Developmental Exposure to Kynurenine Affects Zebrafish and Rat Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Zebrafish
2.2. Rats
2.3. Chemicals
2.4. Biochemical Analysis
2.4.1. Tissue Sampling and Preparation
2.4.2. Determination of Tryptophan, KYN, and KYNA in Animal Plasma
2.4.3. Determination of KYN and KYNA in Zebrafish Medium
2.5. Behavioral Tests in Zebrafish
2.5.1. Determination of the Maximum Tolerated Concentration
2.5.2. Locomotor Activity
2.5.3. Light–Dark Transition Assay
2.6. Behavioral Tests in Rats
2.7. Statistical Analysis
3. Results
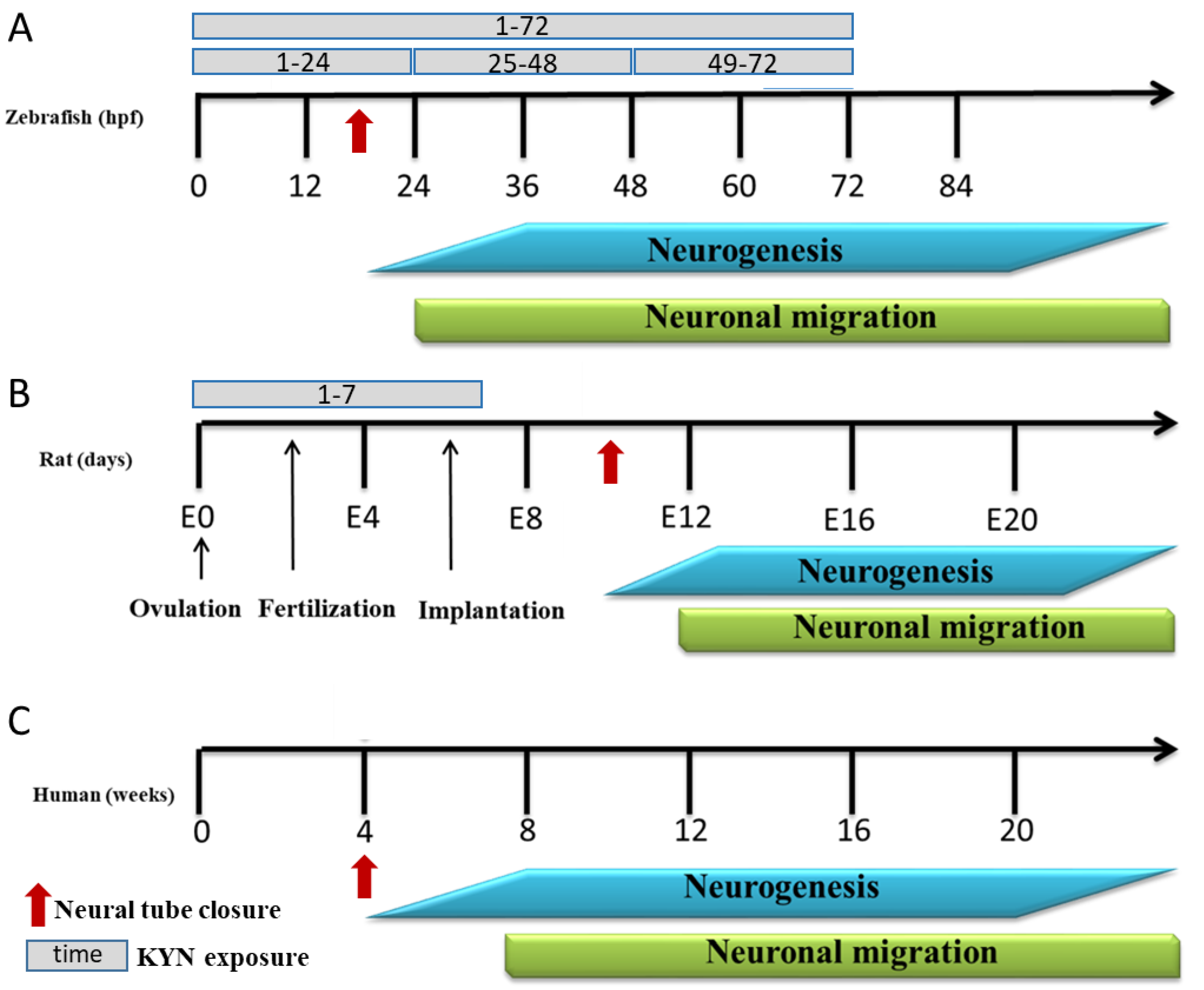
3.1. The Effect of Incubation with KYN at Different Periods on the Behavior of 5-Day-Old Larval Zebrafish
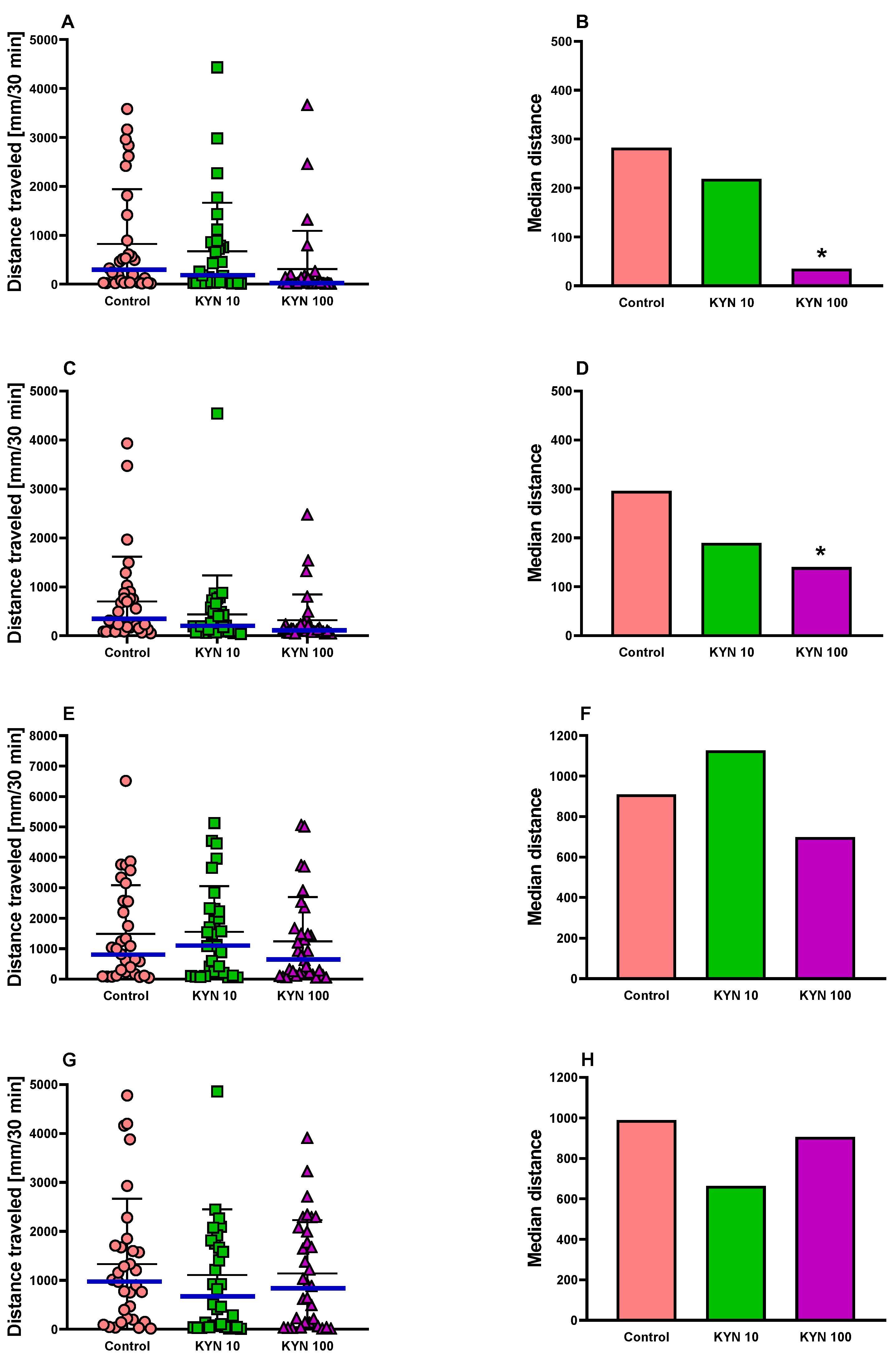
3.1.1. Locomotor Activity in Zebrafish
3.1.2. Light–Dark Transition Assay in Zebrafish
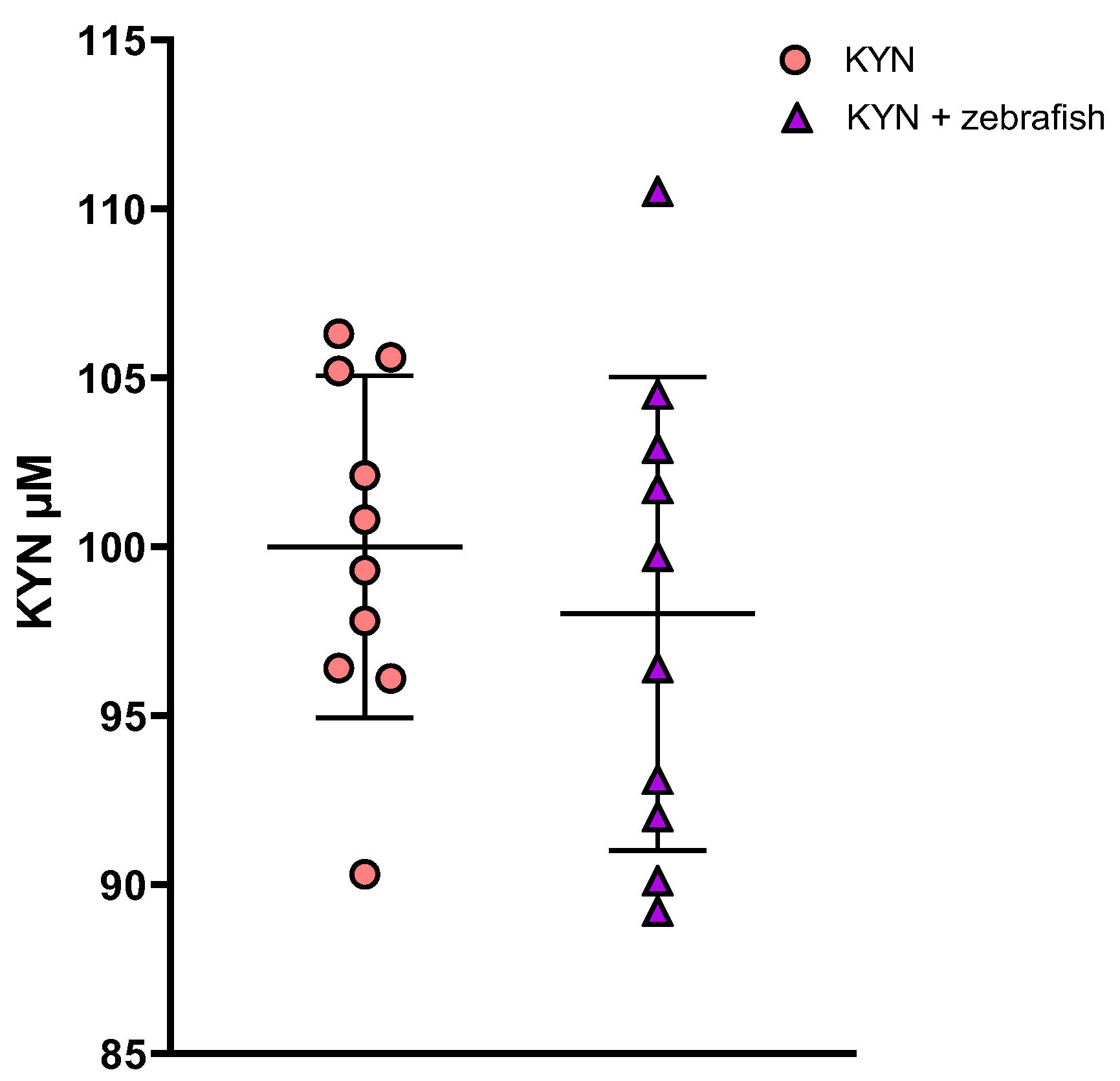
3.1.3. Determination of KYN and KYNA in Zebrafish Medium
3.2. The Effect of KYN on the Level of Tryptophan, KYN and KYNA in Rat Plasma
3.3. The Effect of KYN on the Body Mass Gain of Young and Adult Rats
3.4. The Effect of KYN on the Locomotor Activity of Adult Rats
3.5. The Effect of KYN on the Anxiety-like Behavior of Adult Rats
3.6. The Effect of KYN on the Spatial Memory and Associative Learning of Adult Rats
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, D.; Liu, J.; Yu, W.; Li, C.; Huang, L.; Mao, W.; Lu, Z. Tryptophan intake, not always the more the better. Front. Nutr. 2023, 10, 1140054. [Google Scholar] [CrossRef] [PubMed]
- King, N.J.; Thomas, S.R. Molecules in focus: Indoleamine 2,3-dioxygenase. Int. J. Biochem. Cell Biol. 2007, 39, 2167–2172. [Google Scholar] [CrossRef] [PubMed]
- Capece, L.; Lewis-Ballester, A.; Marti, M.A.; Estrin, D.A.; Yeh, S.R. Molecular basis for the substrate stereoselectivity in tryptophan dioxygenase. Biochemistry 2011, 50, 10910–10918. [Google Scholar] [CrossRef] [PubMed]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef]
- Leklem, J.E. Quantitative aspects of tryptophan metabolism in humans and other species: A review. Am. J. Clin. Nutr. 1971, 24, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A. The tryptophan utilization concept in pregnancy. Obstet. Gynecol. Sci. 2014, 57, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Teshigawara, T.; Mouri, A.; Kubo, H.; Nakamura, Y.; Shiino, T.; Okada, T.; Morikawa, M.; Nabeshima, T.; Ozaki, N.; Yamamoto, Y.; et al. Changes in tryptophan metabolism during pregnancy and postpartum periods: Potential involvement in postpartum depressive symptoms. J. Affect. Disord. 2019, 255, 168–176. [Google Scholar] [CrossRef]
- Erhardt, S.; Schwieler, L.; Imbeault, S.; Engberg, G. The kynurenine pathway in schizophrenia and bipolar disorder. Neuropharmacology 2017, 112, 297–306. [Google Scholar] [CrossRef]
- Notarangelo, F.M.; Pocivavsek, A. Elevated kynurenine pathway metabolism during neurodevelopment: Implications for brain and behavior. Neuropharmacology 2017, 112, 275–285. [Google Scholar] [CrossRef]
- Schwarcz, R.; Bruno, J.P.; Muchowski, P.J.; Wu, H.Q. Kynurenines in the mammalian brain: When physiology meets pathology. Nat. Rev. Neurosci. 2012, 13, 465–477. [Google Scholar] [CrossRef]
- Stone, T.W. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol. Rev. 1993, 45, 309–379. [Google Scholar] [PubMed]
- Stone, T.W. Kynurenic acid blocks nicotinic synaptic transmission to hippocampal interneurons in young rats. Eur. J. Neurosci. 2007, 25, 2656–2665. [Google Scholar] [CrossRef] [PubMed]
- Hilmas, C.; Pereira, E.F.; Alkondon, M.; Rassoulpour, A.; Schwarcz, R.; Albuquerque, E.X. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: Physiopathological implications. J. Neurosci. 2001, 21, 7463–7473. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W. Does kynurenic acid act on nicotinic receptors? An assessment of the evidence. J. Neurochem. 2020, 152, 627–649. [Google Scholar] [CrossRef] [PubMed]
- Oxenkrug, G.; van der Hart, M.; Roeser, J.; Summergrad, P. Anthranilic acid: A potential biomarker and treatment target for schizophrenia. Ann. Psychiatry Ment. Health 2016, 4, 1059. [Google Scholar]
- Wang, J.; Simonavicius, N.; Wu, X.; Swaminath, G.; Reagan, J.; Tian, H.; Ling, L. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J. Biol. Chem. 2006, 281, 22021–22028. [Google Scholar] [CrossRef]
- Shore, D.M.; Reggio, P.H. The therapeutic potential of orphan GPCRs, GPR35 and GPR55. Front. Pharmacol. 2015, 6, 69. [Google Scholar] [CrossRef]
- DiNatale, B.C.; Murray, I.A.; Schroeder, J.C.; Flaveny, C.A.; Lahoti, T.S.; Laurenzana, E.M.; Omiecinski, C.J.; Perdew, G.H. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol. Sci. 2010, 115, 89–97. [Google Scholar] [CrossRef]
- Yamamoto, J.; Ihara, K.; Nakayama, H.; Hikino, S.; Satoh, K.; Kubo, N.; Iida, T.; Fujii, Y.; Hara, T. Characteristic expression of aryl hydrocarbon receptor repressor gene in human tissues: Organ-specific distribution and variable induction patterns in mononuclear cells. Life Sci. 2004, 74, 1039–1049. [Google Scholar] [CrossRef]
- Murray, I.A.; Patterson, A.D.; Perdew, G.H. Aryl hydrocarbon receptor ligands in cancer: Friend and foe. Nat. Rev. Cancer 2014, 14, 801–814. [Google Scholar] [CrossRef]
- Stone, T.; Williams, R.O. Interactions of IDO and the kynurenine pathway with cell transduction Systems and metabolism at the inflammation–cancer interface. Cancers 2023, 15, 2895. [Google Scholar] [CrossRef] [PubMed]
- Turska, M.; Paluszkiewicz, P.; Turski, W.A.; Parada-Turska, J. A review of the health benefits of food enriched with kynurenic acid. Nutrients 2022, 14, 4182. [Google Scholar] [CrossRef] [PubMed]
- Solvay, M.; Holfelder, P.; Klaessens, S.; Pilotte, L.; Stroobant, V.; Lamy, J.; Naulaerts, S.; Spillier, Q.; Frédérick, R.; De Plaen, E.; et al. Tryptophan depletion sensitizes the AHR pathway by increasing AHR expression and GCN2/LAT1-mediated kynurenine uptake, and potentiates induction of regulatory T lymphocytes. J. Immunother. Cancer 2023, 11, e006728. [Google Scholar] [CrossRef] [PubMed]
- Goeden, N.; Notarangelo, F.M.; Pocivavsek, A.; Beggiato, S.; Bonnin, A.; Schwarcz, R. Prenatal dynamics of kynurenine pathway metabolism in mice: Focus on kynurenic acid. Dev. Neurosci. 2017, 39, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Pershing, M.L.; Bortz, D.M.; Pocivavsek, A.; Fredericks, P.J.; Jørgensen, C.V.; Vunck, S.A.; Leuner, B.; Schwarcz, R.; Bruno, J.P. Elevated levels of kynurenic acid during gestation produce neurochemical, morphological, and cognitive deficits in adulthood: Implications for schizophrenia. Neuropharmacology 2015, 90, 33–41. [Google Scholar] [CrossRef]
- Pocivavsek, A.; Wu, H.Q.; Elmer, G.I.; Bruno, J.P.; Schwarcz, R. Pre- and postnatal exposure to kynurenine causes cognitive deficits in adulthood. Eur. J. Neurosci. 2012, 35, 1605–1612. [Google Scholar] [CrossRef]
- Pocivavsek, A.; Thomas, M.A.; Elmer, G.I.; Bruno, J.P.; Schwarcz, R. Continuous kynurenine administration during the prenatal period, but not during adolescence, causes learning and memory deficits in adult rats. Psychopharmacology 2014, 231, 2799–2809. [Google Scholar] [CrossRef]
- Pocivavsek, A.; Elmer, G.I.; Schwarcz, R. Inhibition of kynurenine aminotransferase II attenuates hippocampus-dependent memory deficit in adult rats treated prenatally with kynurenine. Hippocampus 2019, 29, 73–77. [Google Scholar] [CrossRef]
- Alexander, K.S.; Pocivavsek, A.; Wu, H.Q.; Pershing, M.L.; Schwarcz, R.; Bruno, J.P. Early developmental elevations of brain kynurenic acid impair cognitive flexibility in adults: Reversal with galantamine. Neuroscience 2013, 238, 19–28. [Google Scholar] [CrossRef]
- Pershing, M.L.; Phenis, D.; Valentini, V.; Pocivavsek, A.; Lindquist, D.H.; Schwarcz, R.; Bruno, J.P. Prenatal kynurenine exposure in rats: Age-dependent changes in NMDA receptor expression and conditioned fear responding. Psychopharmacology 2016, 233, 3725–3735. [Google Scholar] [CrossRef]
- Milosavljevic, S.; Smith, A.K.; Wright, C.J.; Valafar, H.; Pocivavsek, A. Kynurenine aminotransferase II inhibition promotes sleep and rescues impairments induced by neurodevelopmental insult. Transl. Psychiatry 2023, 13, 106. [Google Scholar] [CrossRef]
- Marszalek-Grabska, M.; Stachniuk, A.; Iwaniak, P.; Gawel, K.; Sumara, A.; Kocki, T.; Fornal, E.; Milart, P.; Paluszkiewicz, P.; Turski, W. Unexpected content of kynurenine in mother’s milk and infant formulas. Sci. Rep. 2022, 12, 6464. [Google Scholar] [CrossRef] [PubMed]
- Forrest, C.M.; Khalil, O.S.; Pisar, M.; Darlington, L.G.; Stone, T.W. Prenatal inhibition of the tryptophan-kynurenine pathway alters synaptic plasticity and protein expression in the rat hippocampus. Brain Res. 2013, 1504, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pisar, M.; Forrest, C.M.; Khalil, O.S.; McNair, K.; Vincenten, M.C.; Qasem, S.; Darlington, L.G.; Stone, T.W. Modified neocortical and cerebellar protein expression and morphology in adult rats following prenatal inhibition of the kynurenine pathway. Brain Res. 2014, 1576, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Khalil, O.S.; Pisar, M.; Forrest, C.M.; Vincenten, M.C.; Darlington, L.G.; Stone, T.W. Prenatal inhibition of the kynurenine pathway leads to structural changes in the hippocampus of adult rat offspring. Eur. J. Neurosci. 2014, 39, 1558–1571. [Google Scholar] [CrossRef]
- Erhardt, S.; Pocivavsek, A.; Repici, M.; Liu, X.C.; Imbeault, S.; Maddison, D.C.; Thomas, M.A.R.; Smalley, J.L.; Larsson, M.K.; Muchowski, P.J.; et al. Adaptive and behavioral changes in kynurenine 3-monooxygenase knockout mice: Relevance to psychotic disorders. Biol. Psychiatry 2017, 82, 756–765. [Google Scholar] [CrossRef]
- Mu, J.; Zhou, Z.; Sang, Q.; Wang, L. The physiological and pathological mechanisms of early embryonic development. Fundam. Res. 2022, 2, 859–872. [Google Scholar] [CrossRef]
- DeSesso, J.M.; Scialli, A.R.; Holson, J.F. Apparent lability of neural tube closure in laboratory animals and humans. Am. J. Med. Genet. 1999, 87, 143–162. [Google Scholar] [CrossRef]
- Rice, D.; Barone, S., Jr. Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ. Health Perspect. 2000, 108, 511–533. [Google Scholar]
- Clarke, J. Role of polarized cell divisions in zebrafish neural tube formation. Curr. Opin. Neurobiol. 2009, 19, 134–138. [Google Scholar] [CrossRef]
- Pla, P.; Monsoro-Burq, A.H. The neural border: Induction, specification and maturation of the territory that generates neural crest cells. Dev. Biol. 2018, 444, S36–S46. [Google Scholar] [CrossRef] [PubMed]
- Gawel, K.; Langlois, M.; Martins, T.; van der Ent, W.; Tiraboschi, E.; Jacmin, M.; Crawford, A.D.; Esguerra, C.V. Seizing the moment: Zebrafish epilepsy models. Neurosci. Biobehav. Rev. 2020, 116, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Gawel, K.; Banono, N.S.; Michalak, A.; Esguerra, C.V. A critical review of zebrafish schizophrenia models: Time for validation? Neurosci. Biobehav. Rev. 2019, 107, 6–22. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.Y.; Kwon, H.B.; Ahn, J.C.; Kang, D.; Kwon, S.H.; Park, J.A.; Kim, K.W. Functional and developmental analysis of the blood-brain barrier in zebrafish. Brain Res. Bull. 2008, 75, 619–628. [Google Scholar] [CrossRef]
- Fleming, A.; Diekmann, H.; Goldsmith, P. Functional characterisation of the maturation of the blood-brain barrier in larval zebrafish. PLoS ONE 2013, 8, e77548. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Gao, P.; Zhu, D. Optimization of Zn2+-containing mobile phase for simultaneous determination of kynurenine, kynurenic acid and tryptophan in human plasma by high performance liquid chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2010, 878, 603–608. [Google Scholar] [CrossRef]
- Gawel, K.; Turski, W.A.; van der Ent, W.; Mathai, B.J.; Kirstein-Smardzewska, K.J.; Simonsen, A.; Esguerra, C.V. Phenotypic characterization of larval zebrafish (Danio rerio) with partial knockdown of the cacna1a gene. Mol. Neurobiol. 2020, 57, 1904–1916. [Google Scholar] [CrossRef]
- Nakonieczna, S.; Grabarska, A.; Gawel, K.; Wróblewska-Łuczka, P.; Czerwonka, A.; Stepulak, A.; Kukula-Koch, W. Isoquinoline alkaloids from coptis chinensis Franch: Focus on coptisine as a potential therapeutic candidate against gastric cancer cells. Int. J. Mol. Sci. 2022, 23, 10330. [Google Scholar] [CrossRef]
- Gawel, K.; Kukula-Koch, W.; Banono, N.S.; Nieoczym, D.; Targowska-Duda, K.M.; Czernicka, L.; Parada-Turska, J.; Esguerra, C.V. 6-Gingerol, a major constituent of zingiber officinale rhizoma, exerts anticonvulsant activity in the pentylenetetrazole-induced seizure model in larval zebrafish. Int. J. Mol. Sci. 2021, 22, 7745. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Préau, L.; Fini, J.B.; Morvan-Dubois, G.; Demeneix, B. Thyroid hormone signaling during early neurogenesis and its significance as a vulnerable window for endocrine disruption. Biochim. Biophys. Acta 2015, 1849, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Banono, N.S.; Gawel, K.; De Witte, L.; Esguerra, C.V. Zebrafish larvae carrying a splice variant mutation in cacna1d: A new model for schizophrenia-like behaviours? Mol. Neurobiol. 2021, 58, 877–894. [Google Scholar] [CrossRef]
- Faria, M.; Bellot, M.; Soto, O.; Prats, E.; Montemurro, N.; Manjarrés, D.; Gómez-Canela, C.; Raldúa, D. Developmental exposure to sertraline impaired zebrafish behavioral and neurochemical profiles. Front. Physiol. 2022, 13, 1040598. [Google Scholar] [CrossRef] [PubMed]
- Burton, C.E.; Zhou, Y.; Bai, Q.; Burton, E.A. Spectral properties of the zebrafish visual motor response. Neurosci. Lett. 2017, 646, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Klüver, N.; König, M.; Ortmann, J.; Massei, R.; Paschke, A.; Kühne, R.; Scholz, S. Fish embryo toxicity test: Identification of compounds with weak toxicity and analysis of behavioral effects to improve prediction of acute toxicity for neurotoxic compounds. Environ. Sci. Technol. 2015, 49, 7002–7011. [Google Scholar] [CrossRef]
- Irons, T.D.; MacPhail, R.C.; Hunter, D.L.; Padilla, S. Acute neuroactive drug exposures alter locomotor activity in larval zebrafish. Neurotoxicol. Teratol. 2010, 32, 84–90. [Google Scholar] [CrossRef]
- Souders, C.L., 2nd; Davis, R.H.; Qing, H.; Liang, X.; Febo, M.; Martyniuk, C.J. The psychoactive cathinone derivative pyrovalerone alters locomotor activity and decreases dopamine receptor expression in zebrafish (Danio rerio). Brain Behav. 2019, 9, e01420. [Google Scholar] [CrossRef]
- Peng, X.; Lin, J.; Zhu, Y.; Liu, X.; Zhang, Y.; Ji, Y.; Yang, X.; Zhang, Y.; Guo, N.; Li, Q. Anxiety-related behavioral responses of pentylenetetrazole-treated zebrafish larvae to light-dark transitions. Pharmacol. Biochem. Behav. 2016, 145, 55–65. [Google Scholar] [CrossRef]
- Maeda, H.; Hasumi, A.; Yoshida, K.I. Caffeine-induced bradycardia, death, and anxiety-like behavior in zebrafish larvae. Forensic Toxicol. 2021, 39, 427–436. [Google Scholar] [CrossRef]
- Yang, X.; Lin, J.; Peng, X.; Zhang, Q.; Zhang, Y.; Guo, N.; Zhou, S.; Li, Q. Effects of picrotoxin on zebrafish larvae behaviors: A comparison study with PTZ. Epilepsy Behav. 2017, 70, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Beischlag, T.V.; Luis Morales, J.; Hollingshead, B.D.; Perdew, G.H. The aryl hydrocarbon receptor complex and the control of gene expression. Crit. Rev. Eukaryot. Gene Expr. 2008, 18, 207–250. [Google Scholar] [CrossRef] [PubMed]
- Tanguay, R.L.; Abnet, C.C.; Heideman, W.; Peterson, R.E. Cloning and characterization of the zebrafish (Danio rerio) aryl hydrocarbon receptor. Biochim. Biophys. Acta (BBA)-Gene Struct. Expr. 1999, 1444, 35–48. [Google Scholar] [CrossRef]
- Andreasen, E.A.; Hahn, M.E.; Heideman, W.; Peterson, R.E.; Tanguay, R.L. The zebrafish (Danio rerio) aryl hydrocarbon receptor type 1 is a novel vertebrate receptor. Mol. Pharmacol. 2002, 62, 234–249. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.E.; Karchner, S.I.; Merson, R.R. Diversity as opportunity: Insights from 600 million years of AHR evolution. Curr. Opin. Toxicol. 2017, 2, 58–71. [Google Scholar] [CrossRef]
- Shankar, P.; Dasgupta, S.; Hahn, M.E.; Tanguay, R.L. A review of the functional roles of the zebrafish aryl hydrocarbon receptors. Toxicol. Sci. 2020, 178, 215–238. [Google Scholar] [CrossRef]
- Sugden, W.W.; Leonardo-Mendonça, R.C.; Acuña-Castroviejo, D.; Siekmann, A.F. Genetic dissection of endothelial transcriptional activity of zebrafish aryl hydrocarbon receptors (AHRs). PLoS ONE 2017, 12, e0183433. [Google Scholar] [CrossRef]
- Garcia, G.R.; Bugel, S.M.; Truong, L.; Spagnoli, S.; Tanguay, R.L. AHR2 required for normal behavioral responses and proper development of the skeletal and reproductive systems in zebrafish. PLoS ONE 2018, 13, e0193484. [Google Scholar] [CrossRef]
- Mathew, L.K.; Andreasen, E.A.; Tanguay, R.L. Aryl hydrocarbon receptor activation inhibits regenerative growth. Mol. Pharmacol. 2006, 69, 257–265. [Google Scholar] [CrossRef]
- Kubota, A.; Goldstone, J.V.; Lemaire, B.; Takata, M.; Woodin, B.R.; Stegeman, J.J. Role of pregnane X receptor and aryl hydrocarbon receptor in transcriptional regulation of pxr, CYP2, and CYP3 genes in developing zebrafish. Toxicol. Sci. 2015, 143, 398–407. [Google Scholar] [CrossRef]
- Prasch, A.L.; Tanguay, R.L.; Mehta, V.; Heideman, W.; Peterson, R.E. Identification of zebrafish ARNT1 homologs: 2,3,7,8-tetrachlorodibenzo-p-dioxin toxicity in the developing zebrafish requires ARNT1. Mol. Pharmacol. 2006, 69, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Antkiewicz, D.S.; Peterson, R.E.; Heideman, W. Blocking expression of AHR2 and ARNT1 in zebrafish larvae protects against cardiac toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 2006, 94, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Incardona, J.P.; Day, H.L.; Collier, T.K.; Scholz, N.L. Developmental toxicity of 4-ring polycyclic aromatic hydrocarbons in zebrafish is differentially dependent on AH receptor isoforms and hepatic cytochrome P4501A metabolism. Toxicol. Appl. Pharmacol. 2006, 217, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Mimura, J.; Fujii-Kuriyama, Y. Functional role of AhR in the expression of toxic effects by TCDD. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2003, 1619, 263–268. [Google Scholar] [CrossRef]
- Carney, S.A.; Prasch, A.L.; Heideman, W.; Peterson, R.E. Understanding dioxin developmental toxicity using the zebrafish model. Birth Defects Res. Part A Clin. Mol. Teratol. 2006, 76, 7–18. [Google Scholar] [CrossRef]
- Martin, N.R.; Patel, R.; Kossack, M.E.; Tian, L.; Camarillo, M.A.; Cintrón-Rivera, L.G.; Gawdzik, J.C.; Yue, M.S.; Nwagugo, F.O.; Elemans, L.M.H.; et al. Proper modulation of AHR signaling is necessary for establishing neural connectivity and oligodendrocyte precursor cell development in the embryonic zebrafish brain. Front. Mol. Neurosci. 2022, 15, 1032302. [Google Scholar] [CrossRef]
- Wincent, E.; Kubota, A.; Timme-Laragy, A.; Jönsson, M.E.; Hahn, M.E.; Stegeman, J.J. Biological effects of 6-formylindolo[3,2-b]carbazole (FICZ) in vivo are enhanced by loss of CYP1A function in an Ahr2-dependent manner. Biochem. Pharmacol. 2016, 110–111, 117–129. [Google Scholar] [CrossRef]
- Majewski, M.; Kasica, N.; Jakimiuk, A.; Podlasz, P. Toxicity and cardiac effects of acute exposure to tryptophan metabolites on the kynurenine pathway in early developing zebrafish (Danio rerio) embryos. Toxicol. Appl. Pharmacol. 2018, 341, 16–29. [Google Scholar] [CrossRef]
- Schmidt, R.; Strähle, U.; Scholpp, S. Neurogenesis in zebrafish-from embryo to adult. Neural Dev. 2013, 8, 3. [Google Scholar] [CrossRef]
- Wilson, S.W.; Ross, L.S.; Parrett, T.; Easter, S.S., Jr. The development of a simple scaffold of axon tracts in the brain of the embryonic zebrafish, Brachydanio rerio. Development 1990, 108, 121–145. [Google Scholar] [CrossRef]
- Liu, J.; Kong, W.; Liu, Y.; Ma, Q.; Shao, Q.; Zeng, L.; Chao, Y.; Song, X.; Zhang, J. Stage-related neurotoxicity of BPA in the development of zebrafish embryos. Toxics 2023, 11, 177. [Google Scholar] [CrossRef] [PubMed]
- Schwieler, L.; Trepci, A.; Krzyzanowski, S.; Hermansson, S.; Granqvist, M.; Piehl, F.; Venckunas, T.; Brazaitis, M.; Kamandulis, S.; Lindqvist, D.; et al. A novel, robust method for quantification of multiple kynurenine pathway metabolites in the cerebrospinal fluid. Bioanalysis 2020, 12, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.J.; Li, X.F.; Hu, J.Q.; Ni, X.J.; Lu, H.Y.; Wang, J.J.; Huang, X.N.; Lin, C.X.; Shang, D.W.; Wen, Y.G. A simple HPLC-MS/MS method for determination of tryptophan, kynurenine and kynurenic acid in human serum and its potential for monitoring antidepressant therapy. J. Anal. Toxicol. 2017, 41, 37–44. [Google Scholar] [CrossRef]
- Sadik, A.; Somarribas Patterson, L.F.; Öztürk, S.; Mohapatra, S.R.; Panitz, V.; Secker, P.F.; Pfänder, P.; Loth, S.; Salem, H.; Prentzell, M.T.; et al. IL4I1 Is a Metabolic immune checkpoint that activates the AHR and promotes tumor progression. Cell 2020, 182, 1252–1270.e34. [Google Scholar] [CrossRef] [PubMed]
- Kaya, B.; Doñas, C.; Wuggenig, P.; Diaz, O.E.; Morales, R.A.; Melhem, H.; Swiss IBD. Cohort Investigators; Hernández, P.P.; Kaymak, T.; Das, S.; et al. Lysophosphatidic acid-mediated GPR35 signaling in CX3CR1+ macrophages regulates intestinal homeostasis. Cell Rep. 2020, 32, 107979. [Google Scholar] [CrossRef]
- Milligan, G. G protein-coupled receptors not currently in the spotlight: Free fatty acid receptor 2 and GPR35. Br. J. Pharmacol. 2018, 175, 2543–2553. [Google Scholar] [CrossRef] [PubMed]
- Seok, S.H.; Ma, Z.X.; Feltenberger, J.B.; Chen, H.; Chen, H.; Scarlett, C.; Lin, Z.; Satyshur, K.A.; Cortopassi, M.; Jefcoate, C.R.; et al. Trace derivatives of kynurenine potently activate the aryl hydrocarbon receptor (AHR). J. Biol. Chem. 2018, 293, 1994–2005. [Google Scholar] [CrossRef] [PubMed]
- Hahn, B.; Reneski, C.H.; Pocivavsek, A.; Schwarcz, R. Prenatal kynurenine treatment in rats causes schizophrenia-like broad monitoring deficits in adulthood. Psychopharmacology 2018, 235, 651–661. [Google Scholar] [CrossRef]
- Buck, S.A.; Baratta, A.M.; Pocivavsek, A. Exposure to elevated embryonic kynurenine in rats: Sex-dependent learning and memory impairments in adult offspring. Neurobiol. Learn. Mem. 2020, 174, 107282. [Google Scholar] [CrossRef]
- Takahashi, T.; Nowakowski, R.S.; Caviness, V.S.J. Early ontogeny of the secondary proliferative population of the embryonic murine cerebral wall. J. Neurosci. 1995, 15, 6058–6068. [Google Scholar] [CrossRef]
- Marszalek-Grabska, M.; Walczak, K.; Gawel, K.; Wicha-Komsta, K.; Wnorowska, S.; Wnorowski, A.; Turski, W.A. Kynurenine emerges from the shadows—Current knowledge on its fate and function. Pharmacol Ther. 2021, 225, 107845. [Google Scholar] [CrossRef]
- Bubeníková-Valesová, V.; Horácek, J.; Vrajová, M.; Höschl, C. Models of schizophrenia in humans and animals based on inhibition of NMDA receptors. Neurosci. Biobehav. Rev. 2008, 32, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, H.Y.; Horiguchi, M.; Massey, B.W. The role of serotonin in the NMDA receptor antagonist models of psychosis and cognitive impairment. Psychopharmacology 2011, 213, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Gozzi, A.; Crestan, V.; Turrini, G.; Clemens, M.; Bifone, A. Antagonism at serotonin 5-HT(2A) receptors modulates functional activity of frontohippocampal circuit. Psychopharmacology 2010, 209, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Weisbrod, A.S.; Barry, E.S.; Graham, A.M.; Eklund, M.; Grunberg, N.E. Decreased BDNF in female but not male rats after exposure to stress: A sex-sensitive rat model of stress? Stress 2019, 22, 581–591. [Google Scholar] [CrossRef]
- Goodwill, H.L.; Manzano-Nieves, G.; Gallo, M.; Lee, H.I.; Oyerinde, E.; Serre, T.; Bath, K.G. Early life stress leads to sex differences in development of depressive-like outcomes in a mouse model. Neuropsychopharmacology 2019, 44, 711–720. [Google Scholar] [CrossRef]
- Meier, T.B.; Drevets, W.C.; Teague, T.K.; Wurfel, B.E.; Mueller, S.C.; Bodurka, J.; Dantzer, R.; Savitz, J. Kynurenic acid is reduced in females and oral contraceptive users: Implications for depression. Brain Behav. Immun. 2018, 67, 59–64. [Google Scholar] [CrossRef]
- Mason, M.; Gullekson, E.H. Estrogen-enzyme interactions: Inhibition and protection of kynurenine transaminase by the sulfate esters of diethylstilbestrol, estradiol, and estrone. J. Biol. Chem. 1960, 235, 1312–1316. [Google Scholar] [CrossRef]
- Bao, A.M.; Swaab, D.F. Sex differences in the brain, behavior, and neuropsychiatric disorders. Neuroscientist 2010, 16, 550–565. [Google Scholar] [CrossRef]
- Schwarcz, R.; Rassoulpour, A.; Wu, H.Q.; Medoff, D.; Tamminga, C.A.; Roberts, R.C. Increased cortical kynurenate content in schizophrenia. Biol. Psychiatry 2001, 50, 521–530. [Google Scholar] [CrossRef]
- Wonodi, I.; Stine, O.C.; Sathyasaikumar, K.V.; Roberts, R.C.; Mitchell, B.D.; Hong, L.E.; Kajii, Y.; Thaker, G.K.; Schwarcz, R. Downregulated kynurenine 3-monooxygenase gene expression and enzyme activity in schizophrenia and genetic association with schizophrenia endophenotypes. Arch. Gen. Psychiatry 2011, 68, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Savitz, J. The kynurenine pathway: A finger in every pie. Mol. Psychiatry 2020, 25, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, B.J.; Onishi, K.G.; Zucker, I. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 2014, 40, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Palanza, P.; Parmigiani, S. How does sex matter? Behavior, stress and animal models of neurobehavioral disorders. Neurosci. Biobehav. Rev. 2017, 76, 134–143. [Google Scholar] [CrossRef]
- Jiang, S.; Lin, L.; Guan, L.; Wu, Y. Selection of the male or female sex in chronic unpredictable mild stress-induced animal models of depression. Biomed. Res. Int. 2022, 2022, 2602276. [Google Scholar] [CrossRef]
- Jain, S.; Maltepe, E.; Lu, M.M.; Simon, C.; Bradfield, C.A. Expression of ARNT, ARNT2, HIF1 alpha, HIF2 alpha and Ah receptor mRNAs in the developing mouse. Mech. Dev. 1998, 73, 117–123. [Google Scholar] [CrossRef]
- Ganong, A.H.; Cotman, C.W. Kynurenic acid and quinolinic acid act at N-methyl-D-aspartate receptors in the rat hippocampus. J. Pharmacol. Exp. Ther. 1986, 236, 293–299. [Google Scholar]
- Schwarcz, R.; Stone, T.W. The kynurenine pathway and the brain: Challenges, controversies and promises. Neuropharmacology 2017, 112, 237–247. [Google Scholar] [CrossRef]
- Guidetti, P.; Schwarcz, R. 3-Hydroxykynurenine potentiates quinolinate but not NMDA toxicity in the rat striatum. Eur. J. Neurosci. 1999, 11, 3857–3863. [Google Scholar] [CrossRef]
- Guillemin, G.J. Quinolinic acid, the inescapable neurotoxin. FEBS J. 2012, 279, 1356–1365. [Google Scholar] [CrossRef]
- Venkatesan, D.; Iyer, M.; Narayanasamy, A.; Siva, K.; Vellingiri, B. Kynurenine pathway in Parkinson’s disease—An update. eNeurologicalSci 2020, 21, 100270. [Google Scholar] [CrossRef] [PubMed]
- Sathyasaikumar, K.V.; Tararina, M.; Wu, H.Q.; Neale, S.A.; Weisz, F.; Salt, T.E.; Schwarcz, R. Xanthurenic acid formation from 3-hydroxykynurenine in the mammalian brain: Neurochemical characterization and physiological effects. Neuroscience 2017, 367, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, H.R.; Agarwal, S.; Fulgoni, V.L., 3rd. Tryptophan intake in the US adult population is not related to liver or kidney function but is associated with depression and sleep outcomes. J. Nutr. 2016, 146, 2609S–2615S. [Google Scholar] [CrossRef] [PubMed]
- Castrogiovanni, P.; Musumeci, G.; Trovato, F.M.; Avola, R.; Magro, G.; Imbesi, R. Effects of high-tryptophan diet on pre- and postnatal development in rats: A morphological study. Eur. J. Nutr. 2014, 53, 297–308. [Google Scholar] [CrossRef]
- Musumeci, G.; Loreto, C.; Trovato, F.M.; Giunta, S.; Imbesi, R.; Castrogiovanni, P. Serotonin (5HT) expression in rat pups treated with high-tryptophan diet during fetal and early postnatal development. Acta Histochem. 2014, 116, 335–343. [Google Scholar] [CrossRef]
- Malaspina, D.; Corcoran, C.; Kleinhaus, K.R.; Perrin, M.C.; Fennig, S.; Nahon, D.; Friedlander, Y.; Harlap, S. Acute maternal stress in pregnancy and schizophrenia in offspring: A cohort prospective study. BMC Psychiatry 2008, 8, 71. [Google Scholar] [CrossRef]
- Motlagh, M.G.; Katsovich, L.; Thompson, N.; Lin, H.; Kim, Y.S.; Scahill, L.; Lombroso, P.J.; King, R.A.; Peterson, B.S.; Leckman, J.F. Severe psychosocial stress and heavy cigarette smoking during pregnancy: An examination of the pre- and perinatal risk factors associated with ADHD and Tourette syndrome. Eur. Child. Adolesc. Psychiatry 2010, 19, 755–764. [Google Scholar] [CrossRef]
- O’Donnell, K.J.; Glover, V.; Lahti, J.; Lahti, M.; Edgar, R.D.; Räikkönen, K.; O’Connor, T.G. Maternal prenatal anxiety and child COMT genotype predict working memory and symptoms of ADHD. PLoS ONE 2017, 12, e0177506. [Google Scholar] [CrossRef]
- Schwabe, L.; Bohbot, V.D.; Wolf, O.T. Prenatal stress changes learning strategies in adulthood. Hippocampus 2012, 22, 2136–2143. [Google Scholar] [CrossRef]
- Moura, C.A.; Cagni, F.C.; Costa, L.R.F.; Tiago, P.R.F.; Croyal, M.; Aguesse, A.; Reyes-Castro, L.A.; Zambrano, E.; Bolaños-Jiménez, F.; Gavioli, E.C. Maternal stress during pregnancy in mice induces sex-dependent behavioral alterations in offspring along with impaired serotonin and kynurenine pathways of tryptophan metabolism. Dev. Neurosci. 2022, 44, 603–614. [Google Scholar] [CrossRef]
- Fineberg, A.M.; Ellman, L.M.; Schaefer, C.A.; Maxwell, S.D.; Shen, L.; Chaudhury, N.H.; Cook, A.L.; Bresnahan, M.A.; Susser, E.S.; Brown, A.S. Fetal exposure to maternal stress and risk for schizophrenia spectrum disorders among offspring: Differential influences of fetal sex. Psychiatry Res. 2016, 236, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Olsen, J.; Vestergaard, M.; Obel, C. Attention-deficit/hyperactivity disorder in the offspring following prenatal maternal bereavement: A nationwide follow-up study in Denmark. Eur. Child Adolesc. Psychiatry 2010, 19, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Hao, J.H.; Tao, R.X.; Huang, K.; Jiang, X.M.; Zhu, Y.D.; Tao, F.B. Sex-specific and time-dependent effects of prenatal stress on the early behavioral symptoms of ADHD: A longitudinal study in China. Eur. Child Adolesc. Psychiatry 2015, 24, 1139–1147. [Google Scholar] [CrossRef]
- Van Lieshout, R.J.; Boylan, K. Increased depressive symptoms in female but not male adolescents born at low birth weight in the offspring of a national cohort. Can. J. Psychiatry 2010, 55, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Favaro, A.; Tenconi, E.; Degortes, D.; Manara, R.; Santonastaso, P. Neural correlates of prenatal stress in young women. Psychol. Med. 2015, 45, 2533–2543. [Google Scholar] [CrossRef] [PubMed]
- Iwata, M.; Ota, K.T.; Duman, R.S. The inflammasome: Pathways linking psychological stress, depression, and systemic illnesses. Brain Behav. Immun. 2013, 31, 105–114. [Google Scholar] [CrossRef]
- Rohleder, N. Stress and inflammation: The need to address the gap in the transition between acute and chronic stress effects. Psychoneuroendocrinology 2019, 105, 164–171. [Google Scholar] [CrossRef]
- Takikawa, O.; Tagawa, Y.; Iwakura, Y.; Yoshida, R.; Truscott, R.J. Interferon-gamma-dependent/independent expression of indoleamine 2,3-dioxygenase. Studies with interferon-gamma-knockout mice. Adv. Exp. Med. Biol. 1999, 467, 553–557. [Google Scholar]
- Popov, A.; Abdullah, Z.; Wickenhauser, C.; Saric, T.; Driesen, J.; Hanisch, F.G.; Domann, E.; Raven, E.L.; Dehus, O.; Hermann, C.; et al. Indoleamine 2,3-dioxygenase-expressing dendritic cells form suppurative granulomas following Listeria monocytogenes infection. J. Clin. Investig. 2006, 116, 3160–3170. [Google Scholar] [CrossRef]
- Maes, M.; Leonard, B.E.; Myint, A.M.; Kubera, M.; Verkerk, R. The new “5-HT” hypothesis of depression: Cell-mediated immune activation induces indoleamine 2, 3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 702–721. [Google Scholar]
- Erhardt, S.; Blennow, K.; Nordin, C.; Skogh, E.; Lindström, L.H.; Engberg, G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci. Lett. 2001, 313, 96–98. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Jeon, S.W. Neuroinflammation and the immune-kynurenine pathway in anxiety disorders. Curr. Neuropharmacol. 2018, 16, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Lovick, T.A.; Zangrossi, H., Jr. Effect of estrous cycle on behavior of females in rodent tests of anxiety. Front. Psychiatry 2021, 12, 711065. [Google Scholar] [CrossRef] [PubMed]

| Compound | Mother | Young Offspring | Adult Offspring | ||
|---|---|---|---|---|---|
| Male | Female | Male | Female | ||
| Tryptophan | ↑ | ↓ | = | ↓ | ↓ |
| KYN | ↑ | ↑ | ↑ | = | = |
| KYNA | ↑ | ↓ | = | = | = |
| Spontaneous | Amphetamine | Dizocylpine 0.15 | Dizocylpine 0.3 | ||
|---|---|---|---|---|---|
| 1 | Horizontal activity | = | = | = | ↓ |
| 2 | Total distance (cm) | = | = | = | ↓ |
| 3 | No. of movements | = | = | = | = |
| 4 | Movement time (s) | = | = | = | ↓ |
| 5 | Rest time (s) | = | ↑ | = | ↑ |
| 6 | Vertical activity | = | = | = | = |
| 7 | No. of vertical movements | = | = | = | = |
| 8 | Vertical time (s) | = | ↓ | = | = |
| 9 | Stereotypy counts | = | = | = | ↓ |
| 10 | No. of stereotypy | = | ↓ | = | = |
| 11 | Stereotypy time (s) | = | = | = | ↓ |
| 12 | Margin distance (cm) | = | = | = | = |
| 13 | Margin time (s) | = | = | = | = |
| 14 | Center distance (cm) | = | = | = | = |
| 15 | Center time (s) | = | = | = | = |
| 16 | Horizontal activity + Vertical activity | = | = | = | ↓ |
| 17 | Horizontal activity/Vertical activity | = | = | = | = |
| 18 | No of movements + No of vertical movements | = | = | = | = |
| 19 | No of movements/No of vertical movements | = | = | = | = |
| 20 | Movement time + Vertical time | = | = | = | ↓ |
| 21 | Movement time/Vertical time | = | = | = | = |
| 22 | Horizontal activity + Vertical activity/Stereotypy counts | = | ↑ | = | ↑ |
| 23 | No of movements + No of vertical movements/No of stereotypy | = | = | = | = |
| 24 | Movement time + Vertical time/stereotypy time | = | ↑ | = | = |
| 25 | Marigin distance/Center distance | = | = | = | = |
| 26 | Marigin time/Center time | = | = | = | = |
| Spontaneous | Amphetamine | Dizocylpine 0.075 | Dizocylpine 0.15 | ||
|---|---|---|---|---|---|
| 1 | Horizontal activity | = | = | = | = |
| 2 | Total distance (cm) | = | = | = | = |
| 3 | No. of movements | = | = | = | = |
| 4 | Movement time (s) | = | = | = | = |
| 5 | Rest time (s) | = | = | = | = |
| 6 | Vertical activity | ↓ | = | = | ↓ |
| 7 | No. of vertical movements | ↓ | = | = | ↓ |
| 8 | Vertical time (s) | ↓ | = | = | ↓ |
| 9 | Stereotypy counts | = | = | ↑ | = |
| 10 | No. of stereotypy | = | = | = | = |
| 11 | Stereotypy time (s) | = | = | ↑ | = |
| 12 | Margin distance (cm) | = | = | = | = |
| 13 | Margin time (s) | ↑ | = | = | ↑ |
| 14 | Center distance (cm) | = | = | = | ↓ |
| 15 | Center time (s) | = | = | = | ↓ |
| 16 | Horizontal activity + Vertical activity | = | = | = | = |
| 17 | Horizontal activity/Vertical activity | ↑ | = | = | = |
| 18 | No of movements + No of vertical movements | = | = | = | = |
| 19 | No of movements/No of vertical movements | = | = | = | = |
| 20 | Movement time + Vertical time | ↓ | = | = | = |
| 21 | Movement time/Vertical time | ↑ | = | = | = |
| 22 | Horizontal activity + Vertical activity/Stereotypy counts | = | ↑ | ↓ | = |
| 23 | No of movements + No of vertical movements/No of stereotypy | ↓ | = | = | = |
| 24 | Movement time + Vertical time/Stereotypy time | ↓ | = | = | = |
| 25 | Marigin distance/Center distance | = | = | = | ↑ |
| 26 | Marigin time/Center time | = | = | = | = |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marszalek-Grabska, M.; Gawel, K.; Kosheva, N.; Kocki, T.; Turski, W.A. Developmental Exposure to Kynurenine Affects Zebrafish and Rat Behavior. Cells 2023, 12, 2224. https://doi.org/10.3390/cells12182224
Marszalek-Grabska M, Gawel K, Kosheva N, Kocki T, Turski WA. Developmental Exposure to Kynurenine Affects Zebrafish and Rat Behavior. Cells. 2023; 12(18):2224. https://doi.org/10.3390/cells12182224
Chicago/Turabian StyleMarszalek-Grabska, Marta, Kinga Gawel, Nataliia Kosheva, Tomasz Kocki, and Waldemar A. Turski. 2023. "Developmental Exposure to Kynurenine Affects Zebrafish and Rat Behavior" Cells 12, no. 18: 2224. https://doi.org/10.3390/cells12182224
APA StyleMarszalek-Grabska, M., Gawel, K., Kosheva, N., Kocki, T., & Turski, W. A. (2023). Developmental Exposure to Kynurenine Affects Zebrafish and Rat Behavior. Cells, 12(18), 2224. https://doi.org/10.3390/cells12182224







