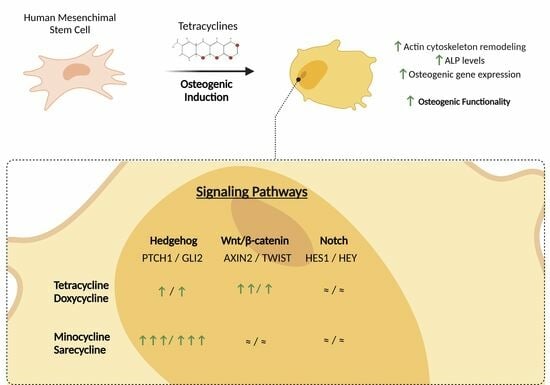
Unveiling the Osteogenic Potential of Tetracyclines: A Comparative Study in Human Mesenchymal Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
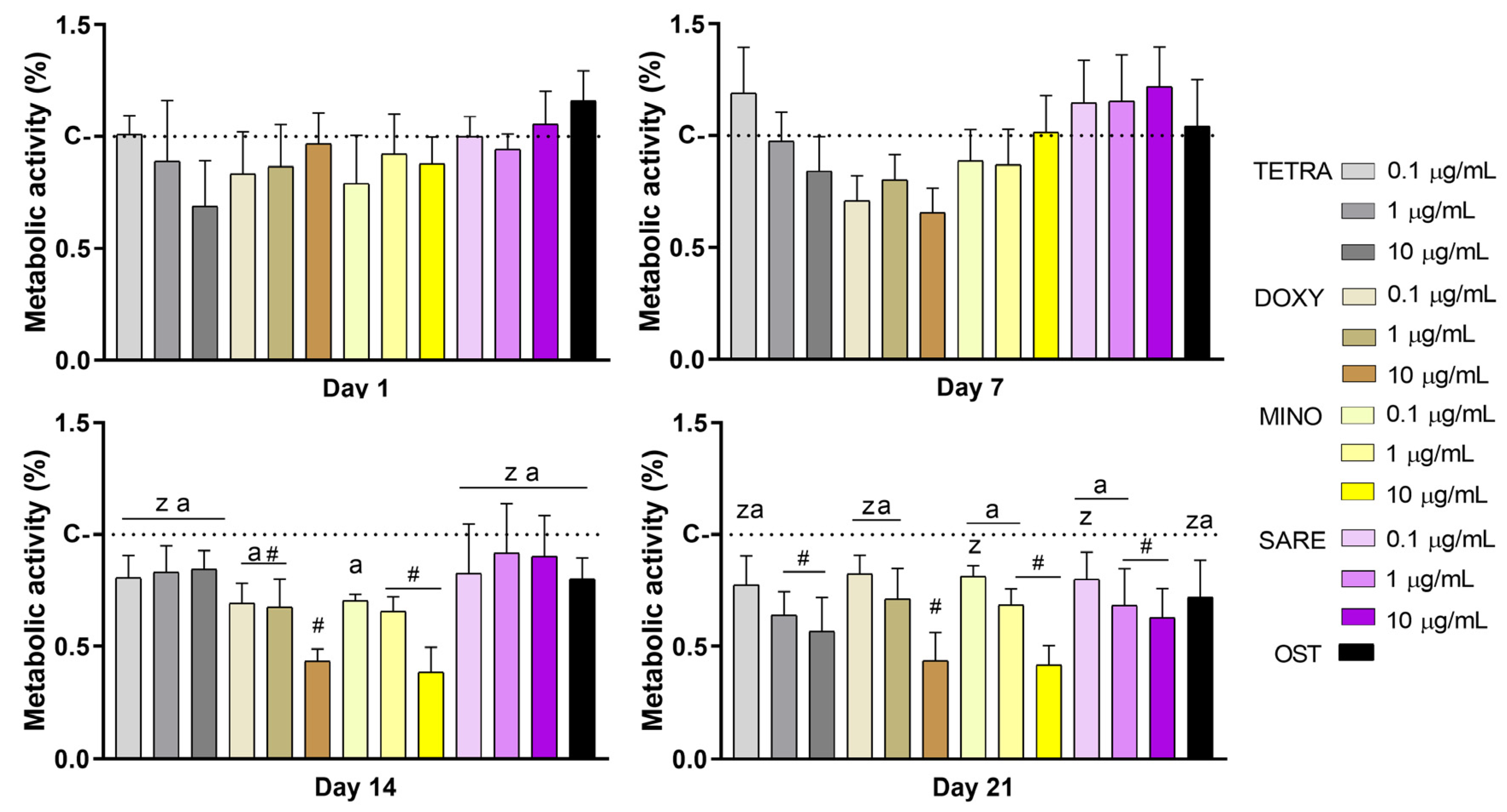
2.1. Metabolic Activity Assay
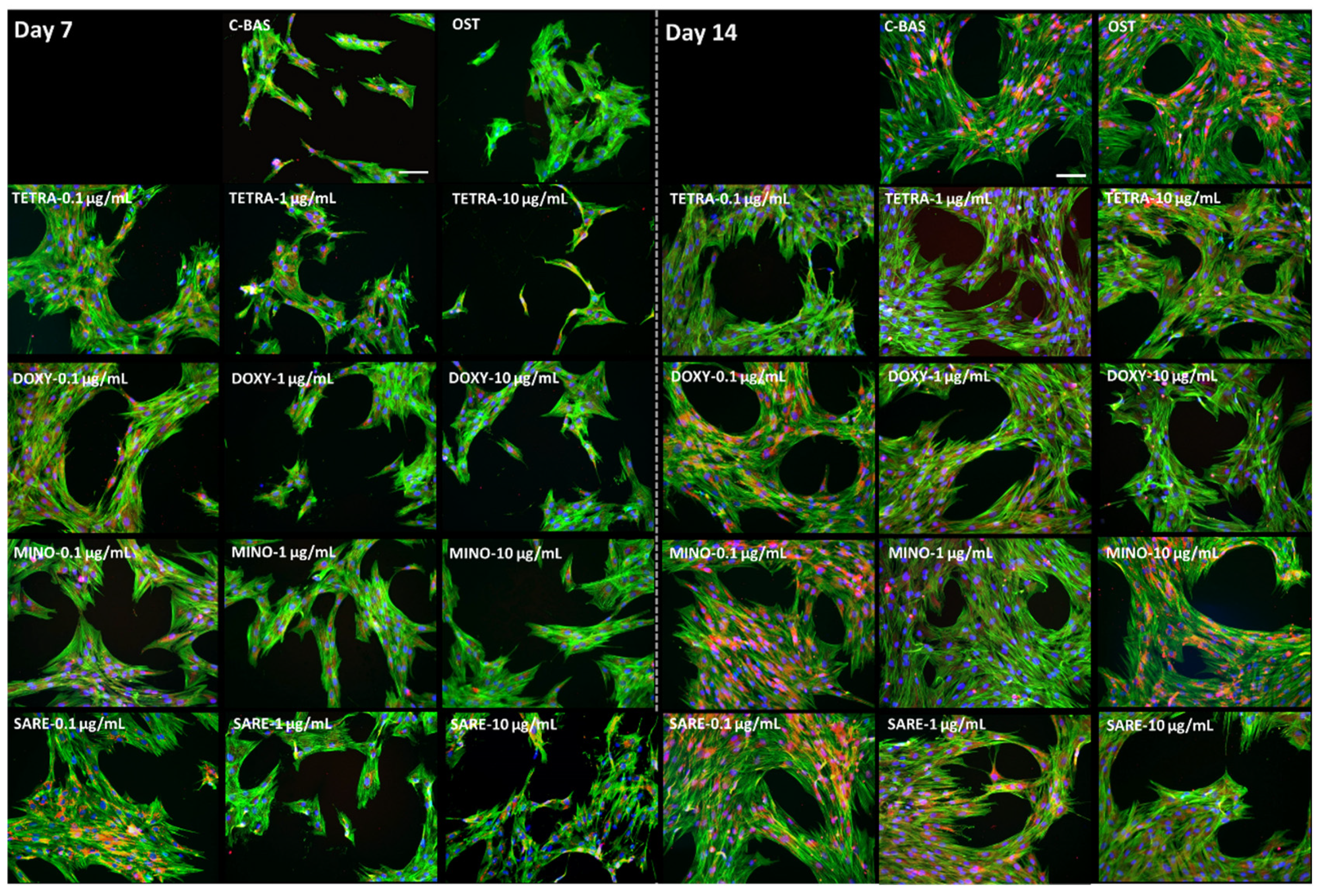
2.2. Cell Morphology
2.3. Assessment of Gene Expression by Quantitative PCR (qPCR)
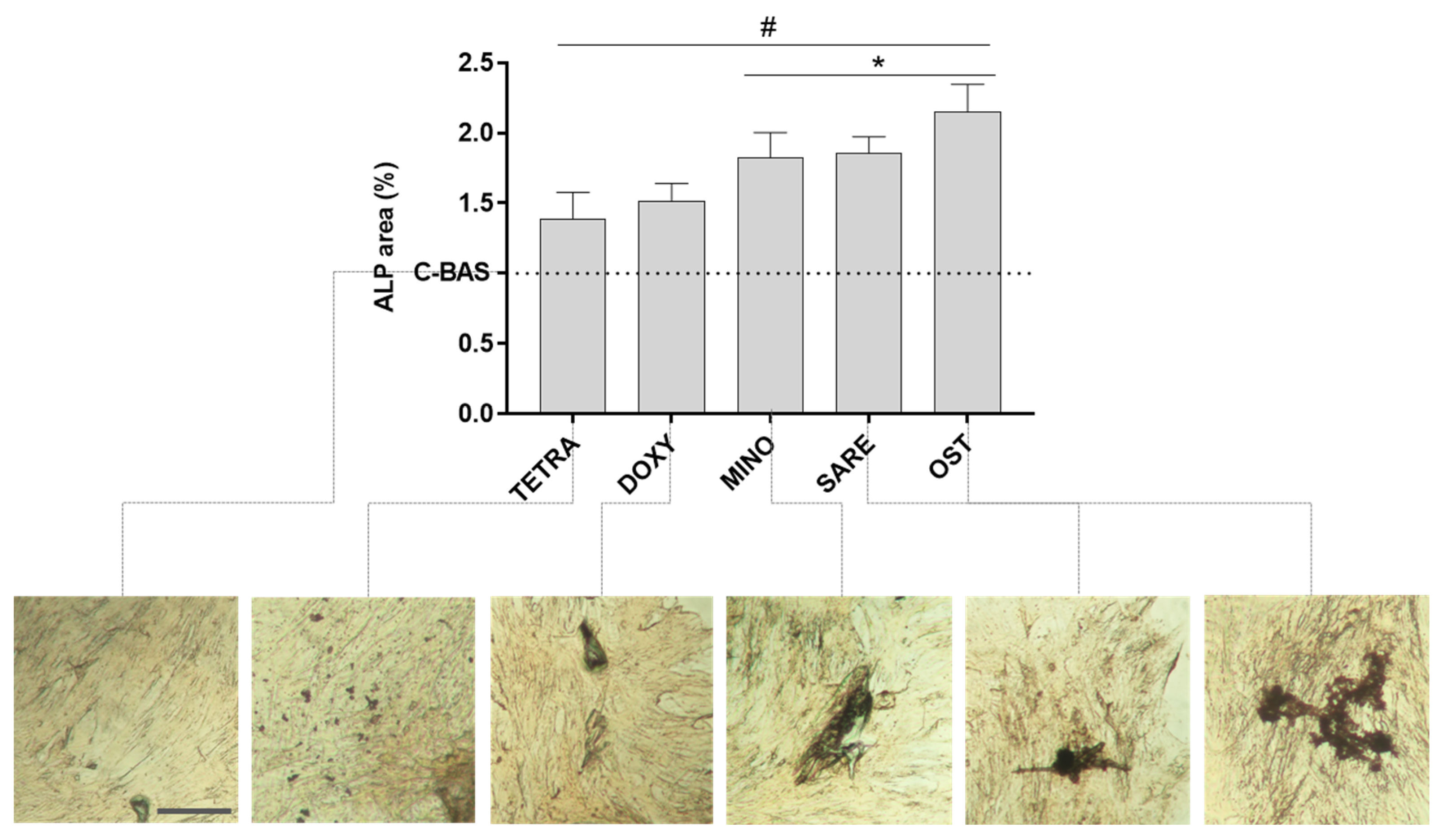
2.4. Alkaline Phosphatase (ALP) Histochemical Staining
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, Y.Y.-H.Y.; Kim, J.; Lee, H.; Shin, W.-R.; Lee, S.; Lee, J.; Park, J.-I.; Jhun, B.H.; Kim, Y.Y.-H.Y.; Yi, S.-J.; et al. Tetracycline Analogs Inhibit Osteoclast Differentiation by Suppressing MMP-9-Mediated Histone H3 Cleavage. Int. J. Mol. Sci. 2019, 20, 4038. [Google Scholar] [CrossRef]
- LaPlante, K.L.; Dhand, A.; Wright, K.; Lauterio, M. Re-establishing the utility of tetracycline-class antibiotics for current challenges with antibiotic resistance. Ann. Med. 2022, 54, 1686–1700. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.L.; Ismail, M.Y. The Antibiotic and Nonantibiotic Tetracyclines. In Comprehensive Medicinal Chemistry II; Elsevier: Amsterdam, The Netherlands, 2007; pp. 597–628. [Google Scholar]
- Bryskier, A. Antibiotics and Antibacterial Agents: Classifications and Structure-Activity Relationship. In Antimicrobial Agents; ASM Press: Washington, DC, USA, 2014; pp. 13–38. [Google Scholar]
- Nguyen, F.; Starosta, A.L.; Arenz, S.; Sohmen, D.; Dönhöfer, A.; Wilson, D.N. Tetracycline antibiotics and resistance mechanisms. Biol. Chem. 2014, 395, 559–575. [Google Scholar] [CrossRef]
- Fuoco, D. Classification Framework and Chemical Biology of Tetracycline-Structure-Based Drugs. Antibiotics 2012, 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Al-kuraishy, H.M.; Al-Gareeb, A.I.; Alqarni, M.; Cruz-Martins, N.; El-Saber Batiha, G. Pleiotropic Effects of Tetracyclines in the Management of COVID-19: Emerging Perspectives. Front. Pharmacol. 2021, 12, 642822. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Mesa, N.; Zarzuelo, A.; Gálvez, J. Minocycline: Far beyond an antibiotic. Br. J. Pharmacol. 2013, 169, 337–352. [Google Scholar] [CrossRef]
- Yang, C.; Gao, J.; Wu, B.; Yan, N.; Li, H.; Ren, Y.; Kan, Y.; Liang, J.; Jiao, Y.; Yu, Y. Minocycline attenuates the development of diabetic neuropathy by inhibiting spinal cord Notch signaling in rat. Biomed. Pharmacother. 2017, 94, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Bunick, C.G.; Keri, J.; Tanaka, S.K.; Furey, N.; Damiani, G.; Johnson, J.L.; Grada, A. Antibacterial mechanisms and efficacy of sarecycline in animal models of infection and inflammation. Antibiotics 2021, 10, 439. [Google Scholar] [CrossRef]
- Golub, L.M.; Elburki, M.S.; Walker, C.; Ryan, M.; Sorsa, T.; Tenenbaum, H.; Goldberg, M.; Wolff, M.; Gu, Y. Non-antibacterial tetracycline formulations: Host-modulators in the treatment of periodontitis and relevant systemic diseases. Int. Dent. J. 2016, 66, 127–135. [Google Scholar] [CrossRef]
- Li, H.; Jiao, S.; Li, X.; Banu, H.; Hamal, S.; Wang, X. Therapeutic effects of antibiotic drug tigecycline against cervical squamous cell carcinoma by inhibiting Wnt/β-catenin signaling. Biochem. Biophys. Res. Commun. 2015, 467, 14–20. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, Q.; Lee, S.; Zhong, W.-l.; Liu, Y.-r.; Liu, H.-j.; Zhao, D.; Chen, S.; Xiao, T.; Meng, J.; et al. Doxycycline reverses epithelial-to-mesenchymal transition and suppresses the proliferation and metastasis of lung cancer cells. Oncotarget 2015, 6, 40667–40679. [Google Scholar] [CrossRef] [PubMed]
- Hirohashi, Y.; Kamijo, S.; Khan, M.; Ikeda, M.; Oki, M.; Matin, K.; Rashed, F.; Aoki, K. Tetracycline, an Appropriate Reagent for Measuring Bone-Formation Activity in the Murine Model of the Streptococcus mutans-Induced Bone Loss. Front. Cell. Infect. Microbiol. 2021, 11, 714366. [Google Scholar] [CrossRef]
- Macri-Pellizzeri, L.; De Melo, N.; Ahmed, I.; Grant, D.; Scammell, B.; Sottile, V. Live Quantitative Monitoring of Mineral Deposition in Stem Cells Using Tetracycline Hydrochloride. Tissue Eng. Part C Methods 2018, 24, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Zegre, M.; Barros, J.; Ribeiro, I.A.C.; Santos, C.; Caetano, L.A.; Gonçalves, L.; Monteiro, F.J.; Ferraz, M.P.; Bettencourt, A. Poly(DL-lactic acid) scaffolds as a bone targeting platform for the co-delivery of antimicrobial agents against S. aureus-C.albicans mixed biofilms. Int. J. Pharm. 2022, 622, 121832. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Ribeiro, I.A.C.; Alves, M.M.; Gonçalves, L.; Almeida, A.J.; Grenho, L.; Fernandes, M.H.; Santos, C.F.; Gomes, P.S.; Bettencourt, A.F. Understanding intracellular trafficking and anti-inflammatory effects of minocycline chitosan-nanoparticles in human gingival fibroblasts for periodontal disease treatment. Int. J. Pharm. 2019, 572, 118821. [Google Scholar] [CrossRef]
- Silva, T.; Grenho, L.; Barros, J.; Silva, J.; Pinto, R.; Matos, A.; Colaço, B.; Fernandes, H.; Bettencourt, A.; Gomes, P.S. Minocycline-releasing PMMA system as a space maintainer for staged bone reconstructions—In vitro antibacterial, cytocompatibility and anti-inflammatory characterization. Biomed. Mater. 2017, 12, 35009. [Google Scholar] [CrossRef]
- Silva, T.; Silva, J.C.; Colaco, B.; Gama, A.; Duarte-Araújo, M.; Fernandes, M.H.; Bettencourt, A.; Gomes, P. In vivo tissue response and antibacterial efficacy of minocycline delivery system based on polymethylmethacrylate bone cement. J. Biomater. Appl. 2018, 33, 380–391. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Z.; Nong, J.; Nix, C.A.; Ji, H.-F.; Zhong, Y. Metal ion-assisted self-assembly of complexes for controlled and sustained release of minocycline for biomedical applications. Biofabrication 2015, 7, 015006. [Google Scholar] [CrossRef] [PubMed]
- Almazin, S.M.; Dziak, R.; Andreana, S.; Ciancio, S.G. The effect of doxycycline hyclate, chlorhexidine gluconate, and minocycline hydrochloride on osteoblastic proliferation and differentiation in vitro. J. Periodontol. 2009, 80, 999–1005. [Google Scholar] [CrossRef]
- Rifkin, B.R.; Vernillo, A.T.; Golub, L.M. Blocking Periodontal Disease Progression by Inhibiting Tissue-Destructive Enzymes: A Potential Therapeutic Role for Tetracyclines and Their Chemically-Modified Analogs. J. Periodontol. 1993, 64, 819–827. [Google Scholar] [CrossRef]
- Bettany, J.; Peet, N.; Wolowacz, R.; Skerry, T.; Grabowski, P. Tetracyclines induce apoptosis in osteoclasts. Bone 2000, 27, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, P.; Zhang, C.; An, B.; Zhu, Z. Tetracyclines Inhibit Rat Osteoclast Formation and Activity In Vitro and Affect Bone Turnover in Young Rats In Vivo. Calcif. Tissue Int. 2010, 86, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Kinugawa, S.; Koide, M.; Kobayashi, Y.; Mizoguchi, T.; Ninomiya, T.; Muto, A.; Kawahara, I.; Nakamura, M.; Yasuda, H.; Takahashi, N.; et al. Tetracyclines Convert the Osteoclastic-Differentiation Pathway of Progenitor Cells To Produce Dendritic Cell-like Cells. J. Immunol. 2012, 188, 1772–1781. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.S.; Fernandes, M.H. Effect of therapeutic levels of doxycycline and minocycline in the proliferation and differentiation of human bone marrow osteoblastic cells. Arch. Oral Biol. 2007, 52, 251–259. [Google Scholar] [CrossRef]
- Gomes, P.S.; Santos, J.D.; Fernandes, M.H. Cell-induced response by tetracyclines on human bone marrow colonized hydroxyapatite and Bonelike®. Acta Biomater. 2008, 4, 630–637. [Google Scholar] [CrossRef]
- Agwuh, K.N.; MacGowan, A. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J. Antimicrob. Chemother. 2006, 58, 256–265. [Google Scholar] [CrossRef]
- Nelson, M.L.; Levy, S.B. The history of the tetracyclines. Ann. N. Y. Acad. Sci. 2011, 1241, 17–32. [Google Scholar] [CrossRef]
- Del Rosso, J.Q. Sarecycline and the Narrow-Spectrum Tetracycline Concept: Currently Available Data and Potential Clinical Relevance in Dermatology. J. Clin. Aesthet. Dermatol. 2020, 13, 45–48. [Google Scholar]
- Monk, E.; Shalita, A.; Siegel, D.M. Clinical applications of non-antimicrobial tetracyclines in dermatology. Pharmacol. Res. 2011, 63, 130–145. [Google Scholar] [CrossRef] [PubMed]
- Caton, J.; Ryan, M.E. Clinical studies on the management of periodontal diseases utilizing subantimicrobial dose doxycycline (SDD). Pharmacol. Res. 2011, 63, 114–120. [Google Scholar] [CrossRef]
- Martin, V.; Grenho, L.; Fernandes, M.H.; Gomes, P.S. Repurposing sarecycline for osteoinductive therapies: An in vitro and ex vivo assessment. J. Bone Miner. Metab. 2023, 41, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Mohamed-Ahmed, S.; Fristad, I.; Lie, S.A.; Suliman, S.; Mustafa, K.; Vindenes, H.; Idris, S.B. Adipose-derived and bone marrow mesenchymal stem cells: A donor-matched comparison. Stem Cell Res. Ther. 2018, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, Y.; Sun, Y.; Wang, B.; Xiong, Y.; Lin, W.; Wei, Q.; Wang, H.; He, W.; Wang, B.; et al. Tissue source determines the differentiation potentials of mesenchymal stem cells: A comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem Cell Res. Ther. 2017, 8, 275. [Google Scholar] [CrossRef]
- Langenbach, F.; Handschel, J. Effects of dexamethasone, ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res. Ther. 2013, 4, 117. [Google Scholar] [CrossRef]
- Coelho, M.; Fernandes, M. Human bone cell cultures in biocompatibility testing. Part II: Effect of ascorbic acid, β-glycerophosphate and dexamethasone on osteoblastic differentiation. Biomaterials 2000, 21, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Jaganathan, B.G. Signaling network regulating osteogenesis in mesenchymal stem cells. J. Cell Commun. Signal. 2022, 16, 47–61. [Google Scholar] [CrossRef]
- Griffin, M.O.; Fricovsky, E.; Ceballos, G.; Villarreal, F. Tetracyclines: A pleitropic family of compounds with promising therapeutic properties. Review of the literature. Am. J. Physiol. Physiol. 2010, 299, C539–C548. [Google Scholar] [CrossRef]
- Griffin, M.O.; Ceballos, G.; Villarreal, F.J. Tetracycline compounds with non-antimicrobial organ protective properties: Possible mechanisms of action. Pharmacol. Res. 2011, 63, 102–107. [Google Scholar] [CrossRef]
- Bettany, J.T.; Wolowacz, R.G. Tetracycline Derivatives Induce Apoptosis Selectively in Cultured Monocytes and Macrophages but not in Mesenchymal Cells. Adv. Dent. Res. 1998, 12, 136–143. [Google Scholar] [CrossRef]
- Malaval, L.; Liu, F.; Roche, P.; Aubin, J.E. Kinetics of osteoprogenitor proliferation and osteoblast differentiation in vitro. J. Cell. Biochem. 1999, 74, 616–627. [Google Scholar] [CrossRef]
- Frank, O.; Heim, M.; Jakob, M.; Barbero, A.; Schäfer, D.; Bendik, I.; Dick, W.; Heberer, M.; Martin, I. Real-time quantitative RT-PCR analysis of human bone marrow stromal cells during osteogenic differentiation in vitro. J. Cell. Biochem. 2002, 85, 737–746. [Google Scholar] [CrossRef]
- Kartsogiannis, V.; Ng, K.W. Cell lines and primary cell cultures in the study of bone cell biology. Mol. Cell. Endocrinol. 2004, 228, 79–102. [Google Scholar] [CrossRef]
- Song, H.; Fares, M.; Maguire, K.R.; Sidén, Å.; Potácová, Z. Cytotoxic Effects of Tetracycline Analogues (Doxycycline, Minocycline and COL-3) in Acute Myeloid Leukemia HL-60 Cells. PLoS ONE 2014, 9, e114457. [Google Scholar] [CrossRef]
- Walters, B.; Uynuk-Ool, T.; Rothdiener, M.; Palm, J.; Hart, M.L.; Stegemann, J.P.; Rolauffs, B. Engineering the geometrical shape of mesenchymal stromal cells through defined cyclic stretch regimens. Sci. Rep. 2017, 7, 6640. [Google Scholar] [CrossRef]
- Pablo Rodríguez, J.; González, M.; Ríos, S.; Cambiazo, V. Cytoskeletal organization of human mesenchymal stem cells (MSC) changes during their osteogenic differentiation. J. Cell. Biochem. 2004, 93, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Whitfield, T.W.; Gordon, J.A.R.; Dobson, J.R.; Tai, P.W.L.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; Lian, J.B. Genomic occupancy of Runx2 with global expression profiling identifies a novel dimension to control of osteoblastogenesis. Genome Biol. 2014, 15, R52. [Google Scholar] [CrossRef]
- Wang, J.S.; Tokavanich, N.; Wein, M.N. SP7: From Bone Development to Skeletal Disease. Curr. Osteoporos. Rep. 2023, 21, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Rosset, E.M.; Bradshaw, A.D. SPARC/osteonectin in mineralized tissue. Matrix Biol. 2016, 52–54, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Whole Aspect of Runx2 Functions in Skeletal Development. Int. J. Mol. Sci. 2022, 23, 5776. [Google Scholar] [CrossRef]
- Ballhause, T.M.; Jiang, S.; Baranowsky, A.; Brandt, S.; Mertens, P.R.; Frosch, K.-H.; Yorgan, T.; Keller, J. Relevance of Notch Signaling for Bone Metabolism and Regeneration. Int. J. Mol. Sci. 2021, 22, 1325. [Google Scholar] [CrossRef]
- Luo, Z.; Shang, X.; Zhang, H.; Wang, G.; Massey, P.A.; Barton, S.R.; Kevil, C.G.; Dong, Y. Notch Signaling in Osteogenesis, Osteoclastogenesis, and Angiogenesis. Am. J. Pathol. 2019, 189, 1495–1500. [Google Scholar] [CrossRef] [PubMed]
- Dao, D.Y.; Yang, X.; Flick, L.M.; Chen, D.; Hilton, M.J.; O’Keefe, R.J. Axin2 regulates chondrocyte maturation and axial skeletal development. J. Orthop. Res. 2009, 28, 89–95. [Google Scholar] [CrossRef]
- Goodnough, L.H.; Chang, A.T.; Treloar, C.; Yang, J.; Scacheri, P.C.; Atit, R.P. Twist1 mediates repression of chondrogenesis by β-catenin to promote cranial bone progenitor specification. Development 2012, 139, 4428–4438. [Google Scholar] [CrossRef] [PubMed]
- Galli, C.; Piemontese, M.; Lumetti, S.; Manfredi, E.; Macaluso, G.; Passeri, G. The importance of WNT pathways for bone metabolism and their regulation by implant topography. Eur. Cells Mater. 2012, 24, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.S.; Resende, M.; Fernandes, M.H. Doxycycline restores the impaired osteogenic commitment of diabetic-derived bone marrow mesenchymal stromal cells by increasing the canonical WNT signaling. Mol. Cell. Endocrinol. 2020, 518, 110975. [Google Scholar] [CrossRef]
- Gomes, N.; Paula, A.; Nunes, N.; Góes, P.; Dutra, P. Doxycycline induces bone repair and changes in Wnt signalling. Int. J. Oral Sci. 2017, 9, 158–166. [Google Scholar] [CrossRef]
- Baht, G.S.; Silkstone, D.; Nadesan, P.; Whetstone, H.; Alman, B.A. Activation of hedgehog signaling during fracture repair enhances osteoblastic-dependent matrix formation. J. Orthop. Res. 2014, 32, 581–586. [Google Scholar] [CrossRef]
- Luo, M.; Huang, H.-X.; Huang, H.; Li, Z.-T.; Lai, Y.-Y. [Hedgehog signaling pathway and osteoporosis]. Zhongguo Gu Shang 2014, 27, 169–172. [Google Scholar]
- Nakamura, T.; Naruse, M.; Chiba, Y.; Komori, T.; Sasaki, K.; Iwamoto, M.; Fukumoto, S. Novel Hedgehog Agonists Promote Osteoblast Differentiation in Mesenchymal Stem Cells. J. Cell. Physiol. 2015, 230, 922–929. [Google Scholar] [CrossRef]
- Kwon, Y.; Park, C.; Lee, J.; Park, D.H.; Jeong, S.; Yun, C.-H.; Park, O.-J.; Han, S.H. Regulation of Bone Cell Differentiation and Activation by Microbe-Associated Molecular Patterns. Int. J. Mol. Sci. 2021, 22, 5805. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, W.; Huang, J.; He, B.-C.; Zuo, G.-W.; Zhang, W.; Luo, Q.; Shi, Q.; Zhang, B.-Q.; Wagner, E.R.; et al. Insulin-like growth factor 2 (IGF-2) potentiates BMP-9-induced osteogenic differentiation and bone formation. J. Bone Miner. Res. 2010, 25, 2447–2459. [Google Scholar] [CrossRef]
- Lai, S.-W.; Chen, J.-H.; Lin, H.-Y.; Liu, Y.-S.; Tsai, C.-F.; Chang, P.-C.; Lu, D.-Y.; Lin, C. Regulatory Effects of Neuroinflammatory Responses Through Brain-Derived Neurotrophic Factor Signaling in Microglial Cells. Mol. Neurobiol. 2018, 55, 7487–7499. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, J.A.; Sucher, A.J.; Chahine, E.B.; Shihadeh, K.C. Omadacycline: A New Tetracycline Antibiotic. Ann. Pharmacother. 2019, 53, 486–500. [Google Scholar] [CrossRef] [PubMed]
- Rusu, A.; Buta, E.L. The Development of Third-Generation Tetracycline Antibiotics and New Perspectives. Pharmaceutics 2021, 13, 2085. [Google Scholar] [CrossRef] [PubMed]


| Tetracycline-HCl | Doxycycline-HCl | Minocycline-HCl | Sarecycline-HCl | |
|---|---|---|---|---|
| Chemical Structure | 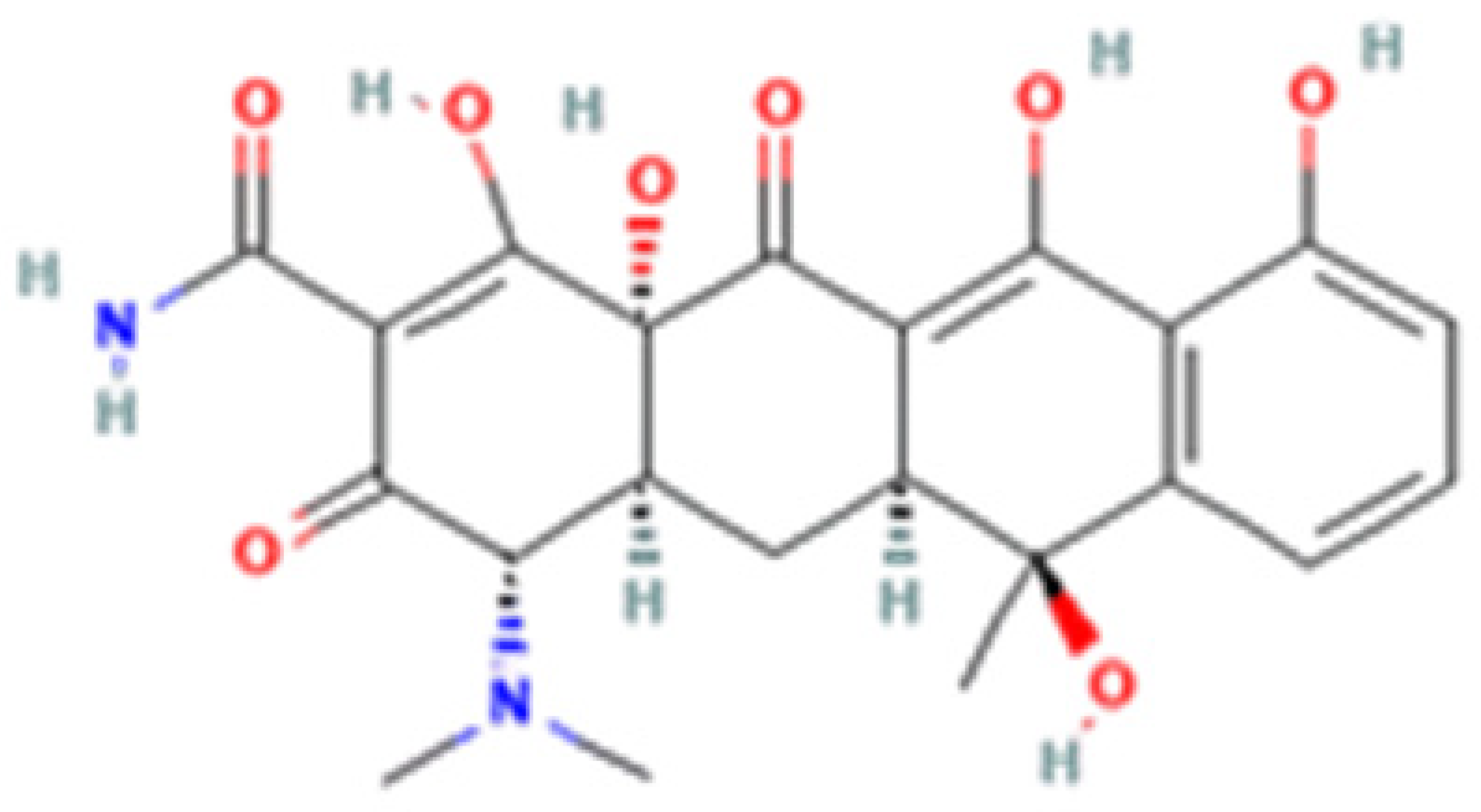 | 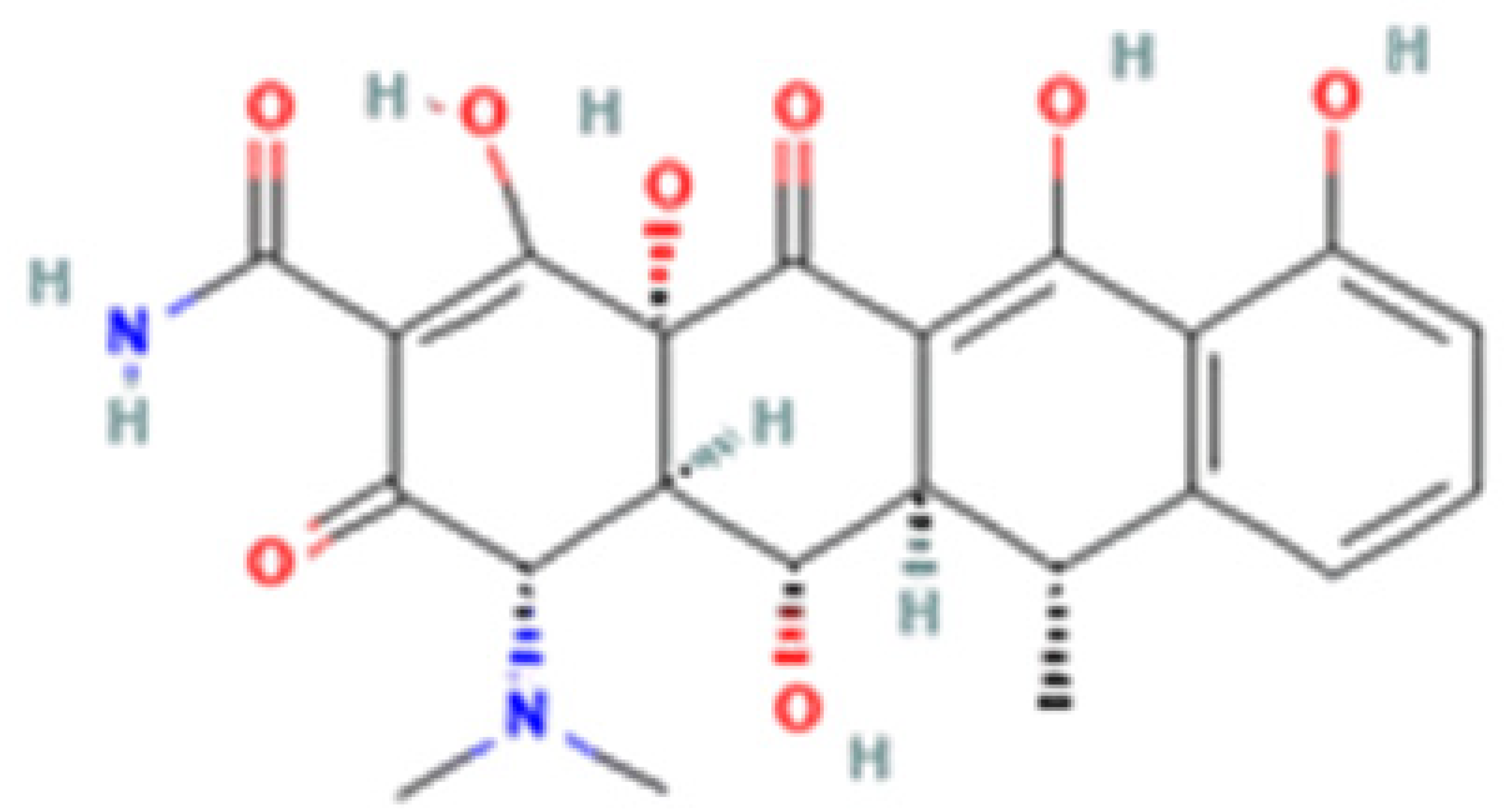 | 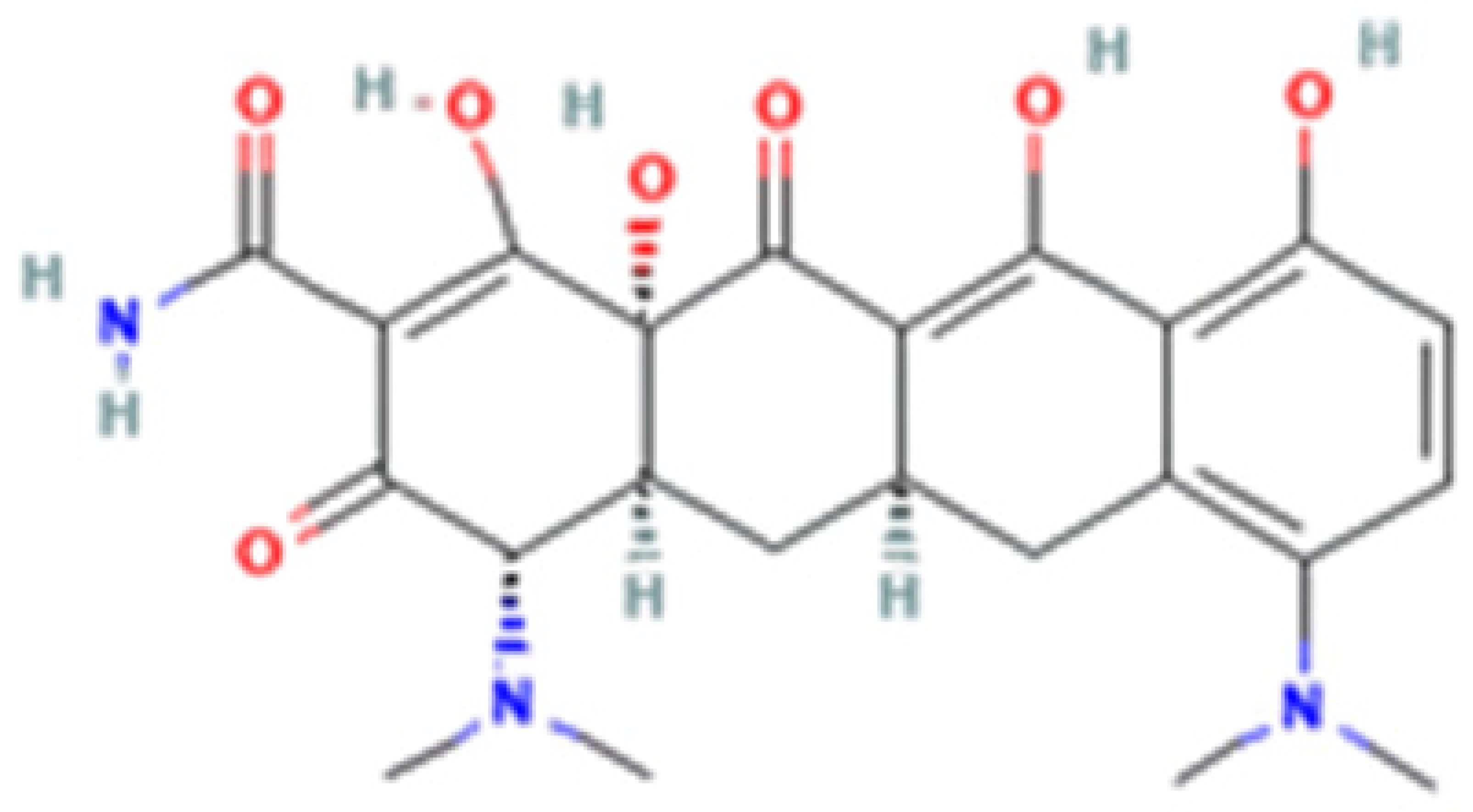 |  |
| Generation | 1st Generation | 2nd Generation | 3rd Generation | |
| Modification (to TC) | ___ | C5 (R4) addition of OH C6 (R2) removal of OH | C7 (R1) addition of N(CH3)2 C6 (R2 and R3) removal of OH and CH3 | C7 (R1) addition of methoxy-methyl-amino-methyl group C6 (R2 and R3) removal of OH and CH3 |
| Obtained from/Batch | Sigma-Aldrich/T8032 | Sigma-Aldrich/D3447 | Sigma-Aldrich/M9511 | Adooq Bioscience/P005672 |
| Gene | Assay ID | Gene | Assay ID |
|---|---|---|---|
| ACTB | qHsaCED0036269 | PTCH1 | qHsaCEP0055042 |
| RUNX2 | qHsaCED0044067 | GLI2 | qHsaCEP0057630 |
| COL1A1 | qHsaCED0043248 | HEY1 | qHsaCED0046240 |
| BGLAP | qHsaCED0038437 | HES1 | qHsaCED0006922 |
| SP7 | qHsaCED0003759 | AXIN2 | qHsaCID0017930 |
| SPARC | qHsaCID0010332 | TWIST1 | qHsaCED0003856 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, V.; Bettencourt, A.F.; Santos, C.; Fernandes, M.H.; Gomes, P.S. Unveiling the Osteogenic Potential of Tetracyclines: A Comparative Study in Human Mesenchymal Stem Cells. Cells 2023, 12, 2244. https://doi.org/10.3390/cells12182244
Martin V, Bettencourt AF, Santos C, Fernandes MH, Gomes PS. Unveiling the Osteogenic Potential of Tetracyclines: A Comparative Study in Human Mesenchymal Stem Cells. Cells. 2023; 12(18):2244. https://doi.org/10.3390/cells12182244
Chicago/Turabian StyleMartin, Victor, Ana Francisca Bettencourt, Catarina Santos, Maria Helena Fernandes, and Pedro Sousa Gomes. 2023. "Unveiling the Osteogenic Potential of Tetracyclines: A Comparative Study in Human Mesenchymal Stem Cells" Cells 12, no. 18: 2244. https://doi.org/10.3390/cells12182244
APA StyleMartin, V., Bettencourt, A. F., Santos, C., Fernandes, M. H., & Gomes, P. S. (2023). Unveiling the Osteogenic Potential of Tetracyclines: A Comparative Study in Human Mesenchymal Stem Cells. Cells, 12(18), 2244. https://doi.org/10.3390/cells12182244