The Role of Inflammation in Age-Related Macular Degeneration—Therapeutic Landscapes in Geographic Atrophy
Abstract
:1. Introduction
2. Method
3. The Role of Inflammation in the Pathogenesis of Geographic Atrophy in AMD
3.1. Complement Pathway
3.2. Microglia and Neutrophils
3.3. Oxidative Stress Mediated Inflammatory Responses
3.4. Lipid Metabolism and Inflammation in AMD
4. The Current Landscape of Dry AMD Therapeutic Strategies: Slowing down the Progression
4.1. Complement Inhibition
4.2. Neuroprotection
4.3. Visual Cycle Modulation
4.4. Glyco-Immune Modulation
5. Future Possible Therapeutic Strategies to Treat Geographic Atrophy: Vision Restoration
5.1. Cell Therapy
5.2. Prosthesis
5.3. Optogenetics
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Seddon, J.M. Macular Degeneration Epidemiology: Nature-Nurture, Lifestyle Factors, Genetic Risk, and Gene-Environment Interactions—The Weisenfeld Award Lecture. Investig. Ophthalmol. Vis. Sci. 2017, 58, 6513–6528. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.; Jolly, J.K.; Couldridge, C.; Xue, K.; MacLaren, R.E. Quality of Life Measures in Patients with Retinal Degeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3295. [Google Scholar]
- Ismayilova, I.; Turdaliyeva, B.; Aldasheva, N.; Veselovskaya, N. Assessing the quality of life in age-related macular degeneration patients: A cross-sectional study in Kazakhstan. Acta Biomed. 2022, 93, e2022299. [Google Scholar]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Q.; Welchowski, T.; Schmid, M.; Mauschitz, M.M.; Holz, F.G.; Finger, R.P. Prevalence and incidence of age-related macular degeneration in Europe: A systematic review and meta-analysis. Br. J. Ophthalmol. 2020, 104, 1077–1084. [Google Scholar] [CrossRef]
- Owsley, C.; McGwin, G., Jr.; Clark, M.E.; Jackson, G.R.; Callahan, M.A.; Kline, L.B.; Witherspoon, C.D.; Curcio, C.A. Delayed Rod-Mediated Dark Adaptation Is a Functional Biomarker for Incident Early Age-Related Macular Degeneration. Ophthalmology 2016, 123, 344–351. [Google Scholar] [CrossRef]
- Brandl, C.; Zimmermann, M.E.; Herold, J.M.; Helbig, H.; Stark, K.J.; Heid, I.M. Photostress Recovery Time as a Potential Predictive Biomarker for Age-Related Macular Degeneration. Transl. Vis. Sci. Technol. 2023, 12, 15. [Google Scholar] [CrossRef]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Ferris, F.L., 3rd; Wilkinson, C.P.; Bird, A.; Chakravarthy, U.; Chew, E.; Csaky, K.; Sadda, S.R.; Beckman Initiative for Macular Research Classification Committee. Clinical classification of age-related macular degeneration. Ophthalmology 2013, 120, 844–851. [Google Scholar] [CrossRef]
- Chen, M.; Xu, H. Parainflammation, chronic inflammation, and age-related macular degeneration. J. Leukoc. Biol. 2015, 98, 713–725. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, J.; Ding, Y.; Huang, H.; Li, Y.; Chen, W. Predicting late-stage age-related macular degeneration by integrating marginally weak SNPs in GWA studies. Front. Genet. 2023, 14, 1075824. [Google Scholar] [CrossRef] [PubMed]
- Joachim, N.; Mitchell, P.; Burlutsky, G.; Kifley, A.; Wang, J.J. The Incidence and Progression of Age-Related Macular Degeneration over 15 Years: The Blue Mountains Eye Study. Ophthalmology 2015, 122, 2482–2489. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog. Retin. Eye Res. 2017, 60, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.H.; Mullins, R.F.; Hageman, G.S.; Johnson, L.V. A role for local inflammation in the formation of drusen in the aging eye. Am. J. Ophthalmol. 2002, 134, 411–431. [Google Scholar] [CrossRef]
- Tan, P.L.; Rickman, C.B.; Katsanis, N. AMD and the alternative complement pathway: Genetics and functional implications. Hum. Genom. 2016, 10, 23. [Google Scholar] [CrossRef]
- Armento, A.; Ueffing, M.; Clark, S.J. The complement system in age-related macular degeneration. Cell. Mol. Life Sci. 2021, 78, 4487–4505. [Google Scholar] [CrossRef]
- Cipriani, V.; Lorés-Motta, L.; He, F.; Fathalla, D.; Tilakaratna, V.; McHarg, S.; Bayatti, N.; Acar, İ.E.; Hoyng, C.B.; Fauser, S.; et al. Increased circulating levels of Factor H-Related Protein 4 are strongly associated with age-related macular degeneration. Nature Commun. 2020, 11, 778. [Google Scholar] [CrossRef]
- Rus, H.; Cudrici, C.; Niculescu, F. The role of the complement system in innate immunity. Immunol. Res. 2005, 33, 103–112. [Google Scholar] [CrossRef]
- Reynolds, R.; Hartnett, M.E.; Atkinson, J.P.; Giclas, P.C.; Rosner, B.; Seddon, J.M. Plasma complement components and activation fragments: Associations with age-related macular degeneration genotypes and phenotypes. Investig. Opthalmology Vis. Sci. 2009, 50, 5818–5827. [Google Scholar] [CrossRef]
- Gehrs, K.M.; Jackson, J.R.; Brown, E.N.; Allikmets, R.; Hageman, G.S. Complement, age-related macular degeneration and a vision of the future. Arch. Ophthalmol. 2010, 128, 349–358. [Google Scholar] [CrossRef]
- Jiang, K.; To, E.; Cui, J.Z.; Cao, S.; Gao, J.; Matsubara, J.A. Drusen and Pro-inflammatory Mediators in the Post-Mortem Human Eye. J. Clin. Exp. Ophthalmol. 2012, 3, 208. [Google Scholar] [CrossRef] [PubMed]
- Nesargikar, P.; Spiller, B.; Chavez, R. The complement system: History, pathways, cascade and inhibitors. Eur. J. Microbiol. Immunol. 2012, 2, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, L.G.; Igl, W.; Bailey, J.N.; Grassmann, F.; Sengupta, S.; Bragg-Gresham, J.L.; Burdon, K.P.; Hebbring, S.J.; Wen, C.; Gorski, M.; et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet. 2016, 48, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Winkler, T.W.; Grassmann, F.; Brandl, C.; Kiel, C.; Günther, F.; Strunz, T.; Weidner, L.; Zimmermann, M.E.; Korb, C.A.; Poplawski, A.; et al. Genome-wide association meta-analysis for early age-related macular degeneration highlights novel loci and insights for advanced disease. BMC Med Genom. 2020, 13, 120. [Google Scholar] [CrossRef] [PubMed]
- Seddon, J.M.; Gensler, G.; Rosner, B. C-reactive protein and CFH, ARMS2/HTRA1 gene variants are independently associated with risk of macular degeneration. Ophthalmology 2010, 117, 1560–1566. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.; Butani, V.; Boyer, D.S.; Atilano, S.R.; Resende, G.P.; Kim, D.S.; Chakrabarti, S.; Kuppermann, B.D.; Khatibi, N.; Chwa, M.; et al. Complement factor H polymorphism in age-related macular degeneration. Ophthalmology 2007, 114, 1327–1331. [Google Scholar] [CrossRef] [PubMed]
- Zareparsi, S.; Branham, K.E.; Li, M.; Shah, S.; Klein, R.J.; Ott, J.; Hoh, J.; Abecasis, G.R.; Swaroop, A. Strong association of the Y402H variant in complement factor H at 1q32 with susceptibility to age-related macular degeneration. Am. J. Hum. Genet. 2005, 77, 149–153. [Google Scholar] [CrossRef]
- Alexander, P.; Gibson, J.; Cree, A.J.; Ennis, S.; Lotery, A.J. Complement factor I and age-related macular degeneration. Mol. Vis. 2014, 20, 1253–1257. [Google Scholar]
- van de Ven, J.P.; Nilsson, S.C.; Tan, P.L.; Buitendijk, G.H.; Ristau, T.; Mohlin, F.C.; Nabuurs, S.B.; Schoenmaker-Koller, F.E.; Smailhodzic, D.; Campochiaro, P.A.; et al. A functional variant in the CFI gene confers a high risk of age-related macular degeneration. Nat. Genet. 2013, 45, 813–817. [Google Scholar] [CrossRef]
- Geerlings, M.J.; Kremlitzka, M.; Bakker, B.; Nilsson, S.C.; Saksens, N.T.; Lechanteur, Y.T.; Pauper, M.; Corominas, J.; Fauser, S.; Hoyng, C.B.; et al. The Functional Effect of Rare Variants in Complement Genes on C3b Degradation in Patients With Age-Related Macular Degeneration. JAMA Ophthalmol. 2017, 135, 39–46. [Google Scholar] [CrossRef]
- Hallam, T.M.; Marchbank, K.J.; Harris, C.L.; Osmond, C.; Shuttleworth, V.G.; Griffiths, H.; Cree, A.J.; Kavanagh, D.; Lotery, A.J. Rare Genetic Variants in Complement Factor I Lead to Low FI Plasma Levels Resulting in Increased Risk of Age-Related Macular Degeneration. Invest. Ophthalmol. Vis. Sci. 2020, 61, 18. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, E.L. Contribution of microglia and monocytes to the development and progression of age related macular degeneration. Ophthalmic Physiol. Opt. 2020, 40, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Murenu, E.; Gerhardt, M.-J.; Biel, M.; Michalakis, S. More than meets the eye: The role of microglia in healthy and diseased retina. Front. Immunol. 2022, 13, 1006897. [Google Scholar] [CrossRef]
- Boyce, M.; Xin, Y.; Chowdhury, O.; Shang, P.; Liu, H.; Koontz, V.; Strizhakova, A.; Nemani, M.; Hose, S.; Zigler, J.S.; et al. Microglia-Neutrophil Interactions Drive Dry AMD-like Pathology in a Mouse Model. Cells 2022, 11, 3535. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Padmanabhan, A.; Vaidya, T.; Watson, A.M.; Bhutto, I.A.; Hose, S.; Shang, P.; Stepicheva, N.; Yazdankhah, M.; Weiss, J.; et al. Neutrophils homing into the retina trigger pathology in early age-related macular degeneration. Commun. Biol. 2019, 2, 348. [Google Scholar] [CrossRef]
- Yu, C.; Roubeix, C.; Sennlaub, F.; Saban, D.R. Microglia versus Monocytes: Distinct Roles in Degenerative Diseases of the Retina. Trends Neurosci. 2020, 43, 433–449. [Google Scholar] [CrossRef] [PubMed]
- O’koren, E.G.; Yu, C.; Klingeborn, M.; Wong, A.Y.; Prigge, C.L.; Mathew, R.; Kalnitsky, J.; Msallam, R.A.; Silvin, A.; Kay, J.N.; et al. Microglial Function Is Distinct in Different Anatomical Locations during Retinal Homeostasis and Degeneration. Immunity 2019, 50, 723–737.e7. [Google Scholar] [CrossRef]
- Combadière, C.; Feumi, C.; Raoul, W.; Keller, N.; Rodéro, M.; Pézard, A.; Lavalette, S.; Houssier, M.; Jonet, L.; Picard, E.; et al. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J. Clin. Investig. 2007, 117, 2920–2928. [Google Scholar] [CrossRef]
- Knickelbein, J.E.; Chan, C.-C.; Sen, H.N.M.; Ferris, F.L.; Nussenblatt, R.B. Inflammatory Mechanisms of Age-related Macular Degeneration. Int. Ophthalmol. Clin. 2015, 55, 63–78. [Google Scholar] [CrossRef]
- Nielsen, M.K.; Hector, S.M.; Allen, K.; Subhi, Y.; Sørensen, T.L. Altered activation state of circulating neutrophils in patients with neovascular age-related macular degeneration. Immun. Ageing 2017, 14, 18. [Google Scholar] [CrossRef]
- Romero-Vazquez, S.; Llorens, V.; Soler-Boronat, A.; Figueras-Roca, M.; Adan, A.; Molins, B. Interlink between Inflammation and Oxidative Stress in Age-Related Macular Degeneration: Role of Complement Factor H. Biomedicines 2021, 9, 763. [Google Scholar] [CrossRef] [PubMed]
- Mitter, S.K.; Song, C.; Qi, X.; Mao, H.; Rao, H.; Akin, D.; Lewin, A.; Grant, M.; Dunn, W., Jr.; Ding, J.; et al. Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy 2014, 10, 1989–2005. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Crabb, J.W.; Miyagi, M.; Gu, X.; Shadrach, K.; West, K.A.; Sakaguchi, H.; Kamei, M.; Hasan, A.; Yan, L.; Rayborn, M.E.; et al. Drusen proteome analysis: An approach to the etiology of age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2002, 99, 14682–14687. [Google Scholar] [CrossRef]
- Yuan, X.; Gu, X.; Crabb, J.S.; Yue, X.; Shadrach, K.; Hollyfield, J.G.; Crabb, J.W. Quantitative Proteomics: Comparison of the Macular Bruch Membrane/Choroid Complex from Age-related Macular Degeneration and Normal Eyes. Mol Cell. Proteom. 2010, 9, 1031–1046. [Google Scholar] [CrossRef]
- Thurman, J.M.; Renner, B.; Kunchithapautham, K.; Ferreira, V.P.; Pangburn, M.K.; Ablonczy, Z.; Tomlinson, S.; Holers, V.M.; Rohrer, B. Oxidative stress renders retinal pigment epithelial cells susceptible to complement-mediated injury. J. Biol. Chem. 2009, 284, 16939–16947. [Google Scholar] [CrossRef]
- Bonilha, V.L. Age and disease-related structural changes in the retinal pigment epithelium. Clin. Ophthalmol. 2008, 2, 413–424. [Google Scholar] [CrossRef]
- Biswal, M.R.; Ildefonso, C.J.; Mao, H.; Seo, S.J.; Wang, Z.; Li, H.; Le, Y.Z.; Lewin, A.S. Conditional Induction of Oxidative Stress in RPE: A Mouse Model of Progressive Retinal Degeneration. Adv. Exp. Med. Biol. 2016, 854, 31–37. [Google Scholar]
- Kamceva, G.; Arsova-Sarafinovska, Z.; Ruskovska, T.; Zdravkovska, M.; Kamceva-Panova, L.; Stikova, E. Cigarette Smoking and Oxidative Stress in Patients with Coronary Artery Disease. Open Access Maced. J. Med Sci. 2016, 4, 636–640. [Google Scholar] [CrossRef]
- Bruno, R.S.; Traber, M.G. Vitamin E biokinetics, oxidative stress and cigarette smoking. Pathophysiology 2006, 13, 143–149. [Google Scholar] [CrossRef]
- Hu, M.L.; Quinn, J.; Xue, K. Interactions between Apolipoprotein E Metabolism and Retinal Inflammation in Age-Related Macular Degeneration. Life 2021, 11, 635. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Clark, M.E.; Crossman, D.K.; Kojima, K.; Messinger, J.D.; Mobley, J.A.; Curcio, C.A. Abundant Lipid and Protein Components of Drusen. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6168. [Google Scholar] [CrossRef]
- Curcio, C.A.; Johnson, M.; Rudolf, M.; Huang, J.-D. The oil spill in ageing Bruch membrane. Br. J. Ophthalmol. 2011, 95, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, E.L.; Jobling, A.I.; Greferath, U.; Mills, S.A.; Waugh, M.; Ho, T.; de Iongh, R.U.; Phipps, J.A.; Vessey, K.A. Studying age-related macular degeneration using animal models. Optom. Vis. Sci. 2014, 91, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Landowski, M.; Rickman, C.B. Targeting Lipid Metabolism for the Treatment of Age-Related Macular Degeneration: Insights from Preclinical Mouse Models. J. Ocul. Pharmacol. Ther. 2022, 38, 3–32. [Google Scholar] [CrossRef] [PubMed]
- Clemons, T.E.; Milton, R.C.; Klein, R.; Seddon, J.M.; Ferris, F.L., 3rd. Risk factors for the incidence of Advanced Age-Related Macular Degeneration in the Age-Related Eye Disease Study (AREDS) AREDS report no. 19. Ophthalmology 2005, 112, 533–539. [Google Scholar] [PubMed]
- Mares-Perlman, J.A.; Brady, W.E.; Klein, R.; VandenLangenberg, G.M.; Klein, B.E.K.; Palta, M. Dietary fat and age-related maculopathy. Arch. Ophthalmol. 1995, 113, 743–748. [Google Scholar] [CrossRef]
- Lin, J.B.; Halawa, O.A.; Husain, D.; Miller, J.W.; Vavvas, D.G. Dyslipidemia in age-related macular degeneration. Eye 2022, 36, 312–318. [Google Scholar] [CrossRef]
- Klein, R.; Myers, C.E.; Buitendijk, G.H.; Rochtchina, E.; Gao, X.; de Jong, P.T.; Sivakumaran, T.A.; Burlutsky, G.; McKean-Cowdin, R.; Hofman, A.; et al. Lipids, lipid genes, and incident age-related macular degeneration: The three continent age-related macular degeneration consortium. Am. J. Ophthalmol. 2014, 158, 513–524.e3. [Google Scholar] [CrossRef]
- Colijn, J.M.; den Hollander, A.I.; Demirkan, A.; Cougnard-Grégoire, A.; Verzijden, T.; Kersten, E.; Meester-Smoor, M.A.; Merle, B.M.; Papageorgiou, G.; Ahmad, S.; et al. Increased High-Density Lipoprotein Levels Associated with Age-Related Macular Degeneration: Evidence from the EYE-RISK and European Eye Epidemiology Consortia. Ophthalmology 2019, 126, 393–406. [Google Scholar] [CrossRef]
- Keenan, T.D.; Agrón, E.; Mares, J.A.; Clemons, T.E.; Van Asten, F.; Swaroop, A.; Chew, E.Y.; AREDS Research Group. Adherence to the Mediterranean Diet and Progression to Late Age-Related Macular Degeneration in the Age-Related Eye Disease Studies 1 and 2. Ophthalmology 2020, 127, 1515–1528. [Google Scholar] [CrossRef] [PubMed]
- Hall, N.F.; Martyn, C.N. Could statins prevent age-related macular degeneration? Expert Opin Pharm. 2002, 3, 803–807. [Google Scholar]
- Gehlbach, P.; Li, T.; Hatef, E. Statins for age-related macular degeneration. Cochrane Database Syst. Rev. 2016, Cd006927. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.S.; Grossi, F.V.; El Mehdi, D.; Gerber, M.R.; Brown, D.M.; Heier, J.S.; Wykoff, C.C.; Singerman, L.J.; Abraham, P.; Grassmann, F.; et al. Complement C3 Inhibitor Pegcetacoplan for Geographic Atrophy Secondary to Age-Related Macular Degeneration: A Randomized Phase 2 Trial. Ophthalmology 2020, 127, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.S.; Metlapally, R.; Joshi, P. Pegcetacoplan treatment for geographic atrophy due to age-related macular degeneration: A plain language summary of the FILLY study. Immunotherapy 2022, 14, 995–1006. [Google Scholar] [CrossRef]
- Yehoshua, Z.; de Amorim Garcia Filho, C.A.; Nunes, R.P.; Gregori, G.; Penha, F.M.; Moshfeghi, A.A.; Zhang, K.; Sadda, S.; Feuer, W.; Rosenfeld, P.J. Systemic complement inhibition with eculizumab for geographic atrophy in age-related macular degeneration: The COMPLETE study. Ophthalmology 2014, 121, 693–701. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Westby, K.; Csaky, K.G.; Monés, J.; Pearlman, J.A.; Patel, S.S.; Joondeph, B.C.; Randolph, J.; Masonson, H.; Rezaei, K.A. C5 Inhibitor Avacincaptad Pegol for Geographic Atrophy Due to Age-Related Macular Degeneration: A Randomized Pivotal Phase 2/3 Trial. Ophthalmology 2021, 128, 576–586. [Google Scholar] [CrossRef]
- Dreismann, A.K.; McClements, M.E.; Barnard, A.R.; Orhan, E.; Hughes, J.P.; Lachmann, P.J.; MacLaren, R.E. Functional expression of complement factor I following AAV-mediated gene delivery in the retina of mice and human cells. Gene Ther. 2021, 28, 265–276. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Sahni, J.; Fauser, S.; Geary, R.S.; Schneider, E.; McCaleb, M. Development of IONIS-FB-LRx to Treat Geographic Atrophy Associated with AMD. Investig. Ophthalmol. Vis. Sci. 2020, 61, 4305. [Google Scholar]
- Kauper, K.; McGovern, C.; Sherman, S.; Heatherton, P.; Rapoza, R.; Stabila, P.; Dean, B.; Lee, A.; Borges, S.; Bouchard, B.; et al. Two-year intraocular delivery of ciliary neurotrophic factor by encapsulated cell technology implants in patients with chronic retinal degenerative diseases. Invest. Ophthalmol. Vis. Sci. 2012, 53, 7484–7491. [Google Scholar] [CrossRef]
- Zhang, K.; Hopkins, J.J.; Heier, J.S.; Birch, D.G.; Halperin, L.S.; Albini, T.A.; Brown, D.M.; Jaffe, G.J.; Tao, W.; Williams, G.A. Ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for treatment of geographic atrophy in age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2011, 108, 6241–6245. [Google Scholar] [CrossRef] [PubMed]
- Sajovic, J.; Meglič, A.; Glavač, D.; Markelj, Š.; Hawlina, M.; Fakin, A. The Role of Vitamin A in Retinal Diseases. Int. J. Mol. Sci. 2022, 23, 1014. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, Y.; Ma, L.; Washington, I. Deuterium enrichment of vitamin A at the C20 position slows the formation of detrimental vitamin A dimers in wild-type rodents. J. Biol. Chem. 2011, 286, 7958–7965. [Google Scholar] [CrossRef]
- Dugel, P.U.; Novack, R.L.; Csaky, K.G.; Richmond, P.P.; Birch, D.G.; Kubota, R. Phase ii, randomized, placebo-controlled, 90-day study of emixustat hydrochloride in geographic atrophy associated with dry age-related macular degeneration. Retina 2015, 35, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, P.J.; Dugel, P.U.; Holz, F.G.; Heier, J.S.; Pearlman, J.A.; Novack, R.L.; Csaky, K.G.; Koester, J.M.; Gregory, J.K.; Kubota, R. Emixustat Hydrochloride for Geographic Atrophy Secondary to Age-Related Macular Degeneration: A Randomized Clinical Trial. Ophthalmology 2018, 125, 1556–1567. [Google Scholar] [CrossRef]
- Saad, L.; Washington, I. Can Vitamin A be Improved to Prevent Blindness due to Age-Related Macular Degeneration, Stargardt Disease and Other Retinal Dystrophies? Adv. Exp. Med. Biol. 2016, 854, 355–361. [Google Scholar]
- Therapeutics, A. Developing Next Generation Immuno-Modulators That Harness the Power of Glyco-Immunology. 2023. Available online: https://www.avicedarx.com/ (accessed on 27 April 2023).
- Cehajic-Kapetanovic, J.; Singh, M.S.; Zrenner, E.; MacLaren, R.E. Bioengineering strategies for restoring vision. Nat. Biomed. Eng. 2022, 7, 387–404. [Google Scholar] [CrossRef]
- Schwartz, S.D.; Regillo, C.D.; Lam, B.L.; Eliott, D.; Rosenfeld, P.J.; Gregori, N.Z.; Hubschman, J.P.; Davis, J.L.; Heilwell, G.; Spirn, M.; et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: Follow-up of two open-label phase 1/2 studies. Lancet 2015, 385, 509–516. [Google Scholar] [CrossRef]
- Qiu, T.G. Transplantation of human embryonic stem cell-derived retinal pigment epithelial cells (MA09-hRPE) in macular degeneration. NPJ Regen. Med. 2019, 4, 19. [Google Scholar] [CrossRef]
- Palanker, D.; Huie, P.; Vankov, A.; Aramant, R.; Seiler, M.; Fishman, H.; Marmor, M.; Blumenkranz, M. Migration of Retinal Cells through a Perforated Membrane: Implications for a High-Resolution Prosthesis. Investig. Opthalmology Vis. Sci. 2004, 45, 3266–3270. [Google Scholar] [CrossRef]
- Palanker, D.; Vankov, A.; Huie, P.; Baccus, S. Design of a high-resolution optoelectronic retinal prosthesis. J. Neural Eng. 2005, 2, S105–S120. [Google Scholar] [CrossRef] [PubMed]
- Palanker, D.V.; Le Mer, Y.; Hornig, R.; Buc, G.; Deterre, M.; Bismuth, V.; Sahel, J.A. Restoration of Sight in Geographic Atrophy using a Photovoltaic Subretinal Prosthesis. Investig. Ophthalmol. Vis. Sci. 2019, 60, 970. [Google Scholar]
- Cehajic-Kapetanovic, J.; Eleftheriou, C.; Allen, A.E.; Milosavljevic, N.; Pienaar, A.; Bedford, R.; Davis, K.E.; Bishop, P.N.; Lucas, R.J. Restoration of Vision with Ectopic Expression of Human Rod Opsin. Curr. Biol. 2015, 25, 2111–2122. [Google Scholar] [CrossRef]
- McClements, M.E.; Staurenghi, F.; MacLaren, R.E.; Cehajic-Kapetanovic, J. Optogenetic Gene Therapy for the Degenerate Retina: Recent Advances. Front. Neurosci. 2020, 14, 570909. [Google Scholar] [CrossRef] [PubMed]
- John, M.C.; Quinn, J.; Hu, M.L.; Cehajic-Kapetanovic, J.; Xue, K. Gene-agnostic therapeutic approaches for inherited retinal degenerations. Front. Mol. Neurosci. 2022, 15, 1068185. [Google Scholar] [CrossRef] [PubMed]
- Sahel, J.A.; Audo, I.S.; Boulanger-Scemama, E.; Pagot, C.; Arleo, A.; Martel, J.N.; Degli Esposti, S.; Delaux, A.; de Saint Aubert, J.B.; De Montleau, C.; et al. Optogenetics in the clinic: Safety and efficacy updates on the phase 1/2 clinical trial PIONEER. Investig. Ophthalmol. Vis. Sci. 2022, 63, 1106. [Google Scholar]
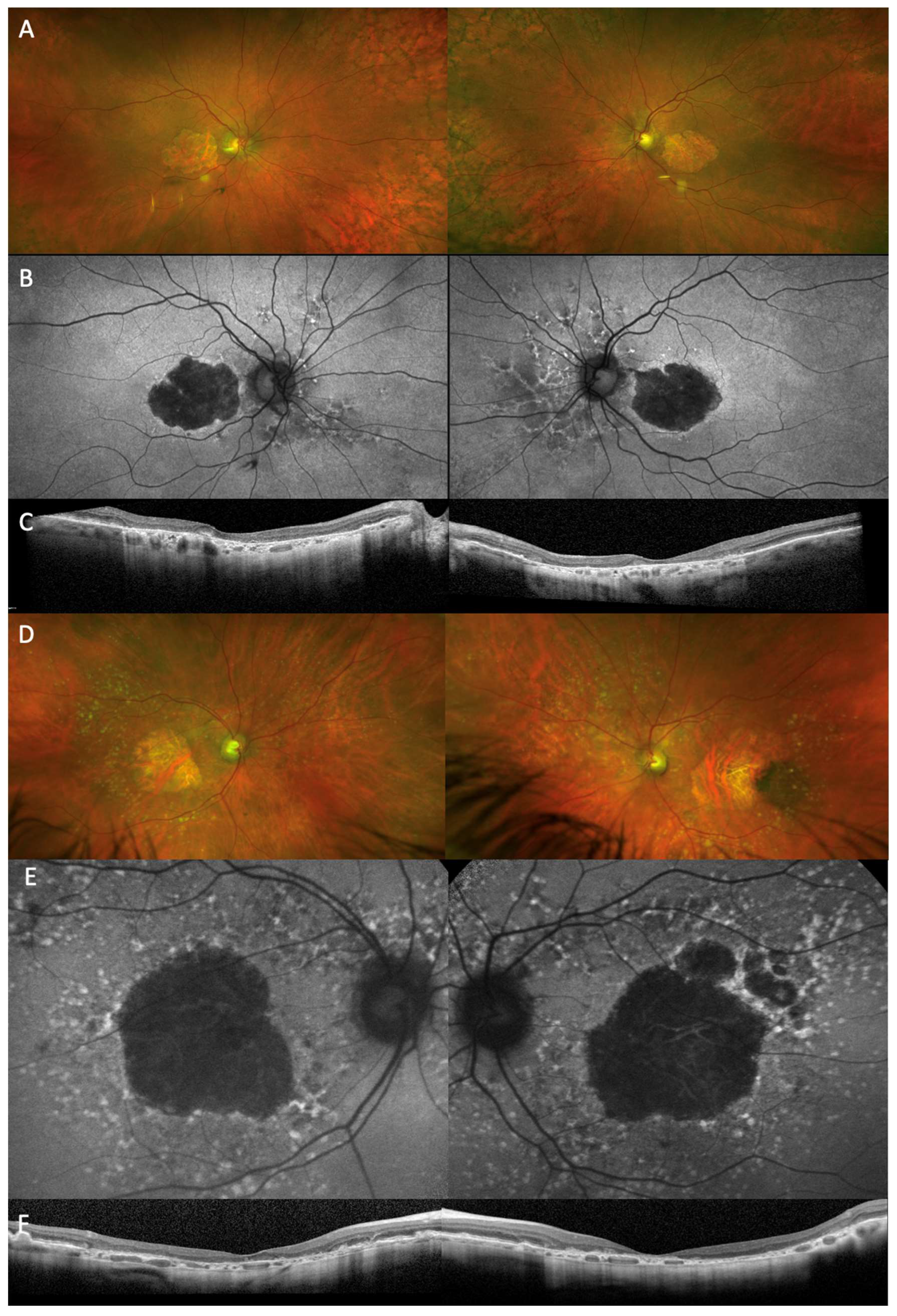
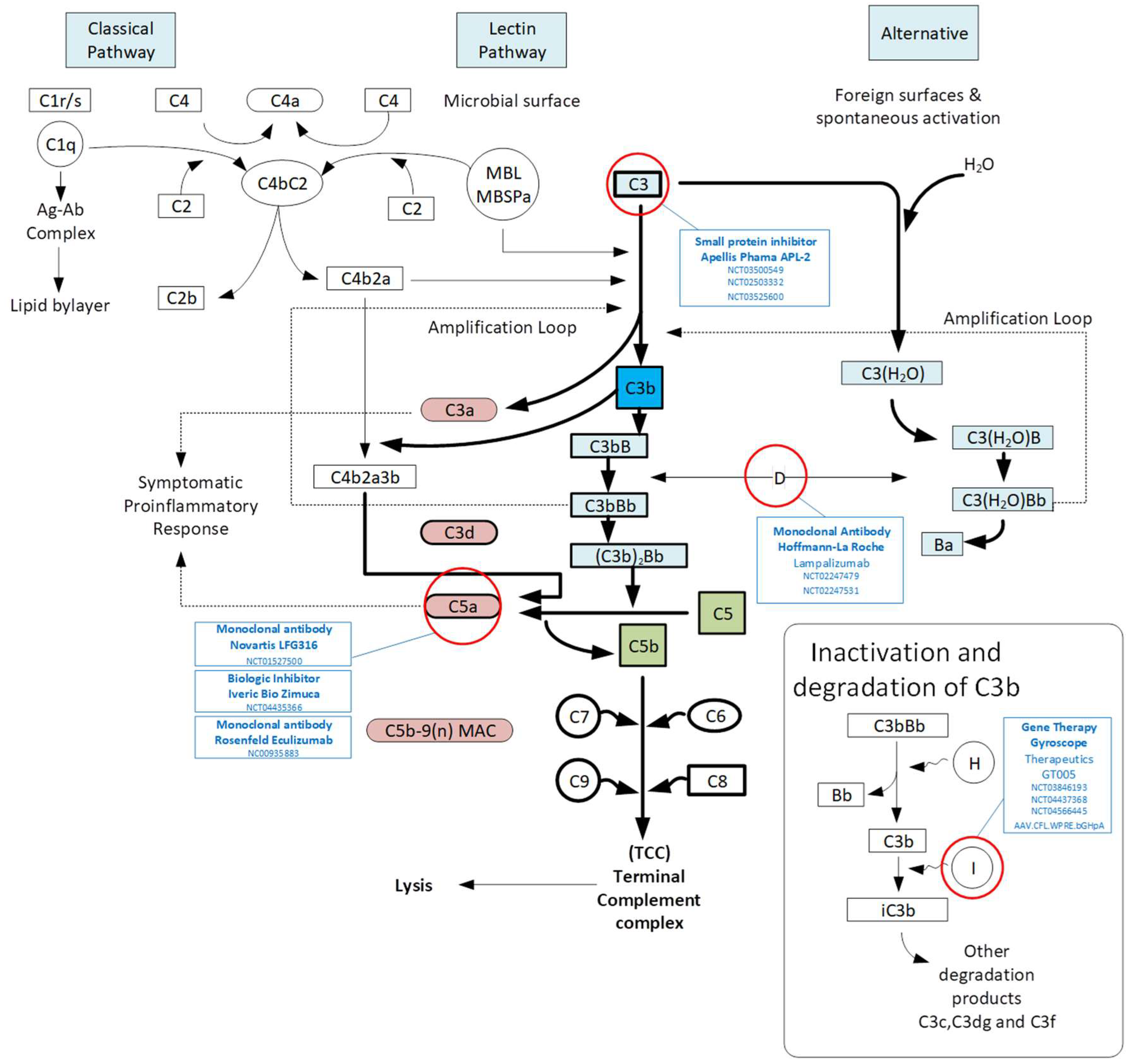

| Approach | Institute/Company | Target/Therapeutic | Phase, NCT, Status, Year and Studies | Outcome/Comment |
|---|---|---|---|---|
| Complement | Apellis Pharma | C3; Small molecule inhibitor APL2 Pegcetacoplan | Phase III, NCT03525600, Active, started 2018, DERBY, OAKS | FDA approved. GA growth rate significantly decreased |
| Iveric Bio | C5; Biologic inhibitor Zimura | Phase III, NCT04435366, Active, started 2020, GATHER2 | FDA approved. GA growth rate significantly decreased | |
| Hoffmann-La Roche | CFD; Monoclonal Ab Lampalizumab | Phase III, NCT02247479, Terminated 2019, CHROMA, SPECTRI | Failed to slow down GA growth | |
| Alexion | CFD; Small molecule inhibitor Danicopan | Phase II, NCT05019521, Recruiting 2021 | Not available yet | |
| Gyroscope Therapeutics | CFI; Gene therapy GT005 | Phase II, NCT03846193, Active, started 2018, EXPLORE, HORIZON | EXPLORE includes rare variants of CFI, HORIZON with GA | |
| Ionis Pharmaceuticals | CFB; Antisense inhibitor IONIS-FB-LRx | Phase II, NCT03815825, Active, started 2019, GOLDEN | Not available yet | |
| National Eye Institute | Rapamycin | Phase II, NCT00766649, Completed 2012, SIRGA | No anatomical or functional benefit | |
| Allegro Ophthalmics | Risuteganib | Phase II, NCT03626636, Completed 2019 | Significant BCVA improvement in dry AMD over 32 weeks | |
| University of Miami | C5; Monoclonal Ab Eculizumab | Phase II, NCT00935883, Completed 2012, COMPLETE | Failed to slow GA progression | |
| Janssen Research | CD59; Gene therapy AAVCAGsCD59 | Phase I, NCT03144999, Completed 2019 | Decreased GA growth rate at 6 months | |
| Visual cycle modulator | Alkeus Pharm | Vitamin A; Small molecule compound ALK-001 | Phase III, NCT03845582, Active, started 2019, SAGA | Not available yet |
| Kubota Vision | RPE65 isomerase; Small molecule inhibitor ACU4429 Emixustat | Phase IIb/III, NCT01802866, Completed 2016, SEATTLE | Did not reduce GA growth | |
| Sirion Therapeutics | Synthetic retinoid compound; Fenretinide | Phase II, NCT00429936, Completed 2010 | Not available yet | |
| Antioxidant | National Eye Institute | Oral dietary supplementation | Phase III, NCT00345176, Completed 2012, ARDES2 | High levels of antioxidants and zinc can decrease risk of advanced AMD by 25% |
| Neuro-protection | Neurotech Pharmaceuticals | CNTF implant NTC-201 | Phase II, NCT00447954, Completed 2009 | Favourable pharmacokinetic profile without systemic exposure |
| Stealth Bio Therapeutics | Mitochondrial supercomplex; Small peptide Elamipretide | Phase II, NCT03891875, Completed 2022, ReCLAIM-2 | Categorical >2 line improvement in low luminance visual acuity in GA | |
| ONL Therapeutics | ONL1204; Apoptosis inhibitor | Phase I, NCT04744662, Active, started 2021 | Not available yet | |
| Allergan | Alpha2-adrenergic receptor agonist, Brimonidine | Phase IIb, NCT02087085, Terminated 2018 | Terminated due to lower than expected GA progression in the control group. | |
| Anti-Inflammatory | Paul Yates | 30S ribosomal subunit, antibiotic Doxycycline | Phase II/III, NCT01782989, Completed 2022 TOGA | Not available yet |
| National Eye Institute | Oral minocycline | Phase II, NCT02564978, Completed 2022 | No significant difference in GA enlargement rate between run-in and treatment phase | |
| Genentech | GM-CSF; Monoclonal Ab Galegenimab FHTR2163 | Phase II, NCT03972709, Terminated 2022, GALLEGO | Terminated because the benefit to risk did not support further treatment | |
| Ocugen | OCU410 Orphan nuclear receptor; Modified gene therapy | Not available | Not available | |
| Photo-biomodulation | LumiThera | Light Delivery system LT-300 | NA, NCT04522999, Completed 2021, LIGHTSITE III, ELECTRO-LIGHT | Positive multi-luminance ERG and visual acuity improvement (58.2% of treated eyes improved >5 letters in 13 months) |
| Glyco-immune | Aviceda Therapeutics | SIGLEC; Conjugated nanoparticle, AVD-104 | FDA approved AVD-104 to initiate Phase II; SIGLEC | Not available yet |
| Cell therapy | Astellas Pharma | hESC-derived RPE (MA09-hRPE) | Phase I/II, NCT01344993, Completed 2021 | Medium-term to long-term safety, graft survival and possible biologic activity |
| National Eye Institute | iPSC- derived RPE | Phase I/II, NCT04339764, Recruiting | Not available yet | |
| Janssen Pharm | IL-12 and IL-23, Monoclonal Ab, CNTO-2476 | Phase II, NCT02659098, Completed 2020, PRELUDE | Mild ocular AE but no demonstrated efficacy in GA area growth or VA | |
| MD stem cells | Bone marrow derived stem cells (BMSC) | NA, NCT03011541, Recruiting, SCOTS2 | Not available yet | |
| Tenpoint Therapeutics | hESC-derived RPE patch graft PF-05206388 | Phase I, NCT01691261, Recruiting | Not available yet | |
| Regenerative Patch Tech | hESC-derived RPE patch graft CPCB-RPE1 | Phase I/II, NCT02590692, Active | Not available yet | |
| Prosthesis | Second Sight Medical | Device Argus II System | NA, NCT02227498, Completed 2020, ArgusII | No significant change in visual acuity and 4 SAE |
| Pixium Vision | Device PRIMA | NA, NCT03392324 PRIAM-FS-US | 3 out of 5 patients had BCVA improvement from 20/460 to 20/550 in 12 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borchert, G.A.; Shamsnajafabadi, H.; Hu, M.L.; De Silva, S.R.; Downes, S.M.; MacLaren, R.E.; Xue, K.; Cehajic-Kapetanovic, J. The Role of Inflammation in Age-Related Macular Degeneration—Therapeutic Landscapes in Geographic Atrophy. Cells 2023, 12, 2092. https://doi.org/10.3390/cells12162092
Borchert GA, Shamsnajafabadi H, Hu ML, De Silva SR, Downes SM, MacLaren RE, Xue K, Cehajic-Kapetanovic J. The Role of Inflammation in Age-Related Macular Degeneration—Therapeutic Landscapes in Geographic Atrophy. Cells. 2023; 12(16):2092. https://doi.org/10.3390/cells12162092
Chicago/Turabian StyleBorchert, Grace A., Hoda Shamsnajafabadi, Monica L. Hu, Samantha R. De Silva, Susan M. Downes, Robert E. MacLaren, Kanmin Xue, and Jasmina Cehajic-Kapetanovic. 2023. "The Role of Inflammation in Age-Related Macular Degeneration—Therapeutic Landscapes in Geographic Atrophy" Cells 12, no. 16: 2092. https://doi.org/10.3390/cells12162092
APA StyleBorchert, G. A., Shamsnajafabadi, H., Hu, M. L., De Silva, S. R., Downes, S. M., MacLaren, R. E., Xue, K., & Cehajic-Kapetanovic, J. (2023). The Role of Inflammation in Age-Related Macular Degeneration—Therapeutic Landscapes in Geographic Atrophy. Cells, 12(16), 2092. https://doi.org/10.3390/cells12162092









