IRE1a-Induced FilaminA Phosphorylation Enhances Migration of Mesenchymal Stem Cells Derived from Multiple Myeloma Patients
Abstract
1. Introduction
2. Results and Discussion
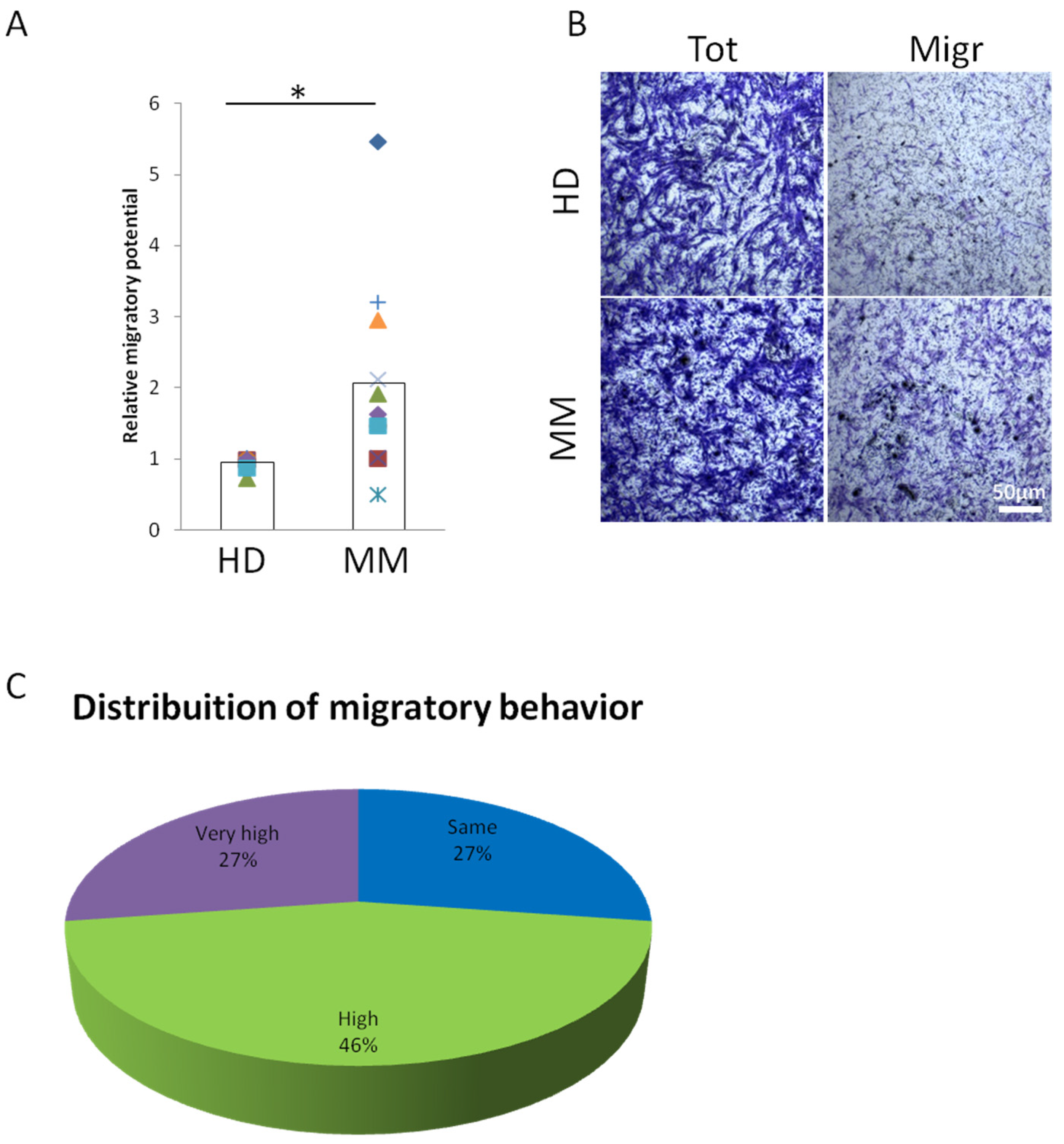
2.1. MM-BMMSCs Exert High Migratory Capability
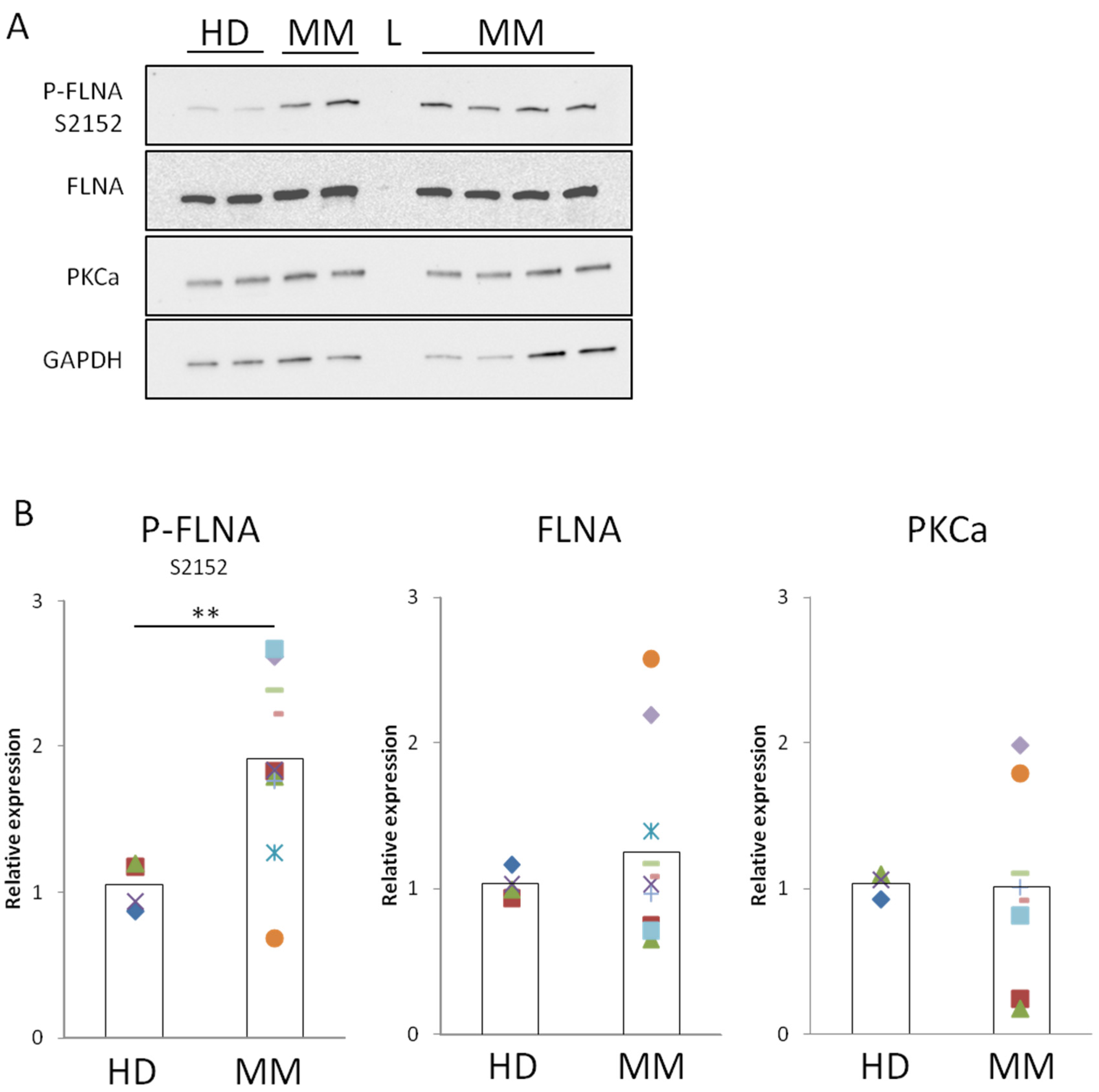
2.2. FLNA Is Phosphorylated in MM-BMMSCs
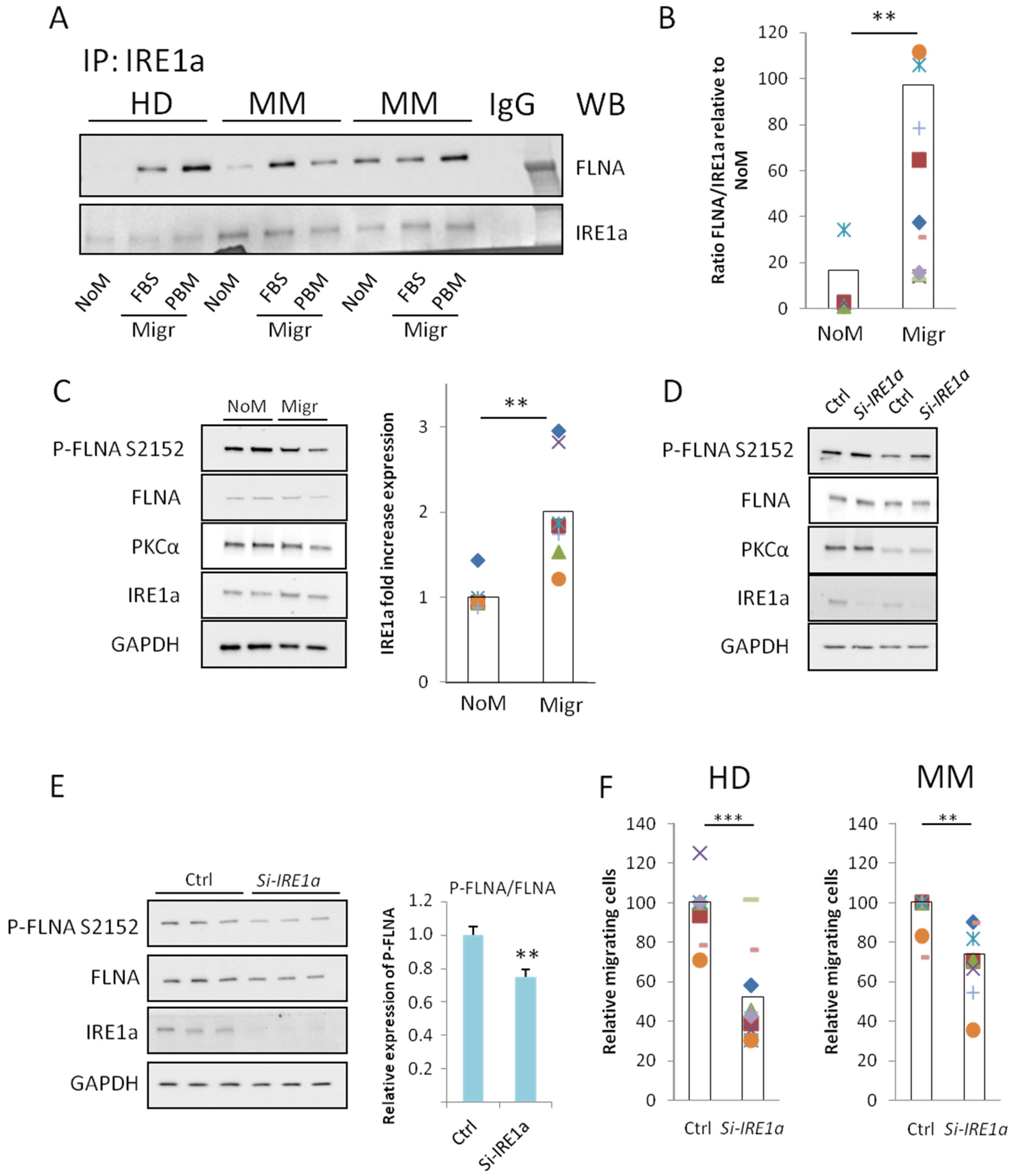
2.3. IRE1a-FLNA Interaction in Non-Migrating and Migrating BMMSCs
2.4. IRE1a Is Required for Efficient Migration of BMMSCs
2.5. Conclusions
3. Material and Methods
3.1. Bone Marrow-Derived Mesenchymal Stem Cell Isolation and Culture
3.2. Western Blot Analysis and Immunoprecipitation
3.3. Migration Experiment
3.4. Si-RNA Transfection
3.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MM | multiple myeloma |
| MGUS | monoclonal gammopathy of uncertain significance |
| HD | healthy donor |
| BMMSC | bone marrow mesenchymal stem cell |
| ER | endoplasmic reticulum |
| UPR | unfolded protein response |
| IRE1a | inositol-requiring enzyme 1 alpha |
| FLNA | FilaminA |
References
- Van de Donk, N.W.C.J.; Pawlyn, C.; Yong, K.L. Multiple myeloma. Lancet 2021, 397, 410–427. [Google Scholar] [CrossRef]
- Morrison, S.J.; Scadden, D.T. The bone marrow niche for haematopoietic stem cells. Nature 2014, 505, 327–33434. [Google Scholar] [CrossRef]
- Raimondi, L.; De Luca, A.; Amodio, N.; Manno, M.; Raccosta, S.; Taverna, S.; Bellavia, D.; Naselli, F.; Fontana, S.; Schillaci, O.; et al. Involvement of multiple myeloma cell-derived exosomes in osteoclast differentiation. Oncotarget 2015, 30, 13772–13789. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, S.; Saieva, L.; Vicario, E.; Pucci, M.; Toscani, D.; Manno, M.; Raccosta, S.; Giuliani, N.; Alessandro, R. Multiple myeloma-derived exosomes are enriched of amphiregulin (AREG) and activate the epidermal growth factor pathway in the bone microenvironment leading to osteoclastogenesis. J. Hematol. Oncol. 2019, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Roccaro, A.M.; Sacco, A.; Maiso, P.; Azab, A.K.; Tai, Y.-T.; Reagan, M.; Azab, F.; Flores, L.M.; Campigotto, F.; Weller, E.; et al. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J. Clin. Investig. 2013, 123, 1542–1555. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, S.; Kurihara, N.; Shiozawa, Y.; Joseph, J.; Taichman, R.; Galson, D.L.; Roodman, G.D. Annexin II interactions with the annexin II receptor enhance multiple myeloma cell adhesion and growth in the bone marrow microenvironment. Blood 2012, 119, 1888–1896. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Najar, M.; Stamatopoulos, B.; Pieters, K.; Pradier, O.; Bron, D.; Meuleman, N.; Lagneaux, L. Immune impairments in multiple myeloma bone marrow mesenchymal stromal cells. Cancer Immunol. Immunother. 2015, 64, 213–224. [Google Scholar] [CrossRef]
- Meads, M.B.; Hazlehurst, L.A.; Dalton, W.S. The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin. Cancer Res. 2008, 14, 2519–2526. [Google Scholar] [CrossRef] [PubMed]
- Alameda, D.; Saez, B.; Lara-Astiaso, D.; Sarvide, S.; Lasa, M.; Alignani, D.; Rodriguez, I.; Garate, S.; Vilas, A.; Paiva, B.; et al. Characterization of freshly isolated bone marrow mesenchymal stromal cells from healthy donors and patients with multiple myeloma: Transcriptional modulation of the microenvironment. Haematologica 2020, 105, e470–e473. [Google Scholar] [CrossRef]
- Fernando, R.C.; Mazzotti, D.R.; Azevedo, H.; Sandes, A.F.; Gil Rizzatti, E.; de Oliveira, M.B.; Alves, V.L.F.; Eugênio, A.I.P.; de Carvalho, F.; Dalboni, M.A.; et al. Transcriptome Analysis of Mesenchymal Stem Cells from Multiple Myeloma Patients Reveals Downregulation of Genes Involved in Cell Cycle Progression, Immune Response, and Bone Metabolism. Sci. Rep. 2019, 9, 1056. [Google Scholar] [CrossRef]
- Xu, S.; Evans, H.; Buckle, C.; De Veirman, K.; Hu, J.; Xu, D.; E Menu, E.; A De Becker, A.; Broek, I.V.; Leleu, X.; et al. Impaired osteogenic differentiation of mesenchymal stem cells derived from multiple myeloma patients is associated with a blockade in the deactivation of the Notch signaling pathway. Leukemia 2012, 26, 2546–2549. [Google Scholar] [CrossRef]
- D𠄙Souza, S.; del Prete, D.; Jin, S.; Sun, Q.; Huston, A.J.; Kostov, F.E.; Sammut, B.; Hong, C.-S.; Anderson, J.L.; Patrene, K.D.; et al. Gfi1 expressed in bone marrow stromal cells is a novel osteoblast suppressor in patients with multiple myeloma bone disease. Blood 2011, 118, 6871–6880. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Santini, G.C.; De Veirman, K.; Broek, I.V.; Leleu, X.; De Becker, A.; Van Camp, B.; Vanderkerken, K.; Van Riet, I. Upregulation of miR-135b is involved in the impaired osteogenic differentiation of mesenchymal stem cells derived from multiple myeloma patients. PLoS ONE 2013, 8, e79752. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Løvendorf, M.; Park, J.; Salem, K.Z.; Reagan, M.R.; Manier, S.; Zavidij, O.; Rahmat, M.; Huynh, D.; Takagi, S.; et al. Inhibition of microRNA-138 enhances bone formation in multiple myeloma bone marrow niche. Leukemia 2018, 32, 1739–1750. [Google Scholar] [CrossRef]
- Wang, J.; De Veirman, K.; Faict, S.; Frassanito, M.A.; Ribatti, D.; Vacca, A.; Menu, E. Multiple myeloma exosomes establish a favourable bone marrow microenvironment with enhanced angiogenesis and immunosuppression. J. Pathol. 2016, 239, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Siwecka, N.; Rozpędek-Kamińska, W.; Wawrzynkiewicz, A.; Pytel, D.; Diehl, J.A.; Majsterek, I. The structure, activation and signaling of ire1 and its role in determining cell fate. Biomedicines 2021, 9, 156. [Google Scholar] [CrossRef] [PubMed]
- Harnoss, J.M.; Le Thomas, A.; Shemorry, A.; Marsters, S.A.; Lawrence, D.A.; Lu, M.; Chen, Y.-C.A.; Qing, J.; Totpal, K.; Kan, D.; et al. Disruption of IRE1α through its kinase domain attenuates multiple myeloma. Proc. Natl. Acad. Sci. USA 2019, 116, 16420–16429. [Google Scholar] [CrossRef]
- Mimura, N.; Fulciniti, M.; Gorgun, G.; Tai, Y.-T.; Cirstea, D.; Santo, L.; Hu, Y.; Fabre, C.; Minami, J.; Ohguchi, H.; et al. Blockade of XBP1 splicing by inhibition of IRE1α is a promising therapeutic option in multiple myeloma. Blood 2012, 119, 5772–5781. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, I.; Denko, N.C.; Olson, M.; Van Melckebeke, H.; Lust, S.; Tam, A.; Solow-Cordero, D.E.; Bouley, D.M.; Offner, F.; Niwa, M.; et al. Identification of an Ire1alpha endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood 2011, 117, 1311–1314. [Google Scholar] [CrossRef]
- Yamashita, Y.; Morita, S.; Hosoi, H.; Kobata, H.; Kishimoto, S.; Ishibashi, T.; Mishima, H.; Kinoshita, A.; Backes, B.J.; Yoshiura, K.-I.; et al. Targeting adaptive ire1α signaling and plk2 in multiple myeloma: Possible anti-tumor mechanisms of kira8 and nilotinib. Int. J. Mol. Sci. 2020, 21, 6314. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, L.; De Luca, A.; Fontana, S.; Amodio, N.; Costa, V.; Carina, V.; Bellavia, D.; Raimondo, S.; Siragusa, S.; Monteleone, F.; et al. Multiple myeloma-derived extracellular vesicles induce osteoclastogenesis through the activation of the XBP1/IRE1α axis. Cancers 2020, 12, 2167. [Google Scholar] [CrossRef]
- Xu, G.; Liu, K.; Anderson, J.; Patrene, K.; Lentzsch, S.; Roodman, G.D.; Ouyang, H. Expression of XBP1s in bone marrow stromal cells is critical for myeloma cell growth and osteoclast formation. Blood 2012, 119, 4205–4214. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, G. Mesenchymal stem cell migration and tissue repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef] [PubMed]
- De Becker, A.; Van Riet, I. Homing and migration of mesenchymal stromal cells: How to improve the efficacy of cell therapy? World J. Stem Cells 2016, 8, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Urra, H.; Henriquez, D.R.; Cánovas, J.; Villarroel-Campos, D.; Carreras-Sureda, A.; Pulgar, E.; Molina, E.; Hazari, Y.M.; Limia, C.M.; Alvarez-Rojas, S.; et al. IRE1α governs cytoskeleton remodelling and cell migration through a direct interaction with filamin A. Nat. Cell Biol. 2018, 20, 942–953. [Google Scholar] [CrossRef]
- Tigges, U.; Koch, B.; Wissing, J.; Jockusch, B.M.; Ziegler, W.H. The F-actin cross-linking and focal adhesion protein filamin A is a ligand and in vivo substrate for protein kinase Cα. J. Biol. Chem. 2003, 278, 23561–23569. [Google Scholar] [CrossRef]
- Nakamura, F.; Stossel, T.P.; Hartwig, J.H. The filamins: Organizers of cell structure and function. Cell Adhes. Migr. 2011, 5, 160–169. [Google Scholar] [CrossRef]
- Yue, J.; Huhn, S.; Shen, Z. Complex roles of filamin-A mediated cytoskeleton network in cancer progression. Cell Biosci. 2013, 3, 7. [Google Scholar] [CrossRef]
- Szeto, S.G.Y.; Williams, E.C.; Rudner, A.D.; Lee, J.M. Phosphorylation of filamin A by Cdk1 regulates filamin A localization and daughter cell separation. Exp. Cell Res. 2015, 330, 248–266. [Google Scholar] [CrossRef]
- Li, L.; Lu, Y.; Stemmer, P.M.; Chen, F. Filamin A phosphorylation by Akt promotes cell migration in response to arsenic. Oncotarget 2015, 6, 12009–12019. [Google Scholar] [CrossRef]
- Adamik, J.; Roodman, G.D.; Galson, D.L. Epigenetic-Based Mechanisms of Osteoblast Suppression in Multiple Myeloma Bone Disease. JBMR Plus 2019, 3, e10183. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hendrix, A.; Hernot, S.; Lemaire, M.; De Bruyne, E.; Van Valckenborgh, E.; Lahoutte, T.; De Wever, O.; Vanderkerken, K.; Menu, E. Bone Marrow Stromal Cell-Derived Exosomes as Communicators in Drug Resistance in Multiple Myeloma Cells. Blood 2014, 124, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Meads, M.; Gatenby, R.; Dalton, W. Environment-mediated drug resistance: A major contributor to minimal residual disease. Nat. Rev. Cancer 2009, 9, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Bagratuni, T.; Wu, P.; de Castro, D.G.; Davenport, E.L.; Dickens, N.J.; Walker, B.A.; Boyd, K.; Johnson, D.C.; Gregory, W.; Morgan, G.J.; et al. XBP1s levels are implicated in the biology and outcome of myeloma mediating different clinical outcomes to thalidomide-based treatments. Blood 2010, 116, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, S.R.; Fiala, M.A.; Schroeder, M.A.; Wildes, T.M.; Ghobadi, A.; Vij, R.; Stockerl-Goldstein, K.E. Overexpression of IRE1α at Myeloma Diagnosis Is Associated with Decreased Survival While Downregulation of IRE1α Expression Is Predictive of Therapy Resistance. Blood 2019, 134, 4351. [Google Scholar] [CrossRef]
- Vicinanza, C.; Lombardi, E.; Da Ros, F.; Marangon, M.; Durante, C.; Mazzucato, M.; Agostini, F. Modified mesenchymal stem cells in cancer therapy: A smart weapon requiring upgrades for wider clinical applications. World J. Stem Cells 2022, 14, 54–75. [Google Scholar] [CrossRef]
| Patient | Migration | Stage (R-ISS) | Therapy |
|---|---|---|---|
| M1 | ++ | 2 | Yes |
| M2 | = | 3 | No |
| M3 | + | Np before 2016 | Yes |
| M4 | = | 3 | Yes |
| M5 | = | 2 | No |
| M6 | + | 3 | No |
| M7 | ++ | 3 | Yes |
| M8 | + | 2 | Yes |
| M9 | ++ | 3a | No |
| M10 | + | 3 | Yes |
| M11 | + | 3 | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Da Ros, F.; Kowal, K.; Vicinanza, C.; Lombardi, E.; Agostini, F.; Ciancia, R.; Rupolo, M.; Durante, C.; Michieli, M.; Mazzucato, M. IRE1a-Induced FilaminA Phosphorylation Enhances Migration of Mesenchymal Stem Cells Derived from Multiple Myeloma Patients. Cells 2023, 12, 1935. https://doi.org/10.3390/cells12151935
Da Ros F, Kowal K, Vicinanza C, Lombardi E, Agostini F, Ciancia R, Rupolo M, Durante C, Michieli M, Mazzucato M. IRE1a-Induced FilaminA Phosphorylation Enhances Migration of Mesenchymal Stem Cells Derived from Multiple Myeloma Patients. Cells. 2023; 12(15):1935. https://doi.org/10.3390/cells12151935
Chicago/Turabian StyleDa Ros, Francesco, Kinga Kowal, Carla Vicinanza, Elisabetta Lombardi, Francesco Agostini, Rosanna Ciancia, Maurizio Rupolo, Cristina Durante, Mariagrazia Michieli, and Mario Mazzucato. 2023. "IRE1a-Induced FilaminA Phosphorylation Enhances Migration of Mesenchymal Stem Cells Derived from Multiple Myeloma Patients" Cells 12, no. 15: 1935. https://doi.org/10.3390/cells12151935
APA StyleDa Ros, F., Kowal, K., Vicinanza, C., Lombardi, E., Agostini, F., Ciancia, R., Rupolo, M., Durante, C., Michieli, M., & Mazzucato, M. (2023). IRE1a-Induced FilaminA Phosphorylation Enhances Migration of Mesenchymal Stem Cells Derived from Multiple Myeloma Patients. Cells, 12(15), 1935. https://doi.org/10.3390/cells12151935







