Rat Plantar Fascia Stem/Progenitor Cells Showed Lower Expression of Ligament Markers and Higher Pro-Inflammatory Cytokines after Intensive Mechanical Loading or Interleukin-1β Treatment In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cell Culture Media
- Growth medium was prepared by supplementing Minimum Essential Medium α (αMEM) with 10% fetal bovine serum (FBS) and 1% Penicillin/Streptomycin (PS) (Life Technologies, Hewlett, NY, USA).
- Control medium for the differentiation assays was prepared by supplementing Dulbecco’s Low Glucose Modified Eagle Medium (LG-DMEM) (Sigma-Aldrich, St. Louis, MO, USA) with 10% FBS and 1% PS.
- Osteogenic medium was prepared by supplementing LG-DMEM with 1 nM dexamethasone, 50 µM ascorbic acid, and 20 mM β-glycerophosphate disodium salt hydrate (BGP) (all from Sigma-Aldrich, St. Louis, MO, USA).
- Adipogenic medium was prepared by supplementing LG-DMEM with 500 nM dexamethasone, 0.5 mM isobutylmethylxanthine, 50 µM indomethacin, and 10 µg/mL insulin (all from Sigma-Aldrich, St. Louis, MO, USA).
- Chondrogenic medium was prepared by supplementing LG-DMEM with 100 nM dexamethasone, 50 µg/mL L-ascorbic acid-2-phosphate, 40 µg/mL proline, and 100 µg/mL pyruvate (all from Sigma-Aldrich, St. Louis, MO, USA), 1:100 diluted ITS + Premix (6.25 mg/mL insulin, 6.25 mg/mL transferrin, 6.25 mg/mL selenious acid, 1.25 mg/mL bovine serum albumin, and 5.35 mg/mL linoleic acid) (Becton Dickinson, Franklin Lakes, NJ, USA), 500 ng/mL bone morphogenetic protein (BMP-2) and 10 ng/mL transforming growth factor beta 3 (TGF-β3) (both from R&D Systems, Minneapolis, MN, USA).
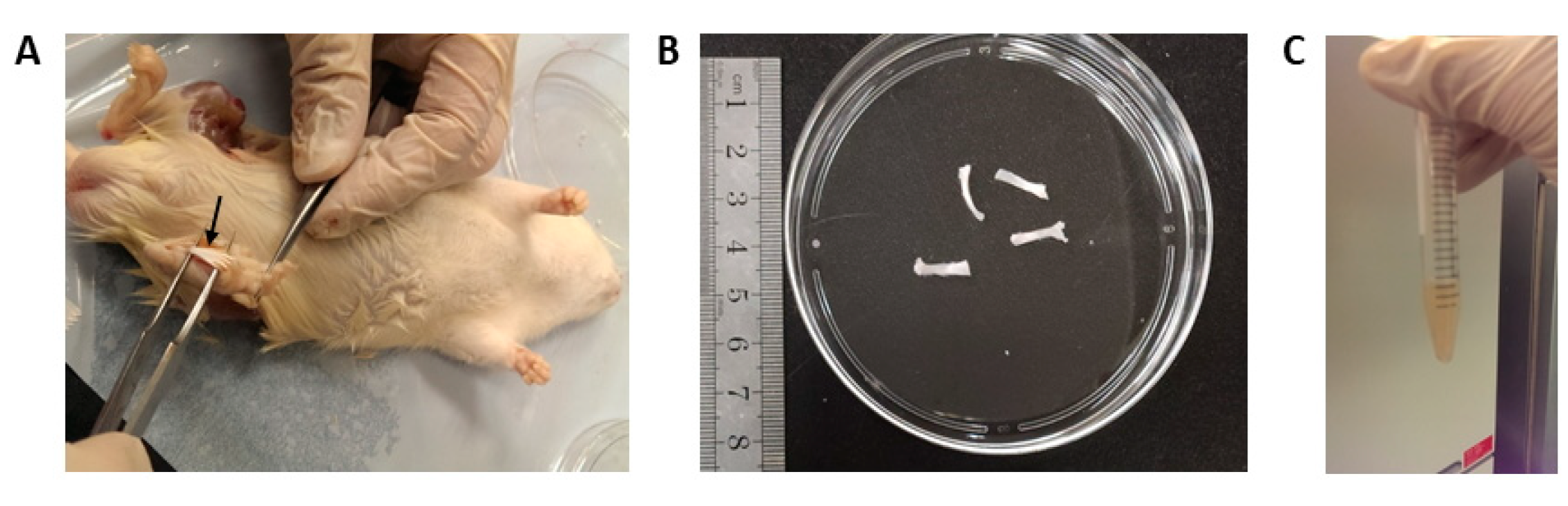
2.3. Isolation and Culture of Rat PFSCs and BMSCs
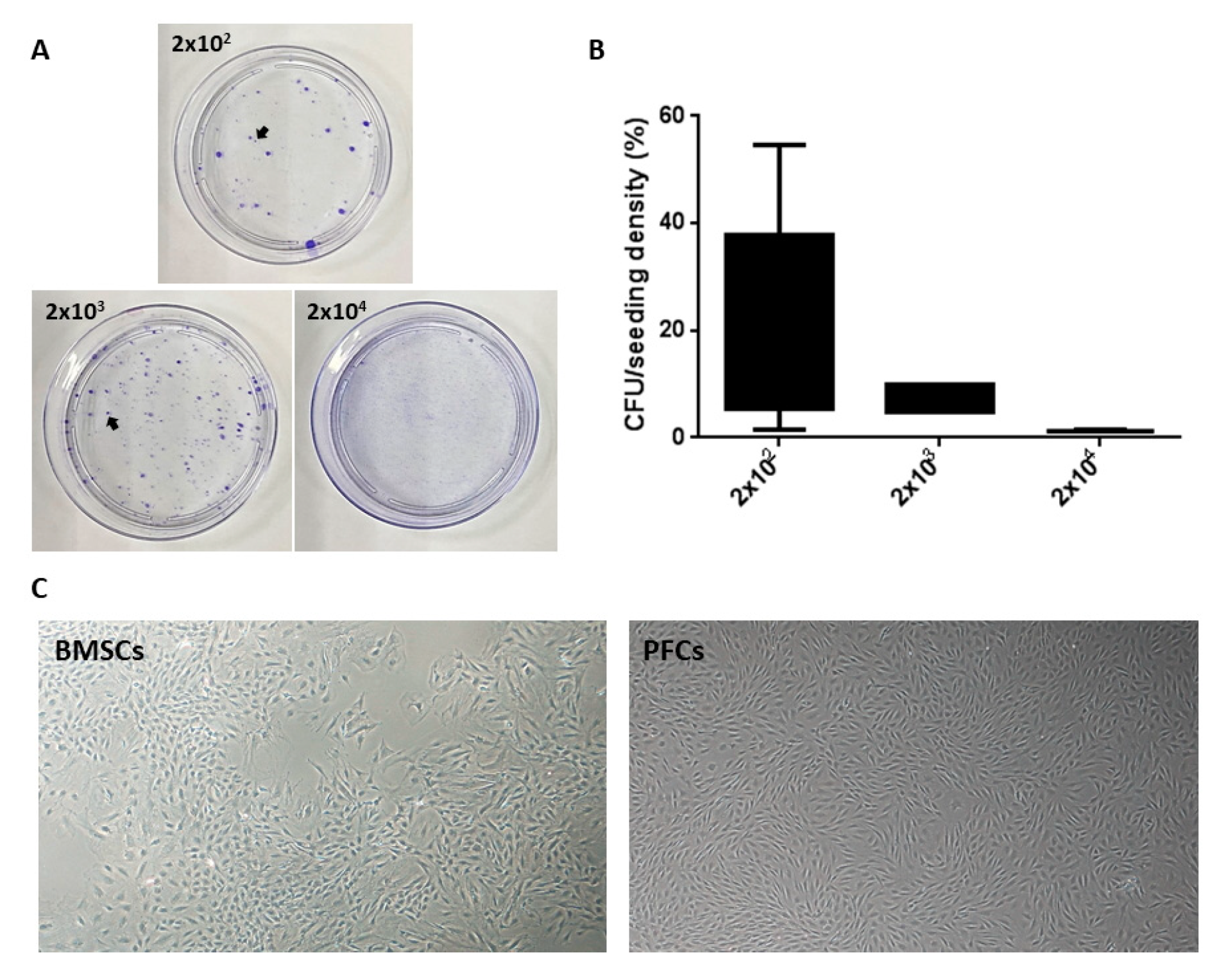
2.4. Colony-Forming Assay
2.5. Cell Counting
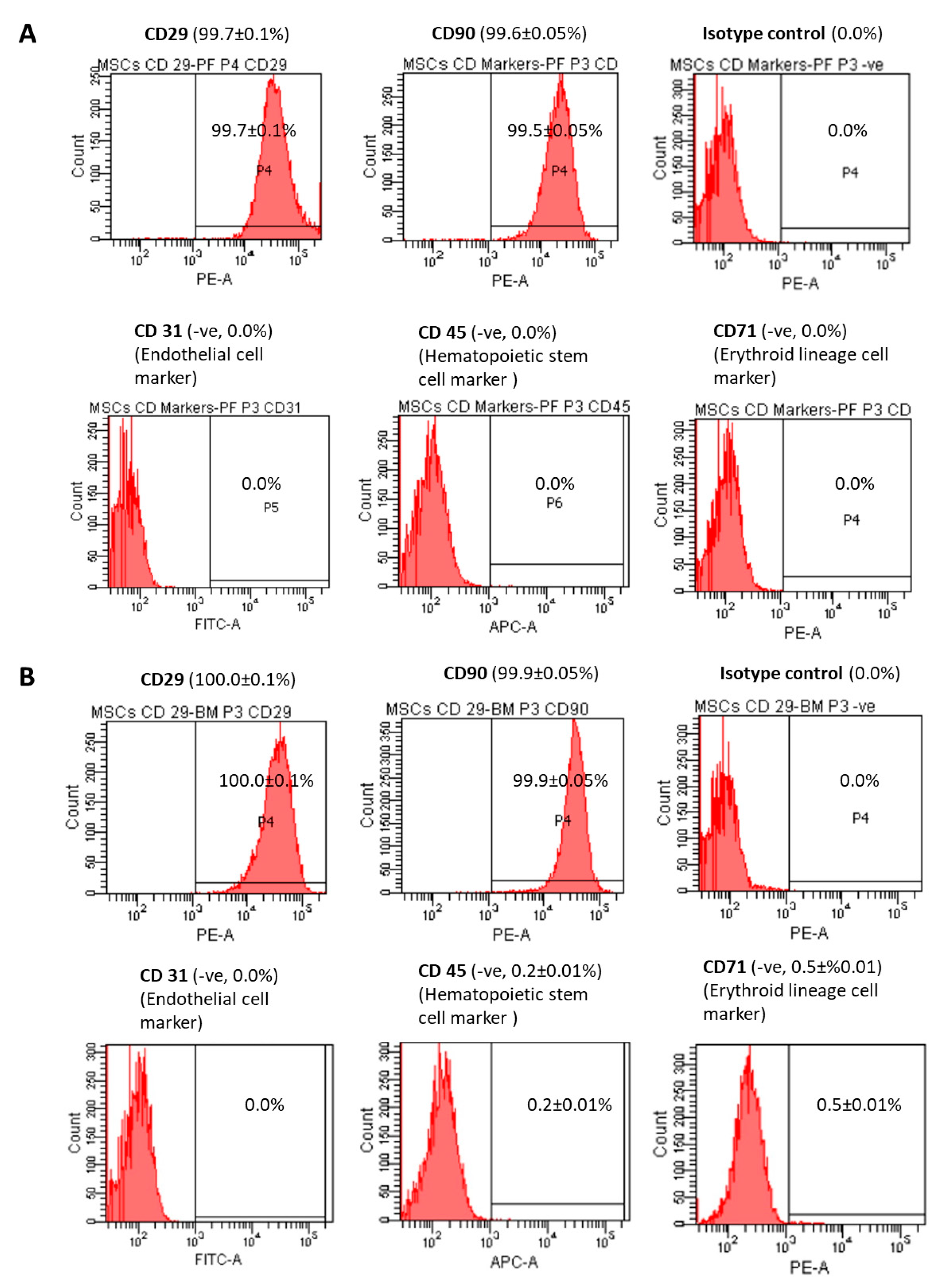
2.6. Analysis of Immunophenotypes
2.7. Immocytochemical Staining of Stemness Markers and Proliferation Marker
2.8. mRNA Expression of Stemness Markers
2.9. Multi-Lineage Differentiation Potential
2.10. Expression of Ligament Markers
2.11. Effects of IL-1β
2.12. Effects of Intensive Mechanical Loading
2.13. Data Analysis
3. Results
3.1. Optimal Seeding Density for PFSC Isolation
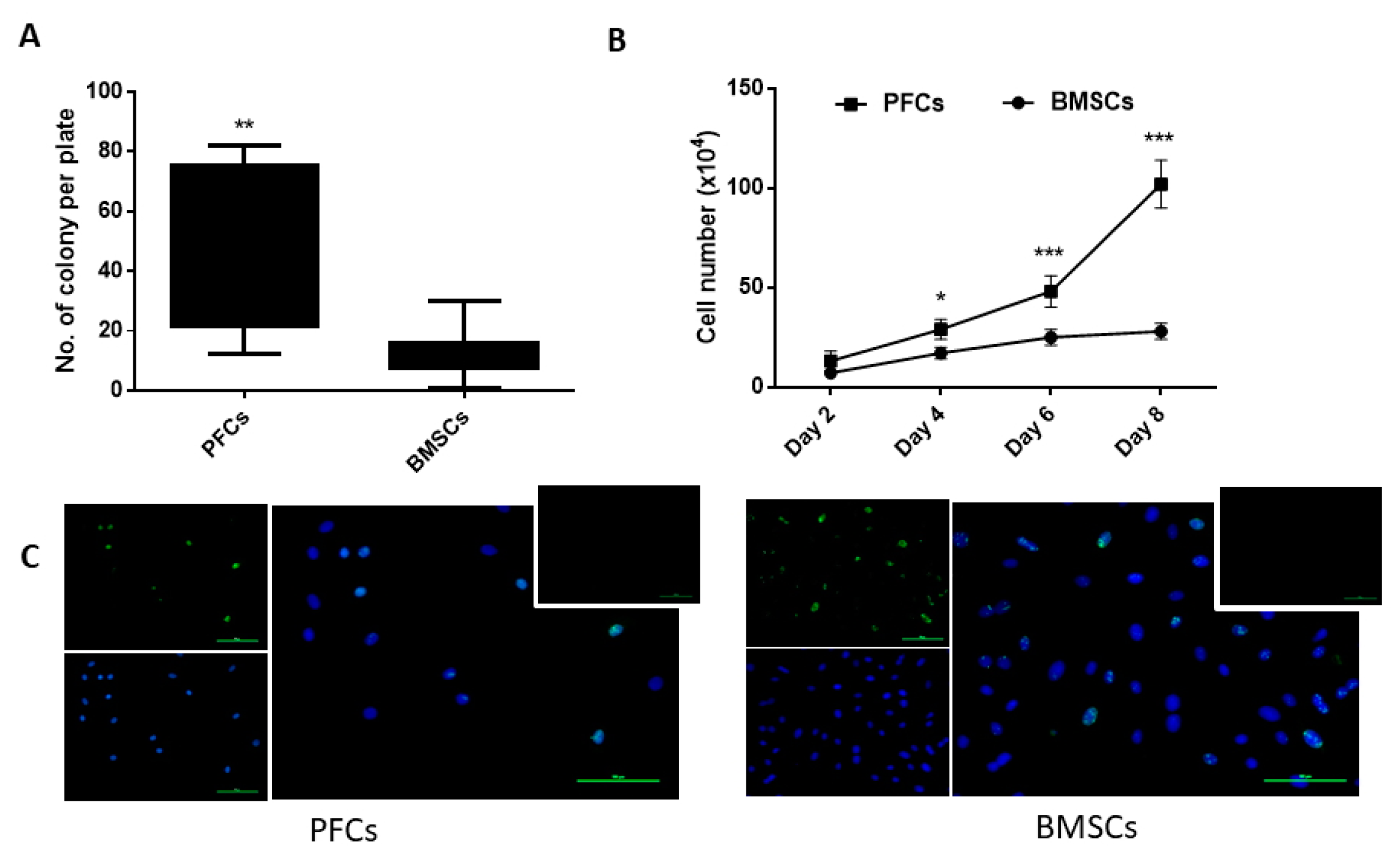
3.2. Clonogenicity
3.3. Proliferative Potential
3.4. Immunophenotypes
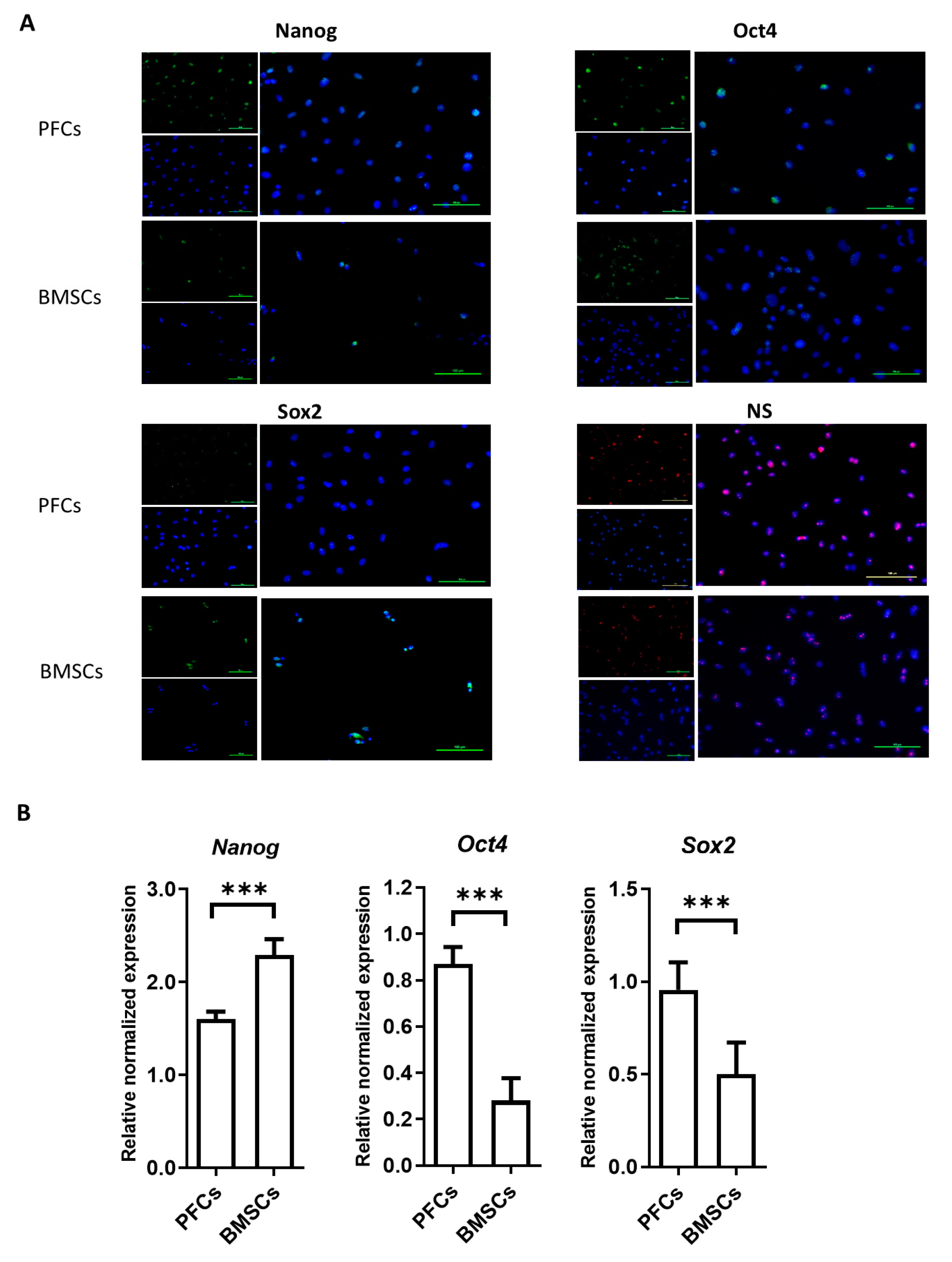
3.5. Protein and mRNA Expression of Stemness Markers
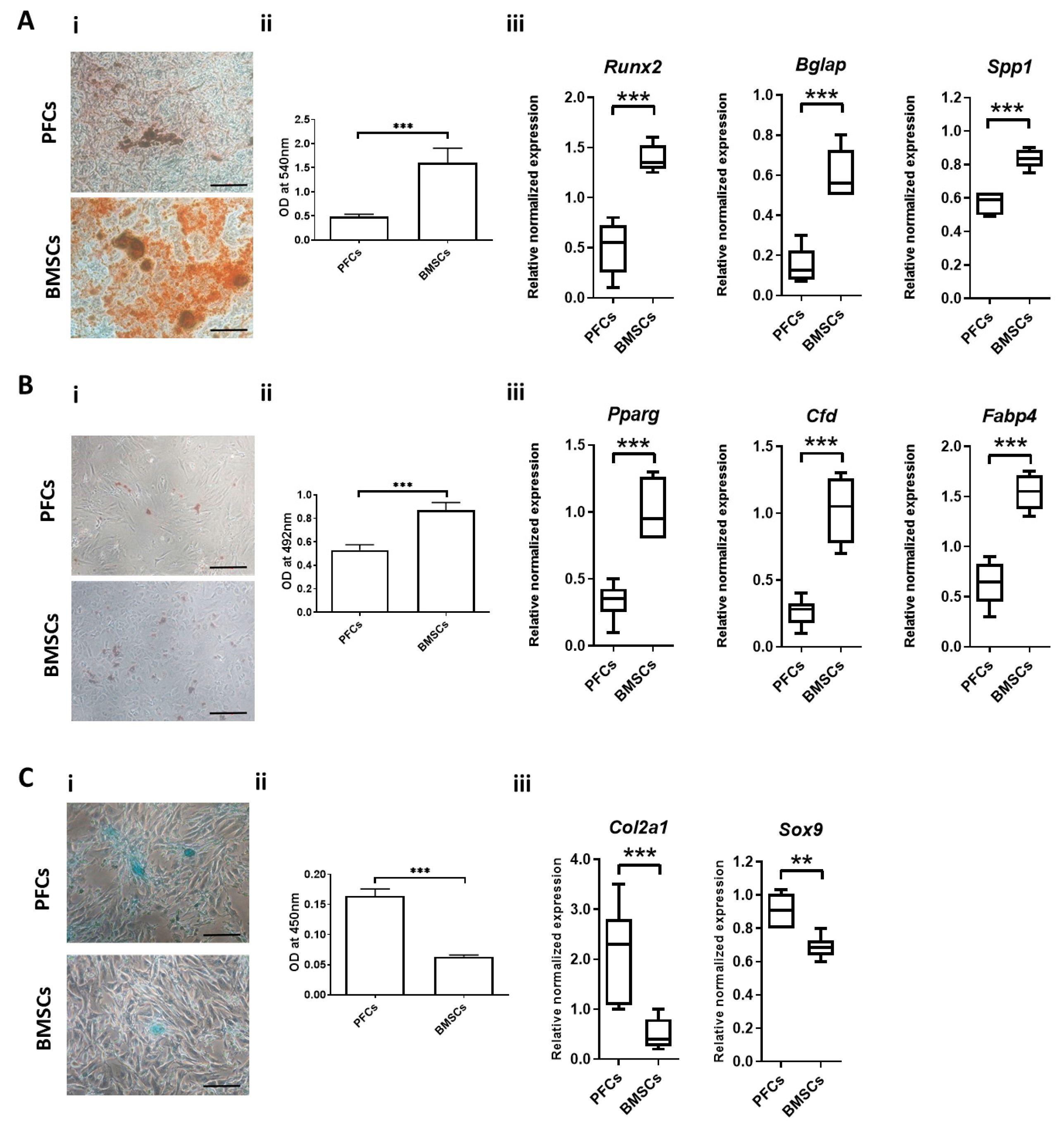
3.6. Multi-Lineage Differentiation Potential
3.6.1. Osteogenic Differentiation
3.6.2. Adipogenic Differentiation
3.6.3. Chondrogenic Differentiation
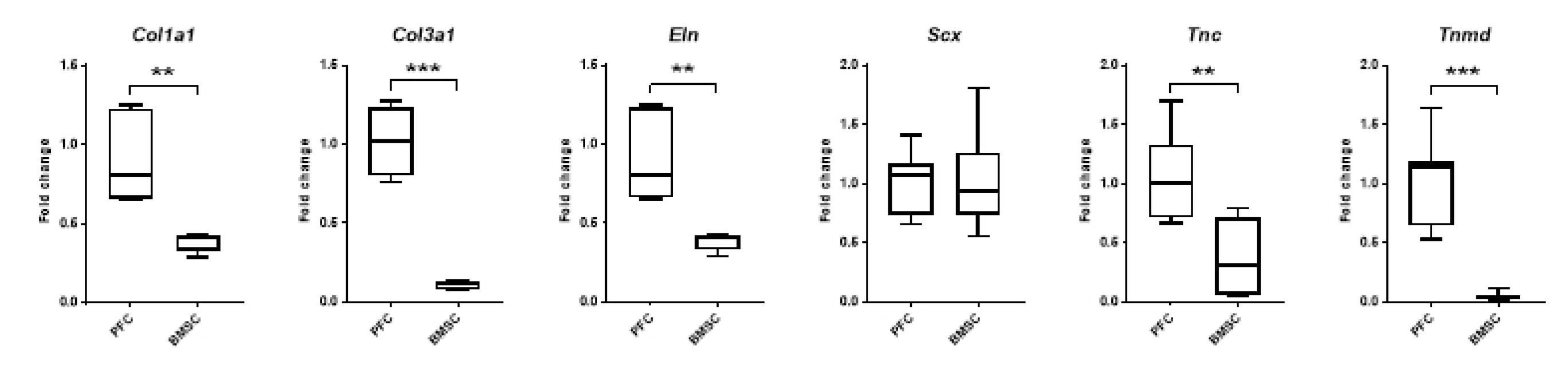
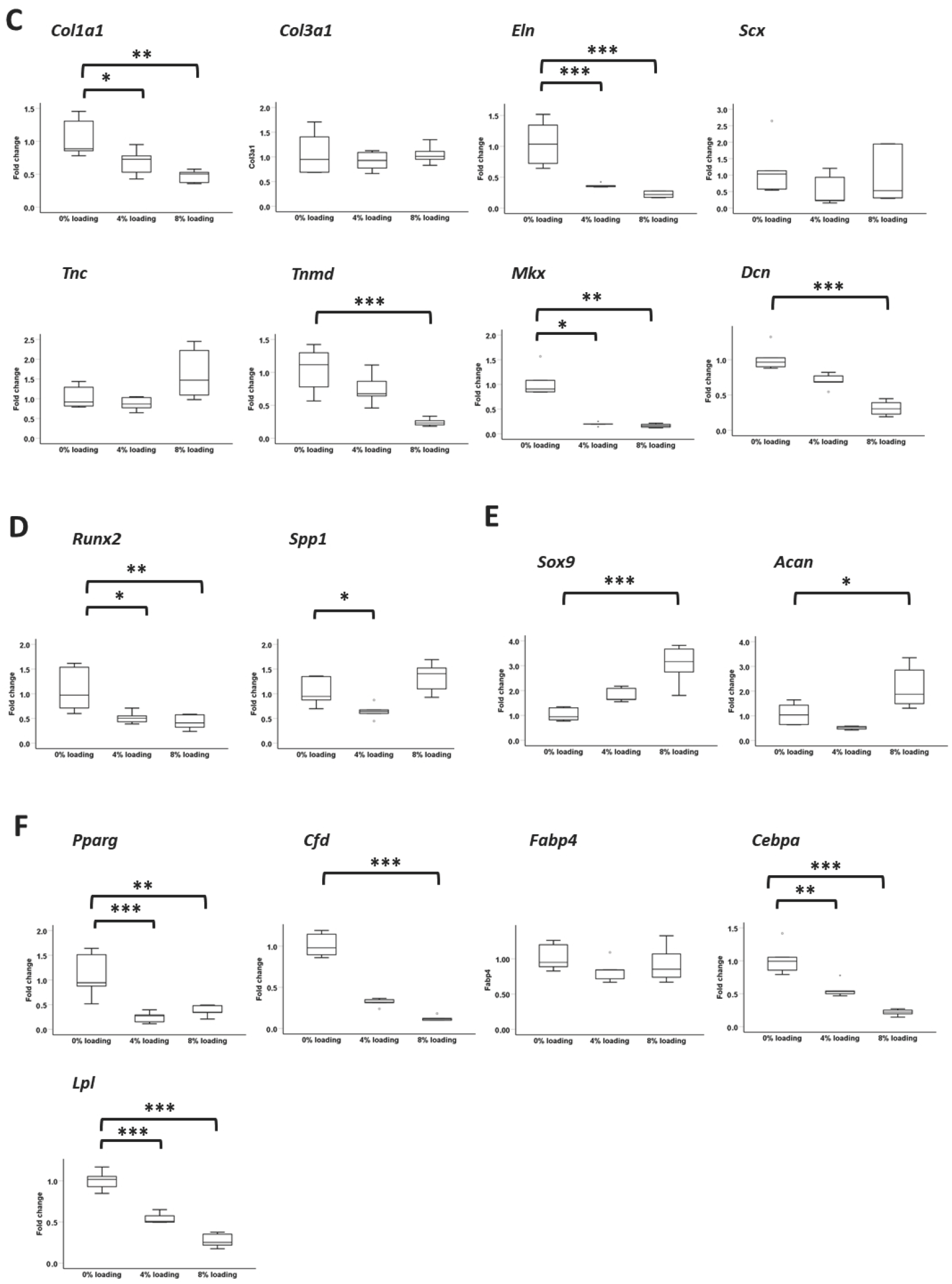
3.6.4. Expression of Ligament Markers
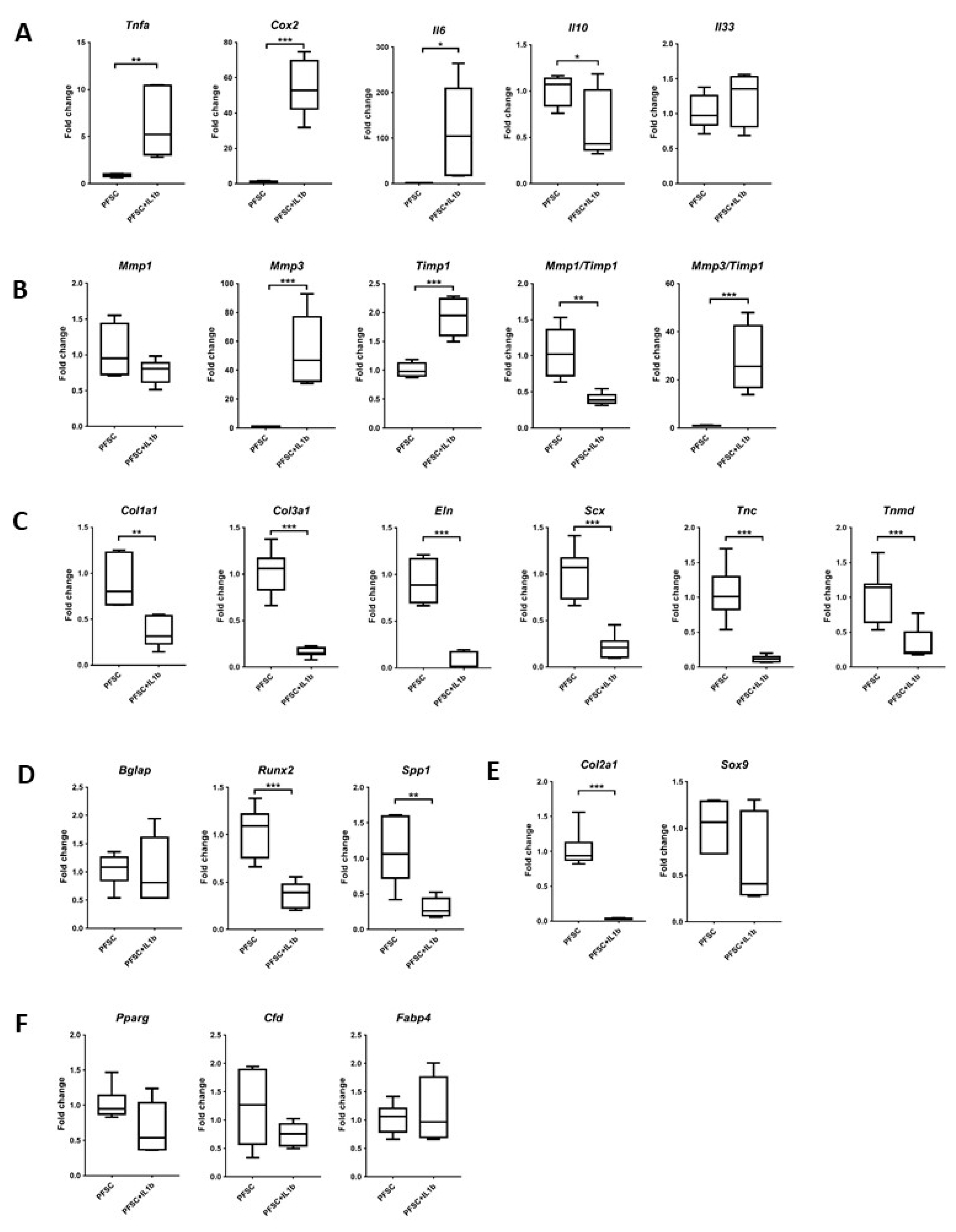
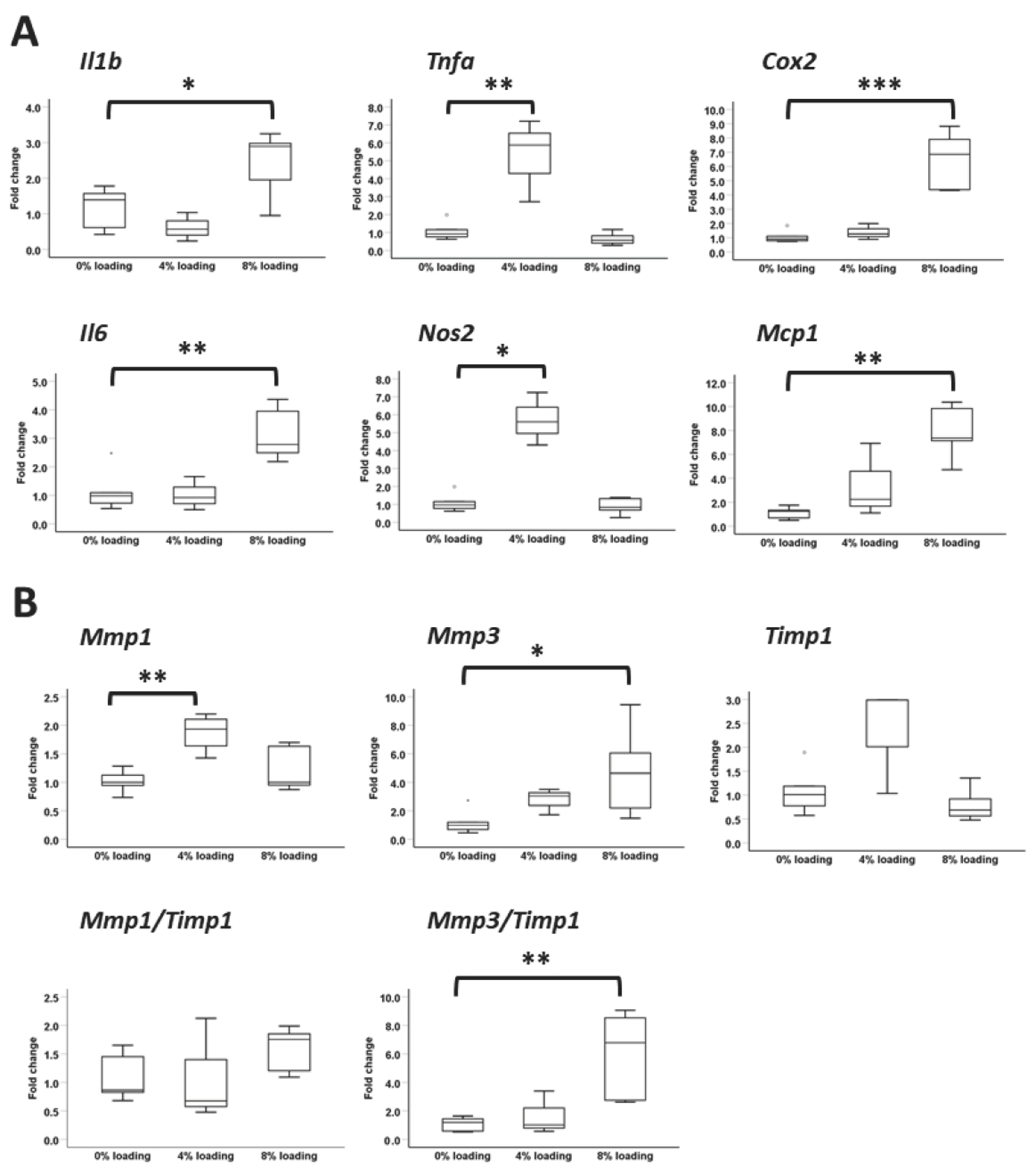
3.7. Effects of IL-1β on the Expression of Ligament, Inflammatory, Matrix-Remodeling, and Non-Ligament Markers
3.8. Effects of Intensive Mechanical Loading on PFSCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Irving, D.B.; Cook, J.L.; Young, M.A.; Menz, H.B. Impact of chronic plantar heel pain on health-related quality of life. J. Am. Podiatr. Med. Assoc. 2008, 98, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Cornwall, M.W.; McPoil, T.G. Plantar fasciitis: Etiology and treatment. J. Orthop. Sports Phys. Ther. 1999, 29, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Crawford, F.; Thomson, C. Interventions for treating plantar heel pain. Cochrane Database Syst. Rev. 2003, 3, CD000416. [Google Scholar]
- Pfeffer, G.; Bacchetti, P.; Deland, J.; Lewis, A.; Anderson, R.; Davis, W.; Alvarez, R.; Brodsky, J.; Cooper, P.; Frey, C.; et al. Comparison of custom and prefabricated orthoses in the initial treatment of proximal plantar fasciitis. Foot Ankle Int. 1999, 20, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Tong, K.B.; Furia, J. Economic burden of plantar fasciitis treatment in the United States. Am. J. Orthop. 2010, 39, 227–231. [Google Scholar]
- Singh, D.; Angel, J.; Bentley, G.; Trevino, S.G. Fortnightly review: Plantar fasciitis. BMJ 1997, 315, 172–175. [Google Scholar] [CrossRef]
- Riddle, D.L.; Pulisic, M.; Sparrow, K. Impact of demographic and impairment-related variables on disability associated with plantar fasciitis. Foot Ankle Int. 2004, 25, 311–317. [Google Scholar] [CrossRef]
- Buchbinder, R. Clinical practice. Plantar fasciitis. N. Engl. J. Med. 2004, 350, 2159–2166. [Google Scholar] [CrossRef]
- Young, C.C.; Rutherford, D.S.; Niedfeldt, M.W. Treatment of plantar fasciitis. Am. Fam. Physician 2001, 63, 467–478. [Google Scholar]
- Li, H.; Lv, H.; Lin, T. Comparison of efficacy of eight treatments for plantar fasciitis: A network meta-analysis. J. Cell Physiol. 2018, 234, 860–870. [Google Scholar] [CrossRef]
- Jarde, O.; Diebold, P.; Havet, E.; Boulu, G.; Vernois, J. Degenerative lesions of the plantar fascia: Surgical treatment by fasciectomy and excision of the heel spur. A report on 38 cases. Acta Orthop. Belg. 2003, 69, 267–274. [Google Scholar] [PubMed]
- Chen, H.; Ho, H.M.; Ying, M.; Fu, S.N. Association between plantar fascia vascularity and morphology and foot dysfunction in individuals with chronic plantar fasciitis. J. Orthop. Sports Phys. Ther. 2013, 43, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Wearing, S.C.; Smeathers, J.E.; Urry, S.R.; Hennig, E.M.; Hills, A.P. The pathomechanics of plantar fasciitis. Sports Med. 2006, 36, 585–611. [Google Scholar] [CrossRef] [PubMed]
- Schepsis, A.A.; Leach, R.E.; Gorzyca, J. Plantar fasciitis. Etiology, treatment, surgical results, and review of the literature. Clin. Orthop. Relat. Res. 1991, 266, 185–196. [Google Scholar] [CrossRef]
- Liptan, G.L. Fascia: A missing link in our understanding of the pathology of fibromyalgia. J. Bodyw. Mov. Ther. 2010, 14, 3–12. [Google Scholar] [CrossRef]
- Lui, P.P. Histopathological changes in tendinopathy--potential roles of BMPs? Rheumatology 2013, 52, 2116–2126. [Google Scholar] [CrossRef]
- Lui, P.P.; Chan, K.M. Tendon-derived stem cells (TDSCs): From basic science to potential roles in tendon pathology and tissue engineering applications. Stem Cell Rev. 2011, 7, 883–897. [Google Scholar] [CrossRef]
- Chang, W.; Callan, K.T.; Dragoo, J.L. The Behavior of Tendon Progenitor Cells from Tendinopathic Tendons: Implications for Treatment. Tissue Eng. Part A 2020, 26, 38–46. [Google Scholar] [CrossRef]
- Still, C., 2nd; Chang, W.T.; Sherman, S.L.; Sochacki, K.R.; Dragoo, J.L.; Qi, L.S. Single-cell transcriptomic profiling reveals distinct mechanical responses between normal and diseased tendon progenitor cells. Cell Rep. Med. 2021, 2, 100343. [Google Scholar] [CrossRef]
- Dakin, S.G.; Buckley, C.D.; Al-Mossawi, M.H.; Hedley, R.; Martinez, F.O.; Wheway, K.; Watkins, B.; Carr, A.J. Persistent stromal fibroblast activation is present in chronic tendinopathy. Arthritis Res. Ther. 2017, 19, 16. [Google Scholar] [CrossRef]
- Rui, Y.F.; Lui, P.P.; Wong, Y.M.; Tan, Q.; Chan, K.M. Altered fate of tendon-derived stem cells isolated from a failed tendon-healing animal model of tendinopathy. Stem Cells Dev. 2013, 22, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.J.; Yin, Z.; Shen, W.L.; Xie, Y.B.; Zhu, T.; Lu, P.; Cai, Y.Z.; Kong, M.J.; Heng, B.C.; Zhou, Y.T.; et al. Pharmacological Regulation of In Situ Tissue Stem Cells Differentiation for Soft Tissue Calcification Treatment. Stem Cells 2016, 34, 1083–1096. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Nie, D.; Rocha, J.L.; Hogan, M.V.; Wang, J.H. Characterization of the structure, cells, and cellular mechanobiological response of human plantar fascia. J. Tissue Eng. 2018, 9, 2041731418801103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Asai, S.; Yu, B. IL-1beta irreversibly inhibits tenogenic differentiation and alters metabolism in injured tendon-derived progenitor cells in vitro. Biochem. Biophys. Res. Commun. 2015, 463, 667–672. [Google Scholar] [CrossRef]
- Millar, N.L.; Murrell, G.A.C.; Mcinnes, I.B. Cytokines in tendon disease: A systematic review. Bone Jt. Res. 2017, 6, 656–664. [Google Scholar]
- Sun, H.B.; Li, Y.; Fung, D.T.; Majeska, R.J.; Schaffler, M.B.; Flatow, E.L. Coordinate regulation of IL-1beta and MMP-13 in rat tendons following subrupture fatigue damage. Clin. Orthop. Relat. Res. 2008, 466, 1555–1561. [Google Scholar] [CrossRef]
- Mobasheri, A.; Shakibaei, M. Is tendinitis an inflammatory disease initiated and driven by pro-inflammatory cytokines such as interleukin 1β? Histol. Histopathol. 2013, 28, 955–964. [Google Scholar]
- Fedorczyk, J.M.; Barr, A.E.; Rani, S.; Gao, H.G.; Amin, M.; Amin, S.; Litvin, J.; Barbe, M.F. Exposure-dependent increases in IL-1beta, substance P, CTGF, and tendinosis in flexor digitorum tendons with upper extremity repetitive strain injury. J. Orthop. Res. 2010, 28, 298–307. [Google Scholar]
- Ko, C.H.; Siu, W.S.; Lau, C.P.; Lau, C.B.; Fung, K.P.; Leung, P.C. Osteoprotective effects of Fructus Ligustri Lucidi aqueous extract in aged ovariectomized rats. Chin. Med. 2010, 5, 39. [Google Scholar] [CrossRef]
- Tan, Q.; Lui, P.P.; Rui, Y.F.; Wong, Y.M. Comparison of potentials of stem cells isolated from tendon and bone marrow for musculoskeletal tissue engineering. Tissue Eng. Part A 2012, 18, 840–851. [Google Scholar] [CrossRef]
- Lui, P.P.; Wong, Y. Higher BMP/Smad sensitivity of tendon-derived stem cells (TDSCs) isolated from the collagenase-induced tendon injury model: Possible mechanism for their altered fate in vitro. BMC Musculoskelet. Disord. 2013, 14, 248. [Google Scholar] [CrossRef] [PubMed]
- Lui, P.P. A practical guide for the isolation and maintenance of stem cells from tendon. Methods Mol. Biol. 2015, 1212, 127–140. [Google Scholar] [PubMed]
- Nichols, A.E.C.; Were, S.R.; Dahlgren, L.A. Transient Scleraxis Overexpression Combined with Cyclic Strain Enhances Ligament Cell Differentiation. Tissue Eng. Part A 2018, 24, 1444–1455. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, K.; Sakai, K.; Kusayama, Y.; Akamatsu, Y.; Sakamaki, K.; Morita, S.; Sasaki, T.; Saito, T.; Sakai, T. The extent of degeneration of cruciate ligament is associated with chondrogenic differentiation in patients with osteoarthritis of the knee. Osteoarthr. Cartil. 2012, 20, 1258–1267. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Comerford, E.J.; Simpson, D.M.; Clegg, P.D.; Canty-Laird, E.G. Identification and Characterization of Canine Ligament Progenitor Cells and Their Extracellular Matrix Niche. J. Proteome Res. 2019, 18, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, G.; Guo, Y.; Tang, H.; Shi, Y.; Bian, X.; Zhu, M.; Kang, X.; Zhou, M.; Lyu, J.; et al. Exosomes from tendon stem cells promote injury tendon healing through balancing synthesis and degradation of the tendon extracellular matrix. J. Cell. Mol. Med. 2019, 23, 5475–5485. [Google Scholar] [CrossRef]
- Liu, K.; Cai, G.L.; Zhuang, Z.; Pei, S.Y.; Xu, S.N.; Wang, Y.N.; Wang, H.; Wang, X.; Cui, C.; Sun, M.C.; et al. Interleukin-1β-Treated Mesenchymal Stem Cells Inhibit Inflammation in Hippocampal Astrocytes Through Exosome-Activated Nrf-2 Signaling. Int. J. Nanomed. 2021, 16, 1423–1434. [Google Scholar] [CrossRef]
- Rui, Y.F.; Lui, P.P.; Ni, M.; Chan, L.S.; Lee, Y.W.; Chan, K.M. Mechanical loading increased BMP-2 expression which promoted osteogenic differentiation of tendon-derived stem cells. J. Orthop. Res. 2011, 29, 390–396. [Google Scholar] [CrossRef]
- Sawangmake, C.; Nantavisai, S.; Osathanon, T.; Pavasant, P. Osteogenic differentiation potential of canine bone marrow-derived mesenchymal stem cells under different β-glycerophosphate concentrations in vitro. Thai J. Vet. Med. 2016, 46, 617–625. [Google Scholar] [CrossRef]
- Tan, Q.; Lui, P.P.; Lee, Y.W. In vivo identity of tendon stem cells and the roles of stem cells in tendon healing. Stem Cells Dev. 2013, 22, 3128–3140. [Google Scholar] [CrossRef]
- Hostettler, K.E.; Gazdhar, A.; Khan, P.; Savic, S.; Tamo, L.; Lardinois, D.; Roth, M.; Tamm, M.; Geiser, T. Multipotent mesenchymal stem cells in lung fibrosis. PLoS ONE 2017, 12, e0181946. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, J.; Wang, J.H. Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC Musculoskelet. Disord. 2010, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.L.B.K.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Hyslop, L.; Stojkovic, M.; Armstrong, L.; Walter, T.; Stojkovic, P.; Przyborski, S.; Herbert, M.; Murdoch, A.; Strachan, T.; Lako, M. Downregulation of NANOG induces differentiation of human embryonic stem cells to extraembryonic lineages. Stem Cells 2005, 23, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Nowling, T.K.; Johnson, L.R.; Wiebe, M.S.; Rizzino, A. Identification of the transactivation domain of the transcription factor Sox-2 and an associated co-activator. J. Biol. Chem. 2000, 275, 3810–3818. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Tsai, R.Y. Turning a new page on nucleostemin and self-renewal. J. Cell Sci. 2014, 127, 3885–3891. [Google Scholar] [CrossRef]
- Siddiqi, S.; Gude, N.; Hosoda, T.; Muraski, J.; Rubio, M.; Emmanuel, G.; Fransioli, J.; Vitale, S.; Parolin, C.; D’Amario, D.; et al. Myocardial induction of nucleostemin in response to postnatal growth and pathological challenge. Circ. Res. 2008, 103, 89–97. [Google Scholar] [CrossRef]
- Kafienah, W.; Mistry, S.; Williams, C.; Hollander, A. Nucleostemin is a marker of proliferating stromal stem cells in adult human bone marrow. Stem Cells 2006, 24, 1113–1120. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Sekiya, I.; Yagishita, K.; Muneta, T. Comparison of human stem cells derived from various mesenchymal tissues: Superiority of synovium as a cell source. Arthritis Rheum. 2005, 52, 2521–2529. [Google Scholar] [CrossRef]
- Baek, J.; Ryu, B.; Kim, J.; Lee, S.G.; Oh, M.S.; Hong, K.S.; Kim, E.Y.; Kim, C.Y.; Chung, H.M. Immunomodulation of Pluripotent Stem Cell-Derived Mesenchymal Stem Cells in Rotator Cuff Tears Model. Biomedicines 2022, 10, 1549. [Google Scholar] [CrossRef] [PubMed]
- Chandrakanthan, V.; Yeola, A.; Kwan, J.C.; Oliver, R.A.; Qiao, Q.; Kang, Y.C.; Zarzour, P.; Beck, D.; Boelen, L.; Unnikrishnan, A.; et al. PDGF-AB and 5-Azacytidine induce conversion of somatic cells into tissue-regenerative multipotent stem cells. Proc. Natl. Acad. Sci. USA 2016, 113, E2306–E2315. [Google Scholar] [CrossRef] [PubMed]
- Bugueño, J.; Li, W.; Salat, P.; Qin, L.; Akintoye, S.O. The Bone Regenerative Capacity of Canine Mesenchymal Stem Cells is Regulated by Site-Specific Multilineage Differentiation. Oral Med. Oral Pathol. Oral Radiol. 2017, 123, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Zaim, M.; Karaman, S.; Cetin, G.; Isik, S. Donor age and long-term culture affect differentiation and proliferation of human bone marrow mesenchymal stem cells. Ann. Hematol. 2012, 91, 1175–1186. [Google Scholar] [CrossRef]
- Rajpar, I.; Barrett, J.G. Multi-differentiation potential is necessary for optimal tenogenesis of tendon stem cells. Stem Cell Res. Ther. 2020, 11, 152. [Google Scholar] [CrossRef]
- Rabadi, D.; Seo, S.; Wong, B.; Chung, D.; Rai, V.; Agrawal, D.K. Immunopathogenesis, early Detection, current therapies and prevention of plantar Fasciitis: A concise review. Int. Immunopharmacol. 2022, 110, 109023. [Google Scholar] [CrossRef]
| Gene | Primer Nucleotide Sequence | Accession Number |
|---|---|---|
| Nanog | (F) GCCACCCACACTTGTGACTA (R) TTCTCGCCTGTGTGAGTTCG | NM_053713 |
| Oct4 | (F) GTCCCTAGGTGAGTCGTCCT (R) TGGAAGCTTAGCCAGGTTCG | NM_001009178 |
| Sox2 | (F) GAGGAGGAGAGCGACTGTTT (R) CTGGCGGAGAATAGTTGGGG | NM_001109181 |
| Runx2 | Primer 1: (F) CACAAGTGCGGTGCAAACTT (R) GCAGCCTTAAATATTACTGCATGG Primer 2: (F) CCGATGGGACCGTGGTT (R) CAGCAGAGGCATTTCGTAGCT | NM_053470 NM_053470.1 |
| Spp1 | (F) CCGAGGTGATAGCTTGGCTT (R) CTCTTCATGCGGGAGGTGAG | NM_012881 |
| Bglap | (F) ATGAGGACCCTCTCTCTGCT (R) AGGTAGCGCCGGAGTCTATT | NM_013414 |
| Pparg | Primer 1: (F) CCTGTTGACCCAGAGCATGG (R) GGTCCACAGAGCTGATTCCG Primer 2: (F) CGGCGATCTTGACAGGAAAG (R) GCTTCCACGGATCGAAACTG | NM_013124 AB019561 |
| Cfd | (F) TGGGGCAATCACCAAGAACA (R) CGAGATCCCCACGTAACCAC | NM_001077642 |
| Fabp4 | (F) TCGTCATCCGGTCAGAGAGT (R) CCAGCTTGTCACCATCTCGT | U75581.1 |
| Col2a1 | (F) GTTCACGTACACTGCCCTGA (R) AAGGCGTGAGGTCTTCTGTG | NM_012929 |
| Sox9 | Primer 1: (F) TGGGAGCGACAACTTTACCA (R) GAGGAGGAGGGAGGGAAAAC Primer 2: (F) AGAGCGTTGCTCGGAACTGT (R) TCCTGGACCGAAACTGGTAAA | XM_001081628 XM_343981.2 |
| Col1a1 | Primer 1: (F) CCCAGCGGTGGTTATGACTT (R) GGGTTTGGGCTGATGTACCA Primer 2: (F) CATCGGTGGTACTAAC (R) CTGGATCATATTGCACA | NM_053304.1 NM_053356.1 |
| Col3a1 | Primer 1: (F) TTCCTGGGAGAAATGGCGAC (R) ACCAGCTGGGCCTTTGATAC Primer 2: (F) TGCAATGTGGGACCTGGTTT (R) GGGCAGTCTAGTGGCTCATC | NM_032085 NM_032085.1 |
| Eln | Primer 1: (F) GGAAAGTTCCTGGTGTCGGT (R) TCCAGCACCATACTTCGCTG Primer 2: (F) GCTTAGGAGTCTCAACAGGTGC (R) CGGAACCTTGGCCTTGACTC | NM_012722 NM_012722.1 |
| Scx | Primer 1: (F) GCCTGTGGGGACCTAAAGAG (R) AGCATGAACACGACAGGGTT Primer 2: (F) AACACGGCCTTCACTGCGCTG (R) CAGTAGCACGTTGCCCAGGTG | NM_001130508 NM_001130508.1 |
| Tnc | Primer 1: (F) CCACAGAAGCTGAACCGGAA (R) CAGTATCCGTCCCATCCACG Primer 2: (F) AAAGCAGCCACCCGCTATTAC (R) GGATCTCCTCTGTCAAGACCTCAA | NM_053861 NM_053861.2 |
| Tnmd | Primer 1: (F) CACCTCAGCAGTGGTCTCTC (R) TGTGCTCCATGCCATAGGTC Primer 2: (F) GTGGTCCCACAAGTGAAGGT (R) GTCTTCCTCGCTTGCTTGTC | NM_022290 NM_022290.1 |
| Tnfa | Primer 1: (F) CAGCCGATTTGCCATTTCATAC (R) GGCTCTGAGGAGTAGACGATAA Primer 2: (F) AAATGGGCTCCCTCTCATCAGTTC (R) TCTGCTTGGTGGTTTGCTACGAC | NM_012675.3 |
| Cox2 | Primer 1: (F) TCTCCAACCTCTCCTACTACAC (R) CTCCACCGATGACCTGATATTT Primer 2: (F) TGTATGCTACCATCTGGCTTCGG (R) GTTTGGAACAGTCGCTCGTCATC | NM_011198.4 S67722.1 |
| Il6 | Primer 1: (F) ATCTGCCCTTCAGGAACAGC (R) AGCCTCCGACTTGTGAAGTG Primer 2: (F) TCCTACCCCAACTTCCAATGCTC (R) TTGGATGGTCTTGGTCCTTAGCC | NM_012589.2 |
| Il10 | (F) TCCGGGGTGACAATAACTGC (R) GCAGCTGTATCCAGAGGGTC | NM_012854.2 |
| Il33 | (F) GACCAGCTATCTCCCATCACT (R) TTGGATACTGCCAAGCAGGG | NM_001014166.1 |
| Mmp1 | (F) CCACTAACATTCGAAAGGGTTT (R) GGTCCATCAAATGGGTTATTG | NM_001134530.1 |
| Mmp3 | (F) ATGGGCCTGGAATGGTCTTG (R) CCCTCCATGAAAAGACTCAGAGG | NM_133523.3 |
| Timp1 | (F) CAGCAAAGGCCTTCGTAAA (R) TGGCTGAACAGGGAAACACT | NM_053819.1 |
| Il1b | (F) CACCTCTCAAGCAGAGCACAG (R) GGGTTCCATGGTGAAGTCAAC | NM_031512.2 |
| Nos2 | (F) TCCTCAGGCTTGGGTCTTGTTAG (R) TTCAGGTCACCTTGGTAGGATTTG | NM_012611.3 |
| Mcp1 | (F) GGCCTGTTGTTCACAGTTGCT (R) TCTCACTTGGTTCTGGTCCAGT | M57441.1 |
| Mkx | (F) TTTACAAGCACCGTGACAACCC (R) ACAGTGTTCTTCAGCCGTCGTC | XM_017600733.2 |
| Dcn | (F) GTTCTGATCTGGGTCTGGACAAAG (R) CTTAAAGGCCCCCTCTTTGATC | NM_024129.1 |
| Acan | (F) CTTGGGCAGAAGAAAGATCG (R) GTGCTTGTAGGTGTTGGGGT | NM_022190.1 |
| Cebpa | (F) AAGGCCAAGAAGTCGGTGGA (R) CAGTTCGCGGCTCAGCTGTT | NM_012524.2 |
| Lpl | (F) GTACAGTCTTGGAGCCCATGC (R) CCAAGCCAGTAATTCTATTGACCTTC | NM_012598.2 |
| Gapdh | (F) CTCAGTTGCTGAGGAGTCCC (R) ATTCGAGAGAAGGGAGGGCT | NM_017008.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siu, W.S.; Ma, H.; Ko, C.H.; Shiu, H.T.; Cheng, W.; Lee, Y.W.; Kot, C.H.; Leung, P.C.; Lui, P.P.Y. Rat Plantar Fascia Stem/Progenitor Cells Showed Lower Expression of Ligament Markers and Higher Pro-Inflammatory Cytokines after Intensive Mechanical Loading or Interleukin-1β Treatment In Vitro. Cells 2023, 12, 2222. https://doi.org/10.3390/cells12182222
Siu WS, Ma H, Ko CH, Shiu HT, Cheng W, Lee YW, Kot CH, Leung PC, Lui PPY. Rat Plantar Fascia Stem/Progenitor Cells Showed Lower Expression of Ligament Markers and Higher Pro-Inflammatory Cytokines after Intensive Mechanical Loading or Interleukin-1β Treatment In Vitro. Cells. 2023; 12(18):2222. https://doi.org/10.3390/cells12182222
Chicago/Turabian StyleSiu, Wing Sum, Hui Ma, Chun Hay Ko, Hoi Ting Shiu, Wen Cheng, Yuk Wa Lee, Cheuk Hin Kot, Ping Chung Leung, and Pauline Po Yee Lui. 2023. "Rat Plantar Fascia Stem/Progenitor Cells Showed Lower Expression of Ligament Markers and Higher Pro-Inflammatory Cytokines after Intensive Mechanical Loading or Interleukin-1β Treatment In Vitro" Cells 12, no. 18: 2222. https://doi.org/10.3390/cells12182222
APA StyleSiu, W. S., Ma, H., Ko, C. H., Shiu, H. T., Cheng, W., Lee, Y. W., Kot, C. H., Leung, P. C., & Lui, P. P. Y. (2023). Rat Plantar Fascia Stem/Progenitor Cells Showed Lower Expression of Ligament Markers and Higher Pro-Inflammatory Cytokines after Intensive Mechanical Loading or Interleukin-1β Treatment In Vitro. Cells, 12(18), 2222. https://doi.org/10.3390/cells12182222







