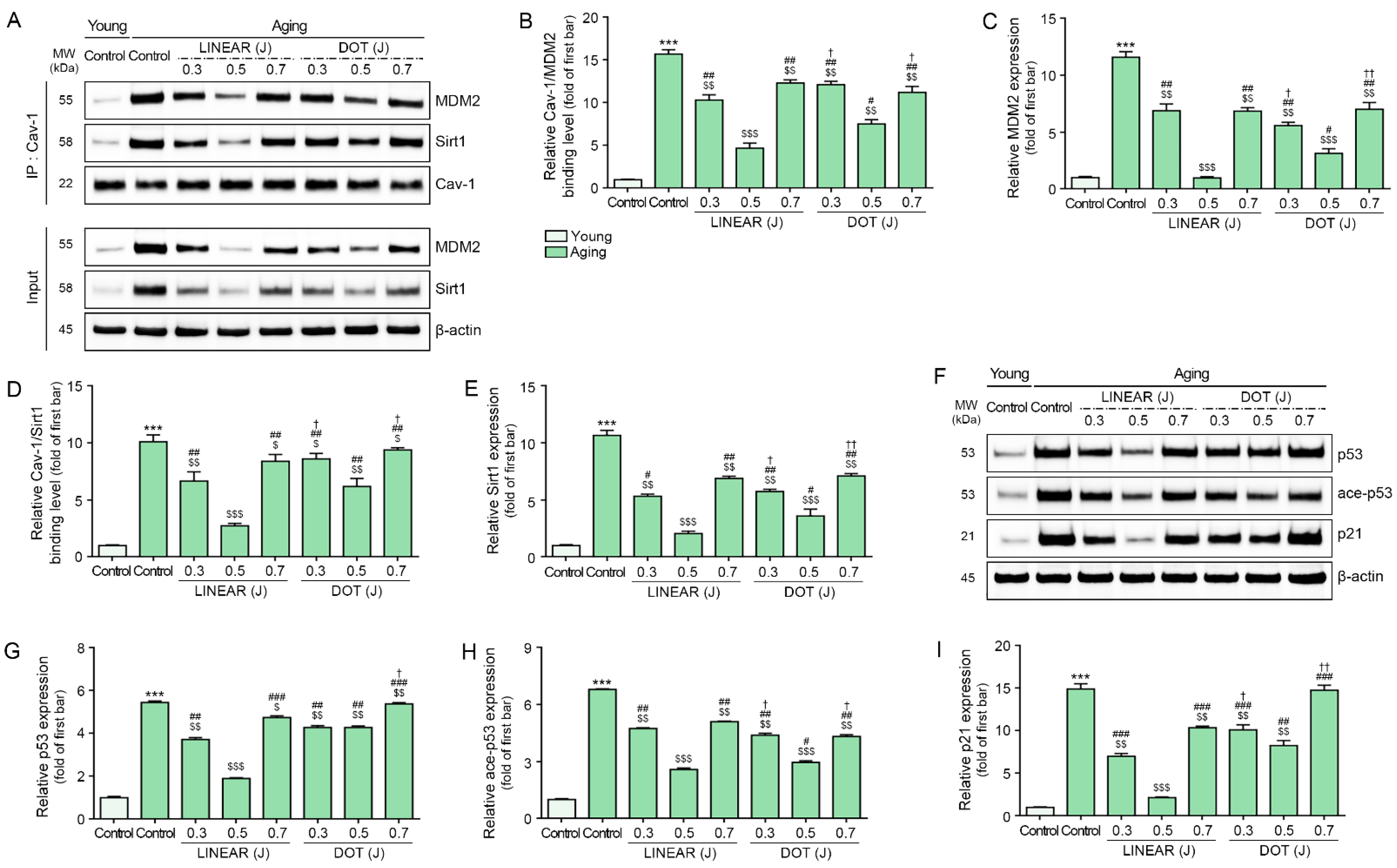
3.4. HIFU Decreases Binding of Cav-1 with MDM2 and Sirt1 and Decreases p53 and p21 Levels
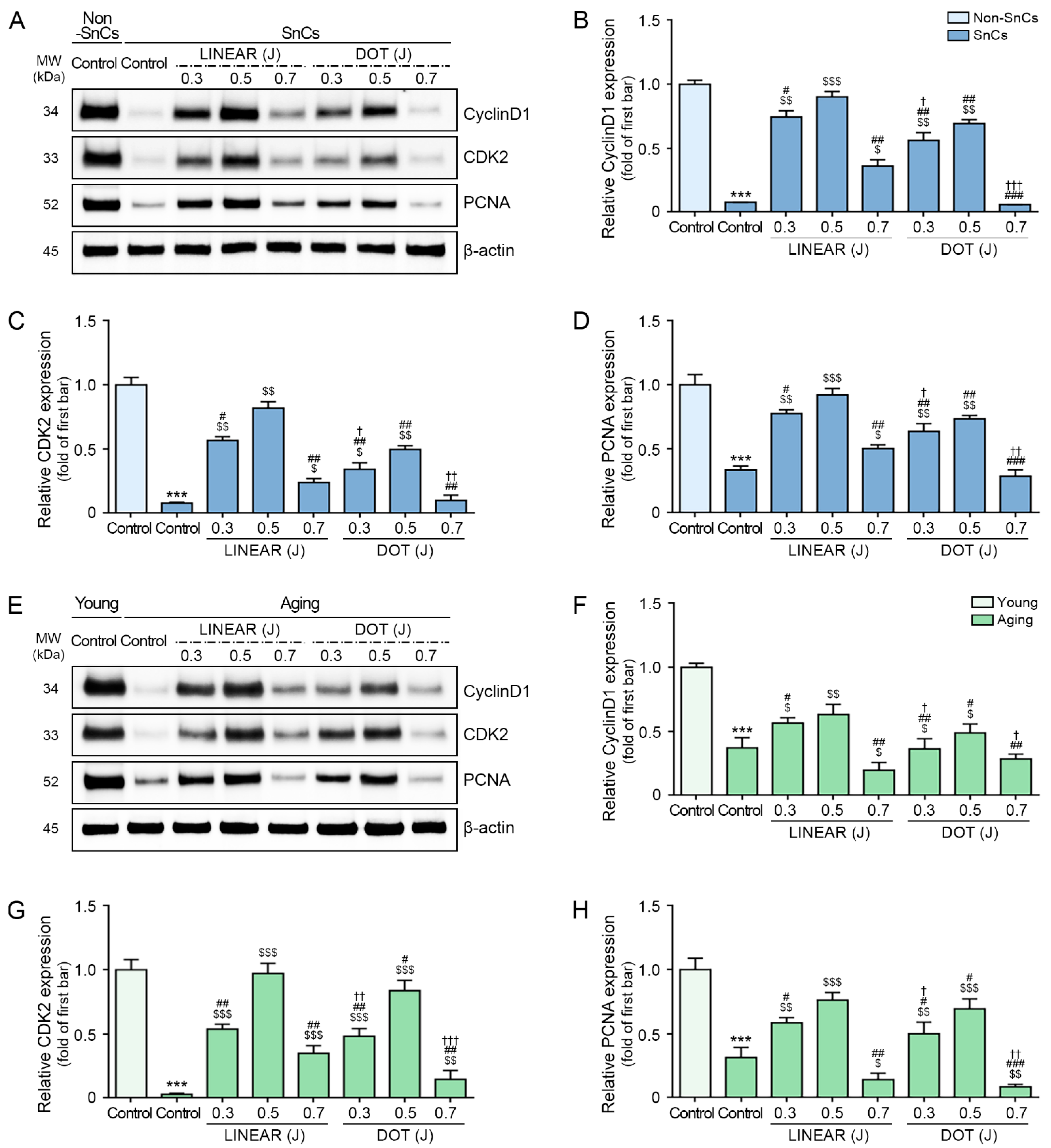
3.5. HIFU Application Decreases Cell Cycle Arrest and Increases Cell Proliferation
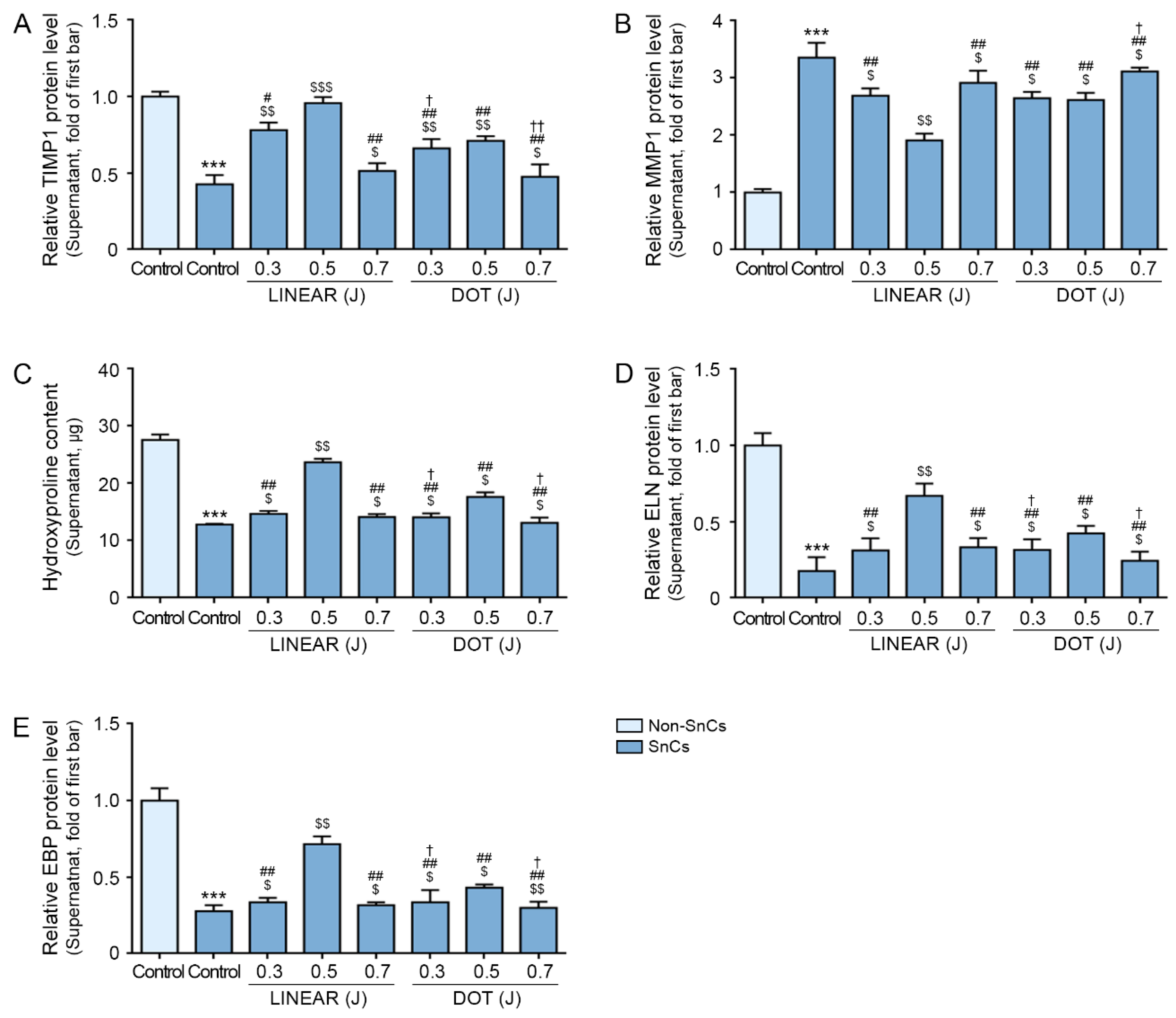
3.6. HIFU Increases TIMP1 Expression and Decreases MMP1 Expression
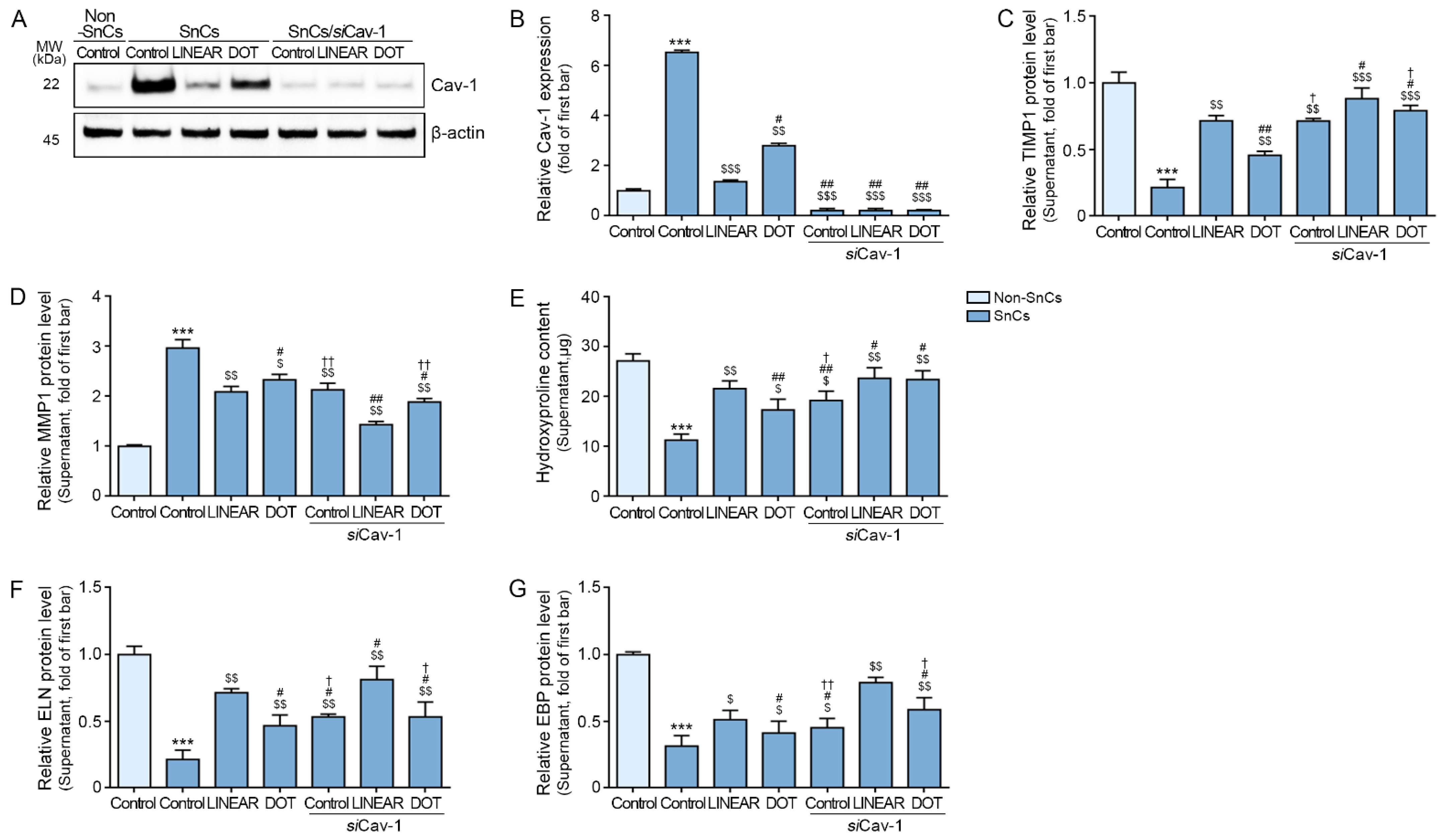
3.7. HIFU Application Induces Decreased MMP Expression and Increased Collagen Synthesis via Cav-1
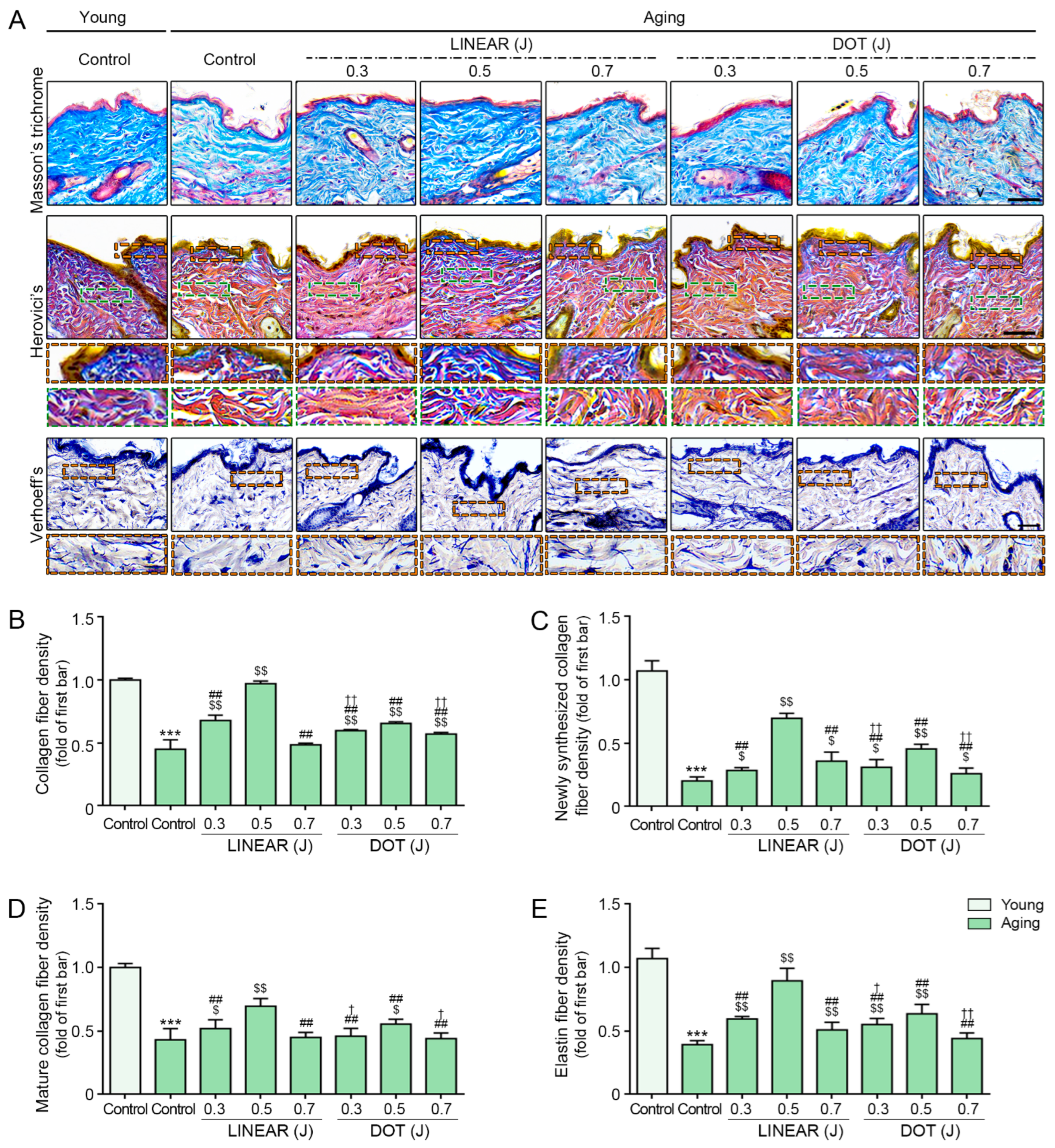
3.8. HIFU Induces Collagen and Elastin Fiber Accumulation
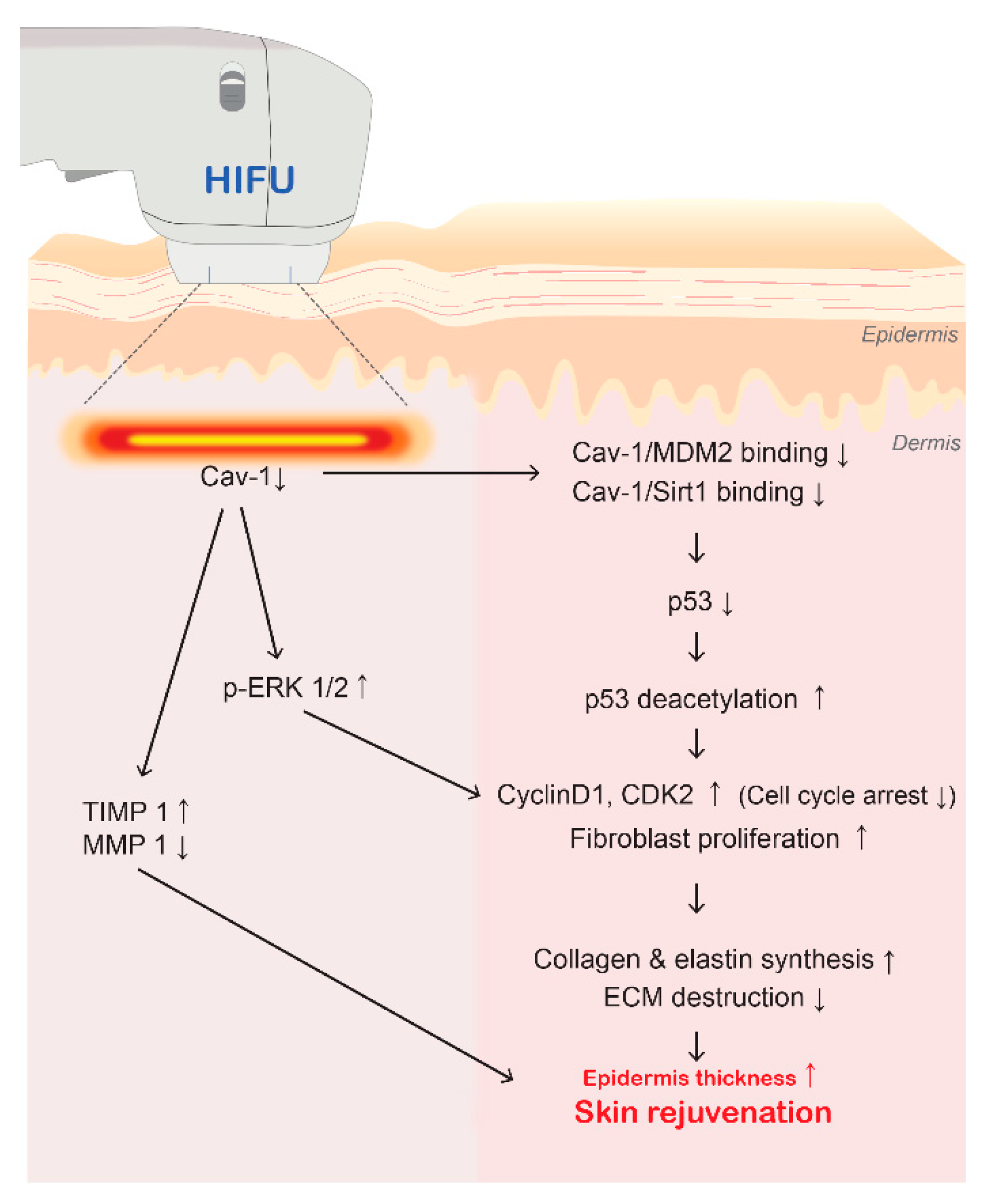
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pike, L.J. Rafts defined: A report on the keystone symposium on lipid rafts and cell function. J. Lipid Res. 2006, 47, 1597–1598. [Google Scholar] [CrossRef]
- Chen, D.; Che, G. Value of caveolin-1 in cancer progression and prognosis: Emphasis on cancer-associated fibroblasts, human cancer cells and mechanism of caveolin-1 expression (Review). Oncol. Lett. 2014, 8, 1409–1421. [Google Scholar] [CrossRef]
- Okamoto, T.; Schlegel, A.; Scherer, P.E.; Lisanti, M.P. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J. Biol. Chem. 1998, 273, 5419–5422. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Razani, B.; Tang, S.; Terman, B.I.; Ware, J.A.; Lisanti, M.P. Angiogenesis activators and inhibitors differentially regulate caveolin-1 expression and caveolae formation in vascular endothelial cells. Angiogenesis inhibitors block vascular endothelial growth factor-induced down-regulation of caveolin-1. J. Biol. Chem. 1999, 274, 15781–15785. [Google Scholar] [CrossRef] [PubMed]
- Galbiati, F.; Volonté, D.; Liu, J.; Capozza, F.; Frank, P.G.; Zhu, L.; Pestell, R.G.; Lisanti, M.P. Caveolin-1 expression negatively regulates cell cycle progression by inducing G(0)/G(1) arrest via a p53/p21(WAF1/Cip1)-dependent mechanism. Mol. Biol. Cell 2001, 12, 2229–2244. [Google Scholar] [CrossRef] [PubMed]
- Hulit, J.; Bash, T.; Fu, M.; Galbiati, F.; Albanese, C.; Sage, D.R.; Schlegel, A.; Zhurinsky, J.; Shtutman, M.; Ben-Ze’ev, A.; et al. The cyclin D1 gene is transcriptionally repressed by caveolin-1. J. Biol. Chem. 2000, 275, 21203–21209. [Google Scholar] [CrossRef]
- Cho, K.A.; Ryu, S.J.; Park, J.S.; Jang, I.S.; Ahn, J.S.; Kim, K.T.; Park, S.C. Senescent phenotype can be reversed by reduction of caveolin status. J. Biol. Chem. 2003, 278, 27789–27795. [Google Scholar] [CrossRef]
- Volonte, D.; Galbiati, F. Caveolin-1, cellular senescence and pulmonary emphysema. Aging 2009, 1, 831–835. [Google Scholar] [CrossRef]
- Volonte, D.; Zhang, K.; Lisanti, M.P.; Galbiati, F. Expression of caveolin-1 induces premature cellular senescence in primary cultures of murine fibroblasts. Mol. Biol. Cell 2002, 13, 2502–2517. [Google Scholar] [CrossRef]
- Park, S.C.; Cho, K.A.; Jang, I.S.; Kim, K.T.; Ryu, S.J. Functional efficiency of the senescent cells: Replace or restore? Ann. N. Y. Acad. Sci. 2004, 1019, 309–316. [Google Scholar] [CrossRef]
- Song, T.; Gu, Y.; Hui, W.; Yang, X.; Liu, Y.; Chen, X. Oxygen-glucose deprivation promoted fibroblast senescence and collagen expression via IL11. Int. J. Mol. Sci. 2022, 23, 12090. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Choi, D.I.; Choi, J.Y.; Kim, S.O.; Cho, K.A.; Lee, J.B.; Yun, S.J.; Lee, S.C. Methyl-β-cyclodextrin up-regulates collagen I expression in chronologically-aged skin via its anti-caveolin-1 activity. Oncotarget 2015, 6, 1942–1953. [Google Scholar] [CrossRef] [PubMed]
- Park, W.Y.; Park, J.S.; Cho, K.A.; Kim, D.I.; Ko, Y.G.; Seo, J.S.; Park, S.C. Up-regulation of caveolin attenuates epidermal growth factor signaling in senescent cells. J. Biol. Chem. 2000, 275, 20847–20852. [Google Scholar] [CrossRef]
- Bentov, I.; Damodarasamy, M.; Plymate, S.; Reed, M.J. Decreased proliferative capacity of aged dermal fibroblasts in a three dimensional matrix is associated with reduced IGF1R expression and activation. Biogerontology 2014, 15, 329–337. [Google Scholar] [CrossRef]
- Sears, R.C.; Nevins, J.R. Signaling networks that link cell proliferation and cell fate. J. Biol. Chem. 2002, 277, 11617–11620. [Google Scholar] [CrossRef] [PubMed]
- Downward, J. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer 2003, 3, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Coleman, M.L.; Marshall, C.J.; Olson, M.F. RAS and RHO GTPases in G1-phase cell-cycle regulation. Nat. Rev. Mol. Cell Biol. 2004, 5, 355–366. [Google Scholar] [CrossRef]
- Massagué, J. G1 cell-cycle control and cancer. Nature 2004, 432, 298–306. [Google Scholar] [CrossRef]
- Dyson, N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998, 12, 2245–2262. [Google Scholar] [CrossRef]
- Sherr, C.J.; Roberts, J.M. CDK inhibitors: Positive and negative regulators of G1-phase progression. Genes Dev. 1999, 13, 1501–1512. [Google Scholar] [CrossRef]
- Huang, W.; Hickson, L.J.; Eirin, A.; Kirkland, J.L.; Lerman, L.O. Cellular senescence: The good, the bad and the unknown. Nat. Rev. Nephrol. 2022, 18, 611–627. [Google Scholar] [CrossRef]
- Bartholomew, J.N.; Volonte, D.; Galbiati, F. Caveolin-1 regulates the antagonistic pleiotropic properties of cellular senescence through a novel Mdm2/p53-mediated pathway. Cancer Res. 2009, 69, 2878–2886. [Google Scholar] [CrossRef]
- Volonte, D.; Zou, H.; Bartholomew, J.N.; Liu, Z.; Morel, P.A.; Galbiati, F. Oxidative stress-induced inhibition of Sirt1 by caveolin-1 promotes p53-dependent premature senescence and stimulates the secretion of interleukin 6 (IL-6). J. Biol. Chem. 2015, 290, 4202–4214. [Google Scholar] [CrossRef]
- Fisher, G.J.; Wang, Z.Q.; Datta, S.C.; Varani, J.; Kang, S.; Voorhees, J.J. Pathophysiology of premature skin aging induced by ultraviolet light. N. Engl. J. Med. 1997, 337, 1419–1428. [Google Scholar] [CrossRef]
- Varani, J.; Warner, R.L.; Gharaee-Kermani, M.; Phan, S.H.; Kang, S.; Chung, J.H.; Wang, Z.Q.; Datta, S.C.; Fisher, G.J.; Voorhees, J.J. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J. Investig. Dermatol. 2000, 114, 480–486. [Google Scholar] [CrossRef]
- Verma, R.P.; Hansch, C. Matrix metalloproteinases (MMPs): Chemical-biological functions and (Q)SARs. Bioorg. Med. Chem. 2007, 15, 2223–2268. [Google Scholar] [CrossRef]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and biological attributes of matrix metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar] [PubMed]
- Weinstein, G.D.; Boucek, R.J. Collagen and elastin of human dermis. J. Investig. Dermatol. 1960, 35, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Tracy, L.E.; Minasian, R.A.; Caterson, E.J. Extracellular matrix and dermal fibroblast function in the healing wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Cokakli, M.; Erdal, E.; Nart, D.; Yilmaz, F.; Sagol, O.; Kilic, M.; Karademir, S.; Atabey, N. Differential expression of Caveolin-1 in hepatocellular carcinoma: Correlation with differentiation state, motility and invasion. BMC Cancer 2009, 24, 65. [Google Scholar] [CrossRef] [PubMed]
- Saket, P.; Shobeihi, S.; Mehrdadi, S. Study of efficacy of esthetic high-intensity focused ultrasound system on Iranian skin for reducing the laxity and wrinkles of aging. J. Cosmet. Dermatol. 2017, 16, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Riggs, K.; Keller, M.; Humphreys, T.R. Ablative laser resurfacing: High-energy pulsed carbon dioxide and erbium:yttrium-aluminum-garnet. Clin. Dermatol. 2007, 25, 462–473. [Google Scholar] [CrossRef]
- El-Domyati, M.; el-Ammawi, T.S.; Medhat, W.; Moawad, O.; Brennan, D.; Mahoney, M.G.; Uitto, J. Radiofrequency facial rejuvenation: Evidence-based effect. J. Am. Acad. Dermatol. 2011, 64, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Wulkan, A.J.; Fabi, S.G.; Green, J.B. Microfocused Ultrasound for Facial Photorejuvenation: A Review. Facial Plast. Surg. 2016, 32, 269–275. [Google Scholar] [PubMed]
- Day, D. Microfocused ultrasound for facial rejuvenation: Current perspectives. Res. Rep. Focus. Ultrasound 2014, 2, 13–17. [Google Scholar] [CrossRef]
- Lee, T.J.; Kim, D.; Kim, T.; Pak, C.J.; Suh, H.P.; Hong, J.P. Rejuvenation of photoaged aged mouse skin using high-intensity focused ultrasound. J. Plast. Reconstr. Aesthet. Surg. 2022, 75, 3859–3868. [Google Scholar] [CrossRef]
- Kim, K.H.; Geronemus, R.G. Nonablative laser and light therapies for skin rejuvenation. Arch. Facial Plast. Surg. 2004, 6, 398–409. [Google Scholar] [CrossRef]
- Herne, K.B.; Zachary, C.B. New facial rejuvenation techniques. Semin. Cutan. Med. Surg. 2000, 19, 221–231. [Google Scholar] [CrossRef]
- Ko, E.J.; Hong, J.Y.; Kwon, T.R.; Choi, E.J.; Jang, Y.J.; Choi, S.Y.; Yoo, K.H.; Kim, S.Y.; Kim, B.J. Efficacy and safety of non-invasive body tightening with high-intensity focused ultrasound (HIFU). Skin Res. Technol 2017, 23, 558–562. [Google Scholar] [CrossRef]
- Pak, C.S.; Lee, Y.K.; Jeong, J.H.; Kim, J.H.; Seo, J.D.; Heo, C.Y. Safety and efficacy of ulthera in the rejuvenation of aging lower eyelids: A pivotal clinical trial. Aesthetic Plast. Surg. 2014, 38, 861–868. [Google Scholar] [CrossRef]
- Suh, D.H.; Oh, Y.J.; Lee, S.J.; Rho, J.H.; Song, K.Y.; Kim, N.I.; Shin, M.K. A intense-focused ultrasound tightening for the treatment of infraorbital laxity. J. Cosmet. Laser Ther. 2012, 14, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Z.B. High-intensity focused ultrasound tumor ablation: Review of ten years of clinical experience. Front. Med. China 2010, 4, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Sklar, L.R.; El Tal, A.K.; Kerwin, L.Y. Use of transcutaneous ultrasound for lipolysis and skin tightening: A review. Aesthetic Plast. Surg. 2014, 38, 429–441. [Google Scholar] [CrossRef]
- Orsi, F.; Arnone, P.; Chen, W.; Zhang, L. High intensity focused ultrasound ablation: A new therapeutic option for solid tumors. J. Cancer Res. Ther. 2010, 6, 414–420. [Google Scholar]
- The European Aesthetic Guide. High Intensity Focused Ultrasound. Available online: http://digital.miinews.com/publication/?i=34631&page=1 (accessed on 12 July 2010).
- Avelar, J. Regional distribution and behavior of the subcutaneous tissue concerning selection and indication for liposuction. Aesthetic Plast Surg. 1989, 13, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Rhim, H.; Choi, M.J.; Lim, H.K.; Choi, D. High-intensity focused ultrasound therapy: An overview for radiologists. Korean J. Radiol. 2008, 9, 291–302. [Google Scholar] [CrossRef]
- Kennedy, J.E.; Ter Haar, G.R.; Cranston, D. High intensity focused ultrasound: Surgery of the future? Br. J. Radiol. 2003, 76, 590–599. [Google Scholar] [CrossRef]
- Joiner, J.B.; Pylayeva-Gupta, Y.; Dayton, P.A. Focused ultrasound for immunomodulation of the tumor microenvironment. J. Immunol. 2020, 205, 2327–2341. [Google Scholar] [CrossRef]
- Diederich, C.J.; Hynynen, K. Ultrasound technology for hyperthermia. Ultrasound Med. Biol. 1999, 25, 871–887. [Google Scholar] [CrossRef]
- Oh, S.; Kim, H.M.; Batsukh, S.; Sun, H.J.; Kim, T.; Kang, D.; Son, K.H.; Byun, K. High-intensity focused ultrasound induces adipogenesis via control of cilia in adipose-derived stem cells in subcutaneous adipose tissue. Int. J. Mol. Sci. 2022, 23, 8866. [Google Scholar] [CrossRef]
- Kang, Y.S.; Ko, Y.G.; Seo, J.S. Caveolin internalization by heat shock or hyperosmotic shock. Exp. Cell Res. 2000, 255, 221–228. [Google Scholar] [CrossRef]
- Wu, Y.H.; Cheng, M.L.; Ho, H.Y.; Chiu, D.T.; Wang, T.C. Telomerase prevents accelerated senescence in glucose-6-phosphate dehydrogenase (G6PD)-deficient human fibroblasts. J. Biomed. Sci. 2009, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Baek, M.O.; Khaliq, S.A.; Parveen, A.; Kim, S.Y.; Kim, J.H.; Kim, I.C.; Yoon, M.S. Antarctic krill extracts enhance muscle regeneration and muscle function via mammalian target of rapamycin regulation. J. Funct. Foods 2023, 103, 105483. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Q.; Xu, C.B. A convenient method for quantifying collagen fibers in atherosclerotic lesions by ImageJ software. Int. J. Clin. Exp. Med. 2017, 10, 14904–14910. [Google Scholar]
- Herovici, C. Picropolychrome: Histological staining technic intended for the study of normal and pathological connective tissue. Rev. Fr. Etud. Clin. Biol. 1963, 8, 88–89. [Google Scholar] [PubMed]
- Anthony, P.P. Manual of histological demonstration techniques. J. Clin. Pathol. 1975, 28, 339. [Google Scholar] [CrossRef]
- Turner, N.J.; Pezzone, M.A.; Brown, B.N.; Badylak, S.F. Quantitative multispectral imaging of Herovici’s polychrome for the assessment of collagen content and tissue remodelling. J. Tissue Eng. Regen. Med. 2013, 7, 139–148. [Google Scholar] [CrossRef]
- Faucz, L.L.; Will, S.E.; Rodrigues, C.J.; Hesse, H.; Moraes, A.C.; Maria, D.A. Quantitative evaluation of collagen and elastic fibers after intense pulsed light treatment of mouse skin. Lasers Surg. Med. 2018, 50, 644–650. [Google Scholar] [CrossRef]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef]
- Trepat, X.; Deng, L.; An, S.S.; Navajas, D.; Tschumperlin, D.J.; Gerthoffer, W.T.; Butler, J.P.; Fredberg, J.J. Universal physical responses to stretch in the living cell. Nature 2007, 447, 592–595. [Google Scholar] [CrossRef]
- Krishnan, R.; Park, C.Y.; Lin, Y.C.; Mead, J.; Jaspers, R.T.; Trepat, X.; Lenormand, G.; Tambe, D.; Smolensky, A.V.; Knoll, A.H.; et al. Reinforcement versus fluidization in cytoskeletal mechanoresponsiveness. PLoS ONE 2009, 4, e5486. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, N.; Zhou, E.H.; Lenormand, G.; Krishnan, R.; Weihs, D.; Butler, J.P.; Weitz, D.A.; Fredberg, J.J.; Kimmel, E. Low intensity ultrasound perturbs cytoskeleton dynamics. Soft Matter 2012, 8, 2438–2443. [Google Scholar] [CrossRef] [PubMed]
- Dynlacht, J.R.; Fox, M.H. The effect of 45 degrees C hyperthermia on the membrane fluidity of cells of several lines. Radiat. Res. 1992, 130, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Fleischmajer, R.; Perlish, J.S.; Timpl, R. Collagen fibrillogenesis in human skin. Ann. N. Y. Acad. Sci. 1985, 460, 246–257. [Google Scholar] [CrossRef]
- Sibilla, S.; Godfrey, M.; Brewer, S.; Budh-Raja, A.; Licia, G. An overview of the beneficial effects of hydrolysed collagen as a nutraceutical on skin properties: Scientific background and clinical studies. Open Nutraceuticals J. 2015, 8, 29–42. [Google Scholar] [CrossRef]
- Vuorio, E.; de Crombrugghe, B. The family of collagen genes. Annu. Rev. Biochem. 1990, 59, 837–872. [Google Scholar] [CrossRef]
- David, M.R.; Jeniffer, L. Skin collagen through the lifestages: Importance for skin health and beauty. Plast. Aesthet. Res. 2021, 8, 2. [Google Scholar]
- Aziz, J.; Shezali, H.; Radzi, Z.; Yahya, N.A.; Abu Kassim, N.H.; Czernuszka, J.; Rahman, M.T. Molecular mechanisms of stress-responsive changes in collagen and elastin networks in skin. Skin Pharmacol. Physiol. 2016, 29, 190–203. [Google Scholar] [CrossRef]
- Rock, M.; Cain, S.A.; Freeman, L.J.; Morgan, A.; Mellody, K.; Marson, A.; Shuttleworth, C.A.; Weiss, A.S.; Kielty, C.M. Molecular basis of elastic fiber formation. Critical interactions and a tropoelastin-fibrillin-1 cross-link. J. Biol. Chem. 2004, 279, 23748–23758. [Google Scholar] [CrossRef]
- Hirai, M.; Ohbayashi, T.; Horiguchi, M.; Okawa, K.; Hagiwara, A.; Chien, K.R.; Kita, T.; Nakamura, T. Fibulin-5/DANCE has an elastogenic organizer activity that is abrogated by proteolytic cleavage in vivo. J. Cell Biol. 2007, 176, 1061–1071. [Google Scholar] [CrossRef]
- Uitto, J.; Li, Q.; Urban, Z. The complexity of elastic fibre biogenesis in the skin-a perspective to the clinical heterogeneity of cutis laxa. Exp. Dermatol. 2013, 22, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.D.; Endicott, S.K.; Province, M.A.; Pierce, J.A.; Campbell, E.J. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J. Clin. Investig. 1991, 87, 1828–1834. [Google Scholar] [CrossRef] [PubMed]
- Langton, A.K.; Halai, P.; Griffiths, C.E.; Sherratt, M.J.; Watson, R.E. The impact of intrinsic ageing on the protein composition of the dermal-epidermal junction. Mech. Ageing Dev. 2016, 156, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Wise, S.G.; Weiss, A.S. Tropoelastin. Int. J. Biochem. Cell Biol. 2009, 41, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Naylor, E.C.; Watson, R.E.; Sherratt, M.J. Molecular aspects of skin ageing. Maturitas 2011, 69, 249–256. [Google Scholar] [CrossRef]
- Baumann, L.; Bernstein, E.F.; Weiss, A.S.; Bates, D.; Humphrey, S.; Silberberg, M.; Daniels, R. Clinical relevance of elastin in the structure and function of skin. Aesthet. Surg. J. Open Forum 2021, 3, ojab019. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, S.; Rhee, D.-Y.; Batsukh, S.; Son, K.H.; Byun, K. High-Intensity Focused Ultrasound Increases Collagen and Elastin Fiber Synthesis by Modulating Caveolin-1 in Aging Skin. Cells 2023, 12, 2275. https://doi.org/10.3390/cells12182275
Oh S, Rhee D-Y, Batsukh S, Son KH, Byun K. High-Intensity Focused Ultrasound Increases Collagen and Elastin Fiber Synthesis by Modulating Caveolin-1 in Aging Skin. Cells. 2023; 12(18):2275. https://doi.org/10.3390/cells12182275
Chicago/Turabian StyleOh, Seyeon, Do-Young Rhee, Sosorburam Batsukh, Kuk Hui Son, and Kyunghee Byun. 2023. "High-Intensity Focused Ultrasound Increases Collagen and Elastin Fiber Synthesis by Modulating Caveolin-1 in Aging Skin" Cells 12, no. 18: 2275. https://doi.org/10.3390/cells12182275
APA StyleOh, S., Rhee, D.-Y., Batsukh, S., Son, K. H., & Byun, K. (2023). High-Intensity Focused Ultrasound Increases Collagen and Elastin Fiber Synthesis by Modulating Caveolin-1 in Aging Skin. Cells, 12(18), 2275. https://doi.org/10.3390/cells12182275




