Current and Future Landscape in Genetic Therapies for Leber Hereditary Optic Neuropathy
Abstract
1. Introduction
2. Clinical Presentation of LHON
3. Molecular Genetics of LHON
4. Treatment Modalities for LHON Disease
4.1. Genetic Therapies in LHON
4.1.1. Allotopic Gene Therapy
Pre-Clinical Allotopic Gene Therapy Studies
Clinical Allotopic Gene Therapy Trials
4.1.2. Gene Editing
4.2. Antioxidant and Neurotrophic Therapies
4.3. Mitochondrial Biogenesis and Replacement Therapies
4.3.1. Mitochondrial Biogenesis
4.3.2. Mitochondrial Replacement Therapy
4.4. Stem Cell Therapy
5. Summaries
6. Future Direction and Expert Opinion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Puomila, A.; Hämäläinen, P.; Kivioja, S.; Savontaus, M.-L.; Koivumäki, S.; Huoponen, K.; Nikoskelainen, E.; Nikoskelainen, E. Epidemiology and penetrance of Leber hereditary optic neuropathy in Finland. Eur. J. Hum. Genet. 2007, 15, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Yu-Wai-Man, P.; Griffiths, P.G.; Brown, D.T.; Howell, N.; Turnbull, D.M.; Chinnery, P.F. The epidemiology of Leber hereditary optic neuropathy in the North East of England. Am. J. Hum. Genet. 2003, 72, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Leber, T. Ueber hereditäre und congenital-angelegte Sehnervenleiden. Albrecht Graefes Arch. Ophthalmol. 1871, 17, 249–291. [Google Scholar] [CrossRef]
- Wallace, D.C.; Singh, G.; Lott, M.T.; Hodge, J.A.; Schurr, T.G.; Lezza, A.M.; Elsas, L.J.; Nikoskelainen, E.K. Mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy. Science 1988, 242, 1427–1430. [Google Scholar] [CrossRef]
- Yu-Wai-Man, P.; Votruba, M.; Moore, A.T.; Chinnery, P.F. Treatment strategies for inherited optic neuropathies: Past, present and future. Eye 2014, 28, 521–537. [Google Scholar] [CrossRef]
- Meyerson, C.; Van Stavern, G.; McClelland, C. Leber hereditary optic neuropathy: Current perspectives. Clin. Ophthalmol. 2015, 9, 1165–1176. [Google Scholar]
- Poincenot, L.; Pearson, A.L.; Karanjia, R. Demographics of a Large International Population of Patients Affected by Leber’s Hereditary Optic Neuropathy. Ophthalmology 2020, 127, 679–688. [Google Scholar] [CrossRef]
- Yu-Wai-Man, P.; Newman, N.J.; Carelli, V.; La Morgia, C.; Biousse, V.; Bandello, F.M.; Clermont, C.V.; Campillo, L.C.; Leruez, S.; Moster, M.L.; et al. Natural history of patients with Leber hereditary optic neuropathy—Results from the REALITY study. Eye 2022, 36, 818–826. [Google Scholar] [CrossRef]
- Mackey, D.A.; Oostra, R.-J.; Rosenberg, T.; Nikoskelainen, E.; Bronte-Stewart, J.; Poulton, J.; Harding, A.E.; Govan, G.; Bolhuis, P.A.; Norby, S. Primary pathogenic mtDNA mutations in multigeneration pedigrees with Leber hereditary optic neuropathy. Am. J. Hum. Genet. 1996, 59, 481. [Google Scholar]
- Tonagel, F.; Wilhelm, H.; Richter, P.; Kelbsch, C. Leber’s hereditary optic neuropathy: Course of disease in consideration of idebenone treatment and type of mutation. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 1009–1013. [Google Scholar] [CrossRef]
- Mackey, D.; Howell, N. A variant of Leber hereditary optic neuropathy characterized by recovery of vision and by an unusual mitochondrial genetic etiology. Am. J. Hum. Genet. 1992, 51, 1218–1228. [Google Scholar]
- Johns, D.R.; Smith, K.H.; Miller, N.R. Leber’s hereditary optic neuropathy: Clinical manifestations of the 3460 mutation. Arch. Ophthalmol. 1992, 110, 1577–1581. [Google Scholar] [CrossRef]
- Huoponen, K.; Vilkki, J.; Aula, P.; Nikoskelainen, E.K.; Savontaus, M. A new mtDNA mutation associated with Leber hereditary optic neuroretinopathy. Am. J. Hum. Genet. 1991, 48, 1147. [Google Scholar]
- Yu-Wai-Man, P.; Griffiths, P.G.; Chinnery, P.F. Mitochondrial optic neuropathies–disease mechanisms and therapeutic strategies. Prog. Retin. Eye Res. 2011, 30, 81–114. [Google Scholar] [CrossRef]
- Jha, R.K.; Dawar, C.; Hasan, Q.; Pujar, A.; Gupta, G.; Vishnu, V.Y.; Thangaraj, K. Mitochondrial Genetic Heterogeneity in Leber’s Hereditary Optic Neuropathy: Original Study with Meta-Analysis. Genes 2021, 12, 1300. [Google Scholar] [CrossRef]
- Harding, A.; Sweeney, M.; Govan, G.; Riordan-Eva, P. Pedigree analysis in Leber hereditary optic neuropathy families with a pathogenic mtDNA mutation. Am. J. Hum. Genet. 1995, 57, 77. [Google Scholar]
- Catarino, C.B.; von Livonius, B.; Priglinger, C.; Banik, R.; Matloob, S.; Tamhankar, M.A.; Castillo, L.; Friedburg, C.; Halfpenny, C.A.; Lincoln, J.A.; et al. Real-World Clinical Experience with Idebenone in the Treatment of Leber Hereditary Optic Neuropathy. J. Neuro-Ophthalmol. 2020, 40, 558. [Google Scholar] [CrossRef]
- Indrieri, A.; Carrella, S.; Romano, A.; Spaziano, A.; Marrocco, E.; Fernandez-Vizarra, E.; Barbato, S.; Pizzo, M.; Ezhova, Y.; Golia, F.M.; et al. miR-181a/b downregulation exerts a protective action on mitochondrial disease models. EMBO Mol. Med. 2019, 11, e8734. [Google Scholar] [CrossRef]
- Weiss, J.N.; Levy, S. Stem Cell Ophthalmology Treatment Study (SCOTS): Bone marrow derived stem cells in the treatment of Dominant Optic Atrophy. Stem Cell Investig. 2019, 6, 41. [Google Scholar] [CrossRef]
- Wong, R.C.B.; Lim, S.Y.; Hung, S.S.C.; Jackson, S.; Khan, S.; Van Bergen, N.J.; De Smit, E.; Liang, H.H.; Kearns, L.S.; Clarke, L.; et al. Mitochondrial replacement in an iPSC model of Leber’s hereditary optic neuropathy. Aging 2017, 9, 1341–1350. [Google Scholar] [CrossRef]
- Ellouze, S.; Augustin, S.; Bouaita, A.; Bonnet, C.; Simonutti, M.; Forster, V.; Picaud, S.; Sahel, J.A.; Corral-Debrinski, M. Optimized allotopic expression of the human mitochondrial ND4 prevents blindness in a rat model of mitochondrial dysfunction. Am. J. Hum. Genet. 2008, 83, 373–387. [Google Scholar] [CrossRef]
- Boddapati, S.V.; D’Souza, G.G.; Erdogan, S.; Torchilin, V.P.; Weissig, V. Organelle-targeted nanocarriers: Specific delivery of liposomal ceramide to mitochondria enhances its cytotoxicity in vitro and in vivo. Nano Lett. 2008, 8, 2559–2563. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Eastwood, J.D.; Alba, D.E.; Velmurugan, S.; Sun, N.; Porciatti, V.; Guy, J.; Yu, H. Gene therapy restores mitochondrial function and protects retinal ganglion cells in optic neuropathy induced by a mito-targeted mutant ND1 gene. Gene Ther. 2022, 29, 368–378. [Google Scholar] [CrossRef]
- Koilkonda, R.D.; Chou, T.H.; Porciatti, V.; Hauswirth, W.W.; Guy, J. Induction of rapid and highly efficient expression of the human ND4 complex I subunit in the mouse visual system by self-complementary adeno-associated virus. Arch. Ophthalmol. 2010, 128, 876–883. [Google Scholar] [CrossRef]
- Cwerman-Thibault, H.; Augustin, S.; Lechauve, C.; Ayache, J.; Ellouze, S.; Sahel, J.A.; Corral-Debrinski, M. Nuclear expression of mitochondrial ND4 leads to the protein assembling in complex I and prevents optic atrophy and visual loss. Mol. Ther. Methods Clin. Dev. 2015, 2, 15003. [Google Scholar] [CrossRef]
- Guy, J.; Qi, X.; Pallotti, F.; Schon, E.A.; Manfredi, G.; Carelli, V.; Martinuzzi, A.; Hauswirth, W.W.; Lewin, A.S. Rescue of a mitochondrial deficiency causing Leber Hereditary Optic Neuropathy. Ann. Neurol. 2002, 52, 534–542. [Google Scholar] [CrossRef]
- van Everdingen, J.A.M.; Pott, J.W.R.; Bauer, N.J.C.; Krijnen, A.M.; Lushchyk, T.; Wubbels, R.J. Clinical outcomes of treatment with idebenone in Leber’s hereditary optic neuropathy in the Netherlands: A national cohort study. Acta Ophthalmol. 2022, 100, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Koilkonda, R.D.; Yu, H.; Chou, T.-H.; Feuer, W.J.; Ruggeri, M.; Porciatti, V.; Tse, D.; Hauswirth, W.W.; Chiodo, V.; Boye, S.L.; et al. Safety and effects of the vector for the Leber hereditary optic neuropathy gene therapy clinical trial. JAMA Ophthalmol. 2014, 132, 409–420. [Google Scholar] [CrossRef]
- Bonnet, C.; Kaltimbacher, V.; Ellouze, S.; Augustin, S.; Bénit, P.; Forster, V.; Rustin, P.; Sahel, J.-A.; Corral-Debrinski, M. Allotopic mRNA localization to the mitochondrial surface rescues respiratory chain defects in fibroblasts harboring mitochondrial DNA mutations affecting complex I or v subunits. Rejuvenation Res. 2007, 10, 127–144. [Google Scholar] [CrossRef]
- Pei, H.; Wan, X.; Hu, W.-K.; Dong, X.-R.; Li, B. Construction and detection of a novel type of recombinant human rAAV2/2-ND4. Eye Sci. 2013, 28, 55–59. [Google Scholar]
- Guy, J.; Feuer, W.J.; Davis, J.L.; Porciatti, V.; Gonzalez, P.J.; Koilkonda, R.D.; Yuan, H.; Hauswirth, W.W.; Lam, B.L. Gene Therapy for Leber Hereditary Optic Neuropathy: Low- and Medium-Dose Visual Results. Ophthalmology 2017, 124, 1621–1634. [Google Scholar] [CrossRef]
- Yang, S.; Ma, S.-Q.; Wan, X.; He, H.; Pei, H.; Zhao, M.-J.; Chen, C.; Wang, D.-W.; Dong, X.-Y.; Yuan, J.-J.; et al. Long-term outcomes of gene therapy for the treatment of Leber’s hereditary optic neuropathy. EBioMedicine 2016, 10, 258–268. [Google Scholar] [CrossRef]
- Biousse, V.; Newman, N.J.; Yu-Wai-Man, P.; Carelli, V.; Moster, M.L.; Vignal-Clermont, C.; Klopstock, T.; Sadun, A.A.; Sergott, R.C.; Hage, R.; et al. Long-Term Follow-Up After Unilateral Intravitreal Gene Therapy for Leber Hereditary Optic Neuropathy: The RESTORE Study. J. Neuro-Ophthalmol. Off. J. N. Am. Neuro-Ophthalmol. Soc. 2021, 41, 309–315. [Google Scholar] [CrossRef]
- Feuer, W.J.; Schiffman, J.C.; Davis, J.L.; Porciatti, V.; Gonzalez, P.; Koilkonda, R.D.; Yuan, H.; Lalwani, A.; Lam, B.L.; Guy, J.; et al. Gene Therapy for Leber Hereditary Optic Neuropathy: Initial Results. Ophthalmology 2016, 123, 558–570. [Google Scholar] [CrossRef] [PubMed]
- Lam, B.L.; Feuer, W.J.; Davis, J.L.; Porciatti, V.; Yu, H.; Levy, R.B.; Vanner, E.; Guy, J. Leber Hereditary Optic Neuropathy Gene Therapy: Adverse Events and Visual Acuity Results of All Patient Groups. Am. J. Ophthalmol. 2022, 241, 262–271. [Google Scholar] [CrossRef]
- Vignal, C.; Uretsky, S.; Fitoussi, S.; Galy, A.; Blouin, L.; Girmens, J.-F.; Bidot, S.; Thomasson, N.; Bouquet, C.; Valero, S. Safety of rAAV2/2-ND4 gene therapy for Leber hereditary optic neuropathy. Ophthalmology 2018, 125, 945–947. [Google Scholar] [CrossRef]
- Vignal-Clermont, C.; Girmens, J.-F.; Audo, I.; Said, S.M.; Errera, M.-H.; Plaine, L.; O’Shaughnessy, D.; Taiel, M.; Sahel, J.-A. Safety of Intravitreal Gene Therapy for Treatment of Subjects with Leber Hereditary Optic Neuropathy due to Mutations in the Mitochondrial ND4 Gene: The REVEAL Study. BioDrugs 2021, 35, 201–214. [Google Scholar] [CrossRef]
- Newman, N.J.; Yu-Wai-Man, P.; Carelli, V.; Moster, M.L.; Biousse, V.; Vignal-Clermont, C.; Sergott, R.C.; Klopstock, T.; Sadun, A.A.; Barboni, P.; et al. Efficacy and Safety of Intravitreal Gene Therapy for Leber Hereditary Optic Neuropathy Treated within 6 Months of Disease Onset. Ophthalmology 2021, 128, 649–660. [Google Scholar] [CrossRef]
- Yu-Wai-Man, P.; Newman, N.J.; Carelli, V.; Moster, M.L.; Biousse, V.; Sadun, A.A.; Klopstock, T.; Vignal-Clermont, C.; Sergott, R.C.; Rudolph, G.; et al. Bilateral visual improvement with unilateral gene therapy injection for Leber hereditary optic neuropathy. Sci. Transl. Med. 2020, 12, eaaz7423. [Google Scholar] [CrossRef]
- Newman, N.J.; Yu-Wai-Man, P.; Carelli, V.; Biousse, V.; Moster, M.L.; Vignal-Clermont, C.; Sergott, R.C.; Klopstock, T.; Sadun, A.A.; Girmens, J.F.; et al. Intravitreal Gene Therapy vs. Natural History in Patients with Leber Hereditary Optic Neuropathy Carrying the m.11778G>A ND4 Mutation: Systematic Review and Indirect Comparison. Front. Neurol. 2021, 12, 662838. [Google Scholar] [CrossRef]
- Wan, X.; Pei, H.; Zhao, M.-J.; Yang, S.; Hu, W.-K.; He, H.; Ma, S.-Q.; Zhang, G.; Dong, X.-Y.; Chen, C.; et al. Efficacy and Safety of rAAV2-ND4 Treatment for Leber’s Hereditary Optic Neuropathy. Sci. Rep. 2016, 6, 21587. [Google Scholar] [CrossRef]
- Liu, H.-L.; Yuan, J.-J.; Zhang, Y.; Tian, Z.; Li, X.; Wang, D.; Du, Y.-Y.; Song, L.; Li, B. Factors associated with rapid improvement in visual acuity in patients with Leber’s hereditary optic neuropathy after gene therapy. Acta Ophthalmol. 2020, 98, e730–e733. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Yuan, J.; Tian, Z.; Liu, H.; Wang, D.; Li, B. Prognostic factors for visual acuity in patients with Leber’s hereditary optic neuropathy after rAAV2-ND4 gene therapy. Clin. Exp. Ophthalmol. 2019, 47, 774–778. [Google Scholar] [CrossRef]
- Liu, H.L.; Yuan, J.J.; Tian, Z.; Li, X.; Song, L.; Li, B. What are the characteristics and progression of visual field defects in patients with Leber hereditary optic neuropathy: A prospective single-centre study in China. BMJ Open 2019, 9, e025307. [Google Scholar] [CrossRef] [PubMed]
- Meunier, I.; Lenaers, G.; Hamel, C.; Defoort-Dhellemmes, S. Hereditary optic neuropathies: From clinical signs to diagnosis. J. Fr. Ophtalmol. 2013, 36, 886–900. [Google Scholar] [CrossRef]
- Riordan-Eva, P.; Sanders, M.; Govan, G.; Sweeney, M.; Costa, J.D.; Harding, A. The clinical features of Leber’s hereditary optic neuropathy defined by the presence of a pathogenic mitochondrial DNA mutation. Brain 1995, 118, 319–337. [Google Scholar] [CrossRef] [PubMed]
- Lam, B.L.; Feuer, W.J.; Schiffman, J.C.; Porciatti, V.; Vandenbroucke, R.; Rosa, P.R.; Gregori, G.; Guy, J. Trial end points and natural history in patients with G11778A Leber hereditary optic neuropathy: Preparation for gene therapy clinical trial. JAMA Ophthalmol. 2014, 132, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Johns, D.R.; Heher, K.L.; Miller, N.R.; Smith, K.H. Leber’s hereditary optic neuropathy. Clinical manifestations of the 14484 mutation. Arch. Ophthalmol. 1993, 111, 495–498. [Google Scholar] [CrossRef]
- Nikoskelainen, E.K.; Huoponen, K.; Juvonen, V.; Lamminen, T.; Nummelin, K.; Savontaus, M.L. Ophthalmologic findings in Leber hereditary optic neuropathy, with special reference to mtDNA mutations. Ophthalmology 1996, 103, 504–514. [Google Scholar] [CrossRef]
- Majander, A.; Bowman, R.; Poulton, J.; Antcliff, R.J.; Reddy, M.A.; Michaelides, M.; Webster, A.R.; Chinnery, P.F.; Votruba, M.; Moore, A.T.; et al. Childhood-onset Leber hereditary optic neuropathy. Br. J. Ophthalmol. 2017, 101, 1505–1509. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ghosh, A. Molecular mechanism of mitochondrial respiratory chain assembly and its relation to mitochondrial diseases. Mitochondrion 2020, 53, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Chial, H.; Craig, J. mtDNA and mitochondrial diseases. Nat. Educ. 2008, 1, 217. [Google Scholar]
- Huoponen, K. Leber hereditary optic neuropathy: Clinical and molecular genetic findings. Neurogenetics 2001, 3, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Valletti, A.; Longo, G.; Bisceglia, L.; Montoya, J.; Emperador, S.; Guerriero, S.; Petruzzella, V. Mitochondrial DNA copy number in affected and unaffected LHON mutation carriers. BMC Res. Notes 2018, 11, 911. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Bisceglia, L.; Russo, L.; Palese, L.L.; D’Agruma, L.; Emperador, S.; Montoya, J.; Guerriero, S.; Petruzzella, V. High Mitochondrial DNA Copy Number Is a Protective Factor from Vision Loss in Heteroplasmic Leber’s Hereditary Optic Neuropathy (LHON). Investig. Ophthalmol. Vis. Sci. 2017, 58, 2193–2197. [Google Scholar] [CrossRef] [PubMed]
- Kirkman, M.A.; Yu-Wai-Man, P.; Korsten, A.; Leonhardt, M.; Dimitriadis, K.; De Coo, I.F.; Klopstock, T.; Chinnery, P.F. Gene-environment interactions in Leber hereditary optic neuropathy. Brain 2009, 132 Pt 9, 2317–2326. [Google Scholar] [CrossRef]
- Ajax, E.T.; Kardon, R. Late-onset Leber’s hereditary optic neuropathy. J. Neuro-Ophthalmol. Off. J. N. Am. Neuro-Ophthalmol. Soc. 1998, 18, 30–31. [Google Scholar]
- Yu-Wai-Man, P. Therapeutic Approaches to Inherited Optic Neuropathies. Semin. Neurol. 2015, 35, 578–586. [Google Scholar] [CrossRef]
- Yu, H.; Koilkonda, R.D.; Chou, T.-H.; Porciatti, V.; Ozdemir, S.S.; Chiodo, V.; Boye, S.L.; Boye, S.E.; Hauswirth, W.W.; Lewin, A.S.; et al. Gene delivery to mitochondria by targeting modified adenoassociated virus suppresses Leber’s hereditary optic neuropathy in a mouse model. Proc. Natl. Acad. Sci. USA 2012, 109, E1238–E1247. [Google Scholar] [CrossRef]
- Bonnet, C.; Augustin, S.; Ellouze, S.; Bénit, P.; Bouaita, A.; Rustin, P.; Sahel, J.-A.; Corral-Debrinski, M. The optimized allotopic expression of ND1 or ND4 genes restores respiratory chain complex I activity in fibroblasts harboring mutations in these genes. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2008, 1783, 1707–1717. [Google Scholar] [CrossRef]
- Gammage, P.A.; Rorbach, J.; Vincent, A.I.; Rebar, E.J.; Minczuk, M. Mitochondrially targeted ZFNs for selective degradation of pathogenic mitochondrial genomes bearing large-scale deletions or point mutations. EMBO Mol. Med. 2014, 6, 458–466. [Google Scholar] [CrossRef]
- Hashimoto, M.; Bacman, S.R.; Peralta, S.; Falk, M.J.; Chomyn, A.; Chan, D.C.; Sahel, J.-A.; Corral-Debrinski, M. MitoTALEN: A General Approach to Reduce Mutant mtDNA Loads and Restore Oxidative Phosphorylation Function in Mitochondrial Diseases. Mol. Ther. 2015, 23, 1592–1599. [Google Scholar] [CrossRef]
- Gammage, P.A.; Viscomi, C.; Simard, M.-L.; Costa, A.S.H.; Gaude, E.; Powell, C.A.; Van Haute, L.; McCann, B.J.; Rebelo-Guiomar, P.; Cerutti, R.; et al. Genome editing in mitochondria corrects a pathogenic mtDNA mutation in vivo. Nat. Med. 2018, 24, 1691–1695. [Google Scholar] [CrossRef]
- Bacman, S.R.; Kauppila, J.H.K.; Pereira, C.V.; Nissanka, N.; Miranda, M.; Pinto, M.; Williams, S.L.; Larsson, N.-G.; Stewart, J.B.; Moraes, C.T. MitoTALEN reduces mutant mtDNA load and restores tRNAAla levels in a mouse model of heteroplasmic mtDNA mutation. Nat. Med. 2018, 24, 1696–1700. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wing, K.; Wang, J.H.; Luu, C.D.; Bender, J.A.; Chen, J.; Wang, Q.; Lu, Q.; Nguyen Tran, M.T.; Young, K.M.; et al. Comparison of CRISPR/Cas Endonucleases for in vivo Retinal Gene Editing. Front. Cell Neurosci. 2020, 14, 570917. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Hung, S.S.C.; Mohd Khalid, M.K.N.; Wang, J.H.; Chrysostomou, V.; Wong, V.H.Y.; Singh, V.; Wing, K.; Tu, L.; Bender, J.A.; et al. Utility of Self-Destructing CRISPR/Cas Constructs for Targeted Gene Editing in the Retina. Hum. Gene Ther. 2019, 30, 1349–1360. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.-R.A.; Yalvac, M.E.; Khoo, B.; Eckardt, S.; McLaughlin, K.J. Adapting CRISPR/Cas9 System for Targeting Mitochondrial Genome. Front. Genet. 2021, 12, 627050. [Google Scholar] [CrossRef]
- Jo, A.; Ham, S.; Lee, G.H.; Lee, Y.I.; Kim, S.; Lee, Y.S.; Shin, J.H.; Lee, Y. Efficient Mitochondrial Genome Editing by CRISPR/Cas9. BioMed Res. Int. 2015, 2015, 305716. [Google Scholar] [CrossRef]
- Mok, B.Y.; de Moraes, M.H.; Zeng, J.; Bosch, D.E.; Kotrys, A.V.; Raguram, A.; Hsu, F.; Radey, M.C.; Peterson, S.B.; Mootha, V.K.; et al. A bacterial cytidine deaminase toxin enables CRISPR-free mitochondrial base editing. Nature 2020, 583, 631–637. [Google Scholar] [CrossRef]
- Storoni, M.; Robert, M.P.; Plant, G.T. The therapeutic potential of a calorie-restricted ketogenic diet for the management of Leber hereditary optic neuropathy. Nutr. Neurosci. 2019, 22, 156–164. [Google Scholar] [CrossRef]
- Chu, C.-C.; Huang, C.-C.; Kao, L.-Y.; Kuo, H.-C.; Yu, T.-N.; Tso, D.-J.; Lee, H.-C.; Wei, Y.-H. Clinical phenotype and the G11778A mutation of mitochondrial DNA in patients with Leber’s hereditary optic neuropathy in Taiwan. Neuro-Ophthalmol. 2001, 26, 207–216. [Google Scholar] [CrossRef]
- Tarnopolsky, M. The mitochondrial cocktail: Rationale for combined nutraceutical therapy in mitochondrial cytopathies. Adv. Drug Deliv. Rev. 2008, 60, 1561–1567. [Google Scholar] [CrossRef]
- Yu-Wai-Man, P.; Soiferman, D.; Moore, D.G.; Burté, F.; Saada, A. Evaluating the therapeutic potential of idebenone and related quinone analogues in Leber hereditary optic neuropathy. Mitochondrion 2017, 36, 36–42. [Google Scholar] [CrossRef]
- Klopstock, T.; Yu-Wai-Man, P.; Dimitriadis, K.; Rouleau, J.; Heck, S.; Bailie, M.; Atawan, A.; Chattopadhyay, S.; Schubert, M.; Garip, A.; et al. A randomized placebo-controlled trial of idebenone in Leber’s hereditary optic neuropathy. Brain 2011, 134, 2677–2686. [Google Scholar] [CrossRef]
- Rudolph, G.; Dimitriadis, K.; Büchner, B.; Heck, S.; Al-Tamami, J.; Seidensticker, F.; Rummey, C.; Leinonen, M.; Meier, T.; Klopstock, T. Effects of idebenone on color vision in patients with leber hereditary optic neuropathy. J. Neuro-Ophthalmol. 2013, 33, 30. [Google Scholar] [CrossRef]
- Klopstock, T.; Metz, G.; Yu-Wai-Man, P.; Büchner, B.; Gallenmüller, C.; Bailie, M.; Nwali, N.; Griffiths, P.G.; von Livonius, B.; Reznicek, L.; et al. Persistence of the treatment effect of idebenone in Leber’s hereditary optic neuropathy. Brain 2013, 136, e230. [Google Scholar] [CrossRef]
- Carelli, V.; La Morgia, C.; Valentino, M.L.; Rizzo, G.; Carbonelli, M.; De Negri, A.M.; Sadun, F.; Carta, A.; Guerriero, S.; Simonelli, F.; et al. Idebenone Treatment in Leber’s Hereditary Optic Neuropathy. Brain 2011, 134, e188. [Google Scholar] [CrossRef]
- Carelli, V.; Carbonelli, M.; Irenaeus, F.; Kawasaki, A.; Klopstock, T.; Lagrèze, W.A.; Sadun, F.; Carta, A.; Guerriero, S.; Simonelli, F.; et al. International consensus statement on the clinical and therapeutic management of Leber hereditary optic neuropathy. J. Neuro-Ophthalmol. 2017, 37, 371–381. [Google Scholar] [CrossRef]
- Enns, G.M.; Kinsman, S.L.; Perlman, S.L.; Spicer, K.M.; Abdenur, J.E.; Cohen, B.H.; Amagata, A.; Barnes, A.; Kheifets, V.; Shrader, W.D.; et al. Initial experience in the treatment of inherited mitochondrial disease with EPI-743. Mol. Genet. Metab. 2012, 105, 91–102. [Google Scholar] [CrossRef]
- Sadun, A.A.; Chicani, C.F.; Ross-Cisneros, F.N.; Barboni, P.; Thoolen, M.; Shrader, W.D.; Kubis, K.; Carelli, V.; Miller, G. Effect of EPI-743 on the clinical course of the mitochondrial disease Leber hereditary optic neuropathy. Arch. Neurol. 2012, 69, 331–338. [Google Scholar] [CrossRef]
- Szeto, H.H. First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br. J. Pharmacol. 2014, 171, 2029–2050. [Google Scholar] [CrossRef] [PubMed]
- Karanjia, R.; Coupland, S.G.; Garcia, M.; Sadun, A.A. Elamipretide (MTP-131) Topical Ophthalmic Solution for the Treatment of Leber’s Hereditary Optic Neuropathy. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2266. [Google Scholar]
- Eells, J.T.; Wong-Riley, M.T.; VerHoeve, J.; Henry, M.; Buchman, E.V.; Kane, M.P.; Gould, L.J.; Das, R.; Jett, M.; Hodgson, B.D.; et al. Mitochondrial signal transduction in accelerated wound and retinal healing by near-infrared light therapy. Mitochondrion 2004, 4, 559–567. [Google Scholar] [CrossRef] [PubMed]
- La Morgia, C.; Carbonelli, M.; Barboni, P.; Sadun, A.A.; Carelli, V. Medical management of hereditary optic neuropathies. Front. Neurol. 2014, 5, 141. [Google Scholar] [CrossRef]
- Dai, Y.; Zheng, K.; Clark, J.; Swerdlow, R.H.; Pulst, S.M.; Sutton, J.P.; Shinobu, L.A.; Simon, D.K. Rapamycin drives selection against a pathogenic heteroplasmic mitochondrial DNA mutation. Hum. Mol. Genet. 2014, 23, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.K.; Datta, S.; McMackin, M.Z.; Cortopassi, G.A. Rescue of cell death and inflammation of a mouse model of complex 1-mediated vision loss by repurposed drug molecules. Hum. Mol. Genet. 2017, 26, 4929–4936. [Google Scholar] [CrossRef]
- Pisano, A.; Preziuso, C.; Iommarini, L.; Perli, E.; Grazioli, P.; Campese, A.F.; Maresca, A.; Montopoli, M.; Masuelli, L.; Sadun, A.A. Targeting estrogen receptor β as preventive therapeutic strategy for Leber’s hereditary optic neuropathy. Hum. Mol. Genet. 2015, 24, 6921–6931. [Google Scholar] [CrossRef]
- Giordano, C.; Montopoli, M.; Perli, E.; Orlandi, M.; Fantin, M.; Ross-Cisneros, F.N.; Caparrotta, L.; Martinuzzi, A.; Ragazzi, E.; Ghelli, A. Oestrogens ameliorate mitochondrial dysfunction in Leber’s hereditary optic neuropathy. Brain 2011, 134, 220–234. [Google Scholar] [CrossRef]
- Weiss, J.N.; Levy, S. Stem Cell Ophthalmology Treatment Study (SCOTS): Bone Marrow-Derived Stem Cells in the Treatment of Stargardt Disease. Medicines 2021, 8, 10. [Google Scholar] [CrossRef]
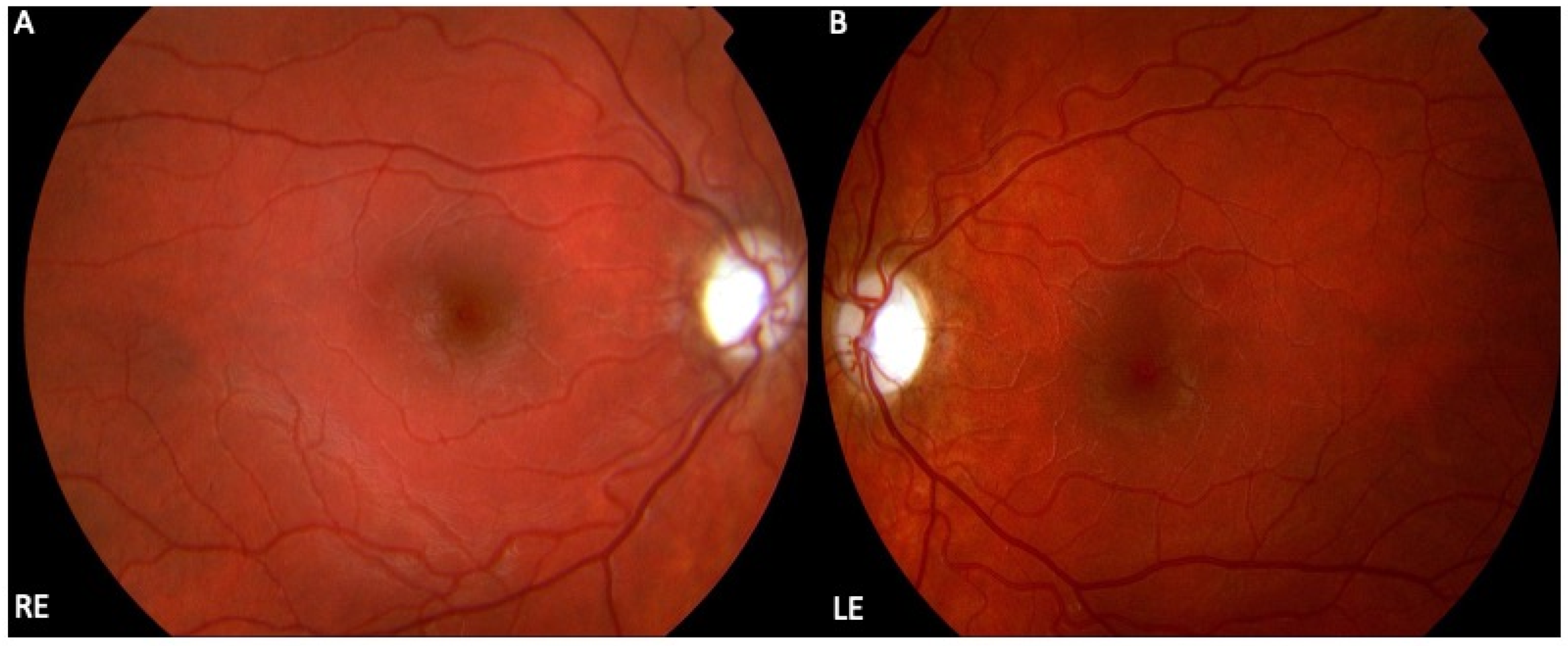


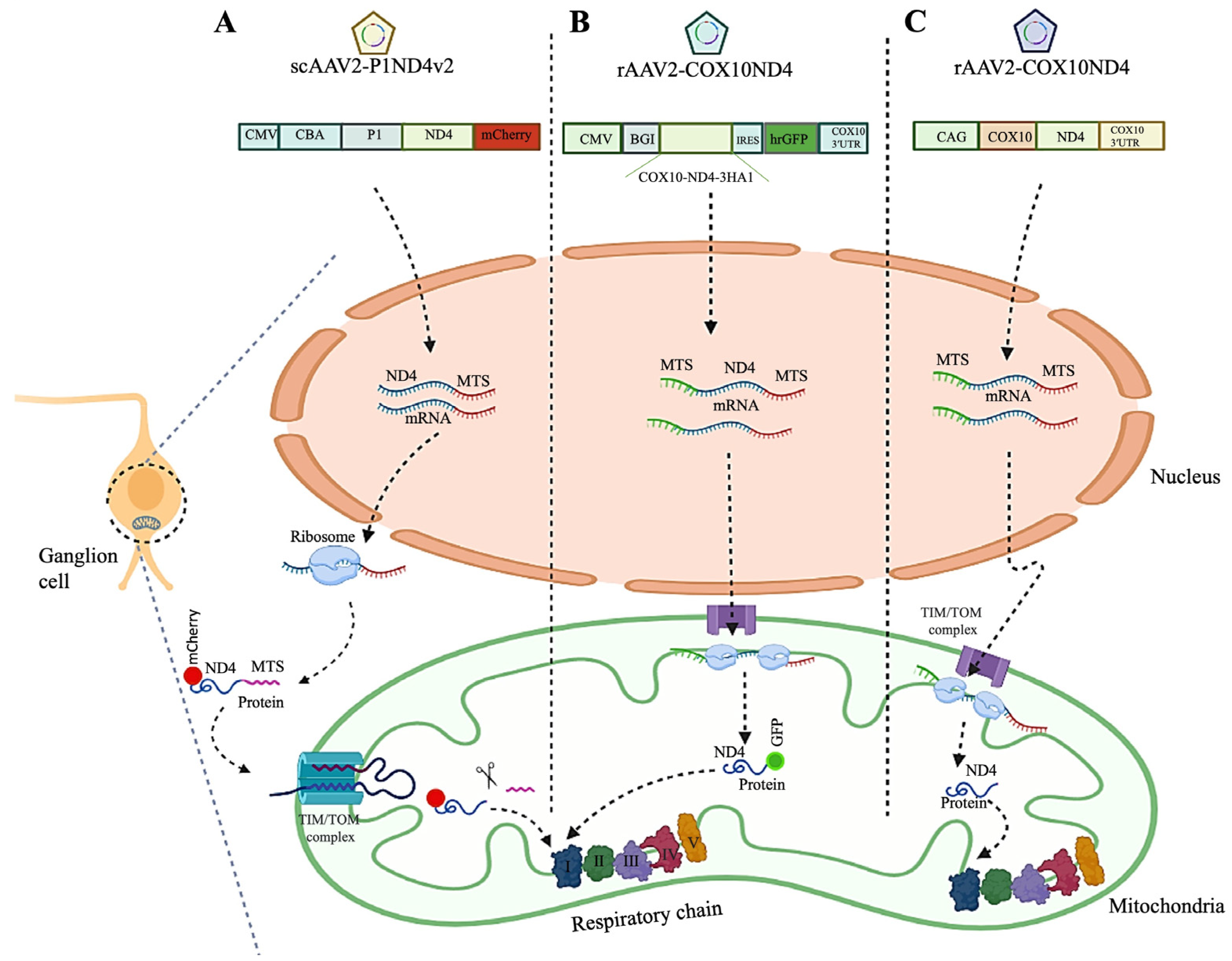
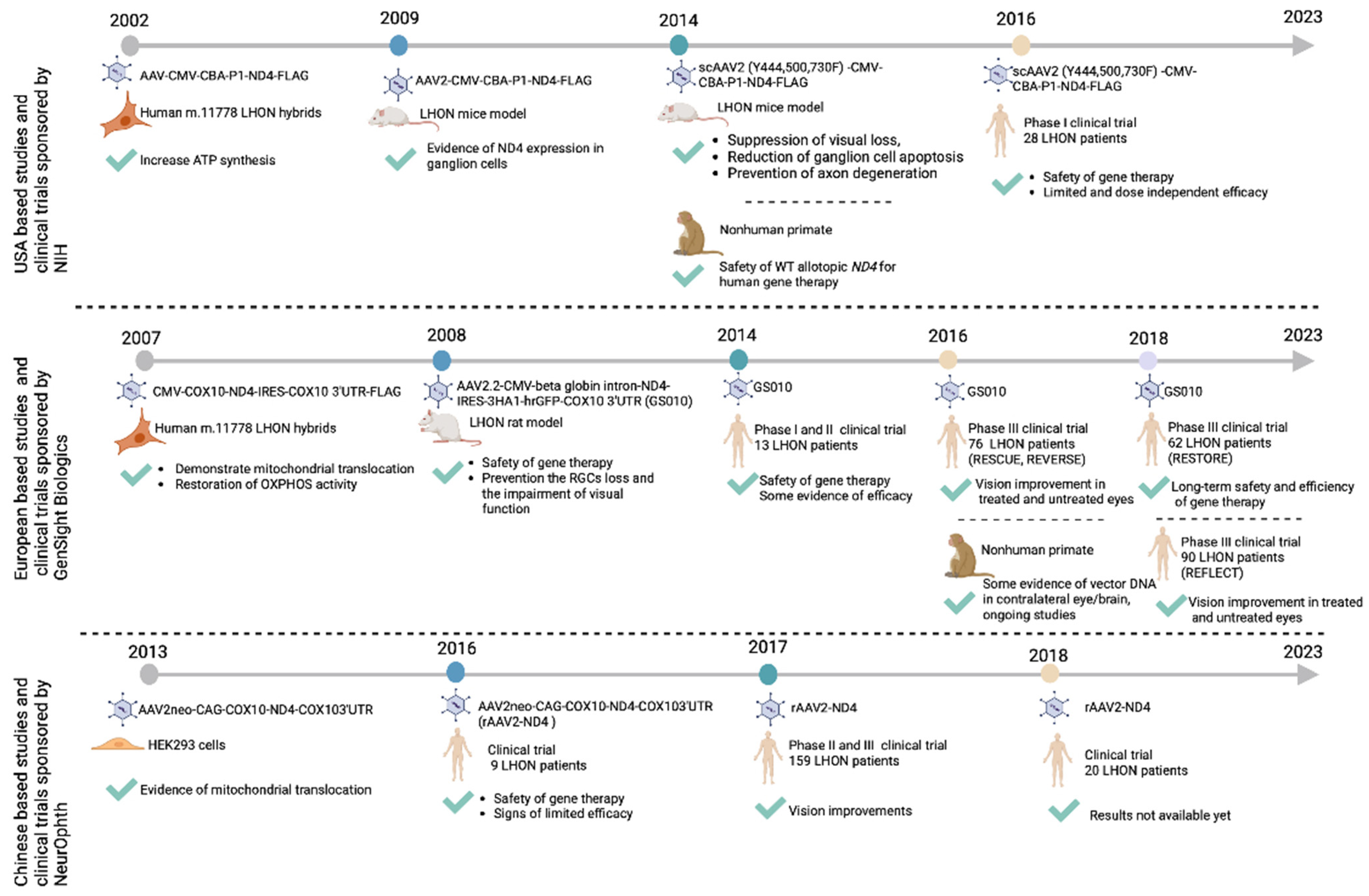
| mtDNA Variation/Complex I Subunit | NT Δ | AA Δ | Patients Carrying the Variant (%) | Variant Penetrance (% in Males) | Recovery of Vision (%) |
|---|---|---|---|---|---|
| m.11778 G > A/ND4 | G > A | R340H | 69 | 82 | 4–25 |
| m.3460 G > A/ND1 | G > A | A52T | 13 | 40–80 | 22–25 |
| m.14484 T > C/ND6 | T > C | M64V | 14 | 68 | 37–65 |
| Clinical Trial Identifier and Name | Phase | Vector | Age Range (Years) | Participants Population | Primary Endpoints | Secondary Endpoints | Results |
|---|---|---|---|---|---|---|---|
| NCT02161380 | I | scAAV2-P1ND4v2 | 15+ | 28 | IAE | Safety, efficacy, and VA change from baseline | No serious safety problems at low and medium doses; asymptomatic uveitis in 2 patients |
| NCT02064569 | I and II | GS010 | 18+ | 19 | IAE | - | Safety and tolerability of intravitreal |
| REVERSE, NCT02652780 | III | GS010 | 15+ | 37 | VA at Week 48 | VA at Week 72 and Week 96, number of eye and subject responders, GCL macular volume, RNFL temporal quadrant and papillo-macular bundle thickness, ETDRS total macular volume, VF MD and foveal threshold, contrast sensitivity, and colour vision | Improvement of BCVA in treated and sham eye |
| RESCUE, NCT02652767 | III | GS010 | 15+ | 39 | VA at Week 48 | VA at Week 72 and Week 96, number of eye and subject responders, GCL macular volume, RNFL temporal quadrant and papillo-macular bundle thickness, ETDRS total macular volume, VFI/MD and foveal threshold, contrast sensitivity, and colour vision | Improvement of BCVA |
| RESTORE, NCT03406104 | III | GS010 | 15+ | 61 | IAE up to 5 years | BCVA, HVF, and OCT, responder analysis, time course of the response, GCL, VFQ-25 and SF-36-v2 up to 5 years | No serious IAE |
| REFLECT, NCT03293524 | III | GS010 | 15+ | 90 | BCVA up to 1.5 year | BCVA, responder analysis, OCT, HVF, contrast sensitivity, VFQ-25 and SF-36-v2 up to 1.5 and 2 years | Improvement of BCVA |
| NCT01267422 | Not Applicable | rAAV2-ND4 | 8 years to 60 years | 9 | BCVA up to 3 years | IOP, neutralizing antibodies, average RNFL thickness, VFI/MD, and VFI, VEP up to 3 | Improvement of BCVA, VF, and VEPs, in both treated and untreated eyes, RNFL thickness relatively unchanged |
| NCT03153293 | II and III | rAAV2-ND4 | 10 years to 65 years | 159 | BCVA up to 1 year | VEP, RNFL, and liver function in plasma up to 1 year | Results unavailable |
| NCT03428178 | Not Applicable | rAAV2-ND4 | 8 years to 60 years | 120 | BCVA up to 1 year | VEP, RNFL, VF MD and VFI, liver, and kidney function in plasma | Results unavailable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shamsnajafabadi, H.; MacLaren, R.E.; Cehajic-Kapetanovic, J. Current and Future Landscape in Genetic Therapies for Leber Hereditary Optic Neuropathy. Cells 2023, 12, 2013. https://doi.org/10.3390/cells12152013
Shamsnajafabadi H, MacLaren RE, Cehajic-Kapetanovic J. Current and Future Landscape in Genetic Therapies for Leber Hereditary Optic Neuropathy. Cells. 2023; 12(15):2013. https://doi.org/10.3390/cells12152013
Chicago/Turabian StyleShamsnajafabadi, Hoda, Robert E. MacLaren, and Jasmina Cehajic-Kapetanovic. 2023. "Current and Future Landscape in Genetic Therapies for Leber Hereditary Optic Neuropathy" Cells 12, no. 15: 2013. https://doi.org/10.3390/cells12152013
APA StyleShamsnajafabadi, H., MacLaren, R. E., & Cehajic-Kapetanovic, J. (2023). Current and Future Landscape in Genetic Therapies for Leber Hereditary Optic Neuropathy. Cells, 12(15), 2013. https://doi.org/10.3390/cells12152013






