Abstract
Head and neck cancers (HNCs) are known to present multiple factors likely to influence their development. This review aims to provide a comprehensive overview of the current scientific literature on the interplay between systemic inflammatory disorders, immunosuppressive treatments and their synergistic effect on HNC risk. Both cell-mediated and humoral-mediated systemic inflammatory disorders involve dysregulated immune responses and chronic inflammation and these inflammatory conditions have been associated with an increased risk of HNC development, primarily in the head and neck region. Likewise, the interaction between systemic inflammatory disorders and immunosuppressive treatments appears to amplify the risk of HNC development, as chronic inflammation fosters a tumor-promoting microenvironment, while immunosuppressive therapies further compromise immune surveillance and anti-tumor immune responses. Understanding the molecular and cellular mechanisms underlying this interaction is crucial for developing targeted prevention strategies and therapeutic interventions. Additionally, the emerging field of immunotherapy provides potential avenues for managing HNCs associated with systemic inflammatory disorders, but further research is needed to determine its efficacy and safety in this specific context. Future studies are warranted to elucidate the underlying mechanisms and optimize preventive strategies and therapeutic interventions.
1. Introduction
Head and neck cancers (HNC) account for 3% of all cancers in the United States, with a fourth of the patients succumbing to the disease every year [1]. There are several classical risk factors associated with HNCs, such as alcohol use, smoking, human papillomavirus (HPV) and Epstein–Barr virus (EBV) infections—and more general risk factors include chronic inflammation, and immunodeficiency (Figure 1).
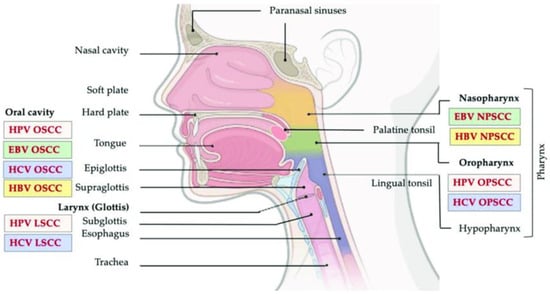
Figure 1.
Head and neck cancer regions. This image shows the relation between the different anatomical regions of the head and neck and viral-associated cancers. Adapted from [2].
An association between chronic systemic inflammation and risk of malignancy has been well established [3,4,5]. Poor oral hygiene and consequent inflammatory periodontal disease may predispose to oral cancers [6,7,8,9]. Interestingly, periodontitis may increase the risk of non-oral cancers such as pancreatic [5] and breast cancers [10]. These observations are likely attributable to increased levels of products of chronic inflammation including known tumor promotors such as interleukin (IL)-6 (IL-6), tumor necrosis factor alpha (TNF-α), IL-12, IL-23, IL-1β and nuclear factor kappa B (NF-κB) as well as transforming growth factor beta (TGF-β) and IL-10 which might inhibit anti-tumor immunity [11,12,13,14].
Likewise, immune deficiency is associated with increased HNC risk. For instance, patients with acquired immunodeficiency syndrome (AIDS) are significantly more likely to develop mucosal head and neck squamous cell carcinomas (SCCs), tend to develop the disease at a younger age and with worse prognosis than human immunodeficiency virus (HIV)-negative patients [15]. Furthermore, it is well known that patients having undergone solid organ transplantation are also at a higher risk of developing HNCs [16].
Moreover, immunosuppression likely predisposes to oncogenesis by suppressing cell-mediated immunity, the primary means by which the immune system eliminates tumor cells. CD4+ Th1 cells and CD8+ T-cells are generally regarded as tumor-suppressant, majorly through the activity of interferon gamma (IFN-γ) [13]. Stimulated by Tumor-Associated Antigens (TAA) presented by the tumor cells, T-cells are activated to secrete, perforins, granzymes cytokines and IFN-γ, which cause direct cell cytotoxicity [17]. Cytolytic T lymphocytes (CTLs) and natural killer cells (NKCs) are also largely regarded to hinder tumors, including HNCs [18]. However, it must be noted that not all T-cells have anti-tumor activity. CD4+CD25+Foxp3+ T cells, regulatory T-cells (T-regs) promote tumor activity by inhibiting the activation of both CD4+ and CD8+ cells. T-regs also secrete pro-tumor cytokines such as TGF-β. Regardless, it must be noted that certain emerging evidence contradicts the pro-tumor activities of T-regs, ergo their oncogenic role remains unclear [13]. Another line of immune cells, IL-17 producing CD4+ T-cells (Th17), are stimulated and expanded by the expression of TGF-β, IL-6 and IL-23 in the tumor micro-environment, and are believed to be implicated in tumorigenesis. While, similarly to T-regs, Th17 cells are also believed to have a pro-tumor effect, contradictory reports leave their exact roles to be inconclusive until further investigation [13].
Patients suffering from auto-immune diseases often face a dual challenge, linked to the chronic inflammation associated with the disease’s flares, but also with the immunosuppression arising from the disease’s treatments, making it rather important to assess their risk to develop HNCs, considering that both chronic inflammation and immunosuppression may be regarded as potential risk factors associated with development of HNCs.
2. Materials and Methods
2.1. HNC Incidence in Systemic Inflammatory Diseases
We defined HNCs as oral, pharyngeal, oro-pharyngeal, lip, nasal cavity and laryngeal cancers—and excluded thyroid, skin and esophageal cancers. Numerous NCBI PubMed searches relating selected system inflammatory conditions and associated reports of head and neck cancers were conducted. Relevant reports and reviews in PubMed results were isolated and reviewed.
2.2. Immune Pathways Review
NCBI Pubmed was mainly used to gather information about the specific immune pathways involved in different systemic diseases, and their treatments. To obtain a more thorough search of pathways, queries using UpToDate® (Waltham, MA, USA), Web of Science™ (Clarivate Plc, PA, USA) and Google Scholar were also conducted.
3. Results
3.1. HNCs and the Immune System
The systemic immune-inflammation index (SII) has emerged as a pertinent biomarker for prognostic assessment across different malignancies. A recent meta-analysis demonstrated a noteworthy correlation between elevated pretreatment SII and unfavorable outcomes, including worsened overall survival, disease-free survival, progression-free survival, as well as higher T and N classifications, among patients diagnosed with head and neck cancer (HNC) [19]. Likewise, another recent systematic review reported an incidence of oral carcinoma in immunosuppressed patients of 200 new cases per 100,000 [20].
Overall, the increased incidence of head and neck cancer in patients with systemic inflammatory diseases can be attributed to a combination of factors, including chronic inflammation, genetic predisposition and epigenetic changes, tissue damage, interactions with viral infections and altered immune responses associated, or not, with immunosuppressive treatments. This complex interplay creates an environment that may favour the initiation and/or progression of cancerous cells within the head and neck region [20].
In fact, inflammation is known to contribute to DNA damage, cell proliferation, angiogenesis and evasion of immune surveillance, which can later promote tumorogenesis. Similarly, altered immune response in systemic inflammatory diseases can lead to dysregulated immune responses, an aspect often impacted by the immunosuppressive treatments usually associated with systemic inflammatory diseases, which weaken the immune system’s ability to identify and eliminate abnormal cells, including cancer cells. In fact, this imbalance can create an environment where the immune system may not effectively target and eliminate early cancerous cells, allowing them to progress into full-blown tumors [20].
One must also consider the likely detrimental impact of carcinogenic cytokines, as pro-inflammatory cytokines may act as signaling molecules which then stimulate cell growth, survival and angiogenesis, fostering an environment conducive to cancer development.
Overall, iinflammatory diseases can be broadly categorized as cell-mediated or humoral, based on the immune mechanisms involved in their pathogenesis. When comparing the risk for head and neck cancer associated with these inflammatory diseases, as well as their response to immunotherapy treatments, there are some notable differences.
- Cell-Mediated Inflammatory Diseases:
Conditions such as oral lichen planus, oral submucous fibrosis and chronic graft-versus-host disease (GVHD) are characterized by cell-mediated immune responses, which is an adaptative immunity response that is based on T-cells. These cells are physically present and actively recognize the antigen and attack the target, which is a slower process [21]. These diseases are generally associated with a higher risk of developing HNC, as the chronic inflammation and tissue damage caused by these diseases can create a favorable environment for the development of malignant changes [22]. GVHD especially, which is related to hematopoietic stem cell transplantation, has been associated with the development of malignancies, namely oral carcinoma [23,24,25,26,27]. Since manifestations of oral squamous cell carcinoma (OSCC) can emerge within a relatively brief latent period, even as early as one year following hematological stem cell transplant (HSCT), it becomes plausible that the activation of the PI3K pathway initiates during the early stages of graft-versus-host disease (GvHD) development (16). The identification of a connection between AKT signaling, which constitutes a downstream element of the PI3K pathway, and the B-cell activity associated with GvHD further strengthens the implication of this pathway in the genesis of GvHD [28]. Moreover, HNC can appear in GVHD patients due to the combination of immunosuppressants used to treat it and radiotherapy, and the oral cancers are normally of squamous nature [29]. Immunotherapy treatments, particularly immune checkpoint inhibitors such as pembrolizumab and nivolumab, have shown promising results in the treatment of HNC associated with cell-mediated inflammatory diseases [30]. These drugs help unleash the body’s immune system to target and destroy cancer cells. However, the response to immunotherapy can vary among individuals, and not all patients may benefit equally [31].
- Humoral Inflammatory Diseases:
Diseases such as Sjögren’s syndrome and systemic lupus erythematosus (SLE) involve humoral immune responses [32,33]. These responses are characterized by the production of antigen-specific antibodies by lymphocytes B, a response that is developed quickly [34]. The inflammatory diseases are not as strongly linked to an increased risk of HNC compared to cell-mediated diseases. However, chronic inflammation and immunological dysregulation may contribute to a slightly elevated risk as we will address in more detail. It is also important to highlight that, contrary to what we can observe in cell-mediated inflammatory diseases, the effectiveness of immunotherapy in treating HNC associated with humoral inflammatory diseases is still not well established [35]. Since these diseases involve complex immune system abnormalities, their interaction with immunotherapy agents may differ from that of cell-mediated diseases. Further research is needed to determine the potential benefits of immunotherapy in this context.
Either way, in this paper we present some examples of the relationship between different autoimmune inflammatory diseases and head and neck cancer, as we also highlight that multifactorial, and individual variations can exist. Hence, regular monitoring, early detection and tailored treatment plans are crucial for managing patients with these conditions and mitigating the risk of cancer development.
3.1.1. Incidence of HNCs in Systemic Sclerosis
While there are contradictory findings, some studies report a strong increased risk of HNCs in systemic sclerosis (SSc) patients. In a cohort of 769 SSc patients in an American institution, Derk et al. reported nine patients with diffuse SSc who developed oral and pharyngeal carcinomas, six of which were SCCs of the tongue. One patient had both pharyngeal and tongue SCC within a 4-year period, while another patient developed three separate tongue SCCs. Altogether, the cohort has a standardized incidence ratio (SIR) of 25 for developing tongue carcinoma, and SIR of 9.63 for oropharyngeal carcinomas [36]. In a review of 2053 Taiwanese patients with SSc, Kuo et al. reports an oral cavity and pharyngeal cancer SIR of 3.67 compared to the general Taiwanese population. This value was obtained by dividing the number of cases of oral cavity and pharyngeal cancers by the expected number among the general Taiwanese population, and the data were collected from the National Death Registry in Taiwan and the Taiwan National Health Insurance Research Dataset (NHIRD) [37].
3.1.2. Incidence of HNCs in Inflammatory Bowel Diseases
Inflammatory bowel diseases (IBD) refers to two autoimmune diseases: ulcerative colitis (UC) and Crohn’s disease (CD). While there are a considerable number of case reports of HNCs arising in patients with IBD, no clear incidence ratios have been reported [38,39].
Related case reports include the development of oral SCC in a CD patient who was treated with azathioprine (AZA) for 9 years [40], neck cavity SCC in a CD patient who was successfully treated with infliximab [41], and tongue SCC in a patient being treated with AZA [42].
A 2015 review of cancerous and precancerous lesions in IBD patients provides an excellent review of many related cohort studies [38], and the incidence of HNCs in IBD patients.
3.1.3. Incidence of HNCs in Systemic Lupus Erythematous
The incidence of HNCs in patients with systemic lupus erythematous (SLE) is more strongly associated. In a study of 8751 patients with SLE, Chang et al. reported a 2.16 higher incidence risk ratio of HNCs in patients compared to controls, with the highest incidence being oral cavity cancer (5 of 11 cases). Of note, SLE comorbidity did not affect survival of patients diagnosed with HNCs [43]. A large meta-analysis of 18 studies including a total of 86,069 SLE patients reported the risk of HNCs, not including thyroid cancer, to be 2.31 fold higher [44]. Furthermore, a meta-analysis of 16 studies including 59,662 SLE patients found that SLE patients have a pooled relative risk of 4.19 for laryngeal cancer and 1.54 for cancer of the oropharynx [45]. Another study of 11,763 Taiwanese patients with SLE reported a nasopharyngeal cancer SIR of 4.18, oropharyngeal and laryngeal SIR of 2.02 relative to the general population [46]. Lastly, in 2021, a cohort of 17,854 Korean patients with SLE showed an increased risk of HNC with an SIR of 2.7, the highest SIR among solid malignancies in this study [47].
Albeit only a few, case reports were also found. These include the case reports of SCC of the palate developing in a SLE patient being treated with prednisone and cyclophosphamide (CYC) [48], and tongue carcinoma developing in a SLE patient during pregnancy, five years after heavy treatment with CYC and mesna [49].
3.1.4. Incidence of HNCs in Rheumatoid Arthritis
While there are case reports, no large review or meta-analysis was found regarding the incidence of HNCs in rheumatoid arthritis (RA) patients. Beattie et al. report oral SCC developing in a RA patient being treated with adalimumab [50]. Chainani-Wu et al. also report a case of oropharyngeal SCC developing in a RA patient being treated with both methotrexate (MTX) and etanercept [51].
A review by Philips et al. of 180 patients with HNCs, reports that the use of TNF inhibitors (TNFi) with both RA and HNC did not affect the risk of HNC recurrence or death. This study, however, did not focus on the occurrence of HNC in RA patients being treated with TNFi [52].
3.1.5. Incidence of HNCs in Dermatomyositis
There are sparse case reports of dermatomyositis (DM) patients developing oral and pharyngeal cancer. These include tongue SCC developing in a young patient with DM being treated with AZA and prednisolone [53], tonsillar SCC developing in a 63 year old male patient with DM being treated with prednisone, MTX and hydroxychloroquine for a year (authors view the DM as a paraneoplastic syndrome) [54], tonsillar carcinoma developing in a Caucasian patient with DM after seven months of treatment with AZA and prednisone [55], pleomorphic adenoma with malignant features developing in a 71 year old Caucasian patient with DM [56], and development of tonsillar SCC in two Korean patients with DM, one after a year of treatment with AZA and prednisolone, and the other after two years of treatment with ‘steroids’ [57]. In a report of 136 hospitalized DM patients, one patient developed hypopharyngeal carcinoma, while 4 developed nasopharyngeal carcinoma (NPC) [58].
There is a strong association of DM patients developing NPC, which seems to be the most prevalent in patients of Eastern Asian. NPC was reported to develop in 12/90 DM patients in a hospital in China [59], 12/104 DM patients in a hospital in Taiwan [60], 5/130 DM patients in hospital in Tunisia [61] and 7/68 DM patients in a multiracial cohort of 68 Asian patients [62]. A review of 1154 NPC cases based in a Hong Kong hospital found 10 patients with concurrent DM diagnosis, 9 of which were made prior to NPC diagnosis [63]. The pathogenesis of NPC may be multifactorial in addition to systemic inflammatory illnesses and its immunosuppressive treatment, as EBV has been significantly associated in DM and SLE patients who developed NPC [64]. Incidence of NPC’s developing in DM patients has also been reported in an Israeli ‘white’ Jewish patients being treated with prednisone and MTX [65].
3.1.6. Incidence of HNCs in Psoriasis
Several studies have explored the incidence of oral, pharyngeal and laryngeal cancer in psoriasis patients. A cohort study of 9773 psoriasis patients in Sweden found SIRs of 2.8 for oral and pharyngeal cancers, and 1.43 for laryngeal cancer [66]. A different cohort study of 6905 psoriasis patients found a SIRs of 1.7 for oral cancer, 2.9 for pharyngeal cancer, and 2.0 for laryngeal cancer. Men had higher risk than females, with SIRs of 2.1 for oral cancer, 4.1 for pharyngeal cancer and 2.4 for laryngeal cancer [67]. Furthermore, a study of 5687 hospitalized patients with psoriasis in Finland reports SIRs of 0.7 for oral cancer, 1.3 for pharyngeal cancer and 2.9 for laryngeal carcinoma [68].
Case reports of HNC occurrence in psoriasis patients can also be found in the literature. For example, Seoane et al. report a case report of a gingival mucinous adenocarcinoma of a minor salivary gland in a patient with a history of psoriasis and chronic gingivitis [69], Carlesimo et al. report two cases of salivary gland tumors developing in two psoriatic patients being treated with TNFi [70], and Yamamoto et al. report the development of oral adenocarcinoma in a psoriasis patient receiving short-term cyclosporine (CsA) therapy [71].
3.2. Pro-Tumor Cytokines and Inflammatory Signaling in Queried Auto-Immune Systemic Diseases
3.2.1. Systemic Sclerosis
Serum and skin presence of TGF-β (cancer-promoting) seems to take place early in pathogenesis of both local and diffuse SSc [72]. Serum IL-6 and C-reactive protein (CRP) (cancer-promoting) are also elevated in SSc and correlate with clinical disease activity [73].
In 54 SSc patients, there were significantly increased serum levels of IL-6, TNF-α, monocyte chemoattractant protein 1 (MCP1) (cancer-promoting), but also reduced IFN-γ and macrophage derived chemokine (MDC) (cancer-suppressive). Peripheral blood mononuclear cells supernatant also showed increased levels of IL-6, IL-8, IL-10, IL-18, macrophage inflammatory protein 1 gamma (MIP-1γ) (cancer-promoting) and TNF-α (cancer-suppressive). Levels of IL-6, IL-10 and MDC could discriminate between limited and diffuse forms of the disease [74]. Increased levels of circulating IL-1 (cancer-promoting), IL-2, IL-2 receptors (IL-2R) (cancer-suppressive), IL-4, IL-8, IL-17 (cancer-promoting) and TNF-α have been reported in patients with SSc [75,76,77,78].
Serum elevations of vascular cell adhesion molecule 1 (VCAM-1) and E-Selectin (cancer-promoting) were noted in SSc and were associated with disease severity [79]. The chemokine platelet activating factor 4 (CXCL4) (cancer-promoting), a signal for chemotaxis, is also increased in patient plasma levels and correlates with disease complications and progression [80]. CXCL4 may also have roles in inhibiting angiogenesis [81].
3.2.2. Systemic Lupus Erythematous
Unlike in other auto-immune diseases, most SLE patients have significant serum acid-labile IFN-α (cancer-suppressive) levels [82], which correlates with disease flares and remission [83]. While one study found that about a quarter of SLE patients have higher levels of TNF-α relative to controls [84], other studies suggest decreased production of TNF-α in a subpopulation of SLE [85,86].
In about half of SLE patients with active disease, serum IL-2 is also increased. This may be normalized with immunosuppressive therapy [87,88]. Serum IL-2R is also elevated in about a third of the patients and it correlates with disease activity and the Westergren erythrocyte sedimentation rate (ESR) [89,90]. Lymphocytes production of activated TGF-β is markedly decreased in active diseases, and less profoundly decreased in treated SLE [91].
Lastly, animal models, specifically NZB/NZW mice who develop an SLE-like illness, also suggest decreased production of TNF-α [92]. Mice with higher production of TNF-α are more resistant to the development of the disease [93].
The role of cytokine expression patterns in SLE triggering HNC is not fully understood and is likely multifactorial. In fact, the cytokine imbalances in SLE can lead to a breakdown of self-tolerance, where the immune system targets normal cells, with IFN-gamma and TNF-alpha contributing to tissue damage, cellular stress and inflammation in various organs. Moreover, immune surveillance, which normally identifies and eliminates abnormal cells, may be compromised in SLE Hence, tissue damage and associated DNA mutations are likely to go undetected, since SLE patients often experience immune dysfunction and suppression, thus weakening the immune system’s ability to detect and eliminate cancerous cells that may arise due to abovementioned DNA mutations.
3.2.3. Rheumatic Arthritis
Serum and synovial TNF-α is a central cytokine in the pathogenesis of RA, which has numerous effects including antigen-presenting cells production of granulocyte macrophage colony stimulating factor (GM-CSF) (cancer-promoting), proliferation of T-cells [94] and possible disruption of regulatory T-cell suppressive effects [95]. Macrophage-produced increases in IL-15, which increase TNF-α production in peripheral blood T cells, is one-local-source of the cytokine [96]. GM-CSF in synovial tissue supernatant, produced by synovial macrophages, may initiate T-cell activation in response to specific antigens in an RA patient [97] and blocking it using a monoclonal antibody reduces disease severity [98].
Lastly, IL-20 (cancer-promoting), a pro-inflammatory cytokine that promotes oral tumor growth, migration and tumor-associated inflammation [99], is also increased in plasma of RA patients [100].
3.2.4. Inflammatory Bowel Diseases
Although primary symptoms of inflammatory bowel diseases (IBD) concern the intestines, extra-intestinal manifestations such as arthralgia, arthritis and cachexia are also present, inferring possible systemic deregulation of inflammatory mediators [101]. Crohn’s disease (CD) can develop at any point of the gastrointestinal tract, more commonly in the end of the small intestine, causing inflammation in all layers in random areas, even if they are adjacent to healthy parts of the intestines. Meanwhile, ulcerative colitis (UC) is confined to the colon and rectum, causing inflammation in continuous areas and only in the inner lining of these areas (Figure 2). Complications associated with CD are anal fissures, strictures, fistulas, bowel obstruction, malabsorption and colon cancer. In UC, the complications include severe dehydration, colon perforation, toxic megacolon and colon cancer [102].
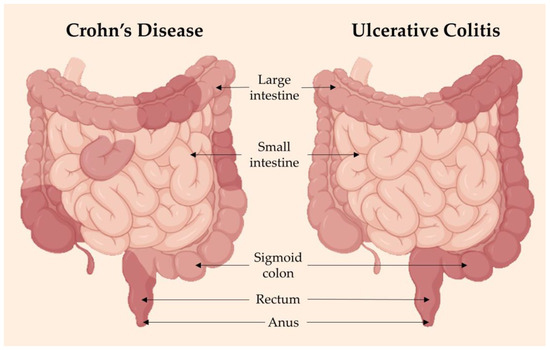
Figure 2.
Inflammatory bowel disease (IBD) is a term referring to two conditions: Crohn’s disease (CD) and ulcerative colitis (UC), both of which are characterized by chronic inflammation and damage of the gastrointestinal tract. CD (left) has inflammation in patches throughout the large bowel, while UC has uniform and continuous inflammation throughout. If not managed well, IBD flare-ups can be aggressive and debilitating to a person’s quality of life. Adapted from [102]. Image made using BioRender software (2023).
However, the underlying pathogenesis of manifestations may be different when comparing UC and CD— whereas CD seems to be primarily a Th1 cytokine mediated disease, with overproduction of IFN-γ, UC seems to be a “Th2-like” mediated disease with overproduction of IL-5 and IL-13 (cancer-promoting), but with normal IFN-γ and IL-4 levels. Furthermore, while involvement of a Th17 response is strongly suggested in CD, this is a drastically smaller source of effector cytokines than the Th1 response [103].
While serum studies of IBD have resulted in contradictory results, significant inflammatory markers have been recognized. For example, one study found increased serum levels of IL-6 and IL-7 (cancer-suppressive) in CD, and increased TNF-α, eotaxin and growth-related oncogene (a chemokine) in UC patients. Both CD and UC also demonstrated increased IL-8 relative to the controls [104]. A review of the studies using R&D Systems enzyme-linked immunosorbent assay (ELISA) suggests that IL-6 seems to be increased in both UC and CD relative to controls. However, testing using other technology is still contradictory [104].
Lastly, sera cytotoxic CD13 antibodies have been reported in 66% of human cytomegalovirus-immunoglobulin-G (HCMV-IgG) positive UC patients and 58% of HCMV-IgG positive CD patients [105]. In a study of 50 CD patients in Turkey, they had elevated ESR and CRP at baseline compared with healthy controls; however, no differences in Th17 levels were found relative to controls or disease activity [106].
3.2.5. Psoriasis
Systemic manifestations of psoriasis range from cardiovascular disease to malignancy, diabetes, IBD and other autoimmune disorders [107,108,109,110,111].
Although there have been multiple studies on the systemic inflammatory markers found in psoriasis patients, different studies report conflicting findings. However, a review of 78 studies found that standardized mean differences (SMD) of IL-6, CRP, TNF-α, E-selectin and intracellular adhesion molecule 1 (ICAM-1) were modestly but significantly higher in patients with severe disease relative to controls. Interestingly, there was no difference in the pooled SMD of serum IL-1β or IL-10 when comparing psoriasis patients to controls [112].
3.2.6. Dermatomyositis
Serum antibodies both favoring and negating the development of malignancy have been found in DM. For instance, antibodies to transcriptional intermediary factor 1 gamma (anti-p155 and anti-p155/140) [113,114], and antibodies to the nuclear matrix protein (anti-MJ or anti-p140) [115,116] were noted in DM patients.
There also seems to be elevations of TNF, IL-1 and IL-6 in both DM and polymyositis (PM) patients [117]. A study of 56 patients with juvenile DM not only correlated serum IFN-signature with DM, but also serum IL-6 [118]. Serum IL-15 (cancer-suppressive), IL-17, B-cell activating factor were found to be elevated in DM relative to healthy patients. Serum IL-18 was found to not only be elevated, but also correlate with disease activity in DM [119].
In one study, the number of anti-inflammatory T-reg cell population, and concentrations of serum TGF-β and IL-10 were also significantly lower in DM patient compared to healthy patients [120]. However, some studies have rather shown an increase in the serum IL-10 in patients with DM [119]. A different study also found significantly elevated levels of serum IL-2R and TNF-R1 in patients with DM [121].
Table 1 is a summarized view of all the above-mentioned cytokines, chemokines and other immune/inflammatory response molecules differently expressed in the diseases studied in this paper, in comparison with those differently expressed in HNC.

Table 1.
Summary of risk of head and neck cancer (HNC), standardized incidence ratio (SIR) values and cytokines, chemokines and other immune/inflammatory response molecules in relation to HNC of patients with Systemic Sclerosis (SSc), Systemic Lupus Erythematous (SLE), Rheumatic Arthritis (RA), Ulcerative Colitis (UC), Crohn’s disease (CD), Psoriasis and Dermatomyositis (DM). Upward arrows indicate increase, downward arrows indicate decrease of compounds.
3.3. Anti-Tumor Cell-Mediated Immunity Pathways in Queried Auto-Immune Systemic Diseases
3.3.1. Rheumatic Arthritis
There may be an imbalance towards increased Th1 and Th17 lymphocytes, and decreased Th2 and regulatory T-cells in patients with RA [122]. However, a study of 107 patients with RA found that 20% were anergic as demonstrated by skin tests [123]. A different study of 14 patients also found depressed cell mediated immunity, specifically delayed hypersensitivity reaction [124]. However, both studies report therapy as a possible source of depressed cell mediated immunity, with the latter study finding an association with use of prednisolone and AZA [124].
A series of studies suggest that while NKCs are increased in the synovial fluid of RA patients, they are found in lower numbers and express lower activity in blood [125,126].
3.3.2. Systemic Sclerosis
A study of 35 SSc patients found upregulation of intracellular IFN-γ and TGF-β (and increased levels of circulating IL-17, IL-6, IL-23 and IL-1α) [127]. Studies of patients with SSc have found reduced antibody-dependent cell mediated cytotoxicity and NKC activity, specially early in the illness of the diffuse-type SSc [128].
Another study of 28 patients with SSc found impaired lymphocyte transformation response relative to controls [129].
3.3.3. Systemic Lupus Erythematous
Animal models, specifically, NZB/NZW mice who develop an SLE-like illness, also suggest increased production of IFN-γ, and those treated with monoclonal antibody to IFN-γ show decreased mortality [92].
One study reported reduced antibody-dependent cell mediated toxicity by peripheral mononuclear cells from patients with SLE [128].
A different study of nine patients also found depressed cell mediated immunity, specifically delayed hypersensitivity reaction [124].
Lastly, a series of studies suggest that patients with SLE have lower number of NKCs, and with decreased activity and lower perforin [130].
3.3.4. Inflammatory Bowel Disease
Unlike UC, CD seems to be primarily a Th1 cytokine mediated disease, with overproduction of IFN-γ [103]. In fact, increased serum levels of IFN-γ have been reported [104]. In a study of 54 IBD patients, authors report increased intracellular IL-2 and IFN-γ in both resting and activated CD4+ and CD8+ cells relative to healthy controls [131].
In a study of 30 patients with CD, decreased cell mediated cytotoxicity was noted relative to healthy controls, and the decrease was weakly associated with disease activity [132]. Furthermore, a different study found significantly decreased circulating NKC activity in CD patients relative to normal controls. The extent of this decrease correlated with disease activity. No such depression was observed in UC patients [133].
3.3.5. Psoriasis
A study found reduced antibody-dependent cell mediated cytotoxicity in patients with psoriasis compared to controls [128]. Decreases in cell-mediated immunity have been reported in other studies. For example, one review reports that while no significant differences in lymphocyte subpopulation frequency were found in psoriasis patients, significant decreases in B-cell response, T-helper function, polymorphonuclear neutrophils (PMN) chemotaxis, PMN and monocyte mediated phagocytosis and lymphokine release were found [134].
While studies suggest increased NKCs in psoriatic plaques, there also seems to be decreased NK and NK-T cells in peripheral blood of psoriatic patients [135,136]. One study even reports decreased capacity of peripheral NKCs (from 45 psoriatic patients) to kill a tumor cell line [137]. A series of studies suggest that peripheral NK-T cells of patients with psoriasis are decreased. This decrease diminishes in response to therapy; however, peripheral NK-T cells do not return to the same level as in normal patients. The reason for this decrease is unclear [136].
Despite the supporting mounting evidence, this decrease in peripheral NKCs in psoriasis patients is still under debate as a series of studies do not report similar findings [138].
3.3.6. Dermatomyositis
Although IFN-γ itself is not detected in high levels in the blood of DM patients, IFN-γ signature (IFN-inducible proteins and transcripts) are highly upregulated [139]. In fact, another study found that the upregulation of IFN-inducible gene expression correlated with disease activity in DM or PM [140]. However, this association with disease activity may be confounded by the presence of specific autoantibodies [119]. Moreover, a study of 50 PM patients and 49 DM patients found a decreased percentage of CD8+ lymphocytes, and decreased IFN-γ expression of CD4+ and CD8+ cells. Similar changes were not noted in PM [141].
It should be noted that the patho-immunology of DM and PM are different in that DM presents abnormal cellular immunity while PM presents with abnormal humoral immunity.
DM seems to be a disease of decreased cellular immunity. Similar to other rheumatic diseases, patients with DM seem to have decreased CD8+ T-cells and NKCs in their peripheral blood [142,143,144]. Other evidence suggests that while DM patients have a depression of circulating lymphocyte counts, there is also a simultaneous increase in B-cell count/humoral immunity activity, suggesting a humoral pathophysiology [145,146]. However, decreased peripheral blood CD4+ and CD8+ cells do correlate with disease activity/therapy, and risk of death in DM patients [147]. Lastly, the mean absolute peripheral blood lymphocyte count in patients with paraneoplastic DM does not seem to statistically differ from DM patients without malignancy [144].
3.4. Effects of Anti-Biological and Immunosuppressive Drugs on Cell Mediated Immunity
3.4.1. Glucocorticoids
By inactivating key transcription factors (e.g., NF-kB, and activator protein 1), upregulating cytokine inhibitory proteins (e.g., I kappa B), and speeding degradation of cytokine mRNAs, glucocorticoids (GCS) inhibit the production of numerous cytokines, including IL-1β, TNF-α, IL-2, IL-3, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12 and IFN-γ [148].
By interfering with expression of adhesion molecules, GCS also impair ability of leukocytes to locate to the site of inflammation, leading to defective cellular immune response [149].
Despite the strong cellular response to GCS, it seems that treatment shifts patients’ cellular activity towards to Th2 profile while inhibiting Th1 profile. While some T-cell types, such as CD4+ and CD8+, undergo apoptosis in presence of GCS, others, such as NKCs, do not [150]. It seems that activated CD4+ Th1 cells are more susceptible to GCS induced apoptosis [151]. In fact, it seems that GCS shifts patients’ cytokine profile from Th1 to Th2 cytokines by more markedly inhibiting Th1 cytokine secretion [152]. This preference towards Th2 cytokine secretion may be resulting from GCS’ increased inhibition of IL-12, relative to IL-10 [153,154].
This preference may also promote selective differentiation of native T-cells to Th17 T-cells [155]. Specific Th17 subsets, specifically those expressing multi-drug resistance type 1 may actually be resistant to suppressive effects of GCS [156].
3.4.2. Azathioprine
Azathioprine (AZA, 1, Figure 3) is a purine analog derivative of 6-mercaptopurine that competitively inhibits enzymes of de novo purine base synthesis. It is included in the disease-modifying antirheumatic drugs (DMARD) used in refractory patients.
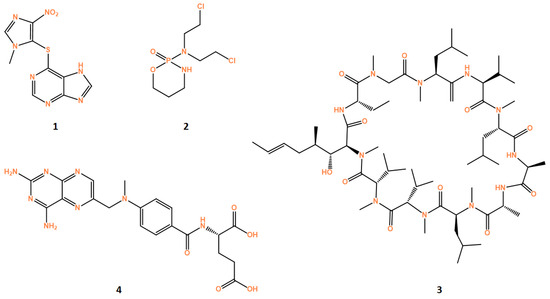
Figure 3.
Structures of drugs azathioprine (1), cyclosporine (2), cyclophosphamide (3) and methotrexate (4). All structures were obtained using ChemDraw software (version 12.0, PerkinElmer, Inc., Waltham, MA, USA).
AZA can selectively reduce peripheral NKCs [157]. It has also been described to lead to a dose-dependent inhibition of T-cell proliferation [158]. It also has been noted to induce T-cell apoptosis in CD patients by blocking Ras-related C3 botulinum toxin substrate 1 (Rac1) and its target genes, such as mitogen-activated protein kinase, NF-kB and B-cell lymphoma-extra-large. This suppression leads to a mitochondrial pathway of apoptosis [159].
One study showed that CD patients who respond to AZA have significantly higher peripheral T-cell apoptosis and lower IFN-γ expression relative to non-responders [160].
AZA does not appear to reduce serum levels of IL-6 or soluble IL-2R [157]. However, a study using cultured splenic mononuclear cells showed not only inhibited Th1 cytokine production from AZA exposure, but also dose dependent decreases in IFN-γ and TNF-α [161].
AZA treatment decreased numbers of CD4+CD69+ and CD4+IL-17+ cells in SLE patients. While systemic autoimmune diseases may already be associated with lymphopenia, AZA induced lymphopenia in SLE patients independent of SLE-associated lymphopenia. Furthermore, AZA-associated lymphopenia in SLE patients resulted in effector T-cells and T-regs with lower proliferation and suppressive activity when compared to that of patients with SLE-associated lymphopenia [162].
AZA-associated lymphopenia has also been reported in IBD patients [163,164].
3.4.3. Cyclophosphamide
Cyclophosphamide (CYC, 2, Figure 3) is an alkylating agent that forms cross-links with DNA, thereby interfering with DNA replication [165]. The dosage and administration schedule of CYC used to treat patients can cause variable immunological activities, which can be used to treat cancer, auto immune diseases and others. High dose CYC (e.g., doses of 100–200 mg/m2 of BSA [166]) are used to treat autoimmune disease, while low doses (1–3 mg/kg) can be used to treat cancer [167].
Evidence regarding use of high dose CYC demonstrates different effects on the immune system, as it is often associated with immunosuppression. CYC administration has been shown to cause increased delayed hypersensitivity skin reactions to specific antigens [168]. Short term administration of 50–250 mg/kg−2/day of CYC produces a dose-dependent lymphopenia and neutropenia [169].
However, the use of a single high dose CYC administration has been shown to eradicate tumor in a CD4+ T-cell dependent manner [166].
It is evident that high dose CYC (200 mg/kg) severely decreases the number of all T-cell subsets by >90%. It also decreased the ratio of CD4+CD25+ to CD4+ T cells. It seems that while both low dose and high dose CYC may have anti-tumor effects, low dose CYC does so through depletion of CD4+CD25+ T-cells and, thus, increased anti-tumor effect, while high dose CYC does so through alkylation and direct cytotoxicity [170].
3.4.4. Cyclosporine
Cyclosporine (CsA, 3, Figure 3) is a calcineurin inhibitor that is used for immunosuppression in many contexts, including refractory RA, severe UC, psoriasis/psoriatic arthritis, DM, SLE and SSc. With the advent of biological agents, the use of CsA has diminished in rheumatic disease [171].
While CsA does not affect already activated T-cells, it can cause a total block of the proliferation of all inactivated T-cells [172]. CsA evidently blocks expression of IL-2, IL-3, IL-4, TNF-α, CD40L, GM-CSF and IFN-γ [172,173,174,175].
It has also been described to lead to a dose dependent inhibition of T-cell proliferation and production of IFN-γ [158]. Furthermore, CsA seems to shift cytokine expression in RA patients from a Th1 to a Th2 profile [176]. However, conflicting evidence is reported, as Rentenaar et al. observed that Th1/Th2 balance in psoriasis patients treated with CsA was not affected [177]. Also in end-stage renal disease patients, CsA seems to increase TGF-β expression [178]. Lastly, studies report CsA inhibition of T-cell cytotoxicity and cytotoxic T-cell lines [179,180].
3.4.5. TNF-α Inhibitors
Several TNFi have been approved to treat numerous rhematic diseases in the Unites States, including etanercept, infliximab, adalimumab, certolizumab and golimumab. While very effective, the risk of malignancy is a significant hypothetical adverse effect of TNFi. This risk has been significantly studied; however, differences in study design, specific cancer type (e.g., lymphoma vs. other), and other challenges have led to contradictory findings [181,182,183,184,185,186,187,188]. While one study found no increase in risk of recurrence of HNCs in patients being treated with TNFi [52], no major study seems to have prospectively studied the risk for initial HNC.
As expected, TNFi increases expression of FoxP3 cells and the suppressive functions of CD4+CD25+ cells. However, there is question of exactly which subsets of T-regs are increased by TNFi, and whether that is a significant mechanism for immunosuppression [189]. Studies have demonstrated that whether alone or in combination with other rheumatic disease treatments, TNFi therapy decreases IFN-γ, and Th1 cytokine expression [190,191,192,193,194,195]. Decreases in Th17 and increases in T-regs are also seen ensuing TNFi therapy [190,195].
However, the influence of TNFi on immune system is more complicated. While one study found decreases in Th17 cells and IFN-γ producing CD8+ T-cells and increases in the proportion of T-regs in response to TNFi therapy, the percentage of IFN-γ-producing NK cells was also increased [190]. Another study found that TNFi therapy in psoriasis and IBD patients led to increased peripheral IFN-γ, IL-17, IL-10 and the proliferative response of CD4+ T-cells to T-cell receptor stimulation, while biopsied target tissues showed decreased Th1/Th17 cytokines and inflammatory genes [196]. A different study found that while in some RA patients TNFi therapy was successful and led to decreased Th1, Th17 and IL-21, in other RA patients therapy was unsuccessful and led to expansion of Th1/Th17 lymphocytes, and increased IL-21 [197]. Another study reports different findings specific to TNFi was used: while etanercept and infliximab led to increased Th1 prevalence 4 weeks after beginning therapy in RA patients, adalimumab led to a stable Th1 prevalence [198].
Study of 21 patients with psoriasis showed that adalimumab downregulates Th1, Th17 and Th22 at 12 weeks relative to the baseline of the patients. At baseline, serum levels of effector cytokines (IFN-γ, IL-17 and IL-22) and proinflammatory cytokines (IL-6, TNF-α) were higher in psoriasis patients relative to healthy controls. All serum cytokines declined significantly by week 12 [191].
In a study of 48 patients UC, 44 of whom were treated with infliximab and 4 with adalimumab, and 26 responded to therapy. In therapy responders, serum decreases in IL-6 were seen in responders at 14 weeks, while an increase in IL-13 and no change in serum levels of IL-1β, IL-4, IL-8, IL-10, IL-12p70, IL-17A, TNF-γ and IFN-γ at week 14 relative to baseline. Among non-responders, serum levels of IFN-γ and IL-12p70 were increased at 14 weeks, while serum levels of IL-1β, IL-4, IL-6, IL-8, IL-10, IL-13, IL-17A and TNF did not change relative to baseline [199].
Another study with 47 psoriasis patients, serum levels of IL-6, TNF-α and proinflammatory adipocytokines (leptin, resistin and visfatin) were higher in psoriasis patients and serum adiponectin (an anti-inflammatory cytokine) was lower in psoriasis patients relative to healthy controls. After 24 weeks of treatment with TNFi, TNF-α and IL-6 were decreased, and only leptin was the only adipocytokine that showed statistically significant decrease [200].
3.4.6. Methotrexate
Methotrexate (MTX, 4, Figure 3) is an analogue of folic acid. One mechanism by which MTX suppresses immunity is by inhibiting dihydrofolate reductase to reduce production of tetrahydrofolate (THF) and de novo production of nucleic acids [201]. While high dose MTX (>1 g/week) is used to treat malignant disease, lower doses (7.5–25 mg/week) may be used to treat rheumatic diseases [202], especially rheumatoid arthritis and psoriasis [203,204].
Several studies suggest that MTX administrations in RA patients favors shift from expression Th1 cytokines to Th2-cytokines, as seen by decreased IFN-γ, IL-12R, CXCR3 and increased IL-10 [205,206,207,208]. MTX also increases production of CD4+CD25+ T-regs [205].
However, conflicting evidence is reported. Rentenaar et al., report unaffected Th1/Th2 balance in psoriasis patients treated with MTX [177]. A different study, wherein SLE was induced in a murine model, found that MTX restores the Th1 dominant immune state of the disease-induced mice to a Th1/Th2 balance comparable to healthy controls [209].
Lastly, a review of 6806 DMARD treatment courses in RA patients found that the overall risk of cancer was increased in patients being treated with MTX, in comparison to the patients being treated with non-biological DMARDS and TNFi [210].
4. Discussion
While some studies report no increased risk of HNCs occurring in patients with systemic inflammatory diseases, many report increased risk, some as high as 25-fold. Despite some existing dispute over the existing data, due to the rarity of HNCs, lack of controls for confounders (e.g., age, sex, alcohol, smoking and betel nut use) in every study or report, and large variability in immunosuppressive treatment regiments make it difficult to arrive on any confident conclusions, significant associations have been reported, and they should be explored in hopes of understanding not only HNCs, but also other devastating cancers of similar etiology in patients suffering from systemic inflammatory diseases.
In fact, a recent systematic review assessed the incidence of oral cancer in patients with systemic immunosuppression and compared it to data from official databases (Globocan, WHO), hence showing an association and possible correlation between immunosuppression and the development of oral cancer. This study not only showed that immunosuppressed patients have a 50 times higher risk of developing oral cancer compared to the general population, but further emphasized the need for establishing tailor-made follow-up clinical protocols for primary prevention and screening in all immunosuppressed patients, based on the cause and severity of the immunosuppression [20].
From the analysis of the articles consulted, it can be understood that there is an increased risk presented in some of the reports may be due to the dual aspect of increased tumor-promoting inflammation due to active under-lying rheumatic disease and decreased anti-tumor cell mediated immunity due to treatment with immunosuppressive regiments. It is evident that several rheumatic diseases display elevated serum levels of inflammatory cytokines, such as IL-2, IL-6, TNF-α and markers of systemic inflammation. Furthermore, many of the major treatments discussed seem to affect cell-mediated immunity—they either downregulate expression of NKCs, CD8+ CTL cells, or thwart the Th1/Th2 balance towards the more anti-inflammatory Th2 profile, which is less effective in suppressing tumors than the Th1 cell mediated profile. It is arguable that combination of chronic elevation of pro-tumor inflammation due to disease flares, and the suppression of anti-tumor inflammatory roles, such as cell mediated immunity and cytotoxicity, by immunosuppressive treatments, may work together to increase the risk of HNCs in patients being treated for systemic inflammatory disorders.
While we have collected a body of evidence that warrants further study on the topic, there are many limitations to this report. For example, we only queried the most common and well-understood inflammatory disorders and their treatments. However, other conditions also lead to chronic systemic inflammation (e.g., tuberculosis, sarcoidosis, bullous pemphigoid, pemphigus vulgaris and ankylosing spondylitis), albeit they may not all be treated with immunosuppressive regiments. Furthermore, we did not investigate every possible treatment regimen used, nor the immune effects of combinatory use of several drugs at once. However, it is commonplace for patients to receive multiple regiments simultaneously to better control disease.
Moreover, many immunological studies we report only show the effects of treatment on the immune system over the short term, endpoints being measured any time between 4 weeks to only 12 months of drug administration. However, many of the rheumatic patients who developed HNCs did so due to often refractory, longstanding disease of several years. It is entirely possible that the short-term immunomodulatory effects of an immunosuppressant are completely different from its stabilized long-term effects.
Other limits of this study include the lack of reports on incidence of HNCs in patients with rheumatic disease. This may be due to report bias, especially with regard to case reports. Lastly, another limitation in this report is the lack of definition of HNCs by other reports—wherein we excluded esophageal, thyroid and skin cancers of the head from our definition, this may not be universal.
5. Conclusions
While no conclusions can be made with certainty, it is alarming that several studies report elevated risk of various HNCs in patients with systemic inflammatory diseases undergoing treatment. We argue that, per the immunological studies presented and the case reports and reviews, this increased risk may be through the combination of chronic increase in pro-tumor inflammatory factors due to disease activity and suppressed anti-tumor cell mediated immunity and cytotoxicity in response to the immunosuppressive treatments of the diseases. Regardless of whatever pro-oncogenic pathophysiology at play, it is warranted for oral- and non-oral physicians to continue investigating this risk, and its legitimacy. Similar to other cancers, HNCs are significantly more likely to be cured when diagnosed early. However, delayed diagnosis of HNCs may necessitate extremely invasive treatment, very poor prognosis, quality of life and healthcare cost. Therefore, it is an apt direction of study to delineate the incidence and pathophysiology of HNCs in systemic inflammatory diseases, to not only earlier diagnose HNCs in susceptible patients but also move towards prevent these devastating cancers.
Author Contributions
Conceptualization, R.A.M. and N.V.; methodology, M.P., R.A.M. and N.V.; formal analysis, M.P., R.A.M. and N.V.; investigation, M.P.; writing—original draft preparation, M.P.; writing—review and editing, R.A.M. and N.V.; supervision, N.V.; project administration, R.A.M. and N.V.; funding acquisition, N.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financed by FEDER—Fundo Europeu de Desenvolvimento Regional through the COMPETE 2020—Operational Program for Competitiveness and Internationalization (POCI), Portugal 2020, and by Portuguese funds through FCT—Fundação para a Ciência e a Tecnologia, in the framework of the projects in CINTESIS, R&D Unit (reference UIDB/4255/2020) and within the scope of the project RISE—LA/P/0053/2020. N.V. also acknowledges the support from FCT and FEDER (European Union), award number IF/00092/2014/CP1255/CT0004 and CHAIR in Onco-Innovation at FMUP.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
M.P. acknowledges FCT for funding her PhD grant (2021.07450.BD).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AIDS | Acquired immunodeficiency syndrome |
| AZA | Azathioprine |
| CCL | chemokine ligands |
| CD | Crohn’s disease |
| CRP | C-reactive protein |
| CsA | Cyclosporine |
| CTLs | Cytolytic T lymphocytes |
| CXCL | Chemokine platelet activating factor |
| CYC | Cyclophosphamide |
| DM | Dermatomyositis |
| DMARD | Disease-modifying antirheumatic drugs |
| EBV | Epstein-Barr virus |
| EGF | Epidermal growth factor |
| ESR | Westerngren erythrocyte sedimentation rate |
| GCS | Glucocorticoids |
| GM-GSF | Granulocyte macrophage colony stimulating factor |
| GRO | growth-related oncogene |
| HCMV-IgC | Human cytomegalovirus-immunoglobulin-G |
| HGF | hepatocyte growth factor |
| HIV | Human immunodeficiency virus |
| HNC | Head and neck cancers |
| HPV | Human papillomavirus |
| IBD | Inflammatory bowel disease |
| ICAM-1 | Intracellular adhesion molecule 1 |
| IFN-α/γ | Interferon alpha/gamma |
| IL | Interleukin |
| IL-2R | Interleukin 2 receptors |
| MAPK-1 | Mitogen-activated protein kinase-activator protein-1 |
| MCP1 | Monocyte chemoattractant protein 1 |
| MDC | Macrophage derived chemokine |
| MIP-1γ | Macrophage inflammatory protein 1 gamma |
| MMP | matrix metalloproteins |
| MTX | Methotrexate |
| NF-κB | Nuclear factor kappa B |
| NKCs | Natural killer cells |
| NPC | Nasopharyngeal carcinoma |
| PGE2 | Prostaglandin E2 |
| PM | Polymyositis |
| PMN | Polymorphonuclear neutrophils |
| RA | Rheumatoid Arthritis |
| SCCs | Squamous cell carcinomas |
| SIR | Standardized incidence ratio |
| SLE | Systemic lupus erythematous |
| SMD | Standardized mean differences |
| SSc | Systemic sclerosis |
| TAA | Tumor-associated antigens |
| TGF-α/β | Transforming growth factor alpha/beta |
| TNF-α | Tumor necrosis factor alpha |
| TNFi | Tumor necrosis factor inhibitors |
| T-regs | Regulatory T-cells |
| UC | Ulcerative colitis |
| VCAM-1 | Vascular cell adhesion molecule 1 |
| VEGF | vascular endothelial growth factor |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Mahmutović, L.; Bilajac, E.; Hromić-Jahjefendić, A. Meet the Insidious Players: Review of Viral Infections in Head and Neck Cancer Etiology with an Update on Clinical Trials. Microorganisms 2021, 9, 1001. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Karin, M.; Lawrence, T.; Nizet, V. Innate immunity gone awry: Linking microbial infections to chronic inflammation and cancer. Cell 2006, 124, 823–835. [Google Scholar] [CrossRef]
- van Kempen, L.C.; de Visser, K.E.; Coussens, L.M. Inflammation, proteases and cancer. Eur. J. Cancer 2006, 42, 728–734. [Google Scholar] [CrossRef]
- Advisory, P.-I. The Link between Periodontal Disease and Cancer: A Review. Available online: https://www.perioimplantadvisory.com/clinical-tips/surgical-techniques/article/16411685/the-link-between-periodontal-disease-and-cancer-a-review (accessed on 23 March 2023).
- Zeng, X.T.; Deng, A.P.; Li, C.; Xia, L.Y.; Niu, Y.M.; Leng, W.D. Periodontal disease and risk of head and neck cancer: A meta-analysis of observational studies. PLoS ONE 2013, 8, e79017. [Google Scholar] [CrossRef]
- Tezal, M.; Sullivan, M.A.; Reid, M.E.; Marshall, J.R.; Hyland, A.; Loree, T.; Lillis, C.; Hauck, L.; Wactawski-Wende, J.; Scannapieco, F.A. Chronic periodontitis and the risk of tongue cancer. Arch. Otolaryngol. Head. Neck Surg. 2007, 133, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Velly, A.M.; Franco, E.L.; Schlecht, N.; Pintos, J.; Kowalski, L.P.; Oliveira, B.V.; Curado, M.P. Relationship between dental factors and risk of upper aerodigestive tract cancer. Oral. Oncol. 1998, 34, 284–291. [Google Scholar] [CrossRef]
- Söder, B.; Yakob, M.; Meurman, J.H.; Andersson, L.C.; Klinge, B.; Söder, P. Periodontal disease may associate with breast cancer. Breast Cancer Res. Treat. 2011, 127, 497–502. [Google Scholar] [CrossRef]
- Gasparoto, T.H.; de Oliveira, C.E.; de Freitas, L.T.; Pinheiro, C.R.; Ramos, R.N.; da Silva, A.L.; Garlet, G.P.; da Silva, J.S.; Campanelli, A.P. Inflammatory events during murine squamous cell carcinoma development. J. Inflamm. 2012, 9, 46. [Google Scholar] [CrossRef]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic Inflammation and Cytokines in the Tumor Microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef] [PubMed]
- Zamarron, B.F.; Chen, W. Dual Roles of Immune Cells and Their Factors in Cancer Development and Progression. Int. J. Biol. Sci. 2011, 7, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Dranoff, G. Cytokines in cancer pathogenesis and cancer therapy. Nat. Rev. Cancer 2004, 4, 11–22. [Google Scholar] [CrossRef]
- Purgina, B.; Pantanowitz, L.; Seethala, R.R. A Review of Carcinomas Arising in the Head and Neck Region in HIV-Positive Patients. Pathol. Res. Int. 2011, 2011, 469150. [Google Scholar] [CrossRef] [PubMed]
- Rabinovics, N.; Mizrachi, A.; Hadar, T.; Ad-El, D.; Feinmesser, R.; Guttman, D.; Shpitzer, T.; Bachar, G. Cancer of the head and neck region in solid organ transplant recipients. Head. Neck 2014, 36, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Franks, A.L.; Slansky, J.E. Multiple associations between a broad spectrum of autoimmune diseases, chronic inflammatory diseases and cancer. Anticancer Res. 2012, 32, 1119–1136. [Google Scholar]
- Whiteside, T.L.; Chikamatsu, K.; Nagashima, S.; Okada, K. Antitumor effects of cytolytic T lymphocytes (CTL) and natural killer (NK) cells in head and neck cancer. Anticancer Res. 1996, 16, 2357–2364. [Google Scholar] [PubMed]
- Wang, Y.-T.; Kuo, L.-T.; Weng, H.-H.; Hsu, C.-M.; Tsai, M.-S.; Chang, G.-H.; Lee, Y.-C.; Huang, E.I.; Tsai, Y.-T. Systemic Immun e–Inflammation Index as a Predictor for Head and Neck Cancer Prognosis: A Meta-Analysis. Front. Oncol. 2022, 12, 899518. [Google Scholar] [CrossRef]
- Patini, R.; Cordaro, M.; Marchesini, D.; Scilla, F.; Gioco, G.; Rupe, C.; D’Agostino, M.A.; Lajolo, C. Is Systemic Immunosuppression a Risk Factor for Oral Cancer? A Systematic Review and Meta-Analysis. Cancers 2023, 15, 3077. [Google Scholar] [CrossRef]
- Zajac, A.J.; Harrington, L.E. Immune Response to Viruses: Cell-Mediated Immunity. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Kumari, P.; Debta, P.; Dixit, A. Oral Potentially Malignant Disorders: Etiology, Pathogenesis, and Transformation Into Oral Cancer. Front. Pharmacol. 2022, 13, 825266. [Google Scholar] [CrossRef]
- de Araújo, R.L.; Lyko Kde, F.; Funke, V.A.; Torres-Pereira, C.C. Oral cancer after prolonged immunosuppression for multiorgan chronic graft-versus-host disease. Rev. Bras. Hematol. Hemoter. 2014, 36, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Curtis, R.E.; Metayer, C.; Rizzo, J.D.; Socié, G.; Sobocinski, K.A.; Flowers, M.E.; Travis, W.D.; Travis, L.B.; Horowitz, M.M.; Deeg, H.J. Impact of chronic GVHD therapy on the development of squamous-cell cancers after hematopoietic stem-cell transplantation: An international case-control study. Blood 2005, 105, 3802–3811. [Google Scholar] [CrossRef]
- Leisenring, W.; Friedman, D.L.; Flowers, M.E.; Schwartz, J.L.; Deeg, H.J. Nonmelanoma skin and mucosal cancers after hematopoietic cell transplantation. J. Clin. Oncol. 2006, 24, 1119–1126. [Google Scholar] [CrossRef]
- Friedman, D.L.; Rovo, A.; Leisenring, W.; Locasciulli, A.; Flowers, M.E.; Tichelli, A.; Sanders, J.E.; Deeg, H.J.; Socie, G. Increased risk of breast cancer among survivors of allogeneic hematopoietic cell transplantation: A report from the FHCRC and the EBMT-Late Effect Working Party. Blood 2008, 111, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Atsuta, Y.; Suzuki, R.; Yamashita, T.; Fukuda, T.; Miyamura, K.; Taniguchi, S.; Iida, H.; Uchida, T.; Ikegame, K.; Takahashi, S.; et al. Continuing increased risk of oral/esophageal cancer after allogeneic hematopoietic stem cell transplantation in adults in association with chronic graft-versus-host disease. Ann. Oncol. 2014, 25, 435–441. [Google Scholar] [CrossRef]
- Sonis, S.T.; Amaral Mendes, R. Could the PI3K canonical pathway be a common link between chronic inflammatory conditions and oral carcinogenesis? J. Oral Pathol. Med. 2016, 45, 469–474. [Google Scholar] [CrossRef]
- Janowiak-Majeranowska, A.; Osowski, J.; Mikaszewski, B.; Majeranowski, A. Secondary Oral Cancer after Systemic Treatment of Hematological Malignancies and Oral GVHD: A Systematic Review. Cancers 2022, 14, 2175. [Google Scholar] [CrossRef] [PubMed]
- Zolkind, P.; Uppaluri, R. Checkpoint immunotherapy in head and neck cancers. Cancer Metastasis Rev. 2017, 36, 475–489. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef]
- Mok, C.C.; Lau, C.S. Pathogenesis of systemic lupus erythematosus. J. Clin. Pathol. 2003, 56, 481–490. [Google Scholar] [CrossRef]
- Srivastava, A.; Makarenkova, H.P. Innate Immunity and Biological Therapies for the Treatment of Sjögren’s Syndrome. Int. J. Mol. Sci. 2020, 21, 9172. [Google Scholar] [CrossRef]
- Cooper, L.; Good-Jacobson, K.L. Dysregulation of humoral immunity in chronic infection. Immunol. Cell Biol. 2020, 98, 456–466. [Google Scholar] [CrossRef]
- Pereira, D.; Martins, D.; Mendes, F. Immunotherapy in Head and Neck Cancer When, How, and Why? Biomedicines 2022, 10, 2151. [Google Scholar] [CrossRef]
- Derk, C.T.; Rasheed, M.; Spiegel, J.R.; Jimenez, S.A. Increased incidence of carcinoma of the tongue in patients with systemic sclerosis. J. Rheumatol. 2005, 32, 637–641. [Google Scholar]
- Kuo, C.F.; Luo, S.F.; Yu, K.H.; Chou, I.J.; Tseng, W.Y.; Chang, H.C.; Fang, Y.F.; Chiou, M.J.; See, L.C. Cancer risk among patients with systemic sclerosis: A nationwide population study in Taiwan. Scand. J. Rheumatol. 2012, 41, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, K.H.; Roda, G.; Brygo, A.; Delaporte, E.; Colombel, J.F. Oral Cancer and Oral Precancerous Lesions in Inflammatory Bowel Diseases: A Systematic Review. J. Crohns Colitis 2015, 9, 1043–1052. [Google Scholar] [CrossRef]
- Giagkou, E.; Christodoulou, D.K.; Katsanos, K.H. Mouth cancer in inflammatory bowel diseases. Oral Dis. 2016, 22, 260–264. [Google Scholar] [CrossRef]
- Vilas-Boas, F.; Magro, F.; Balhau, R.; Lopes, J.M.; Beça, F.; Eloy, C.; Lopes, S.; Macedo, G. Oral squamous cell carcinoma in a Crohn’s disease patient taking azathioprine: Case report and review of the literature. J. Crohns Colitis 2012, 6, 792–795. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Engel, S.H.; Hullar, T.E.; Adkins, D.R.; Thorstad, W.L.; Sunwoo, J.B. Temporal relationship between antitumor necrosis factor-alpha antibody therapy and recrudescence of head and neck squamous cell carcinoma. Laryngoscope 2008, 118, 450–452. [Google Scholar] [CrossRef]
- Li, A.C.; Warnakulasuriya, S.; Thompson, R.P. Neoplasia of the tongue in a patient with Crohn’s disease treated with azathioprine: Case report. Eur. J. Gastroenterol. Hepatol. 2003, 15, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.L.; Hsu, H.T.; Weng, S.F.; Lin, Y.S. Impact of head and neck malignancies on risk factors and survival in systemic lupus erythematosus. Acta Otolaryngol. 2013, 133, 1088–1095. [Google Scholar] [CrossRef]
- Mao, S.; Shen, H.; Zhang, J. Systemic lupus erythematosus and malignancies risk. J. Cancer Res. Clin. Oncol. 2016, 142, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Tong, H.; Xu, G.; Liu, P.; Meng, H.; Wang, J.; Zhao, X.; Tang, Y.; Jin, J. Systemic lupus erythematous and malignancy risk: A meta-analysis. PLoS ONE 2015, 10, e0122964. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Chang, Y.T.; Wang, C.B.; Wu, C.Y. Malignancy in systemic lupus erythematosus: A nationwide cohort study in Taiwan. Am. J. Med. 2010, 123, 1150.e1–1150.e6. [Google Scholar] [CrossRef]
- Han, J.Y.; Kim, H.; Jung, S.Y.; Jang, E.J.; Cho, S.K.; Sung, Y.K. Increased risk of malignancy in patients with systemic lupus erythematosus: Population-based cohort study in Korea. Arthritis Res. Ther. 2021, 23, 270. [Google Scholar] [CrossRef]
- Grimaldo-Carjevschi, M.; López-Labady, J.; Villarroel-Dorrego, M. Squamous cell carcinoma on the palate in a patient with systemic lupus erythematosus: Case report and review of literature. Lupus 2011, 20, 519–522. [Google Scholar] [CrossRef]
- Unsworth, J.D.; Baldwin, A.; Byrd, L. Systemic lupus erythematosus, pregnancy and carcinoma of the tongue. BMJ Case Rep. 2013, 2013, bcr2013008864. [Google Scholar] [CrossRef]
- Beattie, A.; Stassen, L.F.; Ekanayake, K. Oral Squamous Cell Carcinoma Presenting in a Patient Receiving Adalimumab for Rheumatoid Arthritis. J. Oral Maxillofac. Surg. 2015, 73, 2136–2141. [Google Scholar] [CrossRef] [PubMed]
- Chainani-Wu, N.; Chang, C.; Gross, A.J.; Yom, S.S.; Silverman, S., Jr. Oropharyngeal carcinoma arising after methotrexate and etanercept therapy for rheumatoid arthritis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, e261–e263. [Google Scholar] [CrossRef]
- Phillips, C.; Zeringue, A.L.; McDonald, J.R.; Eisen, S.A.; Ranganathan, P. Tumor Necrosis Factor Inhibition and Head and Neck Cancer Recurrence and Death in Rheumatoid Arthritis. PLoS ONE 2015, 10, e0143286. [Google Scholar] [CrossRef]
- Sugiyama, T.; Nakagawa, T.; Inui, M.; Tagawa, T. Tongue carcinoma in a young patient with dermatomyositis: A case report and review of the literature. J. Oral Maxillofac. Surg. 2001, 59, 925–928. [Google Scholar] [CrossRef] [PubMed]
- Adi, A.H.; Alturkmani, H.; Spock, T.; Williams Yohannes, P.; Wargo, S.; Szabo, E.; Gutkind, J.S.; Van Waes, C. Dermatomyositis paraneoplastic syndrome before symptomatic tonsillar squamous cell carcinoma: A case report. Head. Neck 2015, 37, E1–E3. [Google Scholar] [CrossRef] [PubMed]
- Botsios, C.; Ostuni, P.; Boscolo-Rizzo, P.; Da Mosto, M.C.; Punzi, L.; Marchiori, C. Dermatomyositis and malignancy of the pharynx in Caucasian patients: Report of two observations. Rheumatol. Int. 2003, 23, 309–311. [Google Scholar] [CrossRef]
- Holden, C.A.; Davis, R.W.; MacDonald, D.M. Dermatomyositis and salivary pleomorphic adenoma. J. R. Soc. Med. 1983, 76, 787–788. [Google Scholar] [CrossRef]
- Kim, S.W.; Shim, J.S.; Eun, Y.G.; Kwon, K.H. Tonsillar squamous cell carcinoma associated with dermatomyositis: The first 2 cases in Korea. Yonsei Med. J. 2010, 51, 605–608. [Google Scholar] [CrossRef]
- Liu, W.; Gao, Z.; Ni, D.; Li, W.; Li, Y.; Wang, H. [Dermatomyositis/polymyositis associated with head and neck malignancy]. Lin. Chuang Er Bi Yan Hou Ke Za Zhi 2005, 19, 340–341, 344. [Google Scholar]
- Song, X.; Peng, J.; Qiu, Q. Nasopharyngeal carcinoma and dermatomyositis (analysis of 12 cases). Lin. Chuang Er Bi Yan Hou Ke Za Zhi 1998, 12, 401–403. [Google Scholar] [PubMed]
- Peng, J.C.; Sheen, T.S.; Hsu, M.M. Nasopharyngeal carcinoma with dermatomyositis. Analysis of 12 cases. Arch. Otolaryngol. Head. Neck Surg. 1995, 121, 1298–1301. [Google Scholar] [CrossRef]
- Mebazâa, A.; Boussen, H.; Nouira, R.; Rokbani, L.; Ben Osman-Dhahri, A.; Bouaouina, N.; Laouani-Kechrid, C.; Louzir, B.; Zahaf, A.; Kamoun, M.R. Dermatomyositis and malignancy in Tunisia: A multicenter national retrospective study of 20 cases. J. Am. Acad. Dermatol. 2003, 48, 530–534. [Google Scholar] [CrossRef]
- Liu, W.C.; Ho, M.; Koh, W.P.; Tan, A.W.; Ng, P.P.; Chua, S.H.; Tan, S.H.; Tang, M.B. An 11-year review of dermatomyositis in Asian patients. Ann. Acad. Med. Singap. 2010, 39, 843–847. [Google Scholar] [CrossRef]
- Teo, P.; Tai, T.H.; Choy, D. Nasopharyngeal carcinoma with dermatomyositis. Int. J. Radiat. Oncol. Biol. Phys. 1989, 16, 471–474. [Google Scholar] [CrossRef]
- Chen, D.Y.; Chen, Y.M.; Lan, J.L.; Chen, H.H.; Hsieh, C.W.; Wey, S.J.; Lu, J.J. Polymyositis/dermatomyositis and nasopharyngeal carcinoma: The Epstein-Barr virus connection? J. Clin. Virol. 2010, 49, 290–295. [Google Scholar] [CrossRef]
- Abraham, Z.; Rosner, I.; Rozenbaum, M.; Goldenberg, K.; Weller, B. Dermatomyositis and nasopharyngeal carcinoma. J. Dermatol. 1998, 25, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Boffetta, P.; Gridley, G.; Lindelöf, B. Cancer risk in a population-based cohort of patients hospitalized for psoriasis in Sweden. J. Investig. Dermatol. 2001, 117, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Frentz, G.; Olsen, J.H. Malignant tumours and psoriasis: A follow-up study. Br. J. Dermatol. 1999, 140, 237–242. [Google Scholar] [CrossRef]
- Hannuksela-Svahn, A.; Pukkala, E.; Läärä, E.; Poikolainen, K.; Karvonen, J. Psoriasis, its treatment, and cancer in a cohort of Finnish patients. J. Investig. Dermatol. 2000, 114, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Seoane, J.; Varela-Centelles, P.; López-Niño, J.; Vázquez, I.; Abdulkader, I.; García-Caballero, T. Gingival mucinous adenocarcinoma of a minor salivary gland. J. Periodontol. 2010, 81, 626–631. [Google Scholar] [CrossRef]
- Carlesimo, M.; Mari, E.; La Pietra, M.; Orsini, D.; Pranteda, G.; Pranteda, G.; Grimaldi, M.; Arcese, A. Occurrence of salivary gland tumours in two patients treated with biological agents. Int. J. Immunopathol. Pharmacol. 2012, 25, 297–300. [Google Scholar] [CrossRef]
- Yamamoto, T.; Katayama, I.; Nishioka, K. Adenocarcinoma of the mouth in a patient with psoriasis under short-term cyclosporine therapy. Dermatology 1996, 193, 72–73. [Google Scholar] [CrossRef]
- Higley, H.; Persichitte, K.; Chu, S.; Waegell, W.; Vancheeswaran, R.; Black, C. Immunocytochemical localization and serologic detection of transforming growth factor beta 1. Association with type I procollagen and inflammatory cell markers in diffuse and limited systemic sclerosis, morphea, and Raynaud’s phenomenon. Arthritis Rheum. 1994, 37, 278–288. [Google Scholar] [CrossRef]
- Muangchant, C.; Pope, J.E. The significance of interleukin-6 and C-reactive protein in systemic sclerosis: A systematic literature review. Clin. Exp. Rheumatol. 2013, 31, 122–134. [Google Scholar]
- Scala, E.; Pallotta, S.; Frezzolini, A.; Abeni, D.; Barbieri, C.; Sampogna, F.; De Pità, O.; Puddu, P.; Paganelli, R.; Russo, G. Cytokine and chemokine levels in systemic sclerosis: Relationship with cutaneous and internal organ involvement. Clin. Exp. Immunol. 2004, 138, 540–546. [Google Scholar] [CrossRef]
- Needleman, B.W.; Wigley, F.M.; Stair, R.W. Interleukin-1, interleukin-2, interleukin-4, interleukin-6, tumor necrosis factor alpha, and interferon-gamma levels in sera from patients with scleroderma. Arthritis Rheum. 1992, 35, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Takemura, H.; Suzuki, H.; Yoshizaki, K.; Ogata, A.; Yuhara, T.; Akama, T.; Yamane, K.; Kashiwagi, H. Anti-interleukin-6 autoantibodies in rheumatic diseases. Increased frequency in the sera of patients with systemic sclerosis. Arthritis Rheum. 1992, 35, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Kurasawa, K.; Hirose, K.; Sano, H.; Endo, H.; Shinkai, H.; Nawata, Y.; Takabayashi, K.; Iwamoto, I. Increased interleukin-17 production in patients with systemic sclerosis. Arthritis Rheum. 2000, 43, 2455–2463. [Google Scholar] [CrossRef] [PubMed]
- Furuse, S.; Fujii, H.; Kaburagi, Y.; Fujimoto, M.; Hasegawa, M.; Takehara, K.; Sato, S. Serum concentrations of the CXC chemokines interleukin 8 and growth-regulated oncogene-alpha are elevated in patients with systemic sclerosis. J. Rheumatol. 2003, 30, 1524–1528. [Google Scholar]
- Denton, C.P.; Bickerstaff, M.C.; Shiwen, X.; Carulli, M.T.; Haskard, D.O.; Dubois, R.M.; Black, C.M. Serial circulating adhesion molecule levels reflect disease severity in systemic sclerosis. Br. J. Rheumatol. 1995, 34, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- van Bon, L.; Affandi, A.J.; Broen, J.; Christmann, R.B.; Marijnissen, R.J.; Stawski, L.; Farina, G.A.; Stifano, G.; Mathes, A.L.; Cossu, M.; et al. Proteome-wide analysis and CXCL4 as a biomarker in systemic sclerosis. N. Engl. J. Med. 2014, 370, 433–443. [Google Scholar] [CrossRef]
- Vandercappellen, J.; Van Damme, J.; Struyf, S. The role of the CXC chemokines platelet factor-4 (CXCL4/PF-4) and its variant (CXCL4L1/PF-4var) in inflammation, angiogenesis and cancer. Cytokine Growth Factor Rev. 2011, 22, 1–18. [Google Scholar] [CrossRef]
- von Wussow, P.; Jakschies, D.; Hartung, K.; Deicher, H. Presence of interferon and anti-interferon in patients with systemic lupus erythematosus. Rheumatol. Int. 1988, 8, 225–230. [Google Scholar] [CrossRef]
- Yee, A.M.; Yip, Y.K.; Fischer, H.D.; Buyon, J.P. Serum activity that confers acid lability to alpha-interferon in systemic lupus erythematosus: Its association with disease activity and its independence from circulating alpha-interferon. Arthritis Rheum. 1990, 33, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Maury, C.P.; Teppo, A.M. Tumor necrosis factor in the serum of patients with systemic lupus erythematosus. Arthritis Rheum. 1989, 32, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.L.; Chang, K.L.; Chiu, C.C.; Chiang, B.N.; Han, S.H.; Wang, S.R. Defective phagocytosis, decreased tumour necrosis factor-alpha production, and lymphocyte hyporesponsiveness predispose patients with systemic lupus erythematosus to infections. Scand. J. Rheumatol. 1989, 18, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Jacob, C.O.; Fronek, Z.; Lewis, G.D.; Koo, M.; Hansen, J.A.; McDevitt, H.O. Heritable major histocompatibility complex class II-associated differences in production of tumor necrosis factor alpha: Relevance to genetic predisposition to systemic lupus erythematosus. Proc. Natl. Acad. Sci. USA 1990, 87, 1233–1237. [Google Scholar] [CrossRef] [PubMed]
- Draeger, A.M.; Swaak, A.J.; van den Brink, H.G.; Aarden, L.A. T cell function in systemic lupus erythematosus: Normal production of and responsiveness to interleukin 2. Clin. Exp. Immunol. 1986, 64, 80–87. [Google Scholar]
- Sibbitt, W.L., Jr.; Kenny, C.; Spellman, C.W.; Ley, K.D.; Bankhurst, A.D. Lymphokines in autoimmunity: Relationship between interleukin-2 and interferon-gamma production in systemic lupus erythematosus. Clin. Immunol. Immunopathol. 1984, 32, 166–173. [Google Scholar] [CrossRef]
- Campen, D.H.; Horwitz, D.A.; Quismorio, F.P., Jr.; Ehresmann, G.R.; Martin, W.J. Serum levels of interleukin-2 receptor and activity of rheumatic diseases characterized by immune system activation. Arthritis Rheum. 1988, 31, 1358–1364. [Google Scholar] [CrossRef]
- Tokano, Y.; Murashima, A.; Takasaki, Y.; Hashimoto, H.; Okumura, K.; Hirose, S. Relation between soluble interleukin 2 receptor and clinical findings in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 1989, 48, 803–809. [Google Scholar] [CrossRef]
- Ohtsuka, K.; Gray, J.D.; Stimmler, M.M.; Horwitz, D.A. The relationship between defects in lymphocyte production of transforming growth factor-beta1 in systemic lupus erythematosus and disease activity or severity. Lupus 1999, 8, 90–94. [Google Scholar] [CrossRef]
- Jacob, C.O.; van der Meide, P.H.; McDevitt, H.O. In vivo treatment of (NZB X NZW)F1 lupus-like nephritis with monoclonal antibody to gamma interferon. J. Exp. Med. 1987, 166, 798–803. [Google Scholar] [CrossRef]
- Jacob, C.O.; Hwang, F.; Lewis, G.D.; Stall, A.M. Tumor necrosis factor alpha in murine systemic lupus erythematosus disease models: Implications for genetic predisposition and immune regulation. Cytokine 1991, 3, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Brennan, F.M.; Maini, R.N.; Feldmann, M. TNF alpha--a pivotal role in rheumatoid arthritis? Br. J. Rheumatol. 1992, 31, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Valencia, X.; Stephens, G.; Goldbach-Mansky, R.; Wilson, M.; Shevach, E.M.; Lipsky, P.E. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood 2006, 108, 253–261. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Leung, B.P.; Sturrock, R.D.; Field, M.; Liew, F.Y. Interleukin-15 mediates T cell-dependent regulation of tumor necrosis factor-alpha production in rheumatoid arthritis. Nat. Med. 1997, 3, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Alvaro-Gracia, J.M.; Zvaifler, N.J.; Firestein, G.S. Cytokines in chronic inflammatory arthritis. IV. Granulocyte/macrophage colony-stimulating factor-mediated induction of class II MHC antigen on human monocytes: A possible role in rheumatoid arthritis. J. Exp. Med. 1989, 170, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Burmester, G.R.; Weinblatt, M.E.; McInnes, I.B.; Porter, D.; Barbarash, O.; Vatutin, M.; Szombati, I.; Esfandiari, E.; Sleeman, M.A.; Kane, C.D.; et al. Efficacy and safety of mavrilimumab in subjects with rheumatoid arthritis. Ann. Rheum. Dis. 2013, 72, 1445–1452. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Wei, C.C.; Shieh, D.B.; Chan, C.H.; Chang, M.S. Anti-IL-20 monoclonal antibody alleviates inflammation in oral cancer and suppresses tumor growth. Mol. Cancer Res. 2012, 10, 1430–1439. [Google Scholar] [CrossRef]
- Kragstrup, T.W.; Otkjaer, K.; Holm, C.; Jørgensen, A.; Hokland, M.; Iversen, L.; Deleuran, B. The expression of IL-20 and IL-24 and their shared receptors are increased in rheumatoid arthritis and spondyloarthropathy. Cytokine 2008, 41, 16–23. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Surgeons, T. Inflammatory Bowel Disease (IBD). Available online: https://www.thesurgeons.sg/services/inflammatory-bowel-disease/ (accessed on 5 April 2023).
- Strober, W.; Fuss, I.J. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology 2011, 140, 1756–1767. [Google Scholar] [CrossRef]
- Korolkova, O.Y.; Myers, J.N.; Pellom, S.T.; Wang, L.; M’Koma, A.E. Characterization of Serum Cytokine Profile in Predominantly Colonic Inflammatory Bowel Disease to Delineate Ulcerative and Crohn’s Colitides. Clin. Med. Insights Gastroenterol. 2015, 8, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, A.; Boström, L.; Söderberg-Naucler, C. Detection of cytotoxic CD13-specific autoantibodies in sera from patients with ulcerative colitis and Crohn’s disease. J. Autoimmun. 2006, 26, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Sahin, A.; Calhan, T.; Cengiz, M.; Kahraman, R.; Aydin, K.; Ozdil, K.; Korachi, M.; Sokmen, H.M. Serum Interleukin 17 Levels in Patients with Crohn’s Disease: Real Life Data. Dis. Markers 2014, 2014, 690853. [Google Scholar] [CrossRef]
- Gelfand, J.M.; Neimann, A.L.; Shin, D.B.; Wang, X.; Margolis, D.J.; Troxel, A.B. Risk of myocardial infarction in patients with psoriasis. JAMA 2006, 296, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Brauchli, Y.B.; Jick, S.S.; Miret, M.; Meier, C.R. Psoriasis and risk of incident cancer: An inception cohort study with a nested case-control analysis. J. Investig. Dermatol. 2009, 129, 2604–2612. [Google Scholar] [CrossRef]
- Qureshi, A.A.; Choi, H.K.; Setty, A.R.; Curhan, G.C. Psoriasis and the risk of diabetes and hypertension: A prospective study of US female nurses. Arch. Dermatol. 2009, 145, 379–382. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Wajda, A.; Blanchard, J.F. The clustering of other chronic inflammatory diseases in inflammatory bowel disease: A population-based study. Gastroenterology 2005, 129, 827–836. [Google Scholar] [CrossRef]
- Makredes, M.; Robinson, D., Jr.; Bala, M.; Kimball, A.B. The burden of autoimmune disease: A comparison of prevalence ratios in patients with psoriatic arthritis and psoriasis. J. Am. Acad. Dermatol. 2009, 61, 405–410. [Google Scholar] [CrossRef]
- Dowlatshahi, E.A.; van der Voort, E.A.; Arends, L.R.; Nijsten, T. Markers of systemic inflammation in psoriasis: A systematic review and meta-analysis. Br. J. Dermatol. 2013, 169, 266–282. [Google Scholar] [CrossRef]
- Kaji, K.; Fujimoto, M.; Hasegawa, M.; Kondo, M.; Saito, Y.; Komura, K.; Matsushita, T.; Orito, H.; Hamaguchi, Y.; Yanaba, K.; et al. Identification of a novel autoantibody reactive with 155 and 140 kDa nuclear proteins in patients with dermatomyositis: An association with malignancy. Rheumatology 2007, 46, 25–28. [Google Scholar] [CrossRef]
- Targoff, I.N.; Mamyrova, G.; Trieu, E.P.; Perurena, O.; Koneru, B.; O’Hanlon, T.P.; Miller, F.W.; Rider, L.G. A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis Rheum. 2006, 54, 3682–3689. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, D.F.; Chung, L.S.; Christopher-Stine, L.; Zaba, L.; Li, S.; Mammen, A.L.; Rosen, A.; Casciola-Rosen, L. Most patients with cancer-associated dermatomyositis have antibodies to nuclear matrix protein NXP-2 or transcription intermediary factor 1γ. Arthritis Rheum. 2013, 65, 2954–2962. [Google Scholar] [CrossRef] [PubMed]
- Trallero-Araguás, E.; Labrador-Horrillo, M.; Selva-O’Callaghan, A.; Martínez, M.A.; Martínez-Gómez, X.; Palou, E.; Rodriguez-Sanchez, J.L.; Vilardell-Tarrés, M. Cancer-associated myositis and anti-p155 autoantibody in a series of 85 patients with idiopathic inflammatory myopathy. Medicine 2010, 89, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Shinjo, S.K.; de Souza, F.H.; de Moraes, J.C. Dermatomyositis and polymyositis: From immunopathology to immunotherapy (immunobiologics). Rev. Bras. Reumatol. 2013, 53, 101–110. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bilgic, H.; Ytterberg, S.R.; Amin, S.; McNallan, K.T.; Wilson, J.C.; Koeuth, T.; Ellingson, S.; Newman, B.; Bauer, J.W.; Peterson, E.J.; et al. Interleukin-6 and type I interferon-regulated genes and chemokines mark disease activity in dermatomyositis. Arthritis Rheum. 2009, 60, 3436–3446. [Google Scholar] [CrossRef]
- Kao, L.; Chung, L.; Fiorentino, D.F. Pathogenesis of dermatomyositis: Role of cytokines and interferon. Curr. Rheumatol. Rep. 2011, 13, 225–232. [Google Scholar] [CrossRef]
- Antiga, E.; Kretz, C.C.; Klembt, R.; Massi, D.; Ruland, V.; Stumpf, C.; Baroni, G.; Hartmann, M.; Hartschuh, W.; Volpi, W.; et al. Characterization of regulatory T cells in patients with dermatomyositis. J. Autoimmun. 2010, 35, 342–350. [Google Scholar] [CrossRef]
- Mielnik, P.; Chwalinska-Sadowska, H.; Wiesik-Szewczyk, E.; Maslinski, W.; Olesinska, M. Serum concentration of interleukin 15, interleukin 2 receptor and TNF receptor in patients with polymyositis and dermatomyositis: Correlation to disease activity. Rheumatol. Int. 2012, 32, 639–643. [Google Scholar] [CrossRef][Green Version]
- Gary S Firestein, M.; Monica Guma, M. Pathogenesis of Rheumatoid Arthritis. Available online: https://www.uptodate.com/contents/pathogenesis-of-rheumatoid-arthritis (accessed on 29 March 2023).
- Andrianakos, A.A.; Sharp, J.T.; Person, D.A.; Lidsky, M.D.; Duffy, J. Cell-mediated immunity in rheumatoid arthritis. Ann. Rheum. Dis. 1977, 36, 13. [Google Scholar] [CrossRef]
- Toh, B.H.; Roberts-Thomson, I.C.; Mathews, J.D.; Whittingham, S.; Mackay, I.R. Depression of cell-mediated immunity in old age and the immunopathic diseases, lupus erythematosus, chronic hepatitis and rheumatoid arthritis. Clin. Exp. Immunol. 1973, 14, 193–202. [Google Scholar]
- Yanagihara, Y.; Shiozawa, K.; Takai, M.; Kyogoku, M.; Shiozawa, S. Natural killer (NK) T cells are significantly decreased in the peripheral blood of patients with rheumatoid arthritis (RA). Clin. Exp. Immunol. 1999, 118, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Shegarfi, H.; Naddafi, F.; Mirshafiey, A. Natural killer cells and their role in rheumatoid arthritis: Friend or foe? Sci. World J. 2012, 2012, 491974. [Google Scholar] [CrossRef]
- Radstake, T.R.D.J.; van Bon, L.; Broen, J.; Hussiani, A.; Hesselstrand, R.; Wuttge, D.M.; Deng, Y.; Simms, R.; Lubberts, E.; Lafyatis, R. The Pronounced Th17 Profile in Systemic Sclerosis (SSc) Together with Intracellular Expression of TGFβ and IFNγ Distinguishes SSc Phenotypes. PLoS ONE 2009, 4, e5903. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.M.; Harding, B.; Mirick, G.R.; Schneider, J.; Quismorio, F.P.; Friou, G.J. Selective decrease in antibody-dependent cell-mediated cytotoxicity in systemic lupus erythematosus and progressive systemic sclerosis. Clin. Exp. Immunol. 1978, 34, 235–240. [Google Scholar]
- Hughes, P.; Holt, S.; Rowell, N.R.; Allonby, I.D.; Janis, K.; Dodd, J.K. The relationship of defective cell-mediated immunity to visceral disease in systemic sclerosis. Clin. Exp. Immunol. 1977, 28, 233–240. [Google Scholar]
- Mandal, A.; Viswanathan, C. Natural killer cells: In health and disease. Hematol./Oncol. Stem Cell Ther. 2015, 8, 47–55. [Google Scholar] [CrossRef]
- Funderburg, N.T.; Stubblefield Park, S.R.; Sung, H.C.; Hardy, G.; Clagett, B.; Ignatz-Hoover, J.; Harding, C.V.; Fu, P.; Katz, J.A.; Lederman, M.M.; et al. Circulating CD4(+) and CD8(+) T cells are activated in inflammatory bowel disease and are associated with plasma markers of inflammation. Immunology 2013, 140, 87–97. [Google Scholar] [CrossRef]
- Beeken, W.L.; Macpherson, B.R.; Gundel, R.M.; St Andre-Ukena, S.; Wood, S.G.; Sylwester, D.L. Depressed spontaneous cell-mediated cytotoxicity in Crohn’s disease. Clin. Exp. Immunol. 1983, 51, 351–358. [Google Scholar]
- Egawa, S.; Hiwatashi, N. Natural killer cell activity in patients with inflammatory bowel disease. J. Clin. Lab. Immunol. 1986, 20, 187–192. [Google Scholar]
- Ventura, M.; Colizzi, M.; Ottolenghi, A.; Polignano, A.; Sinisi, D.A.; Rantuccio, F.; Antonaci, S.; Bonomo, L. Cell-mediated immune response in psoriasis and psoriatic arthritis. Recent. Prog. Med. 1989, 80, 449–454. [Google Scholar]
- Cameron, A.L.; Kirby, B.; Griffiths, C.E. Circulating natural killer cells in psoriasis. Br. J. Dermatol. 2003, 149, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Tobin, A.M.; Lynch, L.; Kirby, B.; O’Farrelly, C. Natural Killer Cells in Psoriasis. J. Innate Immun. 2011, 3, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Majewski, S.; Wasik, M.; Jabłońska, S.; Kamiński, M.; Fraczykowska, M. Defective natural-killer- and killer-cell activity associated with increased polymorphonuclear leukocyte adherence in psoriasis. Arch. Dermatol. Res. 1986, 278, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Dunphy, S.; Gardiner, C.M. NK Cells and Psoriasis. J. Biomed. Biotechnol. 2011, 2011, 248317. [Google Scholar] [CrossRef]
- Greenberg, S.A. Type 1 interferons and myositis. Arthritis Res. Ther. 2010, 12 (Suppl. S1), S4. [Google Scholar] [CrossRef]
- Walsh, R.J.; Kong, S.W.; Yao, Y.; Jallal, B.; Kiener, P.A.; Pinkus, J.L.; Beggs, A.H.; Amato, A.A.; Greenberg, S.A. Type I interferon-inducible gene expression in blood is present and reflects disease activity in dermatomyositis and polymyositis. Arthritis Rheum. 2007, 56, 3784–3792. [Google Scholar] [CrossRef]
- Aleksza, M.; Szegedi, A.; Antal-Szalmás, P.; Irinyi, B.; Gergely, L.; Ponyi, A.; Hunyadi, J.; Sipka, S.; Zeher, M.; Szegedi, G.; et al. Altered cytokine expression of peripheral blood lymphocytes in polymyositis and dermatomyositis. Ann. Rheum. Dis. 2005, 64, 1485. [Google Scholar] [CrossRef]
- O’Gorman, M.R.; Bianchi, L.; Zaas, D.; Corrochano, V.; Pachman, L.M. Decreased levels of CD54 (ICAM-1)-positive lymphocytes in the peripheral blood in untreated patients with active juvenile dermatomyositis. Clin. Diagn. Lab. Immunol. 2000, 7, 693–697. [Google Scholar] [CrossRef]
- Mitsuo, A.; Morimoto, S.; Nakiri, Y.; Suzuki, J.; Kaneko, H.; Tokano, Y.; Tsuda, H.; Takasaki, Y.; Hashimoto, H. Decreased CD161+CD8+ T cells in the peripheral blood of patients suffering from rheumatic diseases. Rheumatology 2006, 45, 1477–1484. [Google Scholar] [CrossRef][Green Version]
- Viguier, M.; Fouéré, S.; de la Salmonière, P.; Rabian, C.; Lebbe, C.; Dubertret, L.; Morel, P.; Bachelez, H. Peripheral blood lymphocyte subset counts in patients with dermatomyositis: Clinical correlations and changes following therapy. Medicine 2003, 82, 82–86. [Google Scholar] [CrossRef]
- O’Gorman, M.R.; Corrochano, V.; Roleck, J.; Donovan, M.; Pachman, L.M. Flow cytometric analyses of the lymphocyte subsets in peripheral blood of children with untreated active juvenile dermatomyositis. Clin. Diagn. Lab. Immunol. 1995, 2, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Matsumoto, Y.; Ohashi, M.; Sasaki, R. Analysis of lymphocyte subpopulations in peripheral blood in adult and juvenile cases of dermatomyositis. J. Dermatol. 1993, 20, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.X.; Lu, X.; Zu, N.; Lin, B.; Wang, L.Y.; Shu, X.M.; Ma, L.; Wang, G.C. Clinical significance of peripheral blood lymphocyte subsets in patients with polymyositis and dermatomyositis. Clin. Rheumatol. 2012, 31, 1691–1697. [Google Scholar] [CrossRef] [PubMed]
- Brattsand, R.; Linden, M. Cytokine modulation by glucocorticoids: Mechanisms and actions in cellular studies. Aliment. Pharmacol. Ther. 1996, 10 (Suppl. S2), 81–90; discussion 91–92. [Google Scholar] [CrossRef]
- Boumpas, D.T.; Chrousos, G.P.; Wilder, R.L.; Cupps, T.R.; Balow, J.E. Glucocorticoid therapy for immune-mediated diseases: Basic and clinical correlates. Ann. Intern. Med. 1993, 119, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Ashwell, J.D.; Lu, F.W.; Vacchio, M.S. Glucocorticoids in T cell development and function*. Annu. Rev. Immunol. 2000, 18, 309–345. [Google Scholar] [CrossRef]
- Lanza, L.; Scudeletti, M.; Puppo, F.; Bosco, O.; Peirano, L.; Filaci, G.; Fecarotta, E.; Vidali, G.; Indiveri, F. Prednisone increases apoptosis in in vitro activated human peripheral blood T lymphocytes. Clin. Exp. Immunol. 1996, 103, 482–490. [Google Scholar] [CrossRef]
- Franchimont, D.; Louis, E.; Dewe, W.; Martens, H.; Vrindts-Gevaert, Y.; De Groote, D.; Belaiche, J.; Geenen, V. Effects of dexamethasone on the profile of cytokine secretion in human whole blood cell cultures. Regul. Pept. 1998, 73, 59–65. [Google Scholar] [CrossRef]
- Visser, J.; van Boxel-Dezaire, A.; Methorst, D.; Brunt, T.; de Kloet, E.R.; Nagelkerken, L. Differential regulation of interleukin-10 (IL-10) and IL-12 by glucocorticoids in vitro. Blood 1998, 91, 4255–4264. [Google Scholar] [CrossRef]
- Franchimont, D.; Galon, J.; Gadina, M.; Visconti, R.; Zhou, Y.; Aringer, M.; Frucht, D.M.; Chrousos, G.P.; O’Shea, J.J. Inhibition of Th1 immune response by glucocorticoids: Dexamethasone selectively inhibits IL-12-induced Stat4 phosphorylation in T lymphocytes. J. Immunol. 2000, 164, 1768–1774. [Google Scholar] [CrossRef]
- Weaver, C.T.; Harrington, L.E.; Mangan, P.R.; Gavrieli, M.; Murphy, K.M. Th17: An effector CD4 T cell lineage with regulatory T cell ties. Immunity 2006, 24, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, R.; Kozhaya, L.; McKevitt, K.; Djuretic, I.M.; Carlson, T.J.; Quintero, M.A.; McCauley, J.L.; Abreu, M.T.; Unutmaz, D.; Sundrud, M.S. Pro-inflammatory human Th17 cells selectively express P-glycoprotein and are refractory to glucocorticoids. J. Exp. Med. 2014, 211, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Winkelstein, A. The effects of azathioprine and 6 MP on immunity. J. Immunopharmacol. 1979, 1, 429–454. [Google Scholar] [CrossRef]
- Leitner, J.; Drobits, K.; Pickl, W.F.; Majdic, O.; Zlabinger, G.; Steinberger, P. The effects of Cyclosporine A and azathioprine on human T cells activated by different costimulatory signals. Immunol. Lett. 2011, 140, 74–80. [Google Scholar] [CrossRef][Green Version]
- Tiede, I.; Fritz, G.; Strand, S.; Poppe, D.; Dvorsky, R.; Strand, D.; Lehr, H.A.; Wirtz, S.; Becker, C.; Atreya, R.; et al. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J. Clin. Investig. 2003, 111, 1133–1145. [Google Scholar] [CrossRef]
- Cossu, A.; Biancone, L.; Ascolani, M.; Pallone, F.; Boirivant, M. “In vitro” azathioprine-induced changes in peripheral T cell apoptosis and IFN-γ production associate with drug response in patients with Crohn’s Disease. J. Crohn’s Colitis 2013, 7, 441–450. [Google Scholar] [CrossRef]
- Hildner, K.; Märker-Hermann, E.; Schlaak, J.F.; Becker, C.; Germann, T.; Schmitt, E.; Zum Büschenfelde, K.-H.M.; Neurath, M.F. Azathioprine, Mycophenolate Mofetil, and Methotrexate Specifically Modulate Cytokine Production by T Cells. Ann. N. Y. Acad. Sci. 1998, 859, 204–207. [Google Scholar] [CrossRef]
- Gómez-Martín, D.; Díaz-Zamudio, M.; Vanoye, G.; Crispín, J.C.; Alcocer-Varela, J. Quantitative and functional profiles of CD4+ lymphocyte subsets in systemic lupus erythematosus patients with lymphopenia. Clin. Exp. Immunol. 2011, 164, 17–25. [Google Scholar] [CrossRef]
- Al Rifai, A.; Prasad, N.; Shuttleworth, E.; McBurney, H.; Pushpakom, S.; Robinson, A.; Newman, W.; Campbell, S. Natural history of azathioprine-associated lymphopenia in inflammatory bowel disease patients: A prospective observational study. Eur. J. Gastroenterol. Hepatol. 2011, 23, 153–158. [Google Scholar] [CrossRef]
- Stocco, G.; Martelossi, S.; Barabino, A.; Decorti, G.; Bartoli, F.; Montico, M.; Gotti, A.; Ventura, A. Glutathione-S-transferase genotypes and the adverse effects of azathioprine in young patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2007, 13, 57–64. [Google Scholar] [CrossRef]
- Emadi, A.; Jones, R.J.; Brodsky, R.A. Cyclophosphamide and cancer: Golden anniversary. Nat. Rev. Clin. Oncol. 2009, 6, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Naito, T.; Baba, T.; Takeda, K.; Sasaki, S.; Nakamoto, Y.; Mukaida, N. High-dose cyclophosphamide induces specific tumor immunity with concomitant recruitment of LAMP1/CD107a-expressing CD4-positive T cells into tumor sites. Cancer Lett. 2015, 366, 93–99. [Google Scholar] [CrossRef]
- Madondo, M.T.; Quinn, M.; Plebanski, M. Low dose cyclophosphamide: Mechanisms of T cell modulation. Cancer Treat. Rev. 2016, 42, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Turk, J.L.; Parker, D. Effect of cyclophosphamide on immunological control mechanisms. Immunol. Rev. 1982, 65, 99–113. [Google Scholar] [CrossRef]
- Balow, J.E.; Hurley, D.L.; Fauci, A.S. Cyclophosphamide suppression of established cell-mediated immunity. Quantitative vs. qualitative changes in lymphocyte populations. J. Clin. Investig. 1975, 56, 65–70. [Google Scholar] [CrossRef]
- Motoyoshi, Y.; Kaminoda, K.; Saitoh, O.; Hamasaki, K.; Nakao, K.; Ishii, N.; Nagayama, Y.; Eguchi, K. Different mechanisms for anti-tumor effects of low- and high-dose cyclophosphamide. Oncol. Rep. 2006, 16, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Hardinger, K.; Magee, C.C. Pharmacology of Cyclosporine and Tacrolimus. Available online: https://www.uptodate.com/contents/pharmacology-of-cyclosporine-and-tacrolimus (accessed on 30 March 2023).
- Schreiber, S.L.; Crabtree, G.R. The mechanism of action of cyclosporin A and FK506. Immunol. Today 1992, 13, 136–142. [Google Scholar] [CrossRef]
- Suzuki, N.; Kaneko, S.; Ichino, M.; Mihara, S.; Wakisaka, S.; Sakane, T. In vivo mechanisms for the inhibition of T lymphocyte activation by long-term therapy with tacrolimus (FK-506): Experience in patients with Behçet’s disease. Arthritis Rheum. 1997, 40, 1157–1167. [Google Scholar] [CrossRef]
- Wiederrecht, G.; Lam, E.; Hung, S.; Martin, M.; Sigal, N. The mechanism of action of FK-506 and cyclosporin A. Ann. N. Y Acad. Sci. 1993, 696, 9–19. [Google Scholar] [CrossRef]
- Timmerman, L.A.; Clipstone, N.A.; Ho, S.N.; Northrop, J.P.; Crabtree, G.R. Rapid shuttling of NF-AT in discrimination of Ca2+ signals and immunosuppression. Nature 1996, 383, 837–840. [Google Scholar] [CrossRef]
- Kim, W.U.; Cho, M.L.; Kim, S.I.; Yoo, W.H.; Lee, S.S.; Joo, Y.S.; Min, J.K.; Hong, Y.S.; Lee, S.H.; Park, S.H.; et al. Divergent effect of cyclosporine on Th1/Th2 type cytokines in patients with severe, refractory rheumatoid arthritis. J. Rheumatol. 2000, 27, 324–331. [Google Scholar]
- Rentenaar, R.J.; Heydendael, V.M.R.; Diepen, F.N.J.V.; Rie, M.A.d.; Berge, I.J.M.T. Systemic Treatment with Either Cyclosporin A or Methotrexate Does Not Influence the T Helper 1/T Helper 2 Balance in Psoriatic Patients. J. Clin. Immunol. 2004, 24, 361–369. [Google Scholar] [CrossRef]
- Shin, G.T.; Khanna, A.; Ding, R.; Sharma, V.K.; Lagman, M.; Li, B.; Suthanthiran, M. In vivo expression of transforming growth factor-beta1 in humans: Stimulation by cyclosporine. Transplantation 1998, 65, 313–318. [Google Scholar] [CrossRef]
- Zeevi, A.; Eiras, G.; Bach, F.H.; Fung, J.J.; Todo, S.; Starzl, T.; Duquesnoy, R.J. Functional differentiation of human cytotoxic T lymphocytes in the presence of FK 506 and CyA. Transplant. Proc. 1990, 22, 106–109. [Google Scholar] [PubMed]
- Bunjes, D.; Hardt, C.; Röllinghoff, M.; Wagner, H. Cyclosporin A mediates immunosuppression of primary cytotoxic T cell responses by impairing the release of interleukin 1 and interleukin 2. Eur. J. Immunol. 1981, 11, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Askling, J.; Fahrbach, K.; Nordstrom, B.; Ross, S.; Schmid, C.H.; Symmons, D. Cancer risk with tumor necrosis factor alpha (TNF) inhibitors: Meta-analysis of randomized controlled trials of adalimumab, etanercept, and infliximab using patient level data. Pharmacoepidemiol. Drug Saf. 2011, 20, 119–130. [Google Scholar] [CrossRef]
- Askling, J.; van Vollenhoven, R.F.; Granath, F.; Raaschou, P.; Fored, C.M.; Baecklund, E.; Dackhammar, C.; Feltelius, N.; Cöster, L.; Geborek, P.; et al. Cancer risk in patients with rheumatoid arthritis treated with anti-tumor necrosis factor alpha therapies: Does the risk change with the time since start of treatment? Arthritis Rheum. 2009, 60, 3180–3189. [Google Scholar] [CrossRef]
- Dommasch, E.D.; Abuabara, K.; Shin, D.B.; Nguyen, J.; Troxel, A.B.; Gelfand, J.M. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: A systematic review and meta-analysis of randomized controlled trials. J. Am. Acad. Dermatol. 2011, 64, 1035–1050. [Google Scholar] [CrossRef]
- Mariette, X.; Matucci-Cerinic, M.; Pavelka, K.; Taylor, P.; van Vollenhoven, R.; Heatley, R.; Walsh, C.; Lawson, R.; Reynolds, A.; Emery, P. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: A systematic review and meta-analysis. Ann. Rheum. Dis. 2011, 70, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Bongartz, T.; Sutton, A.J.; Sweeting, M.J.; Buchan, I.; Matteson, E.L.; Montori, V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: Systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 2006, 295, 2275–2285. [Google Scholar] [CrossRef]
- Askling, J.; Fored, C.M.; Baecklund, E.; Brandt, L.; Backlin, C.; Ekbom, A.; Sundström, C.; Bertilsson, L.; Cöster, L.; Geborek, P.; et al. Haematopoietic malignancies in rheumatoid arthritis: Lymphoma risk and characteristics after exposure to tumour necrosis factor antagonists. Ann. Rheum. Dis. 2005, 64, 1414–1420. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Michaud, K. The effect of methotrexate and anti-tumor necrosis factor therapy on the risk of lymphoma in rheumatoid arthritis in 19,562 patients during 89,710 person-years of observation. Arthritis Rheum. 2007, 56, 1433–1439. [Google Scholar] [CrossRef]
- Thompson, A.E.; Rieder, S.W.; Pope, J.E. Tumor necrosis factor therapy and the risk of serious infection and malignancy in patients with early rheumatoid arthritis: A meta-analysis of randomized controlled trials. Arthritis Rheum. 2011, 63, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Oppenheim, J.J. Contrasting effects of TNF and anti-TNF on the activation of effector T cells and regulatory T cells in autoimmunity. FEBS Lett. 2011, 585, 3611–3618. [Google Scholar] [CrossRef]
- Aravena, O.; Pesce, B.; Soto, L.; Orrego, N.; Sabugo, F.; Wurmann, P.; Molina, M.C.; Alfaro, J.; Cuchacovich, M.; Aguillón, J.C.; et al. Anti-TNF therapy in patients with rheumatoid arthritis decreases Th1 and Th17 cell populations and expands IFN-γ-producing NK cell and regulatory T cell subsets. Immunobiology 2011, 216, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Luan, L.; Han, S.; Wang, H.; Liu, X. Down-regulation of the Th1, Th17, and Th22 pathways due to anti-TNF-α treatment in psoriasis. Int. Immunopharmacol. 2015, 29, 278–284. [Google Scholar] [CrossRef]
- Ross, S.E.; Williams, R.O.; Mason, L.J.; Mauri, C.; Marinova-Mutafchieva, L.; Malfait, A.M.; Maini, R.N.; Feldmann, M. Suppression of TNF-alpha expression, inhibition of Th1 activity, and amelioration of collagen-induced arthritis by rolipram. J. Immunol. 1997, 159, 6253–6259. [Google Scholar] [CrossRef]
- Eriksson, C.; Rantapää-Dahlqvist, S.; Sundqvist, K.G. Changes in chemokines and their receptors in blood during treatment with the TNF inhibitor infliximab in patients with rheumatoid arthritis. Scand. J. Rheumatol. 2013, 42, 260–265. [Google Scholar] [CrossRef]
- Herman, S.; Zurgil, N.; Machlav, S.; Shinberg, A.; Langevitz, P.; Ehrenfeld, M.; Deutsch, M. Distinct effects of anti-tumor necrosis factor combined therapy on TH1/TH2 balance in rheumatoid arthritis patients. Clin. Vaccine Immunol. 2011, 18, 1077–1082. [Google Scholar] [CrossRef]
- McGovern, J.L.; Nguyen, D.X.; Notley, C.A.; Mauri, C.; Isenberg, D.A.; Ehrenstein, M.R. Th17 cells are restrained by Treg cells via the inhibition of interleukin-6 in patients with rheumatoid arthritis responding to anti-tumor necrosis factor antibody therapy. Arthritis Rheum. 2012, 64, 3129–3138. [Google Scholar] [CrossRef]
- Bosè, F.; Raeli, L.; Garutti, C.; Frigerio, E.; Cozzi, A.; Crimi, M.; Caprioli, F.; Scavelli, R.; Altomare, G.; Geginat, J.; et al. Dual role of anti-TNF therapy: Enhancement of TCR-mediated T cell activation in peripheral blood and inhibition of inflammation in target tissues. Clin. Immunol. 2011, 139, 164–176. [Google Scholar] [CrossRef]
- Talotta, R.; Berzi, A.; Atzeni, F.; Batticciotto, A.; Clerici, M.; Sarzi-Puttini, P.; Trabattoni, D. Paradoxical Expansion of Th1 and Th17 Lymphocytes in Rheumatoid Arthritis Following Infliximab Treatment: A Possible Explanation for a Lack of Clinical Response. J. Clin. Immunol. 2015, 35, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Szalay, B.; Vásárhelyi, B.; Cseh, A.; Tulassay, T.; Deák, M.; Kovács, L.; Balog, A. The impact of conventional DMARD and biological therapies on CD4+ cell subsets in rheumatoid arthritis: A follow-up study. Clin. Rheumatol. 2014, 33, 175–185. [Google Scholar] [CrossRef]
- Dahlén, R.; Magnusson, M.K.; Bajor, A.; Lasson, A.; Ung, K.A.; Strid, H.; Öhman, L. Global mucosal and serum cytokine profile in patients with ulcerative colitis undergoing anti-TNF therapy. Scand. J. Gastroenterol. 2015, 50, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Campanati, A.; Ganzetti, G.; Giuliodori, K.; Marra, M.; Bonfigli, A.; Testa, R.; Offidani, A. Serum levels of adipocytokines in psoriasis patients receiving tumor necrosis factor-α inhibitors: Results of a retrospective analysis. Int. J. Dermatol. 2015, 54, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Seitz, M. Molecular and cellular effects of methotrexate. Curr. Opin. Rheumatol. 1999, 11, 226–232. [Google Scholar] [CrossRef]
- Schröder, O.; Stein, J. Low dose methotrexate in inflammatory bowel disease: Current status and future directions. Am. J. Gastroenterol. 2003, 98, 530–537. [Google Scholar] [CrossRef]
- Cipriani, P.; Ruscitti, P.; Carubbi, F.; Liakouli, V.; Giacomelli, R. Methotrexate: An old new drug in autoimmune disease. Expert. Rev. Clin. Immunol. 2014, 10, 1519–1530. [Google Scholar] [CrossRef]
- Yélamos, O.; Puig, L. Systemic methotrexate for the treatment of psoriasis. Expert. Rev. Clin. Immunol. 2015, 11, 553–563. [Google Scholar] [CrossRef]
- Xinqiang, S.; Fei, L.; Nan, L.; Yuan, L.; Fang, Y.; Hong, X.; Lixin, T.; Juan, L.; Xiao, Z.; Yuying, S.; et al. Therapeutic efficacy of experimental rheumatoid arthritis with low-dose methotrexate by increasing partially CD4+ CD25+ Treg cells and inducing Th1 to Th2 shift in both cells and cytokines. Biomed. Pharmacother. 2010, 64, 463–471. [Google Scholar] [CrossRef]
- Yamaki, K.; Uchida, H.; Harada, Y.; Li, X.; Yanagisawa, R.; Takano, H.; Hayashi, H.; Taneda, S.; Mori, Y.; Yoshino, S. Effect of methotrexate on Th1 and Th2 immune responses in mice. J. Pharm. Pharmacol. 2003, 55, 1661–1666. [Google Scholar] [CrossRef]
- Herman, S.; Zurgil, N.; Langevitz, P.; Ehrenfeld, M.; Deutsch, M. Methotrexate selectively modulates TH1/TH2 balance in active rheumatoid arthritis patients. Clin. Exp. Rheumatol. 2008, 26, 317–323. [Google Scholar] [PubMed]
- Ghoreschi, K.; Mrowietz, U.; Röcken, M. A molecule solves psoriasis? Systemic therapies for psoriasis inducing interleukin 4 and Th2 responses. J. Mol. Med. 2003, 81, 471–480. [Google Scholar] [CrossRef]
- Dayan, M.; Segal, R.; Mozes, E. Cytokine manipulation by methotrexate treatment in murine experimental systemic lupus erythematosus. J. Rheumatol. 1997, 24, 1075–1082. [Google Scholar] [PubMed]
- Solomon, D.H.; Kremer, J.M.; Fisher, M.; Curtis, J.R.; Furer, V.; Harrold, L.R.; Hochberg, M.C.; Reed, G.; Tsao, P.; Greenberg, J.D. Comparative cancer risk associated with methotrexate, other non-biologic and biologic disease-modifying anti-rheumatic drugs. Semin. Arthritis Rheum. 2014, 43, 489–497. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).