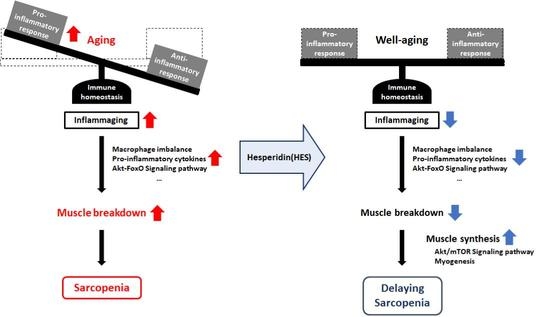
Hesperidin Ameliorates Sarcopenia through the Regulation of Inflammaging and the AKT/mTOR/FoxO3a Signaling Pathway in 22–26-Month-Old Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
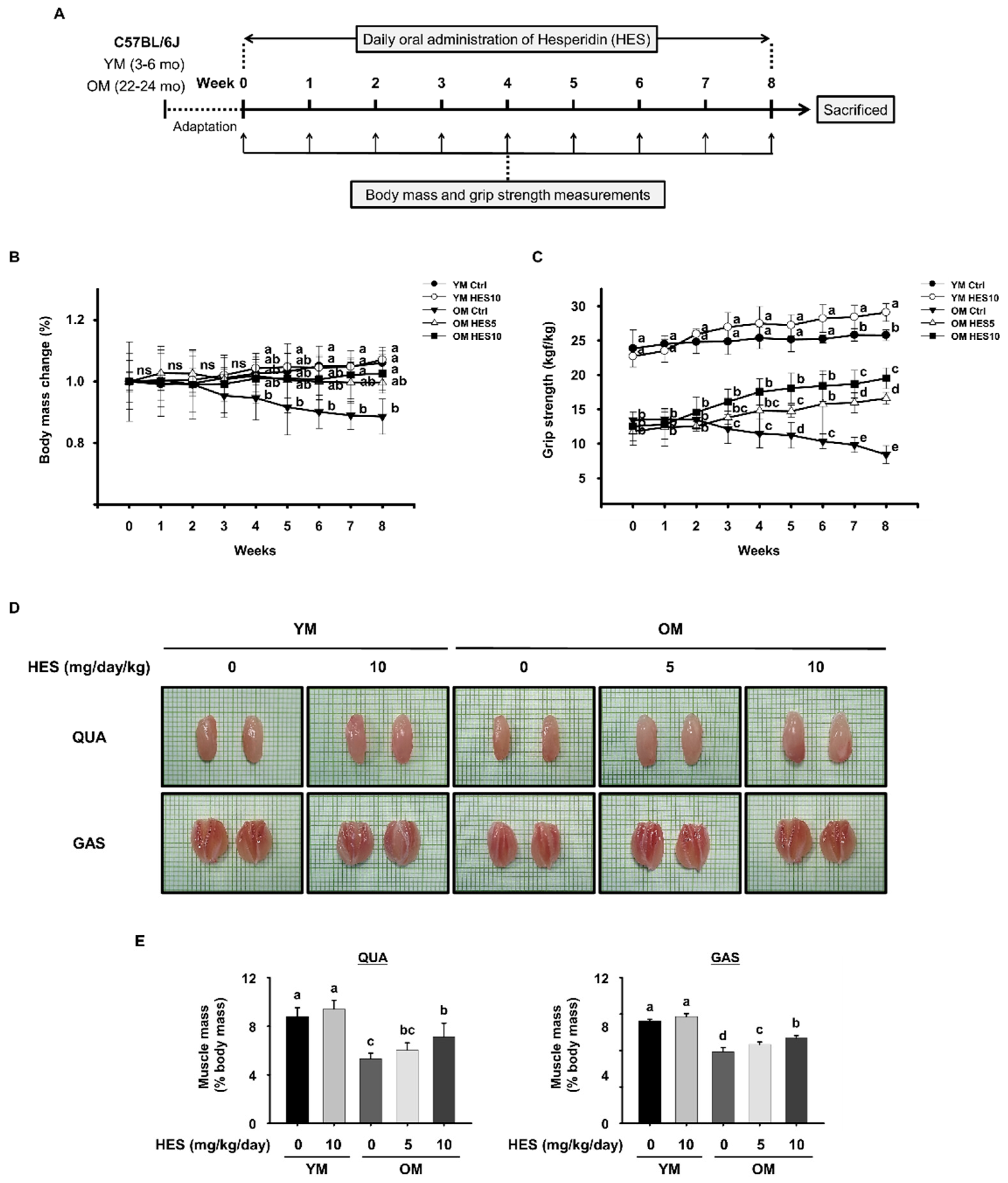
2.2. Animals and Treatments
2.3. Measurement of Grip Strength
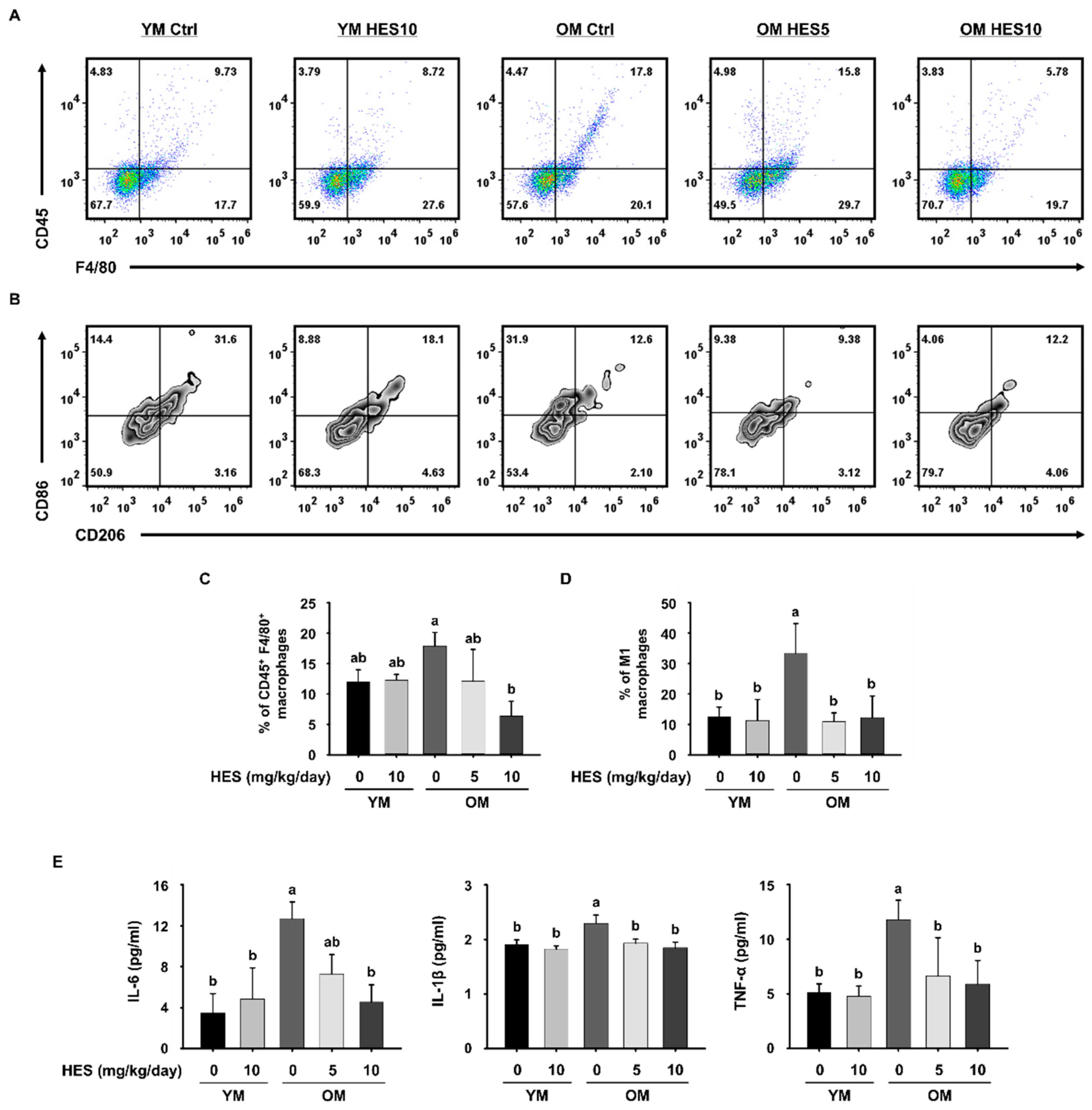
2.4. Flow Cytometry Analysis
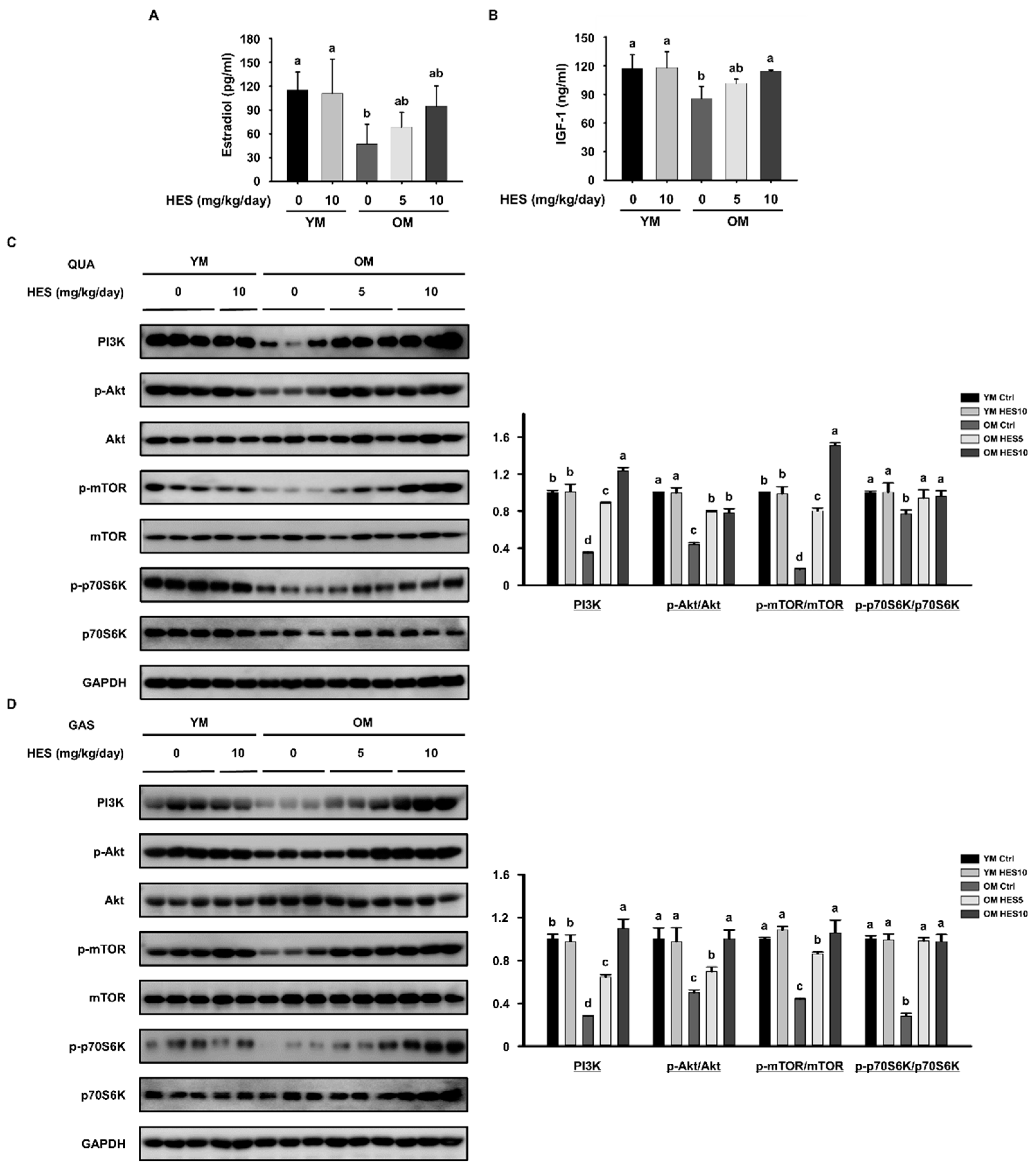
2.5. Serum Analysis
2.6. Western Blotting
2.7. Histological Analysis
2.8. Statistical Analysis
3. Results
3.1. HES Increases the Muscle Strength, Size, and Mass of the Old Mice and Preserves Body Mass
3.2. HES Suppresses Inflammaging through the Regulation of Macrophage Subsets in Old Mice
3.3. HES Reduces the Activation of FoxO3a and E3 Ubiquitin Ligases in Old Mice
3.4. HES Activates the AKT/mTOR Signaling Pathway in Old Mice
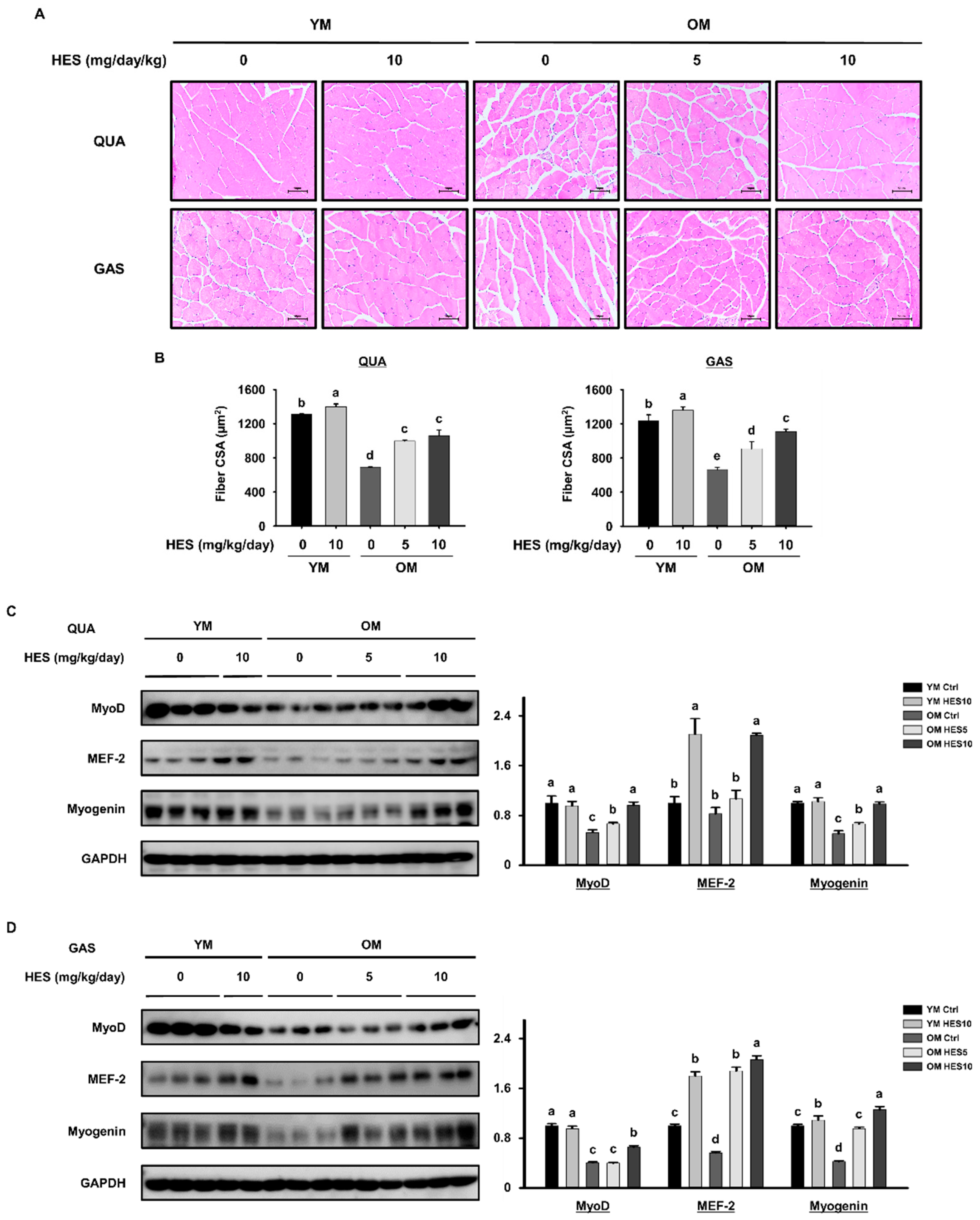
3.5. HES Restores Muscle Fiber Size and the Expression of Myogenic Transcription Factors in Old Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal. Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.F.; Silva, A.J.; Matos Costa, A.; Monteiro, A.M.; Bastos, E.M.; Cardoso Marques, M. Muscle tissue changes with aging. Acta Med. Port. 2013, 26, 51–55. [Google Scholar]
- Miljkovic, N.; Lim, J.Y.; Miljkovic, I.; Frontera, W.R. Aging of skeletal muscle fibers. Ann. Rehabil. Med. 2015, 39, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef] [PubMed]
- Olgun Yazar, H.; Yazar, T. Prevalence of sarcopenia in patients with geriatric depression diagnosis. Ir. J. Med. Sci. 2019, 188, 931–938. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Xie, W.Q.; Luo, Y.X.; Li, Y.D.; Huang, W.H.; Wu, Y.X.; Li, Y.S. High Intensity Interval Training: A Potential Method for Treating Sarcopenia. Clin. Interv. Aging 2022, 17, 857–872. [Google Scholar] [CrossRef]
- Kwak, J.Y.; Kwon, K.S. Pharmacological Interventions for Treatment of Sarcopenia: Current Status of Drug Development for Sarcopenia. Ann. Geriatr. Med. Res. 2019, 23, 98–104. [Google Scholar] [CrossRef]
- Fry, C.S.; Rasmussen, B.B. Skeletal muscle protein balance and metabolism in the elderly. Curr. Aging Sci. 2011, 4, 260–268. [Google Scholar] [CrossRef]
- Breen, L.; Phillips, S.M. Skeletal muscle protein metabolism in the elderly: Interventions to counteract the ‘anabolic resistance’ of ageing. Nutr. Metab. 2011, 8, 68. [Google Scholar] [CrossRef]
- Beyer, I.; Mets, T.; Bautmans, I. Chronic low-grade inflammation and age-related sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 12–22. [Google Scholar] [CrossRef]
- Dalle, S.; Rossmeislova, L.; Koppo, K. The Role of Inflammation in Age-Related Sarcopenia. Front. Physiol. 2017, 8, 1045. [Google Scholar] [CrossRef] [PubMed]
- Rieu, I.; Magne, H.; Savary-Auzeloux, I.; Averous, J.; Bos, C.; Peyron, M.A.; Combaret, L.; Dardevet, D. Reduction of low grade inflammation restores blunting of postprandial muscle anabolism and limits sarcopenia in old rats. J. Physiol. 2009, 587 Pt 22, 5483–5492. [Google Scholar] [CrossRef]
- Marzetti, E.; Calvani, R.; Cesari, M.; Buford, T.W.; Lorenzi, M.; Behnke, B.J.; Leeuwenburgh, C. Mitochondrial dysfunction and sarcopenia of aging: From signaling pathways to clinical trials. Int. J. Biochem. Cell Biol. 2013, 45, 2288–2301. [Google Scholar] [CrossRef]
- Draganidis, D.; Jamurtas, A.Z.; Chondrogianni, N.; Mastorakos, G.; Jung, T.; Grune, T.; Papadopoulos, C.; Papanikolaou, K.; Papassotiriou, I.; Papaevgeniou, N.; et al. Low-Grade Systemic Inflammation Interferes with Anabolic and Catabolic Characteristics of the Aged Human Skeletal Muscle. Oxid. Med. Cell Longev. 2021, 2021, 8376915. [Google Scholar] [CrossRef] [PubMed]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS-) vs. Alternatively Activated Macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef] [PubMed]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef]
- Costantini, A.; Viola, N.; Berretta, A.; Galeazzi, R.; Matacchione, G.; Sabbatinelli, J.; Storci, G.; De Matteis, S.; Butini, L.; Rippo, M.R.; et al. Age-related M1/M2 phenotype changes in circulating monocytes from healthy/unhealthy individuals. Aging 2018, 10, 1268–1280. [Google Scholar] [CrossRef]
- Antuna, E.; Cachan-Vega, C.; Bermejo-Millo, J.C.; Potes, Y.; Caballero, B.; Vega-Naredo, I.; Coto-Montes, A.; Garcia-Gonzalez, C. Inflammaging: Implications in Sarcopenia. Int. J. Mol. Sci. 2022, 23, 15039. [Google Scholar] [CrossRef]
- Cheng, Z. The FoxO-Autophagy Axis in Health and Disease. Trends Endocrinol. Metab. 2019, 30, 658–671. [Google Scholar] [CrossRef]
- Farhan, M.; Silva, M.; Li, S.; Yan, F.; Fang, J.; Peng, T.; Hu, J.; Tsao, M.S.; Little, P.; Zheng, W. The role of FOXOs and autophagy in cancer and metastasis-Implications in therapeutic development. Med. Res. Rev. 2020, 40, 2089–2113. [Google Scholar] [CrossRef]
- Chen, K.; Gao, P.; Li, Z.; Dai, A.; Yang, M.; Chen, S.; Su, J.; Deng, Z.; Li, L. Forkhead Box O Signaling Pathway in Skeletal Muscle Atrophy. Am. J. Pathol. 2022, 192, 1648–1657. [Google Scholar] [CrossRef]
- Zhao, J.; Brault, J.J.; Schild, A.; Cao, P.; Sandri, M.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 2007, 6, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Lee, H.A.; Kim, M.; Lee, E.; Sohn, U.D.; Kim, I. Forkhead box O3 plays a role in skeletal muscle atrophy through expression of E3 ubiquitin ligases MuRF-1 and atrogin-1 in Cushing’s syndrome. Am. J. Physiol. Endocrinol. Metab. 2017, 312, E495–E507. [Google Scholar] [CrossRef] [PubMed]
- Floyd, S.; Favre, C.; Lasorsa, F.M.; Leahy, M.; Trigiante, G.; Stroebel, P.; Marx, A.; Loughran, G.; O’Callaghan, K.; Marobbio, C.M.; et al. The insulin-like growth factor-I-mTOR signaling pathway induces the mitochondrial pyrimidine nucleotide carrier to promote cell growth. Mol. Biol. Cell 2007, 18, 3545–3555. [Google Scholar] [CrossRef]
- Barclay, R.D.; Burd, N.A.; Tyler, C.; Tillin, N.A.; Mackenzie, R.W. The Role of the IGF-1 Signaling Cascade in Muscle Protein Synthesis and Anabolic Resistance in Aging Skeletal Muscle. Front. Nutr. 2019, 6, 146. [Google Scholar] [CrossRef]
- Bodine, S.C.; Stitt, T.N.; Gonzalez, M.; Kline, W.O.; Stover, G.L.; Bauerlein, R.; Zlotchenko, E.; Scrimgeour, A.; Lawrence, J.C.; Glass, D.J.; et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 2001, 3, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Liang, J.; Wu, L.; Zhang, H.; Lv, J.; Chen, N. Exercise-Induced Autophagy Suppresses Sarcopenia through Akt/mTOR and Akt/FoxO3a Signal Pathways and AMPK-Mediated Mitochondrial Quality Control. Front. Physiol. 2020, 11, 583478. [Google Scholar] [CrossRef]
- Horstman, A.M.; Dillon, E.L.; Urban, R.J.; Sheffield-Moore, M. The role of androgens and estrogens on healthy aging and longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 1140–1152. [Google Scholar] [CrossRef]
- Belli, R.; Bonato, A.; De Angelis, L.; Mirabilii, S.; Ricciardi, M.R.; Tafuri, A.; Molfino, A.; Gorini, S.; Leigheb, M.; Costelli, P.; et al. Metabolic Reprogramming Promotes Myogenesis during Aging. Front. Physiol. 2019, 10, 897. [Google Scholar] [CrossRef]
- Ratnaparkhi, A.; Muthu, S.A.; Shiriskar, S.M.; Pissurlenkar, R.R.; Choudhary, S.; Ahmad, B. Effects of hesperidin, a flavanone glycoside interaction on the conformation, stability, and aggregation of lysozyme: Multispectroscopic and molecular dynamic simulation studies? J. Biomol. Struct. Dyn. 2015, 33, 1866–1879. [Google Scholar] [CrossRef]
- Kuroyanagi, M.; Ishii, H.; Kawahara, N.; Sugimoto, H.; Yamada, H.; Okihara, K.; Shirota, O. Flavonoid glycosides and limonoids from Citrus molasses. J. Nat. Med. 2008, 62, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phytother. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Pla-Paga, L.; Companys, J.; Calderon-Perez, L.; Llaurado, E.; Sola, R.; Valls, R.M.; Pedret, A. Effects of hesperidin consumption on cardiovascular risk biomarkers: A systematic review of animal studies and human randomized clinical trials. Nutr. Rev. 2019, 77, 845–864. [Google Scholar] [CrossRef] [PubMed]
- Roohbakhsh, A.; Parhiz, H.; Soltani, F.; Rezaee, R.; Iranshahi, M. Molecular mechanisms behind the biological effects of hesperidin and hesperetin for the prevention of cancer and cardiovascular diseases. Life Sci. 2015, 124, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef]
- Clavel, S.; Siffroi-Fernandez, S.; Coldefy, A.S.; Boulukos, K.; Pisani, D.F.; Derijard, B. Regulation of the intracellular localization of Foxo3a by stress-activated protein kinase signaling pathways in skeletal muscle cells. Mol. Cell Biol. 2010, 30, 470–480. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Zembron-Lacny, A.; Dziubek, W.; Wolny-Rokicka, E.; Dabrowska, G.; Wozniewski, M. The Relation of Inflammaging with Skeletal Muscle Properties in Elderly Men. Am. J. Mens. Health 2019, 13, 1557988319841934. [Google Scholar] [CrossRef]
- Livshits, G.; Kalinkovich, A. Inflammaging as a common ground for the development and maintenance of sarcopenia, obesity, cardiomyopathy and dysbiosis. Ageing Res. Rev. 2019, 56, 100980. [Google Scholar] [CrossRef]
- Sharma, B.; Dabur, R. Role of Pro-inflammatory Cytokines in Regulation of Skeletal Muscle Metabolism: A Systematic Review. Curr. Med. Chem. 2020, 27, 2161–2188. [Google Scholar] [CrossRef]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef] [PubMed]
- Gomez, C.R.; Plackett, T.P.; Kovacs, E.J. Aging and estrogen: Modulation of inflammatory responses after injury. Exp. Gerontol. 2007, 42, 451–456. [Google Scholar] [CrossRef]
- Stitt, T.N.; Drujan, D.; Clarke, B.A.; Panaro, F.; Timofeyva, Y.; Kline, W.O.; Gonzalez, M.; Yancopoulos, G.D.; Glass, D.J. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell 2004, 14, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Bai, Y. IGF-1 activates the P13K/AKT signaling pathway via upregulation of secretory clusterin. Mol. Med. Rep. 2012, 6, 1433–1437. [Google Scholar] [CrossRef]
- Di Iorio, A.; Abate, M.; Di Renzo, D.; Russolillo, A.; Battaglini, C.; Ripari, P.; Saggini, R.; Paganelli, R.; Abate, G. Sarcopenia: Age-related skeletal muscle changes from determinants to physical disability. Int. J. Immunopathol. Pharmacol. 2006, 19, 703–719. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, H.-J.; Jin, H.; Lee, B.-Y. Hesperidin Ameliorates Sarcopenia through the Regulation of Inflammaging and the AKT/mTOR/FoxO3a Signaling Pathway in 22–26-Month-Old Mice. Cells 2023, 12, 2015. https://doi.org/10.3390/cells12152015
Oh H-J, Jin H, Lee B-Y. Hesperidin Ameliorates Sarcopenia through the Regulation of Inflammaging and the AKT/mTOR/FoxO3a Signaling Pathway in 22–26-Month-Old Mice. Cells. 2023; 12(15):2015. https://doi.org/10.3390/cells12152015
Chicago/Turabian StyleOh, Hyun-Ji, Heegu Jin, and Boo-Yong Lee. 2023. "Hesperidin Ameliorates Sarcopenia through the Regulation of Inflammaging and the AKT/mTOR/FoxO3a Signaling Pathway in 22–26-Month-Old Mice" Cells 12, no. 15: 2015. https://doi.org/10.3390/cells12152015
APA StyleOh, H.-J., Jin, H., & Lee, B.-Y. (2023). Hesperidin Ameliorates Sarcopenia through the Regulation of Inflammaging and the AKT/mTOR/FoxO3a Signaling Pathway in 22–26-Month-Old Mice. Cells, 12(15), 2015. https://doi.org/10.3390/cells12152015