Mitochondria in the Spotlight: C. elegans as a Model Organism to Evaluate Xenobiotic-Induced Dysfunction
Abstract
1. Introduction
2. Mitochondrial Dysfunction
2.1. General Aspects
2.2. Human-Related Mitochondrial Disorders Modeled in C. elegans
3. C. elegans and Specific Toxins/Toxicants
3.1. Metals/Metalloid
3.1.1. Arsenic
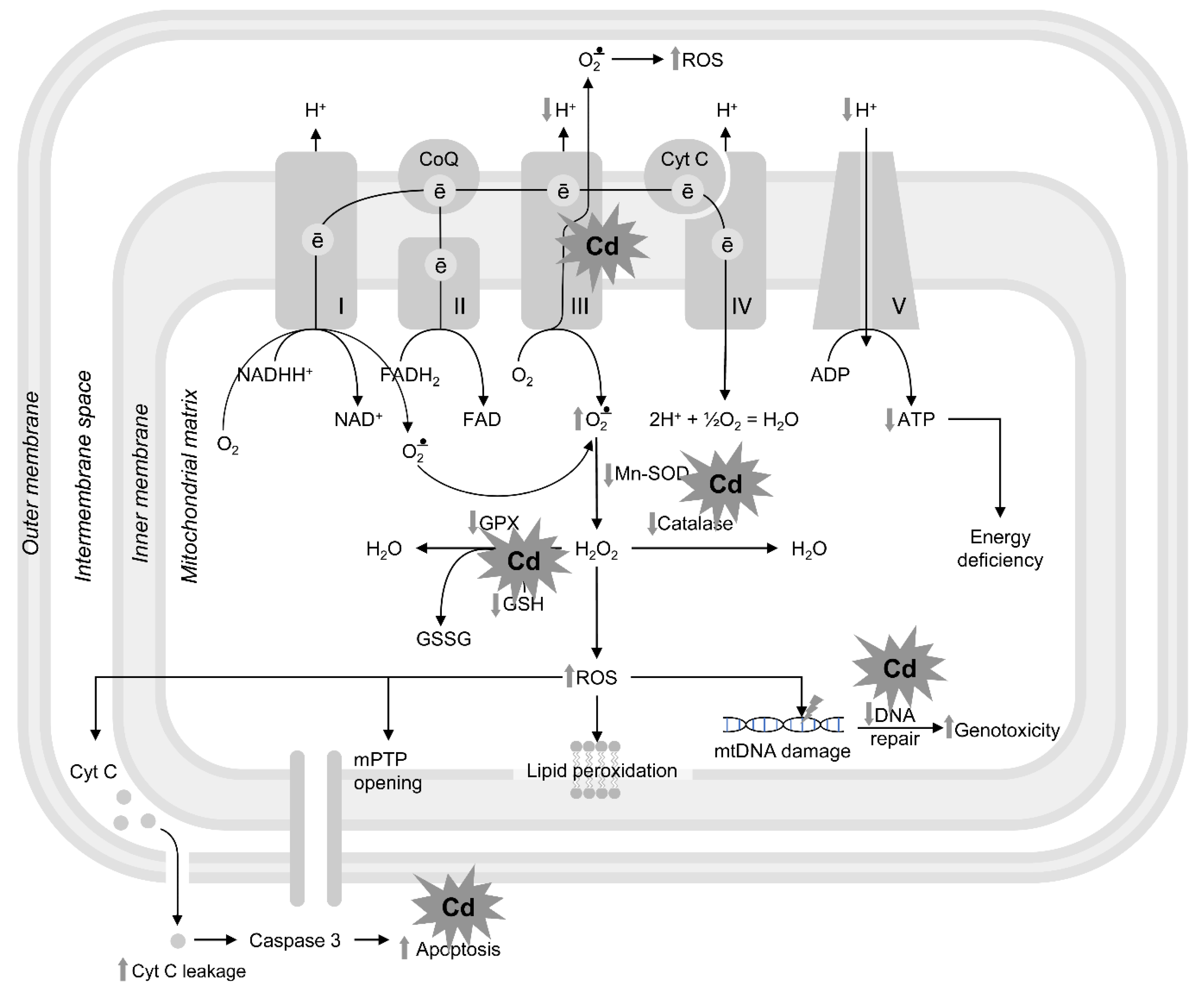
3.1.2. Cadmium
3.1.3. Manganese
3.1.4. Mercury
3.1.5. Iron and Cupper
3.2. Drugs of Abuse
3.3. Pesticides
3.3.1. Paraquat
3.3.2. Rotenone
3.3.3. Thiocarbamates (and Benomyl)
3.3.4. Organophosphates and Carbamates
3.3.5. Pyrethrins and Pyrethroids
3.3.6. Glyphosate
3.3.7. Triazines (Atrazine)
3.3.8. Organochlorines (Lindane)
3.3.9. Neonicotinoids
3.3.10. Other Pesticides
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, K.; Chen, G.; Li, W.; Kepp, O.; Zhu, Y.; Chen, Q. Mitophagy, Mitochondrial Homeostasis, and Cell Fate. Front. Cell Dev. Biol. 2020, 8, 467. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.; Ahn, B.Y.; Byun, K.; Cho, Y.M.; Yu, M.H.; Lee, B.; Hwang, D.; Park, K.S. A systems approach for decoding mitochondrial retrograde signaling pathways. Sci. Signal 2013, 6, rs4. [Google Scholar] [CrossRef] [PubMed]
- Bock, F.J.; Tait, S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef]
- Leung, M.C.; Williams, P.L.; Benedetto, A.; Au, C.; Helmcke, K.J.; Aschner, M.; Meyer, J.N. Caenorhabditis elegans: An emerging model in biomedical and environmental toxicology. Toxicol. Sci. 2008, 106, 5–28. [Google Scholar] [CrossRef]
- Duarte-Hospital, C.; Tete, A.; Brial, F.; Benoit, L.; Koual, M.; Tomkiewicz, C.; Kim, M.J.; Blanc, E.B.; Coumoul, X.; Bortoli, S. Mitochondrial Dysfunction as a Hallmark of Environmental Injury. Cells 2021, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Breton, C.V.; Song, A.Y.; Xiao, J.; Kim, S.J.; Mehta, H.H.; Wan, J.; Yen, K.; Sioutas, C.; Lurmann, F.; Xue, S.; et al. Effects of air pollution on mitochondrial function, mitochondrial DNA methylation, and mitochondrial peptide expression. Mitochondrion 2019, 46, 22–29. [Google Scholar] [CrossRef]
- Martins, A.C.; Gubert, P.; Li, J.; Ke, T.; Nicolai, M.M.; Moura, A.V.; Bornhorst, J.; Bowman, A.B.; Aschner, M. Caenorhabditis elegans as a Model to Study Manganese-Induced Neurotoxicity. Biomolecules 2022, 12, 1396. [Google Scholar] [CrossRef] [PubMed]
- Berks, M. The C. elegans genome sequencing project. C. elegans Genome Mapping and Sequencing Consortium. Genome Res. 1995, 5, 99–104. [Google Scholar] [CrossRef]
- Liu, Y.; Samuel, B.S.; Breen, P.C.; Ruvkun, G. Caenorhabditis elegans pathways that surveil and defend mitochondria. Nature 2014, 508, 406–410. [Google Scholar] [CrossRef]
- He, L.; Tronstad, K.J.; Maheshwari, A. Mitochondrial Dynamics during Development. Newborn 2023, 2, 19–44. [Google Scholar] [CrossRef] [PubMed]
- Mootha, V.K.; Bunkenborg, J.; Olsen, J.V.; Hjerrild, M.; Wisniewski, J.R.; Stahl, E.; Bolouri, M.S.; Ray, H.N.; Sihag, S.; Kamal, M.; et al. Integrated analysis of protein composition, tissue diversity, and gene regulation in mouse mitochondria. Cell 2003, 115, 629–640. [Google Scholar] [CrossRef]
- Nunnari, J.; Suomalainen, A. Mitochondria: In sickness and in health. Cell 2012, 148, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Glancy, B.; Kim, Y.; Katti, P.; Willingham, T.B. The Functional Impact of Mitochondrial Structure Across Subcellular Scales. Front. Physiol. 2020, 11, 541040. [Google Scholar] [CrossRef] [PubMed]
- Onraet, T.; Zuryn, S. C. elegans as a model to study mitochondrial biology and disease. Semin. Cell Dev. Biol. 2023. [Google Scholar] [CrossRef]
- Ploumi, C.; Kyriakakis, E.; Tavernarakis, N. Coupling of autophagy and the mitochondrial intrinsic apoptosis pathway modulates proteostasis and ageing in Caenorhabditis elegans. Cell Death Dis. 2023, 14, 110. [Google Scholar] [CrossRef]
- Waneka, G.; Svendsen, J.M.; Havird, J.C.; Sloan, D.B. Mitochondrial mutations in Caenorhabditis elegans show signatures of oxidative damage and an AT-bias. Genetics 2021, 219, iyab116. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Hunt, C.P.; Leung, M.C.; Bodhicharla, R.K.; McKeever, M.G.; Arrant, A.E.; Margillo, K.M.; Ryde, I.T.; Cyr, D.D.; Kosmaczewski, S.G.; Hammarlund, M.; et al. Exposure to mitochondrial genotoxins and dopaminergic neurodegeneration in Caenorhabditis elegans. PLoS ONE 2014, 9, e114459. [Google Scholar] [CrossRef]
- Yang, W.; Li, J.; Hekimi, S. A Measurable increase in oxidative damage due to reduction in superoxide detoxification fails to shorten the life span of long-lived mitochondrial mutants of Caenorhabditis elegans. Genetics 2007, 177, 2063–2074. [Google Scholar] [CrossRef]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial membrane potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef]
- Soares, A.T.G.; Rodrigues, L.B.L.J.; Salgueiro, W.G.; Dal Forno, A.H.C.; Rodrigues, C.F.; Sacramento, M.; Franco, J.; Alves, D.; Oliveira, R.P.; Pinton, S.; et al. Organoselenotriazoles attenuate oxidative damage induced by mitochondrial dysfunction in mev-1 Caenorhabditis elegans mutants. J. Trace Elem. Med. Biol. 2019, 53, 34–40. [Google Scholar] [CrossRef]
- Angeli, S.; Foulger, A.; Chamoli, M.; Peiris, T.H.; Gerencser, A.; Shahmirzadi, A.A.; Andersen, J.; Lithgow, G. The mitochondrial permeability transition pore activates the mitochondrial unfolded protein response and promotes aging. eLife 2021, 10, e63453. [Google Scholar] [CrossRef] [PubMed]
- Lemasters, J.J. Modulation of mitochondrial membrane permeability in pathogenesis, autophagy and control of metabolism. J. Gastroenterol. Hepatol. 2007, 22 (Suppl. S1), S31–S37. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, P.; Gerle, C.; Halestrap, A.P.; Jonas, E.A.; Karch, J.; Mnatsakanyan, N.; Pavlov, E.; Sheu, S.S.; Soukas, A.A. Identity, structure, and function of the mitochondrial permeability transition pore: Controversies, consensus, recent advances, and future directions. Cell Death Differ. 2023, 30, 1869–1885. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.B.; Samangouei, P.; Kalkhoran, S.B.; Hausenloy, D.J. The mitochondrial permeability transition pore and its role in myocardial ischemia reperfusion injury. J. Mol. Cell Cardiol. 2015, 78, 23–34. [Google Scholar] [CrossRef]
- Rottenberg, H.; Hoek, J.B. The path from mitochondrial ROS to aging runs through the mitochondrial permeability transition pore. Aging Cell 2017, 16, 943–955. [Google Scholar] [CrossRef]
- Ye, X.; Linton, J.M.; Schork, N.J.; Buck, L.B.; Petrascheck, M. A pharmacological network for lifespan extension in Caenorhabditis elegans. Aging Cell 2014, 13, 206–215. [Google Scholar] [CrossRef]
- Wang, C.; Youle, R.J. The role of mitochondria in apoptosis*. Annu. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef]
- Lettre, G.; Hengartner, M.O. Developmental apoptosis in C. elegans: A complex CEDnario. Nat. Rev. Mol. Cell Biol. 2006, 7, 97–108. [Google Scholar] [CrossRef]
- Ryan, K.C.; Ashkavand, Z.; Norman, K.R. The Role of Mitochondrial Calcium Homeostasis in Alzheimer’s and Related Diseases. Int. J. Mol. Sci. 2020, 21, 9153. [Google Scholar] [CrossRef]
- Denton, R.M. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim. Biophys. Acta 2009, 1787, 1309–1316. [Google Scholar] [CrossRef]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Ijomone, O.M.; Aluko, O.M.; Okoh, C.O.A.; Martins, A.C., Jr.; Aschner, M. Role for calcium signaling in manganese neurotoxicity. J. Trace Elem. Med. Biol. 2019, 56, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Sarasija, S.; Norman, K.R. Role of Presenilin in Mitochondrial Oxidative Stress and Neurodegeneration in Caenorhabditis elegans. Antioxidants 2018, 7, 111. [Google Scholar] [CrossRef]
- Chan, S.L.; Mayne, M.; Holden, C.P.; Geiger, J.D.; Mattson, M.P. Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. J. Biol. Chem. 2000, 275, 18195–18200. [Google Scholar] [CrossRef]
- Twig, G.; Shirihai, O.S. The interplay between mitochondrial dynamics and mitophagy. Antioxid. Redox Signal. 2011, 14, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Leboutet, R.; Chen, Y.; Legouis, R.; Culetto, E. Mitophagy during development and stress in C. elegans. Mech. Ageing Dev. 2020, 189, 111266. [Google Scholar] [CrossRef]
- Makarov, M.; Korkotian, E. Differential Role of Active Compounds in Mitophagy and Related Neurodegenerative Diseases. Toxins 2023, 15, 202. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.N.; Leuthner, T.C.; Luz, A.L. Mitochondrial fusion, fission, and mitochondrial toxicity. Toxicology 2017, 391, 42–53. [Google Scholar] [CrossRef]
- Xu, J.; Du, P.; Liu, X.; Xu, X.; Ge, Y.; Zhang, C. Curcumin supplementation increases longevity and antioxidant capacity in Caenorhabditis elegans. Front. Pharmacol. 2023, 14, 1195490. [Google Scholar] [CrossRef]
- Wallace, D.C. Mitochondrial DNA mutations in disease and aging. Environ. Mol. Mutagen. 2010, 51, 440–450. [Google Scholar] [CrossRef]
- Lin, Y.F.; Schulz, A.M.; Pellegrino, M.W.; Lu, Y.; Shaham, S.; Haynes, C.M. Maintenance and propagation of a deleterious mitochondrial genome by the mitochondrial unfolded protein response. Nature 2016, 533, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Kim, E.J.E.; Lee, S.V. Mitochondria-mediated defense mechanisms against pathogens in Caenorhabditis elegans. BMB Rep. 2018, 51, 274–279. [Google Scholar] [CrossRef] [PubMed]
- West, A.P.; Shadel, G.S.; Ghosh, S. Mitochondria in innate immune responses. Nat. Rev. Immunol. 2011, 11, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Rentas, C.; Caldwell, G.A.; Caldwell, K.A. Phenazine derivatives cause proteotoxicity and stress in C. elegans. Neurosci. Lett. 2015, 584, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, L.A.; Manoil, C. Pseudomonas aeruginosa PAO1 kills Caenorhabditis elegans by cyanide poisoning. J. Bacteriol. 2001, 183, 6207–6214. [Google Scholar] [CrossRef] [PubMed]
- van Horssen, J.; van Schaik, P.; Witte, M. Inflammation and mitochondrial dysfunction: A vicious circle in neurodegenerative disorders? Neurosci. Lett. 2019, 710, 132931. [Google Scholar] [CrossRef]
- Felix, M.A.; Duveau, F. Population dynamics and habitat sharing of natural populations of Caenorhabditis elegans and C. briggsae. BMC Biol. 2012, 10, 59. [Google Scholar] [CrossRef]
- Pellegrino, M.W.; Nargund, A.M.; Kirienko, N.V.; Gillis, R.; Fiorese, C.J.; Haynes, C.M. Mitochondrial UPR-regulated innate immunity provides resistance to pathogen infection. Nature 2014, 516, 414–417. [Google Scholar] [CrossRef]
- Deng, P.; Uma Naresh, N.; Du, Y.; Lamech, L.T.; Yu, J.; Zhu, L.J.; Pukkila-Worley, R.; Haynes, C.M. Mitochondrial UPR repression during Pseudomonas aeruginosa infection requires the bZIP protein ZIP-3. Proc. Natl. Acad. Sci. USA 2019, 116, 6146–6151. [Google Scholar] [CrossRef]
- Chikka, M.R.; Anbalagan, C.; Dvorak, K.; Dombeck, K.; Prahlad, V. The Mitochondria-Regulated Immune Pathway Activated in the C. elegans Intestine Is Neuroprotective. Cell Rep. 2016, 16, 2399–2414. [Google Scholar] [CrossRef]
- Meyer, J.N.; Leung, M.C.; Rooney, J.P.; Sendoel, A.; Hengartner, M.O.; Kisby, G.E.; Bess, A.S. Mitochondria as a target of environmental toxicants. Toxicol. Sci. 2013, 134, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.V.; Mesadri, J.; Goncalves, D.F.; Cordeiro, L.M.; Franzen da Silva, A.; Obetine Baptista, F.B.; Wagner, R.; Dalla Corte, C.L.; Soares, F.A.A.; Avila, D.S. Neurotoxicity induced by toluene: In silico and in vivo evidences of mitochondrial dysfunction and dopaminergic neurodegeneration. Environ. Pollut. 2022, 298, 118856. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.V.; Charao, M.F.; Jacques, M.T.; Dos Santos, A.L.A.; Luchese, C.; Pinton, S.; Avila, D.S. Airborne toluene exposure causes germline apoptosis and neuronal damage that promotes neurobehavioural changes in Caenorhabditis elegans. Environ. Pollut. 2020, 256, 113406. [Google Scholar] [CrossRef] [PubMed]
- Herst, P.M.; Rowe, M.R.; Carson, G.M.; Berridge, M.V. Functional Mitochondria in Health and Disease. Front Endocrinol. 2017, 8, 296. [Google Scholar] [CrossRef]
- Burgeois, M.; Goutieres, F.; Chretien, D.; Rustin, P.; Munnich, A.; Aicardi, J. Deficiency in complex II of the respiratory chain, presenting as a leukodystrophy in two sisters with Leigh syndrome. Brain Dev. 1992, 14, 404–408. [Google Scholar] [CrossRef]
- Baysal, B.E.; Ferrell, R.E.; Willett-Brozick, J.E.; Lawrence, E.C.; Myssiorek, D.; Bosch, A.; van der Mey, A.; Taschner, P.E.; Rubinstein, W.S.; Myers, E.N.; et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science 2000, 287, 848–851. [Google Scholar] [CrossRef]
- Hosokawa, H.; Ishii, N.; Ishida, H.; Ichimori, K.; Nakazawa, H.; Suzuki, K. Rapid accumulation of fluorescent material with aging in an oxygen-sensitive mutant mev-1 of Caenorhabditis elegans. Mech. Ageing Dev. 1994, 74, 161–170. [Google Scholar] [CrossRef]
- Senoo-Matsuda, N.; Hartman, P.S.; Akatsuka, A.; Yoshimura, S.; Ishii, N. A complex II defect affects mitochondrial structure, leading to ced-3- and ced-4-dependent apoptosis and aging. J. Biol. Chem. 2003, 278, 22031–22036. [Google Scholar] [CrossRef]
- Yoon, D.S.; Choi, Y.; Cha, D.S.; Zhang, P.; Choi, S.M.; Alfhili, M.A.; Polli, J.R.; Pendergrass, D.; Taki, F.A.; Kapalavavi, B.; et al. Triclosan Disrupts SKN-1/Nrf2-Mediated Oxidative Stress Response in C. elegans and Human Mesenchymal Stem Cells. Sci. Rep. 2017, 7, 12592. [Google Scholar] [CrossRef]
- Hershberger, K.; Leuthner, T.; Waters, T.; Meyer, J. Caenorhabditis elegans strain sensitivity to sodium arsenite exposure is varied based on age and outcome measured. MicroPubl Biol. 2019, 2019. [Google Scholar] [CrossRef]
- Pallotta, M.T.; Tascini, G.; Crispoldi, R.; Orabona, C.; Mondanelli, G.; Grohmann, U.; Esposito, S. Wolfram syndrome, a rare neurodegenerative disease: From pathogenesis to future treatment perspectives. J. Transl. Med. 2019, 17, 238. [Google Scholar] [CrossRef] [PubMed]
- Boos, J.R.; Jandrain, H.N.; Hagiuda, E.; Taguchi, A.T.; Hasegawa, K.; Fedun, B.L.; Taylor, S.J.; Elad, S.M.; Faber, S.E.; Kumasaka, T.; et al. Structure and biological evaluation of Caenorhabditis elegans CISD-1/mitoNEET, a KLP-17 tail domain homologue, supports attenuation of paraquat-induced oxidative stress through a p38 MAPK-mediated antioxidant defense response. Adv. Redox Res. 2022, 6, 100048. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, D.D.L.; Garcia, A.A.; Lee, L.; Mochly-Rosen, D.; Ferreira, J.C.B. Mitochondrial Fusion, Fission, and Mitophagy in Cardiac Diseases: Challenges and Therapeutic Opportunities. Antioxid. Redox Signal. 2022, 36, 844–863. [Google Scholar] [CrossRef]
- Serasinghe, M.N.; Chipuk, J.E. Mitochondrial Fission in Human Diseases. Handb. Exp. Pharmacol. 2017, 240, 159–188. [Google Scholar] [CrossRef] [PubMed]
- Labrousse, A.M.; Zappaterra, M.D.; Rube, D.A.; van der Bliek, A.M.C. elegans dynamin-related protein DRP-1 controls severing of the mitochondrial outer membrane. Mol. Cell 1999, 4, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Luz, A.L.; Godebo, T.R.; Smith, L.L.; Leuthner, T.C.; Maurer, L.L.; Meyer, J.N. Deficiencies in mitochondrial dynamics sensitize Caenorhabditis elegans to arsenite and other mitochondrial toxicants by reducing mitochondrial adaptability. Toxicology 2017, 387, 81–94. [Google Scholar] [CrossRef]
- Kim, I.; Lemasters, J.J. Mitophagy selectively degrades individual damaged mitochondria after photoirradiation. Antioxid. Redox Signal. 2011, 14, 1919–1928. [Google Scholar] [CrossRef]
- Vinish, M.; Prabhakar, S.; Khullar, M.; Verma, I.; Anand, A. Genetic screening reveals high frequency of PARK2 mutations and reduced Parkin expression conferring risk for Parkinsonism in North West India. J. Neurol. Neurosurg. Psychiatry 2010, 81, 166–170. [Google Scholar] [CrossRef]
- Hartman, J.H.; Gonzalez-Hunt, C.; Hall, S.M.; Ryde, I.T.; Caldwell, K.A.; Caldwell, G.A.; Meyer, J.N. Genetic Defects in Mitochondrial Dynamics in Caenorhabditis elegans Impact Ultraviolet C Radiation- and 6-hydroxydopamine-Induced Neurodegeneration. Int. J. Mol. Sci. 2019, 20, 3202. [Google Scholar] [CrossRef]
- Bornhorst, J.; Chakraborty, S.; Meyer, S.; Lohren, H.; Brinkhaus, S.G.; Knight, A.L.; Caldwell, K.A.; Caldwell, G.A.; Karst, U.; Schwerdtle, T.; et al. The effects of pdr1, djr1.1 and pink1 loss in manganese-induced toxicity and the role of alpha-synuclein in C. elegans. Metallomics 2014, 6, 476–490. [Google Scholar] [CrossRef]
- Chakraborty, S.; Chen, P.; Bornhorst, J.; Schwerdtle, T.; Schumacher, F.; Kleuser, B.; Bowman, A.B.; Aschner, M. Loss of pdr-1/parkin influences Mn homeostasis through altered ferroportin expression in C. elegans. Metallomics 2015, 7, 847–856. [Google Scholar] [CrossRef]
- Diaz Rizo, O.; Casanova Diaz, A.O.; Torres Ramos, A.G.; Ramos Lopez, D. Heavy metals concentration, pollution indexes, and health risk assessment of urban road dust in the historical center of Havana, Cuba. Environ. Monit. Assess. 2023, 195, 349. [Google Scholar] [CrossRef]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef]
- Kumari, B.; Bharti, V.K. Recent advancements in toxicology, modern technology for detection, and remedial measures for arsenic exposure: Review. Biotechnol. Genet. Eng. Rev. 2022, 1–43. [Google Scholar] [CrossRef]
- Kesari, V.P.; Kumar, A.; Khan, P.K. Genotoxic potential of arsenic at its reference dose. Ecotoxicol. Environ. Saf. 2012, 80, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.H.; Li, C.Y.; Cheng, Y.Y.; Guo, H.R. Arsenic in Drinking Water and Incidences of Leukemia and Lymphoma: Implication for Its Dual Effects in Carcinogenicity. Front. Public Health 2022, 10, 863882. [Google Scholar] [CrossRef]
- Paulelli, A.C.C.; Martins, A.C., Jr.; Batista, B.L.; Barbosa, F., Jr. Evaluation of uptake, translocation, and accumulation of arsenic species by six different Brazilian rice (Oryza sativa L.) cultivars. Ecotoxicol. Environ. Saf. 2019, 169, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Nuvolone, D.; Stoppa, G.; Petri, D.; Voller, F. Long-term exposure to low-level arsenic in drinking water is associated with cause-specific mortality and hospitalization in the Mt. Amiata area (Tuscany, Italy). BMC Public Health 2023, 23, 71. [Google Scholar] [CrossRef]
- Wongsasuluk, P.; Tun, A.Z.; Chotpantarat, S.; Siriwong, W. Related health risk assessment of exposure to arsenic and some heavy metals in gold mines in Banmauk Township, Myanmar. Sci. Rep. 2021, 11, 22843. [Google Scholar] [CrossRef]
- Freire, B.M.; Gonzaga, R.G.; Pedron, T.; Monteiro, L.R.; Lange, C.N.; Pedreira Filho, W.D.R.; Batista, B.L. Occupational exposure to potentially toxic elements in the foundry industry: An integrated environmental and biological monitoring. Environ. Sci. Pollut. Res. Int. 2021, 28, 34630–34641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jin, B.; Liu, L.; Li, H.; Zheng, X.; Li, M.; He, R.; Wang, K. Glutathione Might Attenuate Arsenic-Induced Liver Injury by Modulating the Foxa2-XIAP Axis to Reduce Oxidative Stress and Mitochondrial Apoptosis. Biol. Trace Elem. Res. 2023, 1–12. [Google Scholar] [CrossRef]
- Li, Y.; Liang, K.; Yuan, L.; Gao, J.; Wei, L.; Zhao, L. The role of thioredoxin and glutathione systems in arsenic-induced liver injury in rats under glutathione depletion. Int. J. Environ. Health Res. 2022, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Sener, I.; Yabanli, M.; Yozukmaz, A. Effects of inorganic arsenic species on the antioxidant enzyme system of the Amazon Sword Plant (Echinodorus amazonicus Rataj). J. Water Health 2022, 20, 1576–1586. [Google Scholar] [CrossRef]
- Maiti, S.; Chatterjee, A.K. Effects on levels of glutathione and some related enzymes in tissues after an acute arsenic exposure in rats and their relationship to dietary protein deficiency. Arch. Toxicol. 2001, 75, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Flora, S.J. Arsenic-induced oxidative stress and its reversibility. Free Radic. Biol. Med. 2011, 51, 257–281. [Google Scholar] [CrossRef]
- Schmeisser, S.; Schmeisser, K.; Weimer, S.; Groth, M.; Priebe, S.; Fazius, E.; Kuhlow, D.; Pick, D.; Einax, J.W.; Guthke, R.; et al. Mitochondrial hormesis links low-dose arsenite exposure to lifespan extension. Aging Cell 2013, 12, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Luz, A.L.; Godebo, T.R.; Bhatt, D.P.; Ilkayeva, O.R.; Maurer, L.L.; Hirschey, M.D.; Meyer, J.N. From the Cover: Arsenite Uncouples Mitochondrial Respiration and Induces a Warburg-like Effect in Caenorhabditis elegans. Toxicol. Sci. 2016, 152, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Hartman, J.H.; Smith, L.L.; Gordon, K.L.; Laranjeiro, R.; Driscoll, M.; Sherwood, D.R.; Meyer, J.N. Swimming Exercise and Transient Food Deprivation in Caenorhabditis elegans Promote Mitochondrial Maintenance and Protect Against Chemical-Induced Mitotoxicity. Sci. Rep. 2018, 8, 8359. [Google Scholar] [CrossRef]
- Chavan, H.; Christudoss, P.; Mickey, K.; Tessman, R.; Ni, H.M.; Swerdlow, R.; Krishnamurthy, P. Arsenite Effects on Mitochondrial Bioenergetics in Human and Mouse Primary Hepatocytes Follow a Nonlinear Dose Response. Oxid. Med. Cell Longev. 2017, 2017, 9251303. [Google Scholar] [CrossRef]
- Nogawa, K.; Suwazono, Y.; Nishijo, M.; Sakurai, M.; Ishizaki, M.; Morikawa, Y.; Watanabe, Y.; Kido, T.; Nakagawa, H. Increase of lifetime cadmium intake dose-dependently increased all cause of mortality in female inhabitants of the cadmium-polluted Jinzu River basin, Toyama, Japan. Environ. Res. 2018, 164, 379–384. [Google Scholar] [CrossRef]
- Martins, A.C.; Urbano, M.R.; Almeida Lopes, A.C.B.; Carvalho, M.F.H.; Buzzo, M.L.; Docea, A.O.; Mesas, A.E.; Aschner, M.; Silva, A.M.R.; Silbergeld, E.K.; et al. Blood cadmium levels and sources of exposure in an adult urban population in southern Brazil. Environ. Res. 2020, 187, 109618. [Google Scholar] [CrossRef] [PubMed]
- Godt, J.; Scheidig, F.; Grosse-Siestrup, C.; Esche, V.; Brandenburg, P.; Reich, A.; Groneberg, D.A. The toxicity of cadmium and resulting hazards for human health. J. Occup. Med. Toxicol. 2006, 1, 22. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.R.; Prozialeck, W.C. Cadmium, diabetes and chronic kidney disease. Toxicol. Appl. Pharmacol. 2009, 238, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, A. Mechanisms in cadmium-induced carcinogenicity: Recent insights. Biometals 2010, 23, 951–960. [Google Scholar] [CrossRef]
- Rani, A.; Kumar, A.; Lal, A.; Pant, M. Cellular mechanisms of cadmium-induced toxicity: A review. Int. J. Environ. Health Res. 2014, 24, 378–399. [Google Scholar] [CrossRef]
- Patra, R.C.; Rautray, A.K.; Swarup, D. Oxidative stress in lead and cadmium toxicity and its amelioration. Vet. Med. Int. 2011, 2011, 457327. [Google Scholar] [CrossRef]
- Polykretis, P.; Cencetti, F.; Donati, C.; Luchinat, E.; Banci, L. Cadmium effects on superoxide dismutase 1 in human cells revealed by NMR. Redox Biol. 2019, 21, 101102. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, J.; Leonard, S.S.; Rao, K.M. Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free Radic. Biol. Med. 2004, 36, 1434–1443. [Google Scholar] [CrossRef]
- Casalino, E.; Calzaretti, G.; Sblano, C.; Landriscina, C. Molecular inhibitory mechanisms of antioxidant enzymes in rat liver and kidney by cadmium. Toxicology 2002, 179, 37–50. [Google Scholar] [CrossRef]
- Branca, J.J.V.; Fiorillo, C.; Carrino, D.; Paternostro, F.; Taddei, N.; Gulisano, M.; Pacini, A.; Becatti, M. Cadmium-Induced Oxidative Stress: Focus on the Central Nervous System. Antioxidants 2020, 9, 492. [Google Scholar] [CrossRef]
- Mao, W.P.; Zhang, N.N.; Zhou, F.Y.; Li, W.X.; Liu, H.Y.; Feng, J.; Zhou, L.; Wei, C.J.; Pan, Y.B.; He, Z.J. Cadmium directly induced mitochondrial dysfunction of human embryonic kidney cells. Hum. Exp. Toxicol. 2011, 30, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Belyaeva, E.A.; Sokolova, T.V.; Emelyanova, L.V.; Zakharova, I.O. Mitochondrial electron transport chain in heavy metal-induced neurotoxicity: Effects of cadmium, mercury, and copper. Sci. World J. 2012, 2012, 136063. [Google Scholar] [CrossRef]
- Skipper, A.; Sims, J.N.; Yedjou, C.G.; Tchounwou, P.B. Cadmium Chloride Induces DNA Damage and Apoptosis of Human Liver Carcinoma Cells via Oxidative Stress. Int. J. Environ. Res. Public Health 2016, 13, 88. [Google Scholar] [CrossRef]
- Leuthner, T.C.; Benzing, L.; Kohrn, B.F.; Bergemann, C.M.; Hipp, M.J.; Hershberger, K.A.; Mello, D.F.; Sokolskyi, T.; Stevenson, K.; Merutka, I.R.; et al. Resistance of mitochondrial DNA to cadmium and Aflatoxin B1 damage-induced germline mutation accumulation in C. elegans. Nucleic Acids Res. 2022, 50, 8626–8642. [Google Scholar] [CrossRef] [PubMed]
- Boland, M.L.; Chourasia, A.H.; Macleod, K.F. Mitochondrial dysfunction in cancer. Front. Oncol. 2013, 3, 292. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Yang, B.; Ke, T.; Li, S.; Yang, X.; Aschner, M.; Chen, P. Mechanisms of Metal-Induced Mitochondrial Dysfunction in Neurological Disorders. Toxics 2021, 9, 142. [Google Scholar] [CrossRef]
- Schouest, K.; Zitova, A.; Spillane, C.; Papkovsky, D. Toxicological assessment of chemicals using Caenorhabditis elegans and optical oxygen respirometry. Environ. Toxicol. Chem. 2009, 28, 791–799. [Google Scholar] [CrossRef]
- Preez, G.D.; Fourie, H.; Daneel, M.; Miller, H.; Hoss, S.; Ricci, C.; Engelbrecht, G.; Zouhar, M.; Wepener, V. Oxygen consumption rate of Caenorhabditis elegans as a high-throughput endpoint of toxicity testing using the Seahorse XF(e)96 Extracellular Flux Analyzer. Sci. Rep. 2020, 10, 4239. [Google Scholar] [CrossRef]
- Swain, S.; Wren, J.F.; Sturzenbaum, S.R.; Kille, P.; Morgan, A.J.; Jager, T.; Jonker, M.J.; Hankard, P.K.; Svendsen, C.; Owen, J.; et al. Linking toxicant physiological mode of action with induced gene expression changes in Caenorhabditis elegans. BMC Syst. Biol. 2010, 4, 32. [Google Scholar] [CrossRef]
- Li, L.; Yang, X. The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxid. Med. Cell Longev. 2018, 2018, 7580707. [Google Scholar] [CrossRef]
- Martins, A.C., Jr.; Gubert, P.; Villas Boas, G.R.; Meirelles Paes, M.; Santamaria, A.; Lee, E.; Tinkov, A.A.; Bowman, A.B.; Aschner, M. Manganese-induced neurodegenerative diseases and possible therapeutic approaches. Expert. Rev. Neurother. 2020, 20, 1109–1121. [Google Scholar] [CrossRef] [PubMed]
- Morcillo, P.; Cordero, H.; Ijomone, O.M.; Ayodele, A.; Bornhorst, J.; Gunther, L.; Macaluso, F.P.; Bowman, A.B.; Aschner, M. Defective Mitochondrial Dynamics Underlie Manganese-Induced Neurotoxicity. Mol. Neurobiol. 2021, 58, 3270–3289. [Google Scholar] [CrossRef]
- Brown, S.; Taylor, N.L. Could mitochondrial dysfunction play a role in manganese toxicity? Environ. Toxicol. Pharmacol. 1999, 7, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhou, Z.; Fu, J. Effect of manganese chloride exposure on liver and brain mitochondria function in rats. Environ. Res. 2003, 93, 149–157. [Google Scholar] [CrossRef]
- Galvani, P.; Fumagalli, P.; Santagostino, A. Vulnerability of mitochondrial complex I in PC12 cells exposed to manganese. Eur. J. Pharmacol. 1995, 293, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.F.; Van Raamsdonk, J.M. Modeling Parkinson’s Disease in C. elegans. J. Parkinsons Dis. 2018, 8, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Bernhoft, R.A. Mercury toxicity and treatment: A review of the literature. J. Environ. Public Health 2012, 2012, 460508. [Google Scholar] [CrossRef]
- Martins, A.C.; Ke, T.; Bowman, A.B.; Aschner, M. New insights on mechanisms underlying methylmercury-induced and manganese-induced neurotoxicity. Curr. Opin. Toxicol. 2021, 25, 30–35. [Google Scholar] [CrossRef]
- Ma, L.; Bi, K.D.; Fan, Y.M.; Jiang, Z.Y.; Zhang, X.Y.; Zhang, J.W.; Zhao, J.; Jiang, F.L.; Dong, J.X. In vitro modulation of mercury-induced rat liver mitochondria dysfunction. Toxicol. Res. 2018, 7, 1135–1143. [Google Scholar] [CrossRef]
- Carocci, A.; Rovito, N.; Sinicropi, M.S.; Genchi, G. Mercury toxicity and neurodegenerative effects. Rev. Environ. Contam. Toxicol. 2014, 229, 1–18. [Google Scholar] [CrossRef]
- Xing, X.J.; Rui, Q.; Du, M.; Wang, D.Y. Exposure to lead and mercury in young larvae induces more severe deficits in neuronal survival and synaptic function than in adult nematodes. Arch. Environ. Contam. Toxicol. 2009, 56, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Finley, E.J.; Caito, S.; Slaughter, J.C.; Aschner, M. The Role of skn-1 in methylmercury-induced latent dopaminergic neurodegeneration. Neurochem. Res. 2013, 38, 2650–2660. [Google Scholar] [CrossRef] [PubMed]
- Ruszkiewicz, J.A.; Pinkas, A.; Miah, M.R.; Weitz, R.L.; Lawes, M.J.A.; Akinyemi, A.J.; Ijomone, O.M.; Aschner, M.C. elegans as a model in developmental neurotoxicology. Toxicol. Appl. Pharmacol. 2018, 354, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Carocci, A.; Catalano, A.; Sinicropi, M.S.; Genchi, G. Oxidative stress and neurodegeneration: The involvement of iron. Biometals 2018, 31, 715–735. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Dhorajia, V.V.; Kim, J.; Kim, Y. Mitochondrial iron metabolism and neurodegenerative diseases. Neurotoxicology 2022, 88, 88–101. [Google Scholar] [CrossRef]
- Huang, J.; Chen, S.; Hu, L.; Niu, H.; Sun, Q.; Li, W.; Tan, G.; Li, J.; Jin, L.; Lyu, J.; et al. Mitoferrin-1 is Involved in the Progression of Alzheimer’s Disease Through Targeting Mitochondrial Iron Metabolism in a Caenorhabditis elegans Model of Alzheimer’s Disease. Neuroscience 2018, 385, 90–101. [Google Scholar] [CrossRef]
- Martins, A.C.; Virgolini, M.B.; Tinkov, A.A.; Skalny, A.V.; Tirumala, R.P.; Farina, M.; Santamaria, A.; Lu, R.; Aschner, M. Iron overload and neurodegenerative diseases: What can we learn from Caenorhabditis elegans? Toxicol. Res. Appl. 2022, 6, 23978473221091852. [Google Scholar] [CrossRef]
- Esposito, B.P.; Martins, A.C.; de Carvalho, R.R.V.; Aschner, M. High throughput fluorimetric assessment of iron traffic and chelation in iron-overloaded Caenorhabditis elegans. Biometals 2020, 33, 255–267. [Google Scholar] [CrossRef]
- Schiavi, A.; Salveridou, E.; Brinkmann, V.; Shaik, A.; Menzel, R.; Kalyanasundaram, S.; Nygard, S.; Nilsen, H.; Ventura, N. Mitochondria hormesis delays aging and associated diseases in Caenorhabditis elegans impacting on key ferroptosis players. iScience 2023, 26, 106448. [Google Scholar] [CrossRef]
- Ruiz, L.M.; Libedinsky, A.; Elorza, A.A. Role of Copper on Mitochondrial Function and Metabolism. Front. Mol. Biosci. 2021, 8, 711227. [Google Scholar] [CrossRef]
- Mondola, P.; Damiano, S.; Sasso, A.; Santillo, M. The Cu, Zn Superoxide Dismutase: Not Only a Dismutase Enzyme. Front. Physiol. 2016, 7, 594. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.J.; Shaki, F.; Ghazi-Khansari, M.; Pourahmad, J. Toxicity of copper on isolated liver mitochondria: Impairment at complexes I, II, and IV leads to increased ROS production. Cell Biochem. Biophys. 2014, 70, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Ngamchuea, K.; Batchelor-McAuley, C.; Compton, R.G. The Copper(II)-Catalyzed Oxidation of Glutathione. Chemistry 2016, 22, 15937–15944. [Google Scholar] [CrossRef]
- Fajardo, C.; Martin, C.; Garrido, E.; Sanchez-Fortun, S.; Nande, M.; Martin, M.; Costa, G. Copper and Chromium toxicity is mediated by oxidative stress in Caenorhabditis elegans: The use of nanoparticles as an immobilization strategy. Environ. Toxicol. Pharmacol. 2022, 92, 103846. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, L.; Wang, Y.; Luo, X.; Lu, Y. Copper-induced germline apoptosis in Caenorhabditis elegans: The independent roles of DNA damage response signaling and the dependent roles of MAPK cascades. Chem. Biol. Interact. 2009, 180, 151–157. [Google Scholar] [CrossRef]
- Mashock, M.J.; Zanon, T.; Kappell, A.D.; Petrella, L.N.; Andersen, E.C.; Hristova, K.R. Copper Oxide Nanoparticles Impact Several Toxicological Endpoints and Cause Neurodegeneration in Caenorhabditis elegans. PLoS ONE 2016, 11, e0167613. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, C.; Zhang, H.; Liu, R.; Wang, S.; Pu, Y.; Yin, L. Integrating transcriptomics and behavior tests reveals how the C. elegans responds to copper induced aging. Ecotoxicol. Environ. Saf. 2021, 222, 112494. [Google Scholar] [CrossRef]
- Wolf, F.W.; Heberlein, U. Invertebrate models of drug abuse. J. Neurobiol. 2003, 54, 161–178. [Google Scholar] [CrossRef]
- Engleman, E.A.; Katner, S.N.; Neal-Beliveau, B.S. Caenorhabditis elegans as a Model to Study the Molecular and Genetic Mechanisms of Drug Addiction. Prog. Mol. Biol. Transl. Sci. 2016, 137, 229–252. [Google Scholar] [CrossRef]
- Mitchell, P.; Mould, R.; Dillon, J.; Glautier, S.; Andrianakis, I.; James, C.; Pugh, A.; Holden-Dye, L.; O’Connor, V. A differential role for neuropeptides in acute and chronic adaptive responses to alcohol: Behavioural and genetic analysis in Caenorhabditis elegans. PLoS ONE 2010, 5, e10422. [Google Scholar] [CrossRef] [PubMed]
- Musselman, H.N.; Neal-Beliveau, B.; Nass, R.; Engleman, E.A. Chemosensory cue conditioning with stimulants in a Caenorhabditis elegans animal model of addiction. Behav. Neurosci. 2012, 126, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Katner, S.N.; Neal-Beliveau, B.S.; Engleman, E.A. Embryonic Methamphetamine Exposure Inhibits Methamphetamine Cue Conditioning and Reduces Dopamine Concentrations in Adult N2 Caenorhabditis elegans. Dev. Neurosci. 2016, 38, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Engleman, E.A.; Steagall, K.B., 2nd; Bredhold, K.E.; Breach, M.; Kline, H.L.; Bell, R.L.; Katner, S.N.; Neal-Beliveau, B.S. Caenorhabditis elegans Show Preference for Stimulants and Potential as a Model Organism for Medications Screening. Front. Physiol. 2018, 9, 1200. [Google Scholar] [CrossRef] [PubMed]
- Bettinger, J.C.; McIntire, S.L. State-dependency in C. elegans. Genes. Brain Behav. 2004, 3, 266–272. [Google Scholar] [CrossRef]
- Lee, J.; Jee, C.; McIntire, S.L. Ethanol preference in C. elegans. Genes. Brain Behav. 2009, 8, 578–585. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, S.; Wang, J.; Du, W.; Zhu, L. Assessment on chronic and transgenerational toxicity of methamphetamine to Caenorhabditis elegans and associated aquatic risk through toxicity indicator sensitivity distribution (TISD) analysis. Environ. Pollut. 2021, 288, 117696. [Google Scholar] [CrossRef]
- Emerson, S.; Hay, M.; Smith, M.; Granger, R.; Blauch, D.; Snyder, N.; El Bejjani, R. Acetylcholine signaling genes are required for cocaine-stimulated egg laying in Caenorhabditis elegans. G3 (Bethesda) 2021, 11, jkab143. [Google Scholar] [CrossRef]
- Carvelli, L.; Matthies, D.S.; Galli, A. Molecular mechanisms of amphetamine actions in Caenorhabditis elegans. Mol. Pharmacol. 2010, 78, 151–156. [Google Scholar] [CrossRef]
- Hossain, M.; Wickramasekara, R.N.; Carvelli, L. beta-Phenylethylamine requires the dopamine transporter to increase extracellular dopamine in Caenorhabditis elegans dopaminergic neurons. Neurochem. Int. 2014, 73, 27–31. [Google Scholar] [CrossRef][Green Version]
- Torres Valladares, D.; Kudumala, S.; Hossain, M.; Carvelli, L. Caenorhabditis elegans as an in vivo Model to Assess Amphetamine Tolerance. Brain Behav. Evol. 2020, 95, 247–255. [Google Scholar] [CrossRef]
- Albrecht, P.A.; Fernandez-Hubeid, L.E.; Deza-Ponzio, R.; Romero, V.L.; Gonzales-Moreno, C.; Carranza, A.D.V.; Moran, Y.; Asis, R.; Virgolini, M.B. Reduced acute functional tolerance and enhanced preference for ethanol in Caenorhabditis elegans exposed to lead during development: Potential role of alcohol dehydrogenase. Neurotoxicol. Teratol. 2022, 94, 107131. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.G.; Pierce-Shimomura, J.T.; Kim, H.; VanHoven, M.K.; Thiele, T.R.; Bonci, A.; Bargmann, C.I.; McIntire, S.L. A central role of the BK potassium channel in behavioral responses to ethanol in C. elegans. Cell 2003, 115, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Salim, C.; Kan, A.K.; Batsaikhan, E.; Patterson, E.C.; Jee, C. Neuropeptidergic regulation of compulsive ethanol seeking in C. elegans. Sci. Rep. 2022, 12, 1804. [Google Scholar] [CrossRef]
- Jee, C.; Batsaikhan, E.; Salim, C. Neurobiological Basis of Aversion-Resistant Ethanol Seeking in C. elegans. Metabolites 2022, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Wise, R.A.; Koob, G.F. The development and maintenance of drug addiction. Neuropsychopharmacology 2014, 39, 254–262. [Google Scholar] [CrossRef] [PubMed]
- McIntire, S.L. Ethanol. WormBook 2010, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bettinger, J.C.; Leung, K.; Bolling, M.H.; Goldsmith, A.D.; Davies, A.G. Lipid environment modulates the development of acute tolerance to ethanol in Caenorhabditis elegans. PLoS ONE 2012, 7, e35192. [Google Scholar] [CrossRef]
- Scott, L.L.; Davis, S.J.; Yen, R.C.; Ordemann, G.J.; Nordquist, S.K.; Bannai, D.; Pierce, J.T. Behavioral Deficits Following Withdrawal from Chronic Ethanol Are Influenced by SLO Channel Function in Caenorhabditis elegans. Genetics 2017, 206, 1445–1458. [Google Scholar] [CrossRef]
- Hendler, R.A.; Ramchandani, V.A.; Gilman, J.; Hommer, D.W. Stimulant and sedative effects of alcohol. Curr. Top. Behav. Neurosci. 2013, 13, 489–509. [Google Scholar] [CrossRef]
- Alaimo, J.T.; Davis, S.J.; Song, S.S.; Burnette, C.R.; Grotewiel, M.; Shelton, K.L.; Pierce-Shimomura, J.T.; Davies, A.G.; Bettinger, J.C. Ethanol metabolism and osmolarity modify behavioral responses to ethanol in C. elegans. Alcohol. Clin. Exp. Res. 2012, 36, 1840–1850. [Google Scholar] [CrossRef]
- Bettinger, J.C.; Davies, A.G. The role of the BK channel in ethanol response behaviors: Evidence from model organism and human studies. Front. Physiol. 2014, 5, 346. [Google Scholar] [CrossRef]
- Raabe, R.C.; Mathies, L.D.; Davies, A.G.; Bettinger, J.C. The omega-3 fatty acid eicosapentaenoic acid is required for normal alcohol response behaviors in C. elegans. PLoS ONE 2014, 9, e105999. [Google Scholar] [CrossRef]
- Sterken, M.G.; van Wijk, M.H.; Quamme, E.C.; Riksen, J.A.G.; Carnell, L.; Mathies, L.D.; Davies, A.G.; Kammenga, J.E.; Bettinger, J.C. Transcriptional analysis of the response of C. elegans to ethanol exposure. Sci. Rep. 2021, 11, 10993. [Google Scholar] [CrossRef]
- Williamson, V.M.; Long, M.; Theodoris, G. Isolation of Caenorhabditis elegans mutants lacking alcohol dehydrogenase activity. Biochem. Genet. 1991, 29, 313–323. [Google Scholar] [CrossRef]
- Patananan, A.N.; Budenholzer, L.M.; Eskin, A.; Torres, E.R.; Clarke, S.G. Ethanol-induced differential gene expression and acetyl-CoA metabolism in a longevity model of the nematode Caenorhabditis elegans. Exp. Gerontol. 2015, 61, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Kayser, E.B.; Hoppel, C.L.; Morgan, P.G.; Sedensky, M.M. A mutation in mitochondrial complex I increases ethanol sensitivity in Caenorhabditis elegans. Alcohol. Clin. Exp. Res. 2003, 27, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Morgan, P.G.; Sedensky, M.M. Mutations affecting sensitivity to ethanol in the nematode, Caenorhabditis elegans. Alcohol. Clin. Exp. Res. 1995, 19, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.H.; Sheoran, S.; Richmond, J.E.; Kim, H. Alcohol induces mitochondrial fragmentation and stress responses to maintain normal muscle function in Caenorhabditis elegans. FASEB J. 2020, 34, 8204–8216. [Google Scholar] [CrossRef]
- Kaptan, D.; Penkov, S.; Zhang, X.; Gade, V.R.; Raghuraman, B.K.; Galli, R.; Sampaio, J.L.; Haase, R.; Koch, E.; Shevchenko, A.; et al. Exogenous ethanol induces a metabolic switch that prolongs the survival of Caenorhabditis elegans dauer larva and enhances its resistance to desiccation. Aging Cell 2020, 19, e13214. [Google Scholar] [CrossRef]
- Ruan, Q.L.; Ju, J.J.; Li, Y.H.; Liu, R.; Pu, Y.P.; Yin, L.H.; Wang, D.Y. Evaluation of pesticide toxicities with differing mechanisms using Caenorhabditis elegans. J. Toxicol. Environ. Health A 2009, 72, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Zongur, A.; Sari, M. Herbicides widely used in the world: An investigation of toxic effects on Caenorhabditis elegans. Biol. Futur. 2023, 74, 171–182. [Google Scholar] [CrossRef]
- Huang, P.; Liu, S.S.; Wang, Z.J.; Ding, T.T.; Tao, M.T.; Gu, Z.W. Study on the characterization of pesticide modes of action similarity and the multi-endpoint combined toxicity of pesticide mixtures to Caenorhabditis elegans. Sci. Total Environ. 2023, 893, 164918. [Google Scholar] [CrossRef] [PubMed]
- Queiros, L.; Martins, A.C.; Krum, B.N.; Ke, T.; Aschner, M.; Pereira, J.L.; Goncalves, F.J.M.; Milne, G.L.; Pereira, P. Assessing the neurotoxicity of the carbamate methomyl in Caenorhabditis elegans with a multi-level approach. Toxicology 2021, 451, 152684. [Google Scholar] [CrossRef] [PubMed]
- Reddam, A.; McLarnan, S.; Kupsco, A. Environmental Chemical Exposures and Mitochondrial Dysfunction: A Review of Recent Literature. Curr. Environ. Health Rep. 2022, 9, 631–649. [Google Scholar] [CrossRef]
- Chin-Chan, M.; Navarro-Yepes, J.; Quintanilla-Vega, B. Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front. Cell Neurosci. 2015, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.R.; Quan, Y.; Sherer, T.B.; Greenamyre, J.T.; Miller, G.W. Paraquat neurotoxicity is distinct from that of MPTP and rotenone. Toxicol. Sci. 2005, 88, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Dinis-Oliveira, R.J.; Remiao, F.; Carmo, H.; Duarte, J.A.; Navarro, A.S.; Bastos, M.L.; Carvalho, F. Paraquat exposure as an etiological factor of Parkinson’s disease. Neurotoxicology 2006, 27, 1110–1122. [Google Scholar] [CrossRef]
- Bastias-Candia, S.; Zolezzi, J.M.; Inestrosa, N.C. Revisiting the Paraquat-Induced Sporadic Parkinson’s Disease-Like Model. Mol. Neurobiol. 2019, 56, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Vaccari, C.; El Dib, R.; de Camargo, J.L.V. Paraquat and Parkinson’s disease: A systematic review protocol according to the OHAT approach for hazard identification. Syst. Rev. 2017, 6, 98. [Google Scholar] [CrossRef]
- Thirugnanam, T.; Santhakumar, K. Chemically induced models of Parkinson’s disease. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 252, 109213. [Google Scholar] [CrossRef] [PubMed]
- Day, B.J.; Patel, M.; Calavetta, L.; Chang, L.Y.; Stamler, J.S. A mechanism of paraquat toxicity involving nitric oxide synthase. Proc. Natl. Acad. Sci. USA 1999, 96, 12760–12765. [Google Scholar] [CrossRef] [PubMed]
- Cocheme, H.M.; Murphy, M.P. Complex I is the major site of mitochondrial superoxide production by paraquat. J. Biol. Chem. 2008, 283, 1786–1798. [Google Scholar] [CrossRef] [PubMed]
- Castello, P.R.; Drechsel, D.A.; Patel, M. Mitochondria are a major source of paraquat-induced reactive oxygen species production in the brain. J. Biol. Chem. 2007, 282, 14186–14193. [Google Scholar] [CrossRef] [PubMed]
- Robb, E.L.; Gawel, J.M.; Aksentijevic, D.; Cocheme, H.M.; Stewart, T.S.; Shchepinova, M.M.; Qiang, H.; Prime, T.A.; Bright, T.P.; James, A.M.; et al. Selective superoxide generation within mitochondria by the targeted redox cycler MitoParaquat. Free Radic. Biol. Med. 2015, 89, 883–894. [Google Scholar] [CrossRef]
- Ferguson, G.D.; Bridge, W.J. The glutathione system and the related thiol network in Caenorhabditis elegans. Redox Biol. 2019, 24, 101171. [Google Scholar] [CrossRef] [PubMed]
- Gonzales-Moreno, C.; Fernandez-Hubeid, L.E.; Holgado, A.; Virgolini, M.B. Low-dose N-acetyl cysteine prevents paraquat-induced mortality in Caenorhabditis elegans. MicroPubl Biol. 2023, 2023. [Google Scholar] [CrossRef]
- Polyak, E.; Ostrovsky, J.; Peng, M.; Dingley, S.D.; Tsukikawa, M.; Kwon, Y.J.; McCormack, S.E.; Bennett, M.; Xiao, R.; Seiler, C.; et al. N-acetylcysteine and vitamin E rescue animal longevity and cellular oxidative stress in pre-clinical models of mitochondrial complex I disease. Mol. Genet. Metab. 2018, 123, 449–462. [Google Scholar] [CrossRef]
- Oh, S.I.; Park, J.K.; Park, S.K. Lifespan extension and increased resistance to environmental stressors by N-acetyl-L-cysteine in Caenorhabditis elegans. Clinics 2015, 70, 380–386. [Google Scholar] [CrossRef]
- Desjardins, D.; Cacho-Valadez, B.; Liu, J.L.; Wang, Y.; Yee, C.; Bernard, K.; Khaki, A.; Breton, L.; Hekimi, S. Antioxidants reveal an inverted U-shaped dose-response relationship between reactive oxygen species levels and the rate of aging in Caenorhabditis elegans. Aging Cell 2017, 16, 104–112. [Google Scholar] [CrossRef]
- Wu, S.; Lei, L.; Song, Y.; Liu, M.; Lu, S.; Lou, D.; Shi, Y.; Wang, Z.; He, D. Mutation of hop-1 and pink-1 attenuates vulnerability of neurotoxicity in C. elegans: The role of mitochondria-associated membrane proteins in Parkinsonism. Exp. Neurol. 2018, 309, 67–78. [Google Scholar] [CrossRef]
- Jadeja, R.N.; Martin, P.M.; Chen, W. Mitochondrial Oxidative Stress and Energy Metabolism: Impact on Aging and Longevity. Oxid. Med. Cell Longev. 2021, 2021, 9789086. [Google Scholar] [CrossRef]
- Bora, S.; Vardhan, G.S.H.; Deka, N.; Khataniar, L.; Gogoi, D.; Baruah, A. Paraquat exposure over generation affects lifespan and reproduction through mitochondrial disruption in C. elegans. Toxicology 2021, 447, 152632. [Google Scholar] [CrossRef]
- Betarbet, R.; Sherer, T.B.; MacKenzie, G.; Garcia-Osuna, M.; Panov, A.V.; Greenamyre, J.T. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat. Neurosci. 2000, 3, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Sherer, T.B.; Betarbet, R.; Testa, C.M.; Seo, B.B.; Richardson, J.R.; Kim, J.H.; Miller, G.W.; Yagi, T.; Matsuno-Yagi, A.; Greenamyre, J.T. Mechanism of toxicity in rotenone models of Parkinson’s disease. J. Neurosci. 2003, 23, 10756–10764. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, J.M.; Pennell, K.D.; Miller, G.W. Parkinson’s disease and pesticides: A toxicological perspective. Trends Pharmacol. Sci. 2008, 29, 322–329. [Google Scholar] [CrossRef]
- Gonzalez-Hunt, C.P.; Luz, A.L.; Ryde, I.T.; Turner, E.A.; Ilkayeva, O.R.; Bhatt, D.P.; Hirschey, M.D.; Meyer, J.N. Multiple metabolic changes mediate the response of Caenorhabditis elegans to the complex I inhibitor rotenone. Toxicology 2021, 447, 152630. [Google Scholar] [CrossRef] [PubMed]
- Ved, R.; Saha, S.; Westlund, B.; Perier, C.; Burnam, L.; Sluder, A.; Hoener, M.; Rodrigues, C.M.; Alfonso, A.; Steer, C.; et al. Similar patterns of mitochondrial vulnerability and rescue induced by genetic modification of alpha-synuclein, parkin, and DJ-1 in Caenorhabditis elegans. J. Biol. Chem. 2005, 280, 42655–42668. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, Z.; Klaunig, J.E. Caenorhabditis elegans neuron degeneration and mitochondrial suppression caused by selected environmental chemicals. Int. J. Biochem. Mol. Biol. 2013, 4, 191–200. [Google Scholar]
- Guven, K.; Power, R.S.; Avramides, S.; Allender, R.; de Pomerai, D.I. The toxicity of dithiocarbamate fungicides to soil nematodes, assessed using a stress-inducible transgenic strain of Caenorhabditis elegans. J. Biochem. Mol. Toxicol. 1999, 13, 324–333. [Google Scholar] [CrossRef]
- Leiphon, L.J.; Picklo, M.J., Sr. Inhibition of aldehyde detoxification in CNS mitochondria by fungicides. Neurotoxicology 2007, 28, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Staub, R.E.; Quistad, G.B.; Casida, J.E. Mechanism for benomyl action as a mitochondrial aldehyde dehydrogenase inhibitor in mice. Chem. Res. Toxicol. 1998, 11, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Staub, R.E.; Quistad, G.B.; Casida, J.E. S-methyl N-butylthiocarbamate sulfoxide: Selective carbamoylating agent for mouse mitochondrial aldehyde dehydrogenase. Biochem. Pharmacol. 1999, 58, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Kara, M.; Oztas, E.; Ramazanogullari, R.; Kouretas, D.; Nepka, C.; Tsatsakis, A.M.; Veskoukis, A.S. Benomyl, a benzimidazole fungicide, induces oxidative stress and apoptosis in neural cells. Toxicol. Rep. 2020, 7, 501–509. [Google Scholar] [CrossRef]
- Caito, S.W.; Valentine, W.M.; Aschner, M. Dopaminergic neurotoxicity of S-ethyl N,N-dipropylthiocarbamate (EPTC), molinate, and S-methyl-N,N-diethylthiocarbamate (MeDETC) in Caenorhabditis elegans. J. Neurochem. 2013, 127, 837–851. [Google Scholar] [CrossRef]
- Negga, R.; Rudd, D.A.; Davis, N.S.; Justice, A.N.; Hatfield, H.E.; Valente, A.L.; Fields, A.S.; Fitsanakis, V.A. Exposure to Mn/Zn ethylene-bis-dithiocarbamate and glyphosate pesticides leads to neurodegeneration in Caenorhabditis elegans. Neurotoxicology 2011, 32, 331–341. [Google Scholar] [CrossRef]
- Negga, R.; Stuart, J.A.; Machen, M.L.; Salva, J.; Lizek, A.J.; Richardson, S.J.; Osborne, A.S.; Mirallas, O.; McVey, K.A.; Fitsanakis, V.A. Exposure to glyphosate- and/or Mn/Zn-ethylene-bis-dithiocarbamate-containing pesticides leads to degeneration of gamma-aminobutyric acid and dopamine neurons in Caenorhabditis elegans. Neurotox. Res. 2012, 21, 281–290. [Google Scholar] [CrossRef]
- Bailey, D.C.; Todt, C.E.; Orfield, S.E.; Denney, R.D.; Snapp, I.B.; Negga, R.; Montgomery, K.M.; Bailey, A.C.; Pressley, A.S.; Traynor, W.L.; et al. Caenorhabditis elegans chronically exposed to a Mn/Zn ethylene-bis-dithiocarbamate fungicide show mitochondrial Complex I inhibition and increased reactive oxygen species. Neurotoxicology 2016, 56, 170–179. [Google Scholar] [CrossRef]
- Todt, C.E.; Bailey, D.C.; Pressley, A.S.; Orfield, S.E.; Denney, R.D.; Snapp, I.B.; Negga, R.; Bailey, A.C.; Montgomery, K.M.; Traynor, W.L.; et al. Acute exposure to a Mn/Zn ethylene-bis-dithiocarbamate fungicide leads to mitochondrial dysfunction and increased reactive oxygen species production in Caenorhabditis elegans. Neurotoxicology 2016, 57, 112–120. [Google Scholar] [CrossRef]
- Legros, H.; Dingeval, M.G.; Janin, F.; Costentin, J.; Bonnet, J.J. Toxicity of a treatment associating dopamine and disulfiram for catecholaminergic neuroblastoma SH-SY5Y cells: Relationships with 3,4-dihydroxyphenylacetaldehyde formation. Neurotoxicology 2004, 25, 365–375. [Google Scholar] [CrossRef]
- Fitzmaurice, A.G.; Rhodes, S.L.; Lulla, A.; Murphy, N.P.; Lam, H.A.; O’Donnell, K.C.; Barnhill, L.; Casida, J.E.; Cockburn, M.; Sagasti, A.; et al. Aldehyde dehydrogenase inhibition as a pathogenic mechanism in Parkinson disease. Proc. Natl. Acad. Sci. USA 2013, 110, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Casida, J.E.; Ford, B.; Jinsmaa, Y.; Sullivan, P.; Cooney, A.; Goldstein, D.S. Benomyl, aldehyde dehydrogenase, DOPAL, and the catecholaldehyde hypothesis for the pathogenesis of Parkinson’s disease. Chem. Res. Toxicol. 2014, 27, 1359–1361. [Google Scholar] [CrossRef] [PubMed]
- Deza-Ponzio, R.; Herrera, M.L.; Bellini, M.J.; Virgolini, M.B.; Herenu, C.B. Aldehyde dehydrogenase 2 in the spotlight: The link between mitochondria and neurodegeneration. Neurotoxicology 2018, 68, 19–24. [Google Scholar] [CrossRef]
- Doorn, J.A.; Florang, V.R.; Schamp, J.H.; Vanle, B.C. Aldehyde dehydrogenase inhibition generates a reactive dopamine metabolite autotoxic to dopamine neurons. Parkinsonism Relat. Disord. 2014, 20 (Suppl. S1), S73–S75. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S.; Sullivan, P.; Holmes, C.; Miller, G.W.; Alter, S.; Strong, R.; Mash, D.C.; Kopin, I.J.; Sharabi, Y. Determinants of buildup of the toxic dopamine metabolite DOPAL in Parkinson’s disease. J. Neurochem. 2013, 126, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Koppaka, V.; Thompson, D.C.; Chen, Y.; Ellermann, M.; Nicolaou, K.C.; Juvonen, R.O.; Petersen, D.; Deitrich, R.A.; Hurley, T.D.; Vasiliou, V. Aldehyde dehydrogenase inhibitors: A comprehensive review of the pharmacology, mechanism of action, substrate specificity, and clinical application. Pharmacol. Rev. 2012, 64, 520–539. [Google Scholar] [CrossRef] [PubMed]
- Marchitti, S.A.; Deitrich, R.A.; Vasiliou, V. Neurotoxicity and metabolism of the catecholamine-derived 3,4-dihydroxyphenylacetaldehyde and 3,4-dihydroxyphenylglycolaldehyde: The role of aldehyde dehydrogenase. Pharmacol. Rev. 2007, 59, 125–150. [Google Scholar] [CrossRef]
- Chen, C.H.; Joshi, A.U.; Mochly-Rosen, D. The Role of Mitochondrial Aldehyde Dehydrogenase 2 (ALDH2) in Neuropathology and Neurodegeneration. Acta Neurol. Taiwan 2016, 25, 111–123. [Google Scholar]
- Masato, A.; Plotegher, N.; Boassa, D.; Bubacco, L. Impaired dopamine metabolism in Parkinson’s disease pathogenesis. Mol. Neurodegener. 2019, 14, 35. [Google Scholar] [CrossRef]
- Meiser, J.; Weindl, D.; Hiller, K. Complexity of dopamine metabolism. Cell Commun. Signal 2013, 11, 34. [Google Scholar] [CrossRef]
- Cai, H.; Liu, G.; Sun, L.; Ding, J. Aldehyde Dehydrogenase 1 making molecular inroads into the differential vulnerability of nigrostriatal dopaminergic neuron subtypes in Parkinson’s disease. Transl. Neurodegener. 2014, 3, 27. [Google Scholar] [CrossRef][Green Version]
- Moshiri, M.; Darchini-Maragheh, E.; Balali-Mood, M. Advances in toxicology and medical treatment of chemical warfare nerve agents. Daru 2012, 20, 81. [Google Scholar] [CrossRef]
- Rajini, P.S.; Melstrom, P.; Williams, P.L. A comparative study on the relationship between various toxicological endpoints in Caenorhabditis elegans exposed to organophosphorus insecticides. J. Toxicol. Environ. Health A 2008, 71, 1043–1050. [Google Scholar] [CrossRef]
- Pearson, J.N.; Patel, M. The role of oxidative stress in organophosphate and nerve agent toxicity. Ann. N. Y. Acad. Sci. 2016, 1378, 17–24. [Google Scholar] [CrossRef]
- Williams, P.L.; Dusenbery, D.B. A promising indicator of neurobehavioral toxicity using the nematode Caenorhabditis elegans and computer tracking. Toxicol. Ind. Health 1990, 6, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.; Williams, P.L. Toxicity testing of neurotoxic pesticides in Caenorhabditis elegans. J. Toxicol. Environ. Health B Crit. Rev. 2014, 17, 284–306. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.C.K.; Meyer, J.N. Mitochondria as a target of organophosphate and carbamate pesticides: Revisiting common mechanisms of action with new approach methodologies. Reprod. Toxicol. 2019, 89, 83–92. [Google Scholar] [CrossRef]
- Han, Y.; Song, S.; Wu, H.; Zhang, J.; Ma, E. Antioxidant enzymes and their role in phoxim and carbaryl stress in Caenorhabditis elegans. Pestic. Biochem. Physiol. 2017, 138, 43–50. [Google Scholar] [CrossRef]
- Zeng, R.; Yu, X.; Tan, X.; Ye, S.; Ding, Z. Deltamethrin affects the expression of voltage-gated calcium channel alpha1 subunits and the locomotion, egg-laying, foraging behavior of Caenorhabditis elegans. Pestic. Biochem. Physiol. 2017, 138, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Shashikumar, S.; Rajini, P.S. Cypermethrin elicited responses in heat shock protein and feeding in Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2010, 73, 1057–1062. [Google Scholar] [CrossRef]
- Duke, S.O. Overview of herbicide mechanisms of action. Environ. Health Perspect. 1990, 87, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Espineira, M.; Tejeda-Benitez, L.; Olivero-Verbel, J. Toxicity of atrazine- and glyphosate-based formulations on Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2018, 156, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.C.; Todt, C.E.; Burchfield, S.L.; Pressley, A.S.; Denney, R.D.; Snapp, I.B.; Negga, R.; Traynor, W.L.; Fitsanakis, V.A. Chronic exposure to a glyphosate-containing pesticide leads to mitochondrial dysfunction and increased reactive oxygen species production in Caenorhabditis elegans. Environ. Toxicol. Pharmacol. 2018, 57, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Burchfield, S.L.; Bailey, D.C.; Todt, C.E.; Denney, R.D.; Negga, R.; Fitsanakis, V.A. Acute exposure to a glyphosate-containing herbicide formulation inhibits Complex II and increases hydrogen peroxide in the model organism Caenorhabditis elegans. Environ. Toxicol. Pharmacol. 2019, 66, 36–42. [Google Scholar] [CrossRef]
- Kronberg, M.F.; Clavijo, A.; Moya, A.; Rossen, A.; Calvo, D.; Pagano, E.; Munarriz, E. Glyphosate-based herbicides modulate oxidative stress response in the nematode Caenorhabditis elegans. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2018, 214, 1–8. [Google Scholar] [CrossRef]
- Adeyemi, J.A.; da Cunha Martins-Junior, A.; Barbosa, F., Jr. Teratogenicity, genotoxicity and oxidative stress in zebrafish embryos (Danio rerio) co-exposed to arsenic and atrazine. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2015, 172–173, 7–12. [Google Scholar] [CrossRef]
- Zhou, R.; Liu, R.; Li, W.; Wang, Y.; Wan, X.; Song, N.; Yu, Y.; Xu, J.; Bu, Y.; Zhang, A. The use of different sublethal endpoints to monitor atrazine toxicity in nematode Caenorhabditis elegans. Chemosphere 2021, 274, 129845. [Google Scholar] [CrossRef]
- Nolan, K.; Kamrath, J.; Levitt, J. Lindane toxicity: A comprehensive review of the medical literature. Pediatr. Dermatol. 2012, 29, 141–146. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, H.; Hua, X.; Wang, Z.; Li, L.; Li, Z.; Xiang, M.; Ding, P. Long-term toxicity of lindane through oxidative stress and cell apoptosis in Caenorhabditis elegans. Environ. Pollut. 2021, 272, 116036. [Google Scholar] [CrossRef]
- Kudelska, M.M.; Holden-Dye, L.; O’Connor, V.; Doyle, D.A. Concentration-dependent effects of acute and chronic neonicotinoid exposure on the behaviour and development of the nematode Caenorhabditis elegans. Pest. Manag. Sci. 2017, 73, 1345–1351. [Google Scholar] [CrossRef]
- Bradford, B.R.; Whidden, E.; Gervasio, E.D.; Checchi, P.M.; Raley-Susman, K.M. Neonicotinoid-containing insecticide disruption of growth, locomotion, and fertility in Caenorhabditis elegans. PLoS ONE 2020, 15, e0238637. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Choi, S.; Kim, K.W. Dithianon exposure induces dopaminergic neurotoxicity in Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2023, 255, 114752. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, W.; Wang, Y.; Liu, H.; Zhang, S.; Ji, X.; Qiao, K. Oxidative stress, intestinal damage, and cell apoptosis: Toxicity induced by fluopyram in Caenorhabditis elegans. Chemosphere 2022, 286, 131830. [Google Scholar] [CrossRef] [PubMed]

| Xenobiotic | Potential Mechanism of Impairment of Mitochondrial Function | Reference |
|---|---|---|
| As | Low-dose arsenite increases ROS formation, promoting upregulation of mitochondrial proteins, increasing the lifespan while higher concentrations reduce longevity. | [86] |
| As | Reduced ATP-like respiration, spare respiratory capacity and augmented proton leak | [87] |
| As | Disrupted mitochondrial function in fusion-deficient worms, suggesting that disruption of pyruvate metabolism and Krebs cycle activity trigger the mitochondrial deficits | [66] |
| Cd | pink-1 strain showed higher levels of mtDNA damage | [103] |
| Cd | Reduced OCR in a dose-dependent manner; Cd exposure is positively associated with worm growth inhibition | [108] |
| Cd | Transcriptional alterations in several genes related to ATP turnover and mitochondrial biogenesis and functioning | [109] |
| Mn | Dysfunctions of mitochondrial genes (e.g., PINK1) | [116] |
| Hg | Loss of dopaminergic neurons, observed in Parkinson’s later in life, following early-life (L1) exposure | [122] |
| Fe | Mitoferrin-1 led to a decrease in mitochondrial Fe content and a reduction in mitochondrial ROS | [126] |
| Fe | Impaired mitochondrial energy production and protein balance | [15] |
| Ethanol | Fragmented mitochondria, probably because of fission from the internal membrane | [169] |
| Ethanol | Dauer larvae survive much longer because during energy depletion EtOH prevents or delays mitochondrial fragmentation and deterioration | [170] |
| Paraquat | Structural damage in mitochondria, ATP depletion, and increased autophagy | [191] |
| Paraquat | Increased number of fragmented mitochondria and reduced membrane potential, Complexes I–IV activity, and pyruvate and lactate levels | [192] |
| Paraquat | Elevated ROS production that leads to oxidative damage to the DNA | [193] |
| Rotenone | Loss of Complex I function including upregulation of mitochondrial Complexes II and V, activation of the glyoxylate pathway, glycolysis, and fatty acid oxidation | [197] |
| Rotenone | Mitochondrial DNA replication is suppressed, pointing out the role of mtDNA biogenesis and mitochondrial content in the process of dopaminergic neuron damage | [198] |
| Thiocarbamates | Mitochondrial Complex I inhibition and increased ROS | [208] |
| Thiocarbamates | Mitochondrial dysfunction and increased ROS production | [209] |
| chlorpyrifos | mtDNA damage after exposure to chlorpyrifos | [227] |
| Organophosphates and carbamates | Oxidative stress altered the antioxidant enzyme activities and their gene expressions | [228] |
| Pyrethrins and Pyrethroids | Oxidative stress by increasing free radicals, decreasing GSH levels, increasing protein carbonyl levels and altering the activities of antioxidant enzymes | [230] |
| Glyphosate | Reduced oxygen consumption, proton gradient, and ATP production | [233] |
| Glyphosate | Inhibition of Complex II and increased hydrogen peroxide levels | [234] |
| Triazines (atrazine) | Mitochondrial unfolded protein response, increased mitochondrial damage and vacuolar degeneration, associated with a decrease in mitochondrial cristae and volume density | [237] |
| Organochlorines (lindane) | Expression of genes related to oxidative stress and cell apoptosis (isp-1, sod-3, ced-3, and cep-1 genes) | [239] |
| Dithianon | Increases in oxidative stress and mitochondrial fragmentation | [242] |
| Fluopyram | Increase in ROS production and decrease in antioxidant enzymes activities, and GSH content | [243] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, A.C.; Virgolini, M.B.; Ávila, D.S.; Scharf, P.; Li, J.; Tinkov, A.A.; Skalny, A.V.; Bowman, A.B.; Rocha, J.B.T.; Aschner, M. Mitochondria in the Spotlight: C. elegans as a Model Organism to Evaluate Xenobiotic-Induced Dysfunction. Cells 2023, 12, 2124. https://doi.org/10.3390/cells12172124
Martins AC, Virgolini MB, Ávila DS, Scharf P, Li J, Tinkov AA, Skalny AV, Bowman AB, Rocha JBT, Aschner M. Mitochondria in the Spotlight: C. elegans as a Model Organism to Evaluate Xenobiotic-Induced Dysfunction. Cells. 2023; 12(17):2124. https://doi.org/10.3390/cells12172124
Chicago/Turabian StyleMartins, Airton C., Miriam B. Virgolini, Daiana Silva Ávila, Pablo Scharf, Jung Li, Alexey A. Tinkov, Anatoly V. Skalny, Aaron B. Bowman, João B. T. Rocha, and Michael Aschner. 2023. "Mitochondria in the Spotlight: C. elegans as a Model Organism to Evaluate Xenobiotic-Induced Dysfunction" Cells 12, no. 17: 2124. https://doi.org/10.3390/cells12172124
APA StyleMartins, A. C., Virgolini, M. B., Ávila, D. S., Scharf, P., Li, J., Tinkov, A. A., Skalny, A. V., Bowman, A. B., Rocha, J. B. T., & Aschner, M. (2023). Mitochondria in the Spotlight: C. elegans as a Model Organism to Evaluate Xenobiotic-Induced Dysfunction. Cells, 12(17), 2124. https://doi.org/10.3390/cells12172124










